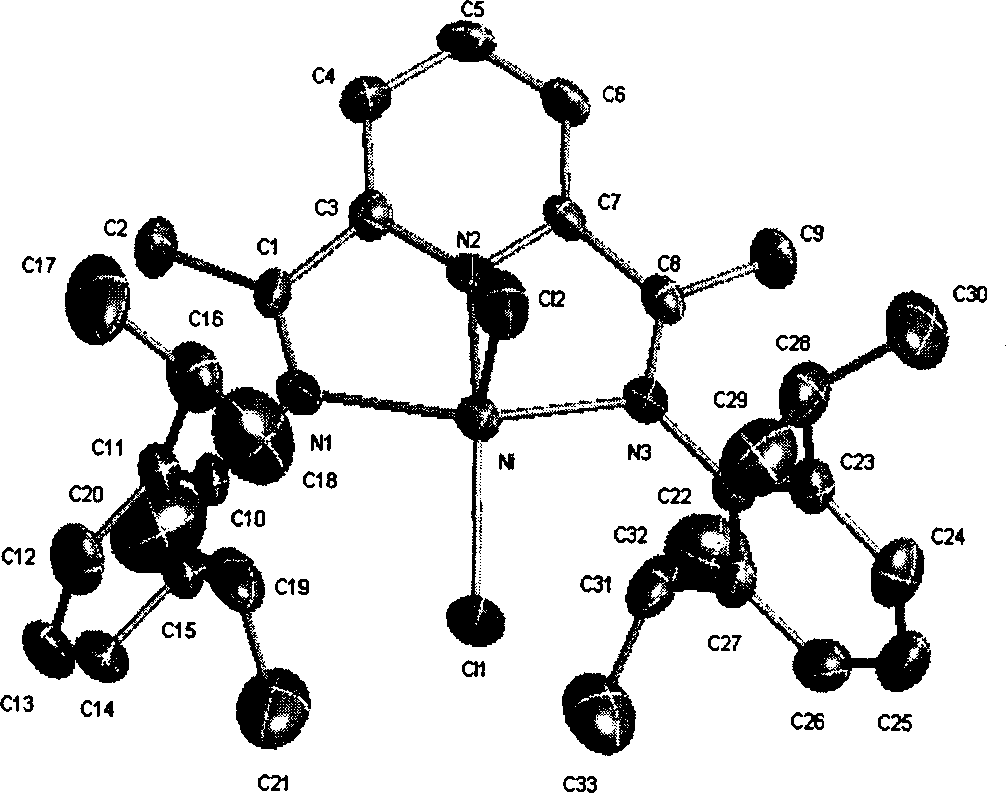
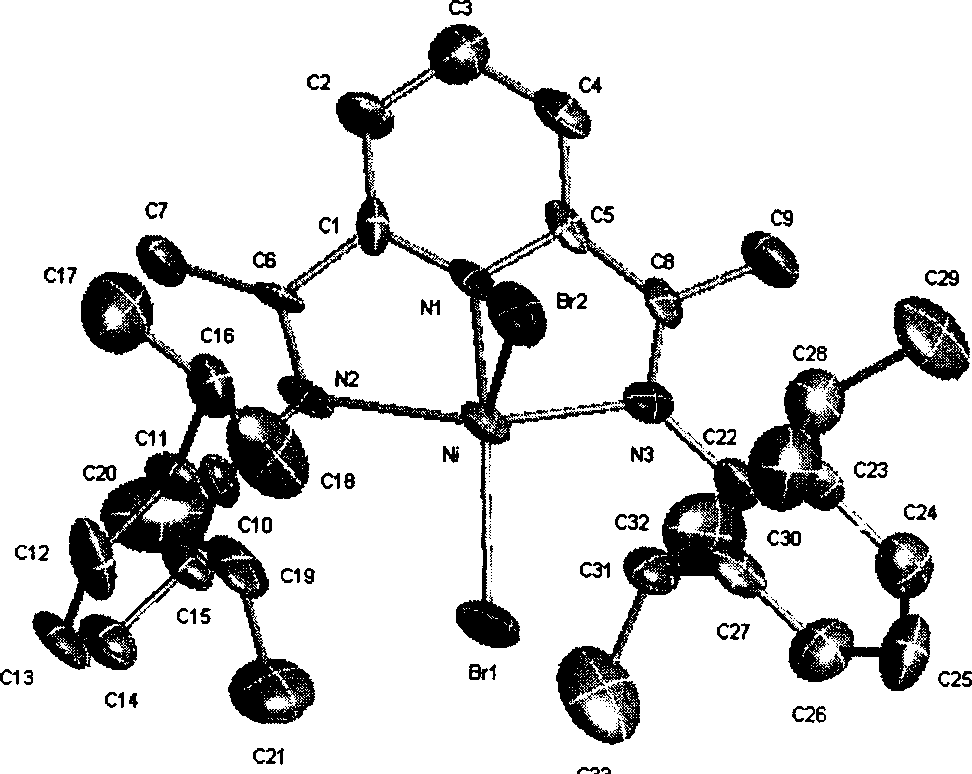
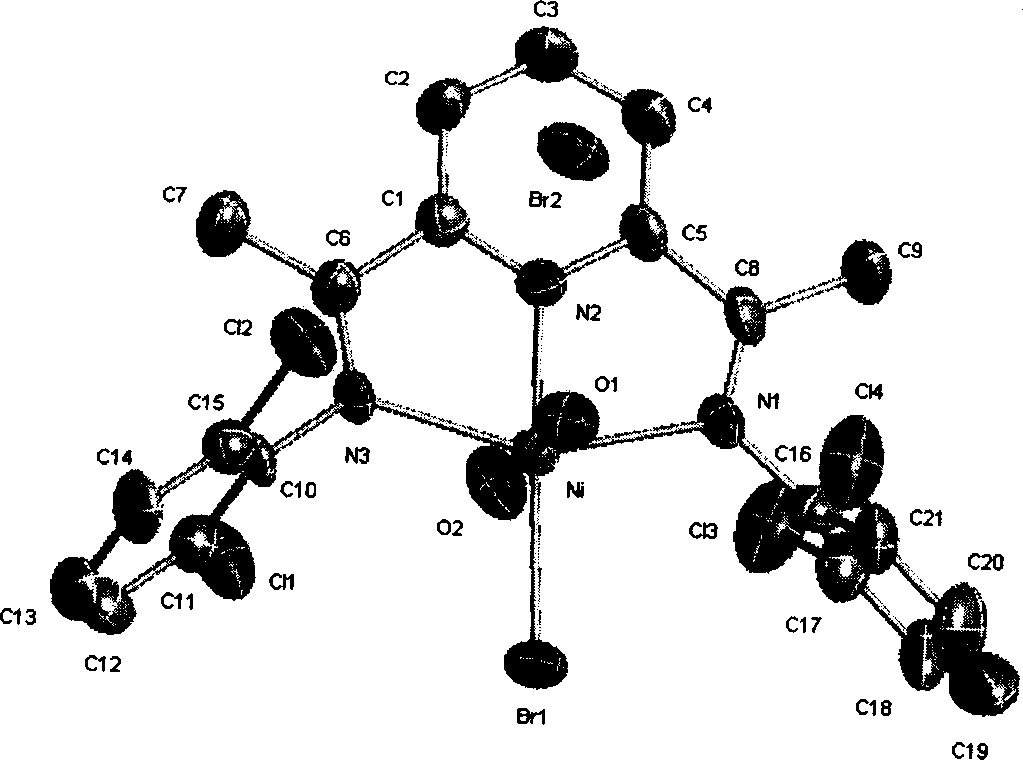
Olefine polymerization catalyst, synthesis method and its use
A technology of olefin polymerization and synthesis method, which is applied in the field of synthesis and olefin polymerization catalyst, and can solve the problems such as tridentate nickel complexes that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment one 2, the preparation of 6-diethyl pyridinedicarboxylate
[0037] Under the protection of nitrogen, dissolve 11g (66mmol) of picolinic acid in 20mL of absolute ethanol, cool in an ice-water bath, and slowly drop into a solution of 12g (63mmol) of p-toluenesulfonic acid, 30mL of absolute ethanol, and 20mL of benzene. Heat to reflux to separate water for 48 hours. After the reaction is complete, spin off most of the solvent, add anhydrous ether, shake and divide into two layers, the upper layer is colorless, and the lower layer is a brownish-red oily liquid. Add solid sodium bicarbonate to neutralize until no bubbles are generated. A large amount of white precipitate appeared, and water was added to dissolve the solid, extracted with ether, the ether layers were combined, dried over anhydrous sodium sulfate, spin-dried, and sucked dry to obtain 12.7 g of white solid, with a yield of 87%. 1H NMR (CDCl3 / TMS, 300 MHz): δ=1.4 (t, J=7.5Hz, 6H); 4.5(q, J...
Embodiment 2
[0038] Example 2 Preparation of 2,6-diacetylpyridine
[0039] Under the protection of nitrogen, add 60mL of absolute ethanol dropwise to 4.75g (200mmol) sodium block, heat to reflux to dissolve the sodium block completely, slowly add dropwise 12.7g (57mmol) diethyl picolinate, 18g (204mmol) acetic acid Ethyl ester in 90mL xylene solution, heat 120-140°C to reflux for 24h, heat up to 160°C to reflux for 36h, add water 40mL, concentrated hydrochloric acid 80mL, oil bath 160°C to reflux for 4h, cool and stand, the solution is separated, the upper organic phase is orange Yellow and transparent, the lower aqueous phase is brown-red turbid, the upper organic phase is separated, dried over anhydrous magnesium sulfate, the solvent xylene is evaporated, the residue is combined with the aqueous phase, refluxed for 4 hours, cooled, neutralized with sodium bicarbonate, extracted with ether, Dry over anhydrous sodium sulfate and purify by column chromatography to obtain 5.8 g o...
Embodiment 3
[0040] Example 3 Preparation of 2,6-diacetylpyridine bis(2,6-diisopropylaniline)
[0041] Under the protection of nitrogen, dissolve 1.5g (9.2mmol) 2,6-diacetylpyridine and 3.3g (18.6mmol) 2,6-diisopropylaniline in 25mL of absolute ethanol, drop a few drops of glacial acetic acid, Refluxed for 24 hours, cooled to room temperature, yellow crystals were precipitated, recrystallized from ethanol, filtered, washed with ethanol, and dried to obtain 3.2 g of yellow solids with a yield of 67%. 1 H NMR (CDCl 3 / TMS, 300MHz): δ=1.2(d, J=6.9Hz, 24H); 2.3(s, 6H); 2.8(sept, J=6.9Hz, 4H); 7.2(m, 6H); 8.0(t, J=7.8, 1H); 8.5 (d, J=7.8, 2H). Mass spectrum (EI): 481 (59.76), 466 (100), 202 (53.37), 467 (36.47), 176 (27.58), 43 (27.47), 186 (25.59), 482 (22.20). IR (KBr) v / cm-1 3059, 2959, 2925, 2867, 1644, 1455, 1365, 1240, 828, 768.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com