Synthesis method and purification method of 5-iodo-2-methylbenzimidazole
A technology of methylbenzimidazole and synthesis method, applied in the direction of organic chemistry, can solve the problems of unstable iodine monochloride, cumbersome operation, expensive price, etc., and achieve simple and efficient purification method, good atom economy, and recovery small loss effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
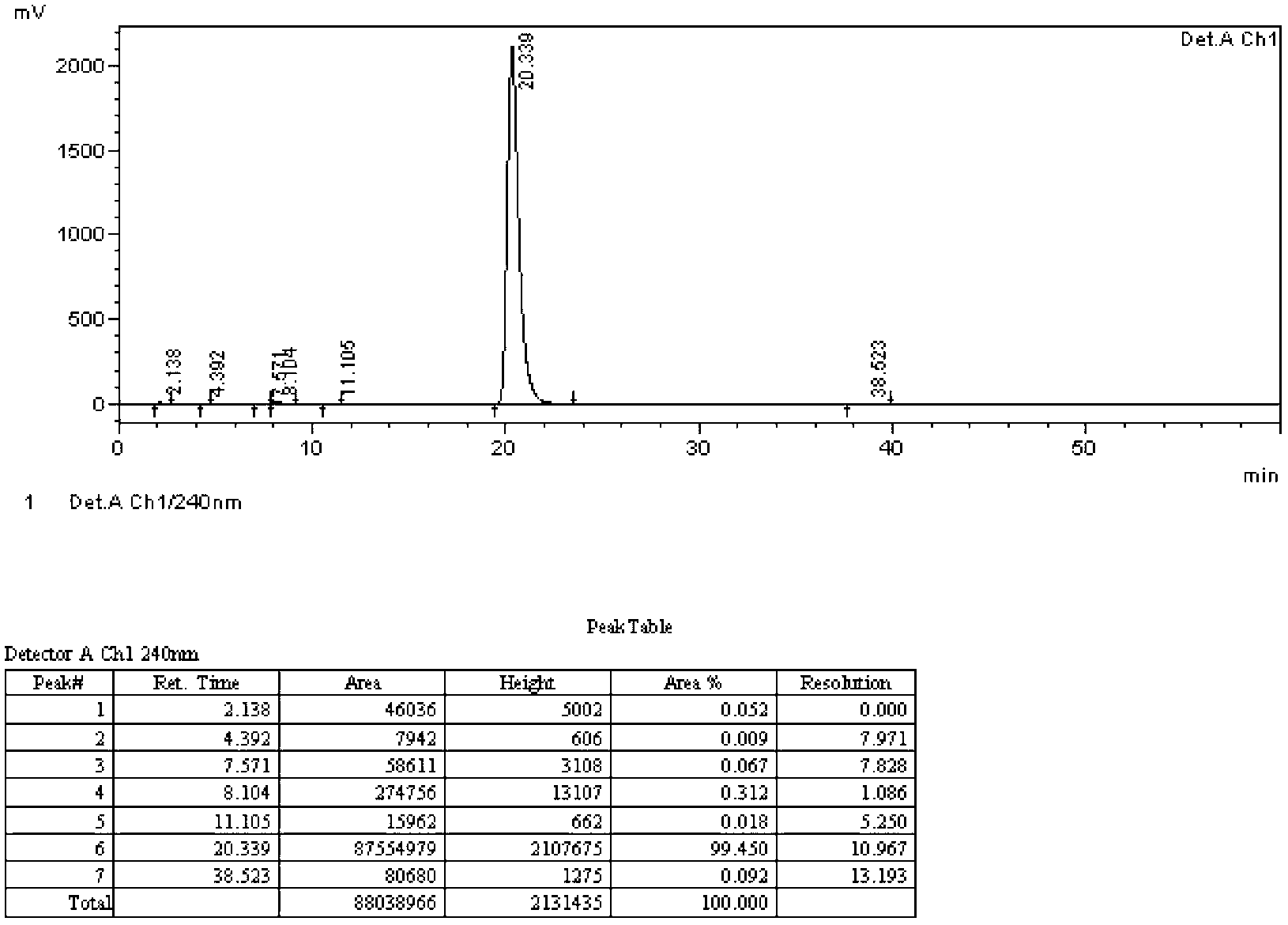
[0066] Iodination reaction: In a 3L four-necked reaction flask, add 720g methanol and 180g water, dropwise add 163g concentrated sulfuric acid, add 150g o-nitroaniline, stir to dissolve, then add 138g iodine, stir to dissolve, and control the temperature at 35°C Add 615g of 30% hydrogen peroxide dropwise, continue to stir at 35°C for 5 hours after the dropwise completion, add 633g of water after the reaction, cool to 5°C, filter with suction, wash the solid with 75g of 5% sodium thiosulfate aqueous solution, and dry in vacuo at 60°C. Obtain 275.3 grams of 4-iodo-2-nitroaniline shown in formula B, such as figure 1 As shown, reddish-brown crystals, yield 96.0%, HPLC purity 99.4%. 1 H-NMR (such as Figure 4 , CDCl 3 ,500MHz,δ5.4(s,br,2H),6.6(d,J=8.5Hz,1H),7.6(dd,J=8.5Hz,2.0Hz,1H),8.4(d,J=2.0Hz, 1H)).
[0067] Reduction reaction: In a 500mL pressure vessel, add 150g methanol, 30g 4-iodo-2-nitroaniline and 0.6g Raney nickel, remove the air, replace with hydrogen until the press...
Embodiment 2
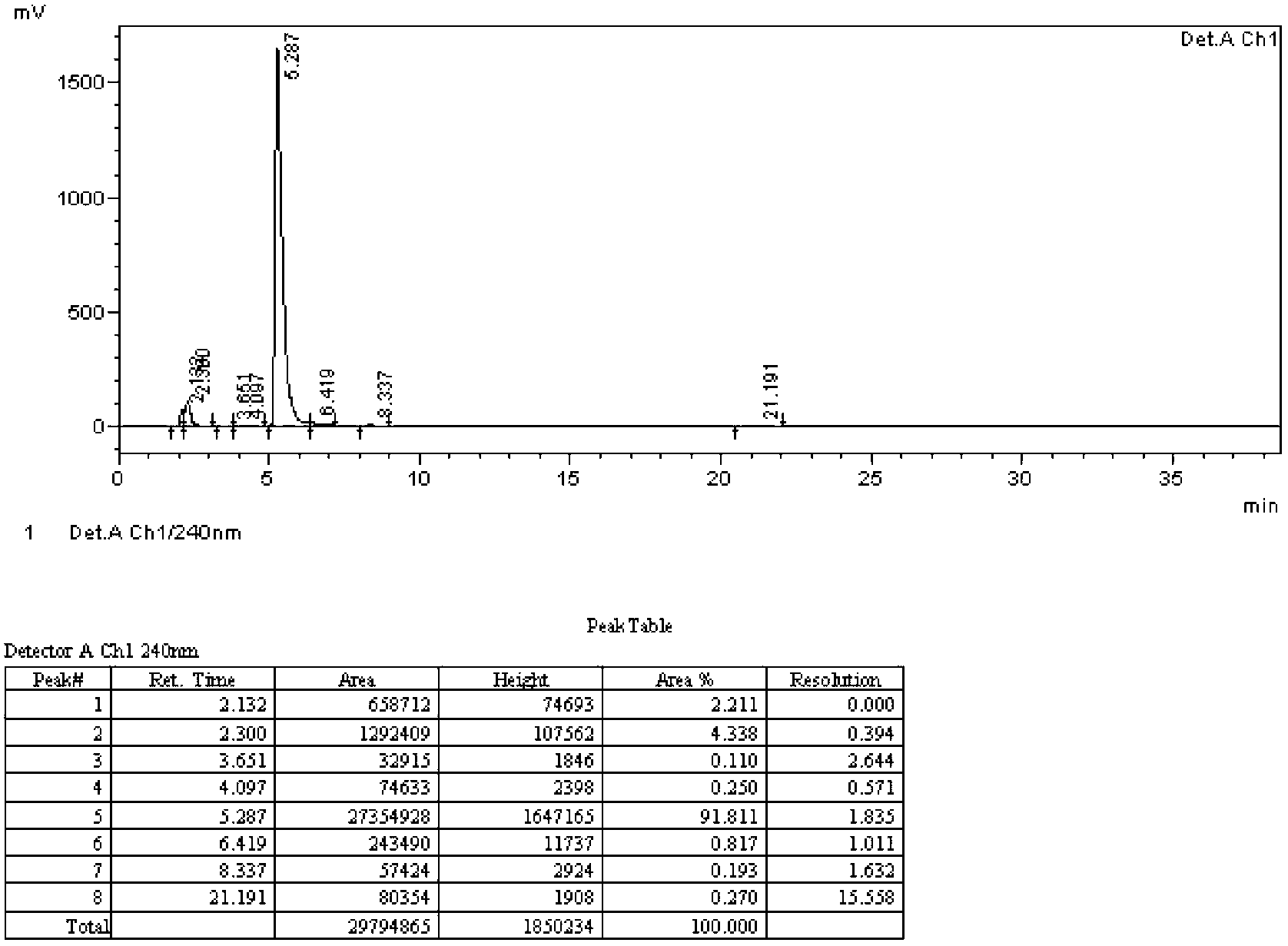
[0070] Iodination reaction: In a 3L four-necked reaction flask, add 900g methanol and 600g water, dropwise add 326g concentrated sulfuric acid, add 150g o-nitroaniline, stir to dissolve, then add 165g iodine, stir to dissolve, and control the temperature at 0°C Add 25g of 30% hydrogen peroxide dropwise, and continue to stir at 0°C for 12 hours after the dropwise completion. After the reaction, add 633g of water, filter with suction, wash the solid with 750g of 5% sodium sulfite aqueous solution, and dry it in vacuum at 60°C to obtain 271.0g as shown in formula B. 4-iodo-2-nitroaniline, reddish-brown crystals, yield 94.5%, HPLC purity 99.2%.
[0071] Reduction reaction: In a 500mL pressure vessel, add 300g methanol, 30g 4-iodo-2-nitroaniline and 4.5g Raney nickel, remove the air, replace with hydrogen until the pressure reaches 0.01MPa, control the reaction temperature at 35°C, Keep the reaction for 8 hours. After the reaction, cool down to 10° C., replace with nitrogen, and fi...
Embodiment 3
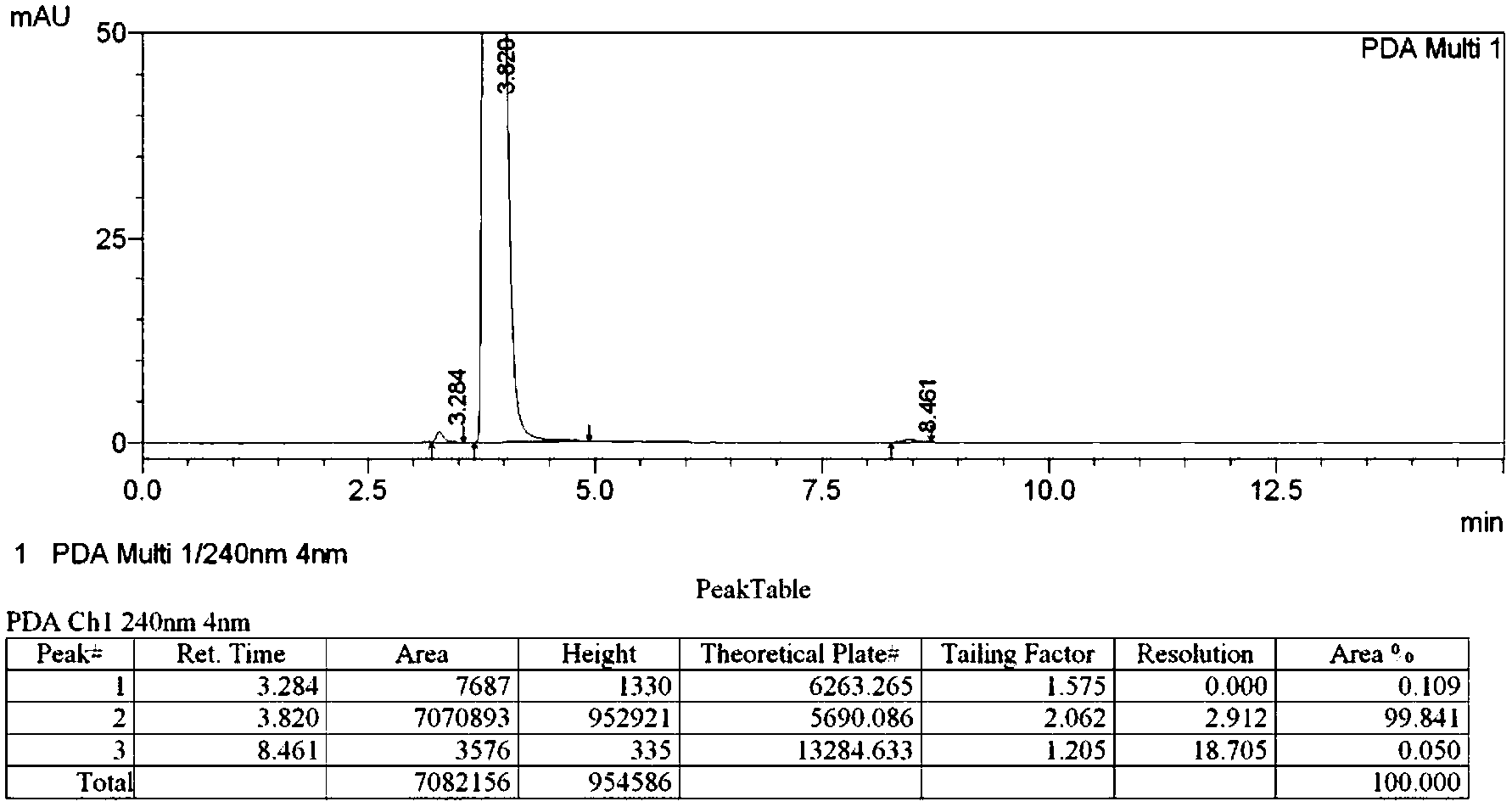
[0074] Iodination reaction: In a 2L four-necked reaction flask, add 321g ethanol and 54g water, add 11g concentrated sulfuric acid dropwise, add 150g o-nitroaniline after dropping, stir to dissolve, then add 152g iodine, stir to dissolve, and control the temperature at 60°C Add 250g of 30% hydrogen peroxide dropwise, and continue to stir at 60°C for 3 hours after the dropwise completion. After the reaction, add 633g of water, cool to 5°C, filter with suction, wash the solid with 375g of 5% aqueous sodium sulfite, and dry it in vacuum at 60°C to obtain 272.4g of 4-iodo-2-nitroaniline represented by formula B, reddish-brown crystal, yield 95.0%, HPLC purity 99.2%.
[0075] Reduction reaction: In a 500mL pressure vessel, add 240g ethanol, 30g 4-iodo-2-nitroaniline and 9g Raney nickel, remove air, replace with hydrogen until the pressure reaches 0.3MPa, control the reaction temperature at 0°C, and keep warm The reaction was carried out for 12 hours. After the reaction was complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com