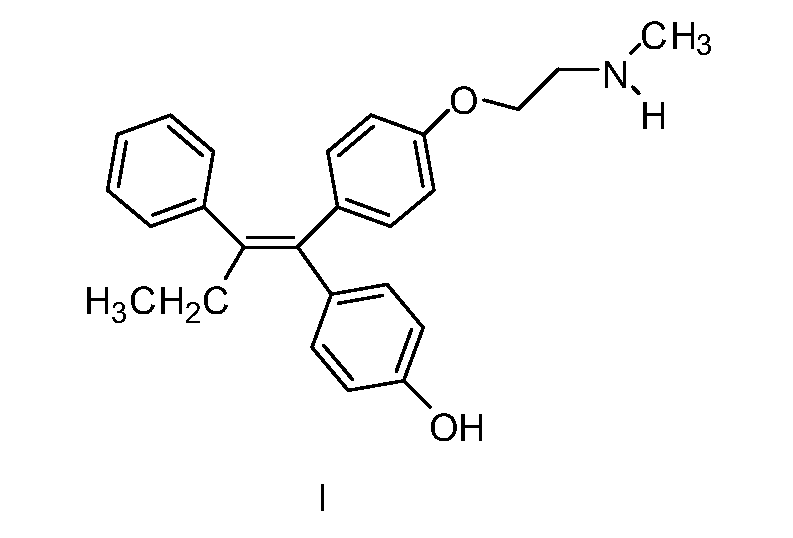
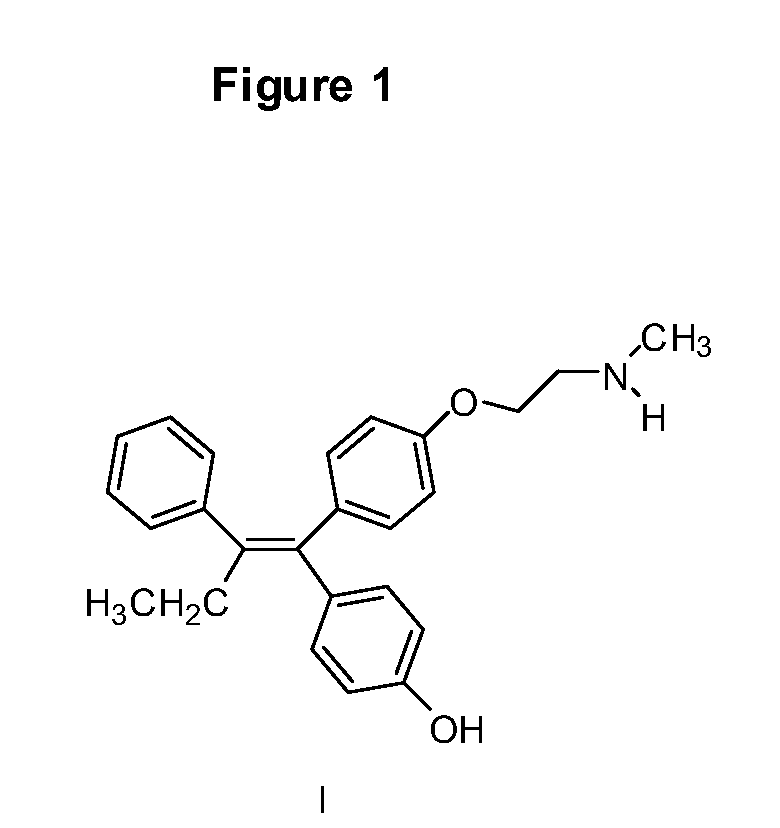
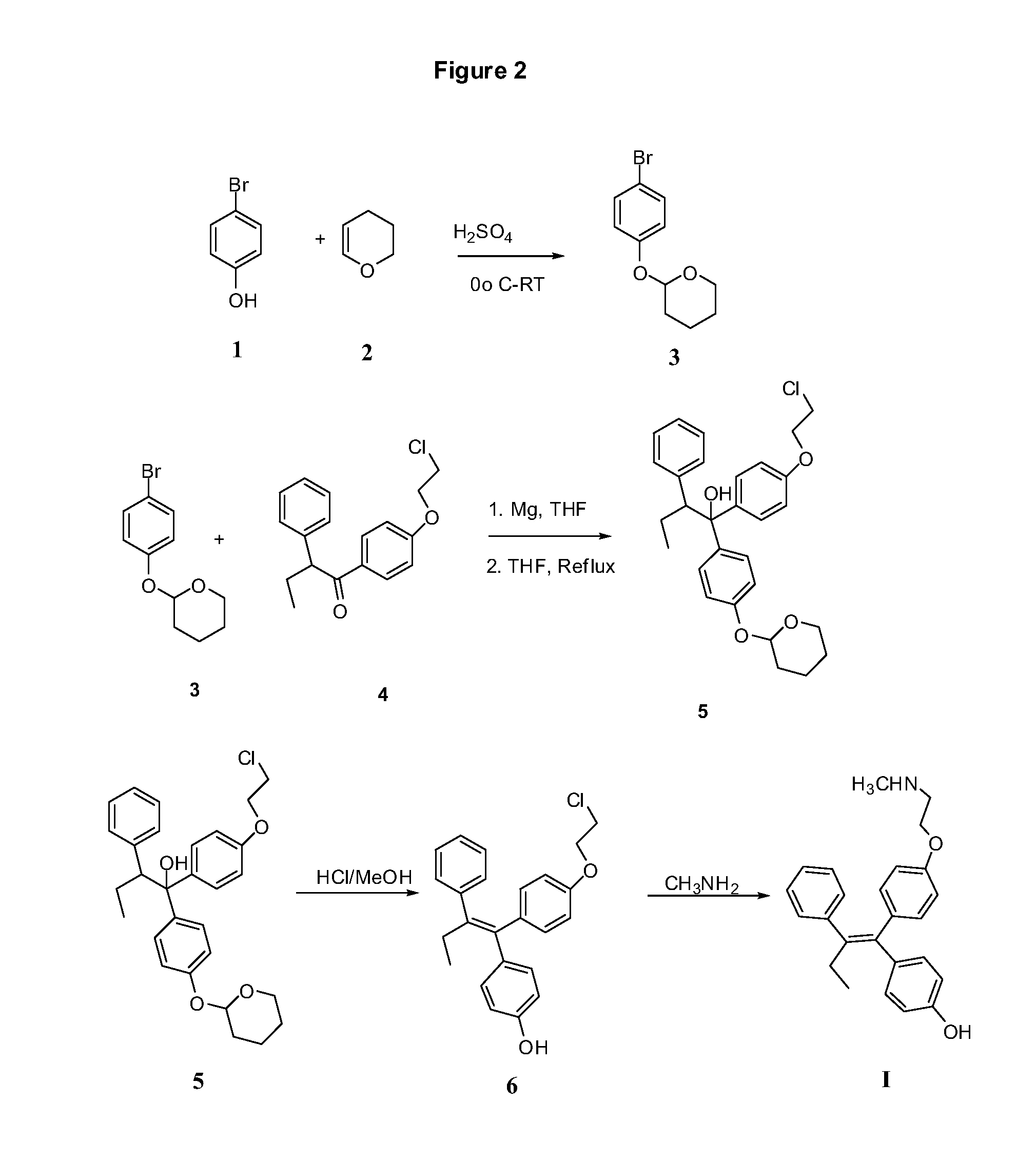
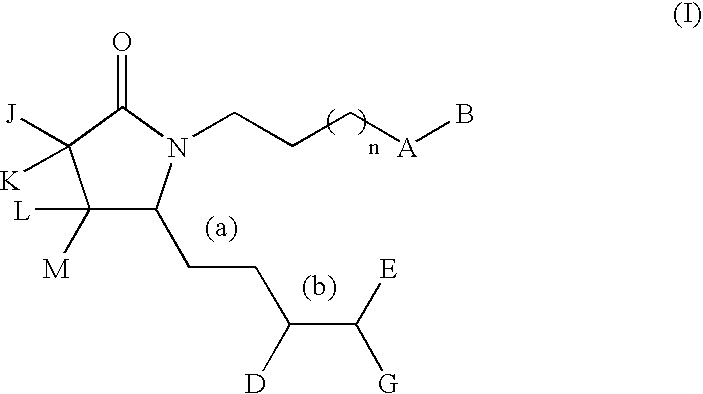
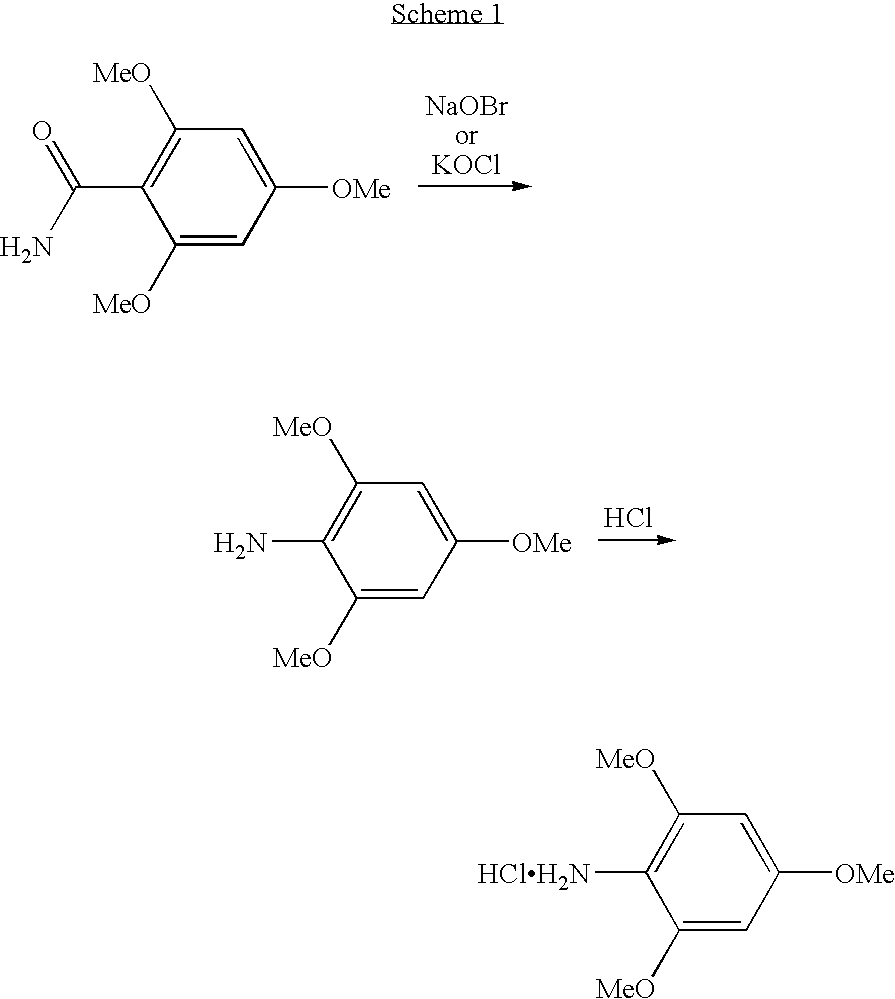
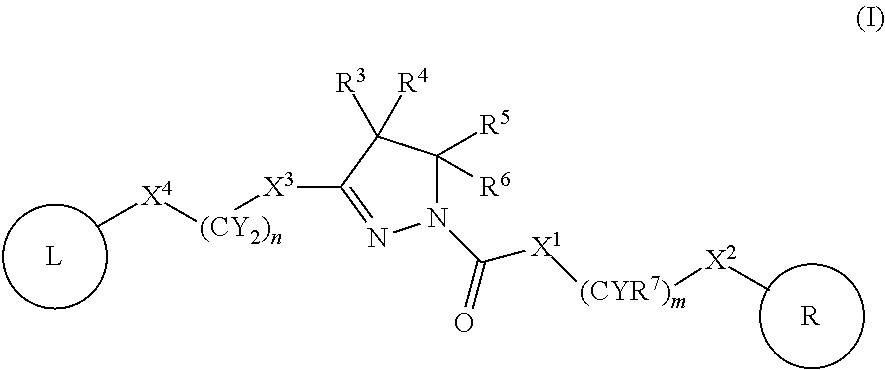
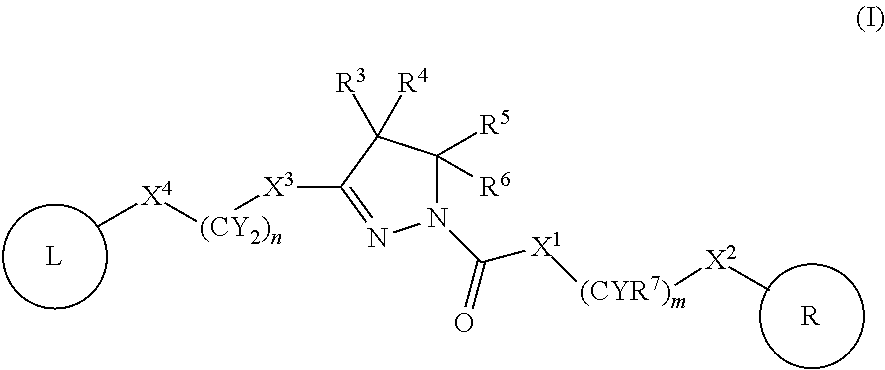
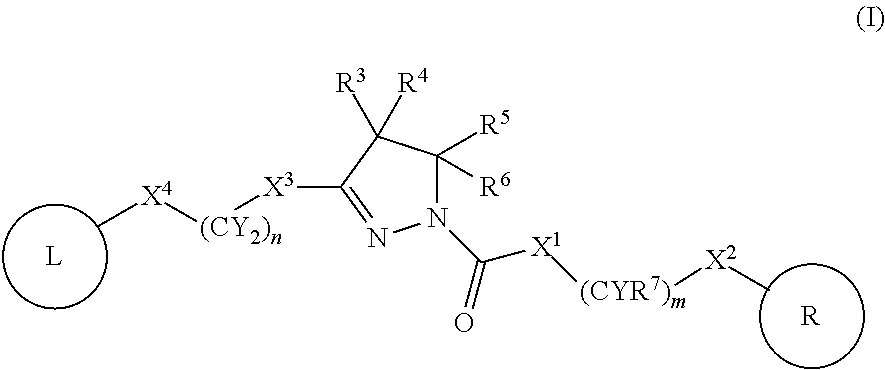
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Fertility Disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ovulation disorders are responsible for approximately 25 percent of female infertility problems. Anovulation (failure to ovulate) and oligoovulation (irregular ovulatory cycles) are among the most common disorders.
Polymer-based compositions for sustained release
InactiveUS20040028733A1Reduced activityReduce aggregationPowder deliveryPeptide/protein ingredientsFollicle-stimulating hormoneSpermatogenesis
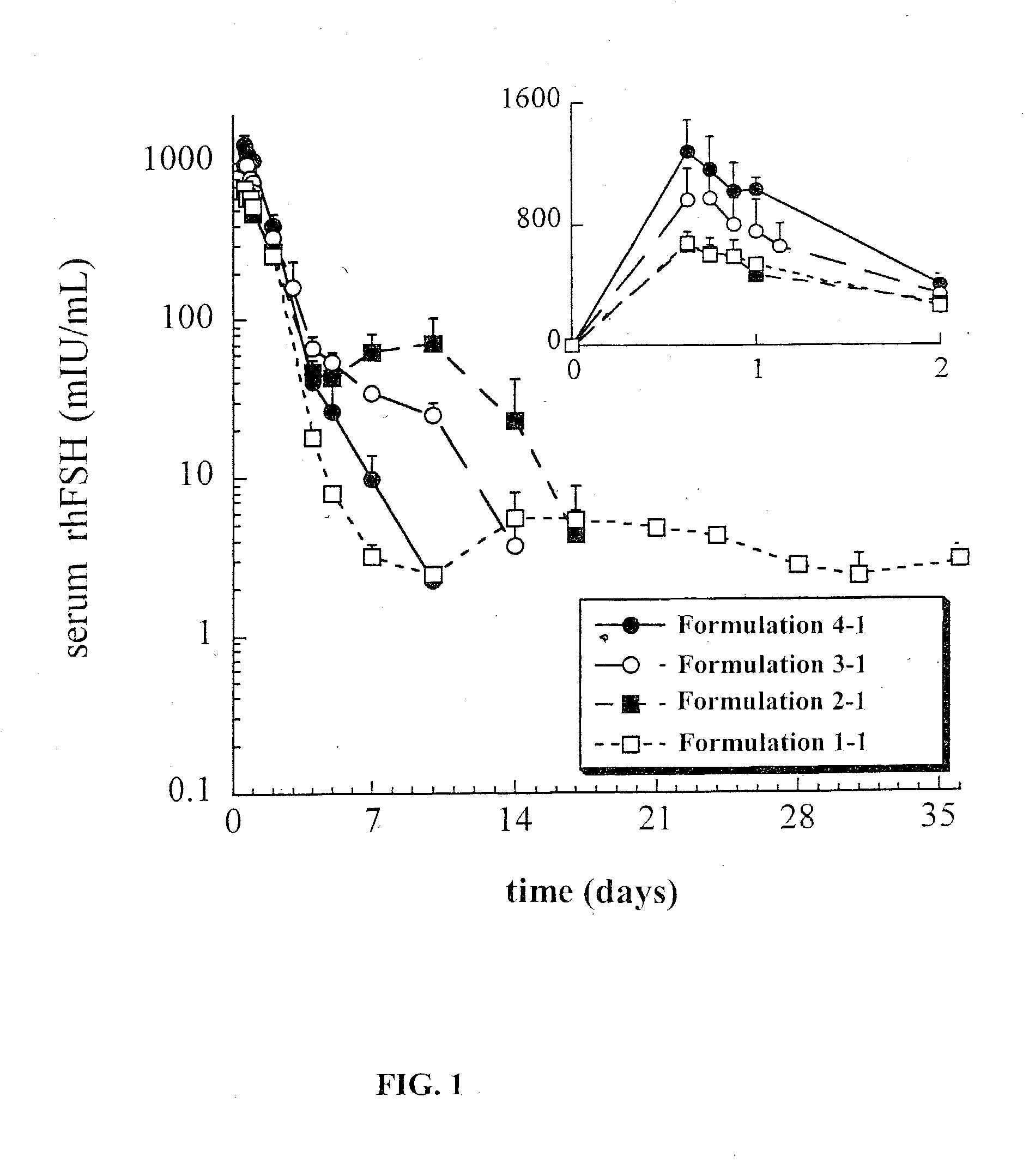
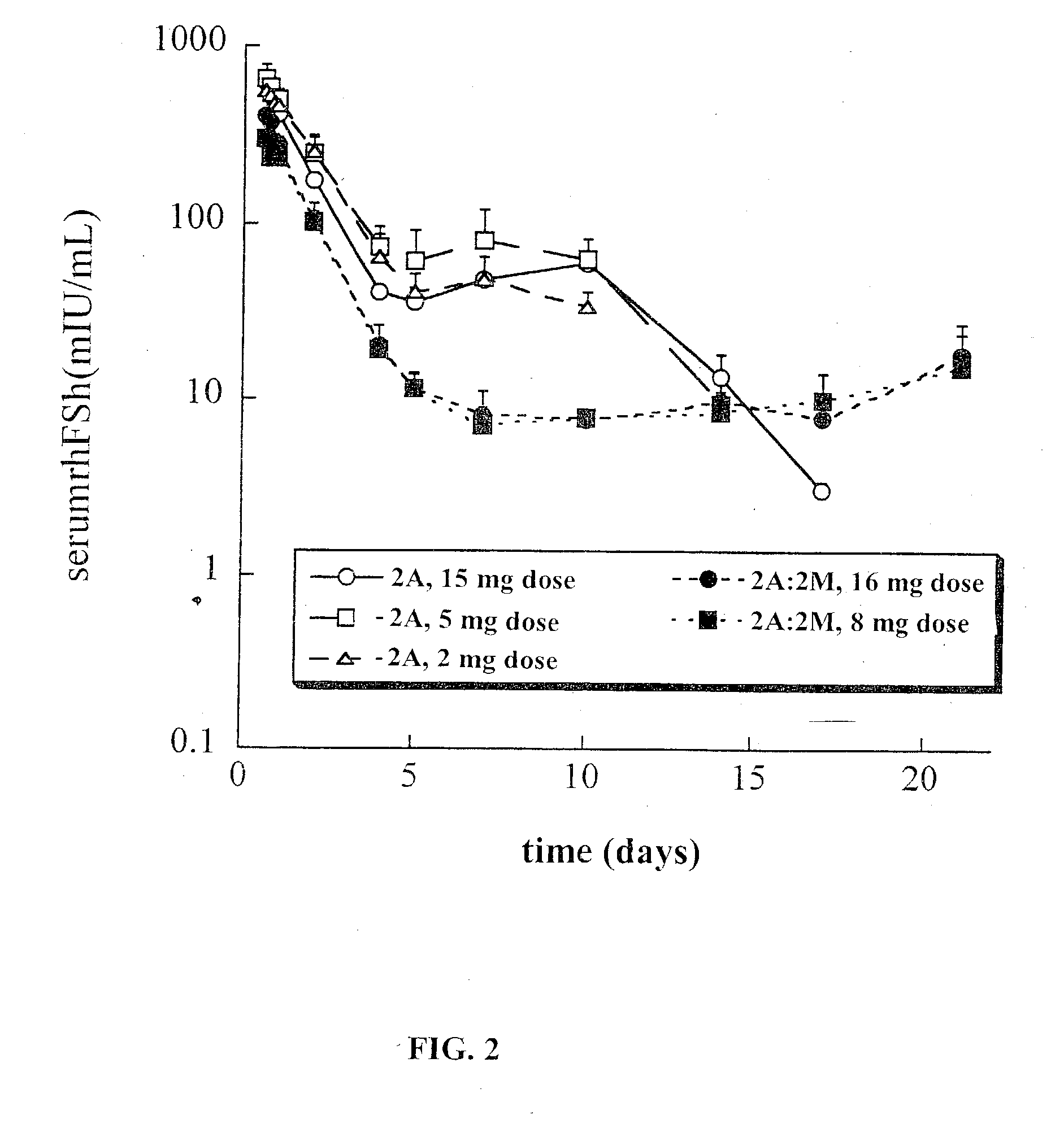
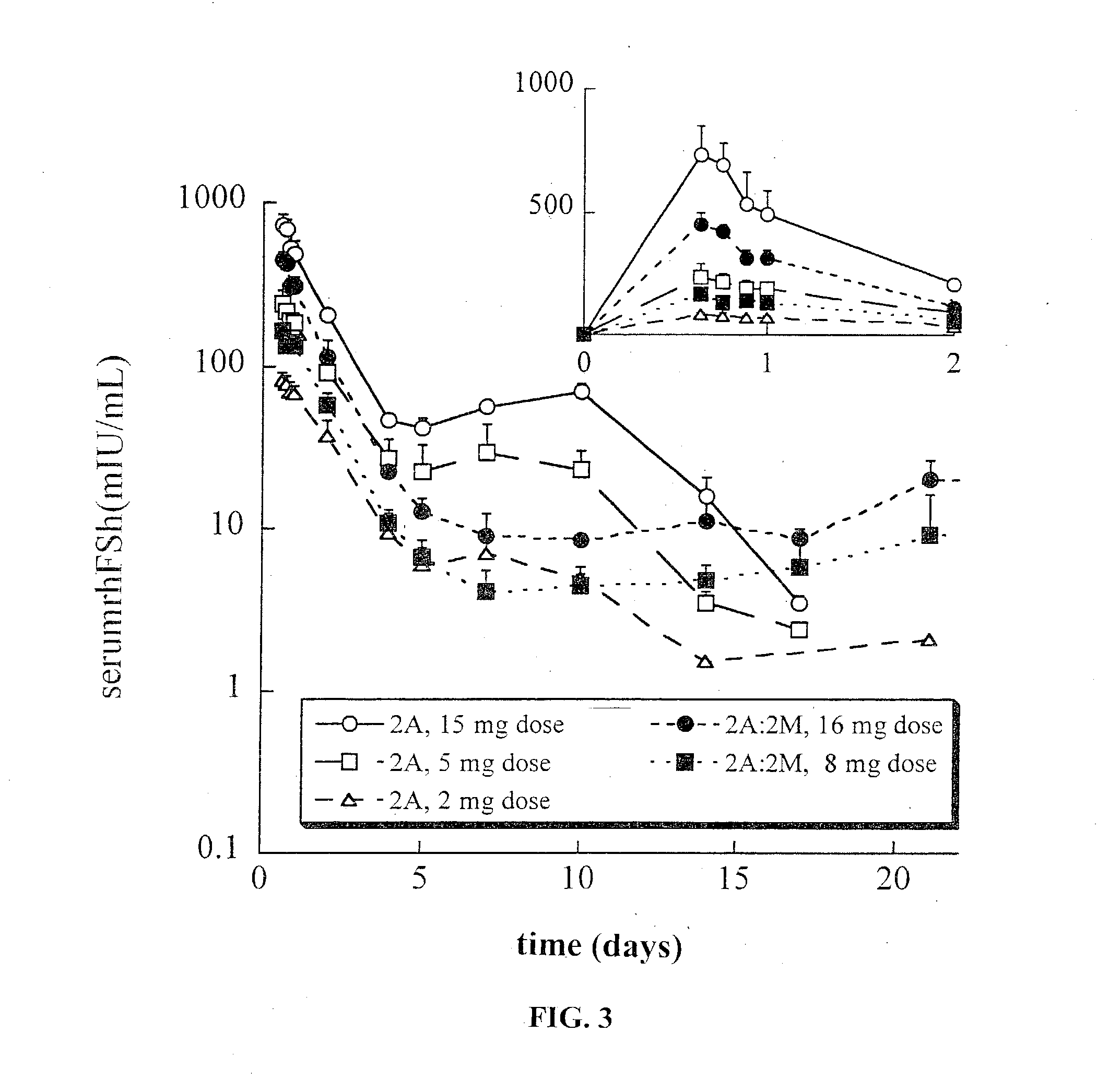
This invention relates to sustained release compositions, and methods of forming and using said compositions, in particular for the sustained release of Follicle Stimulating Hormone (FSH). The sustained release compositions comprise a polymeric matrix of a biodegradable biocompatible polymer and stabilized FSH. The method of the invention for forming a sustained release composition includes, dissolving a biodegradable biocompatible polymer in a polymer solvent to form a polymer solution; adding biologically active stabilized FSH; removing the solvent; and solidifying the polymer to form a polymer matrix containing stabilized FSH dispersed therein. Also described is a method for providing a therapeutically effective amount of stabilized FSH in a patient in need of for a sustained period comprising administering to the patient a dose of the sustained release compositions of the invention. The sustained release composition of FSH can be used to promote maturation of follicles, promote spermatogenesis and to treat fertility disorders.
Owner:MERCK SERONO INT
Treatment of diseases associated with the egr-1 enhancer element
InactiveUS20070099826A1Improve understandingImprove the level ofOrganic active ingredientsPeptide/protein ingredientsFertility DisordersDisease cause
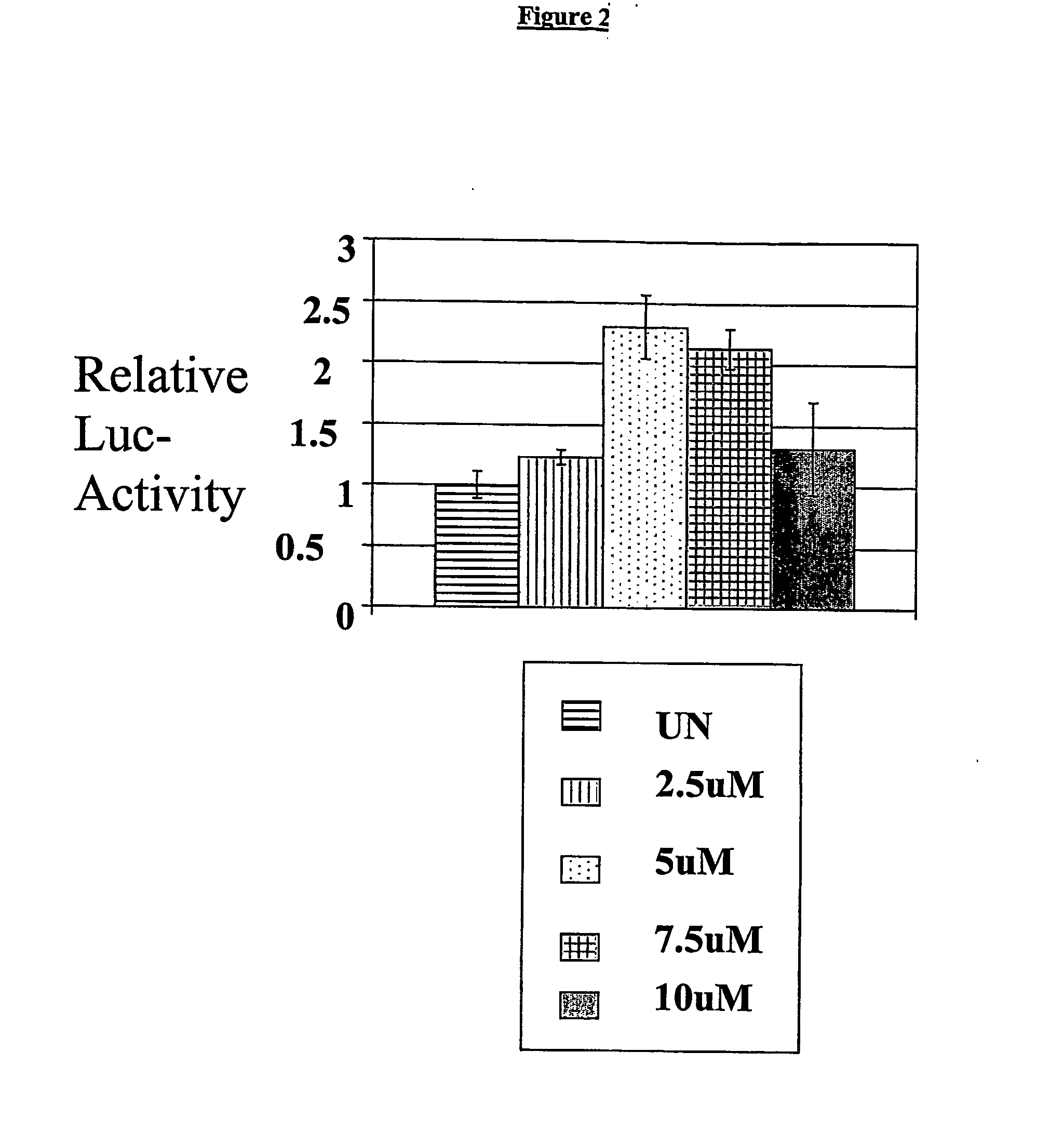
Compounds and methods are provided for treating patients suffering from health condition associated with an expression state of a gene such as fertility disorders, cancer, proliferative diseases, vascular diseases, wounds requiring therapeutic intervention, inflammation, and pulmonary disorders by administering to said patient a compound capable of modulating egr-1 and / or an egr-1 response element consensus sequence thereby altering the expression state of said gene. Also described are new methods for screening compounds to identify effectors of egr-1 and / or egr-1 consensus sequence elements and methods for treating patients by administering such effectors to modulate egr-1 and / or egr-1 consensus sequences to thereby modify expression of genes associated therewith to in turn treat diseases or other physiological conditions associated with such gene expression.
Owner:RESVERLOGIX
Endoxifen methods and compositions in the treatment of mammalian diseases
InactiveUS20120164075A1Particular effectivenessAntibacterial agentsOrganic active ingredientsFertility DisordersLiposome
The present invention provides compositions containing endoxifen, formulations and liposomes of endoxifen, methods of preparation of such agents and formulations, and use of such agents and formulations for the treatment of a subject having or at risk for psychiatric and neurodegenerative diseases, infectious diseases, fertility disorders, osteoporosis, osteoarthritis, and / or cardiovascular diseases. Specifically, the present invention relates to compositions comprising endoxifen for use in the treatment of such disorders or predisposition to such disorders, for use in manufacture of medicaments for treating such disorders, and methods comprising use of such compositions in such treatments.
Owner:JINA PHARMA
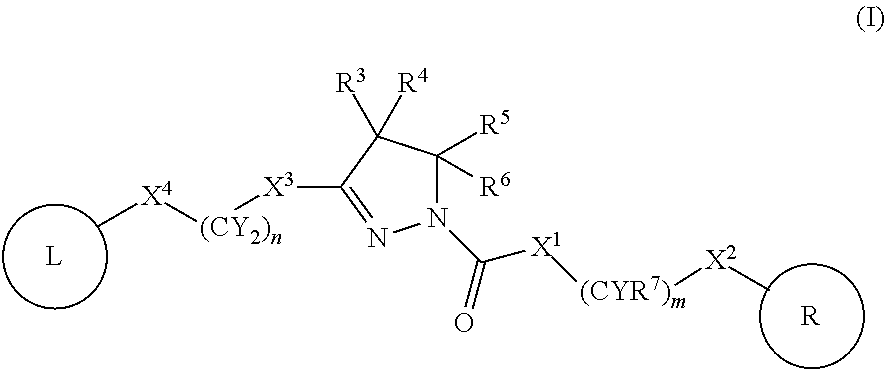
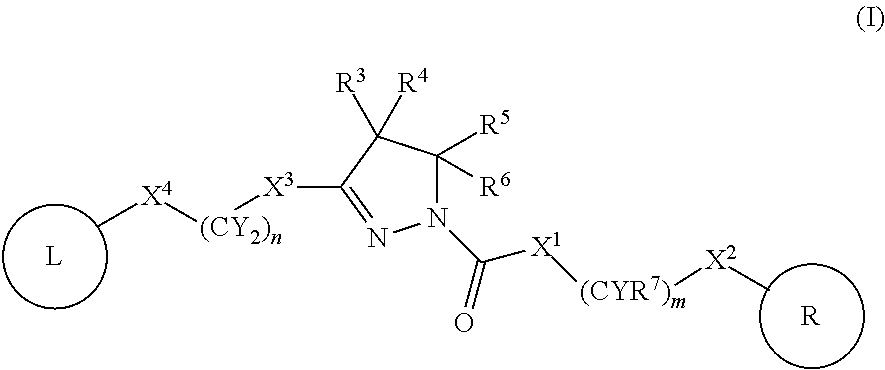
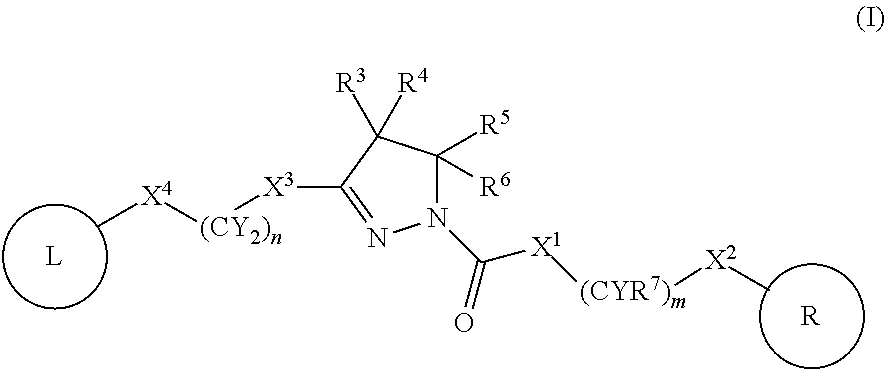
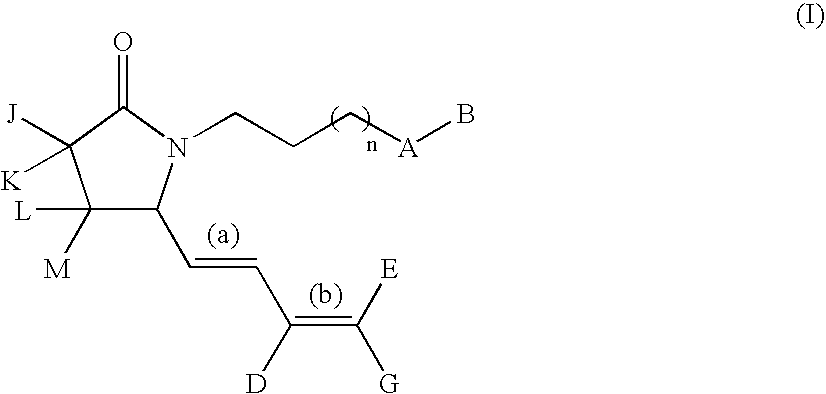
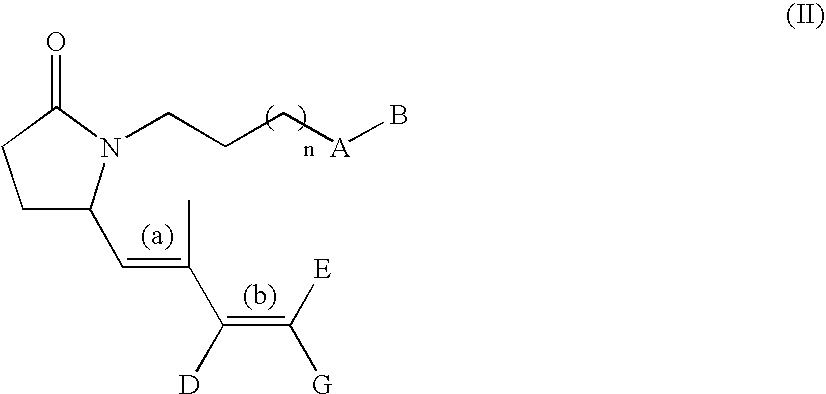
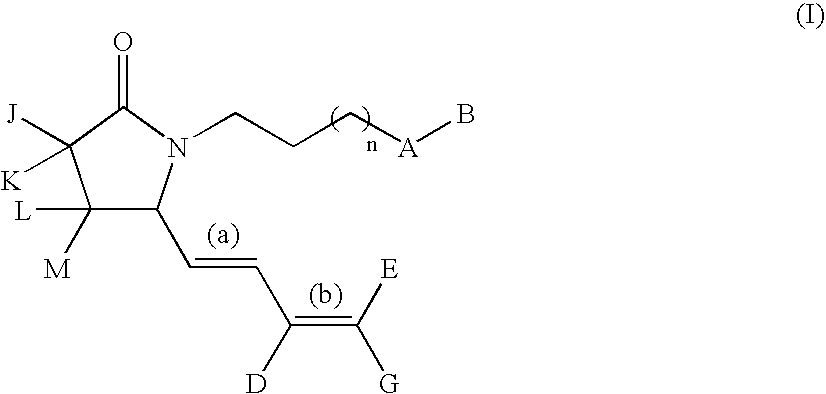
G-lactam derivatives as prostaglandin agonists
Owner:MERCK SERONO SA
Very long chain polyunsaturated fatty acids, methods of production, and uses
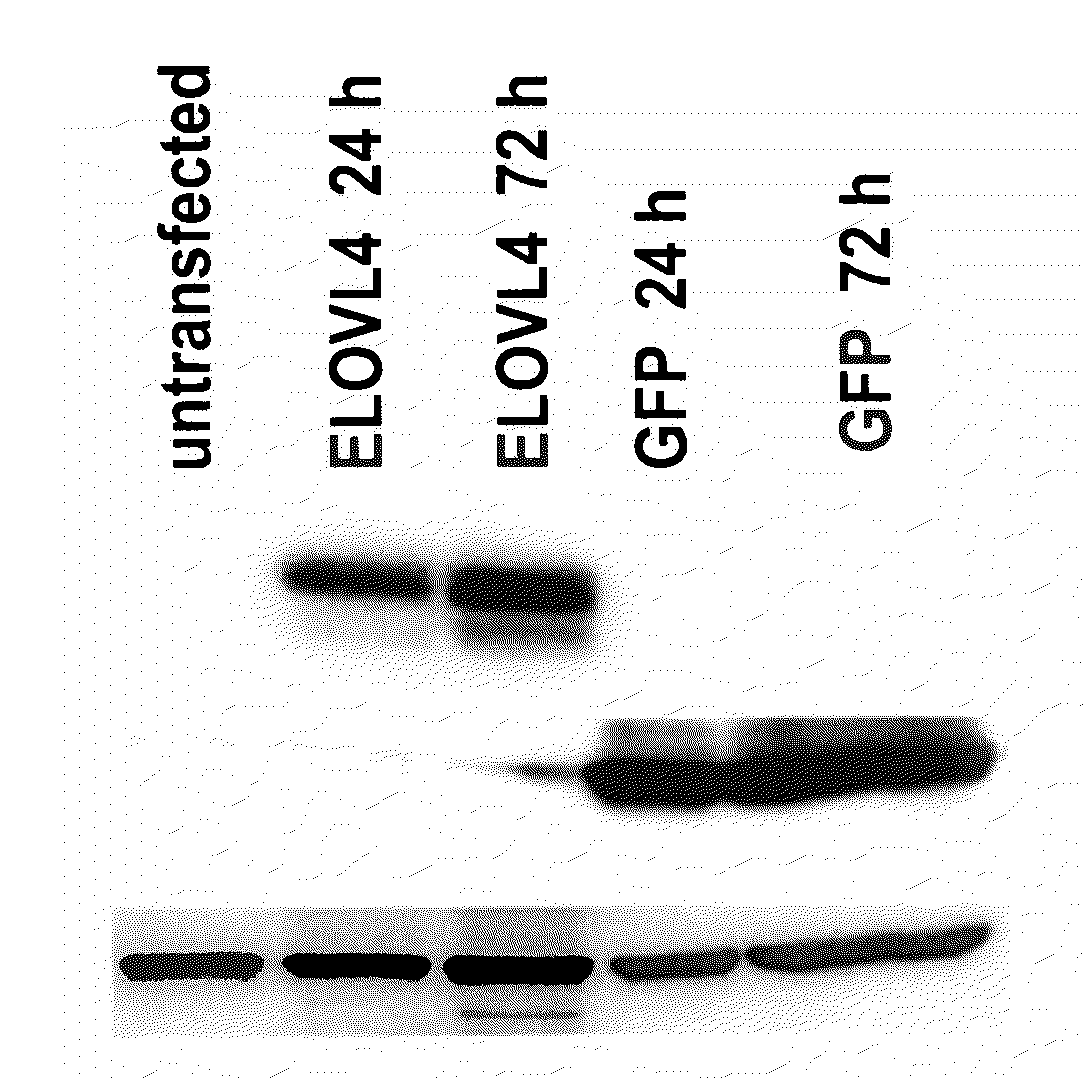
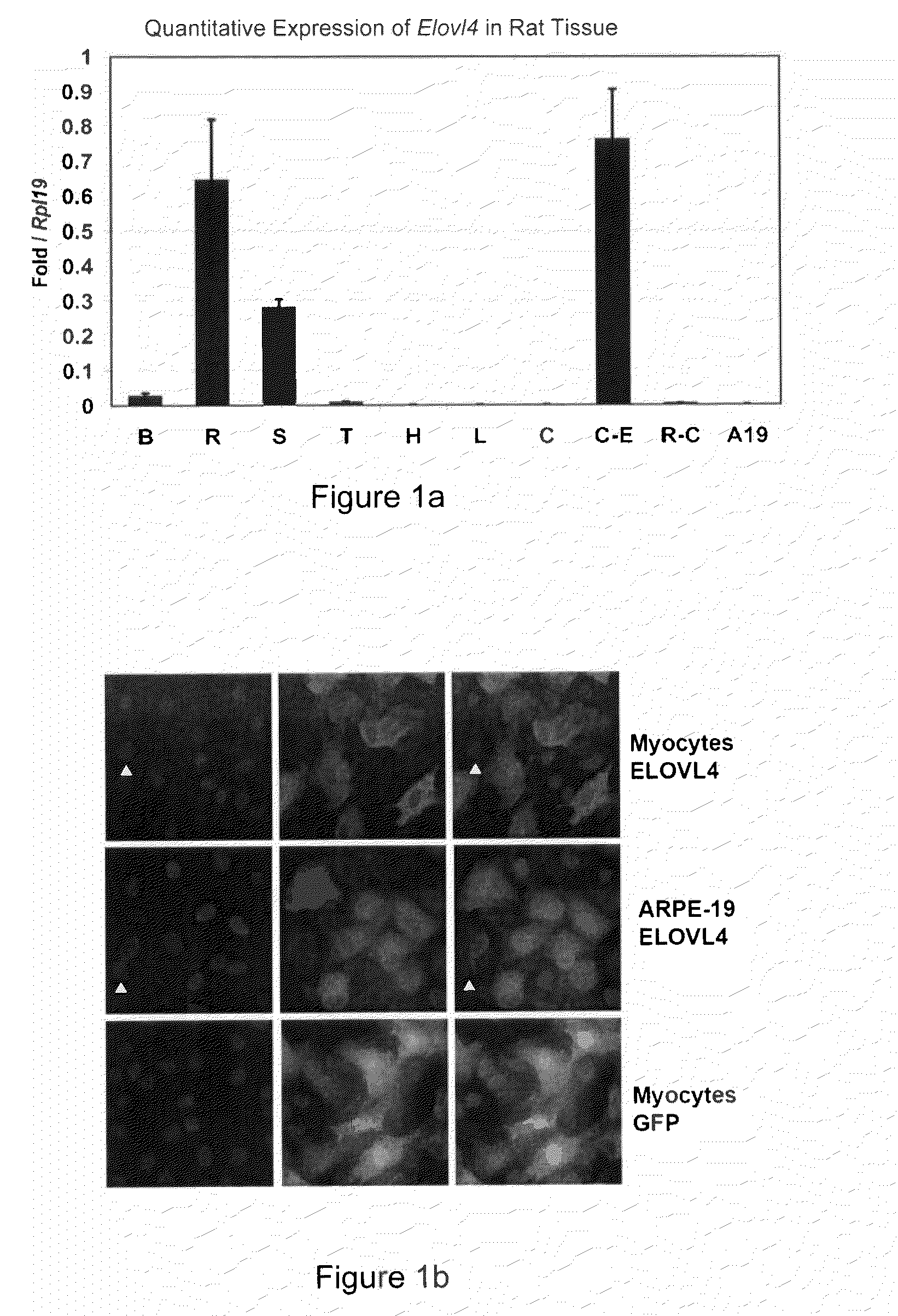
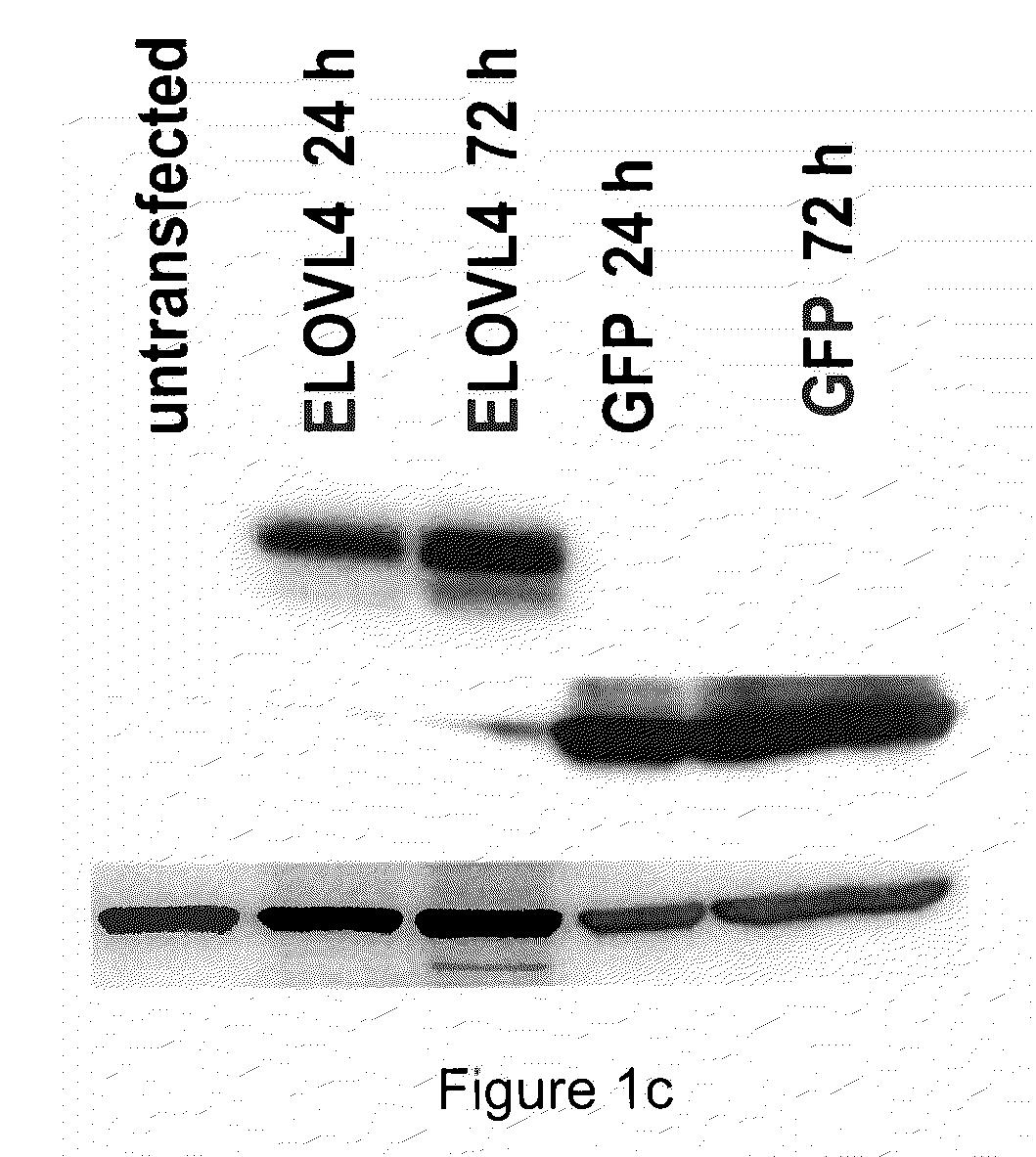
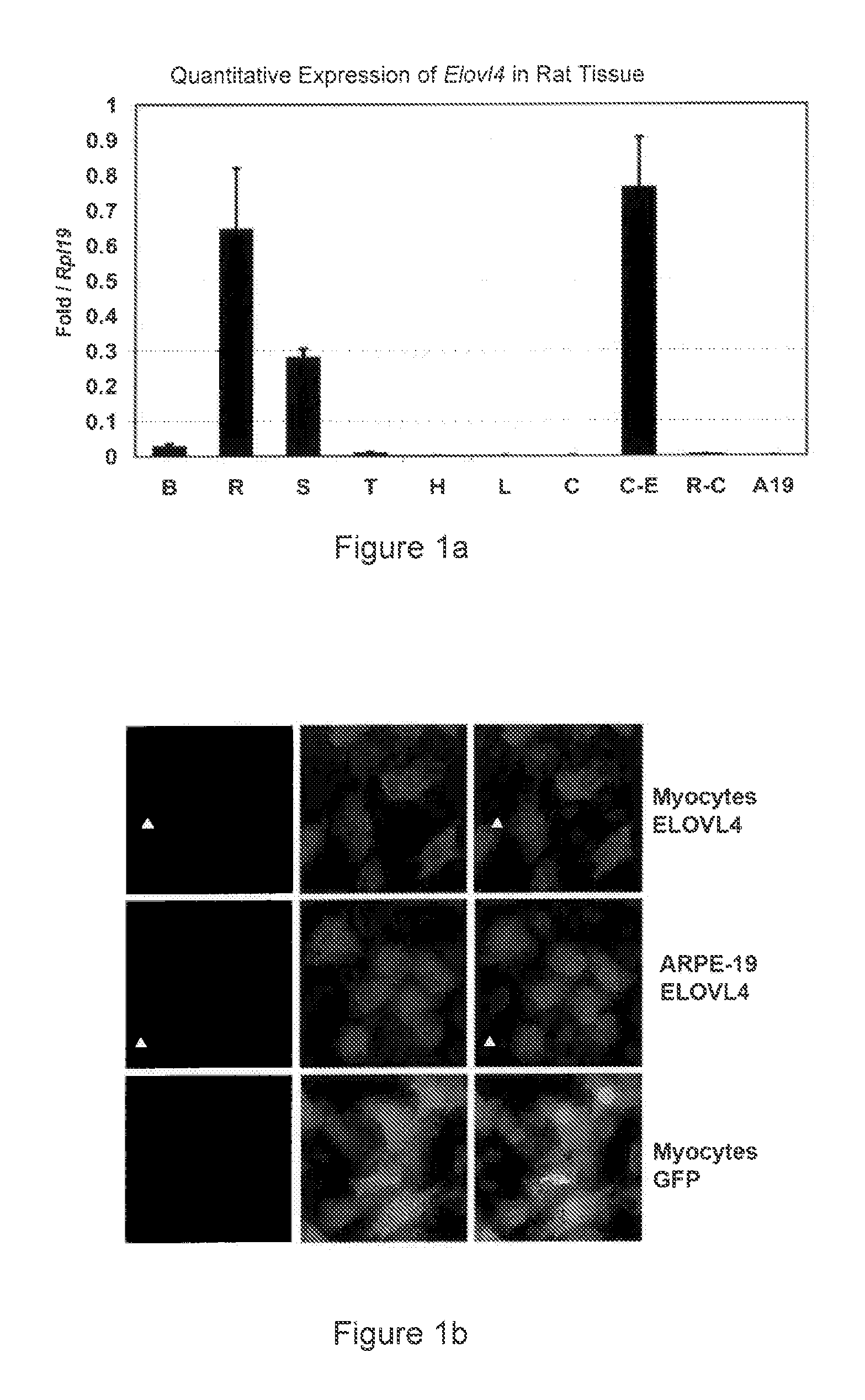
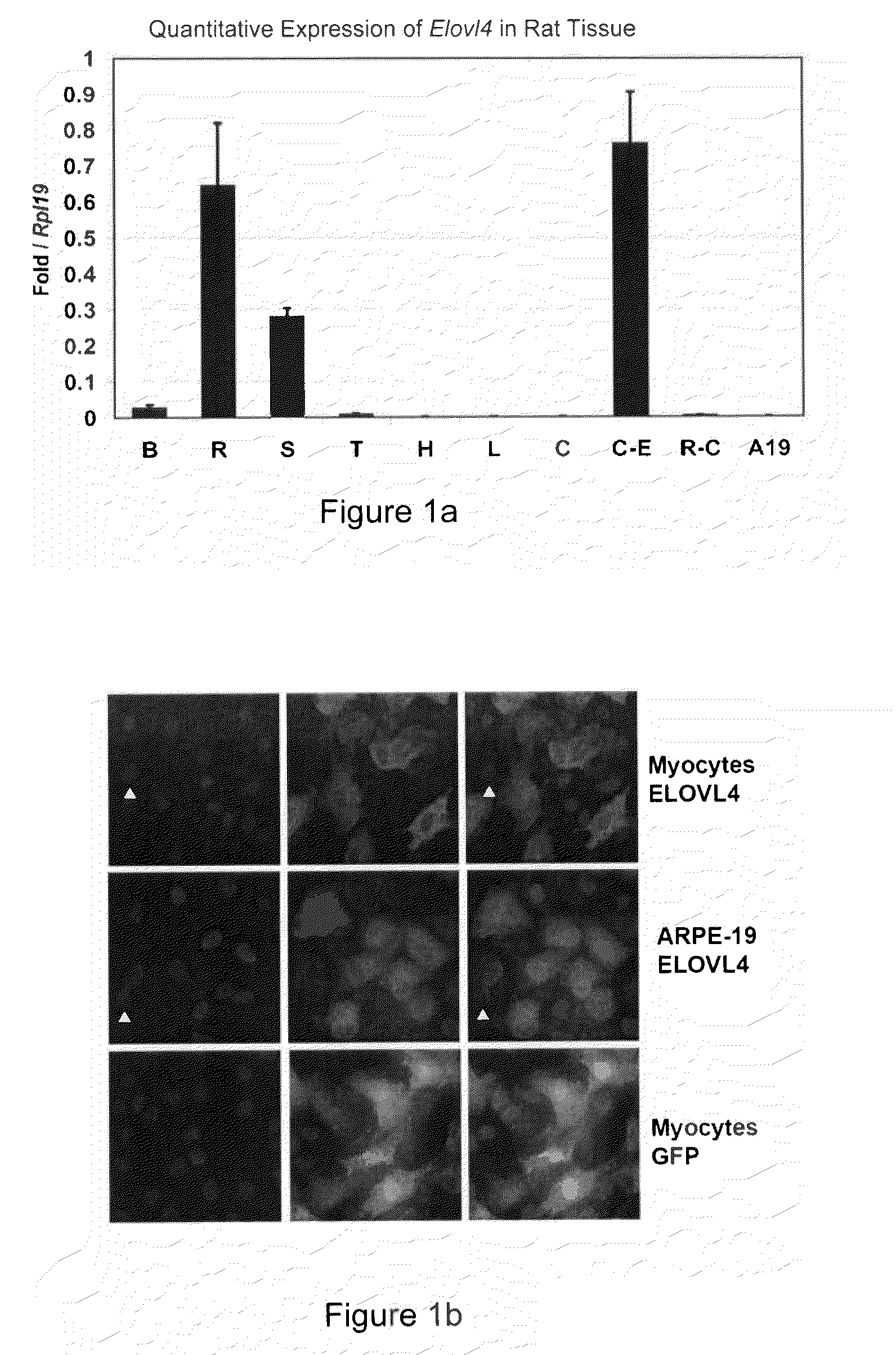
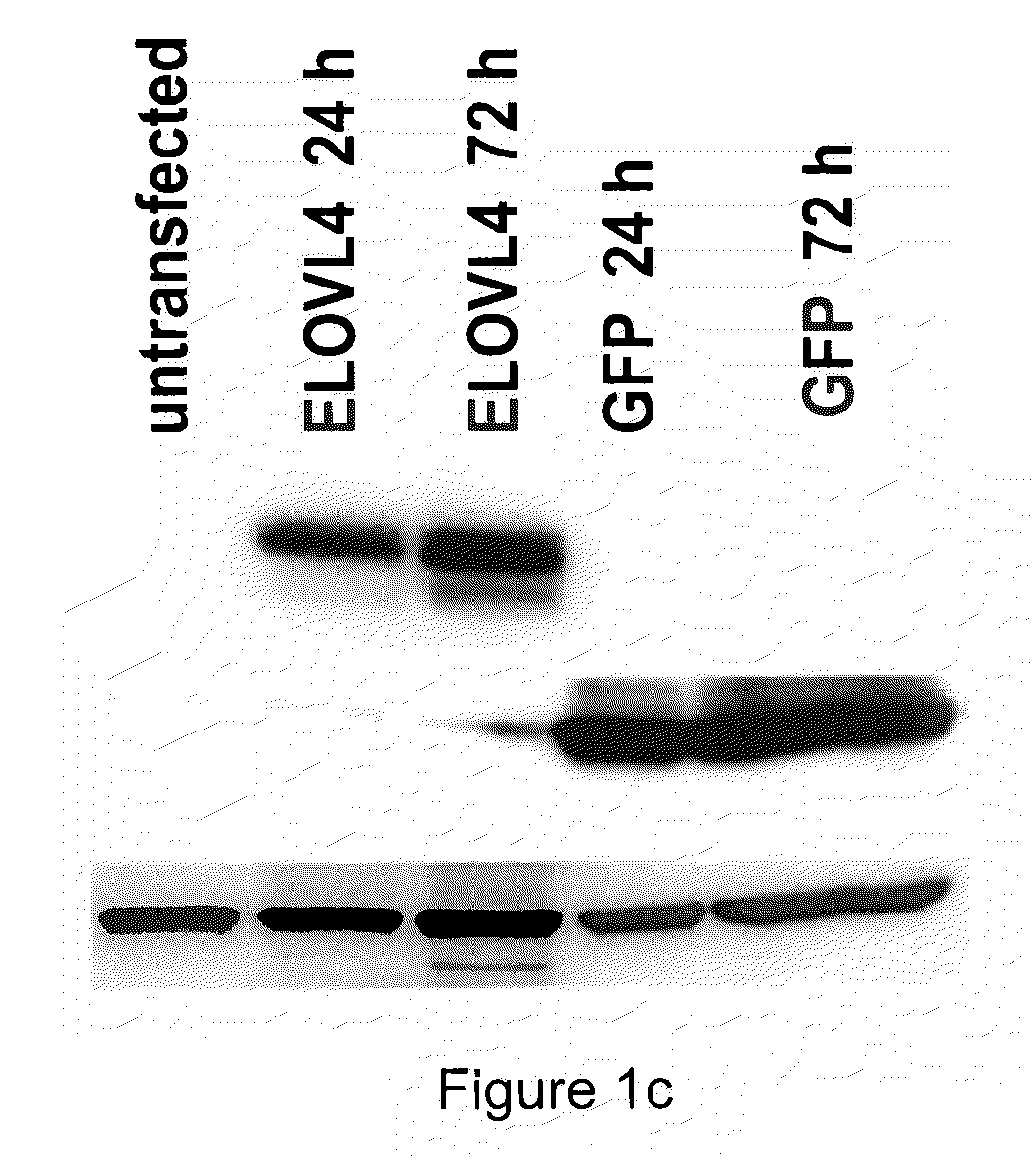
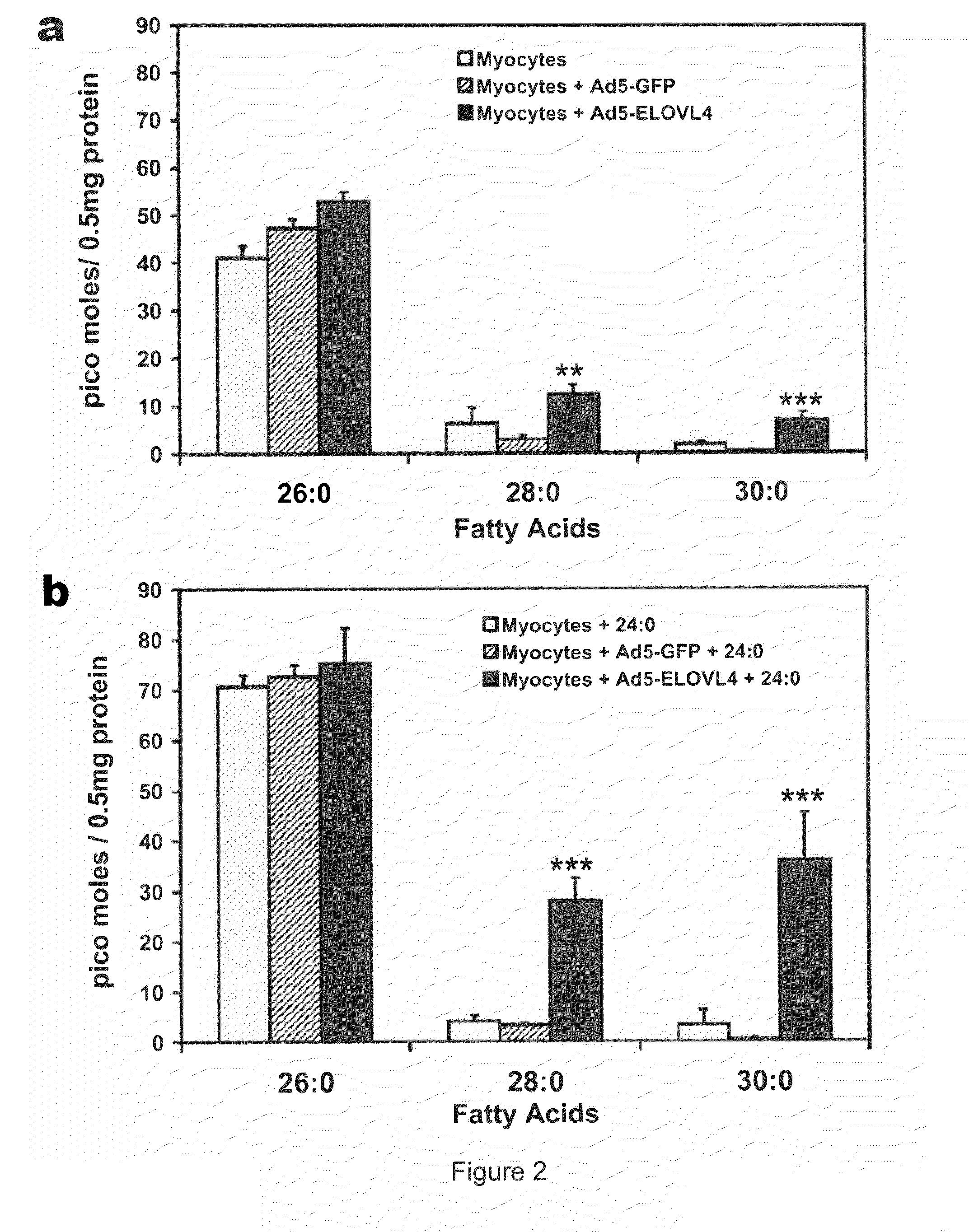
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements and food products) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C38 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a pharmaceutical formulation, a humanized animal milk, an infant formula, a cosmetic item and a biodiesel fuel for example. A pharmaceutical formulation can include, but is not limited to: a drug for treatment of neurodegenerative disease, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method for the treatment of fertility disorders
InactiveUS6319192B1Organic active ingredientsPeptide/protein ingredientsObstetricsHormones regulation
An improvement to the method of intrauterine insemination by the administration of luteinizing hormone-releasing hormone antagonists (LHRH antagonists).
Owner:ZENTARIS IVF
Compositions of very long chain polyunsaturated fatty acids and methods of use
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements, food products, and pharmaceutic compositions) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C40 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a nutritional formulation, a pharmaceutical formulation, a humanized animal milk, an infant formula, and a cosmetic item and for example. A pharmaceutical formulation can include, but is not limited to: a composition for enhancing neural development and function, a drug for treatment of neurodegenerative disease, an ocular disorder, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
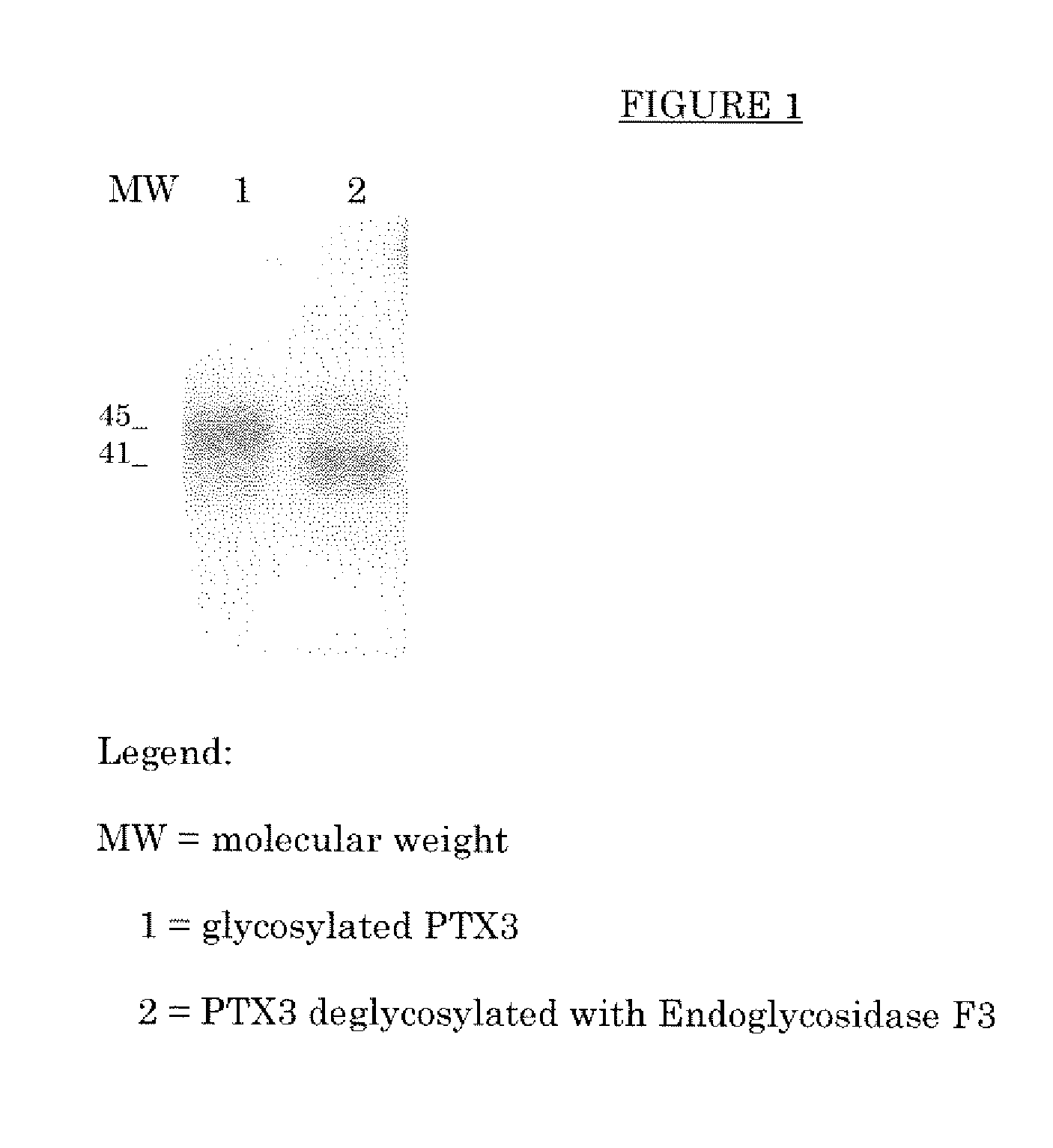
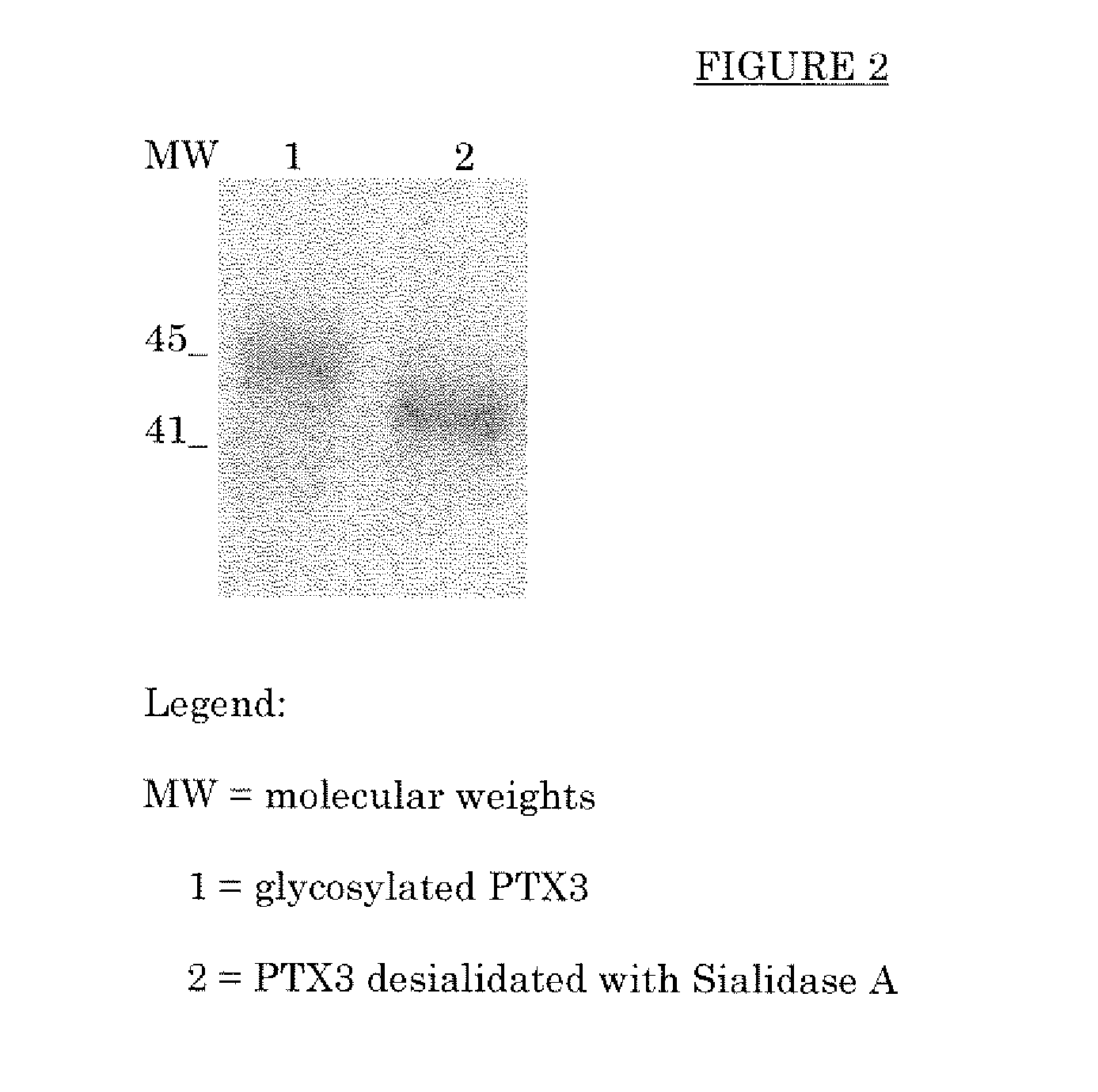
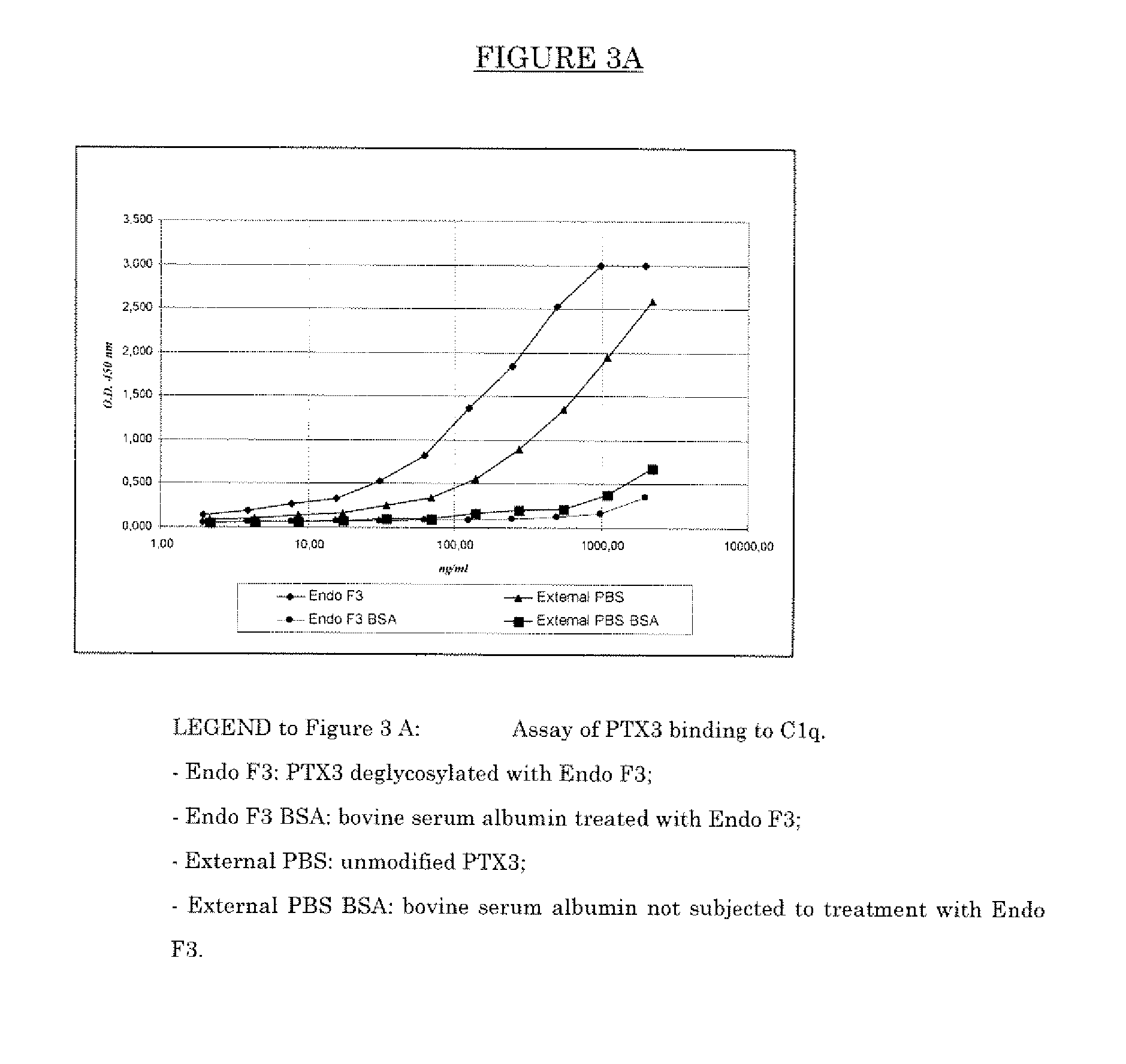
Deglycosylated and desialidated long pentraxin PTX3
InactiveUS7683032B2Improve abilitiesAntibacterial agentsSenses disorderPentraxinsFertility Disorders
Deglycosylated long pentraxin PTX3 and desialidated long pentraxin PTX3 are disclosed, as well as processes for their preparation, pharmacological compositions containing them, and their use for the preparation of a medicament for the treatment of diseases in which the use of the long pentraxin PTX is indicated, particularly infectious and inflammatory diseases and female fertility disorders. These proteins are endowed with therapeutic activity superior to that of glycosylated pentraxin.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
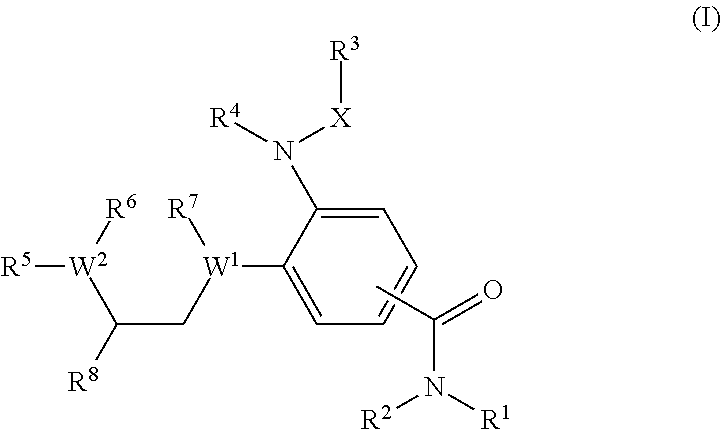
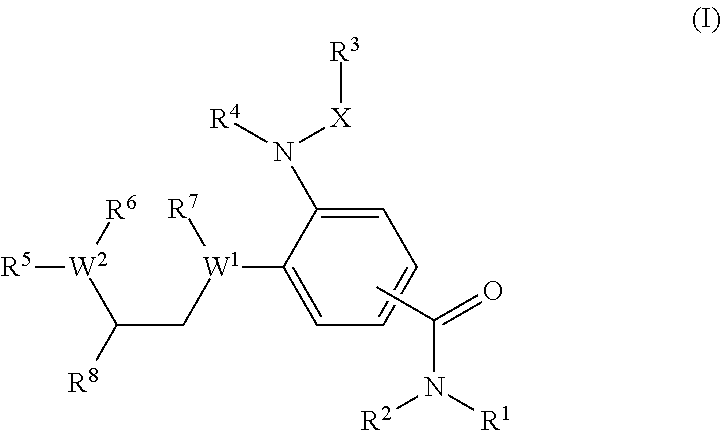
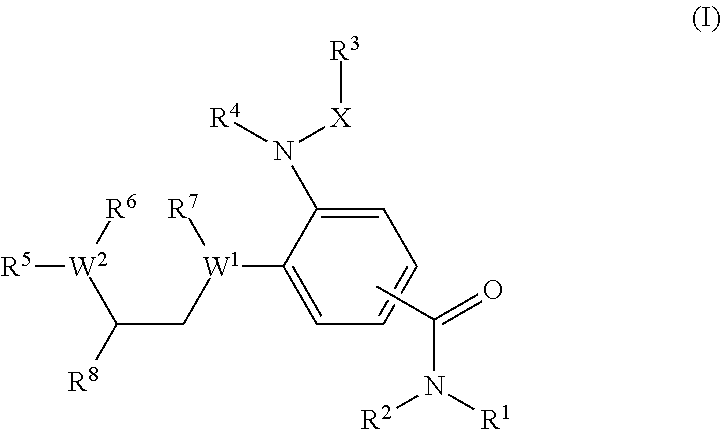
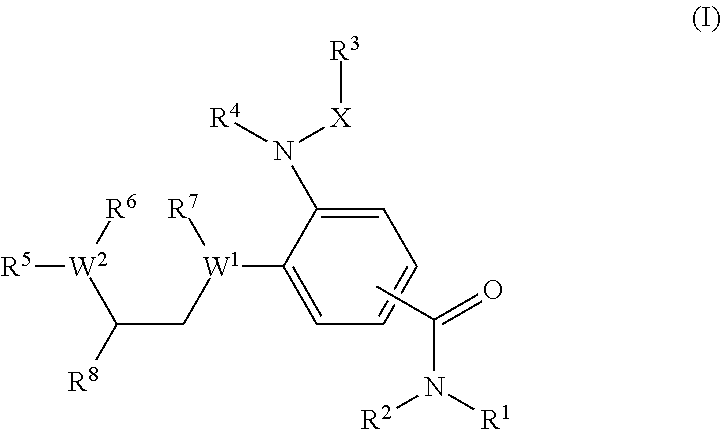
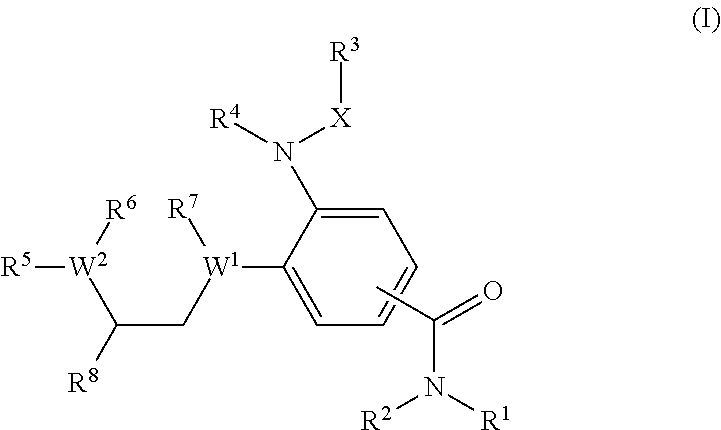
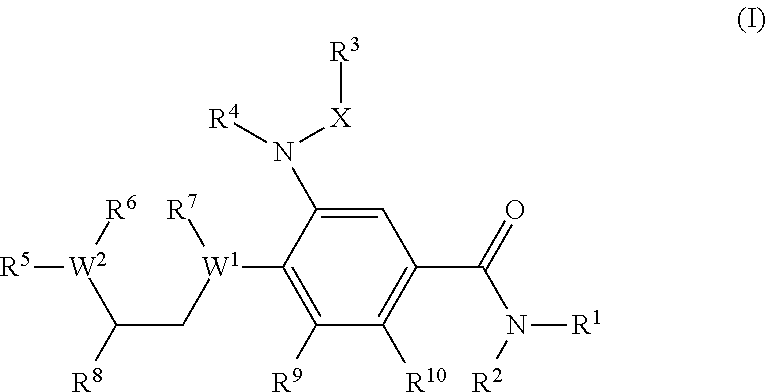
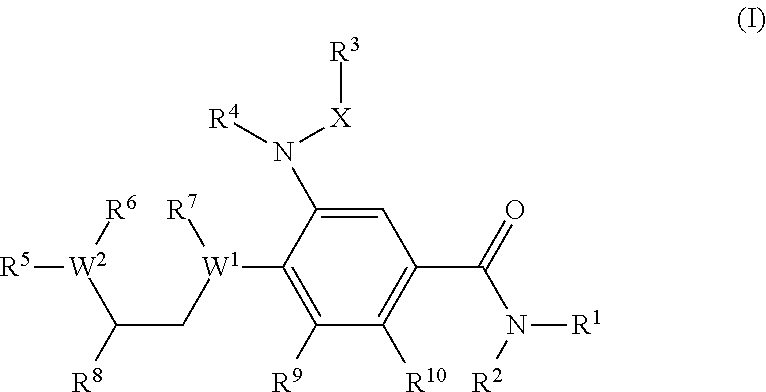
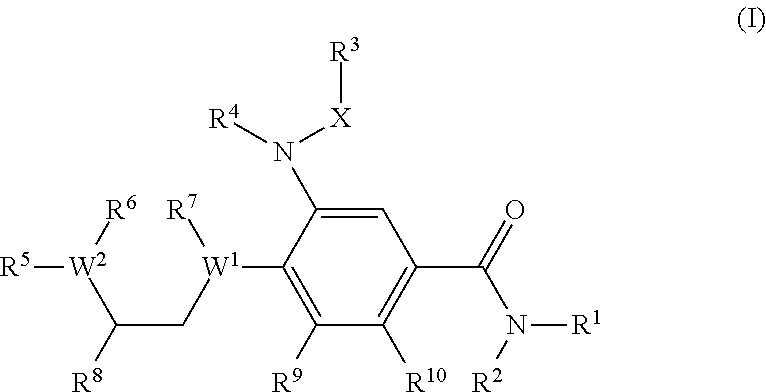
Benzamide derivatives as modulators of the follicle stimulating hormone
ActiveUS20150011562A1Low costReduce usageOrganic active ingredientsAnimal reproductionReceptorAllosteric modulator
Owner:MERCK PATENT GMBH
Very long chain polyunsaturated fatty acids, methods of production, and uses
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements and food products) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C38 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a pharmaceutical formulation, a humanized animal milk, an infant formula, a cosmetic item and a biodiesel fuel for example. A pharmaceutical formulation can include, but is not limited to: a drug for treatment of neurodegenerative disease, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Heterocyclic silicon compounds and their use in the treatment of diseases or conditions associated with gnrh (gonadotropin-releasing hormone)
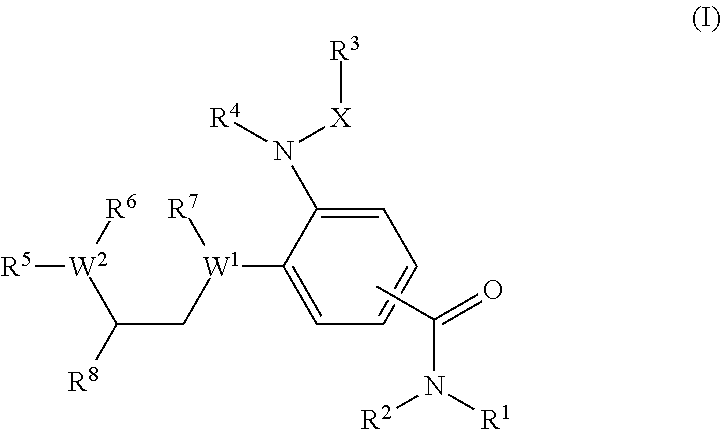
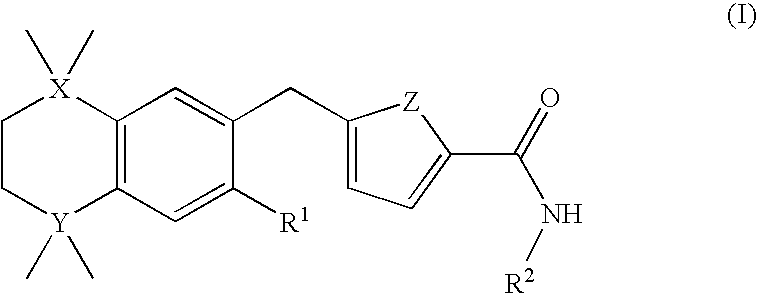
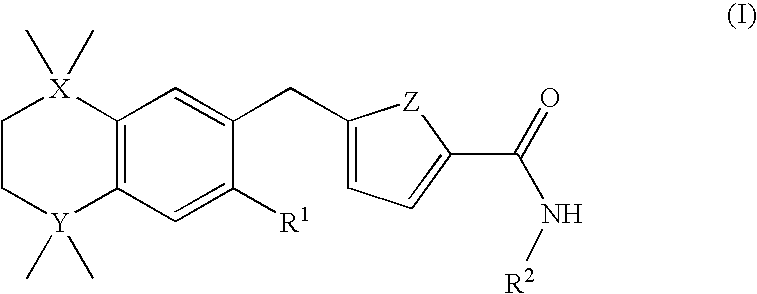
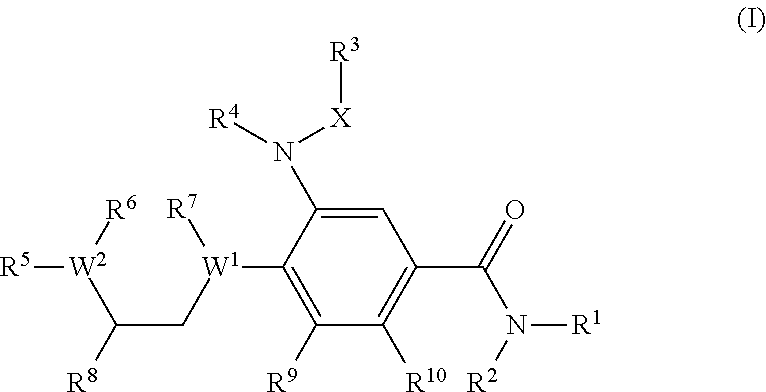
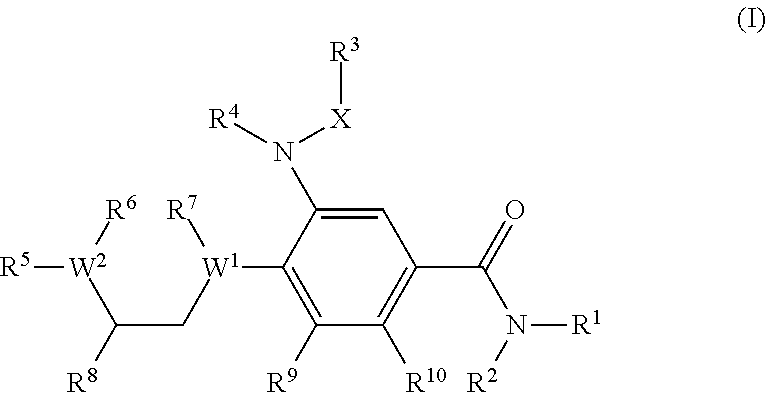
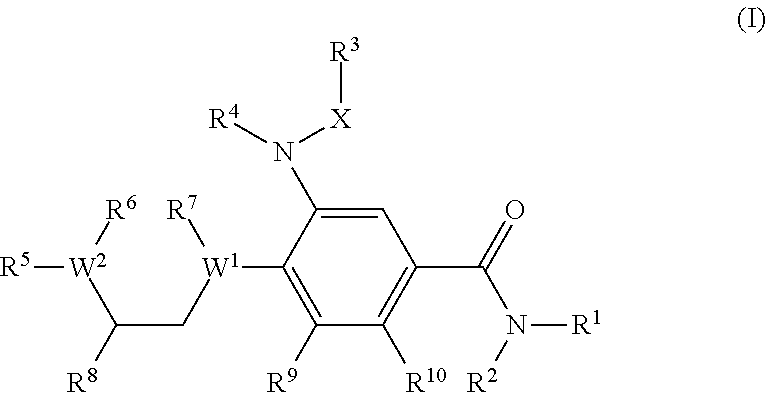
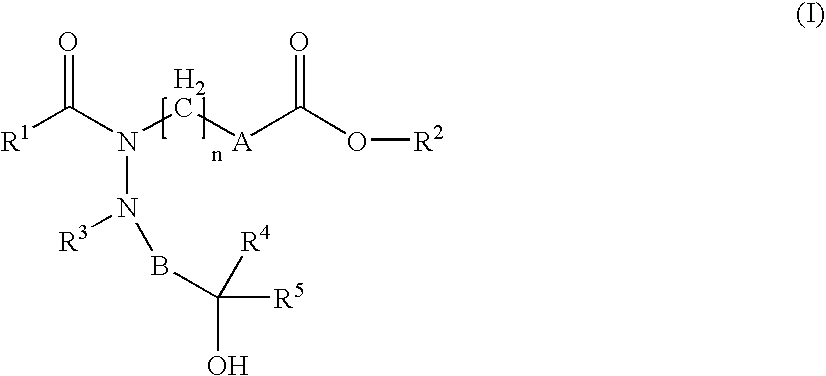
A compound of formula (I) wherein one of X and Y is silicon, and the other is carbon or silicon; Z is oxygen, sulphur or —N(R)—, wherein R is hydrogen or alkyl; R1 is hydrogen, halogen, alkyl, alkenyl, alkynyl, alkoxy or cycloalkyl; and R2 is alkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, -alkyl-cycloalkyl, -alkyl-heterocycloalkyl, -alkyl-aryl or -alkyl-heteroaryl; or a pharmaceutically acceptable salt thereof. The compounds are used in the manufacture of a medicament for the treatment or prevention of a disease or condition associated with GnRH (gonadotropin-releasing hormone), e.g. for the treatment or prevention of progression of cancer (e.g. leukaemia therapy), of a fertility disorder, of HIV infection or AIDS, of Alzheimer's disease of fibrosis, of endometriosis, of uterine fibroids or of uterine leiomyoma.
Owner:PARADIGM THERAPEUTICS
G-lactam derivatives as prostaglandin agonists
The present invention relates γ-lactam derivatives, in particular for use as medicaments, as well as pharmaceutical formulations containing such γ-lactam derivatives. Said γ-lactam derivatives are useful in the treatment and / or prevention of asthma, hypertension, osteoporosis, sexual dysfunction and fertility disorders.
Owner:MERCK SERONO SA
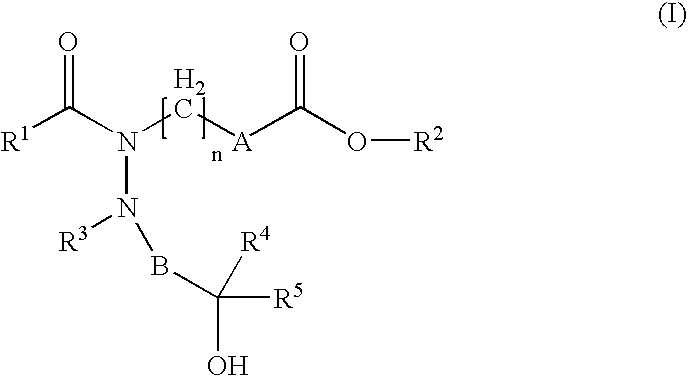
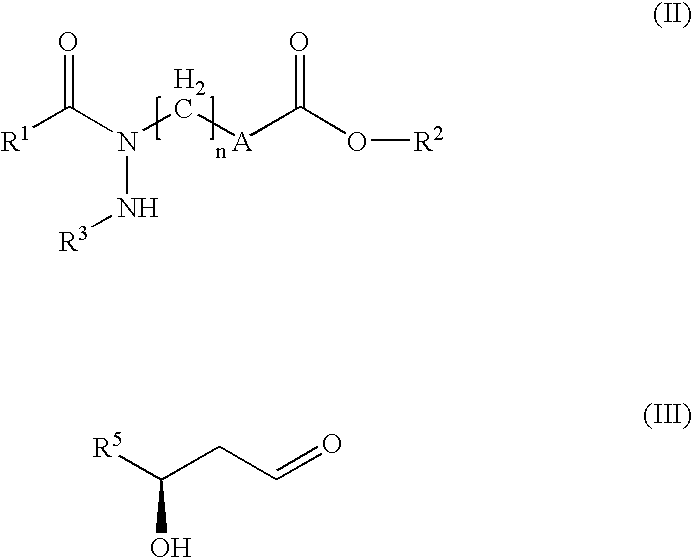
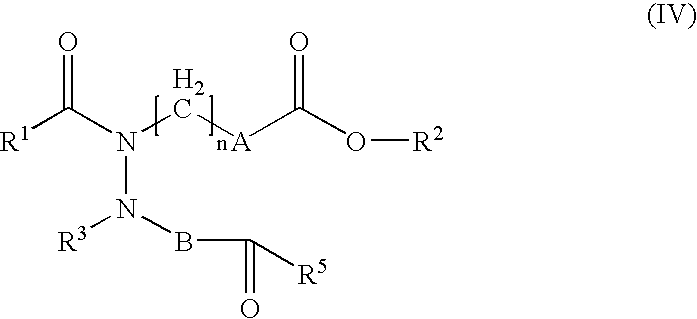
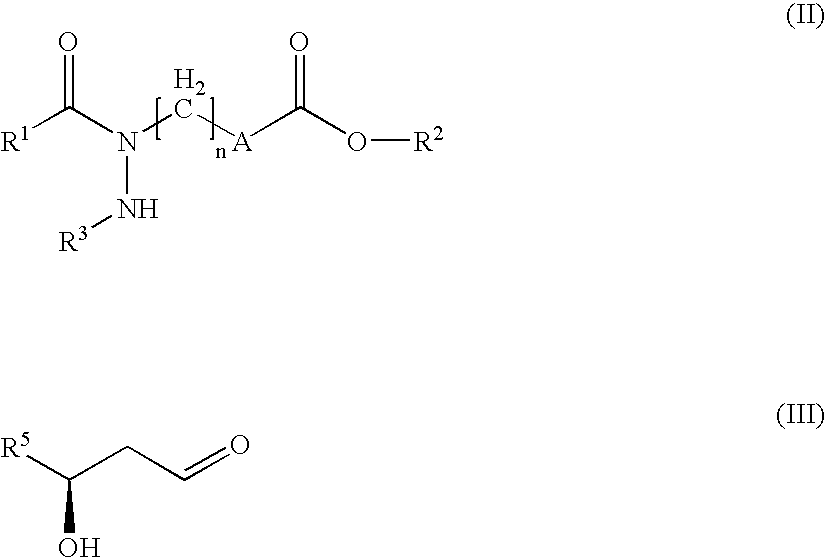
Hydrazide derivatives as prostaglandin receptors modulators
The present invention relates to hydrazide derivatives of Formula I notably for use as pharmaceutically active compounds, as well as to pharmaceutical formulations containing such hydrazide derivatives. Said hydrazide derivatives are useful in the treatment of preterm labor, dysmenorrhea, fertility disorders, asthma, hypertension, undesired blood clotting, preelampsia, eclampsia, an eosinophil disorder, undesired bone loss, renal dysfunction, an immune deficiency disorder, ichthyosis, elevated intraocular pressure, infertility, sexual dysfunction, gastric ulcers and inflammatory disorders.
Owner:LAB SERONO SA
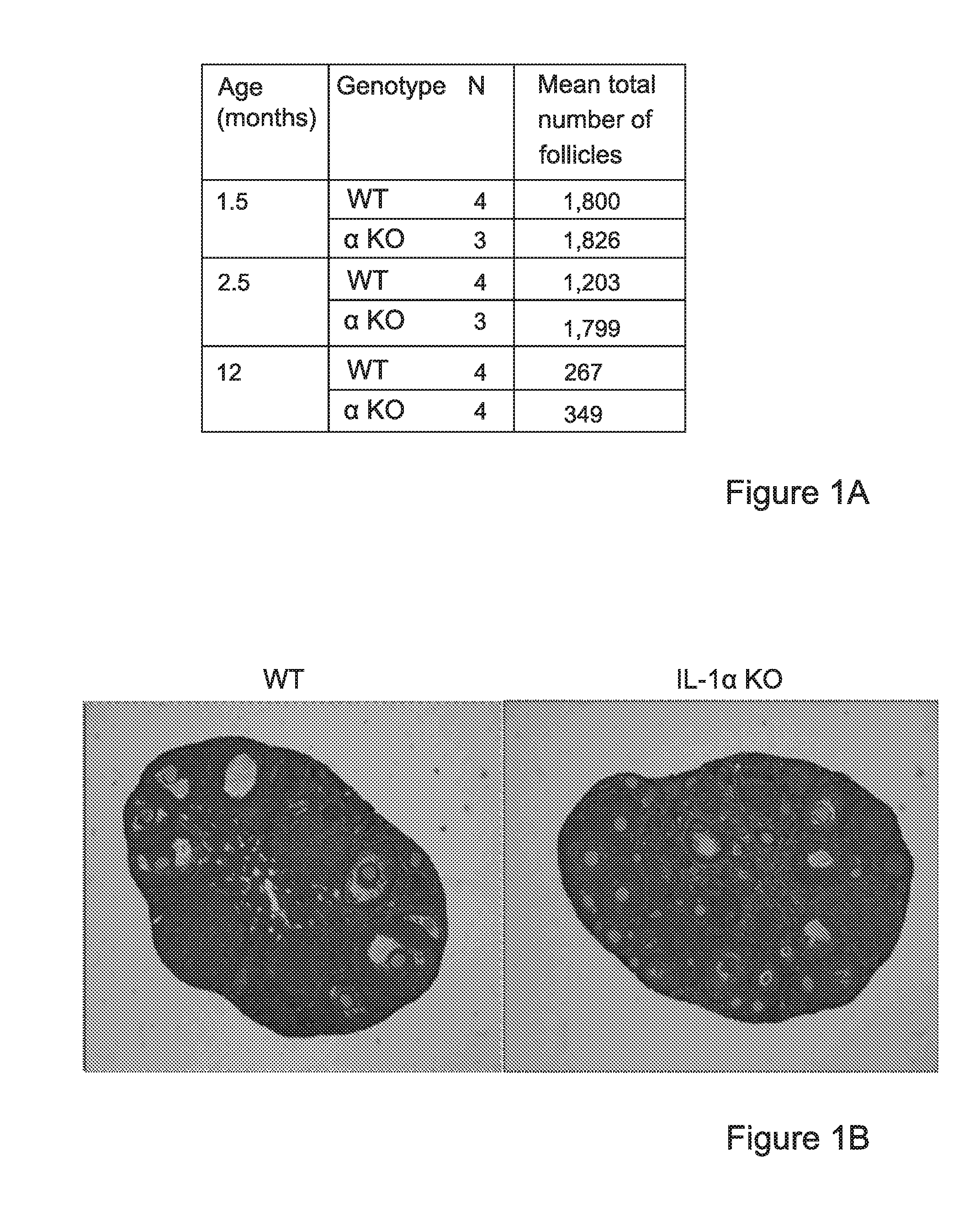
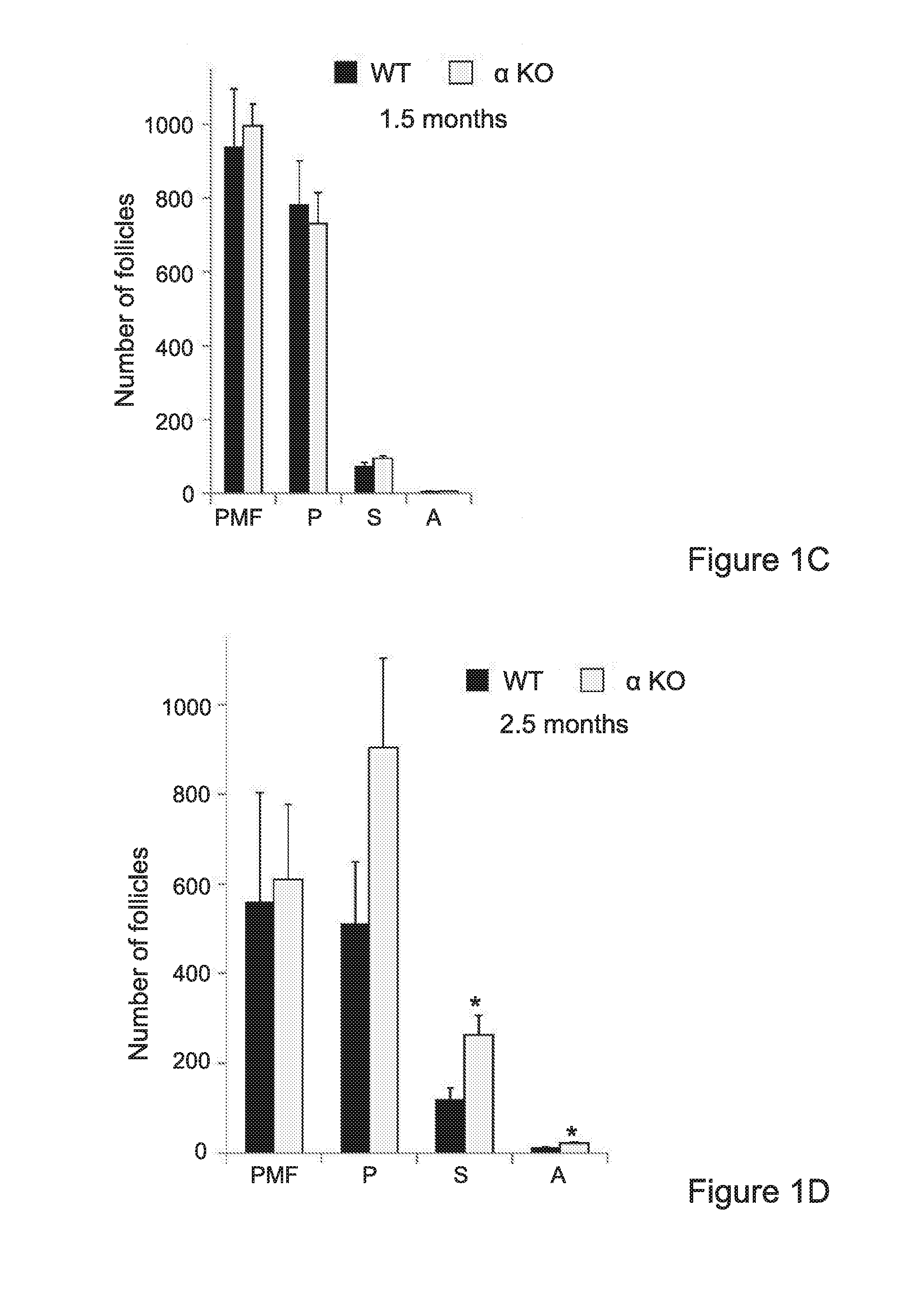
Interleukin-1 (il-1) inhibitors for treating fertility disorders
InactiveUS20160279202A1Improve responseQuality improvementPeptide/protein ingredientsCell culture active agentsFertility DisordersInterleukin 1B
The invention provides IL-1 inhibitors, methods and uses thereof for retaining ovarian follicle reserve in a mammalian subject, treating infertility and improving responsiveness and sensitivity to COH. The invention further provides methods for improving oocyte quality and / or survival, specifically for IVF, and kits for combining COH treatment with the sensitizing IL-inhibitors of the invention.
Owner:RAMOT AT TEL AVIV UNIV LTD +1
LHRH-antagonists in the treatment of fertility disorders
InactiveUS7393834B2Reduces the severe adverse eventsPatient compliance is goodBiocidePeptide/protein ingredientsMenstrual cycleLhrh antagonist
Owner:ZENTARIS IVF
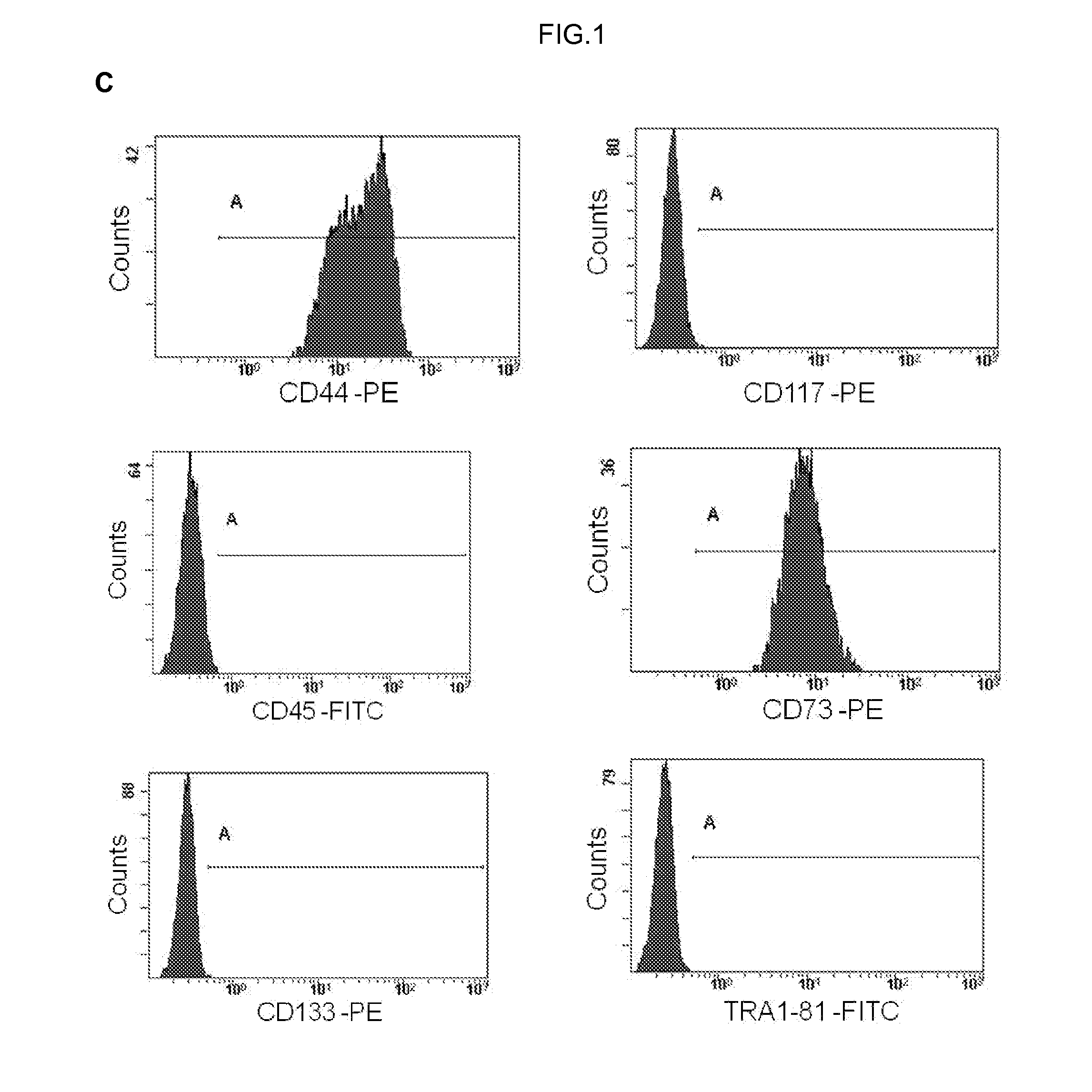
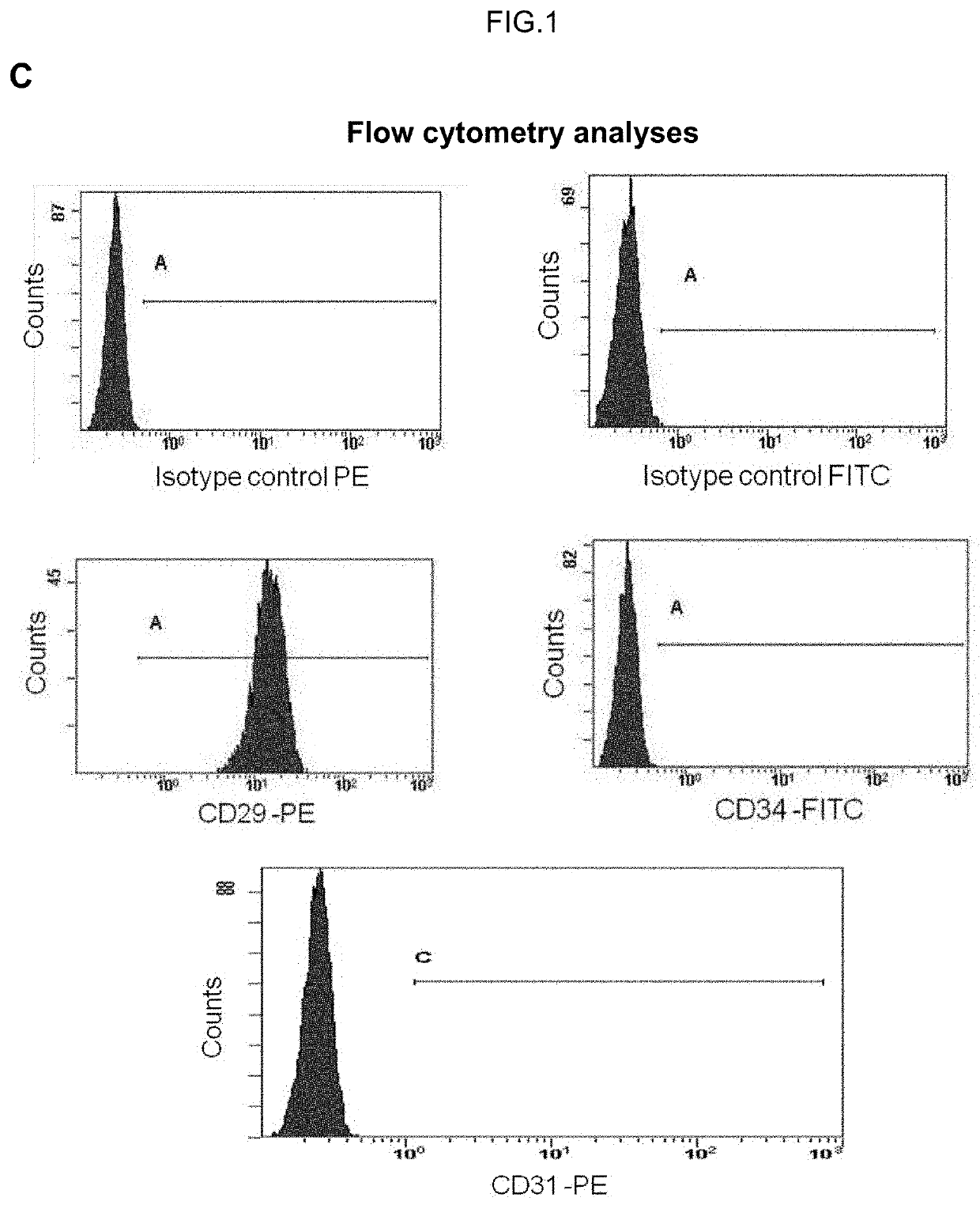
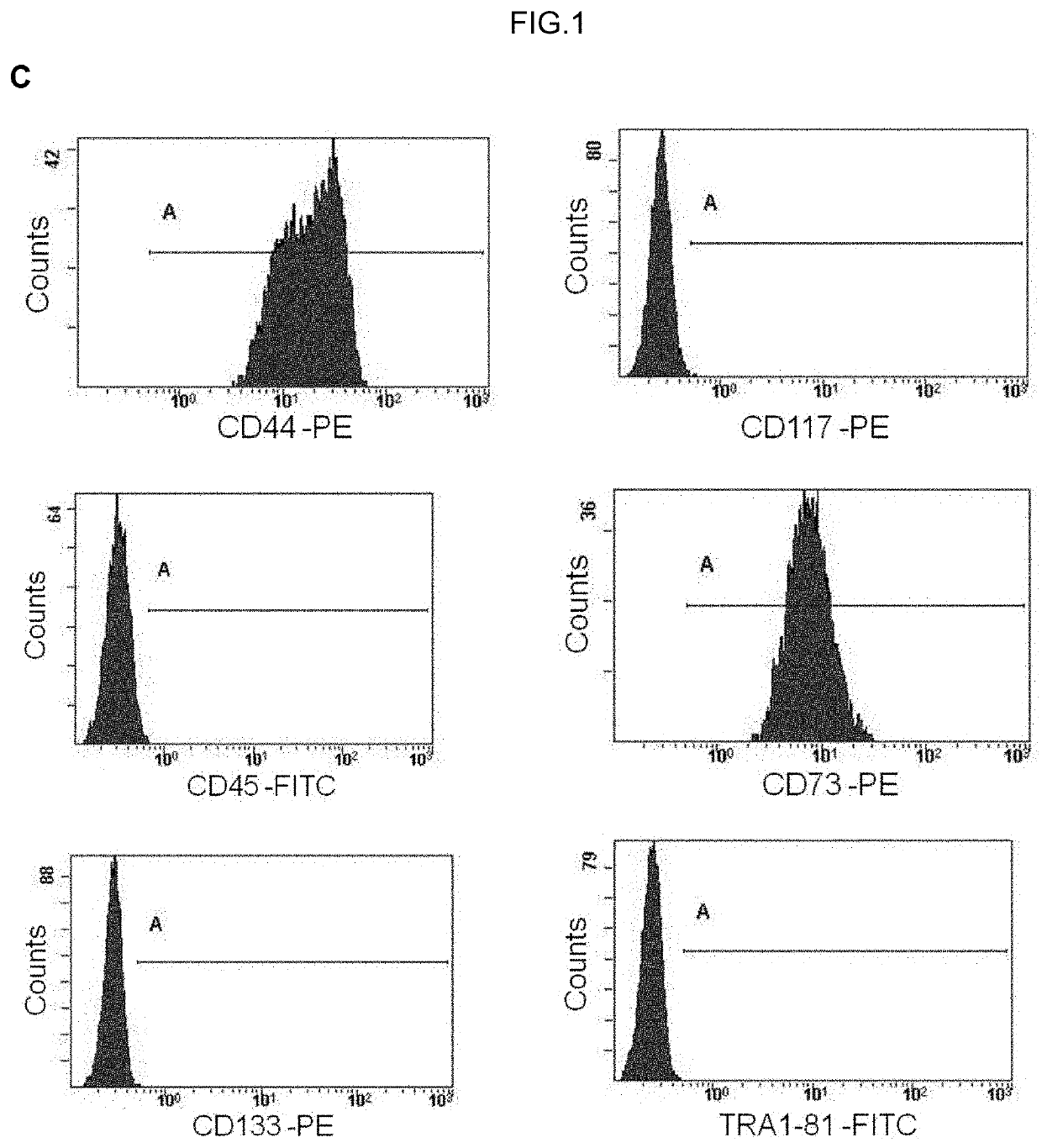
Human uterine cervical stem cell population and uses thereof
InactiveUS20160000835A1High activityIncrease capacityAntibacterial agentsBiocideAutoimmune diseaseFertility Disorders
The present invention relates to a method for isolating stem cells comprising preparing a cell suspension from uterine cervix tissue, to the stem cells isolated by said method, and to the conditioned medium obtained from the culture of said stem cells. The invention also encompasses the use of said stem cells or conditioned medium for treating or preventing cancer, precancerous lesions, inflammatory diseases, autoimmune diseases, chronic pathologies or infectious diseases, diseases associated to tissue loss, or for use in diagnostic, prognostic or treatment of fertility disorders, as well as for cosmetic treatment.
Owner:GISTEM RESERCH SL
LHRH-antagonists in the treatment of fertility disorders
InactiveUS20050049200A1Patient compliance is goodLow costBiocidePeptide/protein ingredientsOvulation timesMenstrual cycle
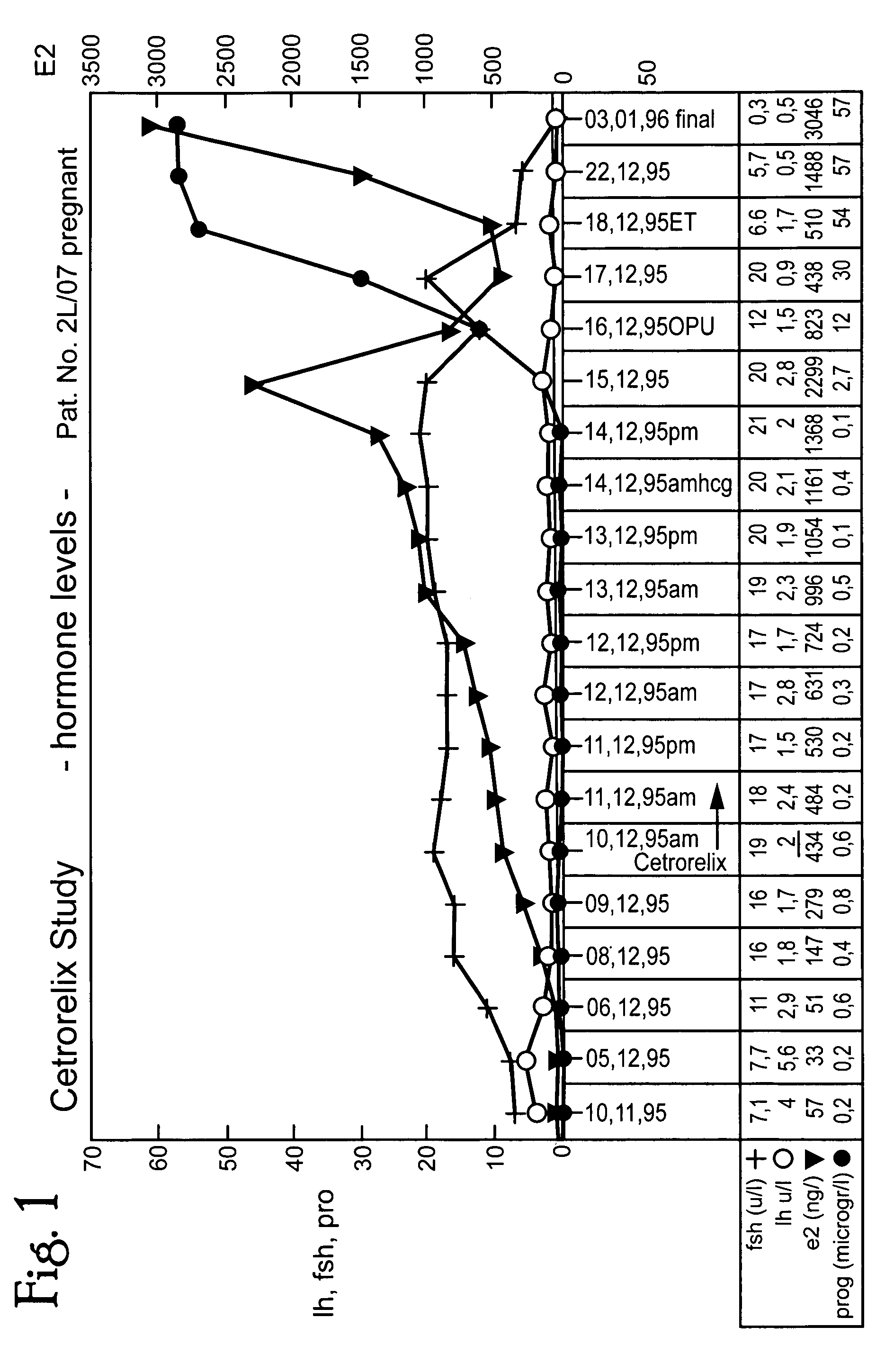
A method of treating infertility disorders by 1) administering an LH-RH antagonist, preferably Cetrorelix, in amounts to selectively suppress endogenous LH but not FSH secretion and 2) inducing follicle growth by administration of exogenous gonadotropin. The selective suppression OF LH allows FSH secretion to be at natural levelS thereby not affecting individual estrogen development. The LH-RH antagonist can be given as a single or dual subcutaneous dose in the range of 1 mg to 10 mg, preferably 2 mg-6 mg. In multiple dosing-posology, LH-RH antagonist can be administered subcutaneously in an amount in the range of 0.1 to 0.5 mg of LH-RH antagonist / day. LH-RH antagonist is applied starting cycle day 1 to 10, preferably on day 4 to 8, and ovulation can be induced between day 9 and 20 of the menstruation cycle by administering rec. LH, native LH-RH, LH-RH agonist or by HCG. In addition rec. LH, native LH-RH or LH-RH agonist can be given to avoid hyperstimulation syndrome and native LH-RH or a LH-RH agonist can be administered to avoid luteal phase stimulation by neutralizing the negative effects of HCG.
Owner:ZENTARIS IVF
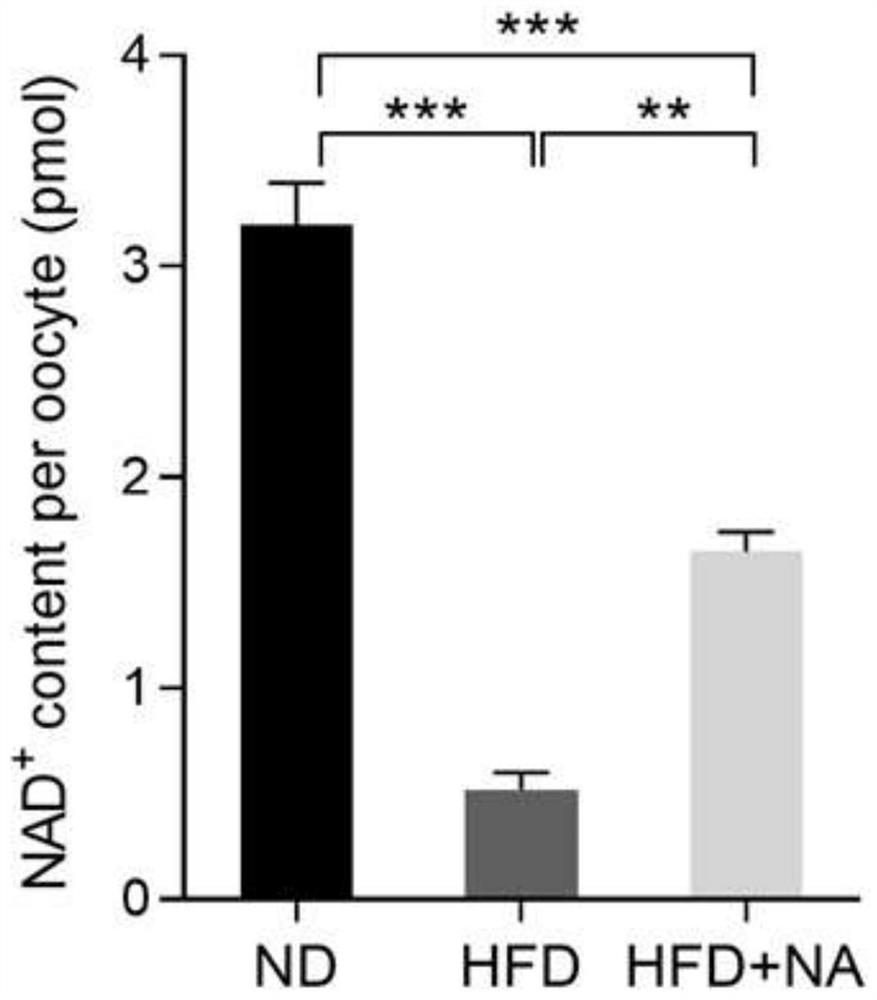
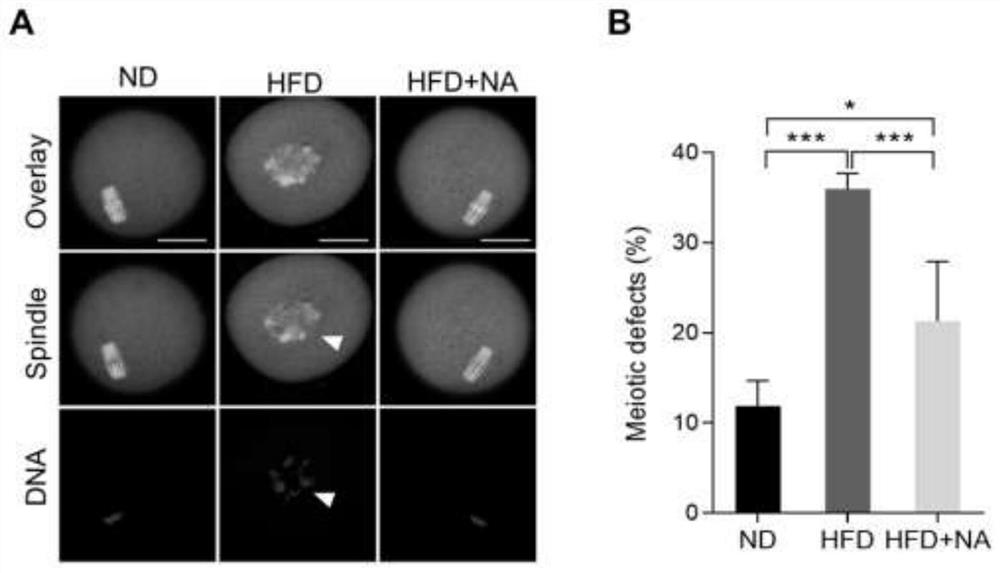
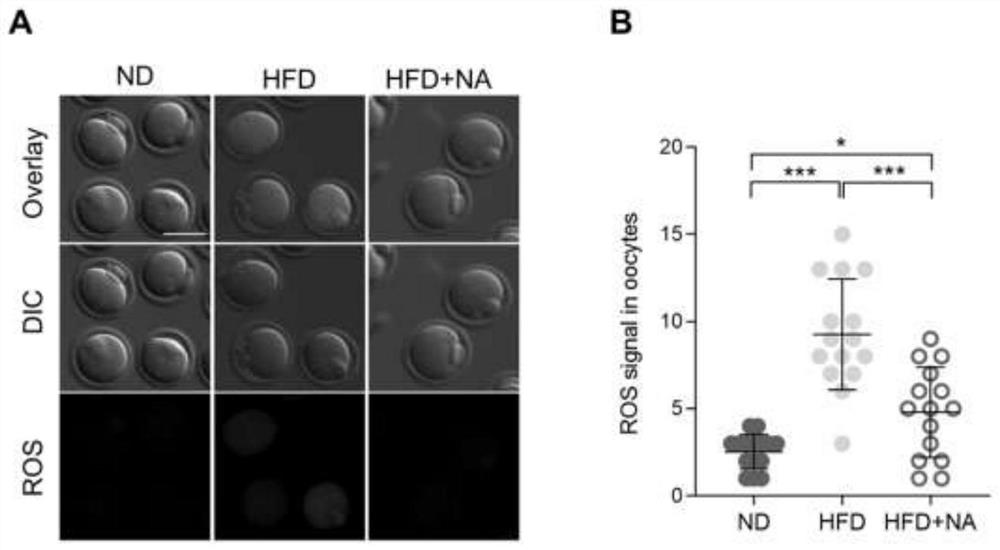
Application of nicotinic acid in preparation of medicine for treating and/or preventing fertility disorder of obese women
PendingCN113413384AIncrease contentReduce the probability of abnormalOrganic active ingredientsMetabolism disorderPhysiologyObesity prevention
The invention discloses application of nicotinic acid in preparation of a medicine for treating and / or preventing fertility disorder of obese women, and belongs to the technical field of medicines. According to the invention, through in-vivo supplement or in-vitro addition of nicotinic acid, fertility of obese women can be effectively improved, abnormal assembly proportion and oxidative stress level of meiosis devices in mature ova are reduced, and early embryos after fertilization are promoted to develop to blastocyst stage; the method is of great significance to prevention and / or treatment of female infertility caused by obesity. Nicotinic acid is an important endogenous substance in an organism, is very stable in structure and property, low in price and easy to obtain, and can play a role when being supplemented in vivo or added in vitro, so that the nicotinic acid is very high in practicability when being used as a medicine or a biological preparation.
Owner:NANJING MEDICAL UNIV
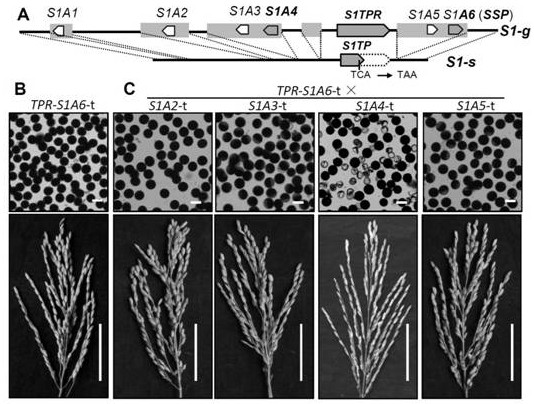
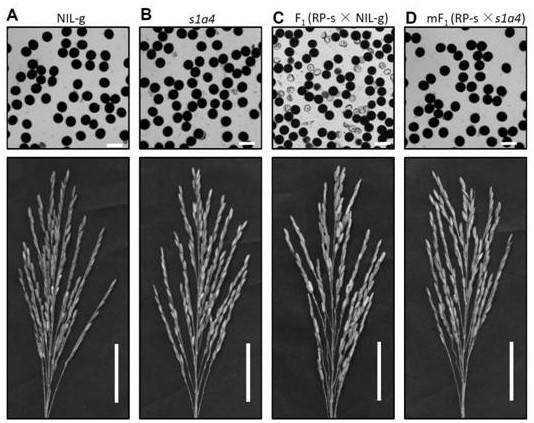
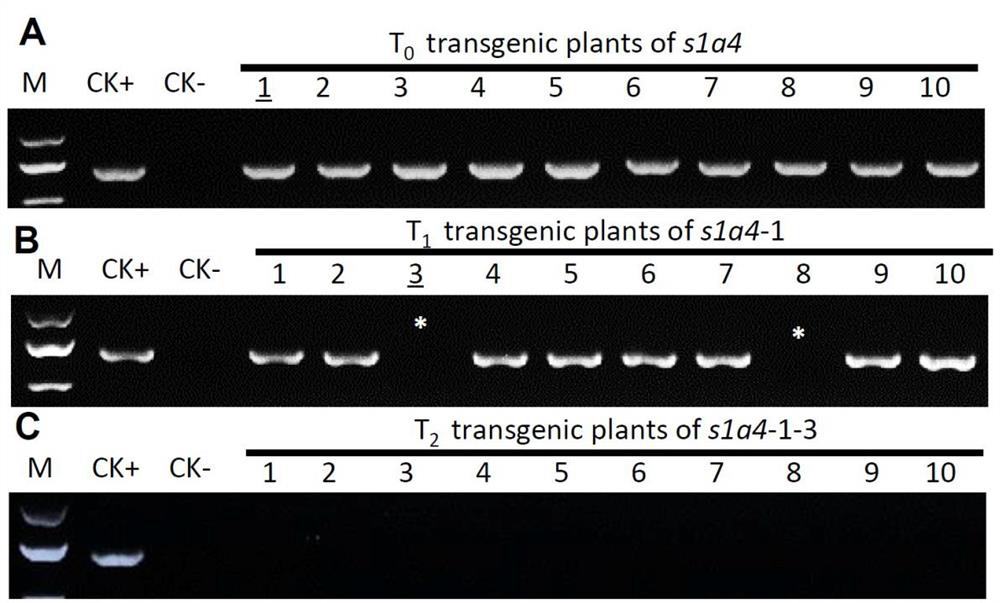
A gene s1a4 controlling sterility in Asian-African rice hybrids and its application
ActiveCN109371038BBreak sterilityElimination of infertilityPlant peptidesFermentationBiotechnologyOryza
The invention belongs to the fields of genetic engineering technology and crop genetic breeding, and specifically discloses a gene for controlling interspecific hybrid sterility between Asian rice and African rice S1A4 and its application. The present invention clones an interspecific hybrid sterility locus between Asian rice and African rice S1 Related African Oryza Specific Genes S1A4 , identified the cause of hybrid gamete sterility S1A4‑S1TPR - S1A6 Three-factor gamete lethal system; for African rice S1A4 The mutant line obtained by site-directed knockout has normal fertility, but the hybrid between this mutant line and O. sativa can eliminate hybrid sterility and effectively break S1 mediated interspecific hybrid fertility disorders. The invention provides knockout by means of genetic engineering S1A4 Gene, a method for efficiently creating hybrid-compatible African cultivated rice varieties (lines), provides potential application value for the use of distant heterosis to improve rice yield in molecular breeding.
Owner:SOUTH CHINA AGRI UNIV
Deuterated Thiazolidinone Analogues as Agonists for Follicle Stimulating Hormone Receptor
InactiveUS20150111933A1Organic active ingredientsBiocideAgonistFollicle-stimulating hormone receptor
Owner:MERCK PATENT GMBH
Purified LH
Recombinant human luteinizing hormone (LH) having a specific bioactivity of from 20,522 to 31,229 IU / mg and obtainable from a process for the purification of recombinant LH from a sample of crude recombinant LH in the supernatant of CHO cells which comprises the combined use of ion-exchange chromatography and reverse phase HPLC. The ion-exchange chromatography and the reverse phase HPLC are performed twice and the final use of a gel permeation column allows the purification from any residual traces of contaminants. The recombinant LH can be formulated into a pharmaceutical composition and used for treating a fertility disorder.
Owner:MERCK SERONO SA
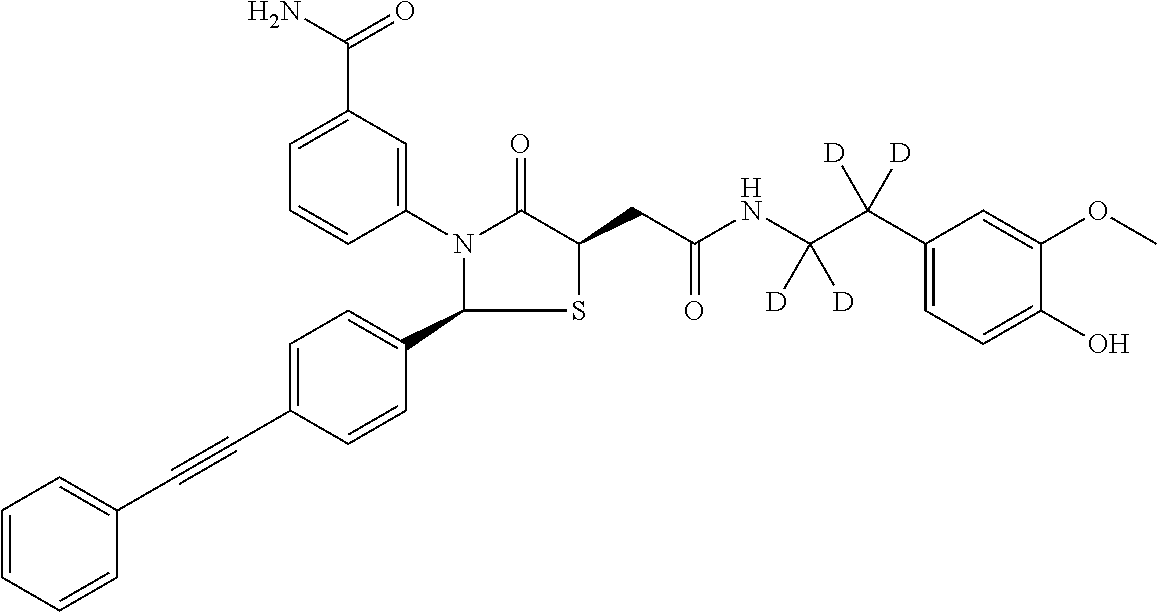
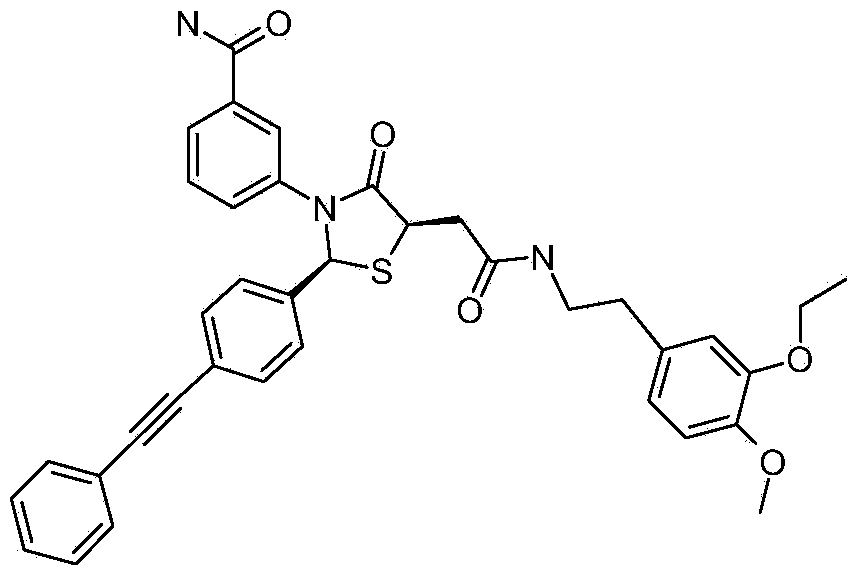
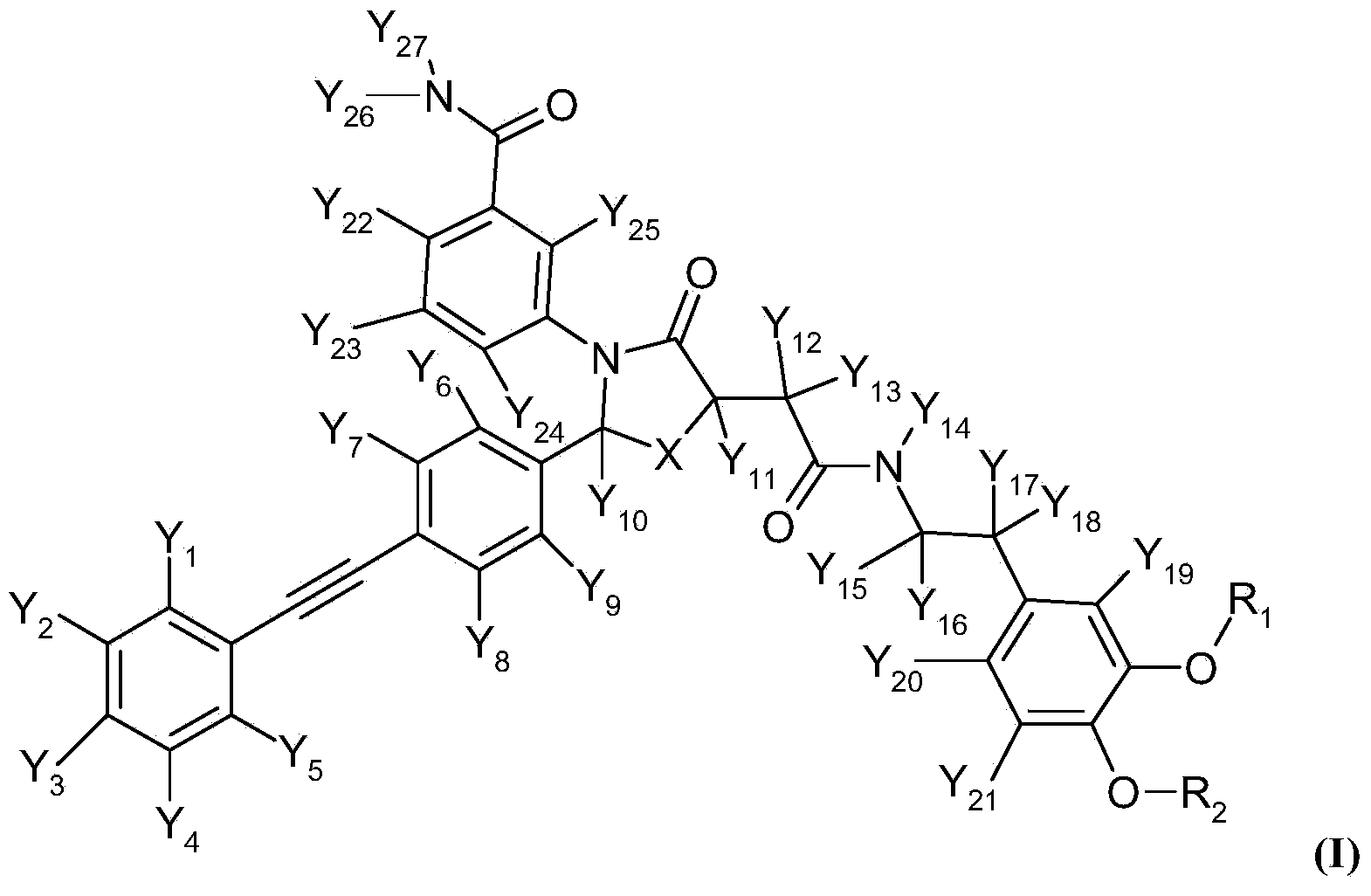
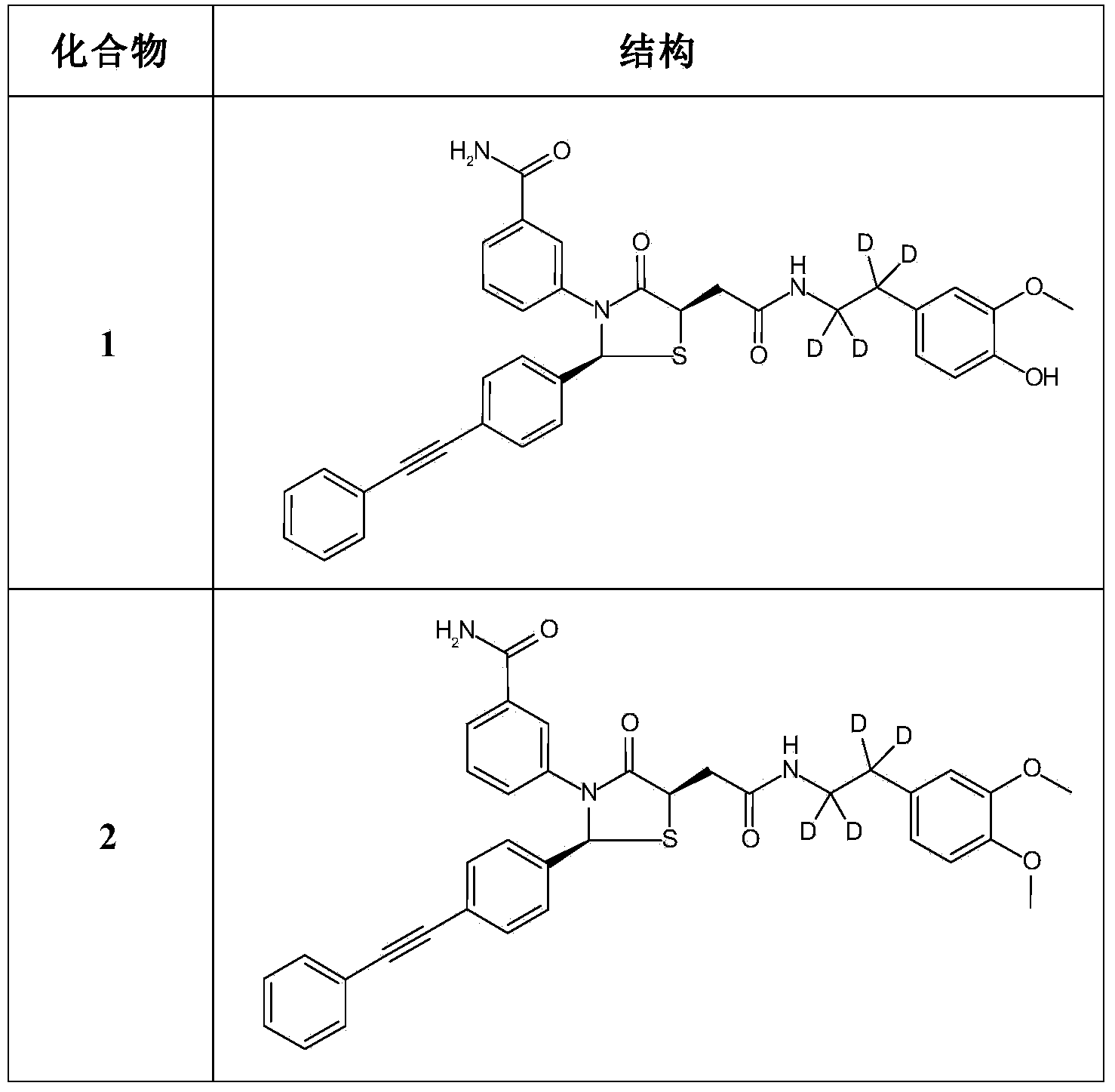
Deuterated thiazolidinone analogues as agonists for follicle stimulating hormone receptor
InactiveCN104114544AOrganic active ingredientsIsotope introduction to heterocyclic compoundsAgonistFertility Disorders
Owner:MERCK PATENT GMBH
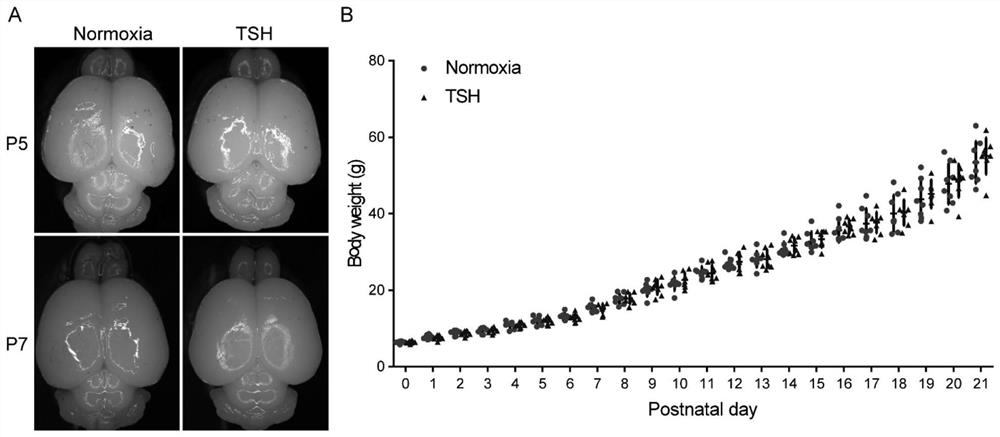
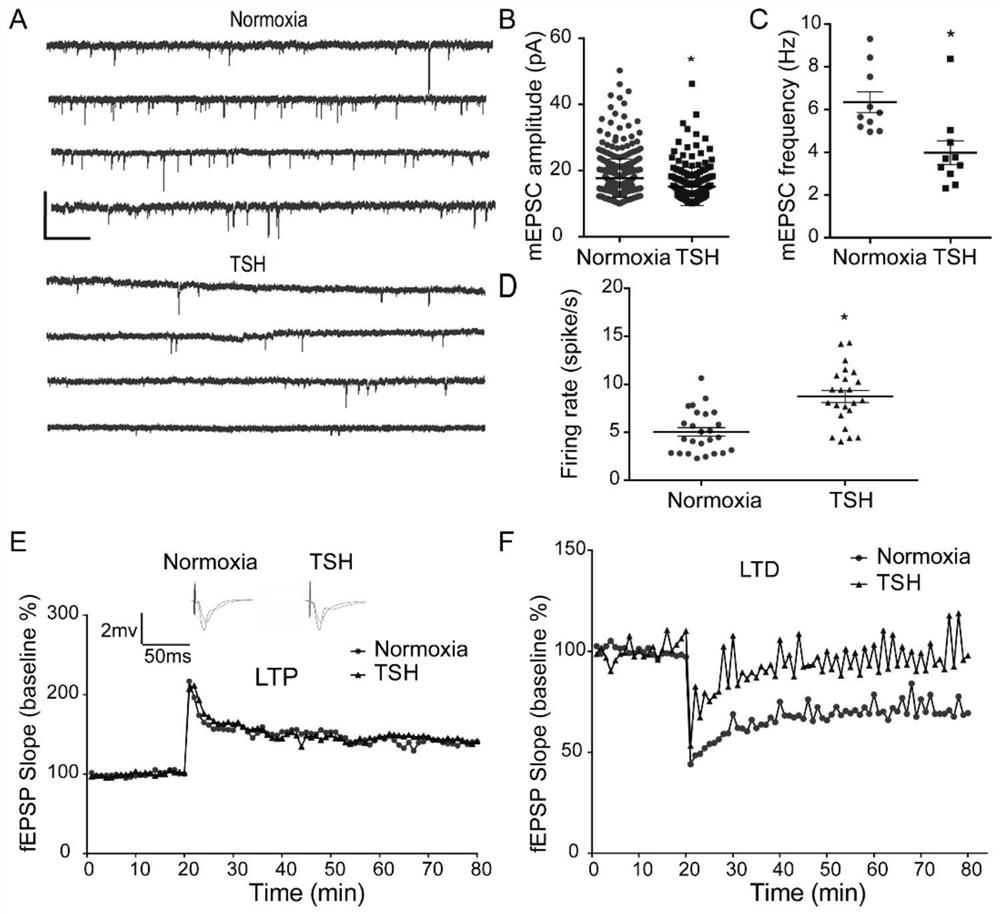
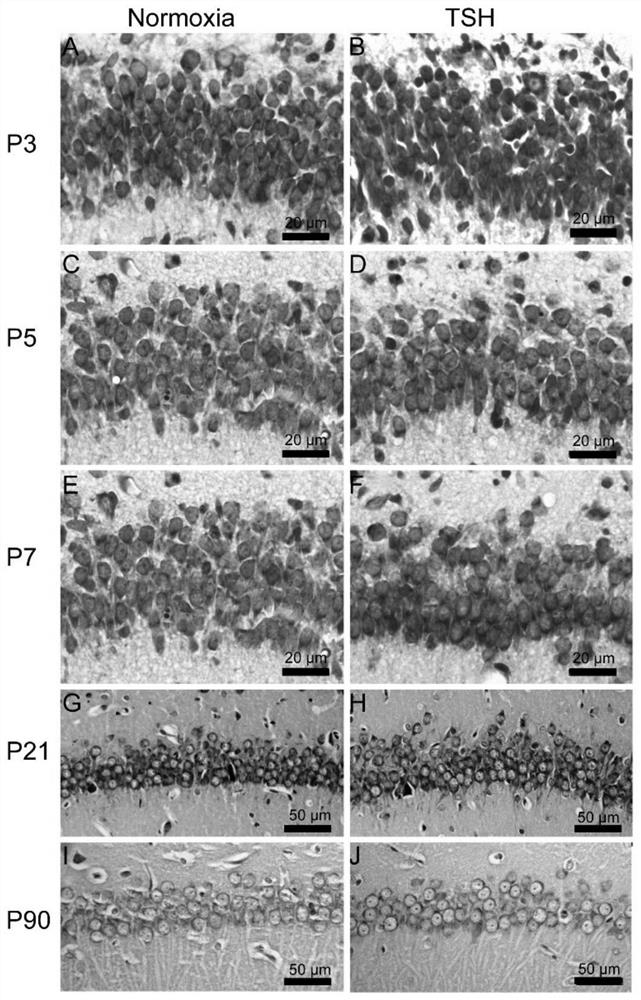
Preparation and application of animal model with broad brain region neuron tree sudden fertility disorder
The invention relates to preparation and application of an animal model with broad brain region neuron tree sudden fertility disorder. Specifically, a preparation method of a non-human mammal model with the broad brain region neuron tree sudden fertility disorder is provided and comprises the following steps: exposing the non-human mammal to a hypoxia condition for 30-40 minutes so as to obtain an animal model with the broad brain region neuron tree sudden fertility disorder, wherein under the hypoxia condition, the oxygen concentration (volume ratio) is reduced from 10-20% to 1-5%. The animal model can be used for researching the broad brain region neuron tree sudden fertility disorder, and can be used for screening and testing specific drugs.
Owner:SHANGHAI EAST HOSPITAL EAST HOSPITAL TONGJI UNIV SCHOOL OF MEDICINE
Human uterine cervical stem cell population and uses thereof
PendingUS20210299184A1High activityIncrease capacityAntibacterial agentsAntipyreticImmunologic disordersCancer prevention
The present invention relates to a method for isolating stem cells comprising preparing a cell suspension from uterine cervix tissue, to the stem cells isolated by said method, and to the conditioned medium obtained from the culture of said stem cells. The invention also encompasses the use of said stem cells or conditioned medium for treating or preventing cancer, precancerous lesions, inflammatory diseases, autoimmune diseases, chronic pathologies or infectious diseases, diseases associated to tissue loss, or for use in diagnostic, prognostic or treatment of fertility disorders, as well as for cosmetic treatment.
Owner:GISTEM RESERCH SL
Hydrazide derivatives as prostaglandin receptors modulators
The present invention relates to hydrazide derivatives of Formula I notably for use as pharmaceutically active compounds, as well as to pharmaceutical formulations containing such hydrazide derivatives. Said hydrazide derivatives are useful in the treatment of preterm labor, dysmenorrhea, fertility disorders, asthma, hypertension, undesired blood clotting, preelampsia, eclampsia, an eosinophil disorder, undesired bone loss, renal dysfunction, an immune deficiency disorder, ichthyosis, elevated intraocular pressure, infertility, sexual dysfunction, gastric ulcers and inflammatory disorders.
Owner:LAB SERONO SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com