Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Clinical marker" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
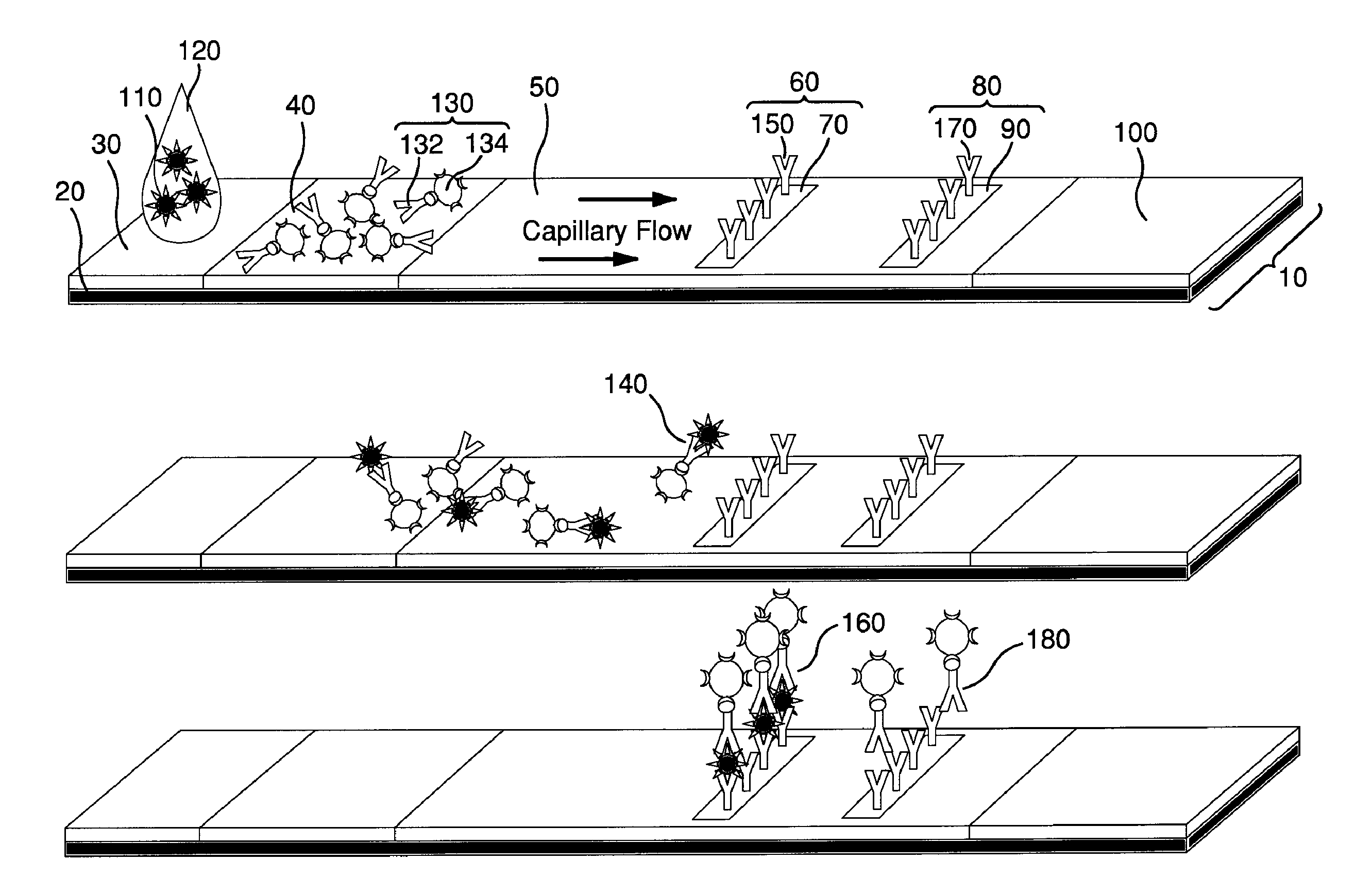
Surface Enhanced Raman Scattering and Multiplexed Diagnostic Assays
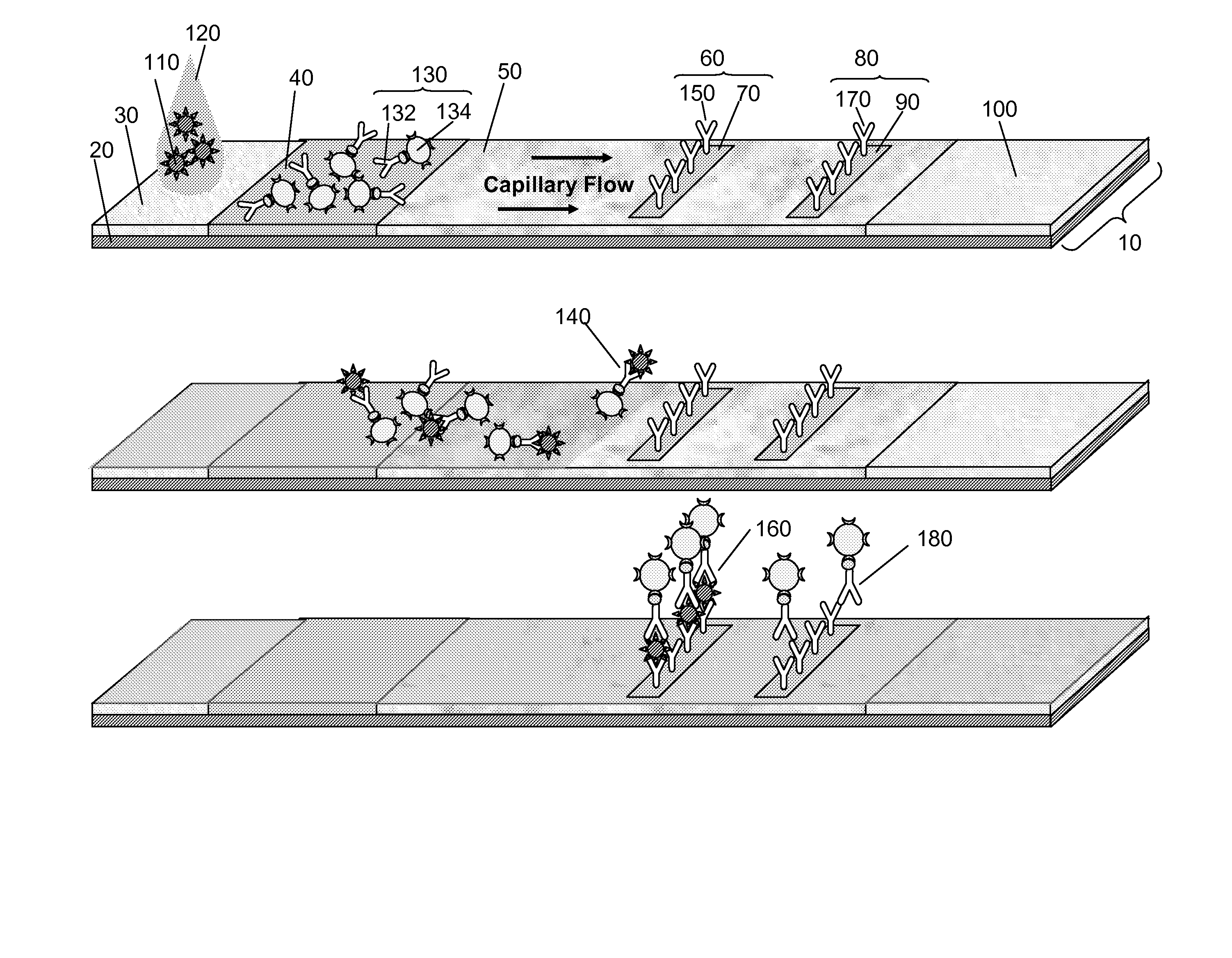
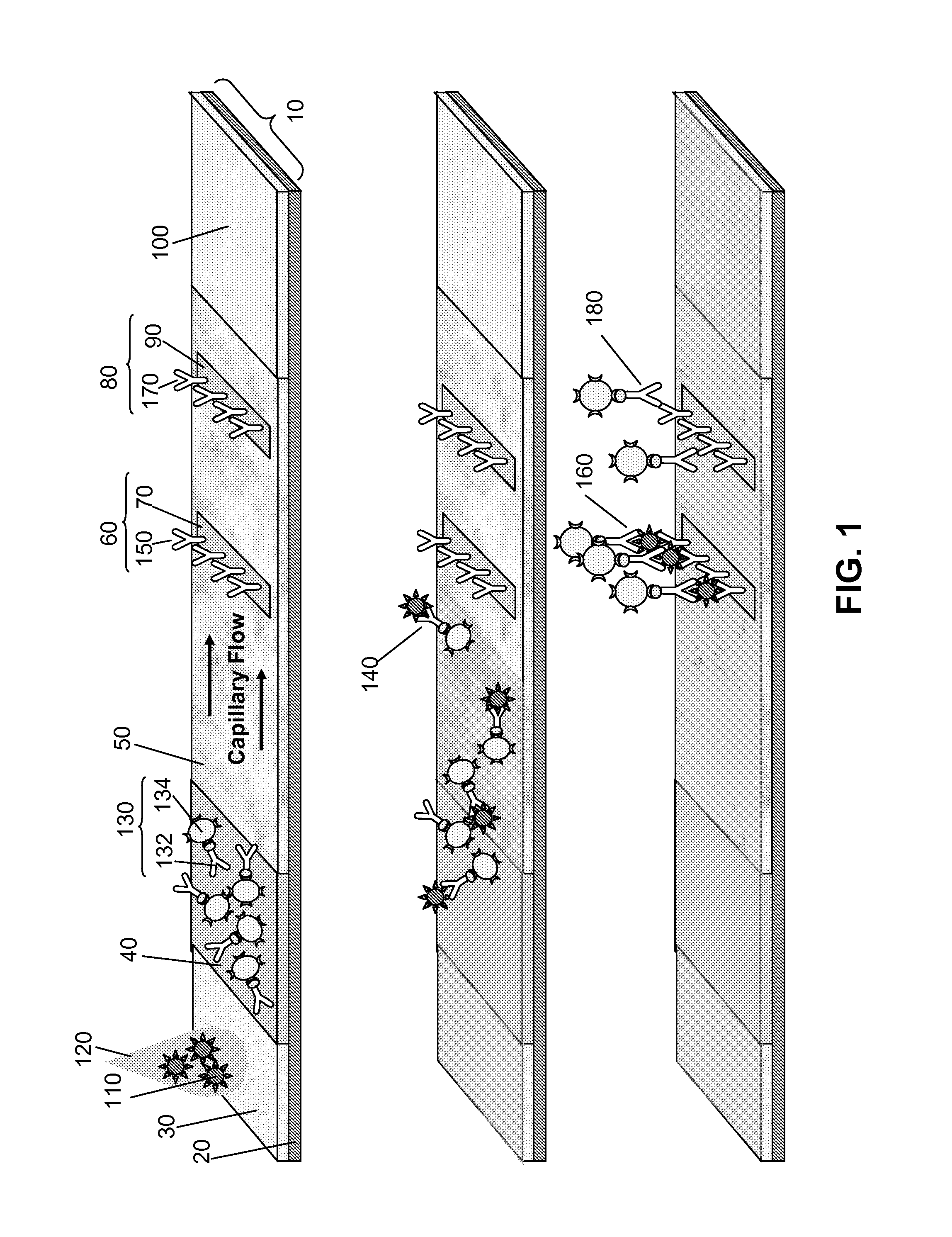
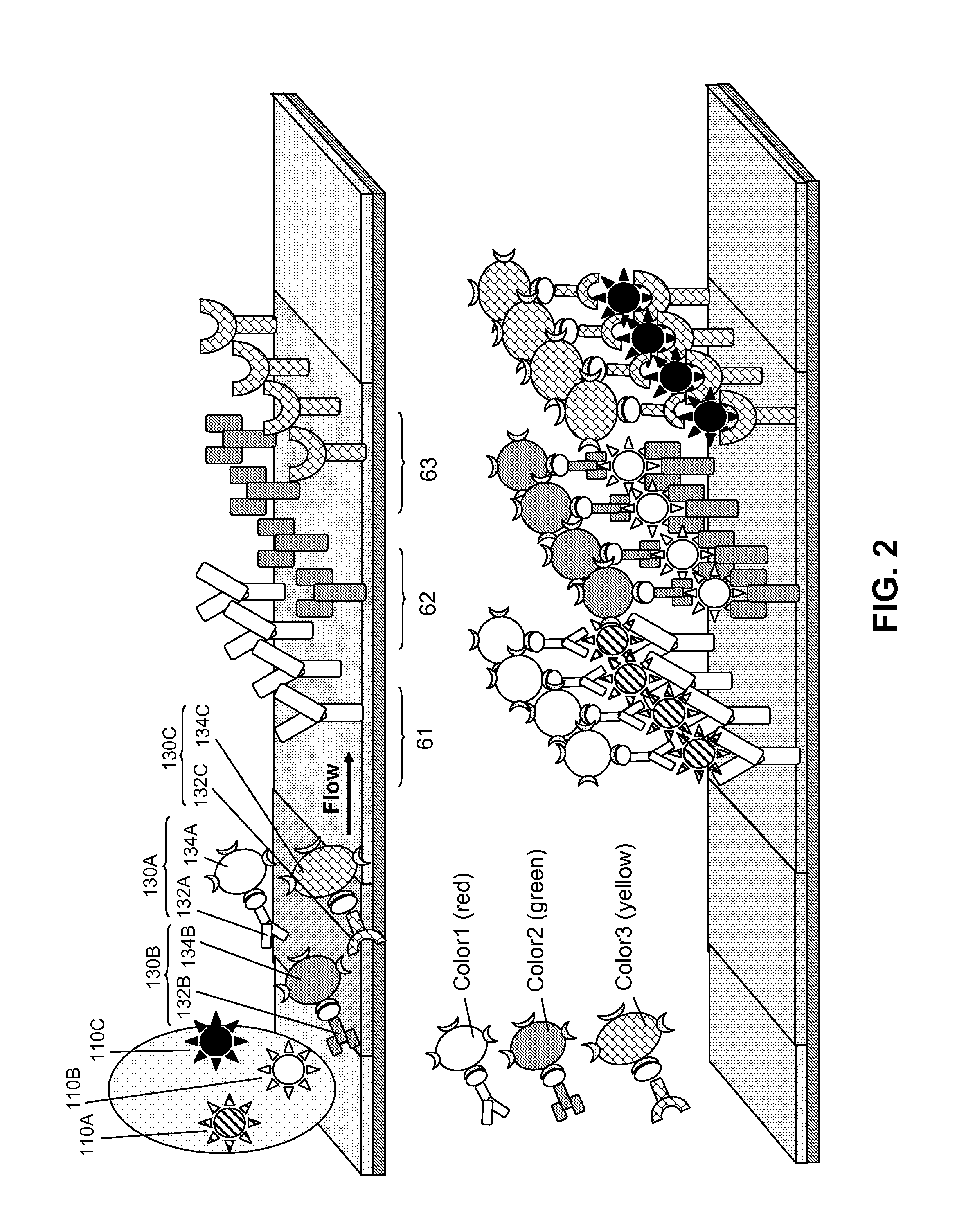
Multiplexed lateral flow assays, related methods, and devices are disclosed which are capable of simultaneously detecting multiple analytes. The assays are preferably immunoassays and can be multiplexed spatially, spectrally, and both spatially and spectrally. Multiplexed assays are disclosed employing quantum dots for applications including the detection of human proteins and the monitoring of microorganisms relevant to water contamination. The multiplexed assays can employ one or more species of Surface Enhanced Raman Scattering nanoparticles, with one or more species having a unique Raman shift spectrum. The invention is widely adaptable to a variety of analytes such as biowarfare agents, human clinical markers, and other substances.
Owner:CALIFORNIA INST OF TECH
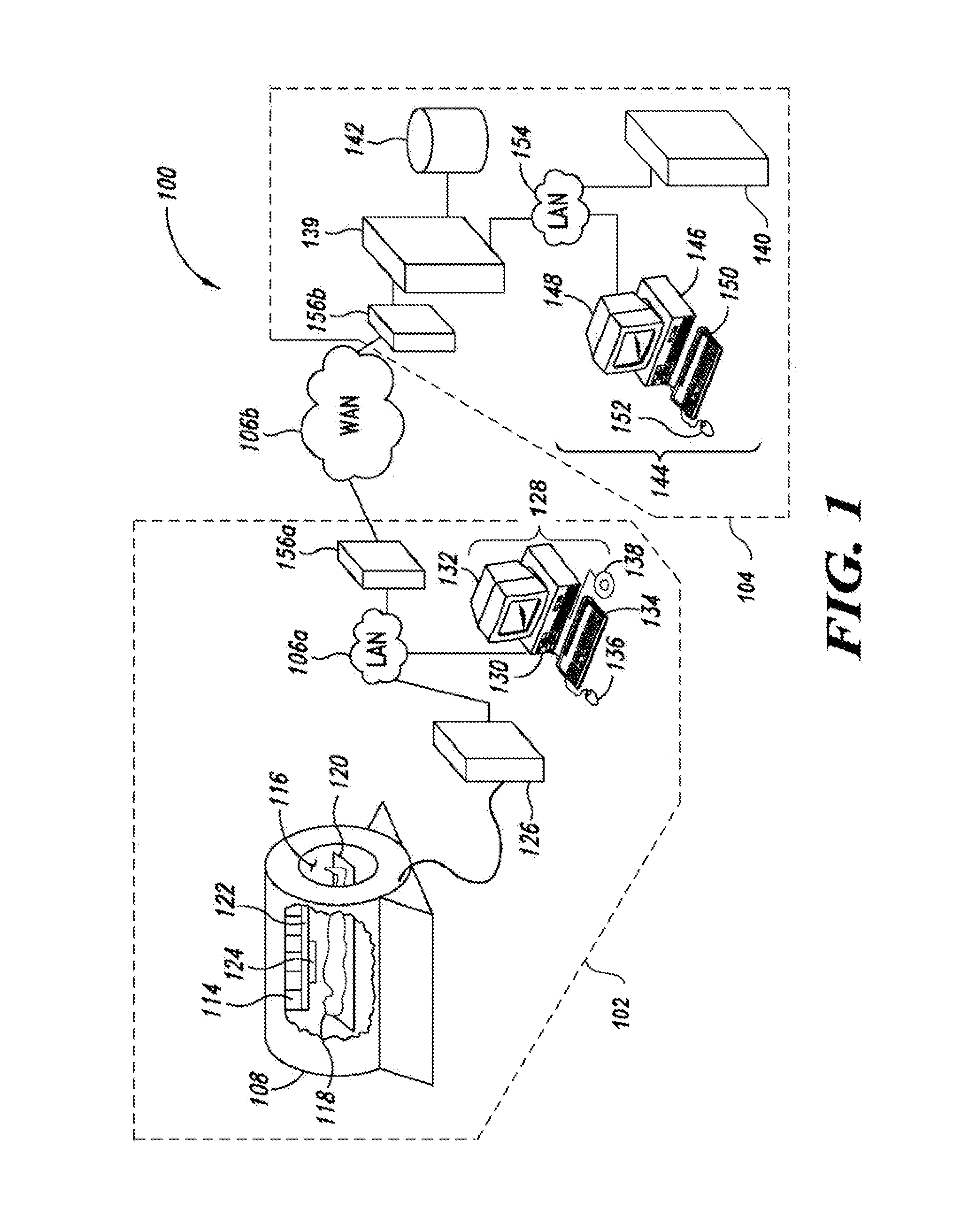
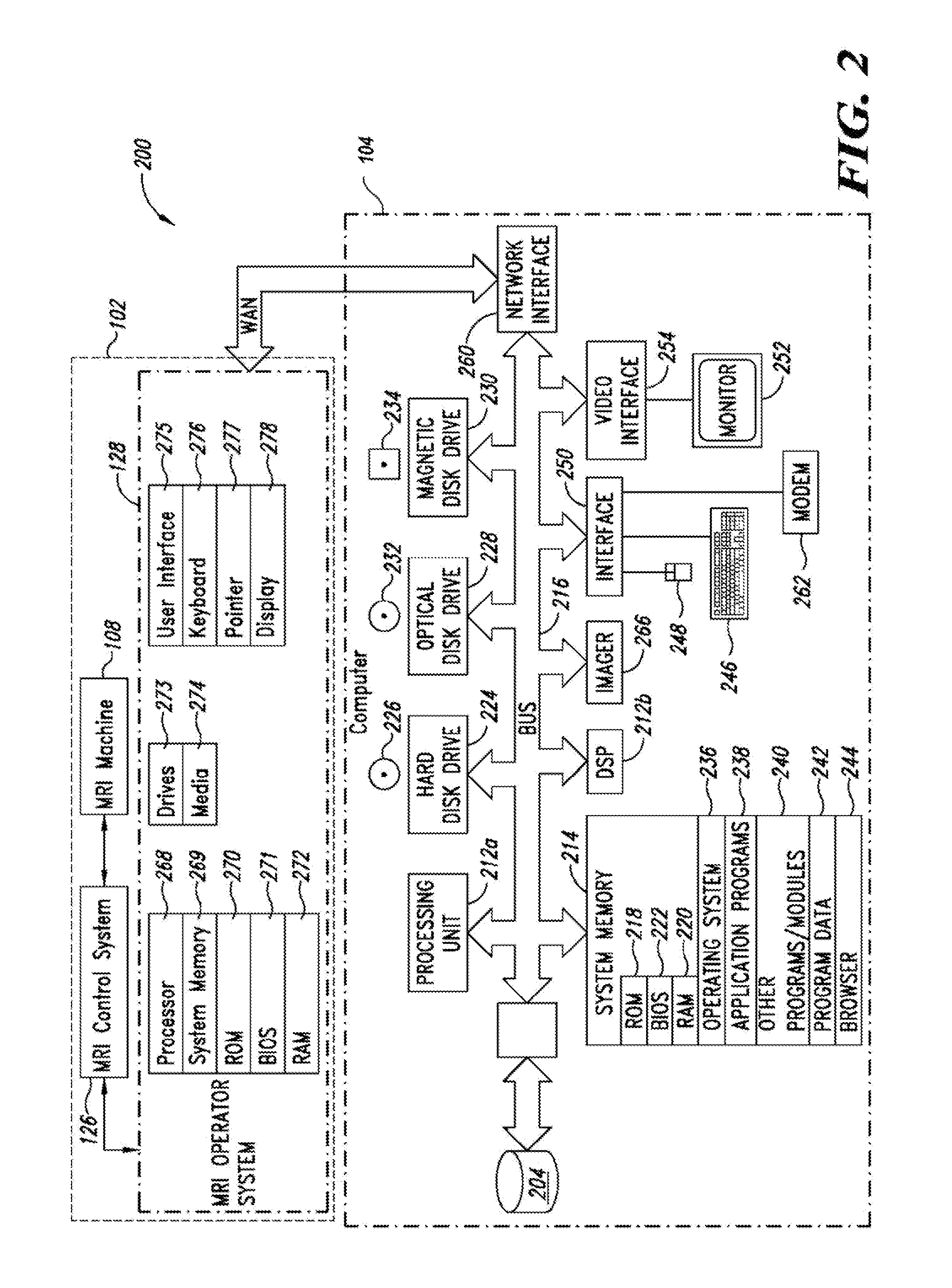
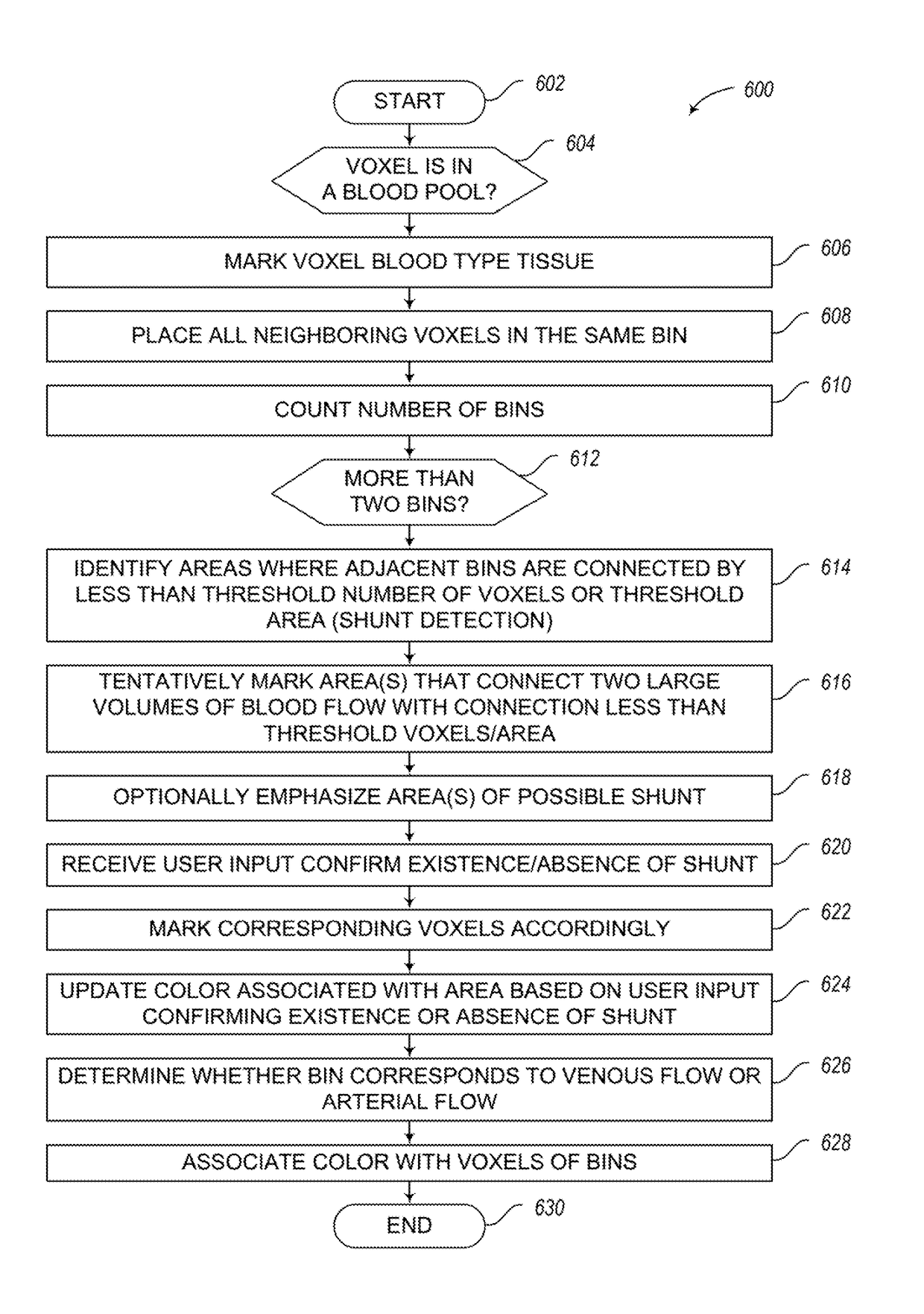
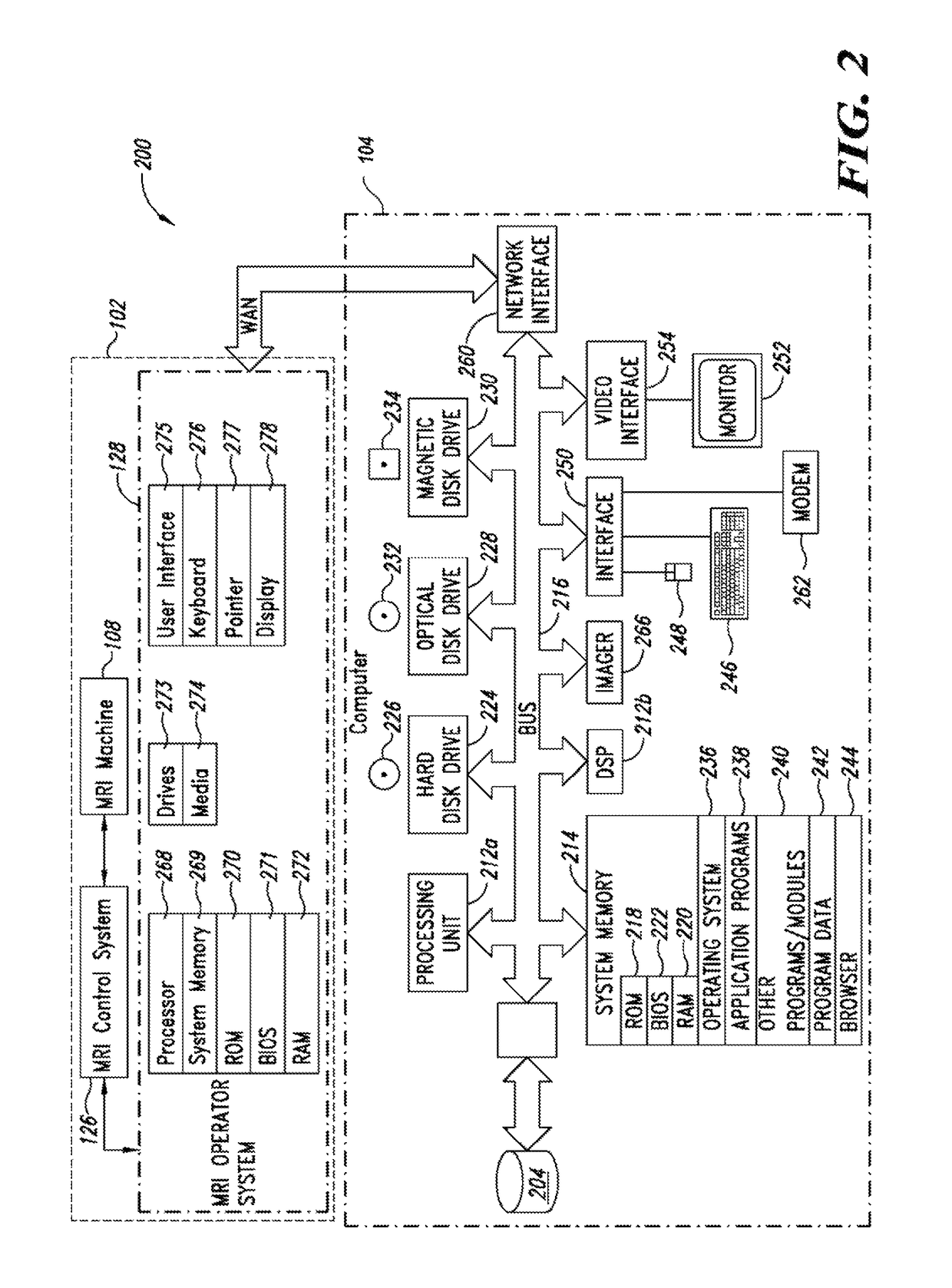
Apparatus, methods and articles for four dimensional (4D) flow magnetic resonance imaging
ActiveUS20160338613A1Low costShorten the lengthImage enhancementMedical imagingAnatomical structuresData set
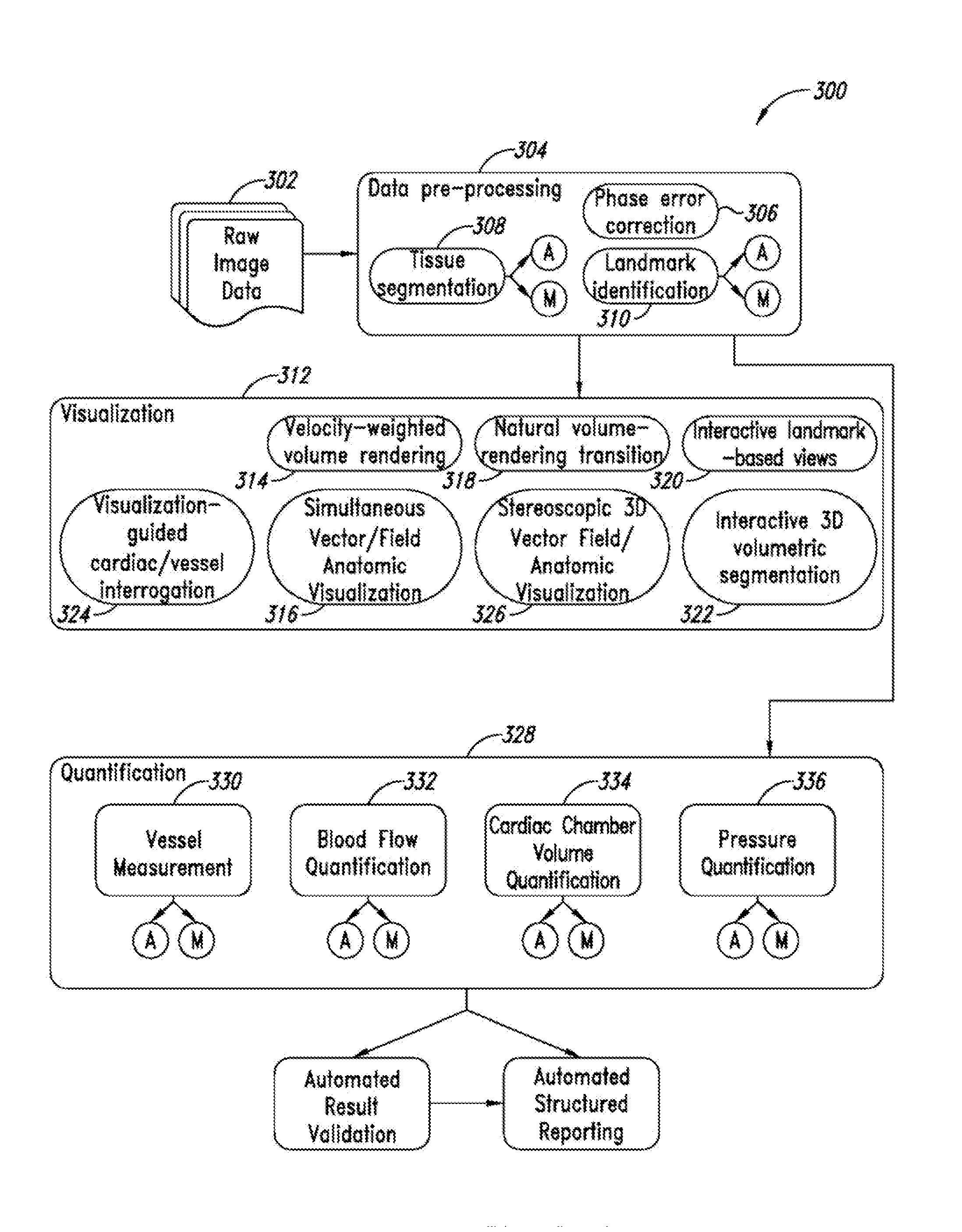
An MRI image processing and analysis system may identify instances of structure in MRI flow data, e.g., coherency, derive contours and / or clinical markers based on the identified structures. The system may be remotely located from one or more MRI acquisition systems, and perform: perform error detection and / or correction on MRI data sets (e.g., phase error correction, phase aliasing, signal unwrapping, and / or on other artifacts); segmentation; visualization of flow (e.g., velocity, arterial versus venous flow, shunts) superimposed on anatomical structure, quantification; verification; and / or generation of patient specific 4-D flow protocols. An asynchronous command and imaging pipeline allows remote image processing and analysis in a timely and secure manner even with complicated or large 4-D flow MRI data sets.
Owner:ARTERYS INC
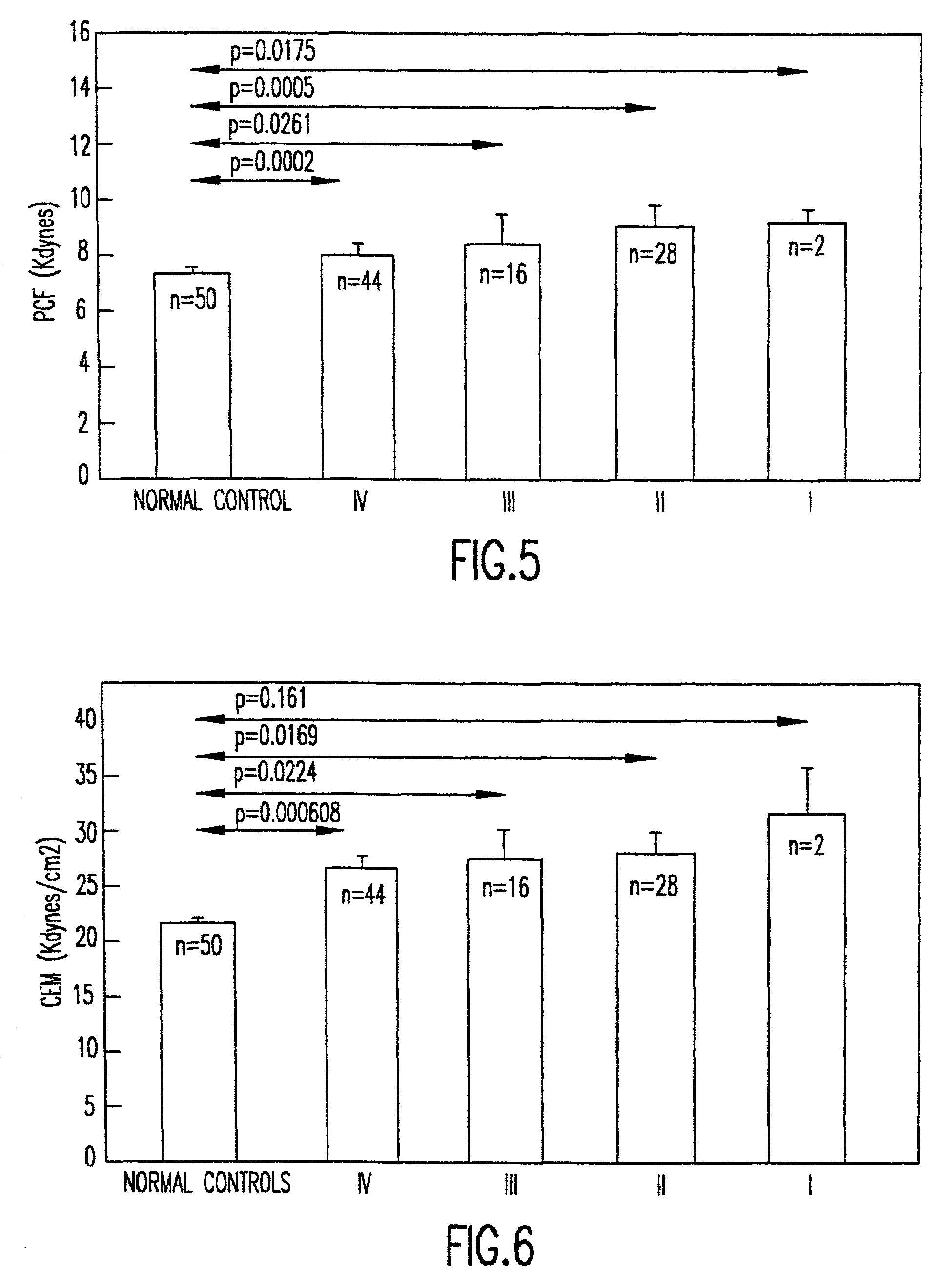
Method of using platelet contractile force and whole blood clot elastic modulus as clinical markers
InactiveUS7192726B1Process is time-consume and expensiveRapid recognition and quantificationMicrobiological testing/measurementDisease diagnosisMedicinePlatelet contraction
Platelet contractile force and / or clot elastic modulus measurements are used to identify patients at risk for atherosclerosis or for bleeding during surgical procedures or other applications. Measurements which are elevated are indicative of atherosclerosis, and measurements which are reduced are indicative of a bleeding risk.
Owner:HEMODYNE
Surface Enhanced Raman Scattering and Multiplexed Diagnostic Assays
InactiveUS20100055721A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismAssay
Multiplexed lateral flow assays, related methods, and devices are disclosed which are capable of simultaneously detecting multiple analytes. The assays are preferably immunoassays and can be multiplexed spatially, spectrally, and both spatially and spectrally. Multiplexed assays are disclosed employing quantum dots for applications including the detection of human proteins and the monitoring of microorganisms relevant to water contamination. The multiplexed assays can employ one or more species of Surface Enhanced Raman Scattering nanoparticles, with one or more species having a unique Raman shift spectrum. The invention is widely adaptable to a variety of analytes such as biowarfare agents, human clinical markers, and other substances.
Owner:CALIFORNIA INST OF TECH
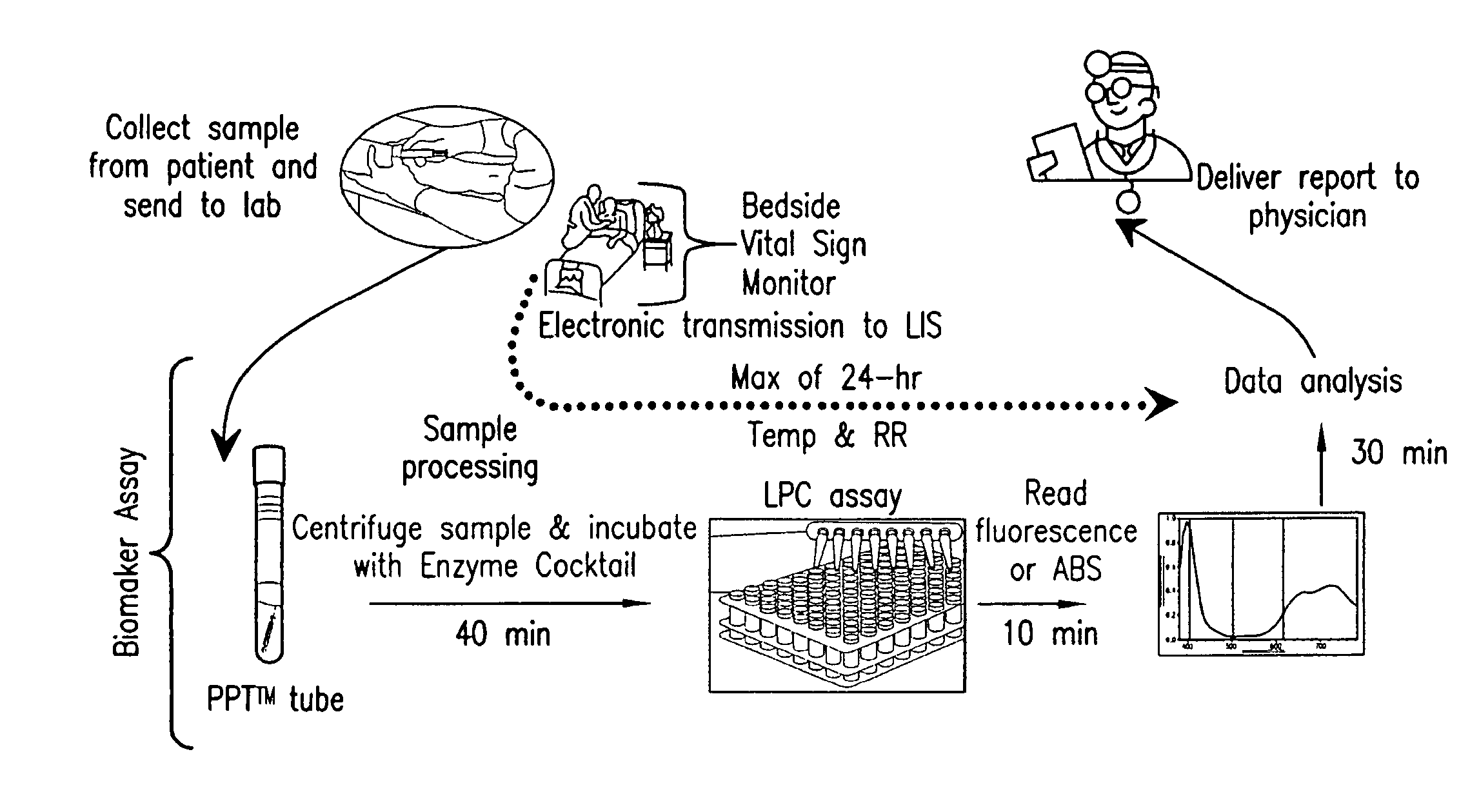
Advanced Detection of Sepsis
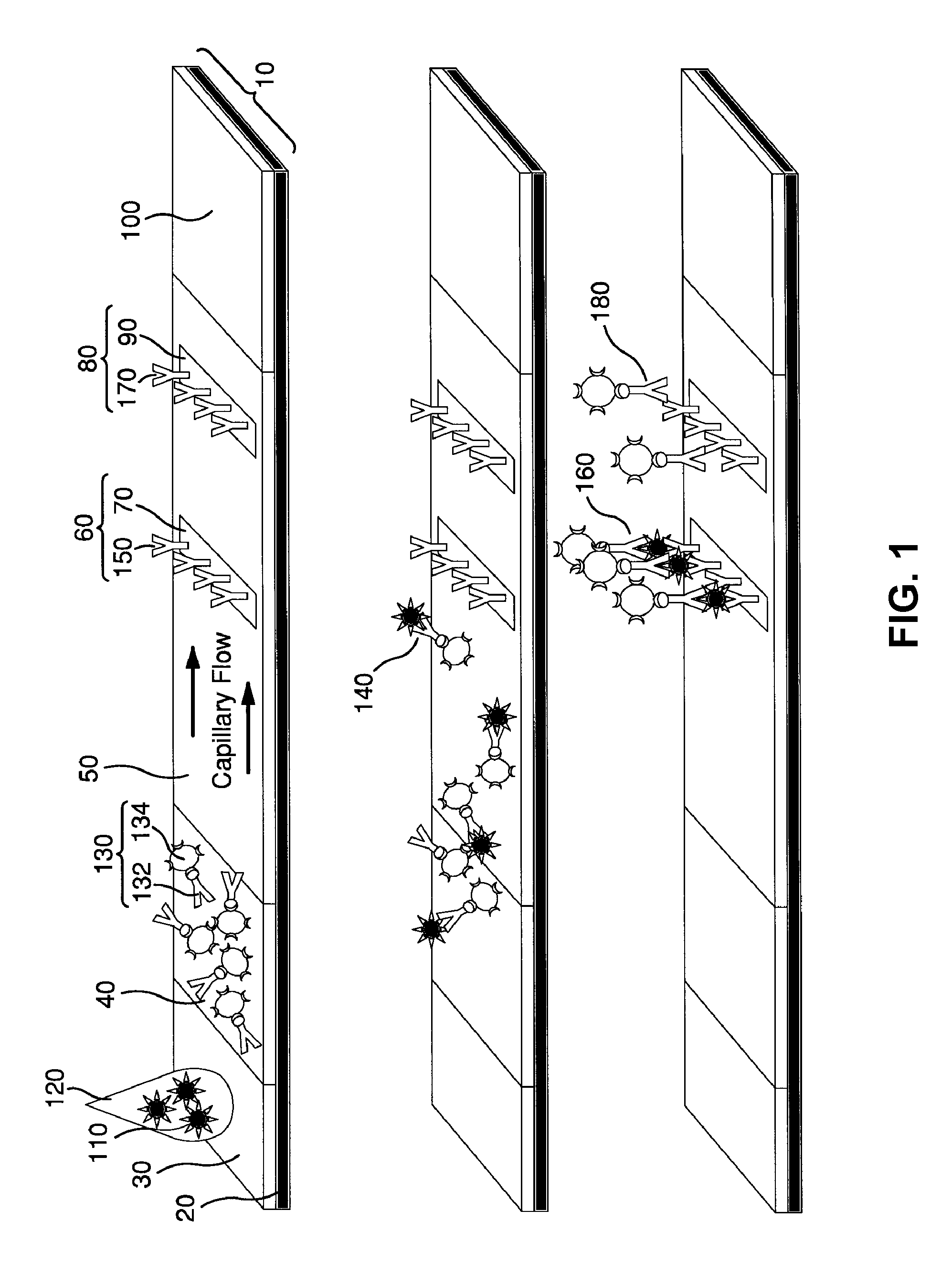
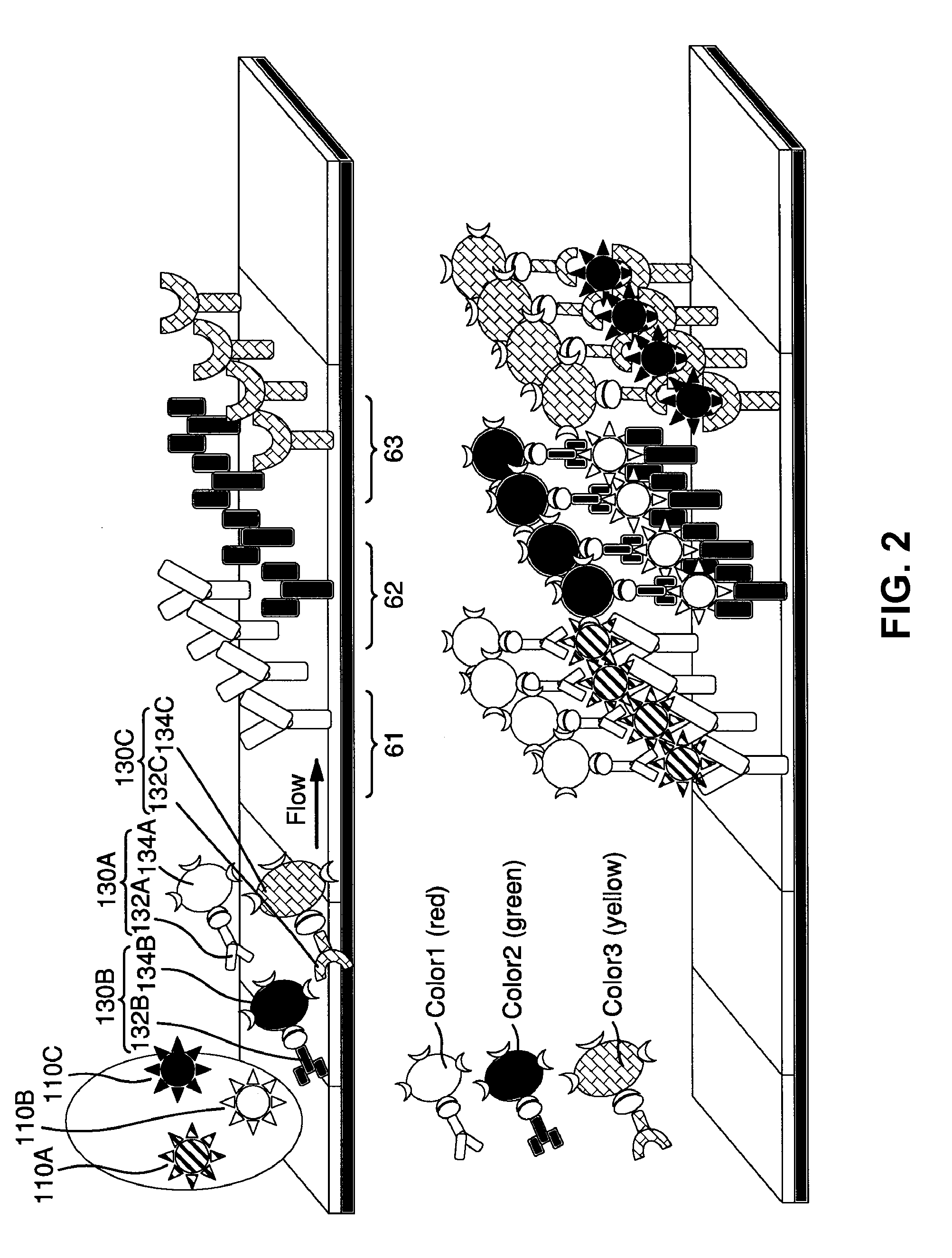
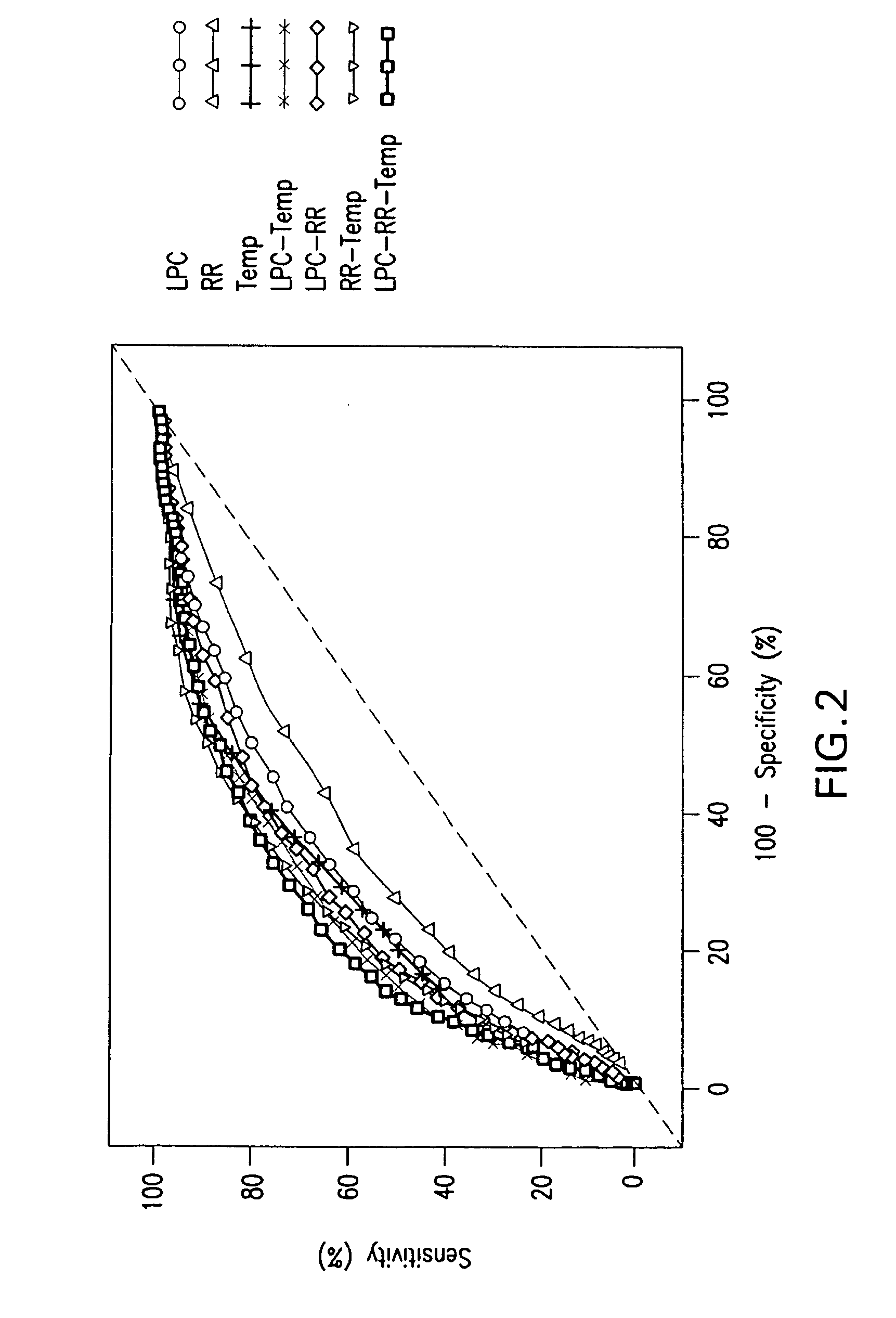
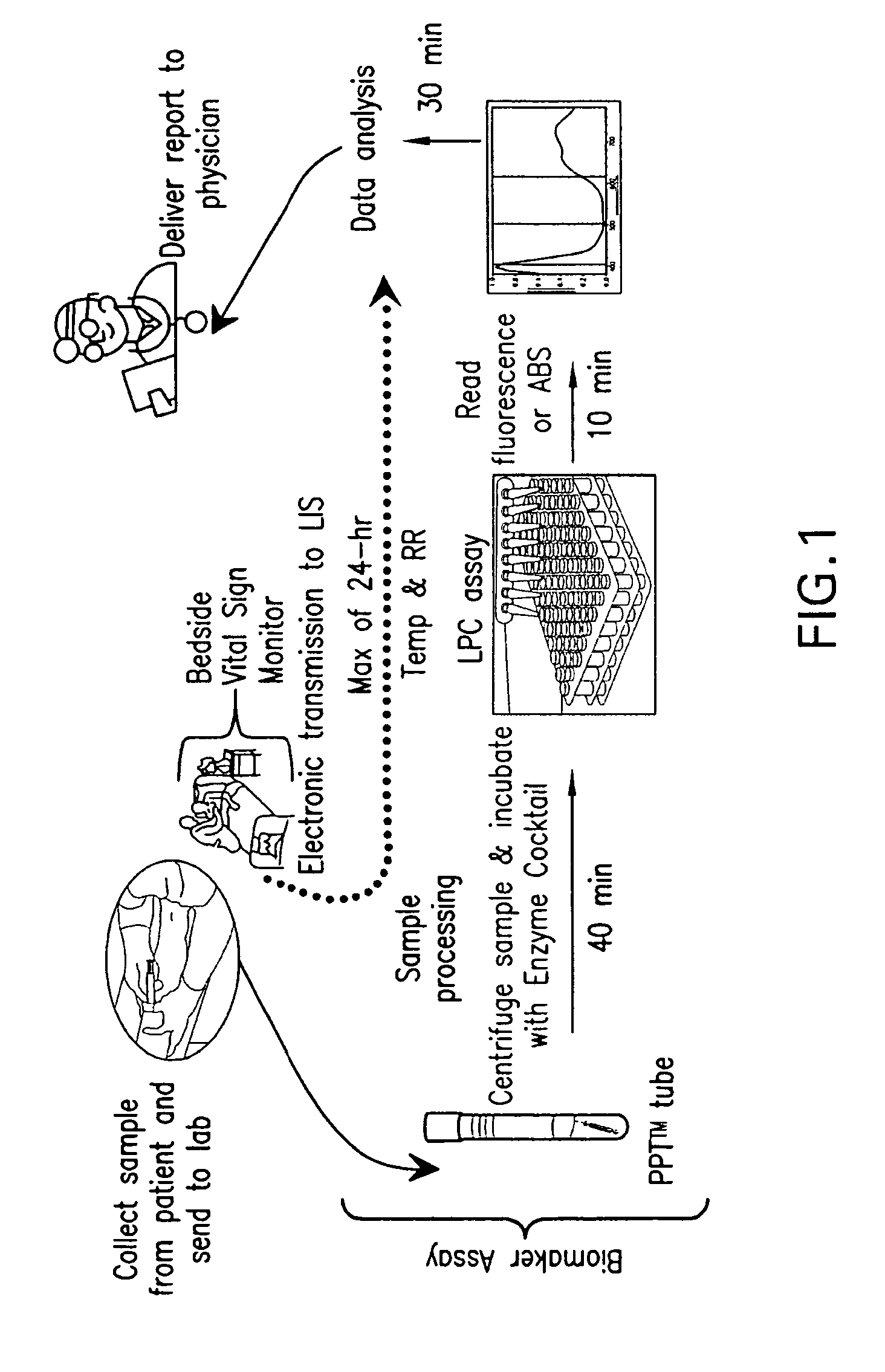
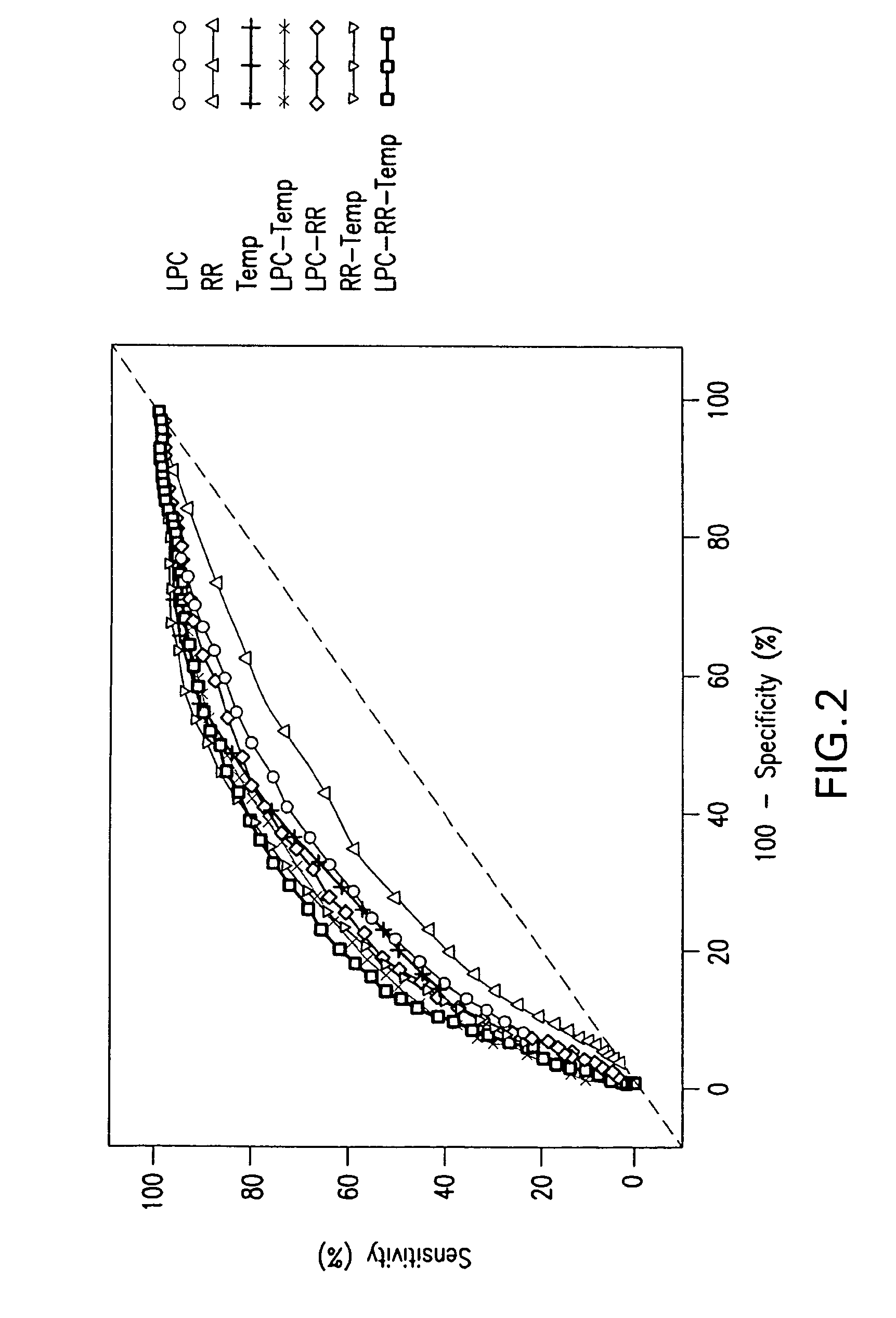
ActiveUS20110118569A1Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsPhospholipinClinical marker
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
Lipidomic biomarkers for atherosclerosis and cardiovascular disease
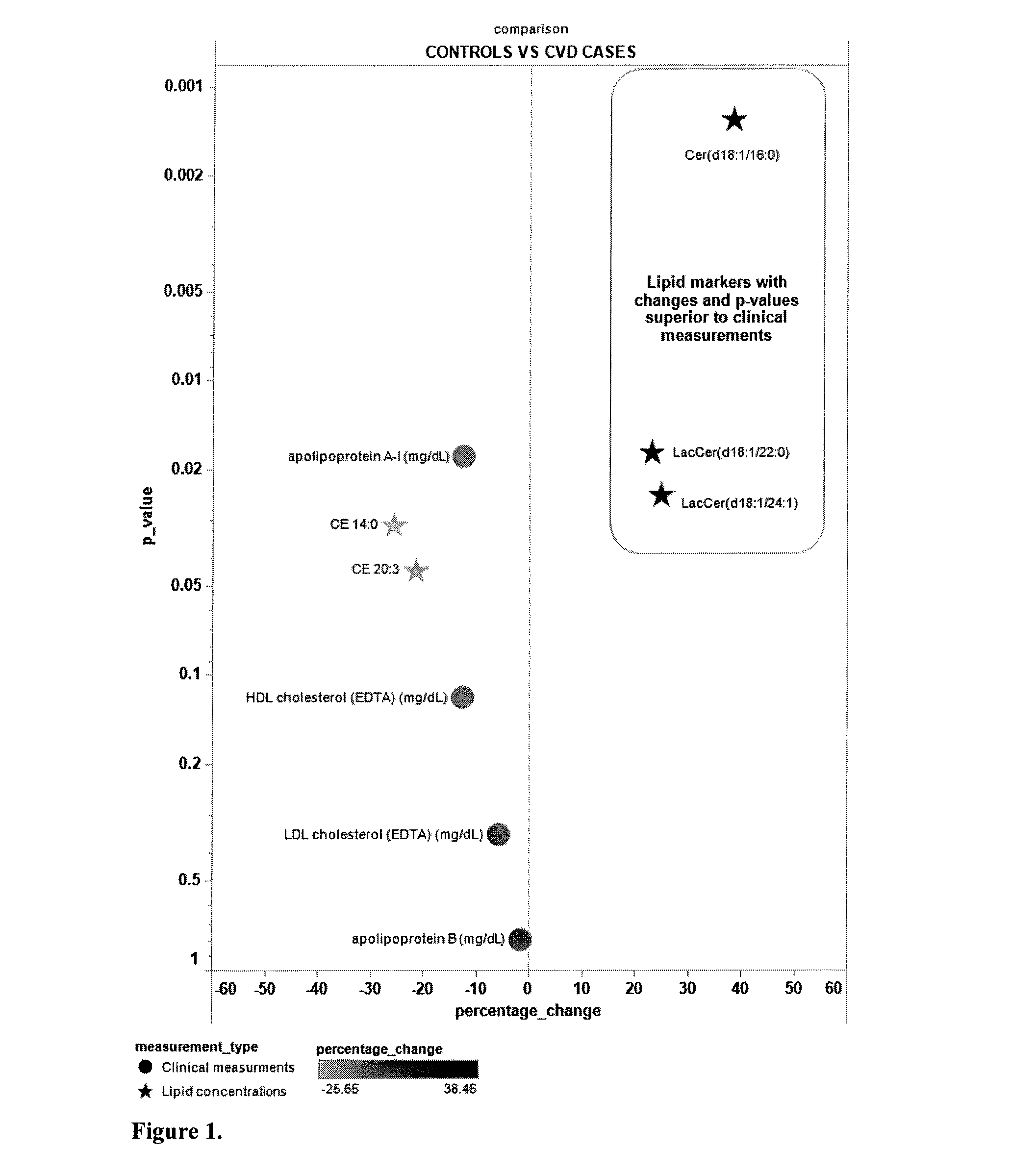
ActiveUS20130045217A1Easy diagnosisSuperior prognostic valueBiocideComponent separationLipidomeClinical marker
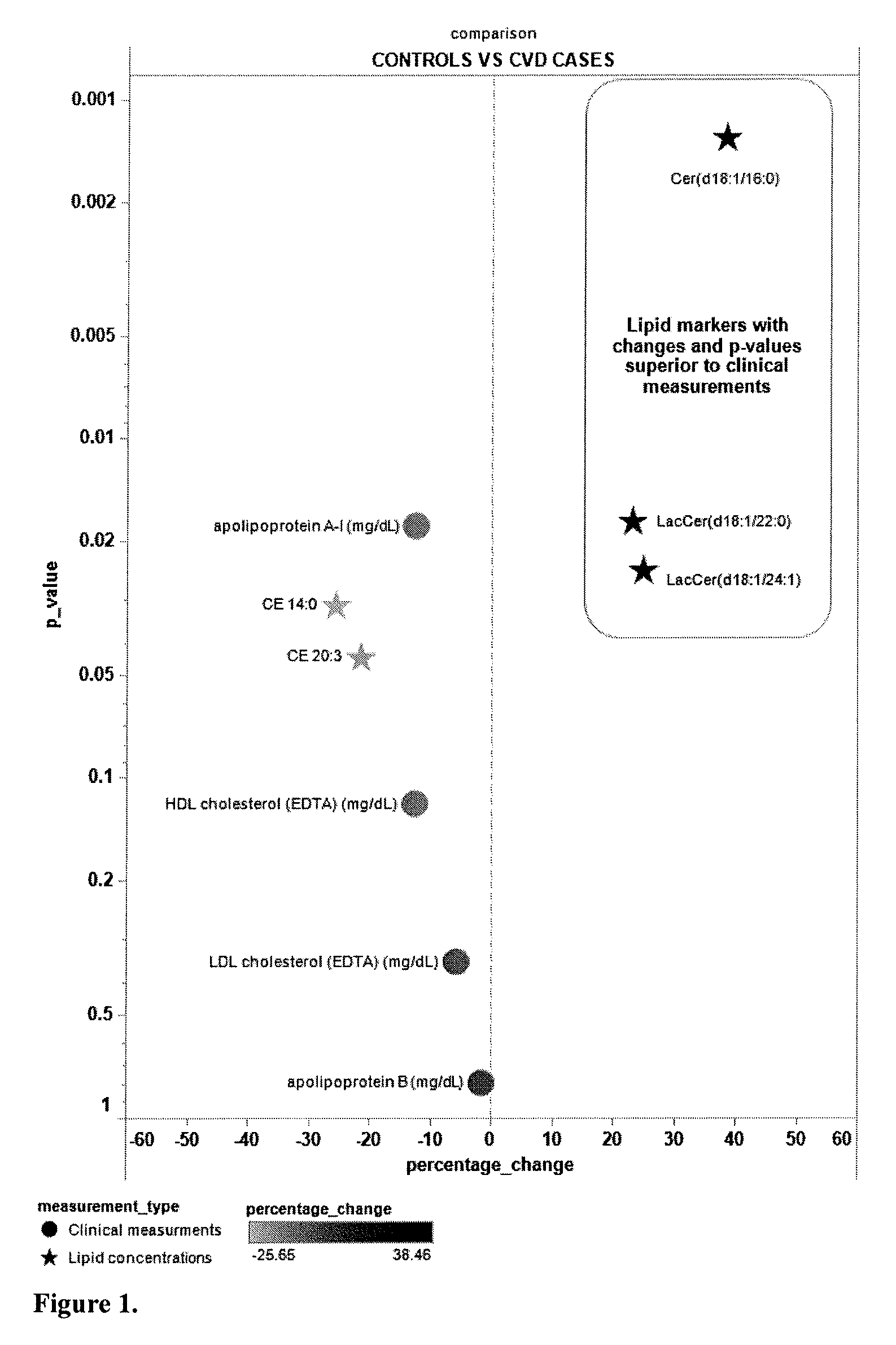
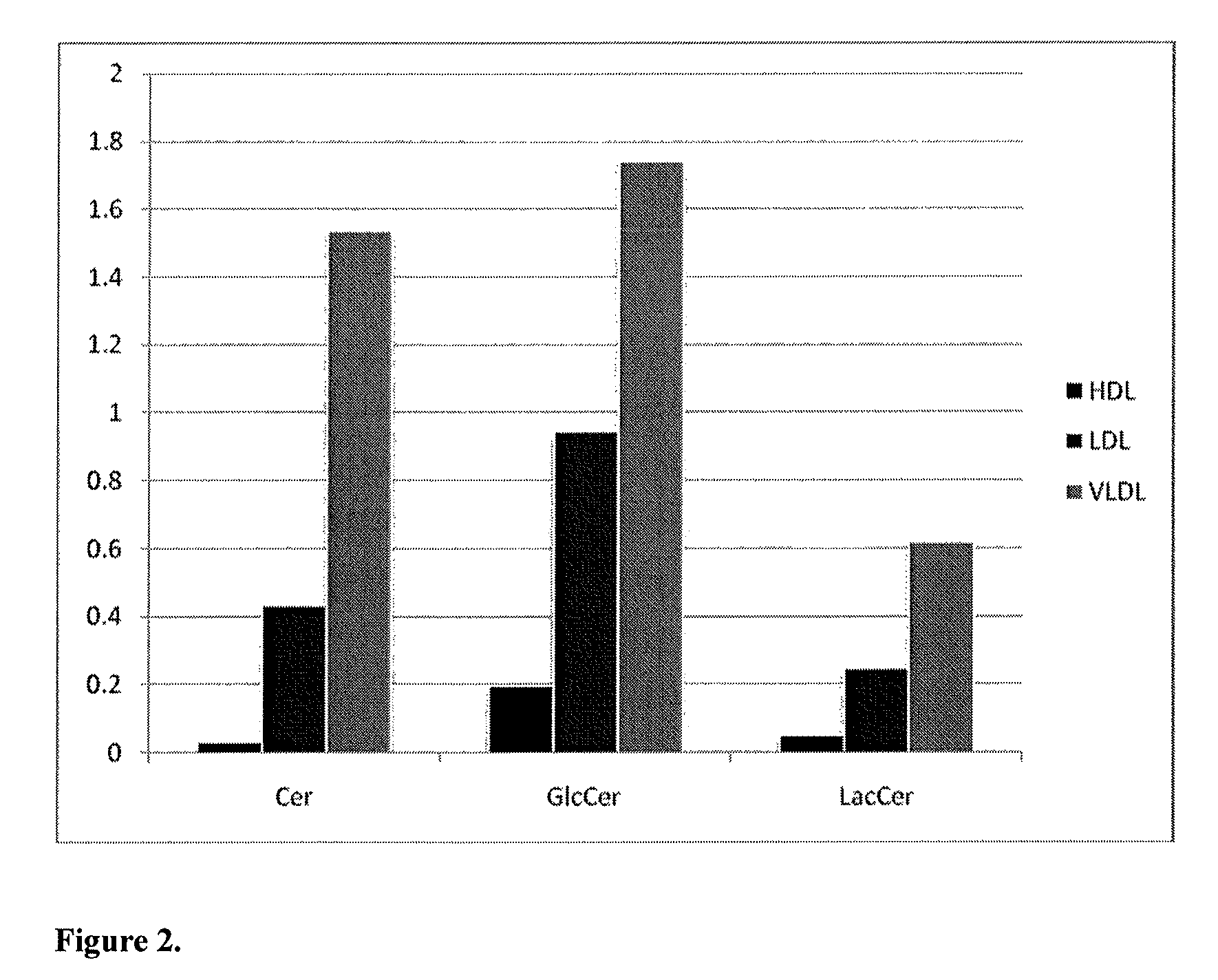
The present invention inter alia provides a method, and use thereof, of diagnosing and / or predicting atherosclerosis or CVD by detecting the lipid concentrations or lipid ratios of a biological sample and comparing it to a control and has identified specific lipid markers that are more specific and sensitive in detecting and predicting atherosclerosis and CVD than currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, preventing and / or treating atherosclerosis or CVD. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of atherosclerosis or CVD.
Owner:ZORA BIOSCIENCES OY
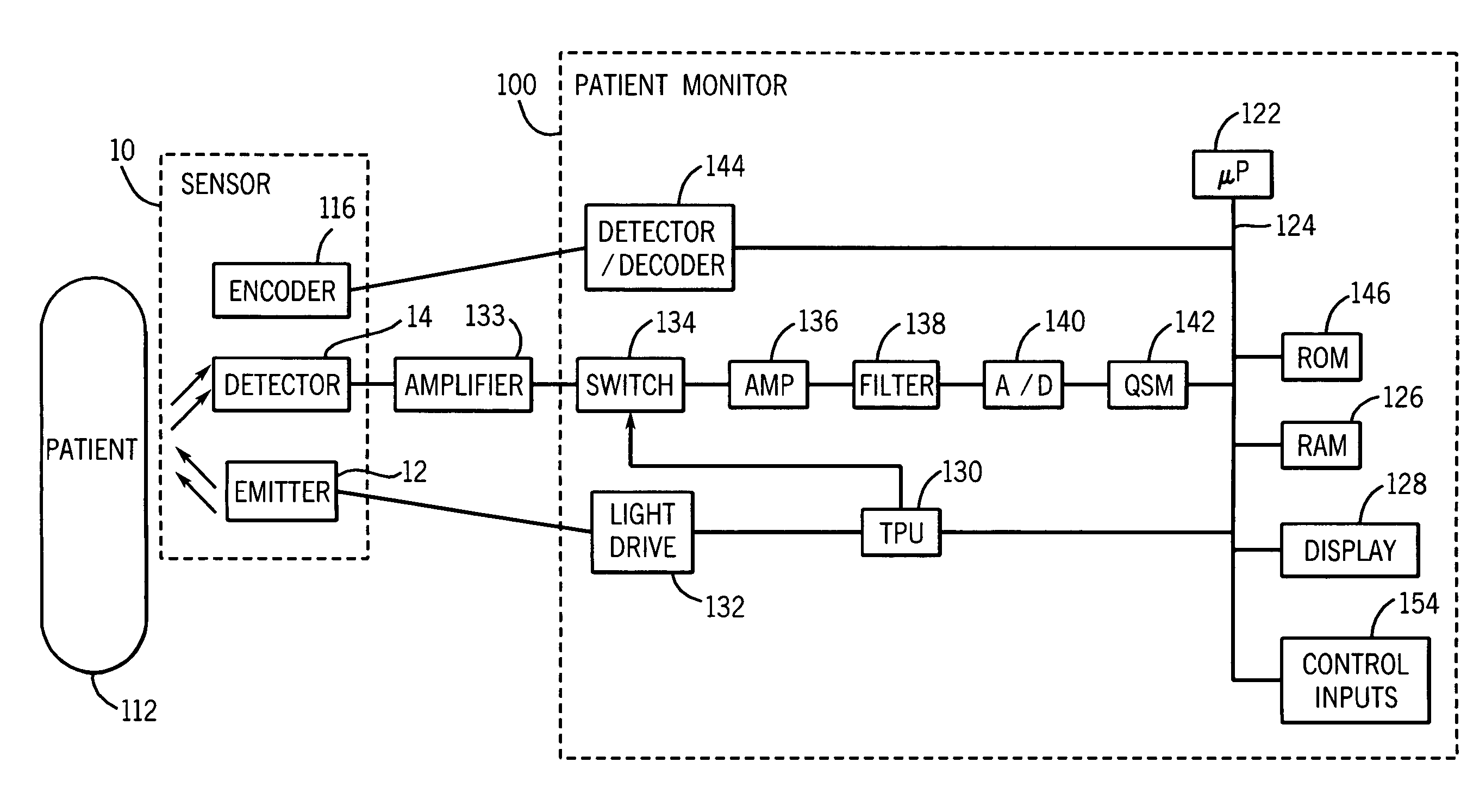
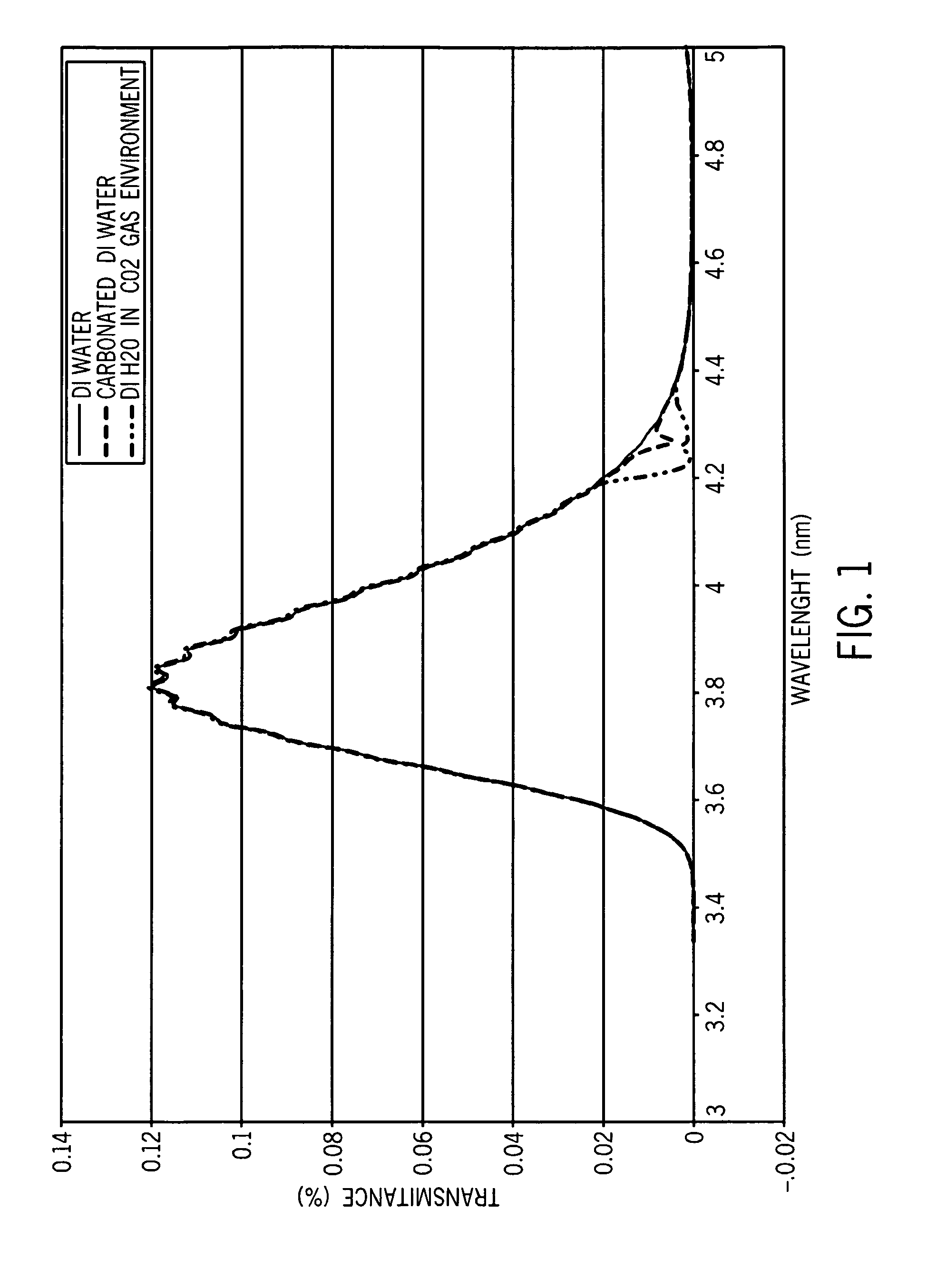
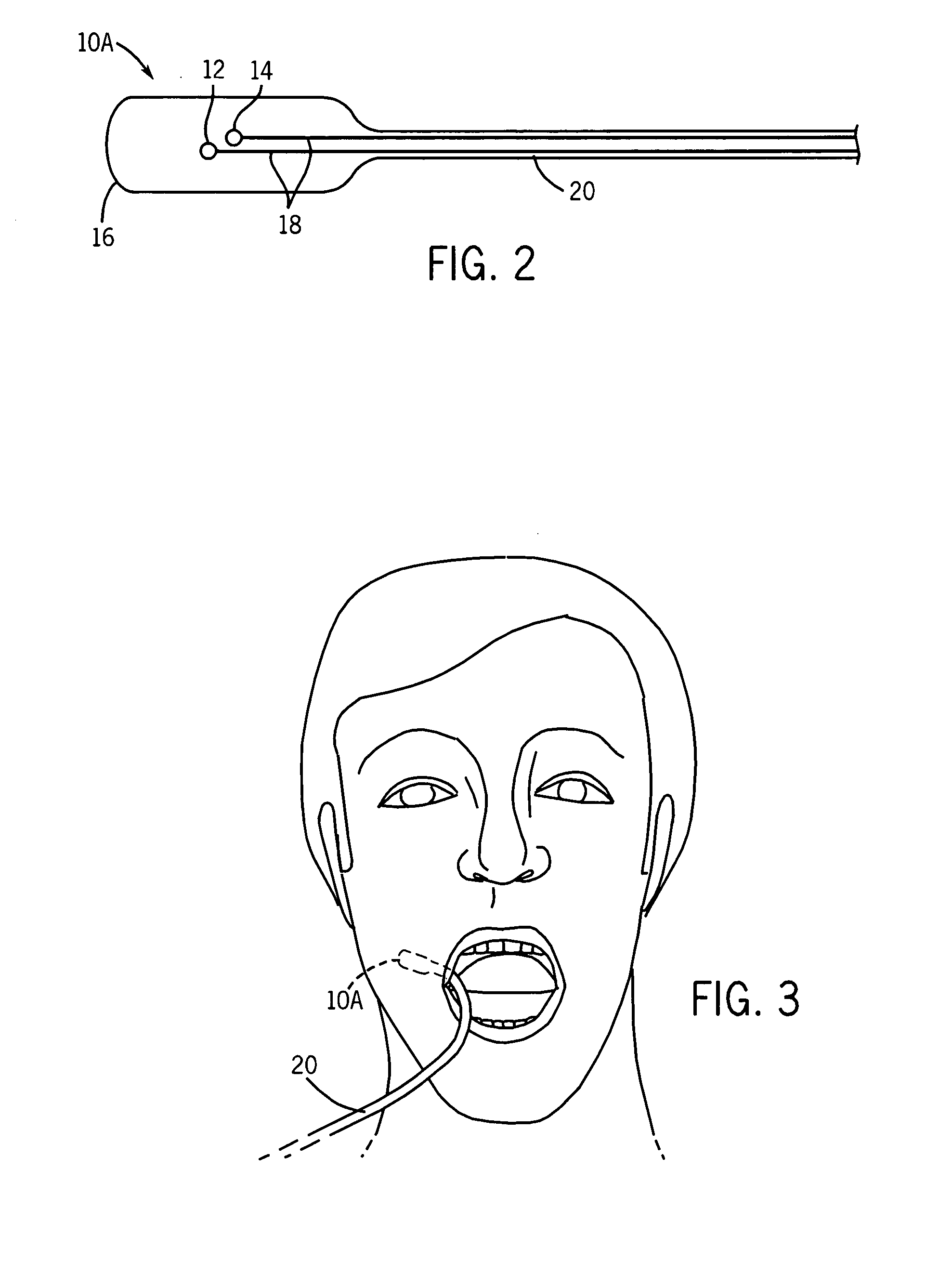
Medical sensor and technique for using the same
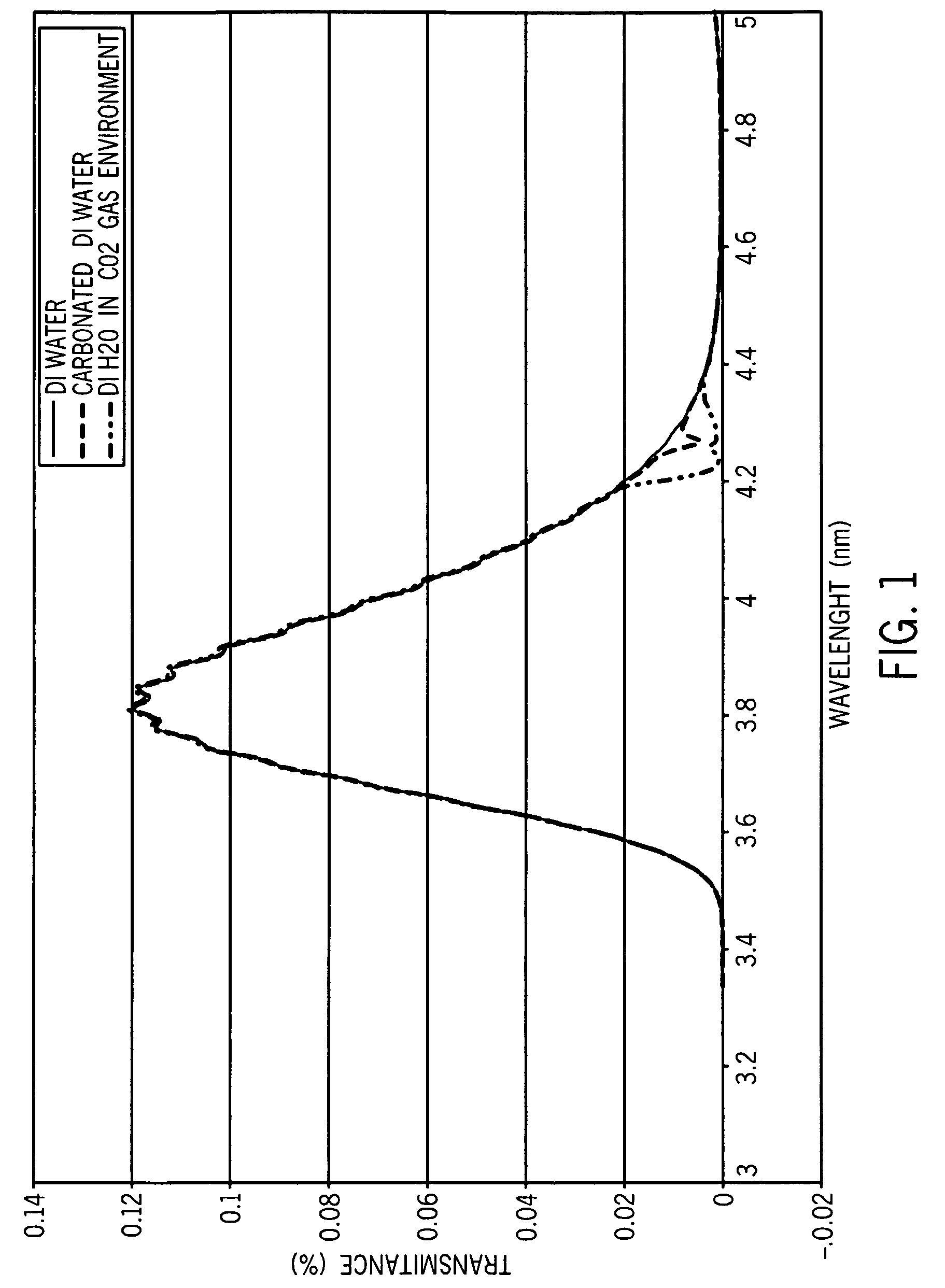
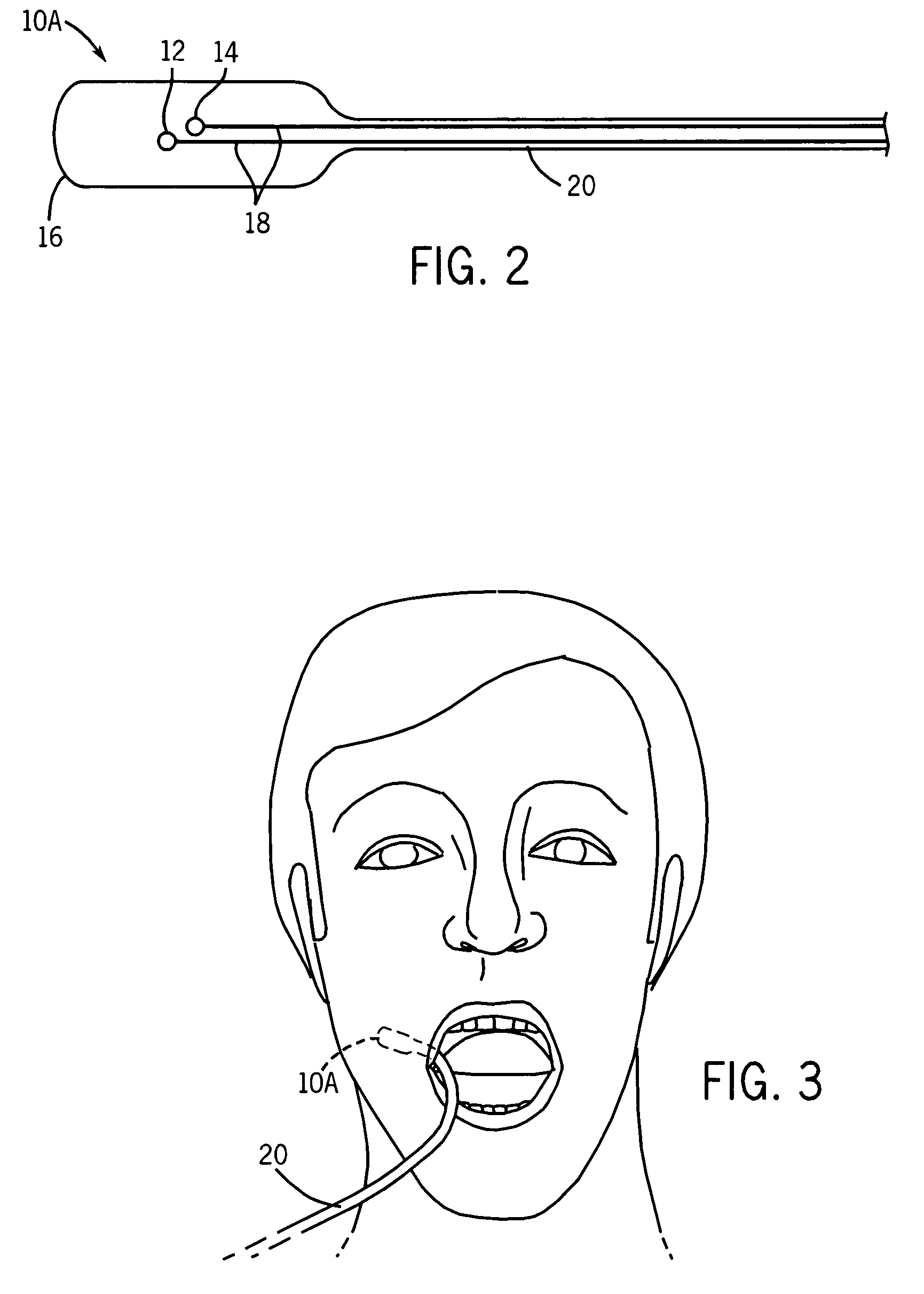
A sensor is provided that is appropriate for optical detection of dissolved carbon dioxide. Such a sensor may be used for transcutaneous detection of carbon dioxide in the tissue. Alternatively, such sensors may be inserted into the tissue. Detection of dissolved carbon dioxide in the tissue may serve as a useful clinical marker for physicians.
Owner:TYCO HEALTHCARE GRP LP
Prediction of an individual's risk of developing rheumatoid arthritis
InactiveUS20090265116A1Useful predictionDisease diagnosisBiological testingAnti ccp antibodiesRecent onset
Methods for predicting the likelihood of development of rheumatoid arthritis for individuals that present with recent-onset undifferentiated arthritis. The methods are based on the determination of a set of clinical markers and / or parameters and determining a predicted risk for developing rheumatoid arthritis. Clinical markers and parameters that are decisive for the risk for developing rheumatoid arthritis may include serum levels of C-reactive protein, Rheumatoid factors, anti-CCP antibodies, anti-MCV as well as age, gender, localization of the joint complaints, length of morning stiffness, and number of tender and / or swollen joints or combinations thereof. The method may be performed by a computer. The invention further relates to a computer, a sample analyser and a computer program product for performing the method and a data carrier with the computer program product.
Owner:EXAGEN DIAGNOSTICS
Advanced detection of sepsis
ActiveUS8669113B2Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsClinical markerBiomarker (petroleum)
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
Apparatus, methods and articles for four dimensional (4D) flow magnetic resonance imaging using coherency identification for magnetic resonance imaging flow data
ActiveUS10117597B2Low costThe process is fast and accurateImage enhancementMedical imagingAnatomical structuresData set
Owner:ARTERYS INC
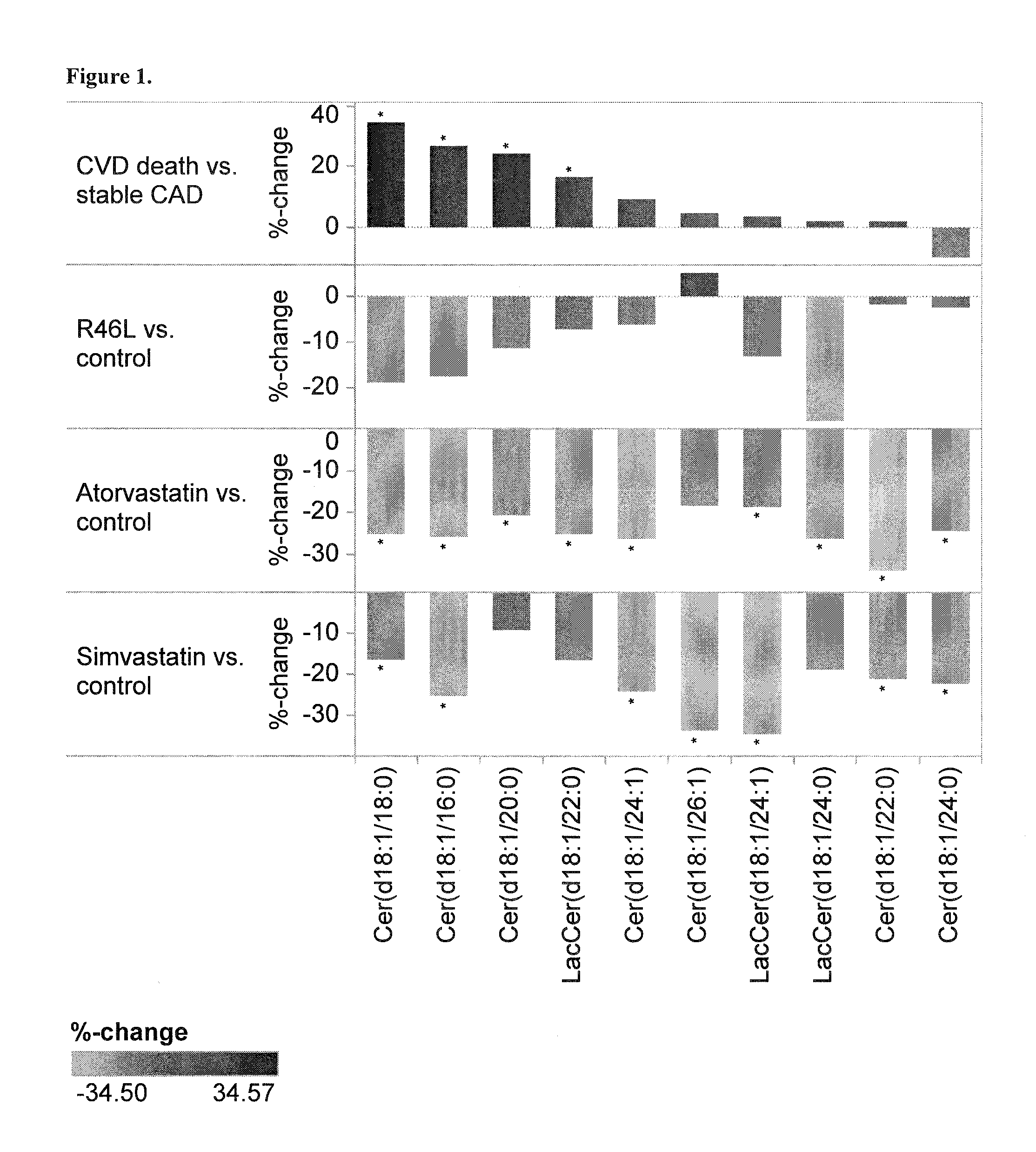
Biomarkers for sensitive detection of statin-induced muscle toxicity
InactiveUS20120258123A1Efficient mechanismSafety assessmentBiocideOrganic chemistryMyopathyConcentration ratio
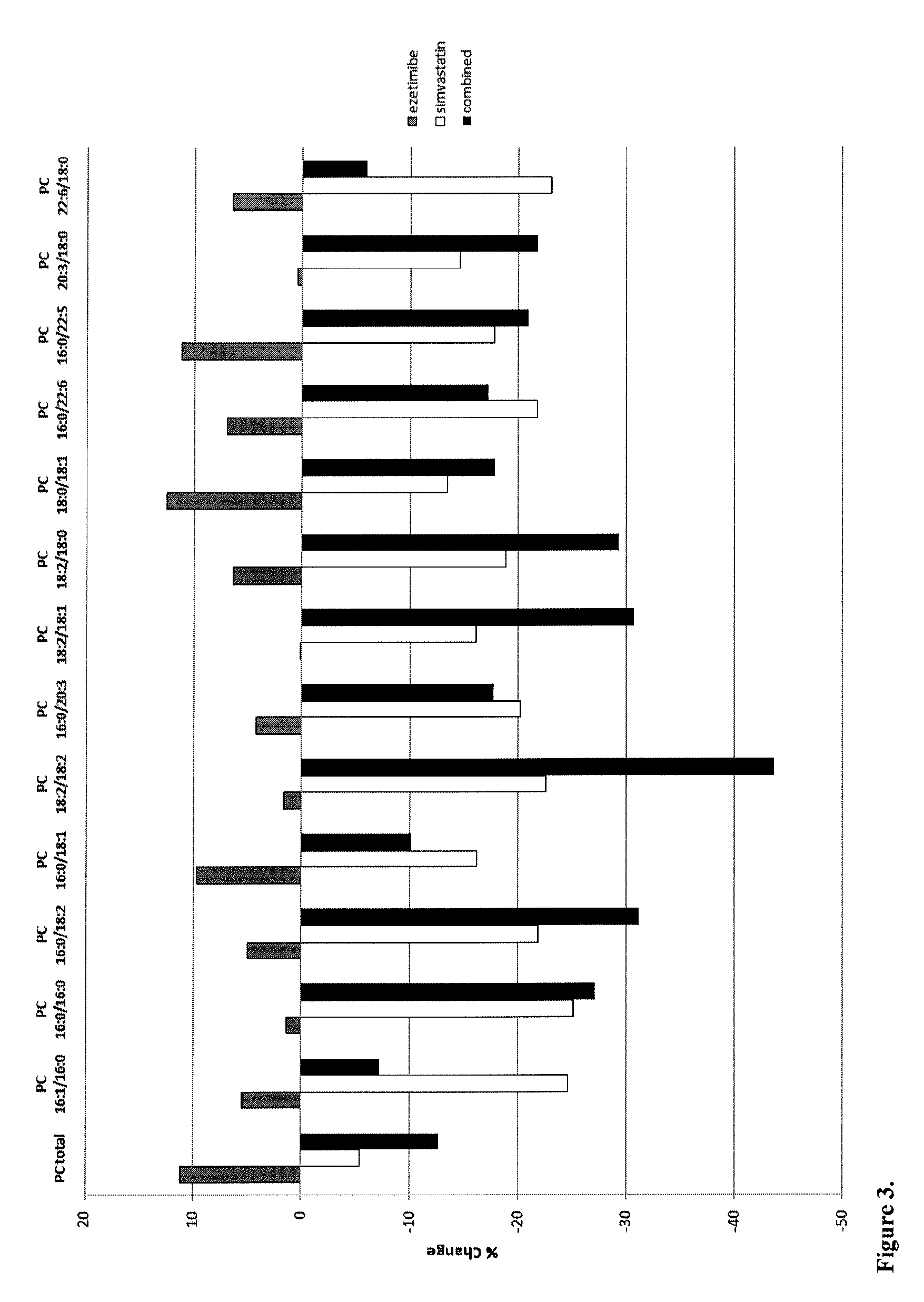
The present invention inter alia provides a method, and uses thereof, of predicting statin-induced muscle toxicity or its complications, such as myalgia, myopathy and rhabdomyolysis, by detecting the lipid concentrations or lipid-lipid concentration ratios of a biological sample and comparing them to a control. This method has identified lipid markers that are more specific and sensitive in detecting these statin-induced muscle toxicity than the currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, statin-induced muscle toxicity. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of statin-induced muscle toxicity.
Owner:ZORA BIOSCIENCES OY
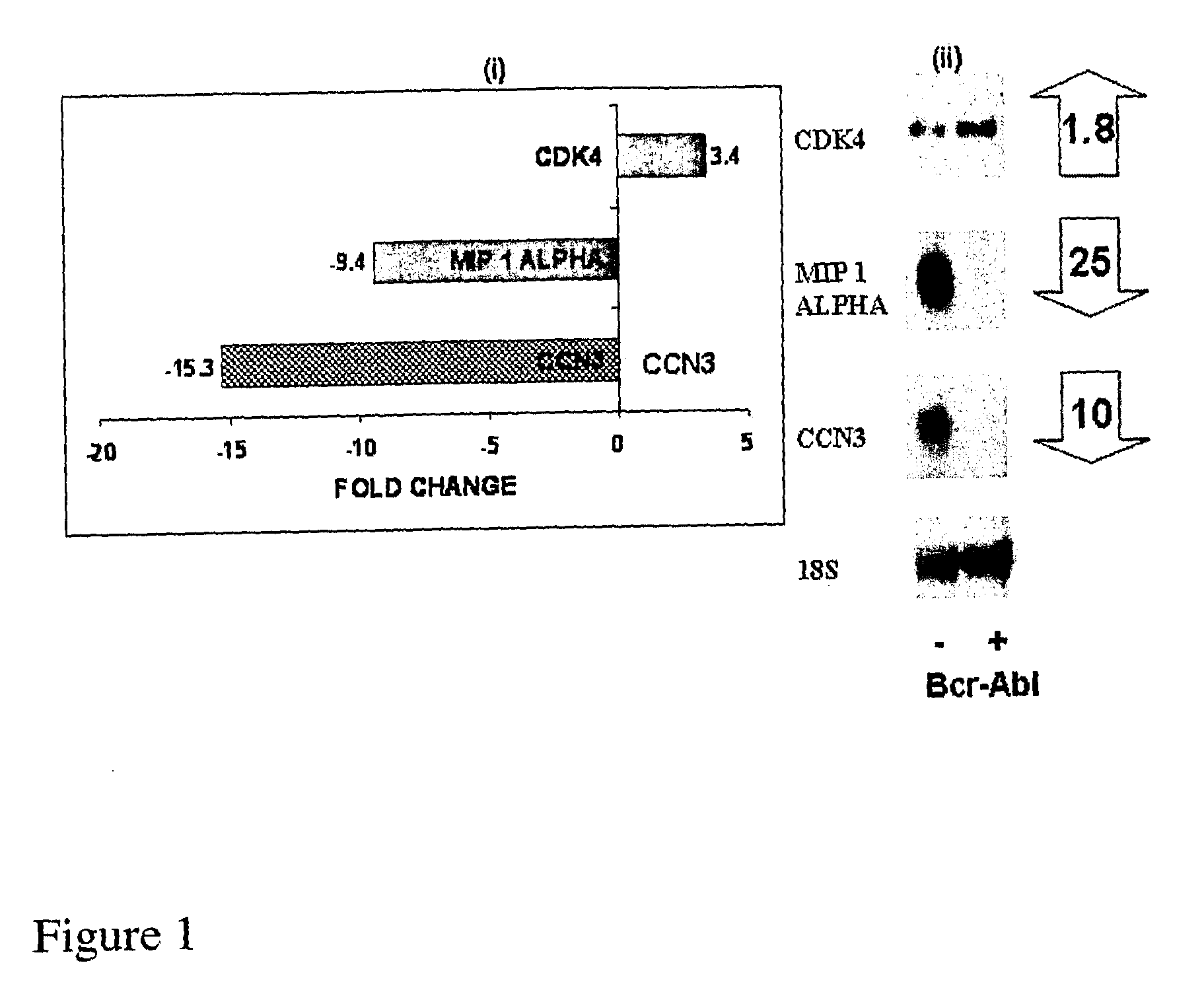
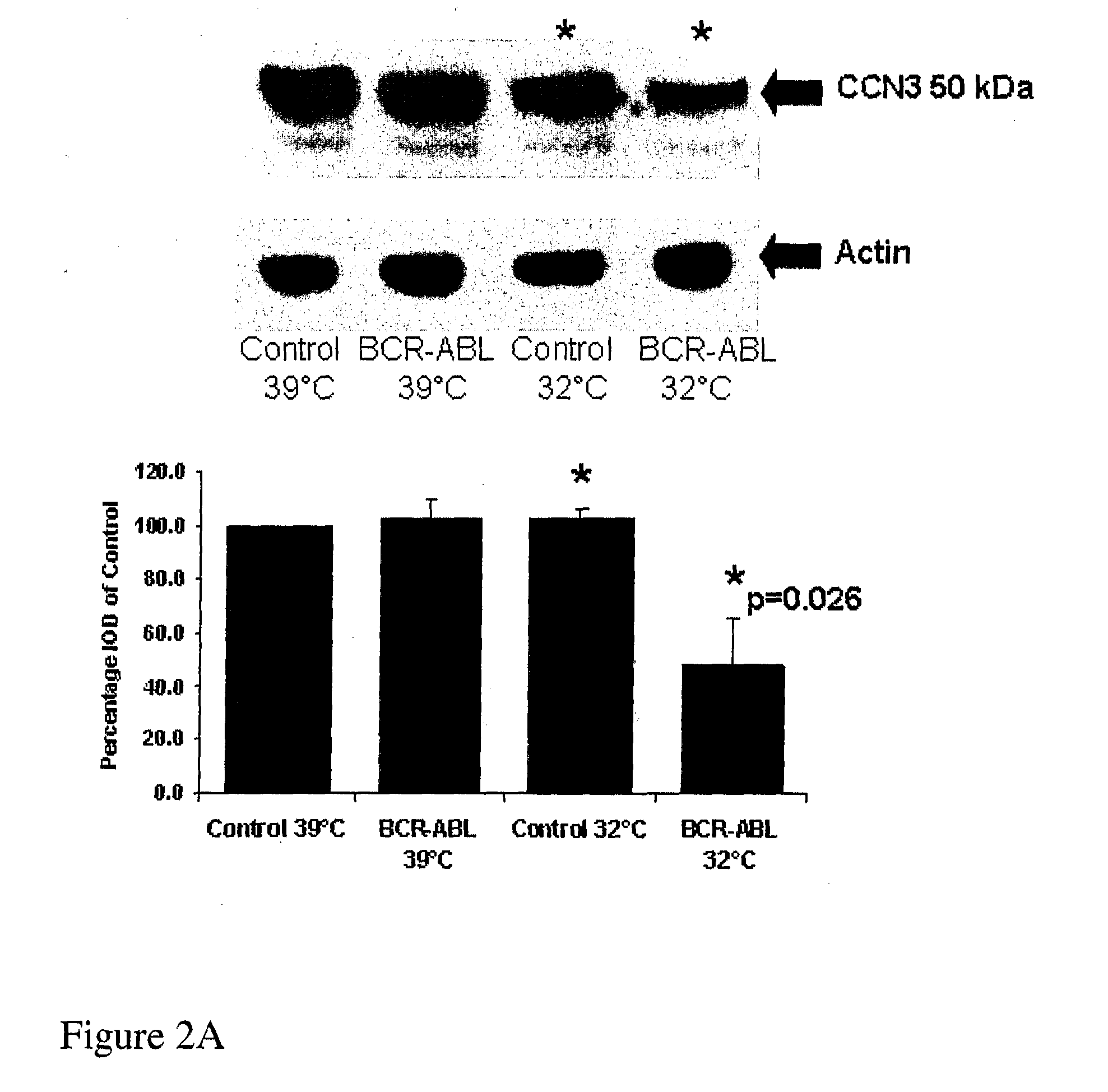
CCN3 peptide
InactiveUS20100004169A1Increase kinase activityActivity be increased and decreasedCompound screeningApoptosis detectionDiseasePeptide sequence
The present application relates to nucleic acid and peptide sequences of CCN3 and derivatives and fragments thereof useful in the treatment of disease, in particular tumours and / or for use as a clinical marker.
Owner:QUEENS UNIV OF BELFAST
Degradable carbon nanotube-containing biosensors and methods for target clinical marker detection
ActiveUS20140255952A1Efficient detectionDetect bone resorptionMicrobiological testing/measurementCatheterCarbon nanotubeClinical marker
The invention relates to carbon nanotube-containing composites as biosensors to detect the presence of target clinical markers, methods of their preparation and uses in the medical field. The invention is particularly suitable for the detection in patient biological specimens of bone markers and tissue markers. The biosensors of the invention include carbon nanotubes deposited on a substrate, gold nanoparticles deposited on the carbon nanotubes and, binder material and biomolecule deposited on the gold-coated carbon nanotubes. The biomolecule is selected to interact with the target clinical markers. The biosensor can be used as an in-situ or an ex-situ device to detect and measure the presence of the target clinical markers.
Owner:UNIVERSITY OF PITTSBURGH
Lipidomic biomarkers for atherosclerosis and cardiovascular disease
The present invention inter alia provides a method, and use thereof, of diagnosing and / or predicting atherosclerosis or CVD by detecting the lipid concentrations or lipid ratios of a biological sample and comparing it to a control and has identified specific lipid markers that are more specific and sensitive in detecting and predicting atherosclerosis and CVD than currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, preventing and / or treating atherosclerosis or CVD. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of atherosclerosis or CVD.
Owner:ZORA BIOSCIENCES OY
Biomarkers for epilepsy
InactiveUS20140315737A1Efficient detectionVariability that can explain a set of SNPs tends to beNucleotide librariesMicrobiological testing/measurementClinical markerBiology
The present invention relates to a group of epilepsy biomarkers comprising (i) SNP rs2740574; (ii) SNP rs1799735; (iii) SNP rs1695; (iv) SNP rs6280; and the locus of the human glutathione S-transferase theta-1 (GSTT1) gene. The invention particularly relates to this group of biomarkers as marker for protection against drug-resistant epilepsy. The group of biomarkers may additionally comprise at least one clinical marker selected from the group comprising (i) a focal seizure with secondary generalization; (ii) age at confirmed diagnosis of epilepsy; (iii) atrophy of hippocampus or medial temporal sclerosis; and (iv) the presence of a brain tumor. The present invention further relates to a method for detecting protection against drug-resistant epilepsy in a subject, a method for monitoring epilepsy therapy, a method of identifying an individual as eligible for an aggressive epilepsy therapy, a composition for detecting protection against drug-resistant epilepsy in a subject, a corresponding kit and a microarray for the analysis of protection against drug-resistant epilepsy.
Owner:UCB PHARMA SA
Medical sensor and technique for using the same
A sensor is provided that is appropriate for optical detection of dissolved carbon dioxide. Such a sensor may be used for transcutaneous detection of carbon dioxide in the tissue. Alternatively, such sensors may be inserted into the tissue. Detection of dissolved carbon dioxide in the tissue may serve as a useful clinical marker for physicians.
Owner:COVIDIEN LP
Biomarkers for Sensitive Detection of Statin-Induced Muscle Toxicity
InactiveUS20140031332A1Weight optimizationSafety assessmentBiocideOrganic active ingredientsLipid formationMyopathy
The present invention inter alia provides a method, and uses thereof, of predicting statin-induced muscle toxicity or its complications, such as myalgia, myopathy and rhabdomyolysis, by detecting the lipid concentrations or lipid ratios of a biological sample and comparing it to a control. This method has identified lipid markers that are more specific and sensitive in detecting these statin-induced muscle toxicity than the currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, statin-induced muscle toxicity. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of statin-induced muscle toxicity.
Owner:ZORA BIOSCIENCES OY
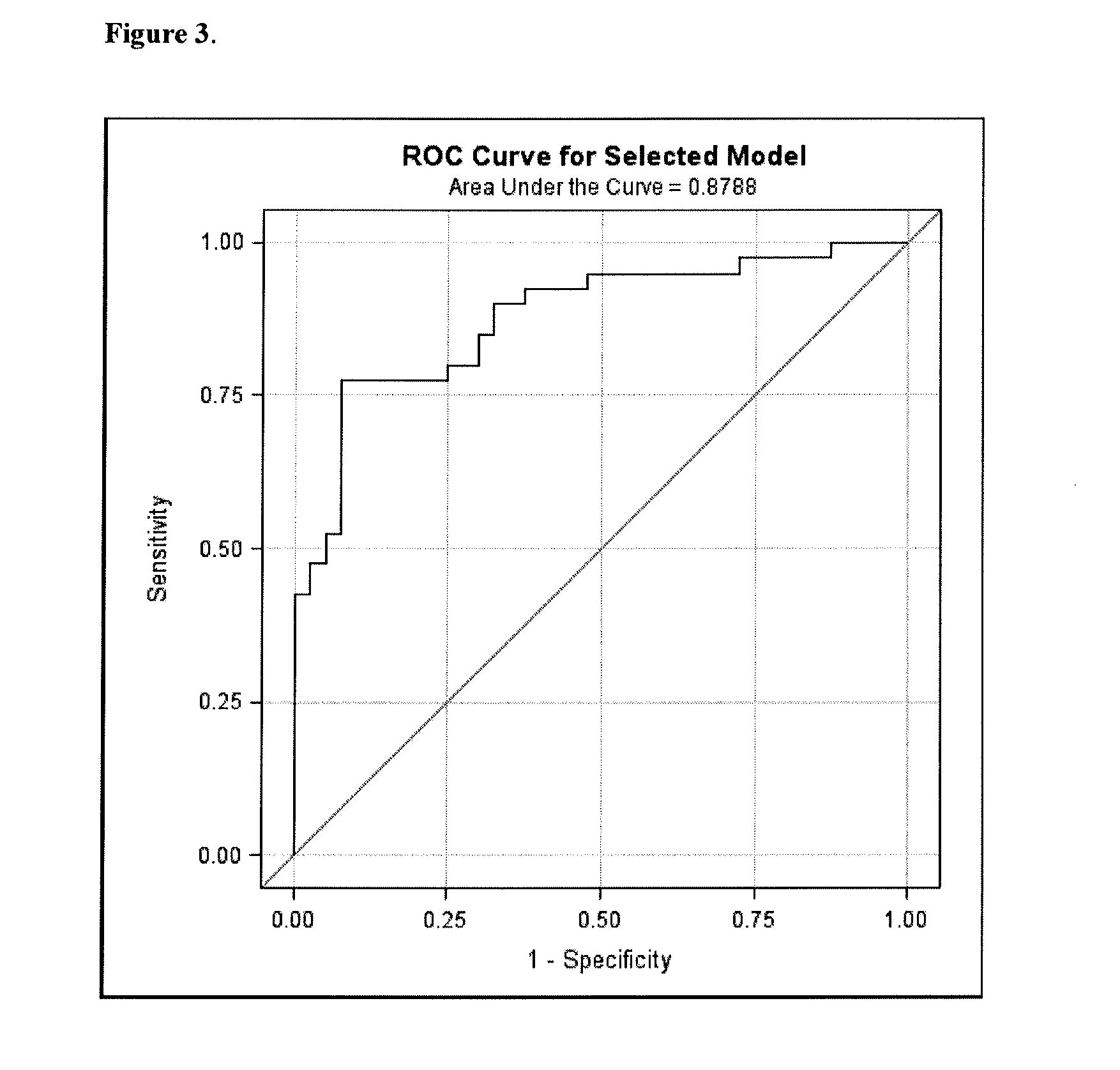
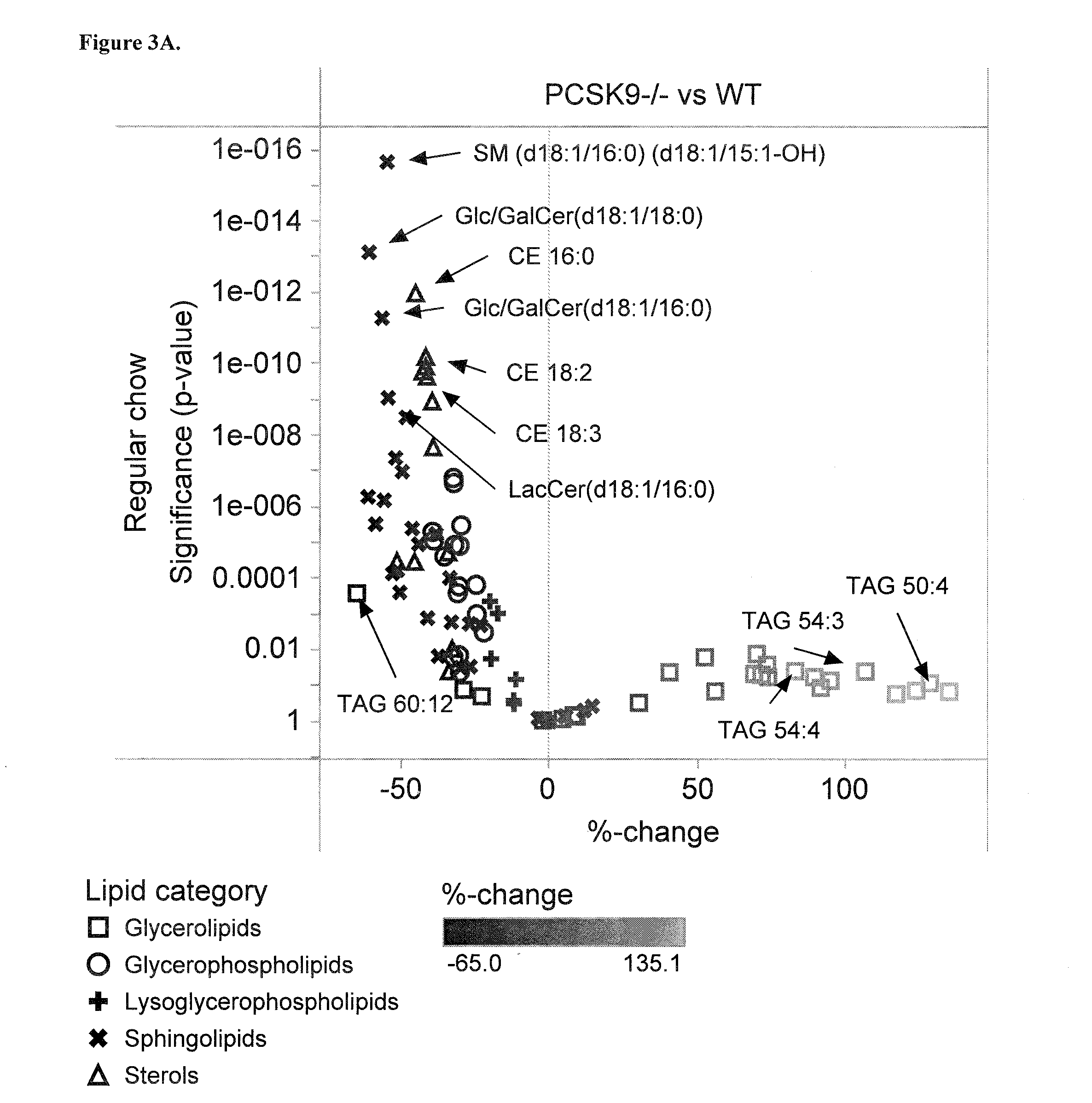
Sensitive Efficacy and Specificity Biomarkers for Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibition
InactiveUS20150160247A1Lower beneficialLower essential lipidCompound screeningApoptosis detectionKexinSubtilisin
The present invention inter alia provides a method, and uses thereof, to measure drug efficacy and specificity of treatment with an inhibitor of Proprotein Convertase Subtilisin / Kexin Type 9 (PCSK9) by detecting the concentrations of lipids and / or lipid-lipid concentration ratios of a biological sample and comparing it to a control. The invention is applicable, inter alia, to determining whether a PCSK9 inhibiting drug is functioning efficiently in lowering serum low-density lipoprotein (LDL) concentration and whether a PCSK9 inhibiting drug displays any adverse side-effects, such as liver toxicity. Provided are lipid markers that are more specific and sensitive in detecting drug efficacy and possible adverse drug-induced side-effects than the currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting and diagnosing of PCSK9 inhibiting drug-induced adverse reactions. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for the determination of PCSK9 inhibiting drug efficacy and drug-induced adverse reactions.
Owner:ZORA BIOSCIENCES OY
Use of masitinib for treatment of cancer in patient subpopulations identified using predictor factors
A method for treating patients afflicted with cancer, wherein the patients are treated with a compound including tyrosine kinase inhibitor, mast cell inhibitor or c-Kit inhibitor, in particular masitinib, optionally in combination with at least one antineoplastic agent. The tyrosine kinase inhibitor, mast cell inhibitor or c-Kit inhibitor, and the optional at least one antineoplastic agent, are administered in a dosage regimen that includes a therapeutically effective amount. Also described are methods for predicting therapeutic response to the treatment in a given patient and therefore identification of applicable patient subpopulations based upon these predictor factors; sometimes referred to as biomarkers. One method is based upon the clinical marker of pain intensity. Another method is based upon gene expression predictive biomarkers assessed via RNA expression in peripheral blood cell samples collected prior to treatment with the compound, which is also used for treating patients afflicted with pancreatic cancer.
Owner:AB SCIENCE
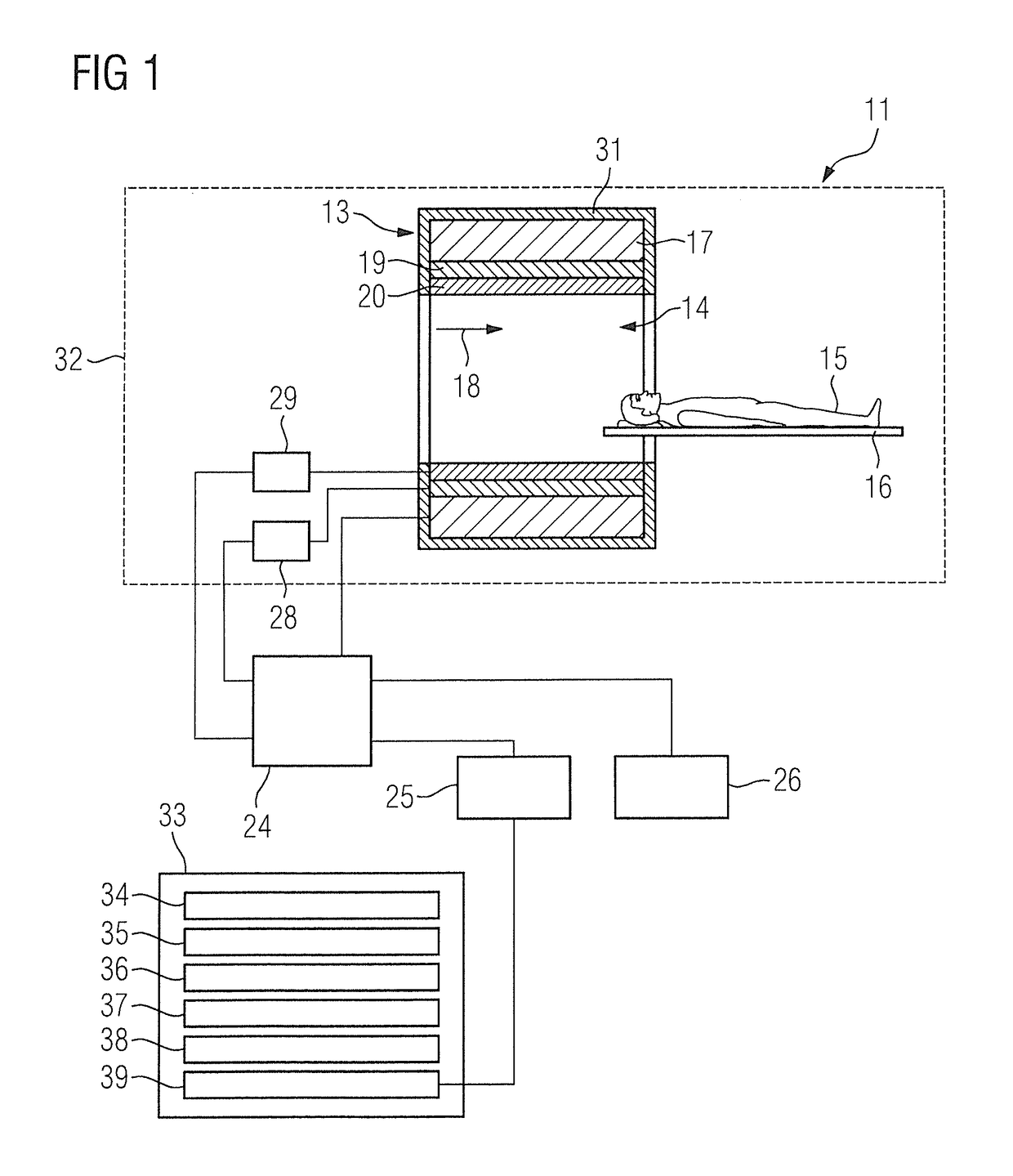
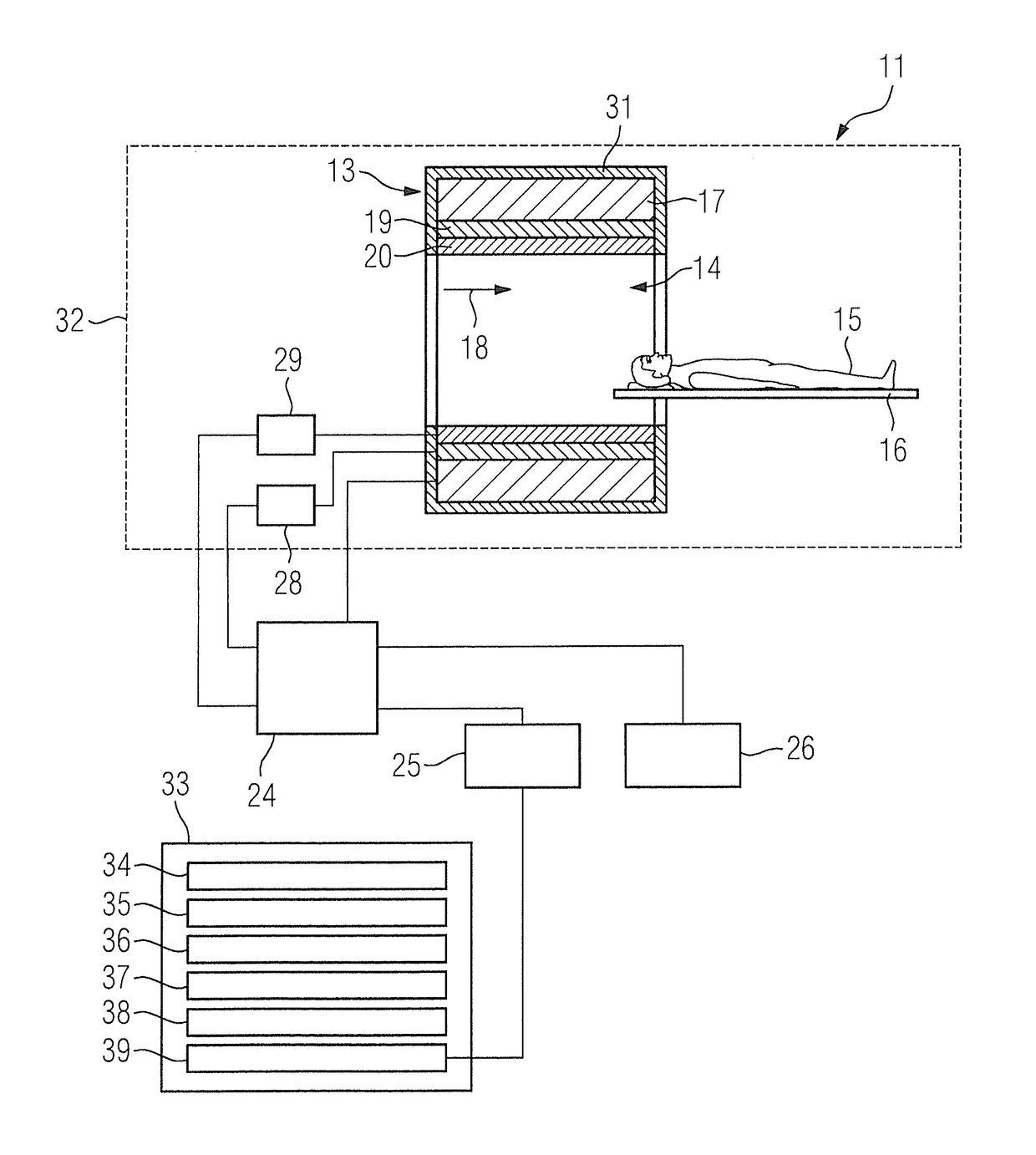
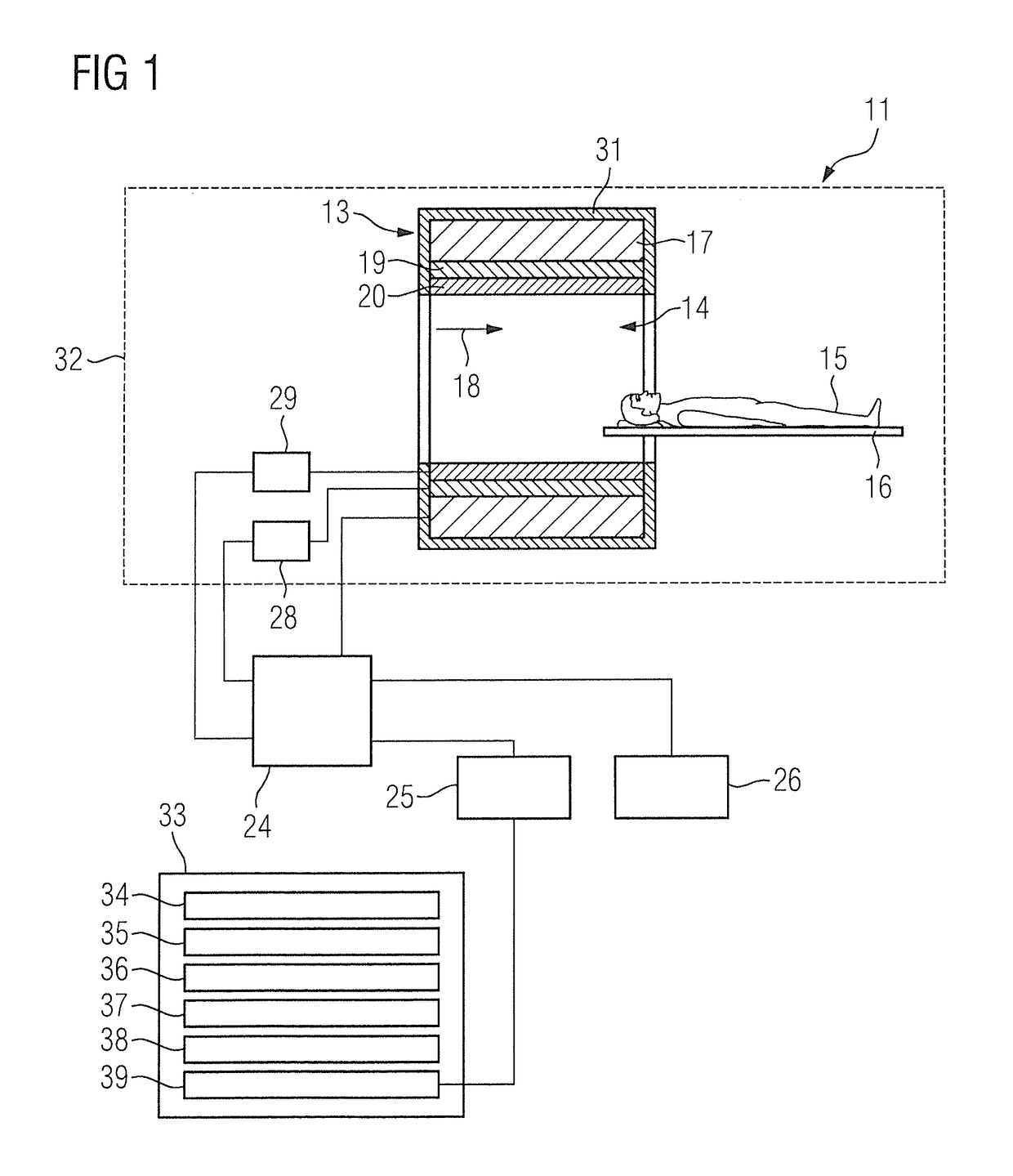
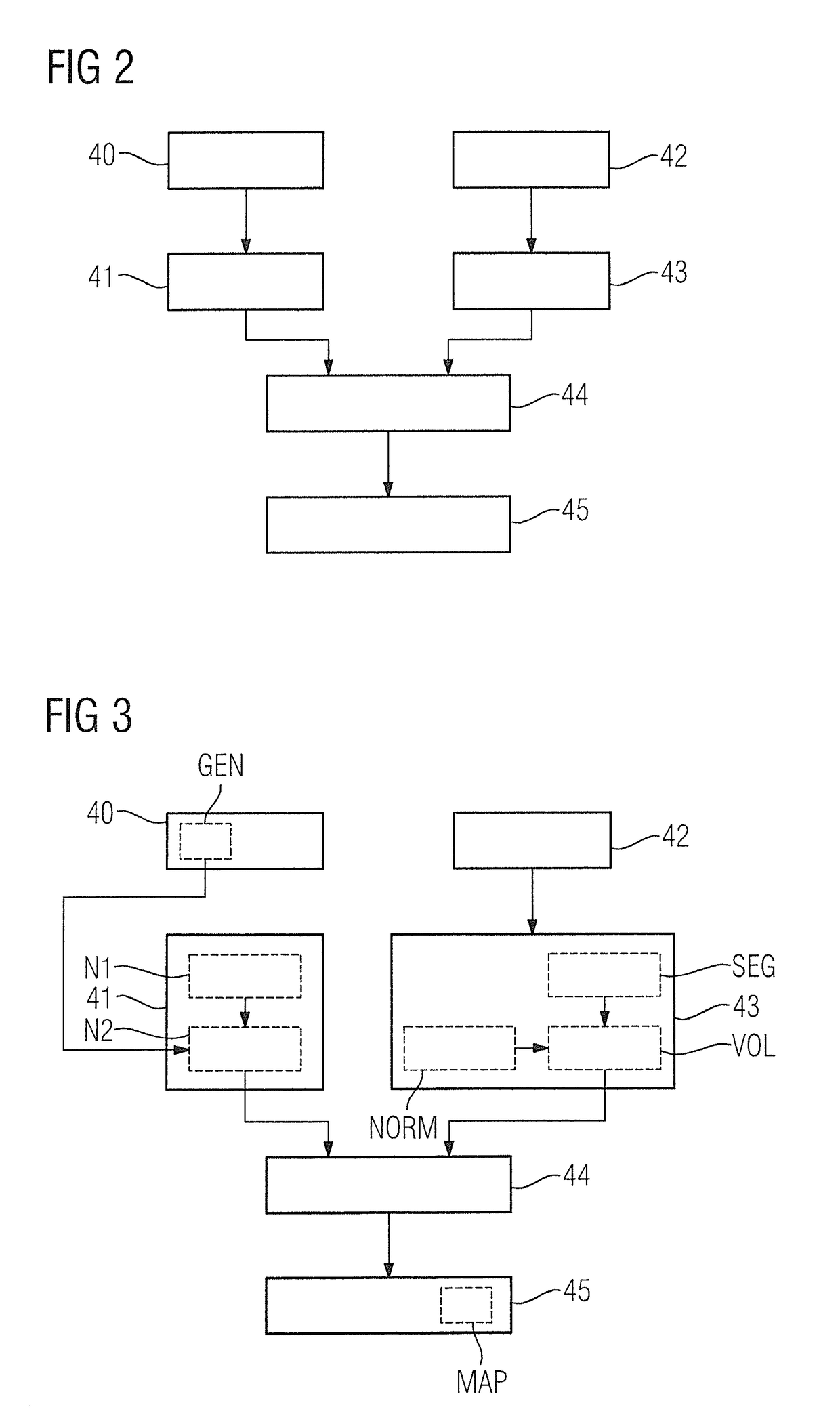
Method, computer and imaging apparatus for evaluating medical image data of an examination subject
ActiveUS10185890B2High sensitivityIncrease valueUltrasonic/sonic/infrasonic diagnosticsImage enhancementClinical markerNormal values
In a method and computer for evaluating medical image data of an examination subject, a clinical marker of the examination subject is acquired that characterizes a status of the examination subject in relation to a physiological parameter, and a normal value range for the physiological parameter is ascertained that is matched to the status of the examination subject as a function of the clinical marker. Medical image data of the examination subject are acquired, and a value of the physiological parameter of the examination subject is determined using the medical image data. This value is compared with the normal value range matched to the status of the examination subject, and the result of the comparison is provided as an output.
Owner:SIEMENS HEALTHCARE GMBH
Clinical marker associated with cerebral aneurysms and application of clinical marker
ActiveCN107557467AImprove reliabilityDetermining the risk of diseaseMicrobiological testing/measurementDNA/RNA fragmentationClinical markerPathology
The invention discloses a clinical marker associated with cerebral aneurysms. The clinical marker comprises one or more of miR-628-3p, miR-505-5p, miR-3074-3p, miR-497-5p, miR-224-5p, miR-181b-5p andlet-7b-5p. The invention also relates to a kit for detecting onset of the cerebral aneurysms or a kit for diagnosing the cerebral aneurysms. The kit contains any one or more of the clinical markers and corresponding primers of the clinical markers. The invention also relates to an application of the kit in a determining method for diagnosing the onset of the cerebral aneurysms or a clinical application in diagnosis of the cerebral aneurysms. The fact that exosome miR-628-3p as a clinical marker of the cerebral aneurysms has quite high accuracy is discovered for the first time. The onset of thecerebral aneurysms can be judged more accurately if the miRNA markers are combined, that is, diagnosis results with higher reliability can be obtained by using the exosome miRNA clinical marker of the cerebral aneurysms and the determining method for diagnosing the onset of the cerebral aneurysms.
Owner:李永东
Method, computer and imaging apparatus for evaluating medical image data of an examination subject
ActiveUS20170132491A1High sensitivityDiagnostic value can be improvedUltrasonic/sonic/infrasonic diagnosticsImage enhancementClinical markerNormal values
In a method and computer for evaluating medical image data of an examination subject, a clinical marker of the examination subject is acquired that characterizes a status of the examination subject in relation to a physiological parameter, and a normal value range for the physiological parameter is ascertained that is matched to the status of the examination subject as a function of the clinical marker. Medical image data of the examination subject are acquired, and a value of the physiological parameter of the examination subject is determined using the medical image data. This value is compared with the normal value range matched to the status of the examination subject, and the result of the comparison is provided as an output.
Owner:SIEMENS HEALTHCARE GMBH
Method for providing dental aligners to a subject showing a non-alignement of its dentition
A personalized method for providing at least one dental aligner; the method being with reference to an actual state of the dentition of the patient and in view of an improvement plan of the alignment targeted over a period of time ranging from 1 to 16 weeks, of the teeth of a subject. The method includes the steps of: (i) providing a 2D image and / or 3D volume of the dentition of a subject showing a non-alignment of its dentition; (ii) recovering clinical markers of the subject; (iii) producing an initial set of manufacturing parameters of the at least one aligner; (iv) building up an image of the 1-16 weeks target of improvement; and (v) directly 3D printing of the at least one personalized aligner fitting with the predicted evolution of intermediate tooth displacement for each tooth of the patient during the improvement plan.
Owner:ORTHOIN3D
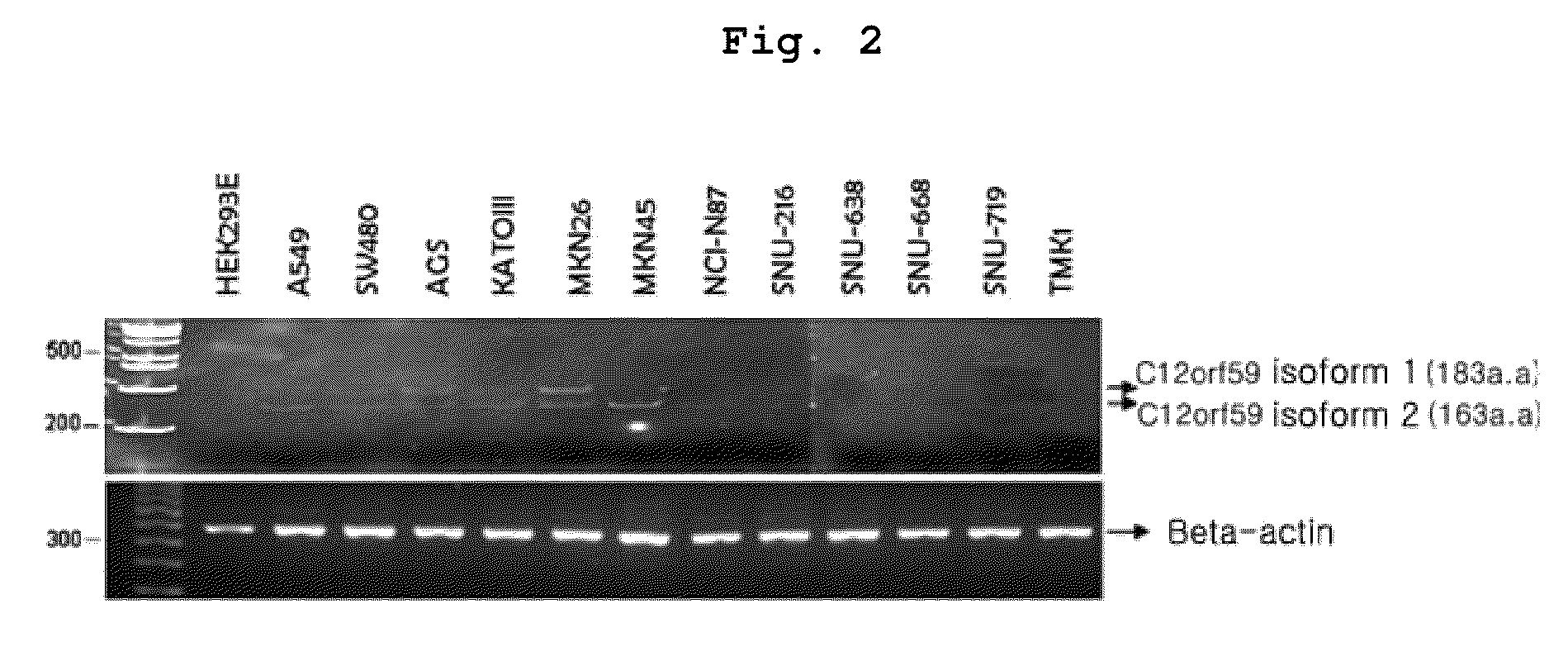
Method of treating cancer with a composition containing a C12ORF59 protein
As explained hereinbefore, the C12orf59 gene of the present invention suppresses cancer cell invasion and inhibits cancer cell survivability, and the over-expression of C12orf59 protein or a fragment thereof inhibits cancer cell invasion, so that C12orf59 gene or a fragment thereof not only can be effectively used for the pharmaceutical composition for preventing and treating cancer but also can be used as a clinical marker for screening a cancer treatment agent candidate, for diagnosing various cancers or for predicting pathological stage. In addition, the C12orf59 gene or a fragment thereof of the present invention can be used for the method for preventing and treating cancer and for the preparation of a pharmaceutical composition for preventing and treating cancer.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
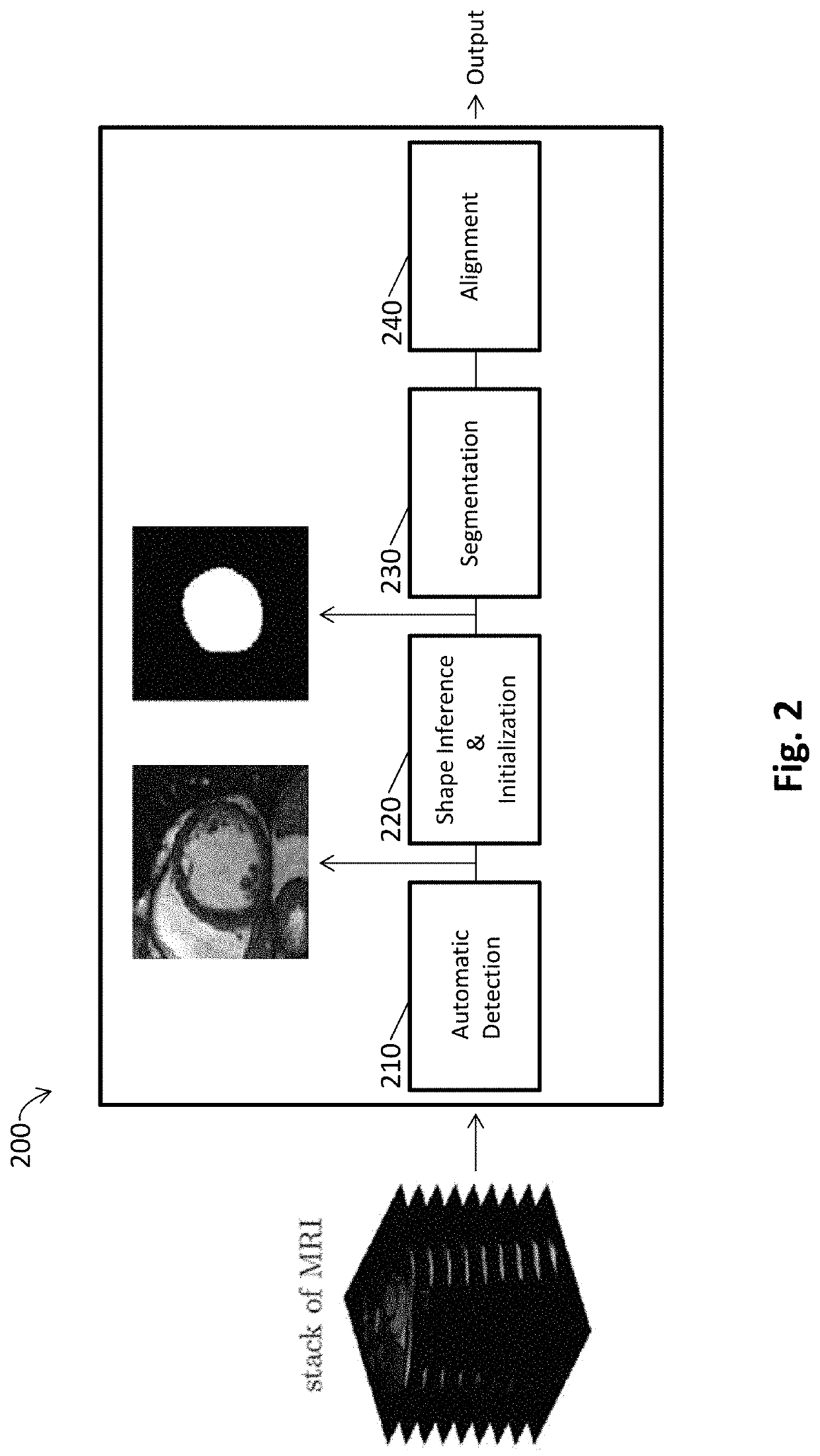
Automated segmentation of organ chambers using deep learning methods from medical imaging
ActiveUS20200118266A1Reduce misalignmentImage enhancementImage analysisClinical markerMedical imaging
A method of detecting whether or not a body chamber has an abnormal structure or function including: (a) providing a stack of images as input to a system comprising one or more hardware processors configured to obtain a stack of medical images comprising at least a representation of the body chamber inside the patient; to obtain a region of interest using a convolutional network trained to locate the body chamber, wherein the region of interest corresponds to the body chamber from each of the medical images; and to infer a shape of the body chamber using a stacked auto-encoder (AE) network trained to delineate the body chamber, wherein the AE network segments the body chamber; (b) operating the system to detect the body chamber in the images using deep convolutional networks trained to locate the body chamber, to infer a shape of the body chamber using a stacked auto-encoder trained to delineate the body chamber, and to incorporate the inferred shape into a deformable model for segmentation; and (c) detecting whether or not the body chamber has an abnormal structure, wherein an abnormal structure is indicated by a body chamber clinical indicia that is different from a corresponding known standard clinical indicia for the body chamber.
Owner:RGT UNIV OF CALIFORNIA
Method for analyzing mucin 1 having siaalpha2-8siaalpha2-3galbeta glycans
InactiveUS20130115612A1Microbiological testing/measurementBiological material analysisGlycanClinical marker
The object of the present invention is to provide a clinical marker capable of distinguishing breast cancer from interstitial pneumonia; and a clinical marker for detecting malignancy or progress level of breast cancer, and for monitoring effects of the treatment of breast cancer. The object can be solved by a method for analyzing mucin 1 having Siaα2-8Siaα2-3Galβ-R, characterized by comprising the step of bringing a first probe specifically binding to a mucin 1 having Siaα2-8Siaα2-3Galβ-R into contact with a sample to be tested.
Owner:YAMAGUCHI UNIV +1
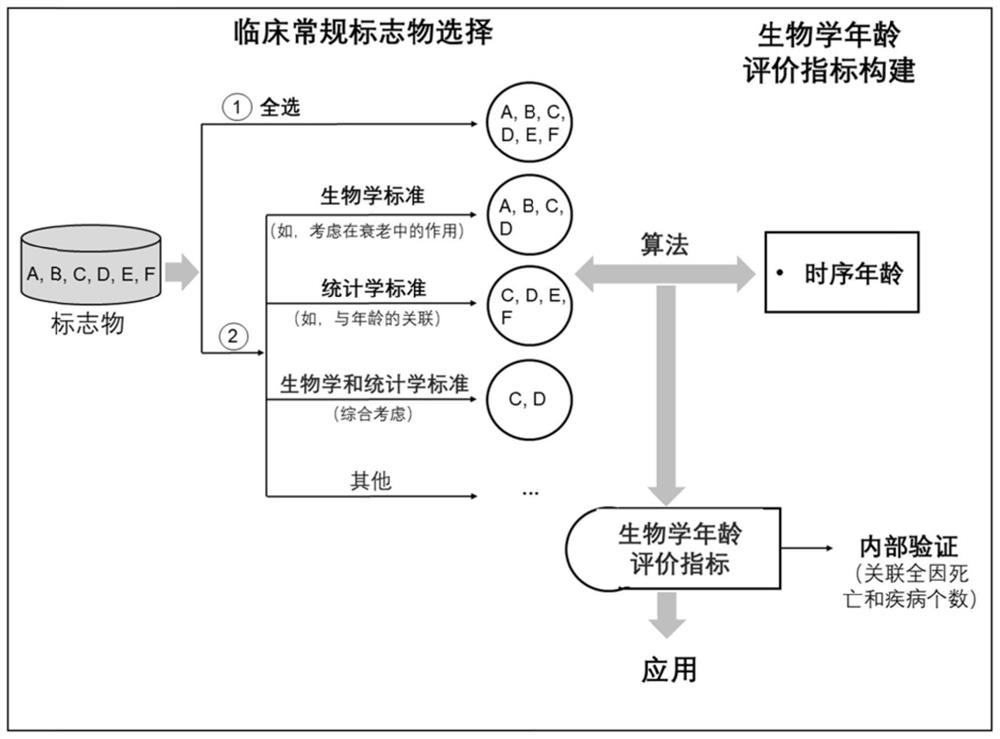
Method for constructing evaluation model for biological age of Chinese population based on clinical markers, and evaluation method
PendingCN111816307AImprove predictive performancePracticalHealth-index calculationDiseaseClinical marker
The invention discloses a method for constructing an evaluation model for the biological age of a Chinese population based on clinical markers. The method comprises the following steps: (1) selectingclinical routine markers representing different pathophysiological systems or functions; (2) constructing a biological age evaluation model by utilizing the selected markers based on a KDM algorithm;(3) evaluating the obtained biological age evaluation model, and if the effect of the model is poor, returning to the steps (1) and (2) and carrying out the next round of model construction operation,or if the effect meets requirements, outputting the constructed biological age evaluation model. The method can be applied to early identification and prevention of aging of people in China and intervention effect evaluation of senile diseases, and has practicability and huge application potential.
Owner:ZHEJIANG UNIV
Lipidomic biomarkers for the prediction of cardiovascular outcomes in coronary artery disease patients undergoing statin treatment
ActiveUS9052327B2Easy diagnosisIncreased and decreased level-asParticle spectrometer methodsMass spectrometric analysisLipidomeLipid formation
Owner:ZORA BIOSCIENCES OY
Biomarkers for sensitive detection of statin-induced muscle toxicity
InactiveUS9541565B2Safety assessmentFacilitate improving patient careBiocideOrganic chemistryMyopathyConcentration ratio
The present invention inter alia provides a method, and uses thereof, of predicting statin-induced muscle toxicity or its complications, such as myalgia, myopathy and rhabdomyolysis, by detecting the lipid concentrations or lipid-lipid concentration ratios of a biological sample and comparing them to a control. This method has identified lipid markers that are more specific and sensitive in detecting these statin-induced muscle toxicity than the currently utilized clinical markers. Also provided is an antibody towards said lipids, and the use thereof for predicting, diagnosing, statin-induced muscle toxicity. The invention additionally relates to kits comprising lipids and / or an antibody thereto, for use in the prediction and / or diagnosis of statin-induced muscle toxicity.
Owner:ZORA BIOSCIENCES OY
Biomarkers for sensitive detection of statin-induced muscle toxicity
InactiveUS9664698B2Safety assessmentFacilitate improving patient careComponent separationHealth-index calculationMyopathySkeletal muscle damage
Owner:ZORA BIOSCIENCES OY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com