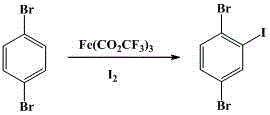Synthesis method for 2,5-dibromo-iodobenzene
A technique for the synthesis of dibromoiodobenzene and its synthesis method, which is applied in the field of preparation of halobenzene compounds and the synthesis of 2,5-dibromoiodobenzene, and can solve problems such as difficult control of monosubstituted products, high production cost, and low yield , to improve iodination reaction activity, avoid ultra-low temperature reaction, and facilitate industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] In a dry 500mL three-neck flask, add 1,4-dibromobenzene (47.2g, 0.2mol) and iron trifluoroacetate (79g, 0.2mol), and dissolve iodine (50.8g, 0.2mol) in 200mL chloroform , slowly added dropwise to the reaction system under reflux conditions, controlled the reaction temperature at 65°C, stirred and reacted for 8 hours, GC detected that the reaction was complete, washed with 200mL saturated aqueous sodium sulfite solution, washed with 500mL water, separated into layers, combined the organic layers, and recovered the solvent under reduced pressure to obtain An off-white solid was recrystallized with 120 mL of isopropanol to obtain 54.6 g of a white solid product, 2,5-dibromoiodobenzene, with a purity greater than 99% (GC), and a yield of 75.4%.
[0013] Product melting point: 38.1-39.6°C, Ms(m / z): 361(M + ).
[0014] 1 HNMR (400MHz, DMSO-d 6 ): 8.127-8.121 (d, 1H; J=2.4Hz), 7.664-7.642 (d, 1H; J=8.8Hz), 7.531-7.504 (dd, 1H; J 1 =8.5Hz;J 2 =2.3Hz).
[0015] 13 CNMR (4...
example 2
[0017] In a dry 2L three-neck flask, add 1,4-dibromobenzene (118g, 0.5mol) and iron trifluoroacetate (197.5g, 0.5mol), and dissolve iodine (94.3g, 0.4mol) in 500mL of chloroform , slowly added dropwise to the reaction system under reflux conditions, the reaction temperature was controlled at 75°C, and after stirring for 10 hours, the reaction was detected by GC. It was washed with 500 mL of saturated aqueous sodium sulfite solution, washed with 2 L of water, separated into layers, combined with the organic layer, and the solvent was recovered under reduced pressure to obtain a The white solid was recrystallized with 250 mL of isopropanol to obtain 139.8 g of the white solid product 2,5-dibromoiodobenzene, with a purity greater than 99% (GC), and a yield of 77.3%.
example 3
[0019] In a dry 1L three-neck flask, add 1,4-dibromobenzene (70.8g, 0.3mol) and iron trifluoroacetate (142.2g, 0.36mol), and dissolve iodine (91.4g, 0.36mol) in 300mL of chloroform , slowly added dropwise to the reaction system under reflux conditions, controlled the reaction temperature at 70°C, stirred and reacted for 10 hours, GC detected that the reaction was complete, washed with 500mL of saturated aqueous sodium sulfite solution, washed with 1L of water, separated into layers, combined the organic layers, and recovered the solvent under reduced pressure to obtain The off-white solid was recrystallized with 150 mL of isopropanol to obtain 82.6 g of a white solid product, 2,5-dibromoiodobenzene, with a purity greater than 99% (GC), and a yield of 76.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com