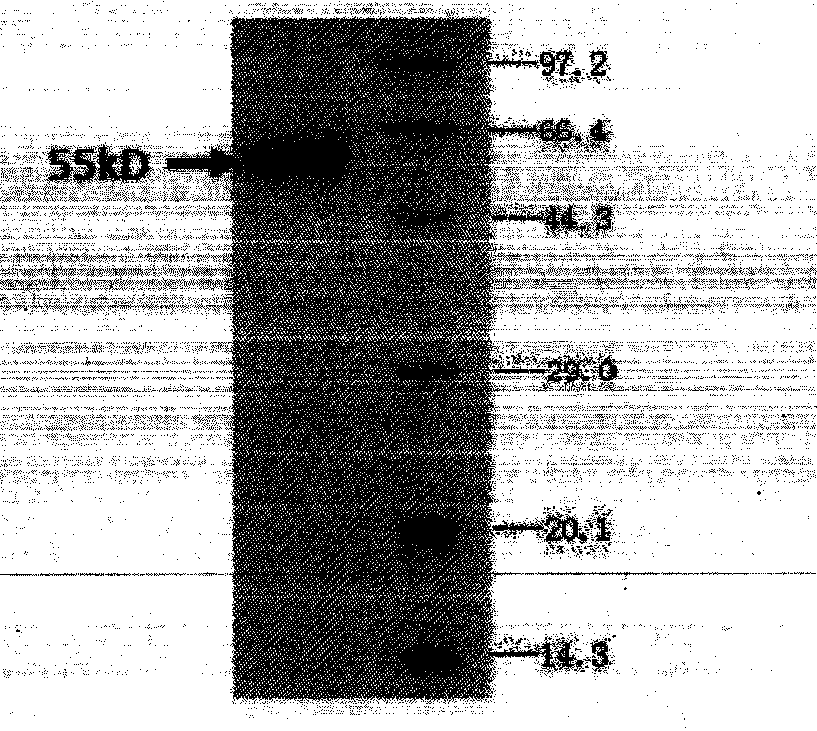Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1102 results about "Phospholipin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Processes for making particle-based pharmaceutical formulations for pulmonary or nasal administration
InactiveUS20070178166A1Improve stabilityStability storage conditionPowder deliverySpray deliveryPowder mixtureNanoparticle
Dry powder pharmaceutical formulations for pulmonary or nasal administration are made to provide an improved respired dose. These formulations may be blends of milled blends and may include a phospholipid, alone or in combination with other excipient materials. In one case, the process includes the steps of (a) providing particles which comprise a pharmaceutical agent, (b) blending the particles with particles of at least one first excipient to form a first powder blend; (c) milling the first powder blend to form a milled blend which comprises microparticles or nanoparticles of the pharmaceutical agent; and (d) blending the milled blend with particles of a second excipient to form a blended dry powder blend pharmaceutical formulation suitable for pulmonary or nasal administration.
Owner:ACUSPHERE INC
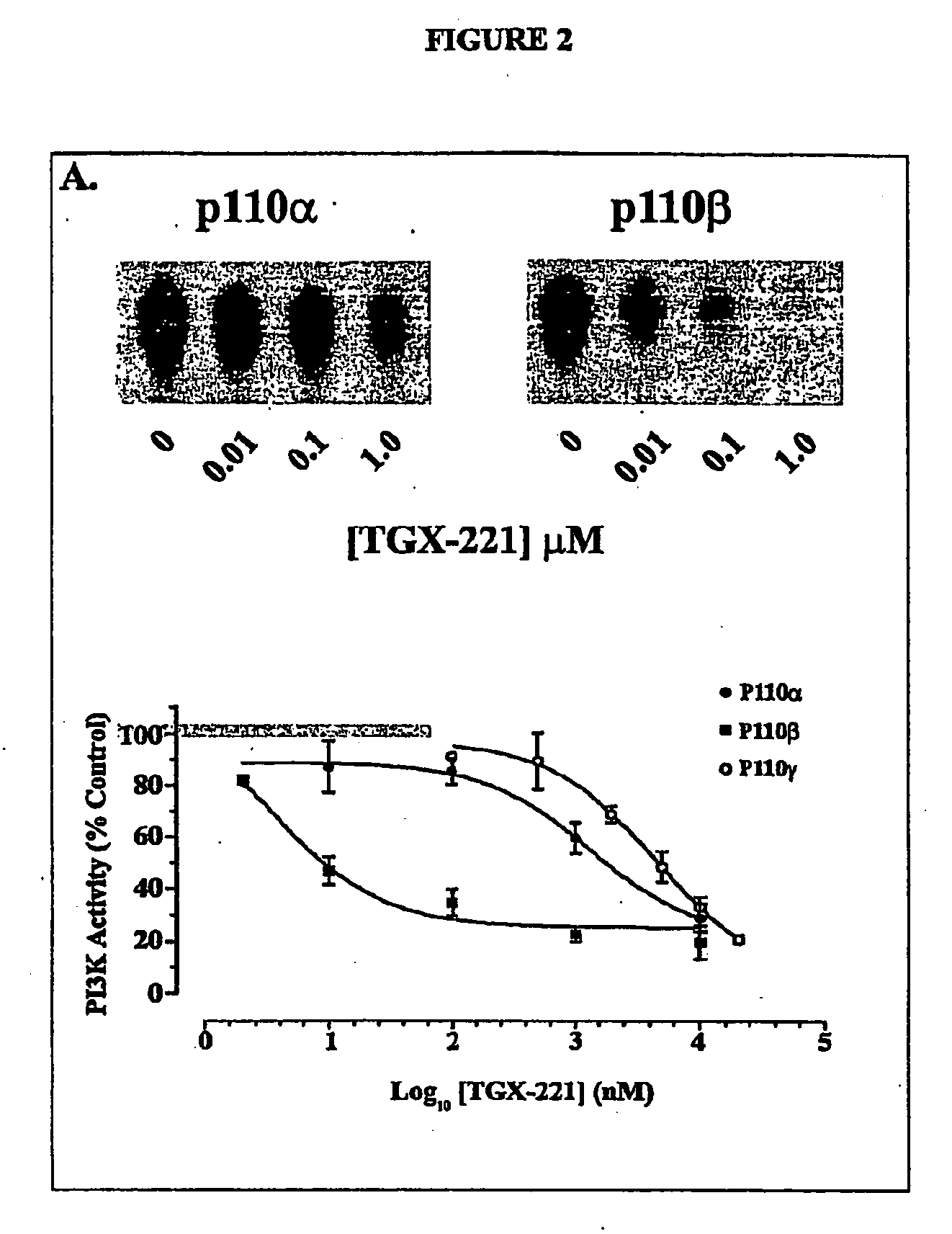
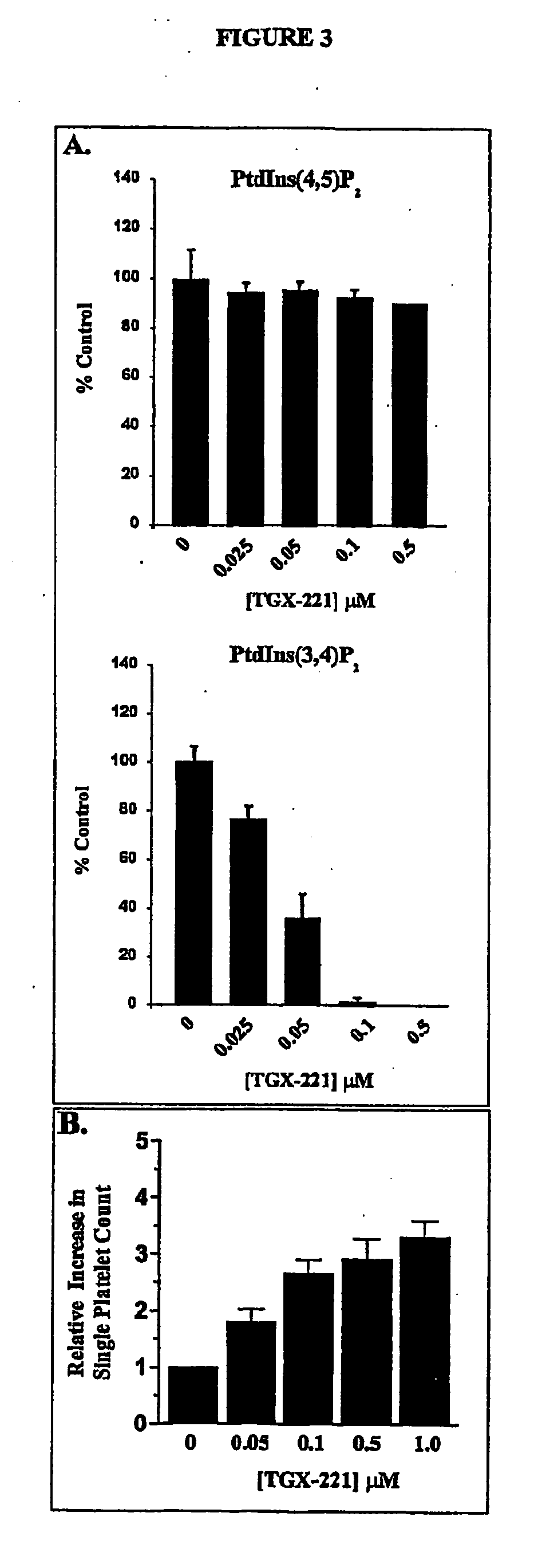
Inhibition of phsphoinostide 3-dinase beta
InactiveUS20060276470A1Specific inhibitionProlonged bleeding timeOrganic active ingredientsOrganic chemistryAntithrombotic treatmentKinase inhibition
The present invention relates to selective inhibitors of phosphoinositide (PI) 3-kinase β, use of the selective inhibitors in anti-thrombotic therapy, and a method for screening compounds useful for the new anti-thrombotic therapy by detecting selective inhibitory activity of PI 3-kinase β of the compound. The invention also relates to novel compounds that are inhibitors of PI 3-kinase.
Owner:ASTRAZENECA AB
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
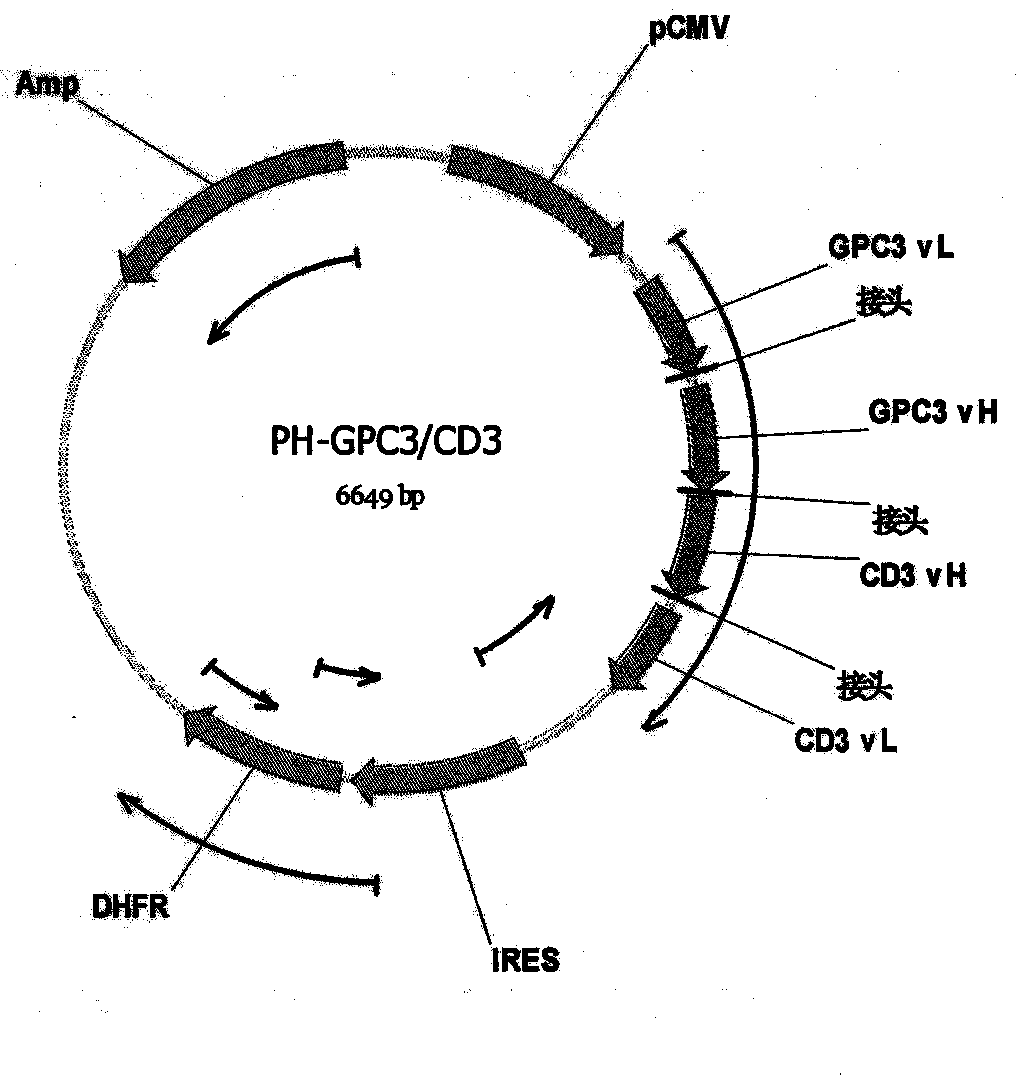
Bispecific antibody aiming at phosphatidylinositols protein polysaccharide-3 and T cell antigen
InactiveCN103833852ABacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenNucleotide
The first aspect of the invention relates to a bispecific antibody, which comprises a first functional domain for specific identification of phosphatidylinositol protein polysaccharide-3, a second domain for specific identification of human T cell antigen CD3, and a connection for connecting the functional domains. The second aspect of the invention relates to a nucleotide sequence encoding the above antibody. The third aspect of the invention relates to a carrier containing the above nucleotide sequence, and includes an expressive vector. The fourth aspect of the invention relates to a eukaryotic or prokaryotic expression system containing the above carrier. The fifth aspect of the invention relates to application of the above antibody to preparation of medicament for treating or preventing tumor.
Owner:SHANGHAI INST OF ONCOLOGY
Delivery System for Topically Applied Compounds
A delivery system for topically applied compounds is disclosed. The delivery system contains a fatty acid, a phospholipid, and an oil, and is activated by the addition of water. The delivery system is admixed with a topically applied compound and water to provide a composition suitable for application to the skin or hair. The relative amounts of delivery system ingredients provide round, flexible vesicles that allow penetration of the topically applied compound to the epidermis and dermis, vesicles having a partially ruptured membrane for a controlled delivery of the topically applied drug to the epidermis and dermis, completely ruptured vesicles in the form of lamellar sheets that allow the topically applied compound to be retained in the stratum corneum, and mixtures thereof.
Owner:LIPO CHEM
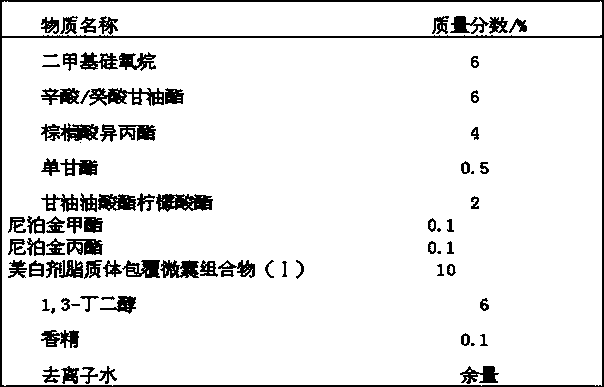
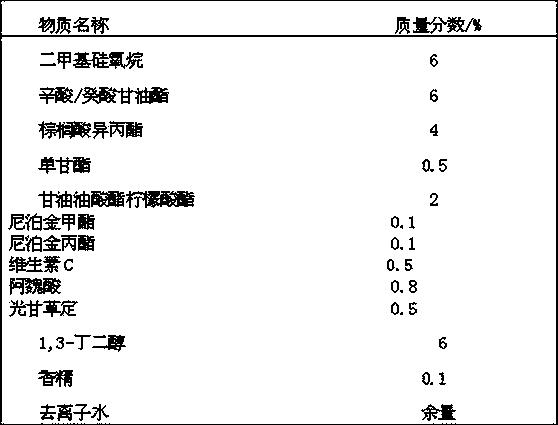
Whitening agent liposome coating micro-capsule composition as well as preparation method and application thereof
ActiveCN103432009AOvercome defects that are difficult to be absorbed by the skinOvercome the defect of being easily oxidized and discoloredCosmetic preparationsToilet preparationsPhospholipinPolyol
The invention discloses a whitening agent liposome coating micro-capsule composition as well as a preparation method and an application thereof. The whitening agent liposome coating micro-capsule composition comprises 5-30% of whitening agent, 2-50% of polyhydric alcohol, 1-30% of phospholipids, 1-30% of grease, 1-10% of emulsifying agent and 1-30% of deionized water, wherein the whitening agent is coated by lecithin which is very similar to a skin lipid structure and is prone to penetrating into the deep layer of skin, thus the defect that the whitening agent is unlikely to be absorbed by the skin is overcome; the whitening agent coated by lecithin is isolated from oxygen in air, thus the detect that the whitening agent is prone to being oxidized and discoloring is solved; when whitening products containing the whitening agent liposome coating micro-capsule composition are used, the whitening agent is slowly released and has a long-time whitening effect.
Owner:GUANGDONG IND TECHN COLLEGE +1
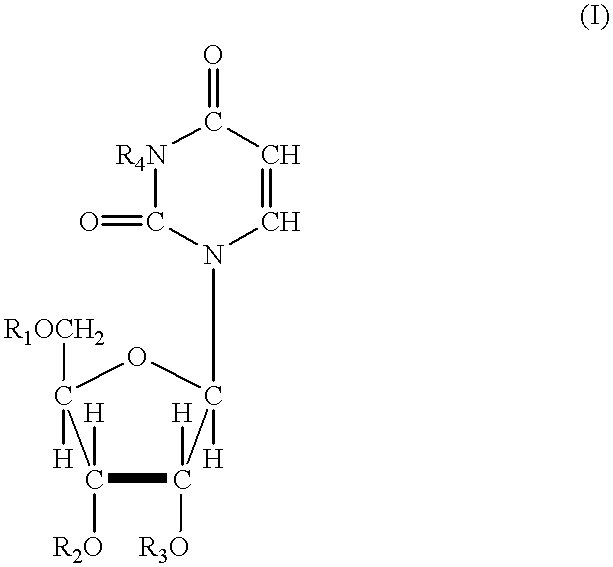
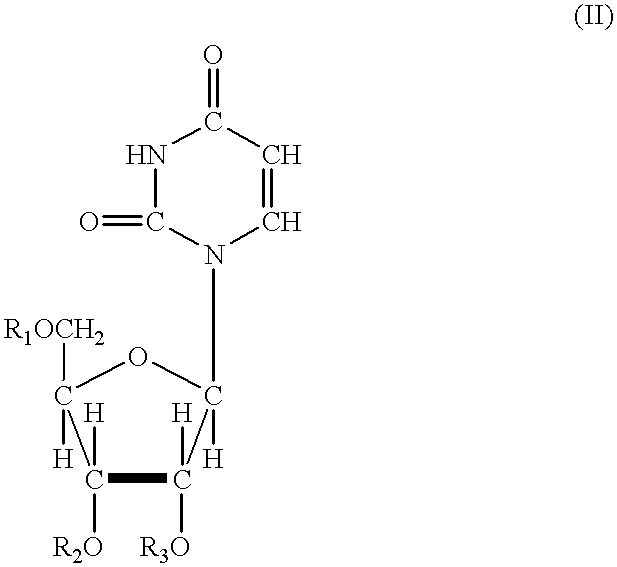
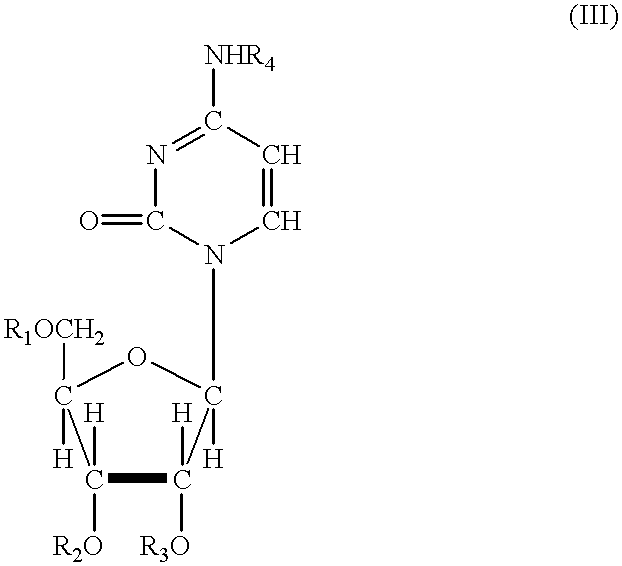
Acylated uridine and cytidine and uses thereof
ActiveUS6258795B1No untoward pharmaceutical effectEfficient managementBiocideSugar derivativesDiseaseDiabetes mellitus
The invention relates to compositions comprising acyl derivatives of cytidine and uridine. The invention also relates to methods of treating hepatopathies, diabetes, heart disease, cerebrovascular disorders, Parkinson's disease, infant respiratory distress syndrome and for enhancement of phospholipid biosynthesis comprising administering the acyl derivatives of the invention to an animal.
Owner:WELLSTAT THERAPEUTICS
Frozen confection product
InactiveUS20150282502A1Heat shock stableImproved sensorial textureLighting and heating apparatusFrozen sweetsAdditive ingredientWhey protein
The present invention relates to a frozen confection product comprising one or more proteins and a whey protein phospholipid concentrate. Preferably the frozen confection product is prepared by using a standard freezing step followed by low temperature extrusion and by acidifying the ingredient mix for preparing the frozen confection product. The present invention also relates to a method of preparing such product.
Owner:NESTEC SA
Cosmetic or pharmaceutical, low-viscosity oil-in-water emulsions containing phospholipids
InactiveUS20050124705A1Good lookingImprove combabilityCosmetic preparationsHair removalPhospholipidViscosity
The invention is a low viscosity oil-in-water emulsion that is readily atomizable for use in cosmetic and dermatological applications and particularly for spray or aerosol applications. The oil-in-water emulsion includes at least one phospholipid and at least one oil-in water emulsifier. In some embodiments, the emulsion may also include a water-in-oil emulsifier. The invention also includes a process for preparing the emulsion.
Owner:BEIERSDORF AG
Hydraulic fluids
InactiveUS20070078068A1Guaranteed to workGood environmental characteristicsGroup 5/15 element organic compoundsLiquid carbonaceous fuelsPhospholipidHydraulic fluid
Invention relates to an aqueous hydraulic fluid comprising, in addition to water, at least one phospholipid lubricant, wherein the hydraulic fluid comprises less than 20% by weight of an oil selected from a mineral oil, a synthetic hydrocarbon oil or any mixture thereof. Preferably the or each phospholipid lubricant comprises a plant-derived lecithin.
Owner:NICHE PRODS
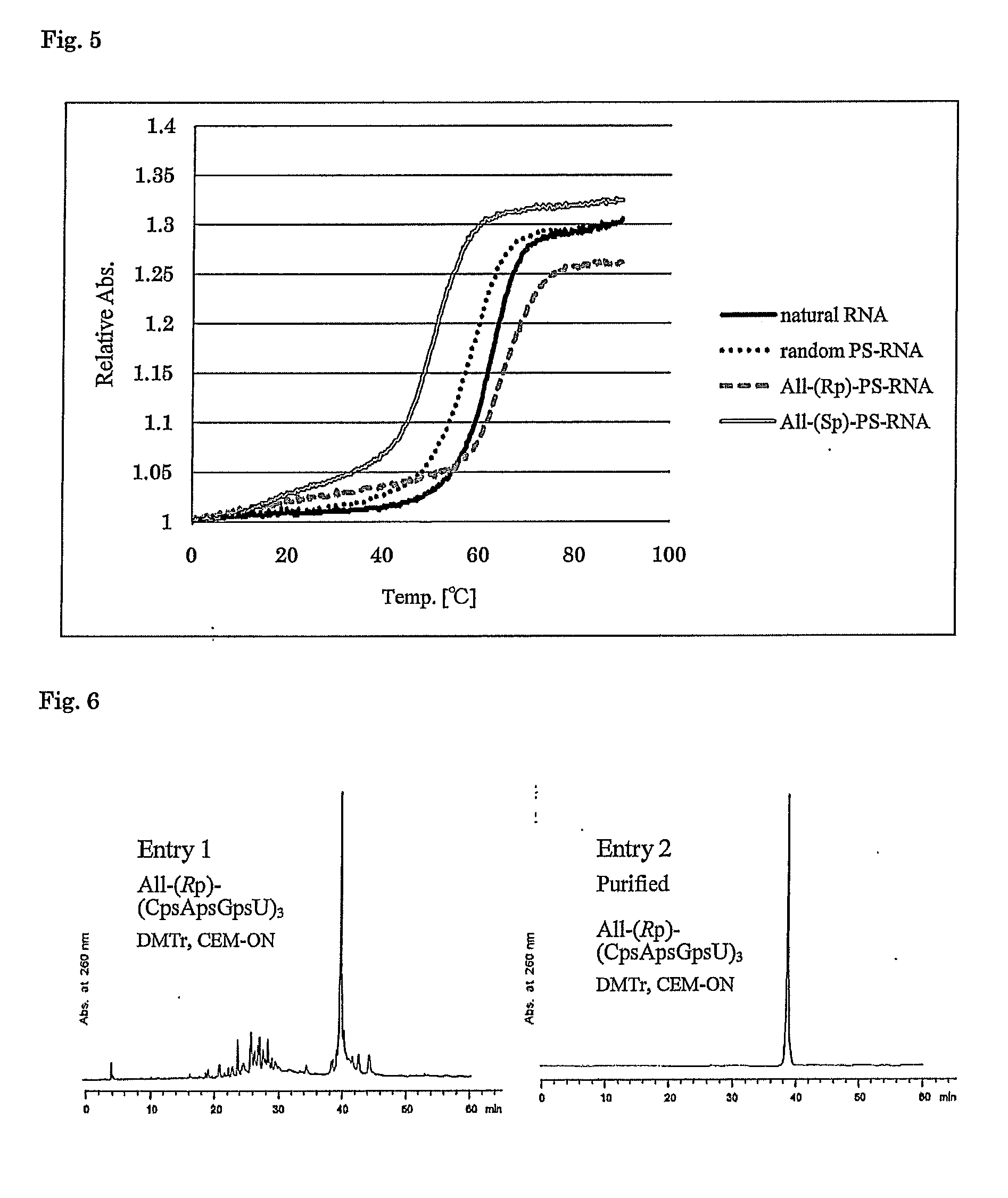
Method for preparing ribonucleoside phosphorothioate
ActiveUS8859755B2Efficient synthesisImprove efficiencySugar derivativesBulk chemical productionPhospholipinRibonucleoside
Owner:WAVE LIFE SCI LTD
Crosslinked cosmetic or pharmaceutical phospholipid-containing gels and emulsions based on ethylene oxide-containing or propylene oxide-containing emulsifiers
InactiveUS20050142153A1Good lookingImprove combabilityCosmetic preparationsHair removalPhospholipidOil phase
The invention is a process for making a cosmetic or pharmaceutical, phospholipid-containing crosslinked microemulsion gel or crosslinked oil-in-water emulsion. The process includes the use of at least one crosslinker that is added to at least one of the water phase and the oil phase of the emulsion, or the is added after the water phase and oil phase are mixed.
Owner:BEIERSDORF AG
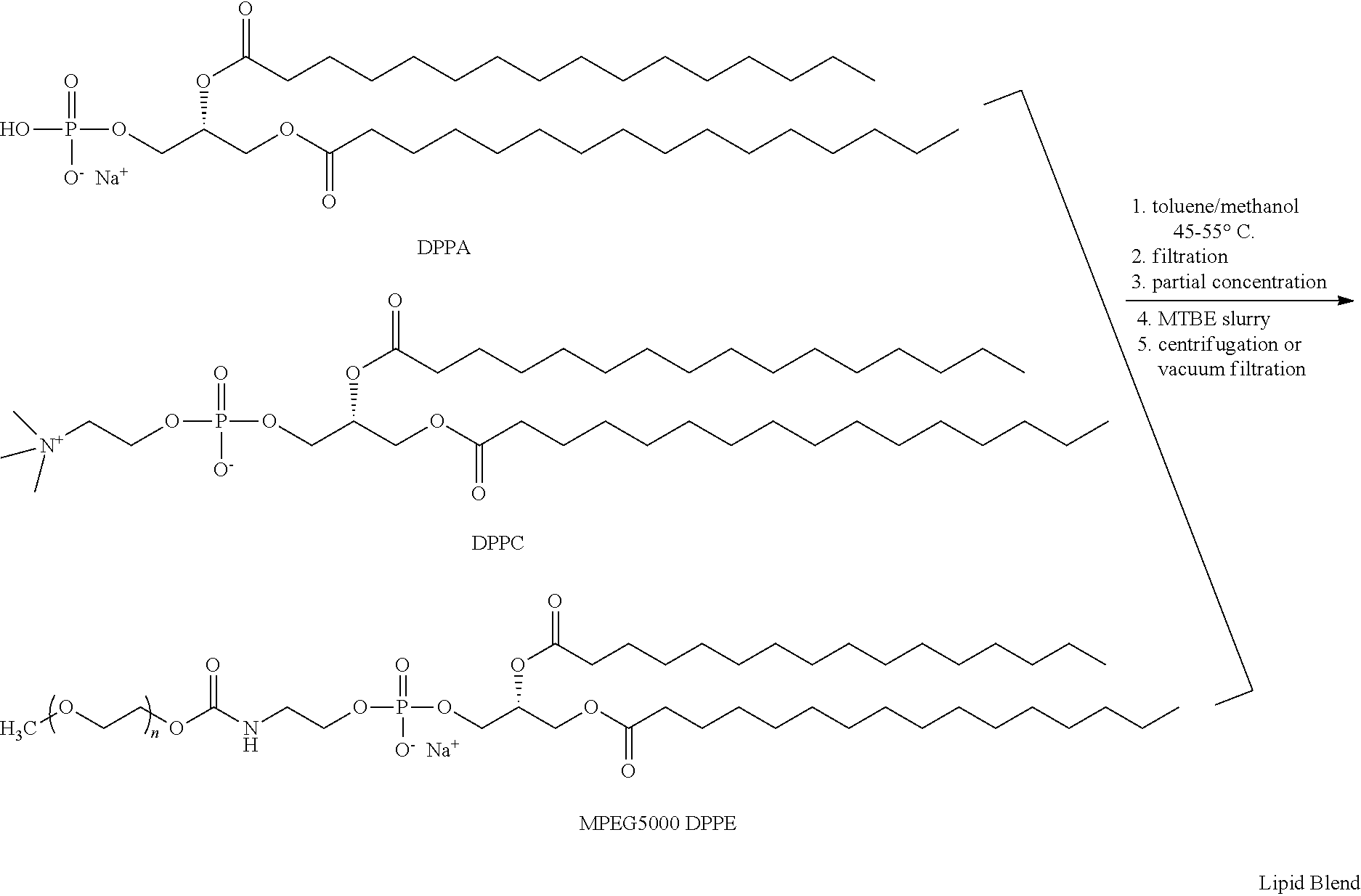
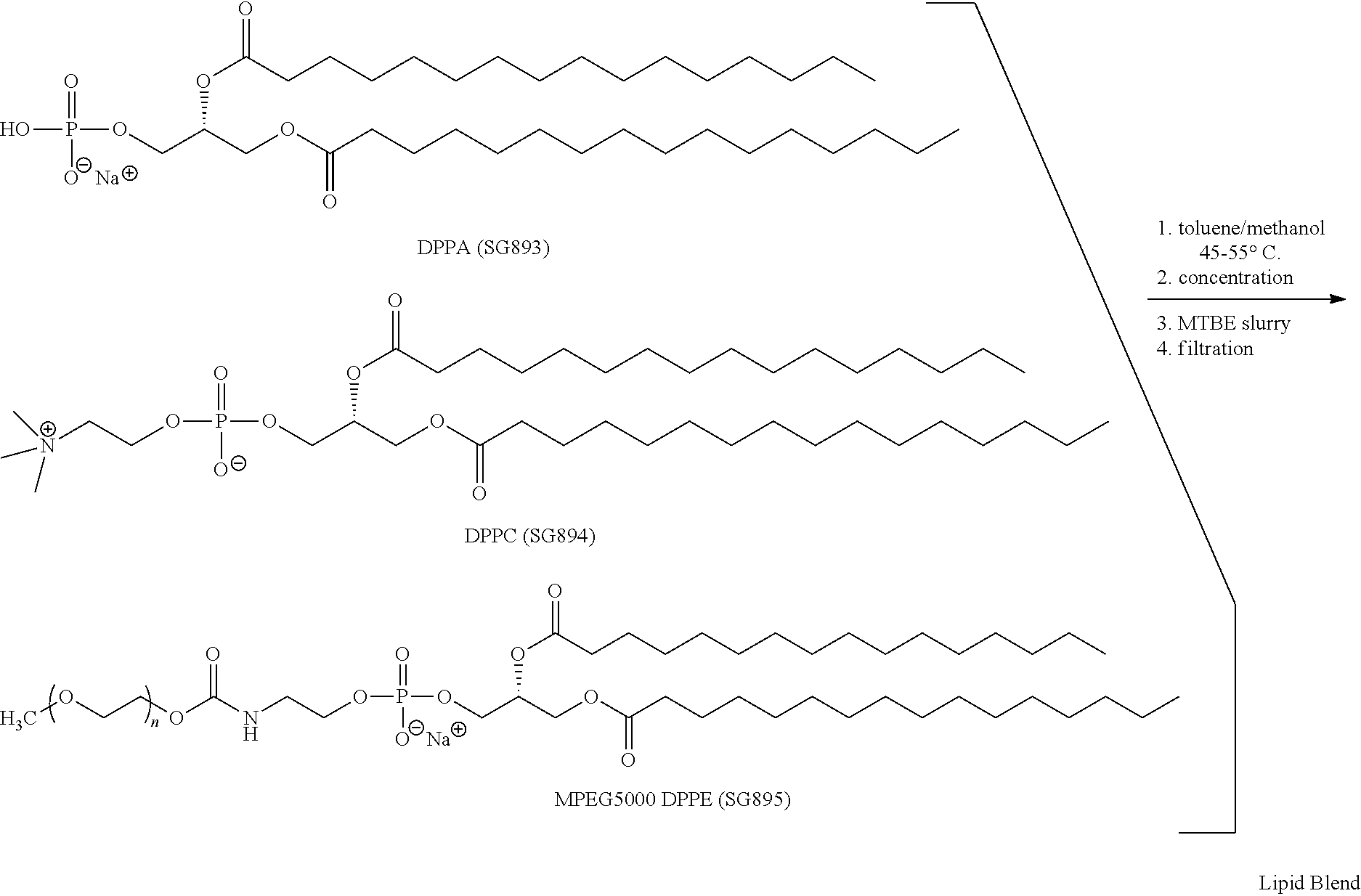
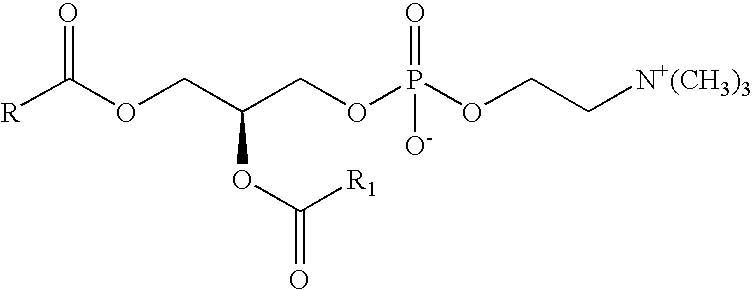
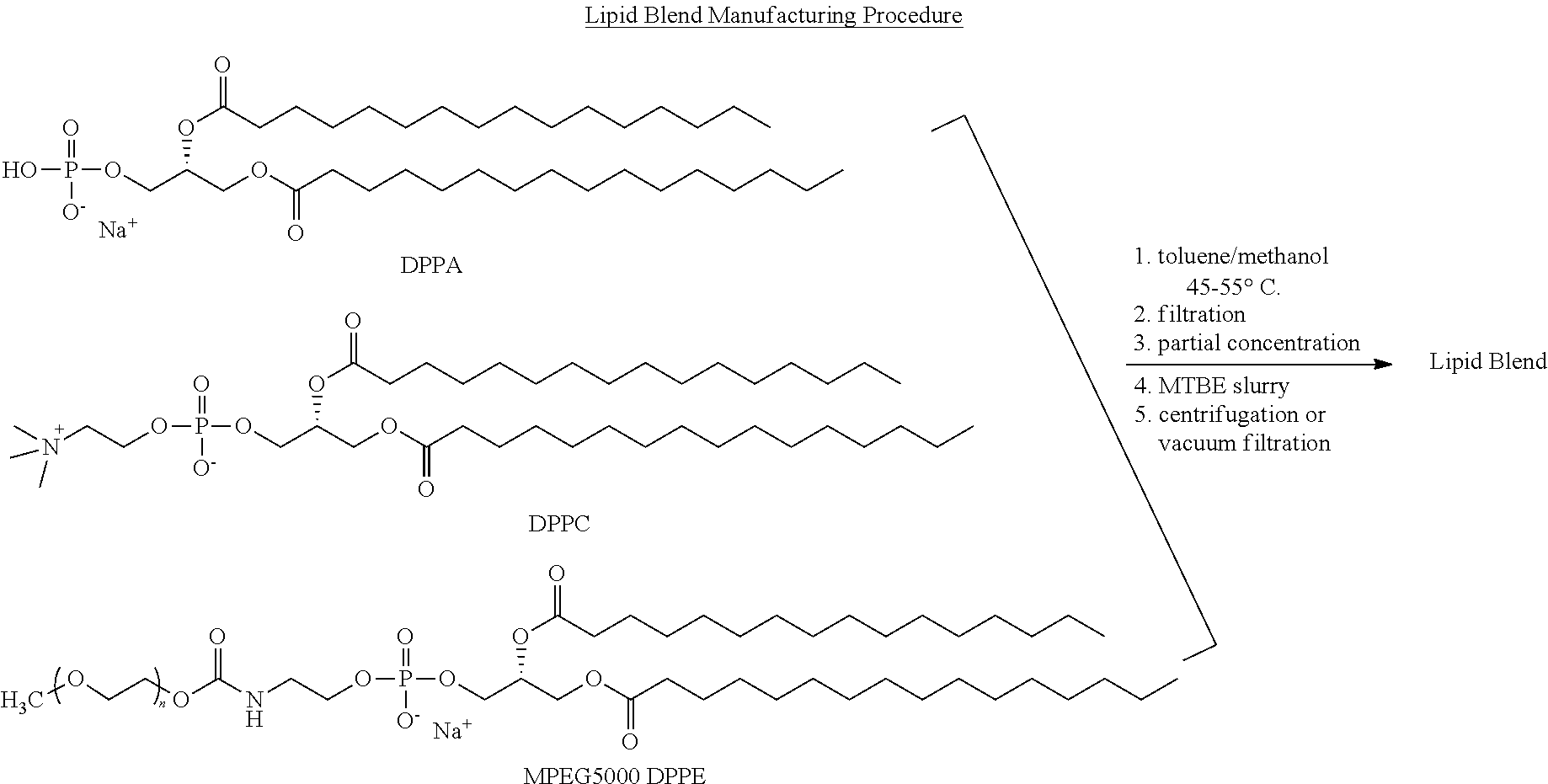
Preparation of a lipid blend and a phospholipid suspension containing the lipid blend
InactiveUS8084056B2Ultrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsLipid formationUltrasound contrast media
The present invention describes processes for the preparation of a lipid blend and a uniform filterable phospholipid suspension containing the lipid blend, such suspension being useful as an ultrasound contrast agent.
Owner:LANTHEUS MEDICAL IMAGING INC
Breviscapine-phosphotide compound and its preparing process
InactiveCN1359682AImprove absorption rateImprove absorptionOrganic active ingredientsUnknown materialsRefluxYolk
A breviscapine-phosphotide composition in the form of oral preparation or injection is prepared from breviscapine and soybean (or egg yolk, or synthetic) lecithine in a wt ratio of 1:(0.5-50) through heating, reflux, washing and drying. Its advantages are high stability and durability, and quickly taking its curative effect.
Owner:SHENYANG PHARMA UNIVERSITY
Environmentally friendly grease composition
InactiveUS20050197260A1Group 5/15 element organic compoundsAdditivesEnvironmentally friendlyGlycerol
An environmentally friendly grease composition is provided comprising (a) a vegetable oil and (b) a cellulose fiber. The grease may further comprise glycerin; a rust inhibitor; and / or a fatty acid ester, lecithin, phosphatidyl choline, or a combination thereof.
Owner:MONTANA STATE UNIVERSITY
Method for preparing ribonucleoside phosphorothioate
ActiveUS20130184450A1Improve efficiencyEfficient synthesisSugar derivativesBulk chemical productionRibonucleosideCombinatorial chemistry
Owner:WAVE LIFE SCI LTD
Feed additive, feed and preparation method thereof for preventing nutritional fatty liver of fish
The invention provides a feed additive, feed and preparation method thereof for preventing nutritional fatty liver of fish. Optimized compound of choline chloride, bile acid, carnitine, betaine and phospholipids is added into pellet feed for feeding fish, which can obviously reduce the settlement of fat in liver of fish caused by nutrient induction, avoid the generation of fatty liver diseases and promote the healthy growth of fish.
Owner:ZHEJIANG KESHENG FEED CO LTD
Advanced Detection of Sepsis
ActiveUS20110118569A1Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsPhospholipinClinical marker
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
Biodegradable film having honeycomb structure
ActiveUS20060189911A1Easy to operateSatisfied with the effectSurgerySurgical drugsPolymer sciencePhospholipid
The invention relates to a honeycomb film made of a biodegradable polymer which has biodegradation properties as an anti-adhesion membrane for preventing adhesion after an operation, is excellent in handling properties, and exhibits a satisfactory adhesion preventive effect stably over a desired period of time, and to a production process of the same. Above all, the invention provides an anti-adhesion membrane of a honeycomb film made of polylactic acid as a biodegradable polymer and a phospholipid and a production process of the same.
Owner:TEIJIN LTD
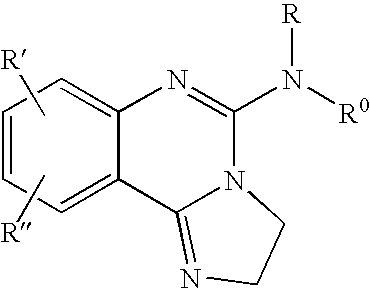
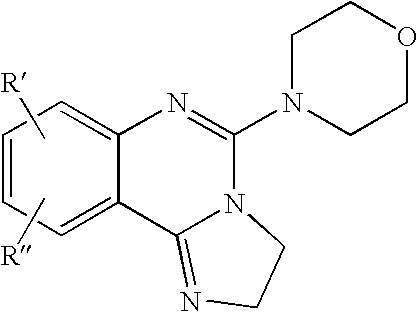
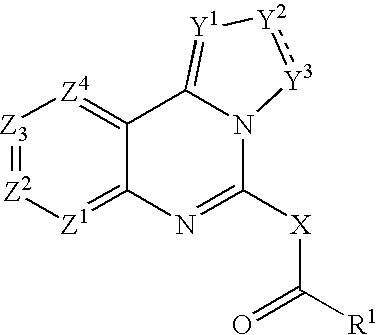
Fused azole-pyrimidine derivatives
The present invention relates to hovel fused azolepyrimidine derivatives, processes for preparing them and pharmaceutical preparations containing them. The fused azolepyrimidine derivatives of the present invention exhibit enhanced potency for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K and particularly with PI3K-γ activity. More specifically, the azole derivatives of the present invention are useful for treatment and prophylaxis of diseases as follows: inflammatory and immunoregulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases, autoixnmune pathologies such as rheumatoid arthritis, and Graves' disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. The compounds of the present invention are also useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
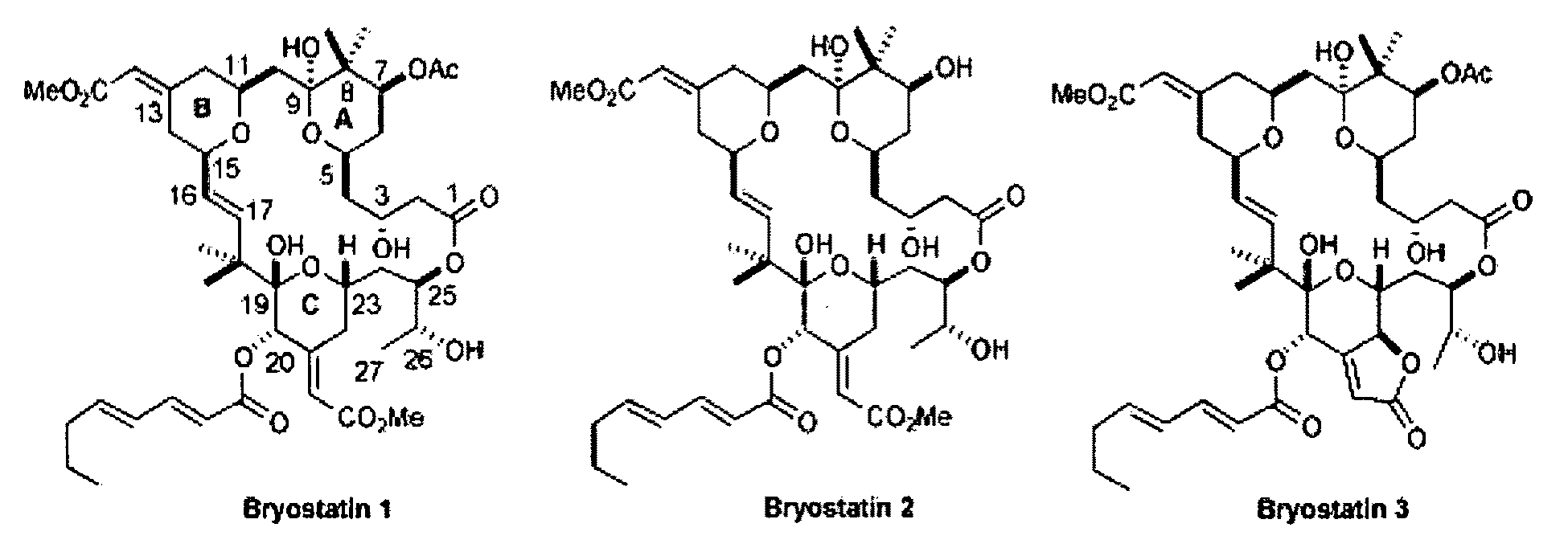
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
Crosslinked cosmetic or pharmaceutical phospholipid-containing gels and emulsions based on ethylene oxide-containing or propylene oxide-containing emulsifiers
Owner:BEIERSDORF AG
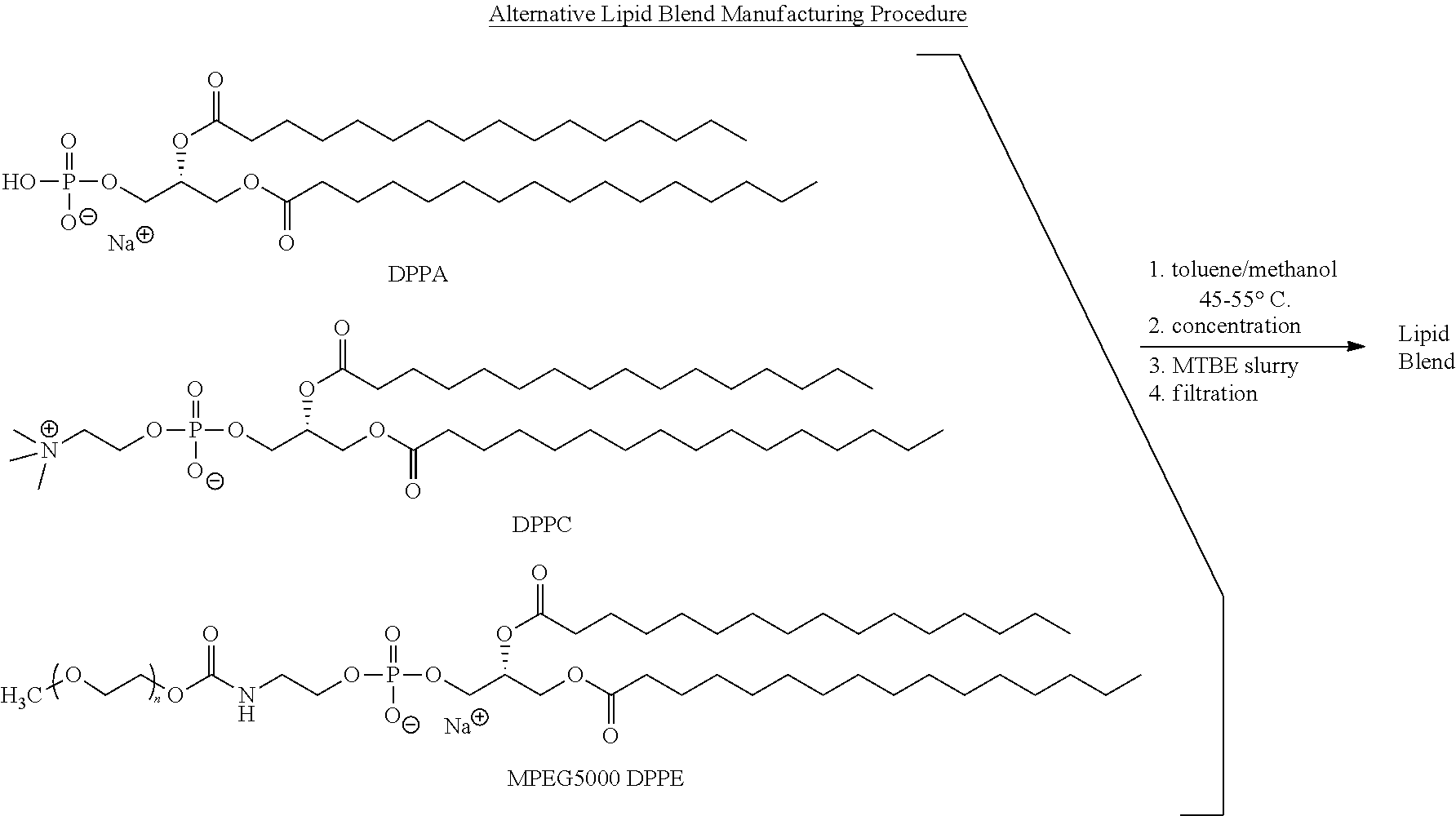
Preparation of a lipid blend and a phospholipid suspension containing the lipid blend
InactiveUS20120027688A1Ultrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsPhospholipinUltrasound contrast media
The present invention describes processes for the preparation of a lipid blend and a uniform filterable phospholipid suspension containing the lipid blend, such suspension being useful as an ultrasound contrast agent.
Owner:LANTHEUS MEDICAL IMAGING INC
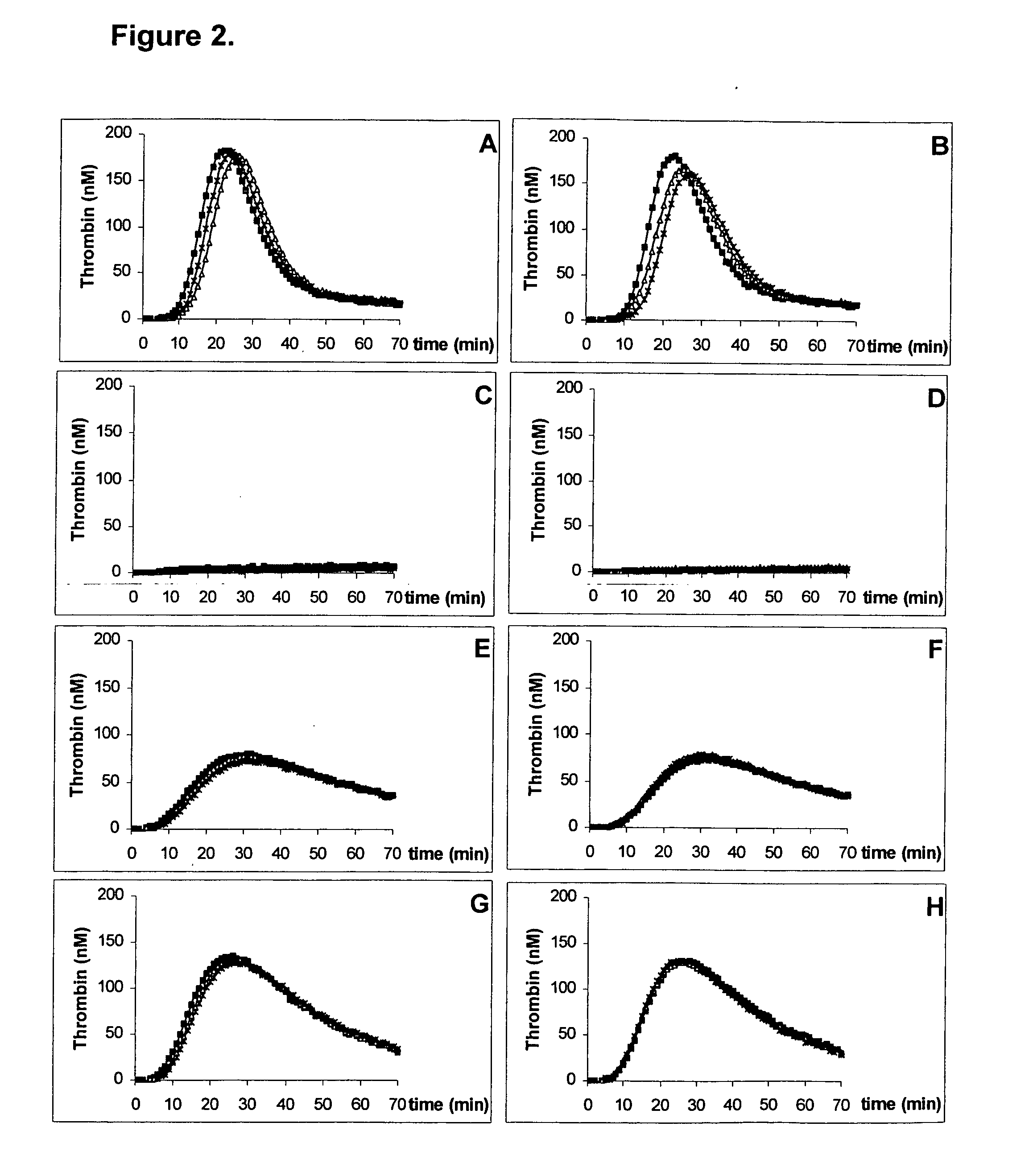
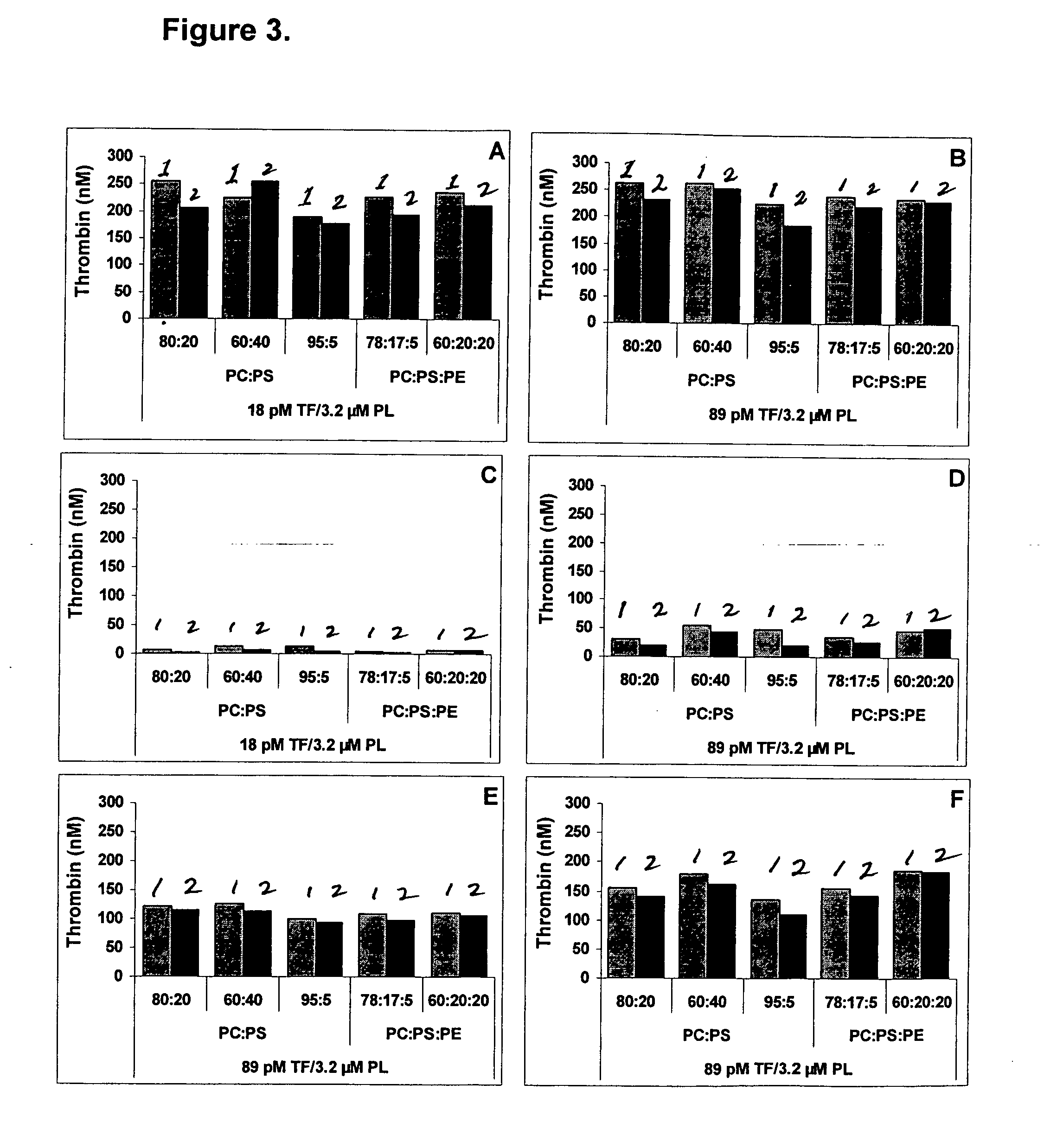
Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
InactiveUS20050221414A1Simple and efficient and fast and reproducible assayConvenient typeMicrobiological testing/measurementBiological material analysisTissue factorThrombin activity
The invention provides a kit for measuring the thrombin generation in a sample of a patient's blood or plasma, or in a sample of clotting factors. The kit contains lyophilized tissue factor / phospholipid-complex and a lyophilized mixture containing a thrombin-substrate and CaCl2. The invention also provides processes for preparing the reagents for the kit. The kit can be used in a method for measuring the thrombin generation in a sample, wherein it is possible to detect changes in thrombin generation kinetics, for example after administration of inhibitor bypassing agents to a patient who has developed inhibitors to an exogenous clotting factor such as Factor VIII.
Owner:BAXTER INT INC +1
Method for extracting squalene by using camellia oleosa seeds as raw materials and tea oil and squalene soft capsules
InactiveCN102146014AWide variety of sourcesPromote regenerationHydrocarbon active ingredientsAntipyreticChromatographic separationCamellia oleifera
The invention discloses a method for extracting squalene by using camellia oleosa seeds as raw materials and tea oil and squalene soft capsules and relates to the technical field of biomedicine. The extraction method comprises the following process flows of material preparation, grinding, leaching, concentration, methyl esterification, complexant-borax reaction, macroporous resin chromatographic separation and purification, concentration, extraction, washing, concentration, preparation of squalene crude oil and supercritical carbon dioxide extraction, so that squalene essential oil with the squalene content of more than or equal to 80 percent is obtained. The tea oil and squalene soft capsules are prepared from the following raw materials in percentage by weight: 87 to 90 percent of squalene essential oil with the squalene content of more than or equal to 80 percent, 3 to 5 percent of polyethylene glycol 400, 2 to 3 percent of beewax and 2 to 5 percent of phospholipid. The raw material resources are wide. The prepared tea oil and squalene soft capsules not only can be used for medicine, but also can be used for food therapy, are particularly suitable for administration of treatment and food therapy of patients suffering from hepatitis, fatty liver, acute and chronic inflammation, tumor, chemotherapy, diabetes mellitus and cardio-cerebrovascular disease and have no toxic or side effects.
Owner:李文东
Long-circulating liposome capable of avoiding accelerated blood clearance (ABC) phenomenon, and preparation method and application thereof
InactiveCN104644555APromote escapeIncrease serum stabilityOrganic active ingredientsAntipyreticLipid formationDisease
The invention relates to a long-circulating liposome capable of avoiding an accelerated blood clearance (ABC) phenomenon, a medicine preparation containing the long-circulating liposome, and a preparation method and an application of the long-circulating liposome. The long-circulating liposome provided by the invention comprises phospholipid, auxiliary lipid and poly carboxyl betaine lipid, wherein the long-circulating liposome and the preparation thereof can be applied to medicines for treating related diseases. According to the long-circulating liposome for avoiding the ABC phenomenon provided by the invention, the defect that a common liposome is easily recognized by a reticular endothelium system is overcome; a long-circulating effect of blood is reached; meanwhile, the ABC phenomenon caused by multiple intravenous injections of the PEG-modified long-circulating liposome is avoided; the medicine effect of a therapeutic medicine is effectively improved; and the long-circulating liposome has a high application prospect in the field of medicines.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Solid nano pharmaceutical formulation and preparation method thereof
A method of preparing low water-soluble medicine into solid nanometer pharmaceutical formulation is disclosed. According to the characters of molecular aggregates such as supramolecular chemical micelles and vesicles, the formulation, which based on the hydroxypropyl-beta-cyclodextrin and phospholipid, is prepared under the condition of hyperthermia sterilization and decompression. Such nanometer formulation is sterile particle or powder with loose porosity. For directly intravenous use, the formulation has targeting activity, sustained release and long circulating characters. While as a solid oral product, it is fast-release, fast-effective, and improved bioavailability characters, and is readily melted in mouth. The formulation utilizes secure accessories, traditional equipments and methods, thus, it is suited to be used and manufactured widely. Also disclosed is intravenous formulation of anticancer paclitaxel, which characterized that there has no polyoxyethylenated castor oil in it. Such intravenous formulation is nonallergic so that it has higher security and efficiency compared to present commercially available paclitaxel formulations.
Owner:LIU YUNGING +3
Full dry-mixing infant formula milk powder and preparation method of full dry-mixing infant formula milk powder
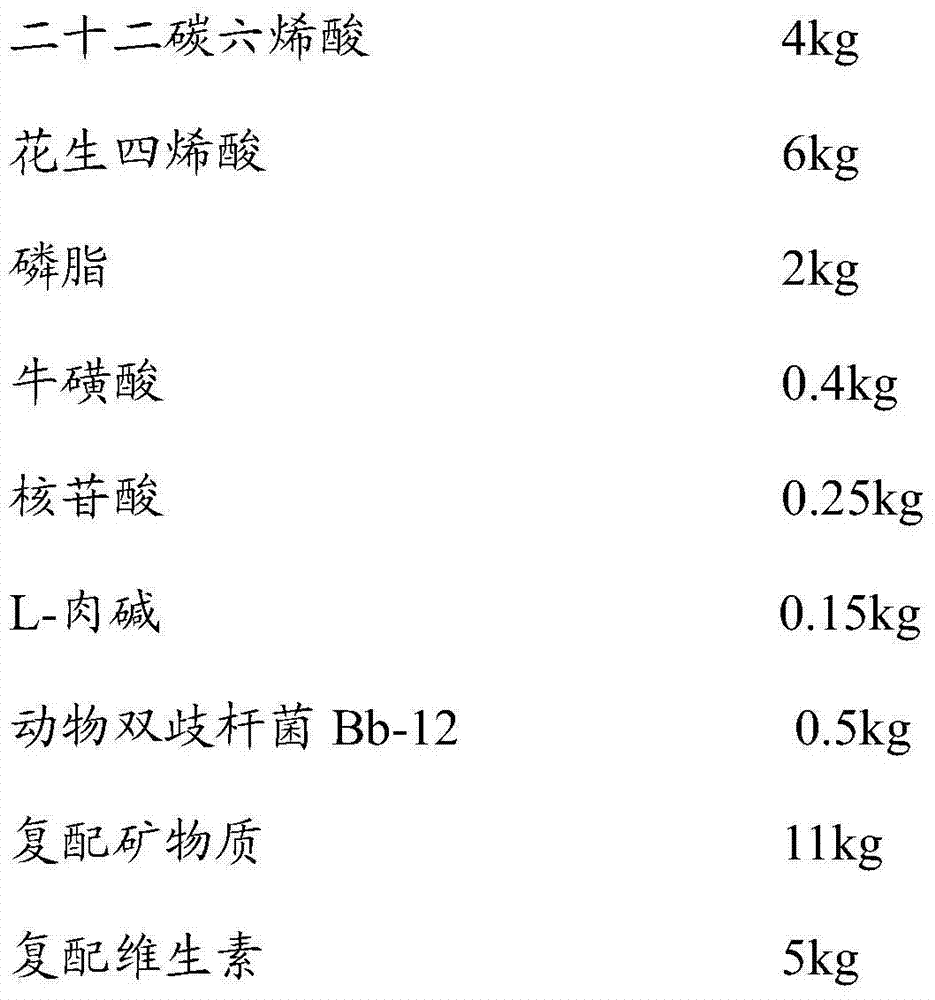
PendingCN104757123APromote digestion and absorptionMeet the needs of comprehensive growthMilk preparationBiotechnologyNucleotide
The invention provides full dry-mixing infant formula milk powder and a preparation method of the full dry-mixing infant formula milk powder. Auxiliaries of the infant formula milk powder comprise whey protein concentrate, galactooligosaccharide, fructo-oligose, docosahexaenoic acid, arachidonic acid, phospholipid, nucleotide, taurine, L-carnitine, probiotics, compound vitamin and compound mineral substances; the full dry-mixing infant formula milk powder is characterized in that the auxiliaries are pre-mixed with demineralized whey powder or skim milk powder to form a pre-mixture firstly, and the pre-mixture is totally mixed with other main ingredients to prepare the full dry-mixing infant formula milk powder. The full dry-mixing infant formula milk powder provided by the invention simulates humanisation productive infant formula milk powder, so that the digestive absorption of infants can be improved, and the infant intelligence development and the immunity of the infants can be improved. The infant formula milk powder prepared by using the method adopts uniform effective components and active components, stable in storage and transportation and low in production address selection requirement and suitable for being energetically popularized.
Owner:合生元(长沙)营养食品有限公司
Soybean phospholipid pressed candies and preparation method thereof
The invention provides soybean phospholipid pressed candies and a preparation method thereof. The soybean phospholipid pressed candies are prepared from the following raw materials in percentage by weight: 40 to 60 percent of sweetener or white granulated sugar and sweetener, 10 to 20 percent of soybean phospholipid powder, 10 to 30 percent of soybean milk powder, 1 to 10 percent of fruit powder,1 to 10 percent of vegetable powder, 5 to 35 percent of malto dextrin, 0 to 8 percent of complex vitamin and minerals, 0 to 10 percent of soybean compound nutritional factor mixture, 0 to 3 percent of flavoring essence, 0 to 0.3 percent of coloring agent and 0.5 to 2.5 percent of magnesium stearate. In the soybean phospholipid pressed candies, the added soybean phospholipid contains various active substances such as orgorganophosphorus, choline, inositol and the like, also contains essential fatty acid required by a human body, does not contain any cholesterol, and provides guarantee for metabolism and health life of the human body. The soybean phospholipid pressed candies have effects of resisting oxidation and ageing, reducing blood fat, promoting blood circulation, beautifying, eliminating brain fatigue, improving memory and the like, are suitable to be eaten by children and middle-aged and elderly people daily to promote mental development of the children and prevent dementia of the middle-aged and elderly people, and are also suitable to be eaten by workers daily to have the effects of regulating intestines and stomach and the like.
Owner:JIUSAN OILS & GRAINS IND GRP CO LTD
Method for preparing soybean separate protein by ultra-filter film separation technology
InactiveCN1615722AImprove flavorImprove solubilityVegetable proteins working-upEgg whiteSeparation technology
The preparation process of separate soybean protein includes the following steps: low temperature defatting bean, the first leaching, separating protein liquid from bean dregs, the second leaching, separating protein liquid from bean dregs, deairing, acid depositing, filterig with ultrafiltering film to obtain concentrated liquid, water washing, neutralizing, sterilizing, spray drying and spraying phospholipid to obtain the separate soybean protein product.
Owner:哈高科大豆食品有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com