Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Isoamyl nitrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for continuously synthesizing arylboronic acid ester by utilizing microreactor
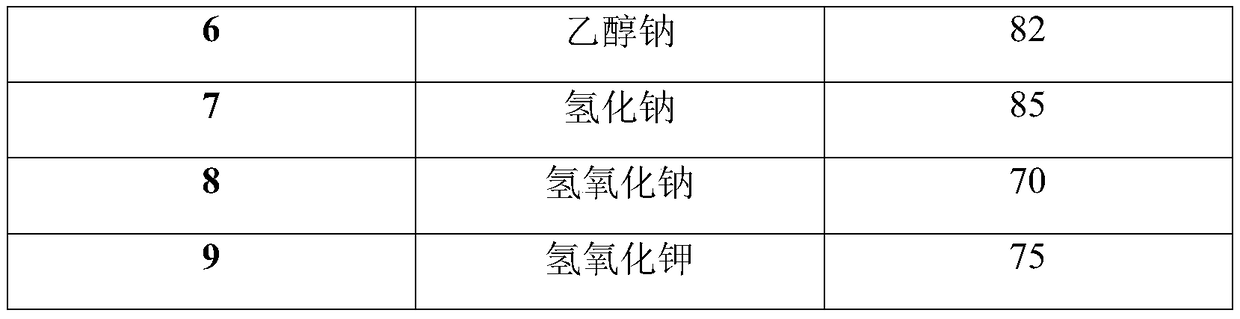
InactiveCN103275112ADegree of reductionShort reaction timeGroup 3/13 element organic compoundsOrganic synthesisN-butyl nitrite
The invention relates to a method for continuously synthesizing arylboronic acid ester by utilizing a microreactor, which belongs to the technical field of green organic synthesis application. The method comprises the following steps of: preheating substituted arylamine, tert-butyl nitrite, bisdiborane in a continuous-flow micro-channel reactor system by using substituted arylamine, acetonitrile, tert-butyl nitrite and bisdiborane as starting materials; and mixing the substituted arylamine with the bisdiborane and then reacting the obtained mixture with the tert-butyl nitrite, wherein in the reaction, the molar ratio of the substituted arylamine to the bisdiborane is (1:0.5)-(1:1.25), the molar ratio of the substituted arylamine to isoamyl nitrite is (1:1.1)-(1:1.5), the reaction temperature is 60 DEG C-120 DEG C, the reaction time is 50 seconds-3600 seconds, and the effective conversion ratio of the substituted arylamine is 50%-90%. The continuous-flow microreactor, which is capable of strengthening the mixing effect, the mass transfer effect and the heat transfer effect, is especially suitable for carrying out homogeneous reaction of the method. Moreover, the method has the characteristics of stable temperature control, safe process and less waste material.
Owner:JINAN SHAOYUAN MEDICAL TECH
Preparation method for modified carbon-fiber-reinforced polyether ether ketone composite material
InactiveCN105219018ADoes not significantly affect strengthIncrease surface active pointsFiberCarbon fibers
The invention provides a preparation method for a modified carbon-fiber-reinforced polyether ether ketone composite material. The method comprises the steps that 1, carbon fiber tows are infiltrated in an extracting solution of organic solvent to obtain degummed carbon fibers, the degummed carbon fibers are infiltrated in a mixed solution of water and p-phenylenediamine, isoamyl nitrite is added, and then stirring, filtering and washing are performed; 2, carbon nanoparticles are dispersed in water through an ultrasonic method, the carbon fibers are infiltrated in a dispersion solution of the carbon nanoparticles, isoamyl nitrite is added, and then stirring, filtering and washing are performed; 3, mixed raw materials of polyether ether ketone and processing aids and the modified carbon fibers are placed in a two-screw extruder, melting extruding and pelletizing are performed, and then the composite material is obtained. According to the preparation method for the modified carbon-fiber-reinforced polyether ether ketone composite material, the carbon fibers are activated through a diazonium salt reaction, the carbon nanoparticles are loaded on the surfaces of the carbon fibers, therefore, the carbon fiber surface activity is improved, and the infiltration performance of the fibers and resin is improved; the modified carbon fibers and the PEEK resin have the better mechanical bonding force, and the mechanical properties such as the stretching property of the composite material can be effectively improved.
Owner:SHANGHAI LEVSON ENTERPRISE GRP
Method for preparing functionalized graphene through ball milling
The invention discloses a method for preparing functionalized graphene through ball milling. According to the method, graphite, organic molecules containing anilino groups and isoamyl nitrite are used as raw materials and subjected to ball milling so as to prepare graphene covalently modified by organic molecules. The method for preparing the functionalized graphene in the invention has the advantages that the functionalized graphene is prepared from graphite through one-step reaction, so preparation process is simple; different organic molecules containing anilino groups can be used so as to realize surface functionalization of graphene; different organic molecules and graphene are connected via covalent bonds, so graphene has good dispersibility in different systems (water, organic solvents and matrix materials); and the method is simple to operate, low in cost, high in efficiency, free of pollution and capable of realizing large-scale preparation. The functionalized graphene prepared by using the one-step method has good dispersibility, which is beneficial for processing of the functionalized graphene in a solvent, and has wide application prospects in the fields of optoelectronic devices, new energy sources and new materials.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Reverse Kleiner method for manufacturing nitrogen dioxide, nitric oxide, nitric acid, metallic ascorbates and alkyl ascorbates of vitamin C
In this invention new chemical reactions, new chemical processes are established, and these chemical reactions and chemical processes can be used with the system designed to produce nitrogen dioxide, nitric oxide, nitric acid as well as metallic ascorbates or alkyl ascorbates, either as main or as secondary products. Ascorbic acid solution is reacted at room temperature or at elevated temperature with either sodium nitrite or potassium nitrite or calcium nitrite or alkyl nitrite such as isobutyl nitrite or barium nitrite or silver nitrite solution. All the second reactants except alkyl nitrites such as isoamyl nitrite or isopropyl nitrite or isobutyl nitrite, as well as the first reactant, ascorbic acid, are in aqueous solutions. The reaction vessel contains the ascorbic acid solution; into this solution, under, certain pressure, is delivered the choosen aqueous nitrite solution. Gas mixture of nitrogen dioxide and nitric oxide is produced by addition of the choosen nitrite solution. The generated and collected gas mixture is then mixed with oxygen, thus the nitric oxide in the gas mixture converts—by reacting with oxygen—into nitrogen dioxide, then this homogeneous gas is dissolved in water, thus giving us nitric acid. In this chemical reaction system two sets of chemical reactions take place; one on the surface of the solution(s) that produces the main part of the gas mixture, and this is the major part of the chemical reaction system. In the liquid phase of the reaction processes form the metallic ascorbates as well as the alkyl ascorbates. All the same can be done with isoascorbic acid; the chemical reactions will go somewhat slower.
Owner:KLEINER BELA
Preparation method of polyaniline-graphene hollow microspheres
The invention discloses a preparation method of polyaniline-graphene hollow microspheres. Firstly, isoamyl nitrite and sulfanilic acid are utilized to sulfonate graphene, the obtained sulfonated graphene is used as a Pickering emulsifier, water dispersion liquid of the obtained sulfonated graphene serves as a water phase, and toluene or an aniline monomer serves as an oil phase to perform emulsification, an oil-in-water (O / W) emulsion is obtained, then an oxidizing agent ammonium persulfate is added to trigger aniline polymerization, and the polyaniline-graphene hollow microspheres can be obtained after the oil phase toluene is removed. The polyaniline-graphene hollow microspheres are prepared by adopting a Pickering emulsion method. The preparation method is simple and efficient, and the prepared polyaniline-graphene hollow microsphere material with a three-dimensional hollow structure has larger specific surface area and a shorter ion and electron transmission path compared with a polyaniline-graphene composite material with a two-dimensional structure and has a greater potential application value on the aspects of catalysis, micro-reactors, sensing, energy storage materials and the like.
Owner:JIANGNAN UNIV
Biomass carbon-based solid acid catalyst and preparation method and application thereof
ActiveCN108940313AHigh catalytic activityImprove stabilityPhysical/chemical process catalystsOrganic chemistryPtru catalystPorous carbon
The invention provides a preparation method and an application of a biomass carbon-based solid acid catalyst. The preparation method comprises the following steps: A) impregnating biomass of a nitrogen-containing compound in an acid solution, and filtering to obtain a solid product; B) carbonizing the solid product to obtain a porous carbon carrier; and C) reacting the porous carbon carrier with p-aminobenzenesulfonic acid and isoamyl nitrite, and sulfonating to obtain a catalyst. In the preparation method provided by the invention, the biomass of the nitrogen-containing compound is impregnated and carbonized to obtain the porous carbon carrier; and after carbonization, diversified surface chemical compositions have better sulfonic acid group loading property compared with a highly carbonized surface, so that the Bronsted acid loading amount is increased, and the conversion efficiency of various types of sugar is improved. The adjustable and controllable carbon-based carrier porous structure formed in the preparation method provided by the invention enables sulfonic acid groups to be firmly loaded inside pore passages, and the pore passages are mesopores and macropores after loading, thereby being beneficial to contact of a substrate with acid sites, and maintaining better recycling property. The biomass carbon-based solid acid catalyst prepared by the invention has high catalytic activity, good stability and high catalytic conversion efficiency.
Owner:UNIV OF SCI & TECH OF CHINA
Universal methyl alcohol diesel
InactiveCN101376848AEasy to stratifySolve technical problems such as poor cold start at low temperatureLiquid carbonaceous fuelsEthyl acetateOleic Acid Triglyceride
The invention discloses universal carbinol diesel oil the composition prescription of which is as follows: 60 to 85 portions of diesel oil, 10 to 30 portions of carbinol, 0.5 to 3.0 portions of oleic acid, 1.0 to 2.0 portions of ethyl acetate, 0.5 to 1.5 portions of sherwood oil, 0.5 to 1.2 portions of cyclopentyl-dicyclopentadienyl iron, 0.3 to 1.5 portions of isoamyl nitrite, 0.5 to 1.5 portions of normal butanol as well as 0.5 to 1.2 portions of isoamylol; the mixture ratio is volume portion. The cetane value of the product achieves 53 and the flash point thereof achieves 80 DEG C; the tail gas exhaustion can meet the requirements of Euro III Standard, thus completely solving the technical difficulties like easy carbinol-oil separation, ill low temperature cool starting and needing not to change any device to locomotives, ships and commercial diesel engine; the universal carbinol diesel oil can be directly used or mixed, is not restrained by seasons and regions; the oil saving rate can achieve 10 to 15 percent; besides, the needed materials and auxiliaries are broad in sources, is low in price and is remarkable in economic benefits. The universal carbinol diesel oil can be popularized and applied.
Owner:上海龙津石油有限公司
Functional diaphragm of lithium-sulfur battery and preparation method thereof
InactiveCN109904372AImprove electrochemical performanceReduce Shuttle EffectCell component detailsMass ratioMetal-organic framework
The invention relates to a functional diaphragm of a lithium-sulfur battery, in particular to a functional diaphragm which is modified by amino functionalization by adopting a metal organic frameworkmaterial MIL-125. The preparation method of the diaphragm comprises the following steps: dissolving a proper amount of metal organic framework material MIL-125 and p-phenylenediamine in the deionizedwater, adding isoamyl nitrite, stirring for 12-24 h at the temperature of 50-80 DEG C, and obtaining an amino functional metal organic framework material MIL-125 after the washing and drying; the amino functional metal organic framework material MIL-125, a conductive agent super p and binder polyvinylidene fluoride being in a mass ratio of 8:1:1, grinding and dispersing, adding 1-methyl-2-pyrrolidone (NMP) solution until the powder becomes black and viscous, and coating the black and viscous powder on the commonly used diaphragm 2400 to obtain the functional diaphragm. The prepared functionaldiaphragm has a porous structure, and can inhibit the diffusion of polysulfide through physical adsorption, reduce the shuttle effect, and improve the dispersion transfer efficiency, thereby improvingthe conductivity.
Owner:INT ACAD OF OPTOELECTRONICS AT ZHAOQING SOUTH CHINA NORMAL UNIV
Preparation method of hydroxylated graphene powder with controllable conductive performance
The invention discloses a preparation method of hydroxylated graphene powder with a controllable conductive performance. The electrical resistivity of the powder is controlled in a range of 10<-4>ohm.m-10<5>ohm.m. The method comprises the following steps: ultrasonically treating oxidized graphene in distilled water to obtain a uniformly dispersed oxidized graphene aqueous solution; adding hydrazine hydrate and ammonium water, and condensing and refluxing in an oil bath to obtain a turbid liquid of graphene and water; then, adding amino phenyl alcohol and isoamyl nitrite and condensing and refluxing to obtain a hydroxylated graphene aqueous solution; filtering to obtain a neutral solution; and freezing and drying to obtain the hydroxylated graphene powder with the controllable conductive performance. The hydroxylation degree of graphene is controlled by controlling the rate of charge of amino phenyl alcohol and oxidized graphene so as to control the conductivity of the hydroxylated graphene powder. The hydroxylated graphene powder is safe, environmental friendly, is free of inert gas protection and foreign ions, is fluffy and is not agglomerated, is high in degree of purity, is excellent in quality and is good in solubleness.
Owner:AVIC BEIJING INST OF AERONAUTICAL MATERIALS
Preparation method of solid strong acid catalyst and preparation method of furfural
InactiveCN107185590AEasy to prepareLarge specific surface areaOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSulfanilic acidPorous carbon
The invention provides a preparation method of a solid strong acid catalyst. The preparation method comprises the following steps of mixing calcium citrate porous carbon, sulfanilic acid, isoamyl nitrite and water; heating to perform sulfonation reaction, so as to obtain the solid strong acid catalyst. Compared with the prior art, the preparation method of the solid strong acid catalyst disclosed by the method has the advantages that the preparation method is simple, the specific surface area is large, the sulfonation temperature is low, the acid strength is high, the hydrothermal stability is high, the solid strong acid catalyst can be repeatedly used for multiple times; and the relatively high yield rate of furfural is produced at a relatively low usage amount of catalyst.
Owner:UNIV OF SCI & TECH OF CHINA
Synthesis method of sodium azide
InactiveCN105129752AThorough responseEasy to controlHydrazoic-acids/azides/halogen-azidesSynthesis methodsIsoamyl alcohol
The invention discloses a synthesis method of sodium azide. According to the synthesis method, sodium nitrite and isoamyl alcohol are taken as the initial raw materials; sodium nitrite and isoamyl carry out reactions, the reaction product is isoamyl nitrite, isoamyl nitrite is dropwise added into alkaline hydrazine hydrate to carry out reactions, and the reaction product is sodium azide crystal. In the provided synthesis method, liquid-liquid homogenous reactions are carried out, the process is easy to control, the operation is stable and safe, the yield is improved by 5% or more, the comprehensive cost is low, and the synthesis method can be applied to industrial massive production.
Owner:ANHUI LANGXI LIANKE IND
Alcohol group fuel for cleaning vehicle
ActiveCN103525487AClean and energy savingSimple compositionLiquid carbonaceous fuelsIsobutanolPropanoic acid
Alcohol group fuel for a cleaning vehicle is prepared from, by weight, 25-45% of arene, 25-69% of naphtha, 5-30% of denatured alcohol, 0.1-0.3% of stabilizer and 0.7-0.9% of fuel powerful agent in a mixed mode; the denatured alcohol is a mixed reactant of methyl alcohol, acetone, isopropanol / isobutanol, dimethyl ether and sodium chloride; the stabilizer is a mixture of oleic acid, cyclic-propionic acid and diethylamine or propylamine; the powerful agent is a mixture of isoamyl nitrite and tetranitromethane. For the alcohol group fuel, a gasoline engine does not need to be modified, the power and the fuel consumption of the alcohol group fuel are equal to those of gasoline, the alcohol group fuel and the gasoline are soluble at any rate and the use quality is not affected; the alcohol group fuel has the advantages of being clean, capable of saving energy and capable of effectively reducing harmful gas emission in automobile tail gas by over 50%, wherein the emission of CO, HC and NOX is reduced by over 90%. The alcohol group fuel for the cleaning vehicle is simple in composition, wide in raw material source and low in production cost; water resistance is strong and combustion performance is good; the storage time is long, and the problems of layering and going bad are avoided; the alcohol group fuel for the cleaning vehicle is worth being produced in mass, popularized and used.
Owner:贵州渝奉实业有限公司
Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine
ActiveCN110003227AAvoid it happening againLow priceOrganic chemistryCarbon–oxygen bondHydrazine compound
The present invention discloses a method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine. The method comprises the steps of under alkaline conditions, forming a carbon-oxygen bond through a nucleophilic substitution reaction of 2-chlorine-3-nitro-6-methyl pyridine and guaiacol; under the catalysis of palladium and carbon, carrying out hydrazine hydrate to reduce nitro to generatecorresponding aromatic amine; and carrying out cyclization of the aromatic amine under the action of isoamyl nitrite to synthesize the 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine. As no need to use expensive 2-amino-3-bromine-6-Methylpyridine and 2, 3-dimethoxy phenylboronic acid serving as raw materials, the method has an obvious cost advantage; and meanwhile, the nucleophilic substitution reaction and a diazotization reaction which are simple to operate are adopted in the first two steps of the method, therefore a Suzuki coupling reaction with higher operation requirements and needing corresponding noble metal palladium as a catalyst can be replaced. The method has the advantages of high reaction yield, low production cost, simple post-treatment process and high product purity, and is suitable for process scale up.
Owner:XINXIANG RUNYU NEW MATERIAL TECH CO LTD
Method for synthesizing salazosulfapyridine using pyridazol as raw material
InactiveCN105367489AConvenient sourceImprove conversion rateOrganic chemistryAqueous sodium hydroxideSalicylic acid
The present invention discloses a method for synthesizing salazosulfapyridine. The synthetic method comprises: step 1, conducting a diazotization reaction on pyridazol using a pipe reactor under the action of hydrochloric acid and isoamyl nitrite in a THF solvent to obtain diazonium salt; and step 2, conducting a coupling reaction on the diazonium salt and salicylic acid in a sodium hydroxide aqueous solution. In the synthetic method provided by the present invention, pyridazol is used as the raw material, and salazosulfapyridine is obtained through the diazotization reaction and the coupling reaction successively, . The diazotization reaction and the coupling reaction have a relatively high conversion rate and relatively few side reactions, thereby improving the product purity and ensuring the yield. In addition, pyridazol is easily available in source, so that the production cost is lowered, and the method has relatively huge industrial production value.
Owner:苏州统华药品有限公司
Alcohol group biodiesel
ActiveCN103525481ASimple compositionReduce manufacturing costLiquid carbonaceous fuelsFuel additivesPolyethylene vinyl acetatePropanoic acid
Alcohol group biodiesel comprises 2-5 parts of denatured alcohol, 2-5 parts of polyatomic alcohol, 8-15 parts of bio-oil, 50-65 parts of primary oil, 15-20 parts of kerosene, 1-4 parts of cetane number improver, 0.1-0.3 part of stabilizer and 0.7-0.9 part of powerful agent; the denatured alcohol is a mixed reactant of methyl alcohol, acetone, dimethyl ether, sodium chloride, ferrocene, polyisobutene and polyethylene-vinyl acetate; the stabilizer is a mixture of oleic acid, cyclic-propionic acid and diethylamine or propylamine; the powerful agent is a mixture of isoamyl nitrite and tetranitromethane. The alcohol group biodiesel is used as diesel vehicle fuel, an engine does not need to be modified, the power and the fuel consumption are equal to those of common diesel, and the alcohol group biodiesel and the common diesel are soluble at any ratio; harmful gas emission in automobile tail gas can be effectively reduced; composition is simple and raw materials are wide; the storage time is long, and the problems of layering and going bad are avoided; the alcohol group biodiesel is worth being produced in mass, popularized and used.
Owner:贵州渝奉实业有限公司
Synthetic method for 2,4-difluorobiphenyl
ActiveCN108658726AThe synthesis method is simpleEasy to operateHalogenated hydrocarbon preparationBenzeneDistillation
The invention discloses a synthetic method for 2,4-difluorobiphenyl, and belongs to the technical field of halogenated biphenyl. The method uses 2,4-difluoroaniline and benzene as raw materials, the 2,4-difluoroaniline is diazotized, and the diazotized product and the benzene are subjected to a coupling reaction to obtain the 2,4-difluorobiphenyl; and the method comprises the following steps: adding trifluoroacetic acid, anhydrous magnesium sulfate, a catalyst and the benzene into a reaction kettle, performing cooling under stirring to temperature of 2-6 DEG C, adding isoamyl nitrite, controlling temperature at 6-10 DEG C, adding the 2,4-difluoroaniline dropwise, controlling reaction temperature at 10-25 DEG C in the adding process, after material addition is completed, performing a reaction under stirring for 1-3 h at temperature of 5-12 DEG C, after the reaction is completed, performing suction filtration on the reaction mixture, performing washing, performing drying, and performingreduced-pressure distillation to obtain the 2,4-difluorobiphenyl. The synthetic method disclosed by the invention is simple, and the synthesized 2,4-difluorobiphenyl has a high yield and high purity.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Synthesis method of 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate
ActiveCN109336762AQuick responseMake up for low temperature kinetic energyOrganic compound preparationCarboxylic acid esters preparationMethylanilineMethyl benzene
The invention discloses a synthesis method of 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate. The synthesis method comprises the following steps: in the presence of a catalyst, performing a reaction on 2, 6-diethyl-4-methylaniline and isoamyl nitrite, so as to obtain 2, 6-diethyl-4-methyl phenyl diazonium salt; performing a reaction on the 2, 6-diethyl-4-methyl phenyl diazonium salt and diethyl malonate in an alkaline condition, so as to obtain the 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate. The yield of the 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate can reach about 85%, and the maximum yield of the 2-(2, 6-diethyl-4-methyl benzene) diethyl malonate can reach 92%; a non-water system is adopted, so that the possibility of generation of three wastes is greatly reduced. The isoamyl nitrite is taken as a diazotization reagent, so that in the present of the catalyst, the reaction speed is increased, and the low-temperature kinetic energy is compensated; the produced diazonium salt is relatively stable at low temperature and in the non-water system, less diazonium salt is decomposed, and the yield of a diazotization reaction is near 100%.
Owner:JIANGSU FUDING CHEM
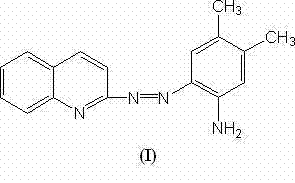
Complexometric reagent as well as preparation method and application thereof
InactiveCN102190617AHigh recovery rateSelectiveOrganic chemistryComponent separationMethylanilineChemical structure
The invention discloses a complexometric reagent as well as a preparation method and application thereof. The reagent has a chemical structure shown as in a novel general formula (I). The method comprises the following steps of: dissolving 2-aminoquinoline into absolute ethyl alcohol; adding sodium amide, heating in a water bath and reflowing; adding isoamyl nitrite, then reflowing and cooling to obtain diazo salt; adding 3,4-dimethylaniline in a diazo salt solution, stirring under the condition of introducing carbon diode, standing overnight, decompressing to evaporate most of ethanol and filtering precipitate to obtain a crude product; and recrystallizing with ethanol for 2-3 times to obtain the required complexometric reagent. The complexometric reagent can generate a stable complex compound with palladium. Therefore, the invention provides a novel method for detecting the palladium, wherein the detection limit can reach ng / L level. The detection result is contrasted by adopting an inductance coupling plasma mass spectroscopy (ICP-MS) method and is proved to be consistent.
Owner:YUXI NORMAL UNIV
Synthetic method for 4-bromo-5-thiazolecarboxylic acid ethyl ester
The invention belongs to the technical field of pharmaceutical intermediates, and relates to a synthetic method for 4-bromo-5-thiazolecarboxylic acid ethyl ester. The method comprises the following steps: mixing copper bromide, acetonitrile and isoamyl nitrite, performing heating to 60 DEG C, adding ethyl 4-aminothiazole-5-carboxylate in batches, after addition is completed, performing a reactionfor 30 min, after the raw materials are completely reacted, cooling the reaction solution to a room temperature, adding the cooled solution into hydrobromic acid, performing extraction by using ethylacetate, washing the organic phase by using water, performing drying by using anhydrous sodium sulfate, performing concentration, and performing treatment by using a chromatographic column to obtain the 4-bromo-5-thiazolecarboxylic acid ethyl ester. The method provided by the invention has low costs of the raw materials and a high yield, and is suitable for scale-up production.
Owner:苏州络森生物科技有限公司
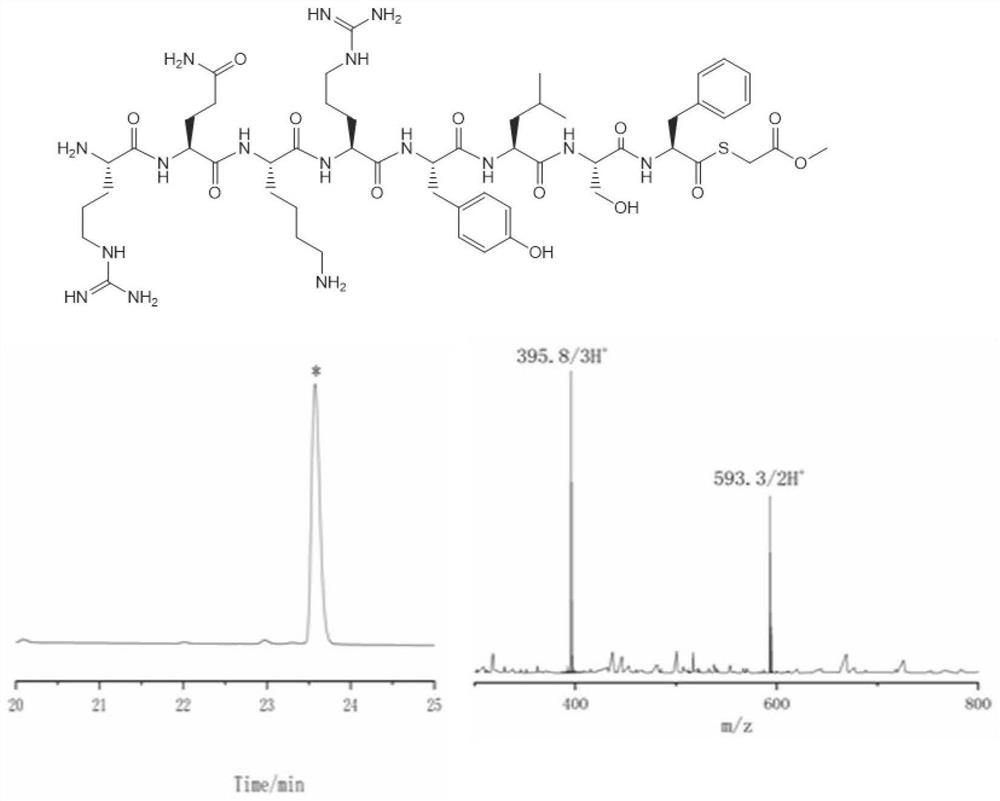
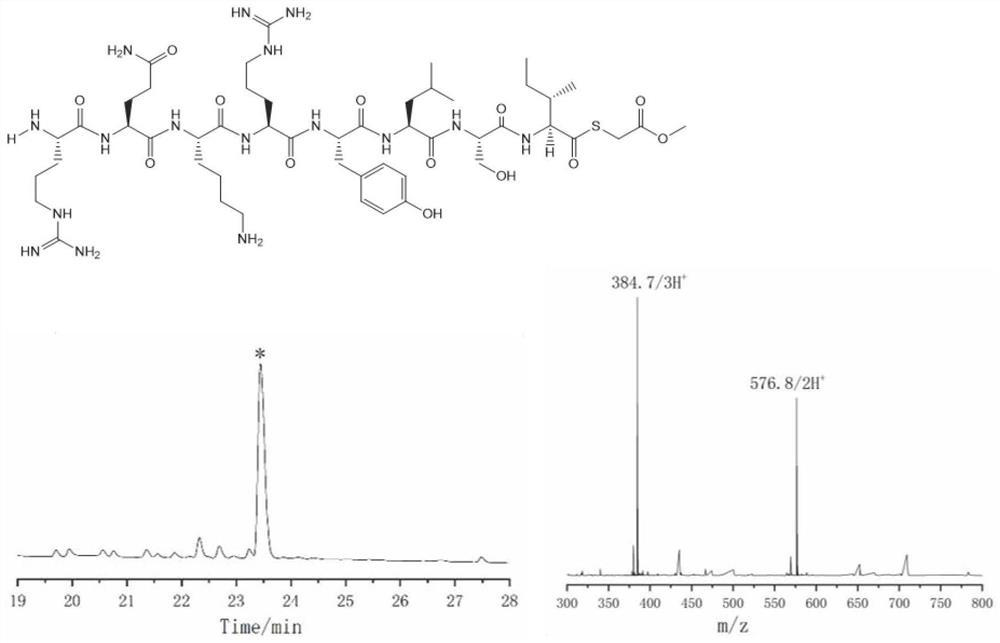
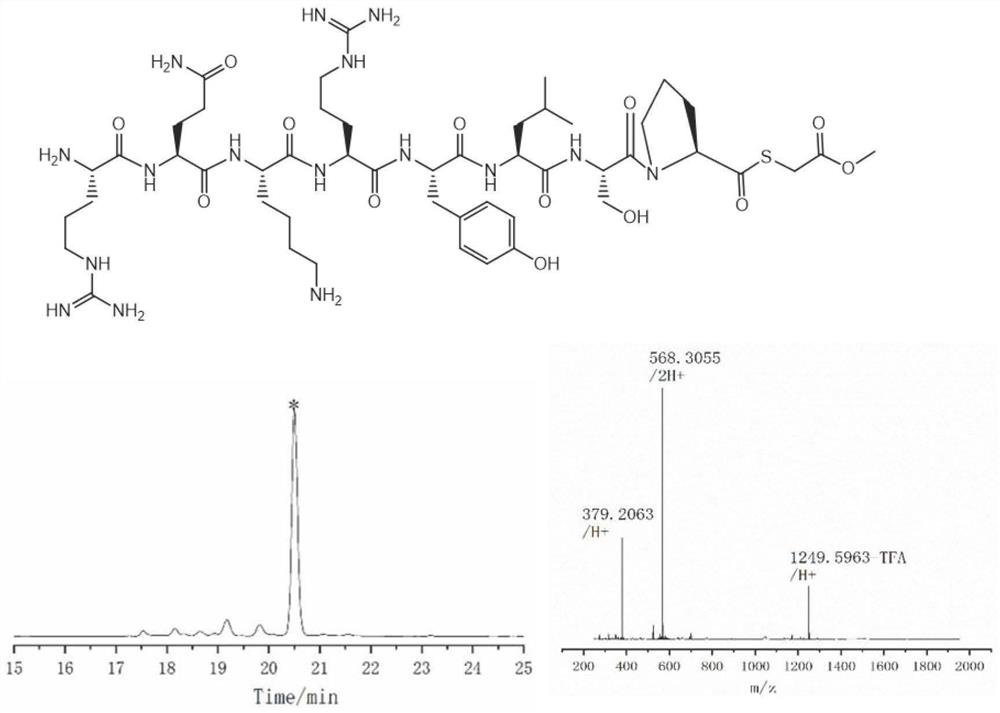
Thioester peptide synthesis method
PendingCN114181276AThe synthesis process is simpleShorten production timePeptide preparation methodsFreeze-dryingAcyl group
The invention discloses a thioester peptide synthesis method, which specifically comprises the following steps: dissolving polypeptide hydrazide in a hydrochloric acid-containing mixed solvent consisting of dimethyl sulfoxide and water, and reacting with isoamyl nitrite in an ice-salt bath to generate acyl azide peptide; adding excessive methyl thioglycolate, and then adding ammonium bicarbonate prepared in advance to adjust the acidity of the solution to be neutral, so that the acyl azide peptide is converted into peptide thioester; pre-cooled trifluoroacetic acid and diethyl ether are sequentially added into a reaction system, so that thioester peptide is crystallized and separated. The thioester peptide is prepared through the low-cost, simple and rapid operation process, and the problem that high-cost high performance liquid chromatography and time-consuming freeze drying operation are needed in traditional thioester peptide synthesis is solved.
Owner:ANHUI UNIVERSITY
A method for preparing functionalized graphene by ball milling
The invention discloses a method for preparing functionalized graphene through ball milling. According to the method, graphite, organic molecules containing anilino groups and isoamyl nitrite are used as raw materials and subjected to ball milling so as to prepare graphene covalently modified by organic molecules. The method for preparing the functionalized graphene in the invention has the advantages that the functionalized graphene is prepared from graphite through one-step reaction, so preparation process is simple; different organic molecules containing anilino groups can be used so as to realize surface functionalization of graphene; different organic molecules and graphene are connected via covalent bonds, so graphene has good dispersibility in different systems (water, organic solvents and matrix materials); and the method is simple to operate, low in cost, high in efficiency, free of pollution and capable of realizing large-scale preparation. The functionalized graphene prepared by using the one-step method has good dispersibility, which is beneficial for processing of the functionalized graphene in a solvent, and has wide application prospects in the fields of optoelectronic devices, new energy sources and new materials.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
The preparation method of iodosulfuron sodium salt intermediate
The invention discloses a preparation method of an intermediate of iodosulfuron-methyl sodium. The intermediate is obtained by performing a reaction on 6-aminosaccharin and potassium iodide in the presence of alkyl nitrite and acetic acid in one step, wherein a molar ratio of the potassium iodide to the 6-aminosaccharin is 1:1 to 1.2:1; a molar ratio of the alkyl nitrite to the 6-aminosaccharin is0.95:1 to 1:1; the alkyl nitrite is ethyl nitrite, isopropyl nitrite, n-butyl nitrite or isoamyl nitrite; and a molar ratio of the acetic acid to the 6-aminosaccharin is 1:1 to 1.2:1. According to the method provided by the invention, the alkyl nitrite is adopted for diazotization, the potassium iodide is added at the same time, so that decomposition and hydrolysis of a diazonium salt can be avoided, and the reaction yield is greatly improved; and the method does not require a large amount of water as a solvent and only adopts a small amount of water to wash an organic layer in the post-treatment, and therefore wastewater produced by adopting the method provided by the invention does not exceed 2 times the weight of the target product.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
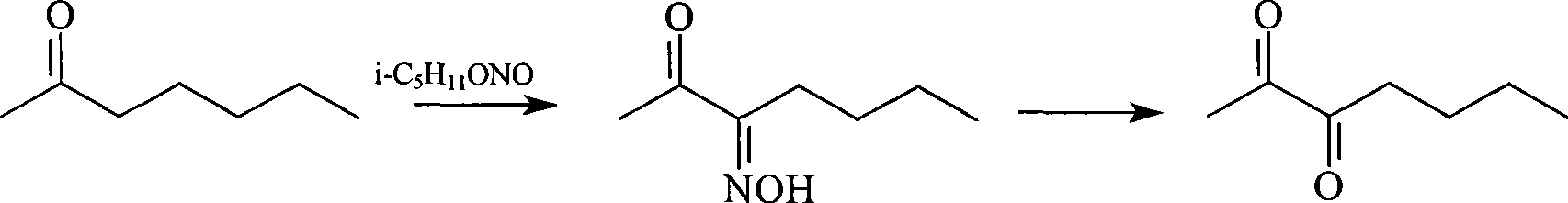
Synthetic method of 2,3-heptane dione
InactiveCN101423466AEasy to operateQuick responseCarbonyl compound preparation by hydrolysisReaction rate2-Heptanone
The invention discloses a method for synthesizing 2, 3-heptanedione, which comprises the following steps: 2, 3-heptanedione monoxime is prepared by taking 2-heptanone as a raw material and dripping isoamyl nitrite in the presence of dilute hydrochloric acid, and then performing hydrolysis. The hydrolysis temperature is between 20 and 60 DEG C, the hydrolysis time is between 4 and 10 hours, and a catalyst for the hydrolysis is the dilute hydrochloric acid. The method has easy operation, quick reaction rate, and high reaction yield.
Owner:上海香料研究所
Alcohol group biodiesel
ActiveCN103525481BSimple compositionReduce manufacturing costLiquid carbonaceous fuelsFuel additivesPolyethylene vinyl acetatePropanoic acid
Alcohol group biodiesel comprises 2-5 parts of denatured alcohol, 2-5 parts of polyatomic alcohol, 8-15 parts of bio-oil, 50-65 parts of primary oil, 15-20 parts of kerosene, 1-4 parts of cetane number improver, 0.1-0.3 part of stabilizer and 0.7-0.9 part of powerful agent; the denatured alcohol is a mixed reactant of methyl alcohol, acetone, dimethyl ether, sodium chloride, ferrocene, polyisobutene and polyethylene-vinyl acetate; the stabilizer is a mixture of oleic acid, cyclic-propionic acid and diethylamine or propylamine; the powerful agent is a mixture of isoamyl nitrite and tetranitromethane. The alcohol group biodiesel is used as diesel vehicle fuel, an engine does not need to be modified, the power and the fuel consumption are equal to those of common diesel, and the alcohol group biodiesel and the common diesel are soluble at any ratio; harmful gas emission in automobile tail gas can be effectively reduced; composition is simple and raw materials are wide; the storage time is long, and the problems of layering and going bad are avoided; the alcohol group biodiesel is worth being produced in mass, popularized and used.
Owner:贵州渝奉实业有限公司
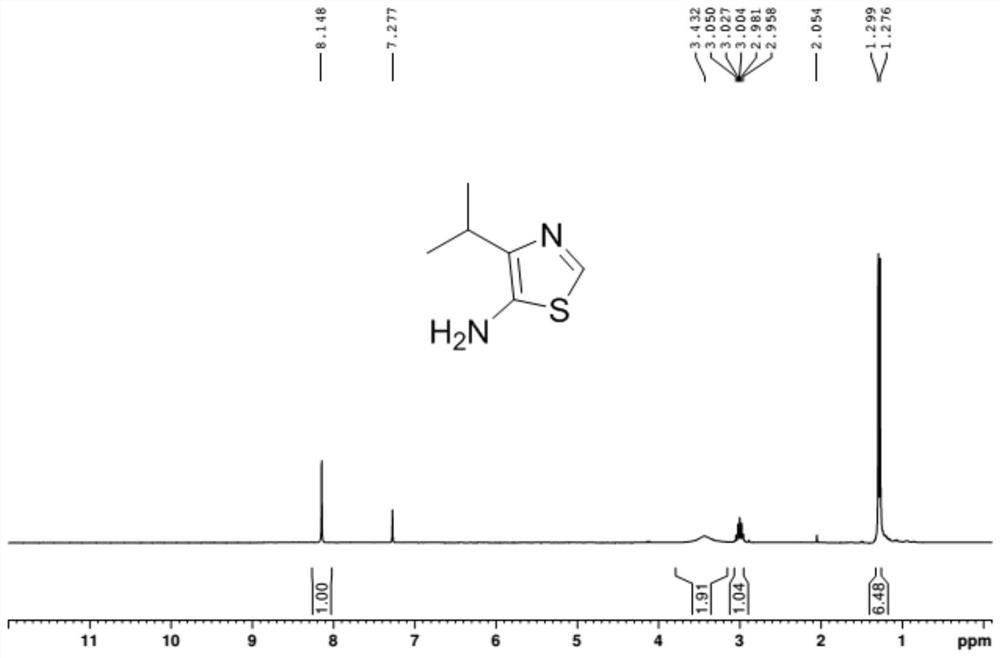
A kind of synthetic method of the amine compound containing sulfur nitrogen heterocycle of medicine intermediate
ActiveCN108084111BFew synthetic stepsImprove creativityOrganic chemistryWater methanolTetrahydrofuran
The invention relates to a method for synthesizing a pharmaceutical intermediate sulfur-containing nitrogen heterocyclic amine compound. The method comprises that ethyl 4-methyl-3-oxo-pentanoate, dichloromethane and sulfonyl chloride as raw materials undergo a reaction, the reaction product is subjected to pH adjustment until pH of 7, the treated reaction product is extracted so that ethyl 2-chloro-4-methyl-3-oxopentanoate is obtained, the ethyl 2-chloro-4-methyl-3-oxopentanoate and thiourea undergo a reaction in the presence of ethanol as a solvent to produce ethyl 2-amino-4-isopropylthiazole-5-formate, isoamyl nitrite is added into ethyl 2-amino-4-isopropylthiazole-5-formate in the presence of tetrahydrofuran as a solvent to obtain 4-isopropylthiazole-5-formate, a NaOH solution is addedinto the 4-isopropylthiazole-5-formate in the presence of anhydrous methanol as a solvent so that 4-isopropylthiazole-5-formic acid is obtained, the 4-isopropylthiazole-5-formic acid and azophosphoryldiphenyl ester undergo a reaction in the presence of tert-butyl alcohol as a solvent and triethylamine, the reaction product is extracted, the extract is washed so that 5-N-BOC-4-isopropylthiazole isobtained, the 5-N-BOC-4-isopropylthiazole and HCl undergo a reaction in the presence of anhydrous methanol as a solvent to produce 4-isopropyl-5-aminothiazole. The method has the advantages of clearprocesses, less waste, high yield, raw material saving and operation easiness.
Owner:烟台宁远药业有限公司
A kind of preparation method of polyaniline-graphene hollow microsphere
The invention discloses a preparation method of polyaniline-graphene hollow microspheres. Firstly, isoamyl nitrite and sulfanilic acid are utilized to sulfonate graphene, the obtained sulfonated graphene is used as a Pickering emulsifier, water dispersion liquid of the obtained sulfonated graphene serves as a water phase, and toluene or an aniline monomer serves as an oil phase to perform emulsification, an oil-in-water (O / W) emulsion is obtained, then an oxidizing agent ammonium persulfate is added to trigger aniline polymerization, and the polyaniline-graphene hollow microspheres can be obtained after the oil phase toluene is removed. The polyaniline-graphene hollow microspheres are prepared by adopting a Pickering emulsion method. The preparation method is simple and efficient, and the prepared polyaniline-graphene hollow microsphere material with a three-dimensional hollow structure has larger specific surface area and a shorter ion and electron transmission path compared with a polyaniline-graphene composite material with a two-dimensional structure and has a greater potential application value on the aspects of catalysis, micro-reactors, sensing, energy storage materials and the like.
Owner:JIANGNAN UNIV
Triflumizole original drug, and preparation method thereof
ActiveCN110105289AMild reaction conditionsEasy to operateOrganic chemistryDrug contentChloroacetic acids
The invention provides a triflumizole original drug, and a preparation method thereof. The triflumizole original drug comprises, by weight, 370 parts to 400 parts of chloroacetic acid, 5 parts to 10 parts of diazabicyclo, 110 parts to 120 parts of n-propanol, 800 parts to 820 parts of sodium n-propoxide, 650 parts to 700 parts of diethyl ether, and 30 parts to 50 parts of concentrated sulfuric acid, 20 parts to 30 parts of isoamyl nitrite, 400 parts to 450 parts of toluene, 600 parts to 700 parts of 2-trifluoromethyl-4-chloroaniline, 410 parts to 440 parts of imidazole, 1000 parts to 1200 parts of cyclohexane, 450 parts to 470 parts of triphosgene, 1400 parts to 1700 parts of chloroform, and 180 parts to 200 parts of triethylamine. The total yield of the triflumizole original drug is higher than 85%, the original drug content is higher than 95%, the water content is less than 0.5, the drug effect is remarkable, and the effect is better.
Owner:JIANGSU HEBEN BIOCHEM
Small-dose combined medicine composition for treating intractable hypertension
InactiveCN113730585ATake a small doseLittle side effectsSalicyclic acid active ingredientsMetabolism disorderSide effectEnzyme Inhibitor Agent
The invention relates to a small-dose combined medicine composition for treating intractable hypertension. The composition comprises the following components in parts by weight: 10-25 parts of an angiotensin transenzyme inhibitor, 5-10 parts of a calcium ion orange resistant agent, 10-25 parts of a diuretic, 10-25 parts of an a receptor blocker, 0.1-0.3 part of nitroglycerin, 1-4 parts of folium ginkgo, 80-130 parts of radix salviae miltiorrhizae, 0.1-0.5 part of estazolam, 0.5-3 parts of diazepam, 1-3 parts of isosorbide dinitrate, 3-8 parts of aspirin, 0.2-0.8 part of isoamyl nitrite, 2-8 parts of atorvastatin calcium, 0.5-3 parts of amlodipine besylate and 8-20 parts of clopidogrel hydrogen sulfate. The medicine composition has small toxic and side effects and can effectively treat intractable hypertension.
Owner:葛荣专
Preparation method of intermediate of iodosulfuron-methyl sodium
The invention discloses a preparation method of an intermediate of iodosulfuron-methyl sodium. The intermediate is obtained by performing a reaction on 6-aminosaccharin and potassium iodide in the presence of alkyl nitrite and acetic acid in one step, wherein a molar ratio of the potassium iodide to the 6-aminosaccharin is 1:1 to 1.2:1; a molar ratio of the alkyl nitrite to the 6-aminosaccharin is0.95:1 to 1:1; the alkyl nitrite is ethyl nitrite, isopropyl nitrite, n-butyl nitrite or isoamyl nitrite; and a molar ratio of the acetic acid to the 6-aminosaccharin is 1:1 to 1.2:1. According to the method provided by the invention, the alkyl nitrite is adopted for diazotization, the potassium iodide is added at the same time, so that decomposition and hydrolysis of a diazonium salt can be avoided, and the reaction yield is greatly improved; and the method does not require a large amount of water as a solvent and only adopts a small amount of water to wash an organic layer in the post-treatment, and therefore wastewater produced by adopting the method provided by the invention does not exceed 2 times the weight of the target product.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
Purification of 2-iodo-6-chloro-9-beta(2',3',5'-trioxyacetyl-D-furanose)purine
InactiveCN100484950CImprove controllabilityImprove product qualitySugar derivativesPurineTetrahydrofuran
Purification of 2-iodine-6-chlorine-9-beta-(2,,3,,5,-acetyl trioxide-D-ribofuranose) purine is carried out by dissolving 2-amino-6-chlorine-9-beta-(2,,3,,5,- acetyl trioxide-D-ribofuranose) purine, iodine, copper iodide and diiodomethane into butylenes oxide, reflux reacting with isoamyl nitrite, decolorizing by active carbon, and re-crystallizing by solvent with double component to obtain purified final product. Its advantages include low cost, short period, stable quality and controllable operation.
Owner:SHANGHAI CHEM REAGENT RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


















![Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/32806ef1-da91-4cfc-aa41-59e5fdcef53f/190424145458.png)
![Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/32806ef1-da91-4cfc-aa41-59e5fdcef53f/190424145503.png)
![Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine Method for synthesizing 2-methyl-8-methoxy benzofuran [2, 3-b] pyridine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/32806ef1-da91-4cfc-aa41-59e5fdcef53f/190424145508.png)