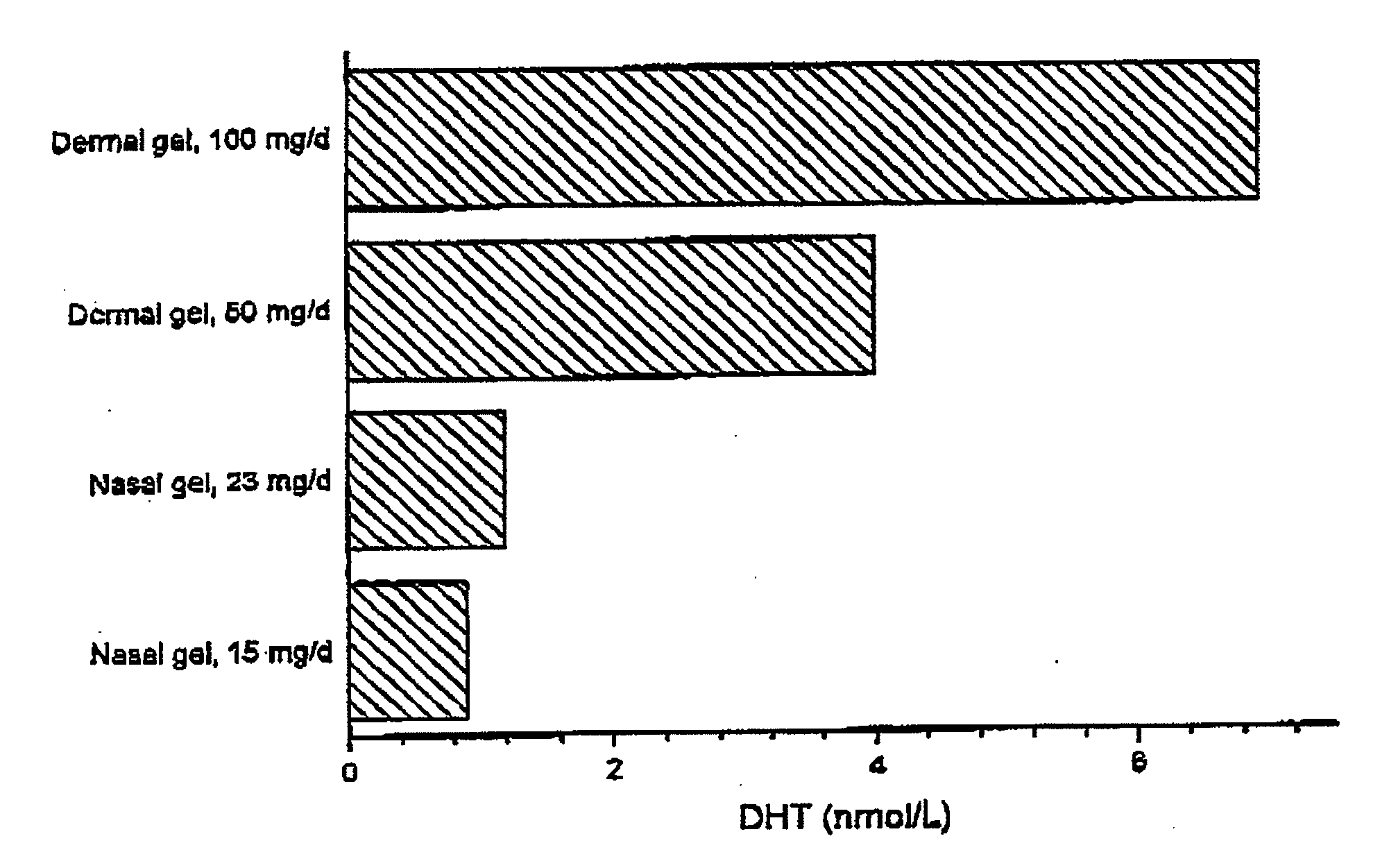
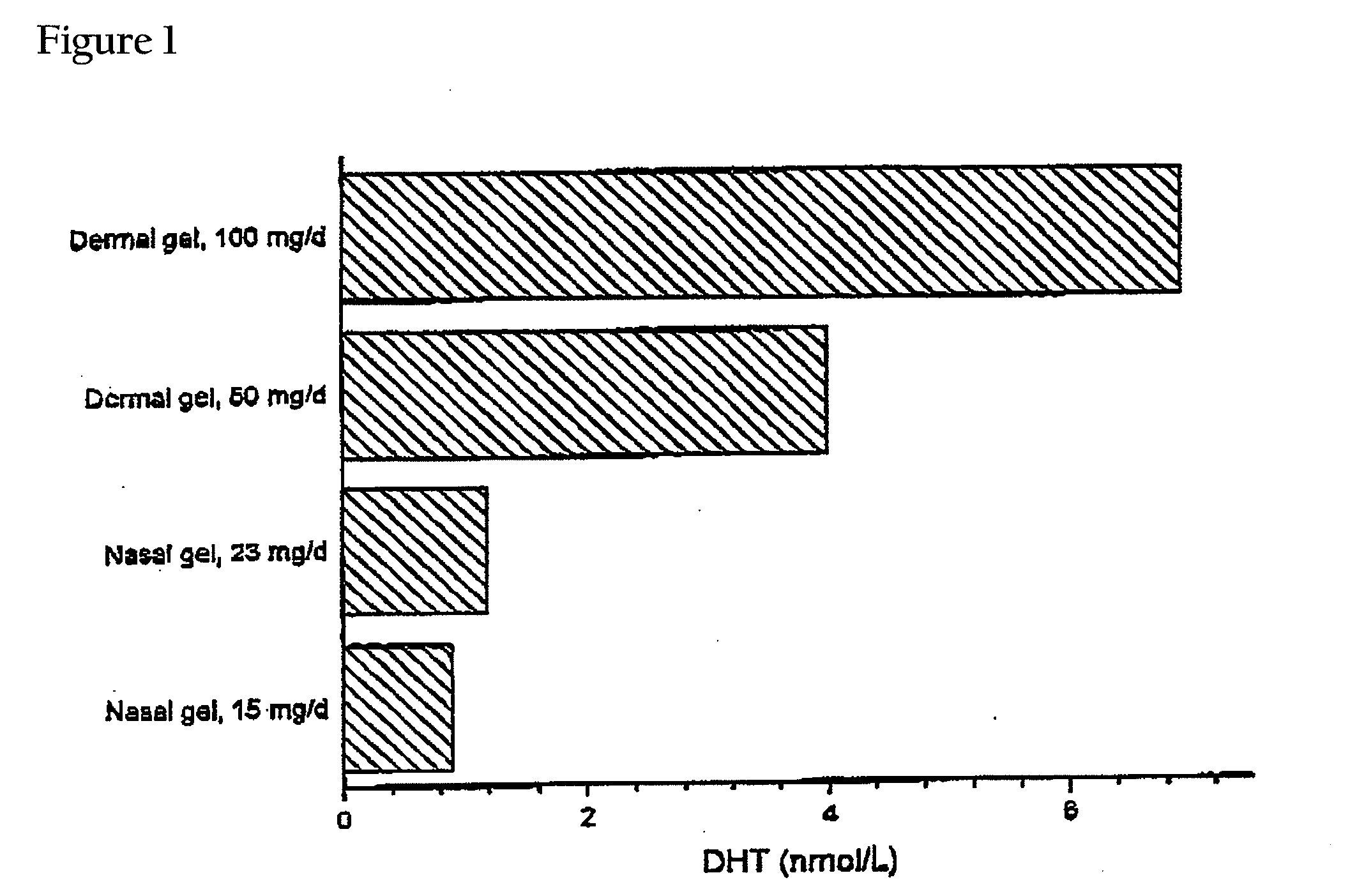
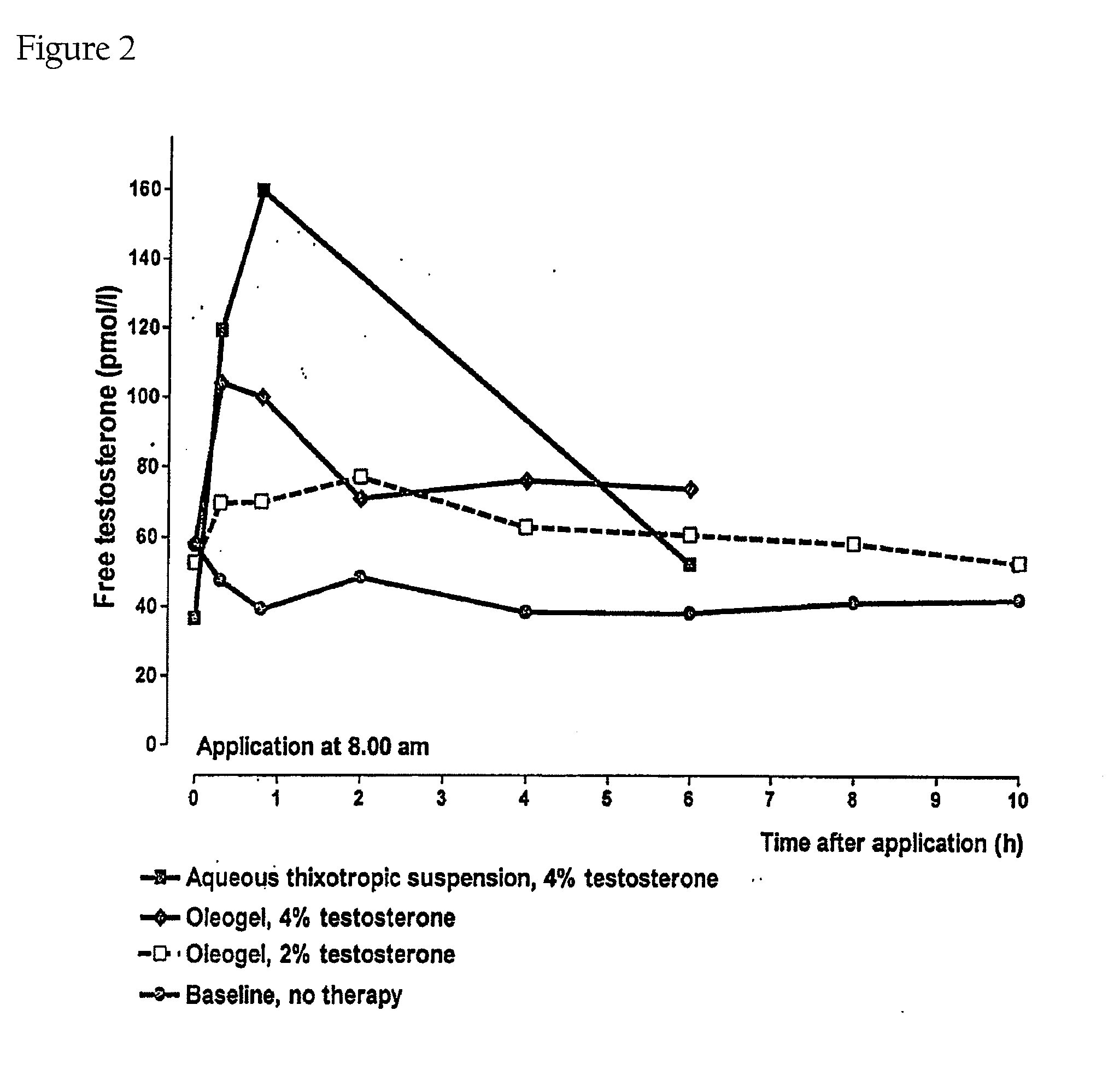
Controlled Release Delivery System for Nasal Applications and Method of Treatment
a delivery system and control technology, applied in the field of neuroendocrinologic disorders, can solve the problems of confusion about the wide range of effects, the underlying mechanism of action is still poorly understood, and the possibility of differential relevance of a molecule's non-genomic action in different cell types, and achieve the effect of modulating brain function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
[0058] The following examples are intended to further illustrate, and not limit, embodiments in accordance with the invention.
example 1
Nasal Administration of Testosterone to Women
[0059] The rapid and relatively high peak concentration of testosterone after application of testosterone was shown to correspond to a signal in the brain. Fourteen healthy, premenopausal women, between thirty-five and forty-five years of age during early follicular phase and who were not taking hormonal contraceptives, received the inventive nasal gel containing 0.9 mg testosterone or a placebo forty minutes before scanning. Scanning was done with functional magnetic resonance imaging (fMRI) using a 1.5 T Siemens Sonata MR scanner (TR 2.29 s, TE 30 ms, 3.5×3.5×3.5 mm voxels) to investigate the regional cerebral blood flow. During scanning, the subjects had to match the emotional expression with faces of different individuals expressing either anger or fear. As shown in FIG. 4, the fMRI data shows that application of the nasal testosterone gel formulation produces rapid effects on the neural emotion circuitry. Although not wishing to be ...
example 2
Effects of Nasal Administered Testosterone on the Sexual Behavior of Female Capuchin Monkeys (Cebus apella)
[0064] The objective of the study was to investigate the effects of nasal administered testosterone on the sexual behavior of female capuchin monkeys (Cebus apella).
[0065] Ten brown tufted capuchins (Cebus apella) were used as subjects as focal animals in this study. Animals were all adult females (>5 years old). All animals were weighted prior to and following the experimental procedures as described in Table 1.
TABLE 1Weight of Female Capuchins Monkeys in the Noseafix Experiment.FemaleBaselineTreat. 1Treat. 1WashoutTreat. 2Treat. 2NumberSept. 23Sept. 30Oct. 03Oct. 08Oct. 10Oct. 1312.1202.0702.2052.1402.1752.11522.2652.3302.5452.5202.3702.39032.3902.3602.3552.3902.3502.34042.3902.3302.3852.3952.3202.34552.5002.1952.2252.3152.3102.24562.5102.4902.6402.3152.4802.61572.5002.4452.6102.6002.4502.46581.7401.7251.7951.7651.7551.76092.6102.5952.6952.7402.7002.745102.5202.3702.3252....
PUM
| Property | Measurement | Unit |
|---|---|---|
| droplet size | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com