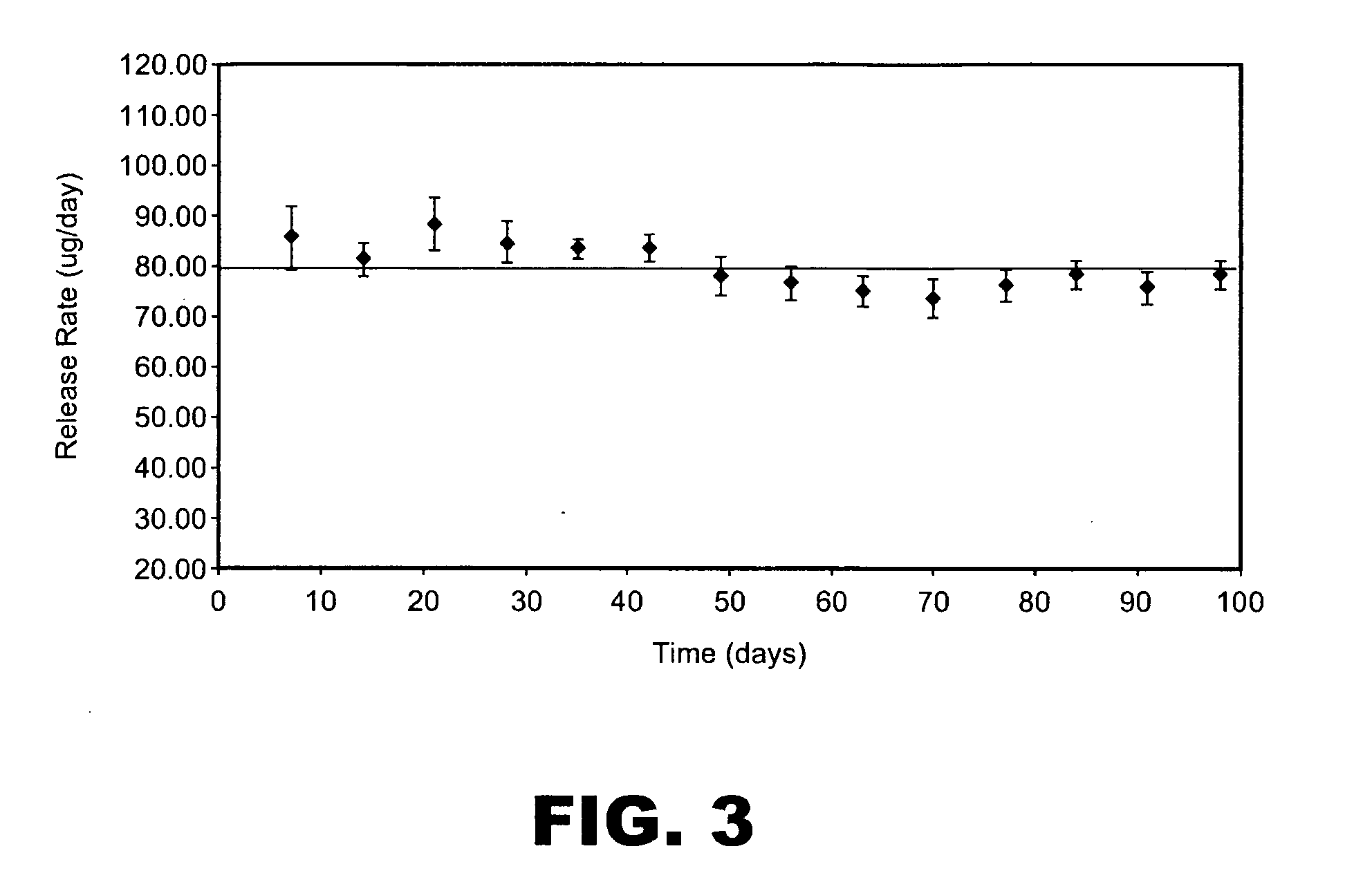
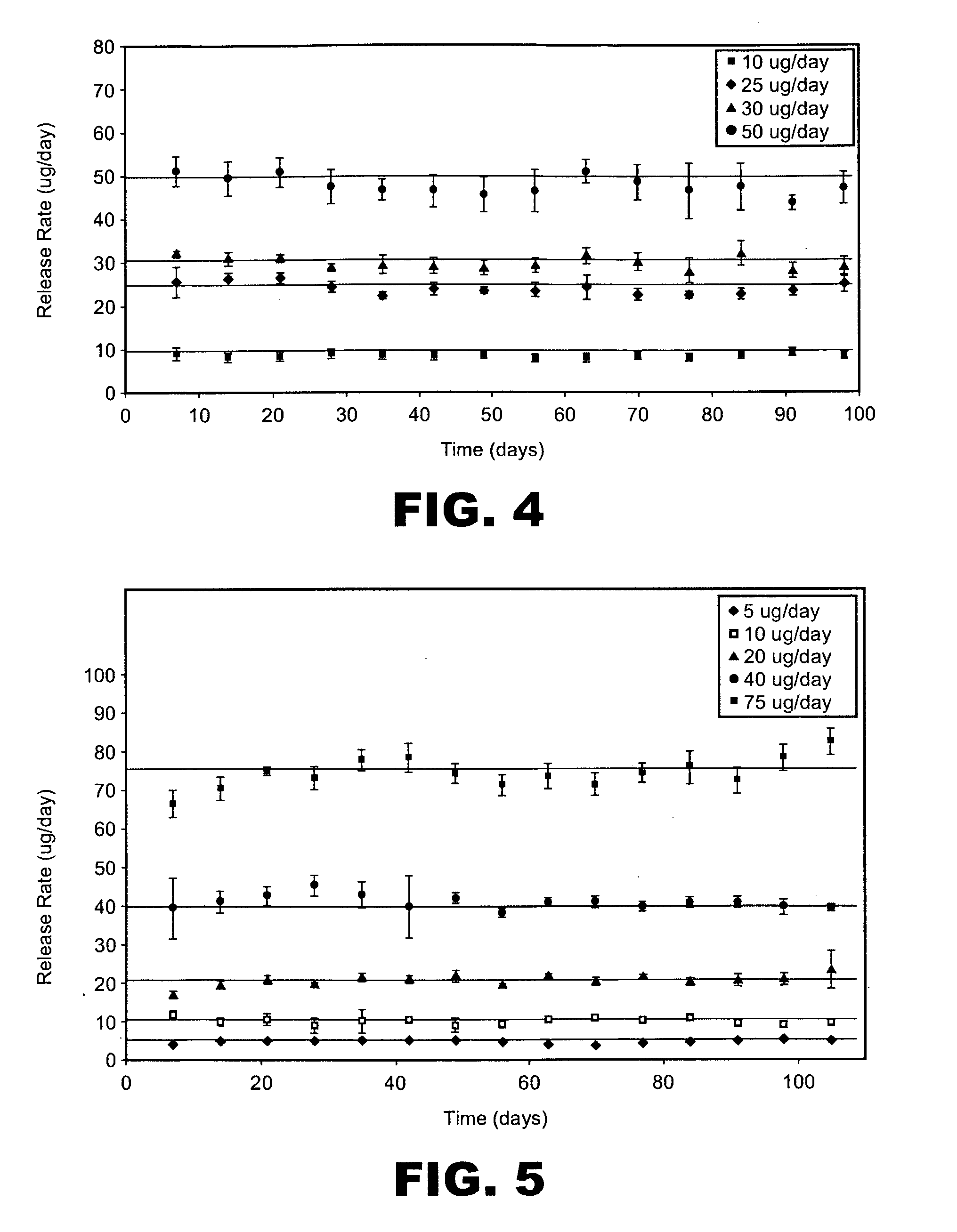
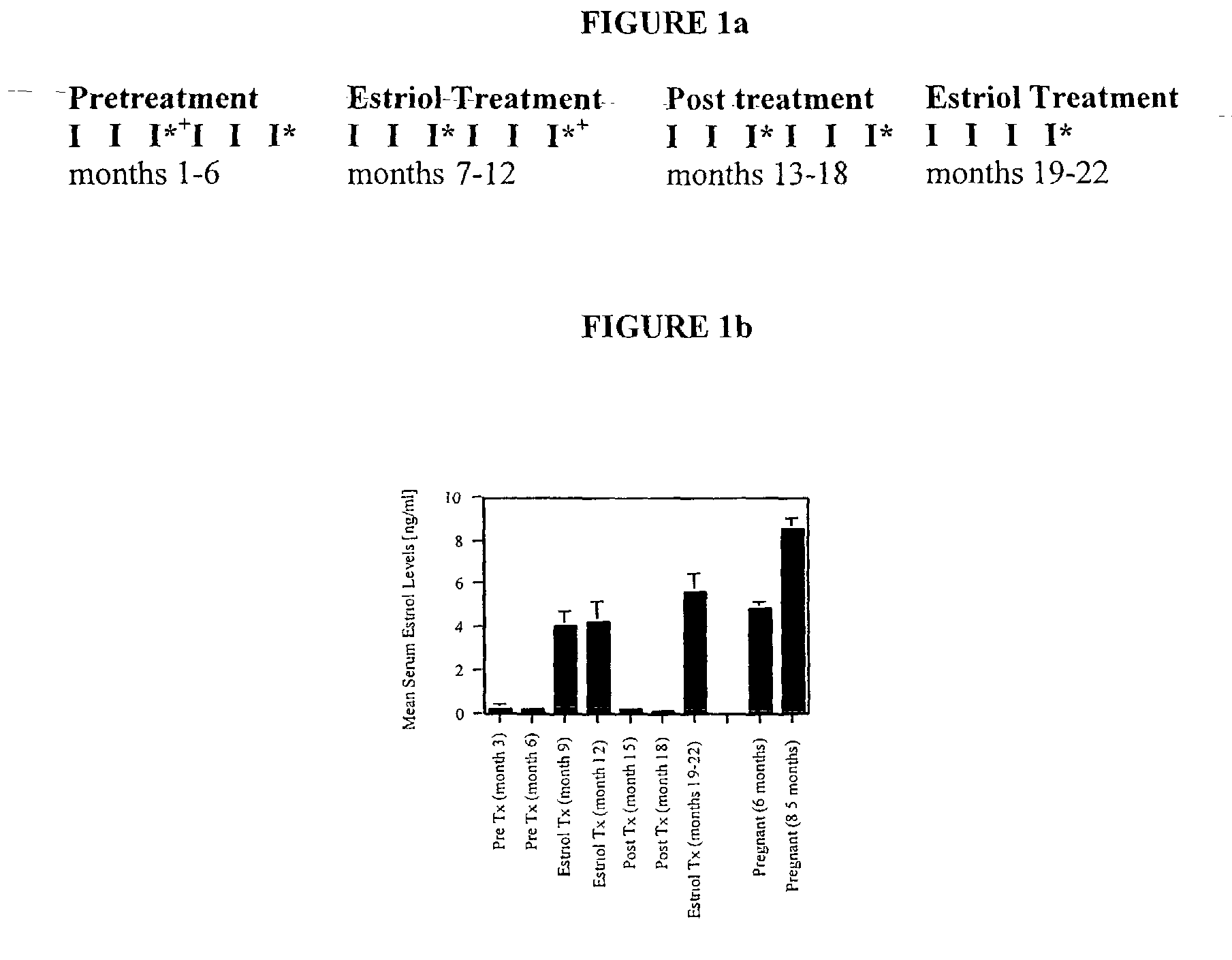
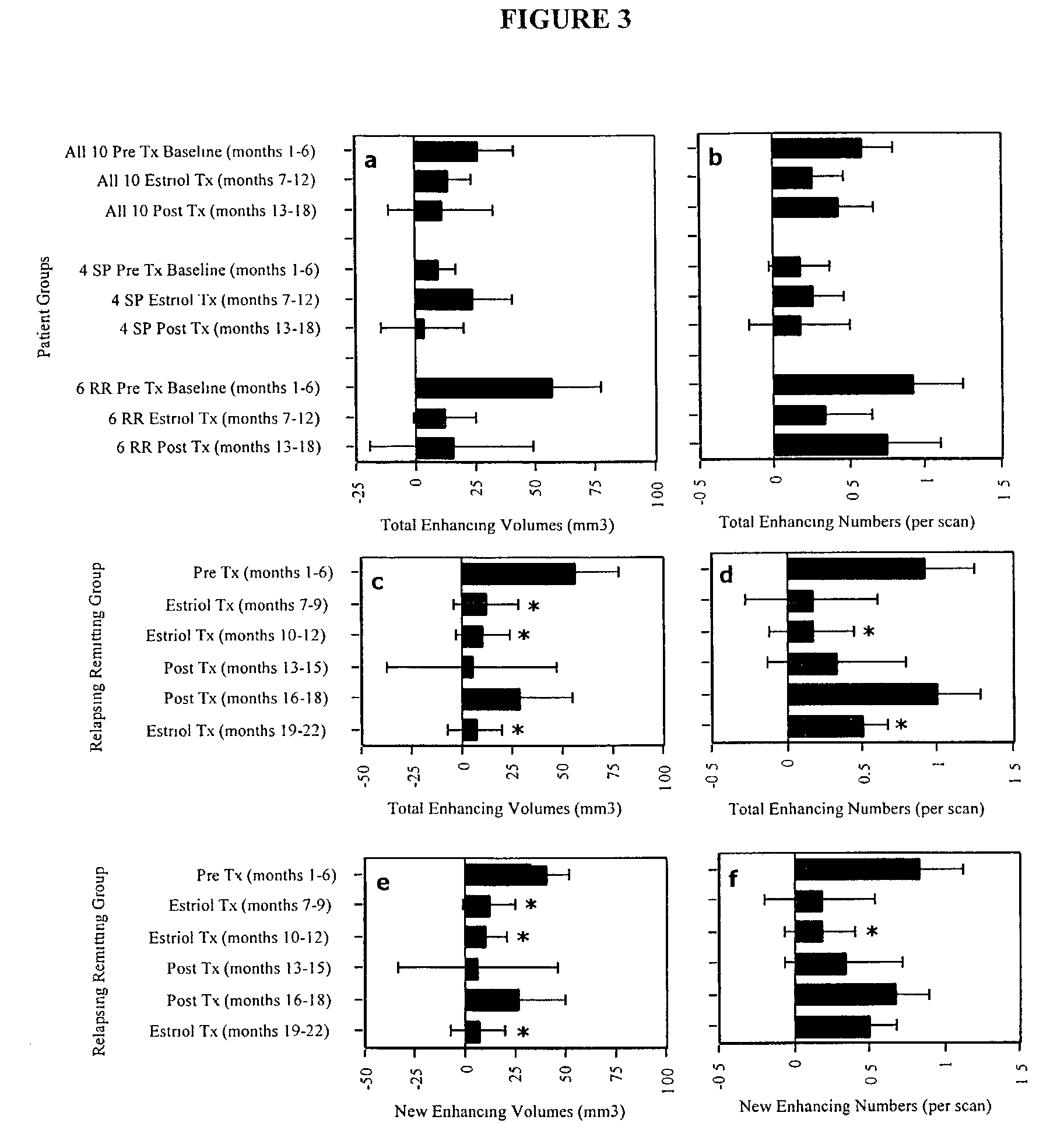
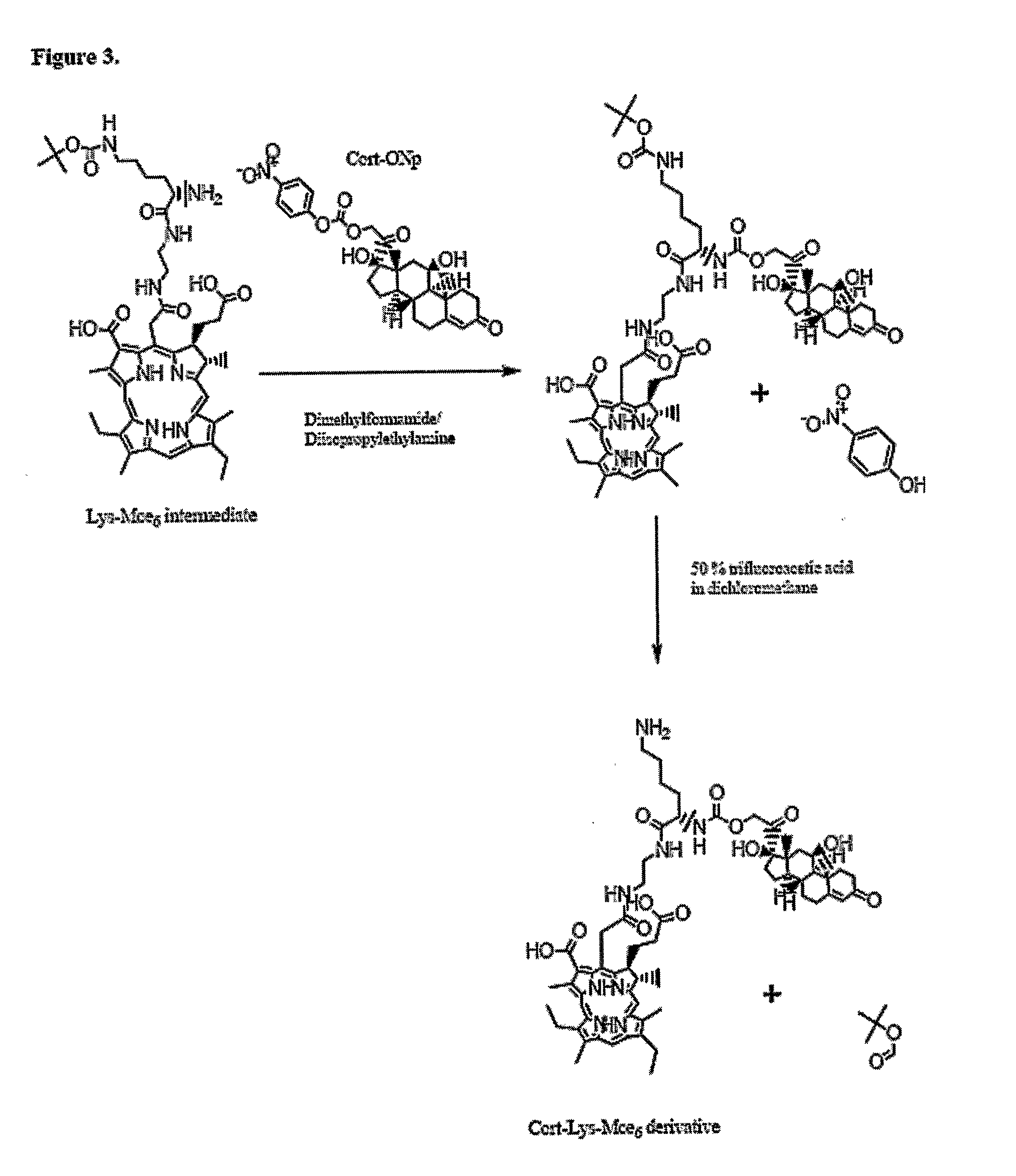
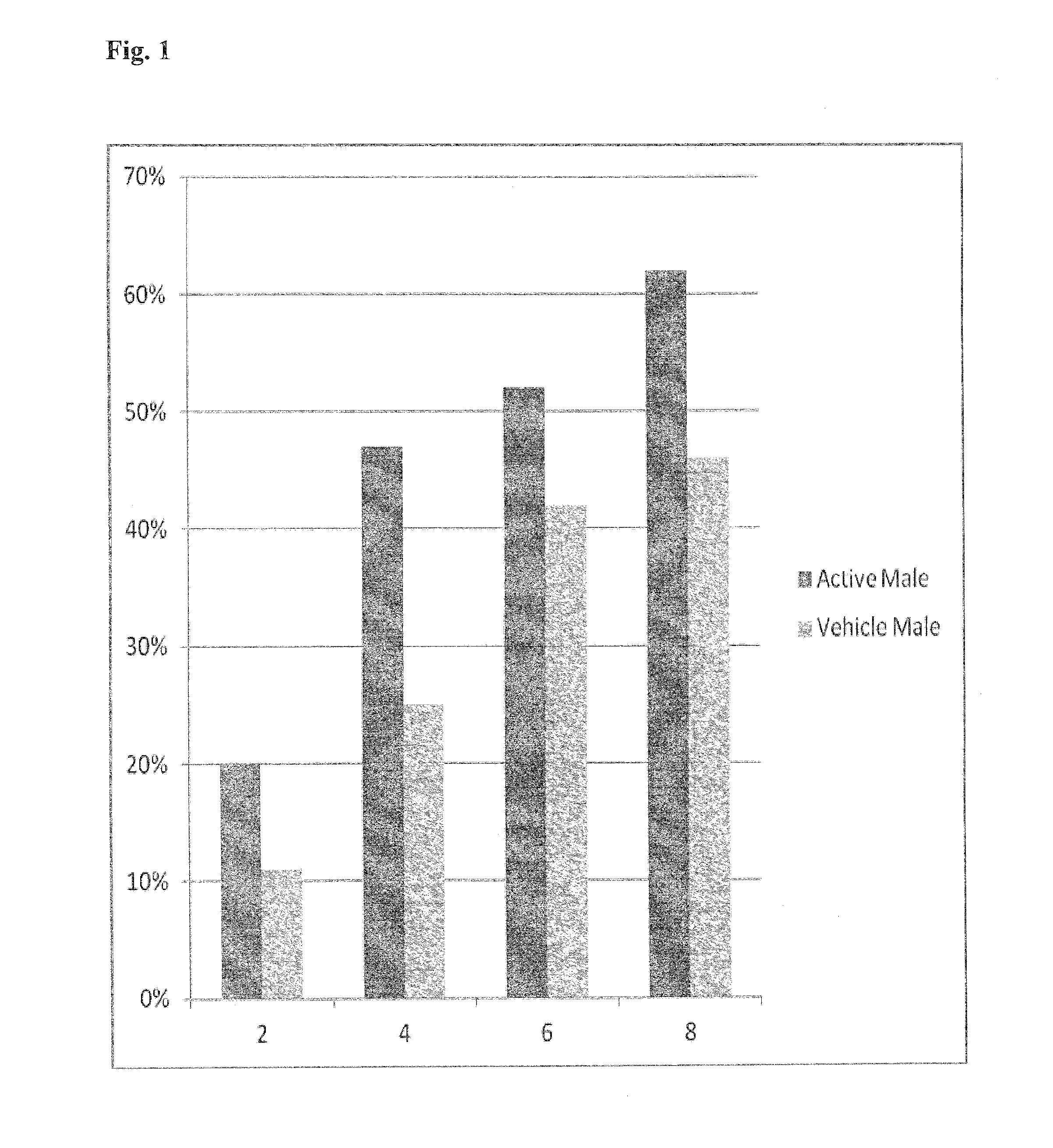
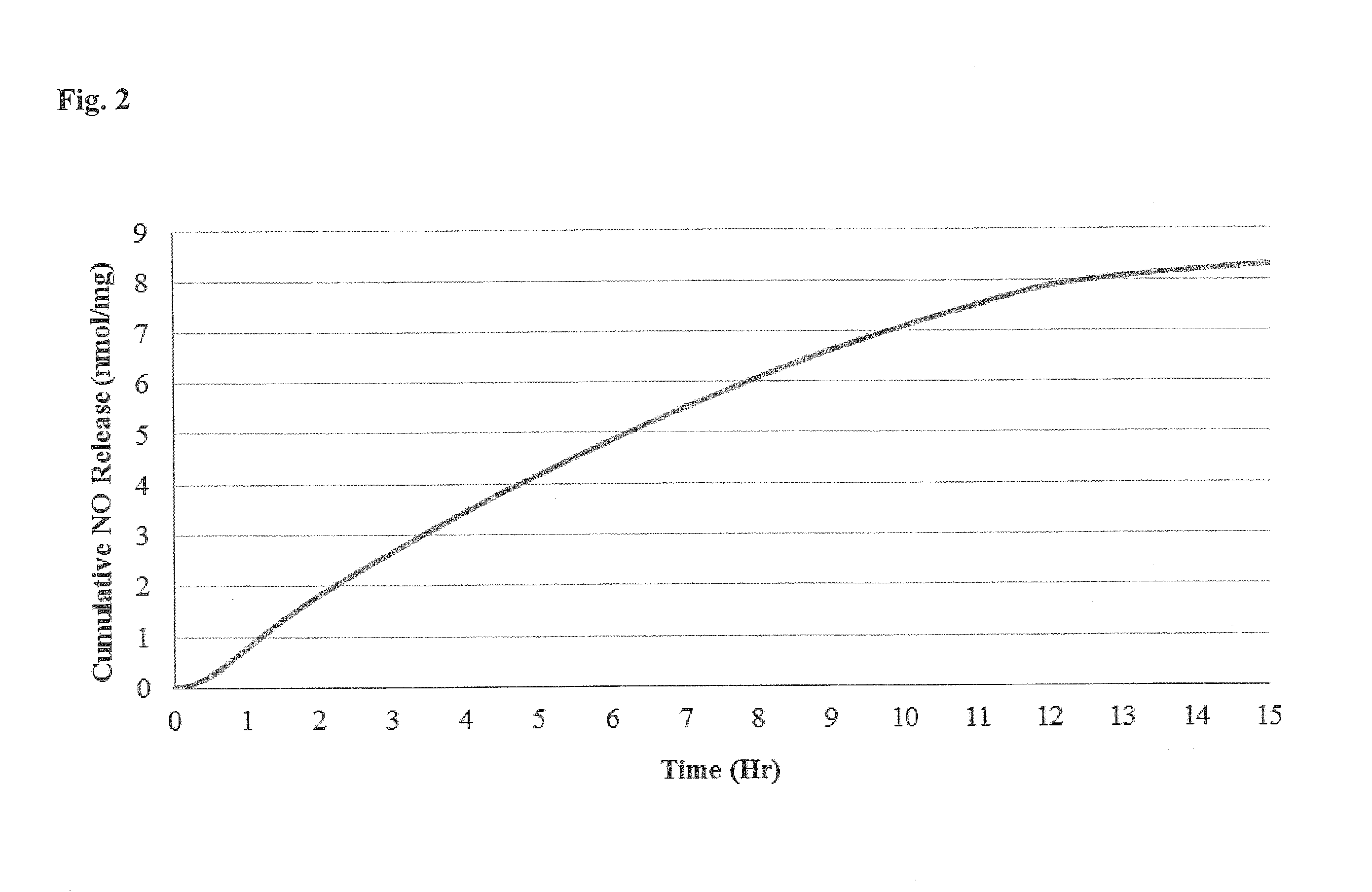
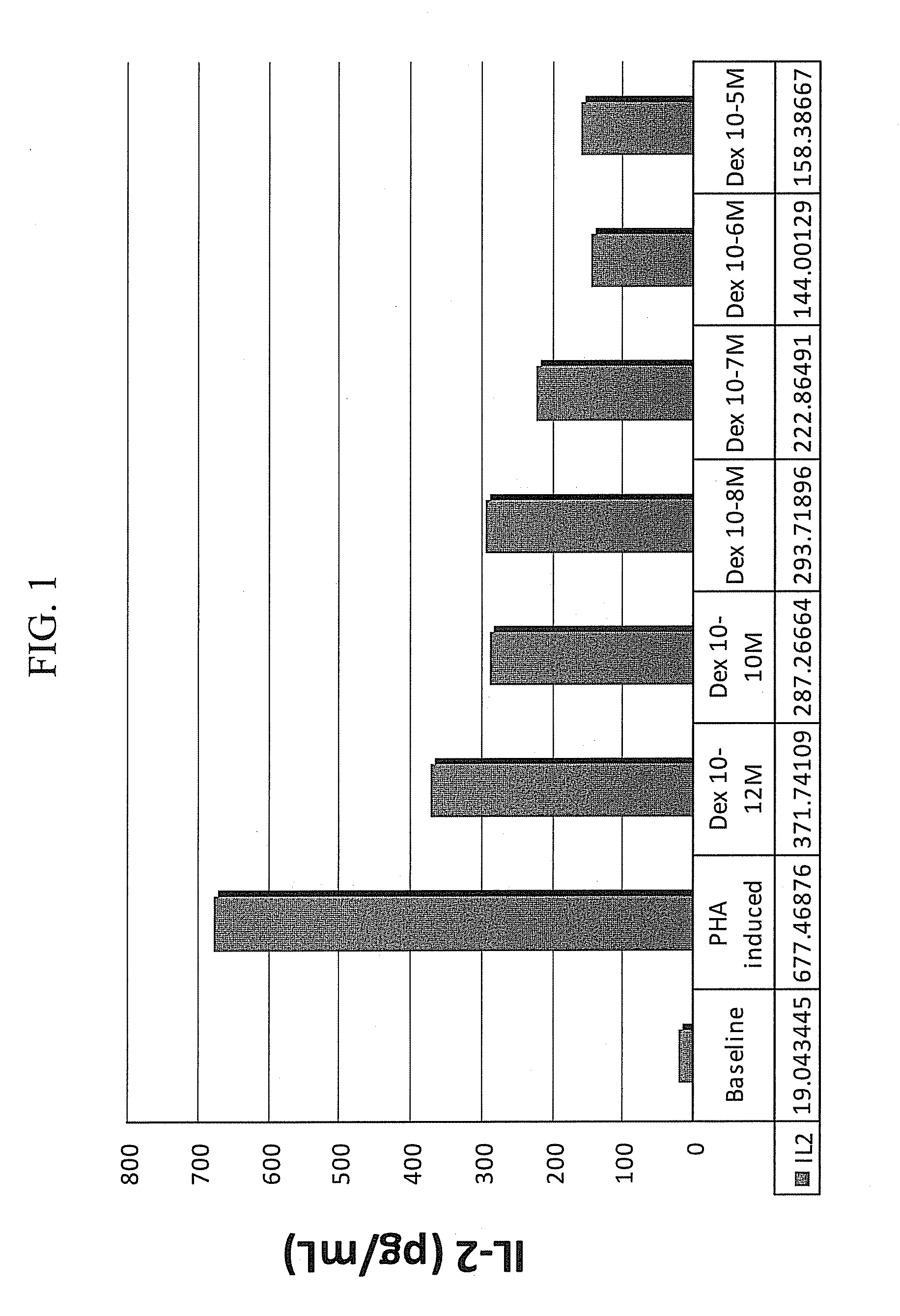
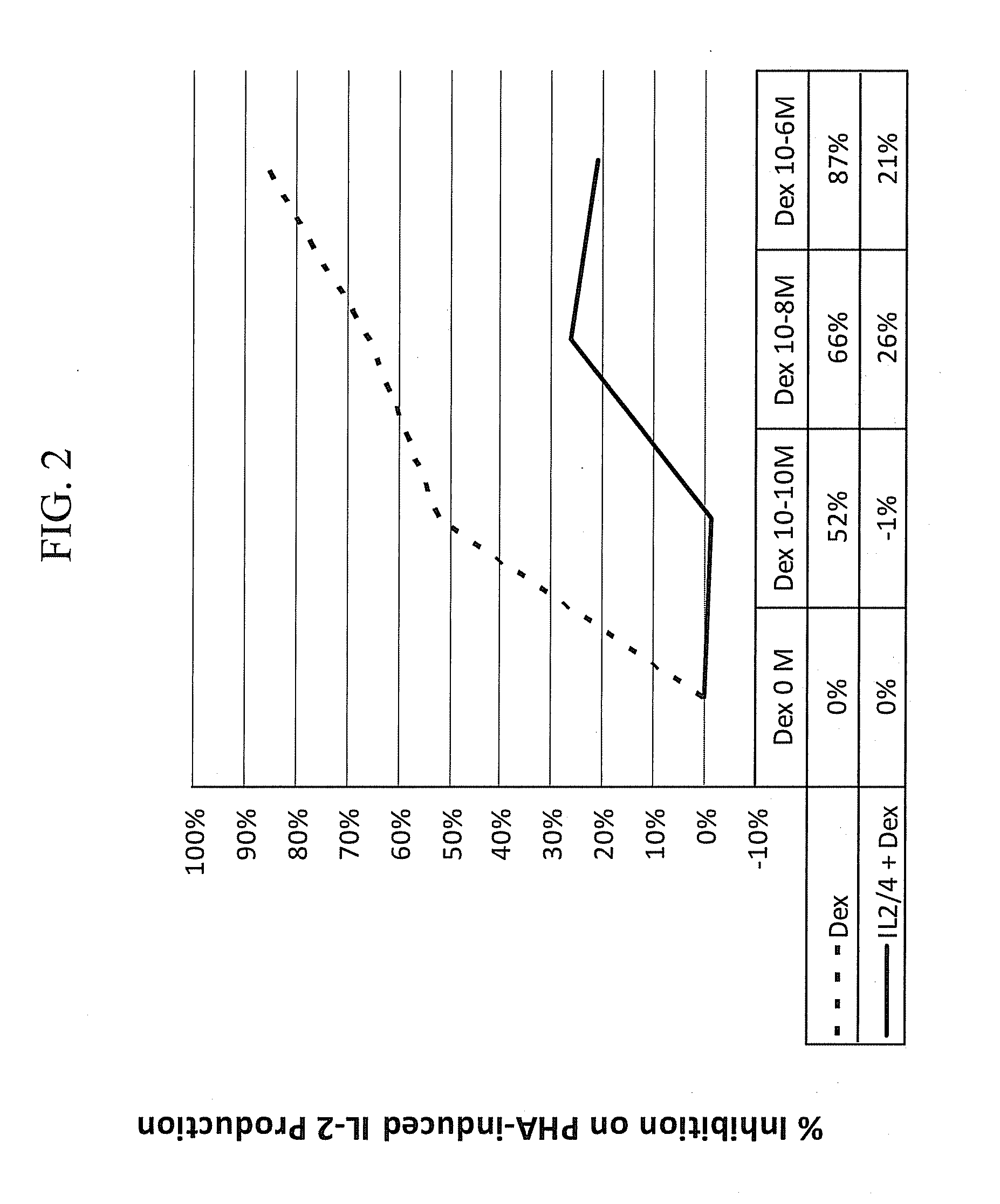
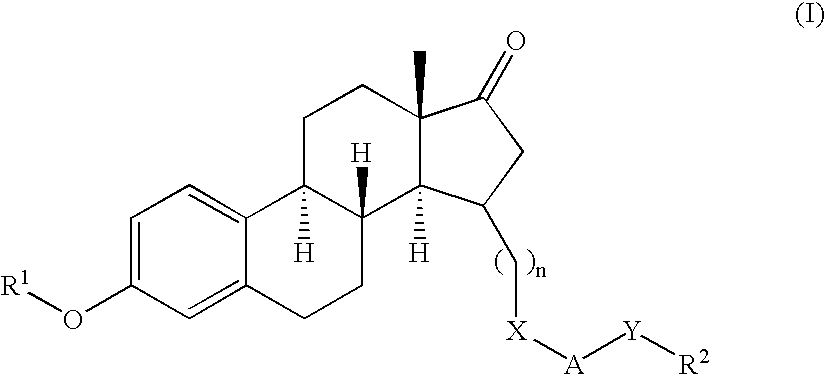
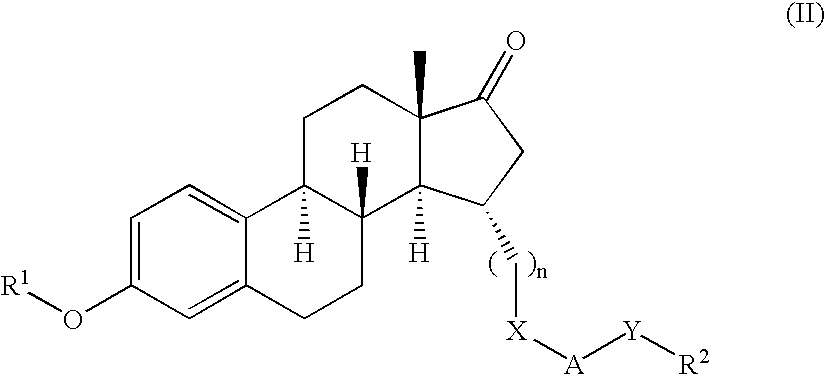
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
222 results about "Steroidal hormones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Steroid hormone, any of a group of hormones that belong to the class of chemical compounds known as steroids; they are secreted by three “steroid glands”—the adrenal cortex, testes, and ovaries—and during pregnancy by the placenta. All steroid hormones are derived from cholesterol.
Methods of generating novel peptides
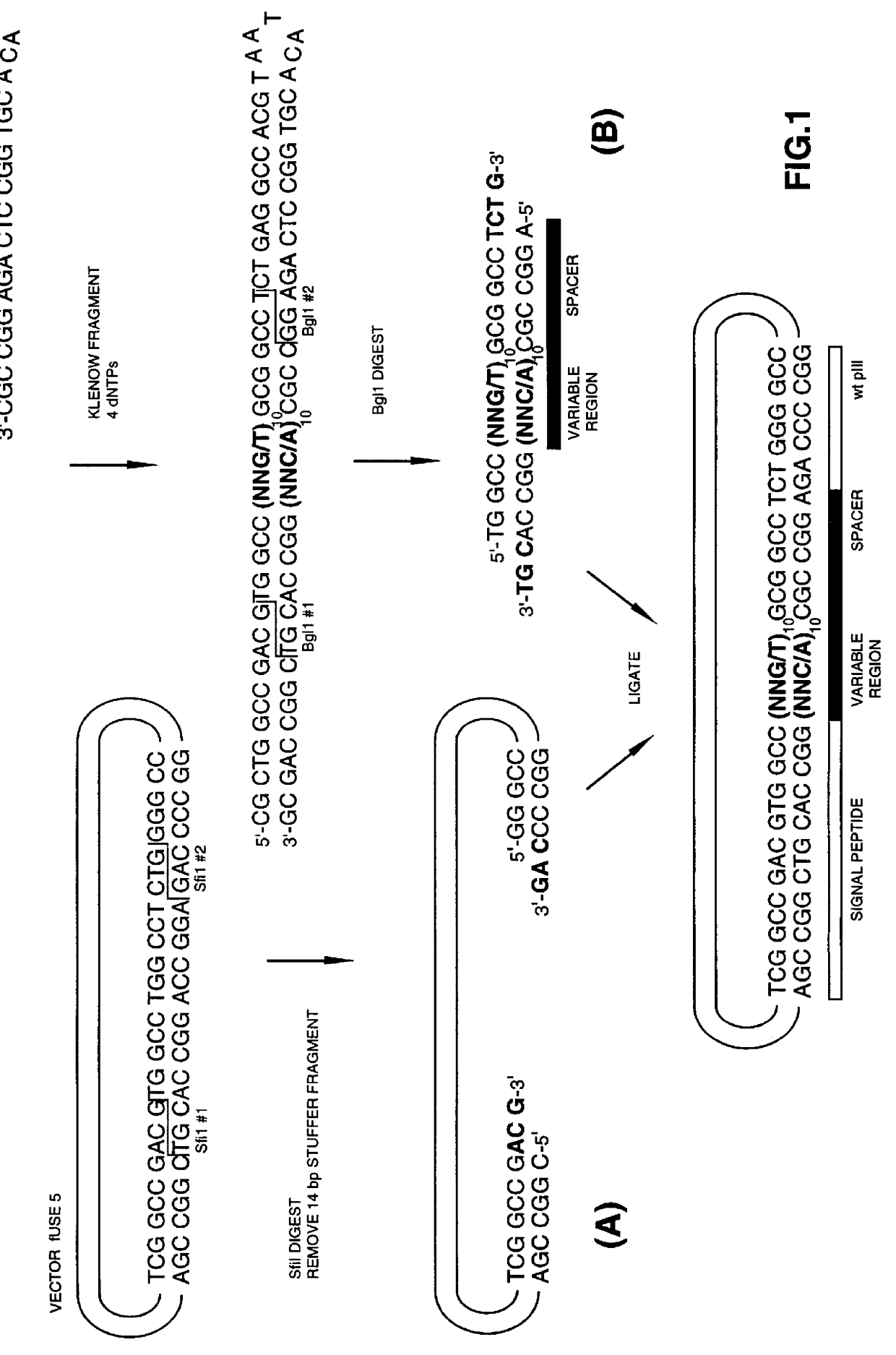
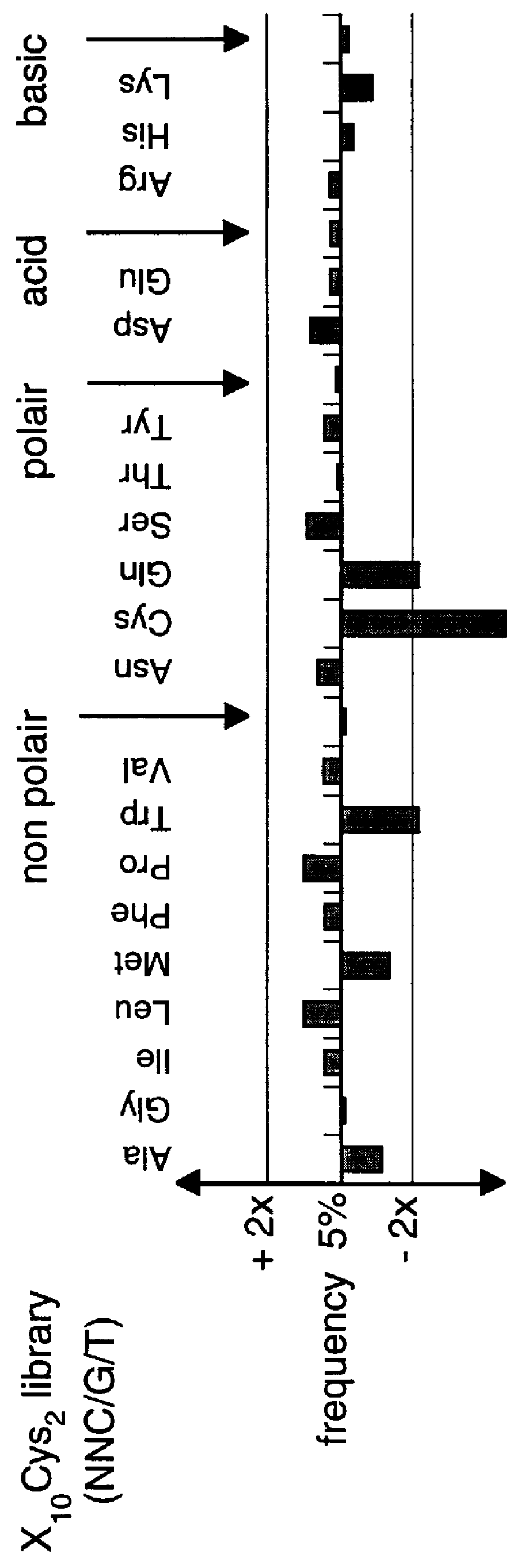
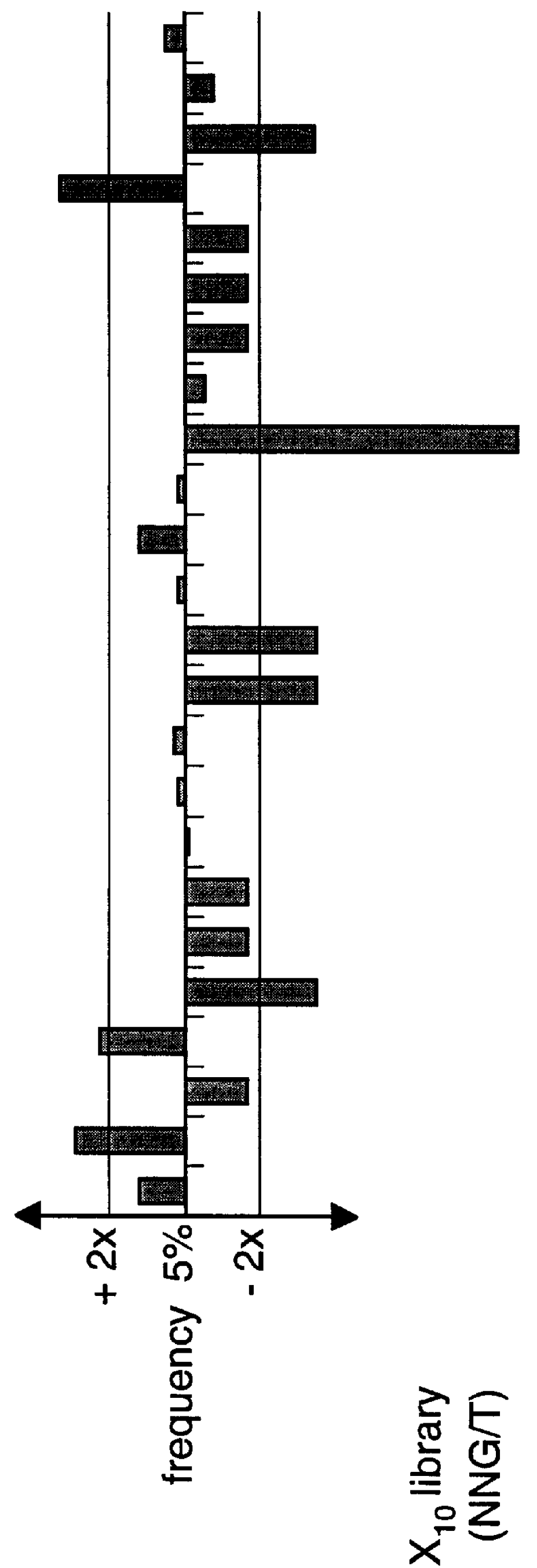
The present invention describes peptides capable of specifically binding to preselected micromolecules or to their natural receptor. The preselected molecules include but are not limited to drugs, vitamins, neuromediators and steroid hormones. Methods of using the phage display libraries to identify peptide compositions in preselected binding interactions are also disclosed. The retrieved peptides mimicking a natural receptor binding site to preselected molecules are used as is or as ligands to re-screen the same or different libraries to find and / or derive new receptor ligands, or are used to elicit the production of antibodies capable of binding to the natural receptor. The two categories of effector molecules (peptides or antibodies) may find diagnostic, therapeutic or prophylactic uses. The peptides directly derived from the phage display libraries may be used as drug detectors or antidotes. The others may be used to identify, target, activate or neutralize the receptor for the preselected micromolecules, the receptor being known or unknown.
Owner:BIOPHAGE
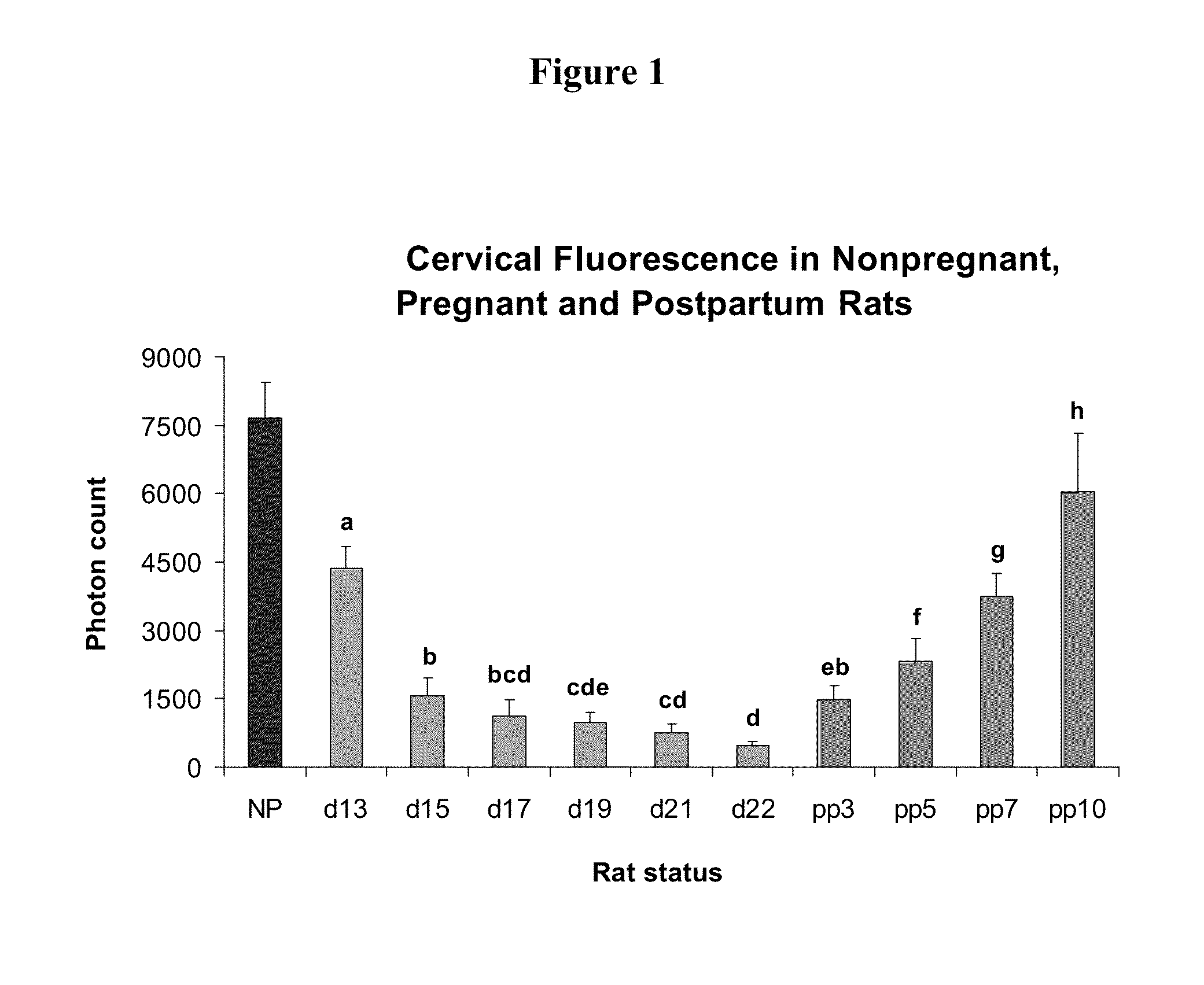
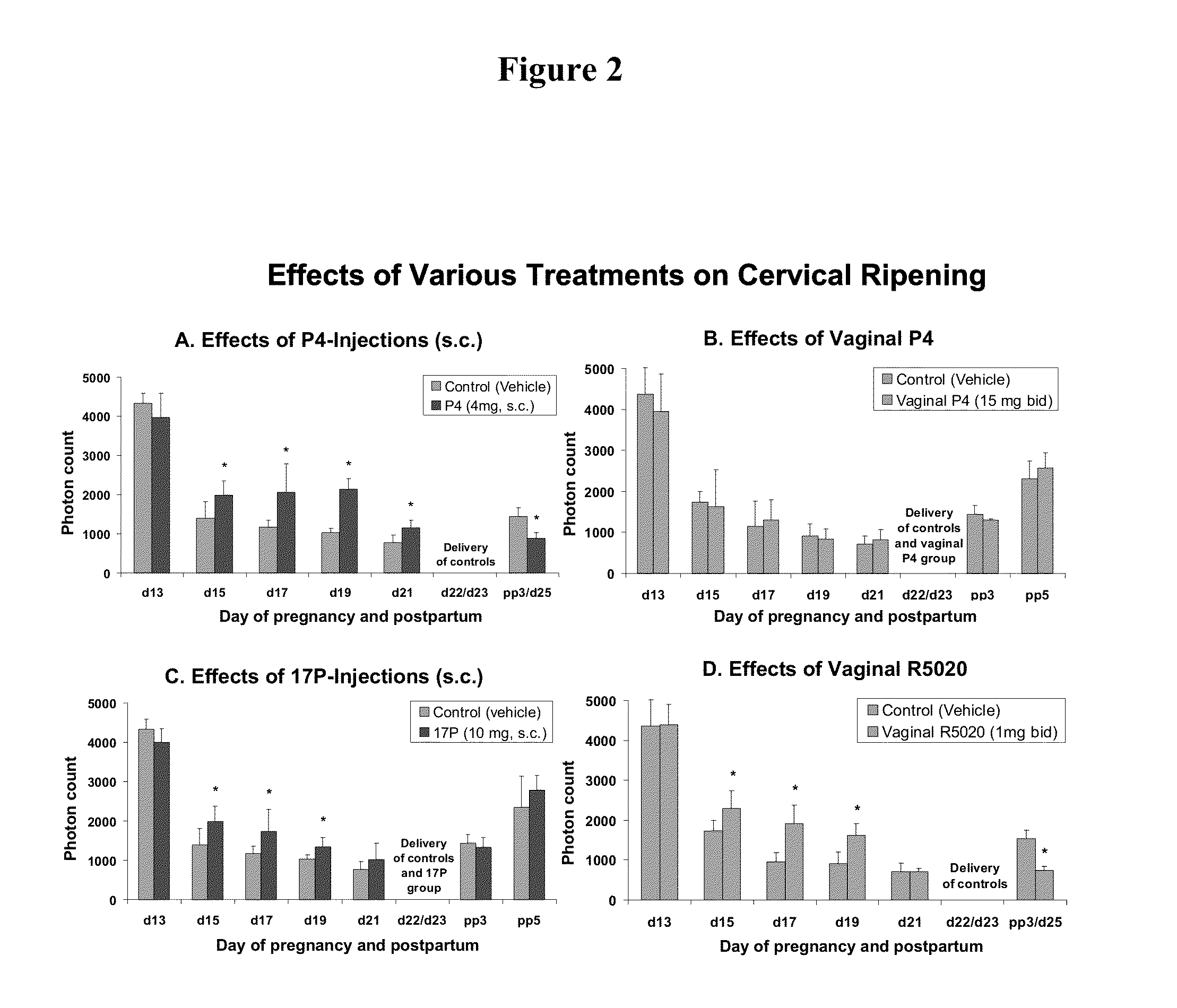
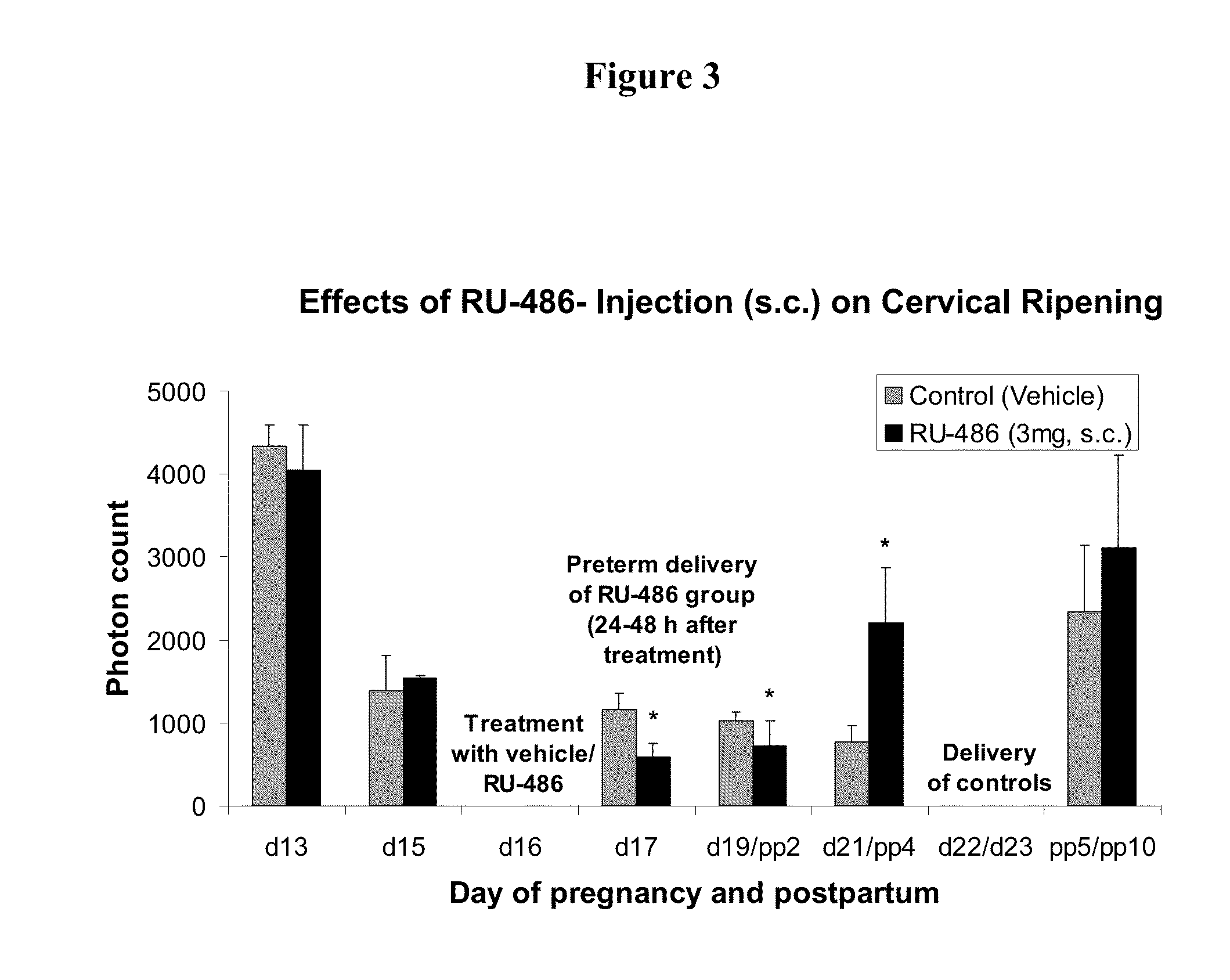
Methods for reducing the occurrence of preterm delivery and other pregnancy-related conditions
The present invention relates to methods and kits for reducing the occurrence of preterm delivery and other pregnancy-related conditions in pregnant female subjects exhibiting one or more risk factors for preterm delivery and other pregnancy-related conditions. For example, the present invention relates to methods for reducing the occurrence of preterm delivery in a pregnant female subject having no history of preterm delivery and exhibiting one or more risk factors for preterm delivery (e.g., smoking during pregnancy). The methods and kits provide for the administration of a steroid hormone to the pregnant female subject.
Owner:LUMARA HEALTH IP
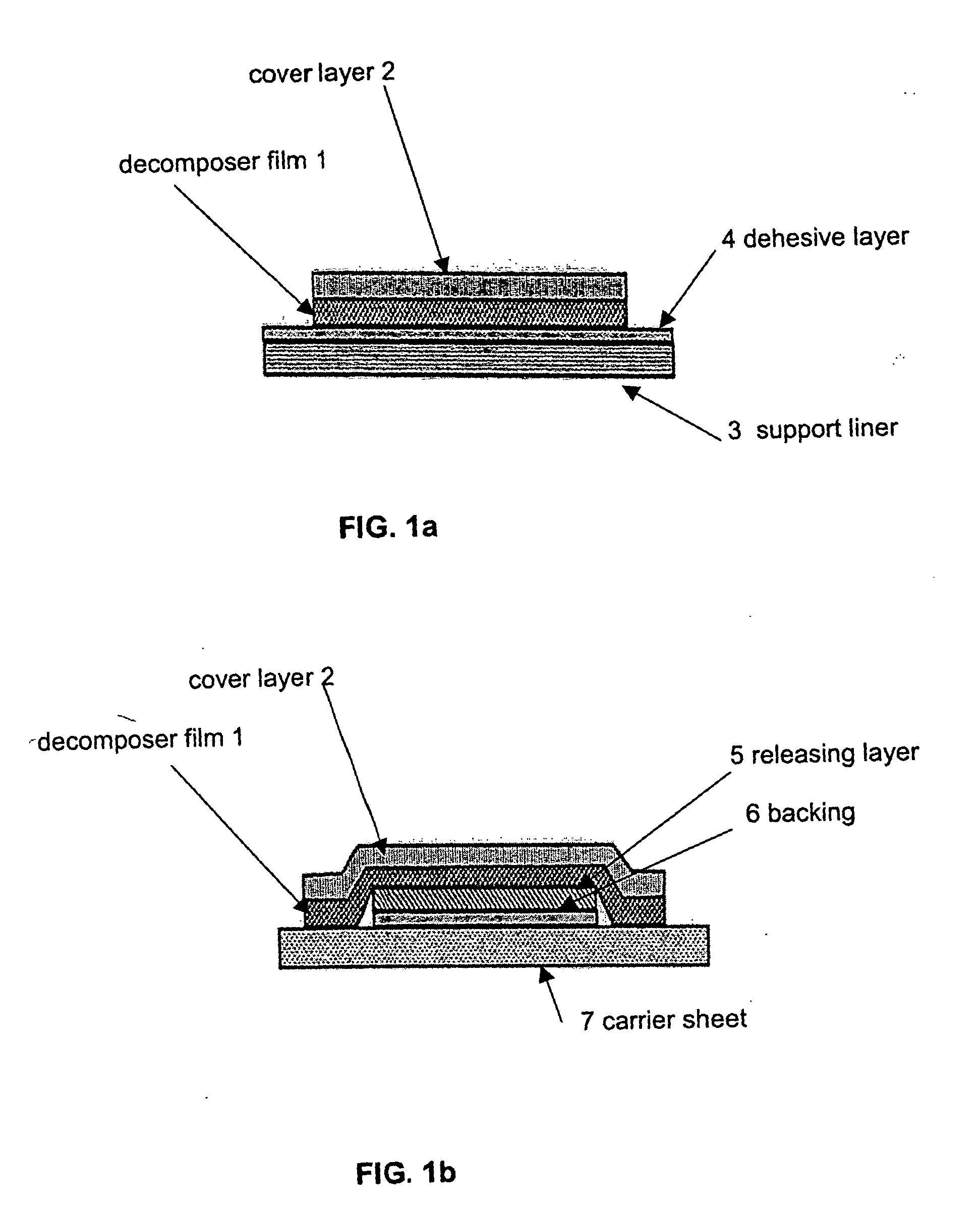
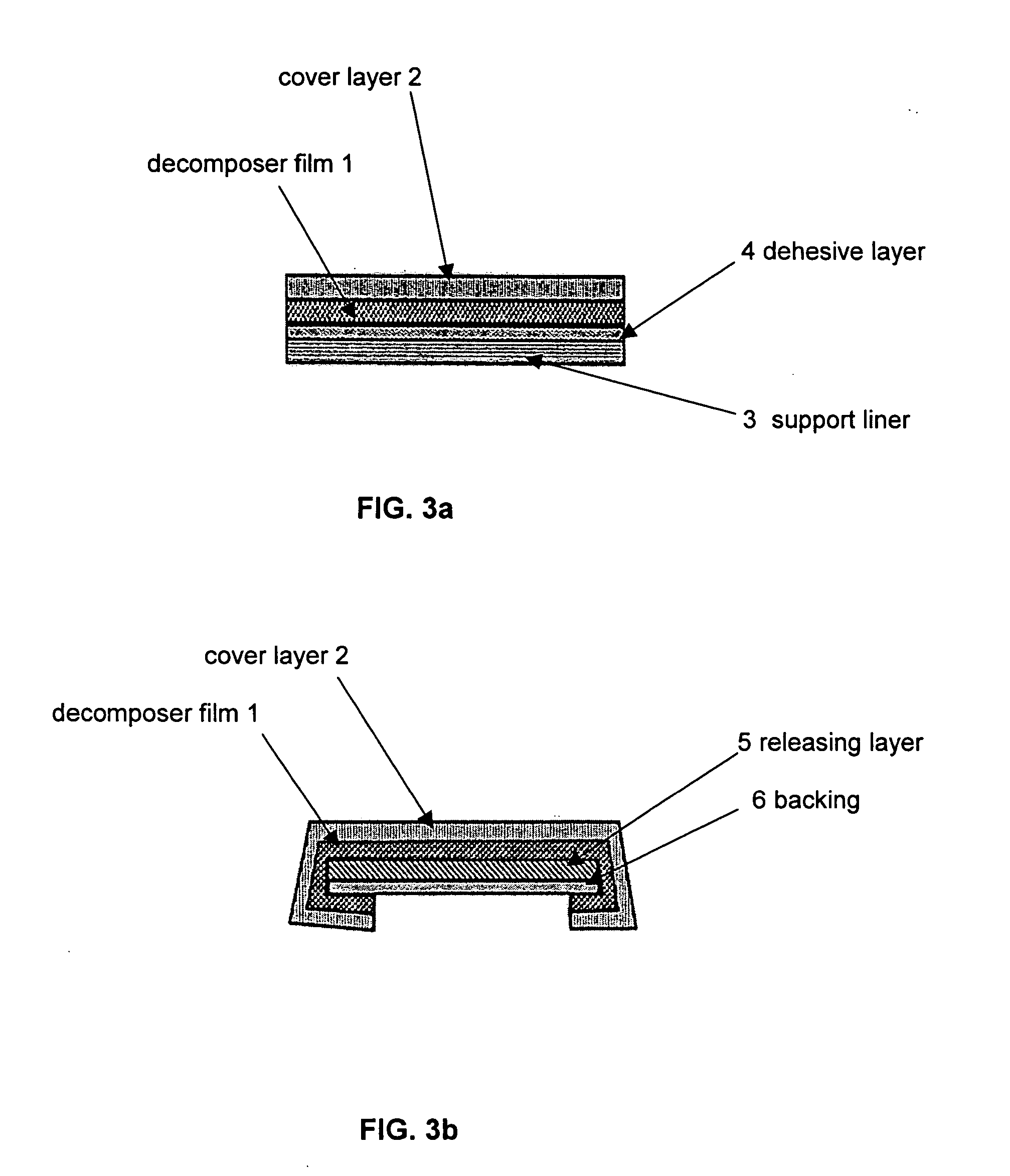
Decomposer film for transdermal patches
InactiveUS20070014839A1Increased internal surface areaAccelerate decomposition reactionOrganic active ingredientsAntipyreticTransdermal patchPolyvinyl alcohol
The decomposer film product has a polymeric decomposer layer, a cover layer for protecting the decomposer film from the surroundings and a releasable support liner, which is removed prior to use. The polymeric decomposer film contains a water-soluble or water-insoluble polymeric adhesive material and a decomposition accelerator, which acts to decompose an effective ingredient, such as a steroid hormone, of a worn or unused transderamal patch, when the effective ingredient releasing layer of the patch adheres to the polymer film, so that the pharmaceutical effective ingredient comes into contact with the decomposition accelerator by diffusion. The decomposition accelerator includes a chemically oxidizing substance, preferably urea peroxide, manganese (III) acetate or iron (III) citrate. The water-insoluble polymeric adhesive material is preferably an acrylates adhesive. The water-soluble polymeric adhesive material is preferably polyvinyl alcohol, polyvinyl pyrrolidone, a cellulose derivative or a polyacrylic acid.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Methods for inhibiting preterm labor and uterine contractility disorders and preventing cervical ripening
InactiveUS20130023505A1Inhibit uterine contractilityInhibit cervical ripeningOrganic active ingredientsPharmaceutical delivery mechanismMyometrial contractilityGynecology
The invention relates to methods and pharmaceutical compositions for inhibiting or preventing preterm birth, inhibiting or delaying cervical ripening, inhibiting myometrial contractility and treating or inhibiting uterine contractility disorders. The methods comprise administering an effective amount of a composition comprising steroid hormones such as soluble progesterone.
Owner:DIGNITY HEALTH
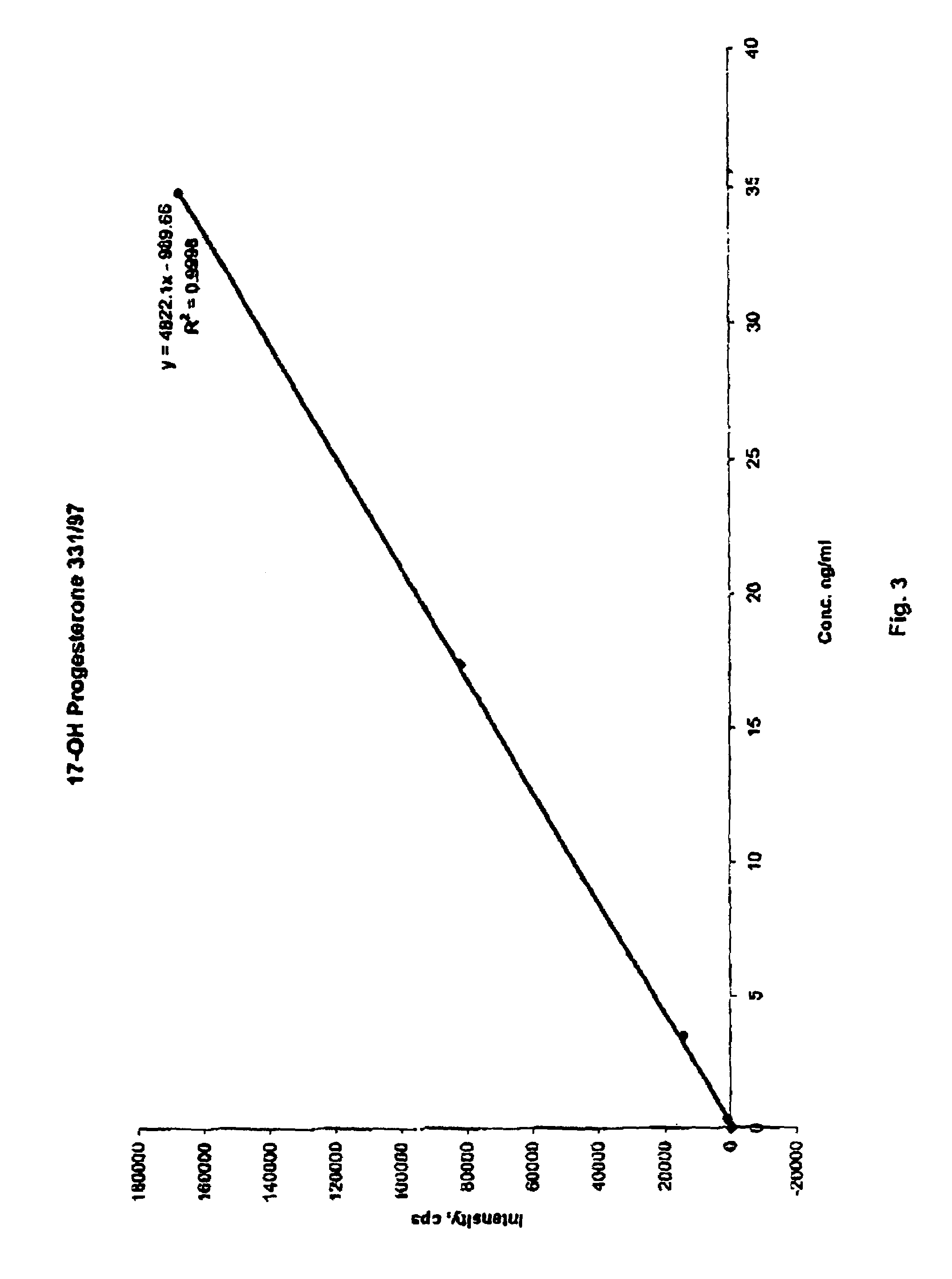
Steroid hormone analysis by mass spectrometry
ActiveUS7473560B2Fast and accurate methodFast and accurate of and quantificationParticle separator tubesComponent separationMedicineMass Spectrometry-Mass Spectrometry
Methods, systems and kits for the simultaneous or sequential analysis of a multitude of steroid hormones by mass spectrometry are disclosed. The methods require minimal sample size and minimal preparation time. The methods include ionizing the hormones and analyzing the hormones by mass spectrometry. In addition, methods, systems and kits for the simultaneous or sequential analysis of steroid hormones are disclosed including ionization of the steroid hormones by photoionization.
Owner:GEORGETOWN UNIV
Highly concentrated drug particles, formulations, suspensions and uses thereof
Highly concentrated drug particle formulations are described, wherein the drug comprises between about 25 wt % and 80 wt % of the particle formulation. The particle formulations of the present invention comprise, for example, macromolecules, such as proteins and / or small molecules (such as steroid hormones). The particle formulation typically further includes one or more additional component, for example, one or more stabilizer (e.g., carbohydrates, antioxidants, amino acids, and buffers). Such concentrated particle formulations can be combined with a suspension vehicle to form suspension formulations. The suspension formulation comprises (i) a non-aqueous, single-phase vehicle, comprising one or more polymer and one or more one solvent, wherein the vehicle exhibits viscous fluid characteristics, and (ii) a highly concentrated drug particle formulation. Devices for delivering the suspension formulations and methods of use are also described. The present invention provides needed improvements in drug formulation and delivery to improve patient compliance and expand drug availability.
Owner:INTARCIA THERAPEUTICS INC
Steroid hormone products and methods for preparing them
ActiveUS7867990B2Improved dissolution profileImprove solubilityPowder deliveryOrganic active ingredientsPhysiologyMechanical energy
The present invention relates to steroid hormone products, such as oral contraceptive products, including at least one steroid active ingredient mixed with an excipient and having improved dissolution and release rate properties. The invention further relates to methods for making such steroid hormone products, wherein a mixture of the hormone and the excipient is subjected to sufficient mechanical energy to form a powder blend wherein the hormone is stabilized by the excipient in substantially non-crystalline form.
Owner:ORTHO MCNEIL PHARM INC
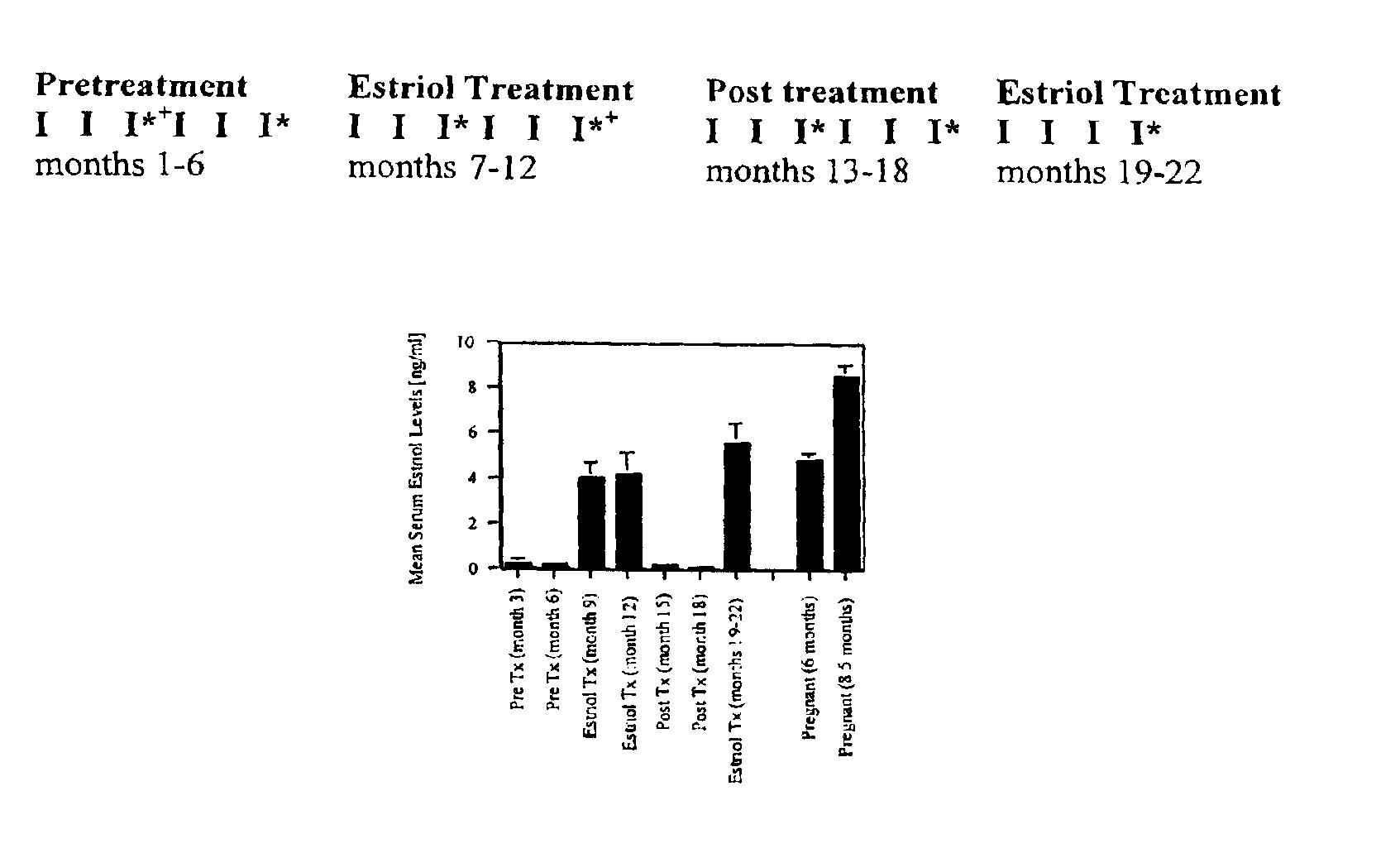
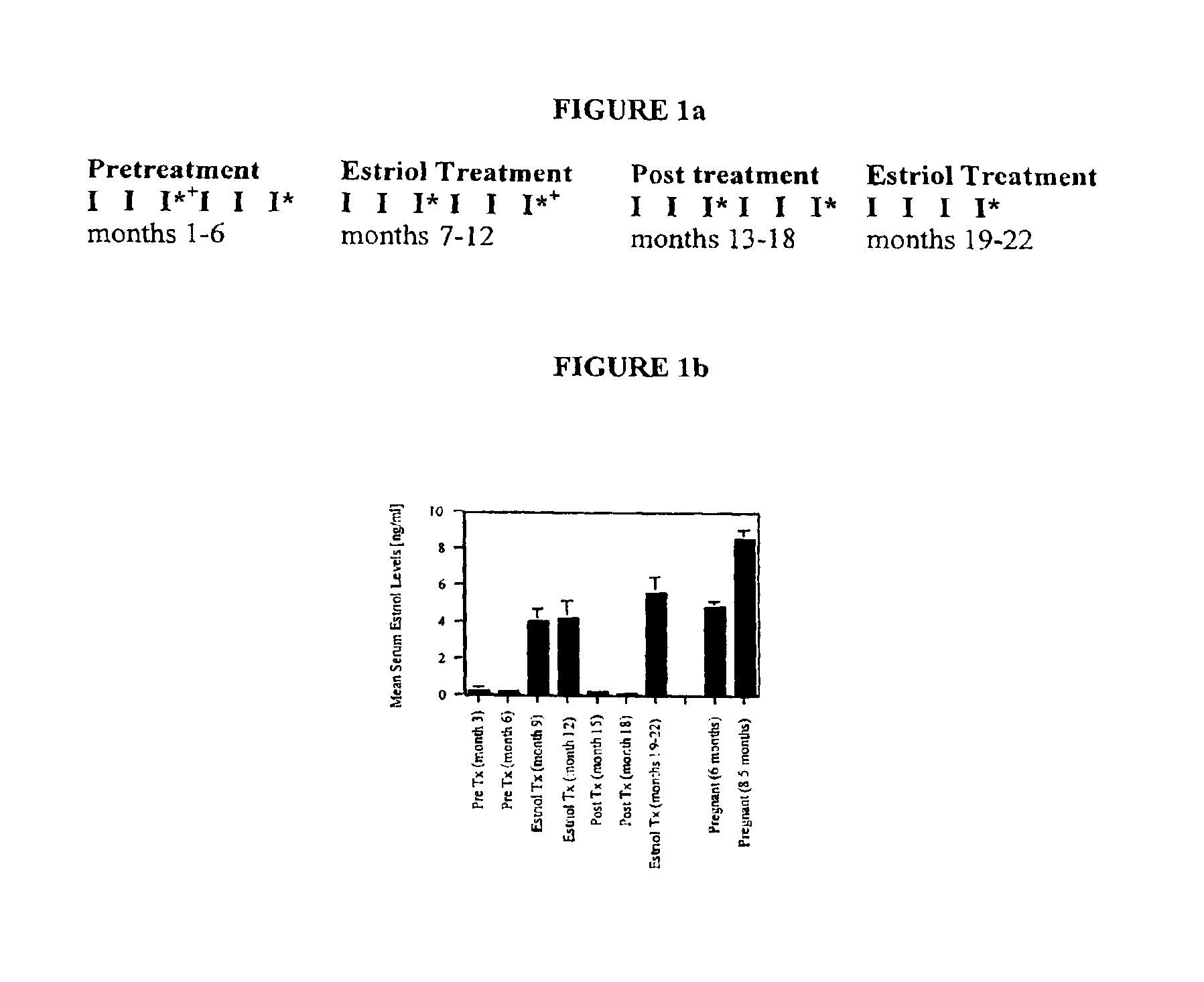
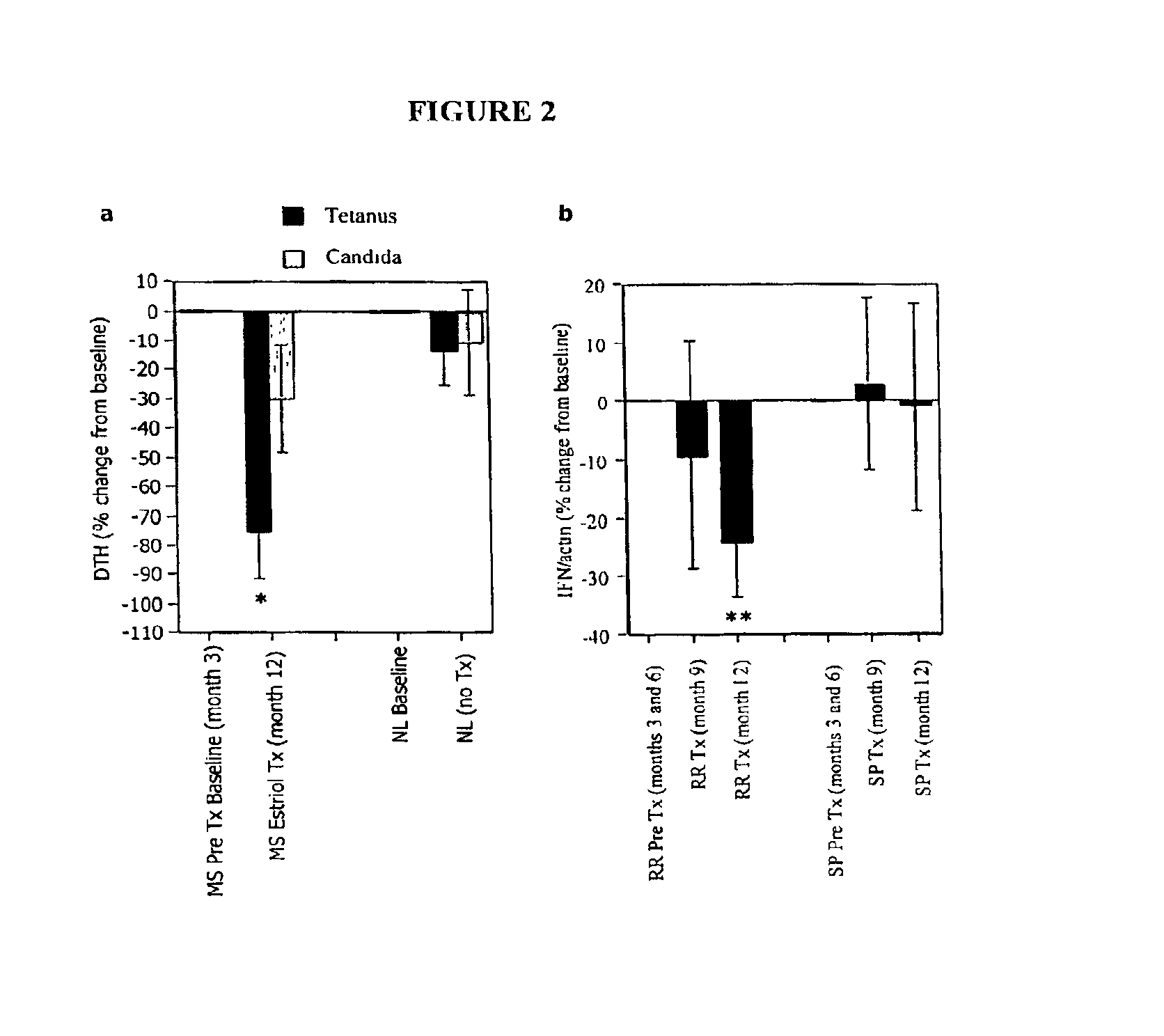
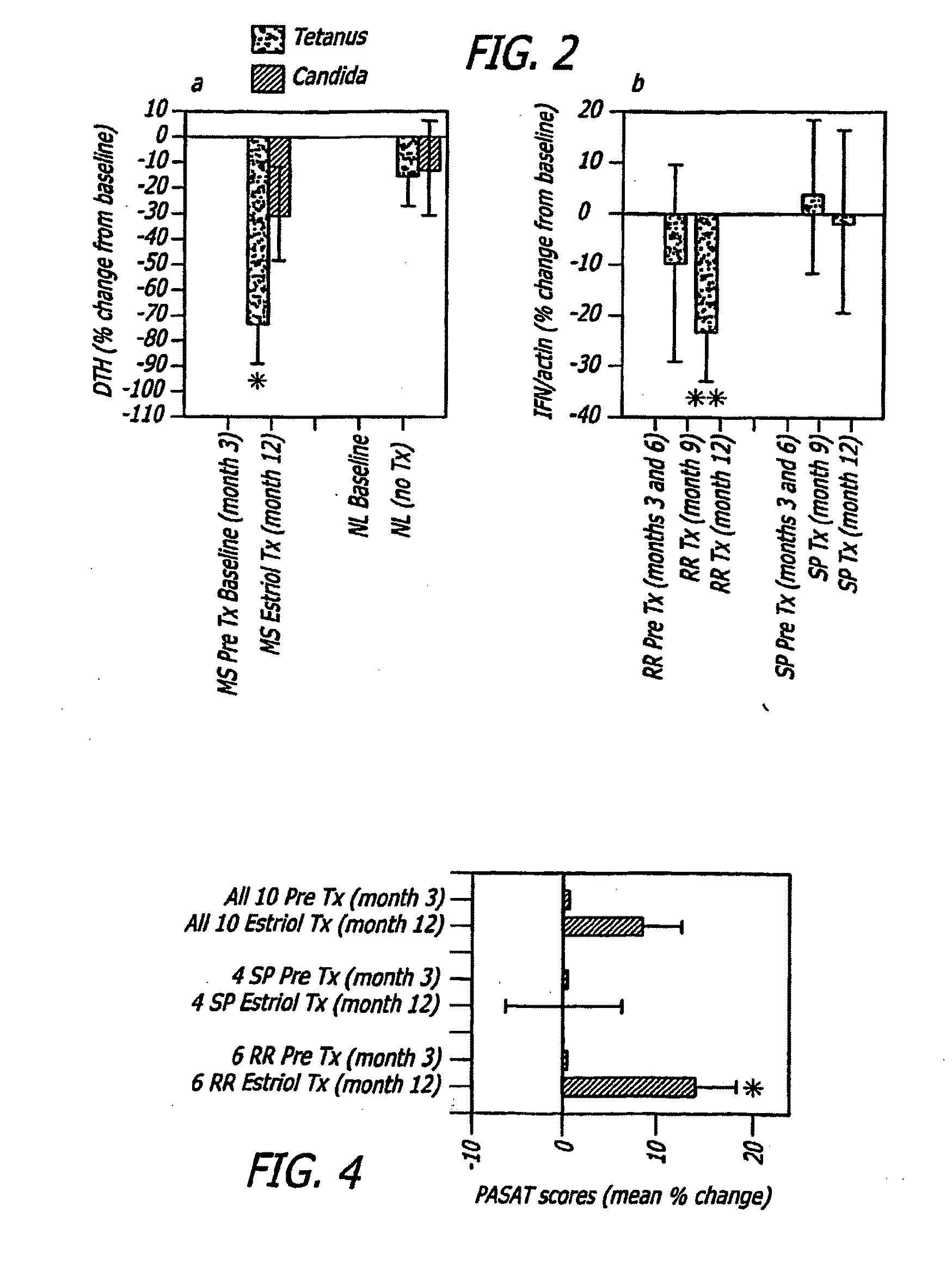
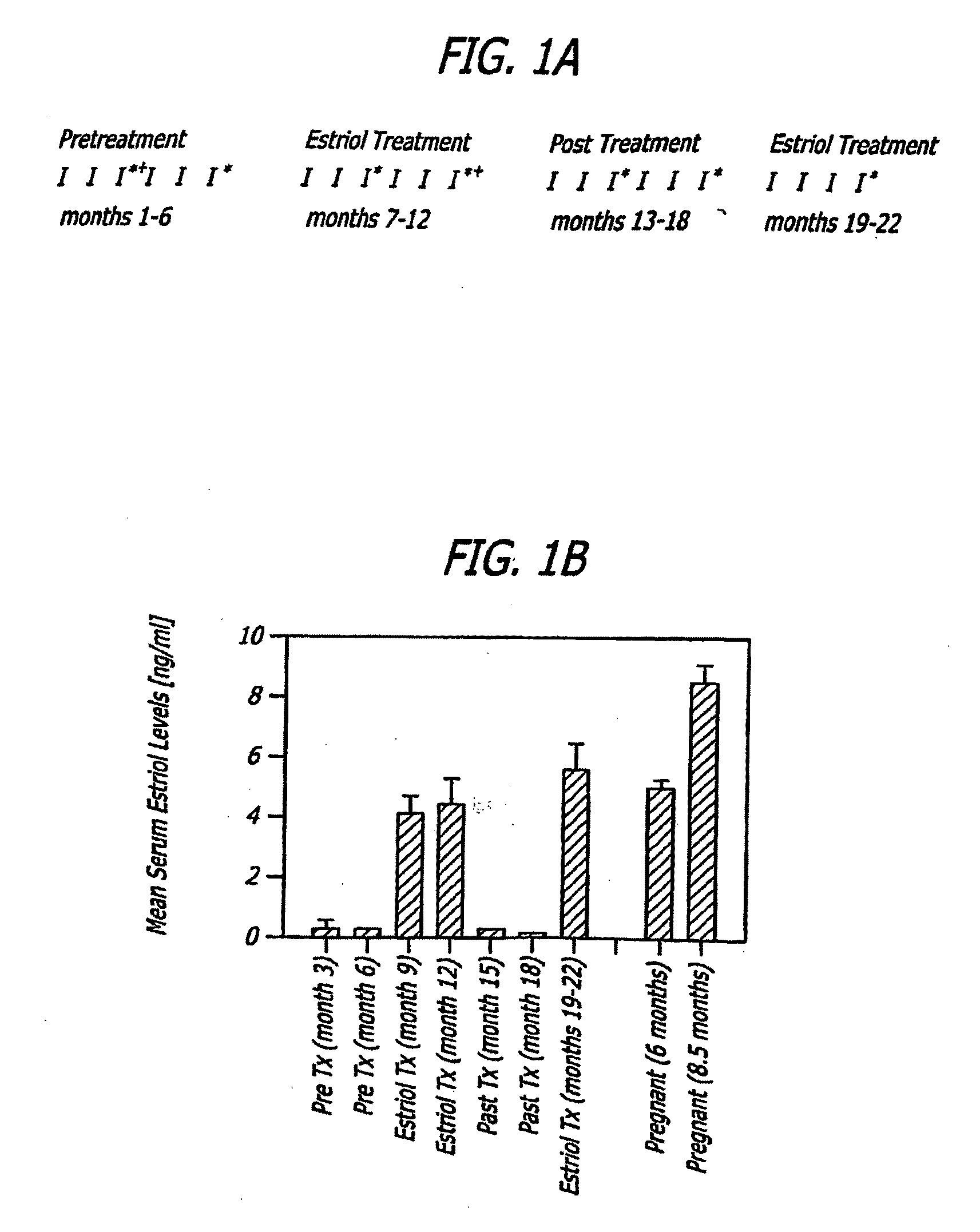
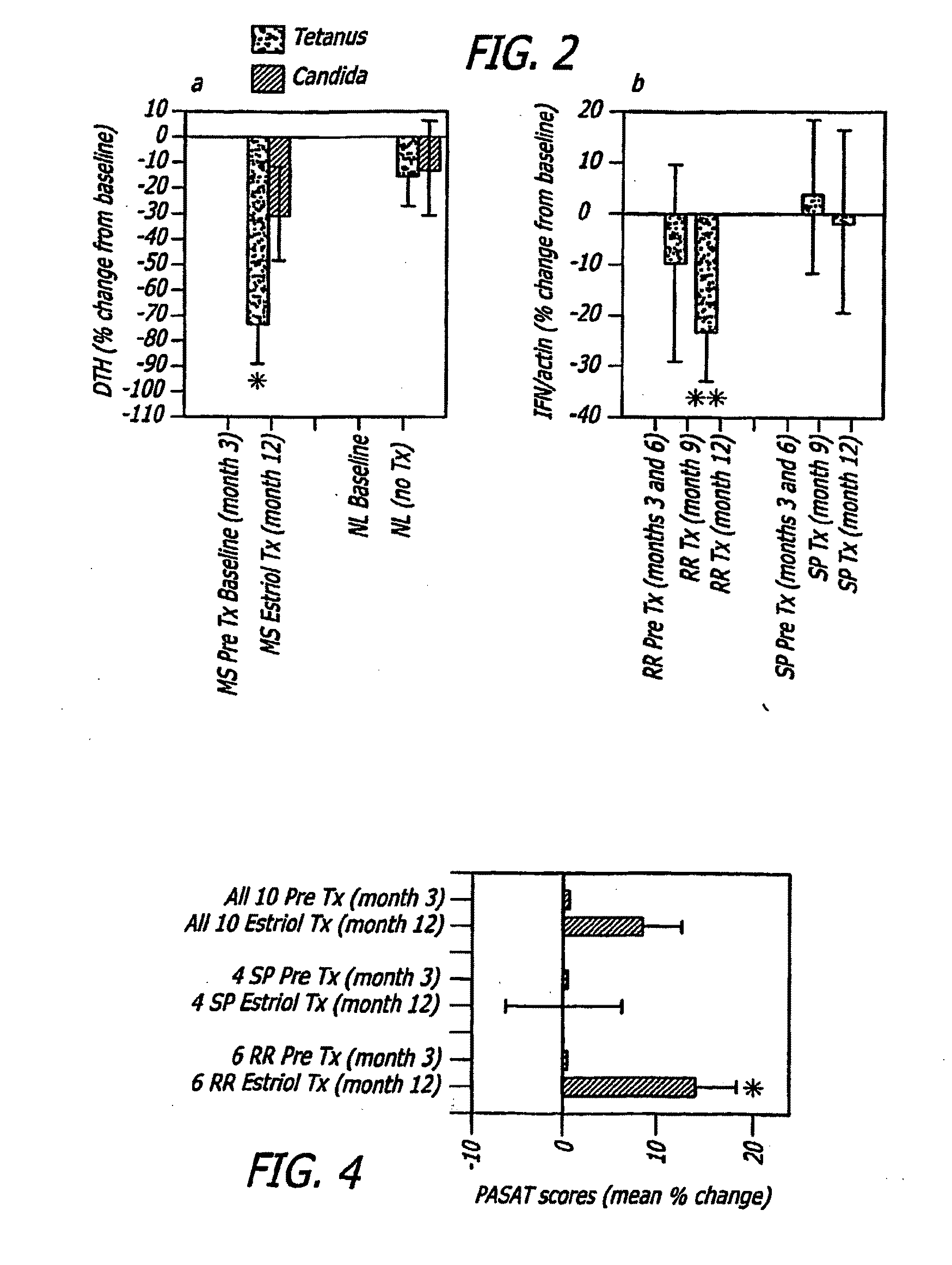
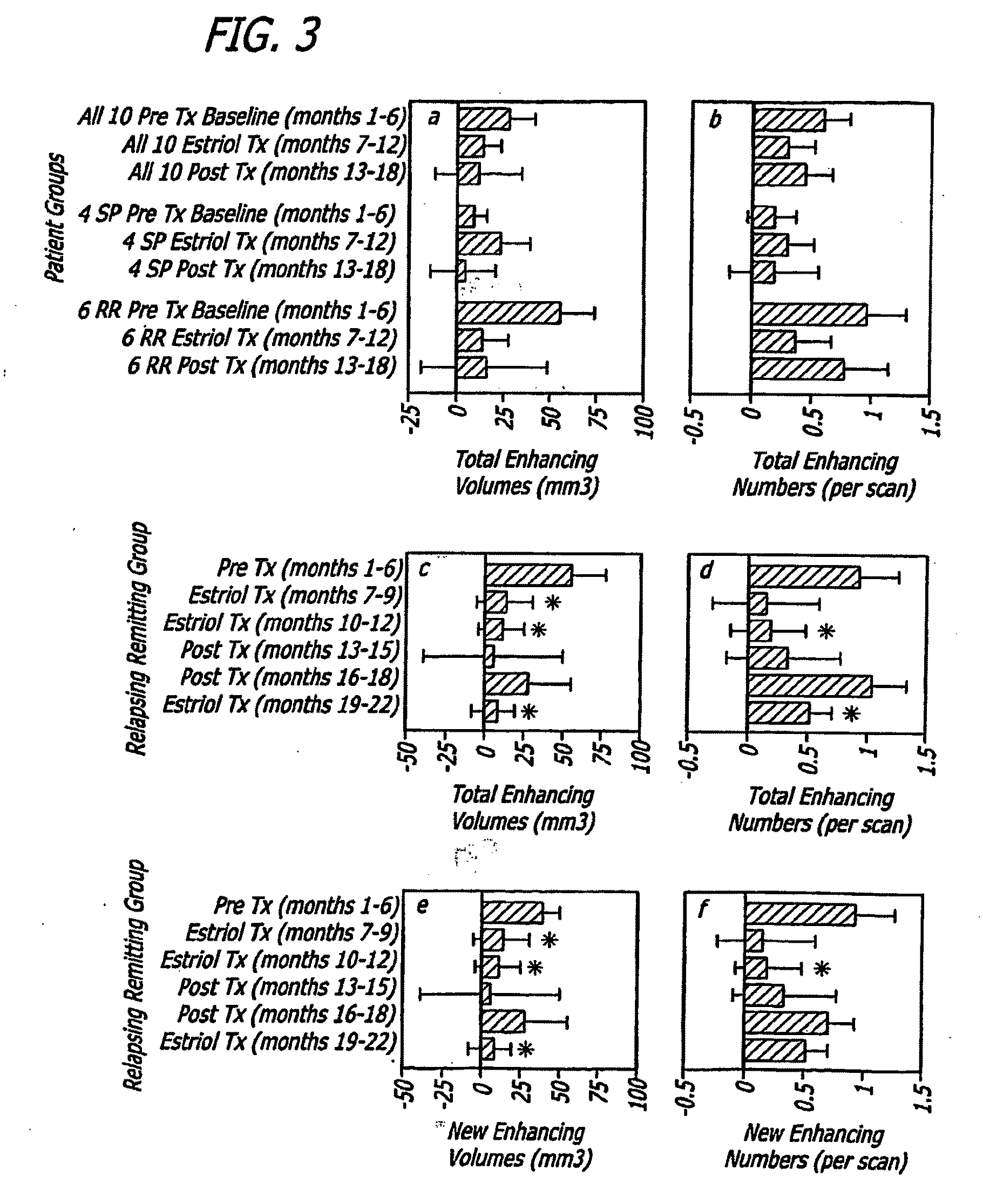
Estriol therapy for multiple sclerosis and other autoimmune diseases
The present invention discloses administering steroid hormones to mammals to treat autoimmune related diseases, more particularly, Th1-mediated (cell-mediated) autoimmune diseases including: multiple sclerosis (MS), rheumatoid arthritis (RA), autoimmune thyroiditis and uveitis. Most preferably the invention is used to treat a patient with a therapeutically effective amount of estriol of 8 milligrams once daily via oral administration to treat the symptoms or prevent the onset of multiple sclerosis.
Owner:RGT UNIV OF CALIFORNIA
Estriol therapy for multiple sclerosis and other autoimmune diseases
Owner:RGT UNIV OF CALIFORNIA
Estriol Therapy for Autoimmune and Neurodegenerative Diseases and Disorders
The present invention discloses administering steroid hormones to mammals to treat autoimmune related diseases, neurodegenerative diseases or disorders, such as Alzheimer's disease, Parkinson's Disease, multiple sclerosis, stroke, ALS Pick's disease, prion disease and Huntington's disease. Most preferably the invention uses estrogens, estranges, estriol or estrogen receptor active agents to prevent or ameliorate clinical symptoms of the diseases and disorders.
Owner:RGT UNIV OF CALIFORNIA
Estriol Therapy for Autoimmune and Neurodegenerative Diseases and Disorders
The present invention discloses administering steroid hormones to mammals to treat autoimmune related diseases, neurodegenerative diseases or disorders, such as Alzheimer's disease, Parkinson's Disease, multiple sclerosis, stroke, ALS Pick's disease, prion disease and Huntington's disease. Most preferably the invention uses estrogens, estranges, estriol or estrogen receptor active agents to prevent or ameliorate clinical symptoms of the diseases and disorders.
Owner:RGT UNIV OF CALIFORNIA
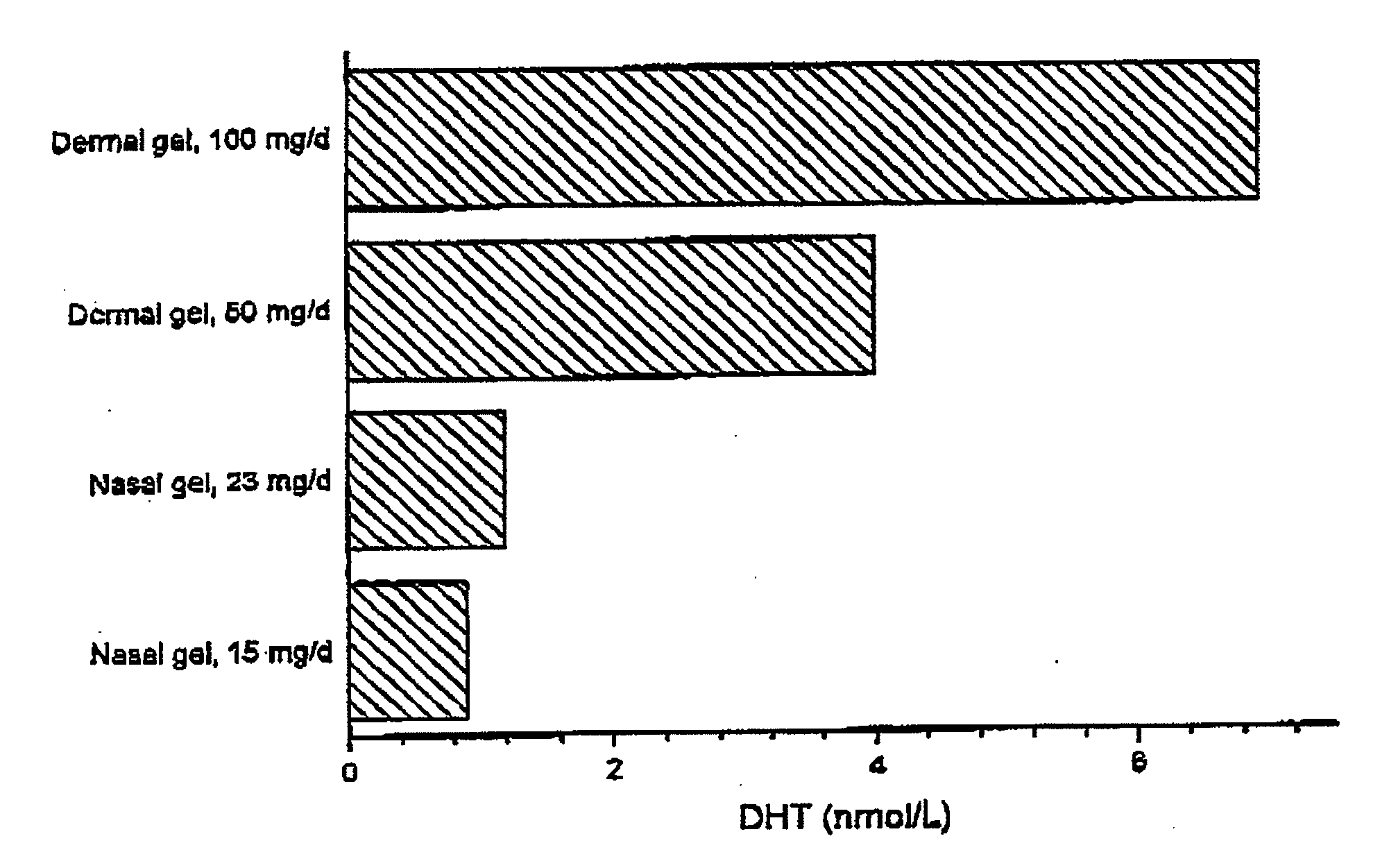
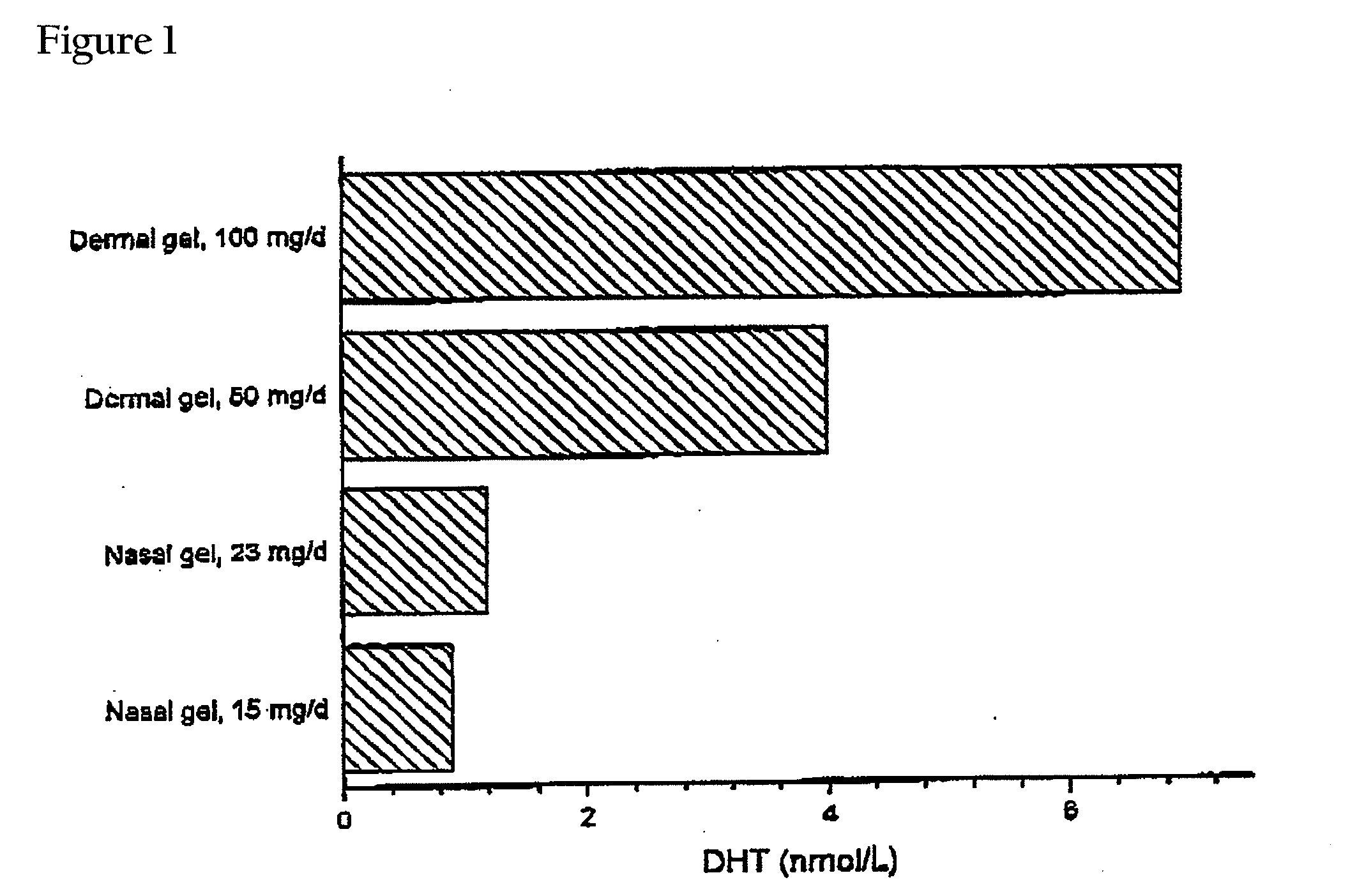
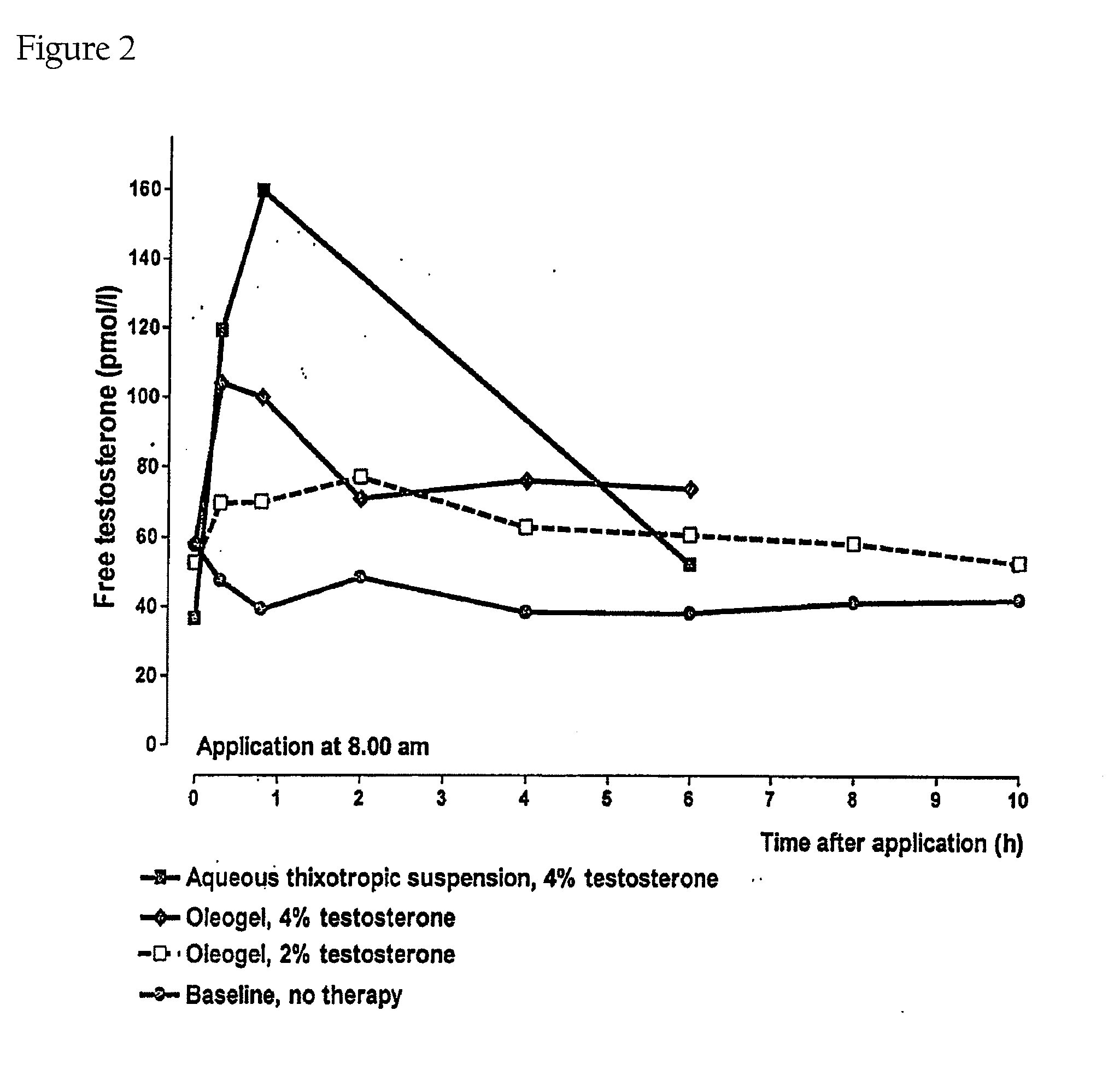
Controlled Release Delivery System for Nasal Applications and Method of Treatment
InactiveUS20070149454A1Improve bioavailabilityImproved profileOrganic active ingredientsBiocideFemale Sexual Arousal DisorderNasal cavity
This invention relates to a gel formulation for nasal administration of a controlled release formulation of hormones to the systemic circulation and / or to the brain. The special lipophilic or partly lipophilic system of the invention leads to higher bioavailability of the active ingredient caused by sustained serum levels in plasma but also leads to a more favorable serum level profile. The special lipophilic or partly lipophilic system also allows for the modulation of brain functioning. The invention also relates to the nasal administration of steroid hormones for treatment of female sexual dysfunction (FSD) or female arousal disorder.
Owner:MATTERN PHARMA
17SS-HSD1 and STS inhibitors
The present invention relates to novel substituted steroid derivatives which represent selectiv inhibitors of the 17β-hydroxysteroid dehydrogenase type I (17β-HSD1) and, in addition, which may represent inhibitors of the steroid sulphatase, as well as to their salts, to pharmaceutical preparations containing these compounds and to processes for the preparation of these compounds. Furthermore, the invention concerns the therapeutic use of said novel substituted steroid derivatives, particularly their use in the treatment, inhibition, prophylaxis or prevention of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I and / or steroid sulphatase enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration.
Owner:ABBVIE PHARMA GMBH
Methods for improved targeting of antibody, antibody fragments, hormones and other targeting agents, and conjugates thereof
InactiveUSRE38008E1Improve localizationReduce productionIn-vivo radioactive preparationsPeptide/protein ingredientsAntibody fragmentsEphA Receptors
Methods for improved targeting of antibody, antibody fragments, peptides hormones, steroid hormones and conjugates thereof are disclosed. Enhanced delivery to target cells of antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, specific for a population of cells of a mammal comprises steps of administering to said mammal an adequate dosage of blocking antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, and administering to said mammal an effective dosage of said antibodies or fragments thereof or other receptor-mediated delivery system, such as peptide, specific for said population of cells. In the preferred embodiment, the specific antibodies are monoclonal antibodies directed toward tumor-associated antigen in man.
Owner:IDEC PHARM CORP
Non-peptide GNRH agents, methods and intermediates for their preparation
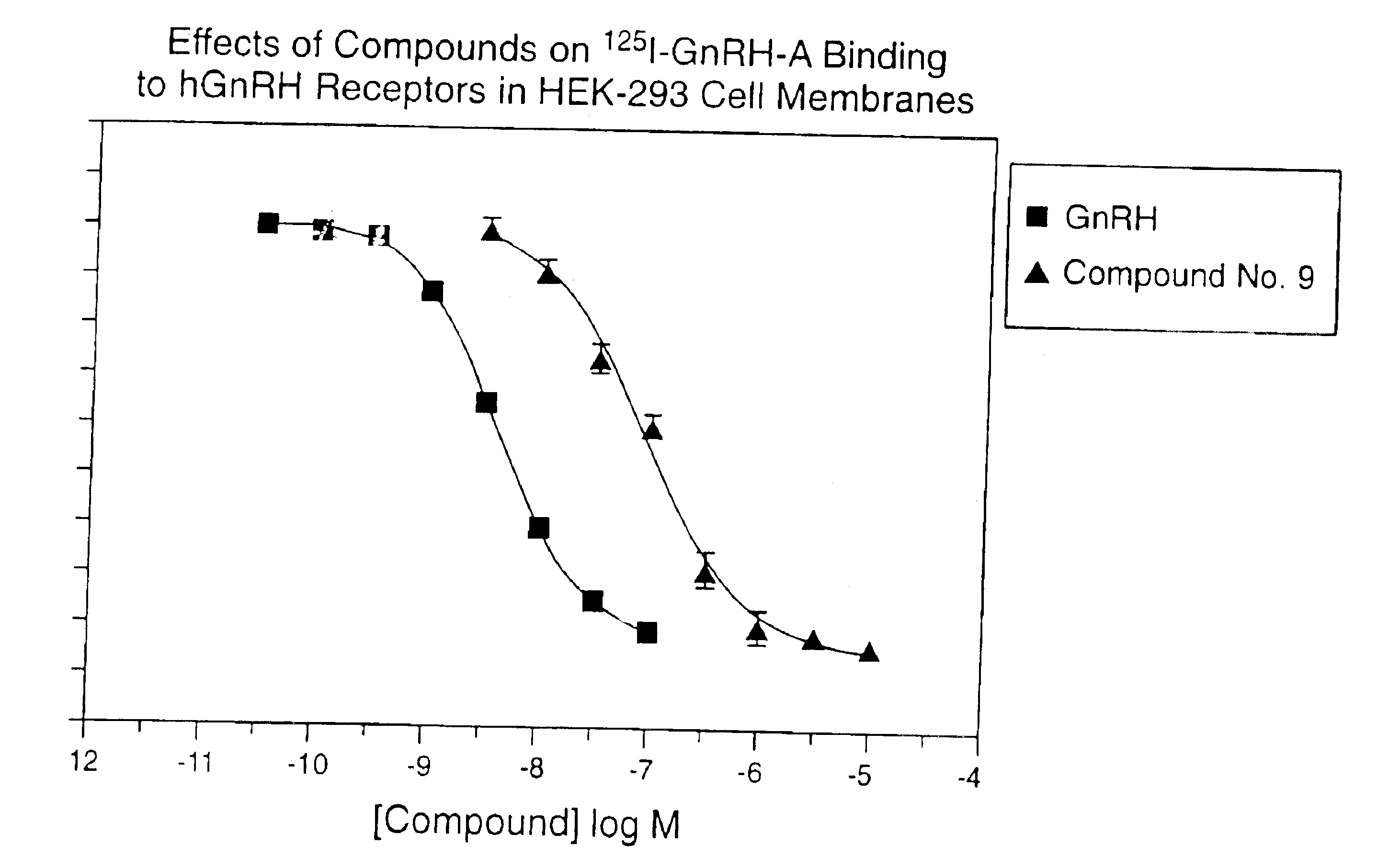
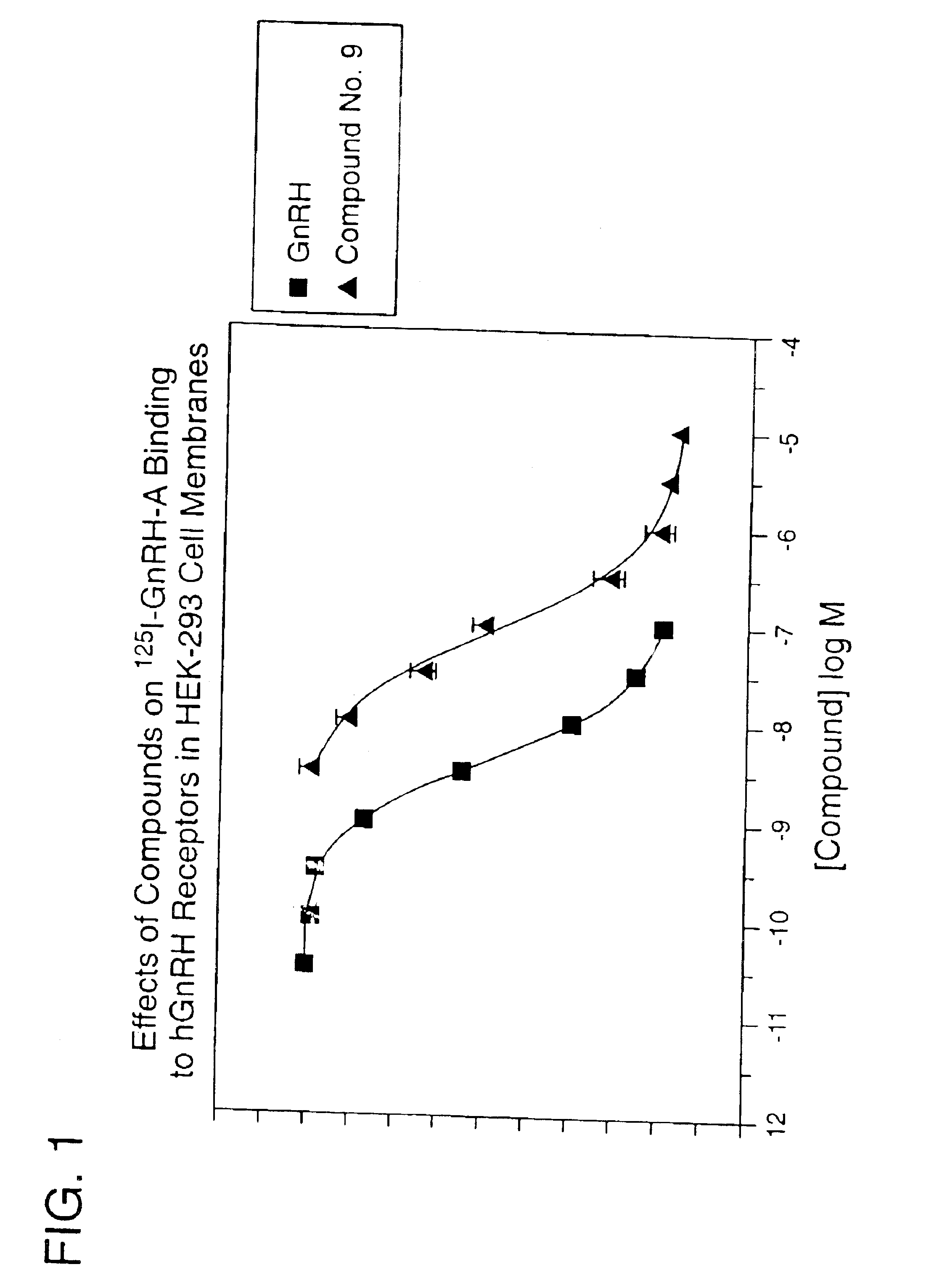
InactiveUS7101878B1Modulate activityGood biodistributionBiocideOrganic chemistrySteroidal hormonesGonadotropin-releasing hormone
Non-peptide GnRH agents capable of inhibiting the effect of gonadotropin-releasing hormone are described. Such compounds and their pharmaceutically acceptable salts, multimers, prodrugs, and active metabolites are suitable for treating mammalian reproductive disorders and steroid hormone-dependent tumors as well as for regulating fertility, where suppression of gonadotropin release is indicated. Methods for synthesizing the compounds and intermediates useful in their preparation are also described.
Owner:AGOURON PHARMA INC
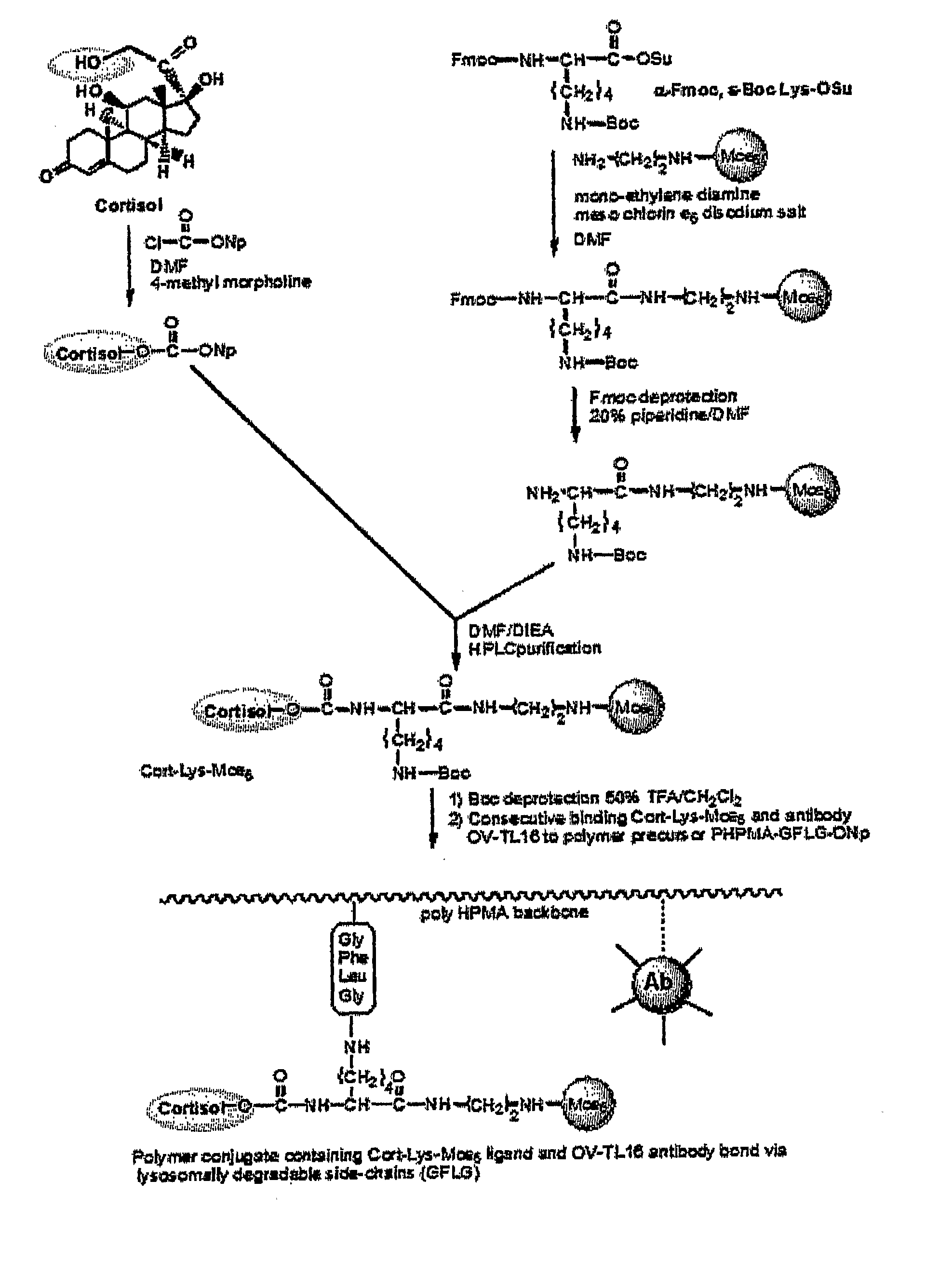
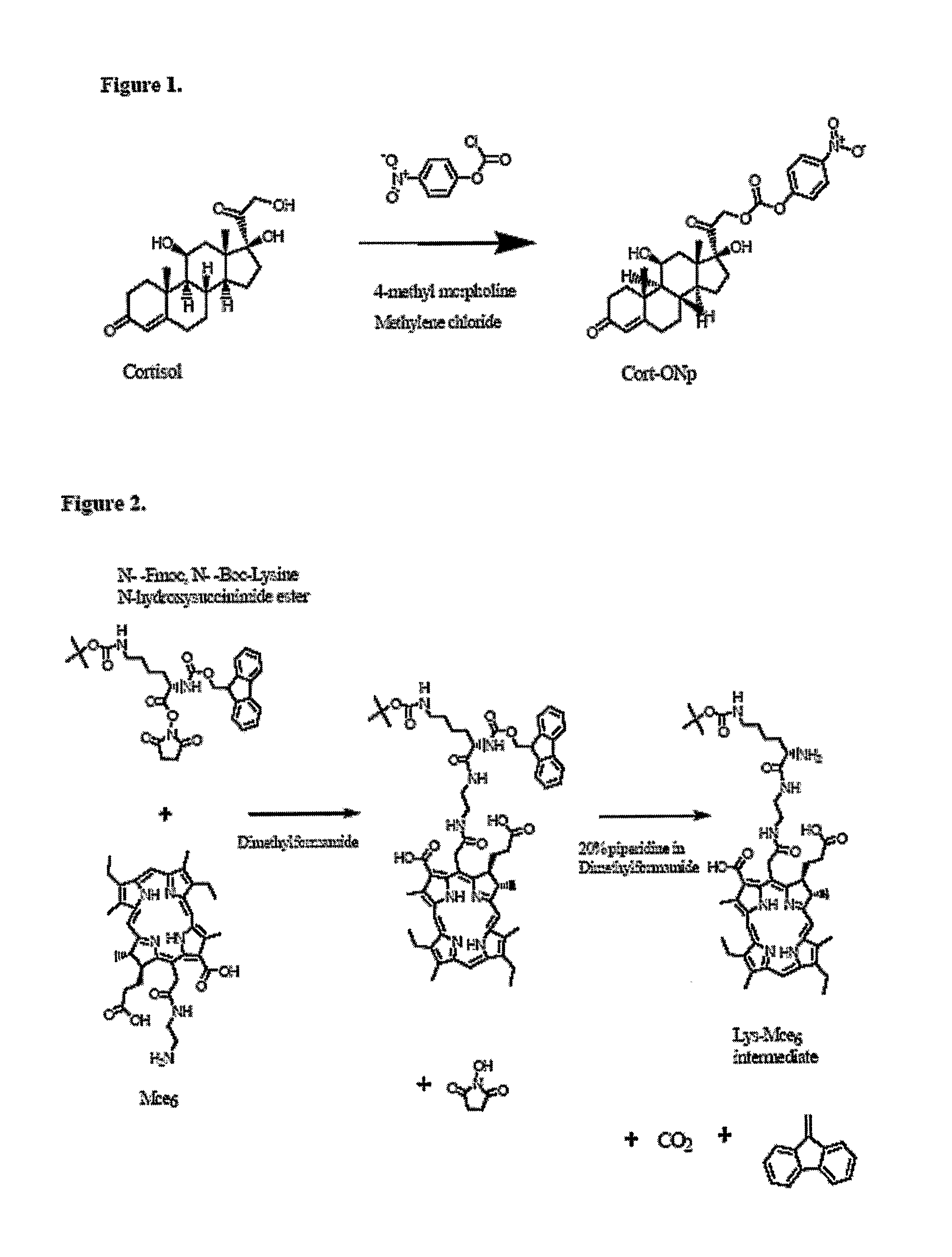
Combined Active and Passive Targeting of Biologically Active Agents
InactiveUS20070287680A1Good curative effectLow in DOPolypeptide with localisation/targeting motifOrganic active ingredientsActive agentEfficacy
Disclosed is a conjugate comprising a biologically active agent (drug) linked to a subcellular targeting moiety that targets a drug specifically to the nucleus. Targeting is achieved by attaching a steroid hormone (or an analog) to the drug. The steroid hormone attached to the drug binds its corresponding receptor, the formation of the receptor-ligand complex results in the internalization of the complex into the nucleus, thus resulting in nuclear translocation of the drug. Also disclosed is a conjugate (comprising the complex of the drug and the steroid hormone) bound to a polymer by spacers allowing for concurrent passive targeting to the tumor cell (afforded by attachment to the polymer by the EPR effect) and nuclear targeting of the conjugate (due to the presence of the steroid). Using a suitable degradable spacer allows for the release of free drug in the tumor and enhances nuclear targeting efficacy. The polymer can be further linked to a cellular targeting molecule, where the targeting molecule directs the polymer to specific cells. One may thus be able to effectively target drugs to the nucleus of tumor cells. With little or modifications, several therapeutic agents can be targeted using the invention.
Owner:UNIV OF UTAH RES FOUND
Phosphate derivatives of pharmaceutical products
InactiveUS20070042999A1Quick conversionReduce solubilityBiocideNervous disorderAnesthetic AgentPhosphate
According to the invention, there is provided a complex of a pharmaceutical compound selected from the group consisting of opioids, hormones, anaethetics and chemotherapeutic agents comprising the reaction product of: (a) one or more phosphate derivatives of one or more opioids, steroid hormones, thyroid hormones, anaesthetics or chemotherapeutic agents having a phenolic, primary alcohol, secondary alcohol or tertiary hydroxyl group; and (b) a complexing agent selected from the group comprising amphoteric surfactants, cationic surfactants, amino acids having nitrogen functional groups and proteins rich in these amino acids.
Owner:VITAL HEALTH SCIENCES PTY LTD
Methods of modulating steroid hormone activity
InactiveUS20150111973A1Reduce sebum productionReduce differentiationBiocideNitro compound active ingredientsSterolSteroidal hormones
Owner:NAVAN INC
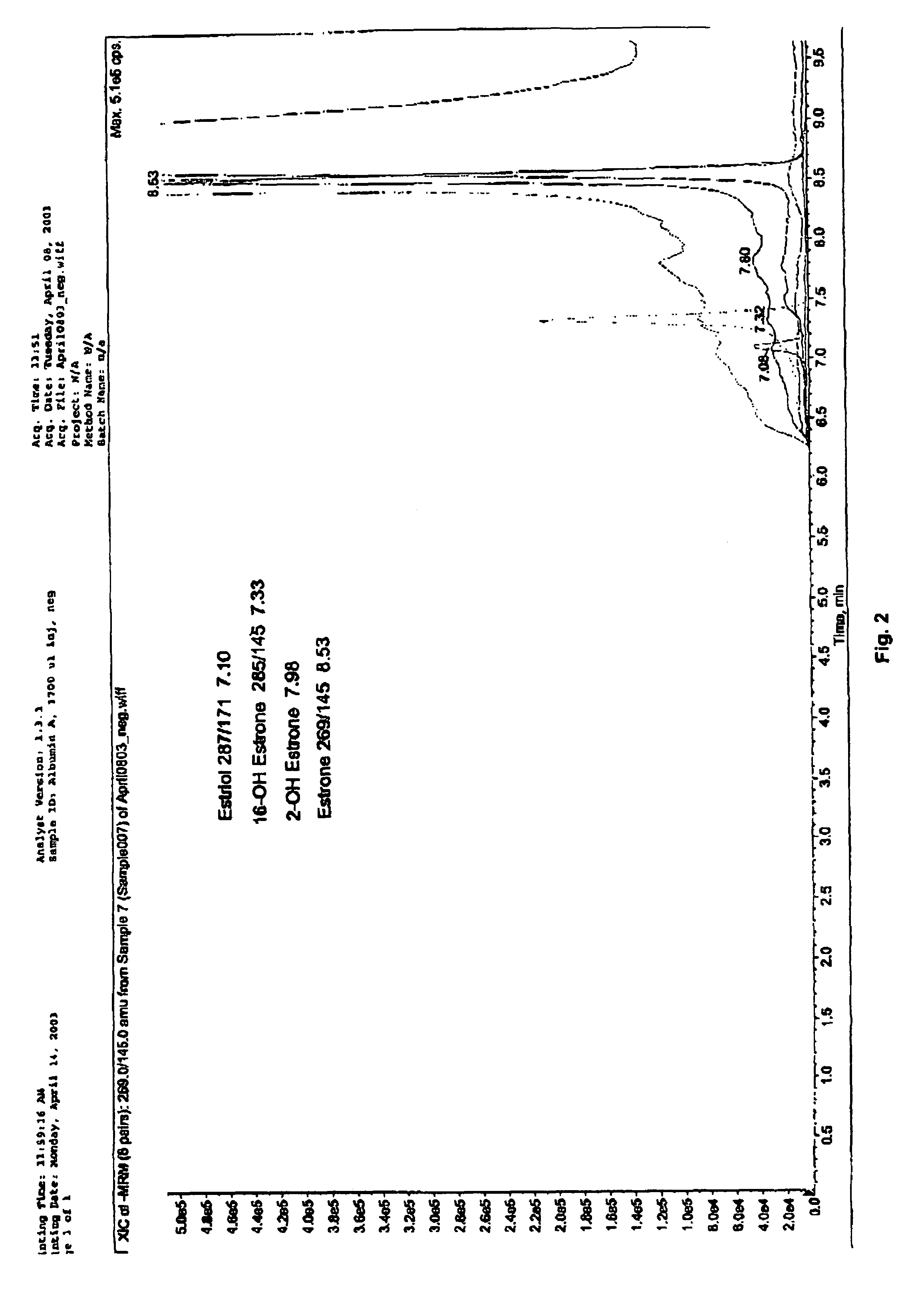
Analysis of steroid hormones in thin tissue sections
Mass-spectrometry based methods of analyzing estrogens and other steroids from biological tissue sections samples are disclosed herein. Methods of detecting a disease state or condition or elevated risk of a disease state or condition in a mammal from tissue sections are also disclosed.
Owner:UNITED STATES OF AMERICA
Pulmonary delivery of 17-hydroxyprogesterone caproate (17-hpc)
InactiveUS20110262502A1Improve responsivenessImprove toleranceAntibacterial agentsOrganic active ingredientsPowder mixtureInhalation
The invention relates to 17-HPC pulmonary formulations for administration by inhalation comprising 17-HPC and a pharmaceutically acceptable excipient. Particle size reduction of 17-HPC is required for the pulmonary delivery, and can be achieved with a surfactant or water without the surfactant. Preferred pulmonary formulations include a powder blend comprising a therapeutically effective amount of at least one steroid hormone (progestogen) as a glucocorticoid sensitizer, and at least one pharmaceutically acceptable excipient, wherein the at least one steroid hormone (progestogen) has a particle size distribution profile ranging from about one nanometer to about ten microns in the powder blend.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Novel 17beta-hydroxysteroid dehydrogenase type I inhibitors
3,15-substituted estrone compounds which act as inhibitors of 17β-hydroxysteroid dehydrogenase type I (17β-HSD1), salts thereof, pharmaceutical preparations containing such compounds, processes for preparing such compounds, and therapeutic uses of such compounds, particularly in the treatment or inhibition of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration, as well as the general use of selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors which possess in addition no or only pure antagonistic binding affinities to the estrogen receptor for the treatment or inhibition of benign gynecological disorders, particularly endometriosis.
Owner:ABBVIE PHARMA GMBH
Combination of slow released anticancer medication
InactiveCN1660437AAddress sensitivityOvercoming toxicityAntineoplastic agentsPharmaceutical active ingredientsWhole bodyTherapeutic effect
A slowly-releasing anticancer composite medicine is composed of the hormone-kind anticancer medicine chosen from steroid-type hormone and hormone antagon for regulating the cell reproduction of hormone dependent tumor and the medicinal additive chosen from biocompatible and biodegradable high-molecular polymer for slowly releasing said anticanser medicine toward tumor.
Owner:SHANDONG LANJIN PHARMA +1
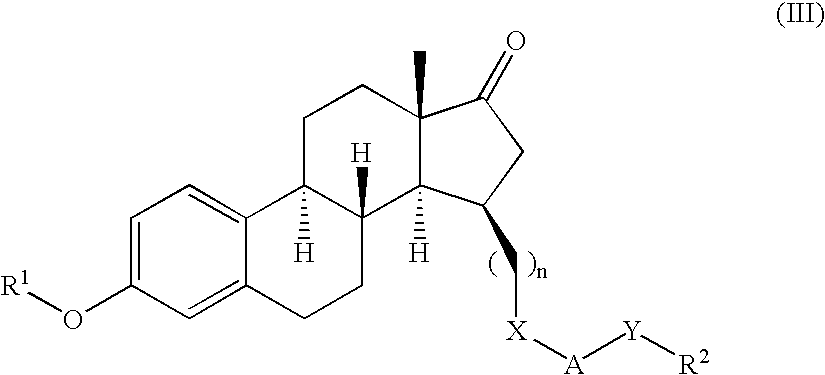
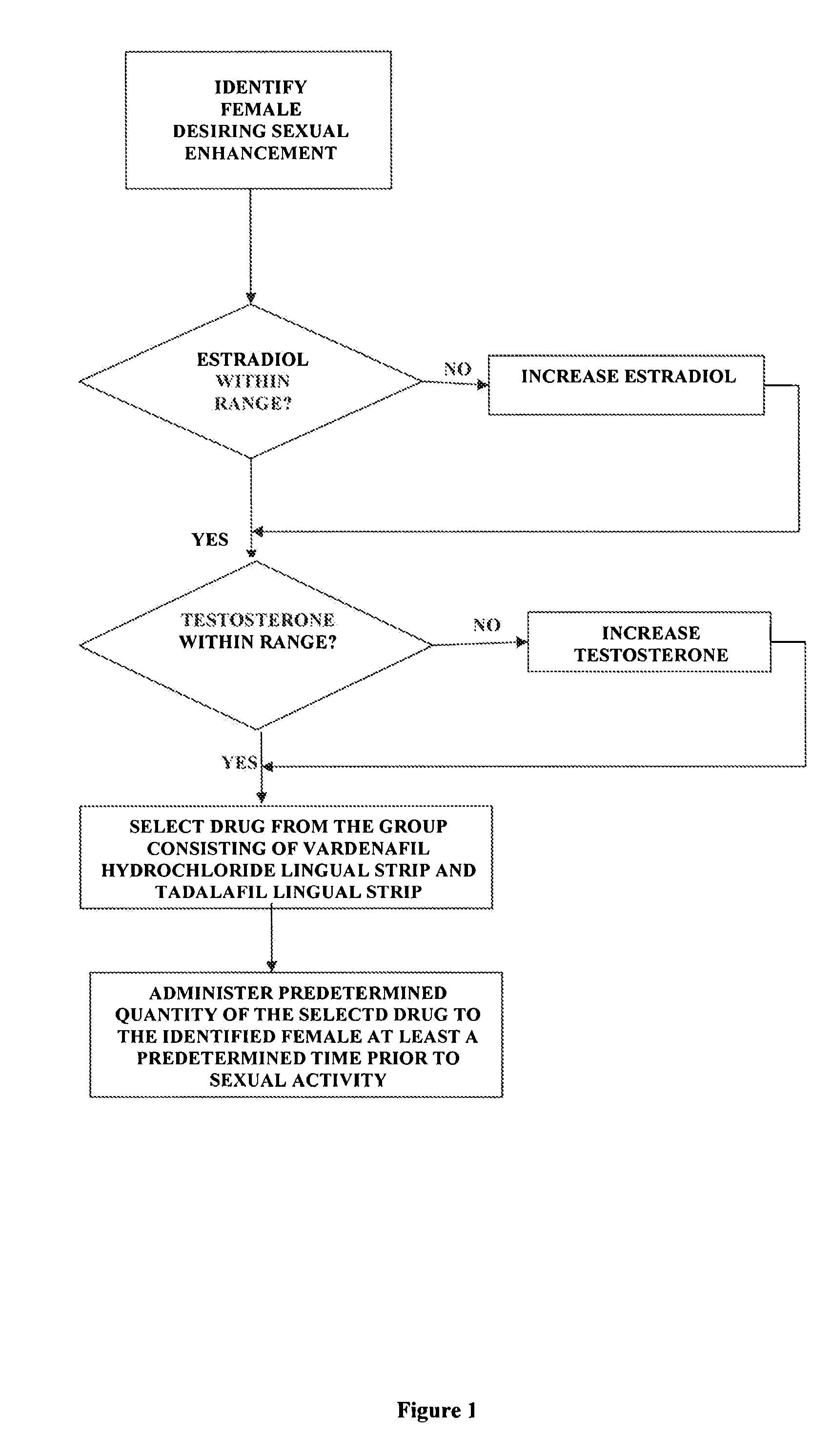
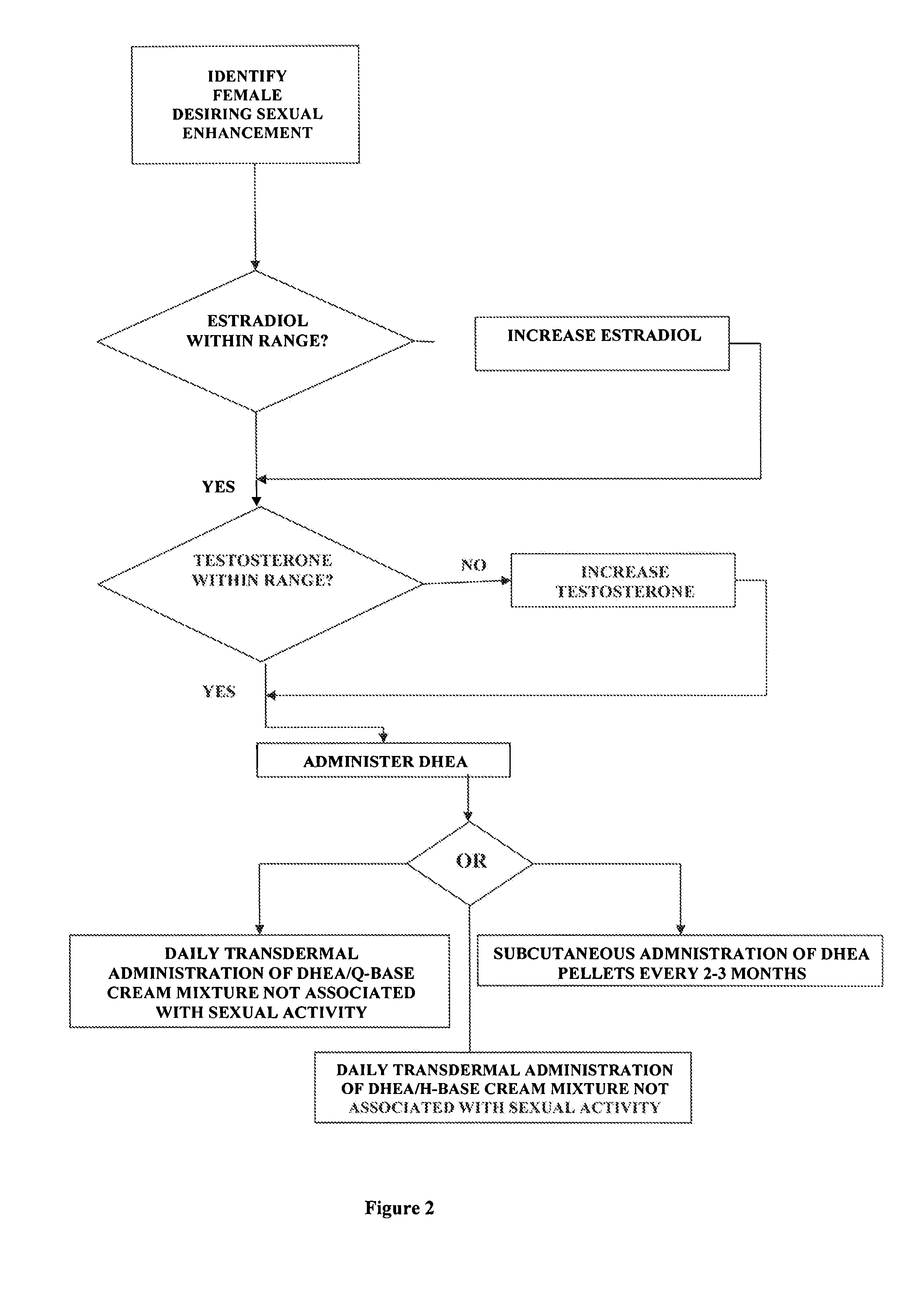
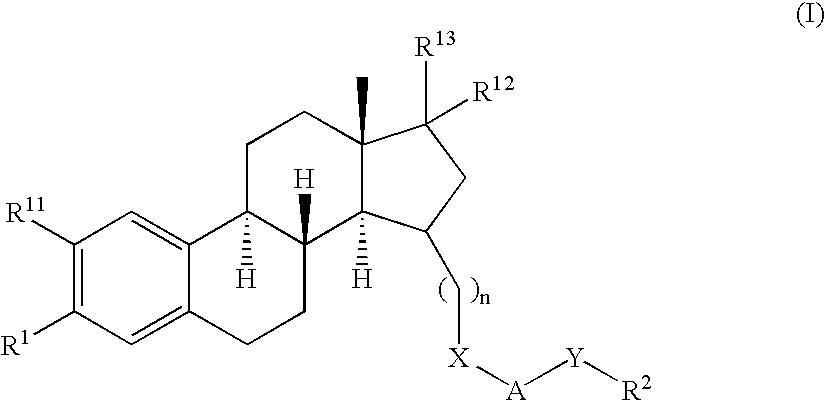
Methods of female sexual enhancement
A method of sexual enhancement in women includes the steps of identifying a woman requesting sexual enhancement, assuring that the woman's blood includes estradiol within a first predetermined range and testosterone within a second predetermined range, and thereafter administrating a drug selected from the group consisting of vardenafil hydrochloride and tadalafil prior to sexual activity. The selected drug may be loaded into a starch strip which is then applied to the woman's tongue. Sexual enhancement in women can also be achieved by transdermal or subcutaneous application of the steroid hormone DHEA.
Owner:LES MEDECINS
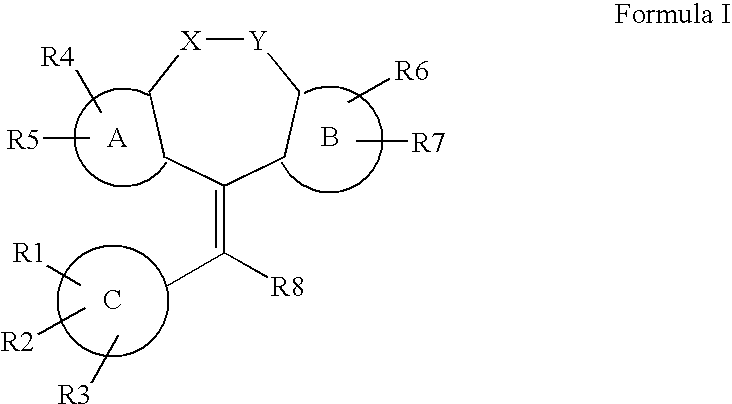
Substituted estratriene derivatives as 17beta hsd inhibitors
InactiveUS20080255075A1Good metabolic stabilityLess inhibitory potentialBiocideOrganic active ingredientsSteroidal hormones17beta-hydroxysteroid dehydrogenase
Substituted estratriene compounds of formula (I) useful in therapy, especially in the treatment or inhibition of a steroid hormone dependent disorder requiring the inhibition of a 17β-hydroxysteroid dehydrogenase (17β-HSD) type 1, type 2 and / or type 3 enzyme, as well as their salts, pharmaceutical compositions containing such compounds and processes for preparing such compounds.
Owner:SOLVAY PHARMA GMBH
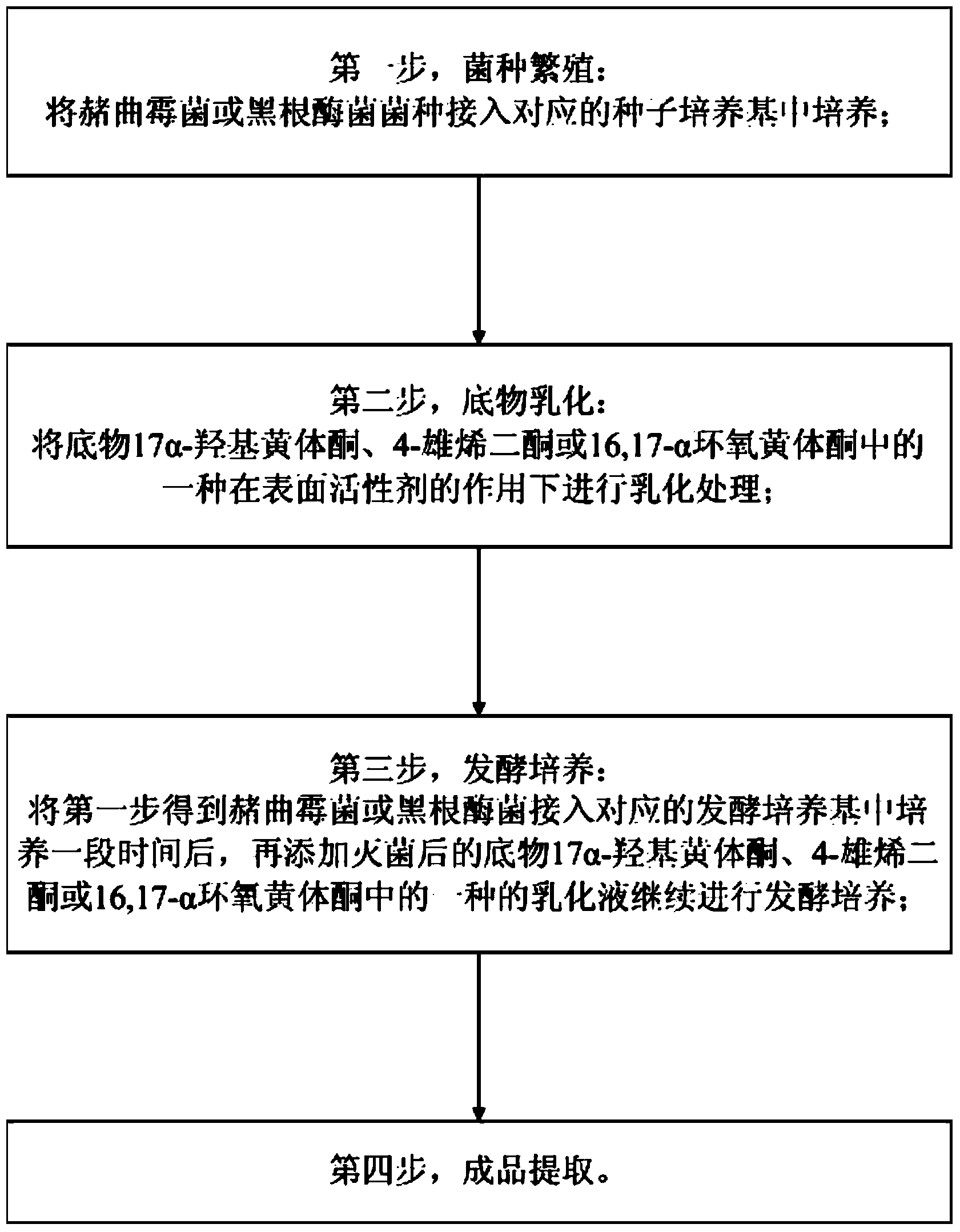
Preparation method for hydroxylation of 11 alpha of important intermediate of steroidal hormone substance
InactiveCN103966299AIncrease profitShorten the production cycleMicroorganism based processesFermentationEpoxyMicrobial transformation
The invention provides a preparation method for hydroxylation of 11 alpha of an important intermediate of a steroidal hormone substance, and aims to solve the problems that the conversion rate is low and the environment is polluted when a microorganism is used for converting steroidal C11 alpha for hydroxylation in the prior art. The preparation method comprises the following steps: strain breeding, wherein a strain of ochratoxin or rhizopus nigricans is inoculated to a corresponding seed medium for cultivation; substrate emulsification, wherein a substrate selected from one of 17-alpha hydroxyl progesterone, 4-androstenedione or 16,17-alpha epoxy progesterone is subjected to emulsification treatment under the action of a surfactant; fermented cultivation, wherein the ochratoxin or rhizopus nigricans is inoculated to a fermented medium to be cultivated for a period of time, and then one of emulsion liquids of sterilized 17-alpha hydroxyl progesterone, 4-androstenedione or 16,17-alpha epoxy progesterone is added for performing continuous fermented cultivation; extracting a finished product. The method has the advantages of high conversion rate, little pollution, environmental protection, low pressure and the like.
Owner:HEBEI ZHONGSHENG BIOTECH
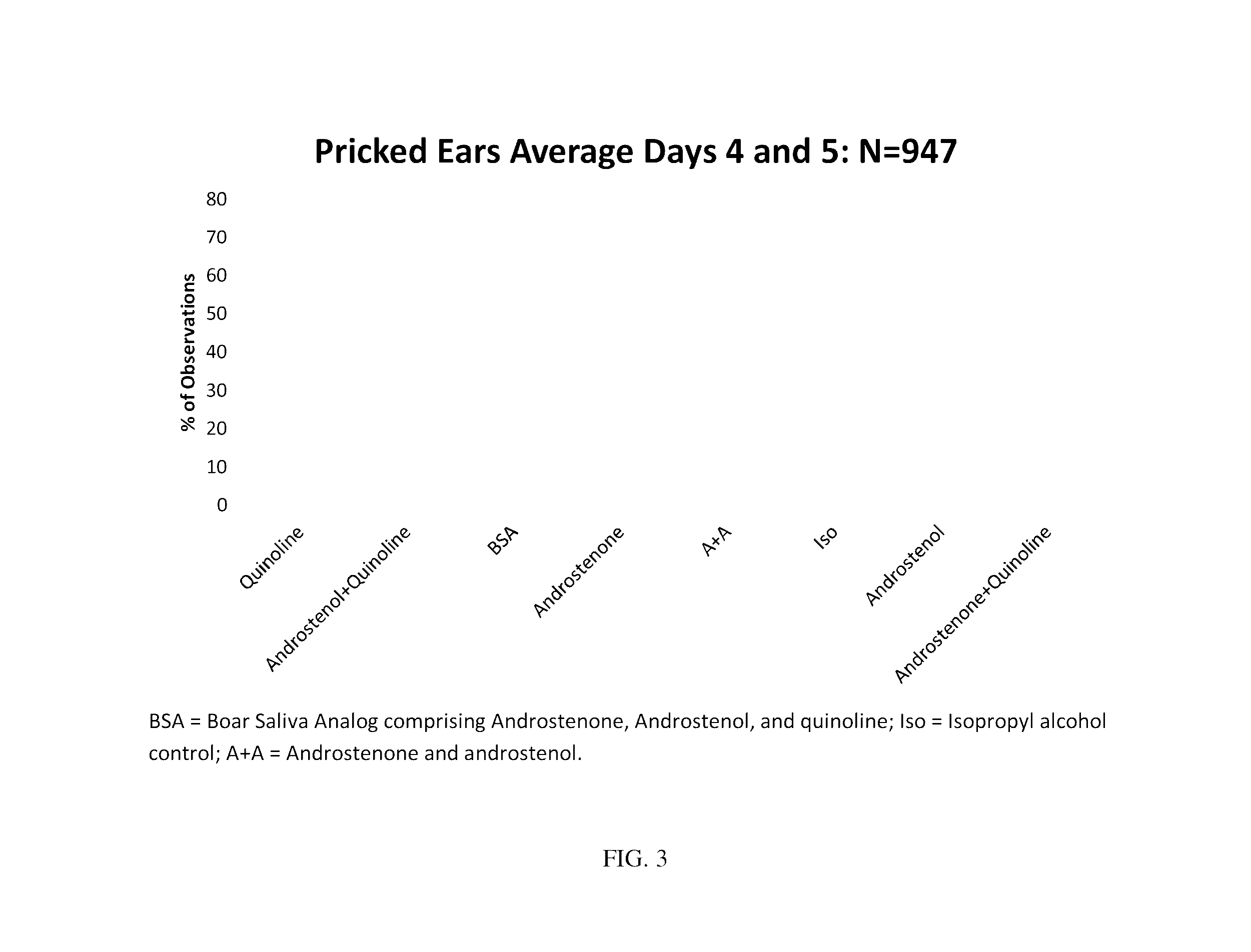
Pheromone composition to stimulate reproduction in female suids and methods of use
ActiveUS9480689B1Inducing productivityInducing reproductivePowder deliverySpray deliveryReproductive successSteroidal hormones
The present disclosure provides for compositions and methods of stimulating reproductive behavior and reproductive success and productivity in a suid, such as pigs. The composition may comprise at least one steroid hormone and a heterocyclic aromatic compound. The method comprises administering the pheromone composition to the suid for a period of time.
Owner:TEXAS TECH UNIV SYST
Nitric oxide manipulation of muscle satellite cell activation
InactiveUS6967102B1Change effectEnhance the beneficial effectHalogenated hydrocarbon active ingredientsCompound screeningWhole bodyBiological activation
The present invention is directed to methods, pharmaceutical compositions and kits for modulating skeletal muscle precursor cell activation. Modulation is effected through the use of nitric oxide (NO), donors of NO, inhibitors of NO activity (NO inhibitor) or regulators of NO production, either locally or systemically. The invention further teaches the use of NO, an NO donor, an NO inhibitor or a regulator of NO production to modulate the effects of steroid hormone on skeletal muscle. The invention further provides a method for identifying a compound which effects a change in activation state of muscle precursor cells. A number of advantages is evident. By allowing skeletal muscle precursor cells to be manipulated directly, the invention enables specific treatments to regenerate and repair muscle.
Owner:UNIVERSITY OF MANITOBA
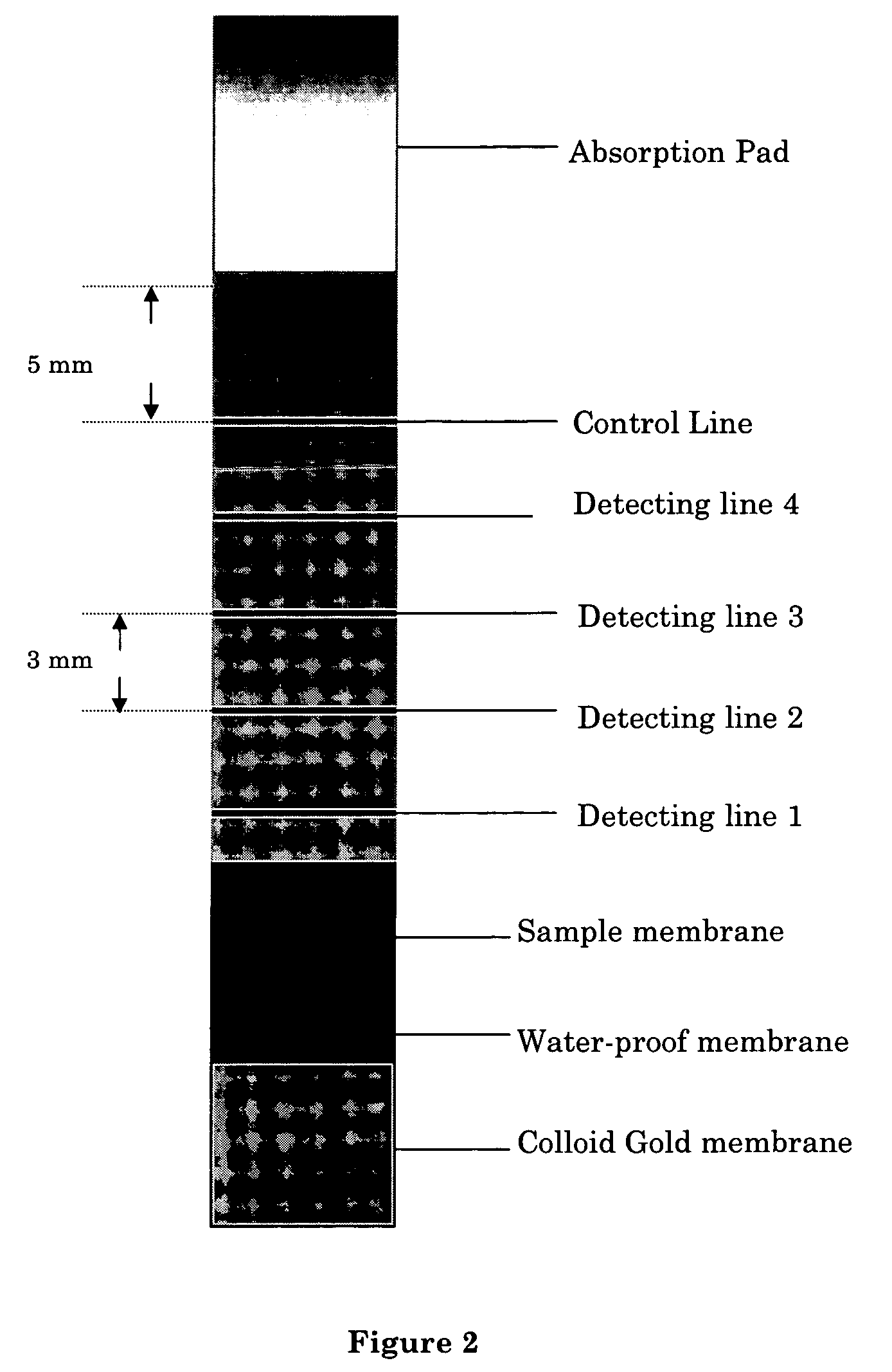
Apparatus and methods for steroid hormone testing
An immuno-chromatographic detection device for detecting an analyte in sample, such as estrogen in a urine or saliva sample, the device comprising (a) a binding membrane having immobilized thereon (i) an test antibody against said analyte in at least one detection region, and (ii) a control antibody against a control antigen known to be present in the sample in a control region, (b) a sample membrane located at a first end of the binding membrane for receiving the sample, wherein the sample membrane is in chromatographic connection with the binding membrane, and (c) a label membrane containing (iii) a labeled antigen that is capable of binding to the test antibody and upon binding with the test antibody exhibits an observable change at the at least one detection region, and (iv) a labeled control antigen that is capable of binding to the control antibody and upon binding with the control antibody exhibits an observable change at the control region, wherein the sample membrane is separated from the label membrane by a waterproof membrane which is removable to allow the sample membrane and label membrane to be connected chromatographically. Also provided are kits comprising the device, method for detecting the analyte, and methods for manufacturing the device and kit.
Owner:NJ INT
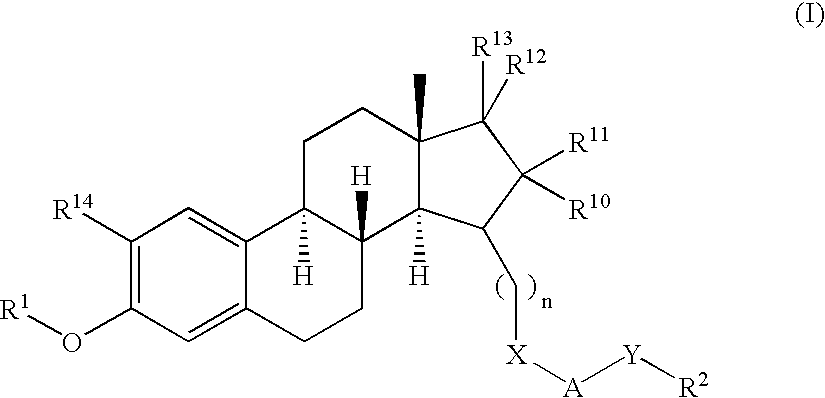
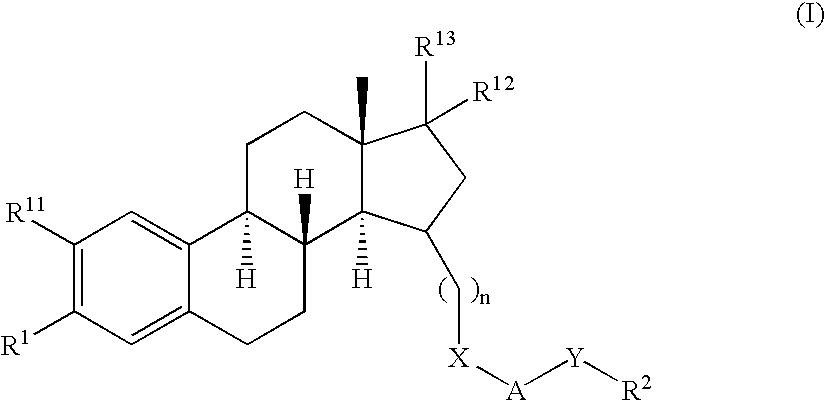
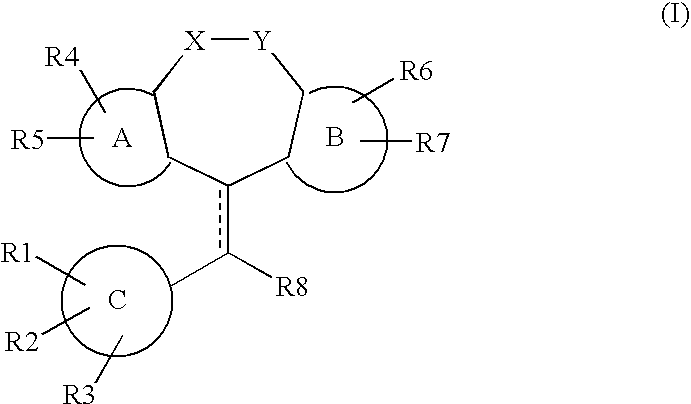
Tricyclic steroid hormone nuclear receptor modulators
InactiveUS20060063759A1Halogenated hydrocarbon active ingredientsBiocideDrug compoundBULK ACTIVE INGREDIENT
The present invention relates to methods of treating pathological disorders susceptible to steroid hormone nuclear receptor modulation comprising administering to a patient in need thereof an effective amount of a compound of the formula (I): or a pharmaceutically acceptable salt thereof. In addition, the present invention provides novel pharmaceutical compounds of Formula (I), including the pharmaceutically acceptable salts thereof, as well as pharmaceutical compositions which comprise as an active ingredient a compound of Formula (I).
Owner:ELI LILLY & CO
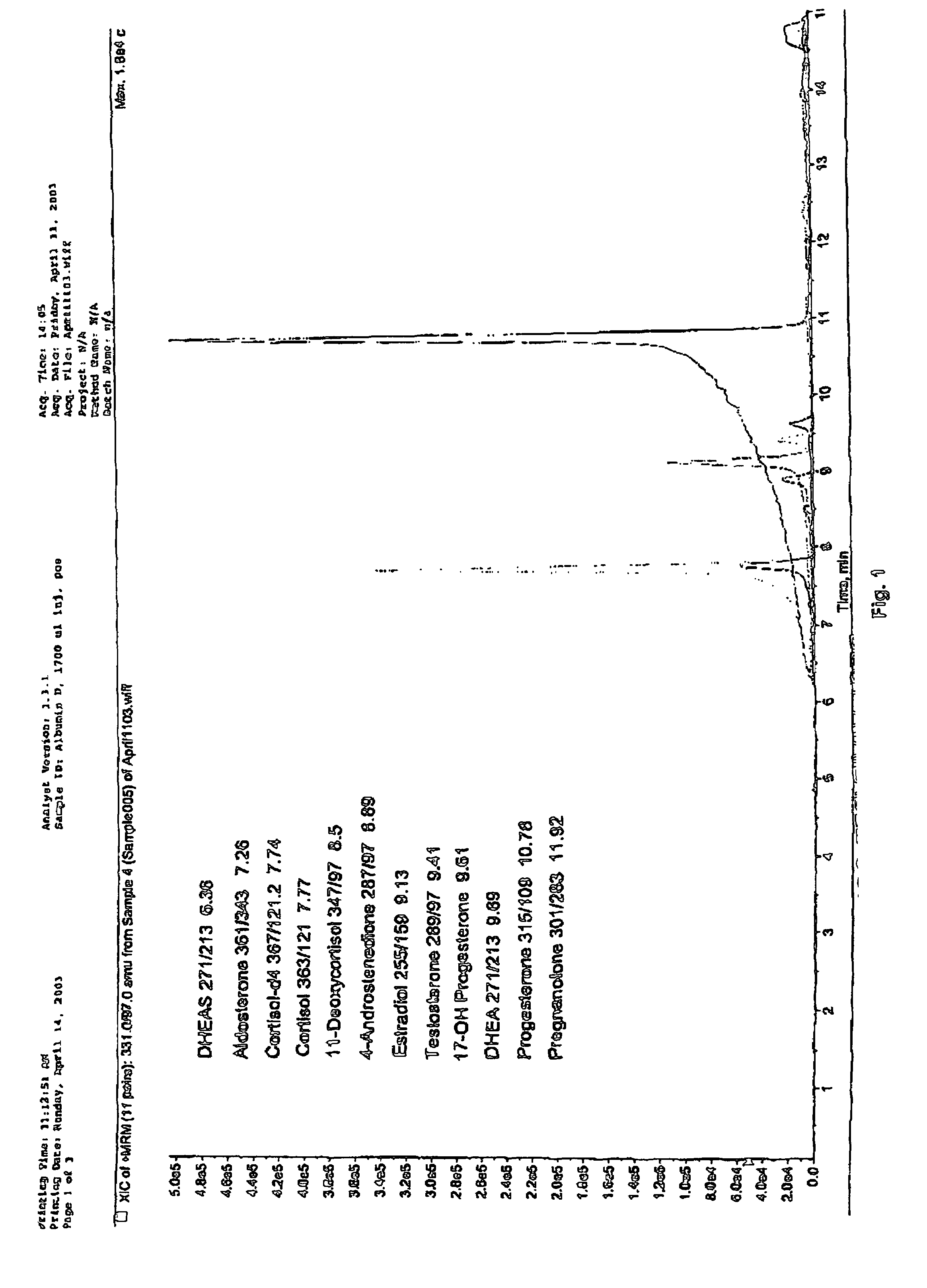
Method for detecting 12 steroid hormones in serum by using ultra-high performance liquid chromatography-tandem mass spectrometry technology
ActiveCN111398446AImprove ionization efficiencyHigh sensitivityComponent separationSolid phase extractionSteroidal hormones
The invention relates to a method for detecting 12 steroid hormones in serum by using an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The method comprises the following steps of: mixing a serum sample with a mixed internal standard solution of all to-be-detected substances, and carrying out one-step liquid-liquid extraction to obtain a to-be-detected solution;adding electrolyte ammonium fluoride into a mobile phase, so that the ionization efficiency of certain target compounds can be effectively improved; and simultaneously detecting the 12 hormones by adopting a mass spectrum positive-negative switching scanning mode. The method has the advantages of multiple detection types, high sensitivity, high specificity, low cost and simple pretreatment process, does not need solid phase extraction or derivation, can complete separation and detection of 12 hormones within 5.0 min, and provides a reliable detection method for the health assessment of clinical endocrine diseases.
Owner:NANJING QLIFE MEDICAL TESTING LAB CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com