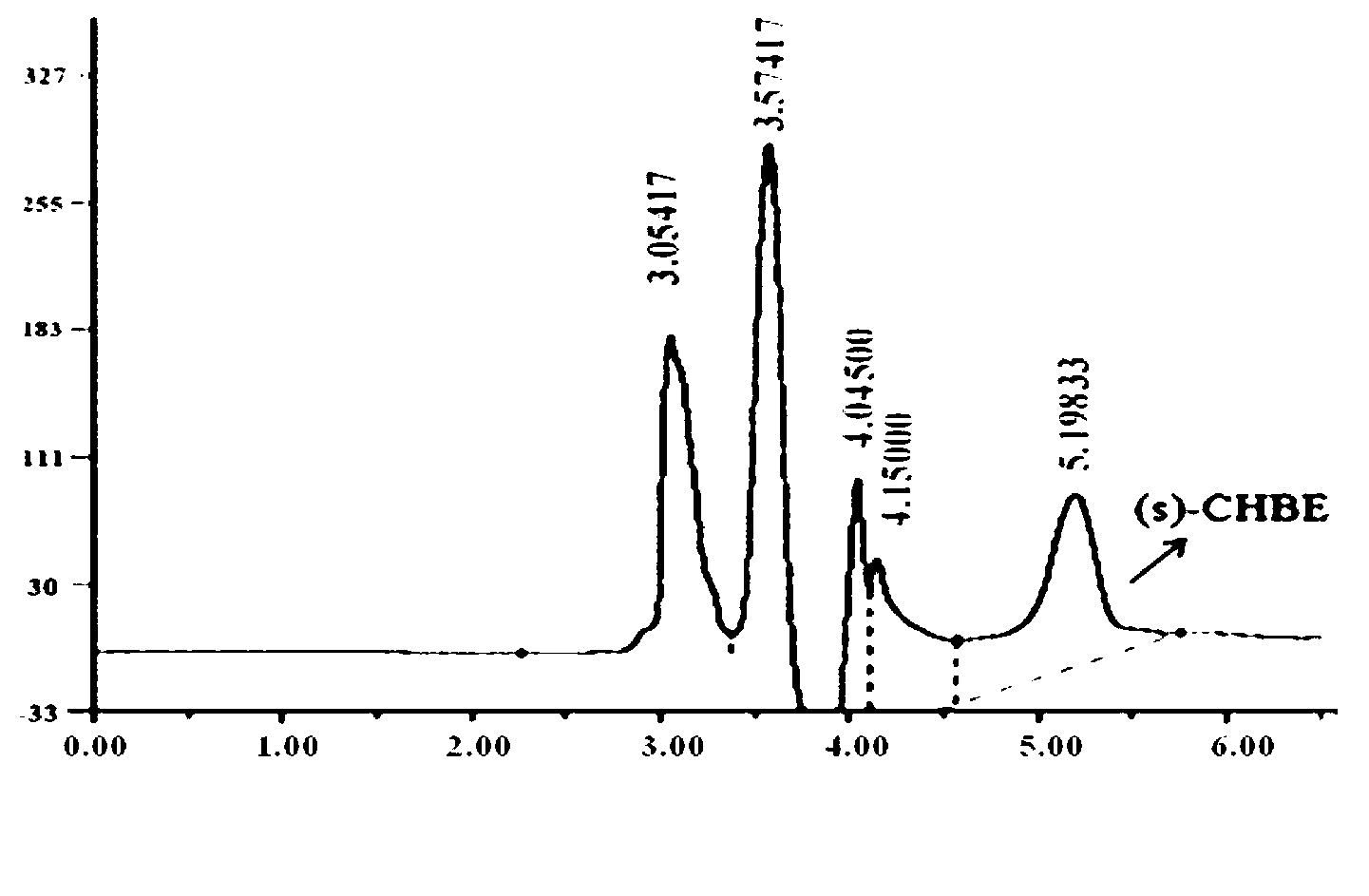
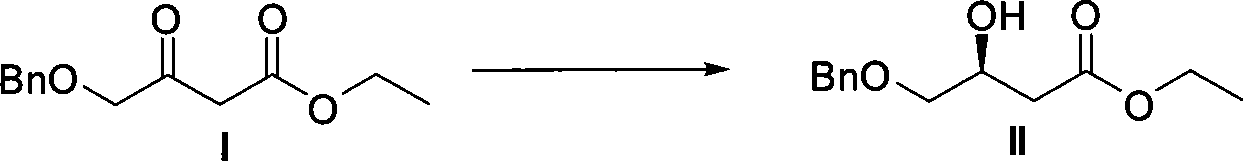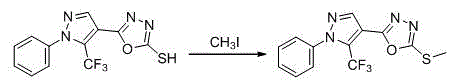Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
823 results about "Ethyl acetoacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B₁; as well as the manufacture of dyes, inks, lacquers, perfumes, plastics, and yellow paint pigments. Alone, it is used as a flavoring for food.
Ratio type fluorescent probe for distinguishing lipid droplets with different polarities as well as preparation method and application thereof
InactiveCN108440475ARealize detectionLow toxicityOrganic chemistryFluorescence/phosphorescenceBenzaldehydeWhole body
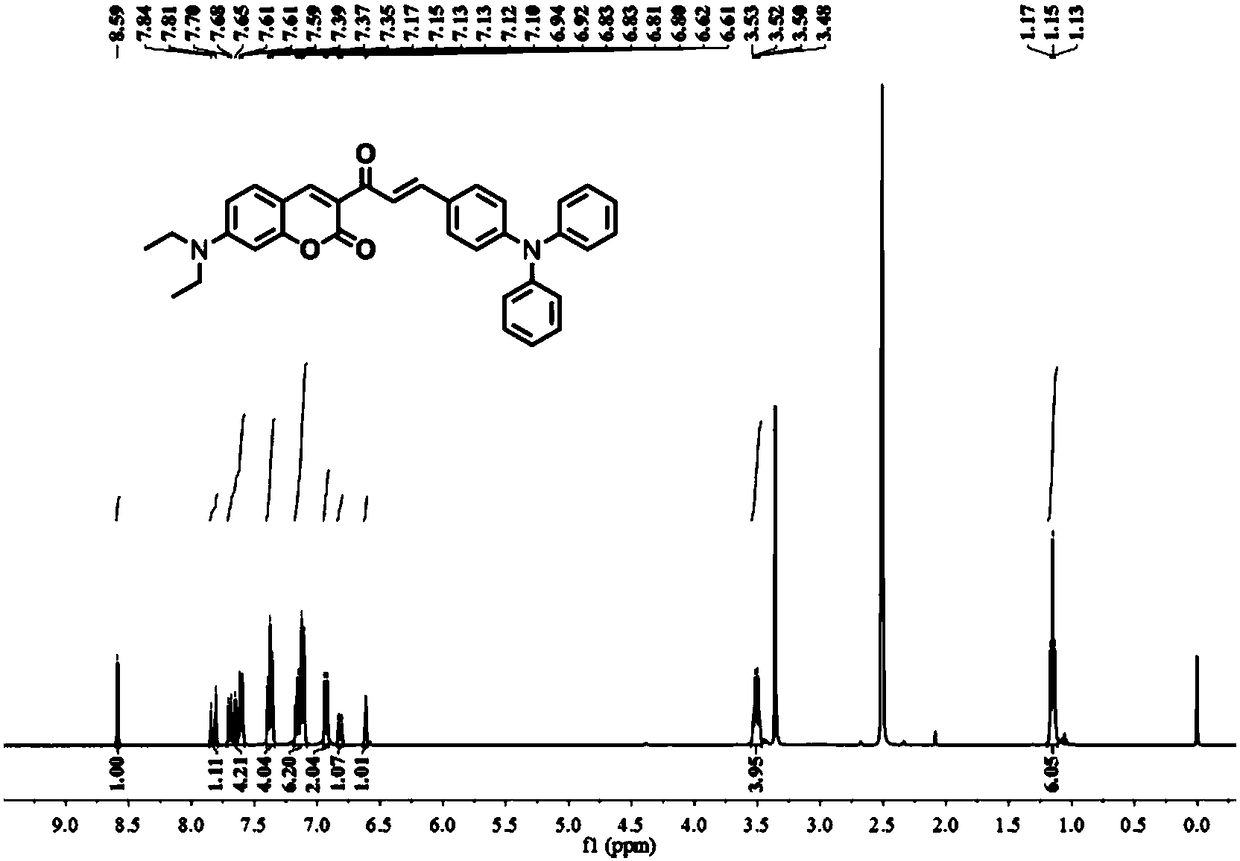
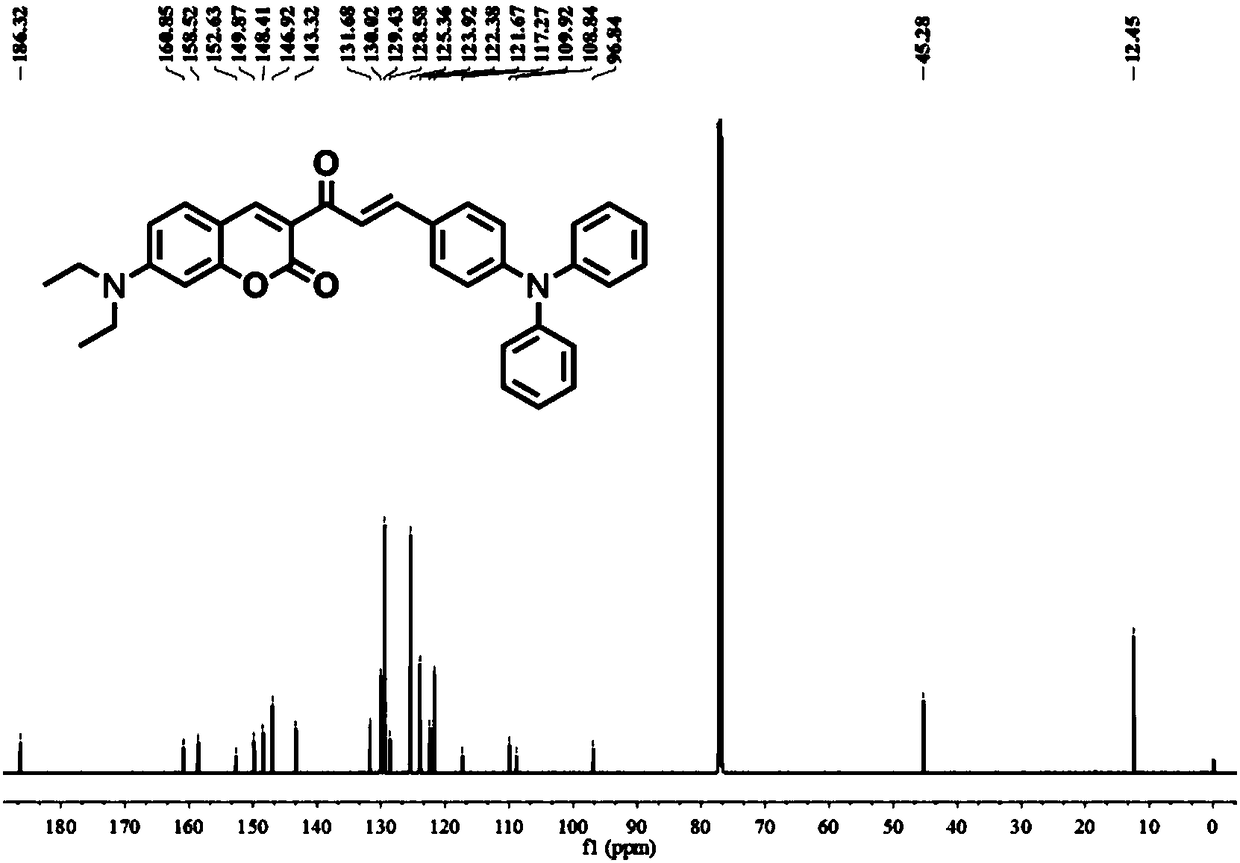
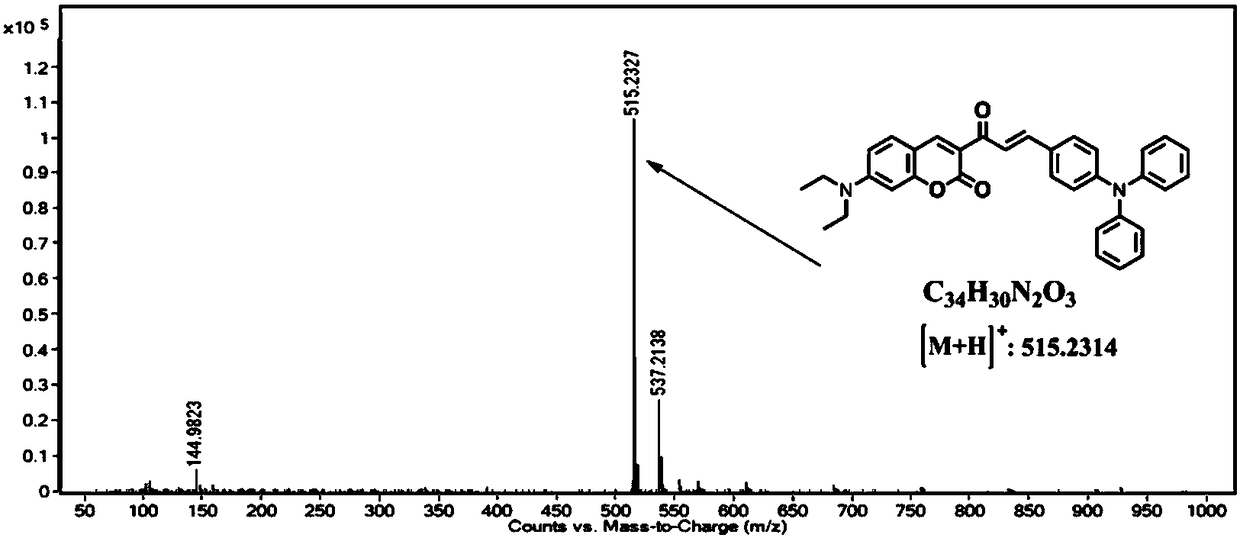
The invention provides a ratio type fluorescent probe for distinguishing lipid droplets with different polarities. A chemical name is (E)-7-(N,N-diethylamino)-3-(3-(4-(diphenylamine)phenyl)acrylyl-2H-benzopyrone; N,N-diethylamino salicylic aldehyde reacts with ethyl acetoacetate to generate 3-acetyl-7-(diethylamino)coumarin; the product reacts with 4-(N,N-Diphenylamino)benzaldehyde (4) to obtain the fluorescent probe. The fluorescent probe has a two-photon property and an aggregation-induced light-emitting property; a whole body of the probe is electrically neutral and can be positioned in thelipid droplets with the different polarities very well. The probe has low toxicity, good optical stability and specific response on the polarities; detection on the polarities of the lipid droletps in organs and living bodies is realized. The invention further provides a synthesis method of the probe; the synthesis method has the advantages of simple steps, convenient for purification and high yield.
Owner:UNIV OF JINAN
Method for preparing pitavastatin calcium raw material medicine using asymmetric hydrogenation
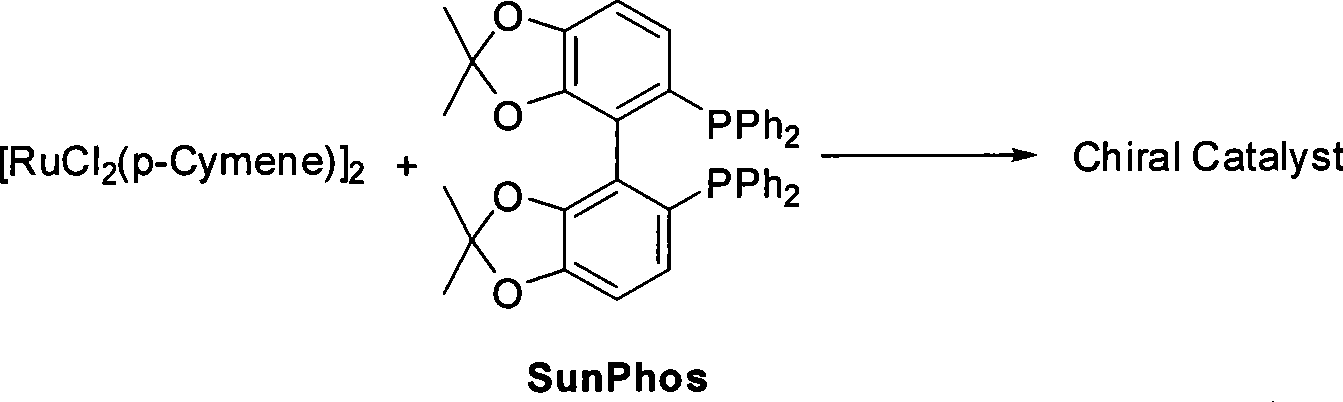
ActiveCN101386592ASolve the problem of expensive chiral side chainsMetabolism disorderAsymmetric synthesesClaisen condensationEthyl butyrate
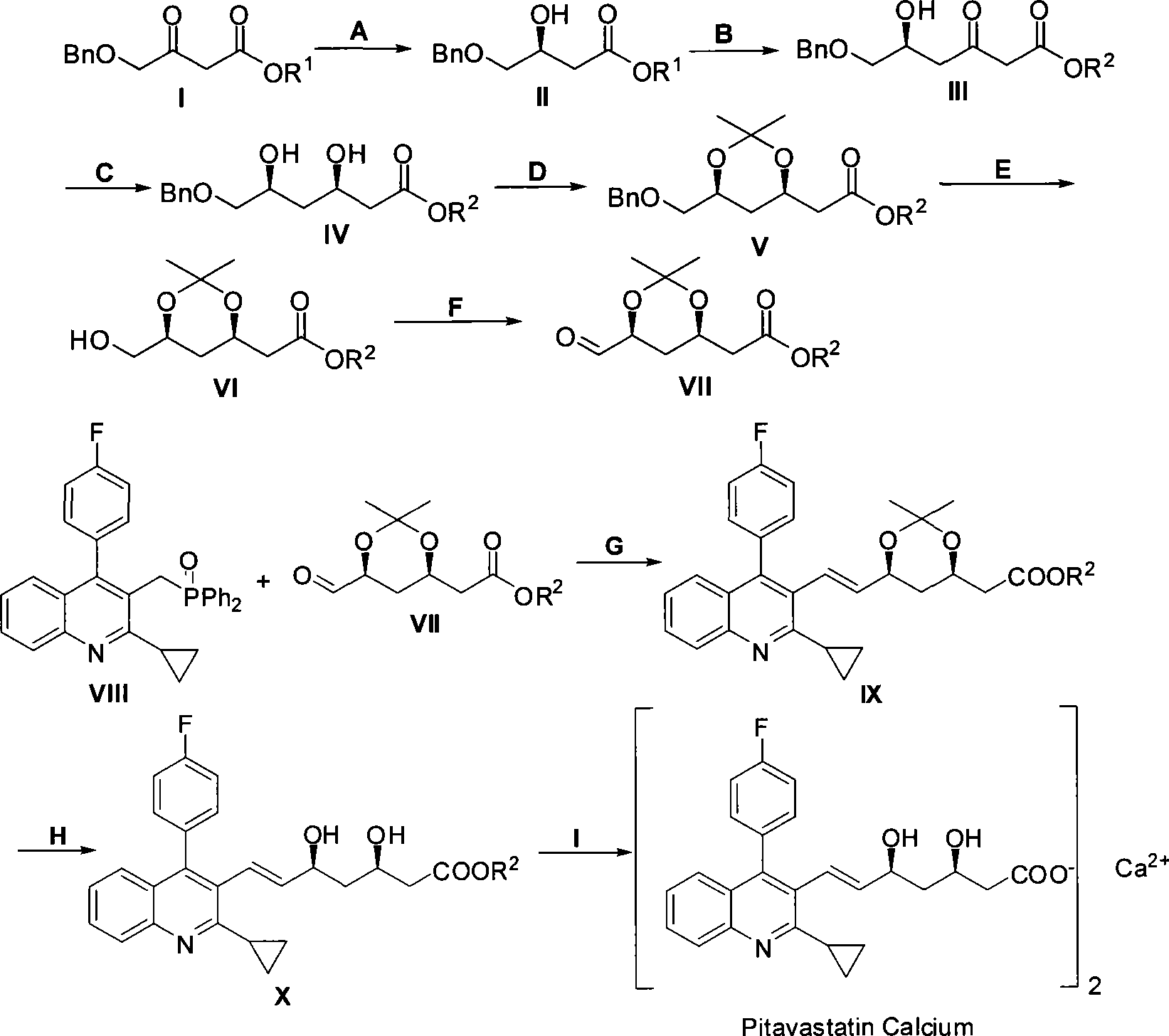
The invention relates to a method for preparing a pitavastatin calcium raw material by asymmetric hydrogenation in the technical field of medicinal chemistry. The method comprises the following steps: performing catalytic hydrogenation on 4-benzyloxy-ethyl-acetoacetate I by using a chiral catalyst to prepare (S)-4-benzyloxy-3-hydroxy-ethyl-butyrate, and then obtaining the pitavastatin calcium through a series of reactions such as Claisen condensation, reduction, hydroxyl protection, removal of benzyl, oxidation and the like. In the invention, the highly-efficient chirality added value is realized by the use of catalytic amount of the chiral catalyst (wherein the ratio of substrate to the catalyst can reach 3, 000 to 1), and by performing the asymmetric hydrogenation and the reduction on non-chiral ketones to generate chiral alcohols compounds directly (wherein the enantioselectivity can be up to 94.3 percent).
Owner:JIANGSU WANBANG BIOPHARMLS +1
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
Flavoured mouth wash composition
InactiveUS20060210491A1Growth inhibitionReduced effectivenessCosmetic preparationsBiocideDamasconePyrophosphate
The present invention provides a flavoured product comprising four or more flavour materials having antimicrobial properties and selected from the group comprising nonanol, decanol, nonanal, decanal, amyl propionate, anethole synthetic, anisic aldehyde, basil oil, benzyl benzoate, benzyl butyrate, benzyl formate, camomile oil, cinnamic aldehyde, cis-3-hexenol, clove bud oil, damascone, ethyl acetoacetate, eucalyptus oil, ginger, isoamyl acetate, menthol laevo, methyl cinnamate, methyl salicylate, orange oil, rosemary oil, tarragon, Tea Tree oil, and peppermint oil; and one or more antimicrobial agents selected from the group comprising triclosan, pyrophosphates, zinc salts, cetylpyridinium chloride, parabens, stannous salts, sodium dodecyl sulphate, chlorhexidine, copper salts, strontium salts, peroxides and sanguinarine.
Owner:QUEST INTERNATIONAL
Rhodamine derivatives and their preparation method and use
InactiveCN102516254AStrong specificityDetection interferenceOrganic chemistryAzo dyesEthylenediamineNon invasive
The invention relates to rhodamine derivatives and their preparation method and use. The rhodamine derivatives have a general formula I and can be utilized for determination of Fe<3+>. The preparation method of the rhodamine derivatives comprises that an intermediate produced by the reaction of rhodamine B and ethylenediamine is utilized as a raw material and undergoes a condensation reaction with acetylacetone or ethyl acetoacetate in the presence of ceric ammonium nitrate as a catalyst to produce the rhodamine derivatives. Results of an analysis of a mixed-ion interference-resistant iron-ion specific identification capacity of the rhodamine derivatives and an analysis of a living cell condition adaptation capacity of the rhodamine derivatives show that the rhodamine derivatives have strong iron ion specificity, a strong other cation-resistant capacity and a strong living cell condition adaptation capacity, wherein iron ion concentration detection sensitivity is 1*10<-6>; reaction stabilization time is 20 minutes; and a pH value of a Tris-HCl buffer solution is 5.5 or 6.0. The rhodamine derivatives can be utilized as iron ion induction agents for a non-invasive micro-metering system and realizes determination of Fe<3+> concentration, a Fe<3+> flowing speed and a Fe<3+> motion direction.
Owner:CAPITAL NORMAL UNIVERSITY +1
Preparation method of graphene/TiO2 fiber
InactiveCN103111274AEasy to recycleNot easy to fall offPhysical/chemical process catalystsFiberTetrahydrofuran
The invention provides a preparation method of a graphene / TiO2 fiber composite material. The method comprises the following steps: ultrasonically dispersing different amounts of graphene into tetrahydrofuran; mxing with ethyl acetoacetate used a chelating agent and tetrabutyl titanate used as a titanium source to form a homogeneous graphene dispersion solution; magnetically stirring the dispersion solution and adding a proper amount of tetrahydrofuran and a small amount of deionized water and reacting; carrying out heating reflux to form a spinning solution; injecting the spinning solution into a spin throwing machine to carry out a spin throwing treatment so as to obtain a precursor fiber; placing the precursor fiber in a procedure heating furnace to carry out a procedure heating treatment at the temperature of 25 to 450 DEG C in a N2 atmosphere; naturally cooling naturally to obtain the graphene / TiO2 fiber. The graphene / TiO2 fiber has excellent photocatalytic performance and is suitable for the treatment of drinking water and the later-period deep treatment of sewage.
Owner:SHANDONG UNIV
Application of alcohol dehydrogenase in catalytic generation of ethyl (R)-4-chloro-3-hydroxy butyrate
InactiveCN103160547AHigh yieldHigh optical activityMicroorganism based processesFermentationHydroxybutyric acidPtru catalyst
The invention discloses application of alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction. By using alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 as a catalyst, ethyl 4-chloroacetoacetate as a substrate and NADH (nicotinamide adenine dinucleotide) as a cofactor, asymmetric reduction is carried out to prepare the ethyl (R)-4-chloro-3-hydroxy butyrate. The invention applies the alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction for the first time, and has favorable effect. The enzyme activity is up to 5.6 U / mg, the yield of the substrate is up to 94%, and the enantiomeric excess value of the product is 100%. The yield is high, and the production cost is greatly lowered.
Owner:NANJING UNIV OF TECH
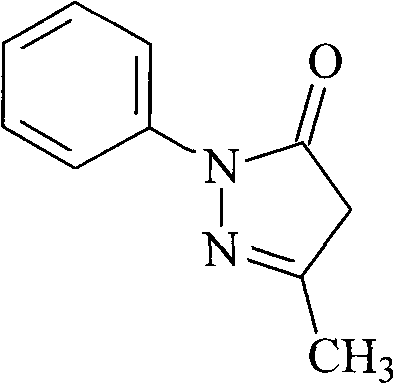
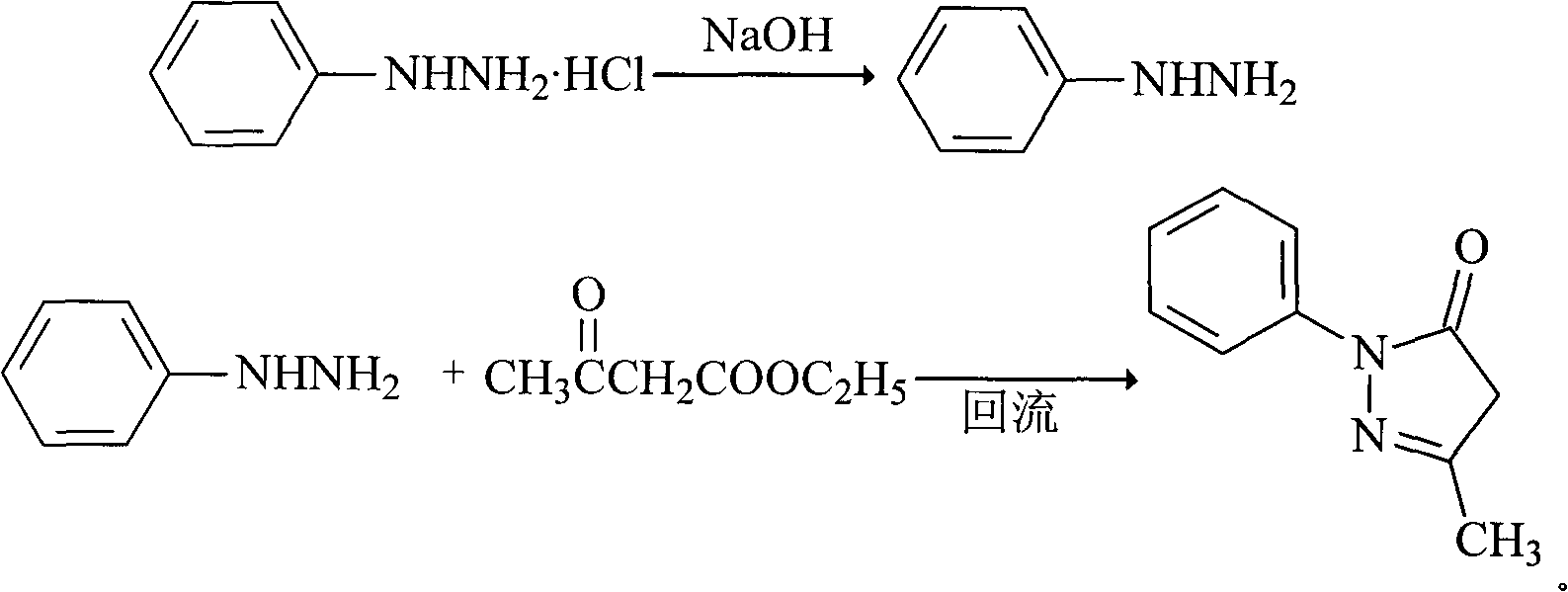
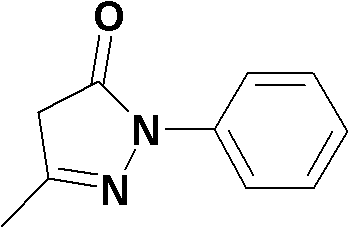
Edaravone compound synthesized by new method
InactiveCN101830852AHigh purityReduce manufacturing costOrganic chemistryAcetic acidPhenylhydrazine hydrochloride
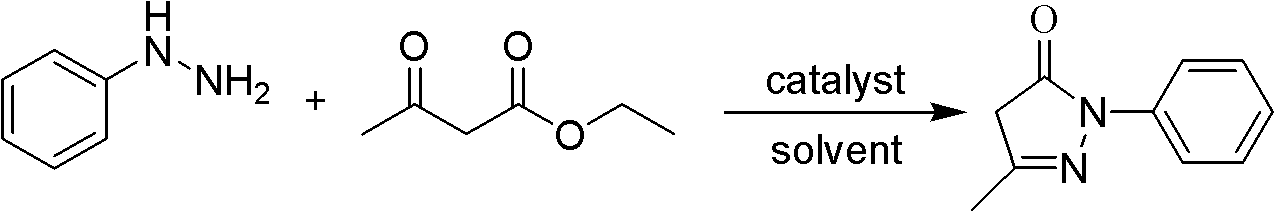
The invention relates to Edaravone synthesized by a new method. The method comprises the following steps of: reacting phenylhydrazine hydrochloride used as initial raw material with sodium hydroxide to generate phenylhydrazine; mixing the phenylhydrazine and ethyl acetoacetate to generate reflux reaction to prepare crude Edaravone; dissolving the crude Edaravone into an isopropanol water solution, adding active carbon for adsorbing and filtering to prepare fine white crystalloid powder. The invention has the advantages of low cost, high product yield and high purity.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Method for synthesizing cosmetic active material hydroxypropyl tetrahydropyrantriol through one-pot method under promoting of rare earth metal coordination compound
InactiveCN110467591ASimple and fast operationSuitable for industrial productionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidOrganic synthesis
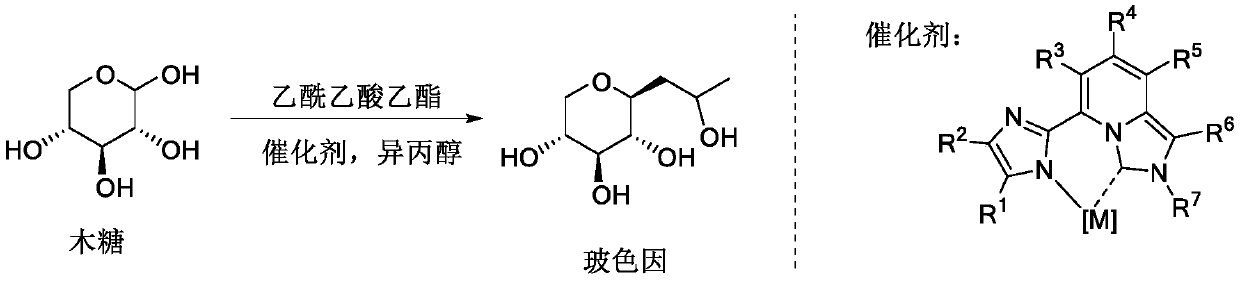
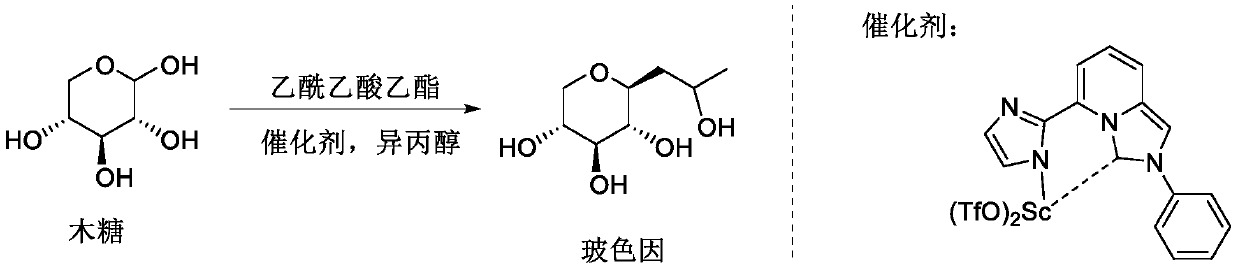
The invention belongs to the fields of organic synthesis, fine chemicals and daily chemicals, and particularly relates to a method for synthesizing hydroxypropyl tetrahydropyrantriol from xylose and ethyl acetoacetate through a one-pot method under the promoting of a rare earth metal coordination compound. According to the present invention, the method has characteristics of easy operation, inexpensive and easily available reagents and good industrial application prospects.
Owner:SHANGHAI COACHCHEM TECH
Method for preparing methyl acetoacetate by using novel composite catalyst
ActiveCN101337890AReduce energy consumptionIncrease production capacityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidBoiling point
The invention discloses a method for preparing methyl acetoacetate by using complex catalyst, which comprises the following steps: carrying out esterification reaction of methanol and diketene to generate crude methyl acetoacetate, wherein two different catalysts are added in the different stages of the esterification reaction; triethylenediamine catalyst is added before the esterification reaction; and diketene can be added directly dropwise without being heated by vapor, thereby overcoming the disadvantage that diketene is added dropwise after heating methanol to the boiling point in the conventional process and reducing the energy consumption; and cooling the generated liquid to 40 DEG C after the reaction, adding concentrated sulfur acid catalyst, keeping the temperature for half an hour, filtering, continuously rectifying the filtrate, and separating to obtain fine methyl acetoacetate with the content larger than 99%. Compared with the conventional intermittent rectification method, the continuous rectification method has greatly improved throughput and more convenient operation.
Owner:安徽天成新材料有限公司
Use of carbonyl reductase in (S)-4-chloro-3 hydroxy butyric ether production
The invention discloses an application of carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 to prepare (S)-4-chloro-3-hydroxybutanoic ethyl ester by asymmetric reduction of 4-chloracetyl ethyl acetate. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is taken as a catalyst, the ethyl 4-chloracetyl ethyl acetate is taken as a substrate, and NADPH is taken as a cofactor, and the (S)-4-chloro-3-hydroxybutanoic ethyl ester is prepared by the asymmetric reduction. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is applied to preparing the (S)-4-chloro-3-hydroxybutanoic ethyl ester by the asymmertric reduction of the 4-chloroacetyl ethyl acetate for the first time, which produces good effect; the yield of the substrate is up to 95%, and the enantiomer excess value of the product is 100%, the yield is high, and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Titanium-containing ceramic paint and protective coating
ActiveUS20140170425A1Maintain good propertiesHigh temperature resistanceLayered productsPaints with free metalsZirconium dioxideAluminium
Disclosed is a titanium-containing ceramic paint, including 100 parts by weight of silica sol, 50 to 100 parts by weight of organic silane, 0.3 to 1 parts by weight of a catalyst, 5 to 20 parts by weight of titanium powder, and 1 to 5 parts by weight of a silicone oil. The organic silane can be methyltrimethoxy silane and / or methyltriethoxy silane. The catalyst can be formic acid, acetic acid, hydrochloric acid, citric acid, methyl formate, ethyl acetoacetate, maleic anhydride, or combinations thereof. The titanium-containing ceramic paint may further include 30 to 50 parts by weight of filler, such as silica, alumina, zirconium dioxide, silicon carbide, aluminum nitride, boron nitride, kaolin, talcum powder, mica powder, silicate of aluminum or zirconium, barium sulfate, metal fiber, stainless powder, or combinations thereof. The titanium-containing ceramic paint may further include 30 to 40 parts by weight of a color powder.
Owner:INNOTEK TECH CHINA
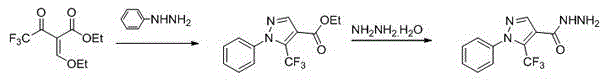
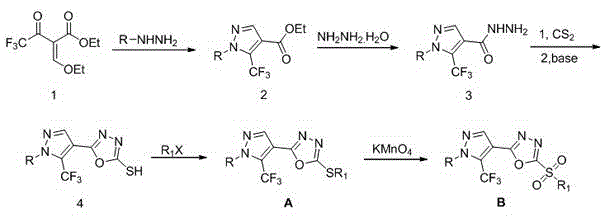
1-substituted-5-trifluoromethyl-4-pyrazol-1,3,4-oxadiazole thioether or sulfone derivatives and application of derivatives
The invention discloses a kind of 1-substituted-5-trifluoromethyl-4-pyrazol-1,3,4-oxadiazole thioether or sulfone derivatives and application of the derivatives. The derivatives have the structures represented by general formulae A and B shown in the specification. 1-substituted-5-trifluoromethyl-4-pyrazol-1,3,4-oxadiazole compounds are synthesized from ethyl trifluoroacetoacetate by the following five reactions: esterification, ring closure, hydrazinolysis, ring closure and substitution. The compounds have certain inhibition effects to plant bacterial diseases or plant fungous diseases and can be used as pesticides and pesticide additives for preventing and controlling the plant bacterial diseases or the plant fungous diseases.
Owner:GUIZHOU UNIV
Preparation method for edaravone
ActiveCN102180834ASimple processThe reaction conditions are mild and easy to controlOrganic chemistryAcetic acidAlcohol
The invention discloses a preparation method for edaravone. The preparation method comprises the following steps of: reacting phenylhydrazine with ethyl acetoacetate in an alcohol solvent under the action of a catalyst to prepare the edaravone; and after the reaction, adding a non-alcohol solvent to cool and crystallize to obtain the edaravone crude product. The high-yield and high-purity edaravone is prepared from the phenylhydrazine and the ethyl acetoacetate which serve as the raw materials in the presence of acid serving as the catalyst. The quality of the edaravone product is high; the process is simple; the reaction condition is mild and easy to control; the aftertreatment is simple; the problem of three wastes is avoided; cost is low; and the preparation method is suitable for industrialized production.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
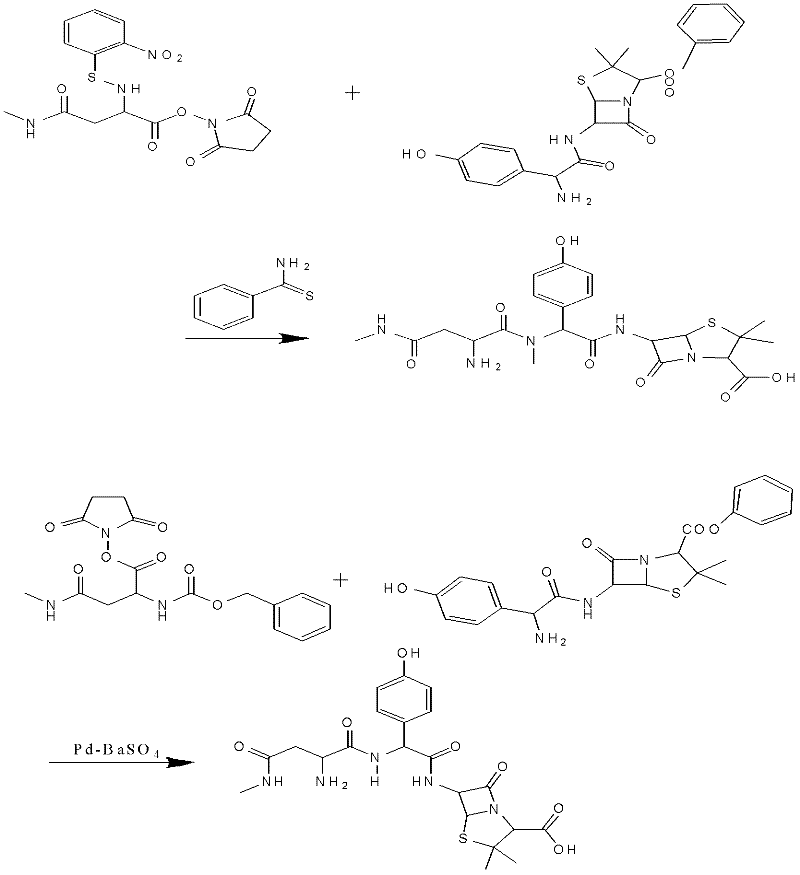
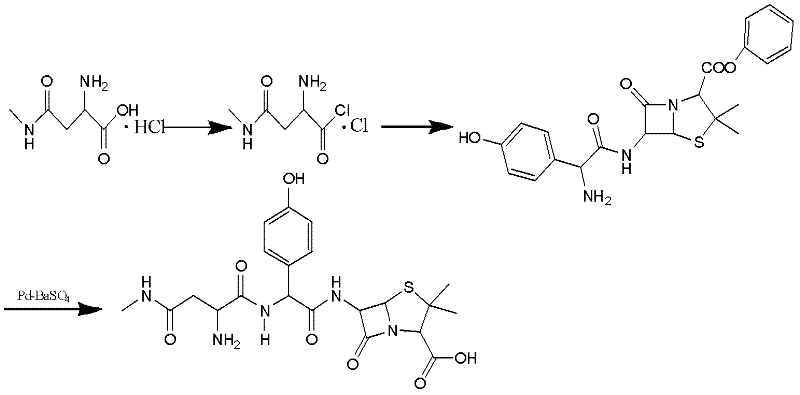
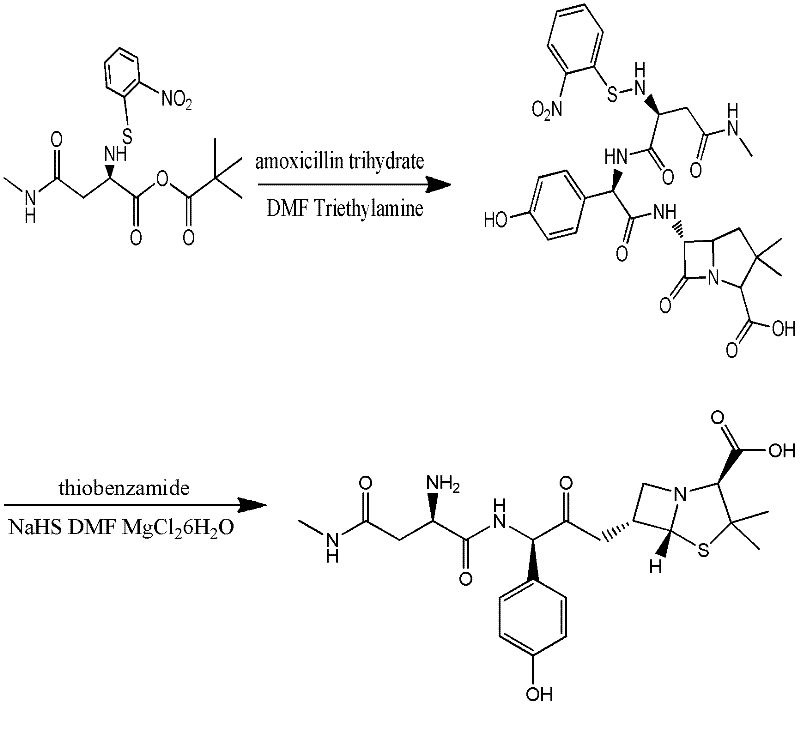
Preparation method for Aspoxicillin
The invention discloses a preparation method for Aspoxicillin. D-aspartic acid is added into a mixture liquid of sulfuryl chloride and carbinol under a low temperature of zero to prepare D-aspartic acid methyl ester hydrochloride; the obtained D-aspartic acid methyl ester hydrochloride and triethylamine are reacted in ethanol to obtain D-aspartic acid methyl ester educt; the D-aspartic acid methyl ester educt and methylamine aqueous liquid with a concentration of 40 percent are reacted in a room temperature to prepare aspartic formamide; the aspartic formamide, ethyl acetoacetate and potassium hydroxide are reacted in isopropanol to prepare diene salt (D-2-amino-3N-methylamino oxo-propionic acid diene formamide); the diene salt and pivaloyl chloride are reacted in acetone under the catalysis of pyridine to obtain active anhydride; then the active anhydride is condensed and further protected by deacidification to obtain a target product-crude product of Aspoxicillin. The preparation method for Aspoxicillin has the advantages of cheap and easily-obtained reagent, lower toxicity and lower environment pressure, stable and simple technological operation and high yield.
Owner:SOUTHWEST JIAOTONG UNIV
Preparation of 2-(3-carboxaldehyde-4-hydroxy phenyl)-4-methyl-5-thiazole ethyl formate
InactiveCN101412699AEmission reductionHigh yieldOrganic chemistryHexamethylenetetramineEthyl acetate
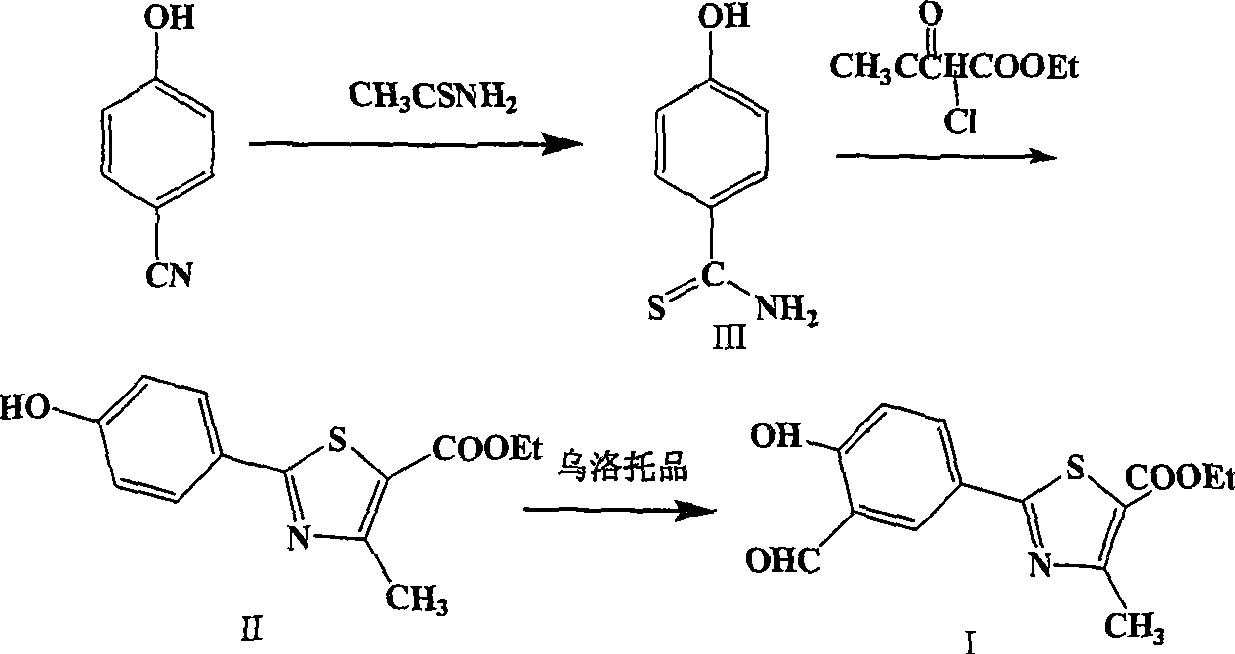
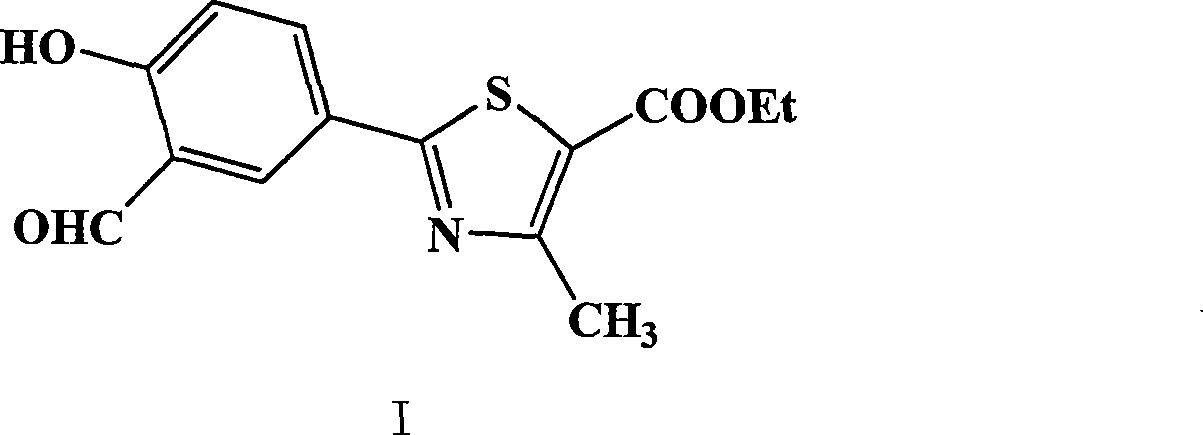
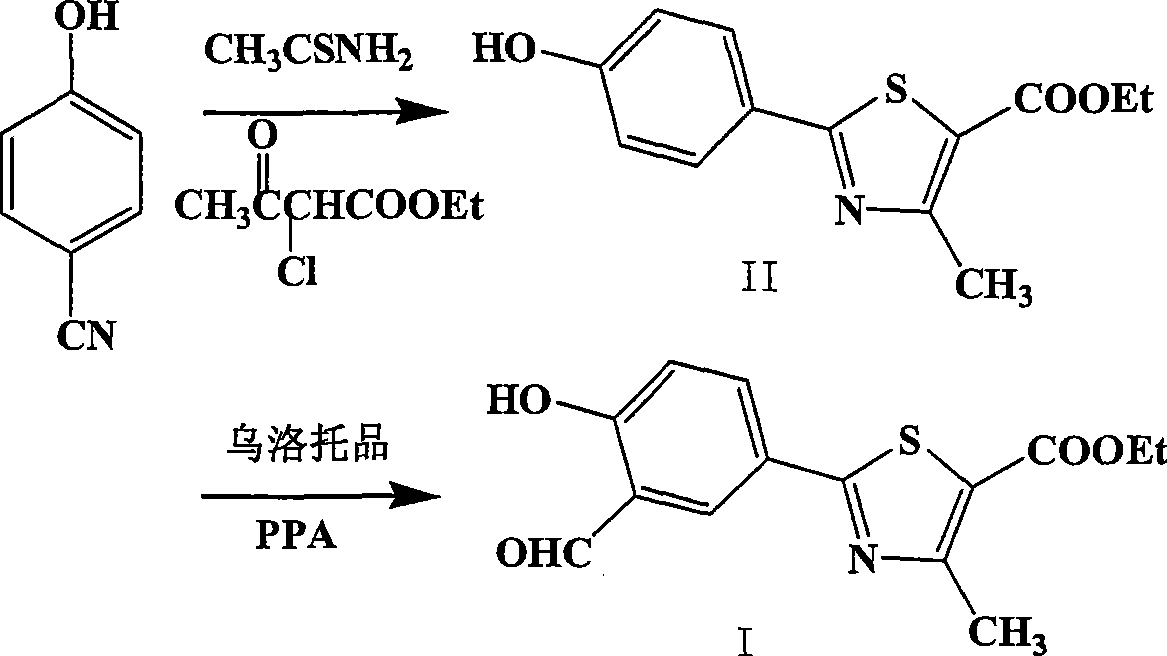
The invention provides a method for preparing 2-(3-formaldehyde base-4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate. The method uses para-cyano-phenol and thioacetamide as raw materials which are reacted with each other to obtain 4-hydroxy-thiobenzamide, and the obtained product is reacted with 2-chloro acetylacetic ether to obtain 2-(4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate, and then is reacted with urotropine to obtain the 2-(3-formaldehyde base-4-hydroxyphenyl)-4-methyl-5-thiazole ethyl formate.
Owner:SHANGHAI INST OF PHARMA IND +1
Prepn of photocatalytic titania fiber material
InactiveCN1772373AThe synthesis process is simpleMeet the requirements of "green chemistry"Physical/chemical process catalystsFiberSteam activation
The preparation process of photocatalytic titania fiber material includes the following steps: synthesizing polyacetyl ethyl acetate-titanium complex as the precursor; dissolving the synthesized polyacetyl ethyl acetate-titanium complex in tetrahydrofuran to compounding spinning liquid of viscosity 5-100 Pa.s, with the ratio between the synthesized polyacetyl ethyl acetate-titanium complex and tetrahydrofuran being 100 g to 400-800 ml; centrifugally spinning the spinning liquid of viscosity 5-100 Pa.s into short precursor fiber; dry spinning the spinning liquid of viscosity 50-100 Pa.s into continuous long precursor fiber; and final water steam activation of the precursor fiber and heat treatment and sintering. The preparation process has simple operation, easy control and low power consumption.
Owner:SHANDONG UNIV
Method for preparing TiO2 film by utilizing sol-gelatin method
ActiveCN101660147AFull coverageImprove surface qualityLiquid surface applicatorsTitanium dioxideCross-linkHeat treated
The invention discloses a method for preparing TiO2 film by utilizing a sol-gelatin method. The method comprises the following steps: (1) dissolving Ti (OC4H9) in ethylene glycol monoemethyl ether, then adding acetylacetic ether and methanamide, adding a pH regulator solution after stirring, continuously stirring the mixture to obtain uniform and transparent TiO2 sol with light yellow color, standing and maturing the mixture for later use; (2) dropwise adding the TiO2 sol after maturing to the surface of a substrate to be coated for film coating, putting the substrate in drying cabinet for predrying, putting the substrate in a cabinet-type high temperature resistance furnace for heat treatment, naturally cooling the substrate to a room temperature, putting an obtained film in a high temperature steam kettle for treatment, and then putting the film in boiling water for treatment to obtain the TiO2 film. In the invention, cross-linking agent formamide is added to a sol precursor solution; steam and hydrothermal joint treatment is carried out on the TiO2 film after heat treatment, therefore, internal stress and surface checkmarks of the TiO2 film prepared by the sol-gelatin method areeliminated or weakened, and the film has the advantages of more smooth and compact surface, complete coverage, favorable surface uniformity, uniform thickness and better practical use performance.
Owner:NORTHWEST INSTITUTE FOR NON-FERROUS METAL RESEARCH
Ceramic paint and coating having stereoscopic effect
The invention relates to ceramic paint having a stereoscopic effect, which is characterized by comprising the following components in parts by weight: 100 parts of silica sol, 50-100 parts of organosilane, 0.3-1 part of catalyst, and 1-20 parts of magnetic powder, wherein the organosilane is methyltrimethoxy silane and / or methyltriethoxy silane; and the catalyst is one or a combination of formic acid, acetic acid, hydrochloric acid, citric acid, methyl formate, ethyl acetoacetate and maleic anhydride. The ceramic paint further comprises 1-5 parts by weight of silicone oil and 5-10 parts by weight of additional organosilane, wherein the additional organosilane is one or a combination of tetramethoxyl silane, tetraethoxy silane, dimethyldimethoxy silane, dimethyldiethoxy silane and phenyltrimethoxy silane. The ceramic paint provided by the invention has favorable cold / hot hardness and wear resistance; and the magnetic powder in the paint can form a coating having multiple stereoscopic patterns, thereby achieving good decorative effect.
Owner:INNOTEK TECH CHINA
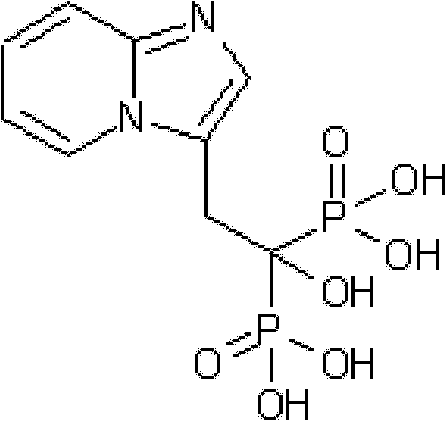
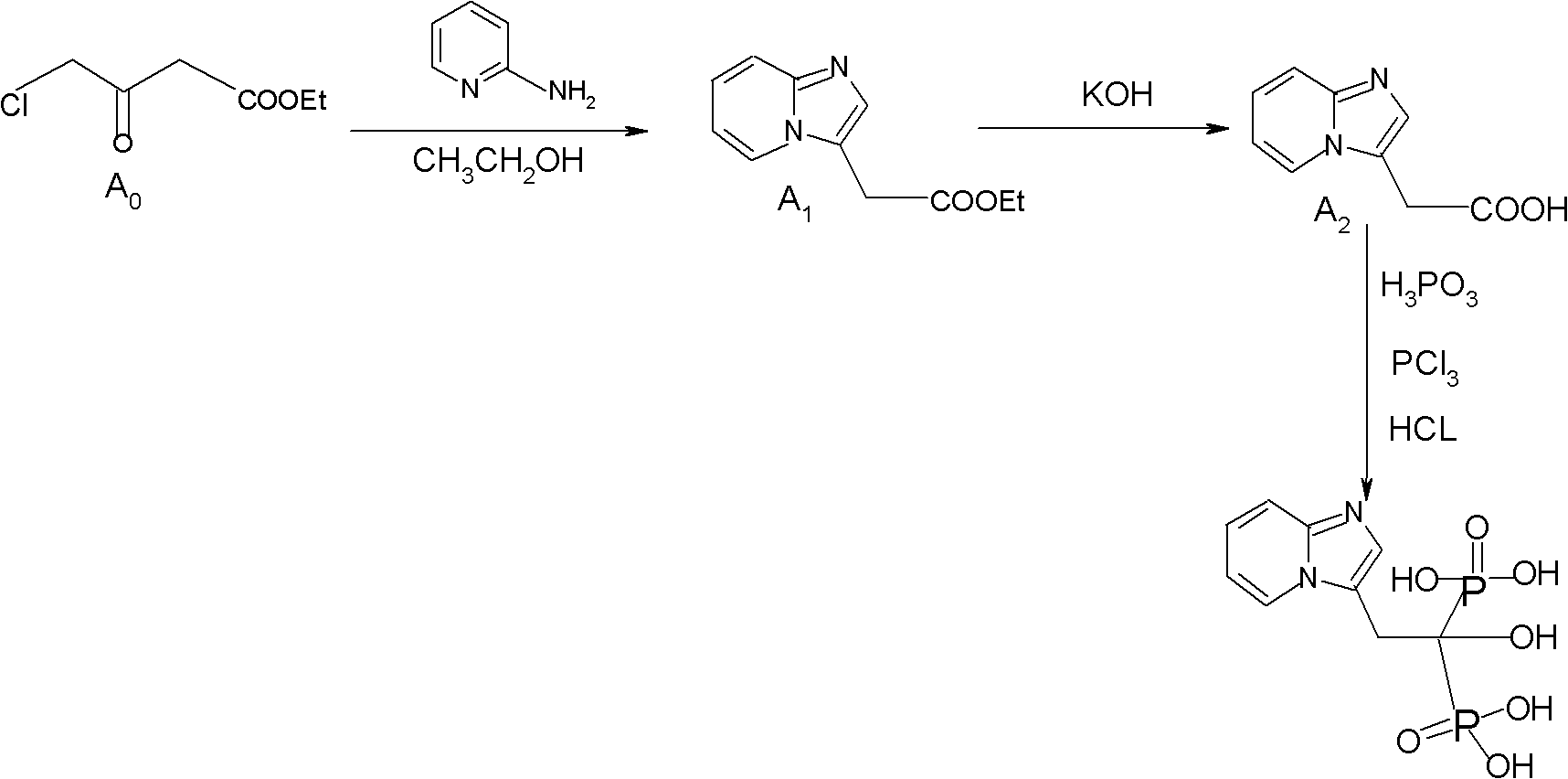
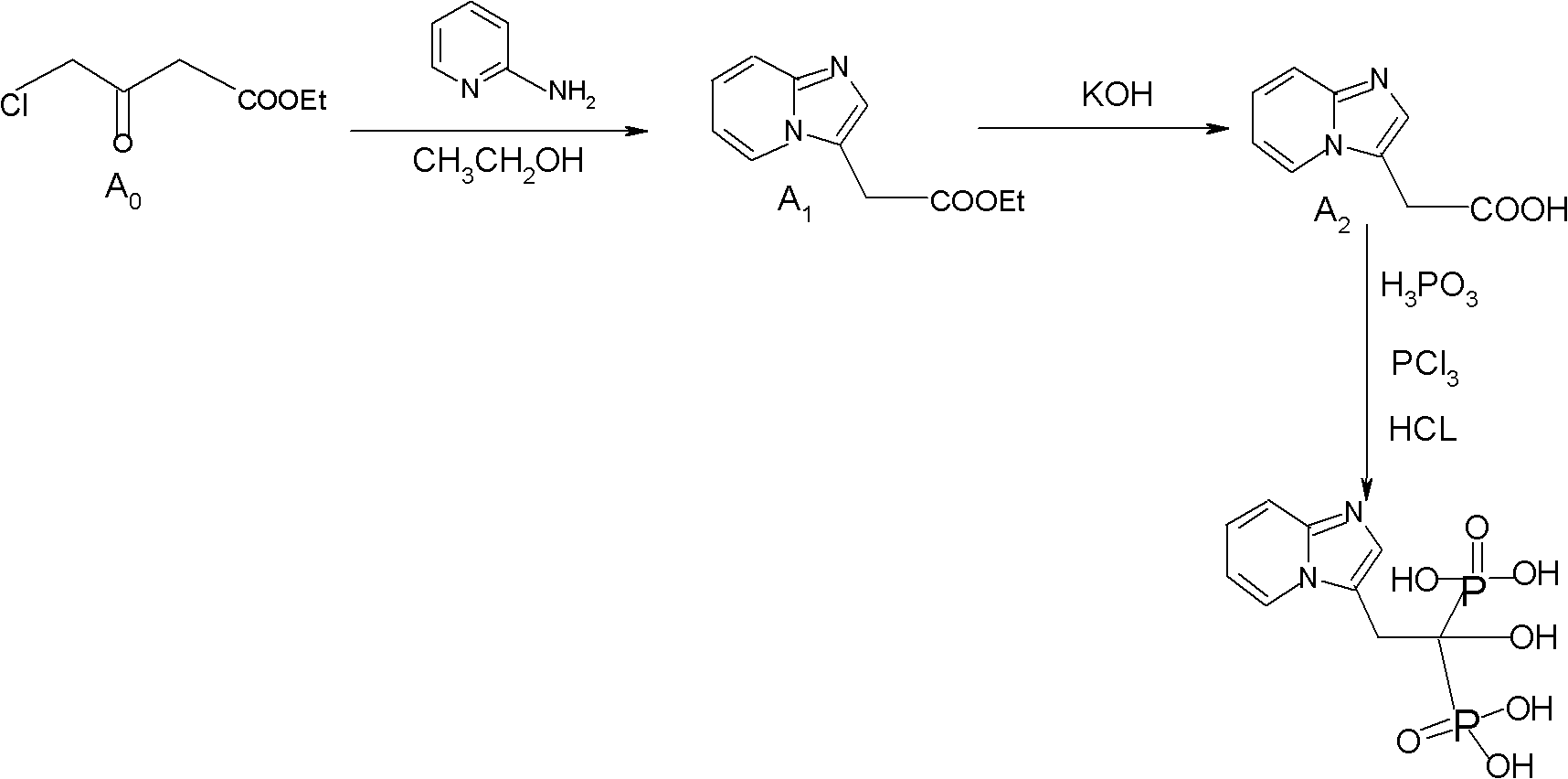
Synthesis method of minodronate midbody and synthesis of minodronate
ActiveCN102153585AAvoid pollutionImprove securityGroup 5/15 element organic compoundsSynthesis methodsFiltration
The invention relates to the field of pharmaceutical chemistry, in particular to a synthesis method of a minodronate midbody and synthesis of minodronate. The preparation method of minodronate includes the following steps: using organic solvent to dissolve 2-aminopyridine, adding 4-acetyl chloride ethyl acetoacetate for reaction, monitoring the reaction solution by TLC(Thin-Layer Chromatography) until spots of 4-acetyl chloride ethyl acetoacetate disappear, concentrating to a dry state, dissolving concentrate in water, washing a water layer to remove impurities, extracting the water layer with the organic solvent, washing extract liquor, separating out an organic layer, conducting filtration, and concentrating the concentrate to be in a dry state, thereby obtaining A1.
Owner:福建太平洋制药有限公司
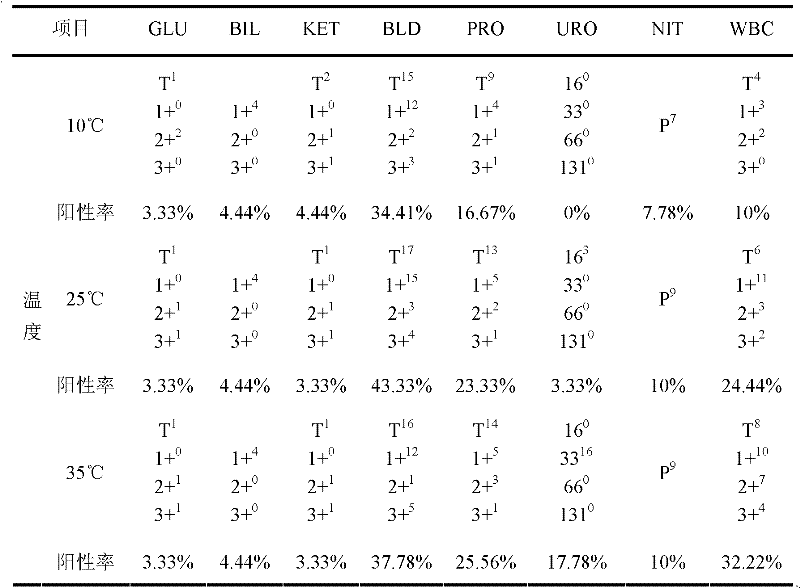
Middle/low concentration positive quality control liquid for urine analysis
ActiveCN102226805AReduce mutual interferenceReduce the impact of quality control effectsBiological testingBovine serum albuminDirect bilirubin
The invention discloses a middle / low concentration positive quality control liquid for urine analysis. The positive quality control liquid comprises a buffer solution with a pH value of 4.5 to 8.5 and a concentration of 20 to 200 mmol / L, a surfactant with the concentration of 20 to 200 mmol / L, a carbohydrate protectant with the concentration of 20 to 200 mmol / L, a protein protectant with the concentration of 20 to 200 mmol / L, anhydrous glucose with the concentration of 300 to 990 mg / L, bovine serum albumin with the concentration of 100 to 1000 mg / L, bovine hemoglobin with the concentration of0.6 to 5 mg / L, esterase with the concentration of 2 to 20 mg / L, acetone or lithium acetoacetate or sodium ethyl acetoacetate or ethyl acetoacetate with the concentration of 50 to 900 mg / L, and a direct bilirubin or naphthylamine salt with the concentration of 3 to 40 mg / L. Quality control objects of corresponding projects can be added as needed. Compared with the prior art, the quality control liquid has the advantages of good stability, high sensitivity, less toxicity of each component, wide source and low production cost.
Owner:URIT MEDICAL ELECTRONICS CO LTD
Solid base catalyst, method for preparing the same and applications
InactiveCN101274274AHigh activitySimple washing and calcination treatmentPreparation by hydrogenolysisMetal/metal-oxides/metal-hydroxide catalystsAcetic anhydrideAdhesive
The invention provides a solid base catalyst and a preparation method thereof for preparing acetylacetone with the solid base catalyst. The solid base catalyst is prepared mainly by the components with the weight composition as follows: carrier: 100 portions of Gamma-Al2O3, load components: 0.5-8 portions of adhesive, 5-35 portions of magnesium compound, 0.5-10 portions of hydroxide and 0.5-5 portions of polyacrylamide; the invention has the beneficial effects which are mainly indicated by that: compared with the catalyst prepared by the traditional dipping method, the solid base catalyst prepared by the method of the invention directly adopts a mixed forming method, has simple preparation method and easy controlling of conditions, avoids the generation of oxide which is generated in the roasting process by adopting nitrate former body, thus generating no environmental pollution problem; when the solid base catalyst of the invention is used for catalysing the ethyl acetoacetate and acetic anhydride to synthesize acetylacetone, the yield is high, the equipment investment is little, and no three-waste exists, thus being beneficial for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
Ketoreductase mutant for producing (S)-4-chloro-3-hydroxy ethyl butyrate
ActiveCN104342412ASignificantly high specific enzyme activityMild reaction conditionsBacteriaMicroorganism based processesEthyl butyrateLactobacillus kefir
The invention relates to a ketoreductase mutant deriving from wild type ketoreductase of lactobacillus kefir. The ketoreductase mutant is capable of converting ethyl-4-chloroacetoacetate to generate (S)-4-chloro-3-hydroxy ethyl butyrate and has one or multiple of mutations in A94S, F147V, L199P and A202V. The ketoreductase mutant provided by the invention has obvious high specific enzyme activity which is improved by 2-20 times in comparison with the wild type ketoreductase; the enzyme can be used for biologically catalyzing to convert the ethyl-4-chloroacetoacetate to generate (S)-4-chloro-3-hydroxy ethyl butyrate; the reaction condition is mild, and the requirement on equipment is low, the production process is free from high temperature or cooling, and the energy consumption is low; since the enzyme catalysis is efficient and unique in selectivity, the method can be used for producing statins key intermediate (S)-4-chloro-3-hydroxy ethyl butyrate without producing byproduct, and the purification is convenient; in addition, the vast majority solvent in the reaction is water, the three-waste emission is low, and the ketoreductase mutant is environmental friendly.
Owner:NANJING LANGEN BIOLOGICAL SCI & TECH
Method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase
InactiveCN103173503AHigh optical purityGenerate efficientlyMicroorganism based processesFermentationHydroxybutyric acidEthyl acetate
The invention discloses a method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase and application thereof in preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester. The recombinant escherichia coli for expressing the gene of the ketoreductase (KRED) is coupled with glucose dehydrogenase (GDH) so that the escherichia coli cell is recombined into a catalyst; efficient preparation of (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester in a single water phase is realized no matter in the presence of or in the absence of coenzyme NAD(P)H; and the molar transformation rate is higher than 92% and the enantiomer excess (e.e.) of the product is 100%.
Owner:JIANGXI NORMAL UNIV +1
Carbonyl reductase expressed recombination engineering bacterium and application thereof
The invention discloses a carbonyl reductase expressed recombination engineering bacterium and application thereof. The engineering bacterium comprises a host cell and a recombinant vector transferred into the host cell, wherein the recombinant vector consists of an original vector and a target gene connected into the original vector, the host cell is Escherichiacoli Rosetta (DE3), the target gene is a carbonyl reductase gene, and the base sequence is shown in SEQ ID NO.1. According to the recombination engineering bacterium, the expression level of a carbonyl reductase is high, bacterial cells which are prepared through induced culture are used for converting N,N-dimethyl-3-oxy-3-(2-thienyl)-l-propylamine hydrochloride into (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)-l-propylamine or converting 4-chloroethyl acetoacetate into (S)-4-chloro-3-hydroxyethyl butyrate, and the two products are higher in both optical purity and conversion rate.
Owner:NINGBO MENOVO PHARMA +1
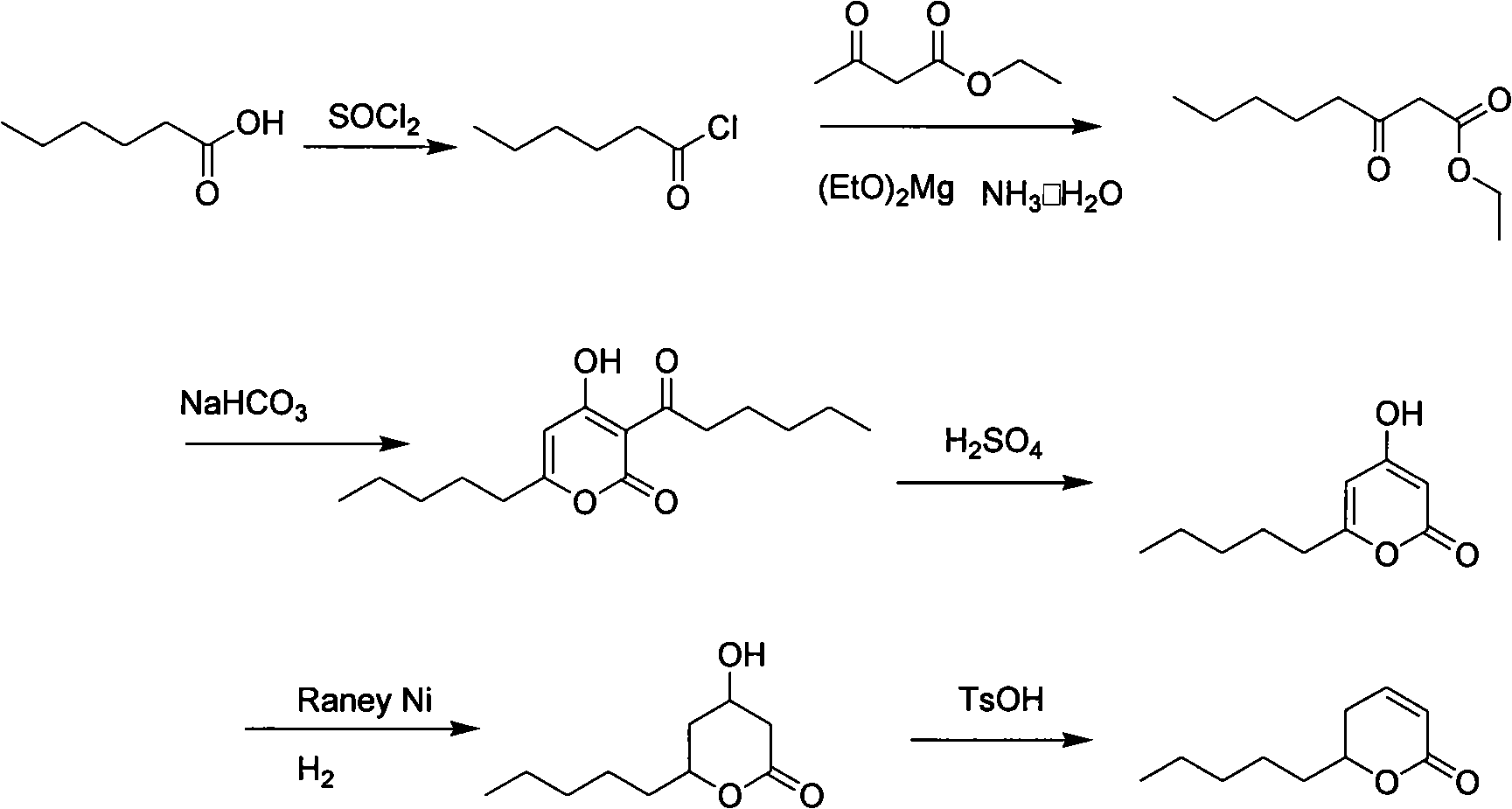
Synthesis method of massoia lactone
ActiveCN102653531AEasy to manufactureEase of industrial productionOrganic chemistryAcetic acidFlavor
The invention provides a synthesis method of massoia lactone. The massoia lactone is a rare spice which has cream fragrance, coconut fragrance, pungent fragrance and celery fragrance. The massoia lactone is prepared from hexanoic acid and acetoacetic ester as raw materials through five steps. All reactions involved in the process steps are classical fine chemical engineering unit reactions, the costs of raw materials and reagents are low, conditions are mild, and the yield of each step is higher. The process route provided by the invention has lower cost and is suitable for industrialized production.
Owner:SHANGHAI AIPU VEGETABLE TECH +1
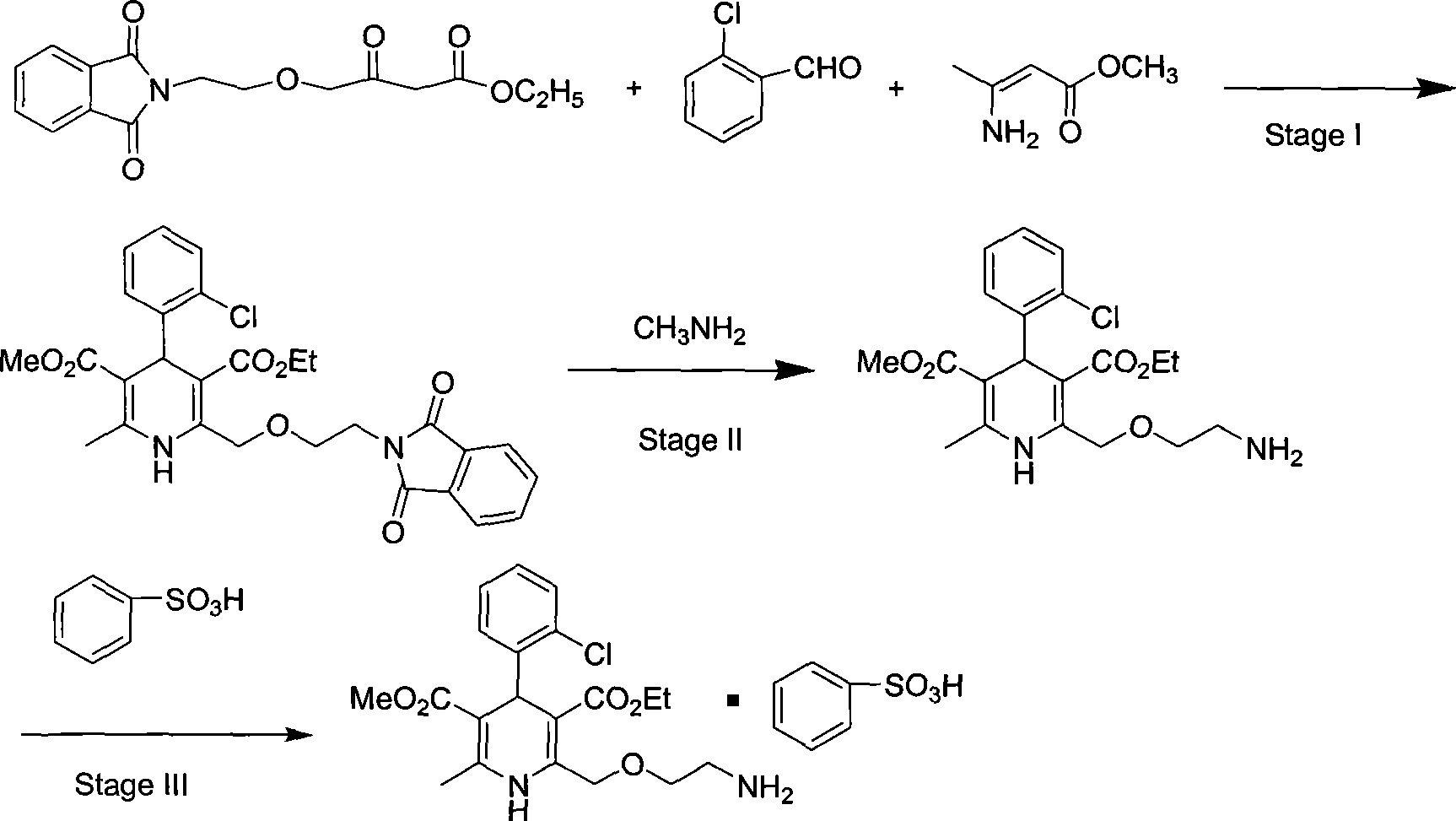
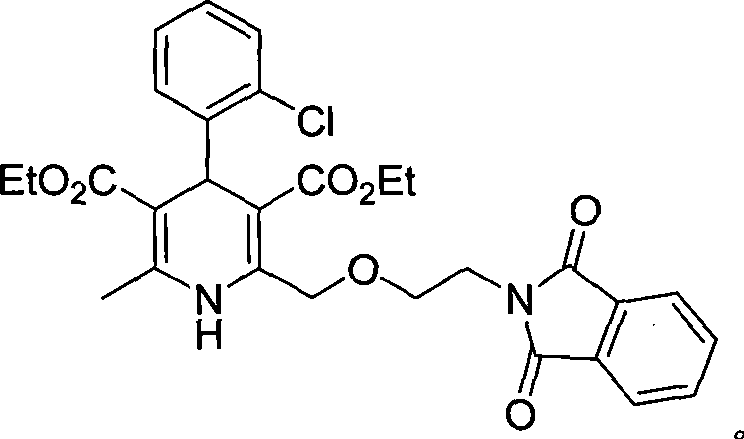
Synthesis of high-purity amlodipine besylate
The present invention relates to a synthesis method of high-purity amlodipine besylate. In particular, o-chlorobenzaldehyde, 4-(2-phthalyl imino radical ethoxy) acetoacetic ester and 3-amino methyl cro-tonate are used as raw materials for ring closure in an alcohol solvent, and when the amount of the 3-amino methyl cro-tonate is three times, the intermediate for ring closure can be prepared; the intermediate is refined by toluene / glacial acetic acid, dissolved in methylamine to form a salt in the aqueous solution, and the high-purity amlodipine besylate can be acquired after recrystallization with ethyl alcohol.
Owner:CHINA RESOURCES SAIKE PHARMA
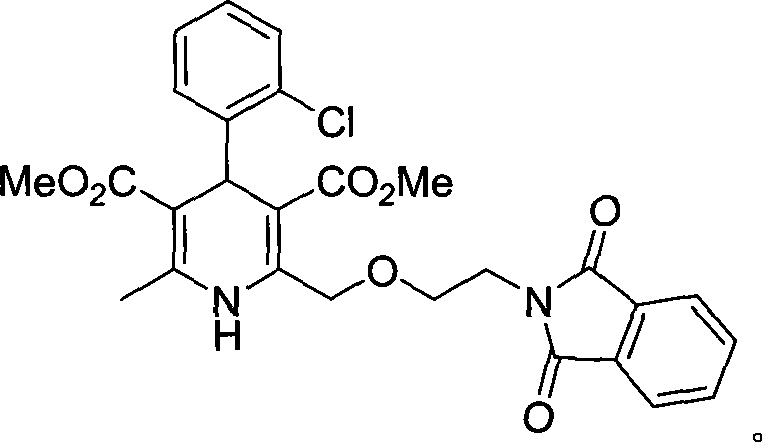
Process for preparing amlodipine benzenesulphonate
InactiveCN1927837ALow costShort reaction pathOrganic chemistryBulk chemical productionBenzaldehydeAmlodipine
The present invention discloses preparation process of Amlodipine benzene sulfonate. The preparation process includes the following steps: 1. the reaction between 4-[2-(tritylamindo)ethoxy] ethyl acetoacetate and amine compound to produce 3-amino-4-[2-( tritylamindo)ethoxy] ethylcrotonate; 2. the reaction between o-chluoro benzaldehyde and methyl acetoacetate under the catalysis of alkali to produce 2-(2-o-chluorobenzal)-ethyl acetoacetate; 3. the reaction of the products in the foregoing steps to obtain 4-(2-chlorophenyl)-6-methyl-2-((2-(tritylamido) ethoxy) methyl)-1, 4-dihydropyridyl-3-ethyl formate-5-methyl formate; and 4. further direct reaction with benzene sulfonic acid to eliminate protecting group and form Amlodipine benzene sulfonate.
Owner:上海开特生物科技有限公司
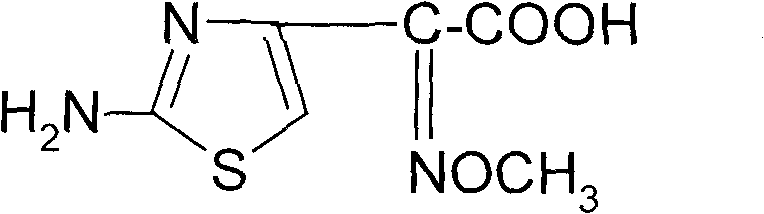
Synthetic method of 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid
ActiveCN101805311AIncrease reaction rateShorten the production cycleOrganic chemistryEthyl acetateHydrolysis
The invention relates to a synthetic method of 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid, which belongs to the synthetic methods of heterocyclic compounds containing 1,3-thiazole ring. The synthetic method is characterized by comprising the following operation steps: 1) homogeneous oximation reaction: preparing 2-hydroxamic ethyl acetoacetate; 2) methylation reaction: preparing 2-methoxyimino ethyl acetoacetate; 3) triphosgene chlorination reaction: preparing 4-chloro-2-methoxyimino ethyl acetoacetate; 4) cyclization reaction: preparing 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid ethyl ester; 5) hydrolysis: preparing a crude product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid; and 6) refining: preparing a product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid. The invention provides an oximating agent system which is applicable to homogeneous nitrosification reaction. The invention provides a triphosgene chlorinating agent which has the advantages of small toxicity, safe and convenient storage, transportation and use, easy control of process operation and high yield. The yield of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid ethyl ester is not less than 95.4%; the yield of the crude product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 94.4%; and the yield of the finished product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 90.5%. The purity of the finished product of the 2-(2-amino-4-thiazolyl)-2-(Z)-methoxyimino acetic acid is not less than 99.06%, and the melting point is 182.1 DEG C-183.9 DEG C. The synthetic method is used for synthesizing raw materials of the third generation of cephalosporins.
Owner:YIYUAN XINQUAN CHEM
Application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate
InactiveCN104726507ADisinhibition effectImprove conversion abilityBacteriaOxidoreductasesHydroxybutyric acidPtru catalyst
The invention discloses an application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate. With aldo-keto reductase of which an amino acid sequence is as shown in SEQ ID NO:2 as a catalyst, with 4-ethyl acetoacetate as a substrate and NADPH as a cofactor, the (R)-4-chlorine-3-hydroxy ethyl butyrate is prepared by asymmetric reduction. The aldo-keto reductase of which the amino acid sequence is as shown in SEQ ID NO:2 is applied to the 4-ethyl acetoacetate to prepare the (R)-4-chlorine-3-hydroxy ethyl butyrate in an asymmetric reduction manner for the first time, so that a good effect is obtained; the enzyme activity can be up to 8.1U / mg; the conversion ratio of the aldo-keto reductase on the substrate can be up to 99.8%; the enantiomer excess value of the product is greater than 995; the yield is high; and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com