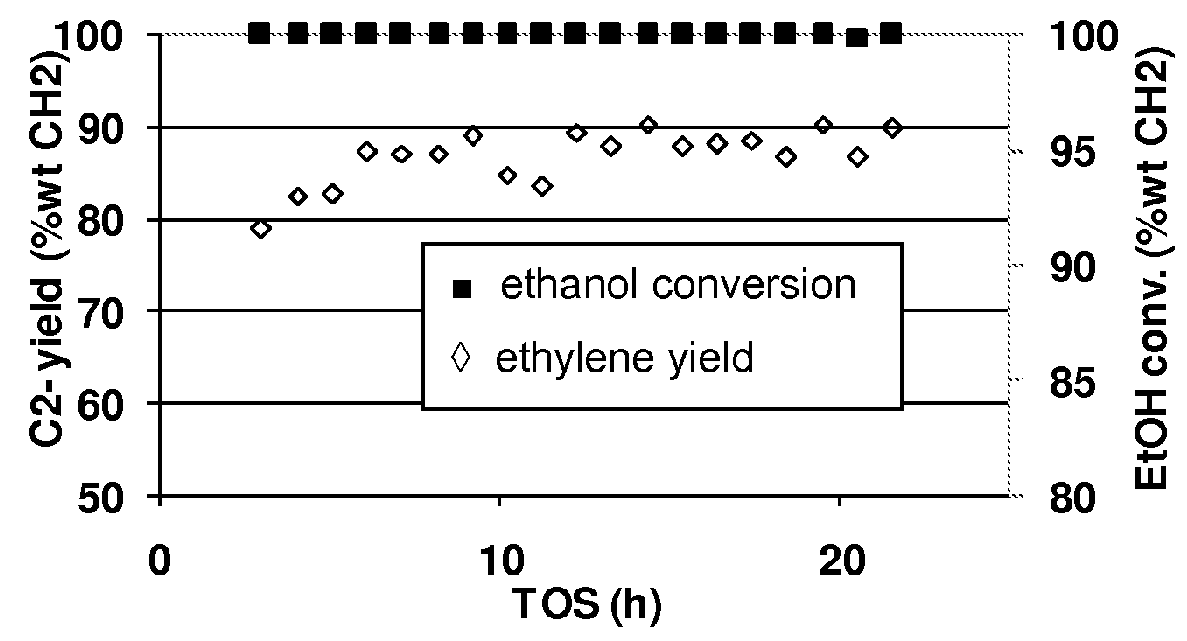
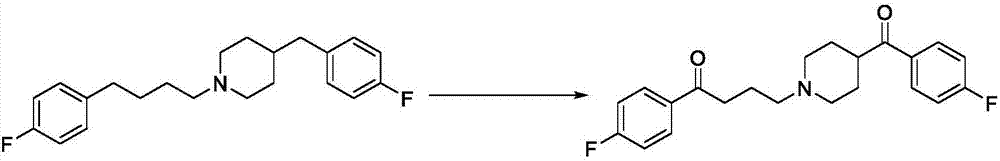
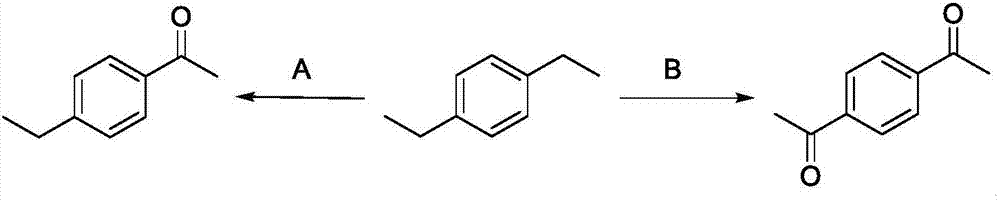
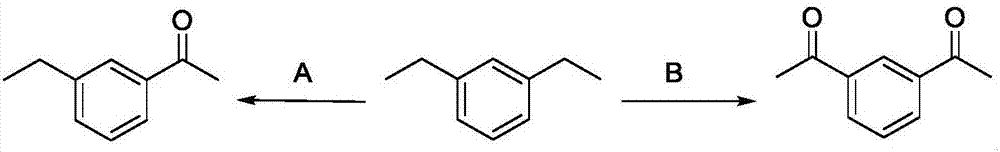
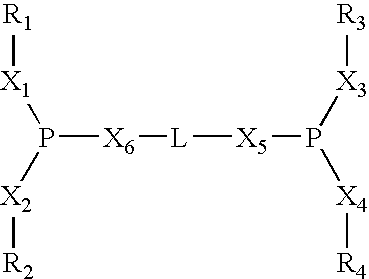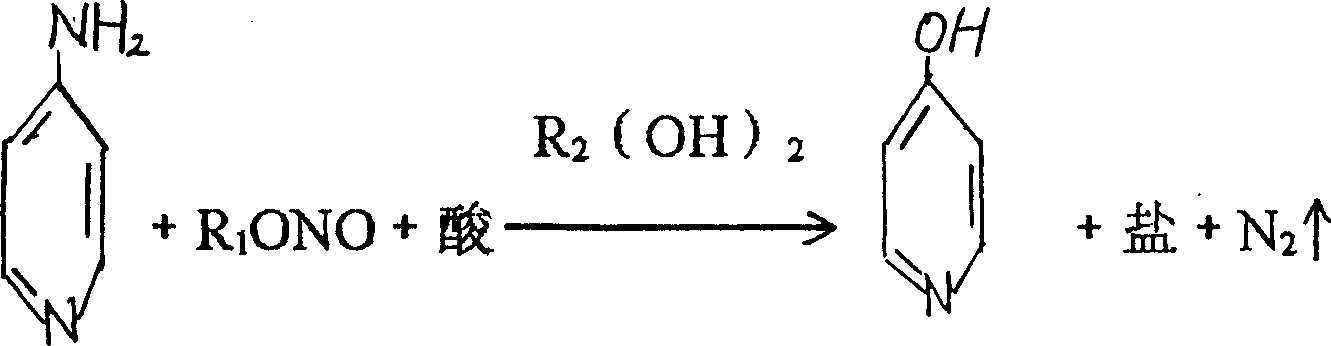Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Alkyl nitrites" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
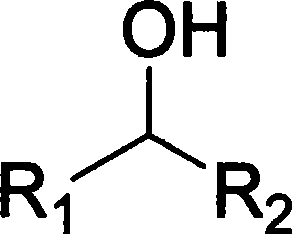
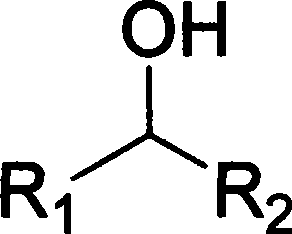
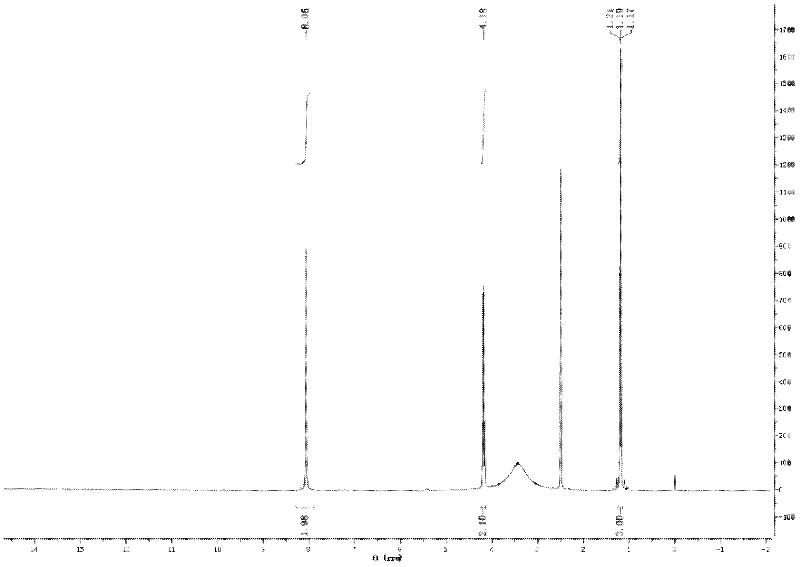
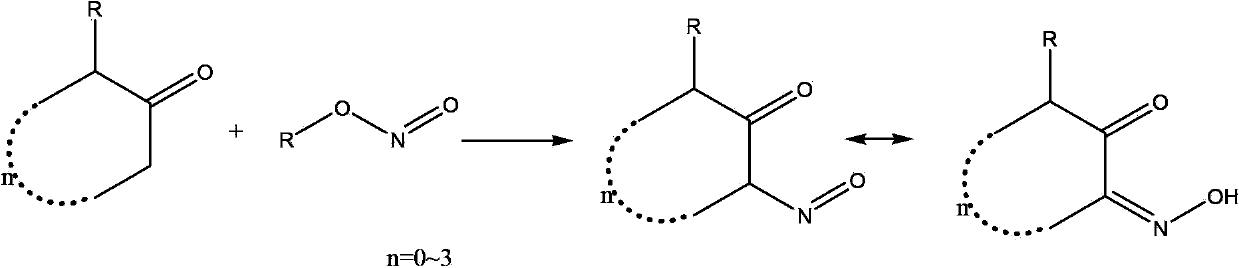
Alkyl nitrites are a group of chemical compounds based upon the molecular structure R-ONO. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds (R-NO₂). The first few members of the series are volatile liquids; methyl nitrite and ethyl nitrite are gaseous at room temperature and pressure. The compounds have a distinctive fruity odor. Another frequently encountered nitrite is amyl nitrite (3-methylbutyl nitrite).
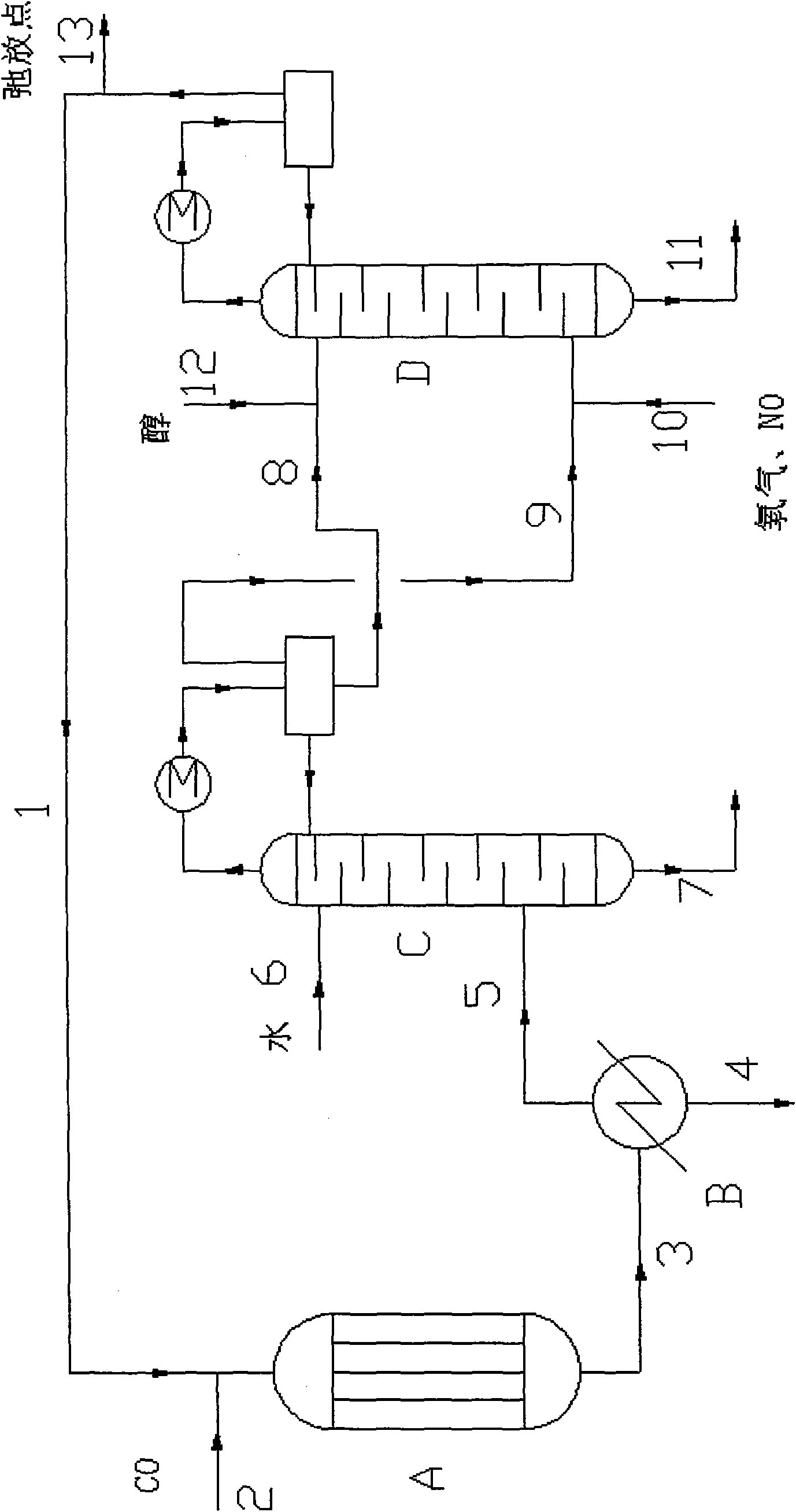
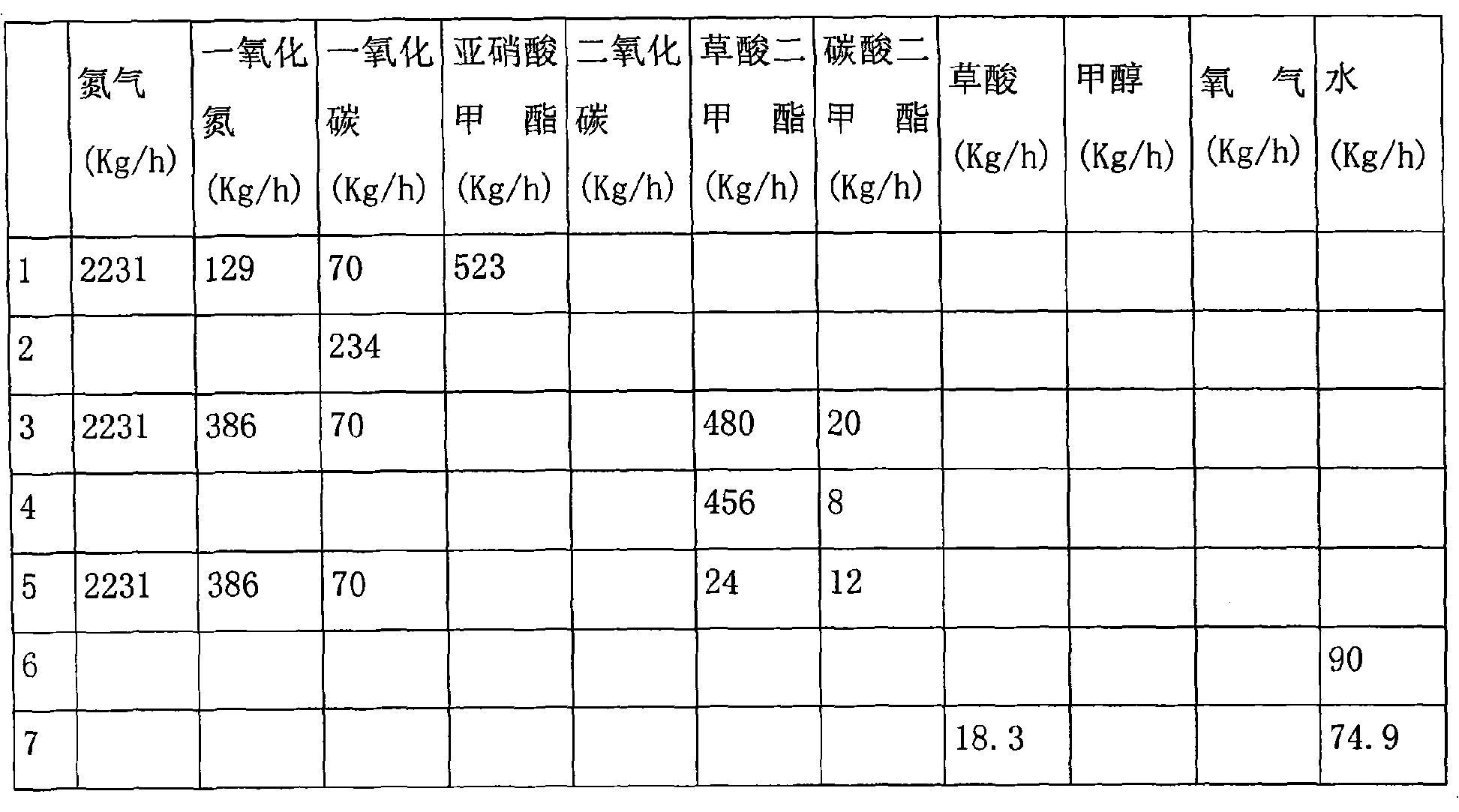
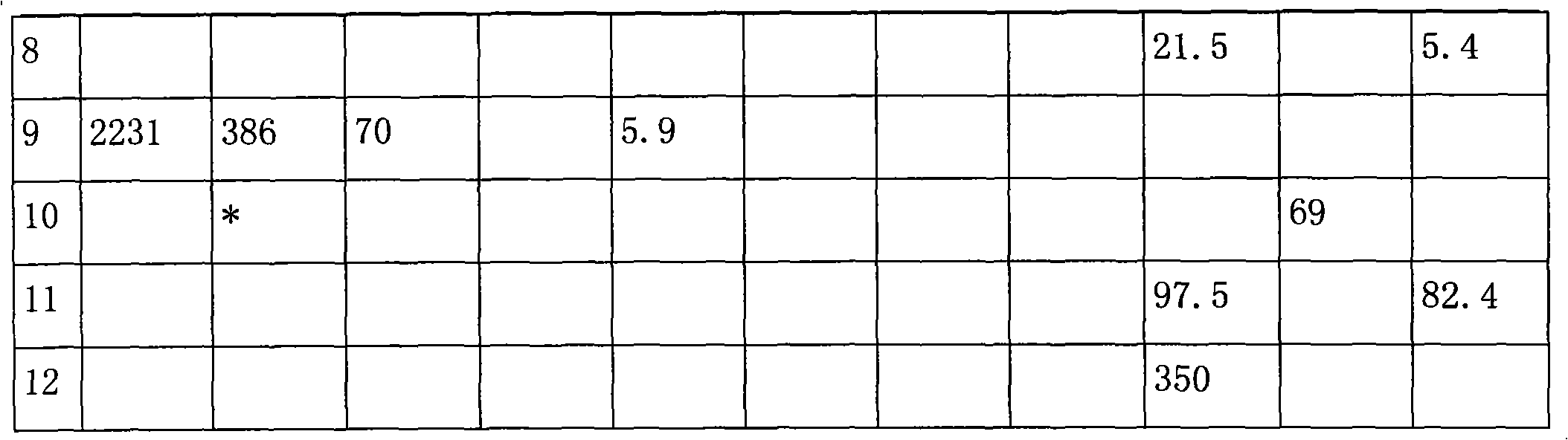
Method for producing oxalic ester with CO coupling
ActiveCN101492370AHigh selectivityImprove conversion ratePreparation by carbon monoxide or formate reactionNitrogen oxidesAlcohol
The invention relates to a method for producing oxalic ester by CO coupling, which mainly solves the problems of the prior art such as low selectivity and low CO single pass conversion in producing the oxalic ester by CO coupling. In the invention, firstly CO and nitrite ester are put into a coupling reactor and then contacted with a catalyst containing Pd; liquid phase reaction effluent II and gas phase reaction effluent III are obtained from effluent I through gas-liquid separation; the gas phase reaction effluent III, O2 and monohydric alcohol of C1-C4 are taken as raw material and put into a regeneration reactor for reaction to generate gas effluent IV containing nitrite ester; the gas effluent IV returns to the coupling reactor for continuous reaction and the mol ratio of nitrogen oxide:O2:monohydric alcohol of C1-C4 is 1:0.3-0.5:1-1.5. The technical proposal of obtaining the oxalic ester product by separating the liquid phase reaction effluent II better solves the problem and can be applicable to the industrial production of oxalic ester.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process of synthesis of compounds having nitrile functions from ethylenically unsaturated compounds
InactiveUS7084293B2High selectivityReduce disadvantagesOrganic compound preparationGroup 8/9/10/18 element organic compoundsButadiene DioxideAlkyl nitrites
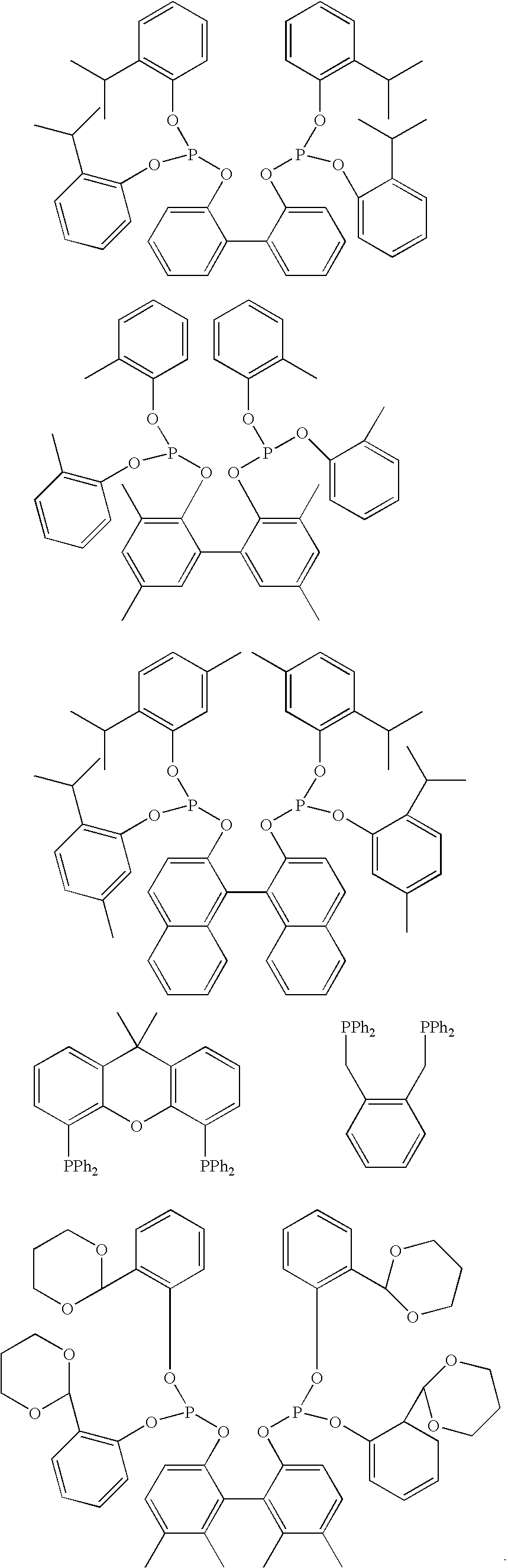
The present invention relates to a process of hydrocyanation of ethylenically unsaturated organic compounds to compounds having at least one nitrile function, particularly the hydrocyanation of diolefins such as butadiene, or of substituted olefins such as alkene nitrites such as pentenenitriles; the subject hydrocyanation is carried out in the presence of a catalytic system comprising a metallic element and mono- and pluri-dentate organophosphorus ligands.
Owner:INV NYLON CHEM AMERICAS LLC
Process for the preparation of C1-C4-alkyl nitrites
ActiveCN101096340AAvoid it happening againReasonable useNitrous acid preparation ester preparationNitrogen oxidesPhysical chemistry
The invention discloses a making method of C1-C4 alkyl ester of nitrous acid, which comprises the following steps: doing the oxidizing reaction and esterifying reaction in two reactors separately; doing the oxidizing reaction in the oxidizing reactor; doing the esterifying reaction in the esterifying tower reactor; inputting nitrogen oxide, oxygen and one or more of inert gas into oxidizing reactor; selecting the nitrogen oxide from one or more composite gas of NO, N2O3, NO2 and N2O4 with NO constantly; making the mole of NO more than the mole of NO2; using 0.15-0.3 m oxygen for each NO m; making the bulk of inert gas at 0-90% corresponding to total gas; inputting the oxidizing reacting product into the esterifying tower reactor to react with C1-C4 alkanol; using 0.8-3.0 m alkanol for each gram atom nitrogen. The invention can transfer heat effectively to reduce by-product, which is smaller than traditional equipment.
Owner:SHANGHAI HUAYI ENERGY CHEM
Catalyst for gas-phase synthesis of oxalate and its preparing process
InactiveCN1381310AHigh reactivityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsNitriteGas phase
A catalyst for gas-phase synthesis of dimethyl (or diethyl) oxalate from CO and nitrite is prepared from alpha-Al2O3 as carrier and Ce and Pd as active components through the dipping method. Its advantages are high reaction activity and selectivity, long service life and easy control of reaction.
Owner:EAST CHINA UNIV OF SCI & TECH
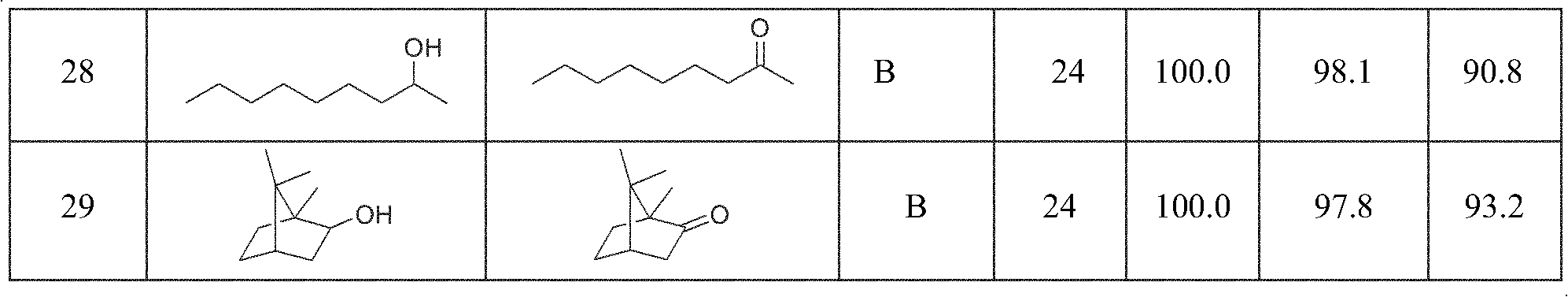
Method for preparing aldehyde or ketone by catalyzing air and oxidizing alcohol
InactiveCN101486621AEasy to separateWide substrate applicabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkyl nitritesKetone
The invention provides a method for preparing aldehyde or ketone through the catalysed air oxidation of alcohol. Calculated by 5mmol of a reaction substrate, 1 percent to 8 percent of 2, 2, 6, 6-tetramethylpiperidine-oxygen free radical (TEMPO) or derivatives thereof, 4 percent to 20 percent of halide-containing compounds, 4 percent to 10 percent of nitrite or nitrite esters are taken as catalysts, 0.1MPa to 0.8MPa of oxygen or air is taken as an oxidizer, the reaction lasts for 1 hour to 36 hours at temperature of 0 DEG C to 80 DEG C and a series of alcohols can be oxidized into aldehyde or ketone with high selectivity. The method has the advantages of fairly cheap and safe reagent adopted, wide applicability of the reaction substrate, mild reaction conditions, convenient separation of products, no pollution to the environment, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for producing oxalic ester by CO coupling
ActiveCN101993365APreparation by carbon monoxide or formate reactionMetal/metal-oxides/metal-hydroxide catalystsAlcoholCoupling
The invention relates to a method for producing oxalic ester by CO coupling, by which the main technical problems of low selectivity, low space-time yield and low oxalic ester utilization rate in producing the oxalic ester by CO coupling in the prior art cen be solved. The invention has the technical scheme comprising the following steps: (a) firstly, CO and nitrous acid ester enter a coupling reactor to contact with a palladium catalyst and react to generate a reaction effluent I containing nitrogen oxide and the oxalic ester; (b) after the reaction effluent I is separated, a reaction effluent II containing the oxalic ester and a gas phase reaction effluent III containing the nitrogen oxide are obtained; (c) the reaction effluent III and O2 and C1-C4 monohydric alcohols enter a regeneration reactor and react to generate an effluent IV; (d) the effluent IV enters a water scrubbing tower and contacts with water to obtain a gas phase effluent V containing the nitrous acid ester and a liquid phase effluent VI; and (e) the gas phase effluent V containing the nitrous acid ester from the step (d) is dried and then returned to the coupling reactor for cycle use. The technical scheme better solves the problems and can be used for industrial production of the oxalic ester.
Owner:CHINA PETROLEUM & CHEM CORP +1
Dehydration of alcohols on acidic catalysts
ActiveUS9249066B2High selectivityMolecular sieve catalystsMolecular sieve catalystKetoneAlkyl nitrites
A dehydration process may include introducing in a reactor an alcohol, and contacting the alcohol with an acidic catalyst to dehydrate the alcohol to make a corresponding olefin. The process may include recovering from the reactor the olefin and water. In the process, an effective amount of a component capable to neutralize a part of the catalyst active site may be introduced. The component may include ammonia, organic ammonium salts, hydrazine, nitriles, amines, amides, imines, di-imines, imides, cyanates, isocyanates, nitrites and nitroso compounds, aldehydes, ketones, carboxylic esters, and their corresponding thio-compounds.
Owner:TOTAL RES & TECH FELUY
Method for directly oxidizing benzyl-position C-H bond into ketone
ActiveCN107011133AEfficient synthesisAtom economy is highCarboxylic acid nitrile preparationOrganic compound preparationSide chainEthyl group
The invention discloses a method for directly oxidizing a benzyl-position C-H bond into ketone, wherein aryl ethyl compounds are catalyzed and oxidized by nitrite ester; a synergistic catalytic system of free radical initiator and nitrite ester is adopted, and a catalytic system of non-metallic catalyst and oxygen is adopted, the oxidization of the C-H bond of a free radical-activated aryl side chain is simple in operation; after completing the reaction, petroleum ether / ethyl acetate at a volume ratio of (50-1):1 is used as an eluent; column chromatography separation is performed to obtain a target product. The catalytic system in the invention uses oxygen as an oxygen source and has high atomic economy; the invention is a non-metallic catalytic system and provides a novel method for avoid metal residues in synthetic drugs; for diethyl aromatic hydrocarbon, the method provided by the invention can be adopted to selectively oxidize diethyl aromatic hydrocarbon into monoketone and diketone; the method of the invention can be adopted to efficiently synthesize tranquillizer lenperone, so that a novel method for synthesizing lenperone is provided.
Owner:UNIV OF SCI & TECH OF CHINA
Preparation method of catalyst used in dilute nitric acid treating and alkyl nitrite generating
ActiveCN104338550ASimple methodEasy to operateMolecular sieve catalystsNitrous acid preparation ester preparationForming gasReaction temperature
The invention relates to a preparation method of a catalyst used in dilute nitric acid treating and alkyl nitrite generating and mainly aims at solving the disadvantages of introducing O2 in the nitrate regeneration process and a series of problems of easy generation of nitric acid, failure in dilute nitric acid treatment, low reaction efficiency, and low nitrite selectivity, and the like in the regeneration process in the prior art. The following technical scheme is adopted: in the presence of an activated carbon coated molecular sieve membrane catalyst, NO reacts with an aqueous solution of alkyl alcohol and nitric acid in a manner of being contacted with the catalyst under the re-circulating conditions in which the reaction temperature is within the range of 60-100 DEG C, a reaction pressure is within the range of 0.28-0.35Mpa, the synthetic gas flow is within the range of 800-1000L / h, the mass fraction of the NO is 8-20%, the mass fraction of the alkyl alcohol is 30-60% in the liquid phase solution and the content of the dilute nitric acid is 1%-10%, due to the reaction, the dilute nitric acid can be completely decomposed into nitrous acid, and the nitrous acid is capable of further reacting with the alkyl alcohol to generate the alkyl nitrite. The catalyst is capable of well being used for catalyzing in the reaction of the dilute nitric acid decomposing and the alkyl nitrite generating with high efficiency, and therefore, the series of problems can be solved. The catalyst can be applied to the regeneration production of the alkyl nitrite in the industry of producing ethylene glycol from coal.
Owner:TONGLIAO GOLD COAL CHEM
Reverse Kleiner method for manufacturing nitrogen dioxide, nitric oxide, nitric acid, metallic ascorbates and alkyl ascorbates of vitamin C
In this invention new chemical reactions, new chemical processes are established, and these chemical reactions and chemical processes can be used with the system designed to produce nitrogen dioxide, nitric oxide, nitric acid as well as metallic ascorbates or alkyl ascorbates, either as main or as secondary products. Ascorbic acid solution is reacted at room temperature or at elevated temperature with either sodium nitrite or potassium nitrite or calcium nitrite or alkyl nitrite such as isobutyl nitrite or barium nitrite or silver nitrite solution. All the second reactants except alkyl nitrites such as isoamyl nitrite or isopropyl nitrite or isobutyl nitrite, as well as the first reactant, ascorbic acid, are in aqueous solutions. The reaction vessel contains the ascorbic acid solution; into this solution, under, certain pressure, is delivered the choosen aqueous nitrite solution. Gas mixture of nitrogen dioxide and nitric oxide is produced by addition of the choosen nitrite solution. The generated and collected gas mixture is then mixed with oxygen, thus the nitric oxide in the gas mixture converts—by reacting with oxygen—into nitrogen dioxide, then this homogeneous gas is dissolved in water, thus giving us nitric acid. In this chemical reaction system two sets of chemical reactions take place; one on the surface of the solution(s) that produces the main part of the gas mixture, and this is the major part of the chemical reaction system. In the liquid phase of the reaction processes form the metallic ascorbates as well as the alkyl ascorbates. All the same can be done with isoascorbic acid; the chemical reactions will go somewhat slower.
Owner:KLEINER BELA
Method for preparing oxalate by using CO gaseous phase process
ActiveCN101993366APreparation by carbon monoxide or formate reactionMetal/metal-oxides/metal-hydroxide catalystsAlkaneGas phase
The invention relates to a method for preparing oxalate by using a CO gaseous phase process, which mainly solves the technical problem of low utilization rate of nitric oxides or nitrite in the prior art. The method comprises the following steps of: a, enabling a nitric oxide mixture, C2-C4 alkane alcohol and air to enter a reactor 1 to generate an effluent I containing C2-C4 alkyl nitrite; separating the effluent to obtain a non-condensable gas effluent II and a non-condensable gas effluent III; b, enabling the effluent III and a first path of CO gas to enter a coupling reactor I to generate an oxalate liquid phase effluent IV and an NO-containing gas phase effluent V; c, enabling the effluent V and methane and oxygen to enter a reactor II to generate an effluent VI containing methyl nitrite, separating to obtain a methyl nitrite effluent VII and then enabling the methyl nitrite effluent VII and a second path of CO gas to enter a coupling reactor II to react to obtain a dimethyl oxalate effluent VIII and an NO-containing gas phase effluent IX; d, returning the effluent IX to be mixed with the effluent V for circular use; and e, separating the dimethyl oxalate effluent VIII to obtain a dimethyl oxalate product I. The technical scheme for obtaining the oxalate product II by separating the oxalate liquid phase effluent IV better solves the problem and can be used in the industrialized production for preparing the oxalate by using the CO gas phase process.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing C1-C4 alkyl nitrites
InactiveCN101993374AMolecular sieve catalystsNitrous acid preparation ester preparationNitric oxideAlkyl nitrites
The invention relates to a method for preparing C1-C4 alkyl nitrites, which mainly solves the technical problems of low target product selectivity and high content of nitric acid as side products in the prior art. The method comprises the following steps of: a, enabling nitric oxide and oxygen to firstly enter a reactor I, reacting to generate an effluent I containing NO2 and unreacted NO; b, enabling the effluent I and C1-C4 alkanols to enter a reactor II, reacting to generate an effluent II containing the nitrites; and c, obtaining the nitrites after separating the effluent II containing the nitrites, enabling the nitrites subjected to the dryness to enter a subsequent reaction unit for use, wherein the nitric oxide contains the NO, and the mol number of the NO is more than that of the NO2, the mol ratio of the NO to the oxygen in the nitric oxide is 4-25:1. The invention better solves the problems, and can be used in the industrialized production for increasing the yield of C1-C4 alkyl nitrites.
Owner:SHANGHAI RES INST OF PETROCHEMICAL TECH SINOPEC
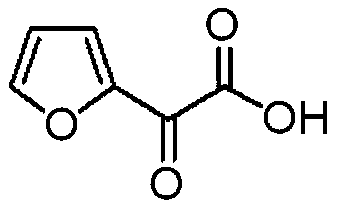
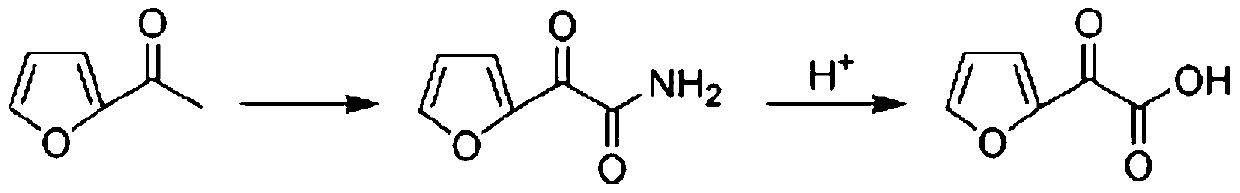
Preparation method of furanone acid
ActiveCN110003151APromote hydrolysis reactionEnhanced leaving activityOrganic chemistryChemical industryFuran
The invention belongs to the technical field of medical chemical industry, and particularly relates to a preparation method of furanone acid. The method comprises the following steps of: adding dropwisely a sodium nitrite aqueous solution into a concentrated sulfuric acid and long-chain alcohol aqueous solution, separating an ester layer to obtain nitrite after the dropwise addition is finished; dissolving 2-acetyl furan in a dilute hydrochloric acid solution, adding concentrated sulfuric acid and a catalyst, controlling temperature, adding dropwisely the nitrite, continuously stirring to react after the dropwise addition is finished, adjusting pH after the reaction is finished, filtering to recover the catalyst, and adding an extracting agent into filtrate to extract long-chain alcohol generated by the reaction and unreacted acetyl furan, wherein a water phase is a furanone acid aqueous solution. By the adoption of the preparation method of the furanone acid, an adopted oxidant only needs to slightly exceed the amount of a substrate, so that generation of toxic gas nitrogen oxide is reduced compared with oxidation of sodium nitrite; and the reaction condition is mild, the use of the catalyst accelerates a hydrolysis process, the side reaction is reduced, the yield is improved and more than 82.5%, and a conversion rate of the 2-acetyl furan reaches more than 95%.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Method for directly converting aldehyde or alcohol into carboxylic acid
InactiveCN108314599ASolve residual problemsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationAlcoholDrugs synthesis
The invention discloses a method for directly converting aldehyde or alcohol into carboxylic acid. The method is characterized by oxidizing CH2OH and CHO under catalysis of N-hydroxyimide compounds orunder combined catalysis of N-hydroxyimide compounds and nitrite compounds in a pure oxygen environment, and directly converting CH2OH and CHO into a carboxylic acid compound. According to the method, the oxygen is used as an oxidant; no metal catalyst is added; the method is environmentally friendly and is high in catalysis efficiency and convenient and simple to operate; the method is differentfrom the previous metal catalysis system and compound catalysis system; the residues of transition metals are caused when the transition metals are used in the existing metal catalysis system process; the method adopts a non-metal catalysis system which is green and environmentally friendly, so that the problem of the residues of the metals is avoided; a novel method is provided for solving the problem of the residues of the transition metals during drug synthesis.
Owner:UNIV OF SCI & TECH OF CHINA
Synthetic method of aminothiazoly loximate
The invention relates to a synthetic method of aminothiazoly loximate, which belongs to the synthetic method of a heterocyclic compound containing 1,3-thiazole ring, and in particular relates to a preparation method of the heterocyclic compound with two double bonds between the annular atoms and without other condensed rings. The method comprises the steps of: (1) using ethyl acetoacetate as a raw material, oximating, alkylating and cyclizing in a same reaction container to obtain ethyl methoxyiminoacetate; (2) modifying a nitrosation reagent in an oximation agent into organic nitrous acid ester by inorganic salt sodium nitrite, wherein organic nitrous acid ester is more suitable for the solvent system and the nitrosation effect is better; (3) hydrolysis: preparing an aminothiazoly loximate coarse product; and (4) refining: preparing an aminothiazoly loximate product. According to the process, the operating process of the reaction is simplified, the production cost is lowered, and the emission of three wastes is reduced. A triphosgene chlorinating agent which is smaller in toxicity, safe and convenient to use, easy to control and operate and high in yield is provided. Aminothiazoly loximate is a raw material for synthesizing third generation cephalosporin, and is an important medical intermediate.
Owner:YIYUAN XINQUAN CHEM
Method for producing C1-C4 alkyl nitrites
ActiveCN101993375AMolecular sieve catalystsNitrous acid preparation ester preparationAlkyl nitritesNitrogen oxide
The invention relates to a method for producing C1-C4 alkyl nitrites, which mainly solves the technical problems of low objective product selectivity and high side reaction product nitric acid content in the prior art. The method comprises the following steps of: a) delivering a nitrogen oxide raw material generated by oxidation of ammonia and air and a first stream of C1-C4 alkanol into a reactor I to generate an effluent I containing C1-C4 alkyl nitrites, and separating the effluent to obtain a gas effluent II and a liquid effluent III; b) delivering the gas effluent II and a first stream of oxygen into a reactor II to contact with an aluminosilicate catalyst to generate an effluent IV; c) delivering a second stream of C1-C4 alkanol and the effluent IV into a reactor III to generate an effluent V by reaction, and separating the effluent to obtain a non-condensed gas effluent VI and an effluent VII containing C1-C4 alkyl nitrites; and d) drying the effluent VII, and then delivering the dried effluent VII to a subsequent reaction unit for use. According to the technical scheme, the method well solves the problems, and can be used for industrial production of the increased C1-C4 alkyl nitrites.
Owner:CHINA PETROLEUM & CHEM CORP +1
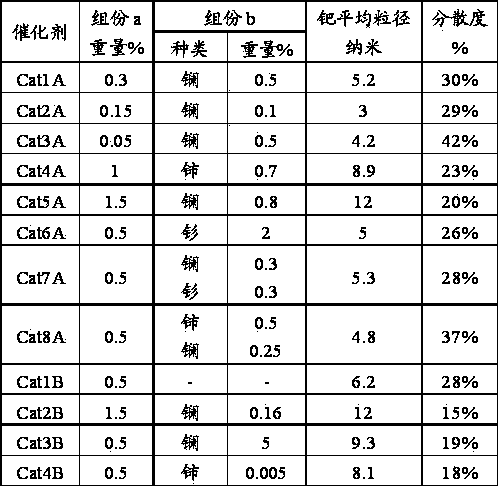
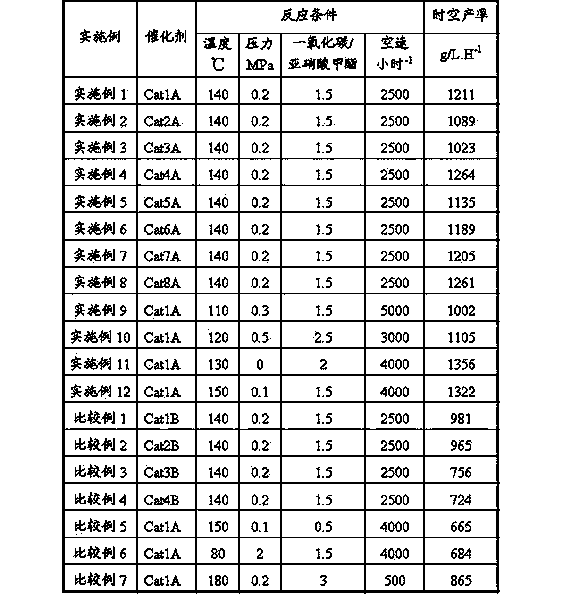
Method for carbon monoxide gas phase synthesis of oxalate
ActiveCN104109092AIncrease profitHigh catalytic activityHeterogenous catalyst chemical elementsPreparation by carbon monoxide or formate reactionRare-earth elementDispersity
The invention relates to a method for carbon monoxide gas phase synthesis of oxalate, and mainly solves the problem of low space time yield of oxalate due to the low dispersion of an active component Pd crystal grain and low microcrystal content in catalysts in the prior art. The method is characterized in that carbon monoxide and a nitrite ester raw material contact with a catalyst under coupling reaction conditions; the catalyst comprises 0.03-3wt% of at least one selected from metallic palladium or palladium oxides, 0.01-3wt% of at least one selected from rare earth elements or rare earth element oxides and 94-99.96wt% of an alumina carrier; the average particle size of metal palladium or palladium oxide crystal grains in the catalyst is 3-12nm; and the dispersity of palladium is greater than 20%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process and production system for synthesizing dimethyl oxalate or diethyl oxalate and coproducing oxalic acid
ActiveCN102001938ASmall scaleHigh speedPreparation from carboxylic acid esters/lactonesPreparation by carbon monoxide or formate reactionGas phaseDiethyl oxalate
The invention discloses a process and a production system for synthesizing dimethyl oxalate or diethyl oxalate and coproducing oxalic acid. The method comprises the following steps of: reacting nitrogen oxide with alcohol in an esterification reactor to form nitrite, adding the nitrite into a gas phase coupling carbonylation reactor, and connecting the gas phase coupling carbonylation reactor with a condenser and an oxalate hydrolysis reactor. After being condensed by the condenser, outlet gas of the gas phase coupling carbonylation reactor is condensed into liquid-phase or solid-phase oxalate; the oxalate in the gas phase enters the oxalate hydrolysis reactor to generate the oxalic acid and the alcohol of corresponding ester; the ester solution of the corresponding ester can enter the subsequent esterification reactor for reaction; and the amount of the oxalate which enters the gas phase can be adjusted by adjusting the temperature of the condenser so as to adjust the distribution of the synthetic amount of the oxalate and the oxalic acid. Nitrogen oxide-containing noncondensable gas which passes through the oxalate hydrolysis reactor is recycled to enter the esterification reactor so as to realize the recycling of the nitrogen oxide. The process and the production system for synthesizing the dimethyl oxalate or the diethyl oxalate and coproducing the oxalic acid have low energy consumption and high productivity and meet the industrial requirement.
Owner:SHANGHAI HUAYI ENERGY CHEM
Method for preparing carbonyl compounds by alcohol catalytic oxidation through oxygen without transition metal
InactiveCN102653504AApplicable productionLow costOrganic compound preparationCarboxylic acid esters preparationCatalytic oxidationAlkyl nitrites
The invention relates to a method for preparing carbonyl compounds by alcohol catalytic oxidation through oxygen without transition metal, wherein N-bromosuccinimide (NBS) or other halogen compounds, and nitrite esters are used as catalysts, oxygen at a pressure of 0.1-0.8 MPa is used as an oxidant, the reaction is carried out at 0-100 DEG C for 1-72 hours, and a series of alcohol can be oxidized into carbonyl compounds with high selectivity. The invention has the characteristics of high yield, relatively mild reaction conditions, easy operation control, low cost, safety, environment-friendly process, no pollution, and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
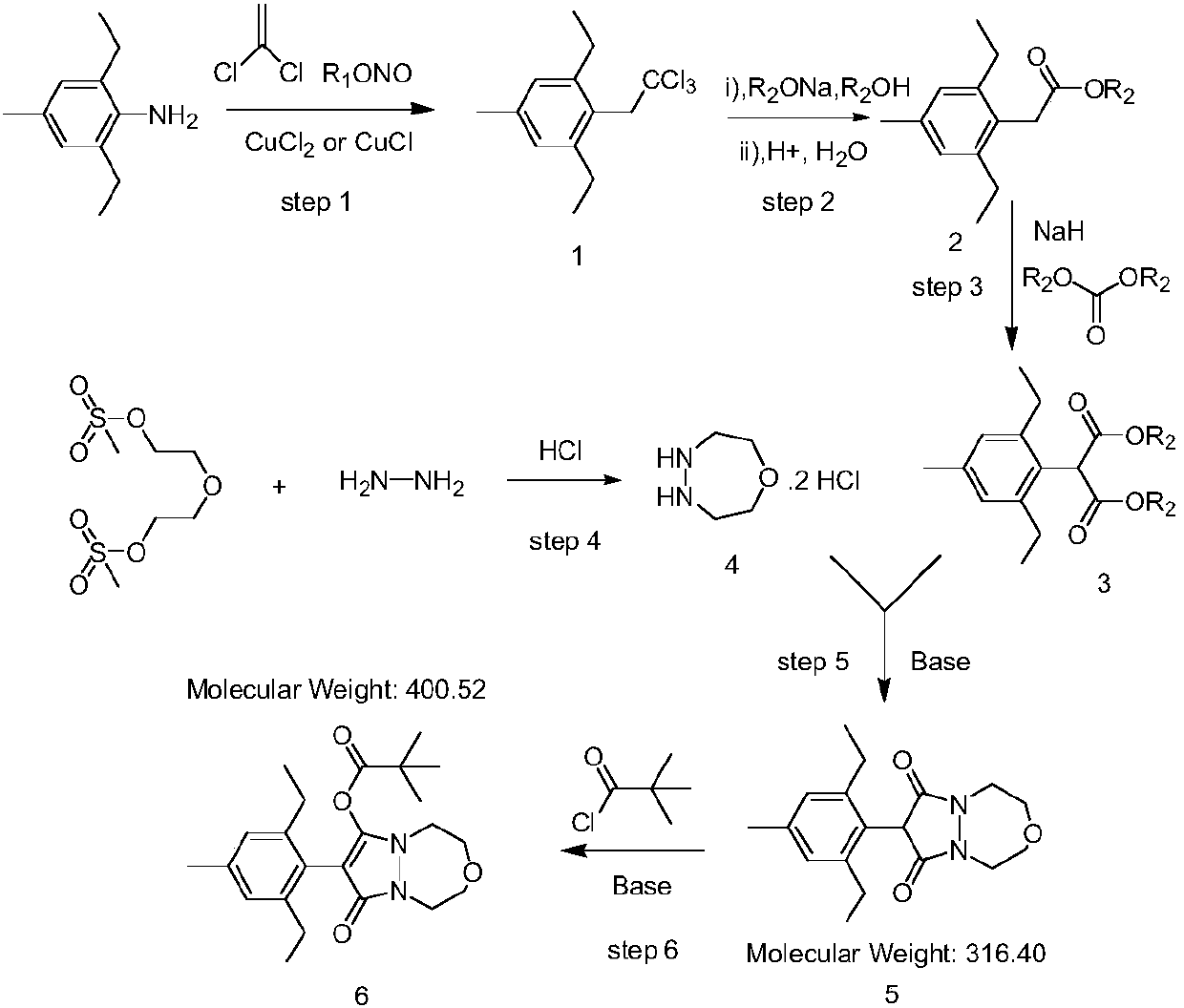
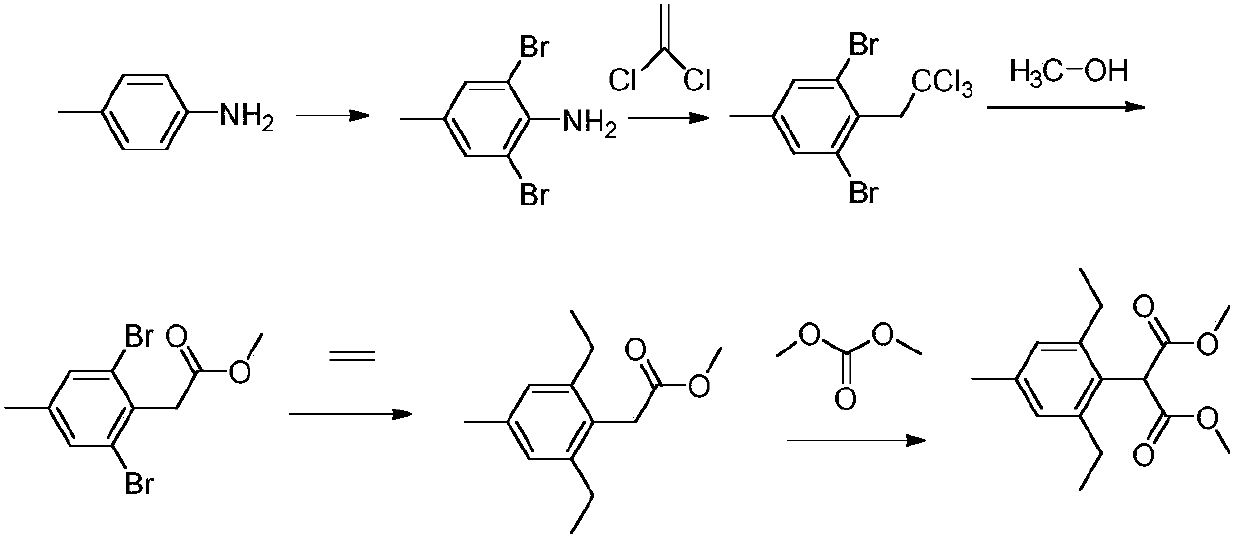
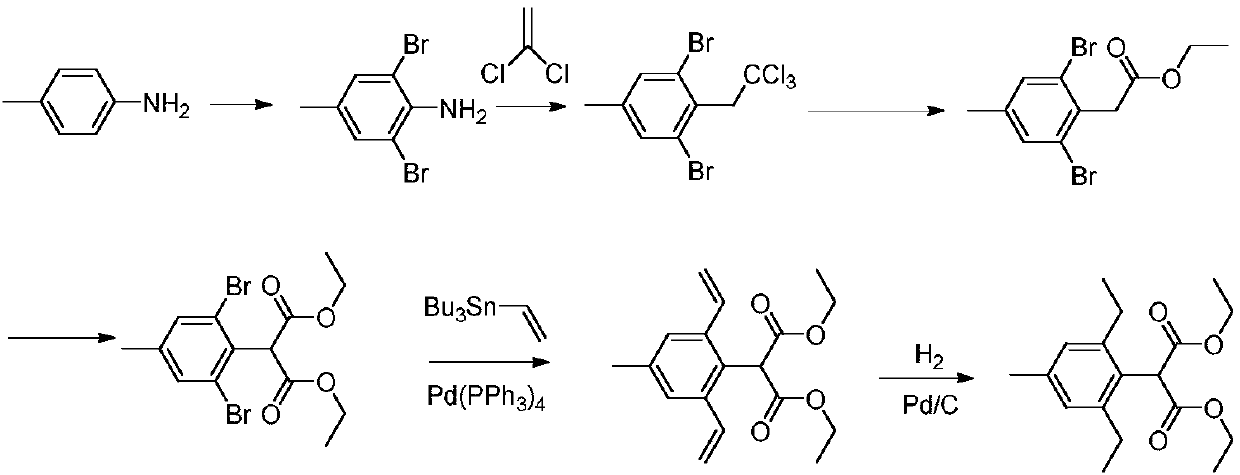
Preparation method of pinoxaden
The invention relates to the field of organic synthesis, in particular to a synthesis method of pinoxaden. The synthesis method comprises the following steps that under catalysis of copper chloride orcuprous chloride, 4-methyl-2,6-diethyl aniline reacts with 1,1-dichloroethylene and nitrous acid ester to generate a compound 1; the compound 1 reacts with sodium alcoholate, and a reaction product is subjected to acidic hydrolysis to obtain a compound 2; the compound 2 reacts with diester carbonate and strong base to obtain an intermediate 3; after diethylene glycol dimethanesulfonate reacts with hydrazine hydrate, salifying with hydrochloric acid is conducted to obtain an intermediate 4; the intermediate 3 and the intermediate 4 are subjected to ring forming under the effect of base to obtain a compound 5; and the compound 5 and pivaloyl chloride react under the effect of the base or base / DMAP to obtain the pinoxaden. The synthesis method of the pinoxaden does not use expensive or toxiccatalysts, does not need to adopt a protection / deprotection strategy, and has the characteristics of low cost and high atomic economy.
Owner:JIANGSU FLAG CHEM IND
Method for preparing ceftaroline side-chain acid
The invention discloses a method for preparing ceftaroline side-chain acid shown as a formula (I). The method comprises the following steps of: performing oximation reaction on 5-amino-1,2,4-thiadiazole-3-acetamide derivative and nitrite under catalysis of concentrated hydrochloric acid, performing esterification reaction with an ethylation reagent in the presence of organic alkali, dissolving in an aqueous solution of inorganic alkali, and thus obtaining the ceftaroline side-chain acid. The method has the advantages of mild reaction conditions, simplicity and convenience in operation, good reaction selectivity, high yield, short production period, low 'three-waste' quantity, easiness in industrialization and high implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +1
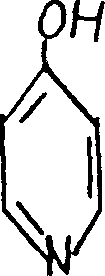
4-hydroxylic pyridine and production process thereof
ActiveCN1560036ASimple production processEasy to operateOrganic chemistryOrganic acidOrganic synthesis
The invention discloses a 4-hydroxy pyridine and its producing method, including the steps: making tetra amido pyridine with nitrite on the condition that inorganic and organic acids exist, so as to obtain 4-hydroxy pyridine. It can act as an organic synthesis intermediate for medicines, pesticides, dyes, etc. The producing process is simple and convenient to operate, the reacting conditions are moderate, the three wastes are a few, the product quality is high and the yield is up to above 90%.
Owner:江苏优普生物化学科技股份有限公司
Method for CO gas phase coupling production of oxalate
ActiveCN104109091AEvenly distributedIncreased space-time yieldHeterogenous catalyst chemical elementsPreparation by carbon monoxide or formate reactionOxalatePtru catalyst
The invention relates to a method for CO gas phase coupling production of oxalate, and mainly solves the problem of low space time yield of oxalate due to the large particle size and low dispersion of an active component Pd crystal grain in catalysts in the prior art. A technical scheme adopted in the invention is characterized in that carbon monoxide and organic nitrite raw materials contact with a palladium-containing catalsty under coupling reaction conditions, and a complexation additive is added into a dipping during the preparation of the catalyst. The technical scheme well solves the problem, and the method can be used in the industrial for the carbon monoxide gas phase coupling production of oxalate.
Owner:CHINA PETROLEUM & CHEM CORP +1
Hydrogenated nitrile rubber composition
InactiveUS7173087B2Improve heat resistanceIncrease resistanceGroup 4/14 element organic compoundsEngine sealsCross-linkPolymer science
A hydrogenated nitrite rubber composition for providing a cross-linked product having a 20% modulus of 10 MPa or more and a thermal conductivity of 0.4 W / m·K or more at 25° C. The hydrogenated nitrile rubber composition includes 100 parts by weight of hydrogenated nitrile rubber having a bound acrylonitrile content of 40% or more, a polymer Mooney viscosity ML1+4 (100° C.) of 75 or less (median) as determined according to JIS K-6395, an iodine value of 23 or less (median), and at least 110 parts by weight in sum total of carbon black as a filler and other gas shielding filler. The hydrogenated nitrile rubber composition can effectively be used as a molding material for sliding applications or for a static sealing members for highly permeable gases. The hydrogenated nitrile rubber composition has a distinguishing carbon dioxide gas shieldability, heat resistance, and wear characteristics.
Owner:EAGLE INDS
Method for removing nitrite ester in tail gas of production of oxalate through CO coupling
ActiveCN103894062AImprove conversion rateImprove technical effectDispersed particle separationHigh concentrationOxalate
The invention relates to a method for removing nitrite ester in tail gas of production of oxalate through CO coupling. The problems of low nitrite ester removal rate and high concentration of nitrogen oxides in the tail gas existing in the prior art are solved in the invention. The nitrite ester-containing tail gas generated in the production of oxalate through CO coupling contacts ammonia under the action of a copper-containing catalyst in a fixed bed reactor under a tail gas volume air speed of 180-10000h<-1> at a reaction temperature of 150-350DEG C under a reaction pressure of 0.1-0.8MPa, and a molar ratio of ammonia to nitrite ester is 0.6-1.2. The above technical scheme well solves the problems, and the method can be used in the industrial production for removing nitrite ester in tail gas of production of oxalate through CO coupling.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing oxalic acid ester through CO gas phase coupling
ActiveCN102219679AHigh selectivityLow in palladiumPreparation by carbon monoxide or formate reactionGas phaseCoupling
The invention relates to a method for producing oxalic acid ester through CO gas phase coupling. The use of the method is aimed to mainly solving the technical problem of low selectivity of the target product in the prior art. A technical scheme is adopted as follows: the method comprises the steps of: taking mixture gas containing nitrite ester and CO as raw materials, and sequentially contacting the raw materials with catalysts A and B in a composite bed reactor under the conditions that the reaction temperature is 100-180 DEG C, the volumetric idle speed is 500-10,000 hour <1>, the reaction pressure is minus 0.08-1.5MPa, and reacting the nitrate ester with the CO in the raw materials to product the oxalic acid ester, wherein the catalysts A and B are both selected from palladium-containing catalysts, the palladium content in the catalyst A is lower than that in the catalyst B, and the weight filling proportion of the catalysts A and B is 0.1-5:1, thus better solving the problem of low selectivity of the target product in the prior art and being used for industrial production of increasing the oxalic acid ester.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for enhancing activity of catalyst for preparing oxalic ester from CO
ActiveCN102649082ARaise the reaction temperatureImprove technical effectMolecular sieve catalystsCatalyst regeneration/reactivationPalladium catalystReaction temperature
The invention relates to a method for enhancing activity of catalyst for preparing oxalic ester from CO, which mainly solves the problem that the space time yield of aiming product oxalic ester is low during the process of preparing oxalic ester by CO in the traditional technology. The invention adopts the technical scheme that mixed gases containing nitrite ester and CO are used as raw materials, and a mole ratio of nitrite ester to CO is larger than (0-1.5):1, under the conditions that the volume space velocity is 500-10,000h -1, the pressure is -0.05-1.5MPa, and the temperature is 60-180 DEG C, the reaction temperature is higher than normal reaction temperature by 3-30 DEG C, and is maintained for 0.1-100 hours, then the temperature is lowered to normal reaction temperature, and raw materials are contacted with the catalyst containing palladium to generate effluent containing oxalic ester; and carrier of the catalyst containing palladium is at least one selected from aluminium oxide, silicon oxide, molecular sieve, magnesium oxide and calcium oxide, the content of palladium is 0.02-0.8 percent in percentage by weight of the catalyst. According to the technical scheme, the problem is better solved, and the method can be applied in the industrial production of oxalic ester prepared by CO.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthetic method for o-cyclodione
InactiveCN103965030AHigh reaction yieldReduce pollutionOximes preparationCarbonyl compound preparation by hydrolysisAlkyl nitritesMethyl group
The invention discloses a synthetic method for o-cyclodione. O-cyclodione normally comprises 1,2-cyclopentanedione, 3-methyl-1,2-cyclopentanedione (methyl cyclopentenolone), 1,2-cyclohexanedione, 1,2-cyclononanedione, etc. 3-methyl-1,2-cyclopentanedione is an important perfume, and 1,2-cyclohexanedione is an important raw material for production of the perfume 5,6,7,8-tetrahydroquinoxaline. According to the invention, cyclic ketone is used as a starting raw material and subjected to nitrosation with nitrite, and an obtained intermediate cyclic ketoxime is subjected to hydrolysis so as to obtain o-cyclodione. The synthetic method provided by the invention has the advantages of simple technological operation, little pollution to the environment, high reaction yield and low production cost and is a promising production process.
Owner:APPLE FLAVOR & FRAGRANCE GRP
Method for producing alkyl nitrite ester
ActiveCN102219697AHigh selectivityAvoid generatingNitrous acid preparation ester preparationReaction temperatureAlkyl nitrites
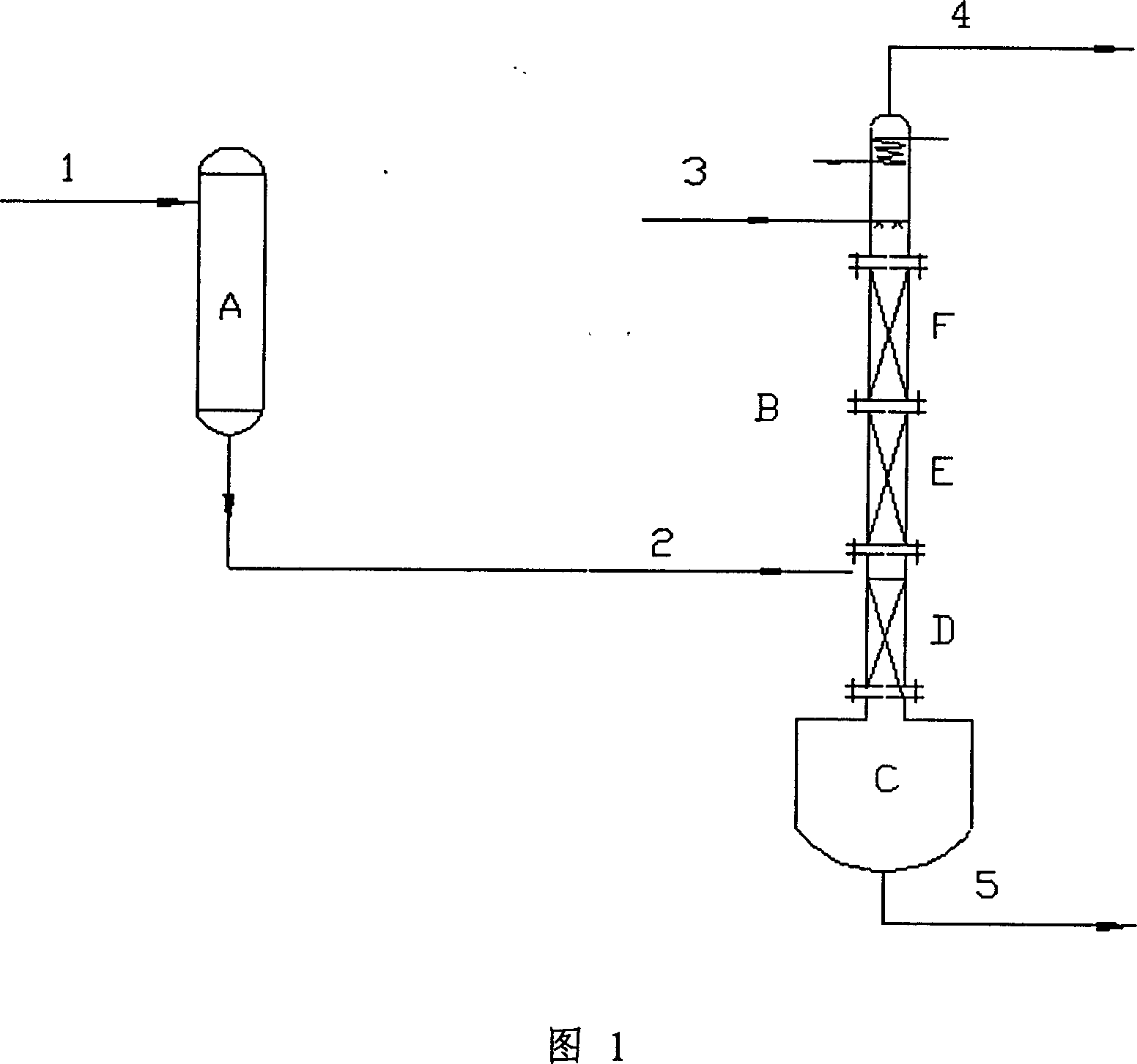
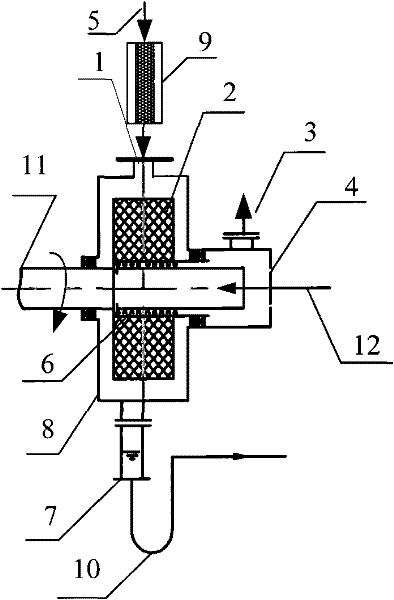
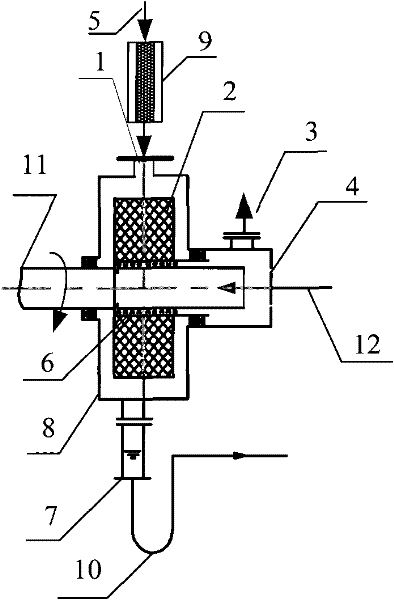
The invention relates to a method for producing alkyl nitrite ester. The use of the method is aimed to mainly solving the technical problem of low selectivity of the target product-the alkyl nitrite ester in the prior art. In the method, a technical scheme is adopted as follows: the method comprises the following steps: (a) firstly, leading nitric oxide and oxygen into a prereactor, contacting with an aluminosilicate catalyst, and reacting to produce effluent I containing NO2 and unreacted NO; and (b) leading the effluent I and raw materials C1-C4 alkanol into a revolving filler bed reactor respectively, and carrying out contact reaction on revolving filler of the revolving filler bed reactor under the conditions that the reaction temperature is at 0-150 DEG C, the mole ratio of the C1-C4 alkanol and the nitric oxide is (1-100):1 and the mol ratio of the nitric oxide and the oxygen is (4-50):1, wherein the revolving filler bed reactor is directly connected with the prereactor (9), a liquid outlet (7) is arranged at the bottom of the revolving filler bed reactor and is communicated by a liquid outlet pipeline (10), a liquid distributor (6) is arranged in a pipeline of a liquid inlet (4), the liquid inlet (4) is communicated by a liquid inlet pipeline (12), and revolving filler (2) is fixed on a motor shaft (11), thus better solving the problem of low selectivity and being used for industrial production of increasing the C1-C4 alkyl nitrite ester.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for catalyzing reduction of dilute nitric acid to prepare nitrites with alcohol, preparation method and application thereof
ActiveCN109529819ALow costReduce contentMetal/metal-oxides/metal-hydroxide catalystsNitrous acid preparation ester preparationChemical industryActivated carbon
The invention discloses a catalyst for catalyzing reduction of dilute nitric acid to prepare nitrites with alcohol, a preparation method and an application thereof, belonging to the technical field ofcoal chemical industry. The catalyst of the invention adopts activated carbon as a carrier, and is composed of a loaded main catalyst and a cocatalyst. The main catalyst is one or two or more components selected from Pt, Pd and Rh, and the cocatalyst is two or more components selected from La2O3, ZrO2, CeO2, CuO and MnO2. The invention also provides the preparation method and the application of the catalyst. The catalyst of the invention can catalyze and reduce the dilute nitric acid waste liquid generated in the process of coal-to-ethylene glycol to be nitrous acid, and then the nitrous acidreacts with alcohol to generate nitrite. Nitrite is an important intermediate in the production of ethylene glycol, so the utilization ratio of a nitrogen source can be improved, the discharge of waste acid liquid is reduced, the NaOH consumption of neutralizing waste liquid is reduced, further the production cost of ethylene glycol is reduced, the economic benefits of enterprises is improved, and a good prospect for industrial application is achieved.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com