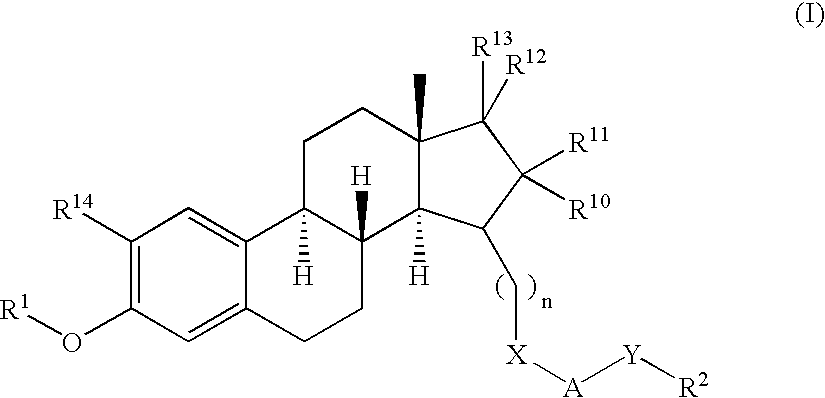
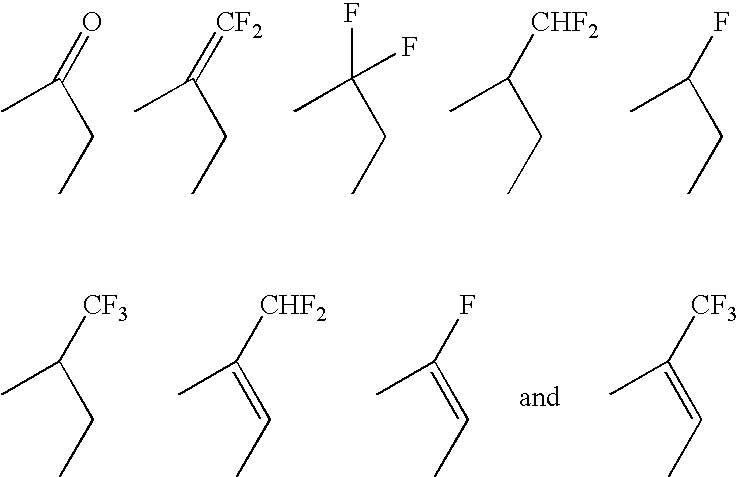
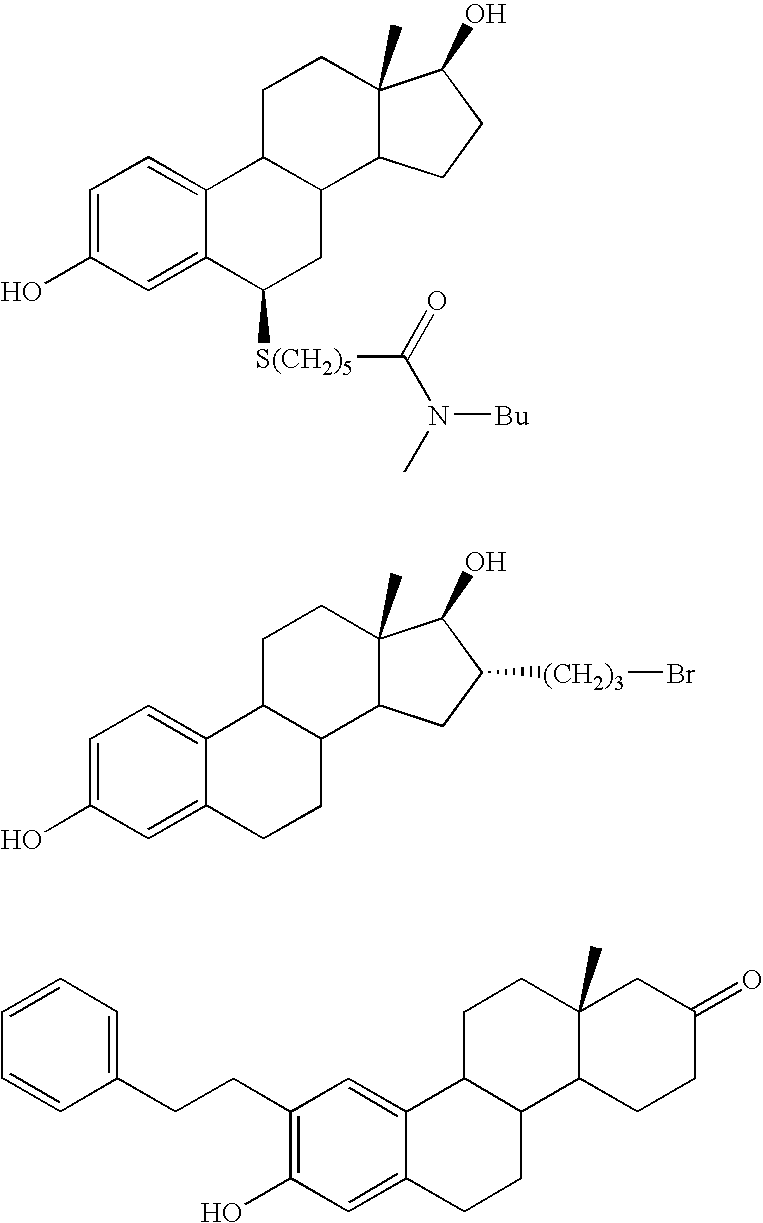
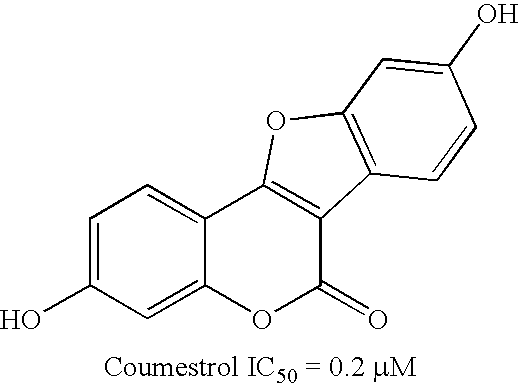
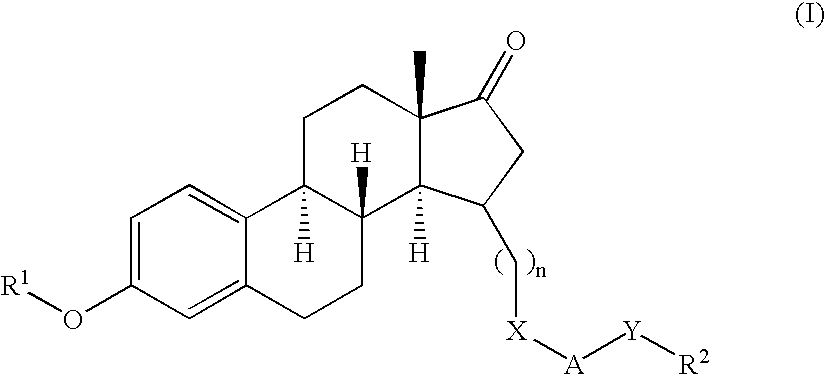
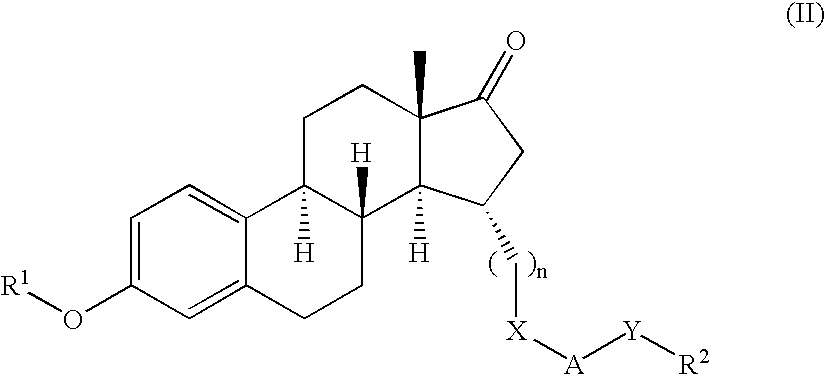
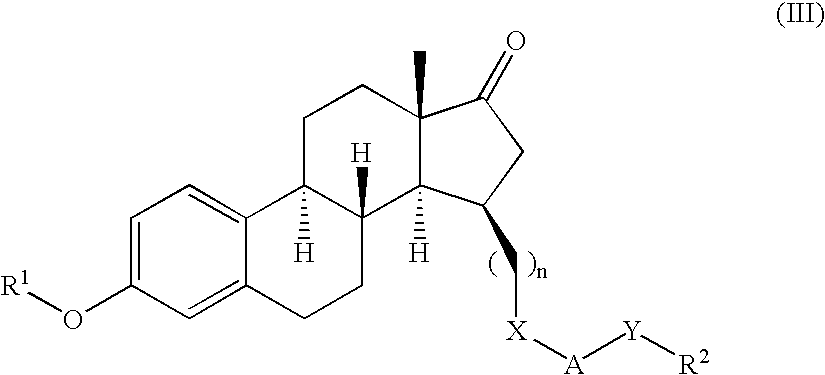
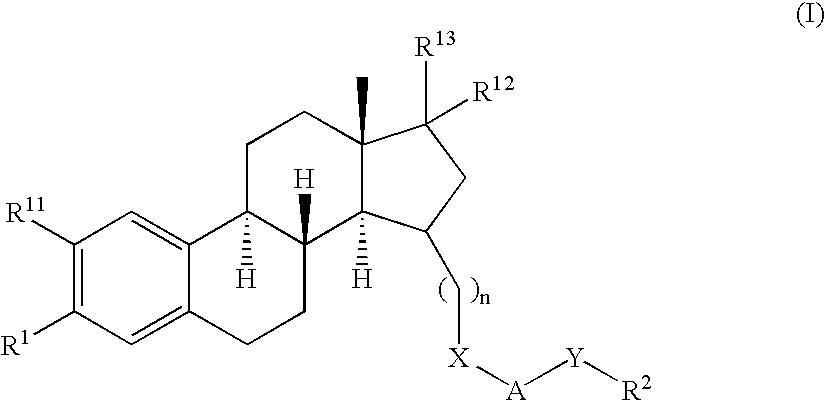
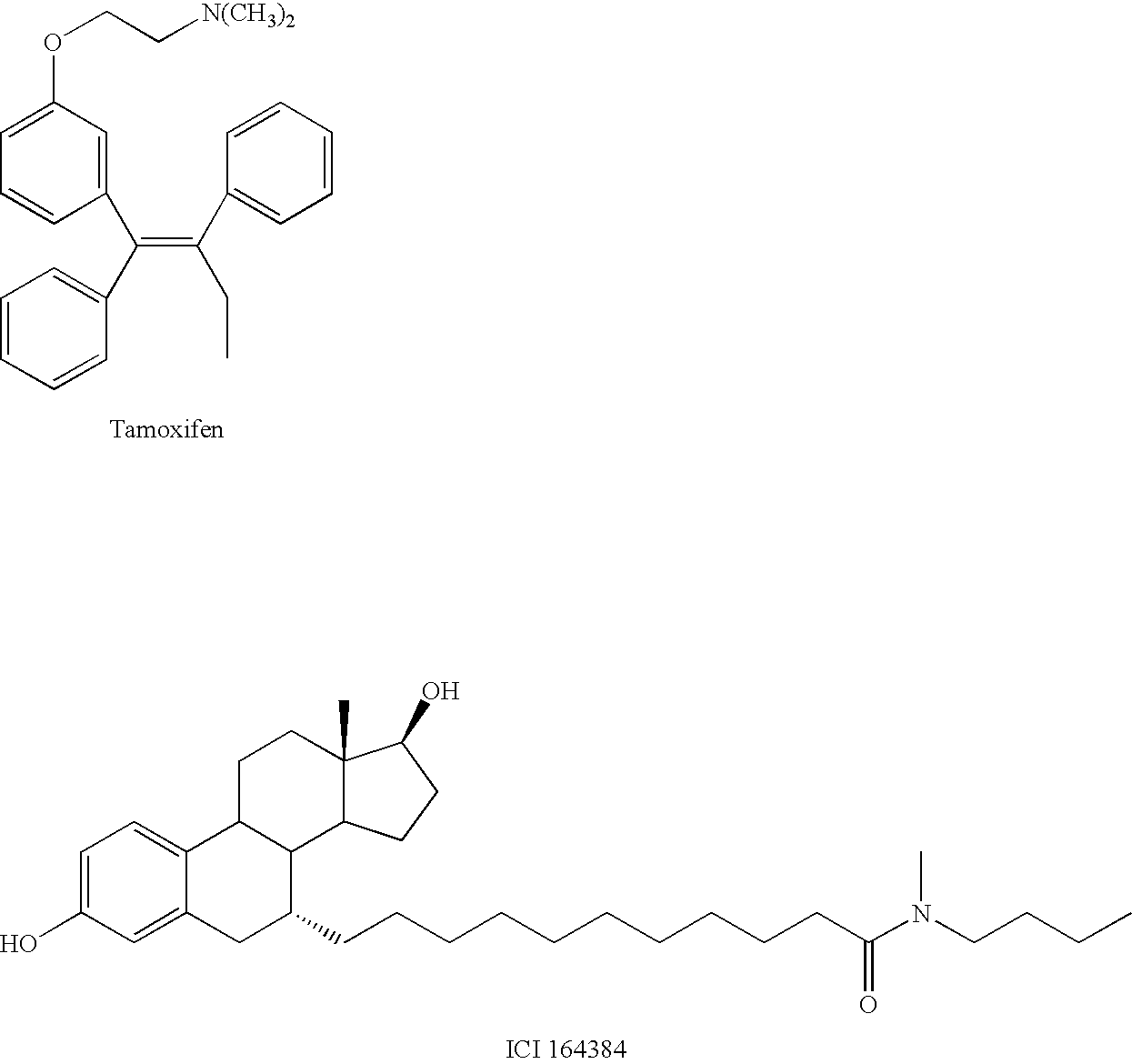
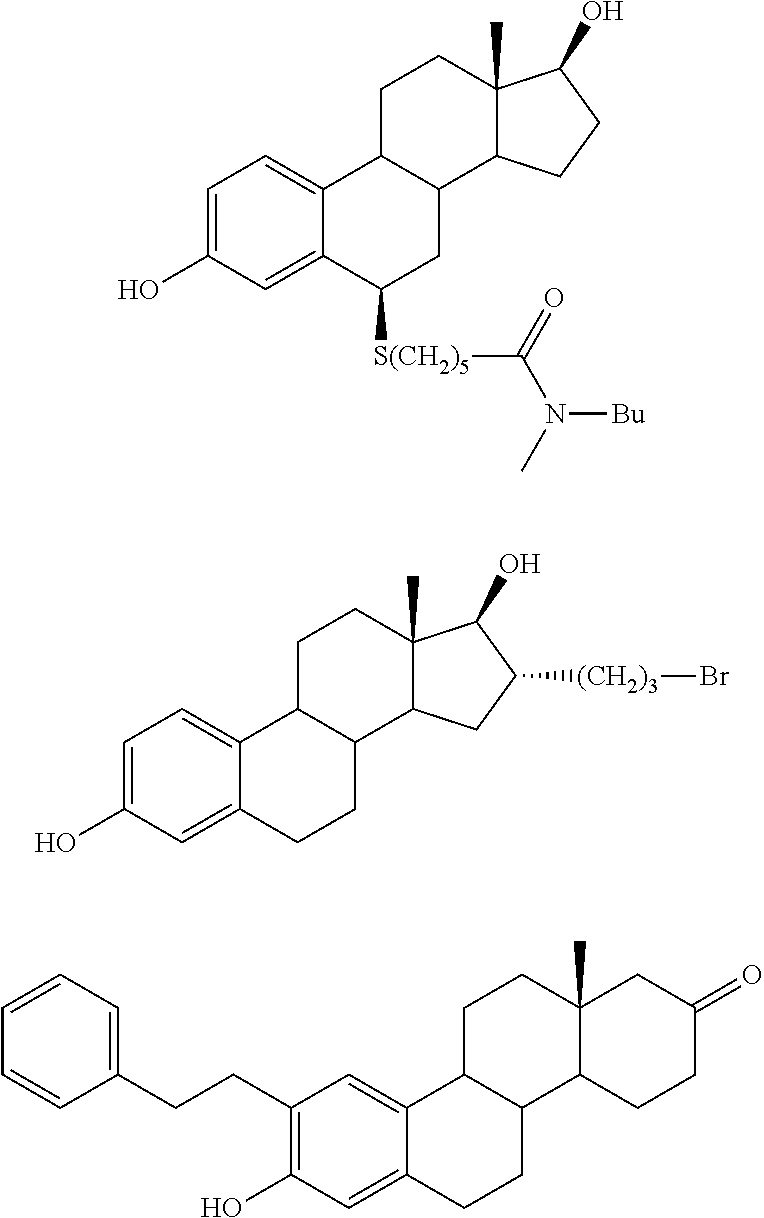
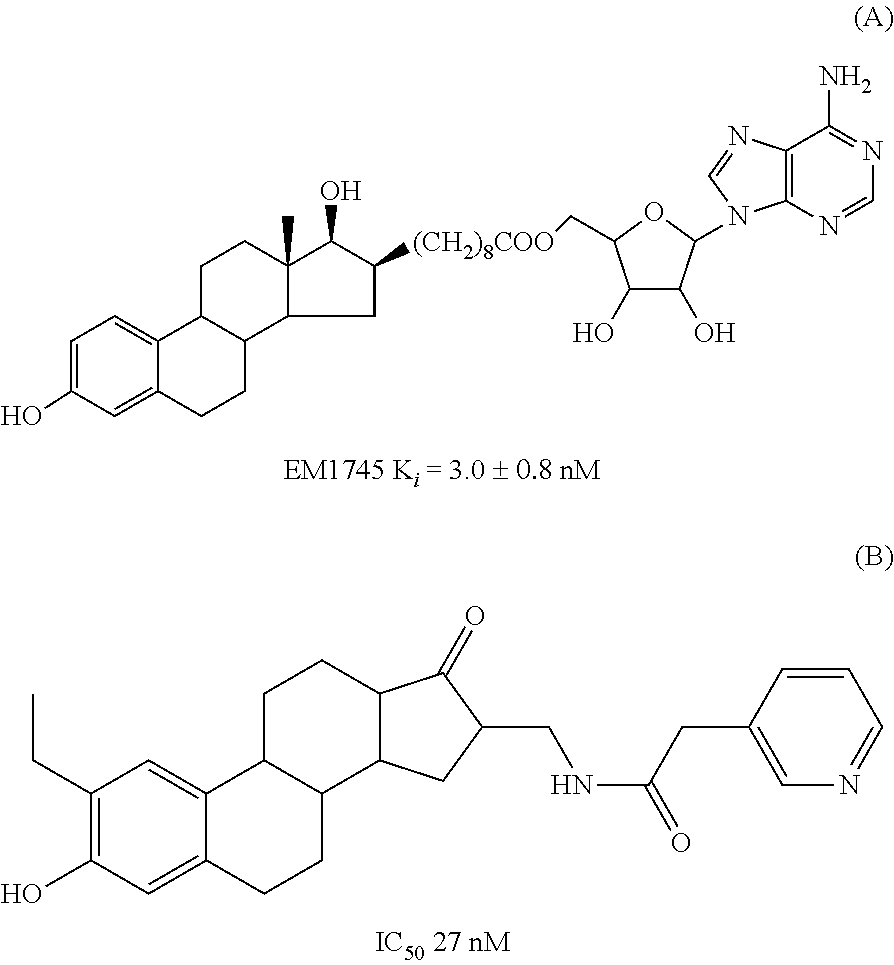
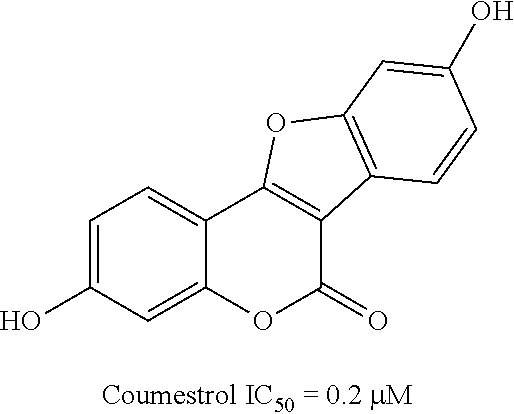
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "17beta-hydroxysteroid dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
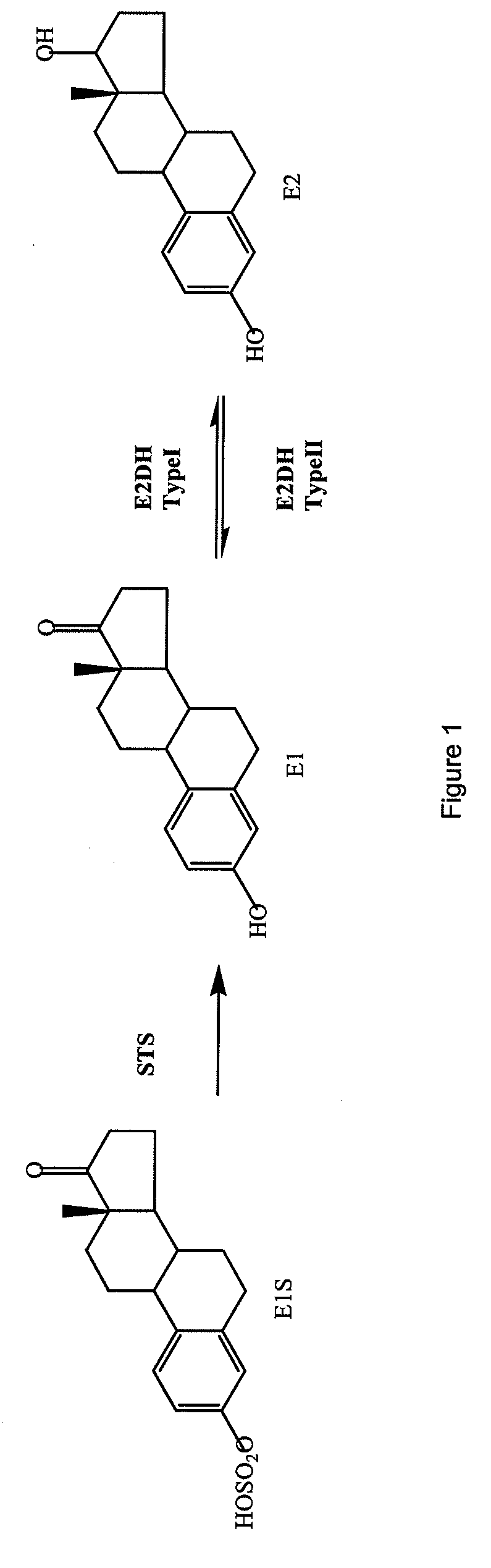
17β-Hydroxysteroid dehydrogenases (17β-HSD, HSD17B) (EC 1.1.1.51), also 17-ketosteroid reductases (17-KSR), are a group of alcohol oxidoreductases which catalyze the reduction of 17-ketosteroids and the dehydrogenation of 17β-hydroxysteroids in steroidogenesis and steroid metabolism. This includes interconversion of DHEA and androstenediol, androstenedione and testosterone, and estrone and estradiol.
Inhibitors of type 5 and type 3 17beta-hydroxysteroid dehydrogenase and methods for their use
Novel methods of medical treatment and / or inhibition of development of diseases are disclosed for diseases that are sensitive to androgenic or estrogenic activity. The treatments utilize inhibitors of type 5 and / or type 3 17beta-hydroxysteroid dehydrogenase. Novel inhibitors of type 5 17beta-hydroxysteroid dehydrogenase are also disclosed, as are novel inhibitors of type 3 17beta-hydroxysteroid dehydrogenase.
Owner:ENDORES & DEV
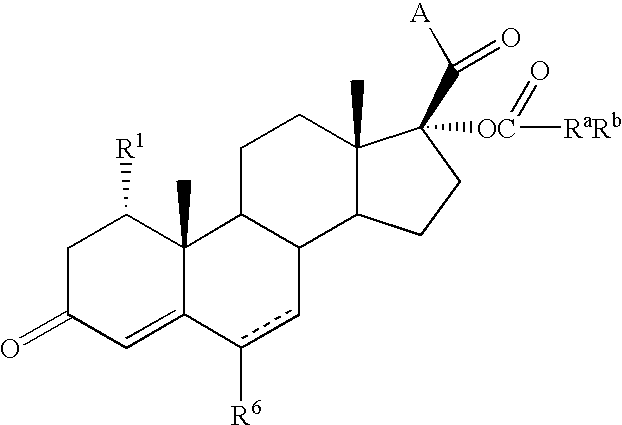
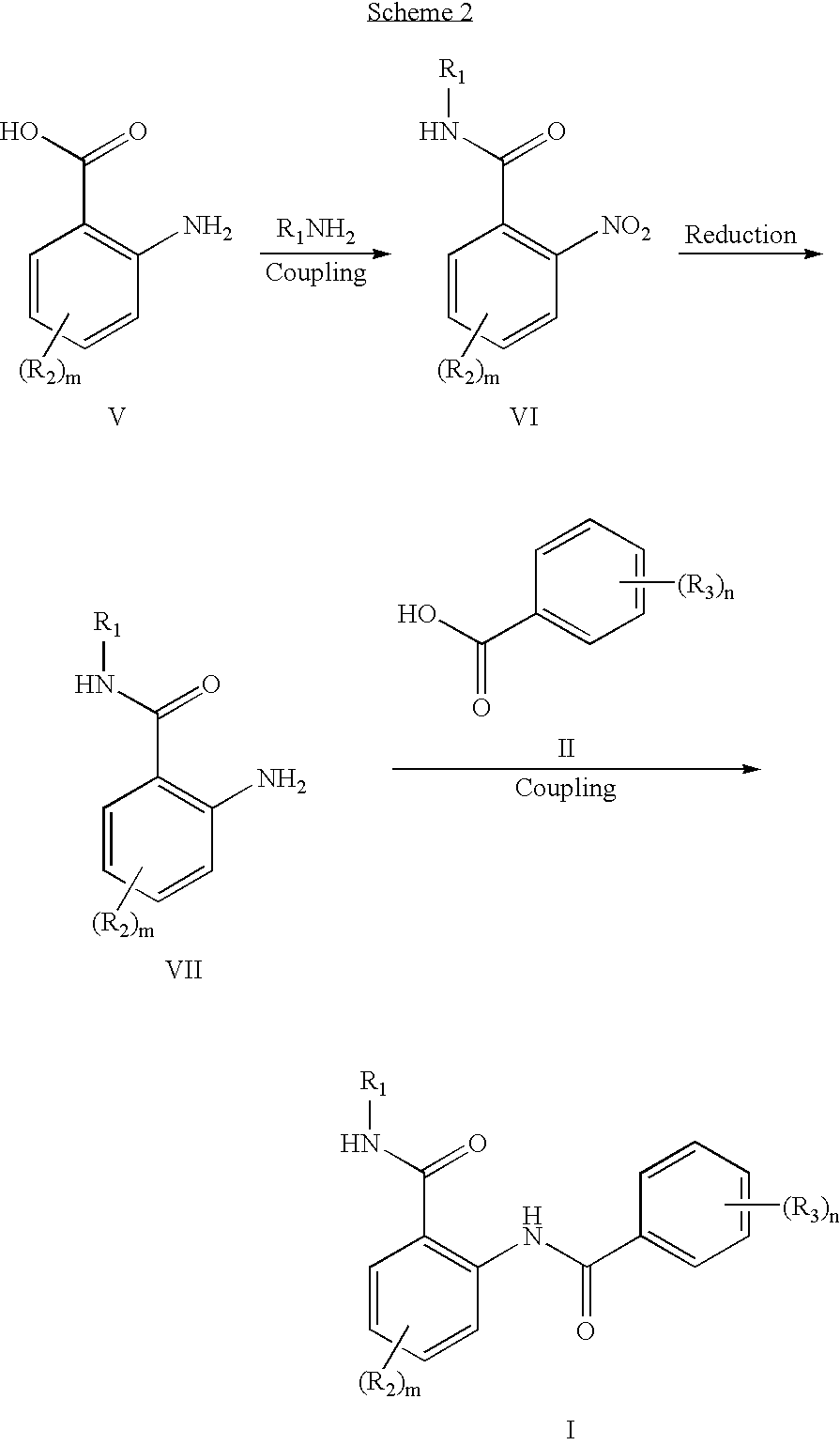
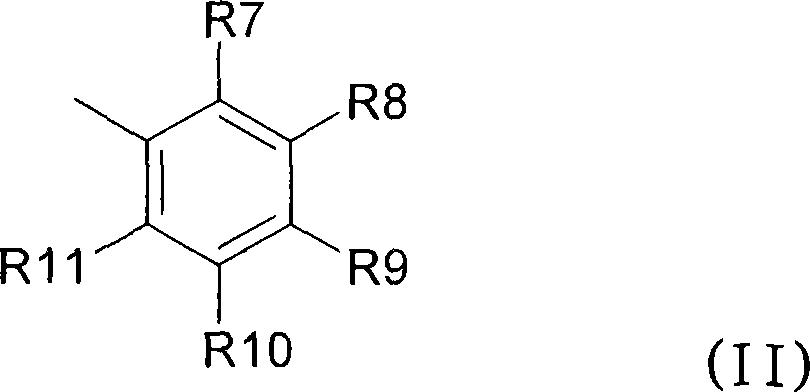
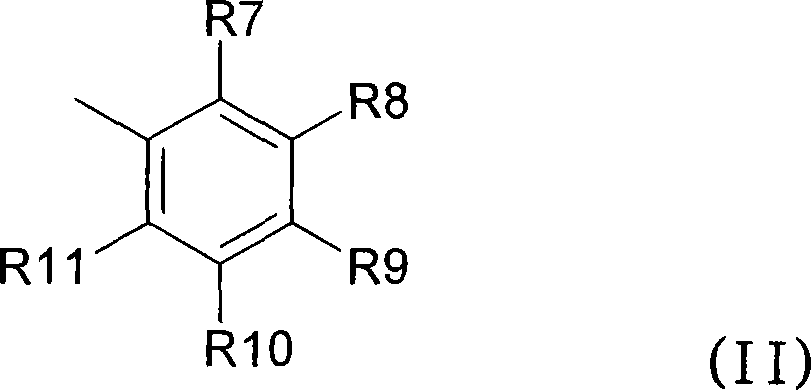
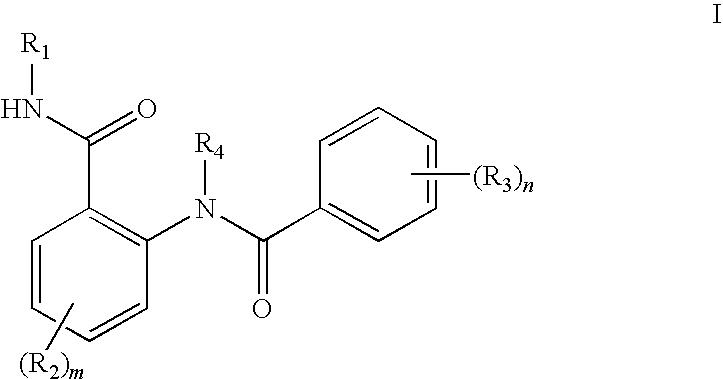
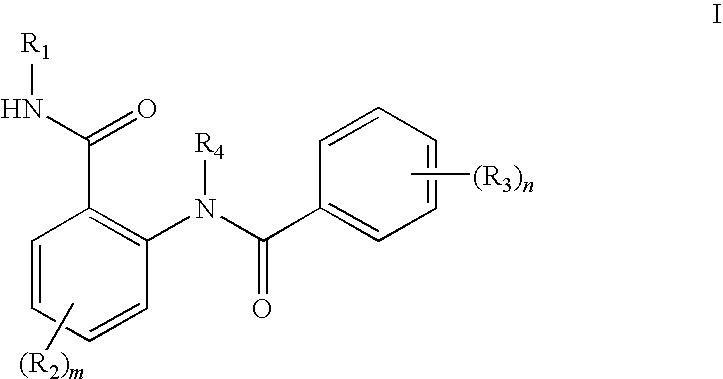
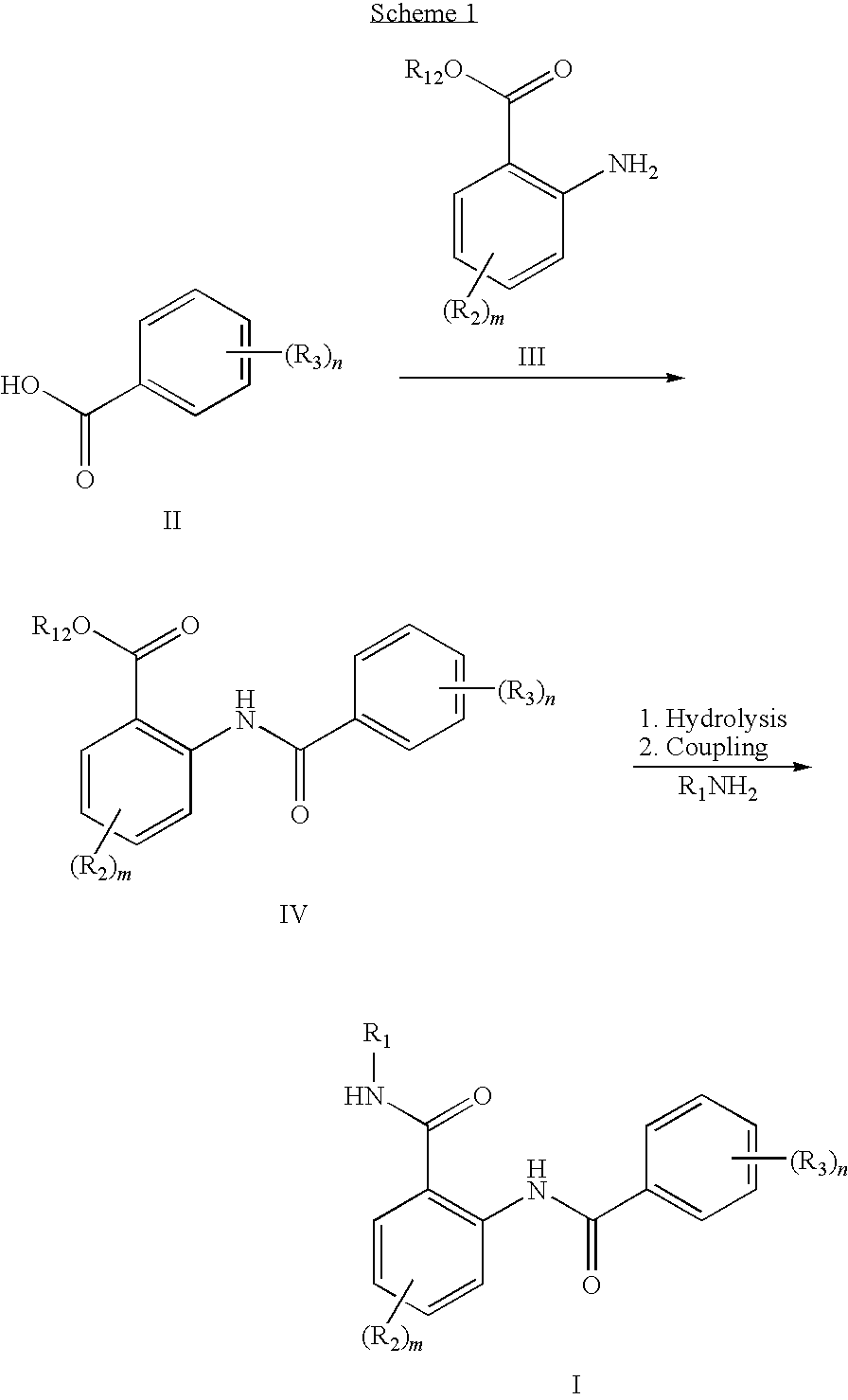
Anthranilic acid derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
ActiveUS20060135619A1Organic active ingredientsNervous disorder17beta-hydroxysteroid dehydrogenaseAnthranilic acid
Anthranilic acid drivatives, methods of using such compounds in the treatment of hormone sensitive diseases such as prostate cancer, and pharmaceutical compositions containing such compounds.
Owner:BRISTOL MYERS SQUIBB CO
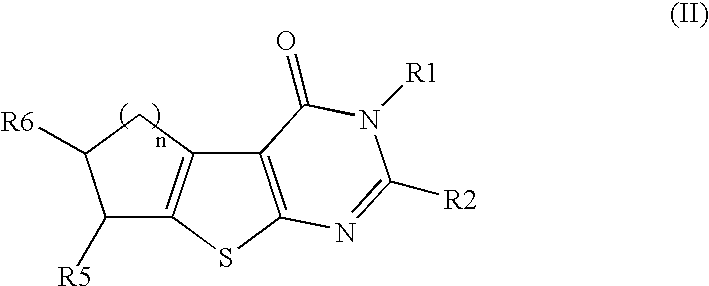
Fused tricyclic compounds as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
Owner:BRISTOL MYERS SQUIBB CO
Fused heterotricyclic compounds as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
Owner:BRISTOL MYERS SQUIBB CO
Use of compositions comprising an estrogenic component for the treatment and prevention of musculoskeletal pain
InactiveUS20060276414A1Increasing cell proliferationHigh affinityOrganic active ingredientsBiocideGynecologyPresent method
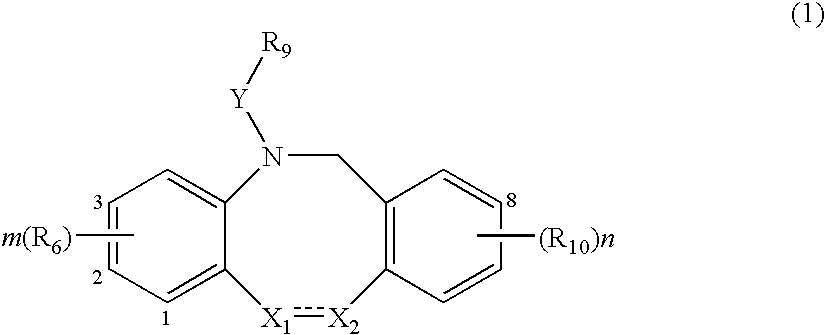
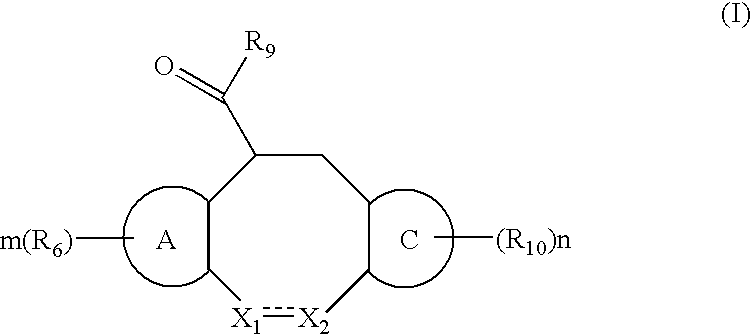
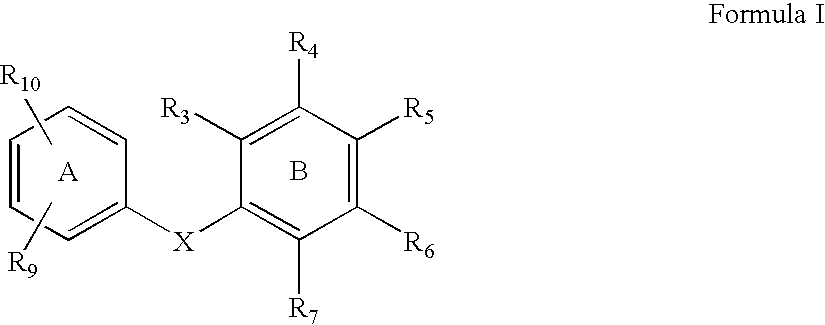
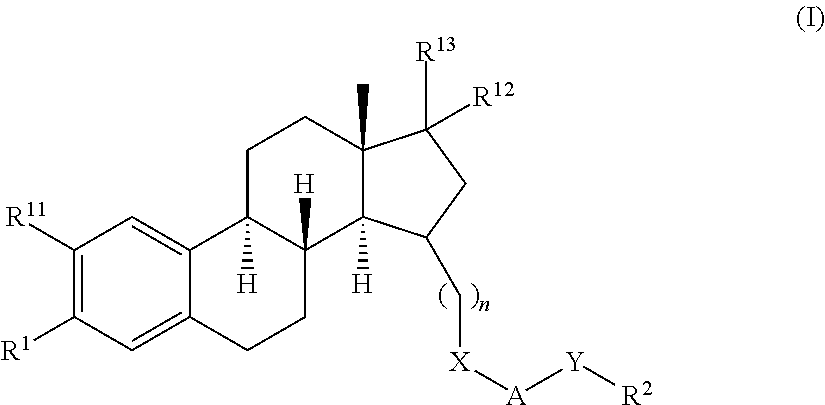
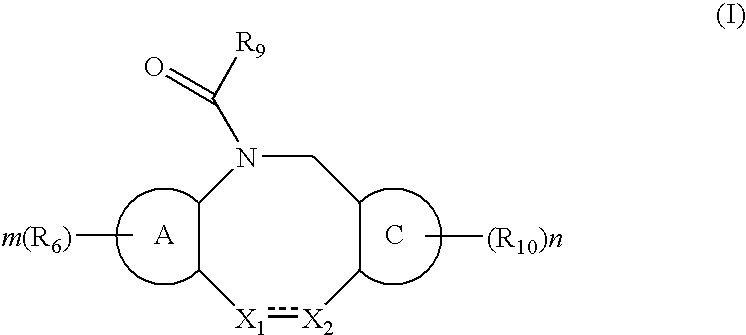
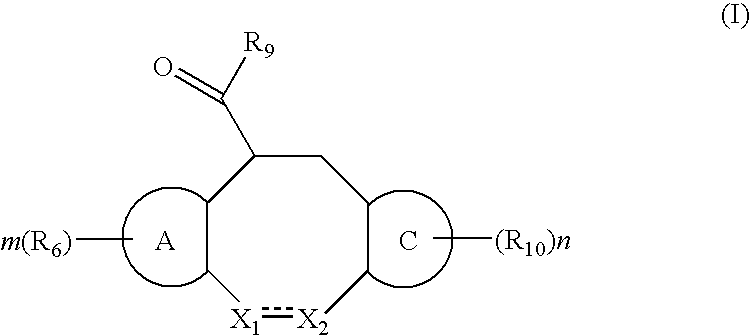
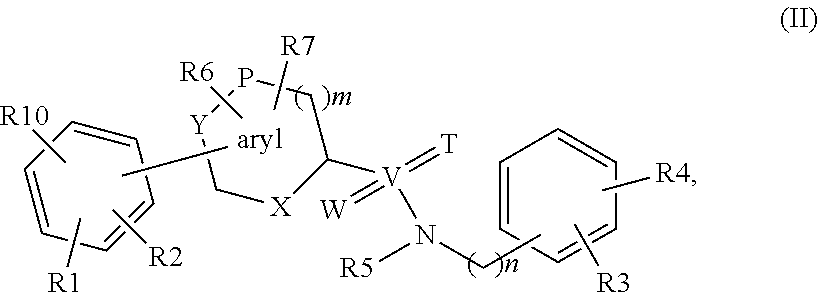
The present invention relates to a method of treating or preventing musculoskeletal pain in a mammal receiving administration of an estrogen. suppressant selected from the group consisting of aromatase inhibitors, GnRH analogues, cyclo-oxy-genase 2 (COX-2) inhibitors, 17β-hydroxysteroid dehydrogenase type 1 inhibitors, progestogens, anti-estrogens and combinations thereof, said method comprising the administration of an effective amount of an estrogenic component, wherein the estrogenic component is selected from the group consisting of: substances represented by the following formula (1) in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors.
Owner:COELINGH BENNINK HERMAN JAN TIJMEN +1
17SS-HSD1 and STS inhibitors
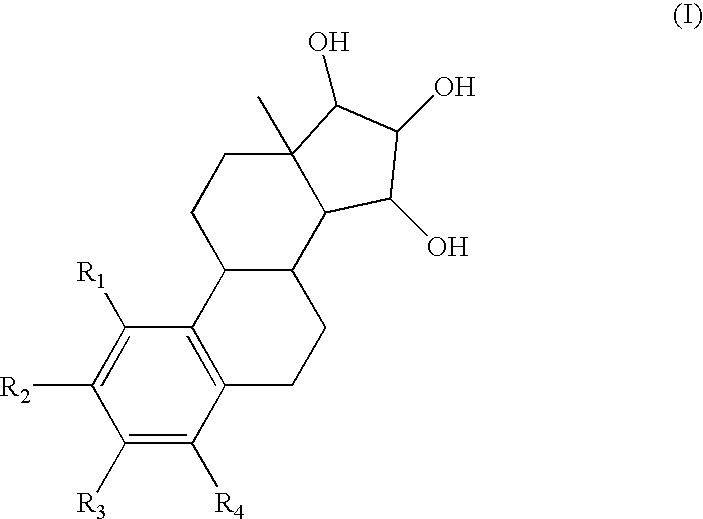
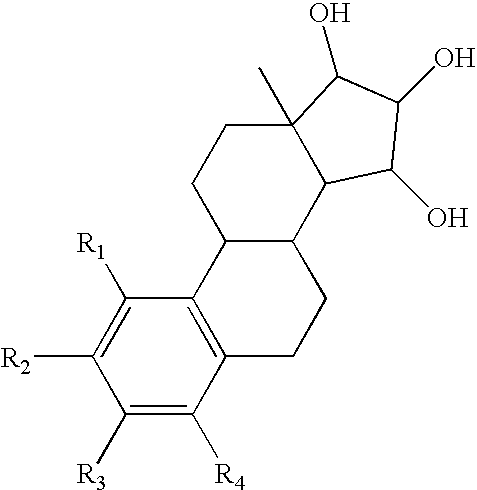
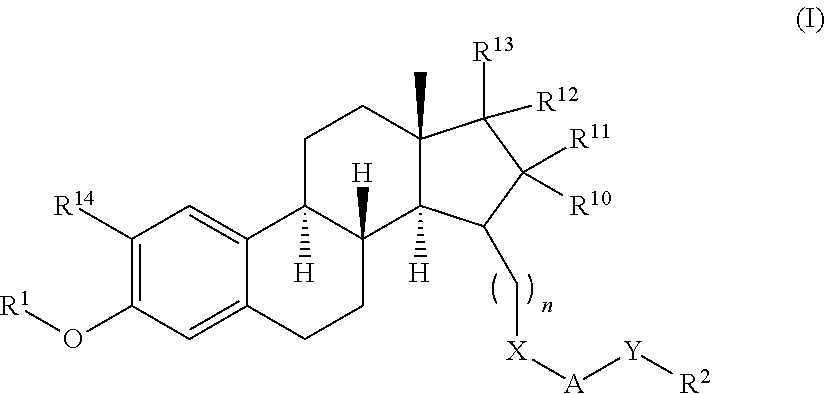
The present invention relates to novel substituted steroid derivatives which represent selectiv inhibitors of the 17β-hydroxysteroid dehydrogenase type I (17β-HSD1) and, in addition, which may represent inhibitors of the steroid sulphatase, as well as to their salts, to pharmaceutical preparations containing these compounds and to processes for the preparation of these compounds. Furthermore, the invention concerns the therapeutic use of said novel substituted steroid derivatives, particularly their use in the treatment, inhibition, prophylaxis or prevention of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I and / or steroid sulphatase enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration.
Owner:ABBVIE PHARMA GMBH
17Beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of hormone-related diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
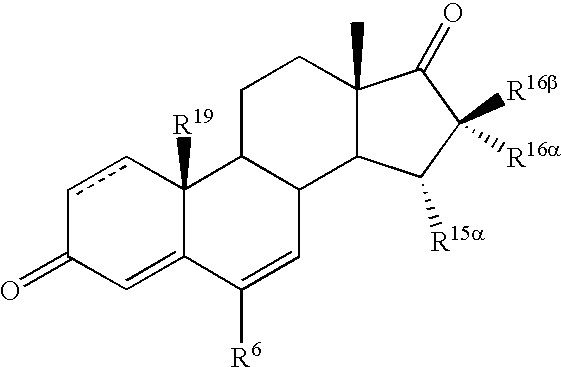
Novel 17beta-hydroxysteroid dehydrogenase type I inhibitors
3,15-substituted estrone compounds which act as inhibitors of 17β-hydroxysteroid dehydrogenase type I (17β-HSD1), salts thereof, pharmaceutical preparations containing such compounds, processes for preparing such compounds, and therapeutic uses of such compounds, particularly in the treatment or inhibition of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration, as well as the general use of selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors which possess in addition no or only pure antagonistic binding affinities to the estrogen receptor for the treatment or inhibition of benign gynecological disorders, particularly endometriosis.
Owner:ABBVIE PHARMA GMBH
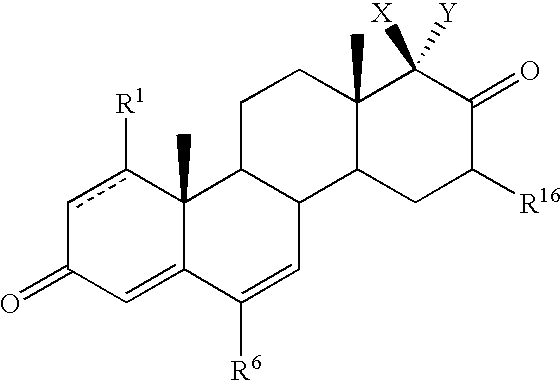
Substituted estratriene derivatives as 17beta hsd inhibitors
InactiveUS20080255075A1Good metabolic stabilityLess inhibitory potentialBiocideOrganic active ingredientsSteroidal hormones17beta-hydroxysteroid dehydrogenase
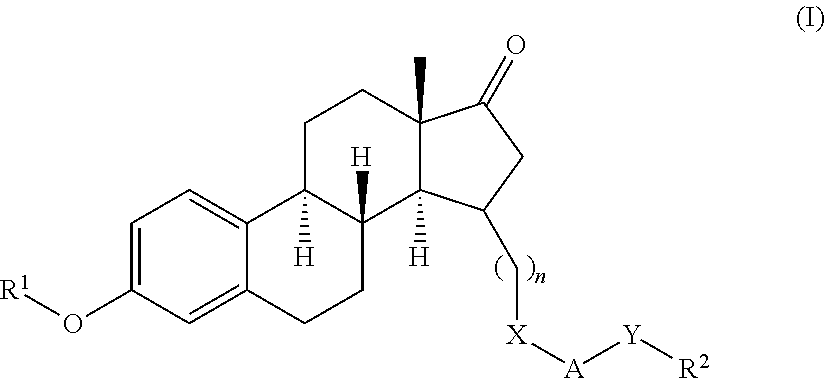
Substituted estratriene compounds of formula (I) useful in therapy, especially in the treatment or inhibition of a steroid hormone dependent disorder requiring the inhibition of a 17β-hydroxysteroid dehydrogenase (17β-HSD) type 1, type 2 and / or type 3 enzyme, as well as their salts, pharmaceutical compositions containing such compounds and processes for preparing such compounds.
Owner:SOLVAY PHARMA GMBH
compound
InactiveUS20090186900A1Inhibition formationInhibit synthesisBiocideOrganic compound preparation17beta-hydroxysteroid dehydrogenaseDisease cause
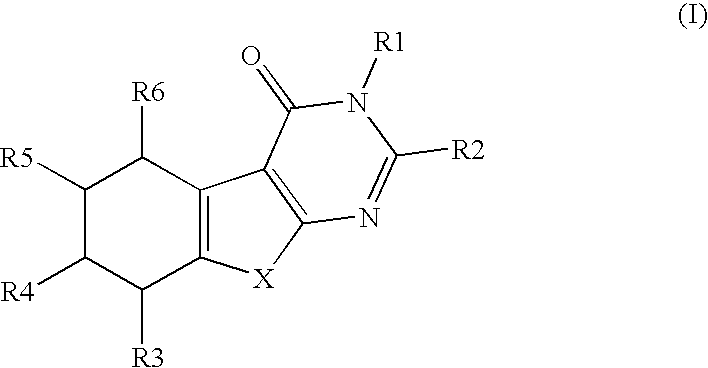
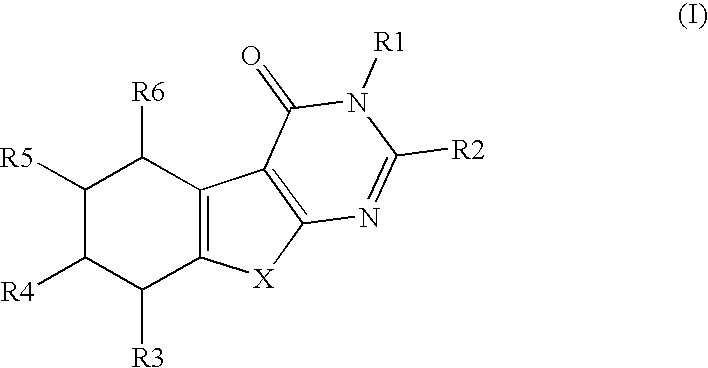
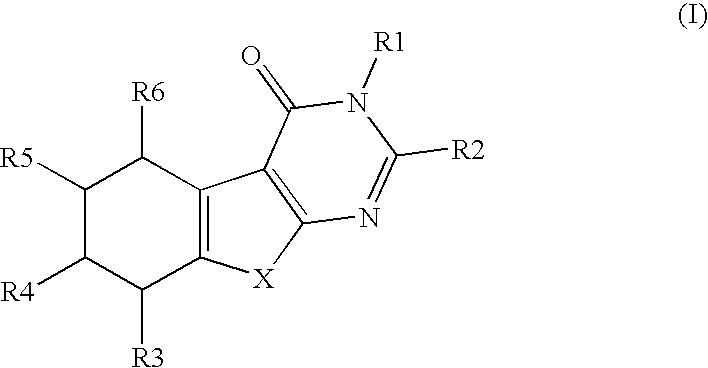
There is provided a compound of Formula Iwherein the various symbols are as defined in the description, and a method of manufacturing a medicament for use in the therapy of a condition or disease associated with 17β-hydroxysteroid dehydrogenase (17β-HSD), comprising a compound of Formula I.
Owner:STRIX LTD
17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of hormone-related diseases
The invention relates to 17beta-hydroxysteroid dehydrogenase type 1 (17betaHSD1) inhibitors, the preparation thereof and the use thereof for the treatment and prophylaxis of hormone-related, especially estrogen-related or androgen-related, diseases.
Owner:UNIV DES SAARLANDES
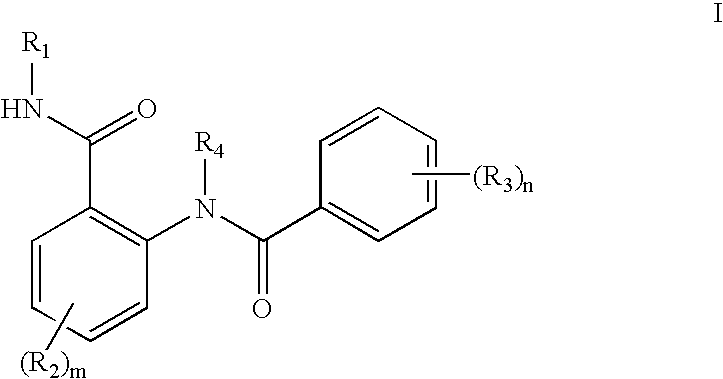
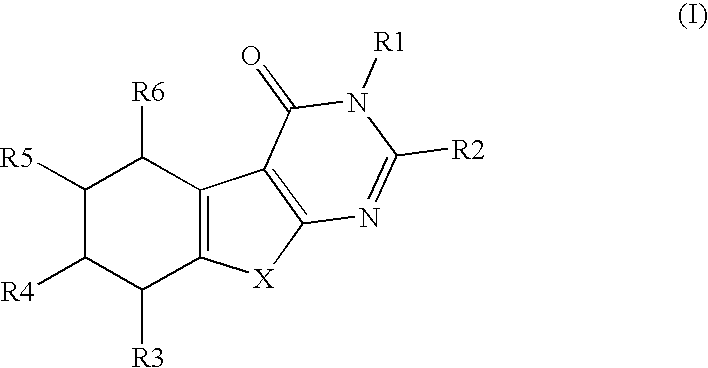
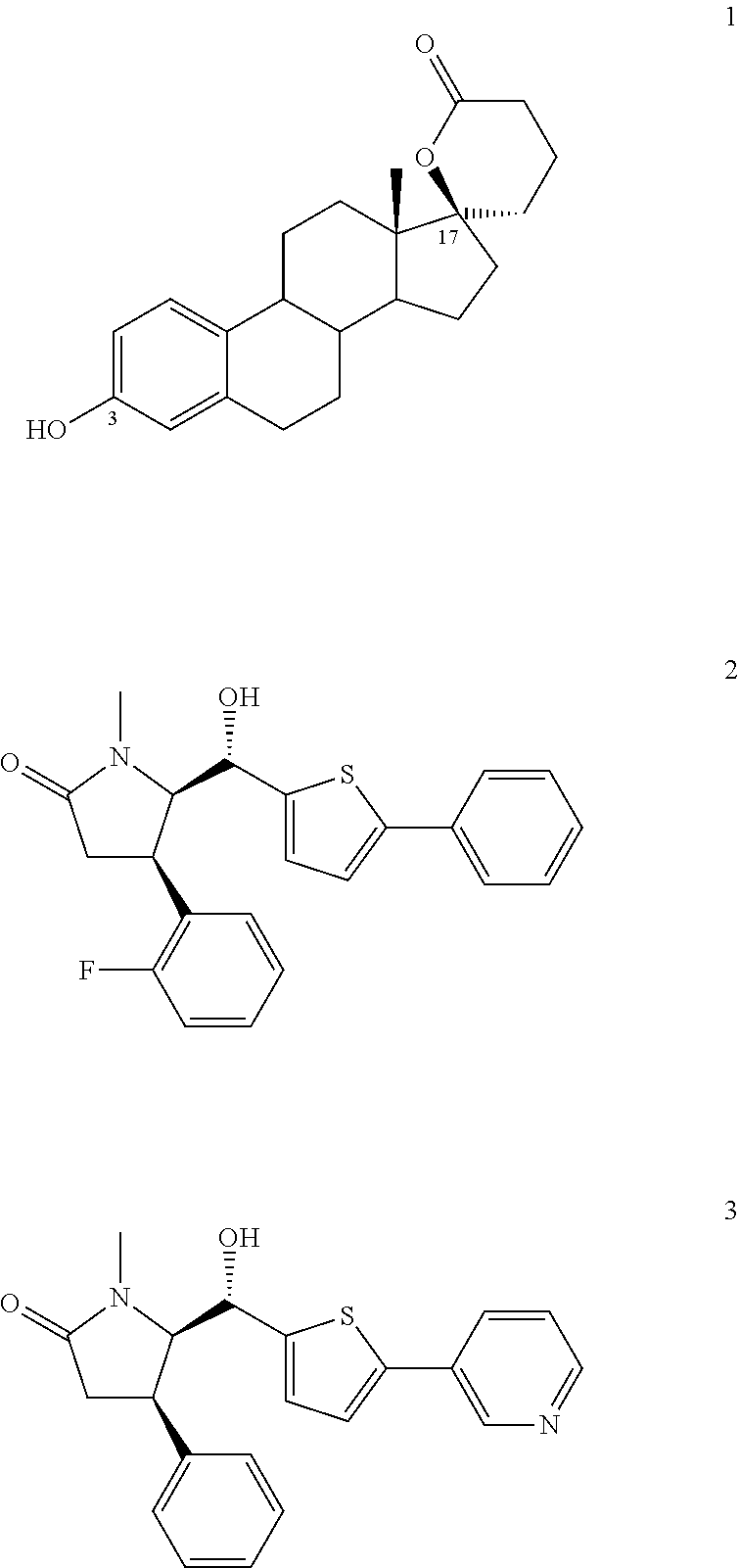
Novel compounds and their use in therapy
InactiveUS20050176742A1Suppress failureOrganic active ingredientsBiocideSteroid prophylaxisSteroidal hormones
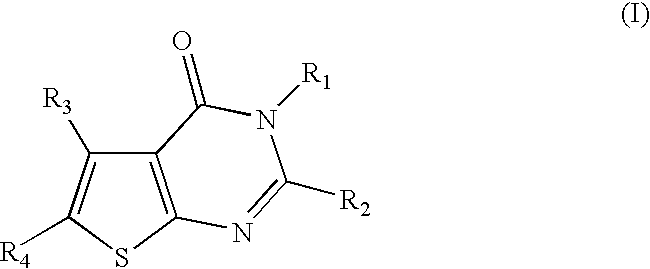
Thiophenepyrimidinone compounds and their use in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring inhibition of 17β-hydroxysteroid dehydrogenase enzymes.
Owner:SOLVAY PHARMA GMBH +1
17β-hydroxysteroid dehydrogenase type 3 inhibitors for the treatment of androgen dependent diseases
InactiveUS6903102B2Reduced activityReduce synthesisBiocideOrganic chemistryMedicineHormone dependence
There are disclosed compounds of the formula (I): prodrugs thereof, or pharmaceutically acceptable salts of the compounds or of said prodrugs which are useful as inhibitors of Type 3 17β-Hydroxysteroid Dehydrogenase. Also disclosed are pharmaceutical compositions containing said compounds and their use for the treatment or prevention of androgen dependent diseases.
Owner:MERCK SHARP & DOHME CORP
Construction and application of Comamonas testosteroni 3,17beta-hydroxysteroid dehydrogenase (3,17beta-HSD) gene reinforced strain
InactiveCN103074360AImprove degradation efficiencyBacteriaMicroorganism based processesMicroorganismEstriol
The invention relates to a construction of a Comamonas testosteroni 3,17beta-HSD gene reinforced strain, and belongs to the microbial field. The construction of the Comamonas testosteroni 3,17beta-HSD gene reinforced strain is characterized in that a recombinant plasmid is pK-Tacpromoter-3,17beta-HSD; and the construction comprises the following steps: carrying out PCR technological amplification, and target segment recovery and purification of a Comamonas testosteroni genome which is a template to connect with a pUCm-T carrier; 2, carrying out PCR technological amplification, and target segment recovery and purification of a plasmid pAH10 which is a template to connect with the pUCm-T carrier; 3, extracting a recombinant plasmid 3,17beta-HSD-T through adopting a column plasmid DNA small-amount extraction kit; and 4, respectively extracting the recombinant plasmid and pK-3,17beta-HSD through adopting the column plasmid DNA small-amount extraction kit. According to the invention, the Comamonas testosteroni 3,17beta-HSD gene reinforced strain is constructed for the first time and is applied, and the estriol degradation efficiency, the cholesterol degradation efficiency and the stosterone degradation efficiency of the Comamonas testosteroni 3,17beta-HSD gene reinforced strain are 30%, 15% and 150% higher than that of a wild Comamonas testosteroni strain respectively.
Owner:CHANGCHUN UNIV OF SCI & TECH
Substituted estratriene derivatives as 17BETA HSD inhibitors
InactiveUS8288367B2Inhibitory activityBiocideOrganic active ingredientsSteroidal hormones17beta-hydroxysteroid dehydrogenase
Owner:SOLVAY PHARMA GMBH
Therapeutically active thiophenepyrimidinone compounds and their use
Thiopheneprymidinone compounds useful in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent disorder, preferably a steroid hormone dependent disease or disorder requiring the inhibition of a 17β-hydroxysteroid dehydrogenase (17β-HSD) such as 17β-HSD type 1, type 2 or type 3 enzyme.
Owner:SOLVAY PHARM BV
Fused heterotricyclic compounds as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
Owner:BRISTOL MYERS SQUIBB CO
Inhibitors of type 5 and type 3 17beta-hydroxysteroid dehydrogenase and methods for their use
InactiveUS20040082556A1Inhibition is effectiveAvoid inhibitionOrganic active ingredientsUrinary disorderDiseaseAndrogen
Novel methods of medical treatment and / or inhibition of development of diseases are disclosed for diseases that are sensitive to androgenic or estrogenic activity. The treatments utilize inhibitors of type 5 and / or type 3 17beta-hydroxysteroid dehydrogenase. Novel inhibitors of type 5 17beta-hydroxysteroid dehydrogenase are also disclosed, as are novel inhibitors of type 3 17beta-hydroxysteroid dehydrogenase.
Owner:ENDORES & DEV
Substituted estratrien derivatives as 17beta hsd inhibitors
InactiveCN101568547AHas inhibitory activityOrally activeOrganic active ingredientsSenses disorderEnzyme17beta-hydroxysteroid dehydrogenase
This invention relates to novel substituted estratrien derivatives of general formula (I) useful in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent disorder requiring the inhibition of a 17beta-hydroxysteroid dehydrogenase (17beta- HSD) type 1, type 2 and / or type 3 enzyme, as well as to their salts, to pharmaceutical preparations containing these compounds and to processes for the preparation of these compounds.
Owner:SOLVAY PHARMA GMBH
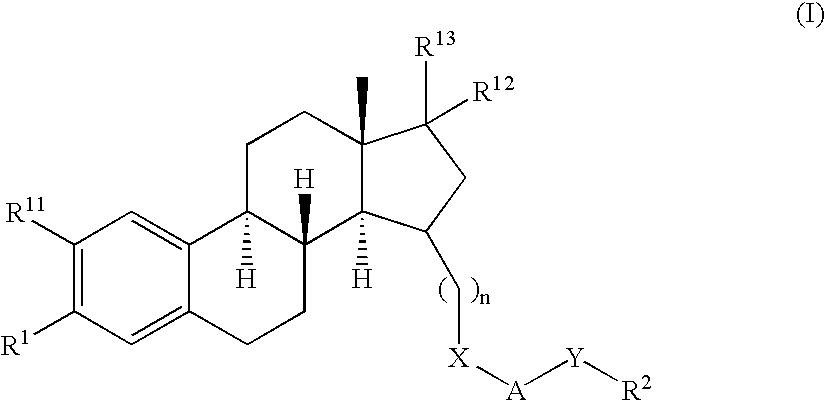
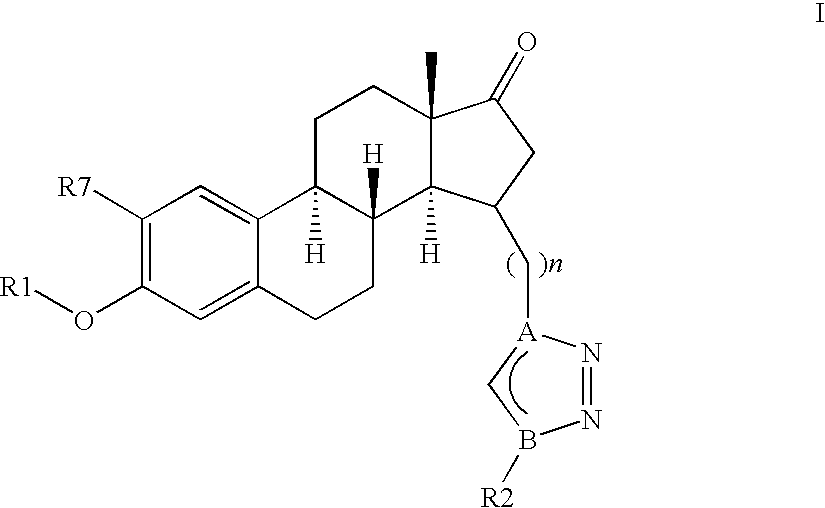
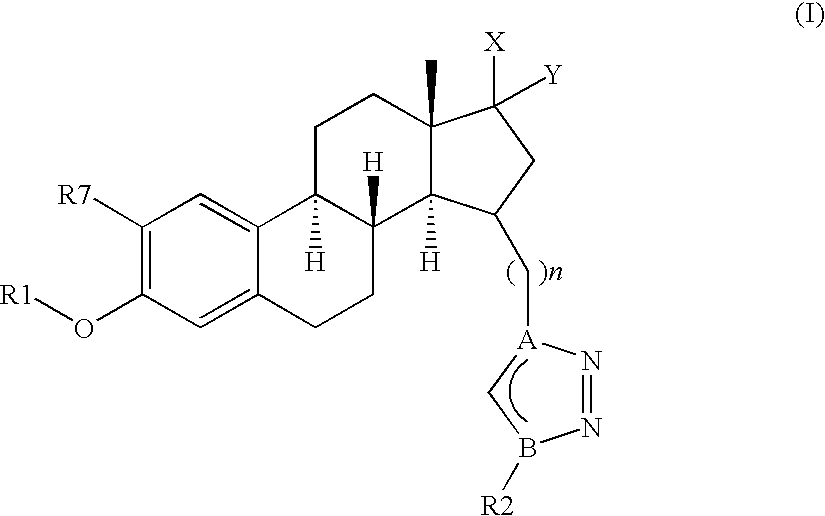
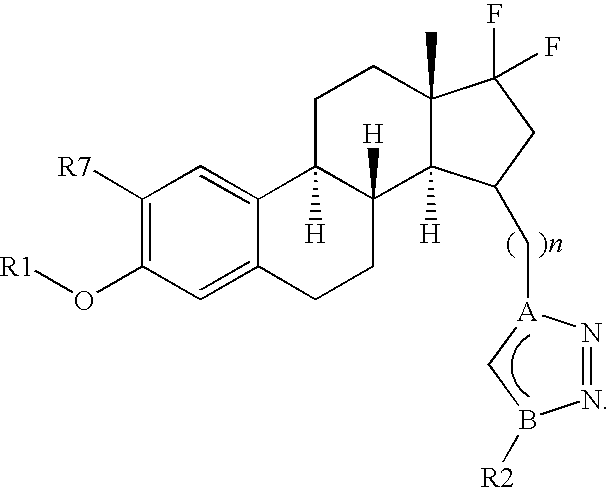
Therapeutically Active Triazoles and Their Use
InactiveUS20080146531A1Organic active ingredientsNervous disorderTriazole antifungalsSteroidal hormones
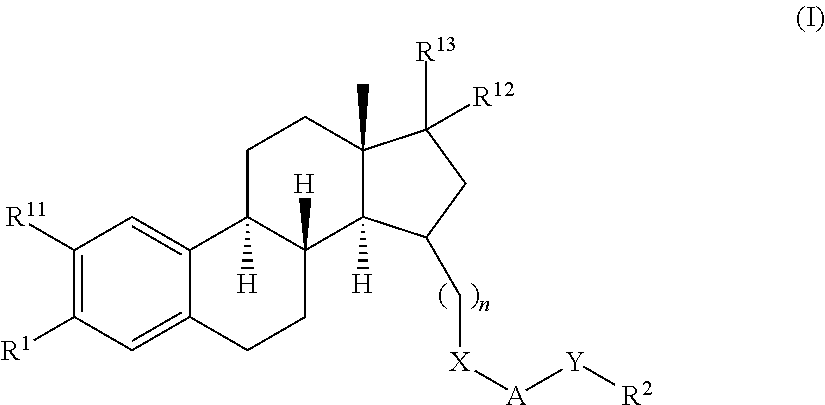
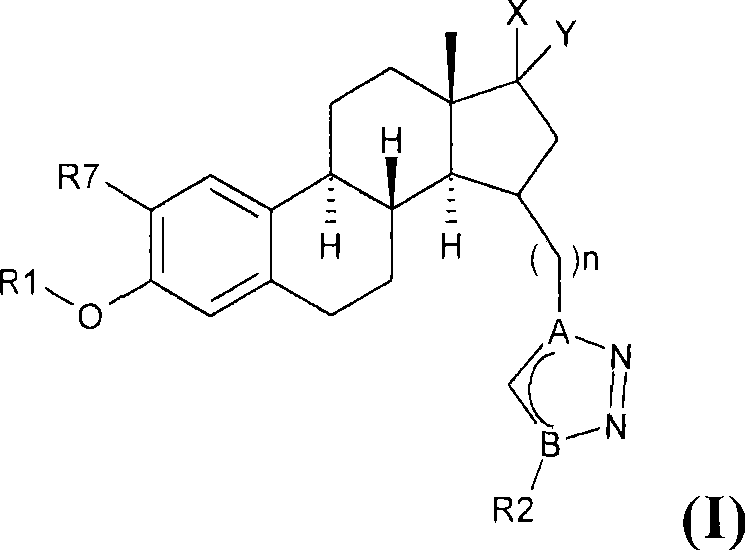
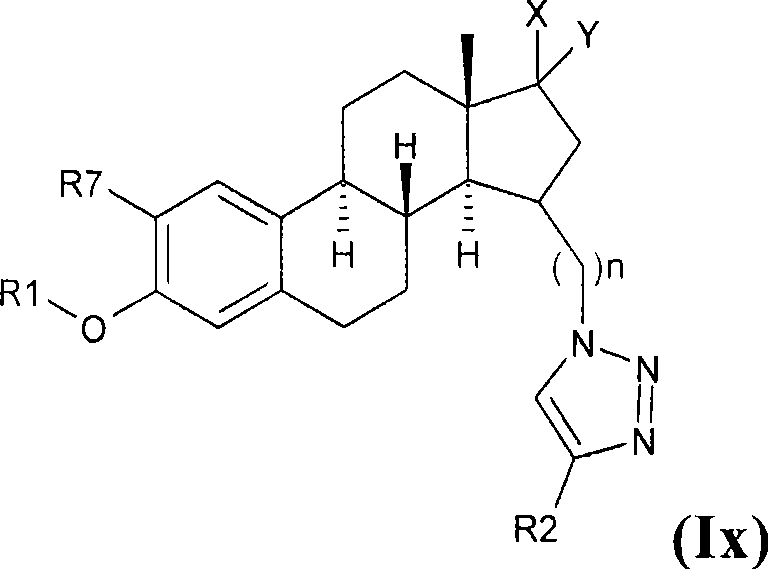
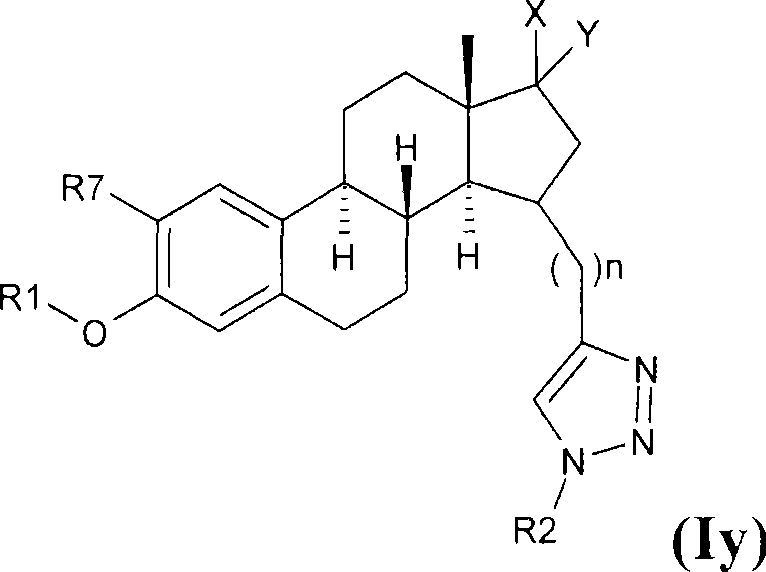
Estratrien-triazoles corresponding to formula (I) (shown below) which are useful in therapy, especially for the treatment and / or prevention or inhibition of a steroid hormone dependent disorder, preferably a steroid hormone dependent disease or disorder requiring the inhibition of a 17β-hydroxysteroid dehydrogenase (17β-HSD) such as 17β-HSD type 1, type 2 or type 3 enzyme.
Owner:ABBVIE PHARMA GMBH
Compounds and their use in therapy
Thiophenepyrimidinone compounds and their use in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring inhibition of 17β-hydroxysteroid dehydrogenase enzymes.
Owner:SOLVAY PHARMA GMBH +1
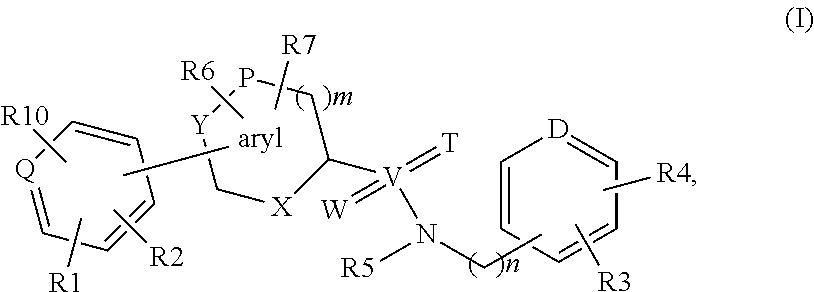
Biaryl derivatives as selective 17beta-hydroxysteroid dehydrogenase type 2 inhibitors
InactiveUS20140057953A1Improve performanceWeaker binding affinityBiocideNervous disorderDiseaseOsteoporosis
The invention relates to selective, non-steroidal 17beta-hydroxysteroid dehydrogenase type 2 (l7beta-HSD2) inhibitors of formula (I), their production and use, notably for the treatment and prophylaxis of sex steroid deficient diseases like osteoporosis in men and women.
Owner:ELEXOPHARM
17-beta HSD1 and STS Inhibitors
Substituted steroid compounds which represent selective inhibitors of 17β-hydroxysteroid dehydrogenase type I (17β-HSD1) and, in addition, which may represent inhibitors of the steroid sulfatase, salts thereof, pharmaceutical preparations containing these compounds, and a process for the preparation of these compounds. Also disclosed is a therapeutic method of using such substituted steroid compounds, particularly in the treatment, inhibition, prophylaxis or prevention of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I and / or steroid sulfatase enzymes and / or requiring lowering of the endogenous 17β-estradiol concentration.
Owner:ABBVIE PHARMA GMBH
Estratriene derivatives and their uses as 17beta-hydroxysteroid dehydrogenase inhibitors
This invention relates to novel estratrien-triazoles of general formula (I) useful in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent disorder, preferably a steroid hormone dependent disease or disorder requiring the inhibition of a 17beta-hydroxysteroid dehydrogenase (17beta-HSD) such as 17beta-HSD type 1, type 2 or type 3 enzyme.
Owner:SOLVAY PHARMA GMBH
17β-hydroxysteroid dehydrogenase type I inhibitors
3,15-substituted estrone compounds which act as inhibitors of 17β-hydroxysteroid dehydrogenase type I (17β-HSD1), salts thereof, pharmaceutical preparations containing such compounds, processes for preparing such compounds, and therapeutic uses of such compounds, particularly in the treatment or inhibition of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration, as well as the general use of selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors which possess in addition no or only pure antagonistic binding affinities to the estrogen receptor for the treatment or inhibition of benign gynecological disorders, particularly endometriosis.
Owner:ABBVIE PHARMA GMBH
A method for screening type I 17β hydroxysteroid dehydrogenase inhibitors using immobilized enzymes
The invention provides a method for screening a I type 17 beta-hydroxysteroid dehydrogenase inhibitor through an immobilized enzyme, and belongs to the technical field of enzymology and enzyme engineering. I type 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD1) has the important function in treating hormone-dependent diseases. At present, a substrate radiolabelling method is mainly adopted in studying the activity of 17 beta-HSD1, due to the fact that the free enzyme of 17 beta-HSD1 is likely to be inactivated, 17 beta-HSD1 is hard to prepare, a fresh placenta is needed for preparing an enzyme source in a new medicine screening experiment of every time, raw materials are hard to obtain, the price is high, and difficulty is brought to the screening work of new medicine. According to the method, an amino-modified silicon ball is adopted as a carrier, a glutaraldehyde crosslinking method is utilized, 17 beta-HSD1 extracted from the placenta is fixed, an external immobilization 17 beta-HSD1 enzyme model is built, androstenedione is adopted as a substrate, a high performance liquid chromatography method is used for detecting products, and the potential 17 beta-HSD1 inhibitor is screened. According to the method, instability of the free enzyme is overcome, operation is easy, the manufacturing cost is low, and repeated using is achieved.
Owner:CHINA PHARM UNIV
Compounds and their use in therapy
Thiophenepyrimidinone compounds and their use in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring inhibition of 17β-hydroxysteroid dehydrogenase enzymes.
Owner:SOLVAY PHARM BV
Therapeutically active 17-nitrogen substituted estratreinthiazole derivatives as inhibitors of 17β-hydroxysteroid dehydrogenase
ActiveUS9663549B2Good metabolic stabilityOrganic active ingredientsSenses disorderDiseaseSteroid prophylaxis
Owner:FORENDO PHARMA LTD
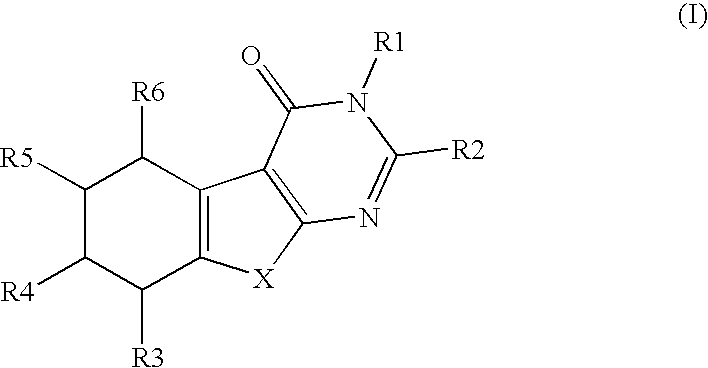
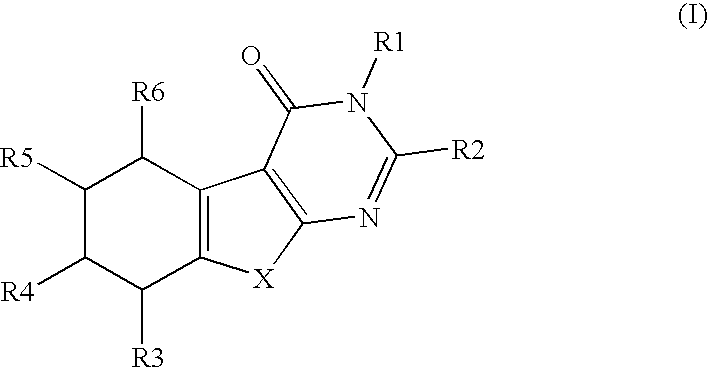
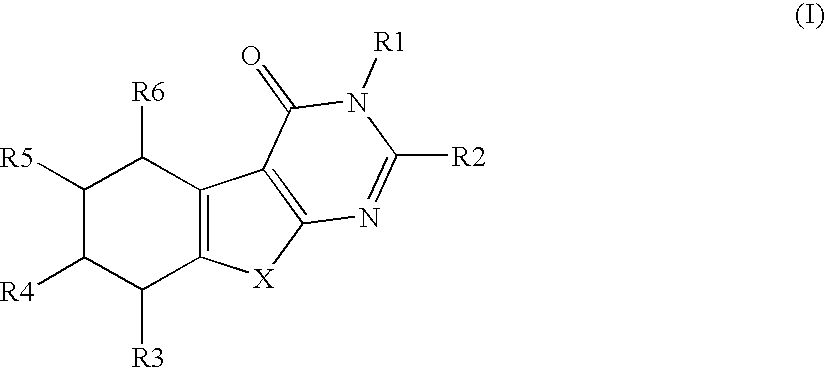
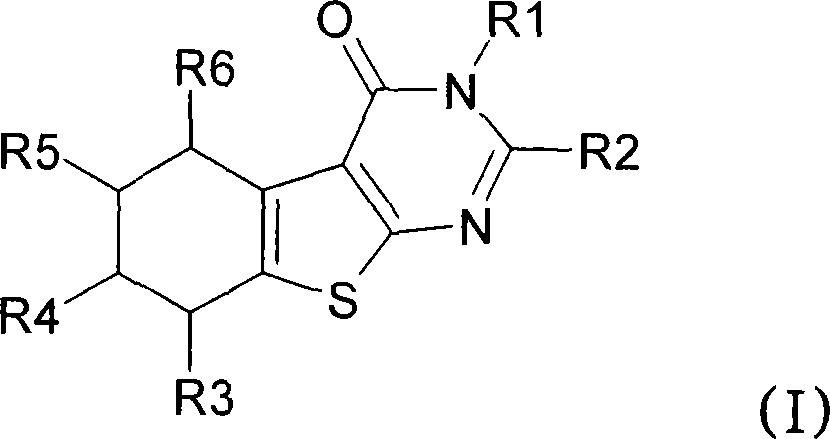
Novel substituted thiophenepyrimidinone derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase
This invention relates to novel substituted thiophenepyrimidinone derivatives and their use in therapy, especially for use in the treatment and / or prevention of a steroid hormone dependent diseases or disorder, such as steroid hormone dependent diseases or disorders requiring inhibition of 17beta-hydroxysteroid dehydrogenase enzymes.
Owner:SOLVAY PHARMA GMBH
Anthranilic acid derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase 3
ActiveUS7569725B2Organic active ingredientsNervous disorderProstate cancer17beta-hydroxysteroid dehydrogenase
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com