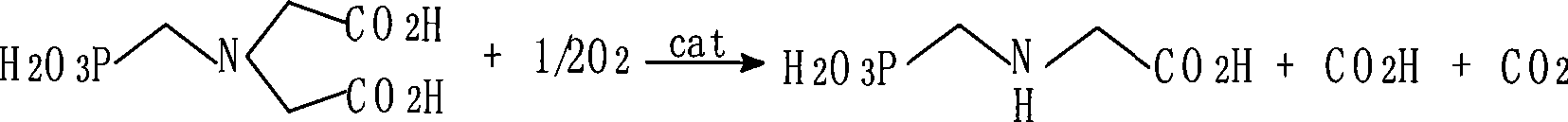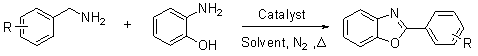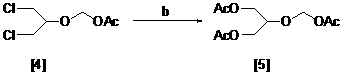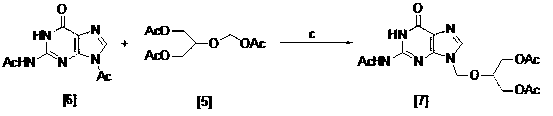Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1239results about How to "Atom economy is high" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
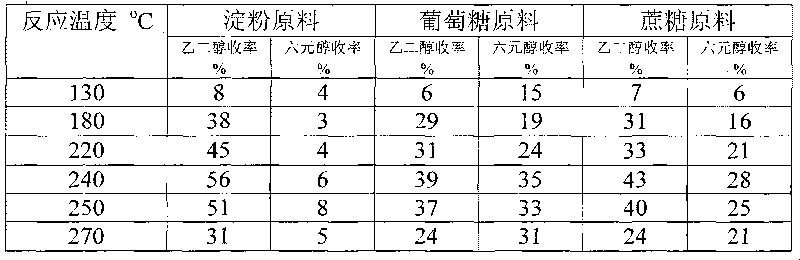
Method for preparing ethanediol from polyhydroxy compounds
ActiveCN101735014ARaw material resources are renewableMeet the requirements of sustainable developmentOrganic compound preparationCatalyst activation/preparationHydrogen pressureCobalt
The invention provides a method for preparing ethanediol from polyhydroxy compounds comprising starch, hemicellulose, cane sugar, glucose, fructose and fructosan. The method comprises the following steps of: taking the polyhydroxy compounds as reaction raw materials, and taking metals, carbides, nitrides, and phosphides of transition metals in families VIII, IX and X, such as ferrum, cobalt, nickel, ruthenium, rhodium, palladium, iridium, platinum, molybdenum and tungsten as catalytic active components to form a polymetallic catalyst; and performing further catalytic conversion under a hydrothermal condition that the temperature is 120 to 300 DEG C and the hydrogen pressure is 1 to 13 MPa to prepare the ethanediol from the polyhydroxy compounds with high efficiency, high selectivity and high yield. The method for preparing the ethanediol from the polyhydroxy compounds has the outstanding advantages of renewable raw materials, environment-friendly reaction process, and atom economical efficiency. Simultaneously, compared with other techniques taking biomasses as the raw materials, the method has the advantages of simple process and high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing ethylene glycol from cellulose
ActiveCN101723802ALow costWide variety of sourcesOrganic compound preparationCatalyst activation/preparationHydrogen pressurePolyethylene glycol
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
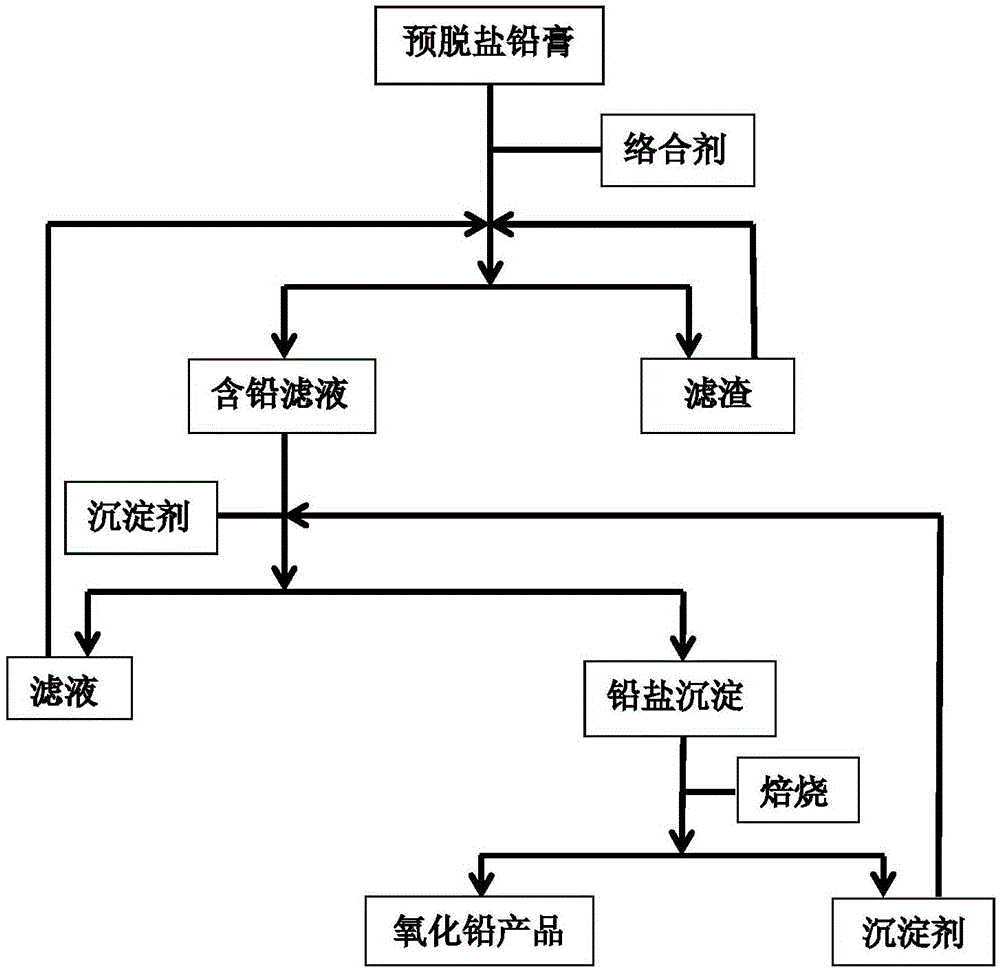
Method for recovering lead oxide from waste lead plaster
ActiveCN104141045AAtom economy is highIn line with the principles of atom economyLead-acid accumulatorsLead monoxideLead saltLead oxide
The invention provides a method for recovering lead oxide from waste lead plaster. The method comprises the following steps: a, dissolving pre-desalted lead plaster by using a complexing agent solution, and reacting all PbOs with a complexing agent to generate complexing ions so as to obtain a lead-containing solution and filter residues; b, adding a precipitating agent into the lead-containing solution, reacting the precipitating agent with the lead complexing ions to generate lead salt precipitation and the regenerated complexing agent; and c, roasting the lead salt precipitation to obtain lead oxide, and regenerating the precipitating agent. The method can be widely suitable for lead plaster formed by mixing various sources, the process conditions are relatively mild, a process is relatively environment-friendly, and the final recovery rate of lead oxide can reach more than 99%, so that the method has very high application values in the industry of recovery treatment of waste lead-acid batteries.
Owner:BEIJING UNIV OF CHEM TECH
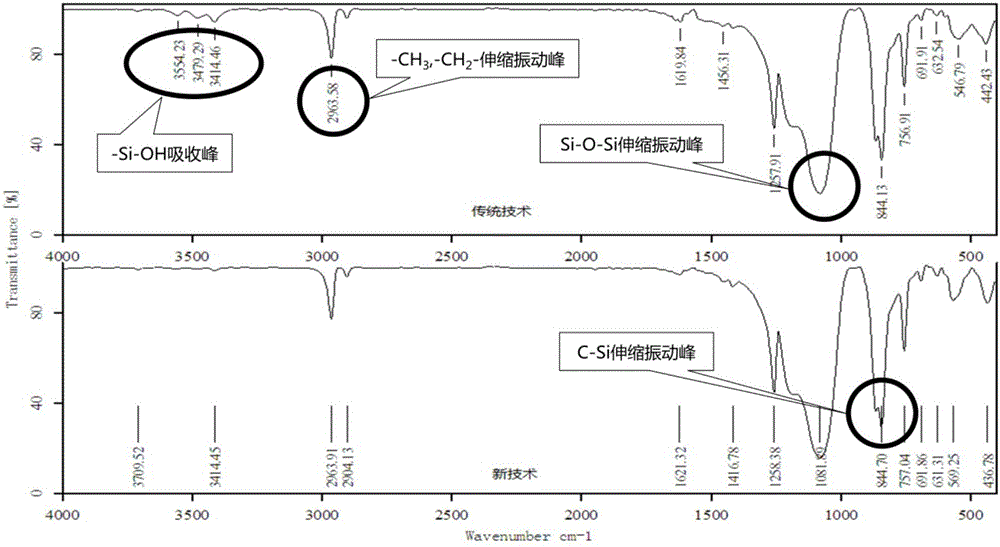
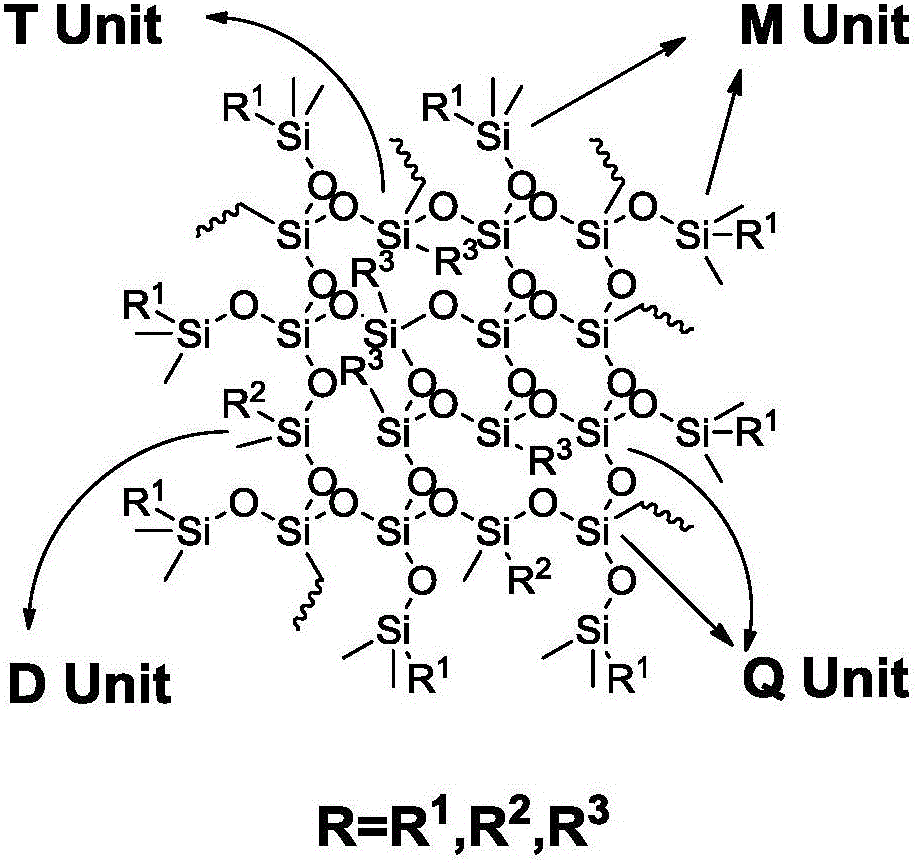
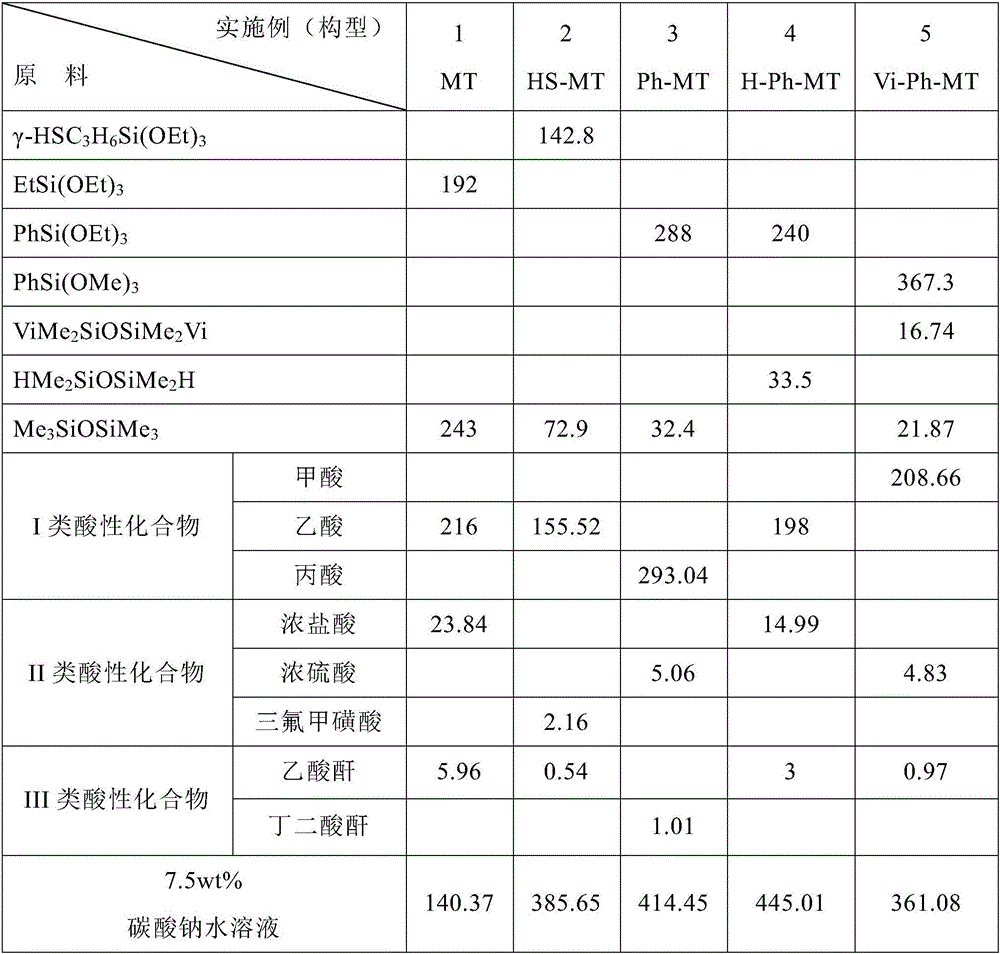
Preparation method of organopolysiloxane resin
The invention discloses a preparation method of organopolysiloxane resin. The preparation method includes the steps of (1), taking alkoxyorganosilane, an end-capping reagent and an acidic compound as preparation raw materials; (2), feeding the preparation raw materials with uniform stirring, wherein the feeding molar ratio of the end-capping reagent to the alkoxyorganosilane to the acidic compound is (0-40):1:(0.2-5); (3), adjusting the temperature at 40-150 DEG C at which condensation polymerization reaction is conducted for 2-20 hours; (4), terminating the condensation polymerization reaction, adjusting a reaction mixture to be neutral, and purifying a reaction product so as to obtain the organopolysiloxane resin. The preparation method of the organopolysiloxane resin has the advantages of environmental protection and no pollution during production, low energy consumption, short period, high product yield and the like and is more excellent in performance.
Owner:GUANGDONG BIOMAX SIANDF NEW MATERIAL CO LTD
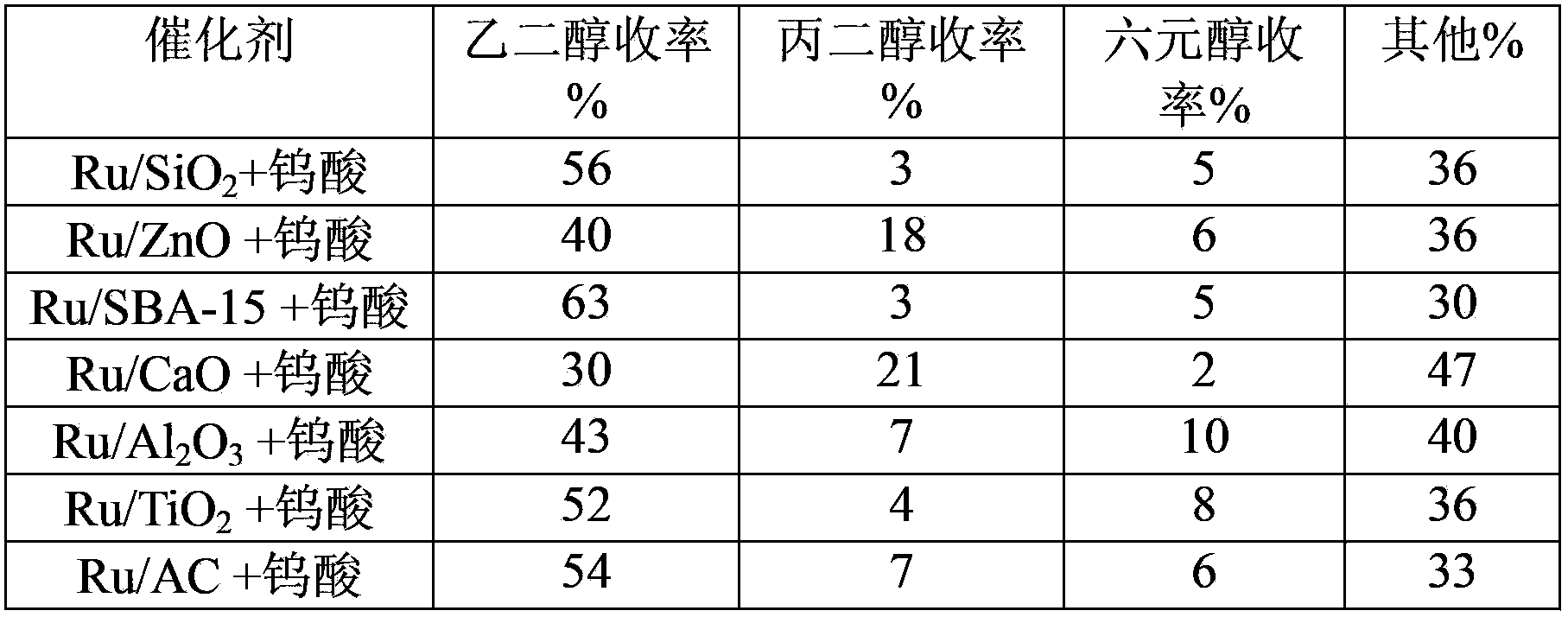
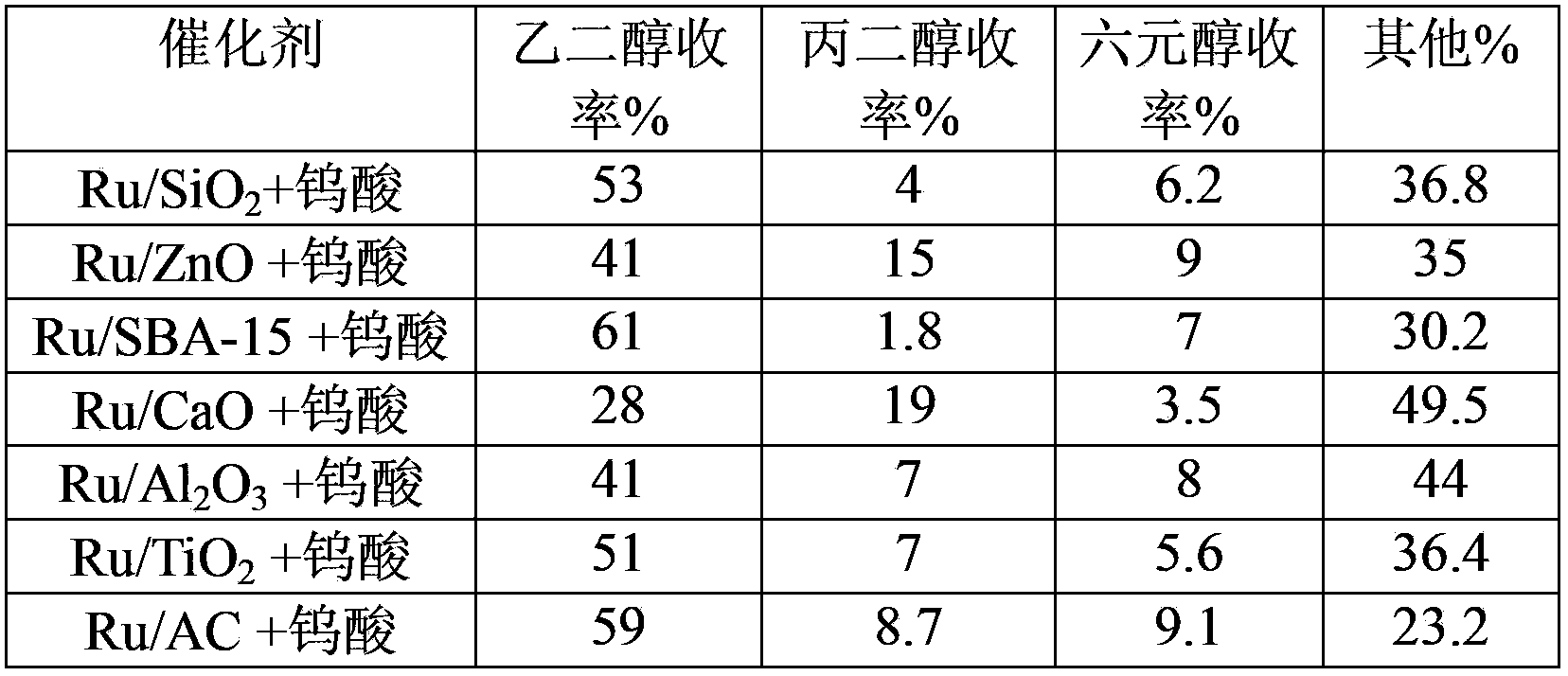
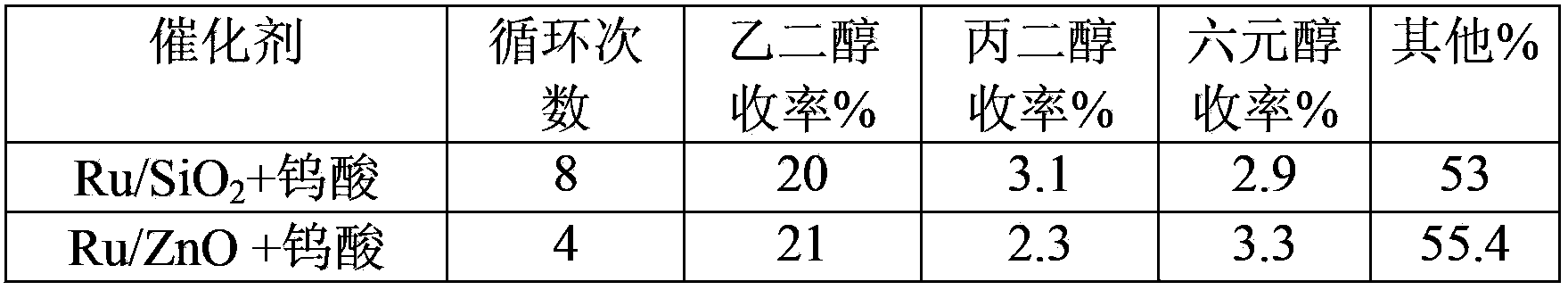
Method of low metal loading catalyst for preparing glycol from carbohydrate
ActiveCN103420797ARaw material resources are renewableMeet the requirements of sustainable developmentMolecular sieve catalystsOrganic compound preparationHydrogen pressureInorganic compound
The invention provides a method of applying a low metal loading catalyst for preparing glycol and propylene glycol from carbohydrate, including cellulose, starch, semi-cellulose, cane sugar, glucose, fructose, fructosan, xylose, and soluble xylo oligosaccharide. In the method, carbohydrate is taken as the raw material, the compound catalyst is composed of catalytic active components selected from one or more components from following components: highly-disperse and low-loading ruthenium, inorganic compounds, organic compounds, and complex of tungsten, or simple substance tungsten, then one-step catalytic conversion process is carried out under the hydrothermal conditions: temperature of 60 to 350 DEG C, and hydrogen pressure 0.1 to 15 MPa, and the high-efficient, high-selective and high yield preparation of glycol and propylene glycol from carbohydrate is achieved. The method takes highly-disperse and high stability low loading Ru-based catalyst as the reaction catalyst, so the usage amount of value metals is reduced, the loss of catalyst carrier is slowed down, and the recycle rate of Ru-base catalyst is increased. The catalyst has the prominent advantages of high activity, high selectivity, and very high cyclicity. Compared to other technologies, which prepare polyol from carbohydrate, the method has the advantages of simple reaction process, high efficiency, good stability of catalyst, and multi-circulation, and has very vast industrial application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
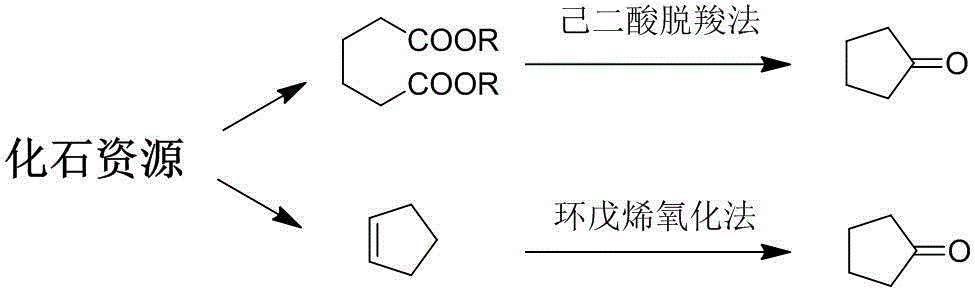
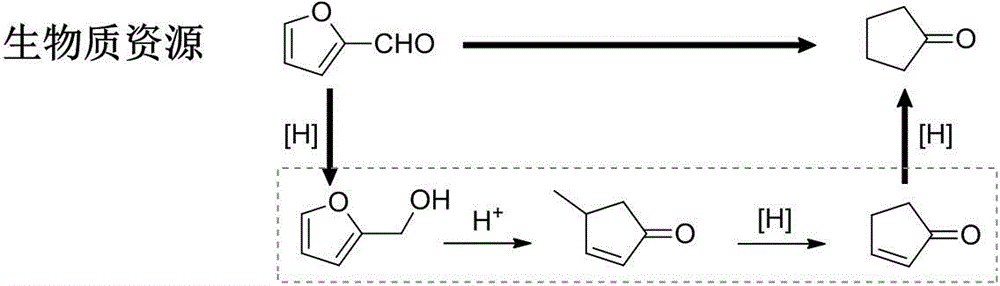
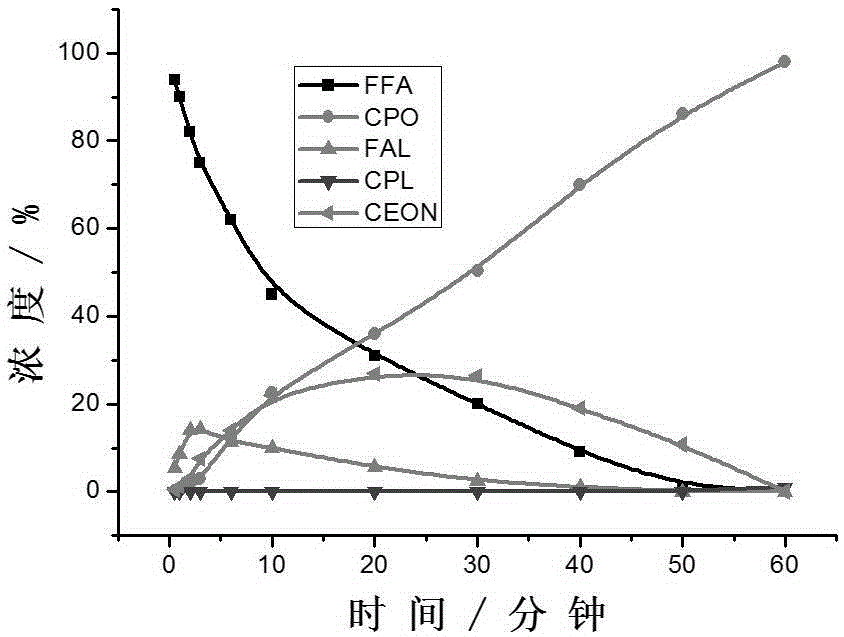
Method for preparing cyclopentanone by taking biomass resource as raw material
InactiveCN105330523AReduce yieldFew stepsMolecular sieve catalystsChemical industryFixed bedFurfural
The invention belongs to the technical field of biomass conversion utilization, and concretely relates to a method for preparing cyclopentanone by taking biomass resource as a raw material. The method comprises taking furfural coming from biomass as the raw material, taking a hydrogen-containing gas as a reducing agent, and in the presence of a metal supported type catalyst, performing hydrogenation rearrangement reaction in a high-pressure reaction kettle or a fixed bed reactor, so as to obtain cyclopentanone in one step, wherein the carrier of the metal supported type catalyst is selected from Nb2O5, H-ZSM-5 molecular sieve, HY molecular sieve, Fe2O3, ZrO2, Al2O3, SiO2, CeO2, MgO, active carbon, and TiO2 in various crystal forms, the active composition is selected from Au, Pt, Ru, Rh, Pd, Ir, Ni and Cu, and the active composition load capacity is 0.1-5% of the catalyst. The method is mild in reaction technological conditions, and cheap and easily-available in raw materials, is capable of realizing quantitative conversion of furfural to cyclopentanone, and belongs to an environment-friendly green chemical technology.
Owner:FUDAN UNIV
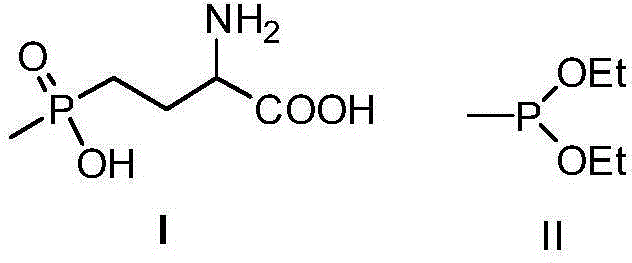
Recycling process of sodium tetrachloroaluminate in phosphinothricin production
ActiveCN105217667ASolve processing problemsReduce pollutionAluminium chloridesWater/sewage treatment by flocculation/precipitationAluminium chlorohydrateSewage treatment
The invention discloses a recycling process of sodium tetrachloroaluminate in phosphinothricin production. The process comprises the following steps that 1, mismatching and precipitation are carried out; 2, solids are filtered; 3, cooling precipitation is carried out; 4, reutilization is carried out; 5, cyclic utilization is carried out; 6, aluminum polychlorid is prepared. Sodium tetrachloroaluminate solid waste is changed into treasure, alchlor obtained after mismatching is recycled, the problem of treatment of sodium tetrachloroaluminate solid waste is solved, pollution to environment is reduced, no three waste is discharged, the atom economy of a reaction is improved, and large-scale production of phosphinothricin is facilitated. A mismatching agent and a poor solvent adopted in the process are low in price and easy to obtain, the whole process is easy and convenient to operate, and especially suitable for industrial production. Further prepared polymerization alchlor can be adopted as a water purifying agent, and is wide in application range and especially suitable for sewage treatment of chemical plants.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method of CF2O-contained monomer liquid crystal compound
ActiveCN103980098AHigh purityQuality improvementLiquid crystal compositionsOrganic compound preparationSolventCopper salt
The invention provides a preparation method of a CF2O-contained monomer liquid crystal compound. The preparation method comprises the following steps of (1) with a compound with a structure as shown in the formula I as a raw material, reacting under the catalytic action of a zinc reagent and copper salt and in the existence of a solvent to generate a compound with a structure as shown in the formula II; (2) enabling the compound with the structure as shown in the formula II and a fluorinated reagent to be subjected to fluoridation in the existence of the solvent to generate a compound with a structure as shown in the formula III; (3) enabling the compound with the structure as shown in the formula III and boric acid as shown in the formula IV to be subjected to SUZUKI coupled reaction in the existence of the solvent to obtain the CF2O-contained monomer liquid crystal compound with a structure as shown in the formula V, wherein the CF2O-contained monomer liquid crystal compound with a structure as shown in the formula V is as shown in the specification.
Owner:SHANGHAI CHEMSPEC CORP +1
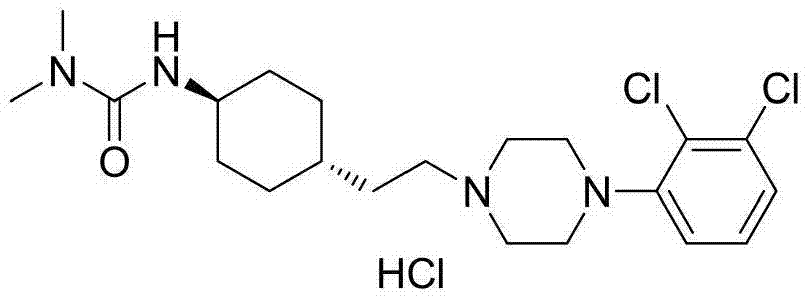
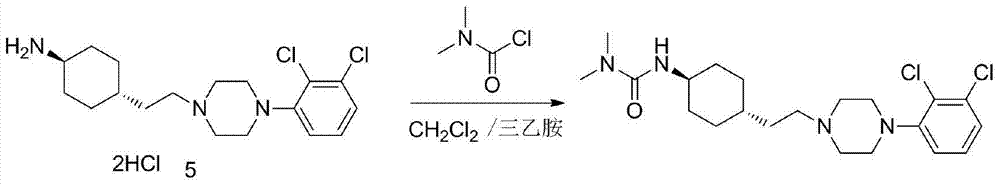
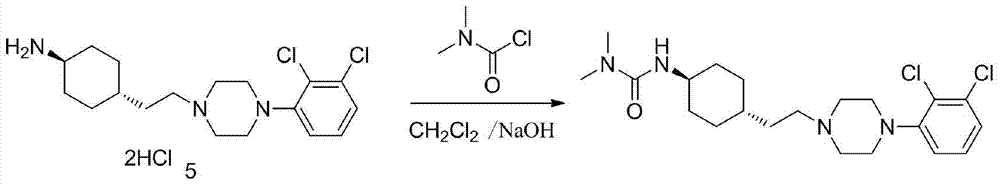
3-cyclohexyl-1,1-dimethylurea compound as well as preparation method and application thereof
ActiveCN104496854AAtom economy is highHigh purityUrea derivatives preparationOrganic compound preparationCariprazineDimethylurea
The invention discloses a 3-cyclohexyl-1,1-dimethylurea compound as well as a preparation method and application thereof. The 3-cyclohexyl-1,1-dimethylurea compound is used for preparing an antischizophrenic drug cariprazine. Compared with the prior art and report literatures, the preparation method of the 3-cyclohexyl-1,1-dimethylurea compound has the remarkable advantages of being free of removal of protecting groups such as Boc group, high in atom economy, low in cost and easy in getting of raw materials, mild in reaction condition, stable in yield, simple and convenient to operate, controllable in product quality, high in product purity, less in three waste pollution and easy to produce industrially. The structure formula of the 3-cyclohexyl-1,1-dimethylurea compound is as shown in (I) in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Allantoin synthesizing process
The invention relates to a new technique to prepare allantoin, in a stirring state, adding hydrogen peroxide solution containing a proper amount of metallic salt catalyst or resin loading metallic salt catalyst to oxalic dialdehyde water solution, to oxidize the oxalic dialdehyde into glyoxalic acid; adding a proper amount of raw material urea to the obtained glyoxalic acid solution and completing catalytic condensation reaction under the condition of heating and stirring; filtering and recovering the catalyst, cooling, pump-filtering, washing, recrystallizing and drying to obtain the finished product allantoin. It has larger yield at 56% or so; reduces the urea dosage; the new catalyst is simple to prepare and has high catalytic activity, able to be repeatedly used for 10 times above; it is a new green technique.
Owner:NINGXIA UNIVERSITY
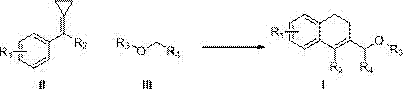
Synthetic method for aromatic[a]carbazole compounds
The invention discloses a synthetic method for aromatic[a]carbazole compounds, belonging to the technical field of organic synthesis. According to a technical scheme in the invention, the synthetic method comprises the following steps: dissolving a 2-phenylindole compound, 2-(naphth-1-yl)indole compound or 2-(thiazol-1-yl)indole compound and a diazo compound in a solvent together; then adding a catalyst and an additive; and carrying out a reaction at 100 to 140 DEG C so as to prepare a benzo[a]carbazole compound, naphtho[a]carbazole compound or thiazolo[a]carbazole compound. According to the invention, 2-substituted indole compounds and the diazo compound are used as raw materials and subjected to a one-pot cascade reaction so as to directly obtain the aromatic[a]carbazole compounds; and the synthetic method is simple and convenient in process, mild in conditions, wide in a substrate adaptability range and suitable for industrial production.
Owner:HENAN NORMAL UNIV
Method for preparing glyphosate
ActiveCN101531677AReduce manufacturing costEliminate generationBiocidePhysical/chemical process catalystsHigh concentrationIminodiacetic acid
The invention discloses a method for preparing glyphosate. N- (Phosphonomethyl) iminodiacetic acid is in the oxygenation reaction with oxygen in the water medium in the presence of catalyzer such as activated carbon, the solid-liquid separation is performed after the reaction to obtain the crystal with glyphosate and the filter cake with activated carbon, the reaction mother liquor is in the later process; the filter cake is added in the glyphosate saturated solution, after heating and dissolving the activated carbon is separated from the glyphosate solution, the glyphosate solution is cooled and crystallized, after separation the solid glyphosate product and the filter solution are obtained, the filter cake is made into the glyphosate saturated solution; the reaction mother liquor passes through a barrier separation device, the glyphosate in the mother liquor is separated from the by-product such as formaldehyde, the glyphosate mother liquor is recycled in the oxygenation reaction, or used as the glyphosate saturated solution. The invention provides the low-cost method for preparing glyphosate by the catalytic oxidation of high-concentration N- (Phosphonomethyl) iminodiacetic acid by the cyclic utilization of the reaction mother liquor, improving the yield of solid glyphosate and solving the environmental protection problem caused by the byproduct such as formaldehyde.
Owner:捷马化工股份有限公司
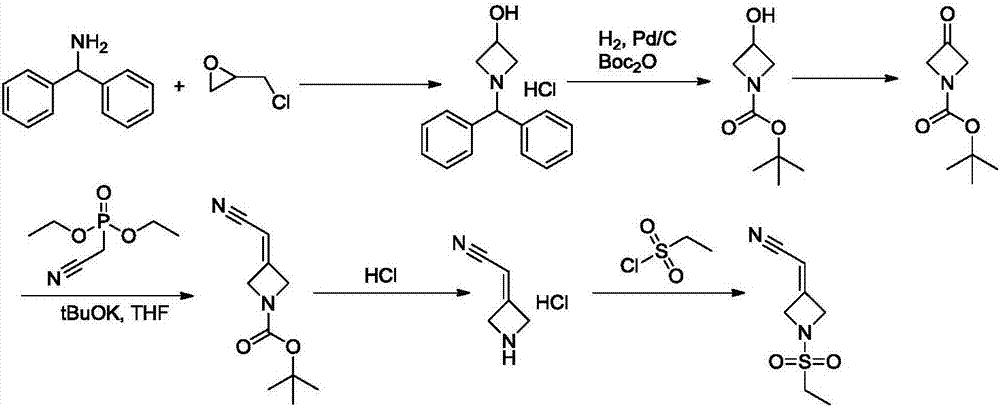
New synthesis methods of JCK inhibitor baricitinib and intermediate thereof
ActiveCN106946917AImprove efficiencyAtom economy is highGroup 3/13 element organic compoundsBulk chemical productionWittig reactionSynthesis methods
The present invention provides a new synthesis method of a baricitinib compound 11. According to the present invention, by using a compound 1 as a staring raw material, the amino group is protected by directly using ethanesulfonyl chloride, and direct cyclization is directly performed by using the effect of an alkali to obtain a key intermediate compound 3 so as to avoid the use of other protection groups and substantially improve the route efficiency and the atomic economy; during the compound 5 preparation, a Wittig reaction is performed by using triphenylphosphine acetonitrile so as to avoid the use of strong alkali and improve the reaction yield; the completely-new neopentyl glycol borate derivative compound 8 has good stability and good crystallinity so as to simplify the separation and purification process; and the route is simple to operate and has the high yield, the purity of the obtained product is high, and the synthesis method is suitable for amplification production. The formulas 1-11 are defined in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Method for preparing 2-substituted benzoxazole compound
The invention discloses a method for preparing a 2-substituted benzoxazole compound. According to the method, a benzylamine compound or a benzaldehyde compound or a benzyl alcohol compound, and o-toluidine-N-methyl-o-phenylenediamine, ortho-aminophenol and o-aminobenzenethiol serve as raw materials, metals palladium, platinum or ruthenium serves as a catalyst, and N,N-dimethylformamide, N,N-dimethylacetamide or N-methylpyrrolidone serves as a solvent. The method comprises the following preparation steps: (1) mixing the raw materials; (2) reacting; (3) separating and extracting; and (4) drying and concentrating. An oxidant and a hydrogen acceptor are not required in the whole preparation process, and the used partial catalysts can be recycled. The method is high in atom economy, simple in aftertreatment and mild in reaction conditions and has a certain industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
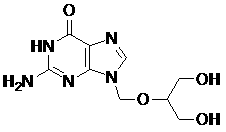
Method for preparing ganciclovir
The invention relates to a method for preparing ganciclovir. The method comprises the following steps of: a, adding paraformaldehyde into 1,3-dichloro-2-propanol [2], reacting under the action of a catalyst to obtain hemiformal [3], and reacting with an acetic anhydride to obtain 1,3-dichloro-2-acetoxylmethoxylpropane [4]; b, making 1,3-dichloro-2-acetoxylmethoxylpropane [4] react with absolute postassium acetate or anhydrous sodium acetate in an organic solvent medium in the presence of a tetraalkyl ammonium bromide catalyst with 1-20 carbon atoms and an acetic anhydride serving as a dehydrating agent to obtain 1,3-diacetoxy-2-acetoxymethoxyl propane [5]; c, performing a condensation reaction on 1,3-diacetoxy-2-acetoxymethoxyl propane [5] and 2,9-diacetyl guanine [6] in an organic solvent medium in the presence of a catalyst and an acetic anhydride serving as a dehydrating agent to obtain triacetyl ganciclovir [7]; and d, hydrolyzing the triacetyl ganciclovir [7] to obtain ganciclovir [1]. The method has the advantages of easy and controllable preparation process, high utilization ratios of raw materials, low cost and high yield of a prepared ganciclovir product.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
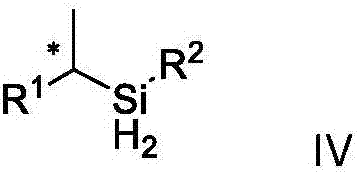
Chirality dihydrogen silane compound and synthetic method and application thereof
ActiveCN107235995AHigh selectivityHigh efficiency and high selectivitySilicon organic compoundsPreparation by oxidation reactionsSilanesEnantio selectivity
The invention discloses a chirality dihydrogen silane compound. The chirality dihydrogen silane compound is as shown in the formula IV. In the formula IV, X represents a chiral carbon atom. The invention further discloses a synthetic method for the chirality dihydrogen silane compound. The method comprises the following steps: using olefin shown in the formula I and silane shown in the formula II as raw materials, and using a chiral CoX2-OIP complex compound as a catalyst, in the existence of a reducing agent, reacting to obtain the chirality dihydrogen silane compound shown in the formula IV. The synthetic method is suitable for different types of the olefins, the reaction condition is moderate, the operation is simple and convenient, and the atomic economy is high. The reaction does not need to be added with any other toxic transition metal ions, the reaction yield is better and is 53%-97% generally, and the enantio-selectivity is higher and is 81%-99% and gt generally. The provided chirality dihydrogen silane compound shown in the formula IV can be used for synthesizing a chiral alcohol compound, a chiral silicon alcohol compound, a chiral polysubstituted silane compound and so on.
Owner:ZHEJIANG UNIV
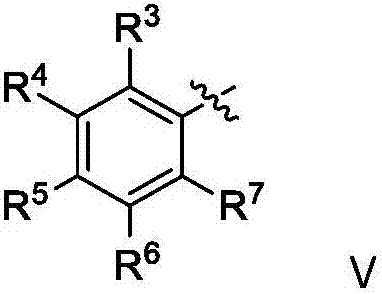
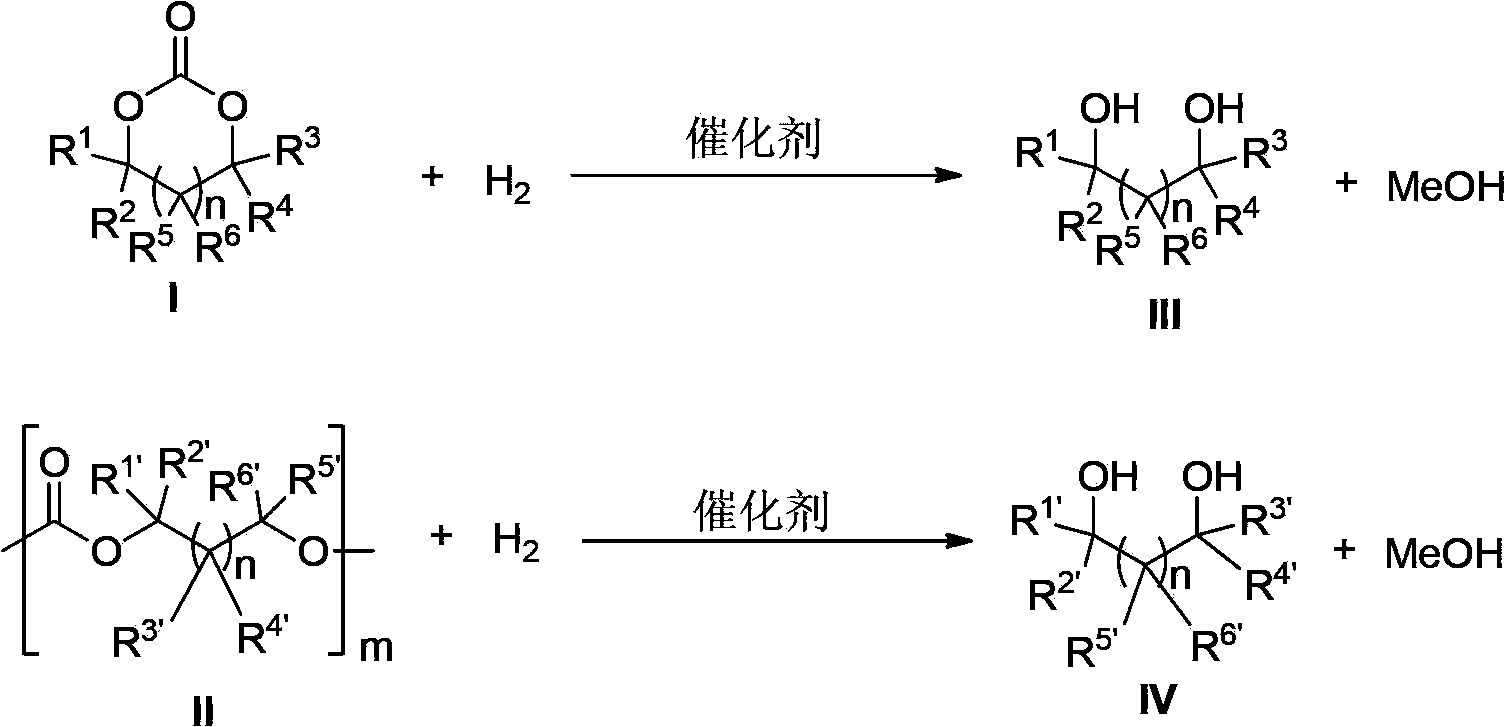
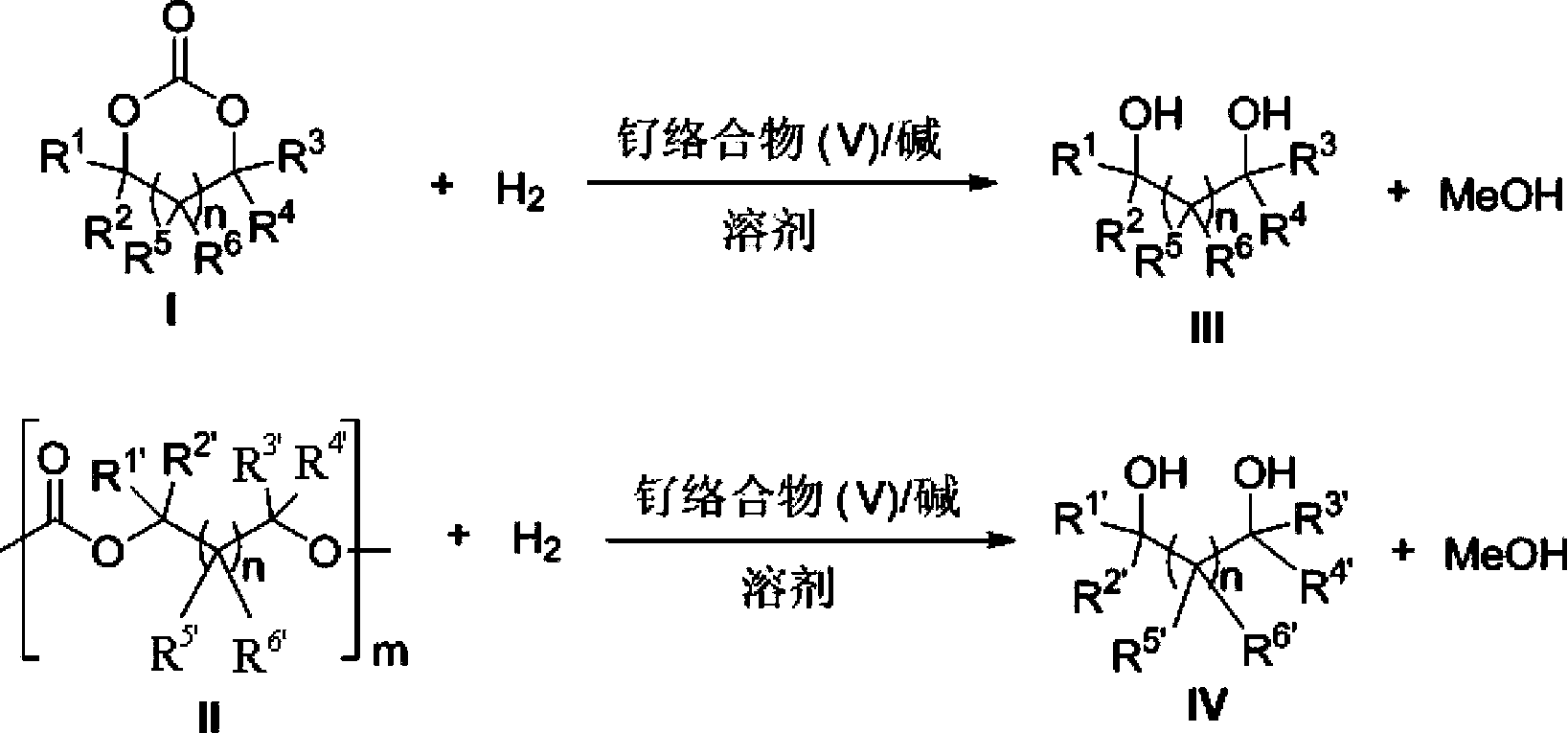
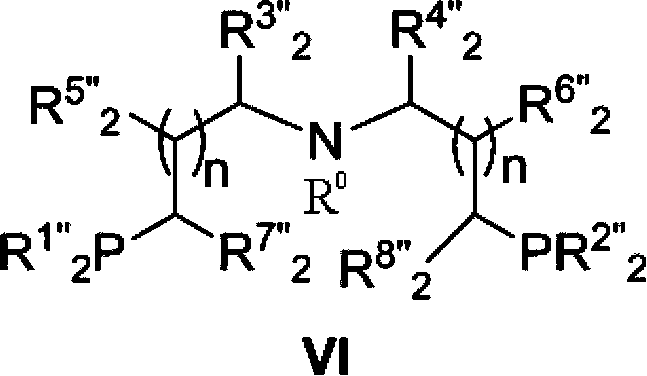
Novel ruthenium complex and method for preparing methanol and diol
ActiveCN103772142AFacilitate large-scale industrial productionImprove efficiencyRuthenium organic compoundsOrganic compound preparationOrganic solventHydrogen atmosphere
The invention provides a method for preparing methanol and diol from cyclic carbonates and polycarbonates. The method comprises: in hydrogen atmosphere, in an organic solvent, and in the presence of a ruthenium complex (Ru(L)XYY') and an alkali, performing hydrogenation reduction reaction on a cyclic carbonate or a polycarbonate, so as to obtain methanol and diol, wherein all groups in the formula are defined in the specification. The invention also provides the ruthenium complex formed by ruthenium and a tridentate amino diphosphine ligand. The invention also provides a method for preparing deuterated methanol and deuterated diol by employing the above preparation method. The method provided by the invention is high in efficiency, high in selectivity, economic, environment-friendly, and simple for operation, can be performed under mild conditions, and has complete atom economy.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
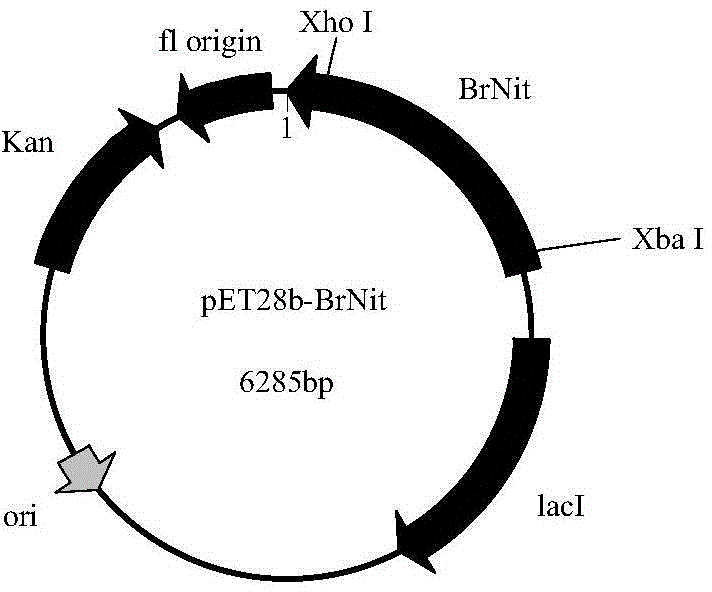
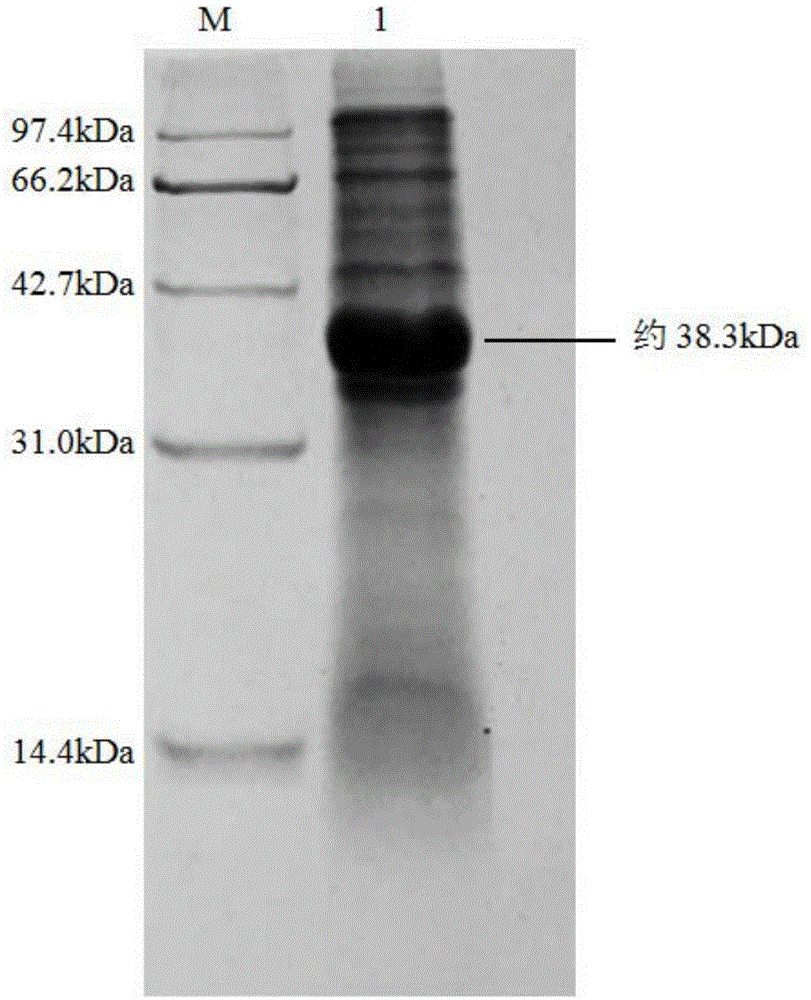
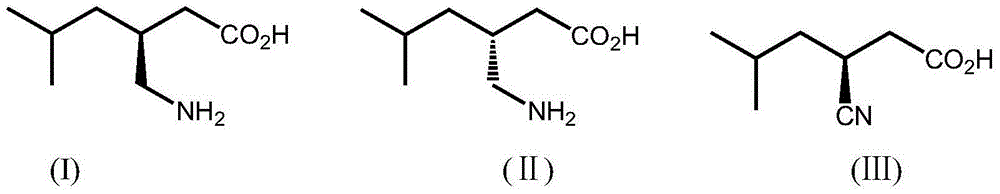
Nitrilase, encoding genes, carrier and application
InactiveCN104962540AAtom economy is highHigh potential for industrial applicationHydrolasesFermentationBrassica rapaBirdsrape Mustard
The invention discloses nitrilase from brassica rapa, encoding genes and an application of the encoding genes to the preparation of pregabalin chiral intermediate (S)-3-cyan-5-methylhexanoic acid through biocatalysis, and an amino acid sequence of the nitrilase is shown as SEQ ID NO:1. The invention provides novel nitrilase for preparing the (S)-3-cyan-5-methylhexanoic acid with high region and high stereoselectivity through hydrolysis, the concentration of hydrolysis substrates of the nitrilase can reach more than 1 M, and an ee (enantiomeric excess) value is kept more than 99 percent. The pregabalin important chiral intermediate (S)-3-cyan-5-methylhexanoic acid can be prepared through the nitrilase; a preparation method of the (S)-3-cyan-5-methylhexanoic acid has the advantages of high atom economy, mild conditions, environmental friendliness and the like and has high industrial application potential.
Owner:ZHEJIANG UNIV OF TECH
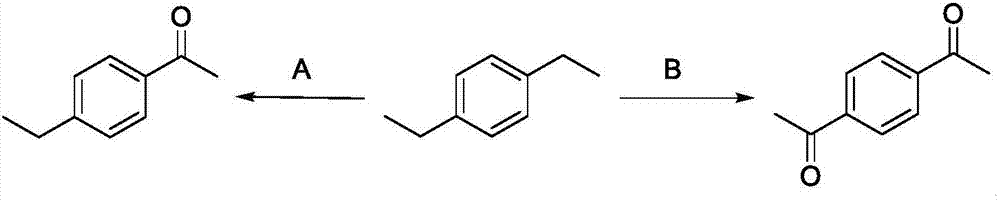
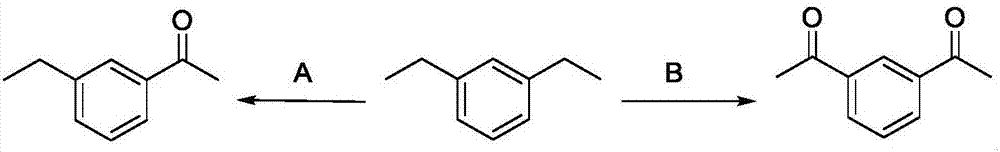
Method for directly oxidizing benzyl-position C-H bond into ketone
ActiveCN107011133AEfficient synthesisAtom economy is highCarboxylic acid nitrile preparationOrganic compound preparationSide chainEthyl group
The invention discloses a method for directly oxidizing a benzyl-position C-H bond into ketone, wherein aryl ethyl compounds are catalyzed and oxidized by nitrite ester; a synergistic catalytic system of free radical initiator and nitrite ester is adopted, and a catalytic system of non-metallic catalyst and oxygen is adopted, the oxidization of the C-H bond of a free radical-activated aryl side chain is simple in operation; after completing the reaction, petroleum ether / ethyl acetate at a volume ratio of (50-1):1 is used as an eluent; column chromatography separation is performed to obtain a target product. The catalytic system in the invention uses oxygen as an oxygen source and has high atomic economy; the invention is a non-metallic catalytic system and provides a novel method for avoid metal residues in synthetic drugs; for diethyl aromatic hydrocarbon, the method provided by the invention can be adopted to selectively oxidize diethyl aromatic hydrocarbon into monoketone and diketone; the method of the invention can be adopted to efficiently synthesize tranquillizer lenperone, so that a novel method for synthesizing lenperone is provided.
Owner:UNIV OF SCI & TECH OF CHINA
Method for preparing epoxy butane
The invention relates to a method for preparing epoxy butane, which comprises the following step: in an isopropyl benzene solution containing 25 wt% of cumene hydroperoxide solute, preparing epoxy butane from butylene oxide by using the cumene hydroperoxide solute as an oxidizer and a titanium-silicon molecular sieve with three-dimensional pore canal structure as a catalyst, wherein the fixed bed reaction conditions are as follows: the mole ratio of butylene to the cumene hydroperoxide solute is (5.0-12.0):1, the weight hourly space velocity of the cumene hydroperoxide is 1.0-5.0 h<-1>, the reaction pressure is 1.0-6.0 MPa, and the temperature is 60.0-120.0 DEG C. The catalyst is the titanium-silicon molecular sieve with three-dimensional pore canal structure; the molecular sieve has hysteresis loop on the low-temperature nitrogen adsorption and desorption isotherm; the average pore size is 2.0-8.0nm, and the specific area is 650.0-1100.0 m<2> / g; and the catalyst has the advantages of favorable activity and high epoxy butane selectivity, and can be widely popularized and applied to industrial production of epoxy butane by butylene epoxidation.
Owner:CHINA PETROLEUM & CHEM CORP
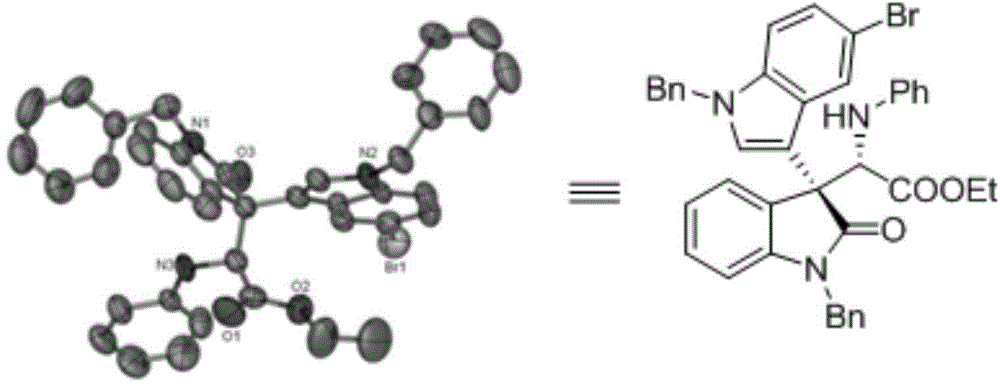
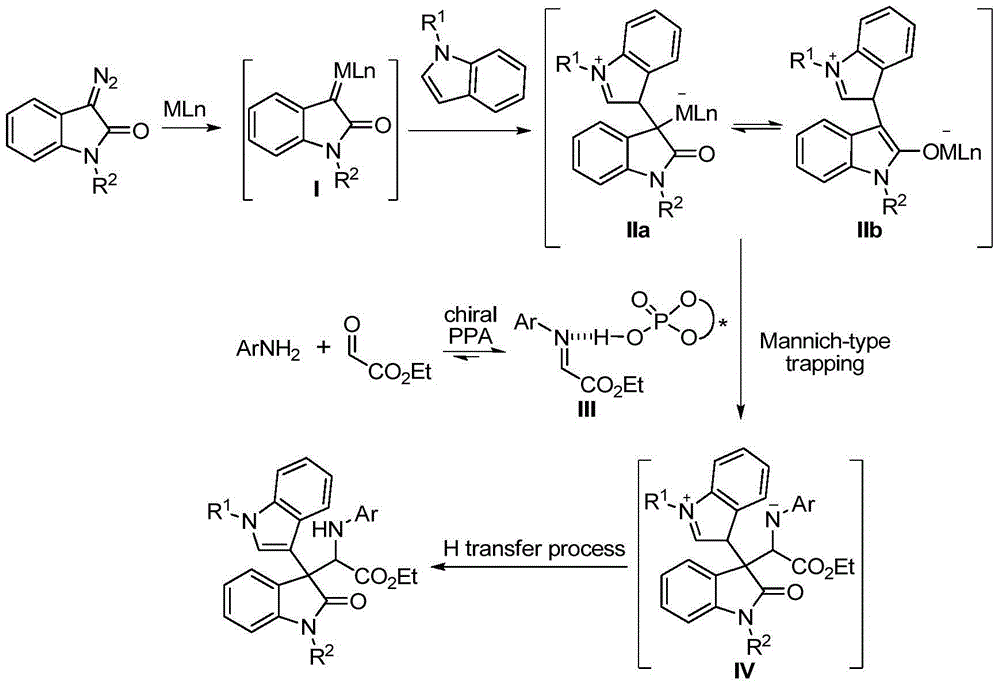
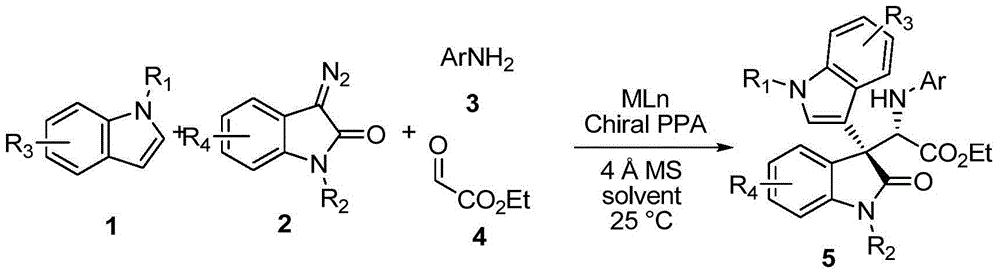
Chiral 3,3-disubstituted oxoindole derivative, and synthetic method and application thereof
ActiveCN104693092AHigh yieldGood diastereoselectivityOrganic chemistryMetabolism disorderChemical synthesisSynthesis methods
The invention discloses a chemical synthetic method of a novel chiral 3,3-disubstituted oxoindole derivative with PTP1B inhibiting action. With diazoisatin, indole, arylamine and aldehyde ester as raw materials, a metal catalyst as catalyst, chiral phosphoric acid as a co-catalyst, an organic solvent as a solvent, and molecular sieve shown in the specification as an additive, a one-step reaction is performed under the condition of 25 degrees centigrade, and the product undergoes column chromatography purification to obtain the target product. The synthetic method has the advantages of high step economy, strong atom economy, high diastereoselectivity and enantioselectivity, high yield, mild reaction condition, and simple and safe operation. The optically pure chiral 3,3-disubstituted oxoindole derivative with two chiral centers synthesized in the invention is an important intermediate in chemical engineering, chemistry and medicines, and has a wide application prospect in the fields of medicines and the chemical engineering.
Owner:广东和博制药有限公司
A method for preparing nitro compounds using nitrogen suboxides
ActiveCN102276471AIncrease profitAtom economy is highNitro compound preparationNitro compoundHydrogen Nitrate
The invention discloses a method for preparing a nitro compound from a low-valent nitric oxide. The method comprises that a low-valent nitric oxide such as nitric oxide, nitrogen dioxide, nitrous oxide, nitrogen trioxide, dinitrogen tetroxide and the like react with an alicyclic compound, an aromatic compound or a heterocyclic compound through promoting effects of molecular oxygen and effects of catalysts comprising a transition metal oxide, a triphenylphosphine metal organic complex, transition metal ion clay and heteropoly acid salts such as aluminosilicate, silicoaluminophosphate, sodium phosphomolybdate and the like. The method can replace the traditional industrial method for preparing a nitro compound from a high-valent (V-valent) nitrogen compound such as nitrogen pentoxide, hydrogen nitrate, nitric acid salts, nitric acid esters and the like. The method improves an atom utilization rate of a reaction in industrial nitration preparation of a nitro compound, and has atom economic characteristics of industrial preparation of a nitro compound.
Owner:彭新华
Preparation method of 3-o-alkylation quinoxaline-2(1H)-ketone compound
ActiveCN108752325AAtom economy is highMild reaction conditionsOrganic chemistryQuinoxalineOrganic synthesis
The invention belongs to the field of organic synthesis, and particularly discloses a preparation method of a 3-o-alkylation quinoxaline-2(1H)-ketone compound. The method includes the steps that a quinoxaline-2(1H)-ketone compound, an ether compound, alkaline, an oxidizing agent and a photocatalyst are added into a reacting bottle and react for 18-30 hours under illumination of a visible light lamp, after the reaction is ended, extracting treatment and concentrating treatment are performed, and the 3-o-alkylation substitution quinoxaline-2(1H)-ketone compound is obtained after column chromatography isolation purification treatment is performed. According to the method, the raw materials are easy to obtain, reaction conditions are simple and convenient to implement, using of metal reagents,high temperature and complex operation are avoided, reaction time is short, energy consumption is low, the compatibility of substrate functional groups is good, the safety and efficiency of the reaction are high, and high application values are obtained.
Owner:QUFU NORMAL UNIV
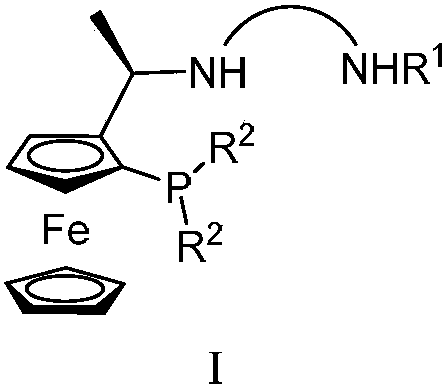
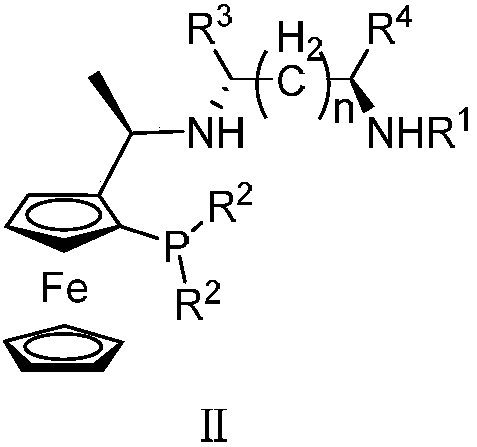
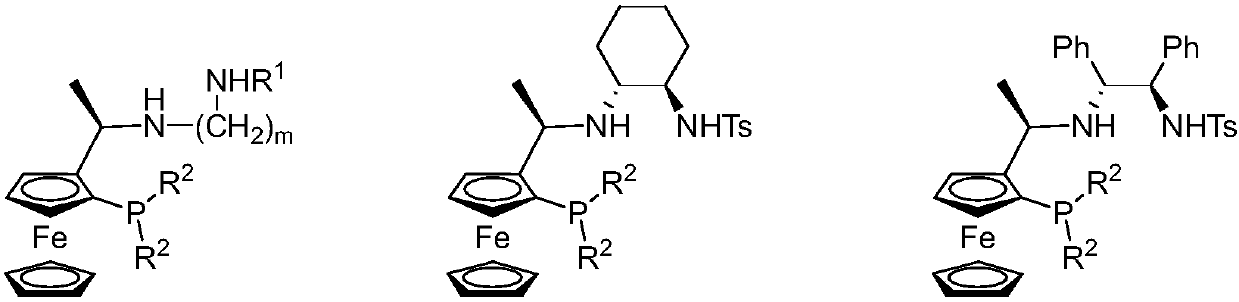
Tridentate nitrogen phosphine ligand and complex and application thereof in asymmetric catalytic hydrogenation of ketone
ActiveCN107722068AHigh enantioselectivityHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetoneOxygen
The invention belongs to the field of organic and medicine synthetic chemistry, and discloses a tridentate nitrogen phosphine ligand. The tridentate nitrogen phosphine ligand has a structure shown ina formula I which is shown in the attached figure, wherein R1 is toluene sulfonyl or 2,4,6-triisopropylbenzenesulfonyl, and R2 is aryl or substituted aryl. The invention also discloses a complex of the tridentate nitrogen phosphine ligand; the complex is prepared by mixing the tridentate nitrogen phosphine ligand and a transition metal complex; the complex is used for asymmetric catalytic hydrogenation of ketone. The tridentate nitrogen phosphine ligand has the advantages that 1, the synthesizing is easy, and the chiral ligand can be prepared by only two to three reaction steps; 2, the ligandis stable, the series of ligand is not sensitive to water and oxygen, and the convenience in storage and use is realized; 3, the catalyzing effect is good, and the catalyst can be used for realizing 100% of conversion and 99% of stereo selectivity on most of suitable primers; 4, the atom economy is high, and the activity of the catalysis system is higher; for most of suitable primers, the conversion number reaches more than 10000, and the maximum conversion number reaches 200000.
Owner:SHENZHEN CATALYS SCI & TECH CO LTD
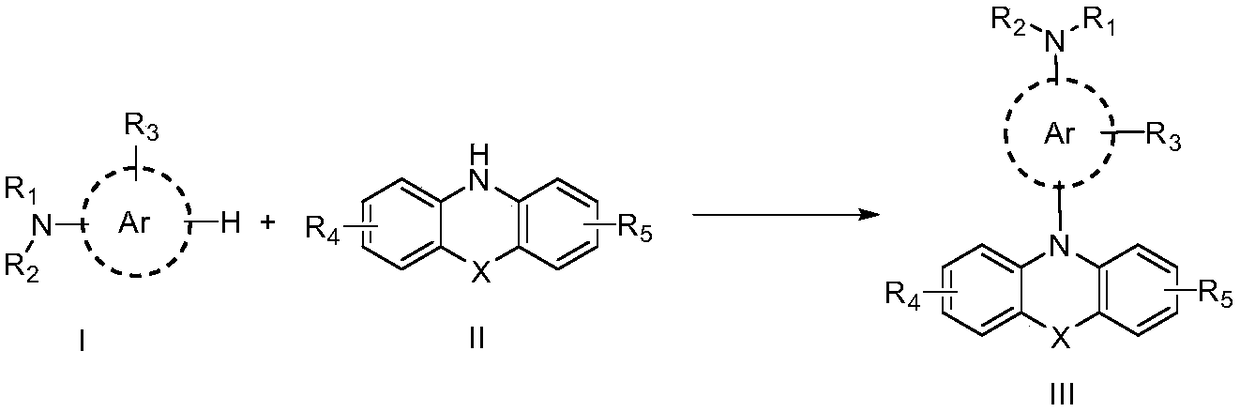
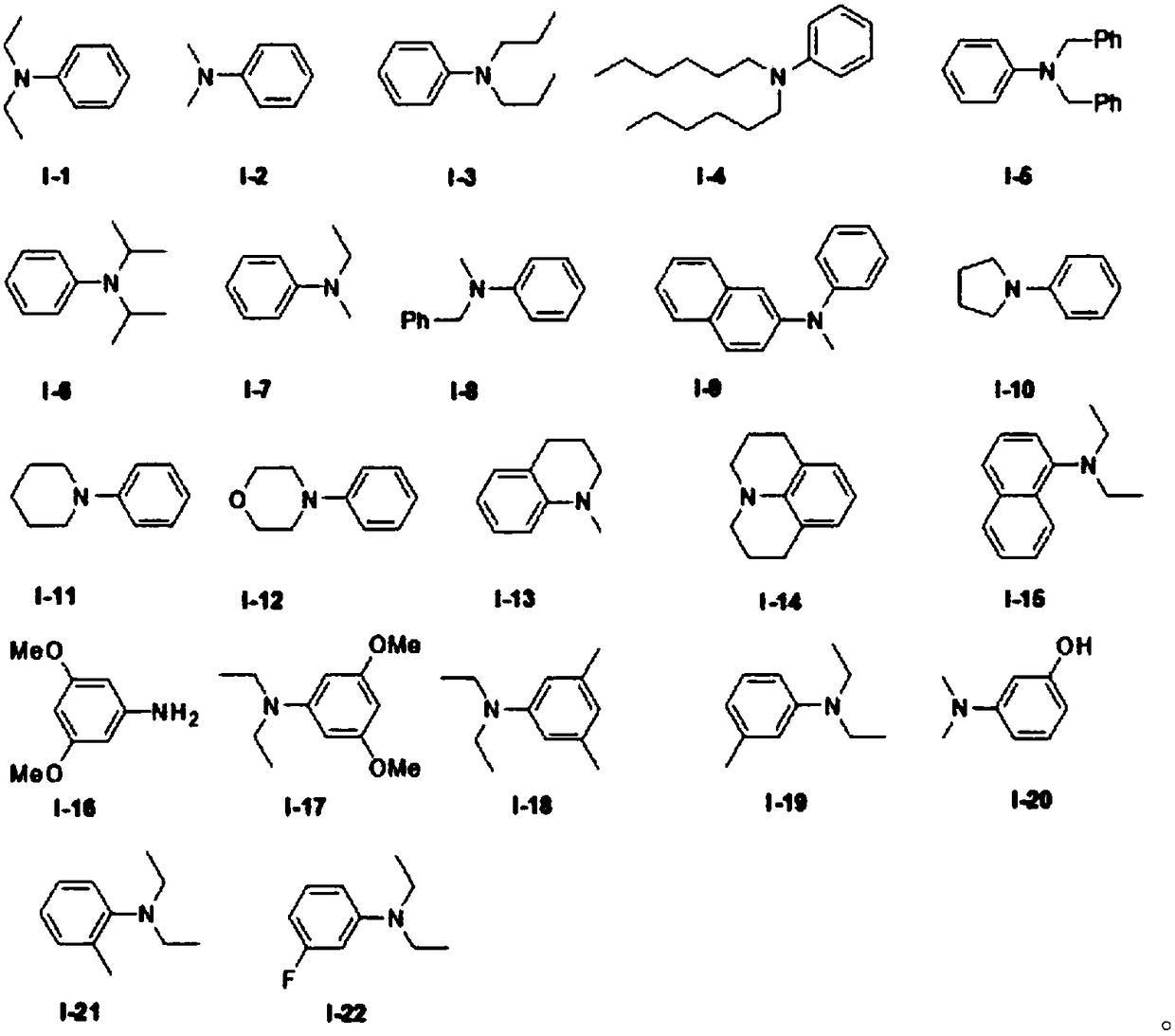
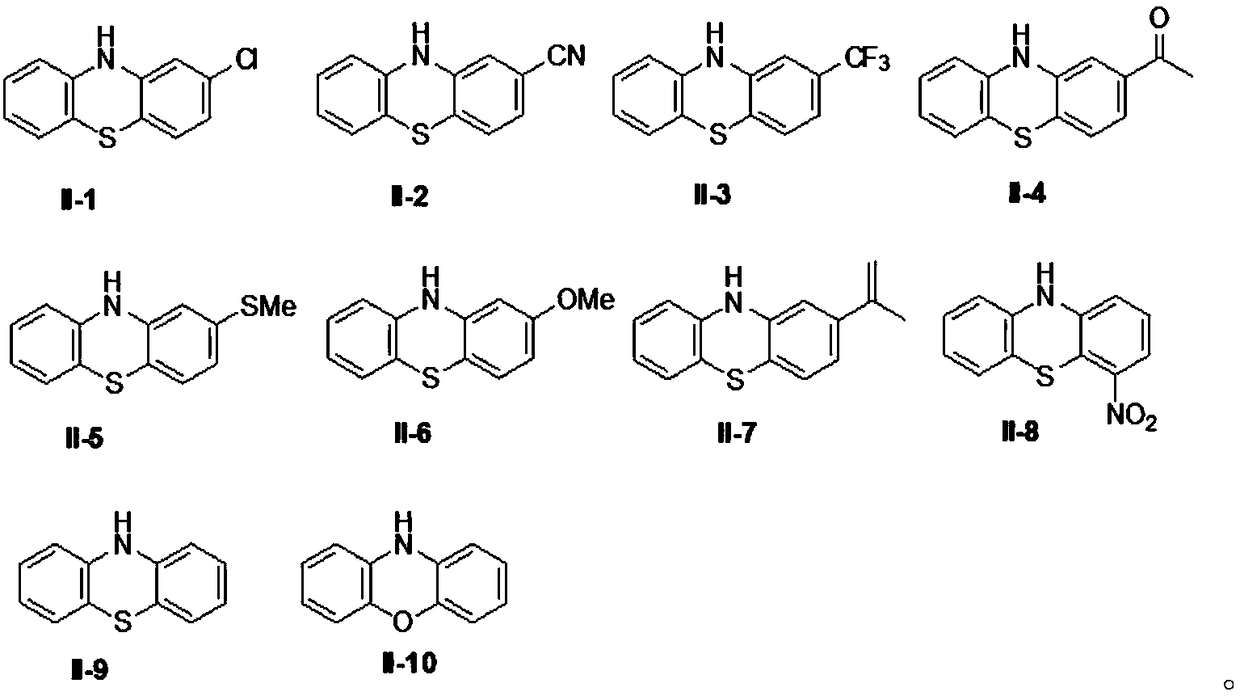
Organic electrosynthesis method of phenothiazine/phenoxazine compound
ActiveCN108863982AReduce processEasy to operateElectrolysis componentsOrganic chemistryPhenoxazineElectrosynthesis
The invention discloses an organic electrosynthesis method of a phenothiazine / phenoxazine compound. The organic electrosynthesis method adopts an organic electrosynthesis means for a compound shown ina formula I and a compound shown in a formula II to prepare the phenothiazine / phenoxazine compound. The organic electrosynthesis method disclosed by the invention has the beneficial effects that a target product shown in a formula III can be obtained by directly activating C-H without need of expensive halogenated raw materials and additional reduction and substitution processes, so that the process flow is obviously shortened, the operation is simplified, the preparation efficiency is improved, and the production cost is reduced; the reaction condition is simple, mild and environmentally friendly, the atom economy is high, the adaptation range of a reaction substrate is wide, and the yield of a target product is high.
Owner:NANCHANG HANGKONG UNIVERSITY
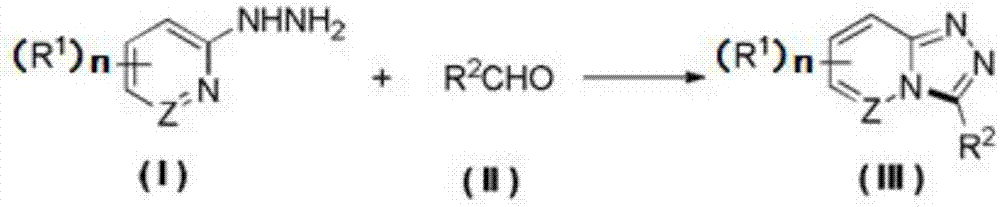
Synthesis method of 1,2,4-triazolohetercyclic compound
ActiveCN107474047AHigh yieldSimple reaction systemOrganic chemistryElectrolysis componentsSynthesis methodsHydrogen molecule
The invention provides a method for synthesizing a 1,2,4-triazolohetercyclic compound. The method comprises the following steps: dissolving a 2-hydrazinohetercyclic compound (I) and an aldehyde compound (II) into acetonitrile A; stirring at 25 DEG C to 80 DEG C and reacting for 1h to 8h; then adding tetrabutylammonium tetrafluoroborate, acetonitrile B and water; taking a graphite carbon rod as an anode and a platinum sheet as a cathode; switching on a power supply and electrifying for 3h to 8h, wherein the current strength is 15mA / mmol to 30mA / mmol according to the amount of substance of the aldehyde compound (II); carrying out post-treatment on a reaction solution to obtain the product 1,2,4-triazolohetercyclic ring compound (III). The method has the advantages of simple reaction system, easiness for obtaining raw materials and high total yield; the 2-hydrazinohetercyclic compound and the aldehyde compound are subjected to electrochemical catalysis to obtain the 1,2,4-triazolohetercyclic compound in one step; only one water molecule and one hydrogen molecule are removed and the atomic economy is extremely high; the formula is shown in the description.
Owner:ZHEJIANG UNIV OF TECH
Method for carrying out reaction on methylenecyclopropane derivative and ether compound C(sp<3>)-H bond
InactiveCN106946817AThe reaction system is efficient and environmentally friendlyAtom economy is highOrganic chemistryOrganic compound preparationChemical compoundOrganic synthesis
The present invention belongs to the technical field of organic synthesis, and particularly relates to a method for carrying out a reaction on a methylenecyclopropane derivative and an ether compound C(sp<3>)-H bond. According to the present invention, a methylenecyclopropane derivative represented by a formula II and an ether compound represented by a formula III are subjected to a reaction in the presence of a free radical initiator to obtain a 1,2-dihydronaphthalene derivative represented by a formula I, wherein the free radical initiator is the mixture comprising one or a plurality of materials selected from TBHP (tert-butyl hydroperoxide), CHP (Cumyl hydroperoxide), and THAP (tert-amyl hydroperoxide). The formulas I, II and III are defined in the specification.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of polythiourea
ActiveCN108570149AHigh molecular weightAtom economy is highFluorescence/phosphorescenceThioureaFluorescence
The invention belongs to the technical field of sulfur-containing organic polymer preparation, and discloses a method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of the polythiourea. The method includes carrying out reaction on amine monomers, isocyanide monomers and elemental sulfur in solvents; cooling, precipitating anddrying reaction products to obtain the polythiourea. The amine monomers are diamine compounds, and the isocyanide monomers are binary isocyanide compounds. The method and the application have the advantages that the method can be implemented under room-temperature and air conditions, is simple and is high in polymerization reaction yield and atom economy, and the polythiourea which is a product is easy to separate; the polythiourea prepared by the aid of the method contains abundant N and S heteroatoms, has special photoelectric properties and has a potential unique application value in the field of biological and chemical fluorescence detection, metallic mercury ion detection and mercury ion removal; the polythiourea is high in metallic mercury ion detection sensitivity, good mercury ionremoval effects can be realized by the polythiourea, and the removal efficiency of the polythiourea is higher than 99.99% and can reach drinking water standards.
Owner:SOUTH CHINA UNIV OF TECH
Dihydropyrrole derivates and method for preparing intermediate pyrrolidine
The invention discloses a method for preparing a dihydropyrrole derivative and an intermediate pyrrolidine thereof, and relates to the preparation of the pyrrolidine and the dihydropyrrole derivative. Firstly, a diazocompound, amine, beta, gamma-unsaturated-alpha-keto ester with the mol ratio of 1.2 to 1.2 to 1 to 0.01 are weighed as raw materials, metal Lewis acid is taken as a catalyst, the diazocompound is decomposed to form metal carbine through a three-component tandem reaction under the catalysis of the metal Lewis acid, ammonium ylide obtained by in-situ formation of the metal carbine and the amine is captured by the beta, gamma-unsaturated-alpha-keto ester through the 1, 4-addition mode, then a corresponding intermediate pyrrolidine derivative is obtained through intra-molecular condensation, finally the pyrrolidine derivative is mixed with an organic solvent, then an organic acid catalyst is added to form a reaction system, and then the corresponding dihydropyrrole derivative is obtained through the water entrainment of a water segregator. The method has the advantages of high atom economy, high efficiency, high yield and so on, and has safe and simple operation. The prepared pyrrolidine and the dihydropyrrole derivative are widely applied to the field of pharmaceutical and chemical industries.
Owner:EAST CHINA NORMAL UNIVERSITY
Multi-component polymerization method based on alkyne and sulfonyl azide as well as sulfonyl-containing polymer and application
ActiveCN109161017AHigh yieldHigh molecular weightFluorescence/phosphorescencePolymer scienceFluorescence
The invention belongs to the technical field of a macromolecular polymer, and discloses a multi-component polymerization method based on alkyne and sulfonyl azide as well as a sulfonyl-containing polymer and application. The multi-component polymerization method comprises the step of performing multi-component polymerization reaction on a binary alkynyl compound, a binary sulfonyl azide compound and a third component compound under the protective atmosphere and through the effect of a univalent copper catalyst to obtain the sulfonyl-containing polymer, wherein the third component compound is acyanophenyl compound containing hydroxyl or amino, a formyl-containing thiophenol potassium compound, an amino-containing benzoyl compound, ammonium salt, ammonia, thiocyanate or selenium cyanate. The method is mild in polymerization conditions, simple in process, high in polymerization efficiency, large in product molecular weight and high in atom economy; furthermore, a functional element containing the complex structure can be constructed in situ simply and efficiently. The obtained sulfonyl-containing polymer is applied in the fields of fluorescence detection, metal ion detection, organicphotoelectric materials and antibacterial materials.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


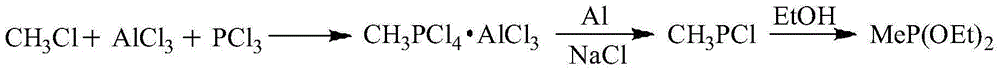
![Synthetic method for aromatic[a]carbazole compounds Synthetic method for aromatic[a]carbazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ceef379-c79e-4323-87be-5b6f2e922b79/BDA0001160801260000011.png)
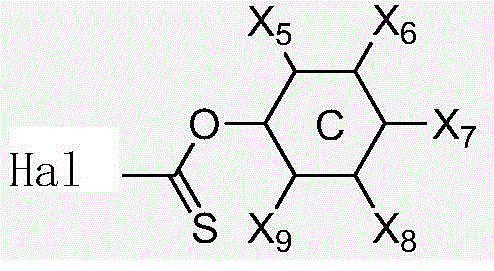
![Synthetic method for aromatic[a]carbazole compounds Synthetic method for aromatic[a]carbazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ceef379-c79e-4323-87be-5b6f2e922b79/BDA0001160801260000021.png)
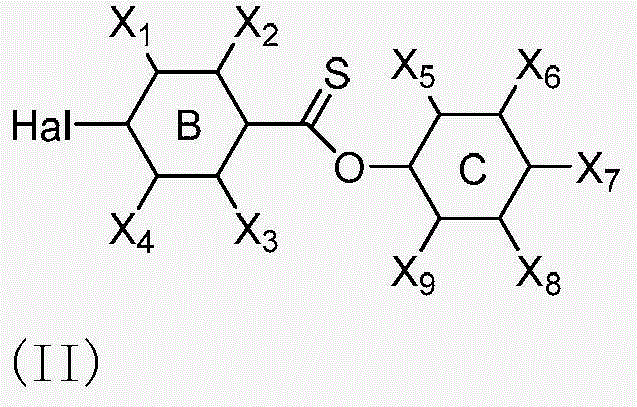
![Synthetic method for aromatic[a]carbazole compounds Synthetic method for aromatic[a]carbazole compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1ceef379-c79e-4323-87be-5b6f2e922b79/BDA0001160801260000022.png)