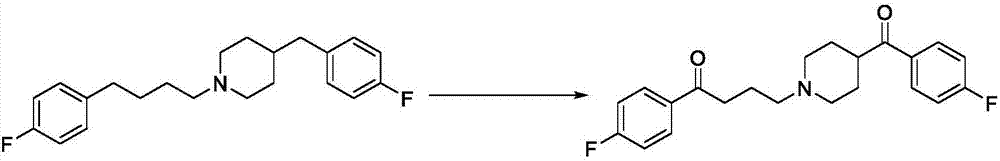
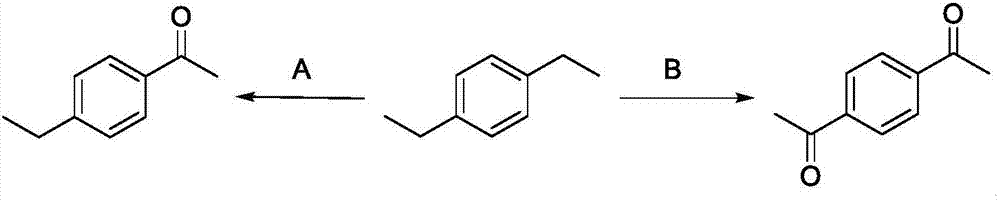
Method for directly oxidizing benzyl-position C-H bond into ketone
A direct, benzylic technology, applied in chemical instruments and methods, preparation of quinone oxides, preparation of organic compounds, etc., can solve the problems of selective oxidation of monoketones and diketones, unstable peroxide oxidants, etc. problems, to achieve the effect of avoiding metal residues, simple operation, and high-efficiency synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] p-Methoxyacetophenone Synthesis
[0021] Add 0.5 equivalent of NHPI to a 25 mL Schlenk reaction tube, dry in vacuum for 15 minutes, cover with an oxygen bulb, add 1 mL of acetonitrile, 1.0 equivalent of tert-butyl nitrite, and 0.5 mmol of p-ethylanisole in sequence under an oxygen atmosphere. Put a polytetrafluoro stopper on the tube and put it in an oil bath, and react at 80°C for 24h. After the reaction was completed, the solvent was concentrated under reduced pressure to remove acetonitrile, and separated by column chromatography, and the eluent was petroleum ether / ethyl acetate (v:v=20:1) to obtain p-methoxyacetophenone. Yield 83%, pale yellow solid; 1 H NMR (CDCl 3 ,400MHz): δ7.92(d,J=9.2Hz,2H),6.92(d,J=8.8Hz,2H),3.85(s,3H),2.54(s,3H); 13 C NMR (CDCl 3 ,100MHz): δ196.7,163.4,130.5,130.3,113.6,55.4,26.3.
Embodiment 2
[0023] 4-Acetoxyacetophenone Synthesis
[0024] Add 1.0 equivalent of NHPI to a 25 mL Schlenk reaction tube, dry in vacuum for 15 minutes, cover with an oxygen bulb, add 1 mL of acetonitrile, 2.0 equivalents of tert-butyl nitrite, and 0.5 mmol of p-acetoxyethylbenzene in an oxygen atmosphere. Put a polytetrafluoro stopper on the reaction tube and put it in an oil bath, and react at 80°C for 24h. After the reaction was completed, the solvent was concentrated under reduced pressure to remove acetonitrile, and separated by column chromatography, the eluent was petroleum ether / ethyl acetate (v:v=10:1) to obtain 4-acetoxyacetophenone. Yield 72%, white solid; 1 H NMR (CDCl 3,400MHz): δ7.96(d,J=6.8Hz,2H),7.16(d,J=6.8Hz,2H),2.56(s,3H),2.30(s,3H); 13 C NMR (CDCl 3 ,100MHz): δ196.8, 168.8, 154.2, 134.5, 129.8, 121.7, 26.5, 21.0.
Embodiment 3
[0026] p-Fluoroacetophenone Synthesis
[0027] Add 0.5 equivalent of NHPI to a 25 mL Schlenk reaction tube, dry in vacuum for 15 minutes, cover with an oxygen bulb, add 1 mL of acetonitrile, 1.0 equivalent of tert-butyl nitrite, and 0.5 mmol of p-fluoroethylbenzene in the reaction tube After adding a polytetrafluoro stopper, put it into an oil bath, and react at 80°C for 24h. After the reaction was completed, the solvent was concentrated under reduced pressure to remove acetonitrile, and separated by column chromatography, and the eluent was petroleum ether / ethyl acetate (v:v=50:1) to obtain p-fluoroacetophenone. Yield 78%, pale yellow oil; 1 HNMR (CDCl 3 ,400MHz):δ7.96(dd,J=8.2Hz,5.4Hz,2H),7.10(t,J=8.4Hz,2H),2.56(s,3H); 13 C NMR (CDCl 3 ,100MHz): δ196.4,166.9,164.4,133.5(d,J=3.0Hz),130.8(d,J=9.8Hz),115.6(d,J=21.9Hz),26.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com