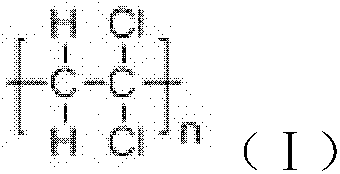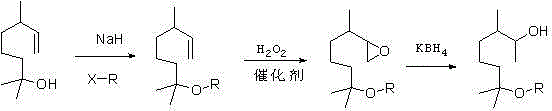Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Dihydromyrcenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dihydromyrcenol is a monoterpenoid that is oct-7-en-2-ol substituted by methyl groups at positions 2 and 6 respectively. It has a role as a fragrance and a metabolite. It is a monoterpenoid and a tertiary alcohol.
Fragrance compositions
InactiveUS20080096790A1Minimally disruptiveEasy to moveCosmetic preparationsToilet preparationsHexyl acetateLemon oil
A method of promoting activated, pleasant moods through the inhalation of energising, non-stressing fragrances (invigorating fragrances) comprising at least 75% by weight, preferably 85% by weight of perfume materials drawn from the following groups:A) At least 10% by weight in total of at least three materials drawn from Group ‘IMP’ comprising: allyl amyl glycolate; benzyl salicylate; bergamot oil; coriander oil; cyclamen aldehyde; 1-(2,6,10-trimethylcyclododeca-2,5,9-trien-1-yl)ethanone; allyl (cyclohexyloxy)acetate; Damascenia 185 SAE; 2,4-dimethylheptan-1-ol; fir balsam; fir needle oil; 3-(4-ethylphenyl)-2,2-dimethylpropanal; ginger oil; guaiacwood; linalyl acetate; litsea cubeba oil; methyl 2,4-dihydroxy-3,6-dimethylbenzoate; nutmeg oil; olibanum oil; orange flower oil; Ozonal AB 7203C; patchouli oil; rose oxide; rosemary oil; sage clary oil; spearmint oil; Tamarine AB 8212E; tarragon oil;B) Optionally up to 90% of materials from the following groups:Group ‘HMR’ comprising:allyl ionone; benzyl acetate; cis-jasmone; citronellol; ethyl linalol; ethylene brassylate; 4-methyl-2-(2-methylpropyl)tetrahydro-2H-pyran-4-ol; geraniol; geranium oil; isoeugenol; lemon oil; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; 3-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; alpha-iso-methyl ionone; 3-methylcyclopentadec-2-en-1-one; cyclopentadecanone; cyclohexadecanolide; gamma-undecalactone.Group ‘HMI’ comprising:1-{[2-(1,1 -dimethylethyl)cyclohexyl]oxy}butan-2-ol; 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1 -{b}]furan; alpha-damascone; dihydromyrcenol; eugenol; 3-(1,3-benzodioxol-5-yl)-2-methylpropanal; 2,4-dimethylcyclohex-3-ene-1-carbaldehyde; mandarin oil; orange oil; 2-(1,1-dimethylethyl)cyclohexyl acetate.Group ‘HMP’ comprising:1-(2,6,6,8-tetramethyltricyclo[5.3.1.0 {1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexyl propionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; cis-3-hexenyl salicylate; damascenone; gamma-decalactone; ethyl acetoacetate; ethyl maltol; ethyl methyl phenylglycidate; hexyl acetate; (3E)-4-methyldec-3-en-5-ol; 2,5,5-trimethyl-6,6-bis(methyloxy)hex-2-ene; 4-(4-hydroxyphenyl)butan-2-one; styrallyl acetate; 2,2,5-trimethyl-5-pentylcyclopentanone; ylang oil. Group ‘RMP’ comprising: anisic aldehyde; (2Z)-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol; benzoin siam resinoid; ethyl vanillin; oxacyclohexadec-12(13)-en-2-one; hexyl salicylate; hydroxycitronellal; jasmin oil; 3-methyl-5-phenylpentan-1-ol; 2-(phenyloxy)ethyl 2-methylpropanoate; alpha-terpineol; vanillin;Group ‘GEN’ comprising:cyclopentadecanolide; oxacyclohexadecan-2-one; hexyl cinnamic aldehyde; ionone beta; isobornyl cyclohexanol; 1-(2,3,8,8-tetramethyl-1,2,3,4,5,6,7(8),8(8a)-octahydronaphthalen-2-yl)ethanone; 4-(1,1-dimethylethyl)phenyl]-2-methylpropanal; linalol; methyl dihydrojasmonate; 2-phenylethanol;provided the following conditions are met:(a) IMPs>=HMPs+HMRs(b) IMPs+HMIs+GENs>=70%(c) (IMP+HMI) / (IMP+HMI+RMP+HMR)>=0.7(d) IMPs / (HMPs+RMPs+IMPs)>=0.5(e) IMPs / [(HMPs+RMPs+IMPs)+(100−TOTAL)]>=0.3wherein ‘IMPs’ indicates the sum of the percentages of materials within Group IMP, and similarly for the remaining groups, the symbol ‘>=’ indicates ‘at least equal to’, and ‘TOTAL’ is the sum of HMPs, HMRs, HMIs, IMPs, RMPs and GENs, provided also that low odour or no odour solvents are excluded from the calculation of these sums is provided which have an invigorating effect when inhaled by a subject.
Owner:GIVAUDAN NEDERLAND SERVICES
Daily-used apple essence and preparation method thereof
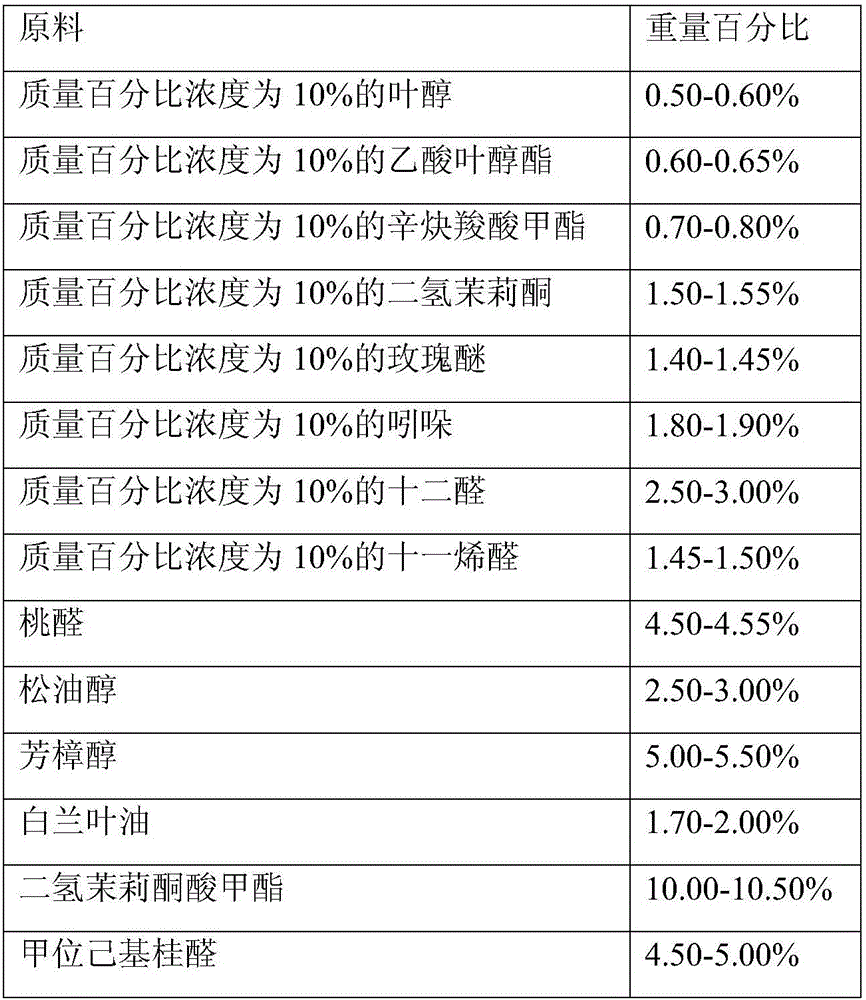
The invention provides daily-used apple essence and a preparation method thereof. The daily-used apple essence consists of components including, by weight, from 0.8 to 1.2% of 2-methyl ethyl butyrate, from 0.8 to 1.2% of acetate isoprene diene ester, from 0.8 to 1.2% of allyl cyclohexanepropionate, from 1.3 to 1.8% of isocyclocitral, from 1.5 to 2.5% of triplal, from 1.5 to 2.5% of pentadecanolide, from 2.0 to 3.0% of hexyl acetate, from 2.5 to 3.5% of jasmine pyran, from 3.5 to 4.5% of allyl heptylate, from 3.5 to 4.5% of peach ketone, from 5.5 to 6.5% of fructone, from 7.5 to 8.5% of peach aldehyde, from 8.0 to 12.0% of dihydromyrcenol, from 10.0 to 14.0% of dihydromyrcenol, from 14.0 to 18.0% of dimethyl benzyl carbinyl butyrate and from 24.0 to 28.0% of high cis butyl cyclohexyl acetate. The preparation method includes (1) preparing materials; (2) stirring the materials; (3) allowing the materials to stand and filtering the materials; (4) detecting obtained products; (5) and filling the products. The apple essence is fresh and fragrant, and is high in natural feeling and rich in fragrance, base fragrance is lasting, and the apple essence can be applied to various daily-used products needing apple tone.
Owner:广东铭康香精香料有限公司
Fragrance compositions
InactiveUS20080096791A1Improve their wellbeingImprove emotional stateCosmetic preparationsToilet preparationsFlavorCyclopentadecanolide
Fragrance composition targeted at delivering well-being benefits through fragrant, low level positive mood stimulation. The composition comprises at least 75% by weight of perfume materials comprising at least 5% by weight of at least three materials drawn from Group ‘HMP’ comprising, for example, 1-(2,6,6,8-tetramethyltricyclo[5.3.1.0{1,5}]undec-8-en-9-yl)ethanone; allyl cyclohexylpropionate; allyl heptanoate; Apple Oliffac S pcmf; 7-methyl-2H-1,5-benzodioxepin-3(4H)-one; cassis base; and optionally up to 95% of materials selected from groups identified as Groups “HMR”, “HMI”, “RMP”, “IMP” and “Gen”, represented respectively by, for example, allyl ionone; dihydromyrcenol; anisic aldehyde; allyl amyl glycolate and cyclopentadecanolide; in specified relationships.
Owner:QUEST INTERNATIONAL
Environment-friendly process for producing dihydromyrcenol by using dihydromyrcene hydration reaction
ActiveCN101684064ASimple processReduce energy consumptionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydroxy group additionHydration reactionFixed bed
The invention discloses an environment-friendly process for producing dihydromyrcenol by using a dihydromyrcene hydration reaction. The process adopts an integrated system consisting of a jet reaction device, an oil-water separation device and a rectification device. A high-speed injector is adopted in a reactor for reinforcing heat transfer and mass transfer of a reaction process; an acid (containing sulphuric acid, phosphoric acid and p-toluenesulfonic acid) is used as a catalyst and is in closed cycle; and an oil-water separator is arranged in the process flow to reduce the heat load of a rectification tower, and meanwhile the catalyst and the water phase in the oil-water separator are cycled and used in the system together so as to avoid the environment problems caused by the discharge of acid waste water. The process of the invention can improve the conversion rate of dihydromyrcene, and reduce the energy consumption and the production cost. Compared with a mechanical stirring reactor or a fixed bed reactor with the same scale, the process can improve the conversion rate of the dihydromyrcene by 1.2 to 4 times, and reduce the energy consumption of a dihydromyrcenol product per ton by more than 55 percent.
Owner:厦门中坤化学有限公司 +1
Novel method for producing dihydromyrcenol
InactiveCN101921176ASimple processContinuous operationOrganic compound preparationPreparation by hydroxy group additionHydration reactionMyrcenol
The invention discloses a novel method for producing dihydromyrcenol. The dihydromyrcenol is continuously produced through direct hydration by adopting a rectification-reaction coupling process and taking dihydromyrcene as a raw material. The process can be used for enhancing the yield of the dihydromyrcenol and the production capacity of equipment and reducing the energy consumption and the production cost; and in addition, compared with other processes, the process is easy to realize the automation of the production process and achieves the purpose of stably producing high-purity dihydromyrcenol products.
Owner:FUZHOU UNIV
Technique for synthesizing dihydromyrcenol
InactiveCN101239885AHigh yieldReduce manufacturing costOxygen-containing compound preparationOrganic compound preparationNickel catalystHydrogen
The invention discloses a dihydromyrcenol synthesis process in which stainless steel plate rectification technology is used to increase the product content; a skeleton nickel catalyst is prepared by hydrogen-filling and washing during catalyst activation so as to improve the activity of the catalyst; and the atomization and aerification effect is solved by mounting a Venturi adjutage during the schizolysis. The dihydromyrcenol synthesized by the process has high purity, good aroma, increased yield during the preparation, and reduced cost.
Owner:ZHEJIANG DONGPING PERFUME
Production method of dihydromyrcenol
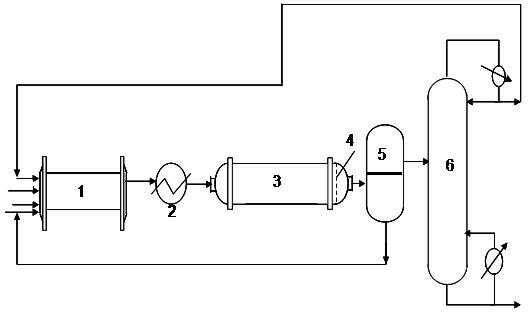
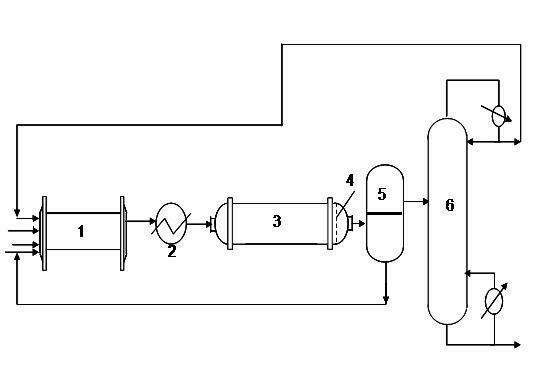
ActiveCN102964215AMild reaction conditionsLower operating temperaturePreparation by hydroxy group additionChemical/physical processesPtru catalystPhysical chemistry
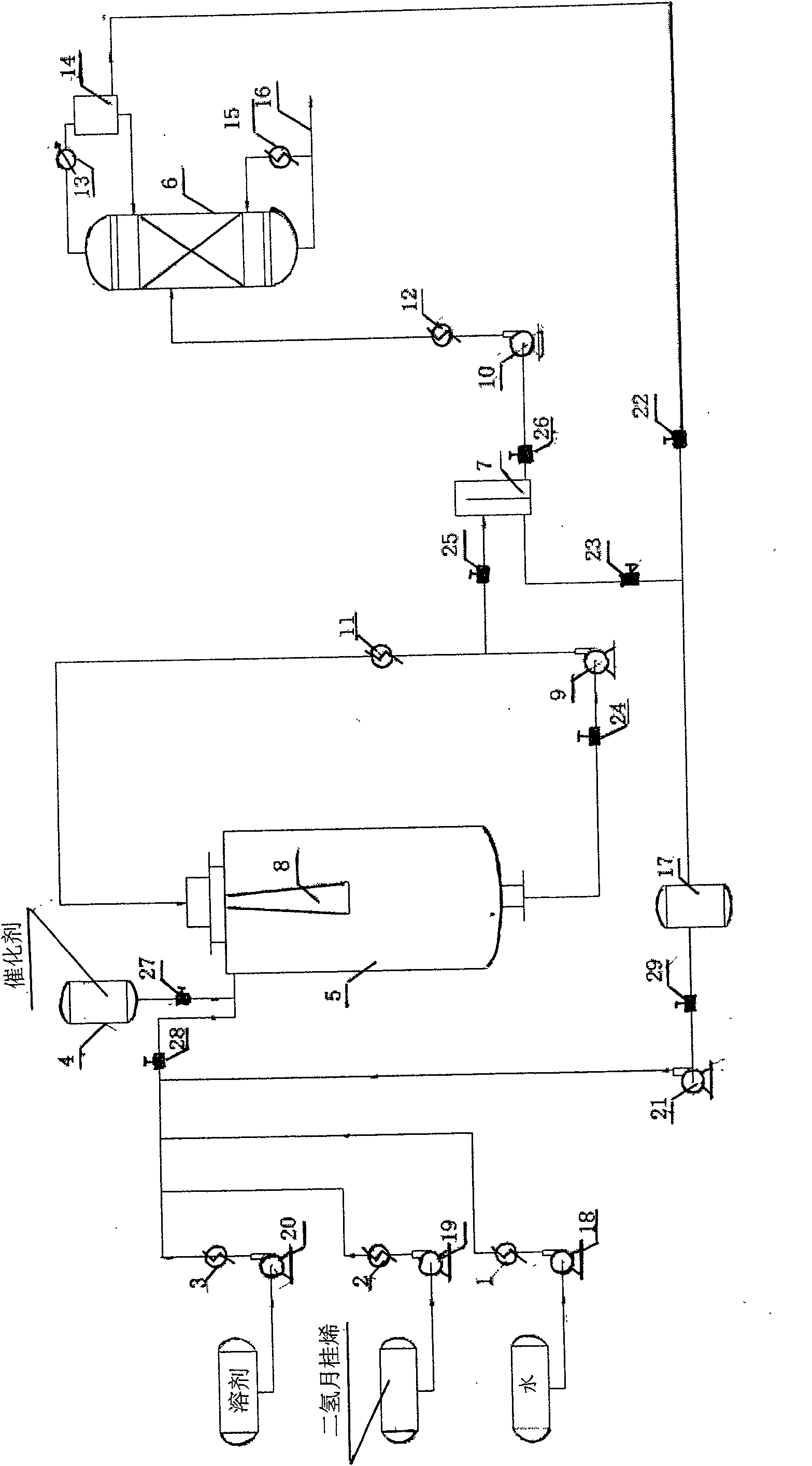
The invention provides a production method of a dihydromyrcenol. The technological processes are shown as the figure and carried out through the following step of: introducing dihydromyrcenol (hereafter referred to as DHM), water and a low-viscosity organic solvent X via a DHM feed pipe 1 and a water and solvent input pipe2 into a forced circulation radiation flow fixed bed (hereafter referred to as IRRFB) reactor 3. The pressure in the reactor is 0.12-0.28MPa; the IRRFB reactor 3 is provided with a barrel-shaped housing; a centre pipe 5 with two sealed ends and apertures covered on a side wall is arranged in the housing; and a catalyst fixed bed layer 4 made of metal nets and filled with catalysts is arranged outside the centre pipe 5. A reaction solution is pumped into the centre pipe 5, is introduced into the catalyst fixed bed layer 4 at a high speed in a radiation manner via the apertures on the side wall, penetrates through the catalyst fixed bed layer 4 to enter the housing of the reactor 3 and carries out a next round of circulation via a first pipeline 6 at the bottom. Firstly, oil and water are separated in a reacted liquid and a product is rectified twice, so that a product with a purity of 99.61% is obtained; and the once conversion rate is 10.6%, and the selectivity is 98.1%.
Owner:NANJING ENETEKS
Polyvinylidene chloride (PVDC) emulsion and preparation method thereof as well as weather-proof barrier coating and application thereof
ActiveCN103626909AIncrease contentEfficient modificationCoatingsCellulose acetateIntermediate structure
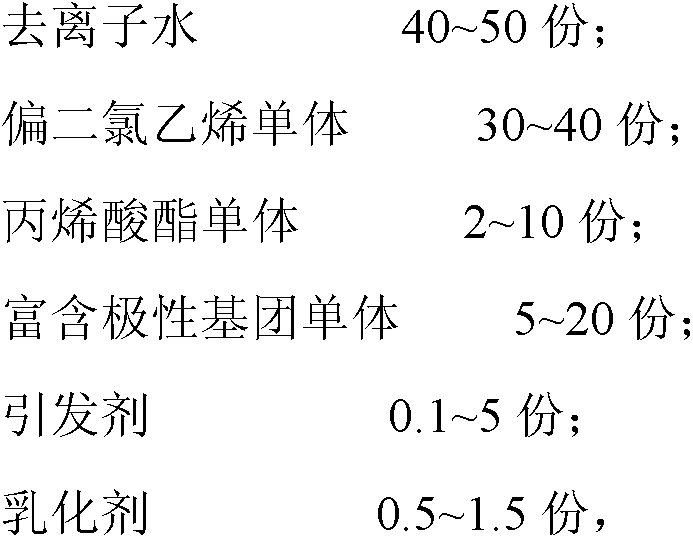
The invention relates to polyvinylidene chloride (PVDC) emulsion, a preparation method of the emulsion, a weather-proof barrier coating including the polyvinylidene chloride emulsion, and the application of the coating. The PVDC emulsion comprises the following components in parts by weight: 40-50 parts of deionized water, 30-40 parts of a vinylidene chloride monomer, 2-10 parts of an acrylic ester monomer, 5-20 parts of a monomer rich in polar groups, 0.1-5 parts of an initiator and 0.5-1.5 parts of an emulsifier, wherein the monomer rich in polar groups is one or more of 2, 2-dimethyl-4-pentene alcohol ester, dihydromyrcene alcohol ester and cellulose acetate; the monomer rich in polar groups is introduced in the raw material formula, so that the content of polar groups of the polymer in the PVDC emulsion can be increased, and the adhesion on the enhancement layer of the intermediate structure is improved.
Owner:SUZHOU FIRST PV MATERIAL CO LTD
Washing essence comprising 2-pentylcyclopentanone
Washing essence comprising 2-pentylcyclopentanone is disclosed. The washing essence comprises following components by mass: 0.1% of ethyl 2-methylbutyrate, 0.1% of ethyl 2-methylvalerate, 0.2% of octyl aldehyde, 0.6% of D-limonene, 0.7% of p-methyl anisole, 1% of dihydromyrcenol, 0.4% of allyl hexanoate, 2% of triplal, 2% of linalool, 0.6% of methyl benzoate, 0.1% of rose oxide, 4% of phenethyl alcohol, 0.2% of camphor, 4% of benzyl acetate, 1.5% of styralyl acetate, 0.4% of terpilenol, 0.7% of decanal, 2% of citronellol, 0.2% of allyl amyl glycolate, 0.1% of citral, 0.5% of geraniol, 0.1% of linalyl acetate, 0.8% of anisic aldehyde, 3% of the 2-pentylcyclopentanone, 1.2% of dipropylene glycol, and the like. Using of the 2-pentylcyclopentanone allows the whole fragrance of the essence to be finer and fuller, and aroma reserving effects to be more durable.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Method for preparing jasmine-fragrant master batch for perfuming composite fiber
The preparation method of jasmin essence mother granules for perfuming composite fibre includes the following steps: using the components of methyl dihydrojasmonate, hexyl cinnamic ester, licarcoal, dihydromyrcenol, leaf alcohol and other 10 components to prepare jasmin essence according to its formula; mixing said jasmin essence together with ethylene vinyl acetate copolymer (EVA) granules and heating to make the essence be eneloped in the EVA resin so as to obtain the invented mother granules with jasmin fragrance. The polyester / polypropylene and polypropylene / polyethylene core-sheath composite aromatic short fibre made up by said mother granules can be used as filling material oil quilt, pillow, cushion and toys, etc. or can be used as dress material and internal decorative material.
Owner:上海香料研究所
Perfume essence with karanal added
The invention discloses a perfume essence with karanal added. The invention is composed of the following ingredients according to the mass percent: 10% Galaxolide, 5% palchouli oil, 5% dihydromyrcenol, 10% imported linalool, 8% phenylethyl alcohol, 6% citronellol, 5% 2-ethyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-Buten-1-ol, 3% rosalin, 20% ambrotone, 1% geraniol, 0.08% 4-Methyl-3-decen-5-ol, 5% ethyl linalool, 6% florol, 0.2% cetalox, 0.03% rose oxide, 14.19% dipropylene glycol and 1.5% karanal. The invention is a classic middle east fragrance type, which is a middle east fragrance perfectly melted with damascus roses full of middle east mystery colors and matched with the light delicate powder.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Preparation method of dihydromyrcenol
InactiveCN104926610AShorten the production cycleHigh yieldPreparation by hydroxy group additionAcetic acidDistillation
The invention discloses a preparation method of dihydromyrcenol, belonging to the technical field of forestry chemical product preparation. The method comprises the following steps: according to a certain ratio, putting 36% of acetic acid and water into a reaction kettle, mixing evenly, and then heating to 80 DEG C to 120 DEG C; adding a catalyst into the reaction kettle, and keeping the material temperature in the kettle within the range of 80 DEG C to 120 DEG C; adding 100 parts of dihydromyrcenol preheated to 80 DEG C to 120 DEG C in advance into the reaction kettle, keeping the material temperature in the kettle within the range of 80 DEG C to 120 DEG C, and performing thermostatic reaction for one hour to 3 hours; performing filtering, standing layering and reduced pressure distillation on a product obtained by the reaction, thus obtaining the dihydromyrcenol. According to the preparation method of the dihydromyrcenol, by adopting the home-made catalyst and matching with the corresponding raw material ratio, the production cycle of the product dihydromyrcenol is shortened, and the yield of the dihydromyrcenol is improved.
Owner:WUZHOU SONGHUA AROMATIC CHEM
Method for continuously producing dihydromyrcenol by using tubular reactor
InactiveCN102659517ASmall volumeLarge specific surface areaPreparation by hydroxy group additionPtru catalystReaction rate
The invention discloses a method for continuously producing dihydromyrcenol by using a tubular reactor. The method comprises the following steps: mixing dihydromyrcene, water and solvent in a mixer; after mixing, heating the mixture to 70-95 DEG C by a pre-heater and pouring the mixture into the tubular reactor; heating and controlling the reaction temperature in the tubular reactor at 100-120 DEG C by adopting a short circuit current; pouring a reaction product in the tubular reactor into a phase splitter; after splitting the phases by the phase splitter, further separating the upper-layer organic phase in a rectifying column; pouring the upper-layer organic phase into the rectifying column after splitting the phases by the phase splitter, causing the dihydromyrcenol as a heavy component to flow to a tower kettle; and extracting the dihydromyrcenol with purity above 95% by the tower kettle. The tubular reactor is adopted according to the method provided by the invention, for reducing liquid back-mixing, reducing energy consumption and avoiding the quick decrease of reaction rate in the later stage of reaction. A fin is arranged on the inner wall of the tubular reactor, for reinforcing the liquid flow, boosting the matter-transferring and heat-transferring of reaction, increasing the reaction rate and meanwhile avoiding the powdering of solid catalyst caused by stirring.
Owner:淮安市产品质量监督检验所
Perfume essence added with musk and fruit ester
InactiveCN103103023AHigh strengthSteady patternCosmetic preparationsToilet preparationsFuranGeraniol
The invention discloses a perfume essence added with musk and fruit ester. The perfume essence mainly comprises the following components by mass percent: 15% of methyl dihydrojasmonate, 2% of alpha-hexylcinnamaldehyde, 4% of lilialdehyde, 40% of ambrotone, 2% of phenylethyl alcohol, 1% of dihydromyrcenol, 1% of cinnamic alcohol, 0.01% of leaf alcohol, 0.8% of vanillyl alcohol, 0.02% of linalool, 1% of geraniol, 5% of ethyl linalool, 6% of florol, 0.2% of dodecahydro-3a, 6, 6, 9a-tetramethyl-naphtol [2, 1-b] furan-3a, 6, 6, 9a-tetrame, 10% of musk and fruit ester and the like. The essence added with musk and fruit ester has the characteristics of stable structure, compound aroma, gradually-released fragrance, novel aroma and lasting fragrance.
Owner:TIANJIN DOUBLE HORSE FLAVOR & FRAGRANCE NEW TECH
Liquid soap
InactiveCN103627548AEasy to cleanSoap detergents with organic compounding agentsSoap detergents with inorganic compounding agentsAlcoholSodium hydroxide
The invention provides a liquid soap. The liquid soap adopts the technical scheme that the liquid soap comprises ethyl alcohol and is characterized by further comprising the following constituents in part by mass: 2 to 2.5 parts of dihydromyrcenol, 5 to 8 parts of Chinese goldthread essence, 0.5 to 0.8 parts of sodium tripolyphosphate, and 1.5 to 1.8 parts of sodium hydroxide. Compared with the prior art, the liquid soap has the benefit that the liquid soap has good cleaning effect by adding the dihydromyrcenol, Chinese goldthread essence, tripolyphosphate and sodium hydroxide into ethyl alcohol.
Owner:王淳
Methoxy elgenol derivative and preparation method thereof
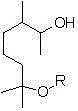
InactiveCN105001061AEfficient synthesisSignificant technological progressOrganic compound preparationEther preparation by ester reactionsMedicinal chemistryRaw material
The invention provides a methoxy elgenol derivative. The methoxy elgenol derivative is characterized in that the general formula of the methoxy elgenol derivative is shown (please see the formula in the instruction), wherein R is methyl or ethyl or propyl or butyl or isobutyl. The invention further provides a preparation method of the methoxy elgenol derivative. Dihydromyrcenol is taken as a raw material, and after etherification and epoxidation, the obtained product is subjected to hydrogenation reduction to obtain the methoxy elgenol similar derivative. According to the methoxy elgenol derivative and the preparation method thereof, the raw material is low in price and easy to obtain, simple and safe effects are achieved, the environmentally friendly effect is achieved, the range of application is wide, post processing is simple, the yield is high, the method for synthesizing the methoxy elgenol derivative is fast and efficient, and the methoxy elgenol derivative has strong and favorable sandalwood odor and cool and refreshing flowery fragrance.
Owner:SHANGHAI INST OF TECH
Cat-driving essence as well as preparation method and application thereof
ActiveCN103911214AEffective in repelling catsEssential-oils/perfumesAnimal repellantsLemon oilYlang-Ylang oil
The invention discloses cat-driving essence as well as a preparation method and an application thereof. The cat-driving essence is obtained by adding a certain amount of bergamot oil, lemon oil, citral, linalyl acetate, sweet orange oil, terpilenol, terpinyl acetate, methyl ortho-aminobenzoate, decanal, geranyl acetate, lemonile, geranium oil, menthol, benzyl acetate, benzyl alcohol, linalool, dihydro jasmine with mass fraction of 10%, hydroxycitronellal, benzpyrole with mass fraction of 10%, dihydromyrcenol, methyl ionone, lyral, oil of daidai leaf, ylang ylang oil, gamma-delta-lacton, leaf alcohol, benzyl benzoate, benzyl propionate, methyl dihydrojasmonate, p-cresyl acetate, jasmonyl, phenylacetic acid, eugenol, myracaldehyde, absolute of jasmine, geraniol, cis-3-hexenyl benzoate, galaxolide with mass fraction of 50% and propylene glycol into a container in sequence, shaking uniformly, standing and ageing for two weeks. The cat-driving essence is remarkable in cat-driving effect.
Owner:福建中益制药有限公司
Cleanser composition
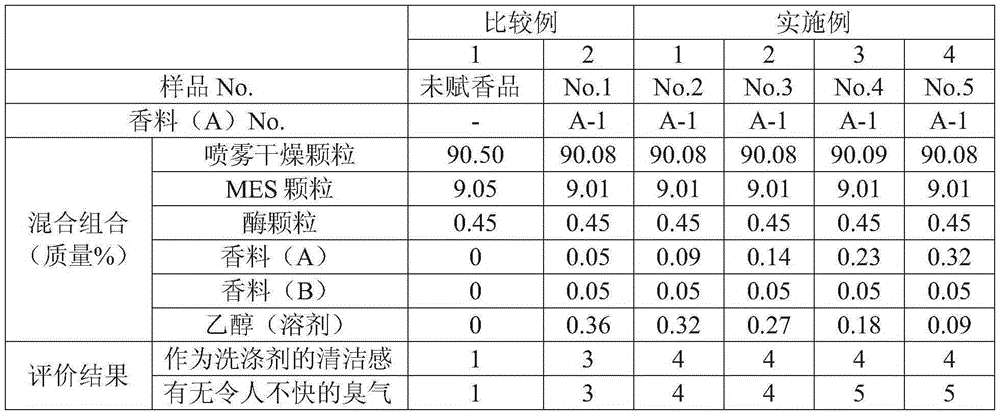
ActiveCN105283531AFragrance RealizationIncrease the fragranceSurface-active detergent compositionsDetergent perfumesCleansers skinIsobornyl acetate
Provided is a cleanser composition characterized by containing a salt of an alpha-sulfo fatty acid alkyl ester, and a fragrance composition, the content of the salt of an alpha-sulfo fatty acid alkyl ester being 1-30 mass% with respect to the total amount of the cleanser composition, and the fragrance composition containing the following fragrance (A), the content of the fragrance (A) being 0.07-0.5 mass% with respect to the total amount of the cleanser composition. Fragrance (A): at least one fragrance ingredient selected from the group consisting of dihydromyrcenol, isobornyl acetate, alpha-hexyl cinnamic aldehyde, lilial, tetrahydrolinalol, amyl salicylate, verdox, vertenex, tricyclodecenyl acetate, tricyclodecenyl propionate, Iso E Super, and Habanolide.
Owner:LION CORP
Dihydromyrcenol polyoxyethylene ether and synthetic method thereof
The invention discloses dihydromyrcenol polyoxyethylene ether and a synthetic method thereof. The dihydromyrcenol polyoxyethylene ether is synthesized from the following raw materials: dihydromyrcenol and ethylene oxide in the mol ratio of 1: (5-150) through two steps, in the presence of a catalyst accounting for 0.03-2% of the total weight of the raw materials dihydromyrcenol and ethylene oxide. The dihydromyrcenol polyoxyethylene ether having a large molecular weight is synthesized through two steps; the product is high in saturability and narrow in molecular weight distribution, and also increased in increase ratio and advantageous for synthesizing products higher in molecular weight. A high-performance polycarboxylic acid water reducing agent prepared from the dihydromyrcenol polyoxyethylene ether provided by the invention has good cement particle dispersity maintaining capability, and remains the advantages such as low dosage, high water-reducing rate, good collapse retaining property and environmental friendless; besides, as the high-performance polycarboxylic acid water reducing agent is enhanced in hydrophobicity in a large monomer structure and increased in molecular steric hindrance, the water reducing agent is remarkably enhanced in adaptability to dinas different in silt content and can be widely applied to high-silt content dinas districts.
Owner:SICHUAN SEDAR CHEM
Lemon essence used in oil-based ink and preparation method of lemon essence
InactiveCN105176684AImprove fragranceGood oil solubilityInksEssential-oils/perfumesSolubilityLimonium
The invention provides lemon essence used in oil-based ink. The lemon essence is prepared from a lemon essence body, maltodextrin and starch sodium octenylsuccinate, wherein the lemon essence body is prepared from triethyl citrate, methyl dihydrojasmonate, peach aldehyde, beta-naphthol ethyl ether, dodecanenitrile, beta-ionone, gamma-decalactone,floropai, allyl cyclohexanepropionate, verdyl acetate, damacscone alpha, vanillin acetate, neryl acetate, undecanal, lauraldehyde, citronellyl formate, geranyl formate, geraniol, citronellol, nerol, citral, capraldehyde, styralyl acetate, citriodora oil, isocyclocitral, triplal, dihydromyrcenol, linalool, allyl hexanoate, ethyl-2-methylbutyrate, terpinolene, Brazil orange oil, lemon oil terpene, octyl aldehyde, paracymene, tangerine aldehyde, vanillic aldehyde, grapefruit oil and glyceryl triacetate. The invention further provides a preparation method of the lemon essence used in the oil-based ink. By means of the lemon essence used in the oil-based ink and the preparation method of the lemon essence, the fragrance retention effect and oil solubility of the essence are improved.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Method for method for synthesizing dihydromyrcenol from dihydro laurene using niobic acid catalyst
ActiveCN101357881AAvoid pollutionAvoid corrosionPreparation by hydroxy group additionMetal/metal-oxides/metal-hydroxide catalystsAlcoholPolyol
The invention relates to a method for synthesizing dihydrogen myrcene alcohol by using dihydrogen myrcene by adopting niobic acid catalyst, which comprises the following steps: niobic acid is used as catalyst; water and polyol are adopted as solvent, and the weight ratio of water and polyol is 1-2.3:1; the weight ratio of polyol complex solvent and dihydrogen myrcene is 1:1-2.5; under the low temperature condition, dihydrogen myrcene alcohol is obtained by the procedures of one-step hydration, filtration, standing and stratification, drying, reduced pressure distillation, etc.
Owner:俞忠华 +2
Environment-friendly regenerated rubber softener
The invention discloses an environment-friendly regenerated rubber softener. The environment-friendly regenerated rubber softener comprises, by weight, 10-12 parts of eugenol, 8-10 parts of limonene, 6-8 parts of carvene, 8-9 parts of ethyl acetate, 5-8 parts of methyl anthranilate, 8-10 parts of prenyl alcohol, 10-12 parts of n-dodecanol, 6-8 parts of dihydromyrcenol, 5-6 parts of terpilenol, 5-8 parts of phenyl-1-hexanol, 6-10 parts of ethyl oleate, 8-10 parts of dioctyl sebacate, 5-6 parts of glycerin monostearate, 6-8 parts of isopropanol, 6-10 parts of acetone and 6-8 parts of n-butyl alcohol. The environment-friendly regenerated rubber softener has the advantages that the regenerated rubber production environment is optimized, quality of regenerated rubber products is improved and physical performance is more excellent. In addition, the regenerated rubber is lower in Mooney viscosity and green and environment friendly and has promising market and industrial prospects.
Owner:张慧玲
Method for preparing dihydromyrcenol through reaction distillation continuity
InactiveCN102617288ARealize continuous productionReduce heat loadOrganic compound preparationChemical industryEffective surfaceFilling materials
The invention relates to a method for preparing dihydromyrcenol through reaction distillation continuity. Corrugated gauze filling materials and solid acid catalysts are filled in a reaction distillation tower, raw materials of dihydromyrcene, water and solvents are respectively preheated to 80 to 120 DEG C, 85 to 95 DEG C and 70 to 90 DEG C to be respectively fed from the upper part, the middle part and the lower part of a reaction section of the reaction distillation tower according to the flow rate ratio of (1-3): (1-3). The tower kettle temperature is controlled to be 105 to 120 DEG C, and the tower top condensing temperature is controlled to be 50 to 70 DEG C. An oil phase and a water phase are separated out from condensed liquid, partial oil phase reaches the tower top to flow back, and the water phase and the rest oil phase respectively return a raw material tank to be cyclically utilized. According to the method, the reaction and the distillation are simultaneously carried out, heat released by the reaction can be effectively utilized for heating materials, and a heat load of a tower kettle is reduced. The corrugated gauze filling materials in the reaction distillation tower and a tubular liquid distributor are favorable for improving the effective surfaces for mass transfer and heat transfer and improving the inter phase contact, dihydromyrcenol products with the purity being 95 percent are obtained from the tower bottom, and the reaction efficiency and the dihydromyrcenol conversion rate are higher.
Owner:淮安市产品质量监督检验所
Magnolia essence and preparation method thereof
InactiveCN107460053AFull of fragranceRich fragranceEssential-oils/perfumesTert butyl phenolDimethyl acetal
The invention discloses a magnolia essence and a preparation method thereof. The magnolia essence is composed of, by weight part, 3-5 parts of allyl hexanoate, 1-3 parts of geranyl formate, 1-3 parts of geranyl acetate, 1-3 parts of butyl acetate, 18-20 parts of benzyl acetate, 8-10 parts of amyl cinnamic aldehyde, 3-5 parts of cinnamic alcohol, 3-5 parts of clove oil, 3-5 parts of terpinyl acetate, 3-5 parts of phenylacetaldehyde dimethyl acetal, 8-10 parts of linalool, 3-5 parts of dihydromyrcenol, 3-5 parts of terpilenol, 2-3 parts of phenethyl alcohol, 3-5 parts of nonanediiol acetate, 8-10 parts of Superfix(1,1,3-Trimethyl-3-phenylindan), 2-3 parts of ethyl butyrate, 2-3 parts of aurantiol, 1-2 parts of o-tert-butyl phenol, 5-6 parts of benzyl salicylate, 5-6 parts of rhodinol, 2-3 parts of benzpyrole, 2-3 parts of fructone, 1-3 parts of amyl acetate and 1-3 parts of allyl amyl glycolate. The preparation method of the magnolia essence comprises the following steps of (1) placing, (2), stirring, (3) standing, (4) filtering, (5) detection and (6) filling. The magnolia essence has fresh and pleasant magnolia flavor and can substitute for magnolia essence oil for being widely applied to products.
Owner:安徽香杰香精科技有限公司
Dihydromyrcenol base silane coupling agent and synthetization method thereof
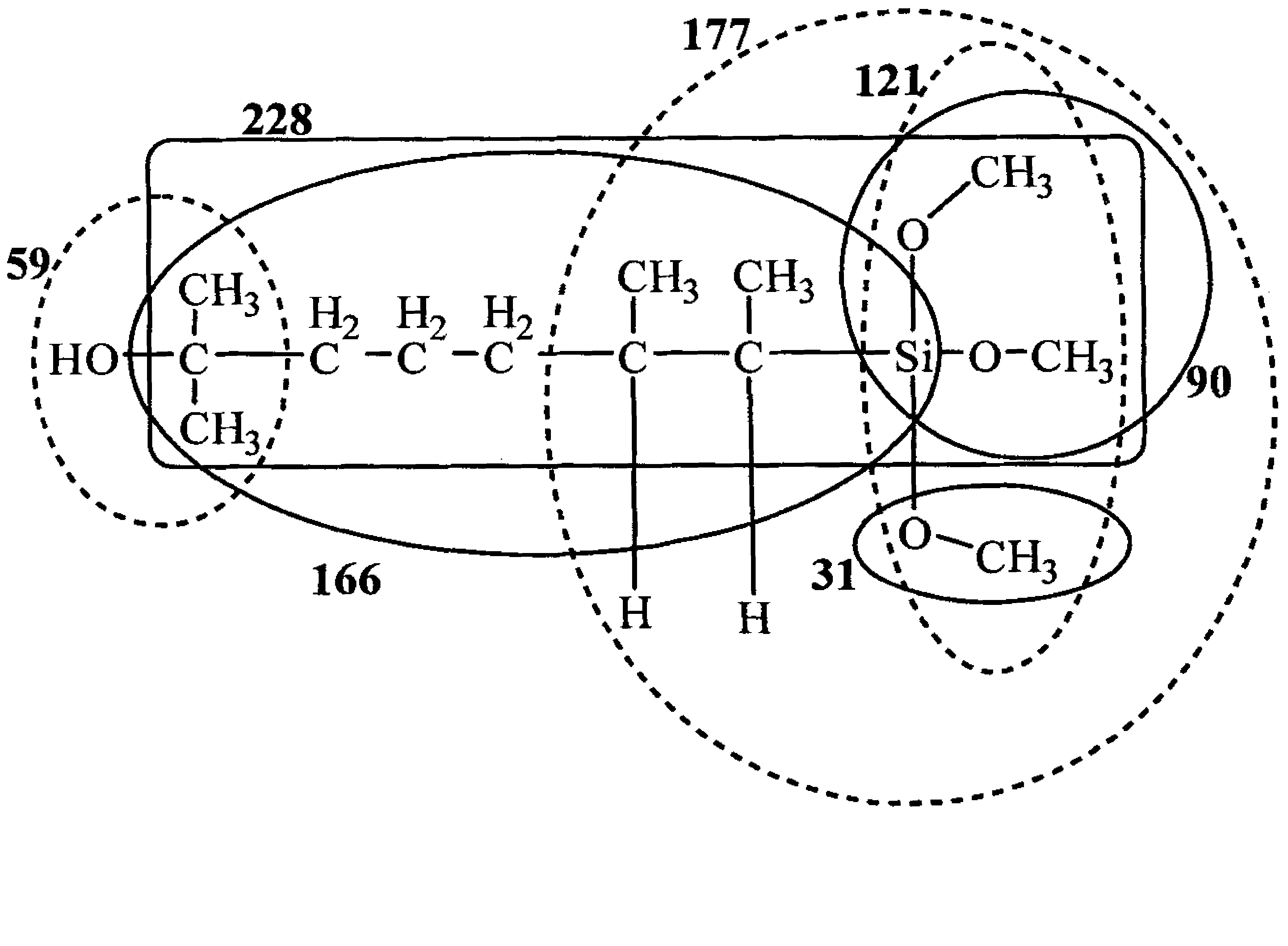
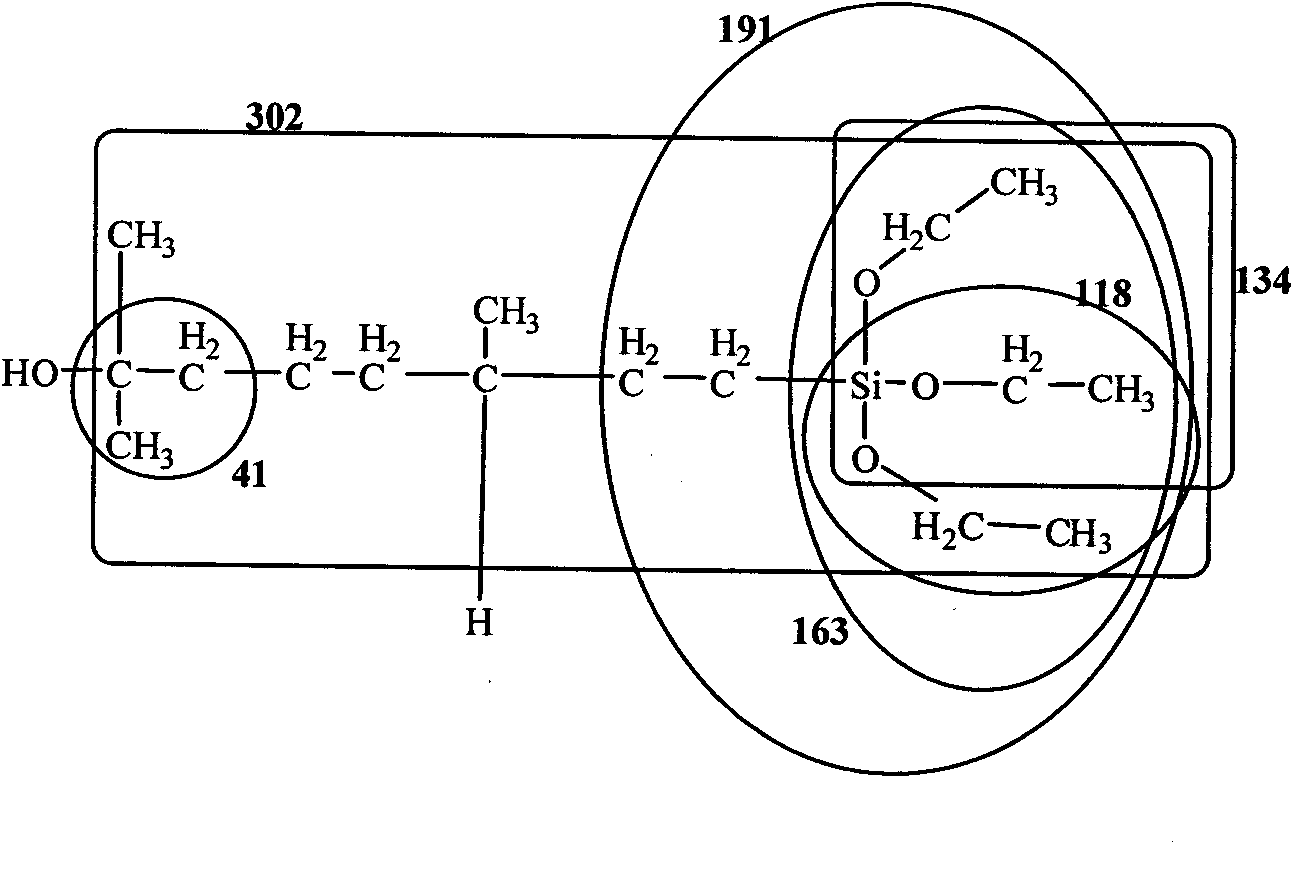
ActiveCN101805365ALong Hydrocarbon Branched Chain StructureUnique coupling propertiesGroup 4/14 element organic compoundsSynthesis methodsCombinatorial chemistry
The invention discloses a dihydromyrcenol base silane coupling agent and a synthetization method thereof. The coupling agent is one of or two of the compounds with the structure disclosed in the formula (I) and the compound with the structure disclosed in the formula (II), wherein R is selected from methyl or ethyl, and R in the formulas (I) and (II) has the same meaning. The synthesis method has simple operation and moderate reaction condition and is suitable for industrial production.
Owner:HANGZHOU ZHIJIANG NEW MATERIAL CO LTD
Cape jasmine flower essence and preparation method thereof
InactiveCN106281689ASweet and strong aromaLong lasting fragranceEssential-oils/perfumesPhenethyl acetateMichelia
The invention provides cape jasmine flower essence. The cape jasmine flower essence is prepared from 10% leaf alcohol, 10% cis-3-hexenyl acetate, 10% methyl 2-nonynoate, 10% dihydrojasmone, 10% rose oxide, 10% indole, 10% dodecyl aldehyde, 10% undecenal, undecan-4-olide, terpineol, linalool, michelia alba leaf oil, methyl dihydrojasmonate, alpha-hexylcinnamaldehyde, benzyl acetate, ionone, methylionone, phenethyl ethanol, phenylacetaldehyde, phenylethyl acetate, styralyl acetate, 1-phenylethyl propionate, tetrahydrogeraniol, rhodinol, dihydromyrcenol, lily aldehyde, benzyl propionate, isoeugenol, eugenol, lyral, galaxolide, ylang-ylang oil, patchouli oil and propylene glycol. The cape jasmine flower essence has sweet and rich fragrance of cape jasmine flowers and coordinated and long-lasting fragrance.
Owner:SHANGHAI INST OF TECH
Environment-friendly regenerated rubber softening agent
Owner:安徽微威环保科技有限公司
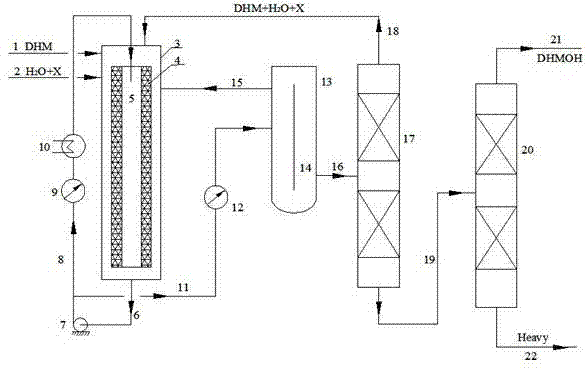
Dihydromyrcenol fixed bed hydration continuous production method
ActiveCN102173978BSimple processSimple and fast operationOrganic compound preparationPreparation by hydroxy group additionFixed bedSolvent
The invention discloses a production method of spice and in particular relates to a dihydromyrcenol fixed bed hydration continuous production method. The method comprises the following steps of: allowing dihydromyrcene, water or aqueous solution of a solvent to enter a feeding preheater respectively through a head tank and a flowmeter to perform preheating; allowing the reaction materials to enter a tubular reactor continuously and then overflow into an oil water separator to be separated; and allowing the lower layer aqueous phase to enter a reaction system continuously and circularly and allowing the oil layer to enter a rectifying tower to perform rectification, wherein the tower is provided with three discharge ports; and materials of different concentrations are discharged from the discharge ports respectively. The method has the advantages of really realizing zero emission of waste water, making the using ratio of pure water close to 100 percent, saving cost of raw materials, exchanging catalysts conveniently and simply, greatly prolonging the service life of the catalysts, solving the problem of escape of the catalysts, expanding easily, fulfilling the aim of zero use of the solvent and saving solvent cost, along with low equipment investment and wide application.
Owner:JIANGSU XINHUA CHEM
Preparation method of high-sensitivity functionalized silver nanoparticle-doped aniracetam MIECS (Molecular Imprinting Electrochemical Sensor)
InactiveCN105628762AHigh sensitivityEasy to manufactureMaterial electrochemical variablesDopantFunctional monomer
The invention discloses a preparation method of a high-sensitivity functionalized silver nanoparticle-doped aniracetam MIECS (Molecular Imprinting Electrochemical Sensor). The preparation method is characterized by taking aniracetam as a template molecule, dihydromyrcenol as a functional monomer, hydrocortisone acetate as a coupling agent and decyl mercaptan functionalized silver nanoparticles as a doping agent, thus preparing the high-sensitivity decyl mercaptan functionalized silver nanoparticle-doped aniracetam MIECS. An analysis method is simple and practical, and the disadvantages of complexity, expensive price of equipment and low sensitivity of a previous analysis method are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Stabilizer-plasticizer for wet mixed mortar and preparation method thereof
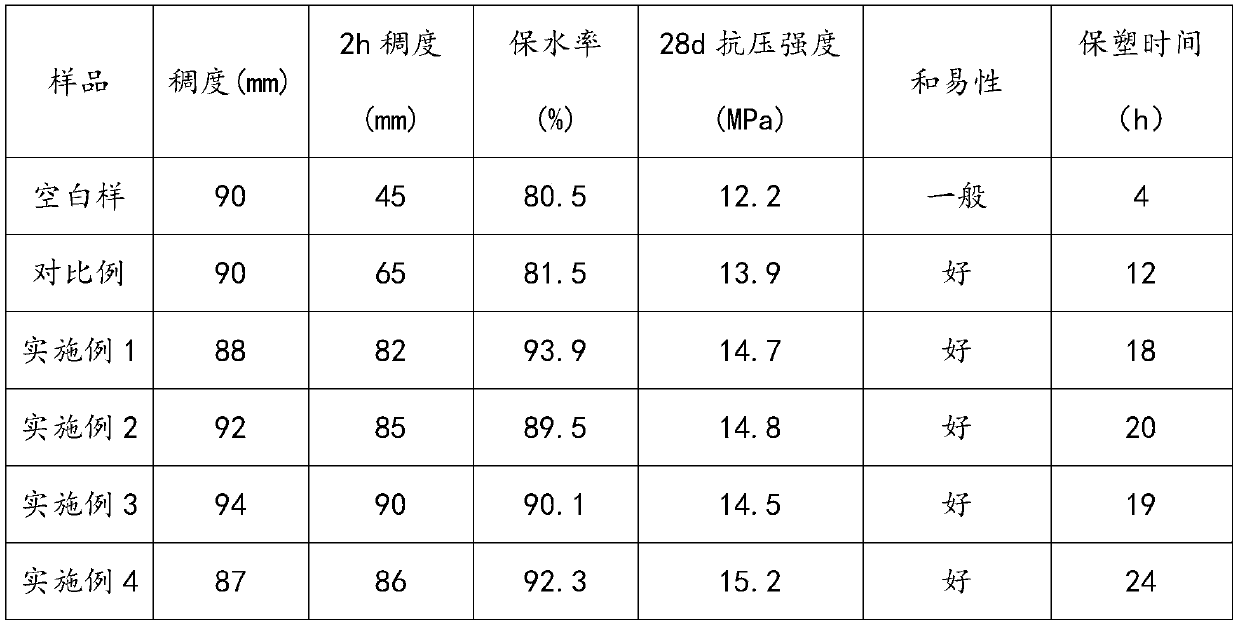
The invention relates to the technical field of building admixtures, specifically to a stabilizer-plasticizer for wet mixed mortar and a preparation method thereof. The preparation method comprises the following steps of: mixing 4-hydroxybutylvinyl polyoxyethylene ether monomer, acrylic acid, methylacryloyloxyethyl succinate and water, and adding hydrogen peroxide; carrying out a reaction, and then sequentially adding an aqueous solution of a reducing agent, an aqueous solution of a cocatalyst, and an aqueous solution composed of acrylic acid, 2-amino-3-p-hydroxyphenylpropionate, 3-dihydromyrcenol hydroxyphenylphosphinyl propionate and a chain transfer agent; and after a reaction is completed, adding polyvinyl alcohol and adjusting a pH value to 7 to 8 to prepare the stabilizer-plasticizer for wet mixed mortar. Compared with the prior art, mortar synthesized by using the stabilizer-plasticizer for wet mixed mortar provided by the invention has the advantages of low consistency loss, good water retention rate, high compressive strength, high long-term stability and the like, so significant progress is made.
Owner:KZJ NEW MATERIALS GROUP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com