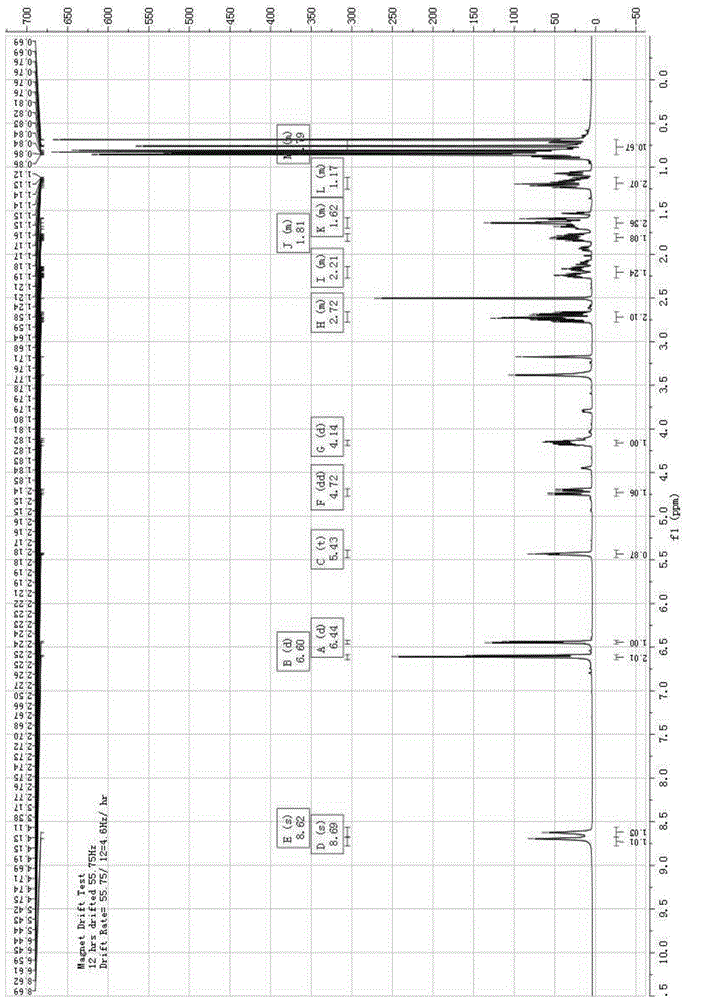
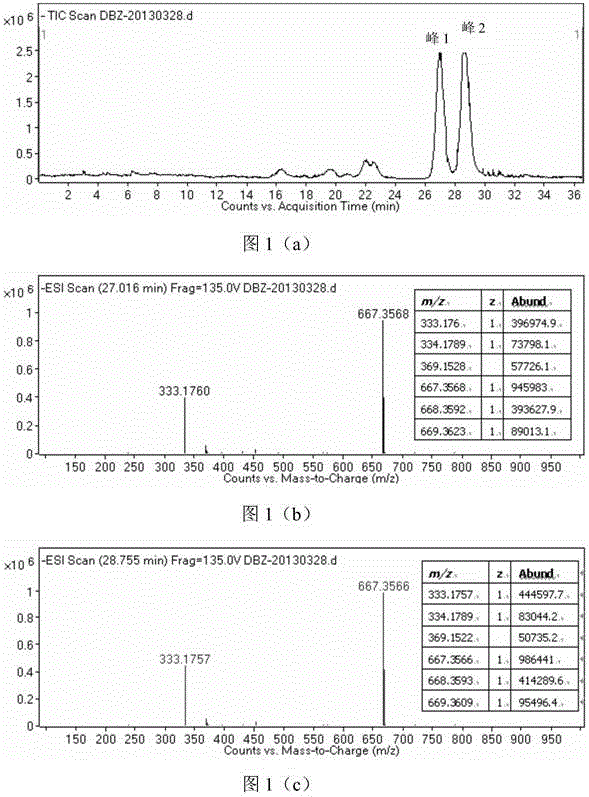
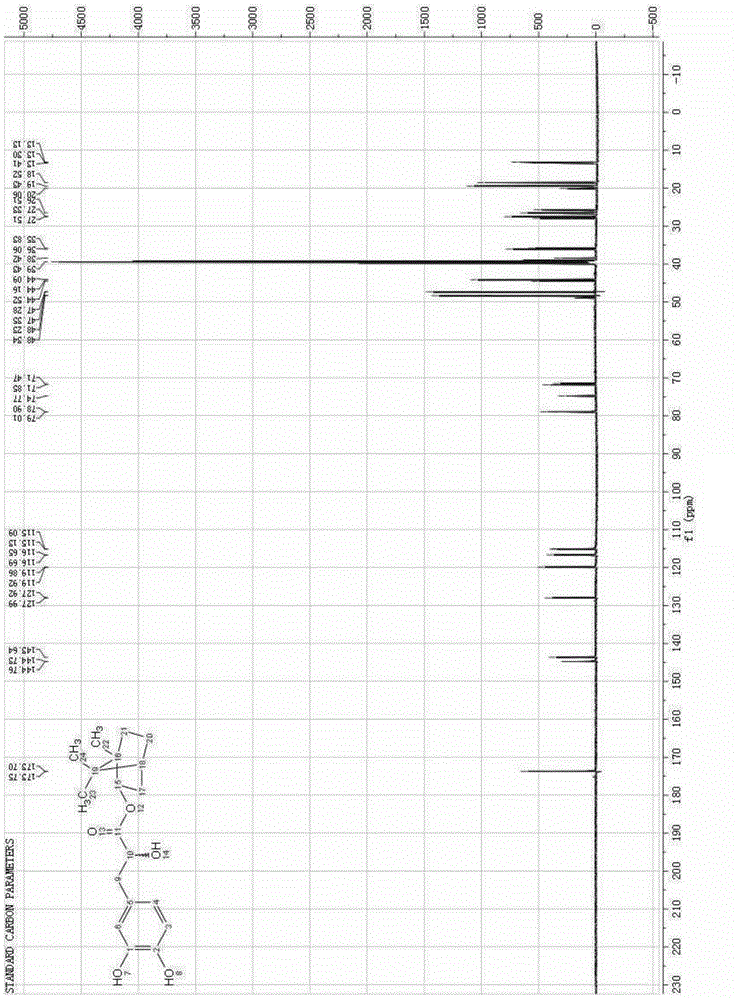
Synthesis method of racemic bornyl beta-(3,4-dihydroxyphenyl)-alpha-hydroxypropionate
A technology of borneyl danshensu and danshensu, which is applied in the field of drug synthesis, can solve problems such as the need to increase the yield, and achieve the effects of easy processing or recycling, reduced production costs, and high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] (1) Synthesis of 3,4-dibenzyloxybenzaldehyde:
[0060] Dissolve 55.2g (0.40mol) of protocatechualdehyde in 600mL of N,N-dimethylformamide, slowly add 116.0g (0.92mol) of benzyl chloride, and weigh anhydrous K 2 CO 3 165.6g (1.2mol) was added to it, stirred and reacted at room temperature for 2 hours, then added K 2 CO 3 55.2g (0.40mol), heated at 130°C for 2 hours, cooled to room temperature, filtered with suction, the filtrate was added to ice water, acidified with dilute hydrochloric acid to produce a yellow precipitate, filtered with suction, washed with ice water to obtain 3,4-dibenzyloxy 122.1 g of benzaldehyde, the yield was 96%.
[0061] (2) Synthesis of Bornyl Chloroacetate:
[0062] Add 400mL of dichloromethane, 77.2g (0.5mol) of borneol, 50.5g (0.5mol) of triethylamine into the reaction vessel, add 56.4g (0.5mol) of chloroacetyl chloride at 0°C, and stir the reaction at room temperature for 3 hours. Add 200mL of water to the solution, wash with 200mL of s...
Embodiment 2
[0076] This embodiment differs from Embodiment 1 in that:
[0077] (1) Synthesis of 3-(3,4-dibenzyloxyphenyl)bornyl glycidate:
[0078] Dissolve 12.2 g (0.225 mol) of sodium methoxide in 150 mL of methanol to obtain solution 1;
[0079] Dissolve 47.7g (0.15mol) of 3,4-dibenzyloxybenzaldehyde and 48.4g (0.21mol) of bornyl chloroacetate in 200mL of dioxane to obtain solution 2;
[0080] Within 1 hour, drop solution 2 into solution 1, keep the reaction temperature at 5-10°C, after the dropwise addition, slowly warm up to room temperature, stir overnight, add the reaction solution into 200mL of ice water, adjust the hydrochloric acid to neutral, Extracted with dichloromethane (200mL×4), combined the organic phases, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized the crude product with ethanol / petroleum ether to obtain 37.6g of off-white solid with a yield of 75%.
[0081] (2) Synthesis of Danshensu Bornyl Ester:
[0082] Dissolve 25....
Embodiment 3
[0084] This embodiment differs from Embodiment 1 in that:
[0085] (1) Synthesis of 3-(3,4-dibenzyloxyphenyl)bornyl glycidate:
[0086] Dissolve 21.6 g (0.225 mol) of sodium tert-butoxide in 150 mL of tert-butanol to obtain solution 1;
[0087]Dissolve 47.7g (0.15mol) of 3,4-dibenzyloxybenzaldehyde and 34.6g (0.15mol) of bornyl chloroacetate in 150mL of dioxane to obtain solution 2;
[0088] Within 1 hour, drop solution 2 into solution 1, keep the reaction temperature at 5-10°C, after the dropwise addition, slowly warm up to room temperature, stir overnight, add the reaction solution into 200mL of ice water, adjust the hydrochloric acid to neutral, Extract with dichloromethane (200 mL×4), combine the organic phases, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and recrystallize the crude product with ethanol / petroleum ether to obtain 49.2 g of off-white solid, with a yield of 64%.
[0089] (2) Synthesis of Danshensu Bornyl Ester:
[0090] Dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com