Synthesis method of mannosidase inhibitor Kifunensine
A mannosidase and synthesis method technology, applied in the field of compound synthesis, can solve the problems of generating a large amount of impurities, high market price, unfavorable purification, etc., and achieve the effects of high overall reaction yield, good industrialization prospects, and cheap and easily available reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] Unless otherwise defined, the technical terms used in the following embodiments have the same meaning as commonly understood by those skilled in the art to which the present invention belongs. The test reagents used in the following examples, unless otherwise specified, are conventional biochemical reagents; the experimental methods, unless otherwise specified, are conventional methods.
[0046] The present invention has chosen the synthesis process under preferred conditions to illustrate the specific synthesis method of the compound molecules involved in the present invention, wherein the substituent R 1 Choose acetyl, substituent R 2 Choose tert-butyldiphenyl, substituent R 3 The pivaloyl group is selected, but the selection of the examples does not imply any limitation of the preparation method of the present invention.
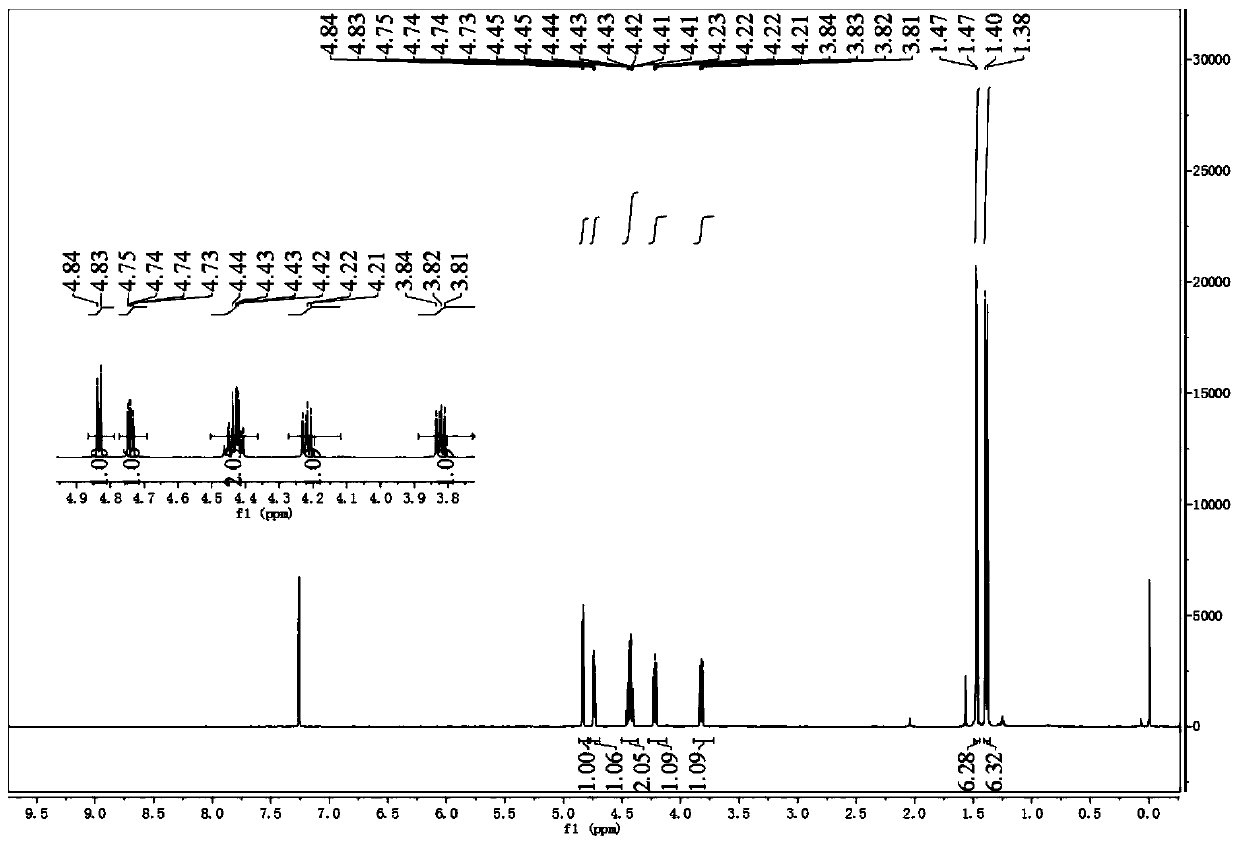
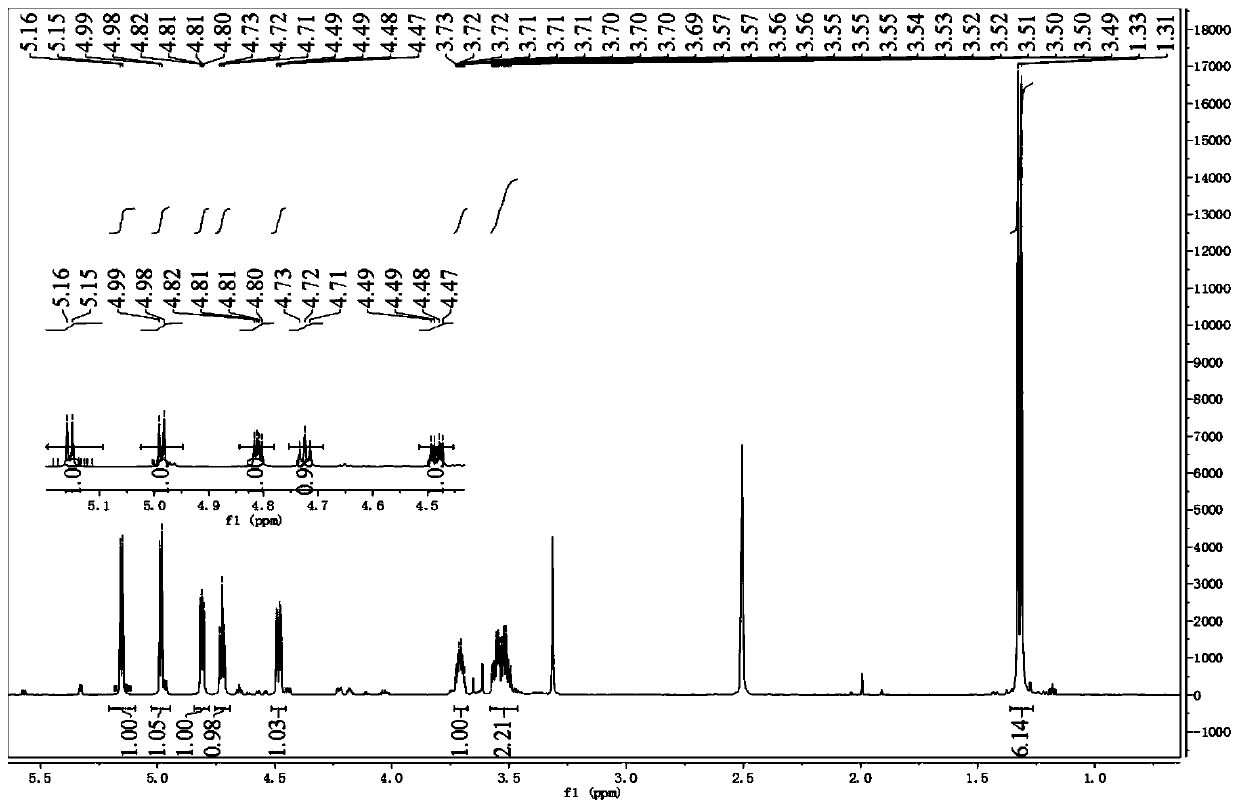
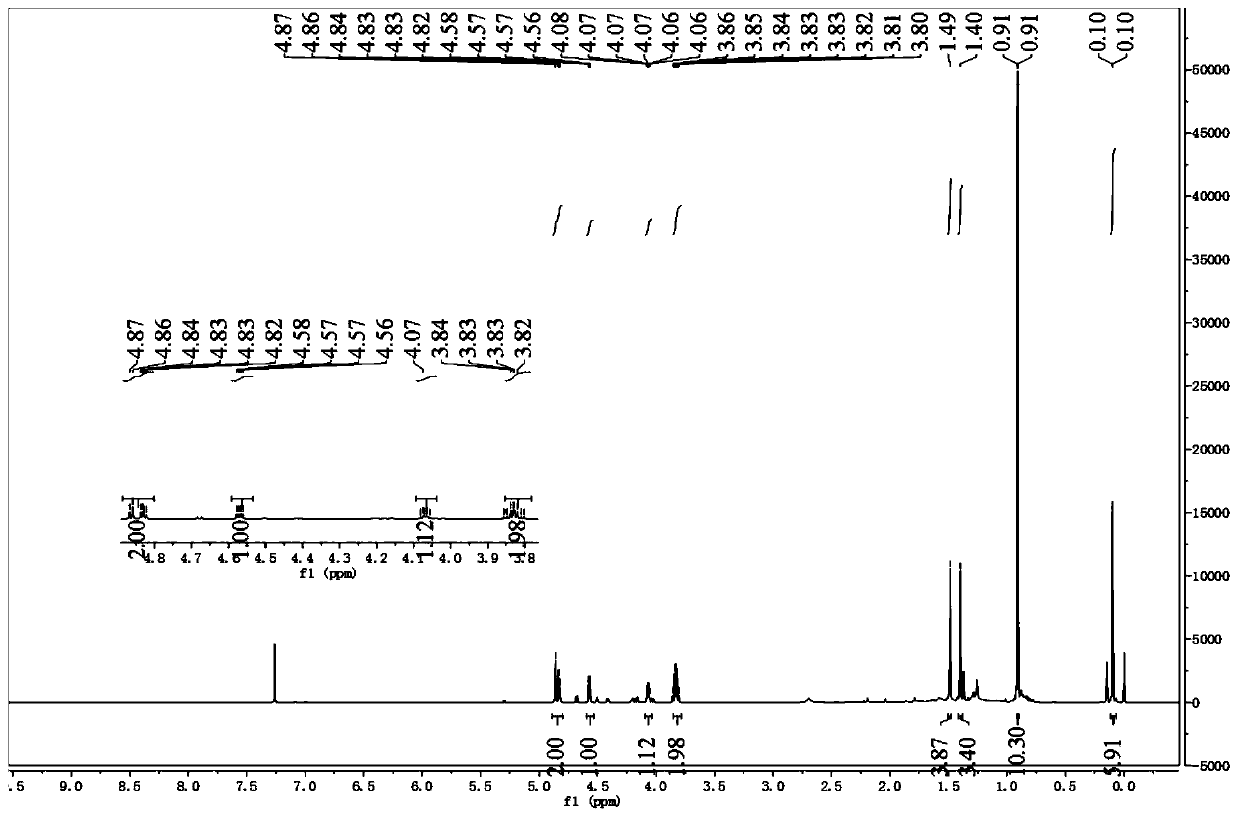
[0047] 1. Synthesis of Intermediate 2
[0048]
[0049]20g (112.3mmol) L-gulonic acid-γ-lactone 1 and 1.93g (11.2mmol) p-toluenesulfonic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com