Method for preparing ceftaroline side-chain acid
A hydrochloric acid aqueous solution, ethoxyiminoacetic acid technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield of amino deprotection reaction, harsh process reaction conditions, low total yield, etc., and achieves great implementation value and social economy. Benefit, good stereo selection, high stereo selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) In a 500mL three-necked flask, mix 5-pivalylamino-1,2,4-thiadiazole-3-acetyl-N-acetamide (50.0g, 0.176mol), 31% hydrochloric acid aqueous solution (2.1 g, 0.0176mol) were mixed in tetrahydrofuran (150g), and isopropyl nitrite (46g, 0.528mol) was added dropwise at -15°C. Add bromoethane (152g, 1.41mol) dropwise, keep the temperature for 5 hours, filter with suction, take the filter cake, and dry it in vacuum to obtain (Z)-5-pivalylamino-1,2,4-thiadiazole- 42.0 g of 3-(1-ethoxyimino-acetyl-N-acetamide), the crude product yield is 70%; white solid, melting point: 182.0-184.2°C.
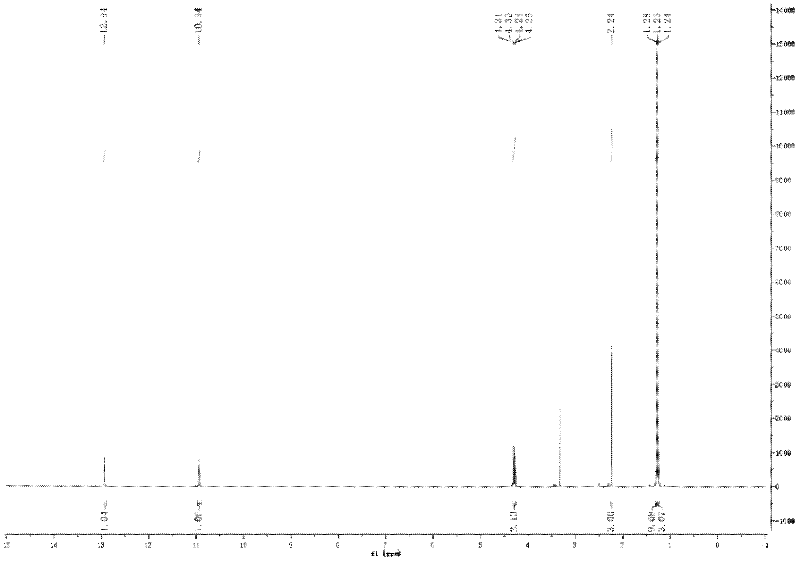
[0052] 1 H-NMR (DMSO, 400MHz): δ (ppm) 1.26 (3H, t, J=8.0Hz, OCH 2 CH 3 ), 1.28 (9H, s, C (CH 3 ) 3 ), 2.24 (3H, s, COCH 3 ), 4.29 (2H, q, J 7.0Hz, OCH 2 CH 3 ), 10.94(H, s, 1H, NH-C=N), 12.94(1H, s, J=11.2Hz, NHCOCH 3 ).
[0053] (2) 42.0 g (0.123 mol) of the crude product obtained in step (1) is mixed with 0.5% NaOH solution (1968 g, 0.246 mol) in a 3L bottle, and incubated for 20 ...
Embodiment 2
[0055] (1) In a 2L three-necked flask, mix 5-pivalylamino-1,2,4-thiadiazole-3-acetyl-N-acetamide (50.0g, 0.176mol), 31% hydrochloric acid aqueous solution (20.7 g, 0.176mol) were mixed in dichloromethane (1000mL), and isopropyl nitrite (76.0g, 0.88mol) was added dropwise at -30°C. ), bromoethane (152g, 1.41mol) was added dropwise at 0°C, and after 10 hours of heat preservation reaction, suction filtration was taken, and the filter cake was taken to obtain 36g of crude product (Z)-5-pivalylamino-1,2,4- Thiadiazole-3-(1-ethoxyimino-acetyl-N-acetamide), crude product yield 60%; white solid, melting point: 181.3-183.5°C.
[0056] (2) Mix 36g (0.105mol) of the crude product obtained in step (1) and 2.5% KOH solution (470.4g, 0.21mol) in a 1L bottle, keep the reaction at 90°C for 20h, cool to 5°C, and wash with 5% dilute hydrochloric acid Adjust the pH to 2-3, filter with suction, and take the filter cake to obtain 13.3 g of ceftaroline side-chain acid, with a total yield of 35%, a...
Embodiment 3
[0058] (1) In a 1L three-necked flask, mix 5-pivalylamino-1,2,4-thiadiazole-3-acetyl-N-acetamide (50.0g, 0.176mol), 31% hydrochloric acid aqueous solution (10.4 g, 0.088mol) were mixed in acetonitrile (50g), and isopropyl nitrite (92.0g, 1.05mol) was added dropwise at 0°C. After 15 hours of insulation reaction, pyridine (69.5g, 0.88mol) was added, Diethyl sulfate (27g, 0.176mol) was added dropwise, and after 1 hour of heat preservation reaction, suction filtration was taken, and the filter cake was taken to obtain 39g of crude product (Z)-5-pivalylamino-1,2,4-thiadiazole- 3-(1-Ethoxyimino-acetyl-N-acetamide), crude product yield 65%; white solid, melting point: 182.4-183.2°C.
[0059] (2) 39g (0.114mol) of the crude product obtained in step (1) and 5% Ba (OH) 2 solution (1949g, 0.57mol) were mixed in a 3L bottle, kept at 100°C for 24h, cooled to 5°C, and heated with 10 % dilute hydrochloric acid to adjust pH to 2-3, suction filtration, take filter cake to obtain 19.8g of ceft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com