Synthesis of high-purity amlodipine besylate
A kind of technology of amlodipine besylate and synthesis method, applied in the field of amlodipine besylate, and can solve the problems such as that the purity of amlodipine besylate product cannot meet requirements and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of impurity E
[0026] (4RS)-4-(2-chlorophenyl)-2-[(2-aminoethoxy)methyl]-6-methyl-1,4-dihydropyridine-3,5-dicarboxylic acid ethyl ester
[0027]
[0028] resolve resolution
[0029] 1, Preparation of ethyl 3-aminobutyrylate
[0030] In a 500ml three-neck flask, add 300ml of ethyl acetoacetate and 150ml of ethanol, cool below 10°C, pass ammonia gas for 5 hours, precipitate crystals, filter to obtain 100g of the product.
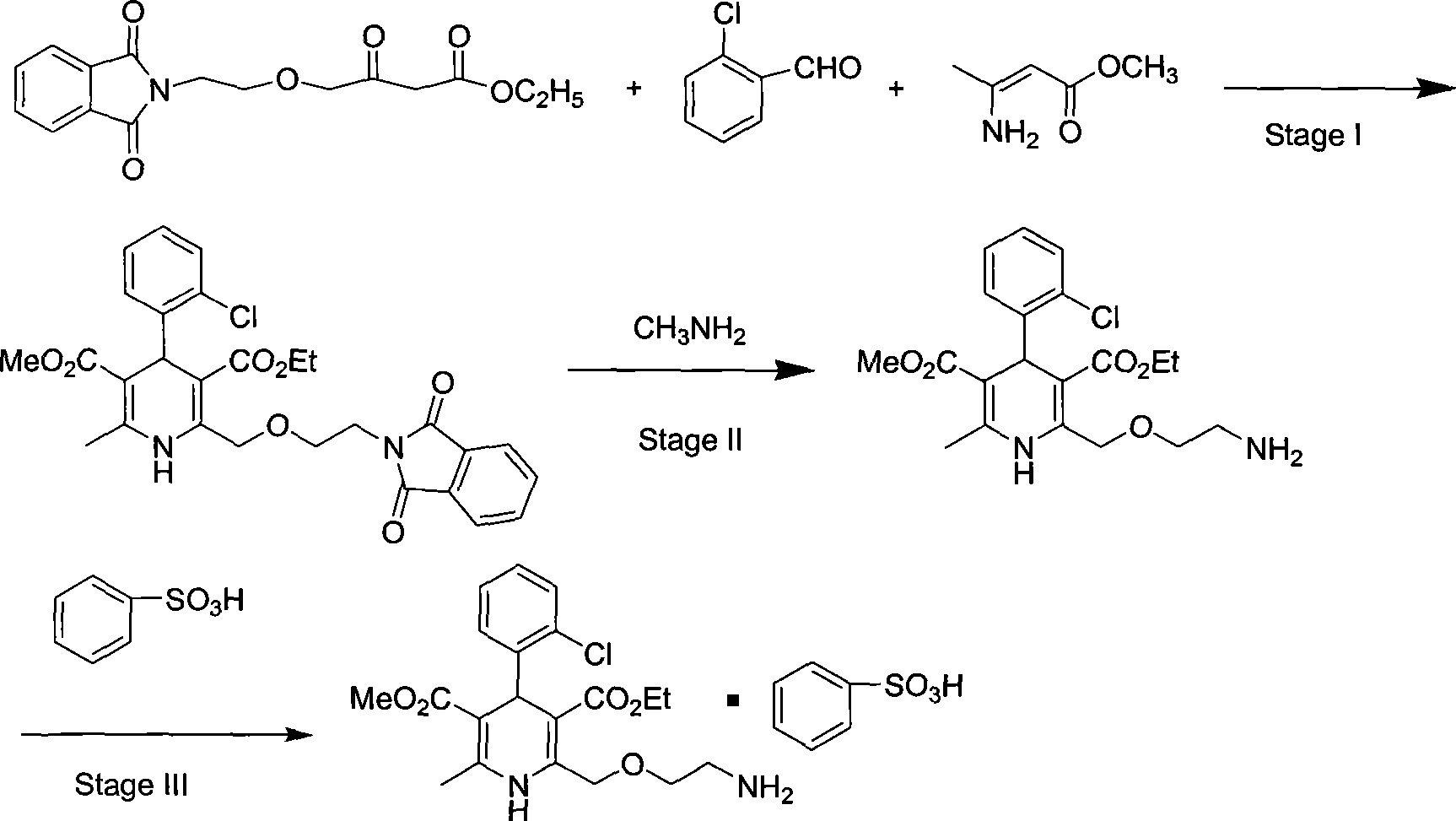
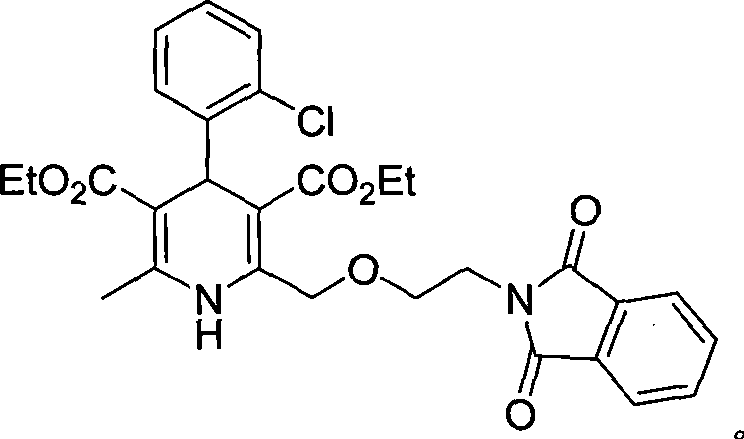
[0031] 2. (4RS)-4-(2-chlorophenyl)-2-[[2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)ethoxy ]Methyl]-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate ethyl ester (impurity E3)
[0032] In a 250ml reaction flask, add 15g of ethyl 3-aminocrotonate, 18g of ethyl 4-(2-phthalimidoethoxy)acetoacetate, 7g of o-chlorobenzaldehyde, 100ml of methanol, Stir and reflux for 20 hours. After completion, recover the solvent under reduced pressure, add 30 ml of glacial acetic acid, stir until crystals are precipitated, and filter to obtain...
Embodiment 2
[0038] Embodiment 2: the preparation of impurity F
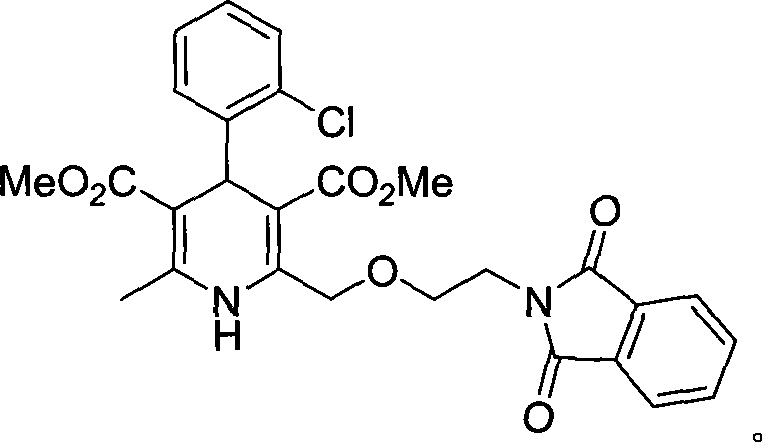
[0039] (4RS)-4-(2-chlorophenyl)-2-[(2-aminoethoxy)methyl]-6-methyl-1,4-dihydropyridine-3,5-dicarboxylic acid methyl ester
[0040]
[0041] resolve resolution
[0042] 1, Synthesis of ethyl 4-bromoacetoacetate (B)
[0043] Add 80g of liquid bromine dropwise to a 500ml chloroform solution containing 58g of methyl acetoacetate at -5°C to 0°C, drop it within 2 hours, react at room temperature for 15 hours, add water 500ml×2 for extraction, separate the organic layer, and dry Chloroform was distilled under reduced pressure (recyclable and used mechanically) to obtain 100 g of the product with a yield of 82%.
[0044] 2, Preparation of 4-[2-(phthalimido) ethoxy] ethyl acetoacetate (C)
[0045] Add 350ml of tetrahydrofuran and 43g of NaH to the three-necked flask, add 97g of phthalimide ethanol in batches, cool to -10°C, add 100g of methyl 4-bromoacetoacetate dropwise, react at -5°C to 0°C for 1 hour, and rise to React at ...
Embodiment 3
[0054] Embodiment 3: the preparation of high-purity amlodipine besylate
[0055] 1, the preparation of phthaloyl amlodipine
[0056] Inhale 50Kg of 4-(2-phthalimidoethoxy)ethyl acetoacetate (content 90%), 200kg of anhydrous methanol, 22Kg of o-chlorobenzaldehyde, and add 3-aminobutyl Acrylic acid methyl ester 54Kgkg, insulation reflux reaction after 20 hours, decompression recovery methanol, recovery is complete. Add 100kg of glacial acetic acid, stir at 12-18°C for about 12 hours, discharge the material in stages and perform rejection filtration in a centrifuge to obtain about 50kg of crude wet product of phthaloyl amlodipine. (Feeding molar ratio 4—(2-phthalimidoethoxy)ethyl acetoacetate: 3-aminobutyrylic acid methyl ester=1:1.12:3.35)
[0057] Use 70 kg of mixed solvent of toluene: glacial acetic acid = 1:1 system to dissolve the above-mentioned crude product by heating, then naturally cool to 20-30 ° C for 1 hour, shake off the filter, rinse with cold toluene, and obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com