Application of alcohol dehydrogenase in catalytic generation of ethyl (R)-4-chloro-3-hydroxy butyrate
A technology of ethyl hydroxybutyrate and alcohol dehydrogenase, which is applied in the biological field to achieve the effects of high yield, high yield and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
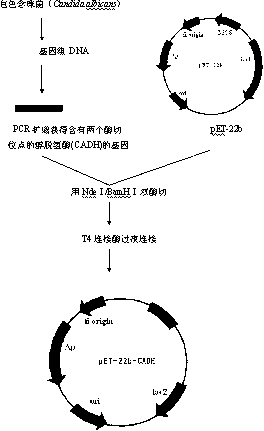
[0030] Embodiment 1: recombinant escherichia coli E. coli Rosetta (pET-22b-CA)
[0031] 1. Acquisition of Alcohol Dehydrogenase Gene
[0032] Candida albicans Candida albicans(purchased from Centraalbureauvoor Schimmelcultures (CBS) Fungal Biodiversiry Centre), medium YPD (g·L -1 ): Yeast extract 10g, peptone 20g, glucose 20g, add distilled water to 1L.
[0033] Candida albicans Candida albicans Inoculated in 5mL YPD liquid medium and cultured at 30°C until the logarithmic growth phase, and the genome was extracted using a genomic DNA extraction kit (Beijing Tianwei Bioengineering Co., Ltd. Yeast Genome Extraction Kit, GD2415 Yeast gDNA Kit).
[0034] The primers used to construct the expression vectors are provided with enzyme cutting sites, and the primer sequences are as follows:
[0035] The upstream primer (CA-sense containing NdeI) is: 5'- GGAATTC CATATGTCAATTCCATCTACTCAGTACG -3'
[0036] The downstream primer (CA-anti containing BamHI) is: 5'- CGC GGATCCTTATGG...
Embodiment 2
[0049] Embodiment 2: recombinant escherichia coli E. coli Fermentation of Rosseta (pET22b-CA)
[0050] pick recombinant bacteria E. coli Add Rosseta (pET-22b-CA) to LB medium containing antibiotics and culture overnight at 37°C with shaking. Then they were inoculated into fresh culture medium according to the inoculum amount of 2%, and cultivated to OD at 37°C. 600 At about 0.6, add IPTG to a final concentration of 0.8 mmol·L -1 , 25°C, 220rpm, after induction of expression for 10 h, centrifuge at 8000 rpm, 4°C for 10 min, discard the supernatant, and precipitate for later use.
[0051]
Embodiment 3
[0053] The precipitate of Example 2 was washed twice with sodium phosphate buffer (100 mmol L-1, pH 6.5), weighed 2 g (wet weight) of Escherichia coli sludge, and suspended in the reaction solution of 25 mL of the total system (pH 6.5 The volume ratio of potassium phosphate buffer to butyl acetate is 1:1). Add isopropanol 150mmol / L, COBE 25g / L, 30°C, 180rpm, 12h. product( R The yield of )-CHBE is 20.5 g / L, the yield of the product is: 82%, and the optical purity e.e% is 100%.
[0054] The detection method of product is as follows (the detection method of product is identical with embodiment 3 in the following examples):
[0055] For the aqueous phase reaction: after the reaction, centrifuge at 8000 rpm for 10 min to separate the organic layer and the aqueous layer. Carefully draw the upper layer of ethyl acetate through the organic membrane, add the internal standard, and save the test sample.
[0056] For water / organic two-phase reaction: after the reaction, centrifuge at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com