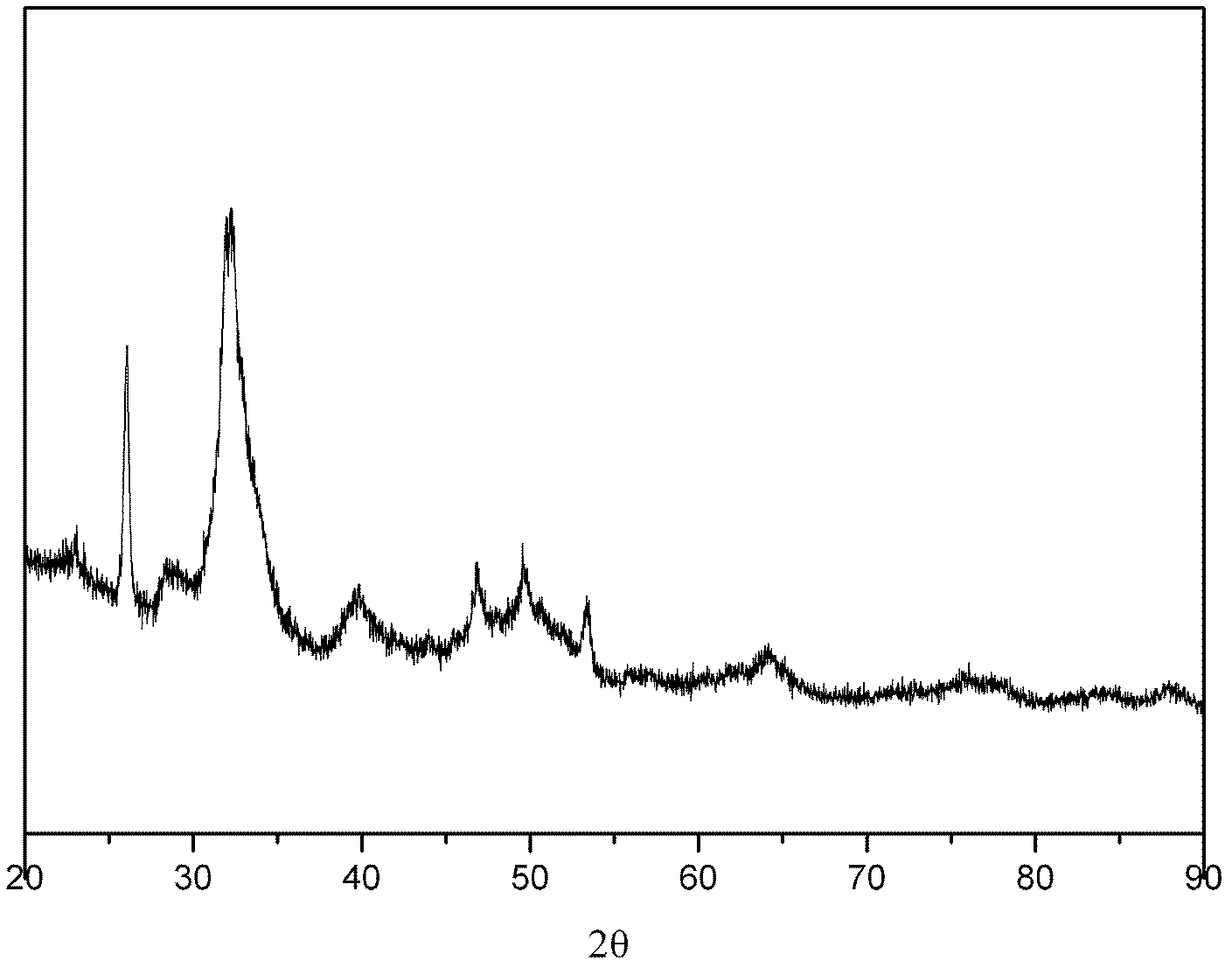
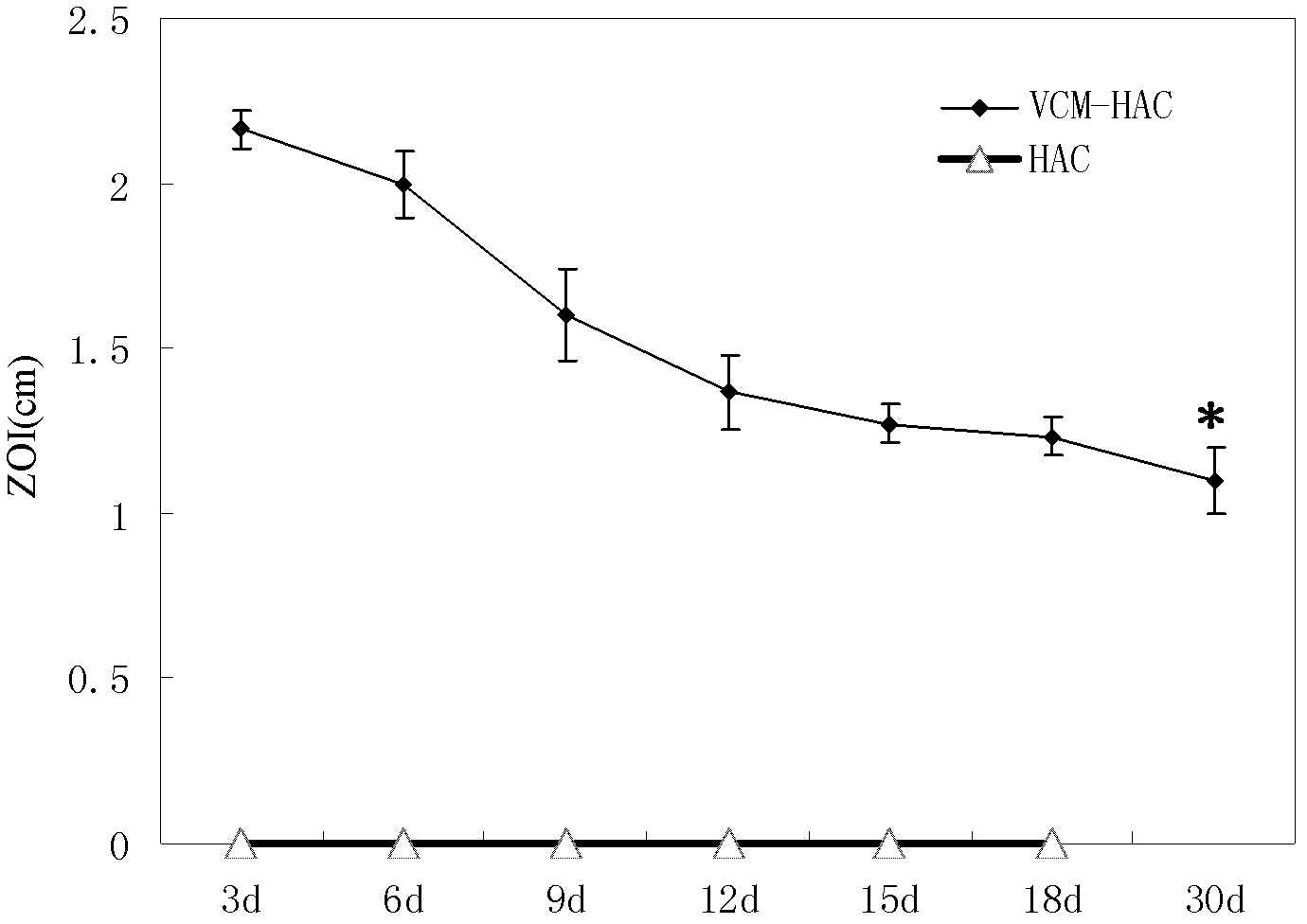
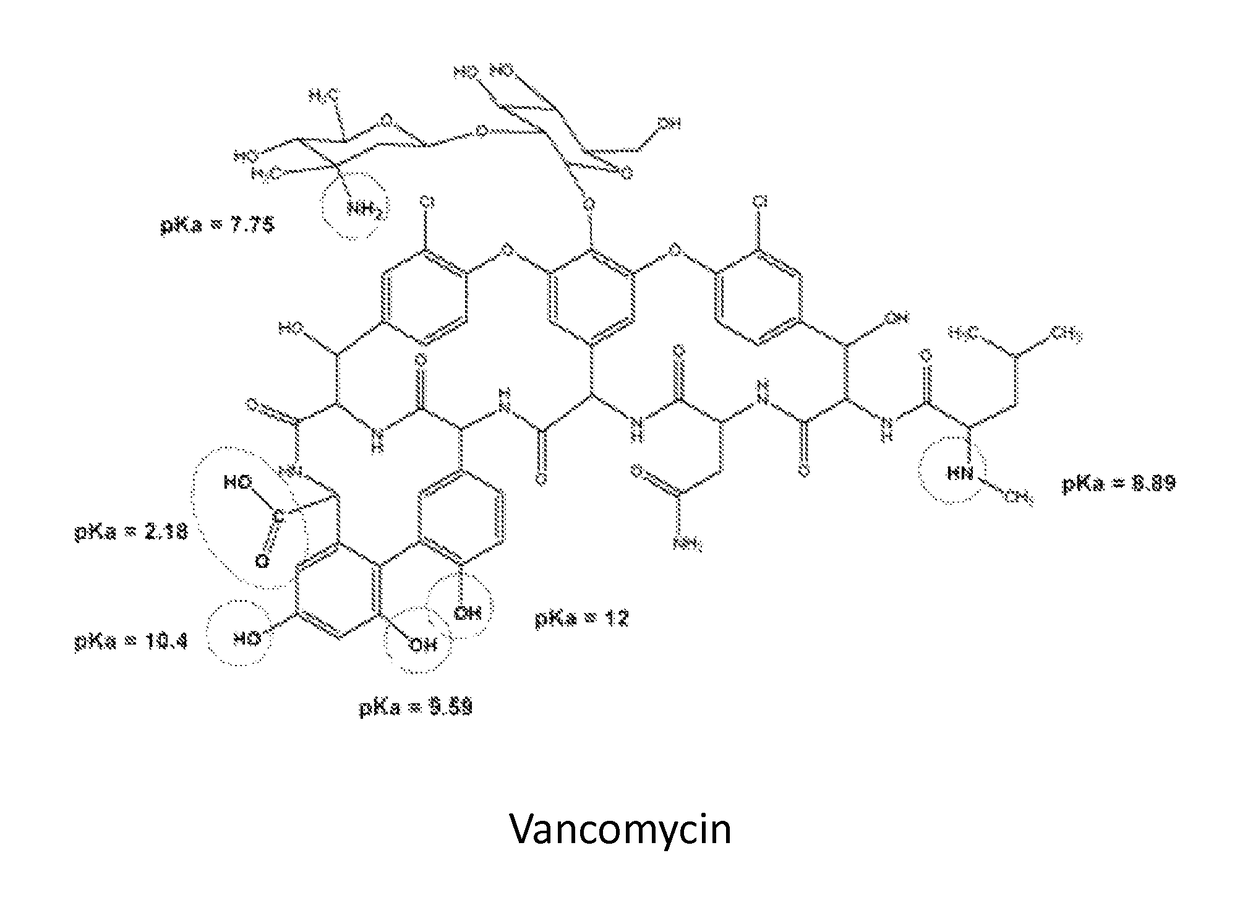
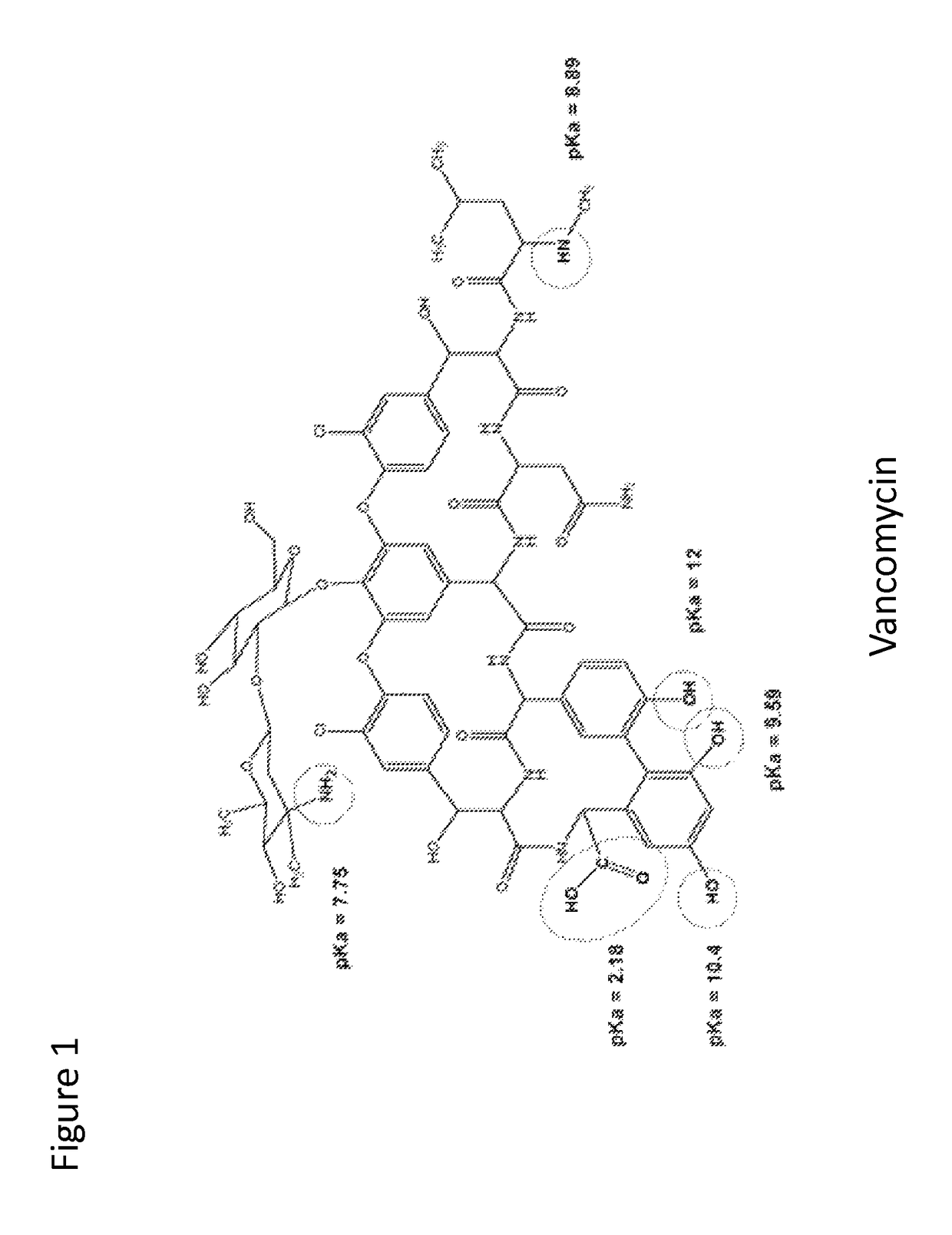
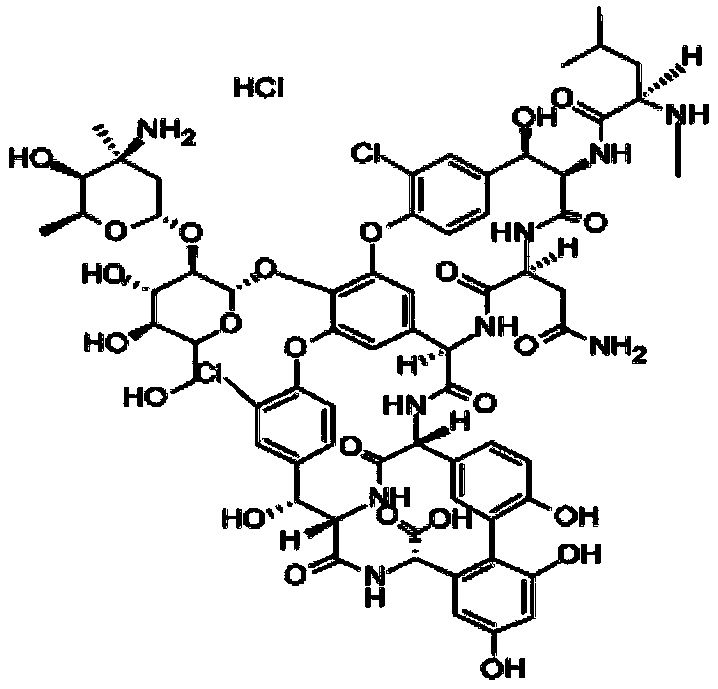
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
330 results about "Vancomycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
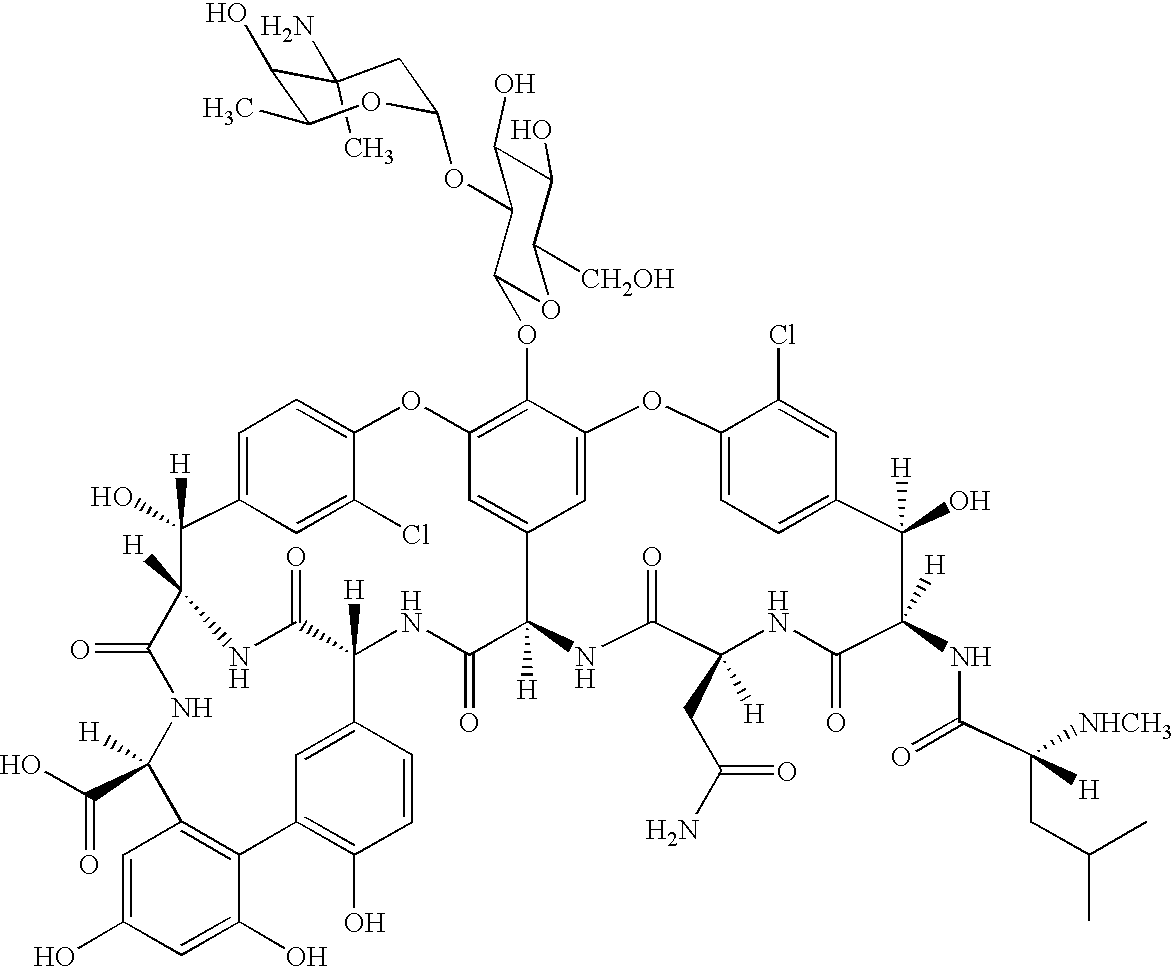
Vancomycin is an antibiotic used to treat infections. This form of vancomycin is used to treat a certain intestinal condition (colitis) caused by bacteria.
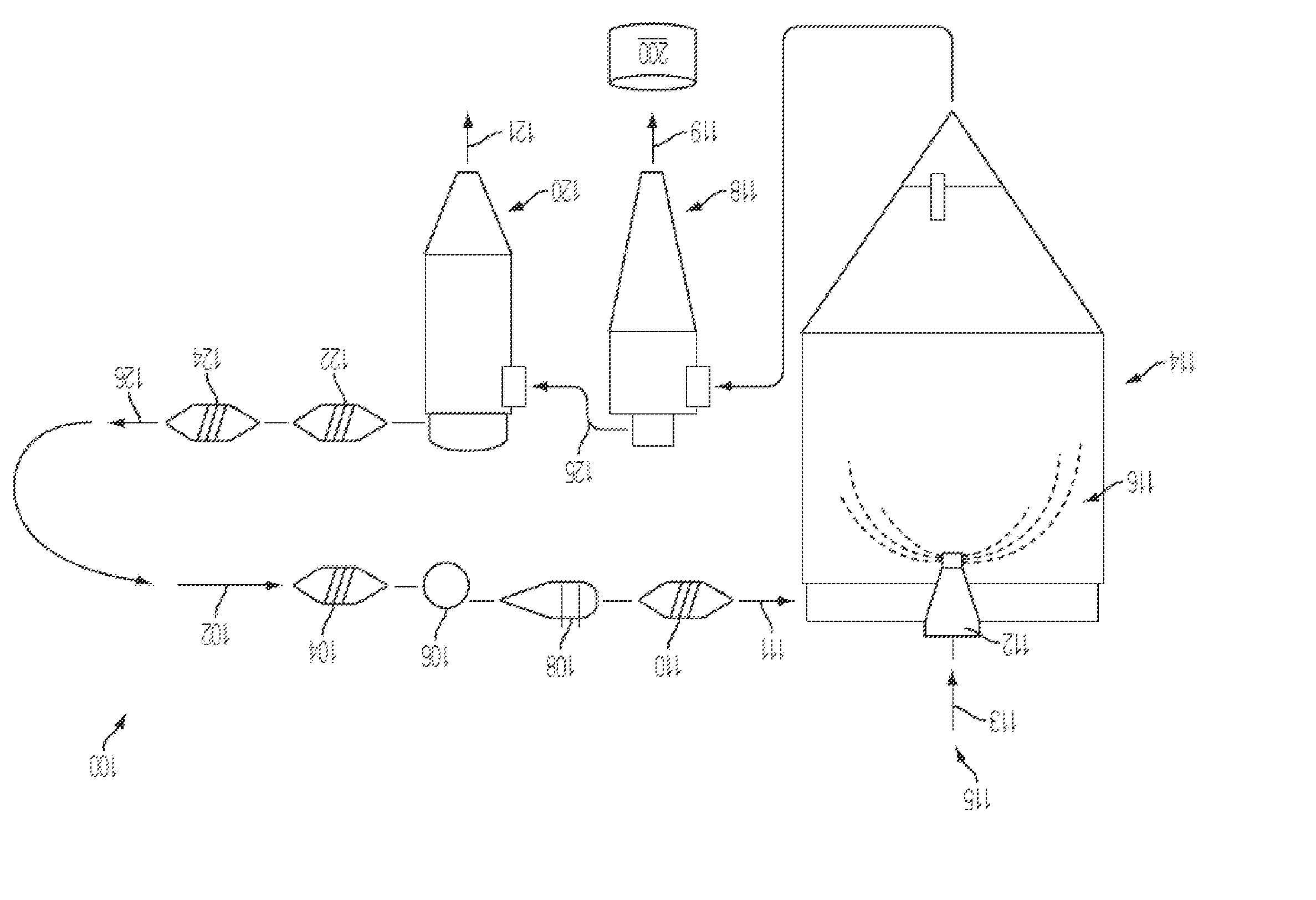
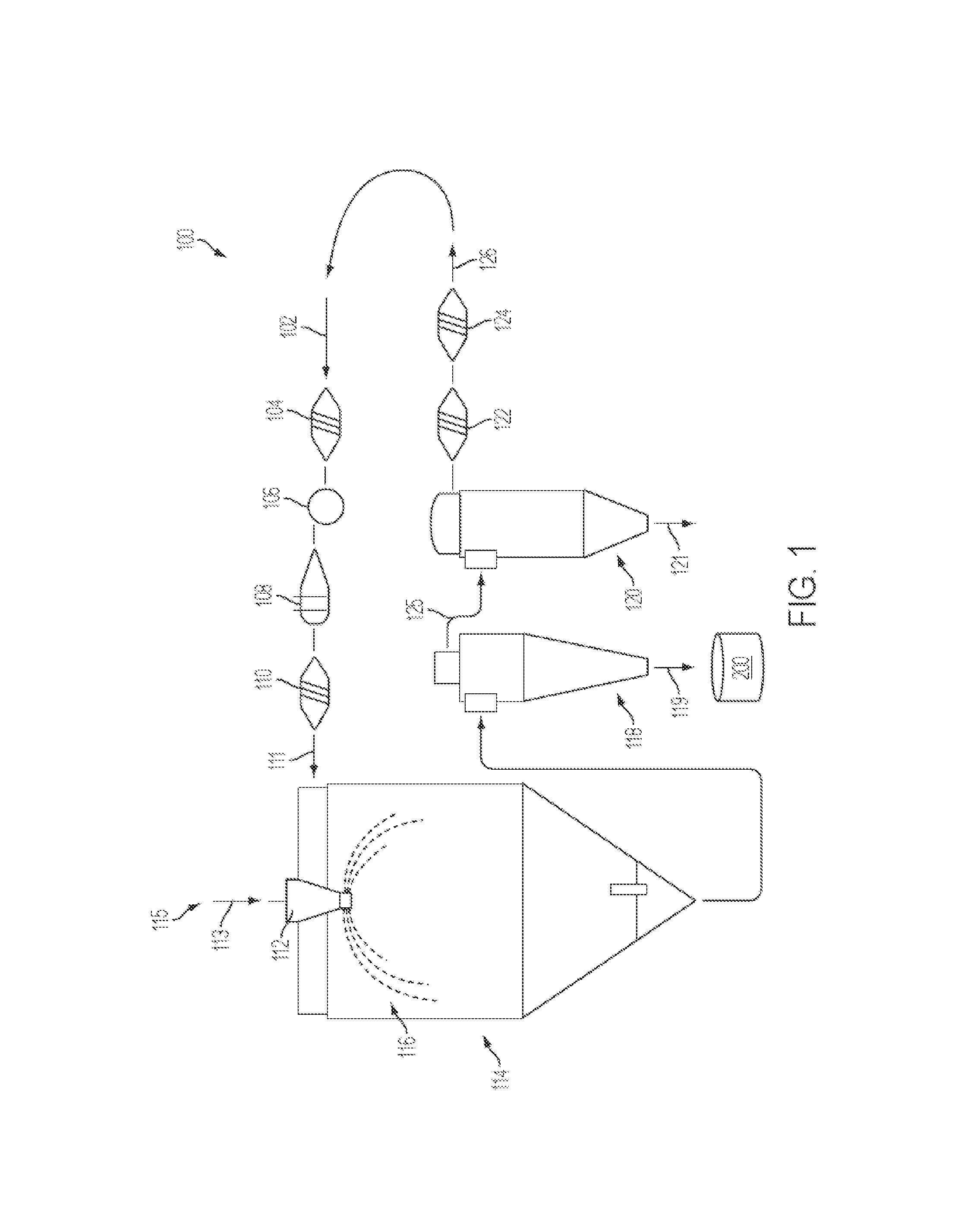
Treatment of pulmonary disorders with aerosolized medicaments such as vancomycin
InactiveUS20100282247A1Reduce in quantityReduce the amount requiredAntibacterial agentsAntimycoticsDiseaseGlycopeptide
A method of administering an aerosolized anti-infective, such as a glycopeptide, to the respiratory system of a patient. A ratio of an amount of the glycopeptide, such as vancomycin, delivered to the pulmonary system of the patient in a 24 hour period to a minimum inhibitory amount for the target organ for the same period is about 2 or more. A system to introduce aerosolized medicament to a patient may include a humidifier coupled to an inspiratory limb of a ventilator circuit wye, where the humidifier supplies heated and humidified air to the patient, and an endotracheal tube having a proximal end coupled to a distal end of the ventilator circuit wye. The system may also include a nebulizer coupled to the endotracheal tube, where the nebulizer generates the aerosolized medicament.
Owner:NEKTAR THERAPEUTICS INC
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Biofunctional magnetic nanoparticles for pathogen detection
InactiveUS20060292555A1High sensitivityEasy to reportNanotechMicrobiological testing/measurementMagnetite NanoparticlesBiology
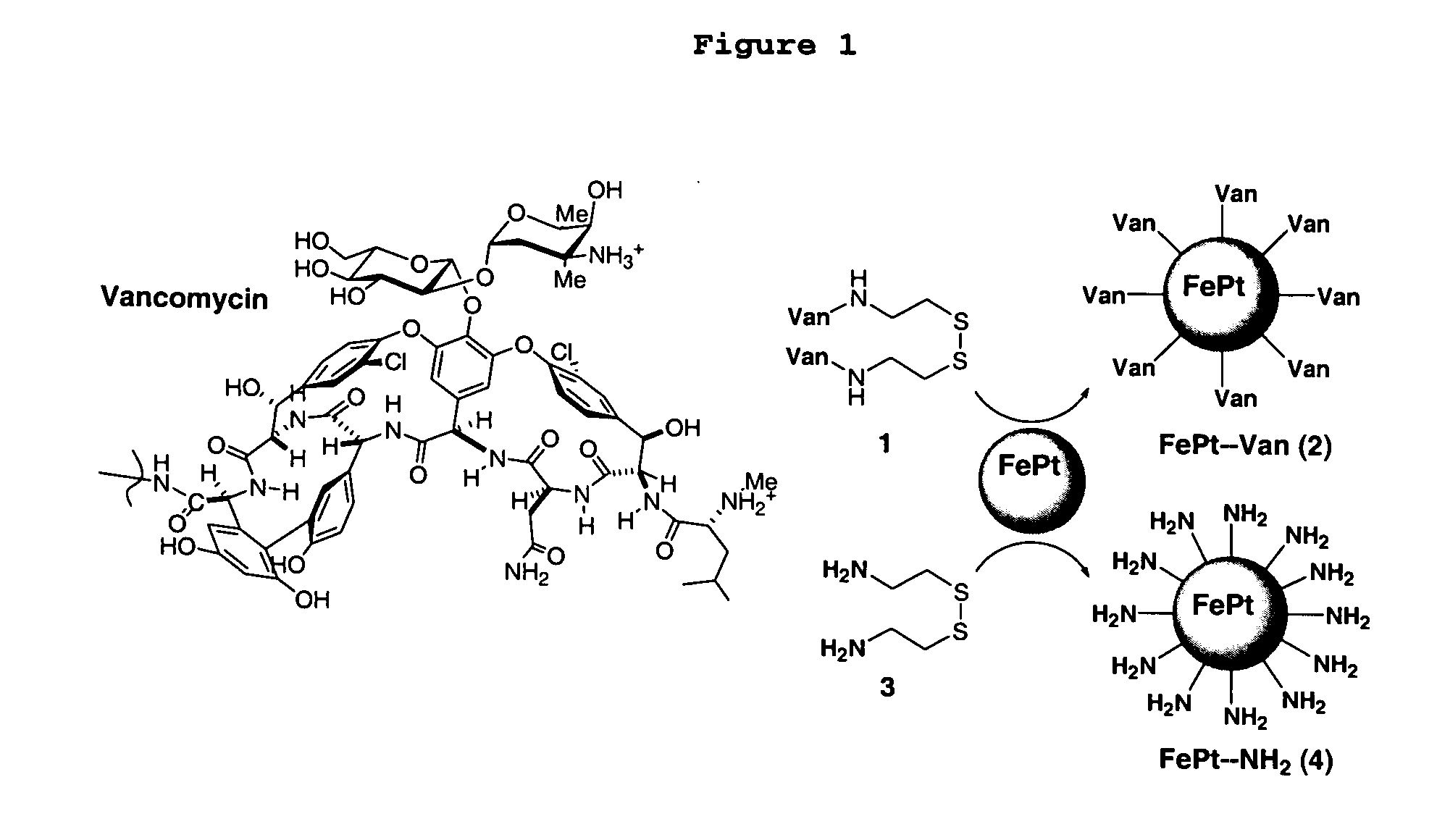
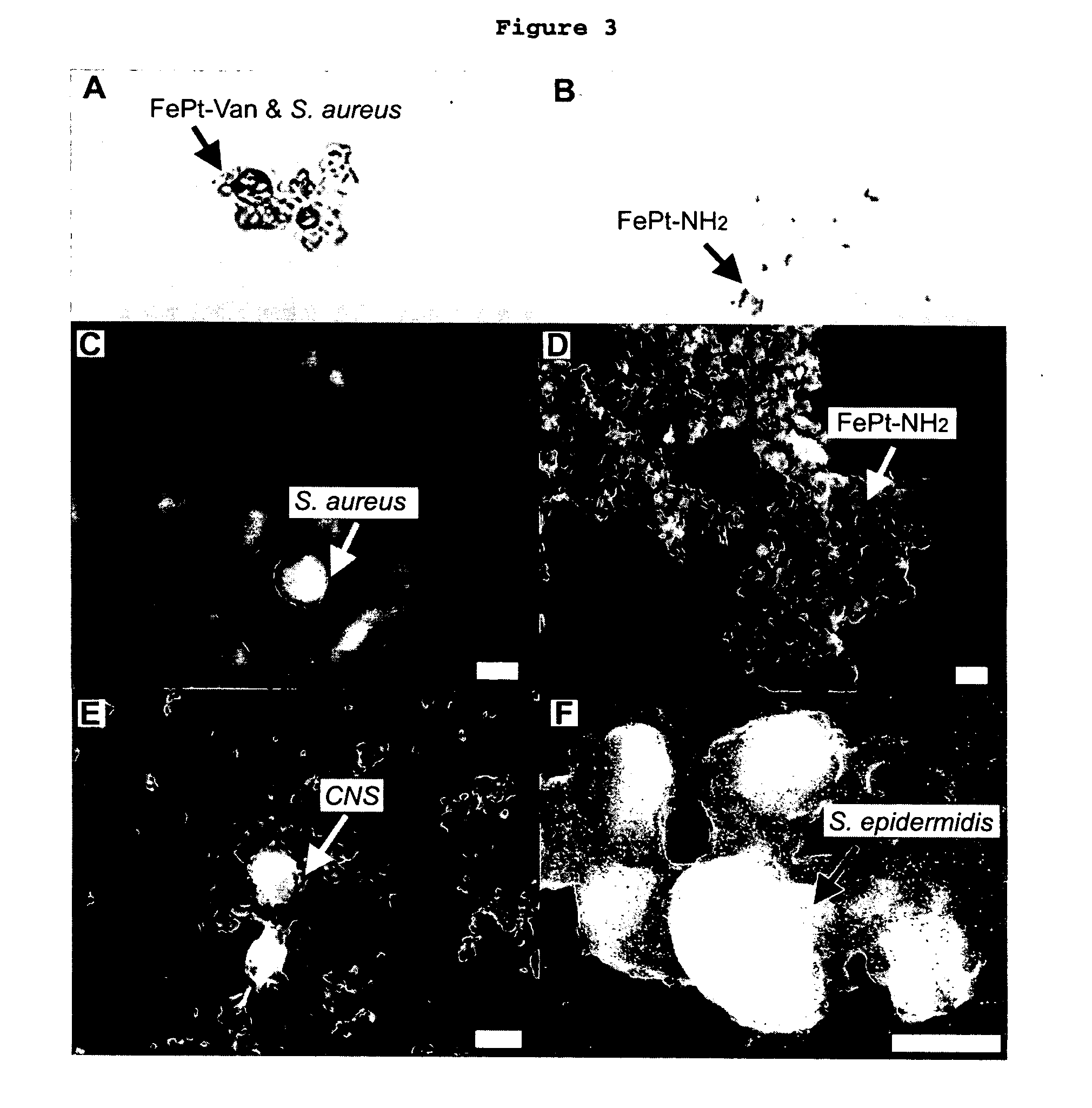
This invention provides a method of detecting pathogens comprising the steps of: (a) contacting a sufficient amount of biofunctional magnetic nanoparticles with an appropriate sample for an appropriate period of time to permit the formation of complexes between the pathogens in the sample and the nanoparticles; (b) using a magnetic field to aggregate said complexes; and (c) detecting said complexes. The method may further comprise the additional step of removing said complexes. The biofunctional magnetic nanoparticles are preferably a conjugate of vancomycin and FePt. The pathogens may be bacteria or viruses, and the sample may be a solid, liquid, or gas. Detection may involve conventional fluorescence assay, enzyme-linked immunosorbent assay (ELISA), optical microscope, electron microscope, or a combination thereof. The sensitivity of detection for the method is at least as low as 10 colony forming units (cfu) of the pathogens in one milliliter of solution within one hour.
Owner:THE HONG KONG UNIV OF SCI & TECH +1
New type antibiotic containing an antibody analog, preparation method and application method thereof
ActiveCN101633699AImprove permeabilityLow immunogenicityAntibacterial agentsPeptide/protein ingredientsNormal cellAntibiotic Y
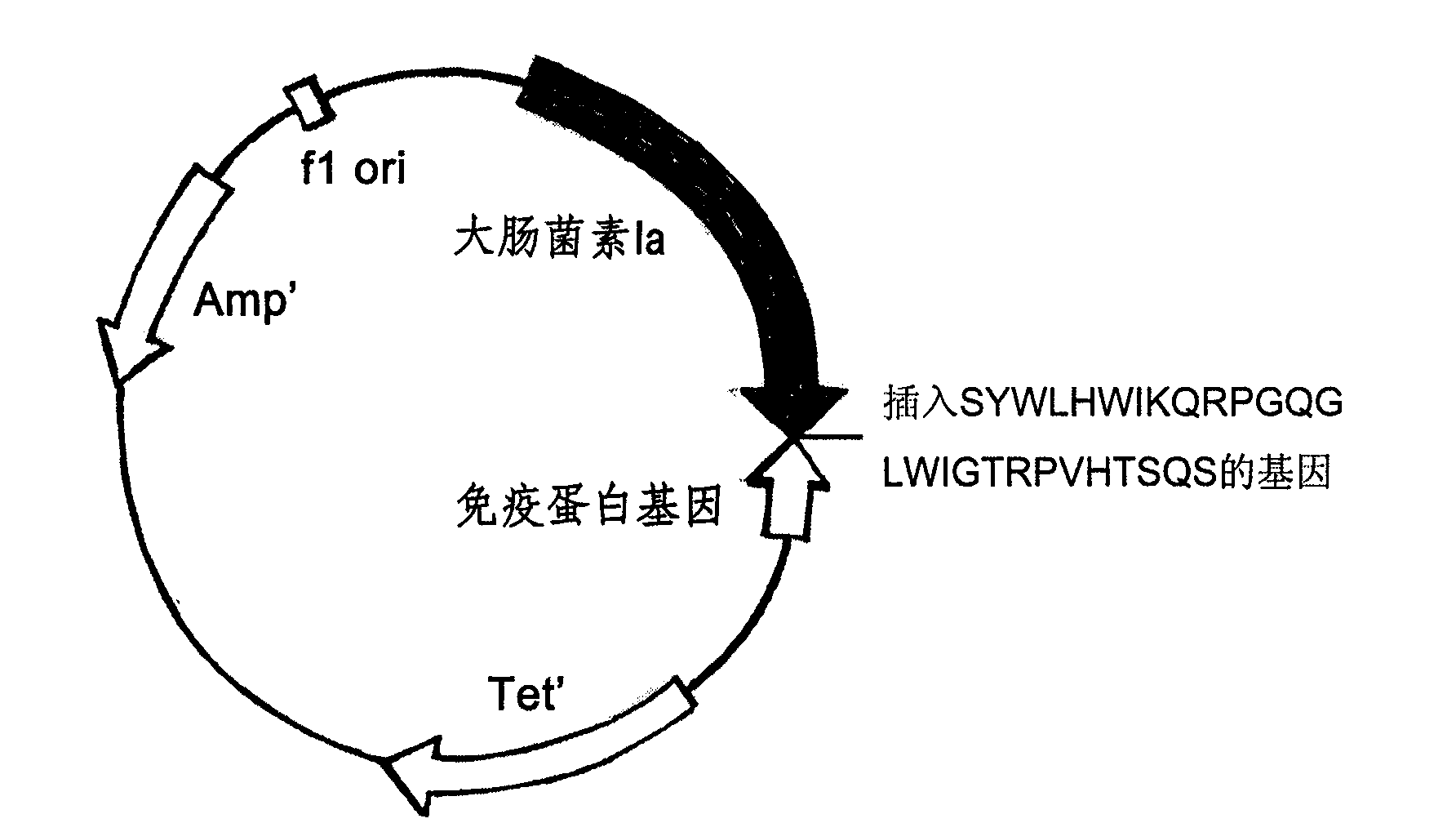
The invention relates to a new type antibiotic containing an antibody analog, a preparation method and an application method thereof, which belong to the field of biological medicaments. The new type antibiotic containing an antibody analog comprises colicin E1, Ia, Ib, A, B, N or a water pore canal domain thereof and an antibody analog, wherein the antibody analog is formed by connecting the carboxyl terminal of VHCDR1 of immunoglobulin with the amidogen terminal of VHFR2 and connecting the carboxyl terminal of VHFR2 with the amidogen terminal of VLCD; and porin is identified specifically by the immunoglobulin. The sterilization capability of the antibiotic is thousands of times of that of commonly used antibiotics. Because the action mechanism is special, pathogen is difficult to produce medicament resistant property; human normal cells are not harmed in the process of sterilization; and the new type antibiotic containing an antibody analog can be used for preparing medicaments of resisting meningococcus, vancocin enterococcus, methicillin staphylomycin or multi-medicament resistant cyanomycosis.
Owner:PHEROMONICIN BIOTECHNOLOGY LTD
Antibacterial agents comprising conjugates of glycopeptides and peptidic membrane associating elements
InactiveUS7078380B2Improve bindingHigh proportionAntibacterial agentsBiocideGlycopeptideAntibacterial activity
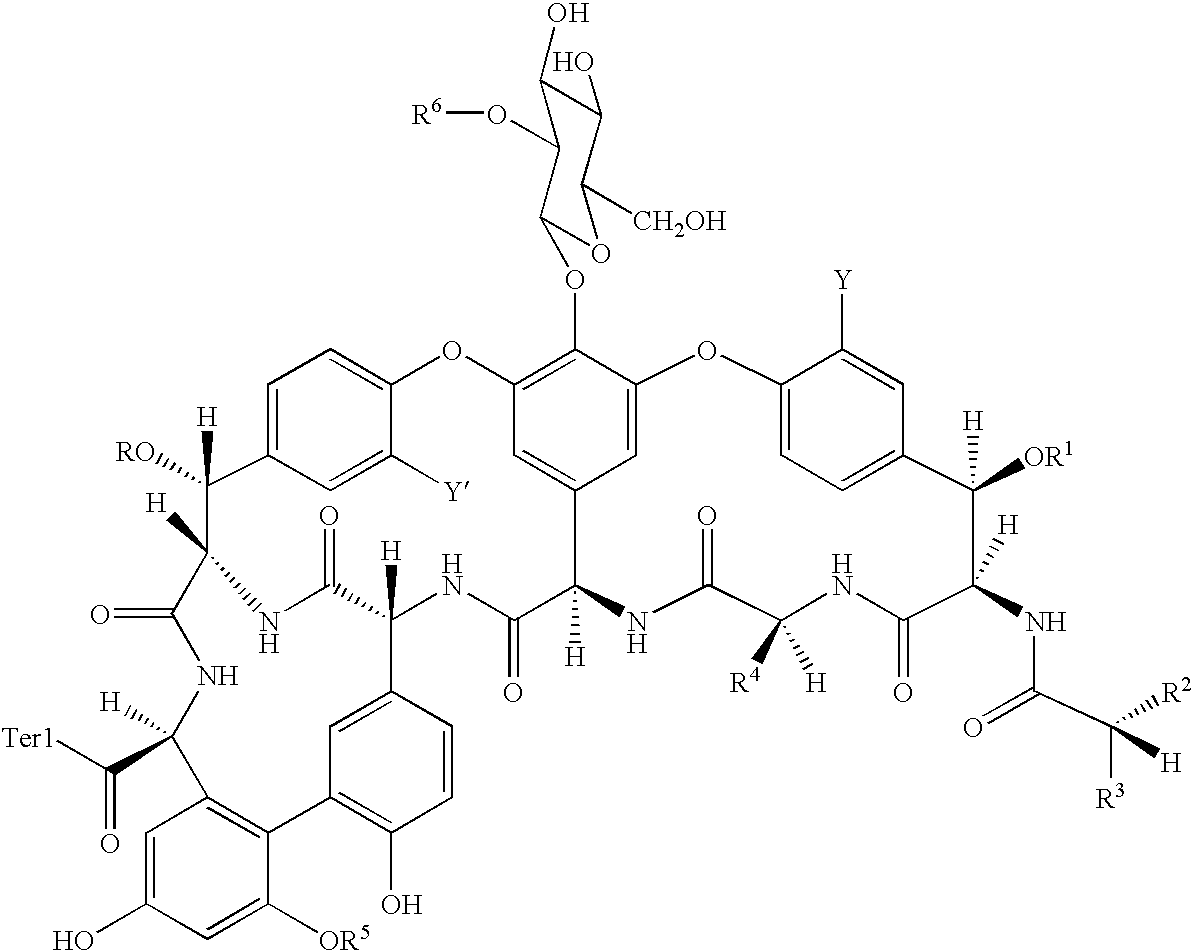
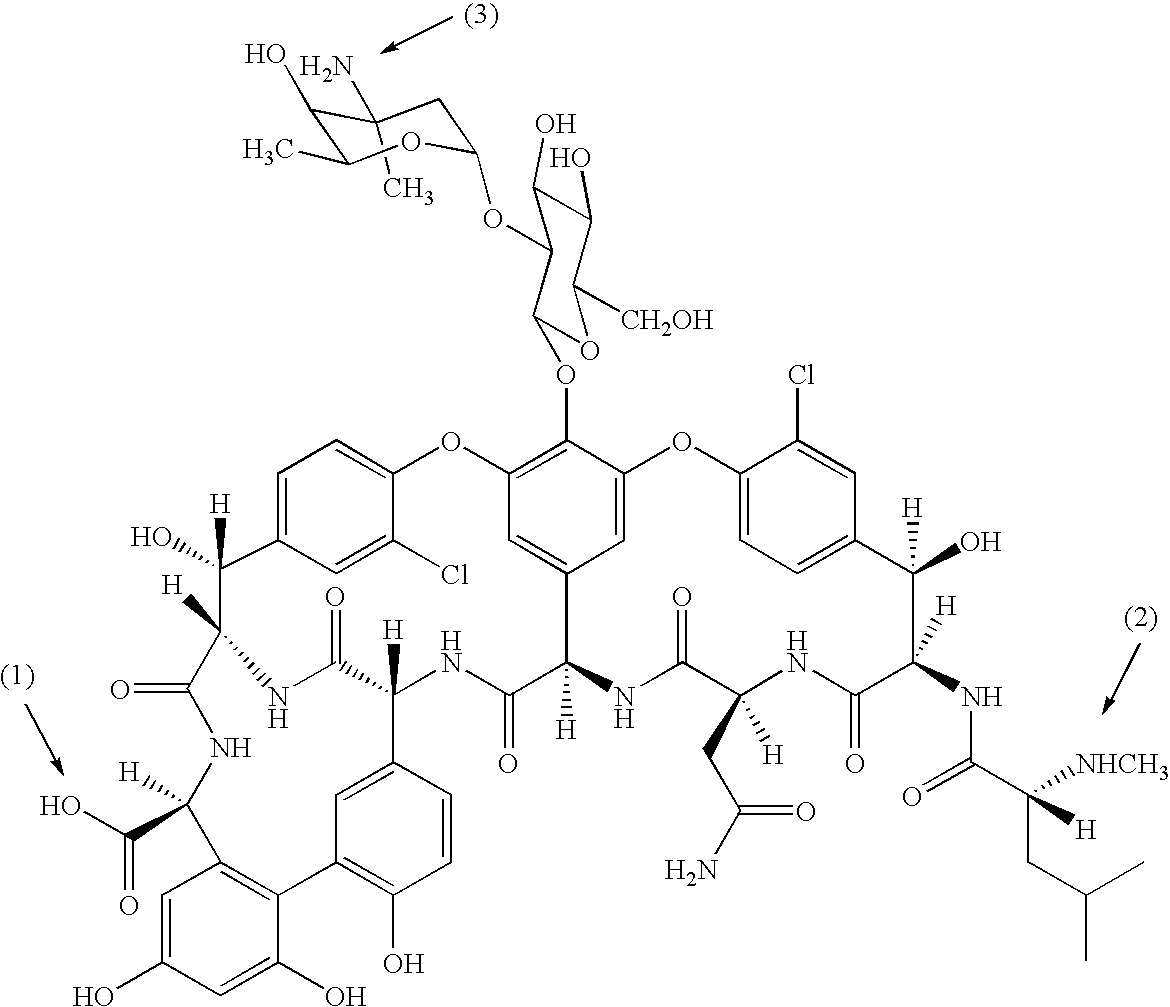
The invention concerns agents with anti-bacterial activity and methods and intermediates for their production. The present invention further concerns the use of such agents for the treatment of bacterial infections in animals, including man. The agents are derivatives of vancomycin-type antibiotics, of structure: V-L-W-X; wherein V is a glycopeptide moiety which inhibits peptidoglycan biosynthesis in bacteria; L is a linking group; W is a peptidic membrane-associating element such as an element based on naturally-occurring animal or bacterial peptide antibiotics; and X is hydrogen or a membrane-insertive element.
Owner:CAMBRIDGE ENTERPRISE LTD
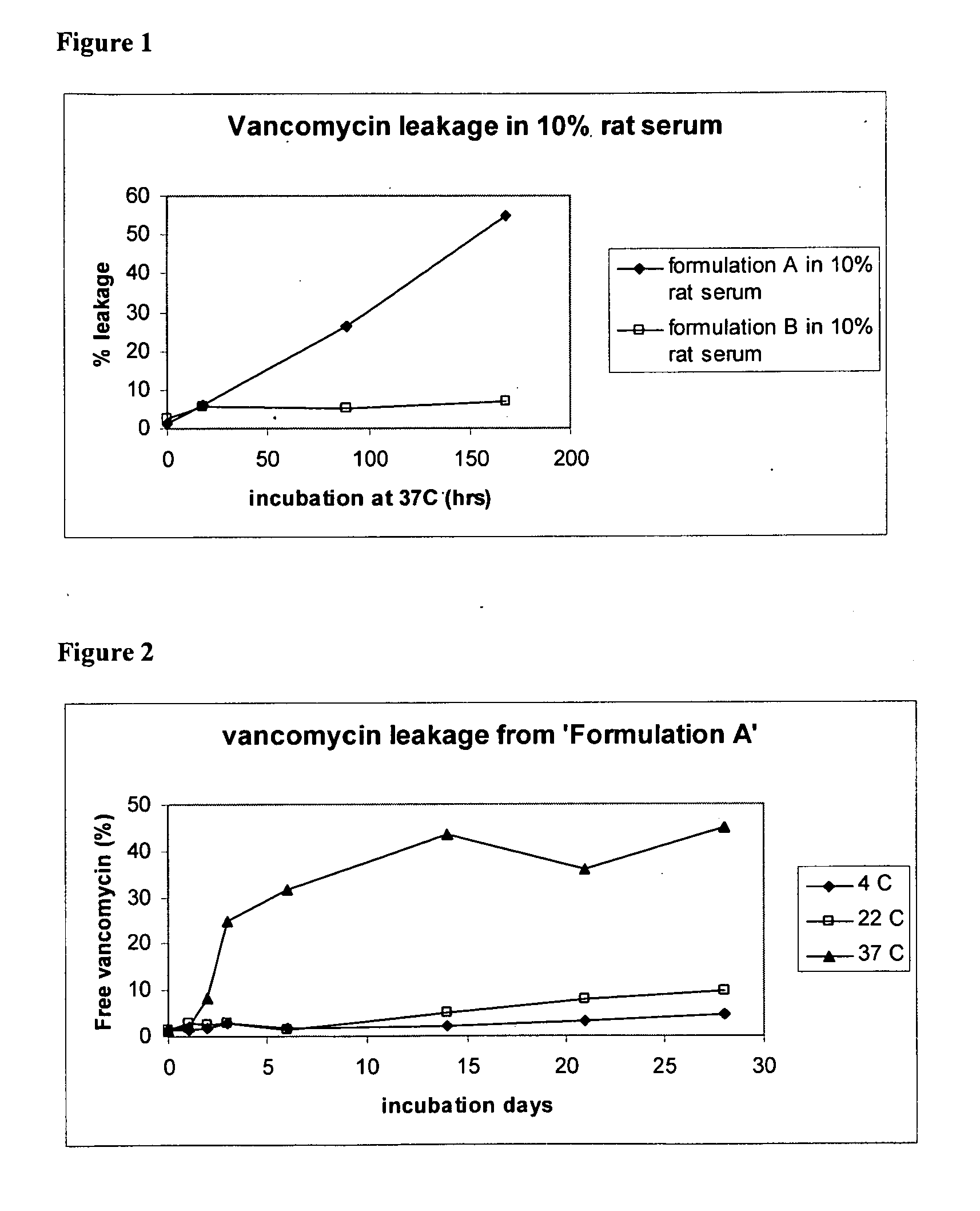
Liposomal Vancomycin Formulations
The present disclosure relates in part to liposomal vancomycin compositions having low lipid to drug ratios and high concentration of vancomycin. The present disclosure also relates in part to methods of making such compositions.
Owner:INSMED INC
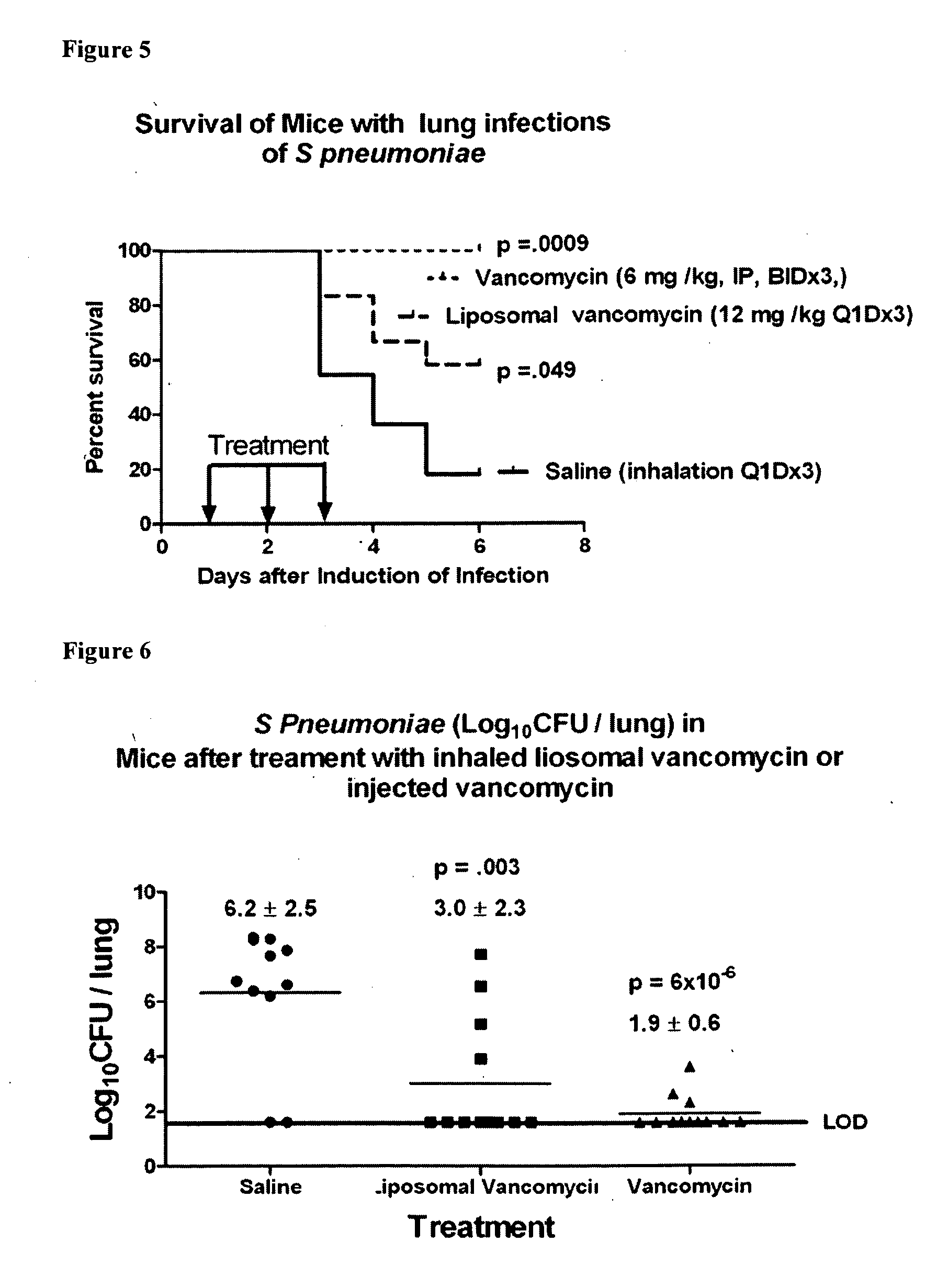
Methods of Treating Pulmonary Disorders using Liposomal Vancomycin Formulations
InactiveUS20090105126A1Reduce infectionShort timeAntibacterial agentsDispersion deliveryHigh concentrationMedicine
The present disclosure relates in part to methods of treating and prevention of pulmonary disorders in a subject in need thereof comprising administering to the subject a liposomal vancomycin compositions having low lipid to drug ratios and high concentration of vancomycin.
Owner:TRANSAVE
Formulations of vancomycin
ActiveUS20160101147A1Improve long-term stabilityAntibacterial agentsBiocidePolyethylene glycolVancomycin
Vancomycin-containing compositions substantially free of precipitation after at least about 12 months of storage at refrigerated or ambient conditions are disclosed. The compositions include vancomycin or a pharmaceutically acceptable salt thereof; a polar solvent including propylene glycol, polyethylene glycol and mixtures thereof; lactic acid, a lactate salt, or mixtures thereof; and optionally, a pH adjuster in an amount sufficient to maintain a pH of the compositions at from about 3 to about 8.
Owner:SCIDOSE PHARMA LLC
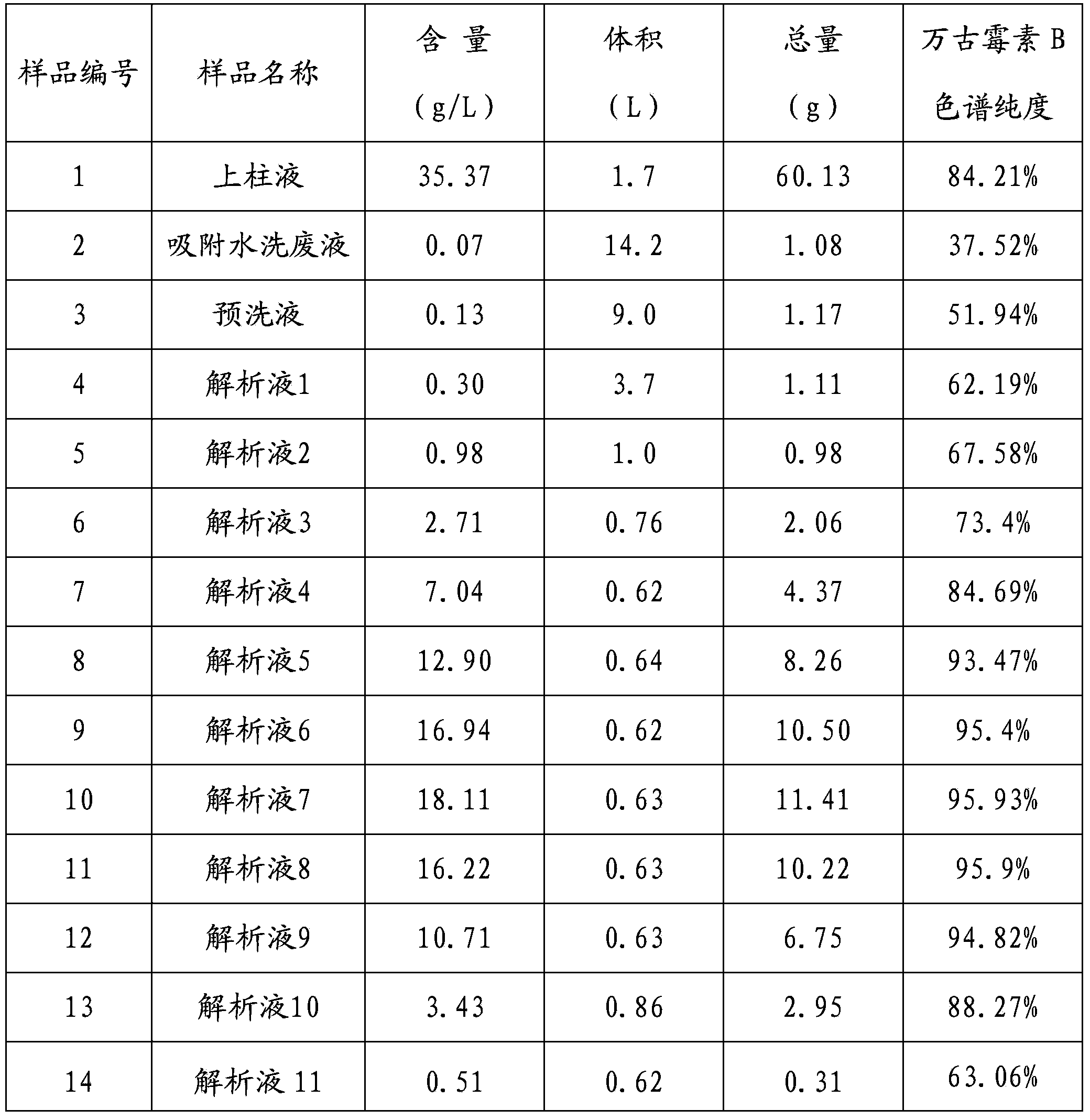
Separation and purification method of vancocin
The invention relates to a method for separating vancomycin. The method comprises the following steps: (1) carrying out the column chromatography elution on the vancomycin fermentation liquor in a hydrochloric acid aqueous solution mobile phase macropore absorptive resin chromatographic column containing alcohol so as to obtain vancomycin aqueous solution; (2) adding NH4HCO3 into the hydrochloricacid vancomycin aqueous solution to form precipitate, adjusting the pH value to between 7.5 and 8.5 through ammonia water, and stirring and standing the solution; and (3) obtaining a vancomycin crudeproduct with the content of vancomycin chromatographic purity not less than 80 percent through filtration separation and alcohol top washing. The method is simple and has high yield. The content of the vancomycin chromatographic purity is not less than 80 percent; and the titer of a vancomycin wet product at least reaches 500 mu g / mg.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
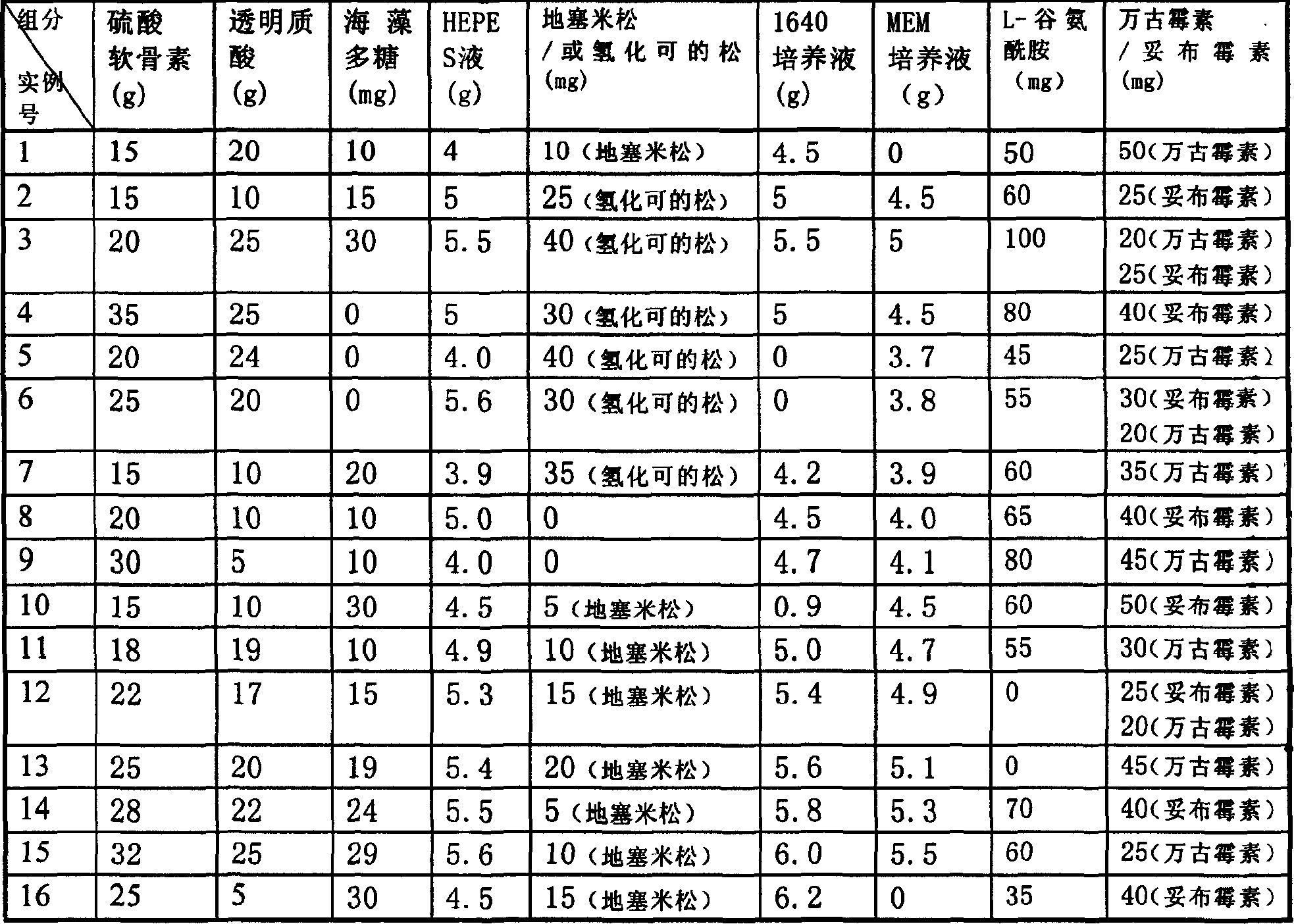
Cornea middle term preserving fluid
Disclosed is a cornea middle term preserving fluid which comprises RPMI 1640 culture liquid, chondroitin sulfate, hyaluronic acid, HEPES cushioning liquid, dexamethasone, low-molecular-weight seaweed polysaccharides, Tobramycin or vancomycin, the preserving fluid has stabilized cell activity during storage period, and higher endothelial cell metabolizing function can be maintained than the US's Opthiol.
Owner:SHENZHEN WATSIN GENETECH
VEGF and vancomycin-supported multilayer slow release microsphere preparation, and preparation method and application thereof
InactiveCN104147594ABiologically activeHas antibacterial propertiesSkeletal disorderSaccharide peptide ingredientsMicrosphereSide chain
The invention discloses a VEGF and vancomycin-supported multilayer local slow release microsphere preparation with sodium alginate and chitosan as a carrier, a preparation method of the microsphere preparation, and an application of the microsphere preparation in the preparation of medicines for treating bone defects, bone tissue regeneration and wound healing, and belongs to the technical field of medicine slow release microspheres. In the invention, a core sphere is prepared through an instillation process, and other multilayer microspheres are prepared according to a positive and negative charge attraction and layer-by-layer self assembling principle. Sodium alginate and chitosan are natural polymer polysaccharides, chitosan is a polycation polymer material, and the side chain structure of chitosan contains a large number of free amino groups; and sodium alginate is a polyanion material, the molecular side chain of sodium alginate contains a large number of carboxyl groups, sodium alginate and chitosan undergo a complexing reaction through attraction of positive and negative charges, and the core-shell multilayer slow release medicine multilayer sphere is formed by sequentially wrapping according to the layer-by-layer self assembling principle, so multilayer slow release microsphere preparation can promote local angiogenesis, improve blood circulation and control local infection.
Owner:JILIN UNIV
Spray Drying Vancomycin
ActiveUS20130009330A1Rapid productionReduce processing timeSaccharide peptide ingredientsCyclic peptide ingredientsVancomycinMannitol
A method and formulation for preparing spray dried vancomycin. In various embodiment, the formulation includes vancomycin HCl (10-20%) and one or more of the following PEG (0-5%), mannitol (0-5%), ethanol (0-10%), and a citrate buffer. Spray dried vancomycin has favorable reconstitution times and water content.
Owner:HOSPIRA
Stabilized vancomycin formulations
InactiveUS20150314002A1Antibacterial agentsOrganic active ingredientsPulmonary infectionLipid formation
In one aspect, the invention provides a stabilized lipid-based glycopeptide antibiotic composition and a process for producing the same. In another aspect, the invention provides methods for treating a bacterial pulmonary infection by administering to a subject in need thereof a therapeutically effective amount of the stabilized lipid-based glycopeptide antibiotic composition.
Owner:INSMED INC
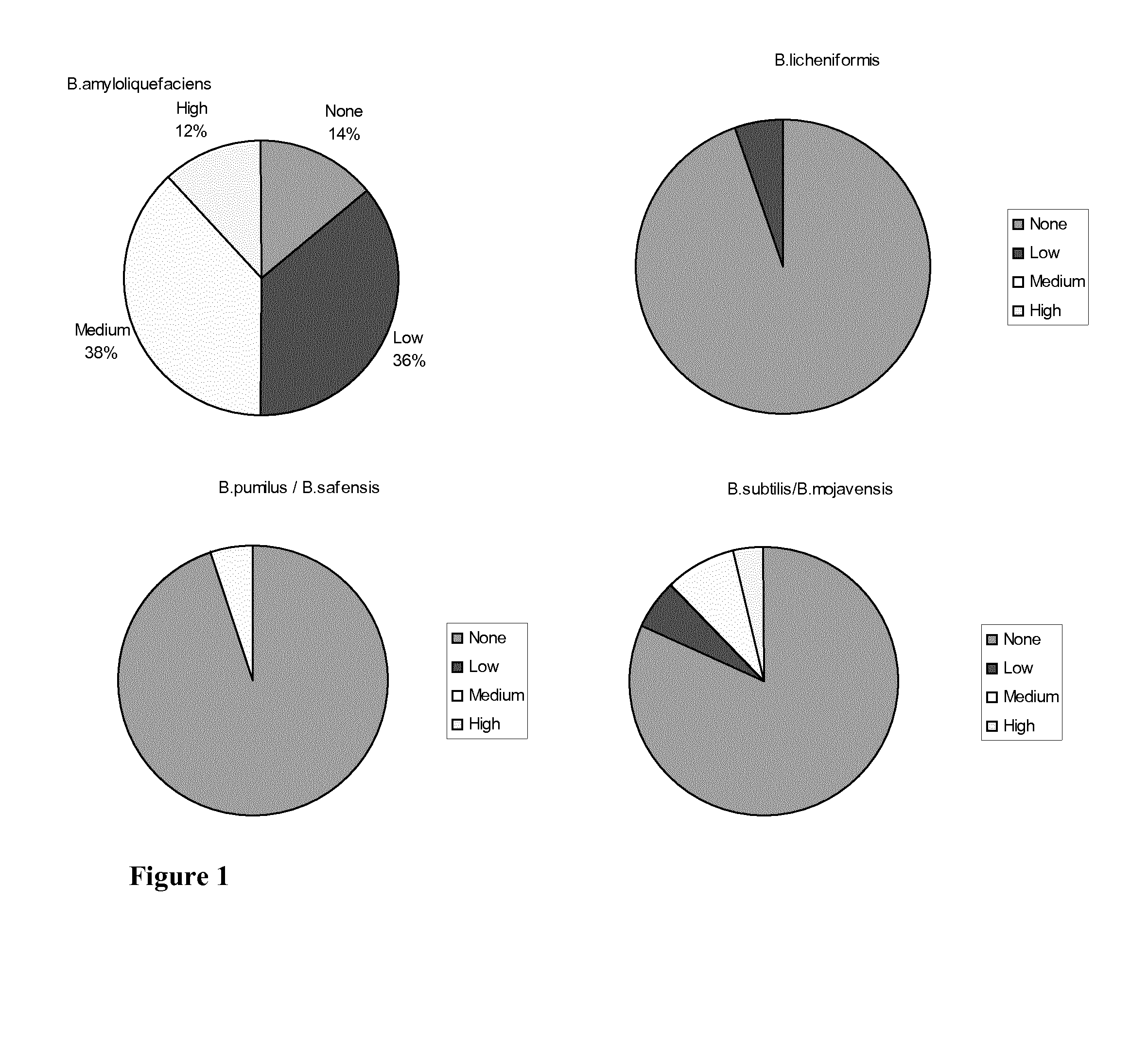
Antibiotic sensitive bacillus strains having antimicrobial effect against e. coli and clostridium perfringens and having high sporulation capacity
A Bacillus strain characterized by (i): sensitivity for ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol; (ii) antimicrobial activity against E. coli and Clostridium perfringens; and (iii) a sporulation percentage of at least 80 when measured after 2 days of incubation. The invention further relates to a method for selecting such strains. Many of the identified strains according to the invention are of the species Bacillus amyloliquefaciens. Some of the Bacillus amyloliquefaciens were further identified as Bacillus amyloliquefaciens subsp. amyloliquefaciens whereas others were identified as amyloliquefaciens subsp. plantarum. A Bacillus strain of the invention may be used as a feed additive to animal feed where it has a probiotic effect.
Owner:CHR HANSEN AS
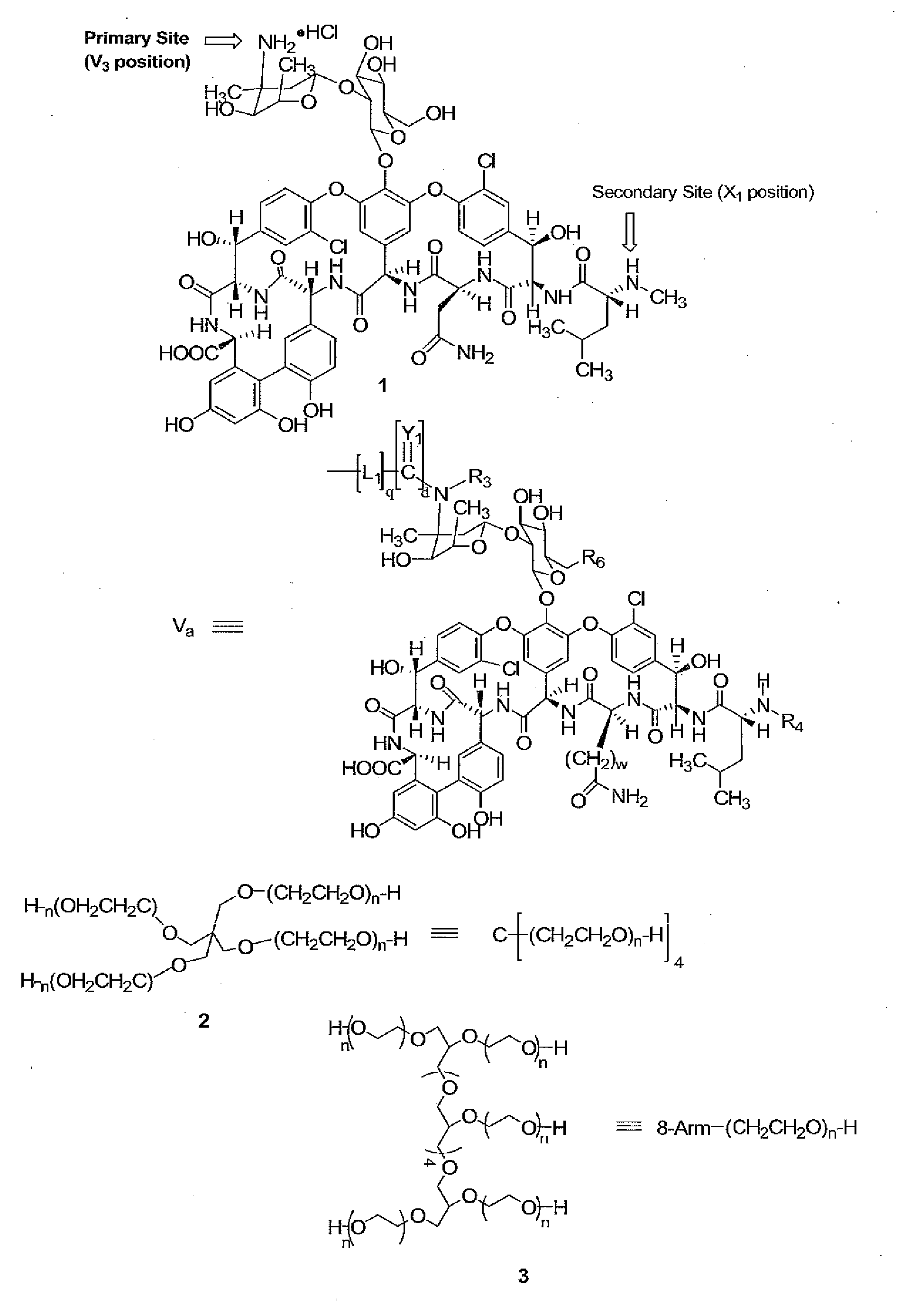
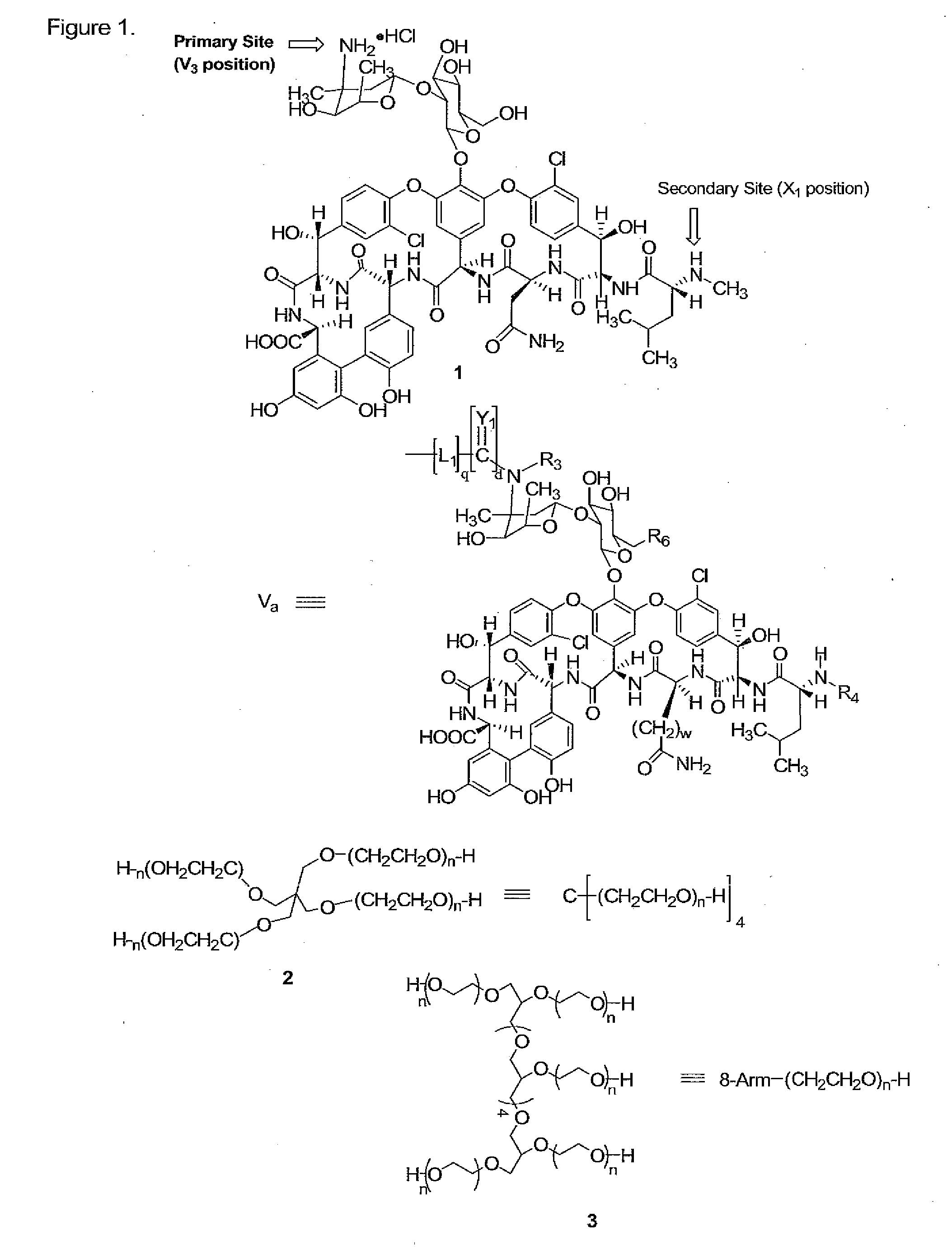
Prodrugs of vancomycin with hydrolysis resistant polymer linkages
InactiveUS20090221471A1Improve cycle lifeAvoid Bacterial ResistanceAntibacterial agentsMicrobiological testing/measurementVancomycinSugar
Vancomycin-polymer conjugates are disclosed. In preferred aspects, polymer residues which are hydrolysis resistant in vitro, are selectively attached to the sugar amino and / or N-methyl amino groups of vancomycin and related compounds. Vancomycin-polymer conjugates made by the methods and methods of treatment using the conjugates are also disclosed.
Owner:ENZON PHARM INC
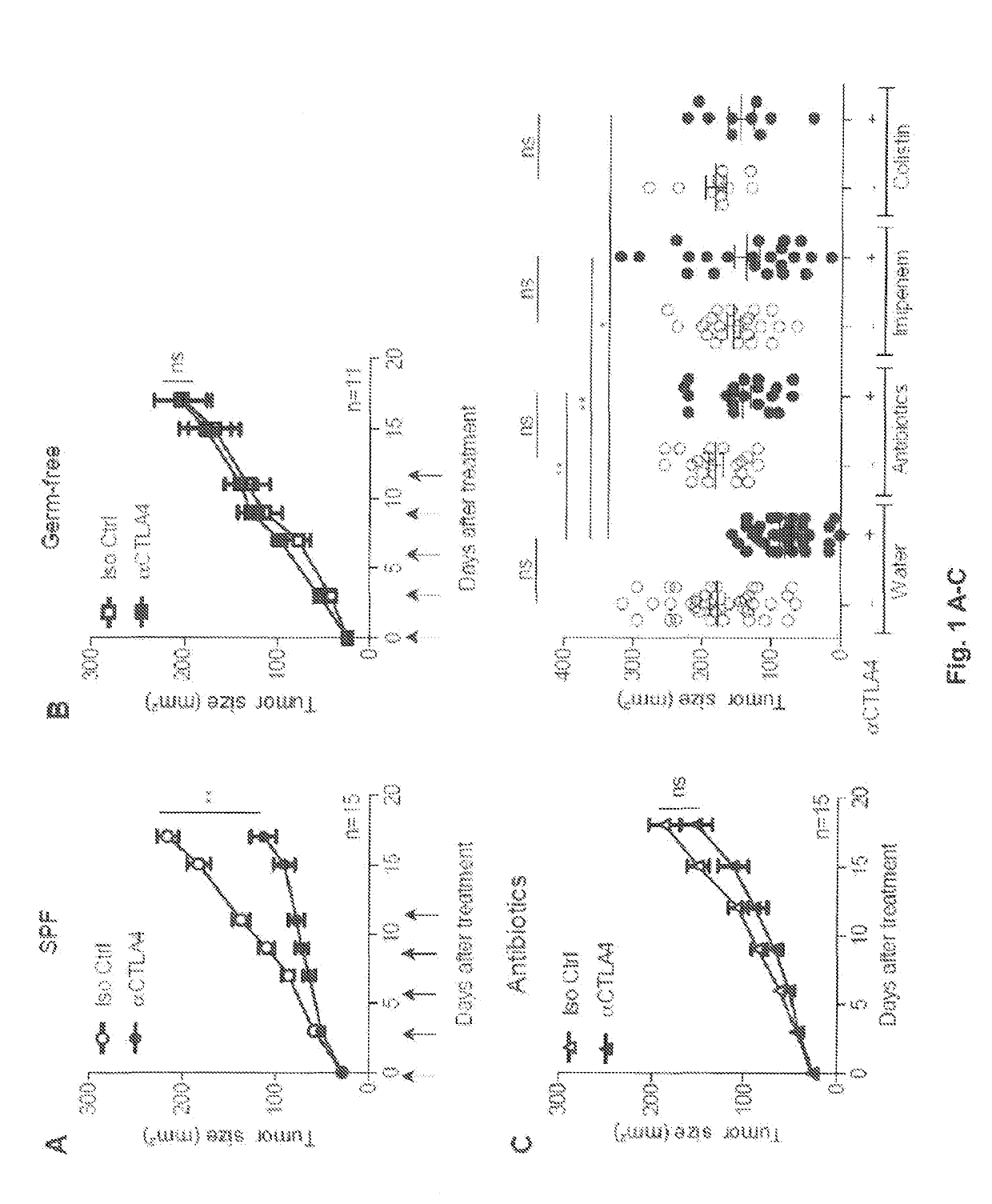
Methods and products for modulating microbiota composition for improving the efficacy of a cancer treatment with an immune checkpoint blocker
PendingUS20190282632A1Pharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsFecesIntestinal microorganisms
The present invention relates to the role of the microbiota in the efficacy of cancer treatments with a drug blocking an. immune checkpoint and provides methods and probiotics to improve the efficacy of such a treatment in patients in need thereof. More particularly, the invention pertains to the use of vancomycin or penicillin to modulate the gut microbiota to potentiate the anticancer effects of anti-CTLA4 molecules. B. fragilis or fecal microbial transplantation of a defined composition enriched in immunogenic Bacteroides spp. can also be used as a probiotic to that aim.
Owner:INSTITUT GUSTAVE ROUSSY +3
Azaphilones compounds in marine fungus HK1-6 and application of azaphilones compounds as MRSA-resistant drug
ActiveCN108101878AAntibacterial agentsOrganic active ingredientsResistant bacteriaSecondary metabolite
The invention relates to azaphilones compounds in marine fungus HK1-6 and an application of the azaphilones compounds as an MRSA-resistant drug, in particular to secondary metabolites 1-4 produced bymarine fungus Penicillium sp.HK1-6 or pharmaceutically acceptable salt. The compounds 1-4 show very high antibacterial activity for MRSA (such as S. aureus ATCC4330 and S. aureus ATCC33591) and VER (such as E. faecalis ATCC51299), MIC is smaller than or equal to 12.5 mu g / mL, the MIC of a compound 2 for E. faecalis ATCC51299 is one times higher than that of positive drug vancomycin, and the compounds have good prospects to be developed into drugs resisting drug-resistant bacteria, lead compounds of drugs and candidate drugs.
Owner:YANGZHOU UNIV
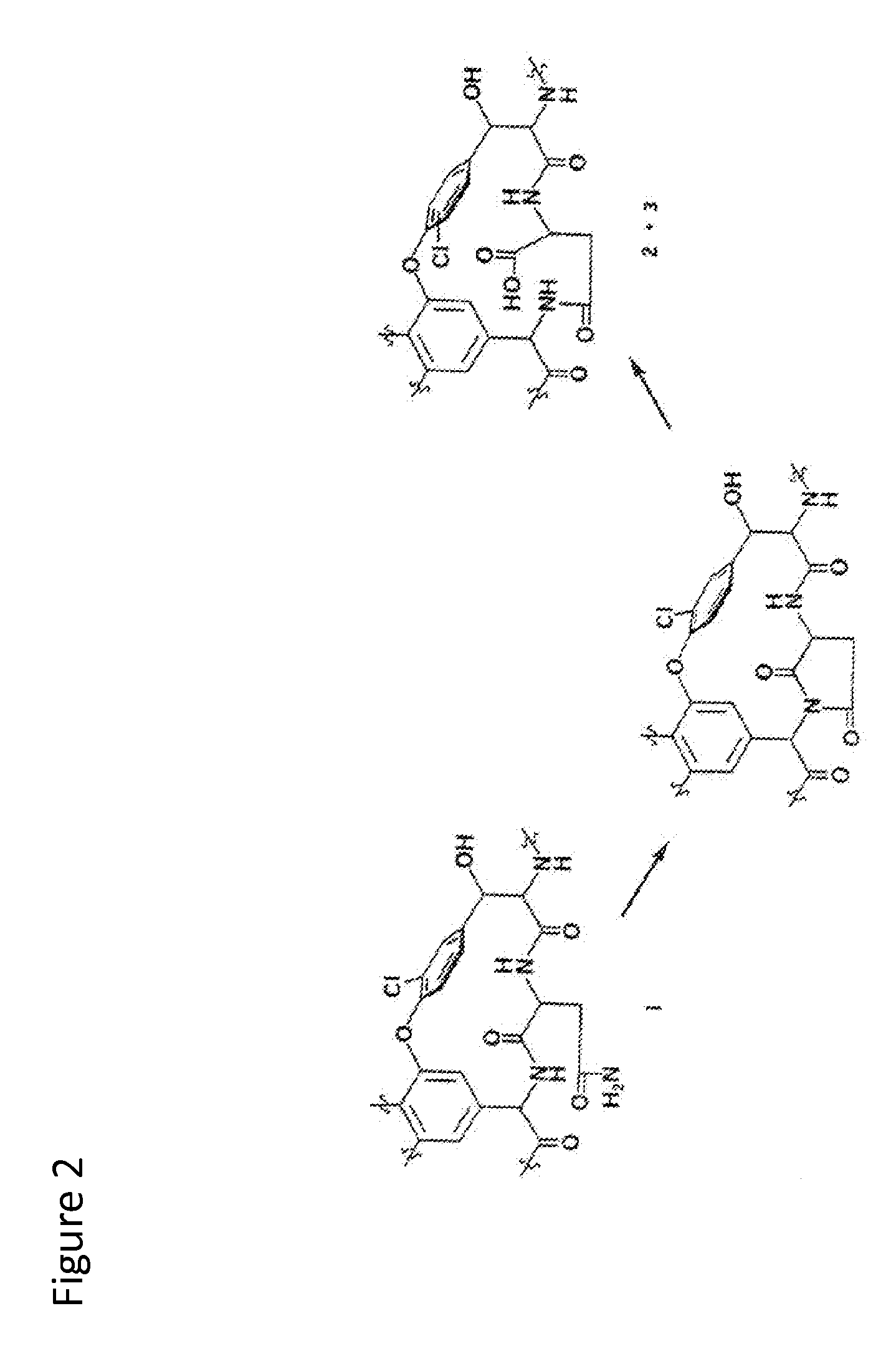
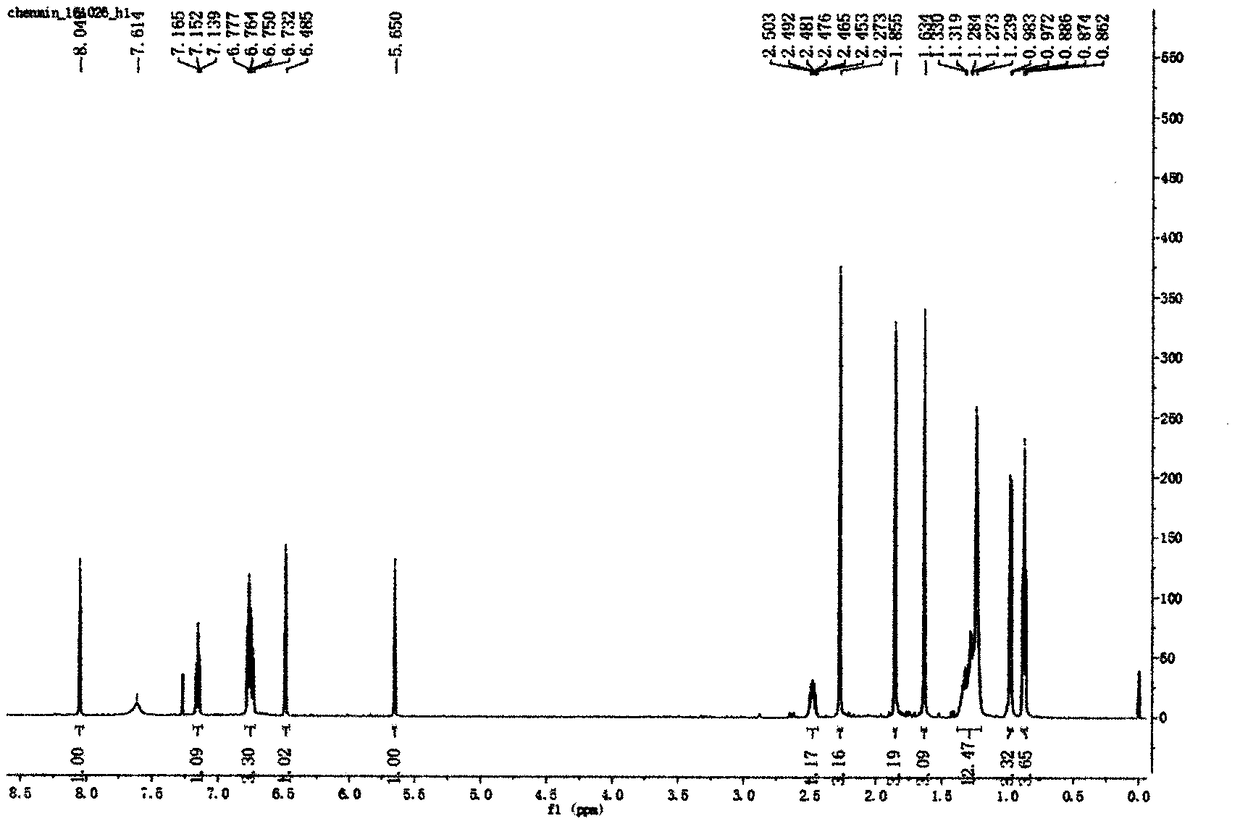
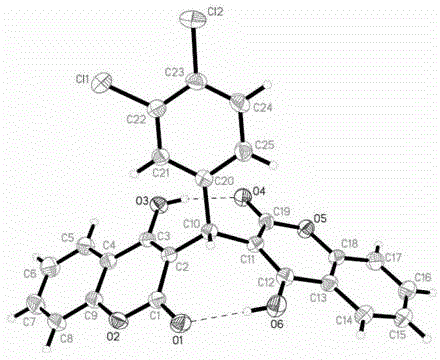
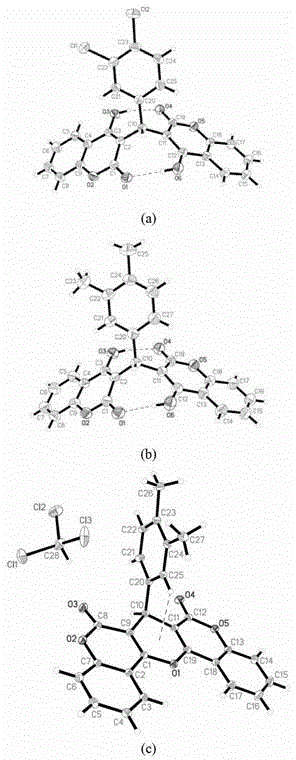
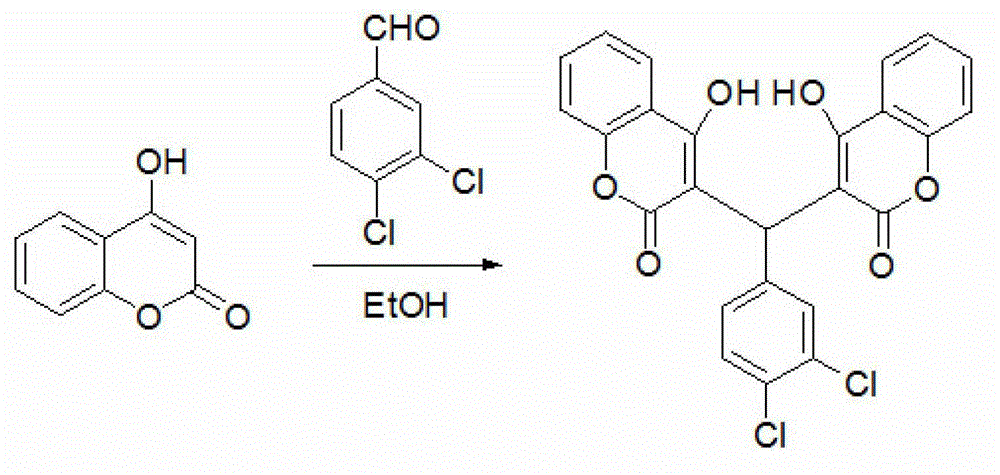
3,3'-(3,4-dichlorobenzylidene)-bis-4-hydroxycoumarin and application thereof in preparation of medicine for resisting multi-drug resistant bacteria
ActiveCN103333148AHas antibacterial application valueLow inhibitory concentrationAntibacterial agentsOrganic chemistryChemical structureCrystal structure
The invention discloses a novel compound 3,3'-(3,4-dichlorobenzylidene)-bis-4-hydroxycoumarin with a strong antibacterial effect which is optimized by transforming the structure of bishydroxycoumarin compounds, and provides a preparation method thereof, wherein the chemical structure and crystal structure are obtained. The novel compound 3,3'-(3,4-dichlorobenzylidene)-bis-4-hydroxycoumarin has an antibacterial application value in preparation of a medicine resisting staphylococcus aureus (ATCC29213), methicillin-resistant staphylococcus aureus (MRSA, XJ7502), USA300 (LAC) and vancomycin-resistant staphylococcus aureus (Mu20ATCC700699).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method for anti-infectious nano collagen/ calcium phosphate bone repair material
The invention discloses a preparation method for an anti-infectious nano collagen / calcium phosphate bone repair material, and belongs to the field of biomedical materials. The method comprises the following steps of: slowly dripping solution containing calcium ions and aqueous solution containing phosphate radical ions into acid-soluble collagen solution, dripping NaOH solution to regulate the pH value, performing freeze drying, grinding to obtain dry powder for later use, dissolving poly-L-lactic acid in 1,4-dioxane, stirring for a certain time, mixing the solution and the dry powder uniformly, finally adding vancomycin, stirring uniformly, removing the solvent by adopting a freeze drying method, and preparing the anti-infectious nano collagen / calcium phosphate bone repair material. The prepared bone repair material has excellent biocompatibility, infection resistance and a micro-structure similar to human body.
Owner:TSINGHUA UNIV +1
Porous bone replacing material and preparation method thereof
The invention provides an antibiotic controlled freed porous bone replacing material and a preparation method thereof. The antibiotic controlled freed porous bone replacing material is characterized by consisting of polycaprolactone, nano tricalcium phosphate, chitosan / vancomycin microsphere with sodium alginate coating, and tobramycin. The preparation method comprises the following steps: dissolving the chitosan in glacial acetic acid solution, dissolving the vancomycin in the glacial acetic acid solution, spraying and drying to prepare the chitosan / vancomycin microsphere; moving the microsphere into the sodium alginate solution; dissolving the microsphere and the nano tricalcium phosphate powder in dichloromethane solution of the polycaprolactone, adding a pore-forming agent, stirring uniformly, extruding and forming, putting the formed material in deionized water after the dichloromethane is volatilized, dissolving out the pore-forming agent, and obtaining the porous material; and immersing the porous material into tobramycin sulfate solution, and drying. The invention has the advantages that the vancomycin and the tobramycin can be released simultaneously in the early stage. The two antibiotics can kill the intractable bacteria of methicillin-resistant Staphylococcus aureus and the like, which are common clinically and infected chronically by bones.
Owner:花沐医疗科技(上海)有限公司
Biofunctional magnetic nanoparticles for pathogen detection
InactiveUS7754444B2High affinityBig ratioNanotechMicrobiological testing/measurementMagnetite NanoparticlesBiology
This invention provides a method of detecting pathogens comprising the steps of: (a) contacting a sufficient amount of biofunctional magnetic nanoparticles with an appropriate sample for an appropriate period of time to permit the formation of complexes between the pathogens in the sample and the nanoparticles; (b) using a magnetic field to aggregate said complexes; and (c) detecting said complexes. The method may further comprise the additional step of removing said complexes. The biofunctional magnetic nanoparticles are preferably a conjugate of vancomycin and FePt. The pathogens may be bacteria or viruses, and the sample may be a solid, liquid, or gas. Detection may involve conventional fluorescence assay, enzyme-linked immunosorbent assay (ELISA), optical microscope, electron microscope, or a combination thereof. The sensitivity of detection for the method is at least as low as 10 colony forming units (cfu) of the pathogens in one milliliter of solution within one hour.
Owner:THE HONG KONG UNIV OF SCI & TECH +1
Novel treatment process of antibiotic wastewater electron beams
InactiveCN106348381ADamage structureLow biological toxicityWater/sewage treatment by irradiationWater/sewage treatment by flocculation/precipitationBiological valueWastewater
The invention discloses a novel treatment process of antibiotic wastewater electron beams. The process is characterized by comprising the following steps of: irradiating electron beams, taking an antibiotic wastewater (500-1000mL) sample, adding 0.5%o-2%o of irradiating synergistic agent, mixing uniformly, conveying by using a tray, and irradiating by using a transmission system under the beam of an electron accelcrator; (2) carrying out coagulating sedimentation, adding 0.2%o - 0.4%o of flocculant into an irradiated water sample, quickly stirring for reacting, adding 0.4%o -0.7%o of coagulant aid, slowly stirring to form alumen ustum, standing for settling for 30 -60min, loading supernate by using a sterile bottle, conserving at temperature of 5oC -8oC, and conveying to the detection place to carry out detection analysis on the concentration of vancomycin and biological value; and (3) carrying out result analysis in which biological value is 0.5%, removal rate is 98.7%, vancomycin in mother solution is lower than a detection limit, and the biotoxicity of wastewater is obviously reduced.
Owner:CGN DASHENG ELECTRON ACCELERATOR TECH
Stabilized vancomycin formulations
InactiveUS10124066B2Antibacterial agentsOrganic active ingredientsLipid formationPulmonary infection
In one aspect, the invention provides a stabilized lipid-based glycopeptide antibiotic composition and a process for producing the same. In another aspect, the invention provides methods for treating a bacterial pulmonary infection by administering to a subject in need thereof a therapeutically effective amount of the stabilized lipid-based glycopeptide antibiotic composition.
Owner:INSMED INC
Anti-infection mineralized collagen and calcium sulfate bone repair material and preparation method thereof
The invention belongs to the technical field of biomedical materials, and discloses an anti-infection mineralized collagen and calcium sulfate bone repair material and a preparation method thereof. The material is composite powder comprising 2-5wt% of mineralized collagen, 5-15wt% of vancomycin, 20-25wt% of calcium sulfate dihydrate and the balance alpha-type calcium sulfate hemihydrate. The alpha-type calcium sulfate hemihydrate is prepared by a hydrothermal method, the mineralized collagen is prepared by a biological mineralization method, and the alpha-type calcium sulfate hemihydrate, the mineralized collagen, the vancomycin and the coagulant calcium sulfate dihydrate are mixed to prepare the anti-infection mineralized collagen and calcium sulfate bone repair material with a certain component ratio. When the liquid / solid ratio is 0.5-0.8mL / g, the material has a fine self-setting performance, is degradable, slowly releases medicines, and is anti-infection. Besides, the material has fine biocompatibility and can promote adhesion and spreading of osteoblasts.
Owner:TSINGHUA UNIV +1
Drug-loading sustained-release support composite body for treating infectious bone defect
InactiveCN108671269AAchieve the purpose of infection controlFor the purpose of infection controlTissue regenerationProsthesisMicrosphereBone formation
The invention relates to a drug-loading sustained-release support composite body for treating infectious bone defect. The drug-loading sustained-release support composite body is prepared by the stepthat vancomycin is encapsulated by polylactic acid-glycolic acid copolymer to prepare sustained release microspheres and loaded to a beta-tricalcium phosphate support. The vancomycin, with broad-spectrum antibacterial capacity, is encapsulated by the polylactic acid-glycolic acid copolymer to prepare into sustained microspheres, and loaded to the beta-tricalcium phosphate support to prepare a novel drug-loading sustained-release anti-infection bone substitute material which can slowly release the vancomycin at a part of the infectious bone defect. When being applied to treating the infectiousbone defect, the material can release the encapsulated vancomycin in an all-dimensional mode for a long time in a partial three-dimensional space of the infectious bone defect after being embedded; thus, the purpose of controlling infection within the focus of infection is achieved; meanwhile, the effects of filling bone defect, promoting bone formation and quickening bone reconstruction are achieved.
Owner:SHANGHAI INST OF TECH +1
Parenteral Combination Therapy For Infective Conditions With Drug Resistant Bacterium
InactiveUS20080188403A1Antibacterial agentsOrganic active ingredientsBacteroidesHospitalized patients
The invention describes a pharmaceutical composition to combat multiple-drug-resistant bacteria in non-ocular infective conditions. Compositions comprising glycopeptides, in particular vancomycin, and cephalosporins, in particular ceftriaxone, are disclosed. Such compositions are found to be useful for parenteral administration for hospitalized patients with serious infections. Specifically, this invention also discloses a pharmaceutical composition further including an excipient such as CVMC agent and is available in dry powder form for reconstitution before injection with a suitable solvent. The pharmaceutical compositions of this invention have been found normally to enhance resistance to precipitation in solutions to be administered parenterally. The invention also gives details of the dosage forms stored in sealed containers to be reconstituted before use. The invention further provides a process to manufacture these compositions and also a method of treating a subject having non-ocular infective conditions due to multi drug resistant bacterium.
Owner:VENUS REMEDIES LTD
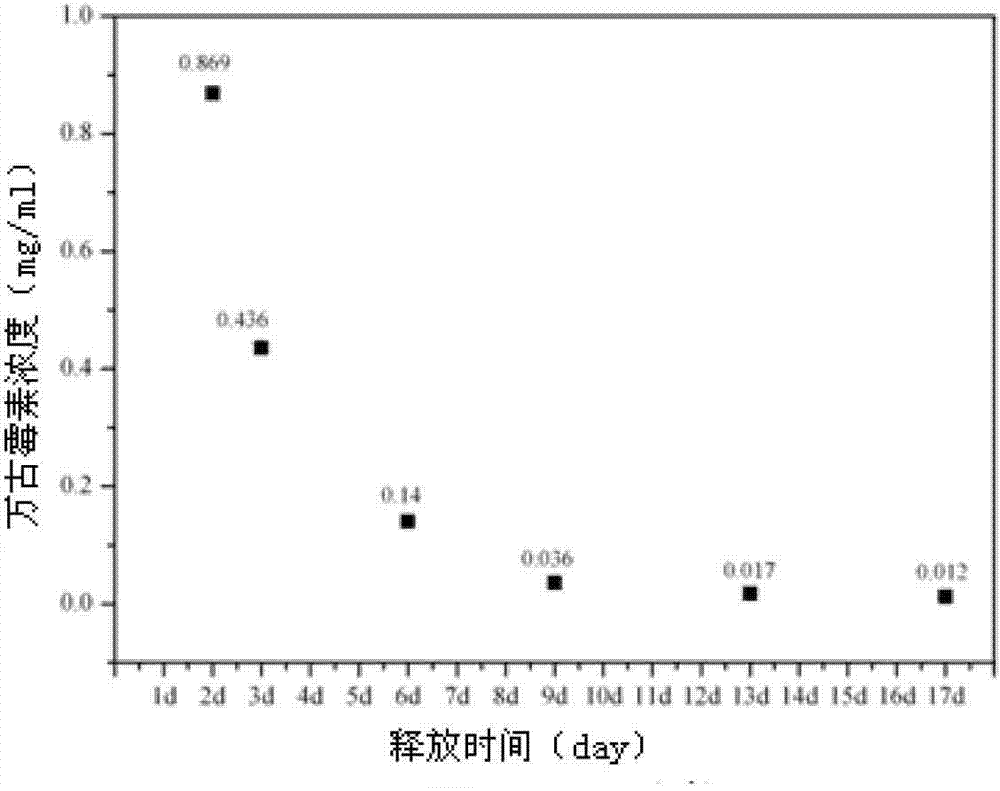
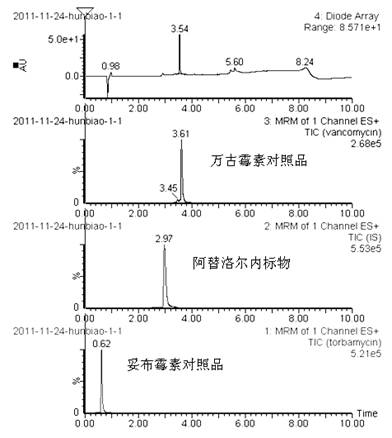
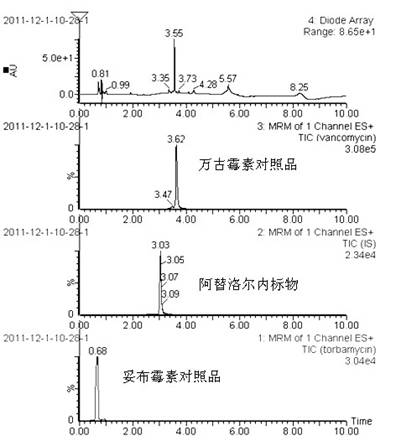
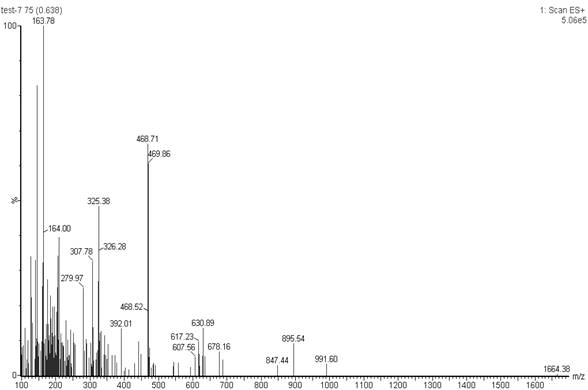
Method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling technique
The invention relates to the field of drugs, in particular to a detection method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid. A method for simultaneously determining content of vancomycin and tobramycin in tissue drainage liquid through an ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry (UPLC-TQD) coupling techniquecomprises the following steps of: 1) preparing reference samples; 2) preparing internal standard solution; 3) preparing sample solution; 4) preparing mobile-phase solution; 5) setting chromatographicconditions; 6) optimizing mass spectrometric conditions; 7) determining the samples; 8) preparing gradient-concentration reference sample solution; 9) preparing a standard curve; and 10) analyzing and computing data. The method has the characteristics that: 1) the method is advanced; 2) the method is rapid and high-efficiency the loss is low; and 3) the clinical application prospect and the effect are good.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Phospholipid depot
ActiveUS20120046220A1Low viscosityPrevent unintended introductionAntibacterial agentsBiocideActive agentVancomycin
The present invention provides a clear depot comprising at least one hydrophilic water-soluble pharmaceutically active agent selected from the group consisting of vancomycin, gentamicin, a pharmaceutically acceptable salt thereof and a mixture thereof, water, a phospholipid, an oil, optionally a pH adjusting agent, and a viscosity modifying agent selected from the group consisting of ethanol, isopropanol, and a mixture thereof, wherein the water present in the depot is no more than about 4 wt % relative to the total weight of the depot and the depot has a pH of between about 3 and about 6, method of making and administering same.
Owner:DR REDDYS LAB SA
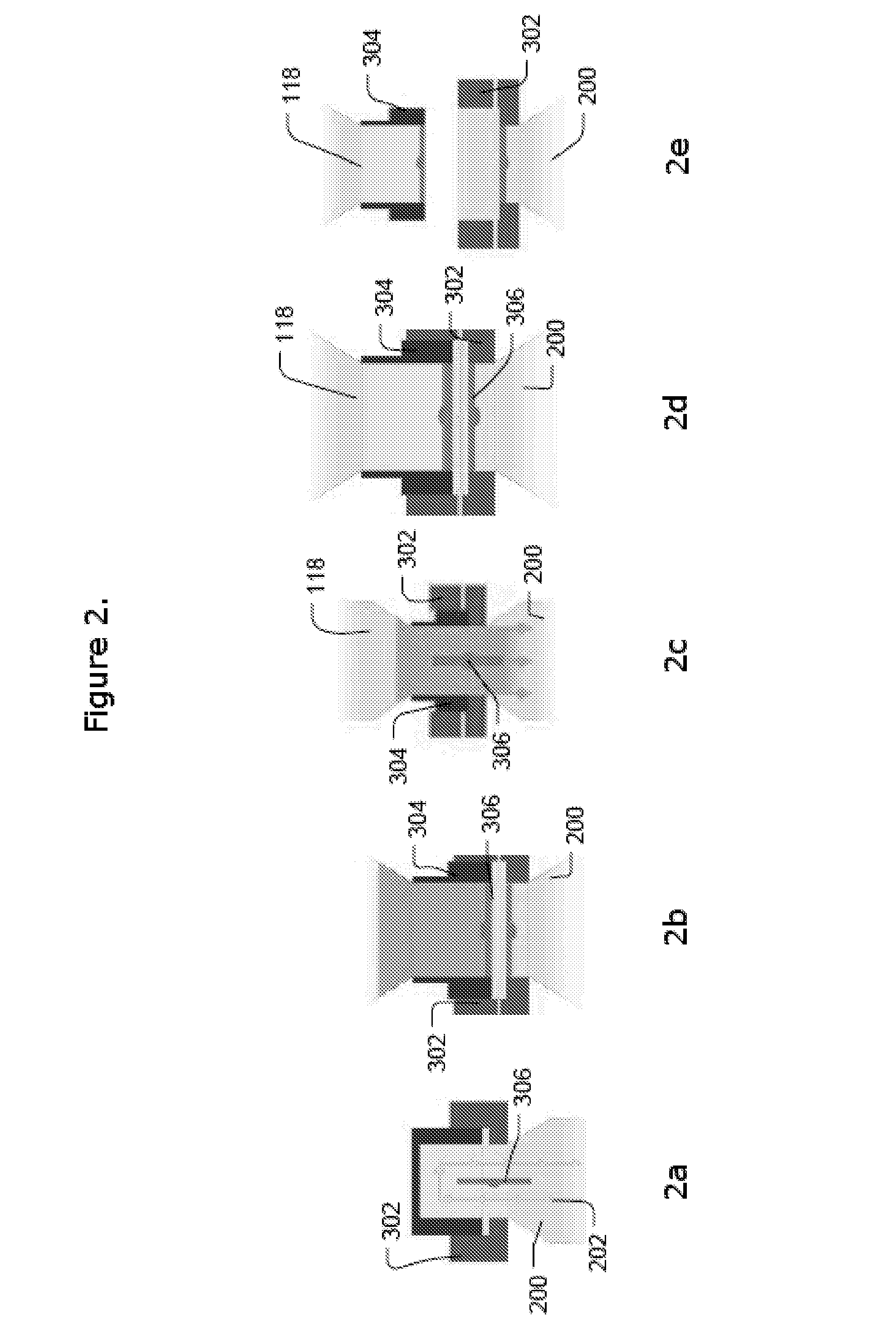
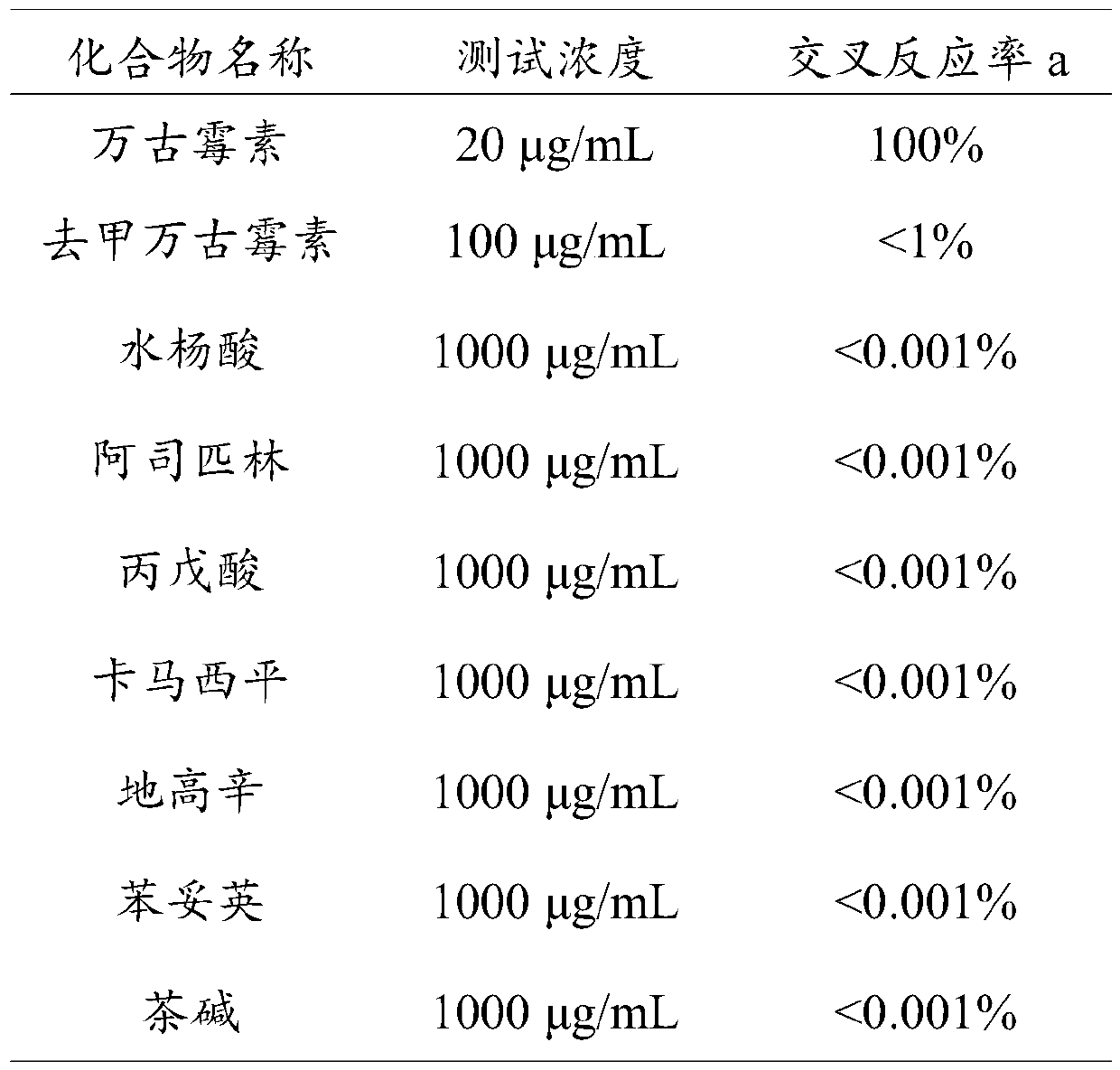
Time-resolved fluorescence immunochromatographic test strip for detecting vancomycin and preparation method and application thereof
InactiveCN109813693AStrong specificityHigh potencySerum albuminBiological testingLap jointBovine serum albumin
The invention provides a time-resolved fluorescence immunochromatographic test strip for detecting vancomycin and a preparation method and application thereof, and belongs to the technical field of detection of drug concentration in in-vitro diagnostic reagents. The test strip comprises a bottom plate, a sample absorption pad, a fluorescent microsphere pad, a nitrocellulose membrane and a water absorption pad, wherein the sample absorption pad, the fluorescent microsphere pad, the nitrocellulose membrane and the water absorption pad are in sequential lap joint and adhere to the bottom plate; the nitrocellulose membrane is coated with vancomycin-carrier protein conjugate; a vancomycin monoclonal antibody with a fluorescent microsphere marker is sprayed on the fluorescent microsphere pad; the vancomycin monoclonal antibody is prepared with vancomycin-bovine serum albumin conjugate as an immunogen. The test strip is free of a cross reaction with various vancomycin analogues, and has the advantages of being high in detection accuracy and high in specificity.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
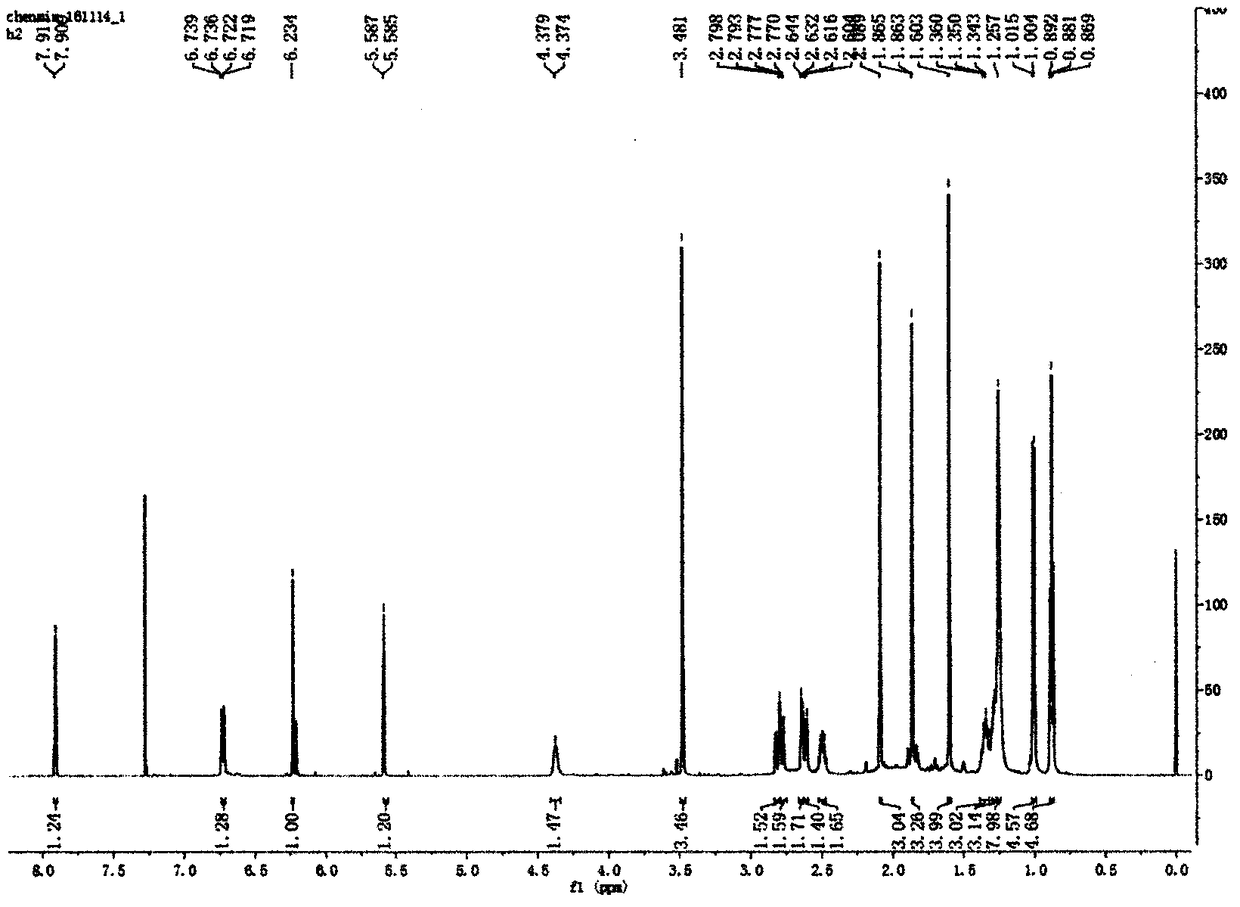
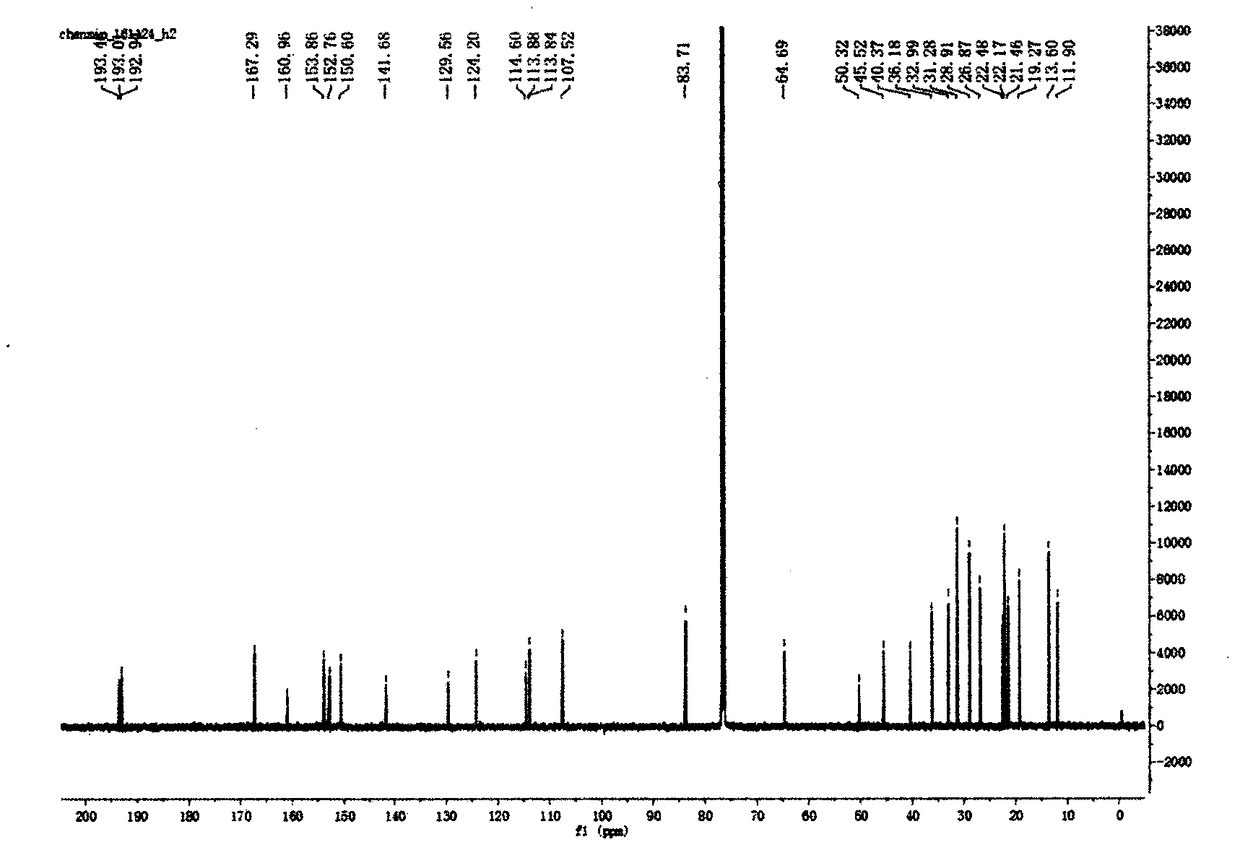
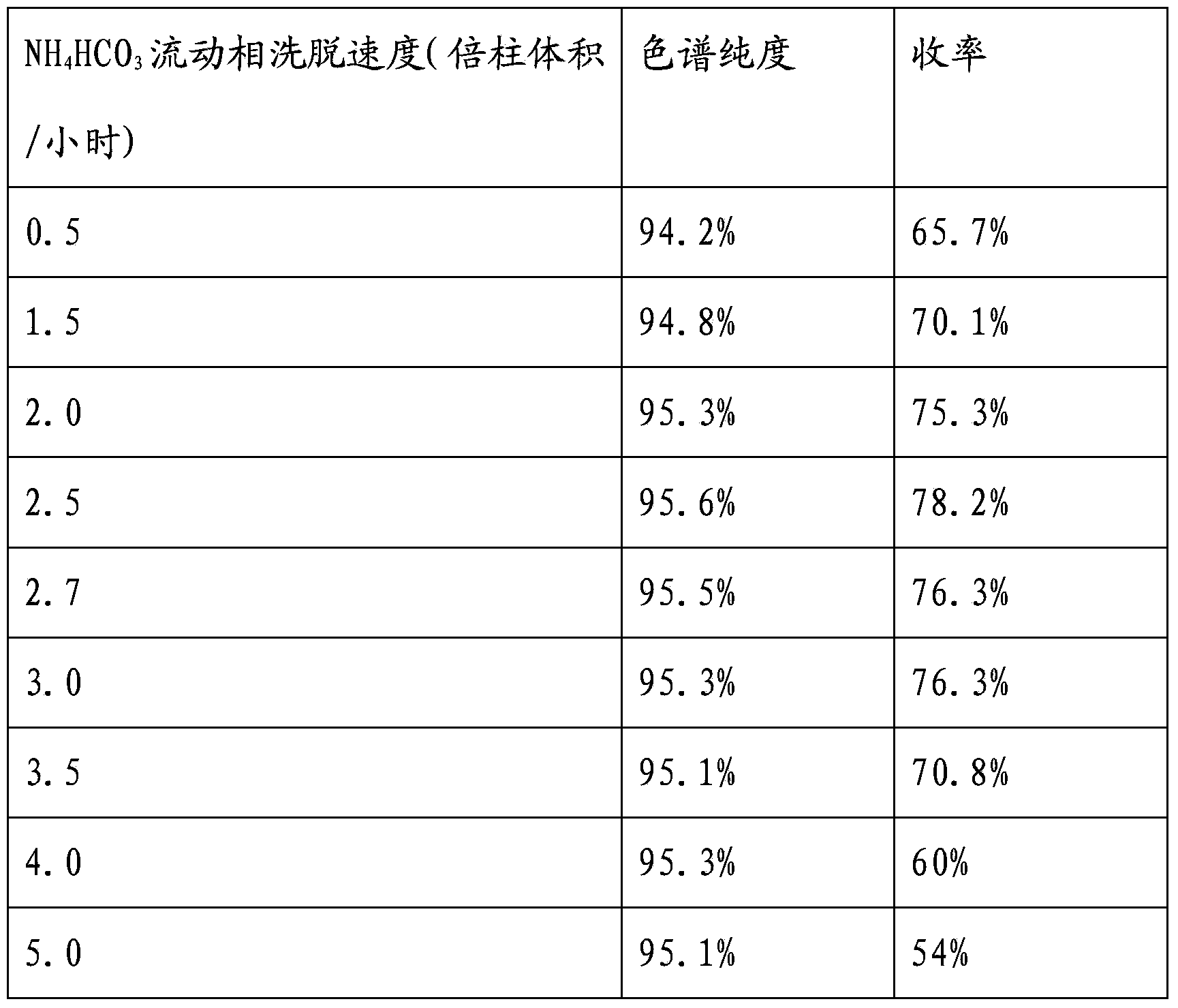
Preparation method of vancomycin with high purity
ActiveCN103408639ASmall granularityLow swelling ratePeptide preparation methodsChromatography liquidVancomycin
The invention belongs to the field of antibiotic preparation technologies, and more specifically relates to a preparation method of vancomycin with high purity. The preparation method comprises following steps: a destaining solution of vancomycin is subjected to column chromatography in a chromatography media, wherein the chromatography media is UniPMM50CAR, the NH4HCO3 mass concentration of a mobile phase of column chromatography is 0.2 to 0.7%; when absorbance rises to 40 at a detection wavelength of 280nm, chromatography liquids are collected segment by segment, the pH value of the chromatography liquids is adjusted to 3.0 to 3.5, the chromatography liquids are preserved under 4 DEG C, and the collected chromatography liquids are mixed. Bubbles in the chromatographic column caused by degradation of carbonate are not likely to generate by using the preparation method. The preparation method is capable of increasing stage number and separation efficiency. The appearance of the obtained vancomycin is improved significantly, purity is as high as 99%, and the vancomycin can be taken orally or by injection.
Owner:LIVZON GROUP FUZHOU FUXING PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com