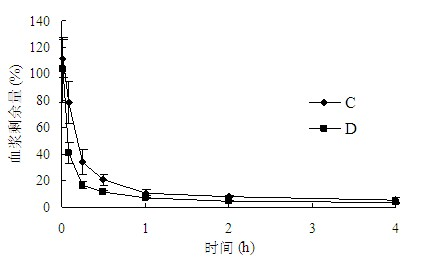
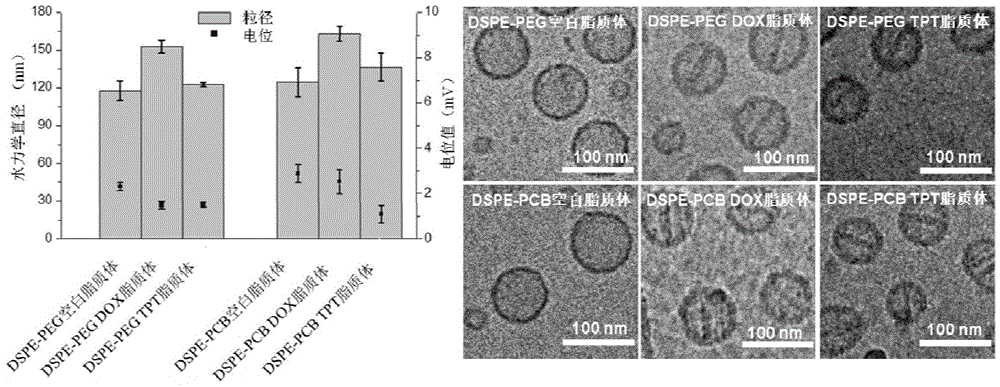
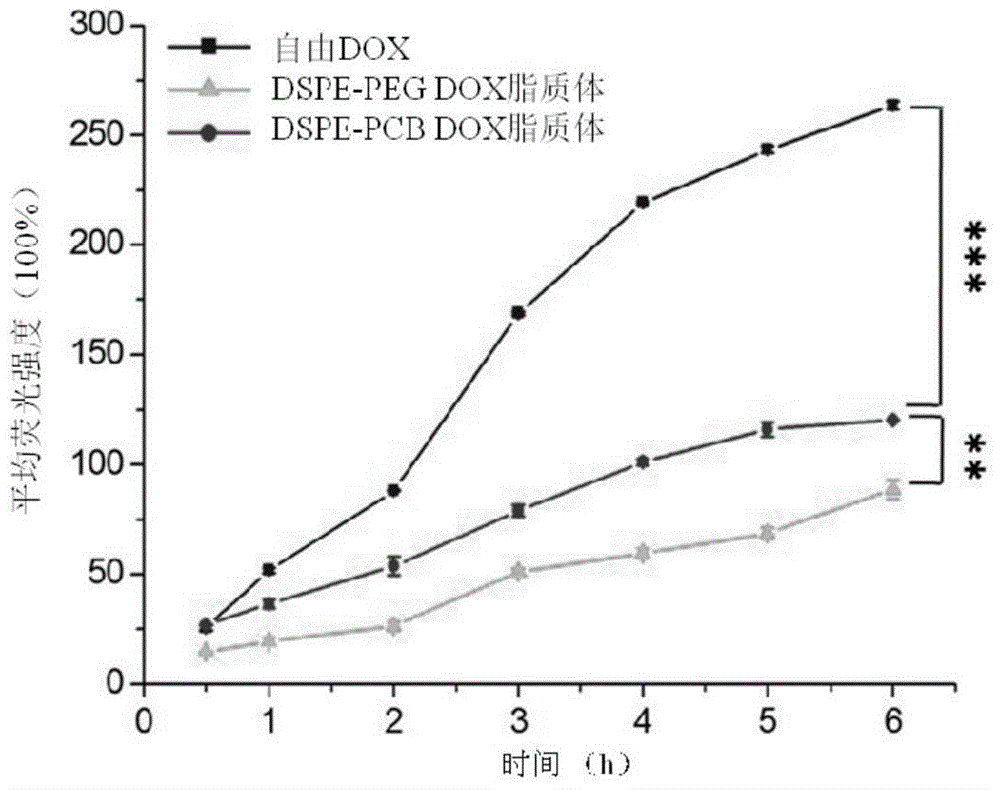
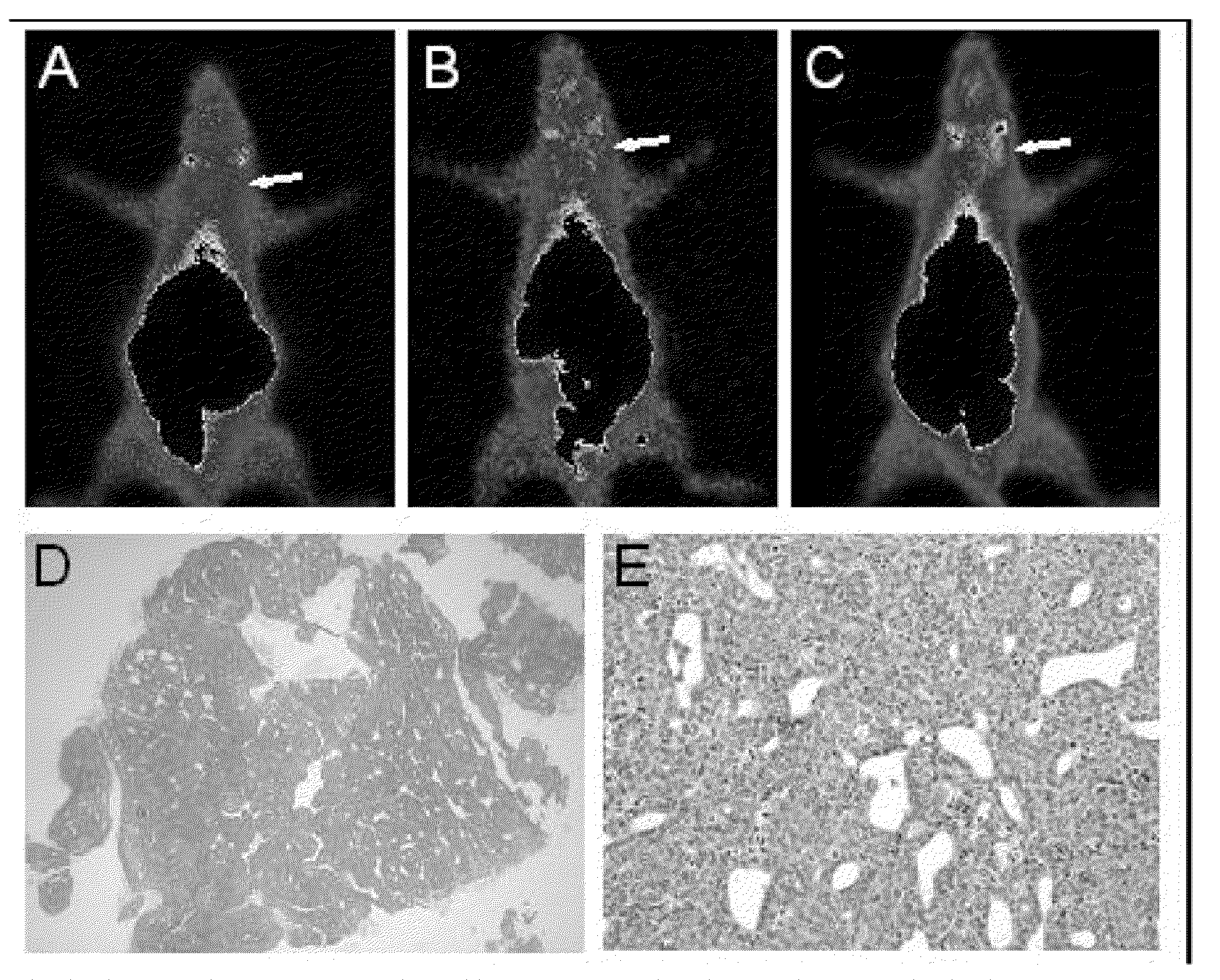
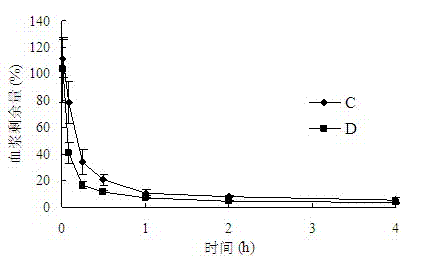
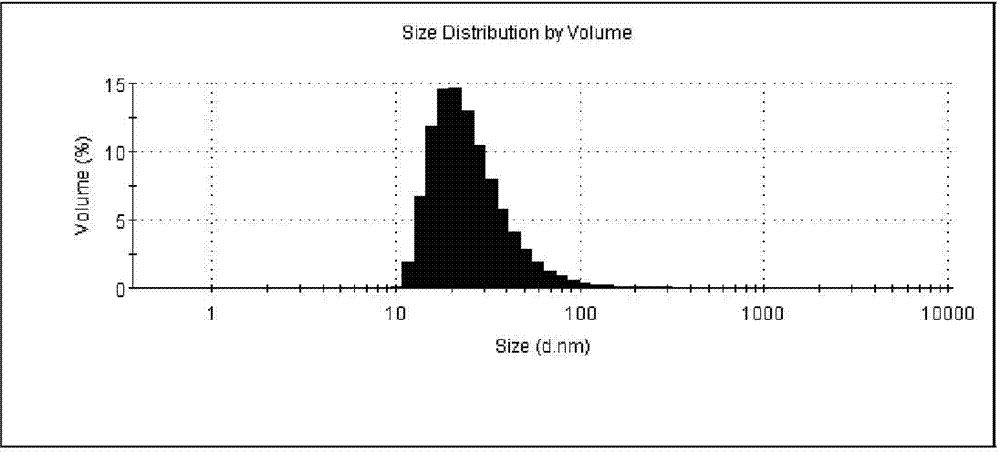
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Blood clearance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clearance is variable in zero-order kinetics because a constant amount of the drug is eliminated per unit time, but it is constant in first-order kinetics, because the amount of drug eliminated per unit time changes with the concentration of drug in the blood. Clearance can refer to the volume of plasma from which the substance is removed (i.e ...
Contrast agents for magnetic resonance imaging
InactiveUS20050260137A1Improve slackImprove abilitiesNanomedicineDiagnostic recording/measuringBlood clearanceSignal-to-noise ratio (imaging)
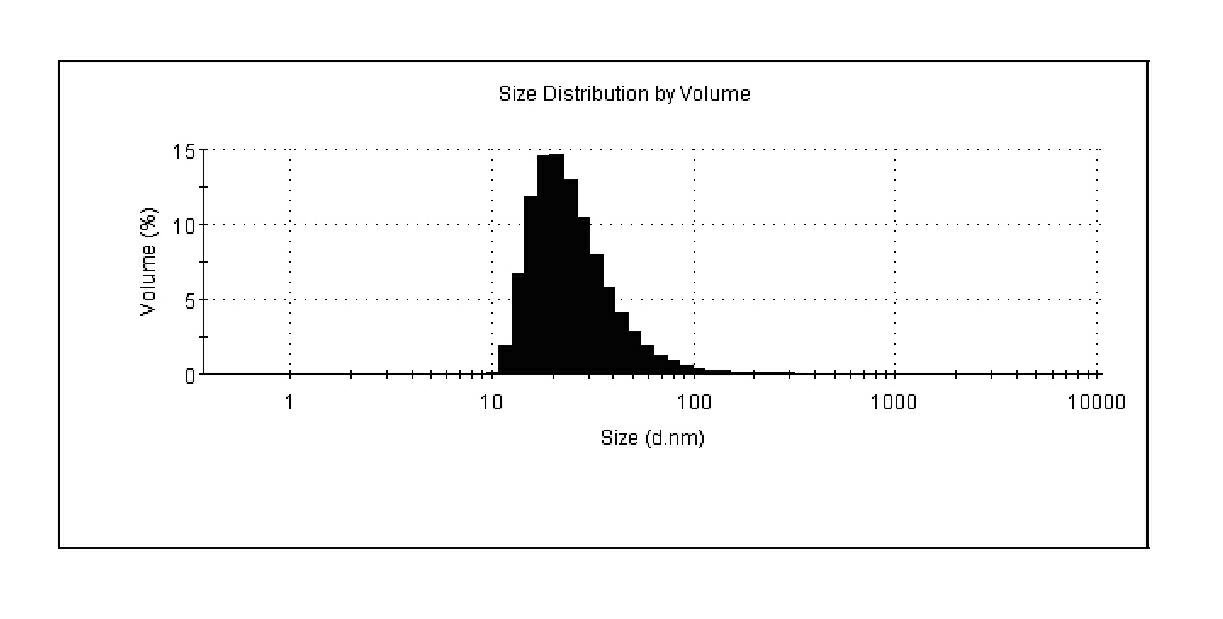
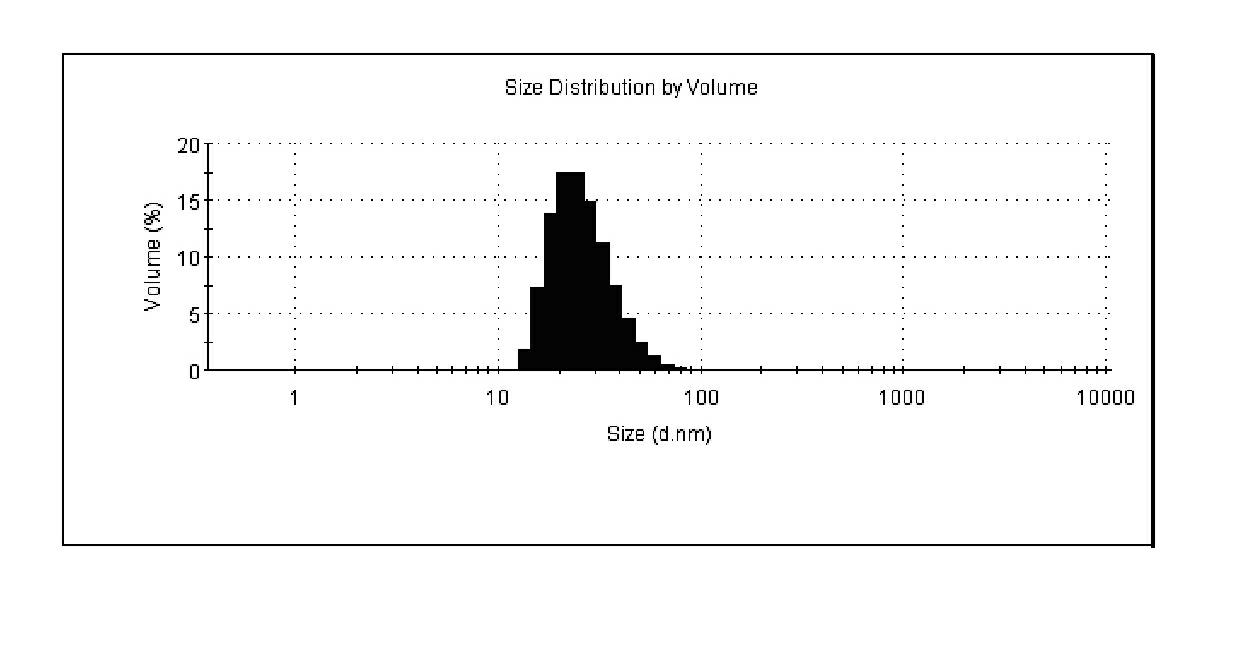
A contrast agent for magnetic resonance imaging having a plurality of nanoparticles. Each of the nanoparticles has: a signal generating core having a diameter of up to 10 nm; at least one organic layer of at least one of a polymer, a monomer, and a surfactant; and a water soluble outer shell of at least one of a polymer, a monomer, and a ligand. The organic layer is adsorbed upon and substantially surrounds and stabilizes the signal generating core. The water soluble outer shell solubilizes and provides biocompatibility for each of the nanoparticles. The contrast agents provide enhanced relaxivity, high signal-to-noise ratios, and targeting abilities. In addition, the contrast agents possess resistance to agglomeration, controlled particle size, blood clearance rate, and biodistribution. Methods of making such contrast agents and nanoparticles are also disclosed.
Owner:PRIAVOID GMBH +1
Low-concentration polyethylene glycol-lipid (PEG-lipid) derivative and application thereof
InactiveCN102813929ARelieve pressureReduce the number of cyclesPharmaceutical non-active ingredientsLiposome VesicleLiposome
The invention belongs to the technical field of medicine, relates to a low-concentration PEG-lipid derivative and an application thereof, particularly to a prescription composition and an application thereof which can relieving or avoiding accelerated blood clearance (ABC) of PEGylation preparations. According to the low-concentration PEG-lipid derivative and the application thereof, the low-content PEG-lipid derivative is used for modifying liquid particle preparations such as emulsions, liposome, vesicles, micro emulsions, micelles and nano-particles, PEG and lipid chain segments are connected through ester bonds, amido bonds or ether bonds in the PEG-lipid derivative, and the molecular weight of the PEG is 200-20000 Dalton. Preparations prepared by the PEG-lipid derivative can only cause slight or cannot cause the ABC, namely, can reduce or avoid the ABC after repeated injection. The low-concentration PEG does not reduce in vitro anti-tumor activity of preparations, and stability of the preparation can be improved if the low-concentration PEG-lipid derivative is added, particularly shaking instability of antagonistic emulsions.
Owner:SHENYANG PHARMA UNIVERSITY
Application of cleavable polyethylene glycol (PEG) lipid derivative to preparation
InactiveCN102068701AEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeated injection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
Long-circulating liposome capable of avoiding accelerated blood clearance (ABC) phenomenon, and preparation method and application thereof
InactiveCN104644555APromote escapeIncrease serum stabilityOrganic active ingredientsAntipyreticLipid formationDisease
The invention relates to a long-circulating liposome capable of avoiding an accelerated blood clearance (ABC) phenomenon, a medicine preparation containing the long-circulating liposome, and a preparation method and an application of the long-circulating liposome. The long-circulating liposome provided by the invention comprises phospholipid, auxiliary lipid and poly carboxyl betaine lipid, wherein the long-circulating liposome and the preparation thereof can be applied to medicines for treating related diseases. According to the long-circulating liposome for avoiding the ABC phenomenon provided by the invention, the defect that a common liposome is easily recognized by a reticular endothelium system is overcome; a long-circulating effect of blood is reached; meanwhile, the ABC phenomenon caused by multiple intravenous injections of the PEG-modified long-circulating liposome is avoided; the medicine effect of a therapeutic medicine is effectively improved; and the long-circulating liposome has a high application prospect in the field of medicines.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
99mTc-LABELED TRIPHENYLPHOSPHONIUM DERIVATIVE CONTRASTING AGENTS AND MOLECULAR PROBES FOR EARLY DETECTION AND IMAGING OF BREAST TUMORS
InactiveUS20090220419A1Radioactive preparation carriersPeptide preparation methodsHalf-lifeBreast fluid
99mTc-labeled triphenylphosphonium contrasting agents that target the mitochondria and are useful for early detection of breast tumors using scintimammographic imaging. 99mTc-Mito10-MAG3 possesses advantageous radiopharmaceutical properties. The uptake in the myocardium is reduced by one to two orders of magnitude compared to 99mTc-MIBI. 99mTc-Mito10-MAG3 exhibits fast blood clearance, with a blood half-life of less than 2 minutes in rats. A diminished myocardial uptake combined with a prompt reduction of cardiovascular blood pool signal to facilitate improved signal-to-background ratios.
Owner:LOPEZ MARCOS +3
Image generation based on limited data set
InactiveUS20100054559A1Increase patient comfortReliable estimateImage enhancementImage analysisData setSonification
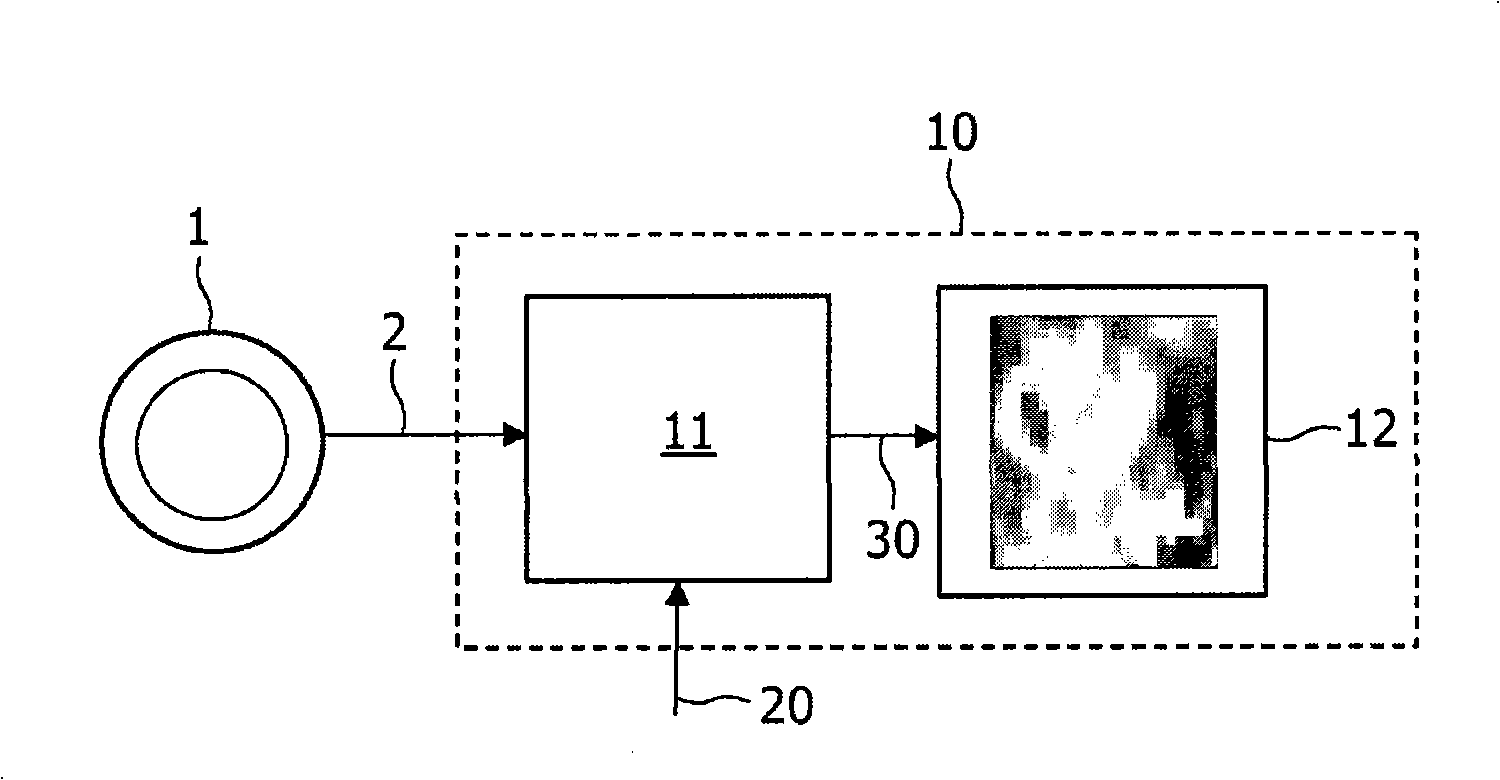
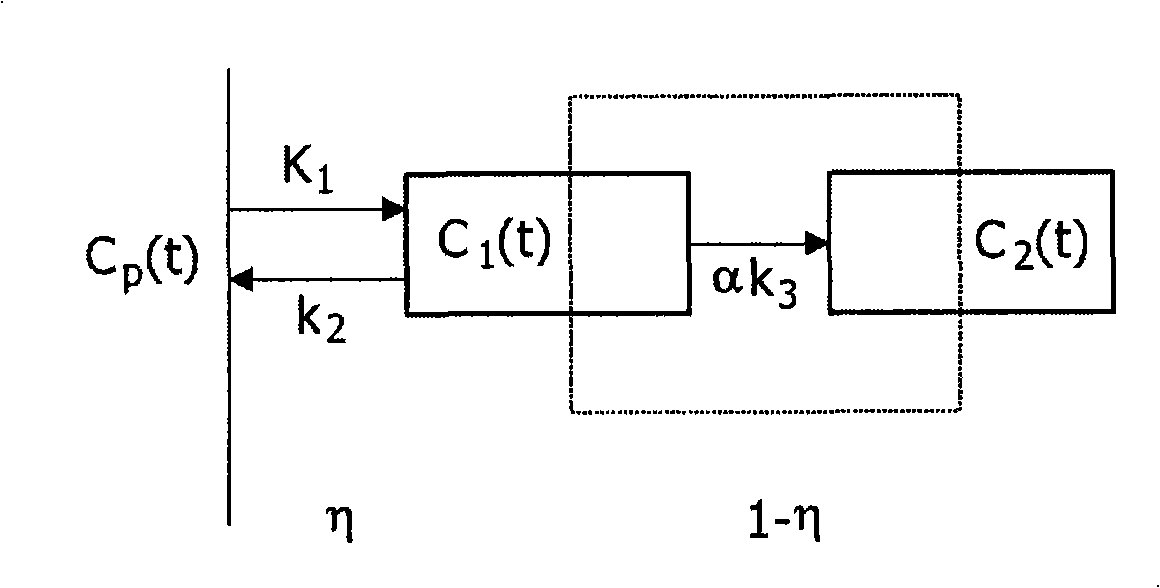
A method, signal processor, device, and system for estimating a parametric or functional image 47 mapping a biological process on the basis of a limited or incomplete sequence of biological process images 40 recorded as a function of time, e.g. by a PET or SPECT scanner after injection of a radio tracer. One or more kinetic parameters 43 are first extracted by applying a pharmacokinetic model 42 (compartmental model of the underlying tracer kinetics) to the sequence of biological process images 40. Additional data 41 are used in the model, comprising at least a predetermined kinetic parameter range (e.g. from the literature), and optionally an input function or a blood clearance function. Next, an iterative algorithm 44 is applied to arrive at a modified sequence of images 45, e.g. by inserting an estimated image into the incomplete sequence of images, utilizing the one or more kinetic parameters 43. After a stop criterion has been fulfilled, the resulting image 47 is finally estimated 46 from the modified sequence of images 45. The method can be used e.g. to estimate a hypoxia parameter k3 image in the case of a FMISO data set where only late-time images are available. The method may be implemented as part of existing PET, SPECT, CT, MR or Ultrasound scanner software, and since only a limited amount of late time post injection images are necessary to provide a reliable result, the method helps to increase patient comfort and clinical throughput.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Modified antibodies and methods of use
InactiveUS20090022658A1Low toxicityRapid blood clearanceAntibody mimetics/scaffoldsRadiation applicationsBlood clearanceBone marrow suppression
Novel compounds, compositions and methods comprising modified antibodies are provided. In preferred embodiments the disclosed modified antibodies comprise antibodies having one or more of the constant region domains altered or deleted to afford beneficial physiological properties such as enhanced target localization and rapid blood clearance. The disclosed compounds are particularly useful for the treatment of neoplastic disorders in myelosuppressed patients.
Owner:BRASLAWSKY GARY R +2
Deproteinated calf blood ingredient brain targeting nanosphere and preparation method thereof
The invention discloses a deproteinated calf blood ingredient brain targeting nanosphere preparation method, specifically comprising: according to the parts by weight, dissolving 5-30 parts of stabilizing agent in distilled water, then dissolving 5-30 parts of pluronic F-68 in distilled water, mixing the two obtained solution, shaking well, adding 50-100 parts of deproteinated calf blood extractive injection, shaking well, after constant volume, adjusting the PH value to 1-4, slowly adding 5-30 parts of polybutylcyanoacrylate while stirring; stirring for 1-6h, adjusting PH value to 6-8, stirring for 1-6h to obtain the colloid solution of deproteinated calf blood ingredient nanosphere, filtering, freeze drying; dissolving the freezedrying powder of deproteinated calf blood ingredient nanosphere, adding 5-30 parts of tween 80 and stirring for 0.5-3h to obtain deproteinated calf blood ingredient brain targeting nanophere. The invention has the advantages of simple preparation method, low cost, easy storage, short time to reach the brain, rapid blood clearance rate and the like.
Owner:WUHAN UNIV OF TECH
Alprostadil lipid nanosphere freeze-drying injection and preparation method thereof
ActiveCN102228446AStable physical stabilityImprove stabilityOrganic active ingredientsPowder deliveryPulmonary vasculatureDisease
The invention discloses an alprostadil lipid nanosphere freeze-drying injection and a preparation method thereof, and belongs to the technical field of medicines. The alprostadil lipid nanosphere freeze-drying injection is prepared from the following raw materials in part by weight: 0.0005 to 0.1 part of alprostadil, 15 to 60 parts of medium-chain oil, 3.0 to 35 parts of emulsifier, 8.5 to 48 parts of polyethylene glycol-12-hydroxy stearate, 22 parts of glycerol, 20 to 200 parts of trehalose, and 20 to 300 parts of cyclodextrin. The particle size of the alprostadil lipid nanosphere freeze-drying injection after redissolution is less than 100nm; and an aseptic filtration way can be used for sterilization, so that the disadvantage of thermal instability of the alprostadil is overcome, and the stability of products is improved. Meanwhile, lipid nanospheres have smaller particle sizes which are less than 100nm, so the alprostadil lipid nanosphere freeze-drying injection is more favorable for the distribution of the alprostadil in in-vivo non-reticuloendothelial system (RES) tissues, reduces pulmonary circulation inactivation and blood clearance, is favorable for circulating in vivo for a long time, and is suitable for the treatment of cardiac and neurosurgical diseases.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Image generation based on limited data set
A method, signal processor, device, and system for estimating a parametric or functional image 47 mapping a biological process on the basis of a limited or incomplete sequence of biological process images 40 recorded as a function of time, e.g. by a PET or SPECT scanner after injection of a radio tracer. One or more kinetic parameters 43 are first extracted by applying a pharmacokinetic model 42 (compartmental model of the underlying tracer kinetics) to the sequence of biological process images 40. Additional data 41 are used in the model, comprising at least a predetermined kinetic parameter range (e.g. from the literature), and optionally an input function or a blood clearance function. Next, an iterative algorithm 44 is applied to arrive at a modified sequence of images 45, e.g. by inserting an estimated image into the incomplete sequence of images, utilizing the one or more kinetic parameters 43. After a stop criterion has been fulfilled, the resulting image 47 is finally estimated 46 from the modified sequence of images 45. The method can be used e.g. to estimate a hypoxia parameter k3 image in the case of a FMISO data set where only late-time images are available. The method may be implemented as part of existing PET, SPECT, CT, MR or Ultrasound scanner software, and since only a limited amount of late time post injection images are necessary to provide a reliable result, the method helps to increase patient comfort and clinical throughput.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
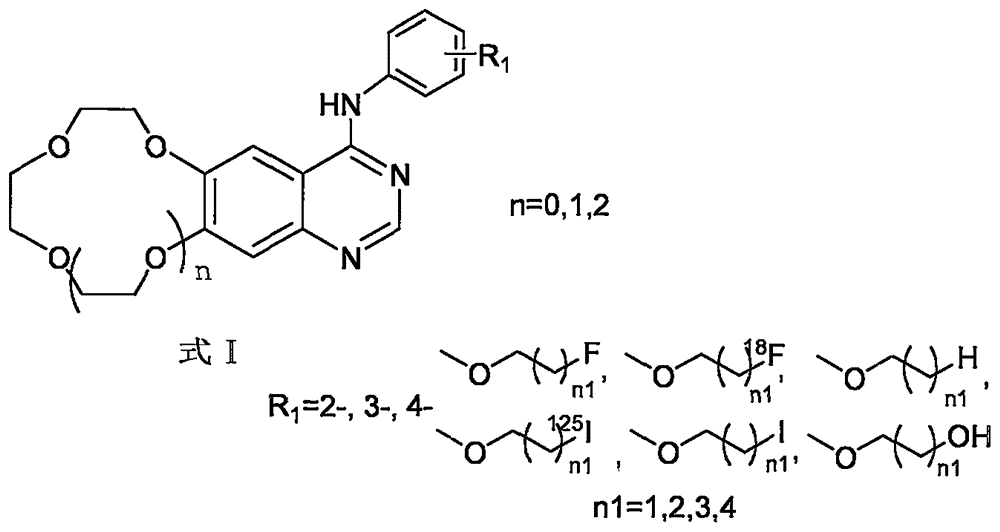
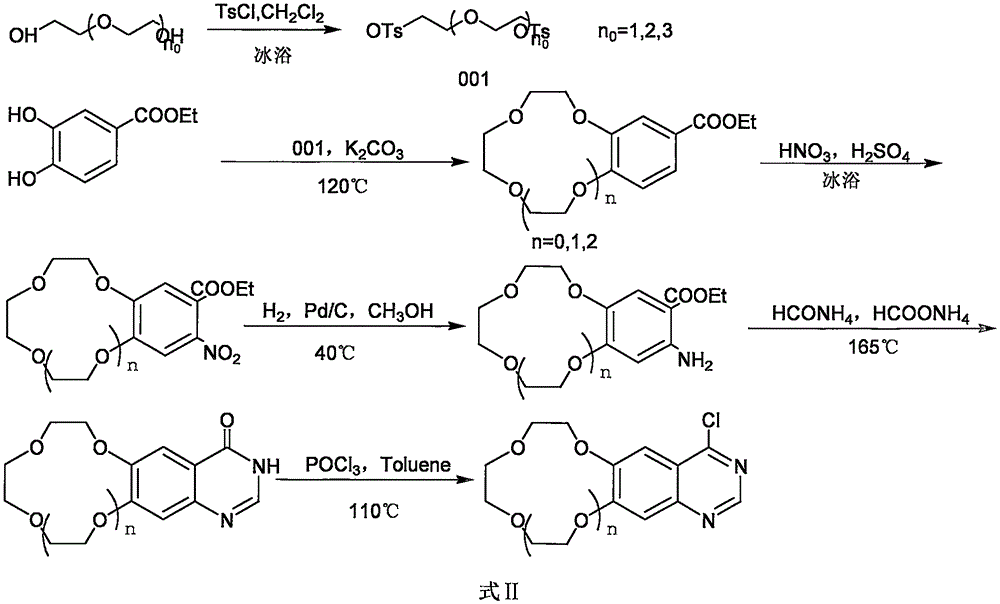
Crown ether cyclic quinazoline compound, preparation method therefor and application thereof in preparing tumor therapy and imaging drug
InactiveCN105384745AGood antitumor activityGood tumor imagingOrganic chemistryRadioactive preparation carriersTumor therapyIsotope
The invention relates to a crown ether cyclic quinazoline derivative and medicinal salts thereof using in tumor therapy and imaging. The crown ether cyclic quinazoline compound is characterized in that a 2-, 3-, 4- substituted aniline is disposed at one end; a 6-, 7- substituted crown ether cyclic quinazoline structure is disposed at the other end; the substituent group R1 is located on the fourth-position aniline of a quinazoline mother ring and is 2-, 3-, 4- substituted alkoxy; and the crown ether cycle is located at the 6, 7 position of the mother ring, and is a 9-crown-3, 12-crown-4 and 15-crown-5, and the structural formula is formula I. The experimental result of antitumor activity in vitro of nonradioactive isotope labeling compounds shows that the compounds are good in inhibitory effect for four kind of cancer cells namely HepG2, A549, sy5y and DU145, and have the potential as cancer treatment medicine; and the body distribution experiment of 18F or 125I labeling compounds show that the compounds are higher in ingestion and certain detention in tumors and faster in blood clearance and have potential applied to tumor imaging.
Owner:BEIJING NORMAL UNIVERSITY
Imaging of activated vascular endothelium using immunomagnetic mri contrast agents
lmmunomagnetic nanoparticles are used as a contrast agent for enhancing medical diagnostic imaging such as magnetic resonance imaging (MRI). The present invention is directed to methods of making targeted MRI contrast agents from immunomagnetic particles, and to methods of using such MRI contrast agents. Typically, such targeted MRI contrast agents provide enhanced relaxivity, improved signal-to-noise, targeting ability, and resistance to agglomeration. Methods of making such MRI contrast agents typically afford better control over particle size, and methods of using such MRI contrast agents typically can afford enhanced blood clearance rates and distribution. The ability to use the contrast agents im MRI provides a tool in the diagnosis and treatment of several disease states.
Owner:VERIDEX LCC
Application of cyclodextrin in preparing radioactive iodine labeling hypericum monogynum medicine preparation
InactiveCN107308455AMentioned water solubilityQuick clearOrganic active ingredientsPharmaceutical delivery mechanismSolventPharmaceutical formulation
The invention belongs to the field of medicine preparations, and in particular relates to application of cyclodextrin in preparing a radioactive iodine labeling hypericum monogynum medicine preparation. The cyclodextrin is applied to preparation of a radioactive iodine labeling hypericum monogynum medicine' preparation first a first time; the cyclodextrin is an oligo ring compound of glucose, is non-toxic to human bodies, hydroxypropyl-beta-cyclodextrin is approved by China as an auxiliary material for intravenous injection; meanwhile, compared with other solvents, the auxiliary material can greatly improve the water solubility of hypericin on premise that the iodine labeling efficiency is ensured, the radioactive iodine labeling hypericum monogynum medicine dissolved in the cyclodextrin is rapid in blood clearance, and the necrosis targeting of the medicine is not different from that of conventional preparations, so that the cyclodextrin can be used as a DMSO (Dimethyl Sulfoxide) substituent applied to the radioactive iodine labeling hypericum monogynum medicine preparation; the method for preparing the radioactive iodine labeling hypericum monogynum medicine preparation is simple in process, low in cost, high in preparation efficiency and applicable to industrial operation.
Owner:南京成至诚医药科技有限公司
Method for eliminating ABC (accelerated blood clearance) phenomenon of PEGtion nano-carrier
InactiveCN103800911AImprove physical stabilityImprove biostabilitySolution deliveryPharmaceutical non-active ingredientsDisulfide bondingMicrosphere
The invention discloses a novel means for eliminating the ABC (accelerated blood clearance) phenomenon of a PEGtion nano-carrier. A nano-carrier is modified by using a PEG-lipid derivative with the PEG end group (OH) being hydroxy, and is formed by connecting PEG with hydroxy as the end group and phospholipid through one of amido bond, ether bond, ester bond and disulfide bond. The nano-carrier comprises an emulsion, micelles, micro-capsules, microspheres and nano-particles. According to the means, the phenomenon of 'accelerated blood clearance' (ABC) of an existing PEGtion nano-carrier can be eliminated, and a compact hydration layer is formed on the carrier surface, so that the physical stability of the carrier can be improved, and the biological stability of the carrier can be improved.
Owner:SHENYANG PHARMA UNIVERSITY
99m Tc-labeled triphenylphosphonium derivative contrasting agents and molecular probes for early detection and imaging of breast tumors
99mTc-labeled triphenylphosphonium contrasting agents that target the mitochondria and are useful for early detection of breast tumors using scintimammographic imaging. 99mTc-Mito10-MAG3 possesses advantageous radiopharmaceutical properties. The uptake in the myocardium is reduced by one to two orders of magnitude compared to 99mTc-MIBI. 99mTc-Mito10-MAG3 exhibits fast blood clearance, with a blood half-life of less than 2 minutes in rats. A diminished myocardial uptake combined with a prompt reduction of cardiovascular blood pool signal to facilitate improved signal-to-background ratios.
Owner:LOPEZ MARCOS +3
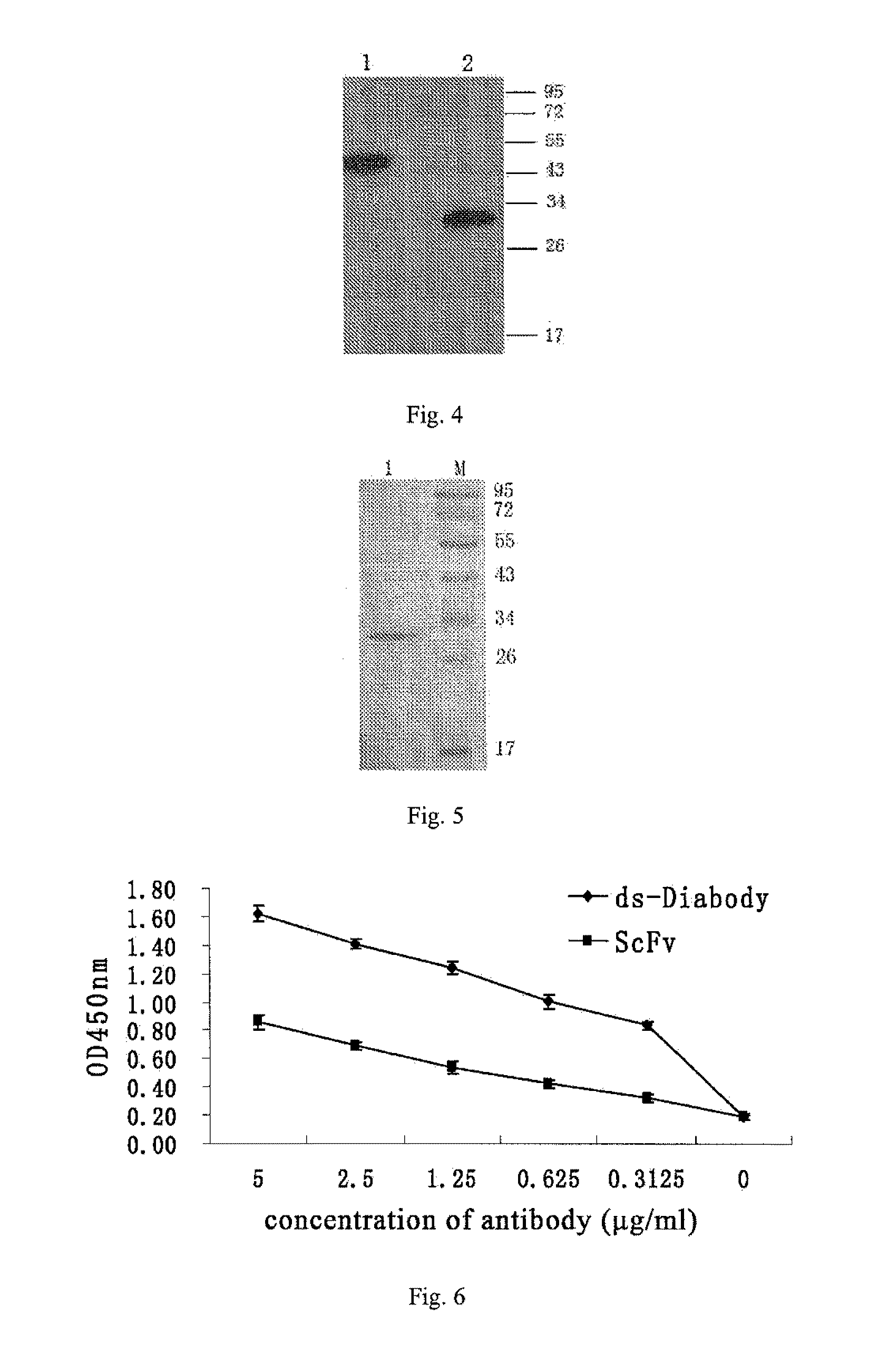
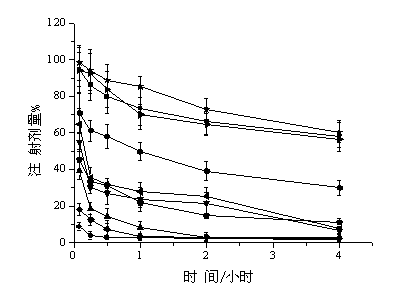
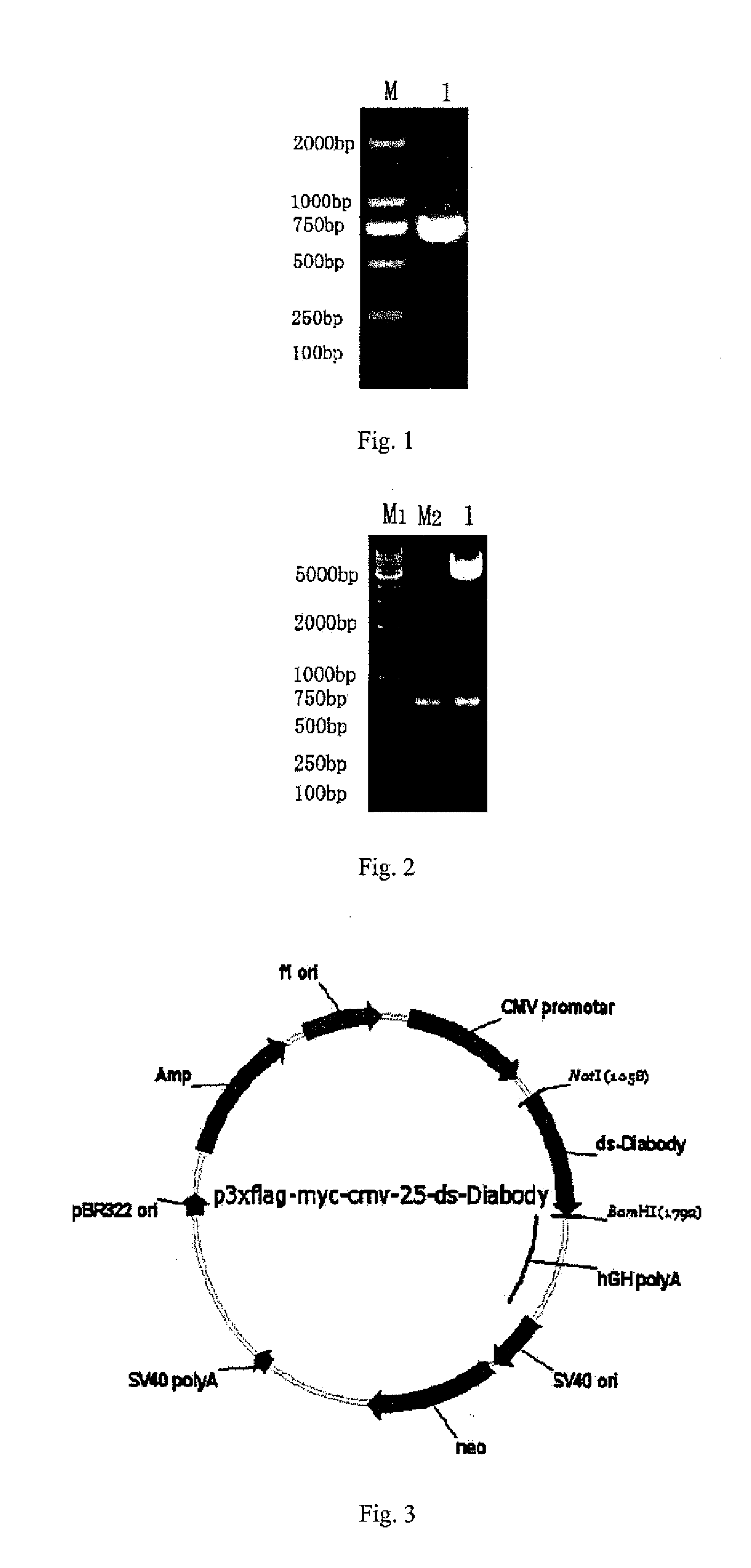
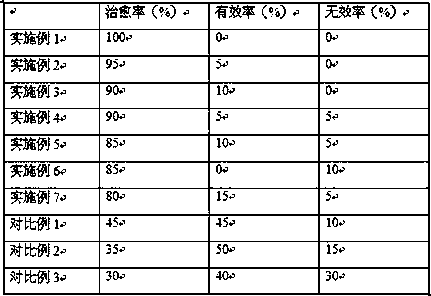
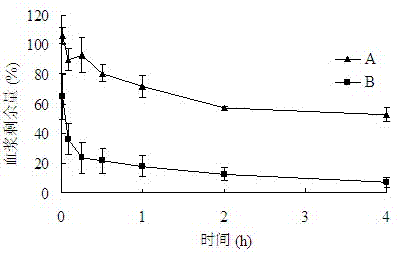
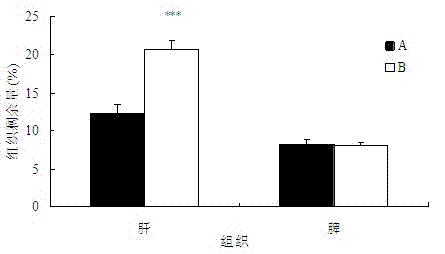
Anti-bFGF humanized double-stranded antibody with stable disulfide bond, preparation method and application thereof
InactiveUS9273131B2Improve stabilityModerate molecular weightImmunoglobulins against growth factorsAntibody ingredientsTumor targetAntigen
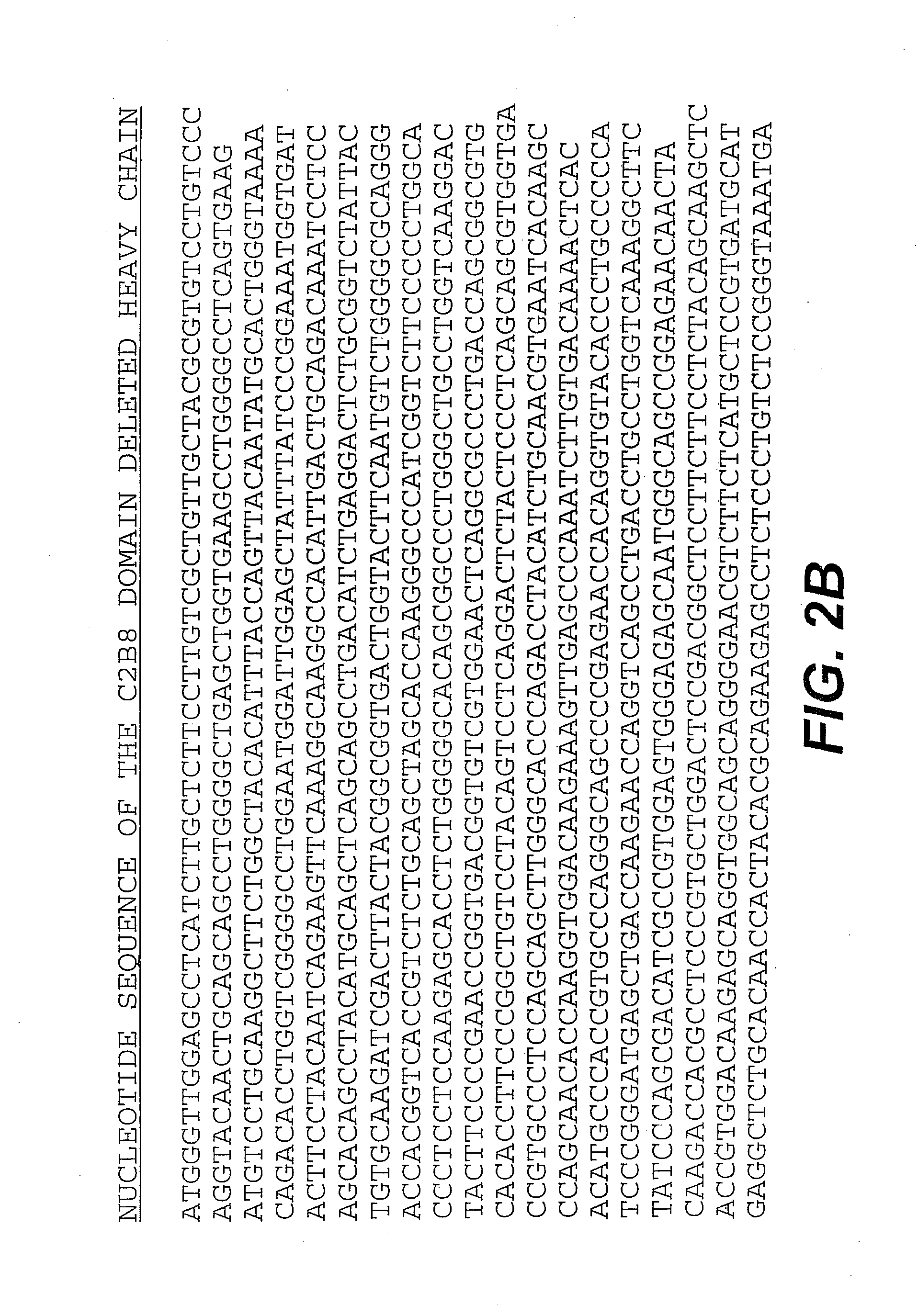
Disclosed are an anti-bFGF humanized double-stranded antibody with stable disulfide bond, the preparation method and the applications thereof. The amino acid sequence of the anti-bFGF humanized ds-Diabody is shown in SEQ ID NO.1. The nucleotide sequence of gene encoding the anti-bFGF humanized ds-Diabody is shown in SEQ ID NO.2. By site-directed mutation, two cysteine residues are introduced into VL and VH domain of anti-bFGF Diabody, thus introducing the disulfide bond and form ds-Diabody. It is shown by experiments that the obtained ds-Diabody has the following advantages: enhanced stability; better affinity when binding to the specific antigen bFGF; moderate relative molecular weight, good tumor targeting, more powerful in tumor tissue penetration, and higher blood clearance rate, showing a broad application prospects in the clinic diagnosis and tumor therapy.
Owner:JINAN UNIVERSITY
In-vitro controlled release drug and preparation method thereof
InactiveCN109364258AControl timeControl part and quantityOrganic active ingredientsPharmaceutical non-active ingredientsBlood clearanceFluorescence
The invention discloses an in-vitro controlled release drug. Red blood cells are used as carriers of the in-vitro controlled release drug, and a target drug and an infrared fluorescent dye are loadedin the red blood cells. By loading the drug in the red blood cells and modifying a photoresponse substance in the red blood cells, after intravenous administration, the targeted release of the targetdrug is realized through the irradiation of laser (808nm) in vitro. The targeted release drug can improve the targeting efficiency of the drug and reduces the blood clearance rate and the poison effect and the like.
Owner:NANTONG UNIVERSITY
Application of Cyclodextrin in the Preparation of Radioactive Iodine Labeled Hypericum Drug Preparations
InactiveCN107308455BMentioned water solubilityQuick clearOrganic active ingredientsPharmaceutical delivery mechanismCyclodextrinPharmaceutical Aids
The invention belongs to the field of pharmaceutical preparations, in particular to the application of cyclodextrin in the preparation of radioactive iodine-labeled hypericum drug preparations. For the first time, cyclodextrin is applied to the preparation of "radioactive iodine-labeled hypericum drug" preparations. Dextrin is an oligocyclic compound of glucose, which is basically non-toxic to the human body. Hydroxypropyl-β-cyclodextrin is an excipient approved for intravenous injection in China. At the same time, compared with other solvents, it can ensure the efficiency of iodine labeling Under the premise, the water solubility of hypericin can be greatly mentioned, and the blood clearance of radioactive iodine-labeled hypericum drugs dissolved in cyclodextrin is fast, and there is no difference in necrosis targeting compared with traditional preparations, so cyclodextrin As a substitute for DMSO, it can be applied to radioactive iodine-labeled hypericum drug preparations; the method for preparing iodine-labeled hypericum drug preparations has simple process, low cost and high preparation efficiency, and is suitable for industrial operation.
Owner:南京成至诚医药科技有限公司
PSA-TPGS (d-alpha-tocopherol polyethylene glycol 1000 succinate) conjugate and application thereof
ActiveCN106075467AOrganic active ingredientsPeptide/protein ingredientsBlood clearanceCritical micelle concentration
PSA is modified on high-purity TPGS (d-alpha-tocopherol polyethylene glycol 1000 succinate), so that the CMC (critical micelle concentration) value and the hemolysis of the TPGS can be reduced; the antitumor activity can also be improved; the TPGS and lipophilic substances are jointly prepared into mixed micelle. The mixed micelle can simultaneously have the respective advantages of PSA and TPGS; firstly, the character of the necessary nutrient element of tumor of the PSA is used for actively targeting to the tumor position; then, the anti-tumor character of the TPGS is used for synergistically killing tumor cells together with medicine; the original advantages of the TPGS are maintained; meanwhile, the CMC value of the TPGS is also reduced. More importantly, the ABC (accelerated blood clearance) phenomenon of PEG carrier crossed injection can also be eliminated.
Owner:SHENYANG PHARMA UNIVERSITY
Composite cefquinome microsphere gel preparation and preparation method thereof
ActiveCN106474087AGood sustained release effectReduce clearanceAntibacterial agentsOrganic active ingredientsGel preparationBlood clearance
The invention discloses a composite cefquinome microsphere gel preparation and a preparation method thereof, and belongs to the technical field of medicines. The composite cefquinome microsphere gel preparation for veterinary use is prepared by adopting a spray drying method to prepare microspheres, adopting cefquinome as the medicine for the microspheres, adopting polylactic acid / polylactic acid-glycolic acid as a coating carrier material, and adopting hyaluronic acid and water as gel bases to prepare the composite cefquinome microsphere gel preparation. The microspheres are of 5-30umd in grain size, which is beneficial to lung targeting of the main medicine cefquinome, the cefquinome can easily gather at a target organ, blood clearance is reduced, long-time circulation is benefited, and pharmacological efficacy is improved. In addition, by the aid of the gel bases, contact of the medicine and interstitial fluid is reduced, the time of the medicine flowing into a bloodstream is prolonged, and a slow-release effect of the preparation is further increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method of avoiding phenomenon of accelerating blood clearance by continuously and repeatedly injecting polyethylene glycol (PEG) lipidosome of epirubicin hydrochloride
InactiveCN103156871AAntibacterial agentsOrganic active ingredientsBlood clearancePolyethylene glycol
The invention belongs to the field of medical technology, relates to a method of avoiding a phenomenon of accelerating blood clearance by continuously and repeatedly injecting polyethylene glycol (PEG) lipidosome of epirubicin hydrochloride, and particularly relates to the influences of the pharmacokinetics behavior of the epirubicin hydrochloride in the second injection of the PEG epirubicin hydrochloride lipidosome by the first injection of blank lipidosome and PEG epirubicin hydrochloride lipidosome and the phenomenon of accelerating blood clearance by continuously and repeatedly injecting the PEG lipidosome. Dosage of PEG epirubicin hydrochloride lipidosome is provided to relieve or avoid the acceleration of the blood clearance on the mentioned basis. By changing the dosage of the epirubicin hydrochloride of the first injection or the continuous injection, the method reaches the purpose of preventing drugs from releasing from the lipidosome and relieving or eliminating the acceleration of the blood clearance.
Owner:SHENYANG PHARMA UNIVERSITY
Modified antibodies and methods of use
InactiveUS20110020222A1Improve localizationReduce dwell timeAntibody mimetics/scaffoldsRadiation applicationsBlood clearanceAntibody
Owner:BIOGEN MA INC
Anti-bfgf humanized double-stranded antibody with stable disulfide bond, preparation method and application thereof
InactiveUS20140350225A1High affinityImprove stabilityImmunoglobulins against growth factorsAntibody ingredientsAntigenTumor target
Disclosed are an anti-bFGF humanized double-stranded antibody with stable disulfide bond, the preparation method and the applications thereof. The amino acid sequence of the anti-bFGF humanized ds-Diabody is shown in SEQ ID NO. 1. The nucleotide sequence of gene encoding the anti-bFGF humanized ds-Diabody is shown in SEQ ID NO. 2. By site-directed mutation, two cysteine residues are introduced into each single-stranded antibody, thus introducing the disulfide bond and form ds-Diabody. It is shown by experiments that the obtained ds-Diabody has the following advantages: enhanced stability; better affinity when binding to the specific antigen bFGF; moderate relative molecular weight, good tumor targeting, more powerful in tumor tissue penetration, and higher blood clearance rate, showing a broad application prospects in the clinic diagnosis and tumor therapy.
Owner:JINAN UNIVERSITY
Compositions and methods for leukocyte-targeting multi-valent imaging probes
InactiveUS20130144035A1High affinityHigh sensitivityPeptide/protein ingredientsGeneral/multifunctional contrast agentsFormyl peptidePeptide ligand
The present application discloses a new multivalent peptide ligand specifically targeting polymorphonuclear leukocytes (PMNs) with favorable pharmacological parameters to monitor sites of inflammation for imaging. The detailed synthesis, characterization, and pharmacological evaluation of the ligands are reported here. Two separate peptide binding ligands for formyl peptide and tuftsin receptors were chosen to link together based on the high expression levels of the two receptors on activated PMNs The heterobivalency and pegylated links were incorporated in the structural design to improve the sensitivity of the detection and to improve the bioavailability along with blood clearance profile, respectively. Two chemical constructs: cFLFLF-(PEG)n-TKPPR-99mTc (n=4, 12) were evaluated in vitro with human PMNs for binding affinity and bioavailability. As a result, FLFLF-(PEG)12-TKPPR99mTc was found to have more favorable pharmacological properties and was therefore used for further in vivo studies. Preliminary in vivo assessment of the agent was performed using Single Gamma Emission Computed Tomography (SPECT) imaging of a mouse model of ear inflammation. The results of these studies indicate cFLFLF-(PEG)12-TKPPR-99mTc may be a desirable imaging agent for binding to PMNs to identify sites of inflammation by SPECT.
Owner:ZEN GROUP
Blood-clearing and heart-nourishing tea and preparation method thereof
InactiveCN107865171AReduce permeabilityReduce brain water contentTea substituesSalvia miltiorrhizaCholesterol excretion
A blood-clearing and heart-nourishing tea and a preparation method thereof belong to the field of health-care teas. The following raw materials are included by weight: 4-6 parts of raw Sanqi, 0.5-2 parts of cooked Sanqi, 4-6 parts of Danshen, 8-11 parts of Ganoderma lucidum and 8-11 parts of Burdock. The synergistic effect of each component promotes lipid and sugar metabolism, accelerates cholesterol excretion, reduces cholesterol absorption, and lowers blood viscosity through the three stages of clearing, nourishing, and regulating, so as to reduce blood fat, blood sugar, and blood pressure, and effectively For the purpose of protecting the cardiovascular and cerebrovascular system, the test drinking effect shows that for low-to-medium symptoms of three highs, drinking this tea for three to six months, 70-100% of triglycerides, low-density cholesterol, blood sugar, and blood pressure indicators will return to normal , the inefficiency is below 10%.
Owner:郗永生
Application of cleavable polyethylene glycol (PEG) lipid derivative in preparation
InactiveCN102068701BEasy to prepareNo radioactive contaminationPowder deliveryPharmaceutical non-active ingredientsLiposome VesicleMethyl carbonate
The invention belongs to the technical field of medicaments, and provides application of a cleavable polyethylene glycol (PEG) lipid derivative to preparation of a PEGylated preparation for relieving or avoiding accelerated blood clearance. In the application, liquid microparticle preparations such as liposome, vesicles, emulsions, microemulsion, micelles, nanoparticles and the like are modified by the cleavable PEG lipid derivative such as PEG-cholesteryl hemisuccinate, PEG-cholesteryl methyl carbonate, PEG-alpha tocopheryl hemisuccinate and the like; and the measurement of variation of preparation elimination in tissues such as animal blood plasma, liver, spleen and the like after a cleavable PEG lipid derivative-modified medicinal preparation is repeatedly injected proves that repeatedinjection of cleavable PEG lipid derivative-modified microparticle preparations only causes light accelerated blood clearance or avoids the accelerated blood clearance, namely the accelerated blood clearance can be relieved or avoided. The invention discloses new application of the cleavable PEG lipid derivative.
Owner:SHENYANG PHARMA UNIVERSITY
A kind of cefquinome microsphere gel composite preparation and preparation method thereof
ActiveCN106474087BGood sustained release effectReduce clearanceAntibacterial agentsOrganic active ingredientsGel preparationBlood clearance
The invention discloses a composite cefquinome microsphere gel preparation and a preparation method thereof, and belongs to the technical field of medicines. The composite cefquinome microsphere gel preparation for veterinary use is prepared by adopting a spray drying method to prepare microspheres, adopting cefquinome as the medicine for the microspheres, adopting polylactic acid / polylactic acid-glycolic acid as a coating carrier material, and adopting hyaluronic acid and water as gel bases to prepare the composite cefquinome microsphere gel preparation. The microspheres are of 5-30umd in grain size, which is beneficial to lung targeting of the main medicine cefquinome, the cefquinome can easily gather at a target organ, blood clearance is reduced, long-time circulation is benefited, and pharmacological efficacy is improved. In addition, by the aid of the gel bases, contact of the medicine and interstitial fluid is reduced, the time of the medicine flowing into a bloodstream is prolonged, and a slow-release effect of the preparation is further increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Alprostadil lipid nanosphere freeze-drying injection and preparation method thereof
ActiveCN102228446BMeet the characteristics of microemulsionOvercoming unstable traditional technical difficultiesPowder deliveryOrganic active ingredientsPulmonary vasculatureDisease
The invention discloses an alprostadil lipid nanosphere freeze-dried injection and a preparation method thereof, belonging to the technical field of medicines. Alprostadil lipid nanosphere freeze-dried injection, which is prepared from the following raw materials in parts by weight: alprostadil 0.0005-0.1, medium-chain oil 15-60, emulsifier 3.0-35, polyethylene glycol Alcohol lauryl hydroxystearate 8.5-48, glycerin 22, trehalose 20-200, cyclodextrin 20-300. The particle size of the reconstituted alprostadil lipid nanosphere freeze-dried injection of the present invention is less than 100nm, can be sterilized by sterile filtration, overcomes the shortcoming of alprostadil thermal instability, and increases the stability of the product. At the same time, because the lipid nanospheres have a smaller particle size below 100nm, it is more conducive to the distribution of alprostadil in non-RES tissues, reducing the inactivation of pulmonary circulation and blood clearance, which is conducive to long-term circulation in the body, and is suitable for the heart and brain. Treatment of neurosurgical diseases.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
A kind of polypeptide PET imaging agent targeting EGFR and its preparation method and application
ActiveCN109350751BHigh purityImprove stabilityRadioactive preparation carriersBlood clearanceRadiology
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Automatic pull-back trigger method for intracoronary imaging devices or systems using blood clearance
PendingCN114053553AU pull back flush syncAvoid image lossImage enhancementImage analysisOptical instrumentationOPHTHALMOLOGICALS
The invention relates to an automatic pull-back trigger method for an intracoronary imaging device or system using blood clearance. Provided herein are one or more devices, systems, methods, and storage media for optical imaging medical devices, such as, but not limited to, optical coherence tomography (OCT), single-mode OCT, and / or multimode OCT devices and systems, and methods and storage media for use therewith, for triggering automatic pullback, including devices or systems for using blood clearance. Examples of applications include imaging, evaluation, and diagnosis of biological subjects, such as, but not limited to, gastrointestinal, cardiac, and / or ophthalmic applications, and are obtained via one or more optical instruments, such as, but not limited to, optical probes, catheters, capsules, and needles (e.g., biopsy needles). Techniques provided herein also improve processing and imaging efficiency, while implementing more accurate images.
Owner:CANON USA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com