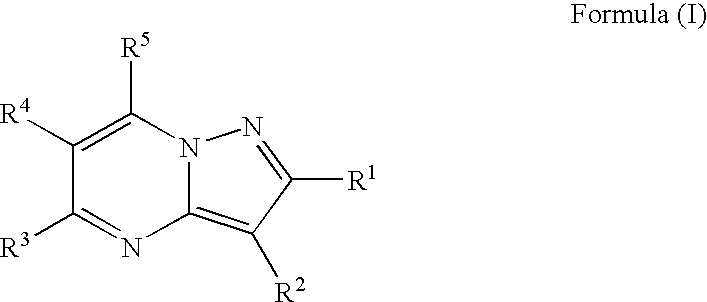
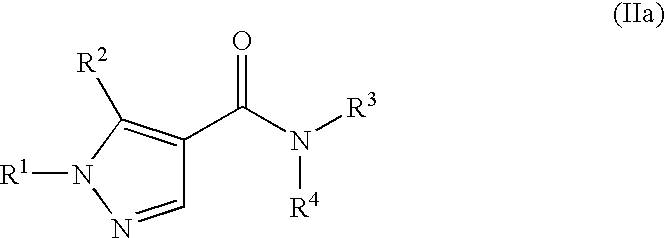
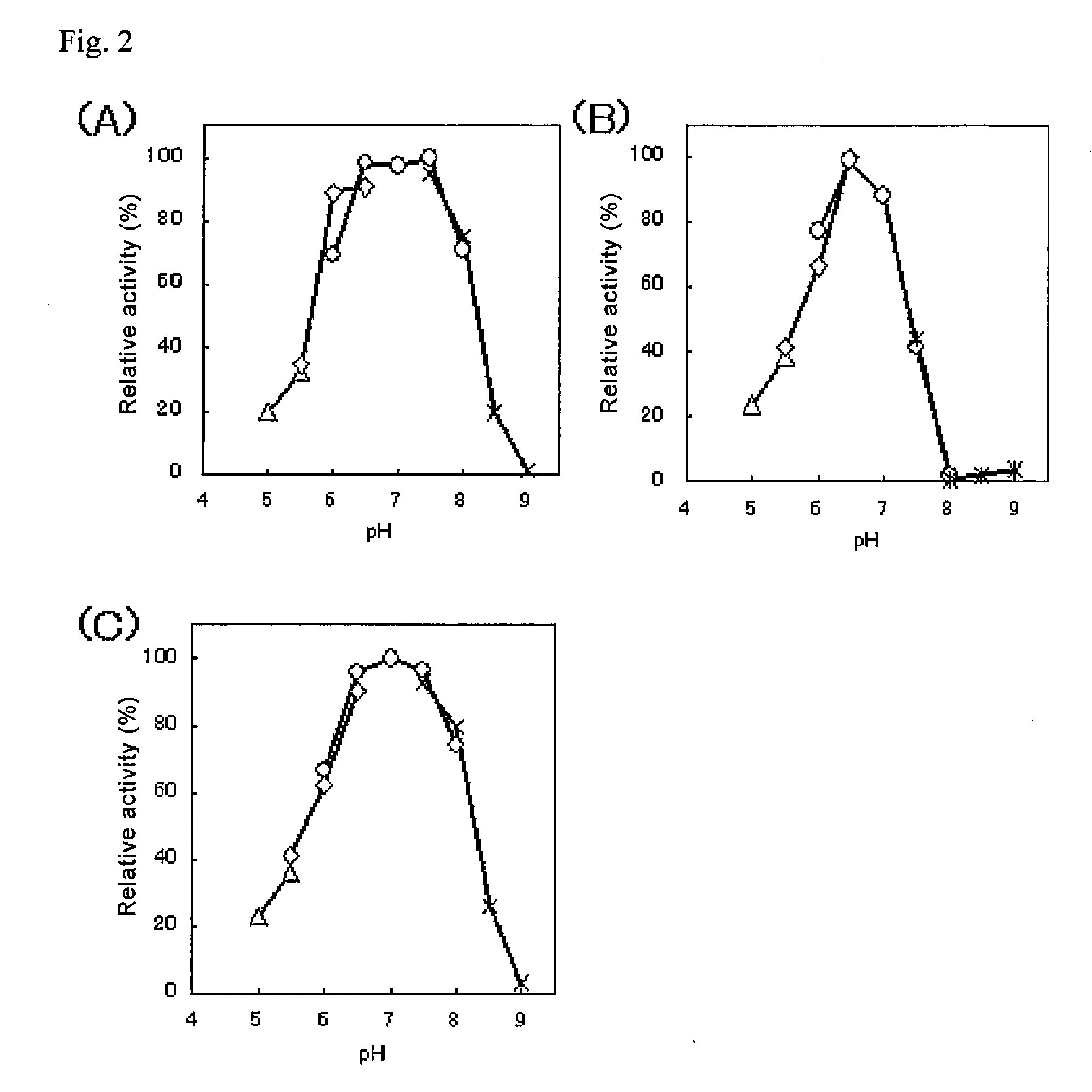
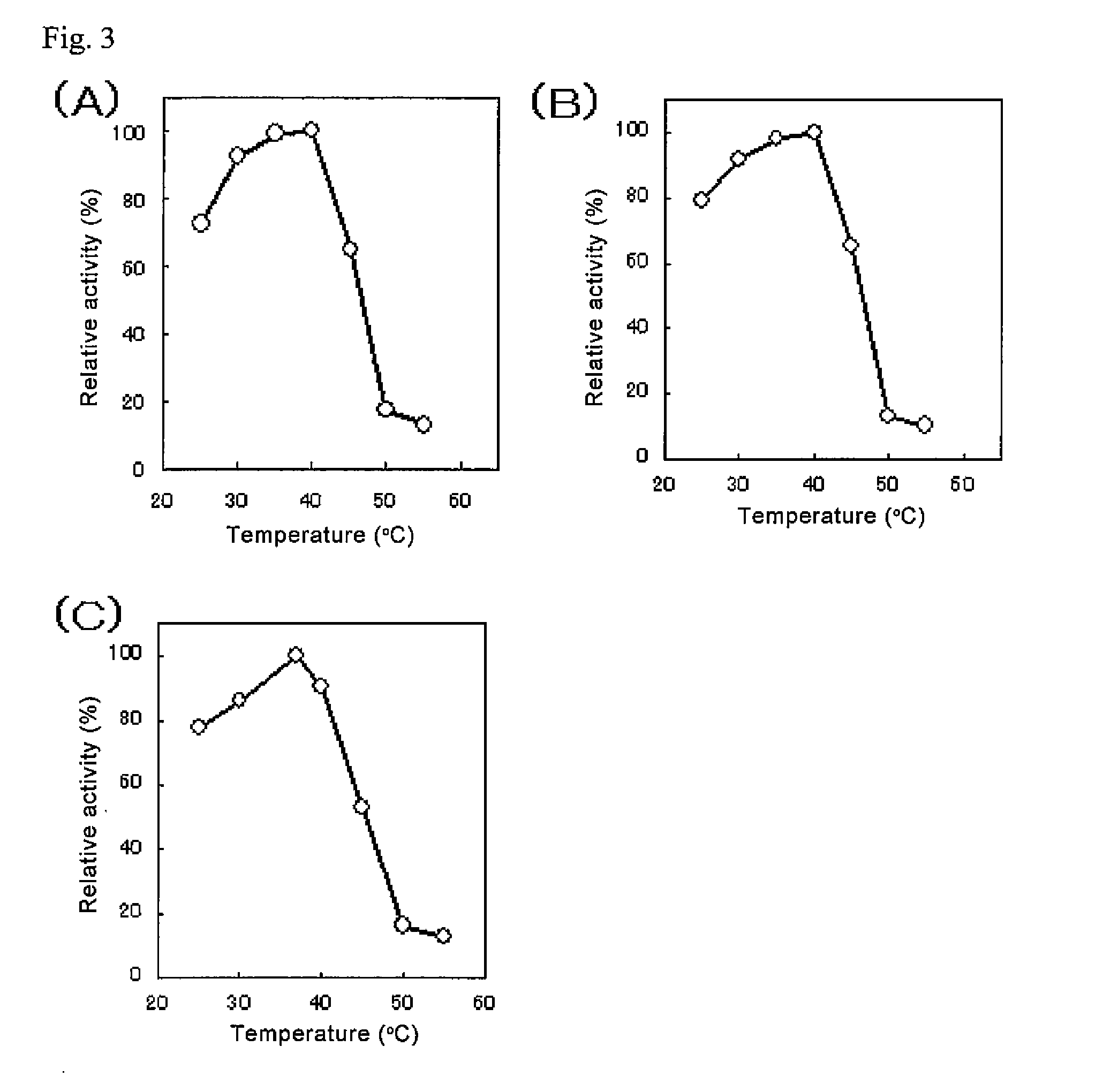
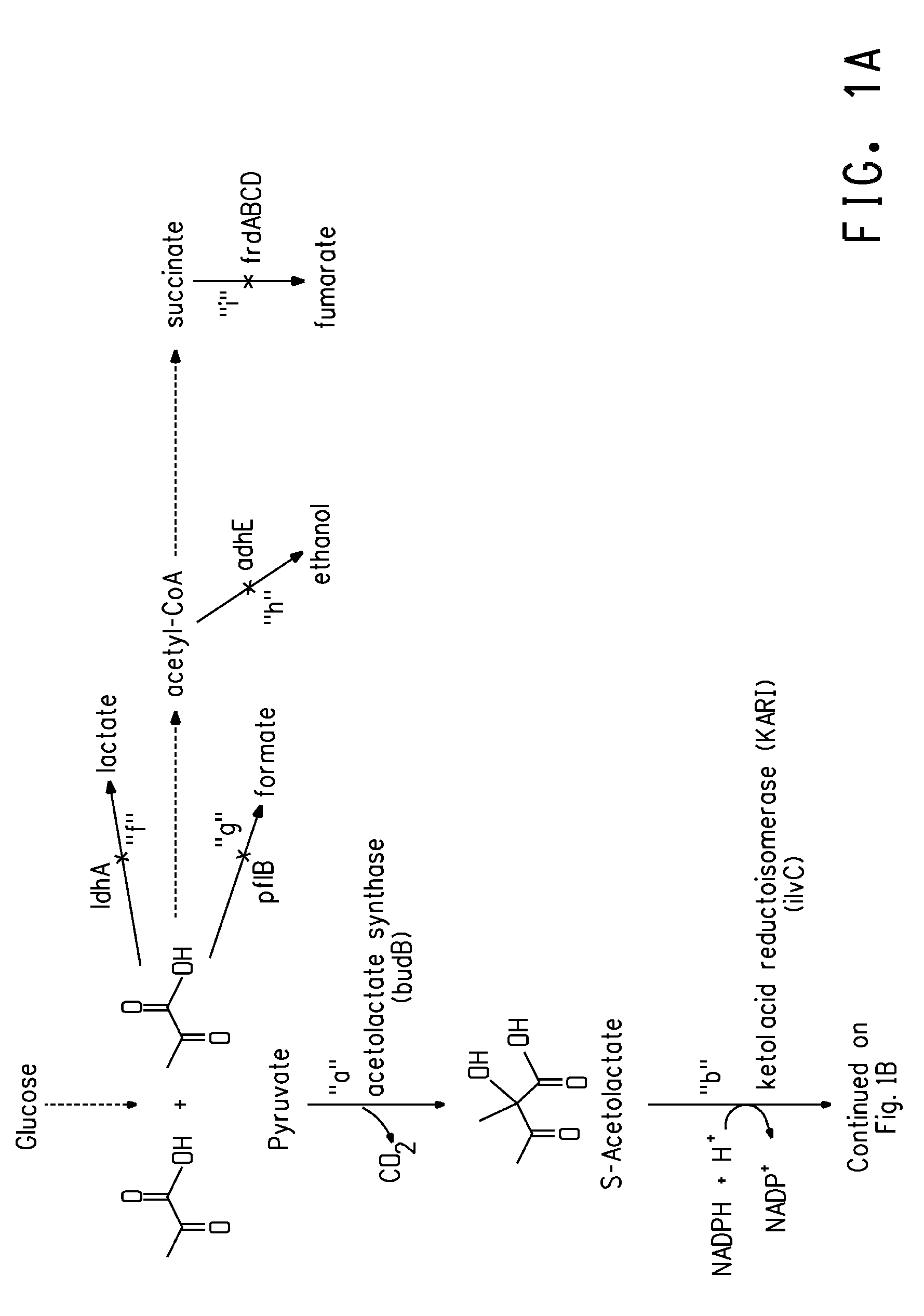
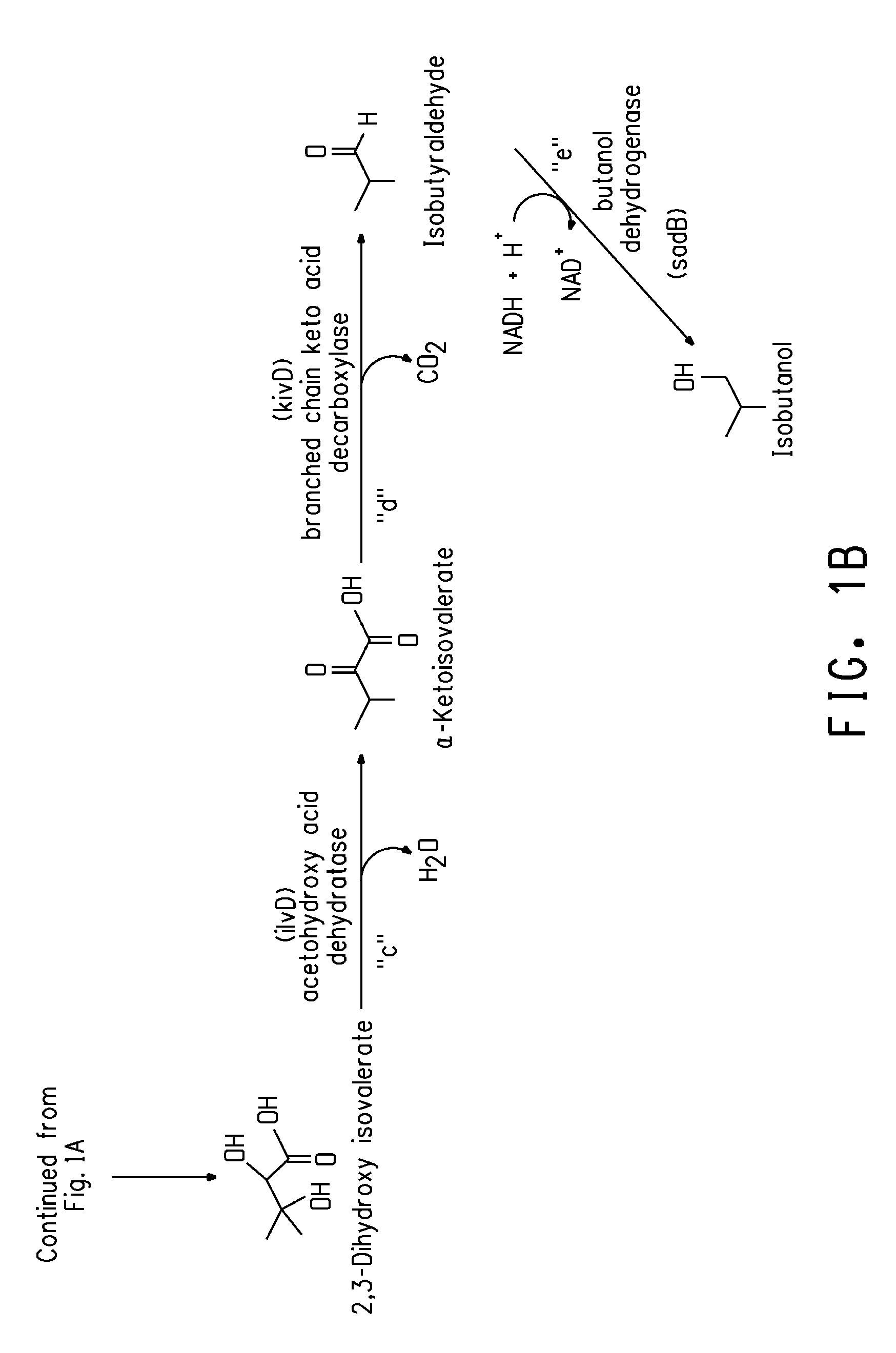
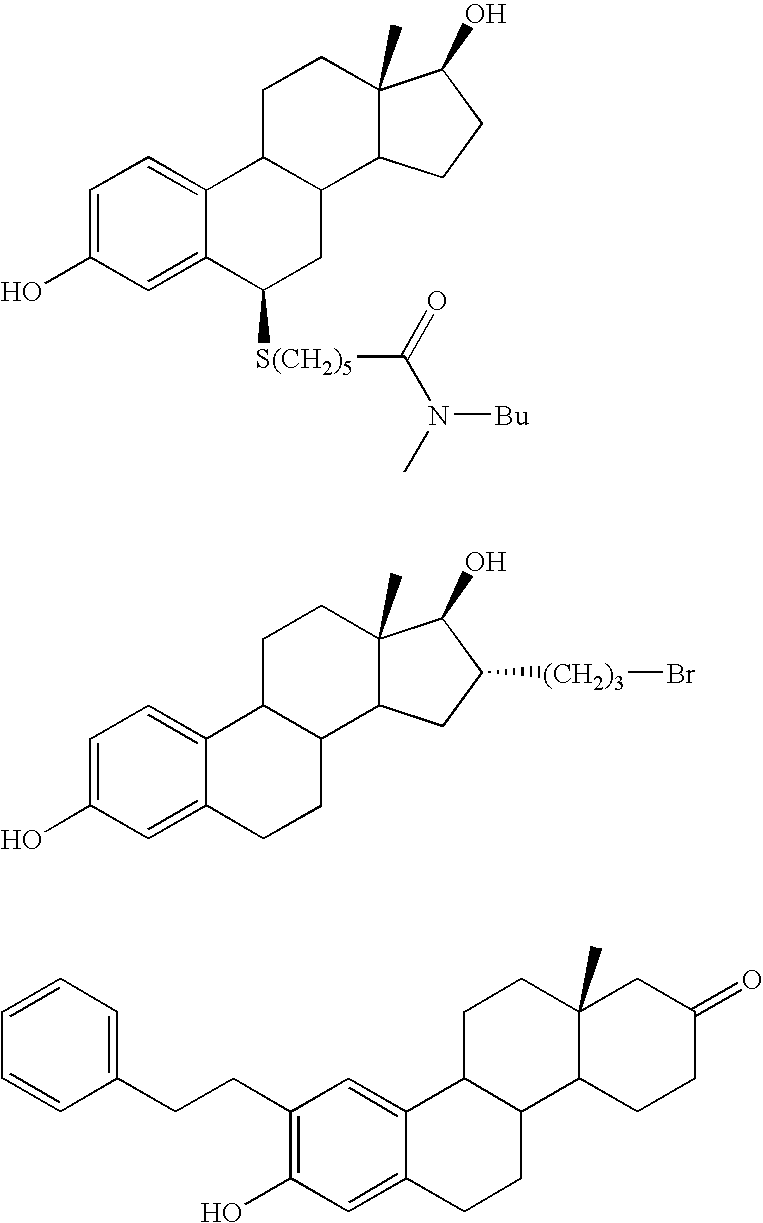
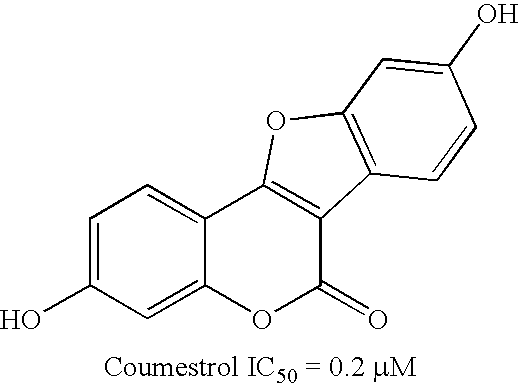
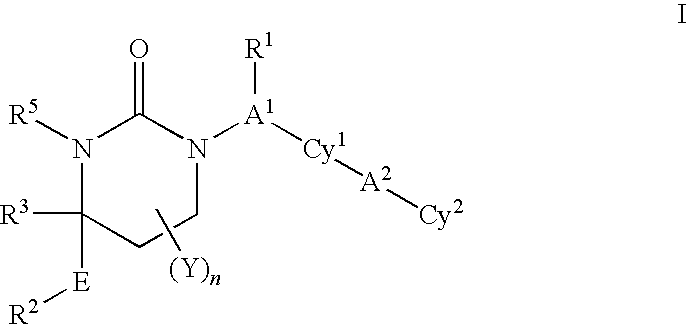
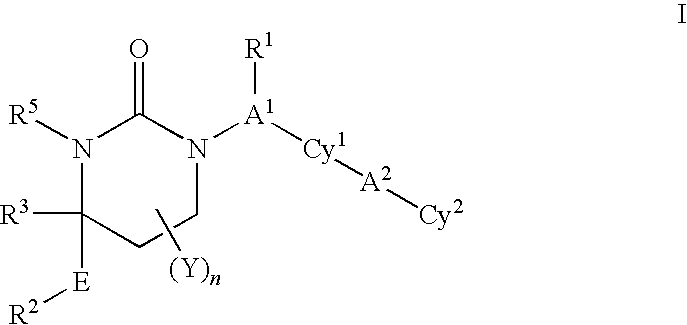
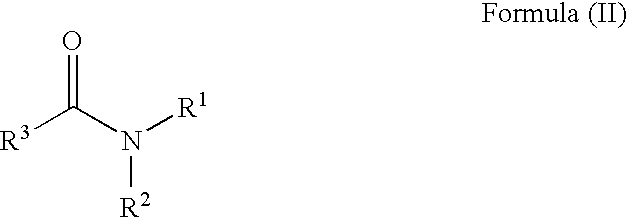Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1687 results about "Acyl CoA dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acyl-CoA dehydrogenases (ACADs) are a class of enzymes that function to catalyze the initial step in each cycle of fatty acid β-oxidation in the mitochondria of cells. Their action results in the introduction of a trans double-bond between C2 (α) and C3 (β) of the acyl-CoA thioester substrate. Flavin adenine dinucleotide (FAD) is a required co-factor in addition to the presence of an active site glutamate in order for the enzyme to function.
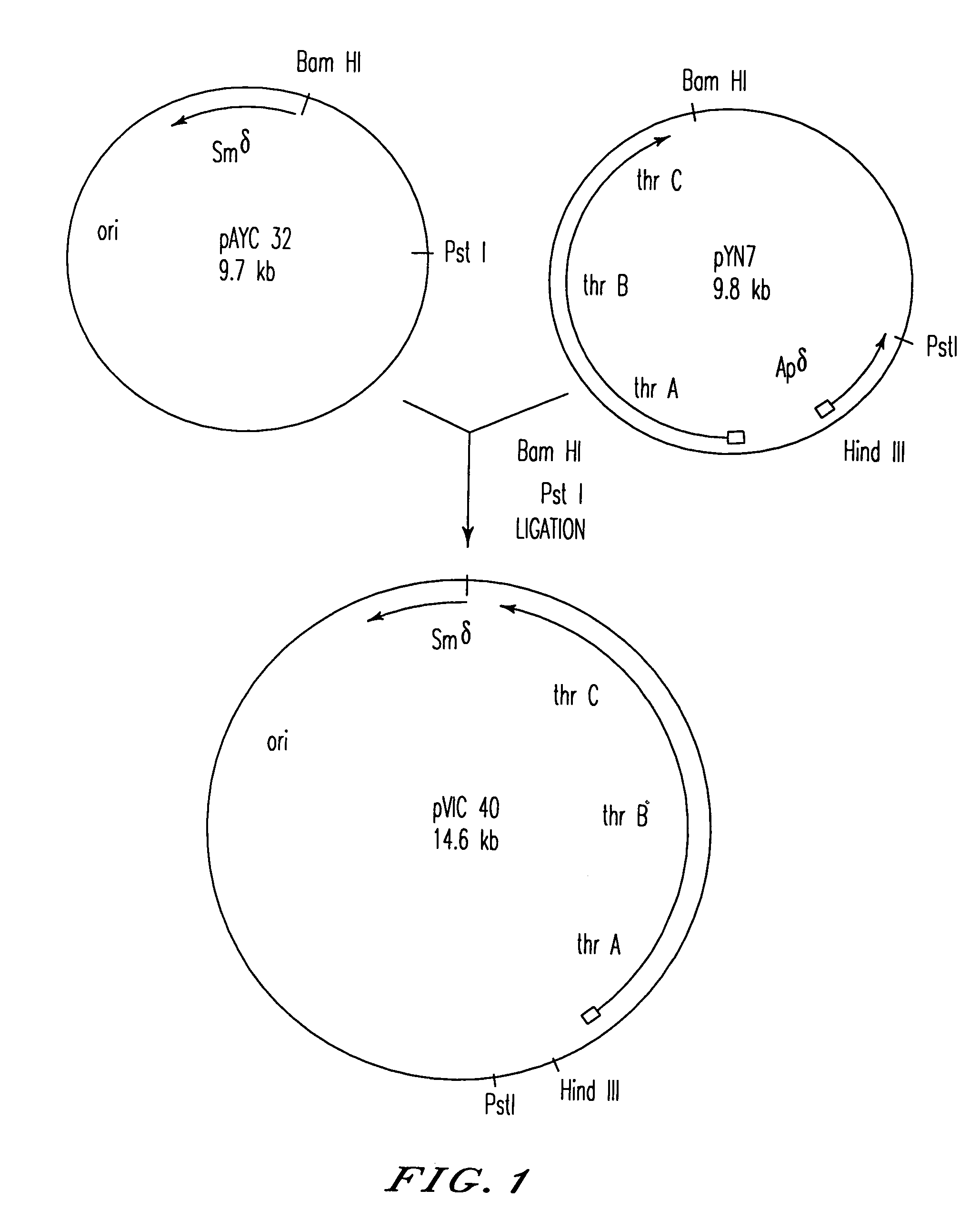
Bacterial strain of Escherichia coli BKIIM B-3996 as the producer of L-threonine
InactiveUS7138266B2High yieldHigh plasmid stabilityBacteriaUnicellular algaeBacilliThreonine dehydrogenase activity
A bacterial strain of Escherichia coli is described which produces L-threonine, and is obtained by a process comprising transduction by bacteriophage P1 which bears a transposon which inactivates threonine dehydrogenase activity, and isolation of a transductant lacking threonine dehydrogenase activity.
Owner:AJINOMOTO CO INC
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase type 1 enzyme
ActiveUS20060149070A1Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryInsulin dependentEnzyme inhibitor
The present invention relates to compounds that are inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome and other diseases and conditions that are mediated by excessive glucocorticoid action.
Owner:ABBVIE INC
Nutraceutical composition and method of use for treatment / prevention of cancer
InactiveUS20070248693A1Function increaseAbility to createBiocideAlgae medical ingredients1,4-BenzoquinonePantothenic acid
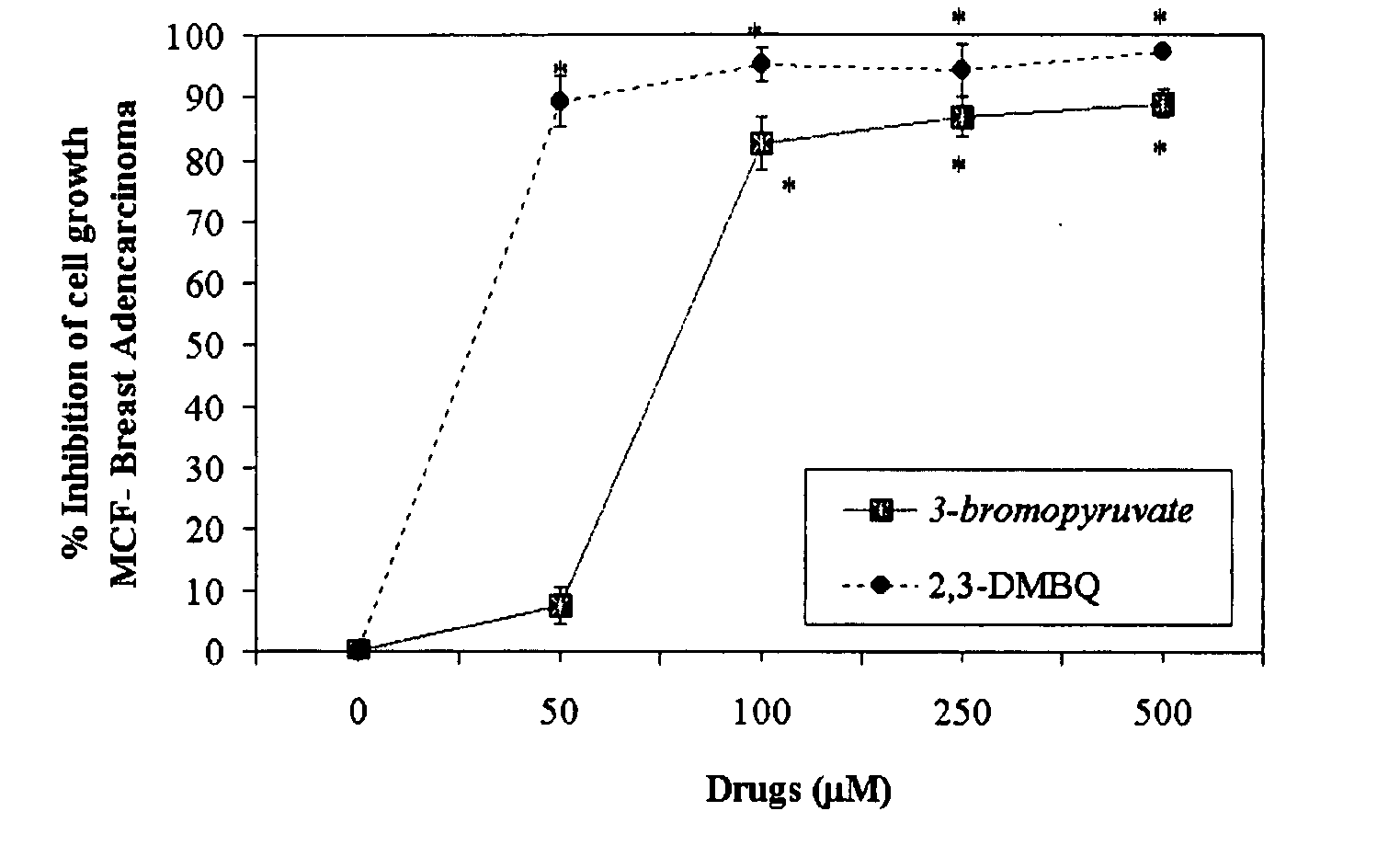
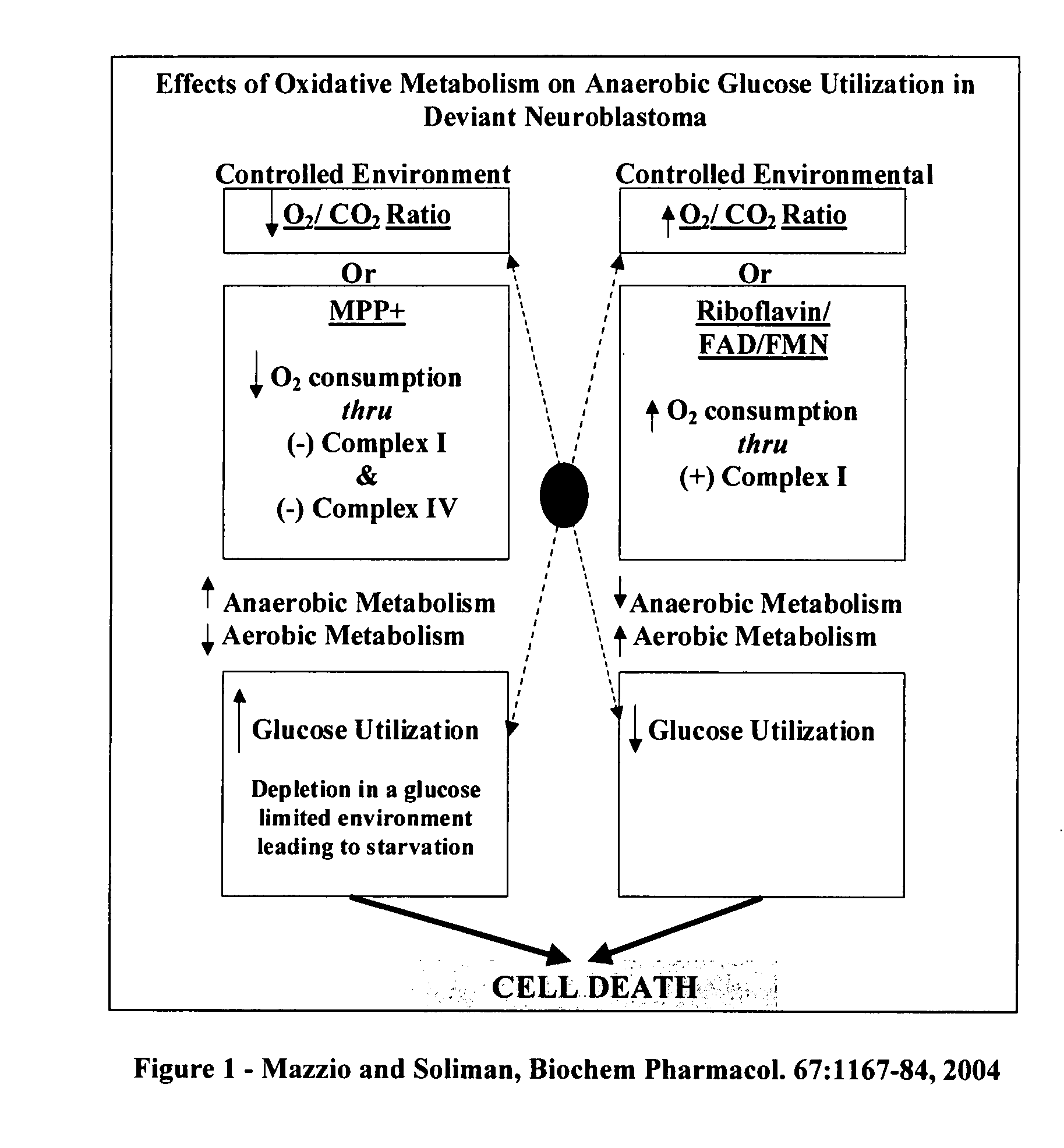
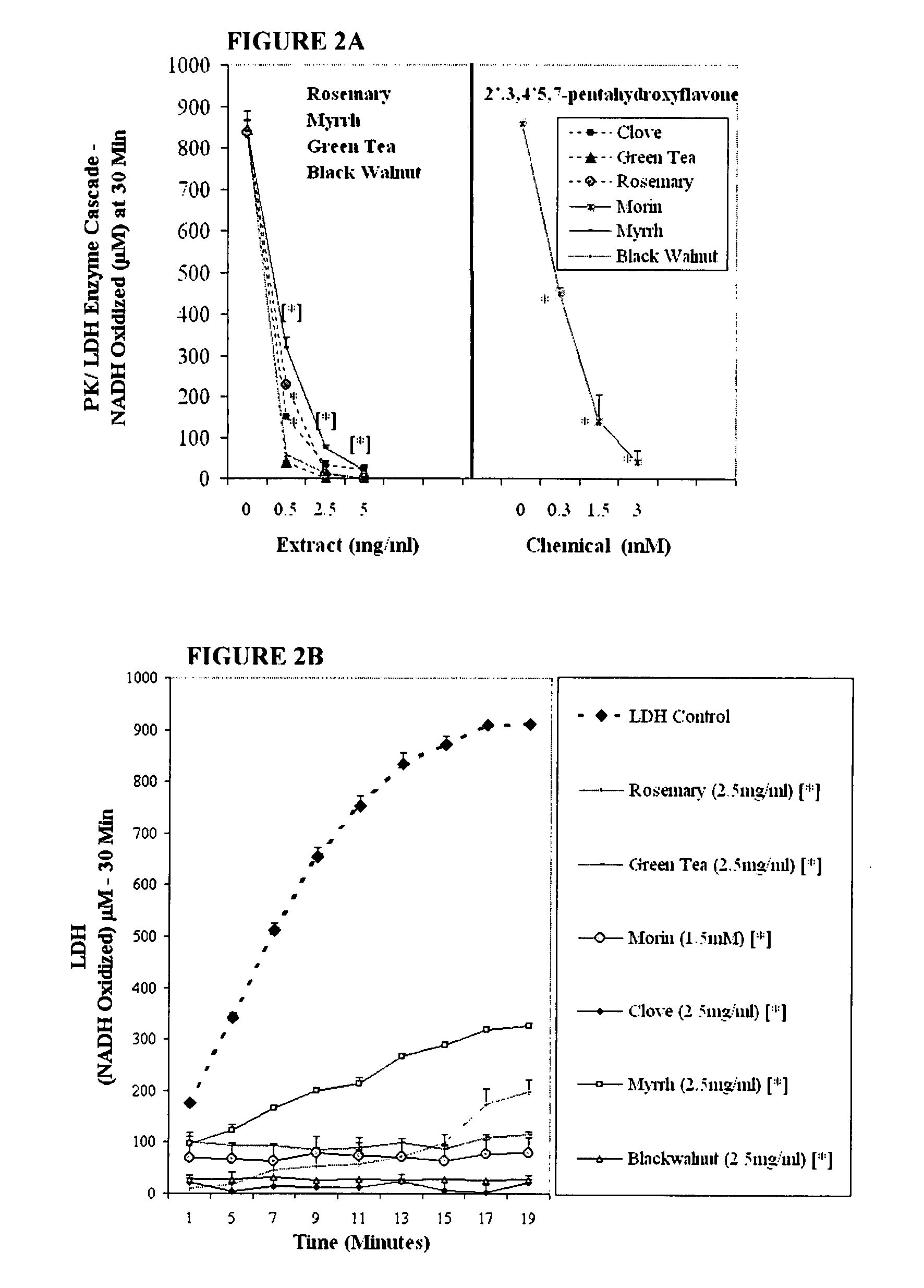
The invention describes a pharmaceutical composition and method for treating cancer comprised of A) 2,3-dimethoxy-5-methyl-1,4-benzoquinone and / or B) at least one of wild yam root, teasel root, balm of gilead bud, bakuchi seed, dichroa root, kochia seed, kanta kari, bushy knotweed rhizome, arjun, babul chall bark, opopanax and bhumy amalaki; optionally one or more of frankincense, garcinia fruit, vitex, dragons blood, mace, sage and red sandalwood with at least c) one compound capable of maximizing oxidative mitochondrial function preferably riboflavin or vitamin B2 derivatives, FAD, FMN, 5-amino-6-(5′-phosphoribitylamino)uracil, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, niacin, vitamin C and pantothenate and / or d) at least one lactic acid dehydrogenase inhibitor (preferably 2′,3,4′5,7-pentahydroxyflavone) and optionally f) an alkalizing agent (aloe vera, chlorella, wheat grass, sodium or potassium bicarbonate, potassium) g) an antiproliferative herb (speranskia or goldenseal) and h) a pharmaceutically acceptable carrier.
Owner:MAZZIO ELIZABETH +1
Lactam compounds and their use as pharmaceuticals
InactiveUS20060116382A1Avoid conversionInhibit productionBiocideSenses disorderDiseaseMineralocorticoid receptor
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
Inhibitors of 11-beta hydroxysteroid dehydrogenase type I
InactiveUS20060235028A1Inhibitory activityBiocideNervous disorder11-beta-Hydroxysteroid DehydrogenasesCompound (substance)
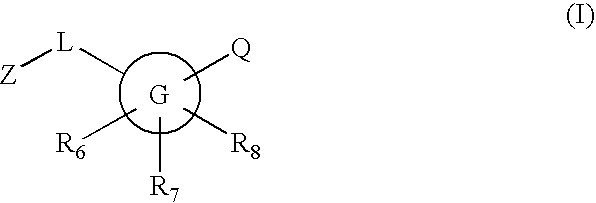
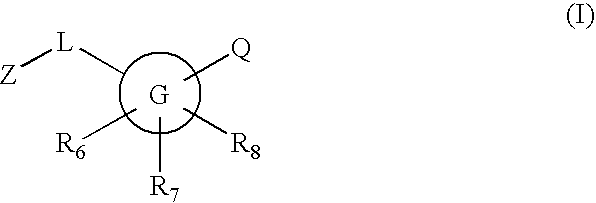
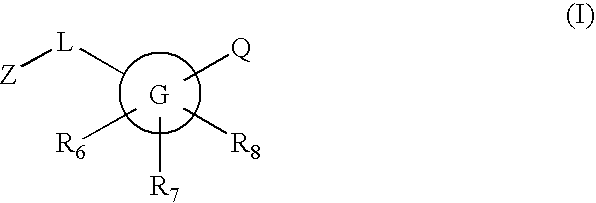
Novel compounds are provided which are 11-beta-hydroxysteroid dehydrogenase type I inhibitors. 11-beta-hydroxysteroid dehydrogenase type I inhibitors are useful in treating, preventing, or slowing the progression of diseases requiring 11-beta-hydroxysteroid dehydrogenase type I inhibitor therapy. These novel compounds have the structure: or stereoisomers or prodrugs or pharmaceutically acceptable salts thereof, wherein G, L, Q, Z, R6, R7, and R8 are defined herein.
Owner:BRISTOL MYERS SQUIBB CO
Circulating mRNA as diagnostic markers
Methods and kits are provided for diagnosing, monitoring, or predicting the conditions of pre-eclaimpsia, fetal chromosomal aneuploidy, and pre-term labor in a pregnant woman, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more mRNA species encoding human chorionic gonadotropin β subunit (hCG-β), human placental lactogen (hPL), human corticotropin releasing hormone (hCRH), KiSS-1 metastasis-suppressor (KISS1), tissue factor pathway inhibitor 2 (TPFI2), placenta-specific 1 (PLAC1), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and comparing the amount of the mRNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Method for producing L-methionine by fermentation
InactiveUS7611873B1High activityIncrease productivityBacteriaSugar derivativesSerine dehydrogenaseThreonine
L-Methionine is produced by culturing a microorganism which is deficient in repressor of L-methionine biosynthesis system and / or enhanced intracellular homoserine transsuccinylase activity is cultured in a medium so that L-methionine should be produced and accumulated in the medium, and collecting the L-methionine from the medium. The microorganism preferably further exhibits reduced intracellular S-adenosylmethionine synthetase activity, L-threonine auxotrophy, enhanced intracellular cystathionine γ-synthase activity and enhanced intracellular aspartokinase-homoserine dehydrogenase II activity. The present invention enables breeding of L-methionine-producing bacteria, and L-methionine production by fermentation.
Owner:AJINOMOTO CO INC
Fused heterotricyclic compounds as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
Owner:BRISTOL MYERS SQUIBB CO
Amido compounds and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE HLDG CORP
L-threonine producing bacterium belonging to the genus Escherichia and method for producing L-threonine
Owner:AJINOMOTO CO INC
Combination therapy using an 11beta-hydroxysteroid dehydrogenase type 1 inhibitor and an antihypertensive agent for the treatment of metabolic syndrome and related diseases and disorders
InactiveUS20060111348A1Reduce riskReduce development riskBiocideMetabolism disorderDyslipidemiaPancreatic hormone
Combination therapy comprising the administration of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor and an antihypertensive agent useful for treating, preventing and reducing the risk of developing insulin resistance, dyslipidemia, obesity, hypertension and other related diseases and disorders.
Owner:VTV THERAPEUTICS LLC
E. coli transformant, method for producing flavin-bound glucose dehydrogenase using the same, and mutant flavin-bound glucose dehydrogenases
ActiveUS20130309750A1Accurate measurementEfficiently obtainedBacteriaOxidoreductasesEscherichia coliMicroorganism
A flavin-bound glucose dehydrogenase (FAD-GDH) with high substrate specificity for D-glucose. A gene encoding a mutant FAD-GDH with its N-terminal region, containing an amino acid sequence corresponding to MKITAAIITVATAFASFASA that exists in the N-terminal region, deleted from the amino acid sequence of a wild-type FAD-GDH derived from Mucor is introduced into E. coli to obtain an E. coli transformant. Subsequently, this E. coli transformant is cultured to obtain an FAD-GDH with a specific N-terminal region deleted. The transformant allows the production of a large amount of GDH in a short time as compared with the original microorganism. An FAD-GDH that is less susceptible to the effects of dissolved oxygen and allows accurate measurement of glucose even in the presence of sugar compounds other than glucose in a sample.
Owner:KIKKOMAN CORP
Deletion mutants for the production of isobutanol
An E. coli host strain was engineered wherein genes adhE, IdhA, frdB, and pfIB were disrupted and novel butanol dehydrogenase gene, sadB, from Achromobacter xylosoxidans, was added to produce the isobutanol production host.
Owner:BUTAMAXTM ADVANCED BIOFUELS
Glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate mutase regulatory sequences for gene expression in oleaginous yeast
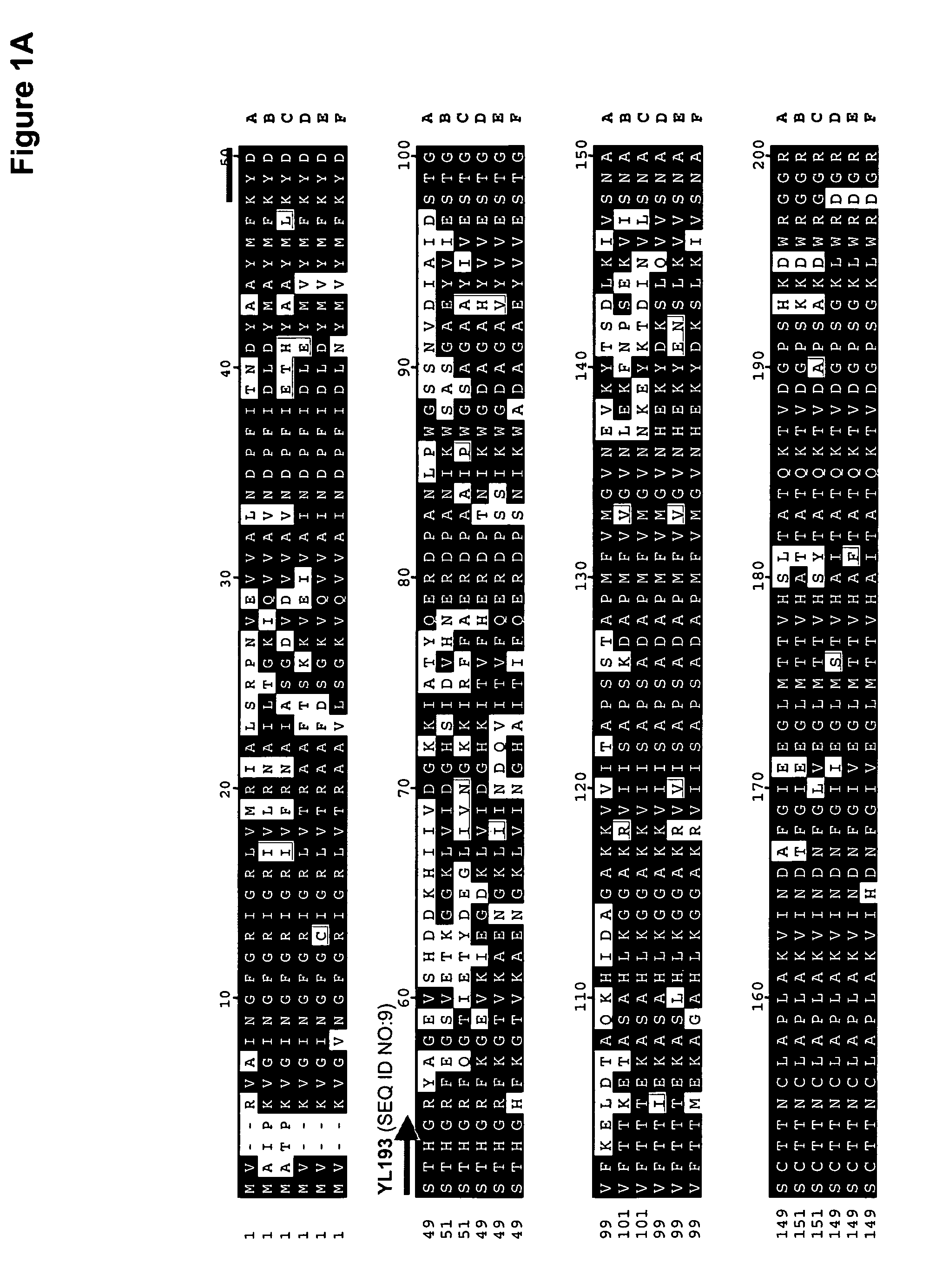
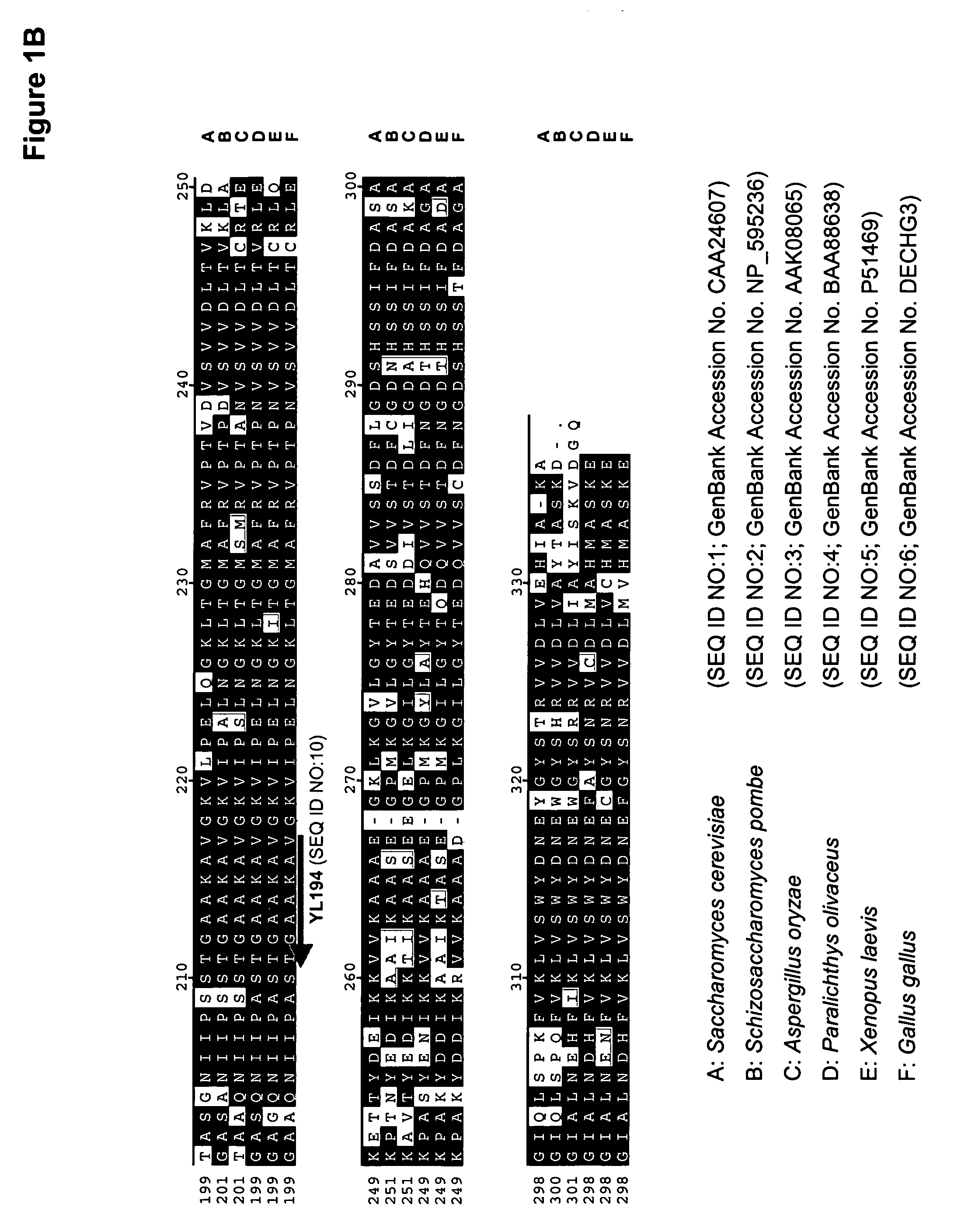
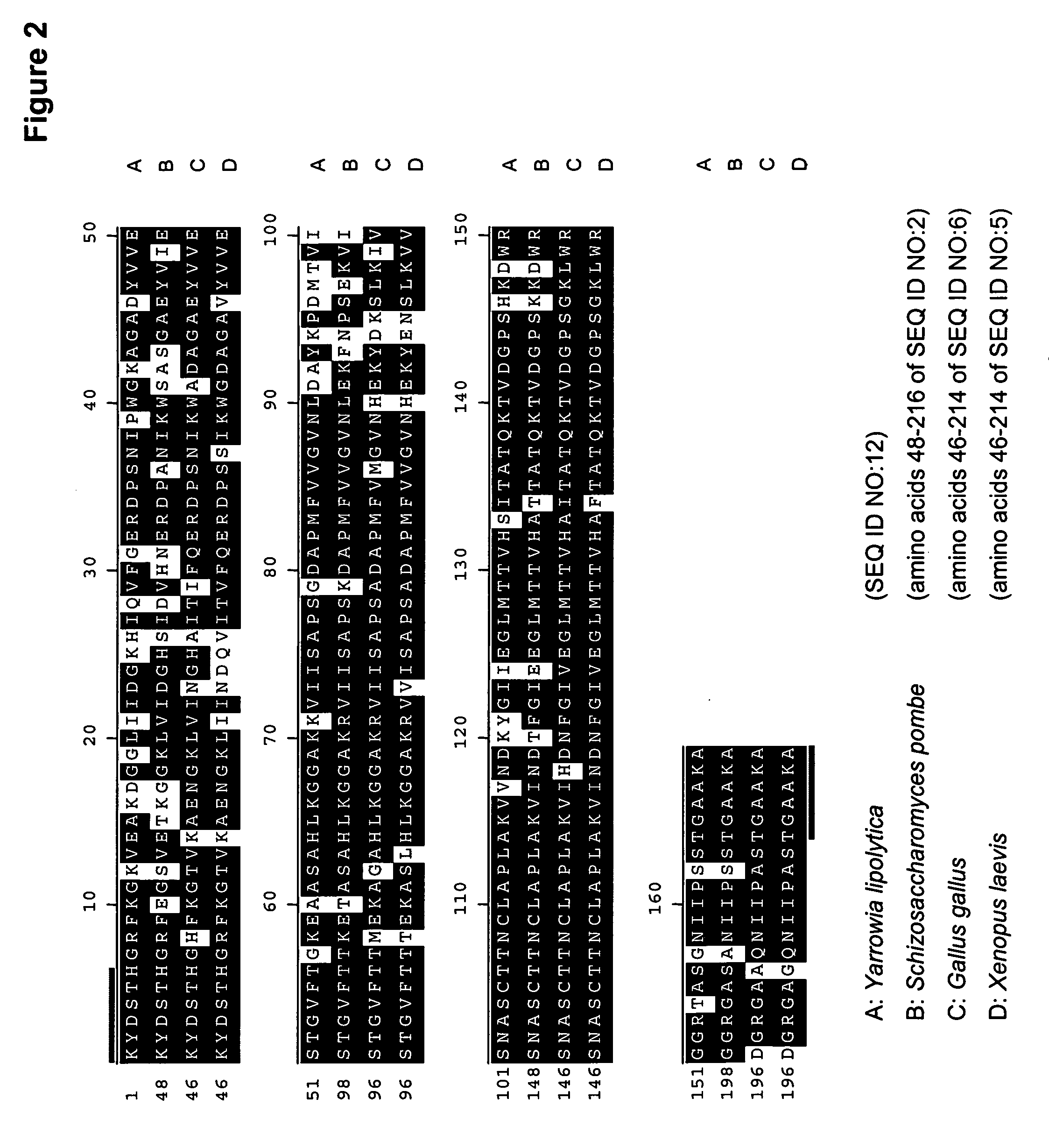
The regulatory sequences associated with the Yarrowia lipolytica glyceraldehyde-3-phosphate dehydrogenase (gpd) and phosphoglycerate mutase (gpm) genes have been found to be particularly effective for the expression of heterologous genes in oleaginous yeast. The promoter regions of the invention, intron and enhancer have been shown to drive high-level expression of genes involved in the production of ω-3 and ω-6 fatty acids.
Owner:EI DU PONT DE NEMOURS & CO
17Beta-Hydroxysteroid Dehydrogenase Type 1 Inhibitors for the Treatment of Hormone-Related Diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
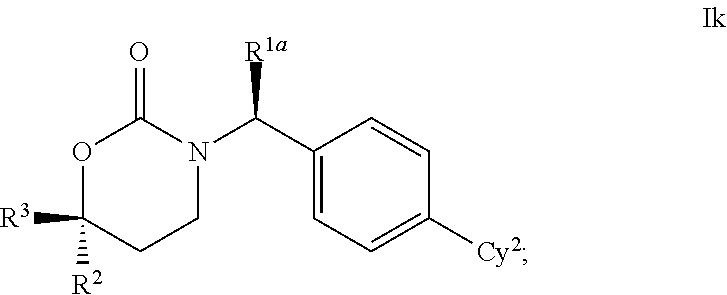
Cyclic Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase 1
This invention relates to novel compounds of the Formula Il Ik, Im3, Im4, Im6-12, In3, In4, In6-12, lo3, lo4, lo6-12, Ip2, Ip4-7, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of Cortisol in a cell or the inhibition of the conversion of cortisone to Cortisol in a cell.
Owner:BOEHRINGER INGELHEIM INT GMBH +1
Methods and materials for identifying polymorphic variants, diagnosing susceptibilities, and treating disease
InactiveUS20080213775A1Increased susceptibilitySugar derivativesMicrobiological testing/measurementDiseaseCarbon metabolism
The invention is directed to materials and methods associated with polymorphic variants in two enzymes involved in folate-dependent and one-carbon metabolic pathways: MTHFD1 (5,10-methylenetetrahydrofolate dehydrogenase, 5,10-methenyltetrahydrofolate cyclohydrolase, 10-formyltetrahydrofolate synthetase) and methylenetetrahydrofolate dehydrogenase (NADP+dependent) 1-like (MTHFD1L). Diagnostic and therapeutic methods are provided involving the correlation of polymorphic variants in MTBFD1, MTHFD1, and other genes with relative susceptibility for various pregnancy-related and other complications.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC +2
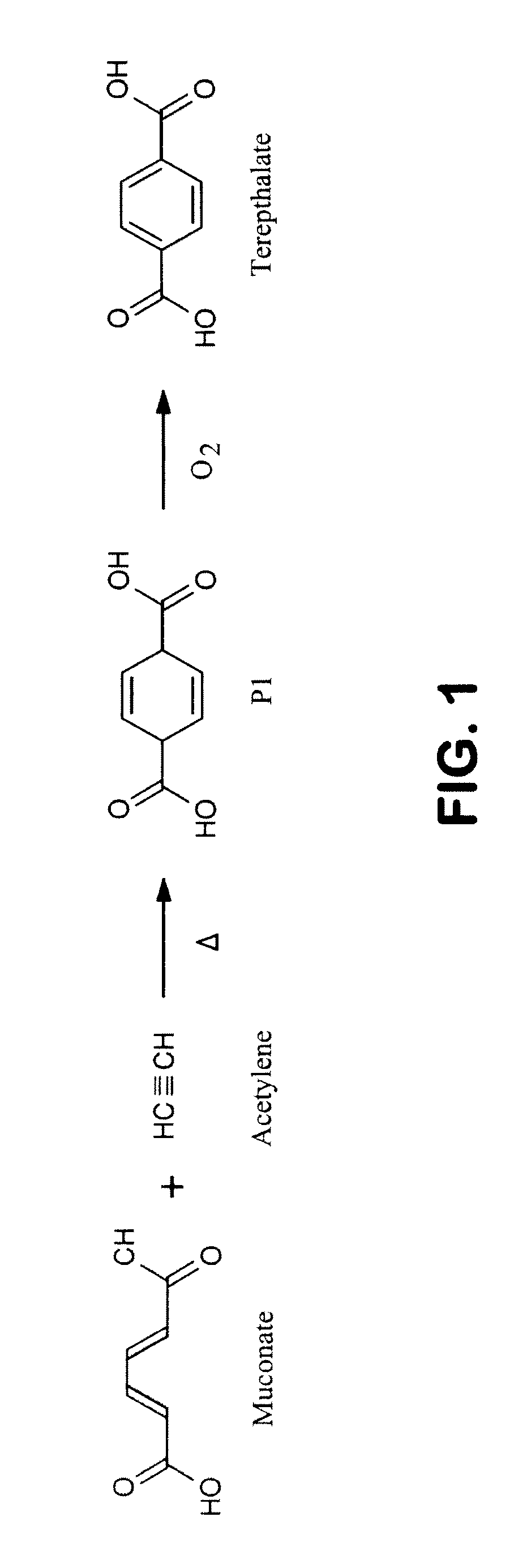
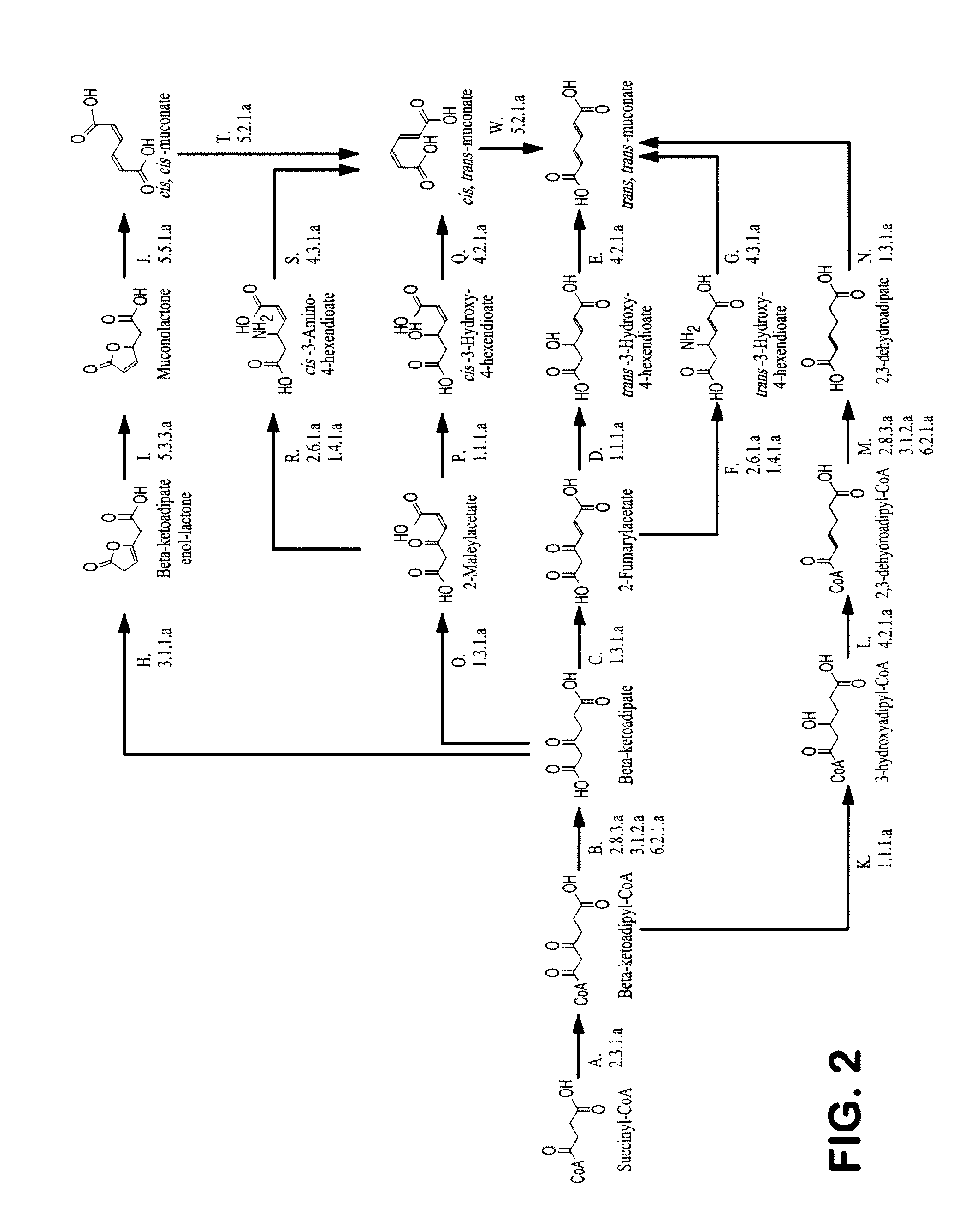
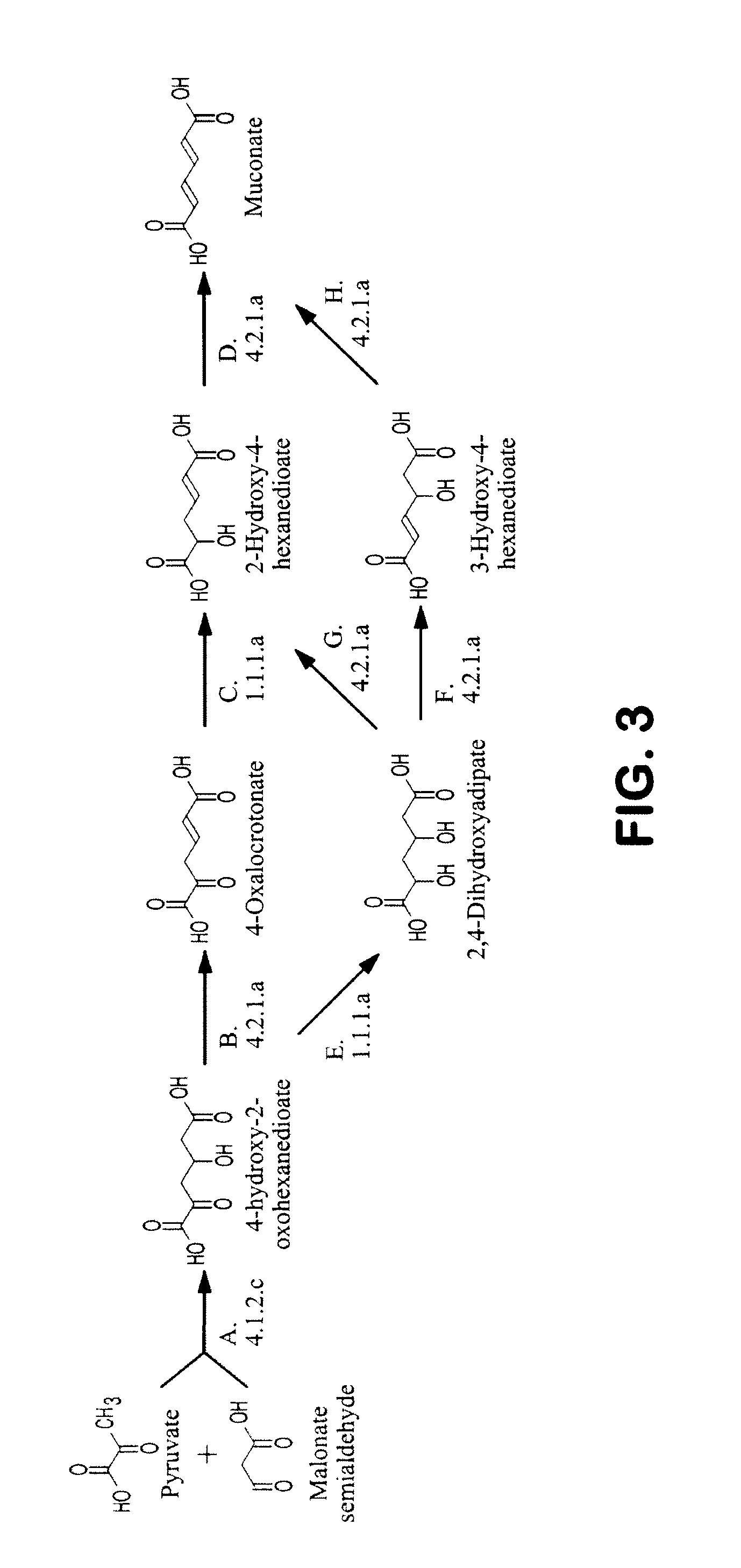
Semi-synthetic terephthalic acid via microorganisms that produce muconic acid
The invention provides a non-naturally occurring microbial organism having a muconate pathway having at least one exogenous nucleic acid encoding a muconate pathway enzyme expressed in a sufficient amount to produce muconate. The muconate pathway including an enzyme selected from the group consisting of a beta-ketothiolase, a beta-ketoadipyl-CoA hydrolase, a beta-ketoadipyl-CoA transferase, a beta-ketoadipyl-CoA ligase, a 2-fumarylacetate reductase, a 2-fumarylacetate dehydrogenase, a trans-3-hydroxy-4-hexendioate dehydratase, a 2-fumarylacetate aminotransferase, a 2-fumarylacetate aminating oxidoreductase, a trans-3-amino-4-hexenoate deaminase, a beta-ketoadipate enol-lactone hydrolase, a muconolactone isomerase, a muconate cycloisomerase, a beta-ketoadipyl-CoA dehydrogenase, a 3-hydroxyadipyl-CoA dehydratase, a 2,3-dehydroadipyl-CoA transferase, a 2,3-dehydroadipyl-CoA hydrolase, a 2,3-dehydroadipyl-CoA ligase, a muconate reductase, a 2-maleylacetate reductase, a 2-maleylacetate dehydrogenase, a cis-3-hydroxy-4-hexendioate dehydratase, a 2-maleylacetate aminoatransferase, a 2-maleylacetate aminating oxidoreductase, a cis-3-amino-4-hexendioate deaminase, and a muconate cis / trans isomerase. Other muconate pathway enzymes also are provided. Additionally provided are methods of producing muconate.
Owner:GENOMATICA INC
Inhibitors of 11-beta hydroxyl steroid dehydrogenase type I and methods of using the same
InactiveUS20060122210A1Improve actionReduce releaseBiocideSenses disorderDiseaseMineralocorticoid receptor
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
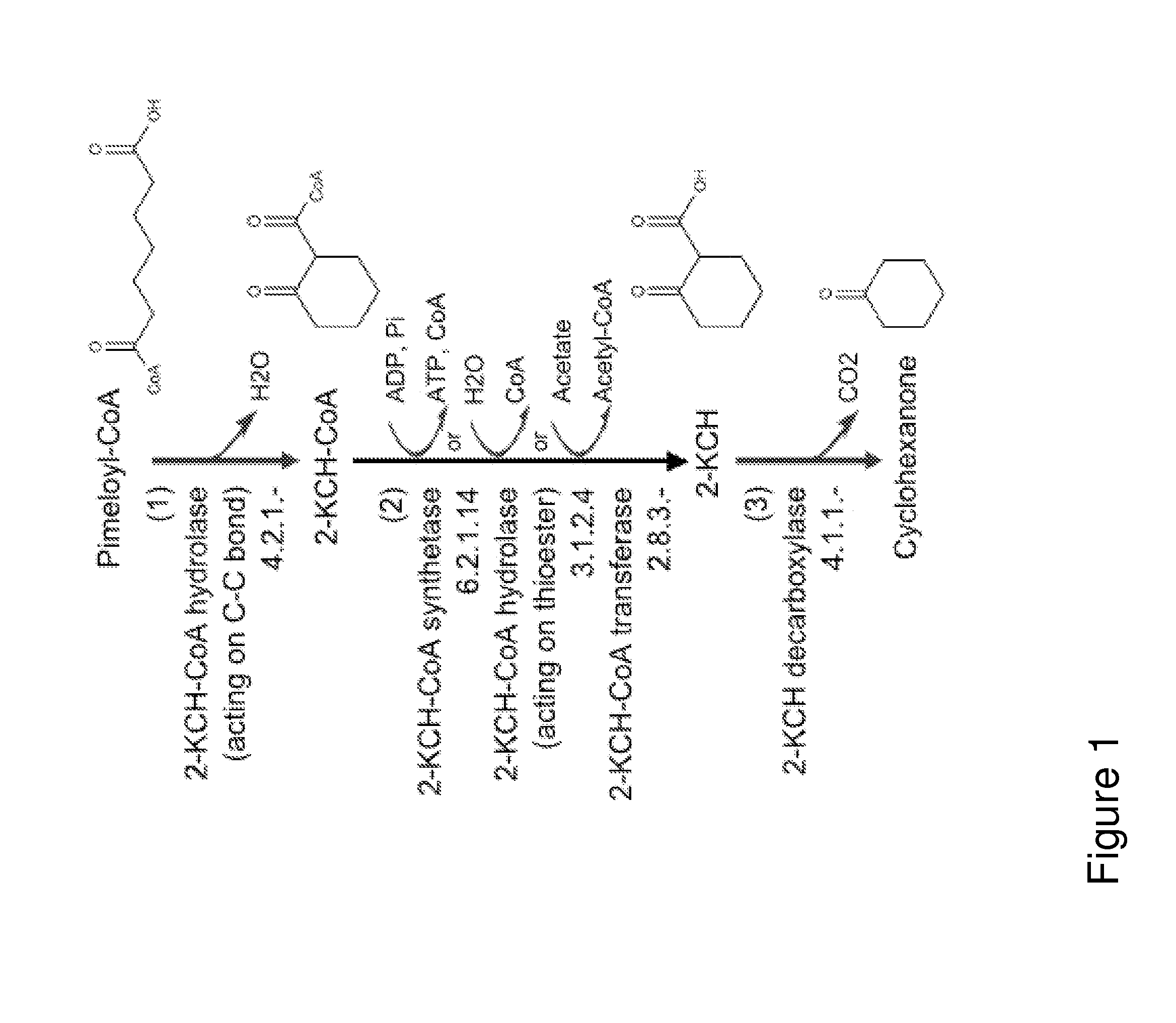
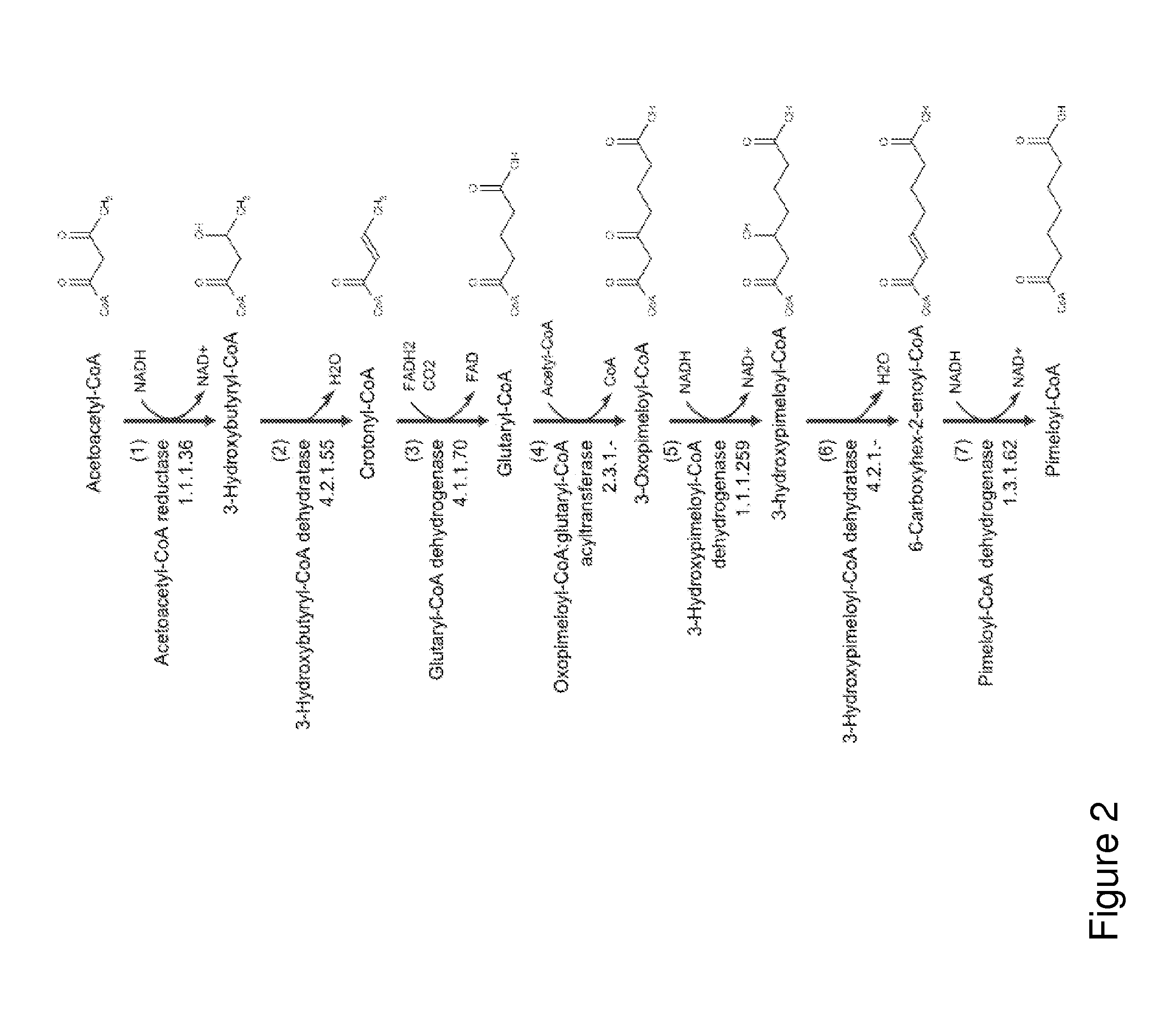
Organisms for the production of cyclohexanone
A non-naturally occurring microbial organism has cyclohexanone pathways that include at least one exogenous nucleic acid encoding a cyclohexanone pathway enzyme. A pathway includes a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on C—C bond), a 2-ketocyclohexane-1-carboxylate decarboxylase and an enzyme selected from a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxyl-CoA transferase, and a 2-ketocyclohexane-1-carboxyl-CoA synthetase. A pathway includes an enzyme selected from a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on C—C bond), a 6-ketocyclohex-1-ene-1-carboxyl-CoA synthetase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA hydrolase (acting on thioester), a 6-ketocyclohex-1-ene-1-carboxyl-CoA transferase, a 6-ketocyclohex-1-ene-1-carboxyl-CoA reductase, a 6-ketocyclohex-1-ene-1-carboxylate decarboxylase, a 6-ketocyclohex-1-ene-1-carboxylate reductase, a 2-ketocyclohexane-1-carboxyl-CoA synthetase, a 2-ketocyclohexane-1-carboxyl-CoA transferase, a 2-ketocyclohexane-1-carboxyl-CoA hydrolase (acting on thioester), a 2-ketocyclohexane-1-carboxylate decarboxylase, and a cyclohexanone dehydrogenase. A pathway includes an adipate semialdehyde dehydratase, a cyclohexane-1,2-diol dehydrogenase, and a cyclohexane-1,2-diol dehydratase. A pathway includes a 3-oxopimelate decarboxylase, a 4-acetylbutyrate dehydratase, a 3-hydroxycyclohexanone dehydrogenase, a 2-cyclohexenone hydratase, a cyclohexanone dehydrogenase and an enzyme selected from a 3-oxopimeloyl-CoA synthetase, a 3-oxopimeloyl-CoA hydrolase (acting on thioester), and a 3-oxopimeloyl-coA transferase. Each these pathways can include a PEP carboxykinase. A method for producing cyclohexanone includes culturing these non-naturally occurring microbial organisms.
Owner:GENOMATICA INC
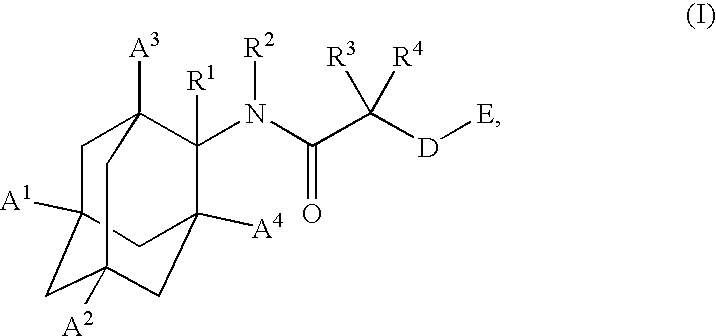
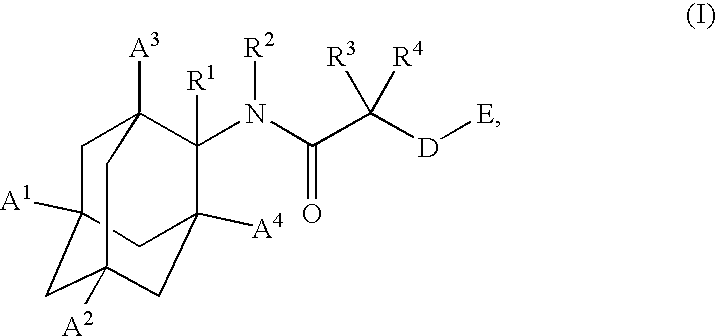
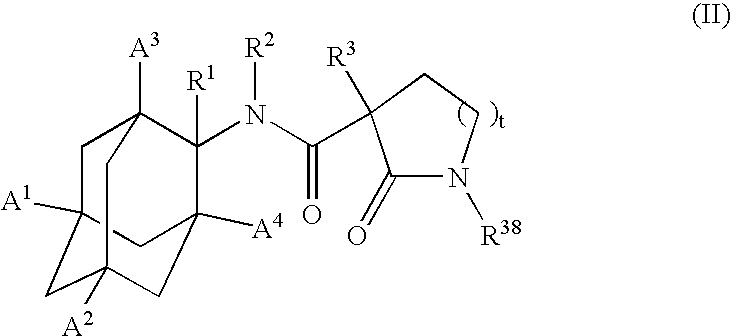
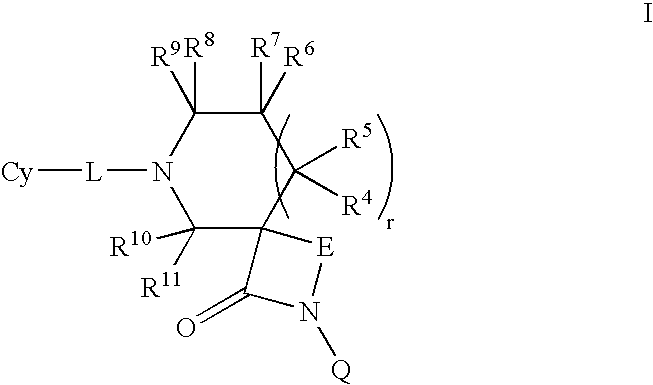
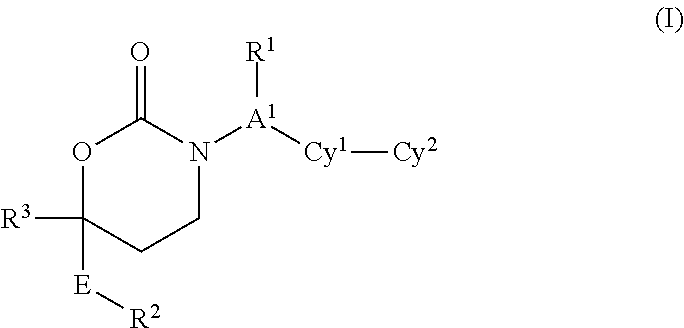
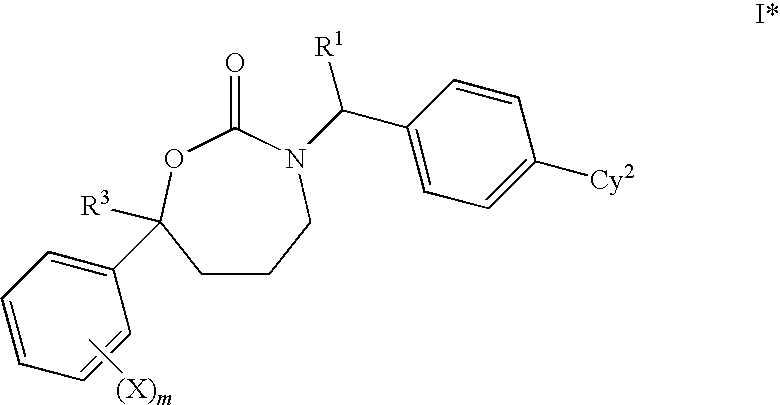
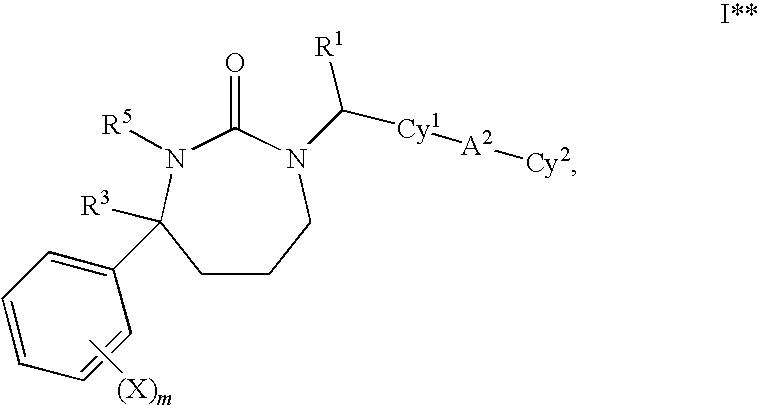
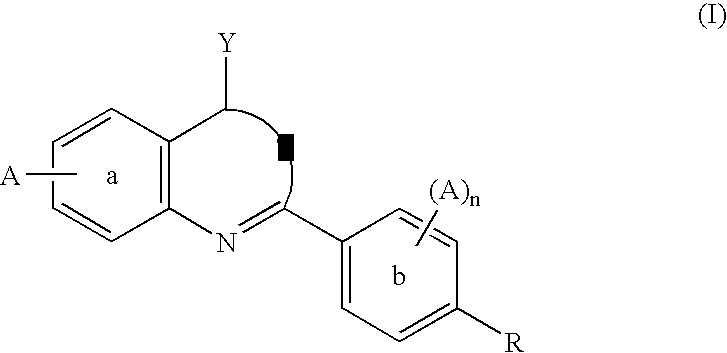
1,3-Oxazepan-2-one and 1,3-diazepan-2-one inhibitors of 11ß-hydroxysteroid dehydrogenase 1
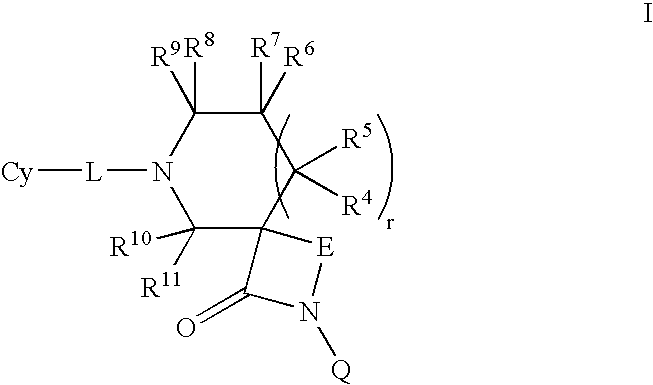
This invention relates to novel compounds of the Formula (I), (I*), (I**), I, Ia, Ib, Ic, Id, Ie, If, Ig, Il1-3, Im1-3, In1-3, or Io1-2, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC
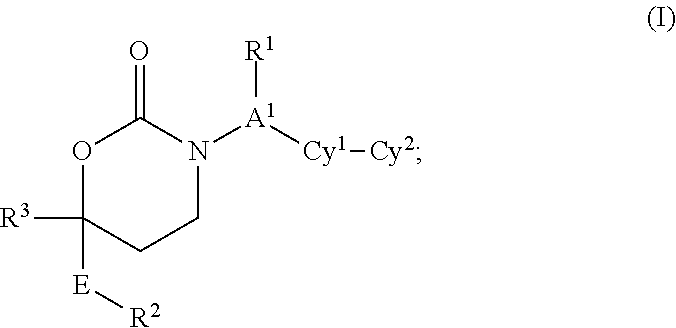
Cyclic Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase 1
This invention relates to novel compounds of the Formula I, Ik, Iq1-21, Ir1-21, Is1-21, It1-7, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11 β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC +1
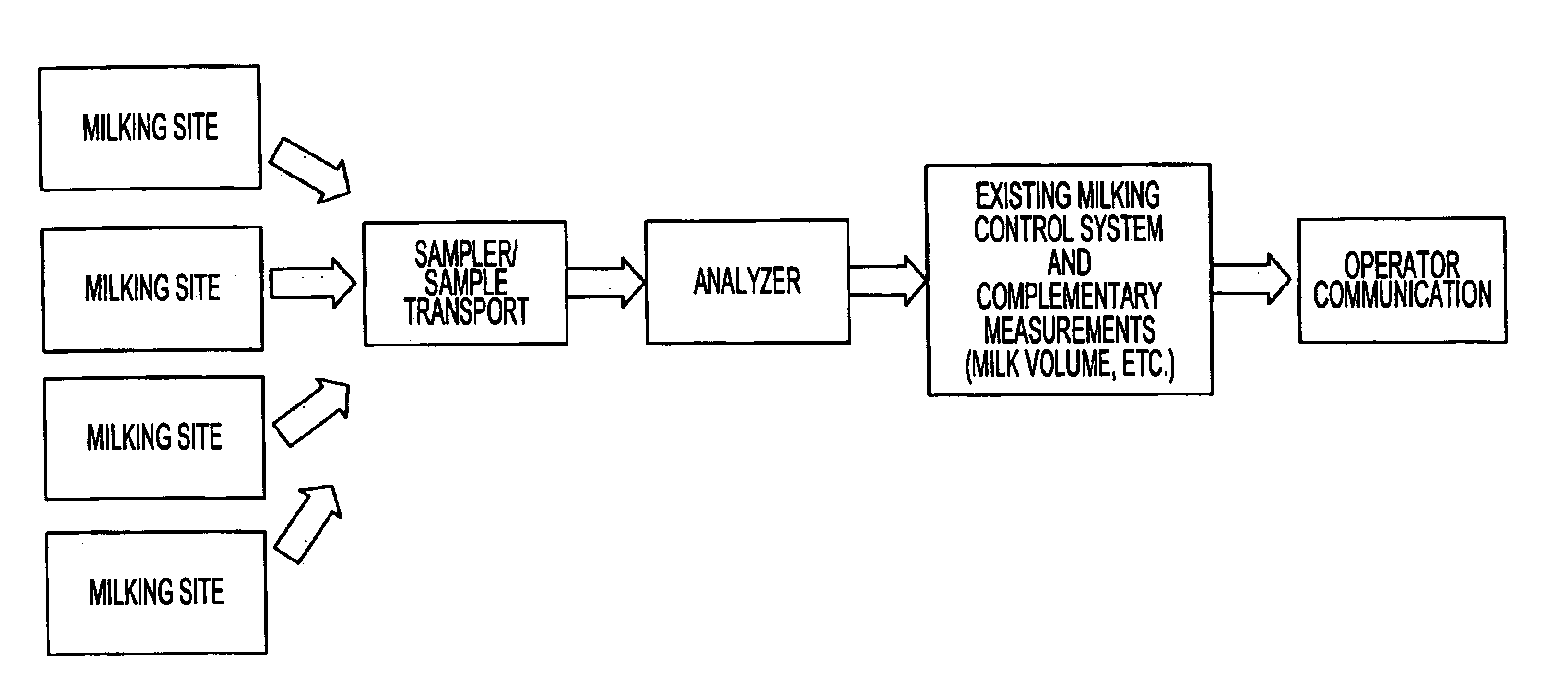
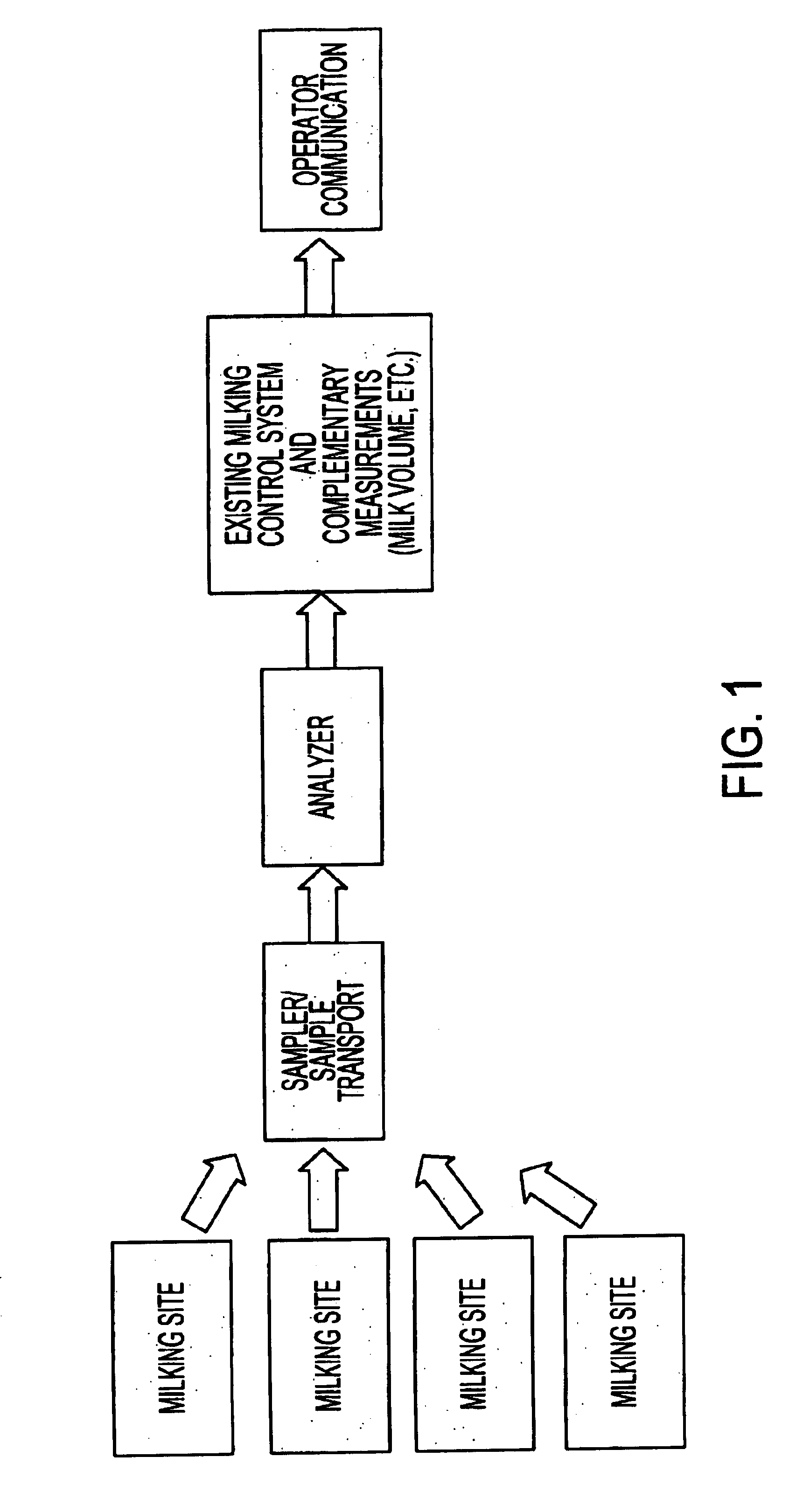
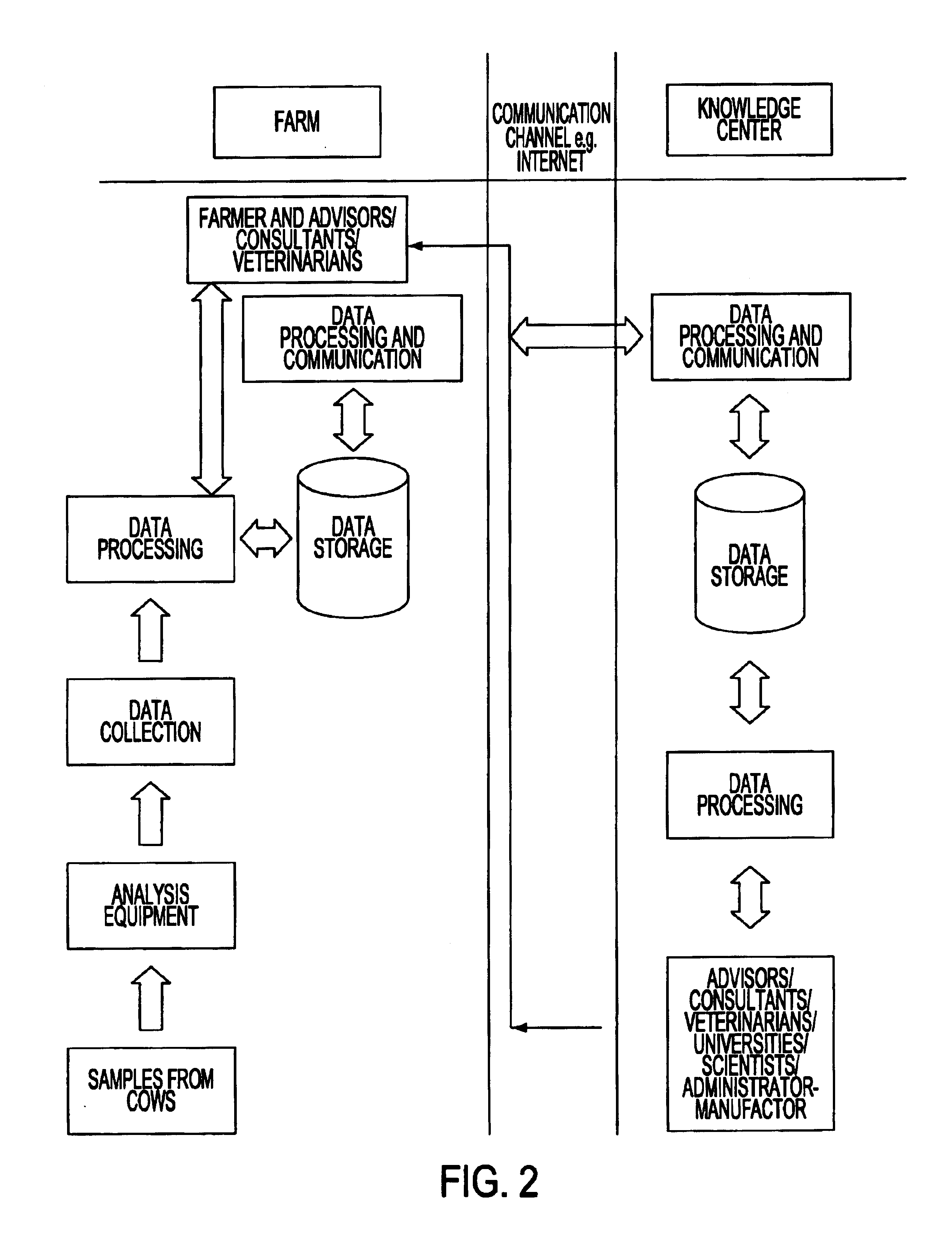
System for optimizing the production performance of a milk producing animal herd
InactiveUS6814025B2Increase productivityIncrease profitabilitySamplingCathetersLactate dehydrogenaseAgricultural science
Owner:LATTEC
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Tetrasubstituted ureas as modulators of 11-beta hydroxyl steroid dehydrogenase type 1
InactiveUS20070293529A1Avoid conversionInhibit productionUrea derivatives preparationBiocideSubstituted ureaPharmaceutical Substances
The present invention relates to tetra-substituted urea compounds which are modulators of 11-β hydroxyl steroid dehydrogenase type 1 (11βHSD1), their pharmaceutical compositions, and methods of using the same.
Owner:INCYTE
Cyclic inhibitors of 11β-hydroxysteroid dehydrogenase 1
This invention relates to novel compounds of a structural formula selected from:pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals, e.g., diabetes mellitus and obesity. Values for the variables in the structural formulas are provided herein. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of cortisol in a cell or the inhibition of the conversion of cortisone to cortisol in a cell.
Owner:VITAE PHARMA INC
Method for producing glucose dehydrogenase from aspergillus oryzae
ActiveUS20080014612A1Efficient productionStable productionSugar derivativesBacteriaAspergillus oryzaeMicrobiology
The present invention effectively produces glucose dehydrogenase derived from Aspergillus oryzae, and provides more practical glucose dehydrogenase. The invention makes it possible to efficiently produce glucose dehydrogenase and to obtain glucose dehydrogenase in more practical manner by using a glucose dehydrogenase gene isolated from Aspergillus oryzae.
Owner:TOYOBO CO LTD
17Beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of hormone-related diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
Cyclic urea inhibitors of 11beta-hydroxysteroid dehydrogenase 1
This invention relates to novel compounds of the Formula (I), (Ia), (Ib), (Ic), (Id), (Ie), (If), (Ig), (Ih)1 (Ij), (Ik), (Il1-3). (Im1-3), (In1-3), (Io1-2), (Ip1-6), (Iq1-6), (Ir1-6) and (Is1-2), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of Cortisol in a cell or the inhibition of the conversion of cortisone to Cortisol in a cell.
Owner:VITAE PHARMA INC
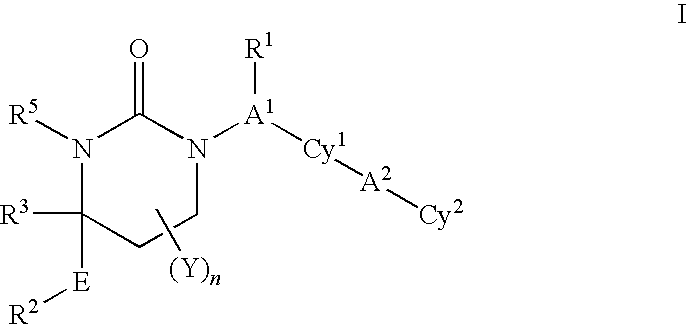
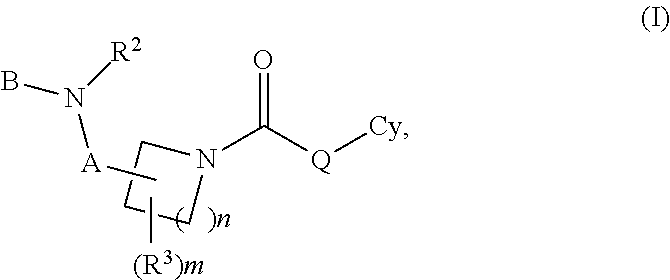
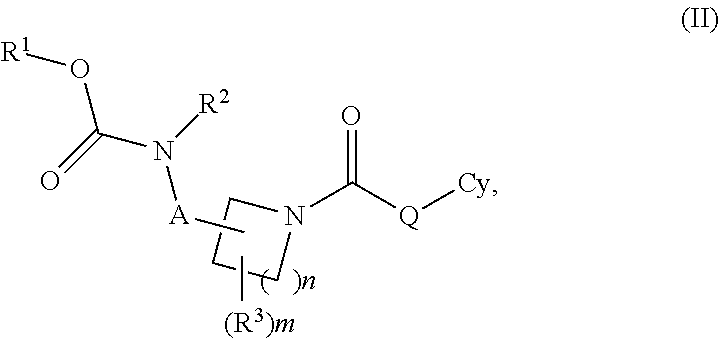
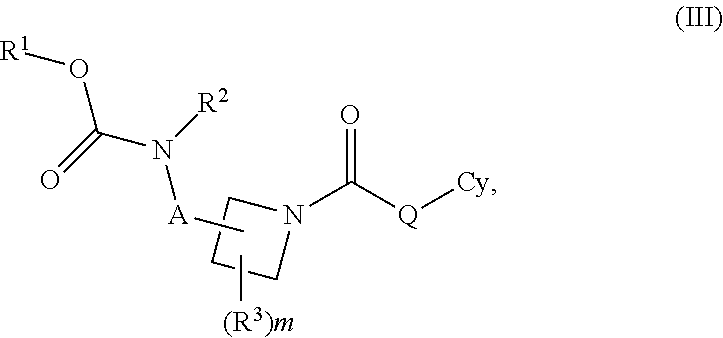
Carbamate And Urea Inhibitors Of 11Beta-Hydroxysteroid Dehydrogenase 1
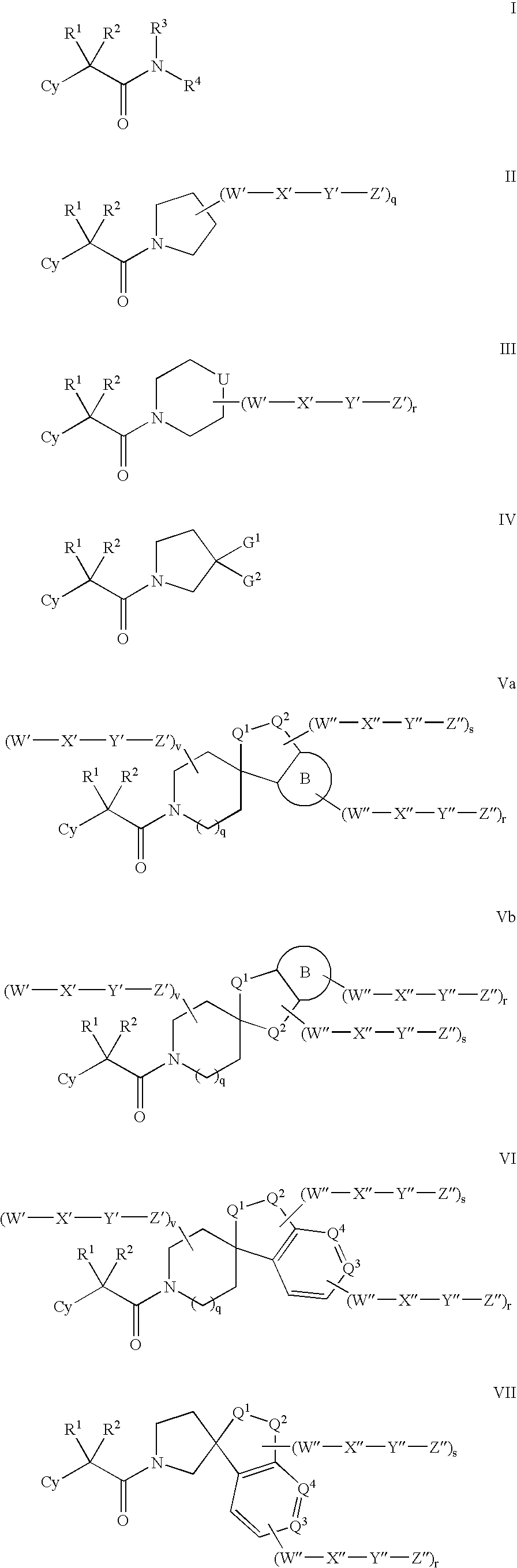
This invention relates to novel compounds of the Formula I, II, III, IHa, NIb, IV, IVa, IVb, IVc, IVd, IVe, V, Va, Vb1 V1, V1a, VIb, VII, Vi1a, VIIb, VIII, V111a, VIIIb, IX, IXa, X, and Xa, pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof, which are useful for the therapeutic treatment of diseases associated with the modulation or inhibition of 11 β-HSD1 in mammals. The invention further relates to pharmaceutical compositions of the novel compounds and methods for their use in the reduction or control of the production of Cortisol in a cell or the inhibition of the conversion of cortisone to Cortisol in a cell.
Owner:VITAE PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com