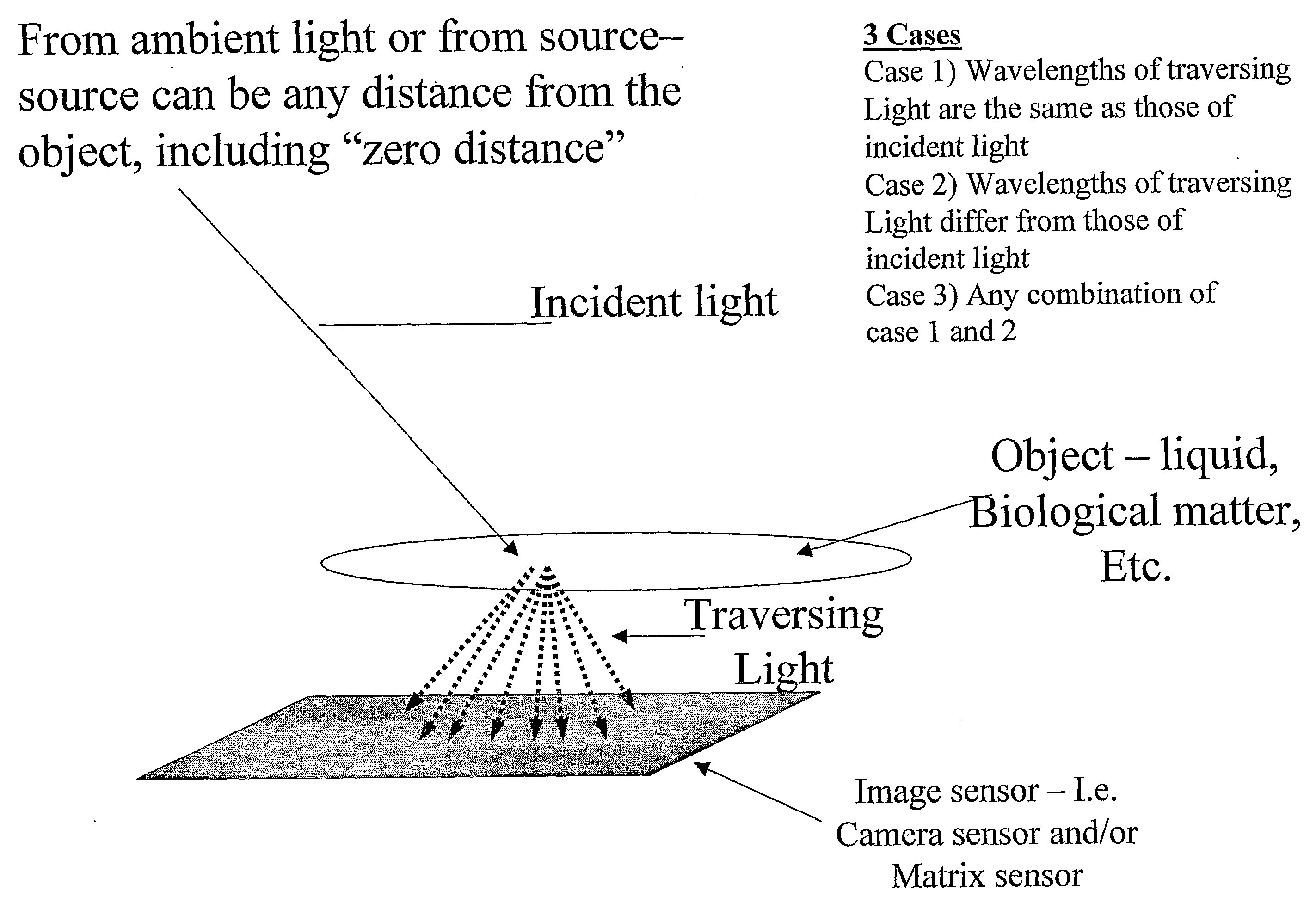
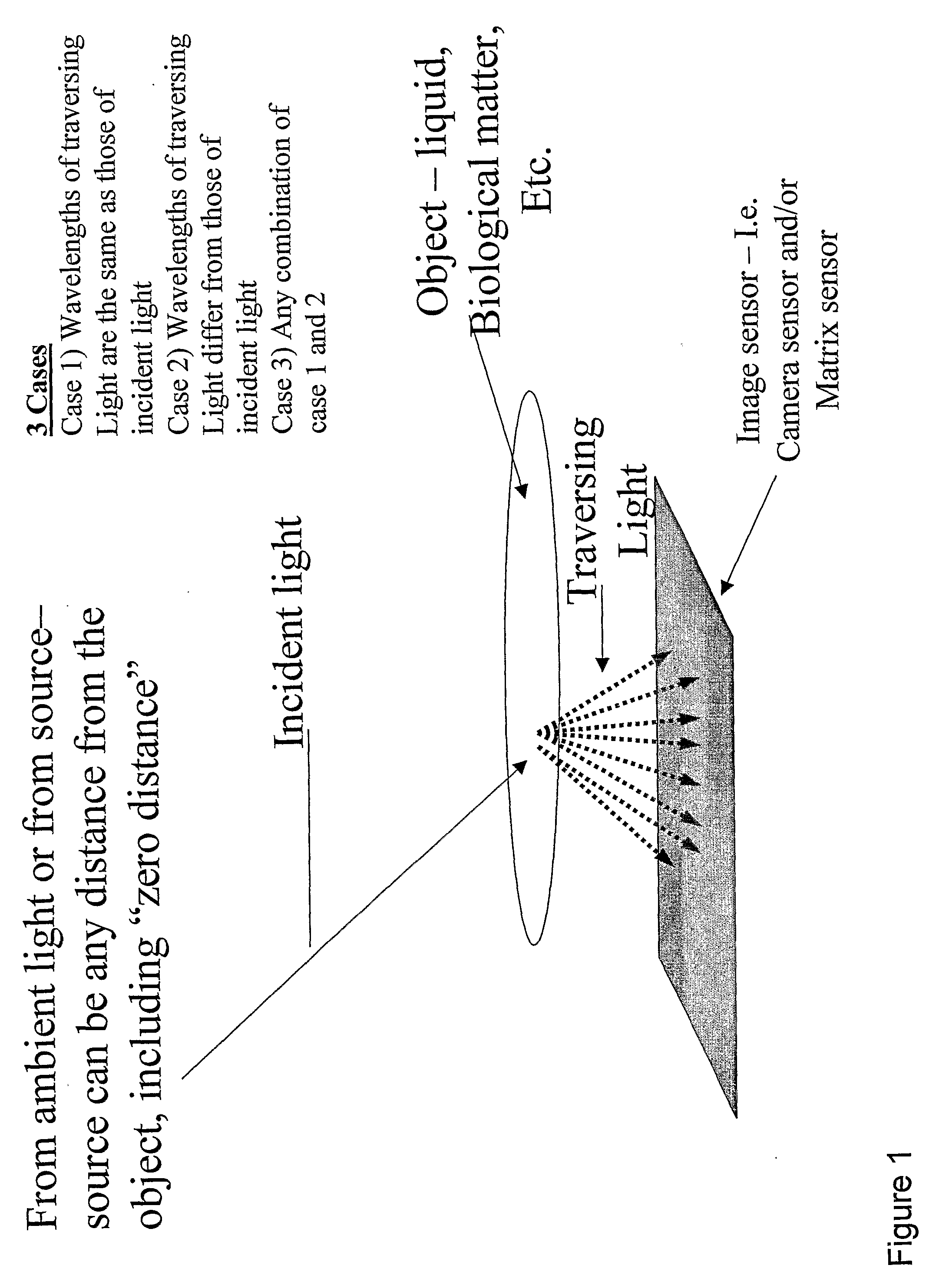
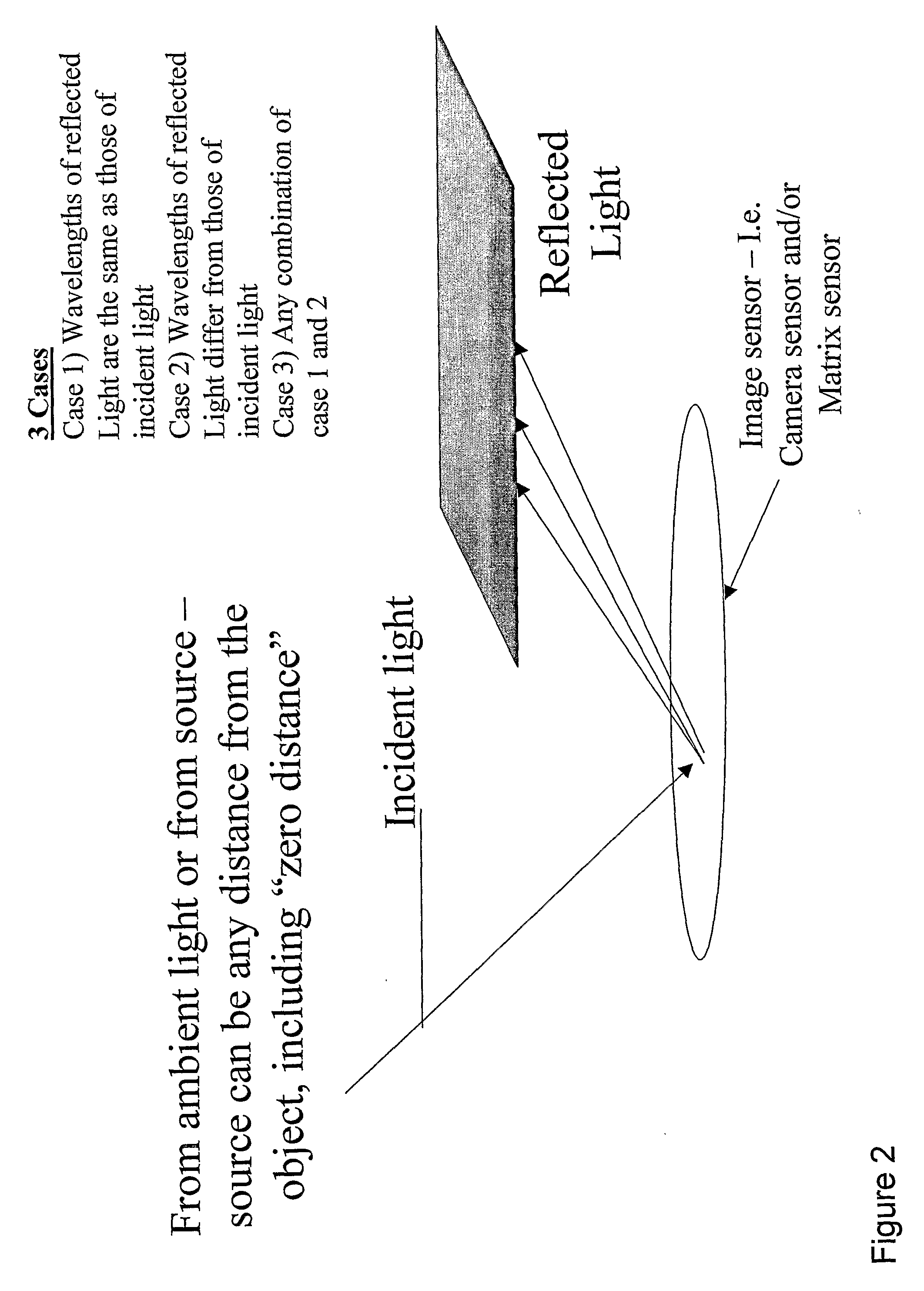
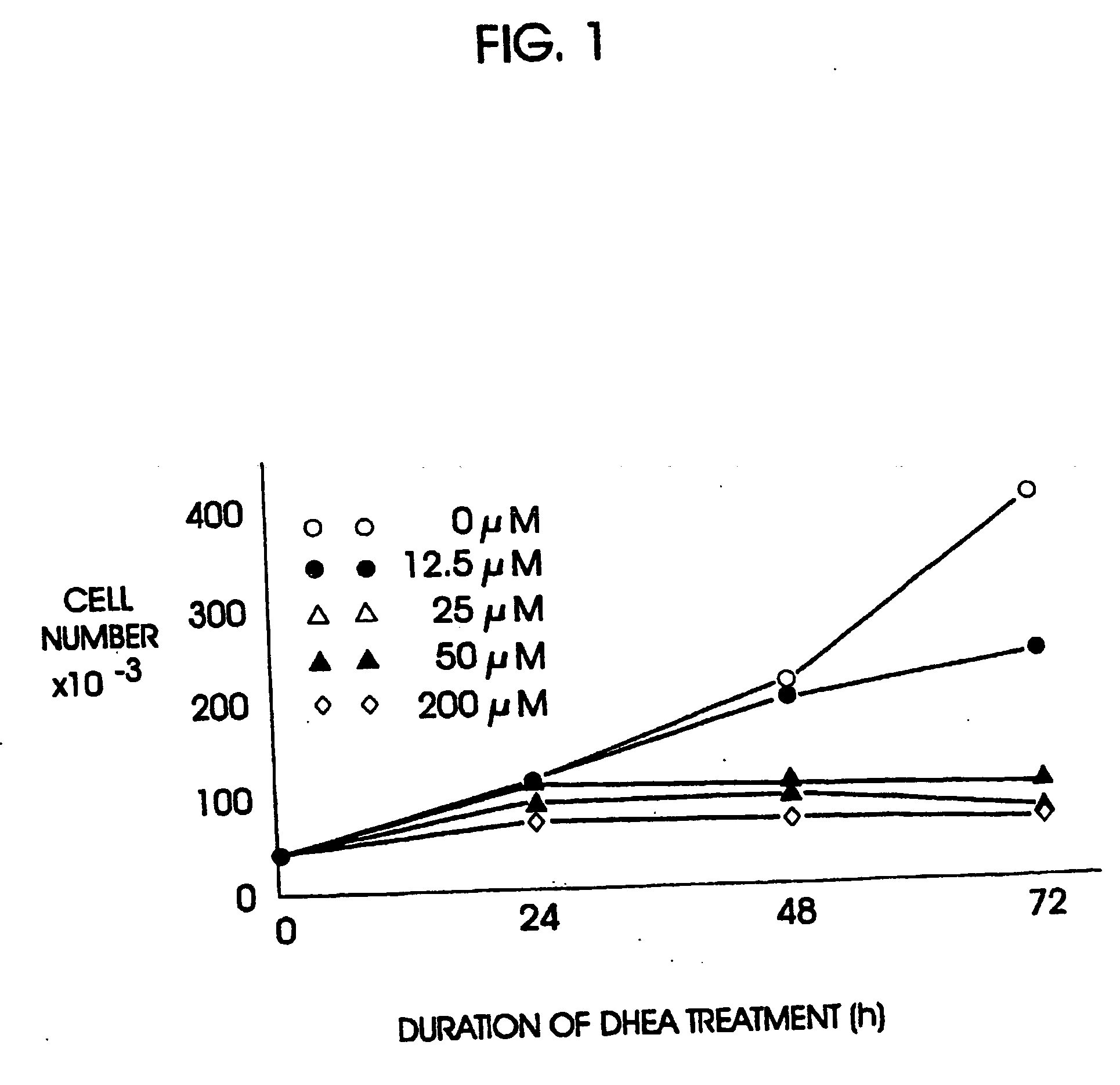
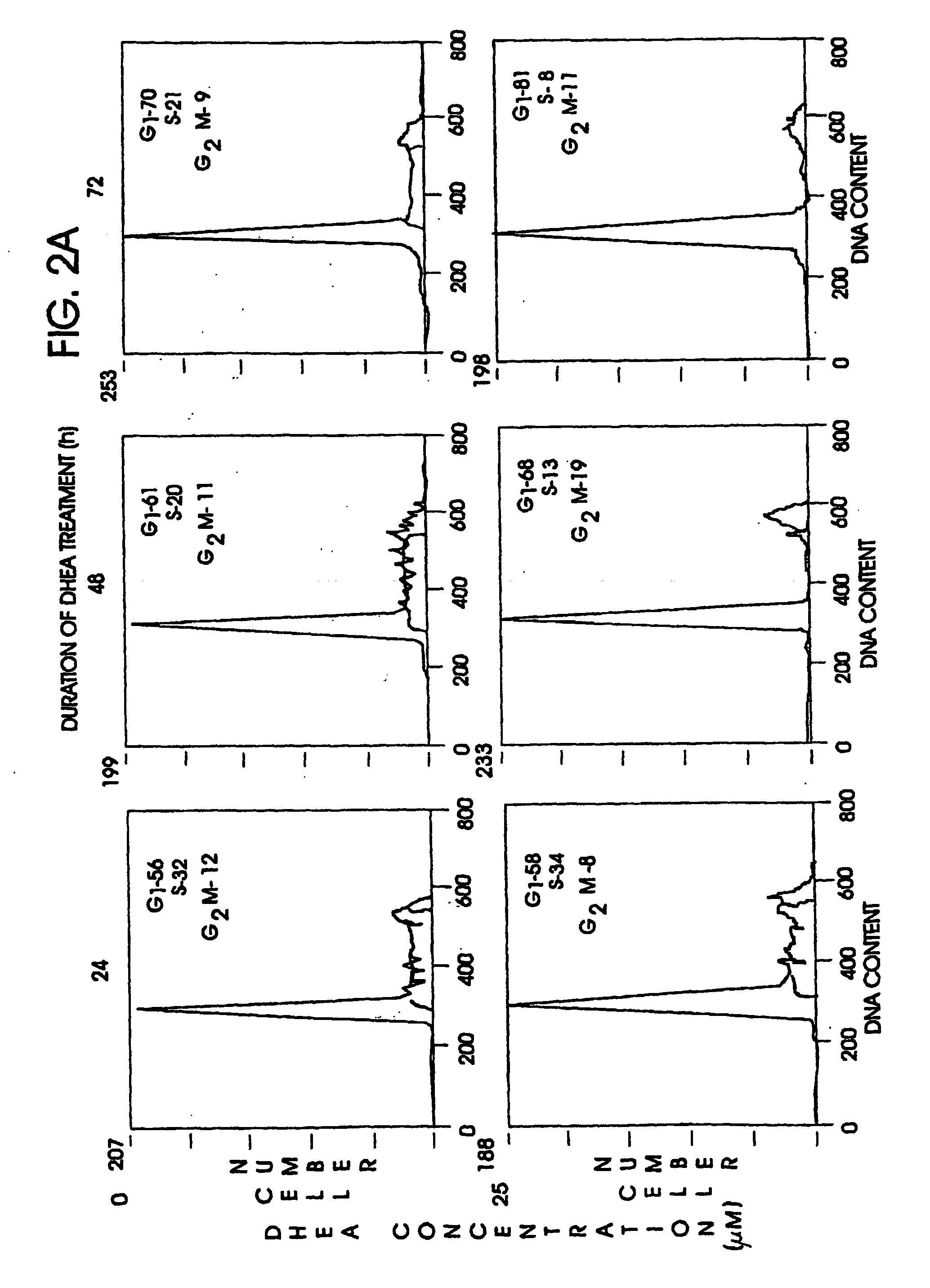
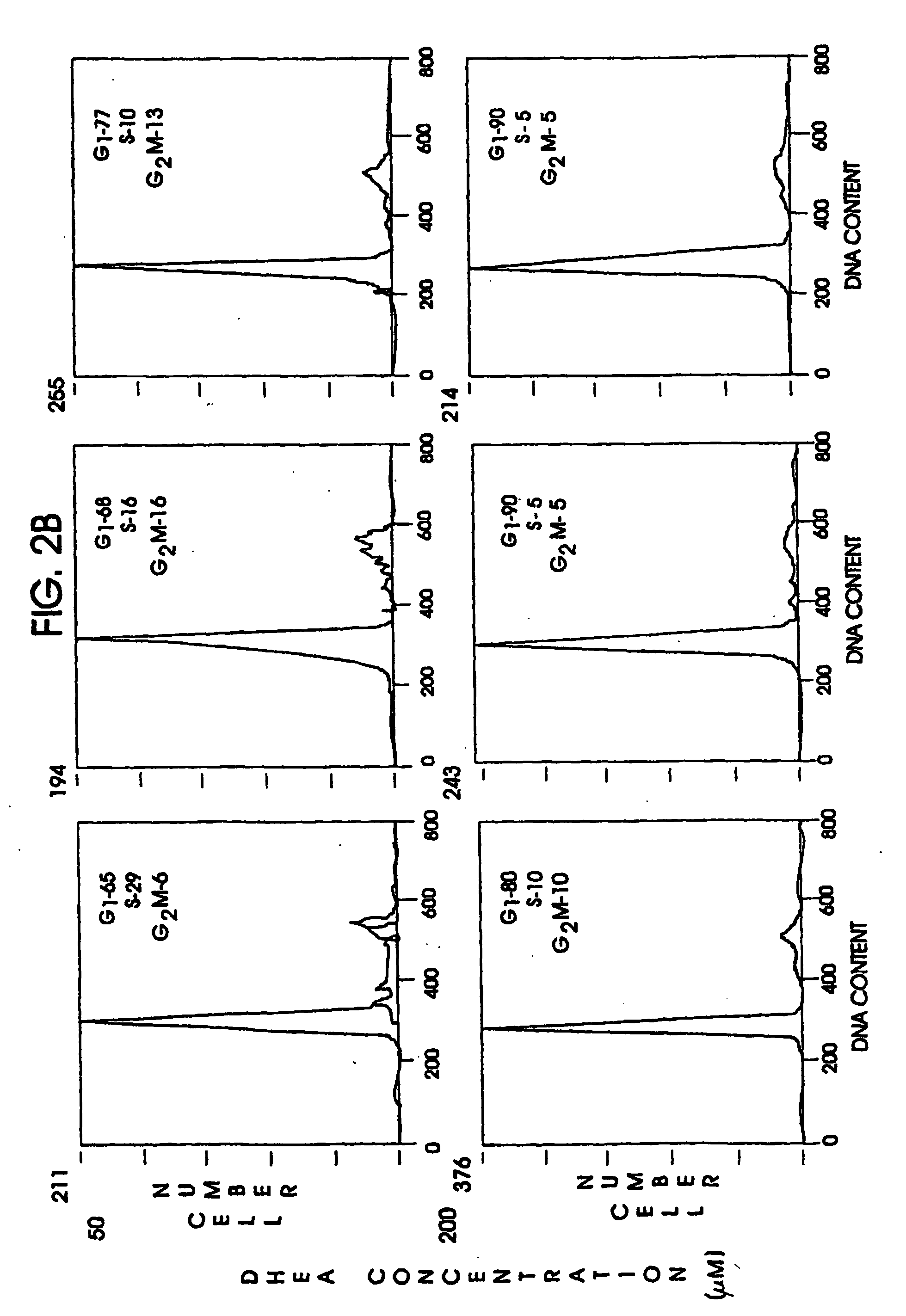
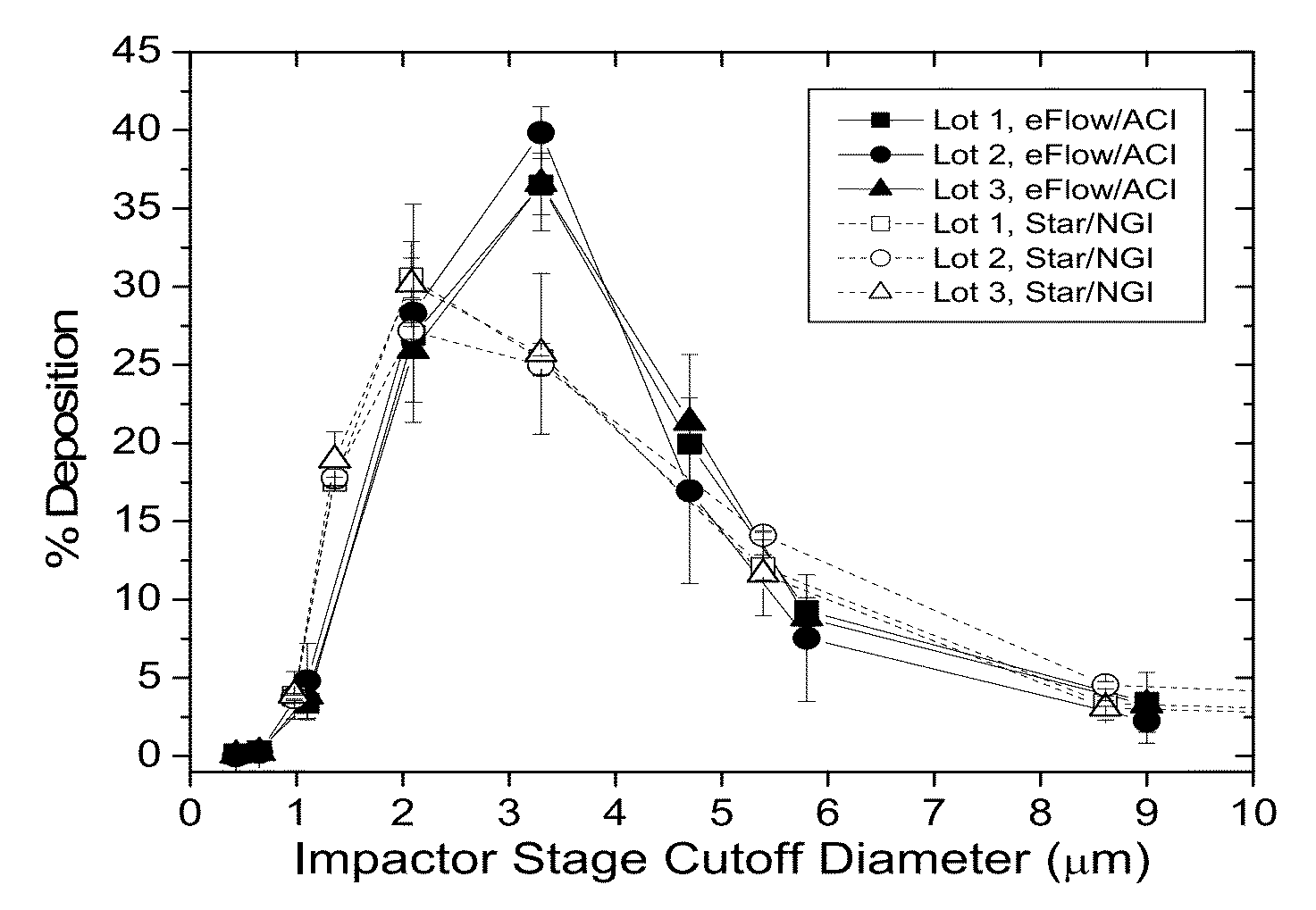
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
345 results about "Pulmonary disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pulmonary diseases, otherwise known as lung diseases, are the third biggest cause of death in America. There are several different types of pulmonary diseases that vary in severity. According to Medline Plus, a few of the main types of pulmonary diseases include emphysema, asthma, pneumonia, tuberculosis and lung cancer.
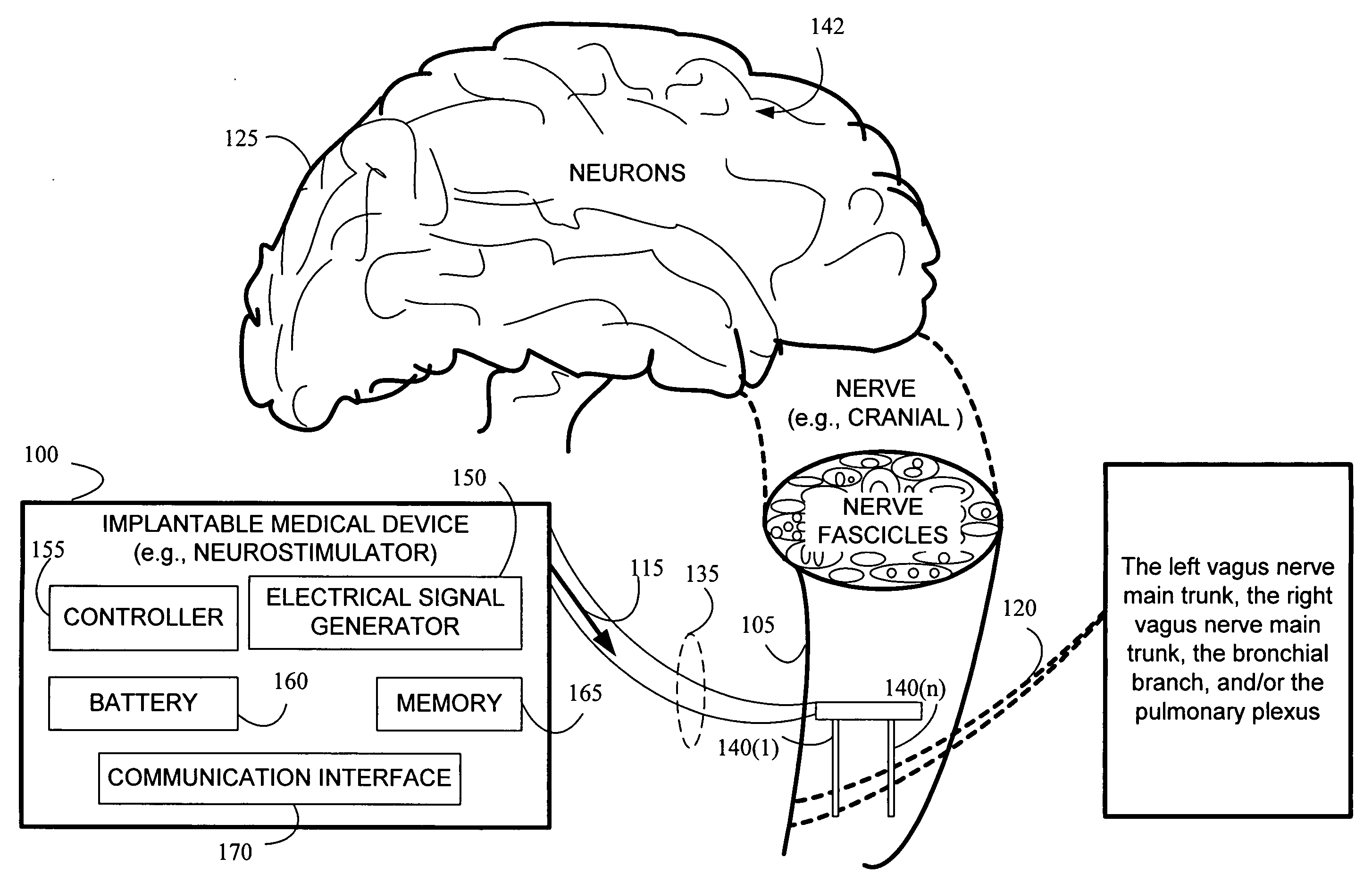
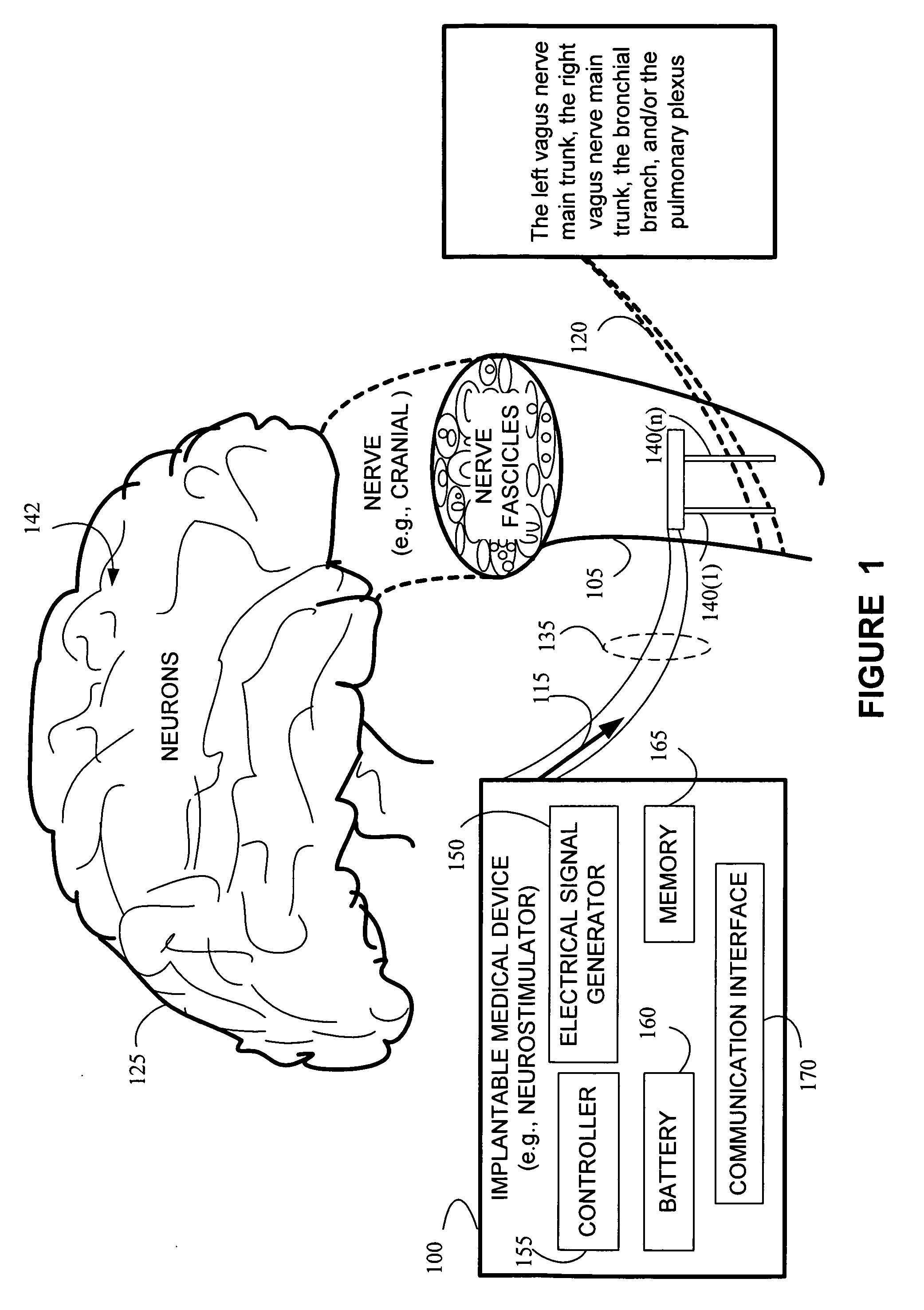
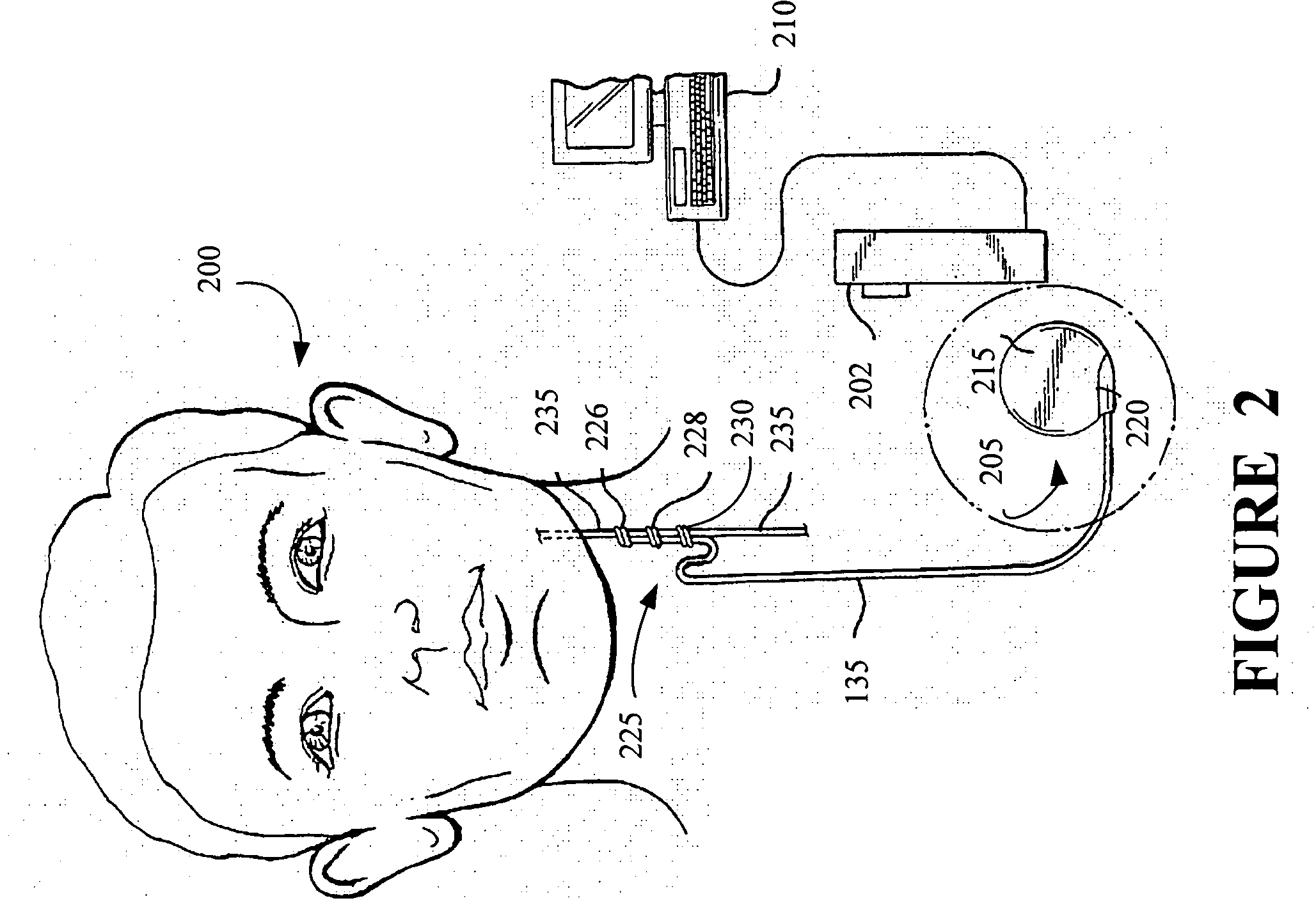
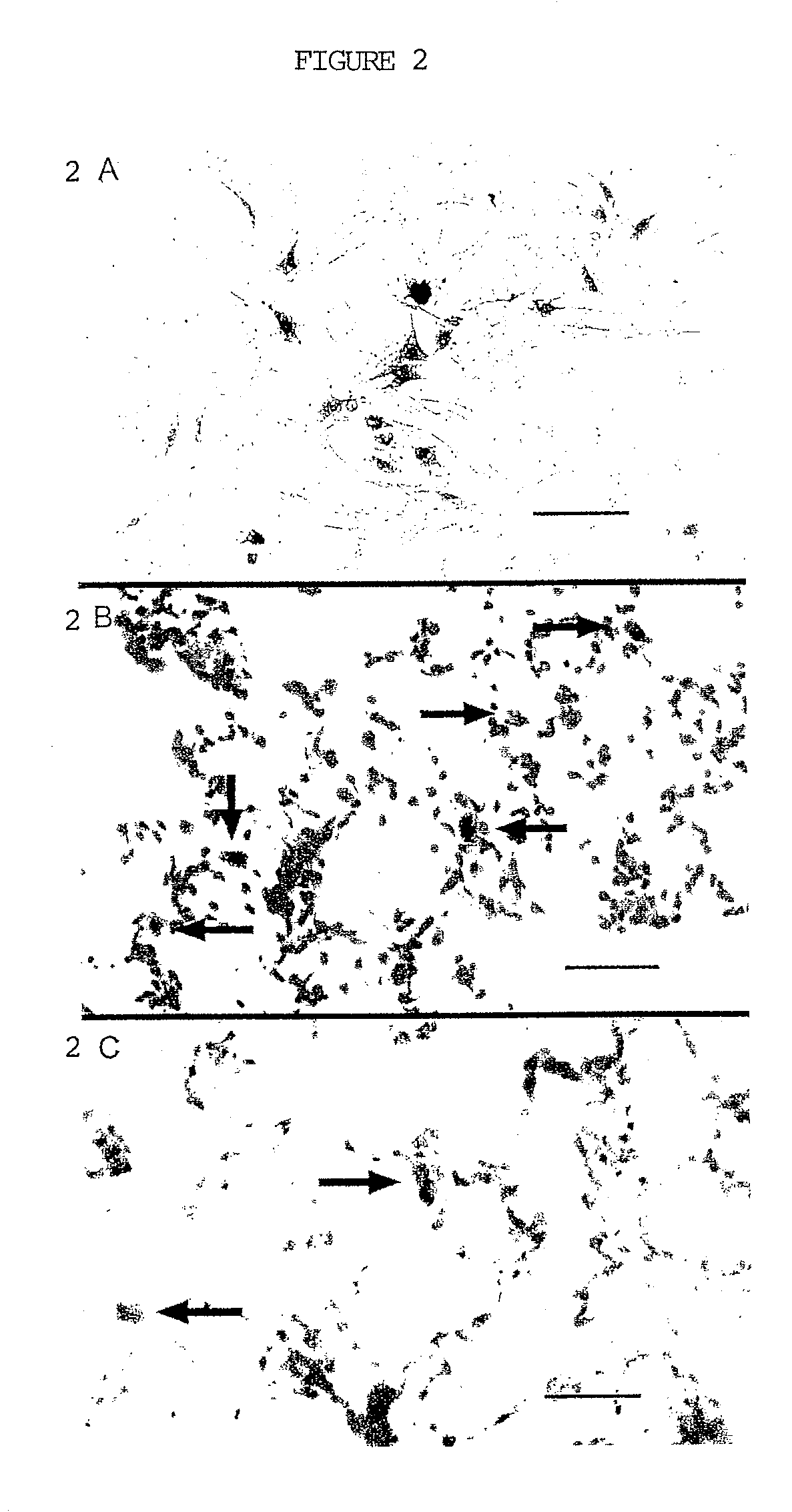
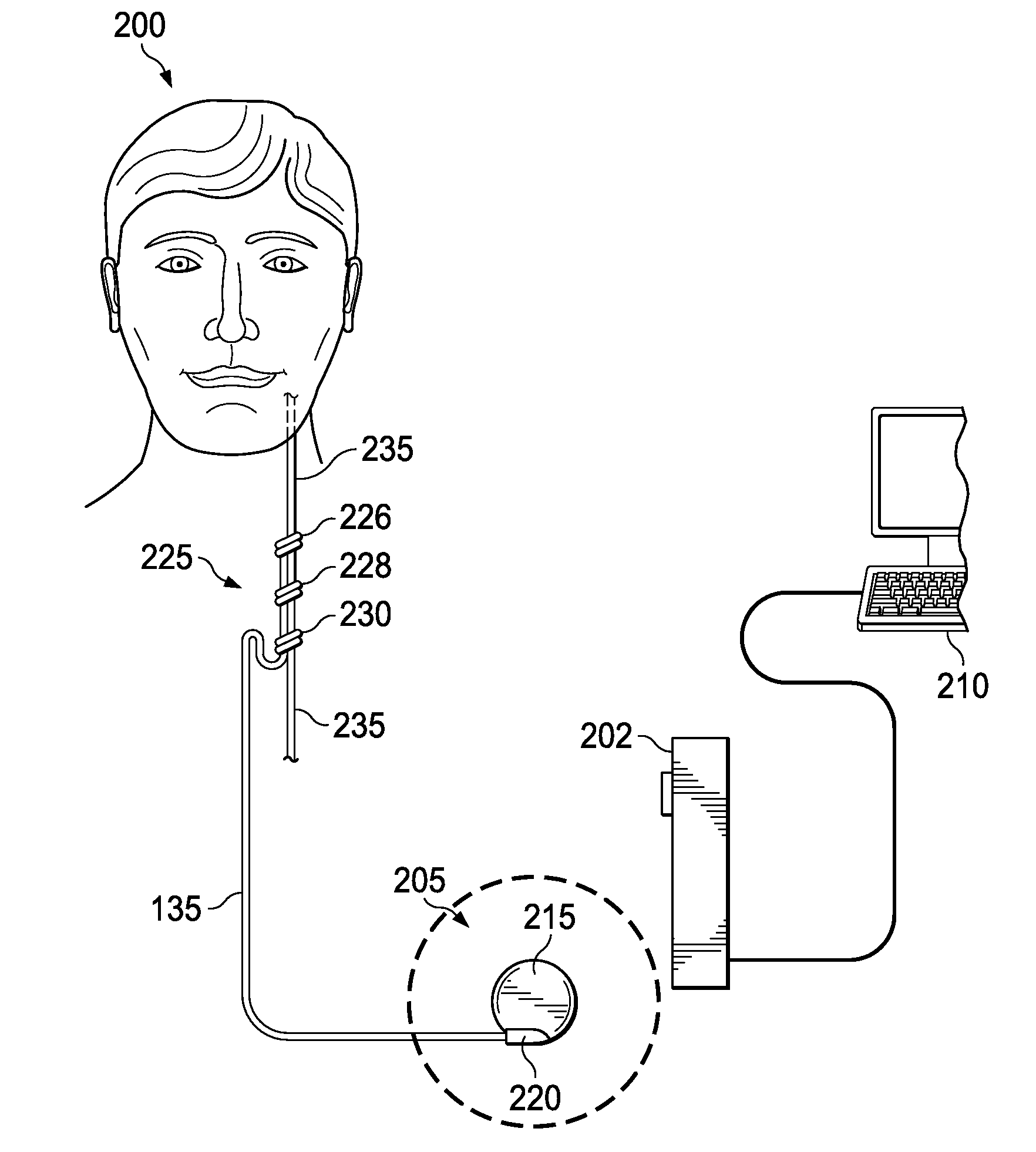
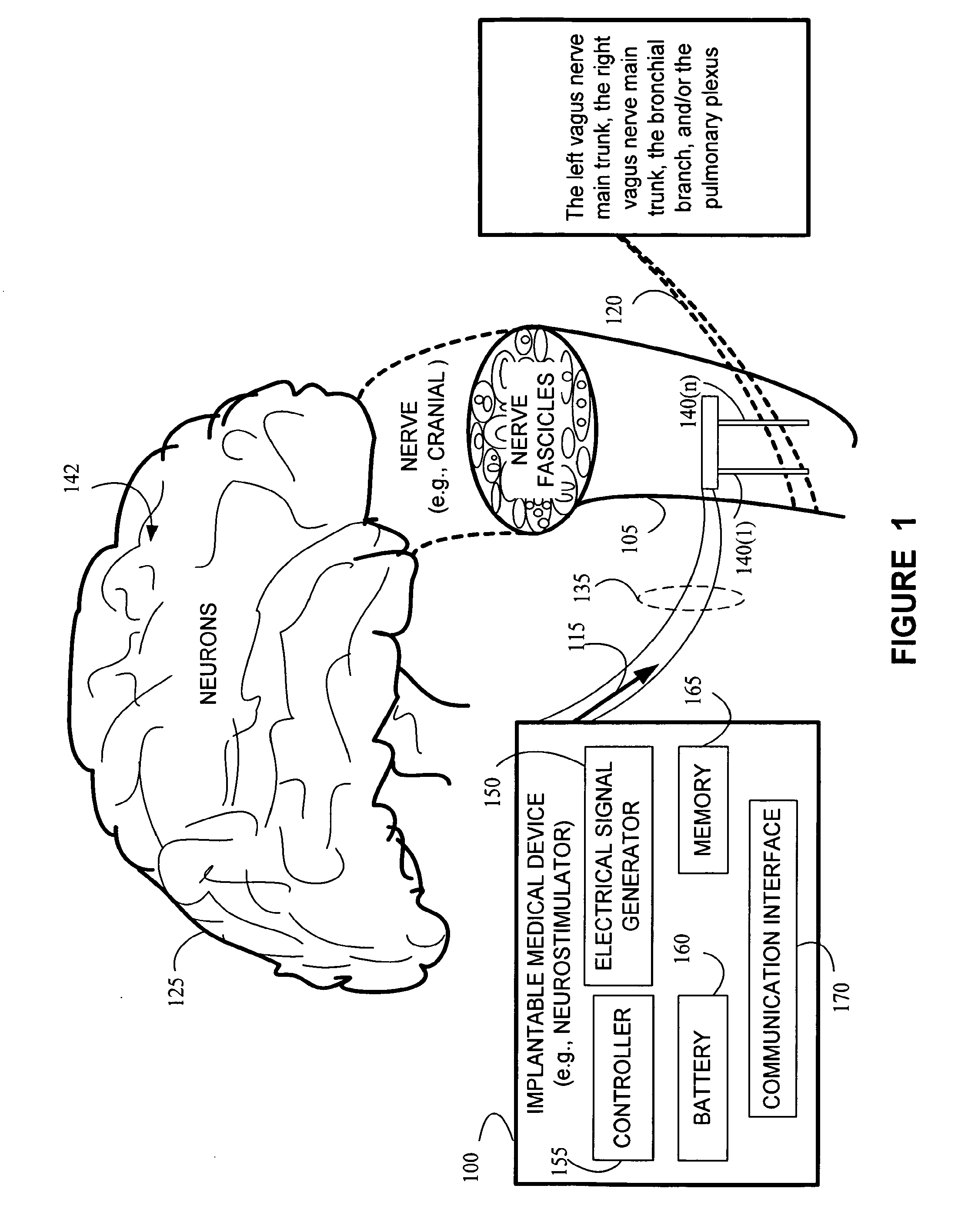
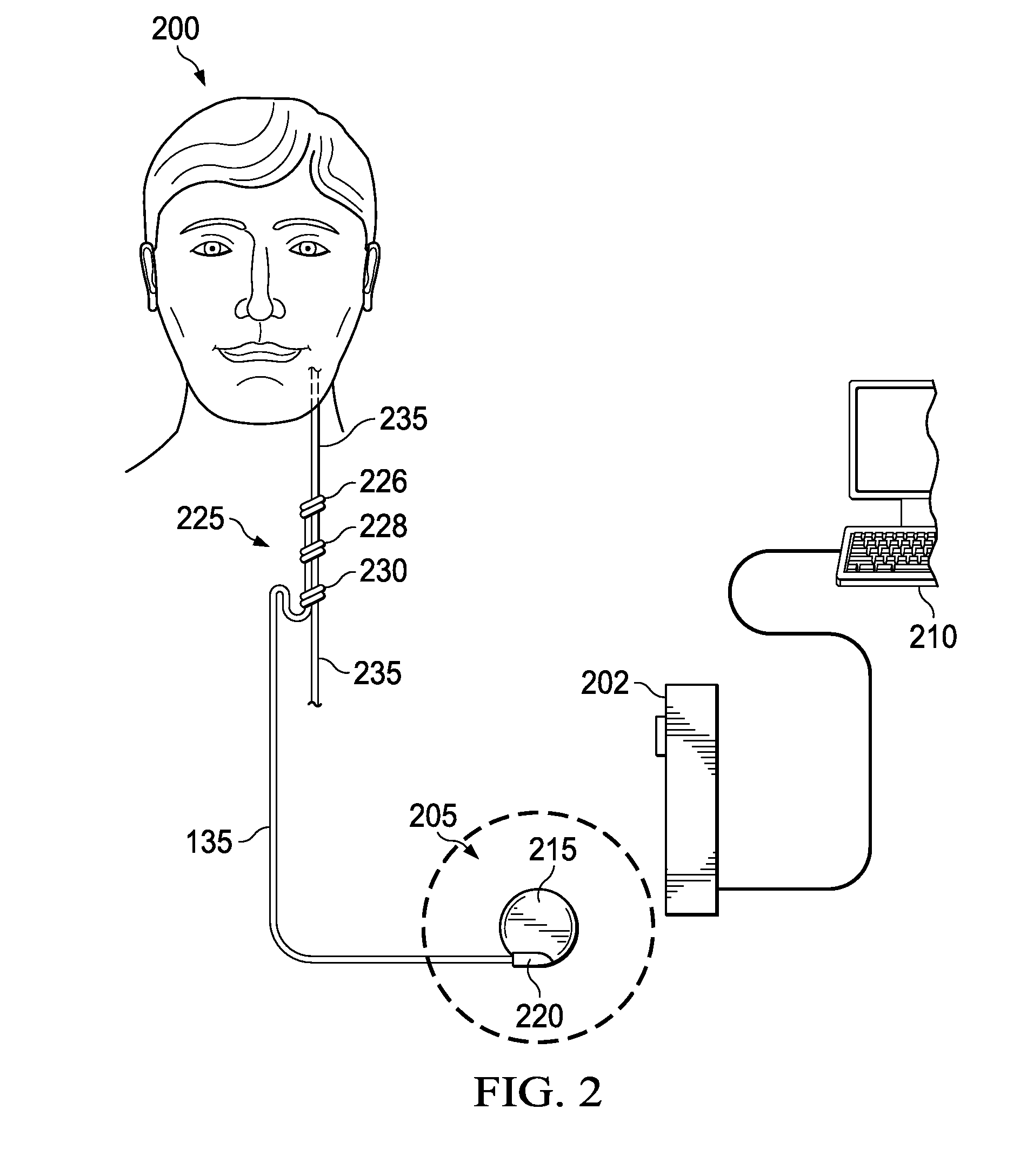
Stimulating cranial nerve to treat pulmonary disorder
A method for stimulating a portion of a vagus nerve of a patient to treat a pulmonary disorder is provided. At least one electrode is coupled to at least one portion of a left vagus nerve and / or a right vagus nerve of the patient. An electrical signal is applied to the portion of the vagus nerve using the electrode to treat the pulmonary disorder. The electrical signal may perform a blocking of an intrinsic neural activity on said at least one portion of the left vagus nerve and said right vagus nerve.
Owner:LIVANOVA USA INC
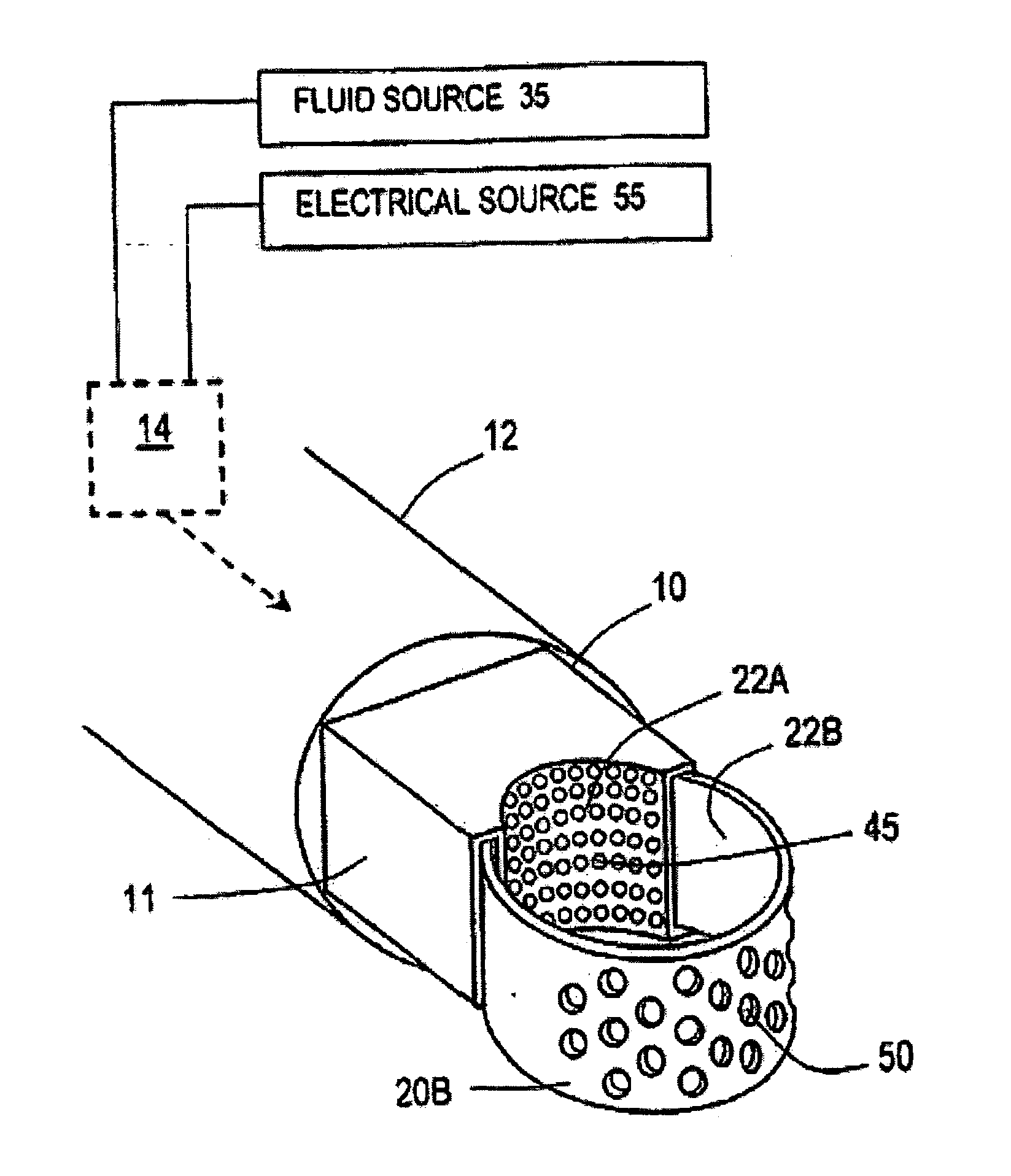
Medical instruments and techniques for treating pulmonary disorders
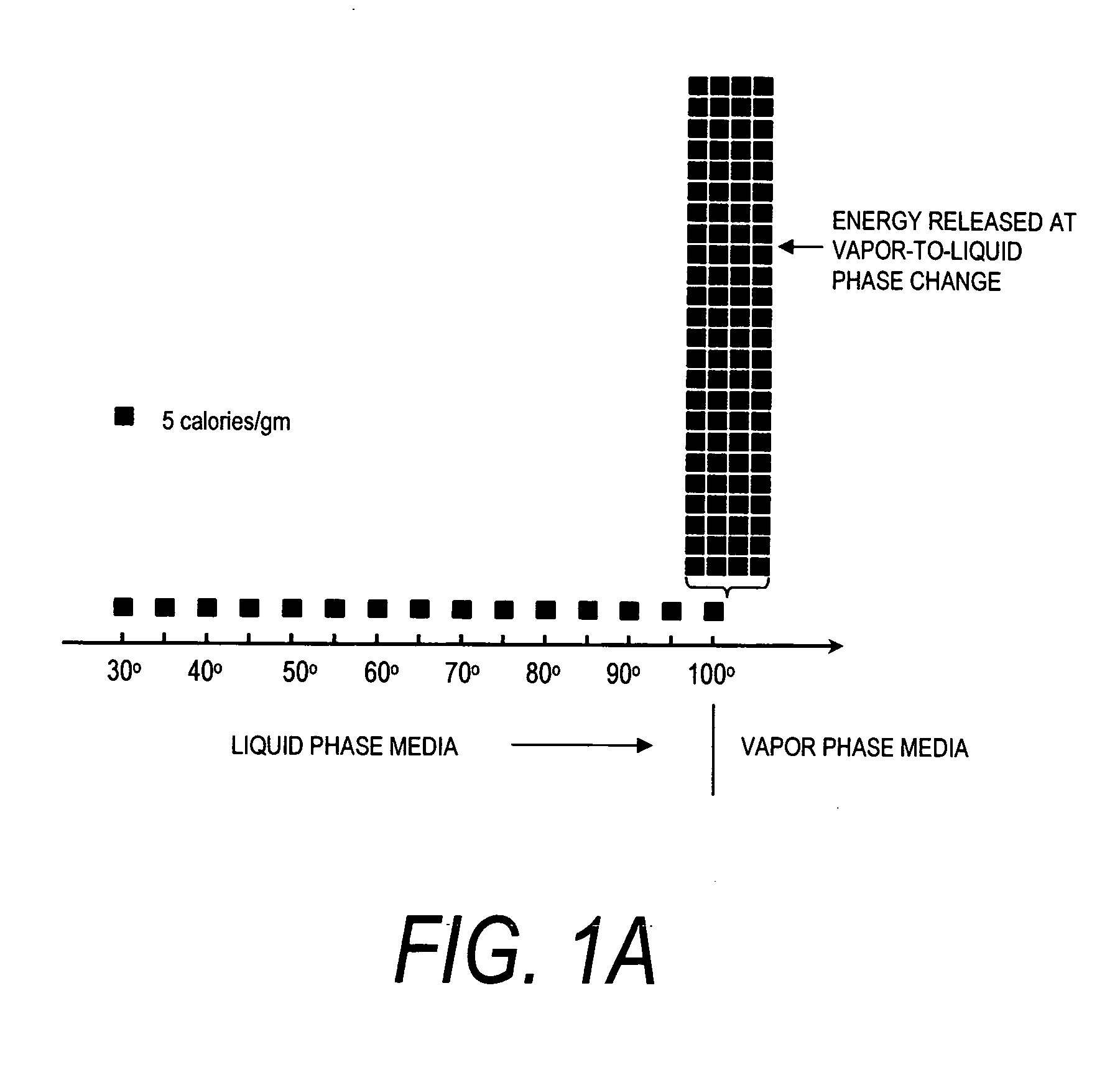
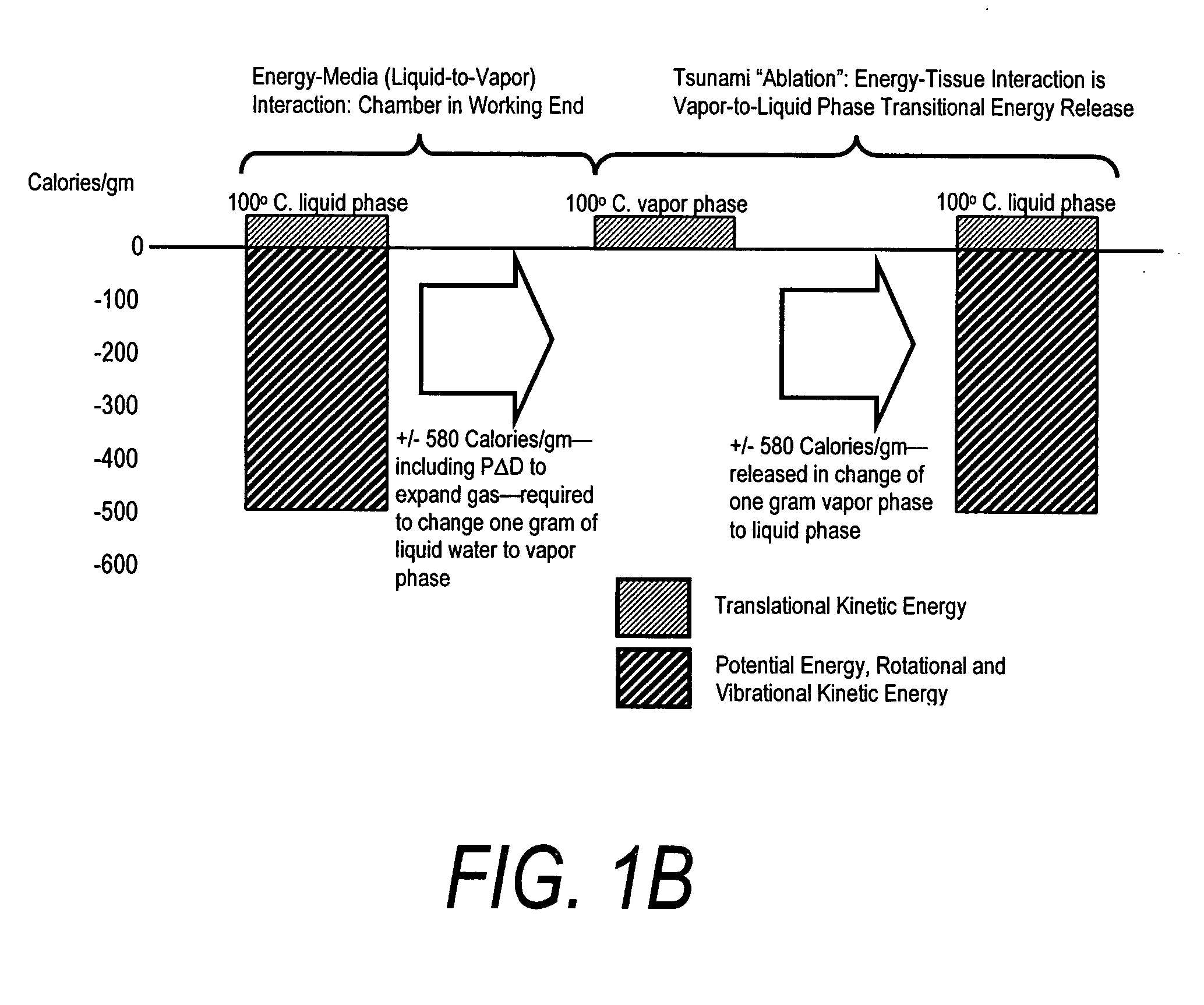
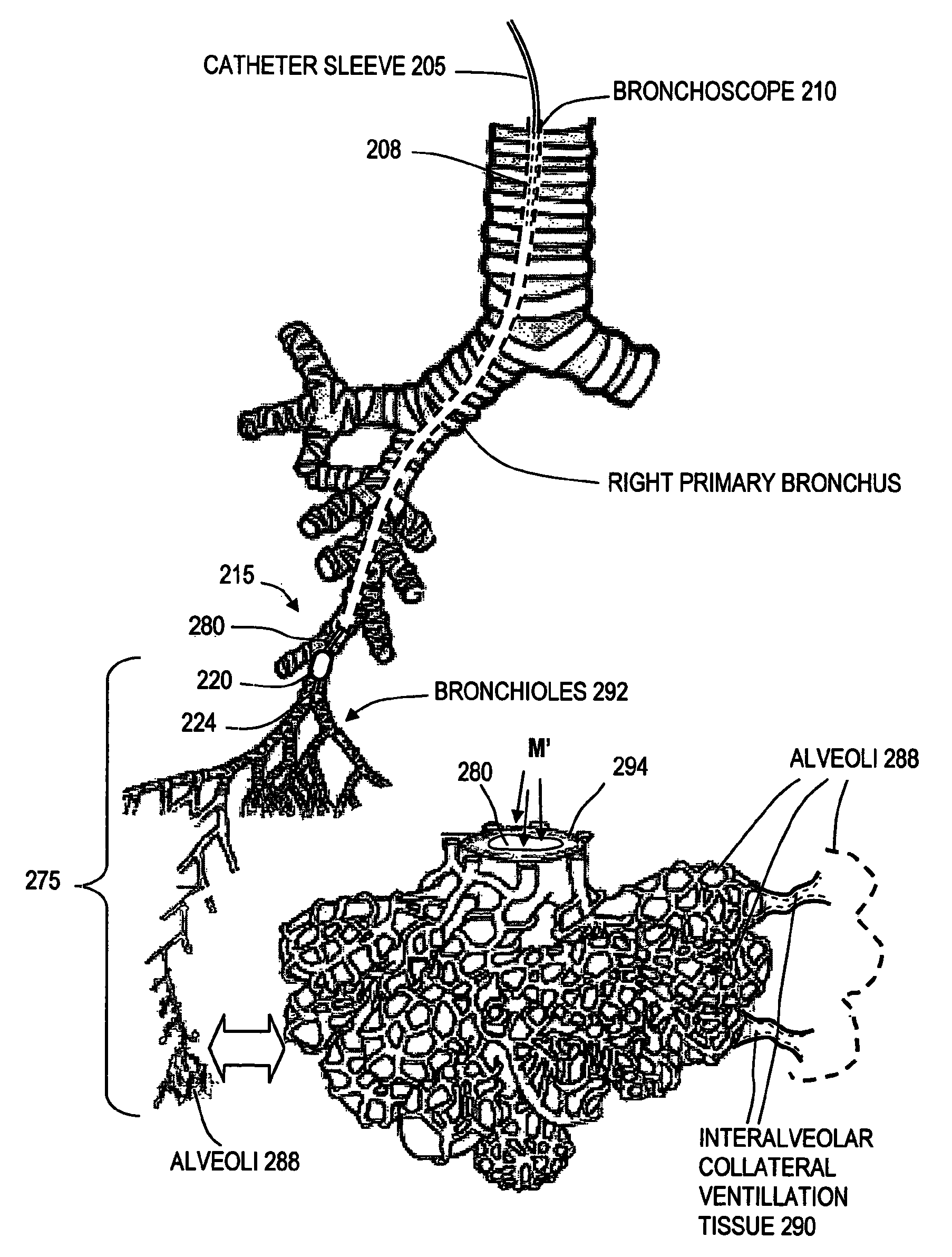
ActiveUS20080132826A1Enhance tissue remodelingReducing lung volumeMedical devicesFluid jet surgical cuttersThermal energyDisease
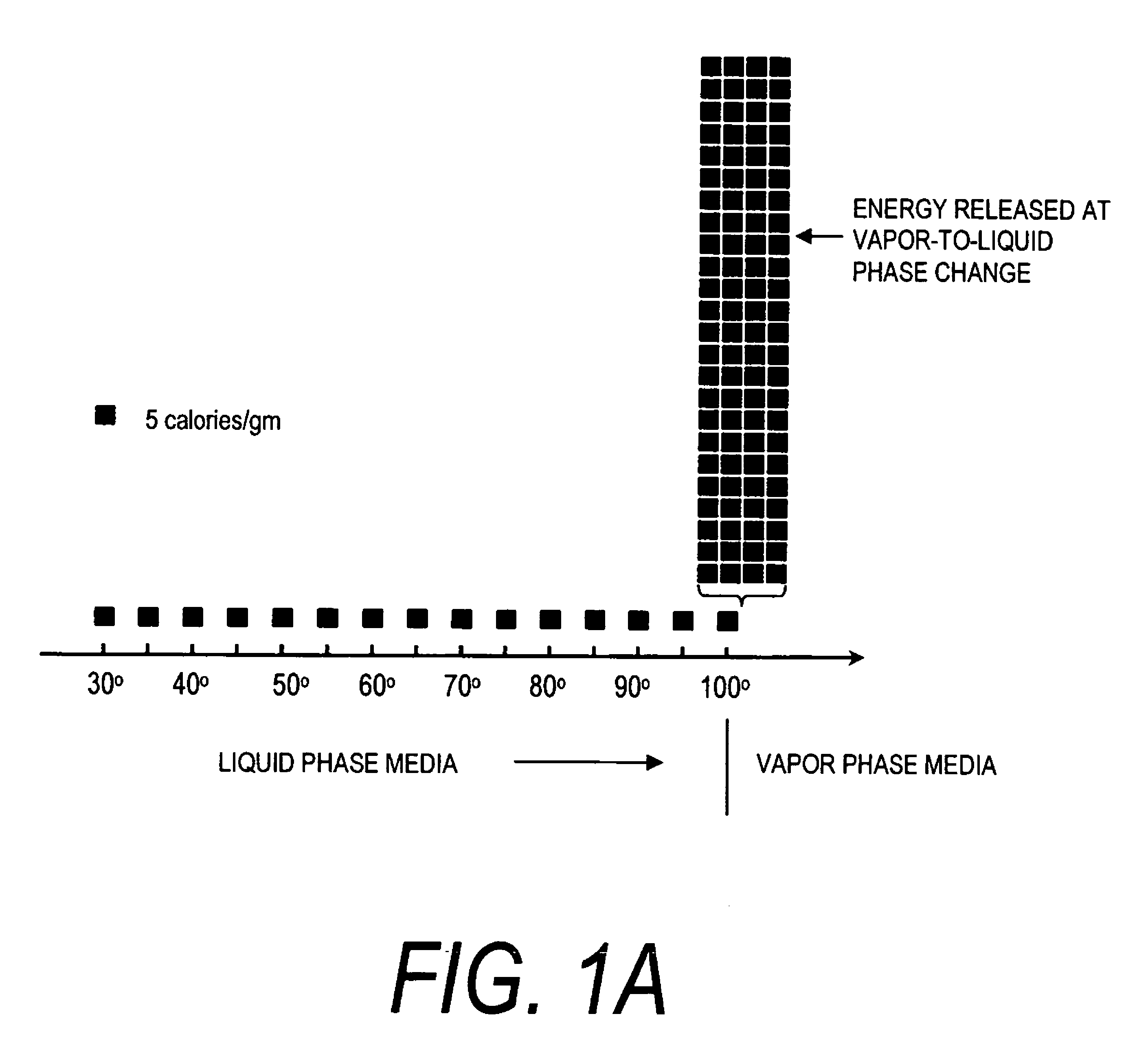
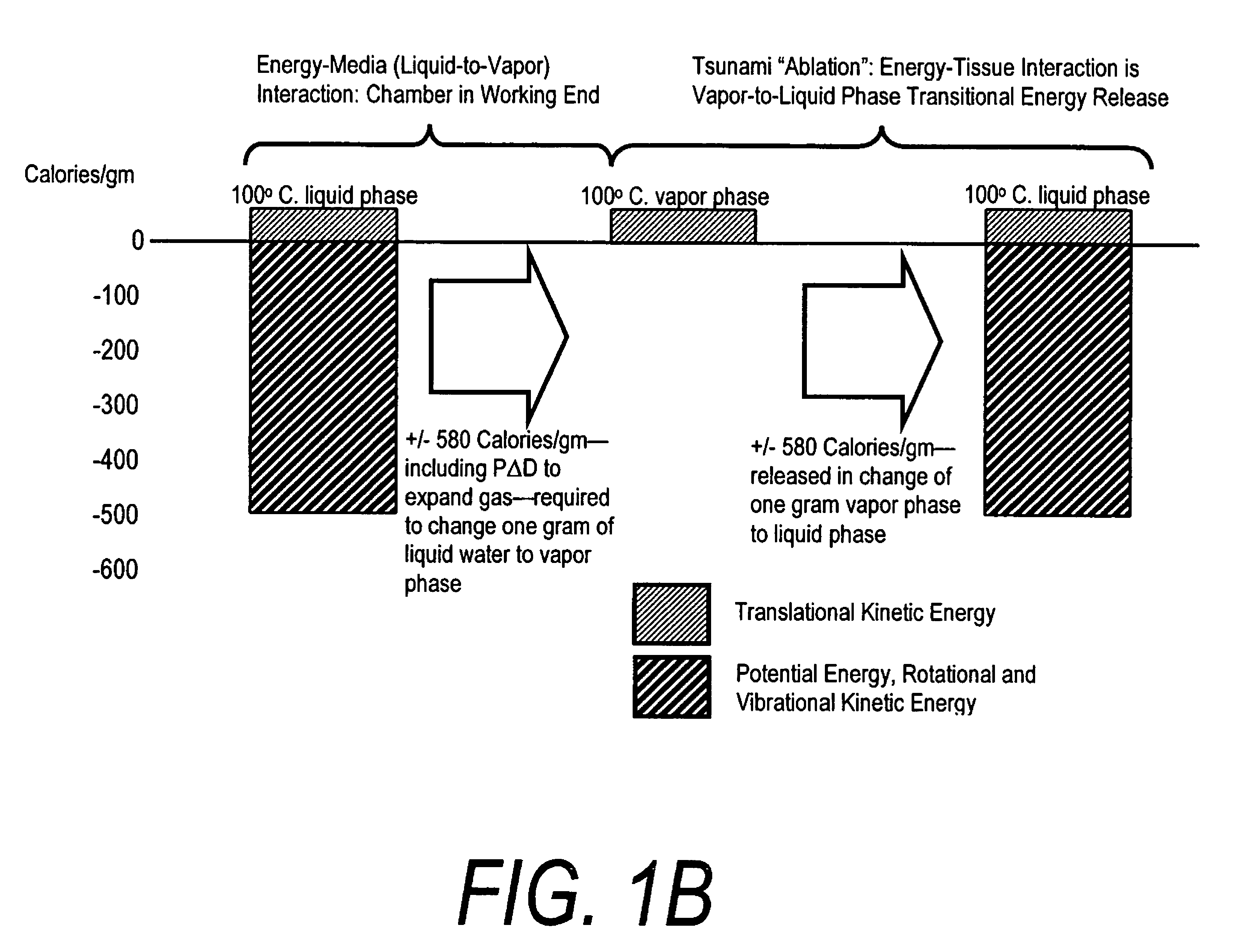
A surgical instrument for delivering energy to lung tissue, for example to cause lung volume reduction. In one embodiment, an elongated catheter has a handle portion that includes an interior chamber that is supplied with a biocompatible liquid media under pressure. An energy source delivers energy to the media to cause a liquid-to-vapor phase change within the interior chamber and ejects a flow of vapor media from the working end of the catheter. The delivery of energy and the flow of vapor are controlled by a computer controller to cause a selected pressure and selected volume of vapor to propagate to the extremities of the airways. Contemporaneously, the vapor undergoes a vapor-to-liquid phase transition which delivers a large amount of energy to airway tissue. The thermal energy delivered is equivalent to the heat of vaporization of the fluid media, which shrinks and collapses the treated airways. The treated tissue is the maintained in a collapsed state by means of aspiration for a short interval to enhance tissue remodeling. Thereafter, the patient's wound healing response causes fibrosis and further remodeling to cause permanent lung volume reduction.
Owner:TSUNAMI MEDTECH
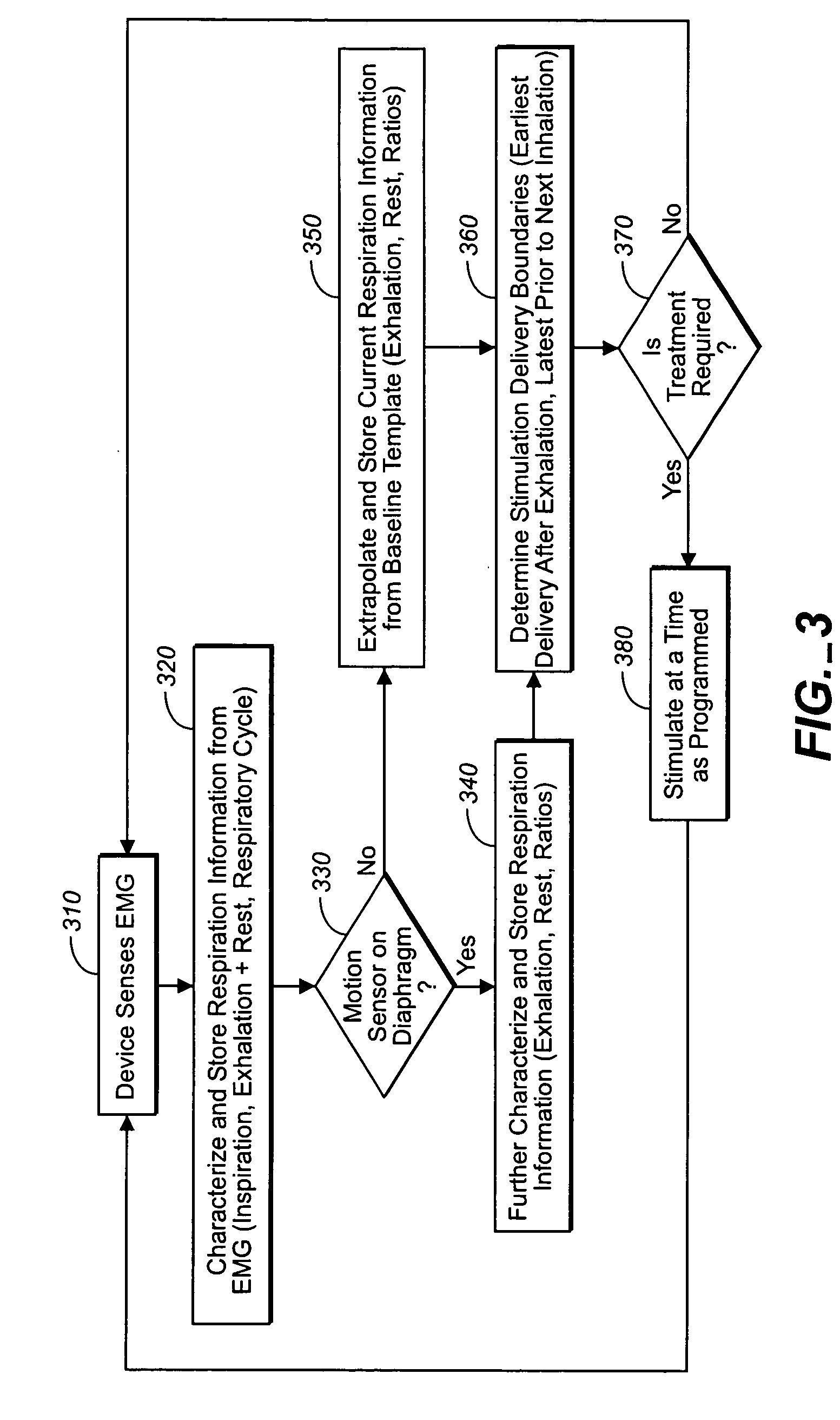
Breathing therapy device and method
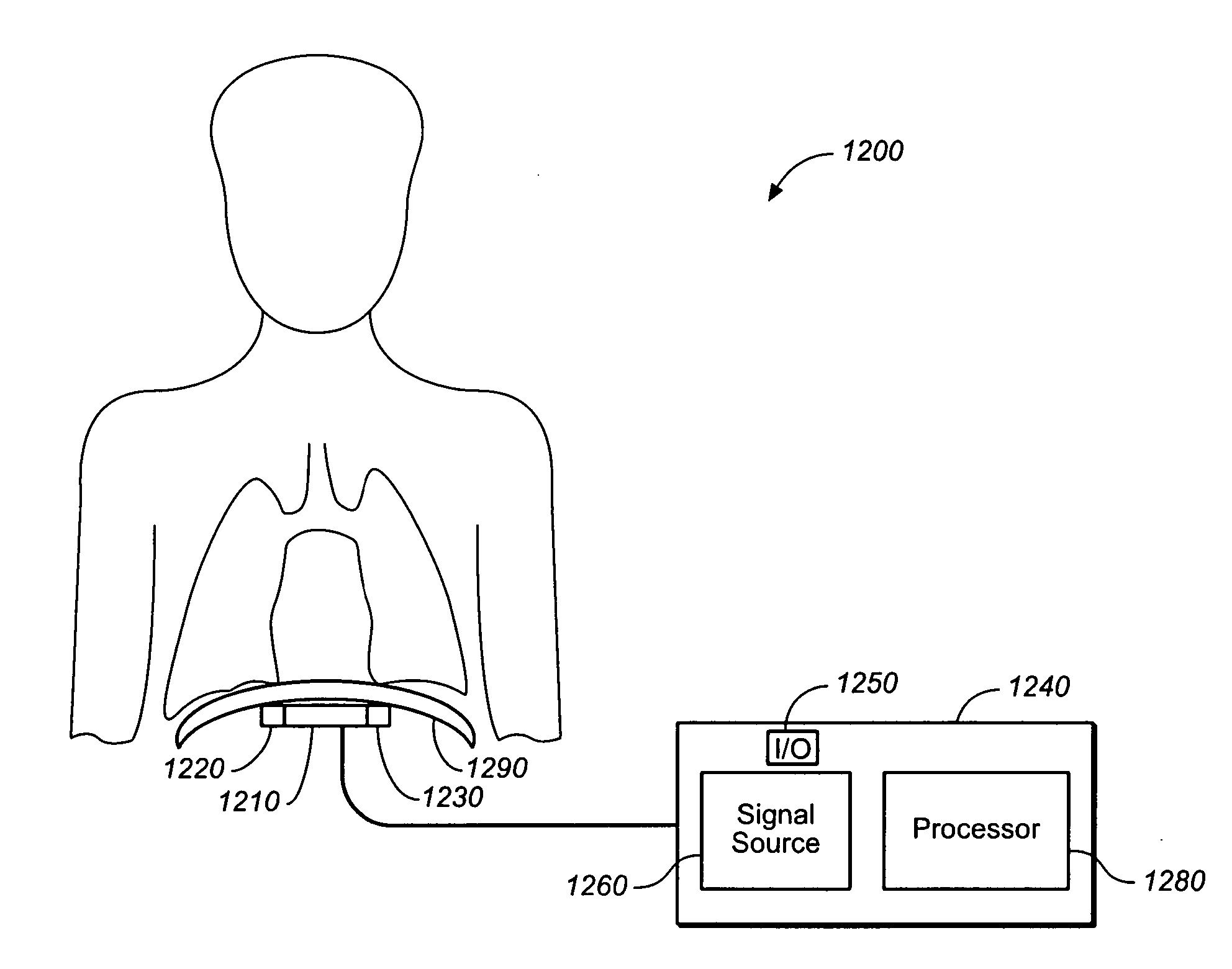
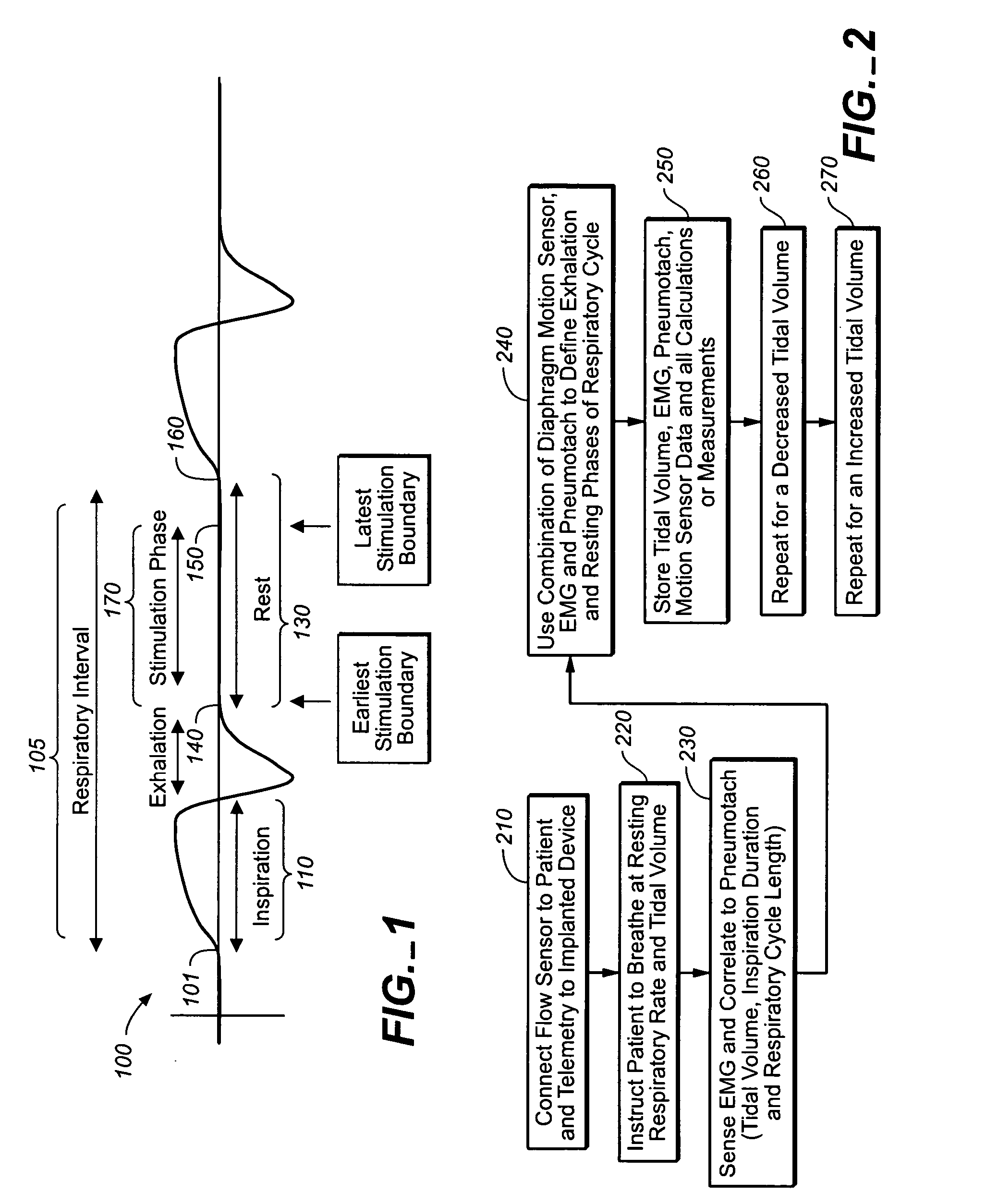
ActiveUS20050085868A1Inhibiting respiratory driveRestricts breathingElectrotherapyElectromyographyCOPDControlled breathing
A device and method is provided for electrically stimulating the diaphragm to control breathing while inhibiting respiratory drive. A stimulation phase is identified. The stimulation phase is a period of time within the breathing cycle in which stimulation will inhibit respiratory drive. The respiratory drive inhibition may be used in a number of applications including but not limited to: improving or remodeling the heart in heart failure patients, treating apnea, chronic obstructive pulmonary disorder (COPD), and hypertension.
Owner:RMX
Compositions, formulations and kit with anti-sense oligonucleotide and anti-inflammatory steroid and/or obiquinone for treatment of respiratory and lung disesase
InactiveUS20070021360A1Decreased airwayOrganic active ingredientsBiocideDiseaseAntiendomysial antibodies
A pharmaceutical composition and formulations comprise preventative, prophylactic or therapeutic amounts of an oligo(s) anti-sense to a specific gene(s) or its corresponding mRNA(s), and a glucocorticoid and / or non-glucocorticoid steroid or a ubiquinone or their salts. The agents, composition and formulations are used for treatment of ailments associated with impaired respiration, bronchoconstriction, lung allergy(ies) or inflammation, and abnormal levels of adenosine, adenosine receptors, sensitivity to adenosine, lung surfactant and ubiquinone, such as pulmonary fibrosis, vasoconstriction, inflammation, allergies, allergic rhinitis, asthma, impeded respiration, lung pain, cystic fibrosis, bronchoconstriction, COPD, RDS, ARDS, cancer, and others. The present treatment is effectively administered by itself for conditions without known therapies, as a substitute for therapies exhibiting undesirable side effects, or in combination with other treatments, e.g. before, during and after other respiratory system therapies, radiation, chemotherapy, antibody therapy and surgery, among others. Each of the agents of this invention may be administered directly into the respiratory system so that they gain direct access to the lungs, or by other effective routes of administration. A kit comprises a delivery device, the agents and instructions for its use.
Owner:EPIGENESIS PHARMA LLC
Medical instruments and techniques for treating pulmonary disorders
ActiveUS7892229B2Enhance tissue remodelingReduce volumeMedical devicesFluid jet surgical cuttersTissue remodelingDisease
A surgical instrument for delivering energy to lung tissue, for example to cause lung volume reduction. In one embodiment, an elongated catheter has a handle portion that includes an interior chamber that is supplied with a biocompatible liquid media under pressure. An energy source delivers energy to the media to cause a liquid-to-vapor phase change within the interior chamber and ejects a flow of vapor media from the working end of the catheter. The delivery of energy and the flow of vapor are controlled by a computer controller to cause a selected pressure and selected volume of vapor to propagate to the extremities of the airways. Contemporaneously, the vapor undergoes a vapor-to-liquid phase transition which delivers a large amount of energy to airway tissue. The thermal energy delivered is equivalent to the heat of vaporization of the fluid media, which shrinks and collapses the treated airways. The treated tissue is the maintained in a collapsed state by means of aspiration for a short interval to enhance tissue remodeling. Thereafter, the patient's wound healing response causes fibrosis and further remodeling to cause permanent lung volume reduction.
Owner:TSUNAMI MEDTECH
Compositions and methods for treating asthma and other lung disorders
InactiveUS20100008997A1Powder deliveryOrganic active ingredientsObstructive Pulmonary DiseasesOxygen
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination treatment methods comprising administration of electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Treatment of pulmonary disorders with aerosolized medicaments such as vancomycin
InactiveUS20100282247A1Reduce in quantityReduce the amount requiredAntibacterial agentsAntimycoticsDiseaseGlycopeptide
A method of administering an aerosolized anti-infective, such as a glycopeptide, to the respiratory system of a patient. A ratio of an amount of the glycopeptide, such as vancomycin, delivered to the pulmonary system of the patient in a 24 hour period to a minimum inhibitory amount for the target organ for the same period is about 2 or more. A system to introduce aerosolized medicament to a patient may include a humidifier coupled to an inspiratory limb of a ventilator circuit wye, where the humidifier supplies heated and humidified air to the patient, and an endotracheal tube having a proximal end coupled to a distal end of the ventilator circuit wye. The system may also include a nebulizer coupled to the endotracheal tube, where the nebulizer generates the aerosolized medicament.
Owner:NEKTAR THERAPEUTICS INC
Compositions and methods for treating asthma and other lung disorders
Provided are compositions and methods for treating or preventing lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis (e.g. nose respiratory tract), and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically generated fluid (including gas-enriched electrokinetically generated fluids) as disclosed herein, the electrokinetically altered aqueous fluid suitable to alter cellular membrane structure or function sufficient to provide for modulation of intracellular signal transduction, wherein treating a lung disorder or a symptom thereof is thereby afforded. Additional aspects relate to therapeutic compositions, and combination treatment methods comprising administration of at least one electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Compositions and methods for treating asthma and other lung disorders
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential and / or conductance, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination therapies comprising administration of the electrokinetically generated fluids with at least one additional therapeutic agent.
Owner:REVALESIO CORP
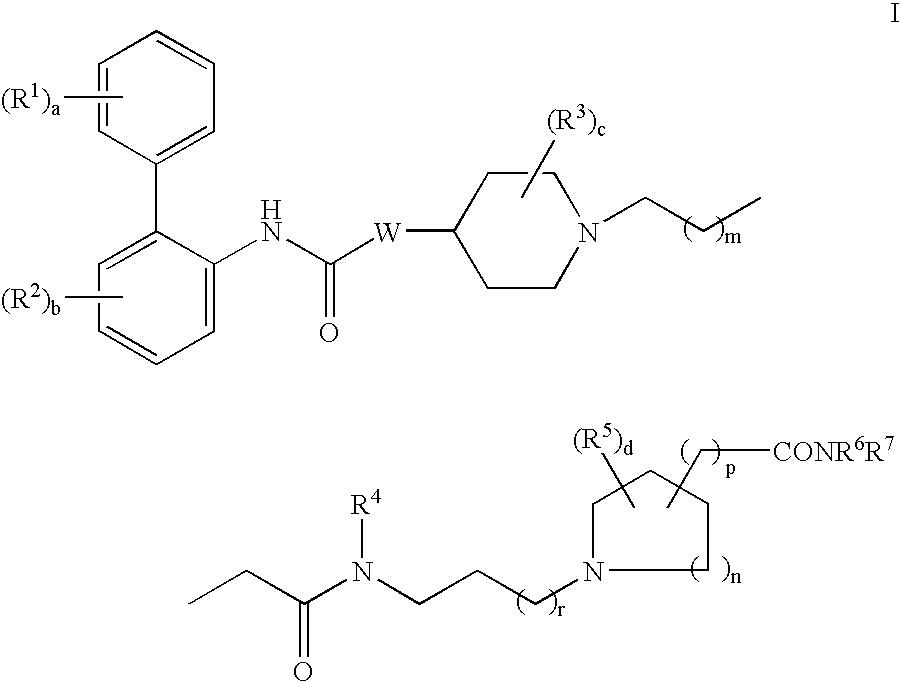
Biphenyl compounds useful as muscarinic receptor antagonists
ActiveUS7288657B2Long duration of actionImprove performanceNervous disorderOrganic chemistryDiseaseCombinatorial chemistry
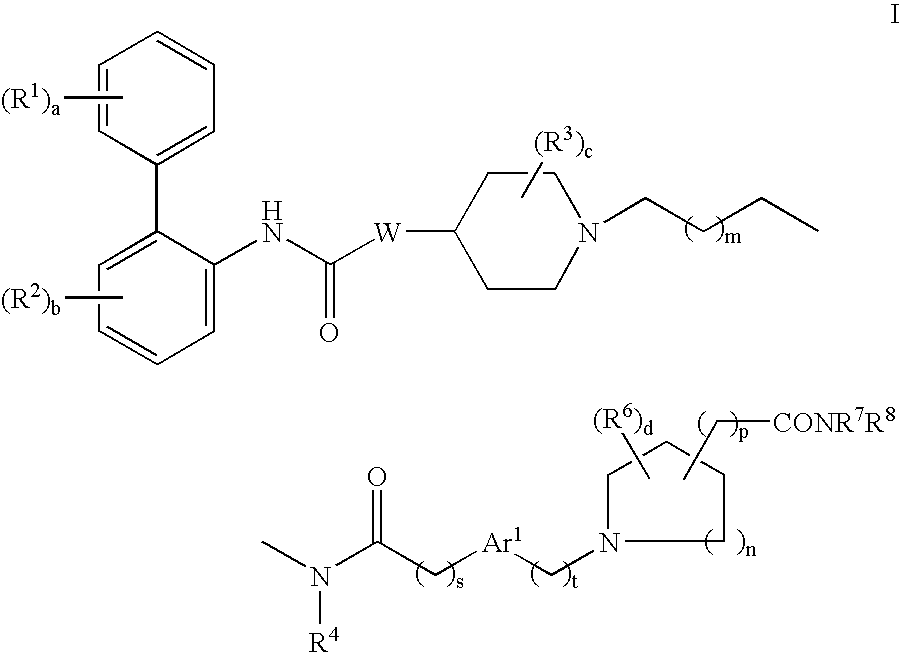
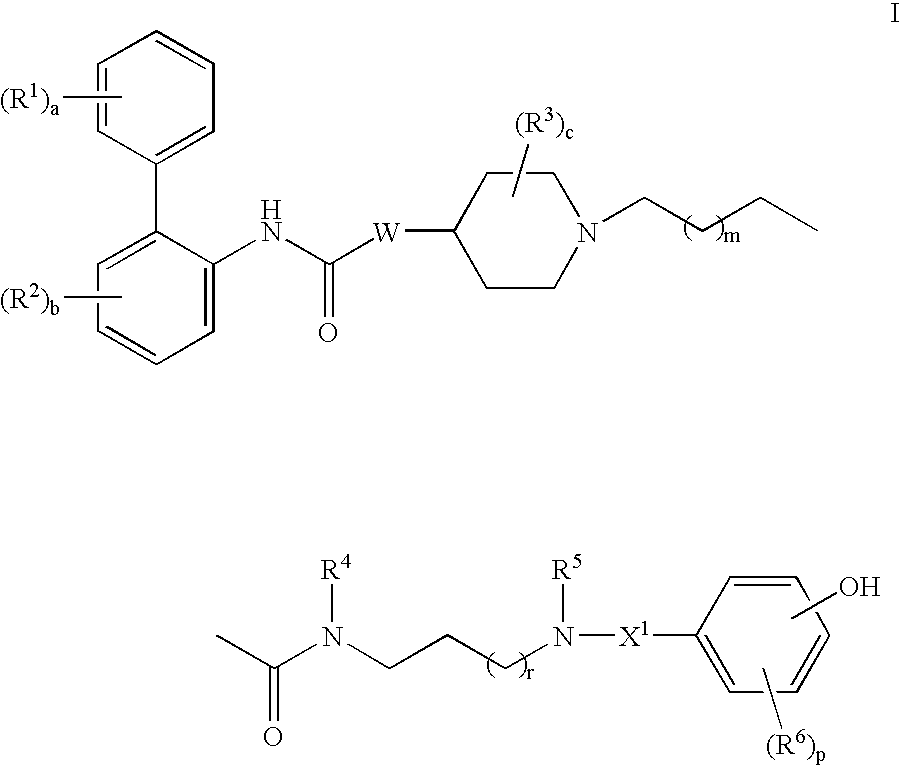
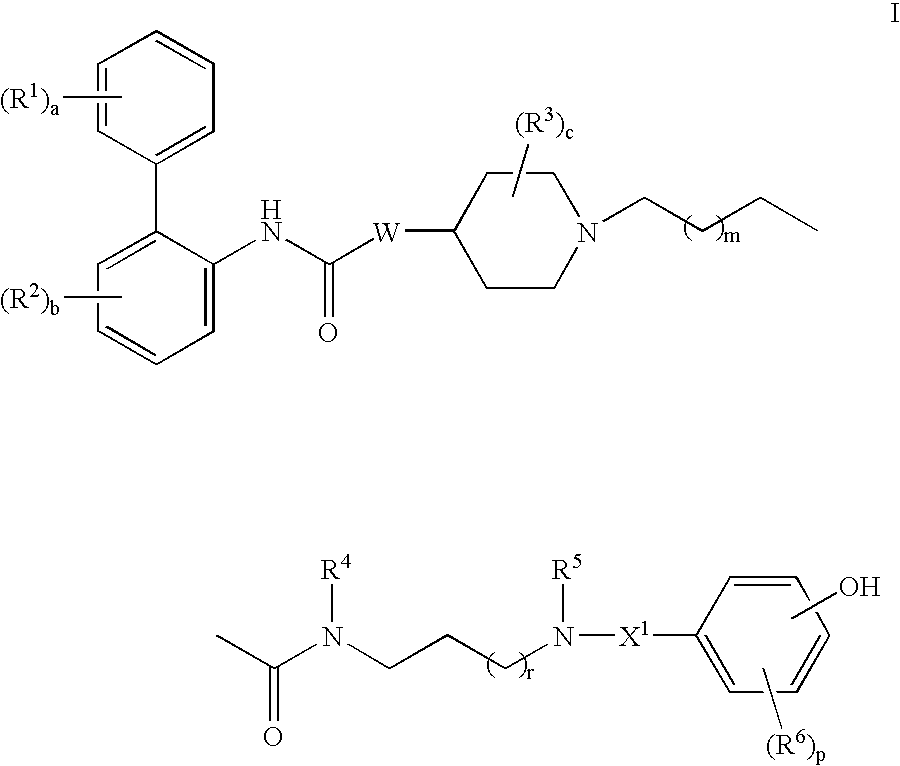
This invention provides compounds of formula I:wherein a, b, c, d, m, n, p, s, t, W, Ar1, R1, R2, R3, R4, R6, R7, and R8 are as defined in the specification. The compounds of formula I are muscarinic receptor antagonists. The invention also provides pharmaceutical compositions containing such compounds, processes and intermediates for preparing such compounds and methods of using such compounds to treat pulmonary disorders.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Optical sensor device and image processing unit for measuring chemical concentrations, chemical saturations and biophysical parameters
ActiveUS20090299154A1New riskReduce in quantityImage enhancementImage analysisNutritional deficiencyAntioxidant
Optical sensor devices, image processing devices, methods and computer readable code computer-readable storage media for detecting biophysical parameters, chemical concentrations, chemical saturations, vital signs and physiological information such as a malignant condition are provided. In some embodiments, the optical sensor includes an array of photodetectors, where each photodetector is configured to detect a spectrum of light. In some embodiments, the image processing device receives a live still or video electronic image, or alternatively, the electronic image is provided from an electronic storage media. Exemplary physiological parameters include but are not limited to a pulse rate, a biophysical or physiological property of skin, a cardiovascular property, a property related to an organ such as the liver or the kidneys, and a temperature fluctuation. In some embodiments, the physiological parameter is indicative of a malady including but not limited to an autoimmune disease, a cancer, a nutritional deficiency, a malignant condition of bone marrow, a present of an infectious microbe such as a fungus, a present of hepatitis, and a cardiovascular disorder a pulmonary disorder. Exemplary chemical concentrations include but are not limited to a chemical saturation, a pH level, a pH level in blood vessels such as capillarys or in skin, a glucose level such as a blood glucose level, a urea nitrogen level such as a blood urea nitrogen level, a CO2 level such as a blood CO2 level or a CO2 saturation level, and an oxygen level such as a blood oxygen level or a blood oxygen saturation level. In some embodiments, the biophysical parameter, physiological parameter or chemical concentration is obtained from reflecting light from tissue from a mammalian subject. Alternatively one or more of these parameters are detected from a food item such as food tissue, a consumable beverage such as an alcoholic beverage, a dairy product, wine, a baked good, a fruit and a vegetable. Exemplary parameters related to food items include but are not limited to a parameter indicative of cooking or spoilage, a pH, a concentration of an antioxidant, and a concentration of an anti-inflammatory agent.
Owner:CNOGA HLDG LTD
1,3,5-triazinane-2,4,6-trione derivatives and uses thereof
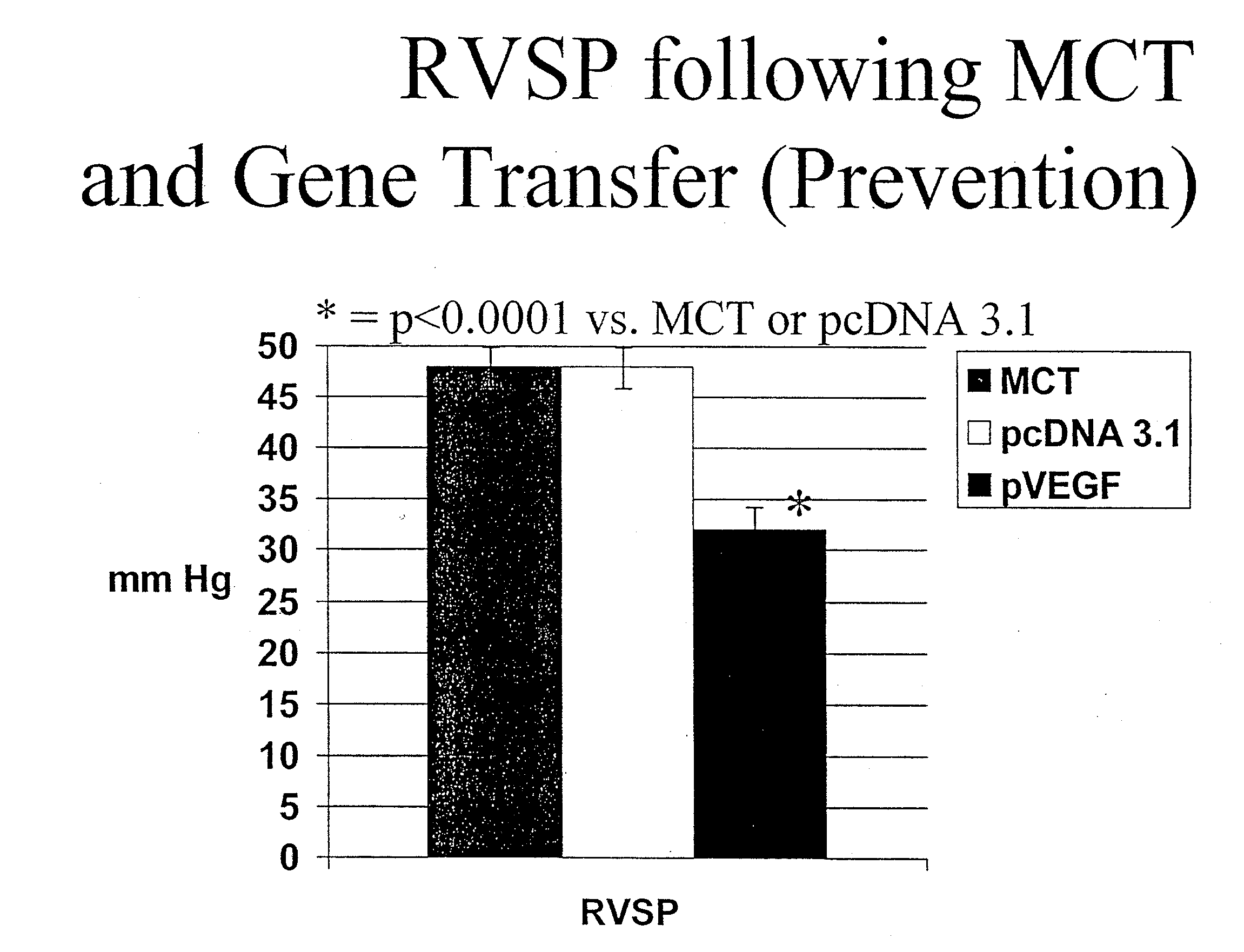
The present invention provides novel 1,3,5-triazinane-2,4,6-trione derivatives, such as compounds of any one of Formulae (I) and (II), and salts thereof, and methods of preparing the compounds. Also provided are compositions including a compound of the invention and an agent (e.g., an siRNA, mRNA, or plasmid DNA). The present invention also provides methods and kits using the compositions for delivering an agent to a subject (e.g., to the liver, spleen, or lung of the subject) or cell and for treating and / or preventing a range of diseases, such as genetic diseases, proliferative diseases, hematological diseases, neurological diseases, liver diseases, and lung diseases.
Owner:MASSACHUSETTS INST OF TECH
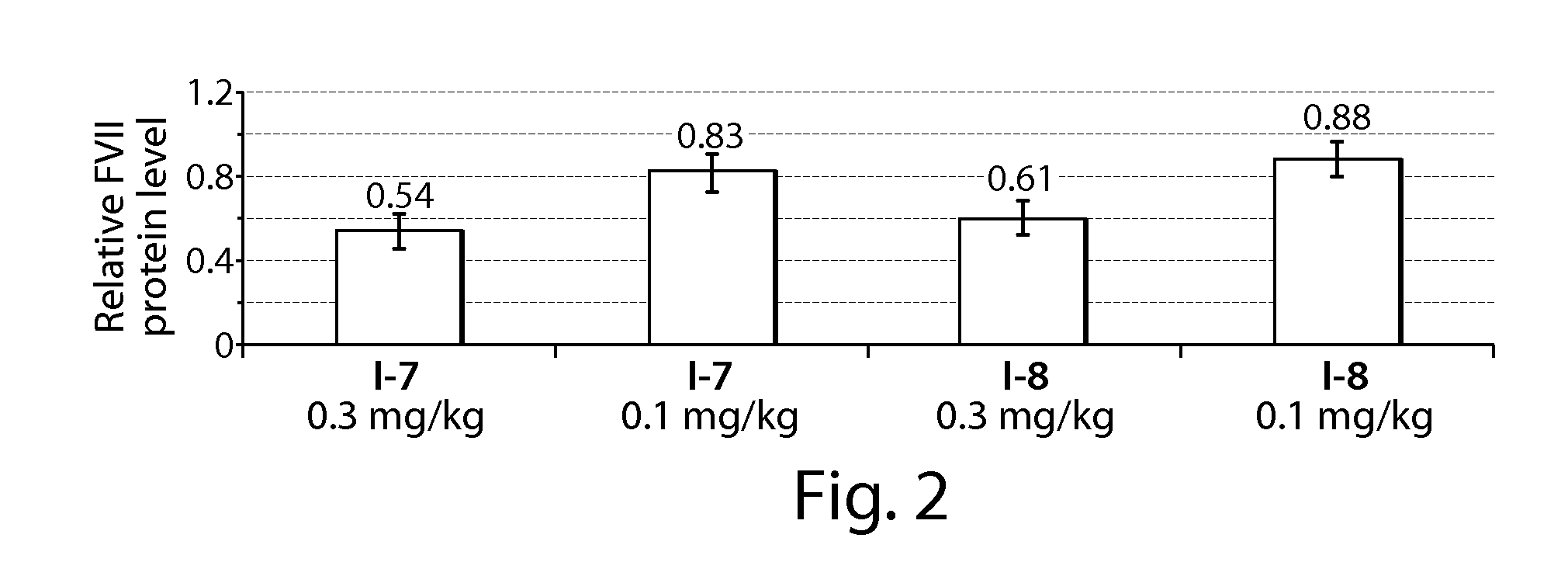
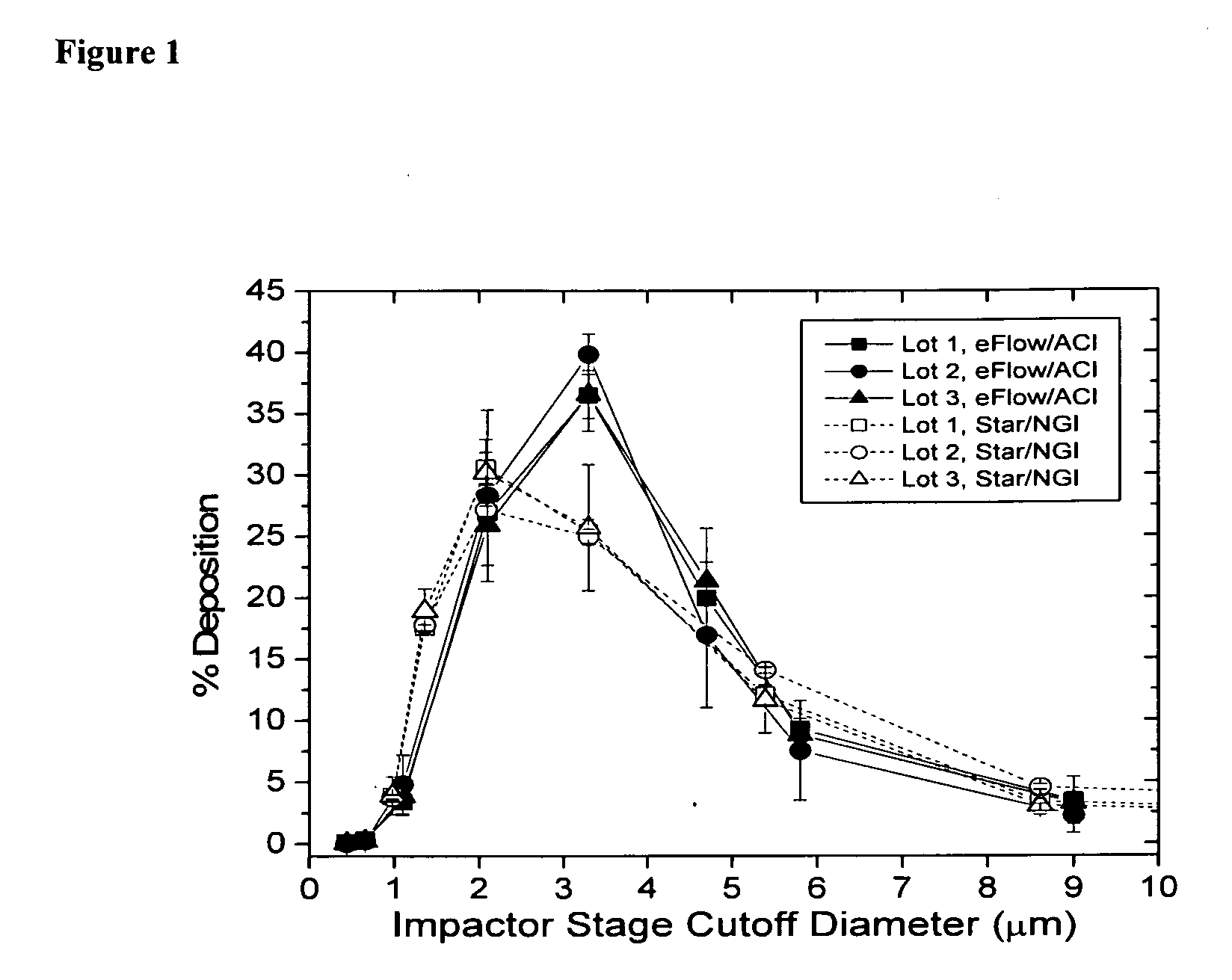
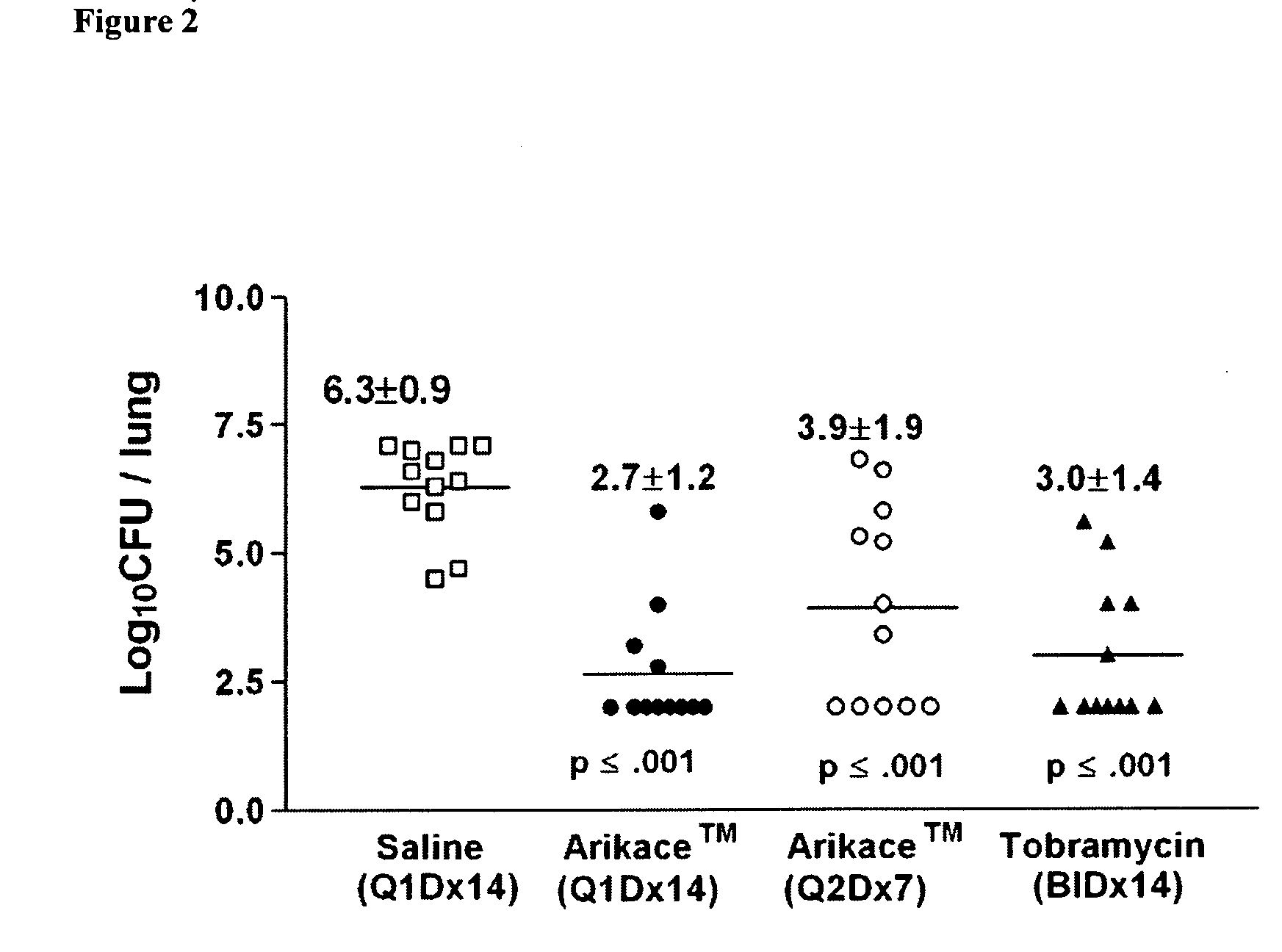
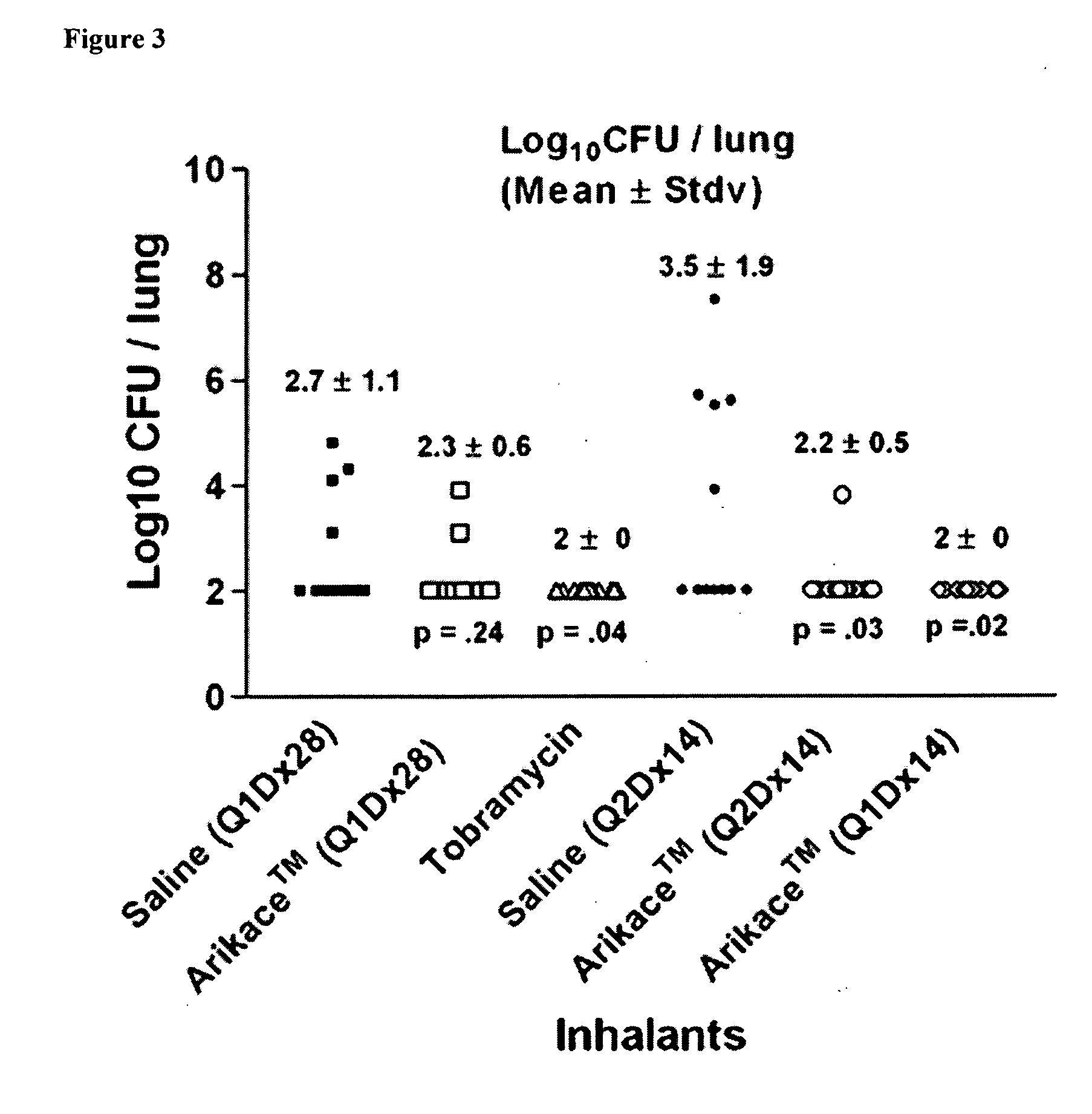
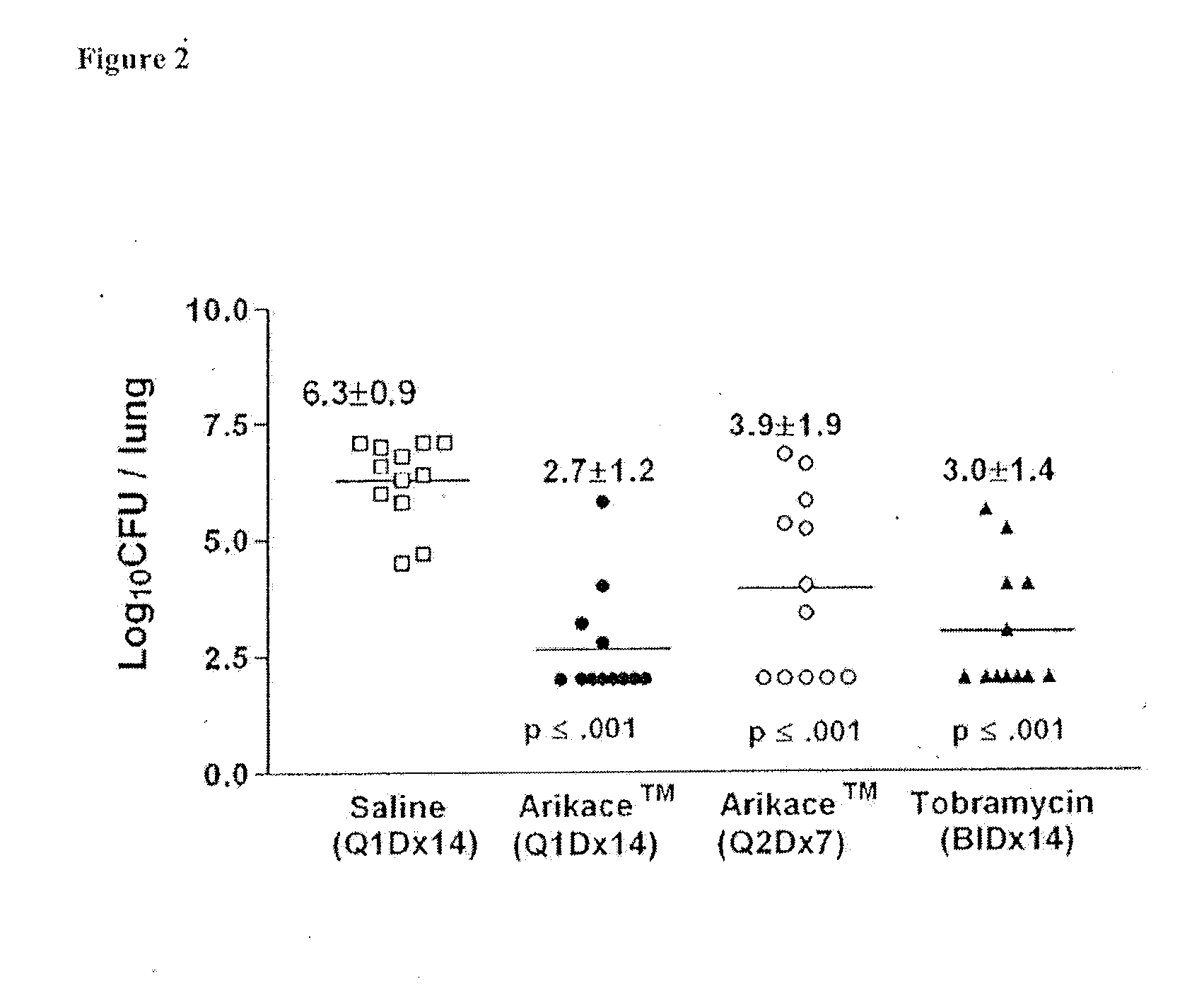
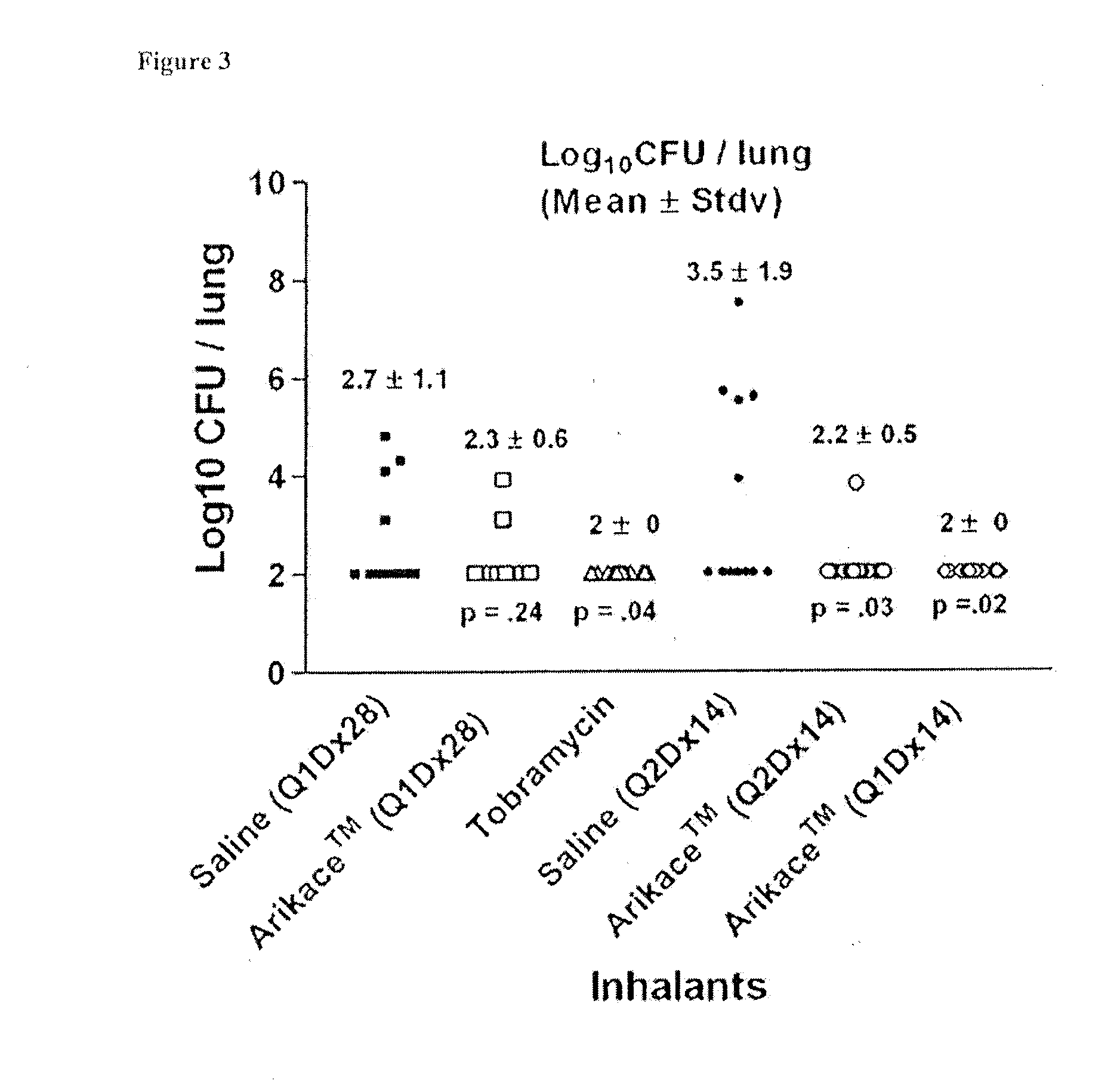
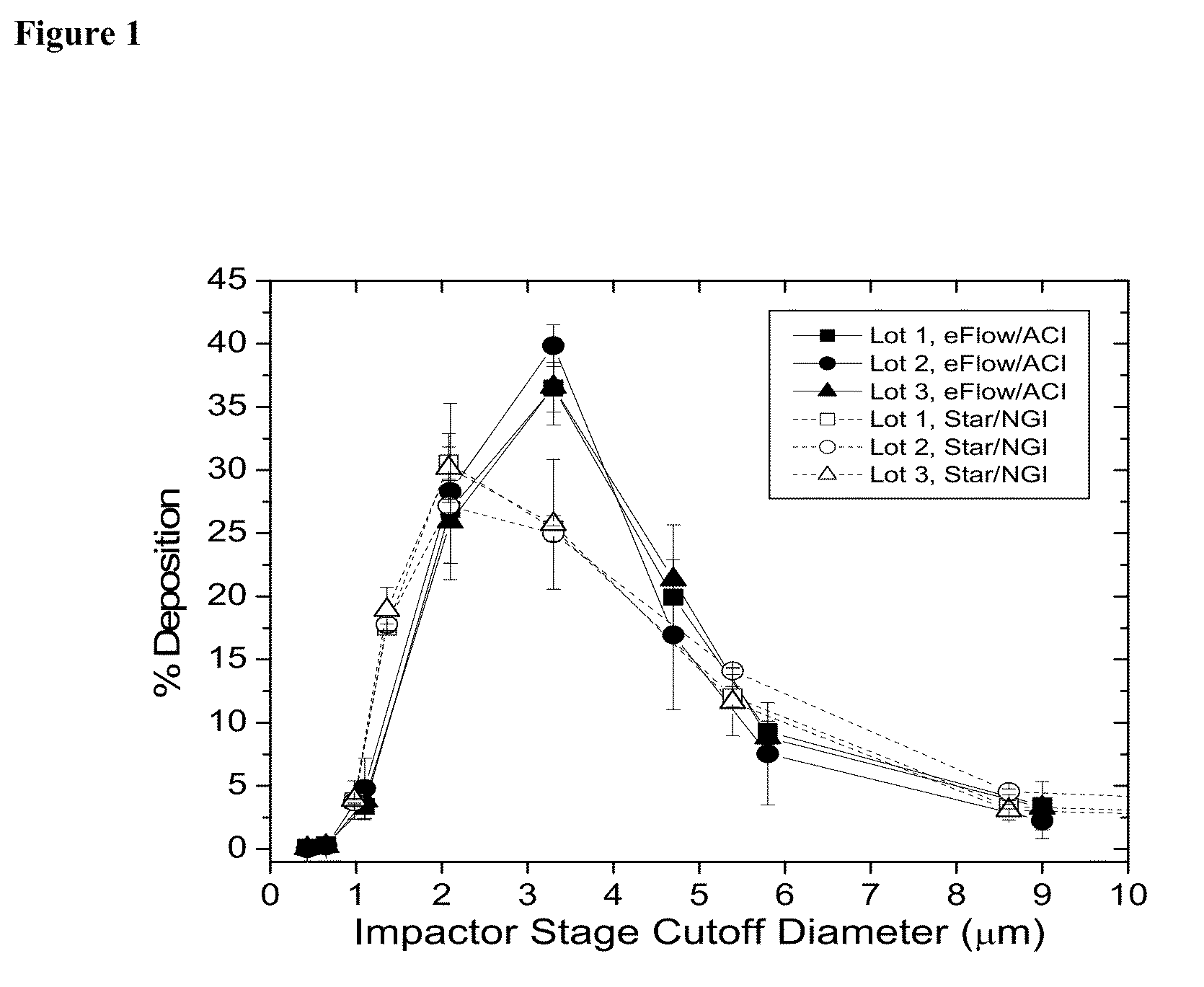
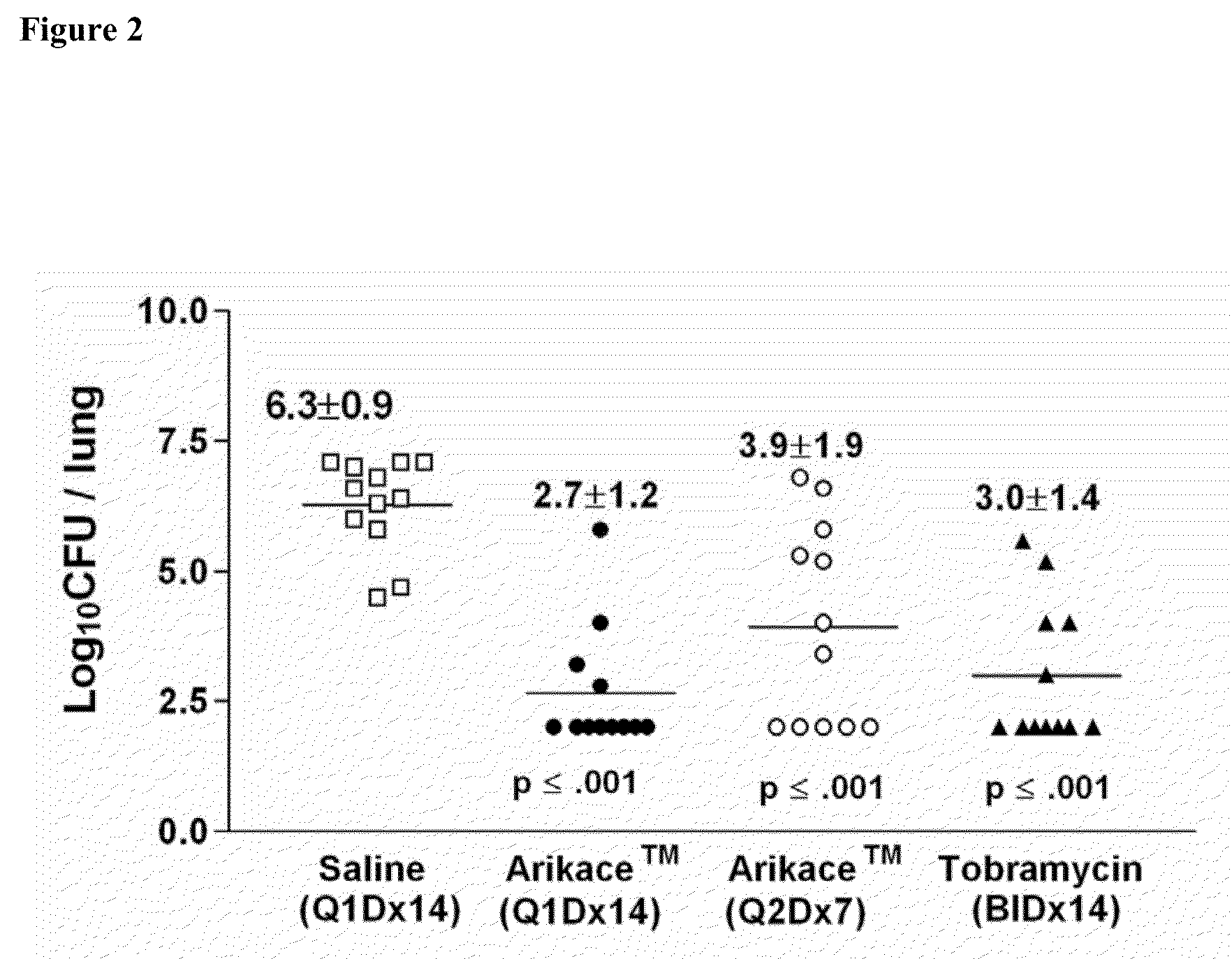
Methods of Treating Pulmonary Disorders with Liposomal Amikacin Formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
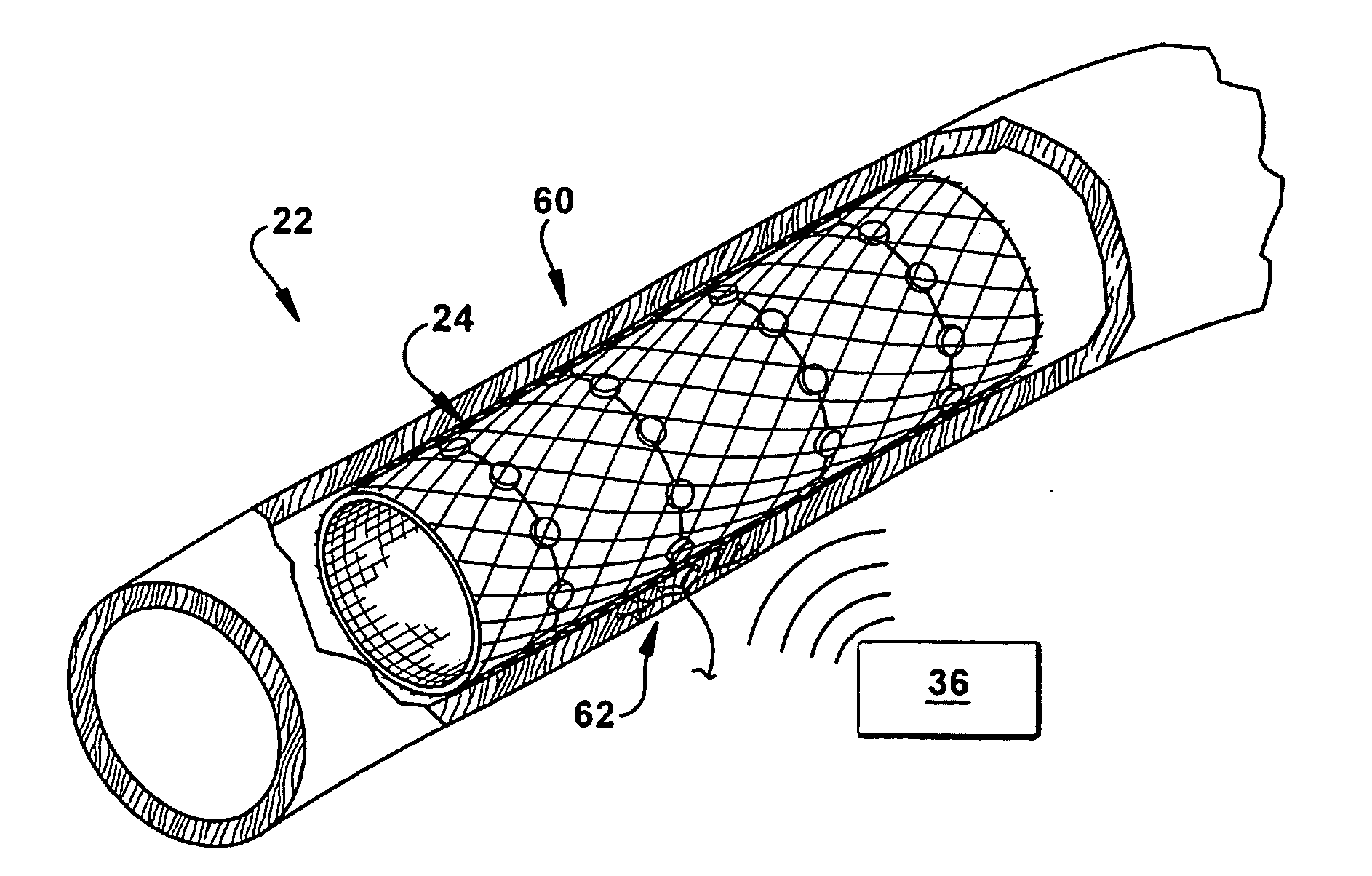
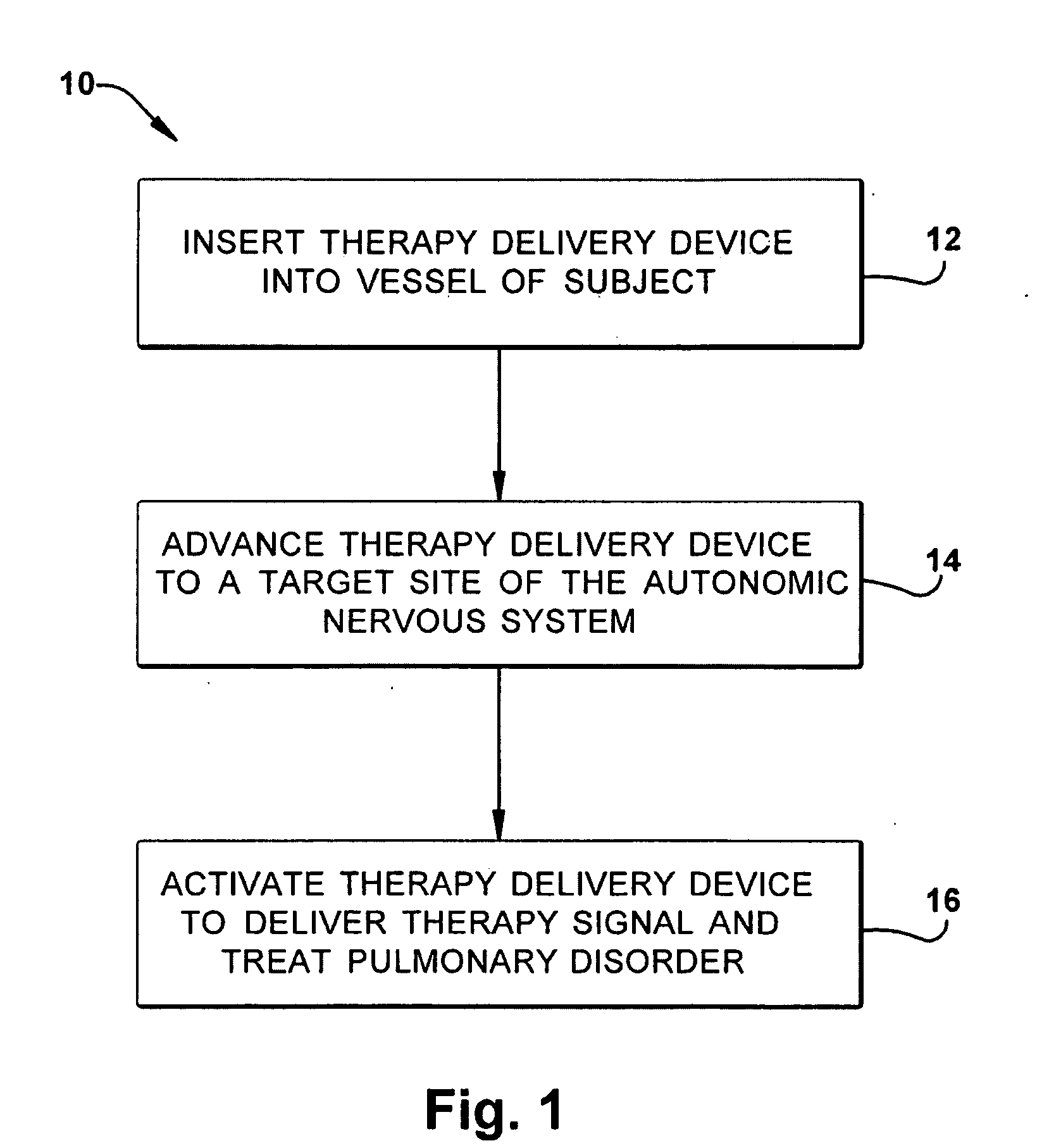
Neuromodulatory methods for treating pulmonary disorders
A method for treating a pulmonary disorder in a subject includes inserting a therapy delivery device into a vessel of the subject, advancing the therapy delivery device to a point adjacent an intraluminal target site of the autonomic nervous system, and activating the therapy delivery device to delivery a therapy signal to the intraluminal target site to treat the pulmonary disorder. The intraluminal target site is in electrical communication with nervous tissue selected from the group consisting of a spinal nerve, a postganglionic fiber of a spinal nerve, a sympathetic chain ganglion, a thoracic sympathetic chain ganglion, a cervical ganglion, a lower cervical ganglion, an inferior cervical ganglion, an intramural ganglion, a splanchnic nerve, an esophageal plexus, a cardiac plexus, a pulmonary plexus, an anterior pulmonary plexus, a posterior pulmonary plexus, a celiac plexus, a hypogastric plexus, an inferior mesenteric ganglion, a celiac ganglion, and a superior mesenteric ganglion.
Owner:THE CLEVELAND CLINIC FOUND
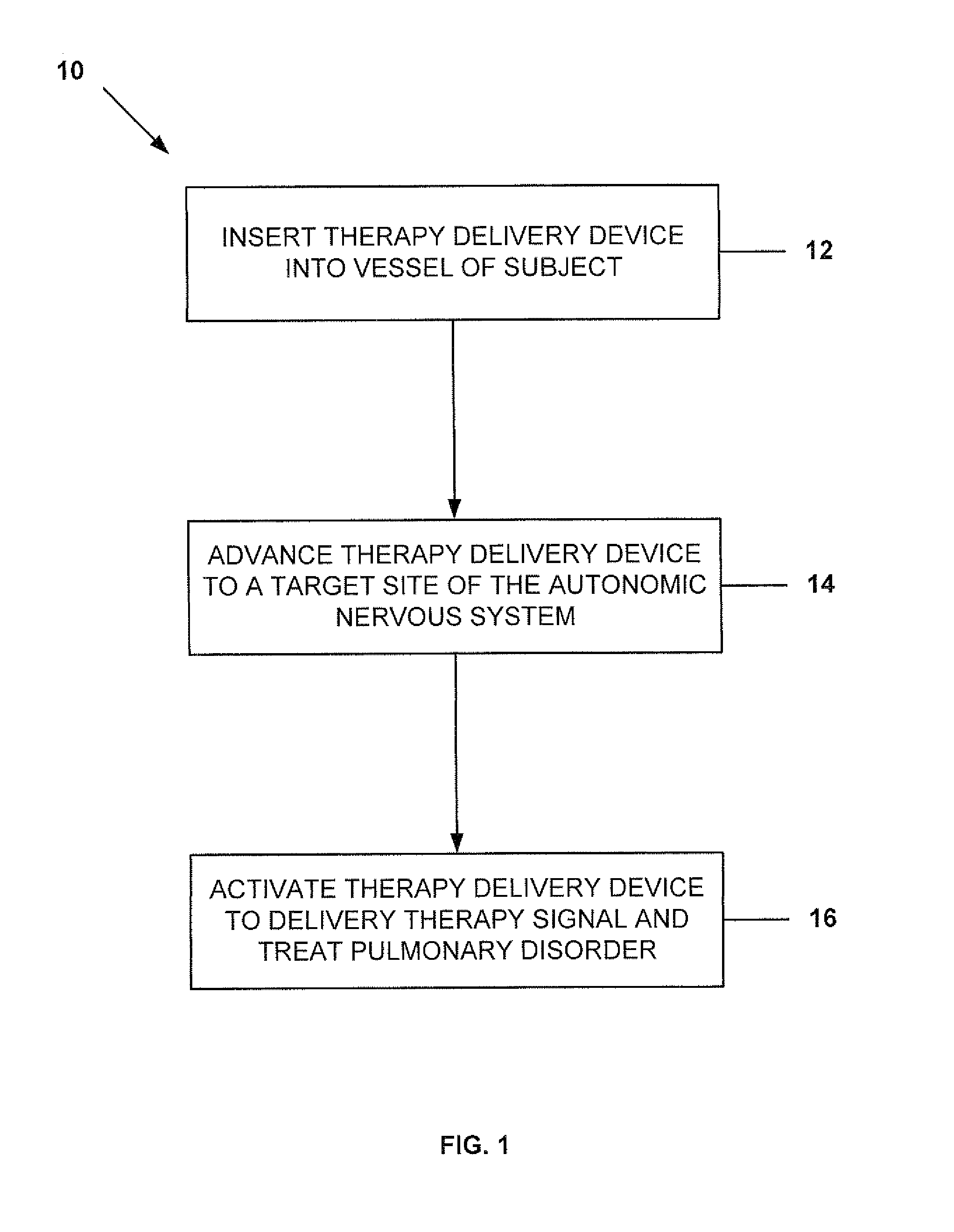
Neuromodulatory methods for treating pulmonary disorders
A method for treating asthma in a subject includes inserting a therapy delivery device into a vessel of a subject. The therapy delivery device is advanced to a point substantially adjacent an intraluminal target site of the autonomic nervous system. Next, the therapy delivery device is activated to deliver a therapy signal to the intraluminal target site to treat the asthma. The intraluminal target site is in electrical communication with nervous tissue selected from the group consisting of a spinal nerve, pre- or post-ganglionic autonomic fibers, a sympathetic chain ganglion, a thoracic sympathetic chain ganglion, a cervical ganglion, a lower cervical ganglion, an inferior cervical ganglion, an intramural ganglion, a splanchnic nerve, an esophageal plexus, a cardiac plexus, a pulmonary plexus, an anterior pulmonary plexus, a posterior pulmonary plexus, a celiac plexus, a hypogastric plexus, an inferior mesenteric ganglion, a celiac ganglion, and a superior mesenteric ganglion.
Owner:THE CLEVELAND CLINIC FOUND
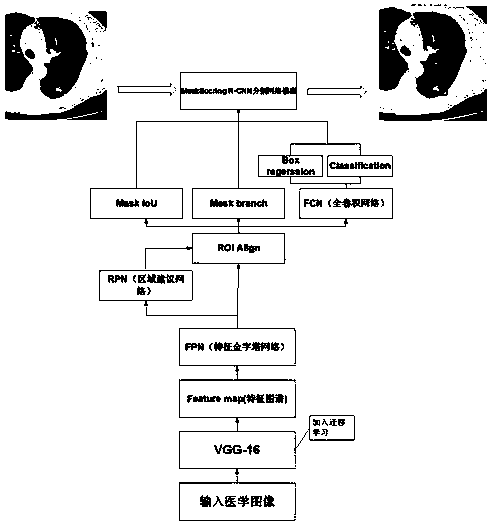
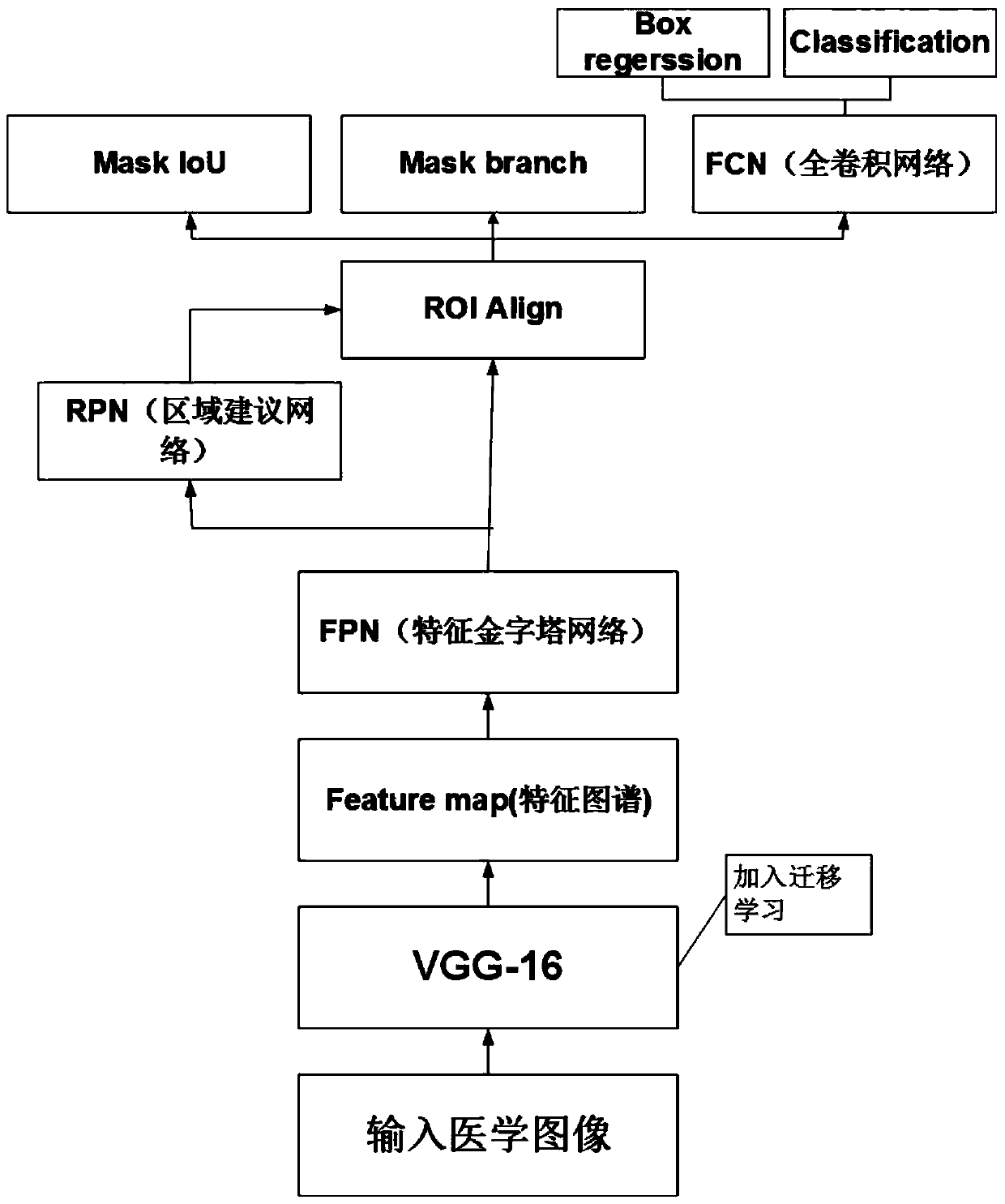
Migration learning lung lesion tissue detection system based on MaskScoring R-CNN network
ActiveCN110599448AMeet high precisionStrong generalizationImage enhancementImage analysisNetwork modelLung cancer
A migration learning lung lesion tissue detection system based on an MaskScoring R-CNN network comprises a storage module for storing four lung diseases including lung cancer, pneumonia, pulmonary tuberculosis and emphysema and further comprises a diagnosis module, and the diagnosis module is in communication connection with the storage module and comprises the following steps of 1) preprocessinga medical image; 2) constructing the MaskScoring R-CNN network model, wherein the step 2) specially comprises 1, constructing a shared convolutional neural network backbone (for feature extraction); 2, carrying out transfer learning on a shared convolutional neural network; 3, constructing an FPN network; 4, constructing an RPN network; 5, constructing an ROIAlign layer; 6, adding the MaskIoU head; and 3) identifying the lung medical image lesion tissue, inputting a to-be-detected lung CT image into the constructed MaskScoring R-CNN network, outputting and obtaining an identified image by thenetwork, framing out and masking the identified lesion tissues, and marking the lesion categories. According to the method, the requirement for high precision of medical image segmentation is met, andthe network can have the good generalization.
Owner:ZHEJIANG UNIV OF TECH
Method for treating pulmonary disorders with liposomal amikacin formulations
Disclosed herein are methods of treating pulmonary disorders comprising administering to the patient an effective dose of a nebulized liposomal amikacin formulation for at least one treatment cycle, wherein: the treatment cycle comprises an administration period of 15 to 75 days, followed by an off period of 15 to 75 days; and the effective dose comprises 100 to 2500 mg of amikacin daily during the administration period.
Owner:INSMED INC
Cell based therapy for the pulmonary system
ActiveUS20080050349A1Increase chances of survivalImprove survivabilityBiocidePeptide/protein ingredientsDiseaseSurgery
Cell based therapy comprises administration to the lung by injection into the blood system of viable, mammalian cells effective for alleviating or inhibiting pulmonary disorders. The cells may express a therapeutic transgene or the cells may be therapeutic in their own right by inducing regenerative effects.
Owner:NORTHERN THERAPEUTICS
Stimulating cranial nerve to treat pulmonary disorder
A method for stimulating a portion of a vagus nerve of a patient to treat a pulmonary disorder is provided. At least one electrode is coupled to at least one portion of a left vagus nerve and / or a right vagus nerve of the patient. An electrical signal is applied to the portion of the vagus nerve using the electrode to treat the pulmonary disorder. The electrical signal may perform a blocking of an intrinsic neural activity on said at least one portion of the left vagus nerve and said right vagus nerve.
Owner:LIVANOVA USA INC
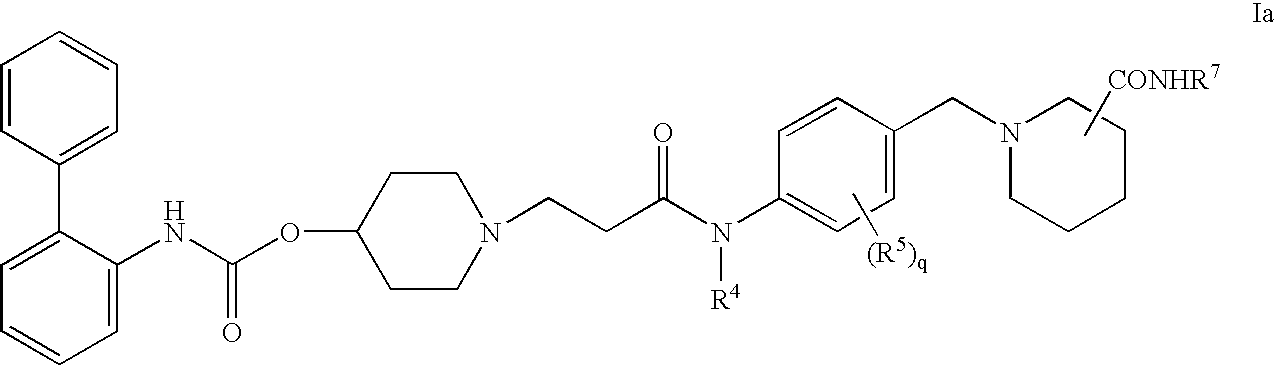
Biphenyl compounds useful as muscarinic receptor antagonists
ActiveUS20050203083A1Improve performanceReduce systemic side effectsBiocideOrganic chemistryCombinatorial chemistryAntagonist
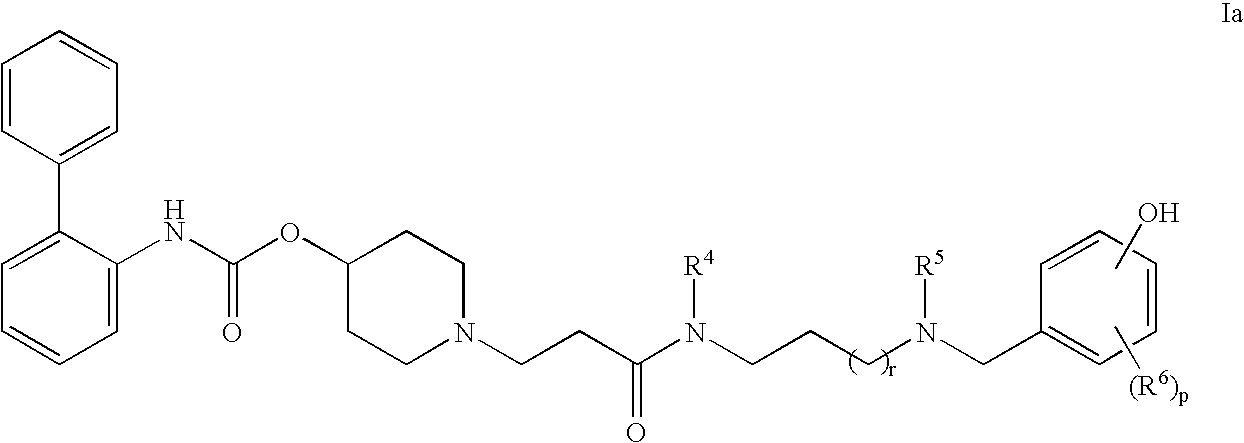
This invention provides compounds of formula I: wherein a, b, c, m, p, r, R1, R2, R3, R4, R5, R6, W and X1 are as defined in the specification. The compounds of formula I are muscarinic receptor antagonists. The invention also provides pharmaceutical compositions containing such compounds, processes and intermediates for preparing such compounds and methods of using such compounds to treat pulmonary disorders.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
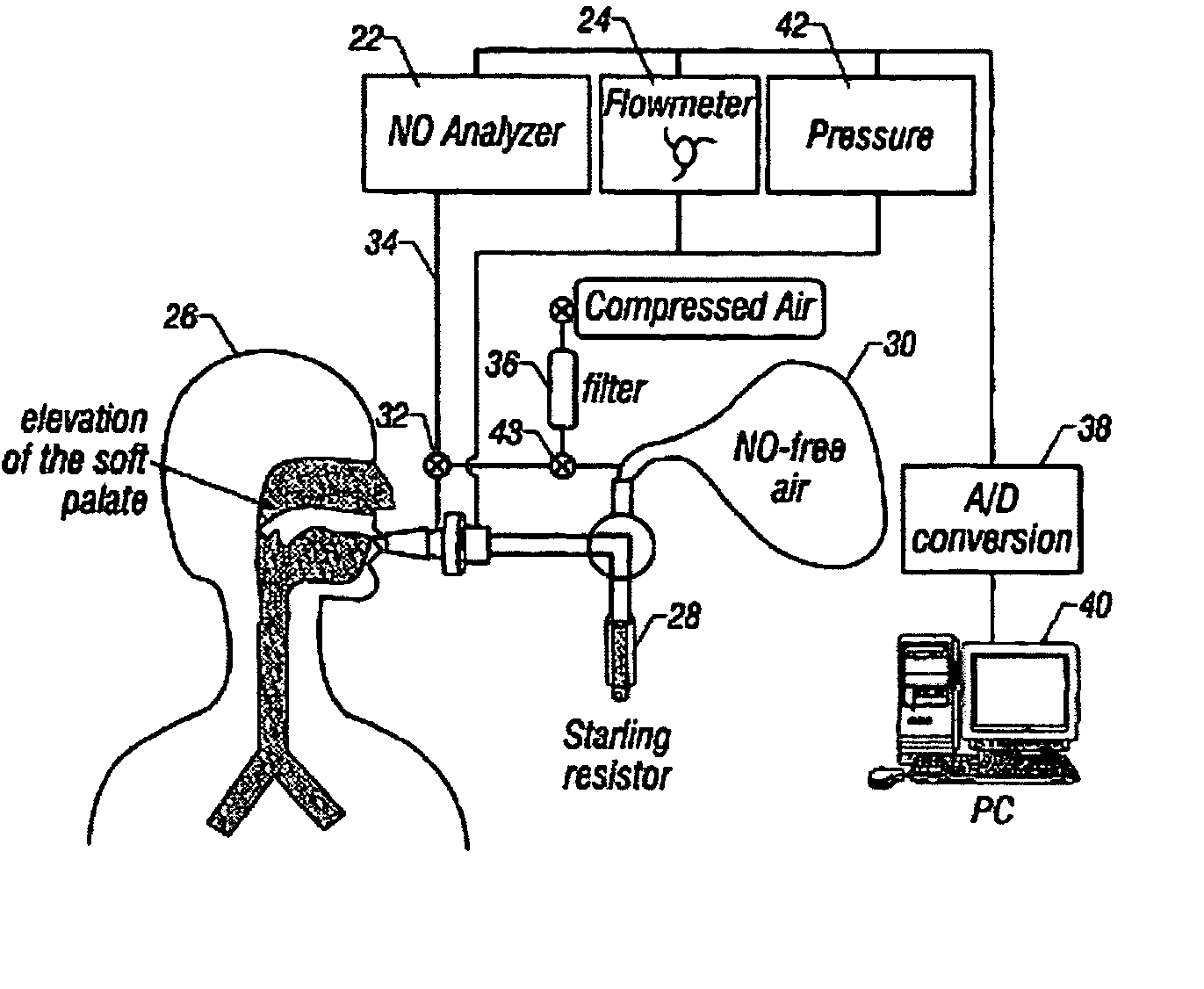
Accurate method to characterize airway nitric oxide using different breath-hold times including axial diffusion of nitric oxide using heliox and breath hold
InactiveUS20070149891A1Easy to operatePrevent nasal contaminationSurgeryRespiratory organ evaluationDiseaseHeliox
An apparatus and method to characterize NO gas exchange dynamics in human lungs comprising the steps of: (1) performing a series of breath hold maneuvers of progressively increasingly breath hold times, each breath hold maneuver comprising a) inhaling a gas, b) holding a breath for a selected time duration, and c) exhaling at a flow rate which is uncontrolled but which is effective to ensure evacuation of the airway space and (2) measuring airway NO parameters during consecutive breath hold maneuvers. As a result disease states of the lungs are diagnosed using the measured airway NO parameters.
Owner:RGT UNIV OF CALIFORNIA
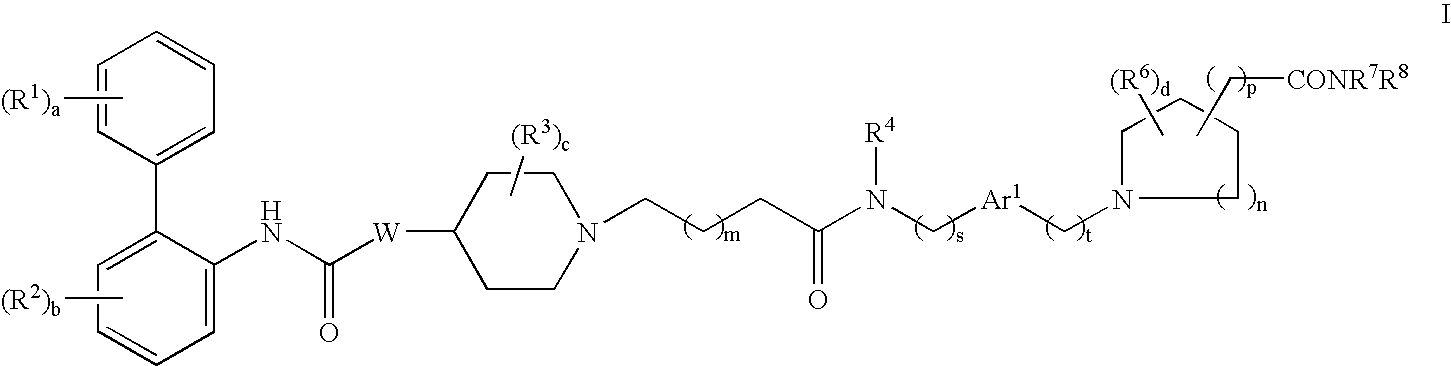
Biphenyl compounds useful as muscarinic receptor antagonists
ActiveUS20050203132A1Improve performanceReduce systemic side effectsBiocideOrganic chemistryBiphenyl compoundCombinatorial chemistry
This invention provides compounds of formula I: wherein a, b, c, d, m, n, p, r, R1, R2, R3, R4, R5, R6, R7, and W are as defined in the specification. The compounds of formula I are muscarinic receptor antagonists. The invention also provides pharmaceutical compositions containing such compounds, processes and intermediates for preparing such compounds and methods of using such compounds to treat pulmonary disorders.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Biphenyl compounds useful as muscarinic receptor antagonists
ActiveUS7560469B2Improve performanceReduce systemic side effectsBiocideOrganic chemistryCombinatorial chemistryReceptor antagonist
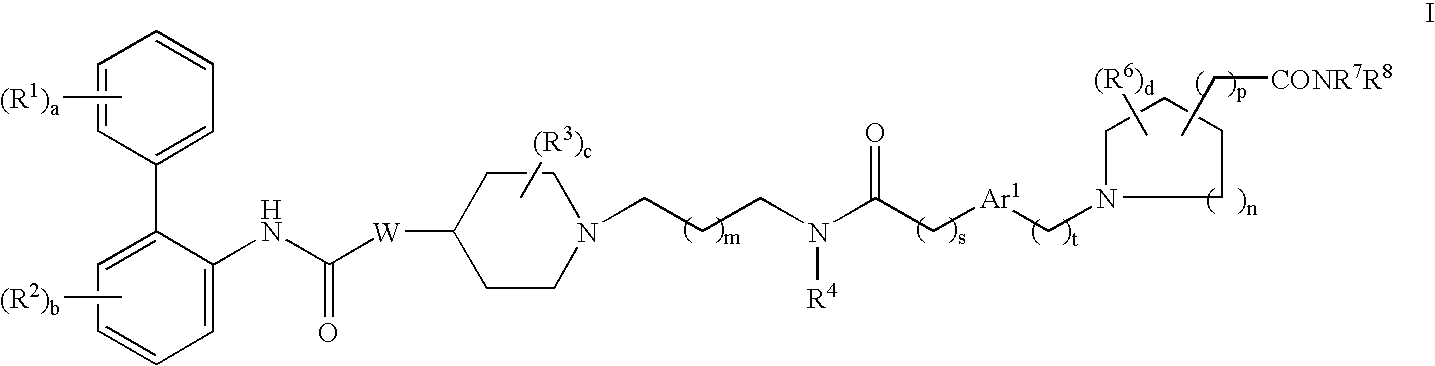
This invention provides compounds of formula I:wherein a, b, c, d, m, n, p, s, t, Ar1, R1, R2, R3, R4, R6, R7, R8, and W are as defined in the specification. The compounds of formula I are mescaline receptor antagonists. The invention also provides pharmaceutical compositions containing such compounds, processes and intermediates for preparing such compounds and methods of using such compounds to treat pulmonary disorders.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Detection methods for disorders of the lung
InactiveUS20060154278A1Induces xenobiotic and redox regulating genesReduce expressionSugar derivativesMicrobiological testing/measurementDisease riskLung cancer
The present invention is directed to prognostic and diagnostic methods to assess lung disease risk caused by airway pollutants by analyzing expression of one or more genes belonging to the airway transcriptome provided herein. Based on the finding of a so called “field defect” affecting the airways, the invention further provides a minimally invasive sample procurement method in combination with the gene expression-based tools for the diagnosis and prognosis of diseases of the lung, particularly diagnosis and prognosis of lung cancer.
Owner:TRUSTEES OF BOSTON UNIV
Biphenyl compounds useful as muscarinic receptor antagonists
ActiveUS7569588B2Long duration of actionImprove performanceBiocideOrganic chemistryStereochemistryBiphenyl compound
This invention provides compounds of formula I:wherein a, b, c, m, p, r, R1, R2, R3, R4, R5, R6, W and X1 are as defined in the specification. The compounds of formula I are muscarinic receptor antagonists. The invention also provides pharmaceutical compositions containing such compounds, processes and intermediates for preparing such compounds and methods of using such compounds to treat pulmonary disorders.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Accurate method to characterize airway nitric oxide using different breath-hold times including axial diffusion of nitric oxide using heliox and breath hold
InactiveUS7427269B2Easy to operatePrevent nasal contaminationRespiratory organ evaluationSensorsDiseaseHeliox
An apparatus and method to characterize NO gas exchange dynamics in human lungs comprising the steps of: (1) performing a series of breath hold maneuvers of progressively increasingly breath hold times, each breath hold maneuver comprising a) inhaling a gas, b) holding a breath for a selected time duration, and c) exhaling at a flow rate which is uncontrolled but which is effective to ensure evacuation of the airway space and (2) measuring airway NO parameters during consecutive breath hold maneuvers. As a result disease states of the lungs are diagnosed using the measured airway NO parameters.
Owner:RGT UNIV OF CALIFORNIA
Treating pulmonary disorders with gaseous agent causing repletion of GSNO
InactiveUS7045152B2Increased GSNO or glutathione (GSH)Avoid failureOrganic active ingredientsPeptide/protein ingredientsDiseaseMedicine
Pulmonary disorders in which the GSNO pool or glutathione pool in the lung is depleted and where reactive oxygen species in lung are increased, are treated by delivering into the lung as a gas, agent causing repletion or increase of the GSNO pool or protection against toxicity and does so independently of reaction with oxygen. Agents include ethyl nitrite, NOCl, NOBr, NOF, NOCN, N2O3, HNO, and H2S. Optionally, N-acetylcysteine, ascorbate, H2S or HNO is administered in addition to other GSNO repleting agent to potentiate the effect of said agent.
Owner:DUKE UNIV
Treatment of diseases associated with the egr-1 enhancer element
InactiveUS20070099826A1Improve understandingImprove the level ofOrganic active ingredientsPeptide/protein ingredientsFertility DisordersDisease cause
Compounds and methods are provided for treating patients suffering from health condition associated with an expression state of a gene such as fertility disorders, cancer, proliferative diseases, vascular diseases, wounds requiring therapeutic intervention, inflammation, and pulmonary disorders by administering to said patient a compound capable of modulating egr-1 and / or an egr-1 response element consensus sequence thereby altering the expression state of said gene. Also described are new methods for screening compounds to identify effectors of egr-1 and / or egr-1 consensus sequence elements and methods for treating patients by administering such effectors to modulate egr-1 and / or egr-1 consensus sequences to thereby modify expression of genes associated therewith to in turn treat diseases or other physiological conditions associated with such gene expression.
Owner:RESVERLOGIX
Composition, formulations and kit for treatment of respiratory and lung disease with non-glucocorticoid steroids and/or ubiquinone and a bronchodilating agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a non-glucocorticoid steroid or analogues, a ubiquinone, or salts thereof, and a second active agent comprising a bronchodilator. The composition is provided in various formulations and in the form of a kit The products of this patent are applied to the prophylaxis and treatment of respiratory, lung and malignant diseases.
Owner:EPIGENESIS PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com