Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
204 results about "Progestogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Progestogens, also sometimes written progestagens or gestagens, are a class of steroid hormones that bind to and activate the progesterone receptor (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy (i.e., progestational), although they are also present at other phases of the estrous and menstrual cycles.
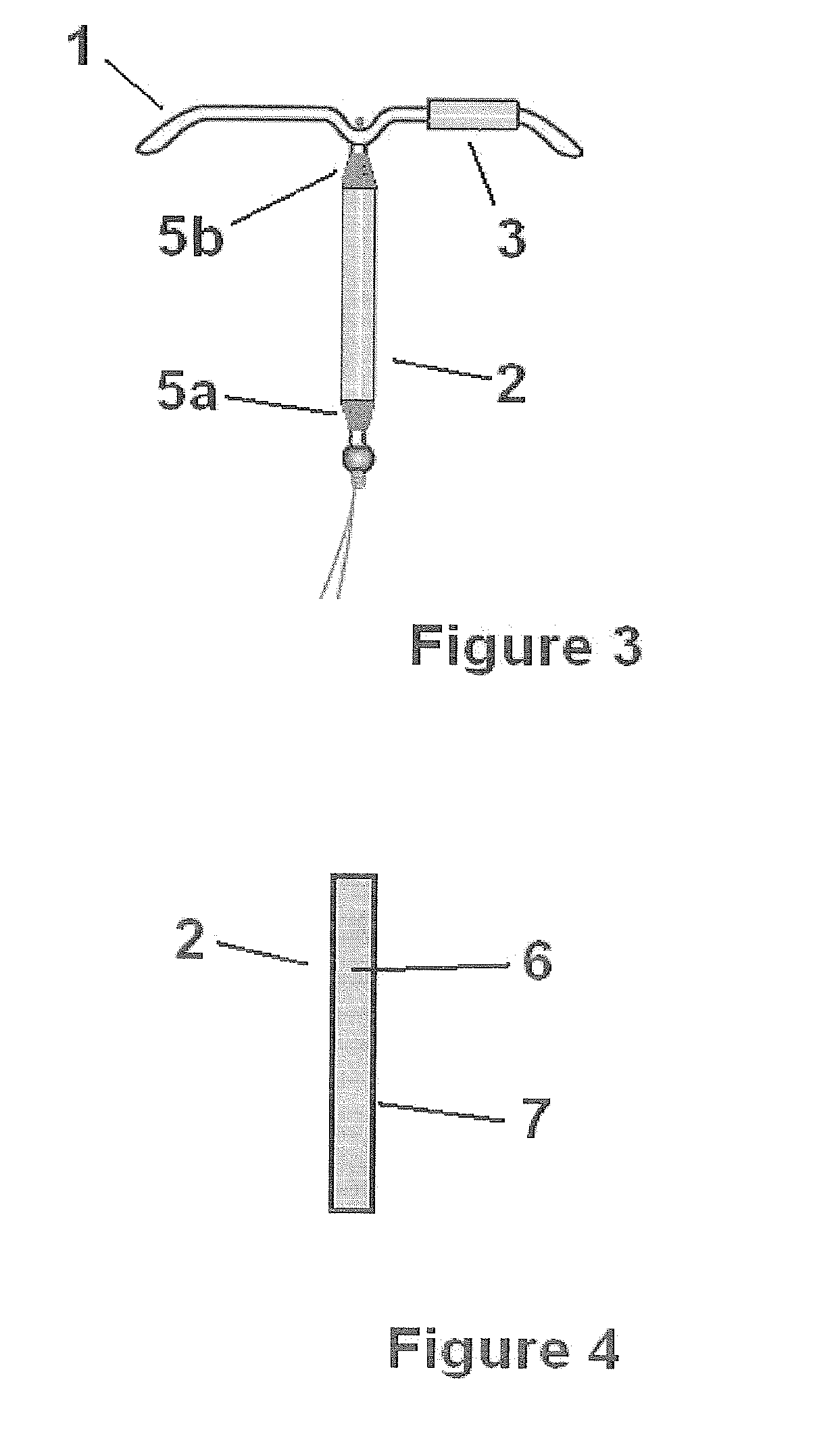
Transdermal estradiol/progestogen agent patch and its production
PCT No. PCT / EP96 / 05759 Sec. 371 Date Sep. 14, 1998 Sec. 102(e) Date Sep. 14, 1998 PCT Filed Dec. 20, 1996 PCT Pub. No. WO97 / 23227 PCT Pub. Date Jul. 3, 1997The invention concerns a transdermal patch for the release through the skin of estradiol and a progestogen agent and a process for its production.
Owner:ROTTA RES
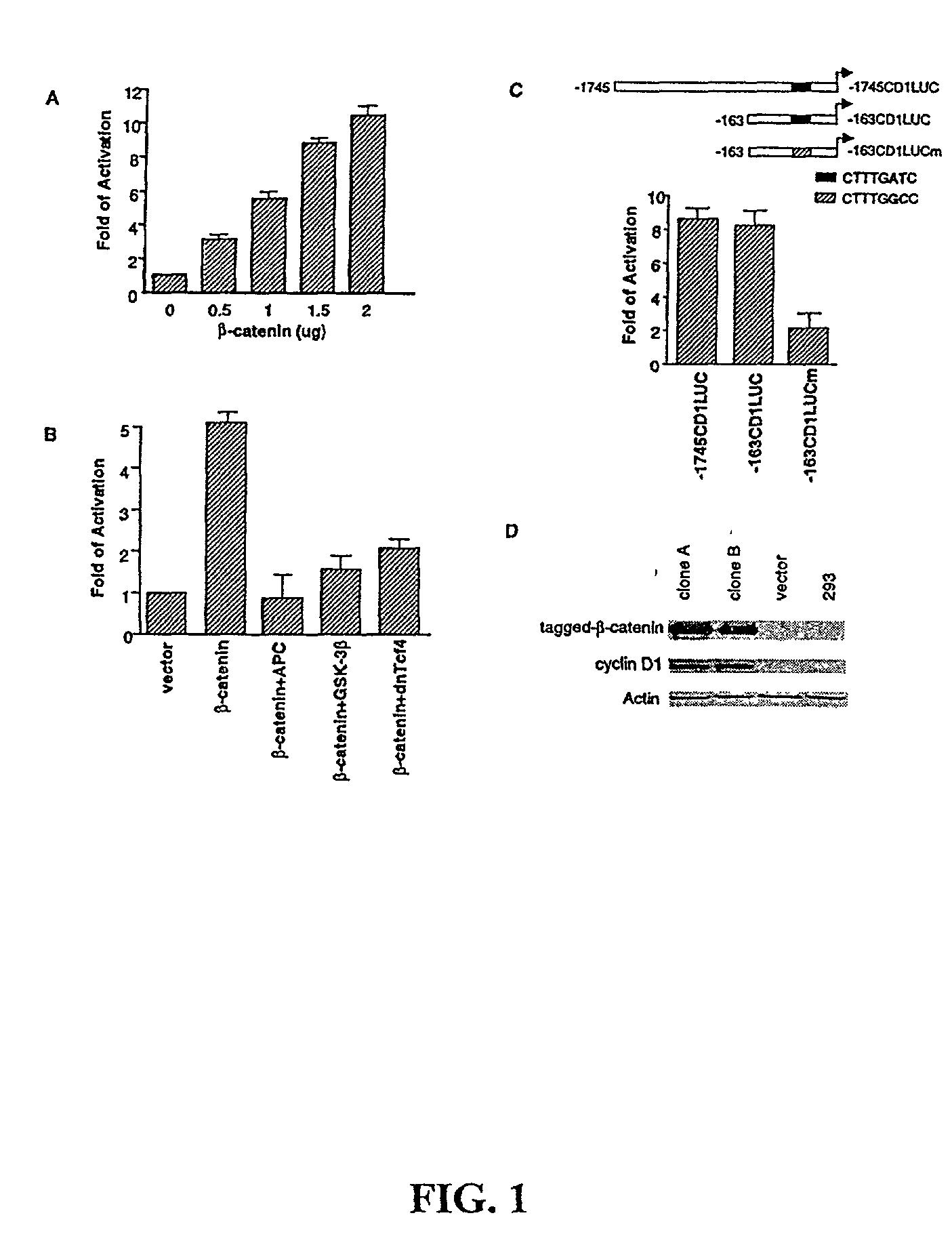
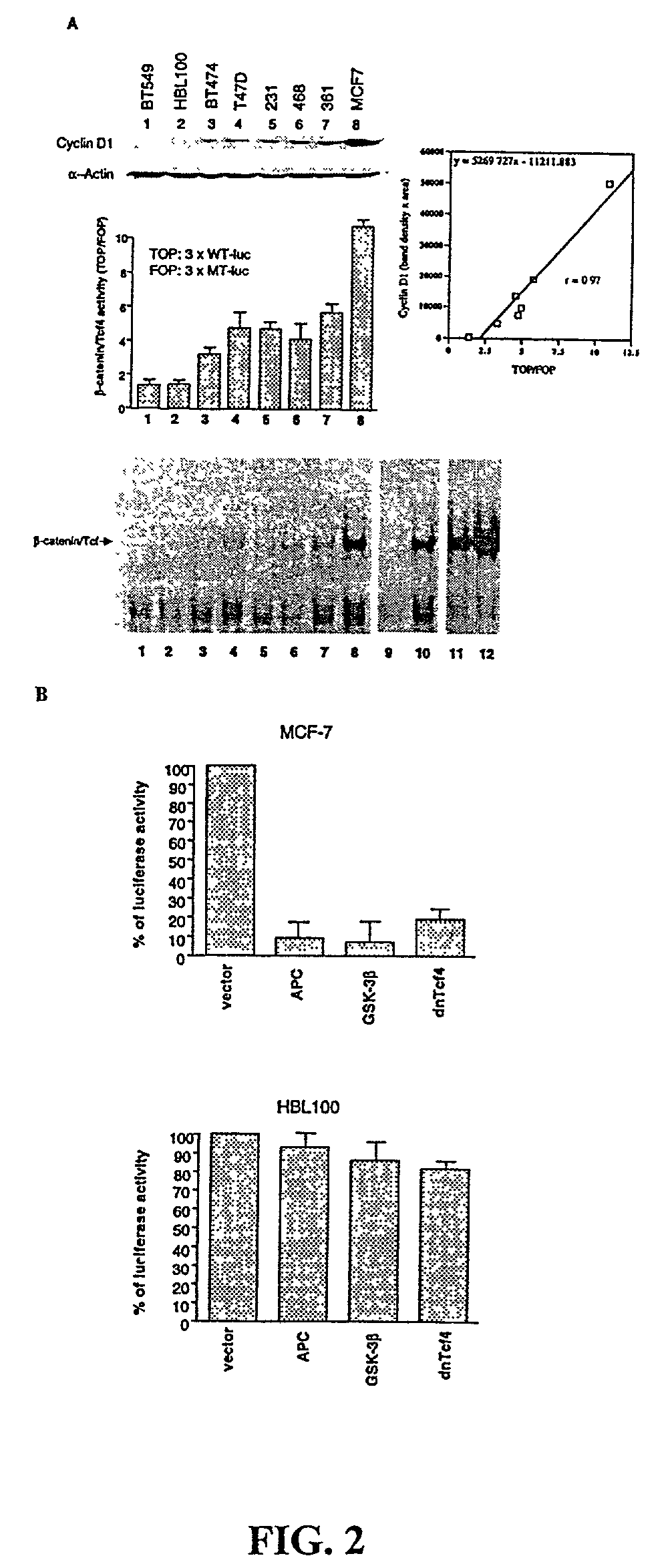
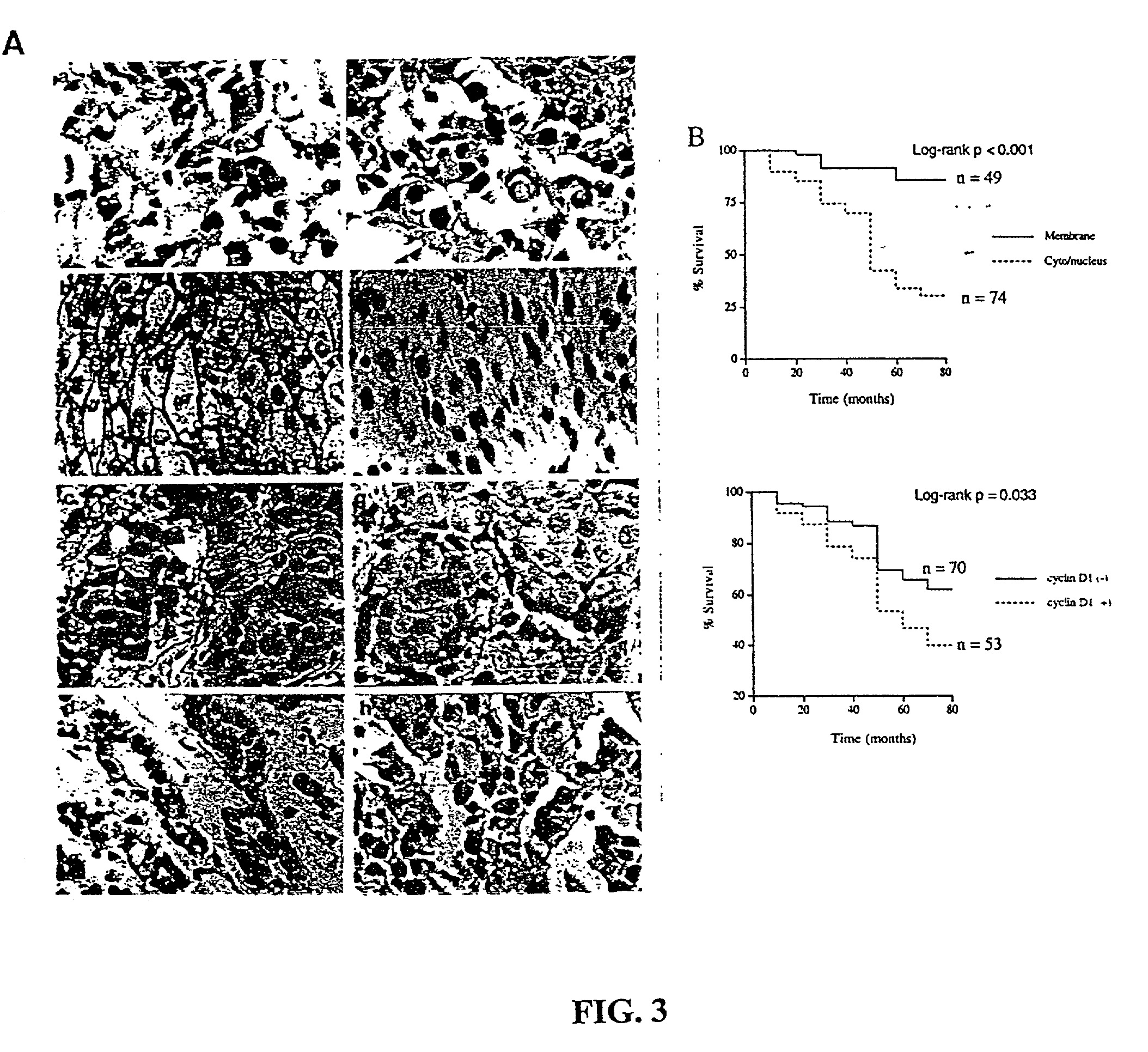
Beta-catenin is a strong and independent prognostic factor for cancer
InactiveUS20030064384A1Poor prognosisStrong and independent prognostic factor in cancerGenetic material ingredientsMicrobiological testing/measurementTransactivationFactor ii
Cyclin D1 is one of the targets of beta-catenin in breast cancer cells. Transactivation of beta-catenin correlated significantly with cyclin D1 expression both in eight breast cell lines in vitro and in 123 patient samples. More importantly, high beta-catenin activity significantly correlated with poor prognosis of the patients and is a strong and independent prognostic factor in breast cancer (p<0.001). Moreover, by multivariate analyses, the inventors found that activated beta-catenin is a strong prognostic factor which provided additional and independent predictive information on patients survival rate even when other prognostic factors, including lymph node metastasis, tumor size, estrogen receptor and progesterone receptor status, were taken into account (p<0.001). This invention demonstrates that beta-catenin is involved in breast cancer formation and / or progression and may serve as a target for breast cancer therapy.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Low-oil pharmaceutical emulsion compositions comprising progestogen
ActiveUS20110262494A1High progesterone solubilityElevation in histamine releaseOrganic active ingredientsNervous disorderIUD with progestogenTG - Triglyceride
Described are sterile, ready-to-use, pharmaceutical oil-in-water emulsion compositions for parenteral administration comprising:0.015 to 0.5% wt / vol progesterone;0.5 to 10% wt / vol oil, wherein the oil comprises at least 85% wt. / wt. triglyceride;0.0425 to 4.1% wt / vol phospholipid;80-99.4% wt / vol aqueous medium;wherein the composition has an osmolality in the range of 200-1000 mOsm / kg.Also described are methods of making such compositions and method of using such compositions in therapeutic or prophylactic treatment, such as treatments comprising intravenous administration of the pharmaceutical composition.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
Hormone replacement therapy method
InactiveUS6551611B2Lower energy requirementsOrganic active ingredientsAerosol deliveryHormone replacementPhysician attending
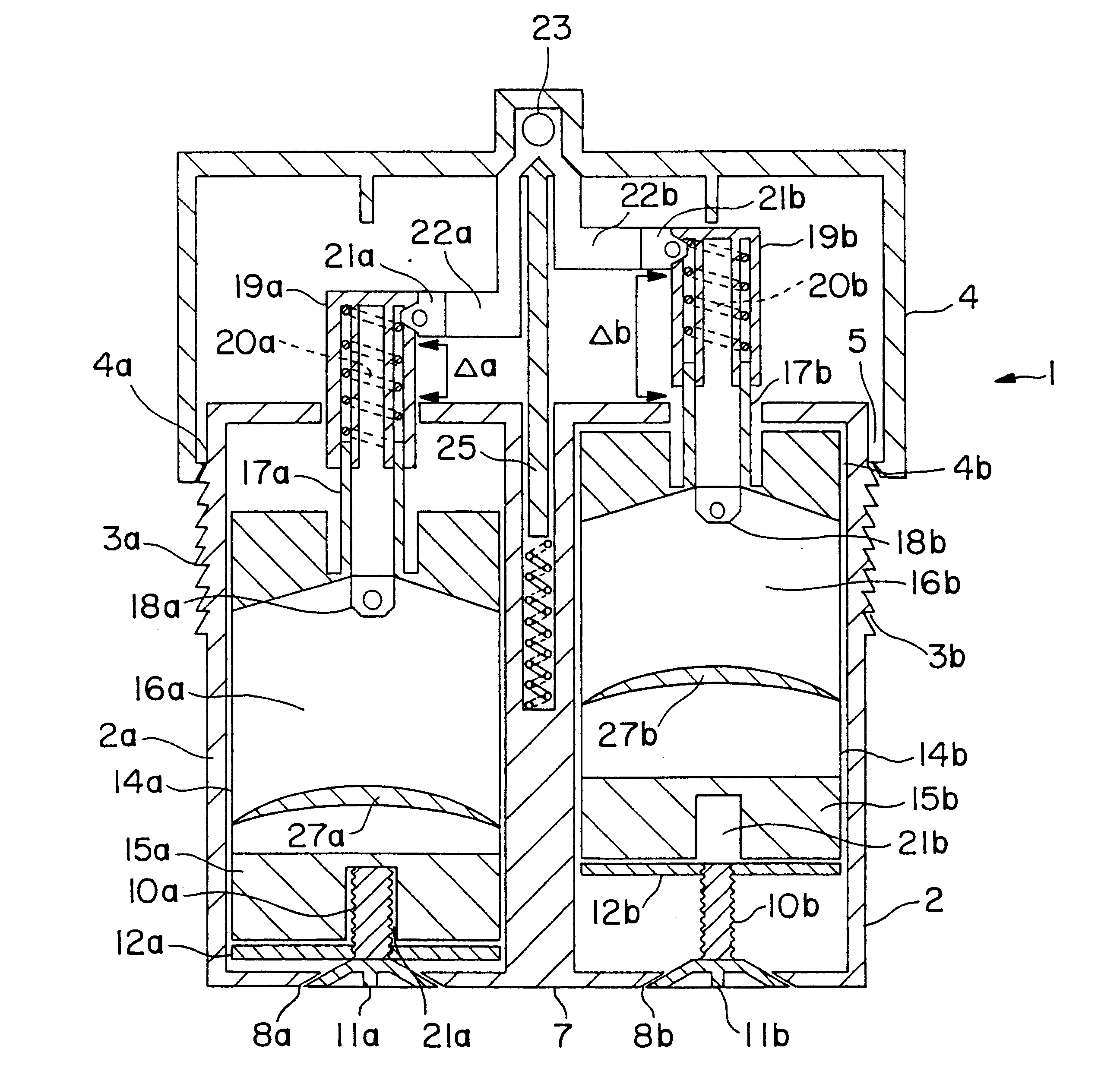
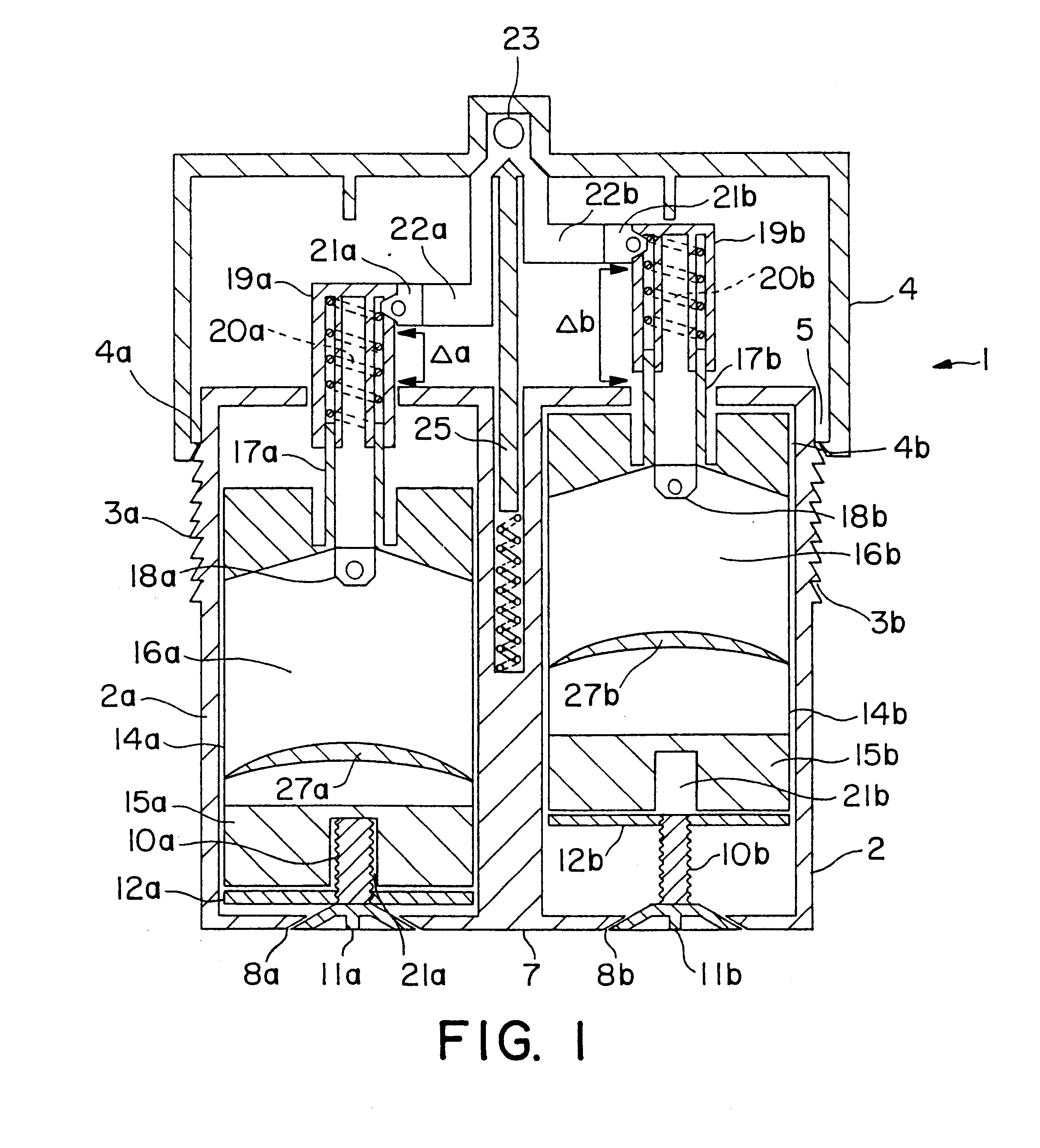
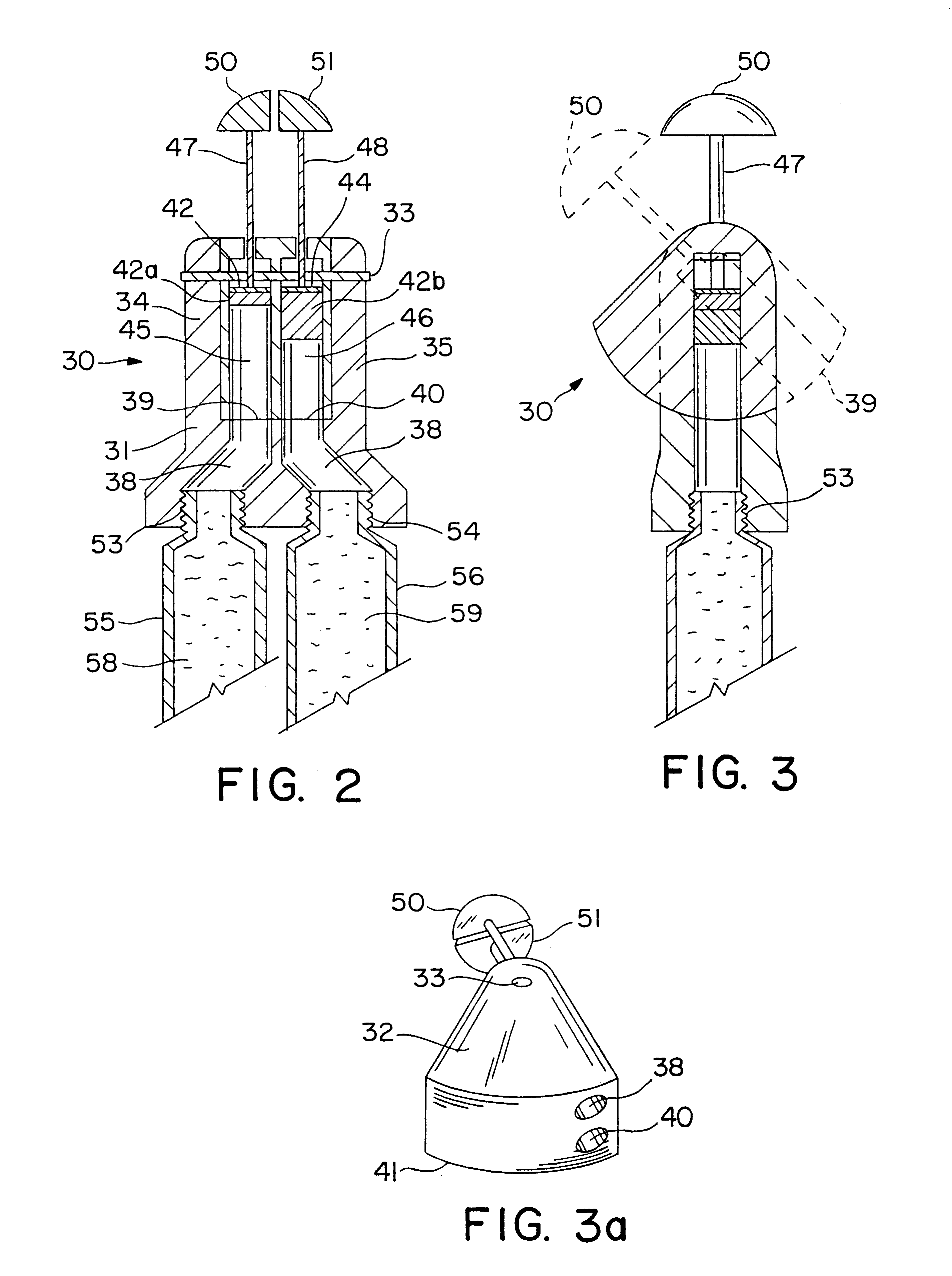
Varying the daily dose of either or both of the estrogen and the progestogen administered for hormone replacement therapy (HRT) is readily and inexpensively accomplished, without the necessity of the physician prescribing a new product each time the daily dose of the estrogen or progestogen is changed, by administering preferably transdermally the estrogen and the progestogen contained in separate extrudable pharmaceutical compositions from a dispenser which contains means, preferably adjustable only by the attending physician or dispensing pharmacist, for varying the volume of either or both of the respective compositions which is dispensed as a single dose from the dispenser in response to a defined digital dispensing manipulation of the dispenser thereby facilitating optimal compliance to a combination of HRT with individually adjusted dosages of the estrogen and progestogen.
Owner:BAYER SCHERING PHARMA AG
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Pharmaceutical composition for use in hormone replacement therapy
ActiveUS8048869B2Interaction can be difficultReliable efficacyBiocideOrganic active ingredientsAnti-ProgestinPresent method
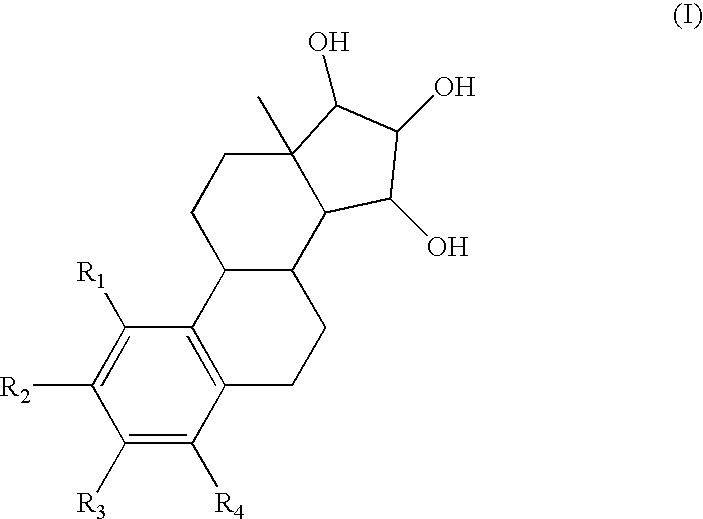
One aspect of the invention is concerned with a method of hormone replacement therapy, which method comprises administering to a person in need of such a therapy an effective amount of an estrogenic component selected from the group consisting of: substances represented by the formulain which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alokxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method, and mixtures thereof; said composition containing virtually no progestogen or anti-progestin.Another aspect of the invention relates to a drug delivery system for enteral or parenteral administration that contains at least 1 μg of the aforementioned estrogenic component and virtually no progestogen or anti-progestin.
Owner:ESTETRA SRL
Use of compositions comprising an estrogenic component for the treatment and prevention of musculoskeletal pain
InactiveUS20060276414A1Increasing cell proliferationHigh affinityOrganic active ingredientsBiocideGynecologyPresent method
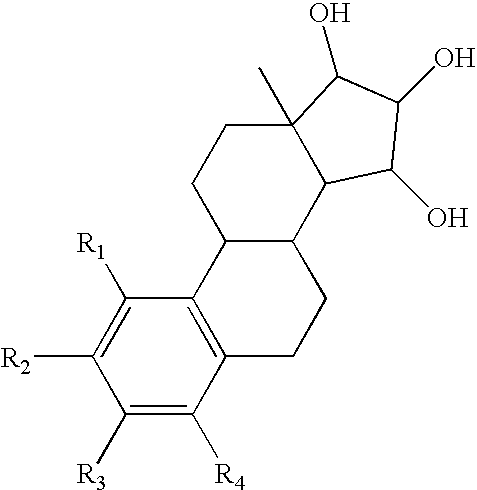
The present invention relates to a method of treating or preventing musculoskeletal pain in a mammal receiving administration of an estrogen. suppressant selected from the group consisting of aromatase inhibitors, GnRH analogues, cyclo-oxy-genase 2 (COX-2) inhibitors, 17β-hydroxysteroid dehydrogenase type 1 inhibitors, progestogens, anti-estrogens and combinations thereof, said method comprising the administration of an effective amount of an estrogenic component, wherein the estrogenic component is selected from the group consisting of: substances represented by the following formula (1) in which formula R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors.
Owner:COELINGH BENNINK HERMAN JAN TIJMEN +1
Pharmaceutical compositions comprising active drugs, contraceptive kits comprising active drugs, and methods of administering the same
ActiveUS20120128733A1Inhibit ovulationBiocideOrganic active ingredientsDrospirenoneProgestogen-only contraception
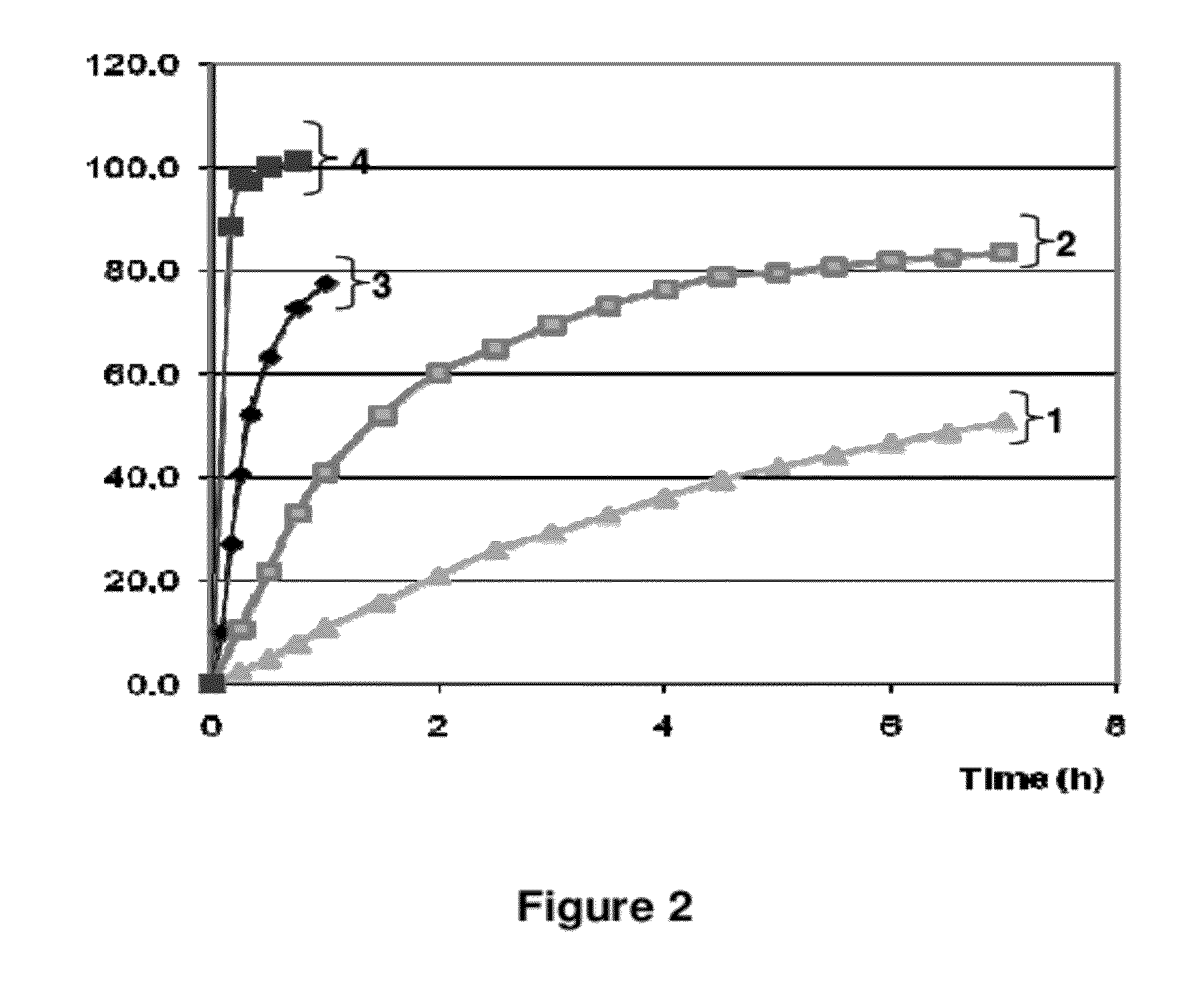
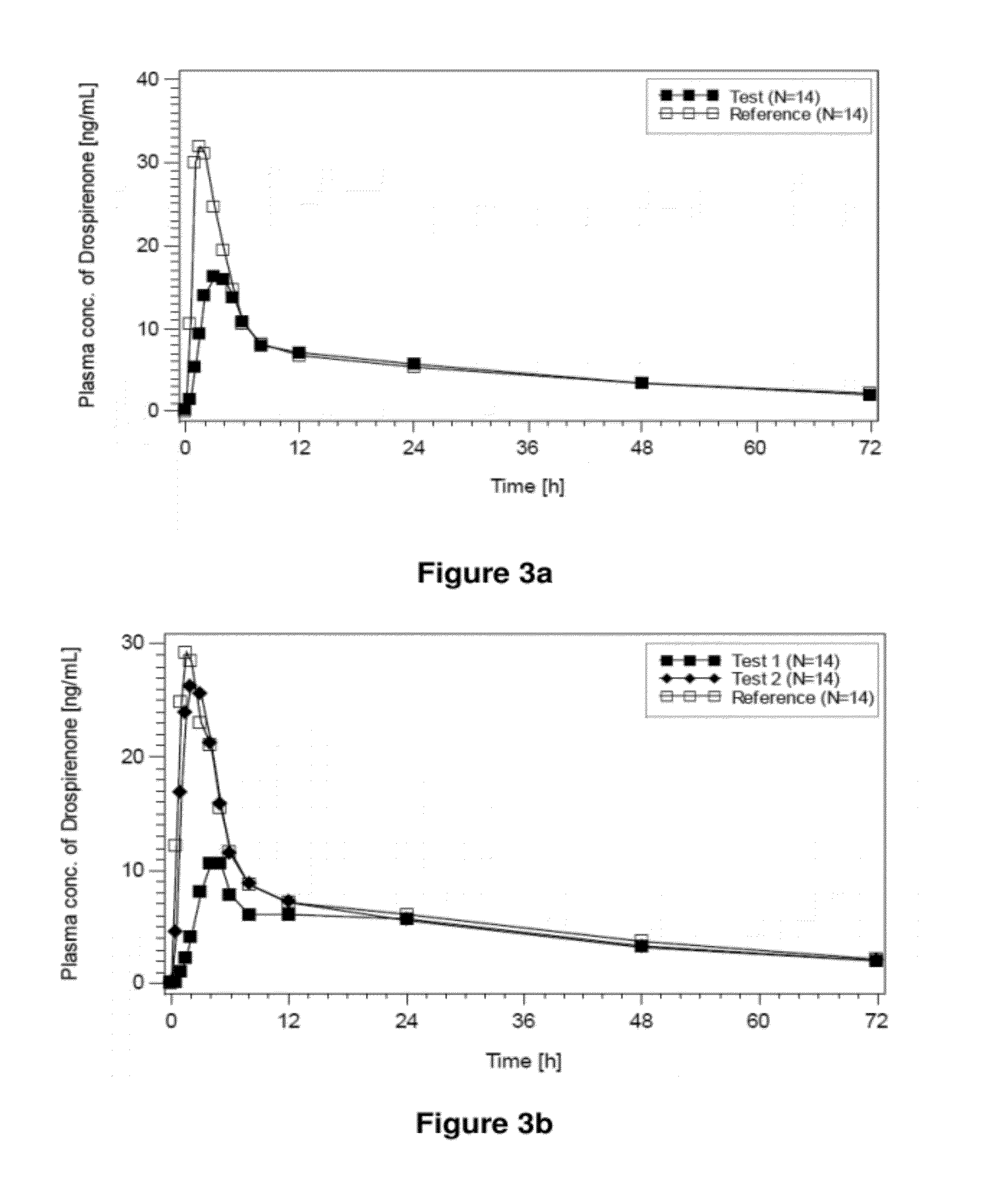
The present invention relates to pharmaceutical compositions and kits comprising pharmaceutical compositions, and methods for administering pharmaceutical compositions comprising active contraceptive drugs in a patient. Specifically, the pharmaceutical compositions may comprise progestogen-only contraceptives (“POC”), such as Drospirenone.
Owner:LAB LEON FARMA
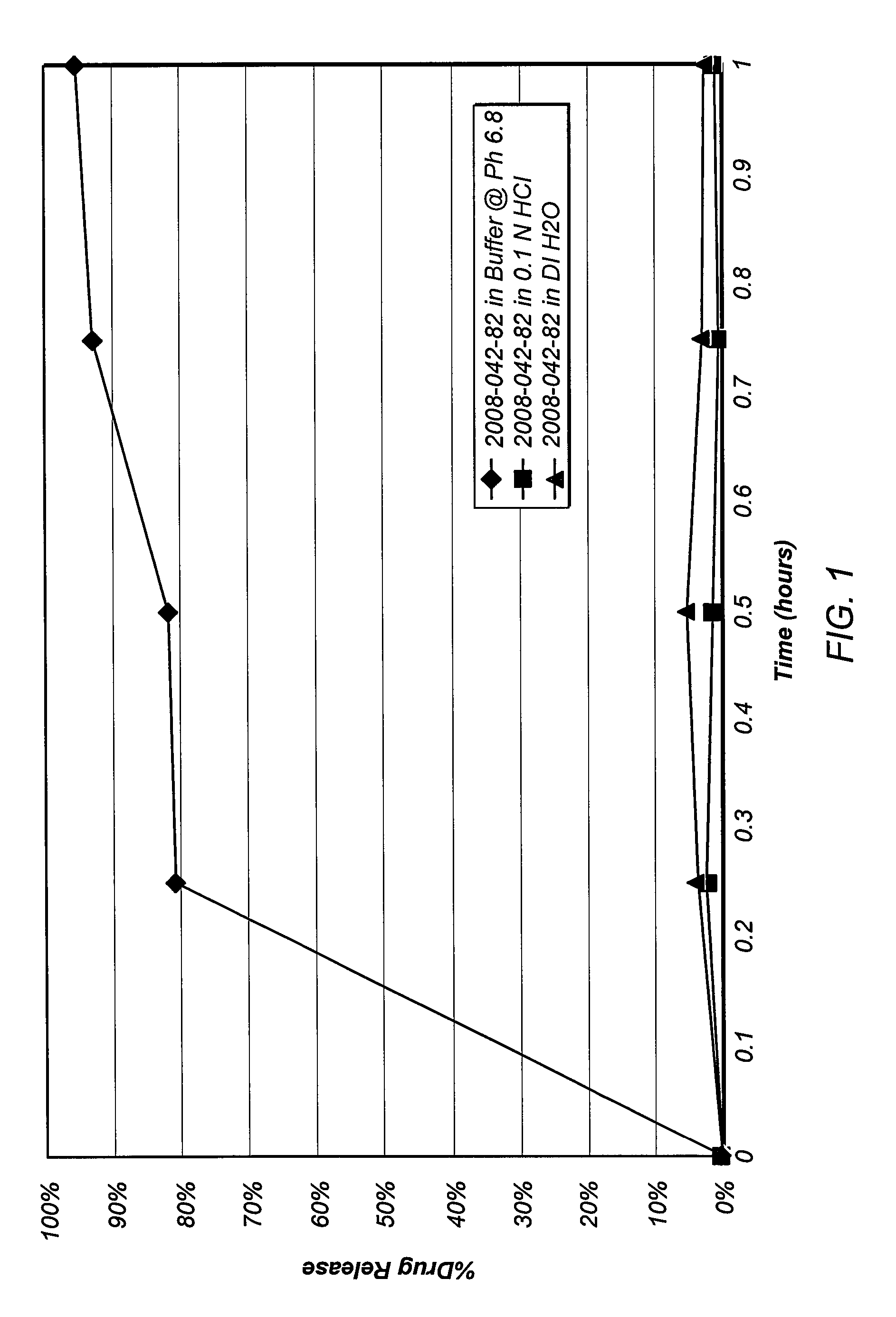
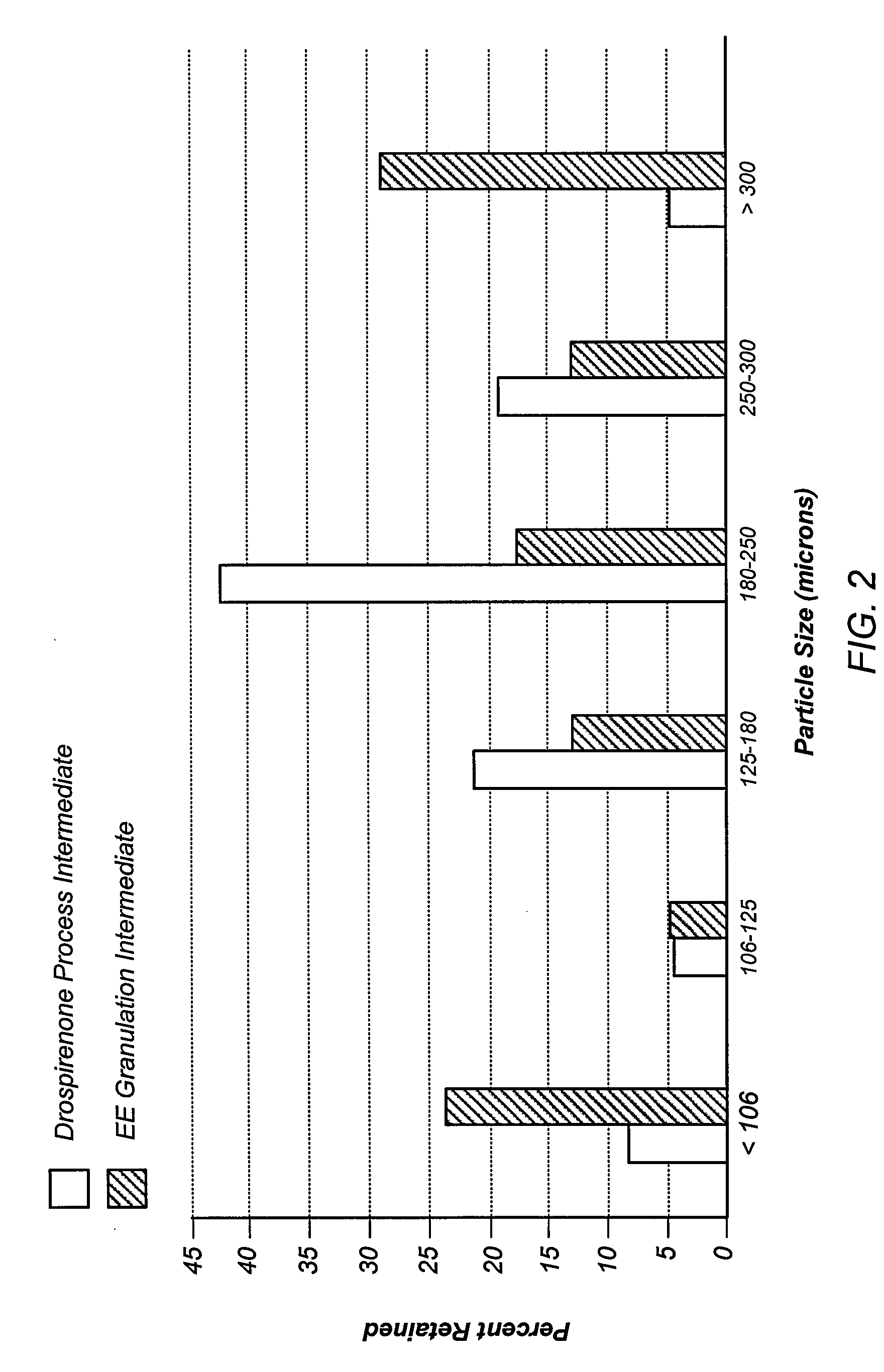
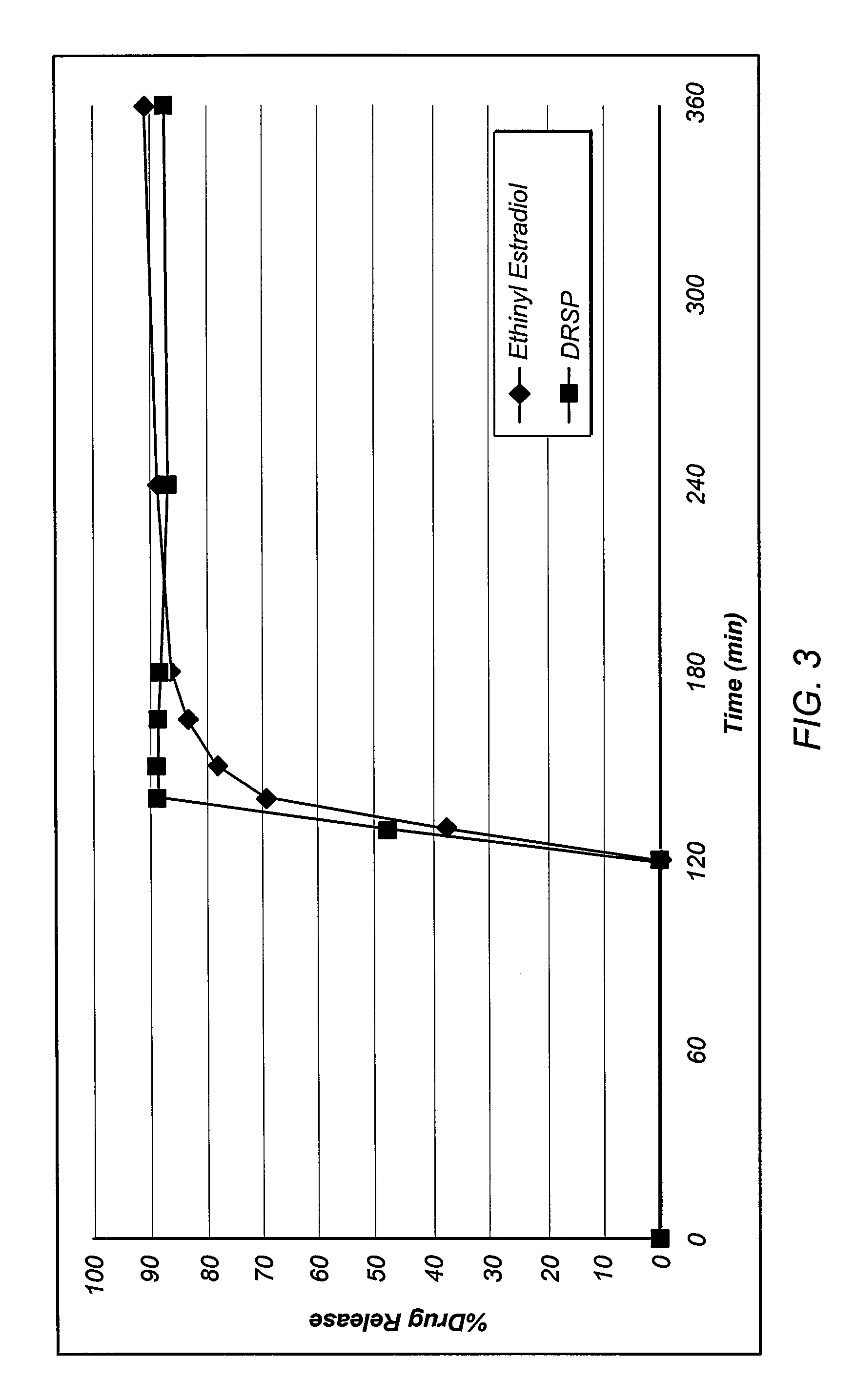
Oral contraceptive dosage forms and methods of making such dosage forms
Disclosed herein are oral dosage forms and methods of their use, in particular oral dosage systems for the delivery of drugs for use as a female oral contraceptive. In an embodiment, an oral dosage form includes a progestogen dispersed in an enteric polymer and an estrogen.
Owner:EVESTRA
Pharmaceutical compositions comprising one or more steroids one or more tetrahydrofolate components and vitamin b12
InactiveUS20050164977A1Avoid problemsBiocideCarbohydrate active ingredientsOral medicationPhysiology
The present invention is concerned with a kit for use in a hormonal contraceptive method or hormone replacement therapy in mammalian females, said kit comprising at least 10 oral dosage units containing at least 1 μg of one or more steroids selected from the group consisting of estrogens and progestogens; at least 0.1 mg of one or more tetrahydrofolate components selected from the group consisting of (6S)-tetrahydrofolic acid, 5-methyl-(6S)-tetrahydrofolic acid, 5-formyl-(6S)-tetrahydrofolic acid, 10-formyl-(6R)-tetrahydrofolic acid, 5,10-methylene-(6R)-tetrahydrofolic acid, 5,10-methenyl-(6R)-tetrahydrofolic acid, 5-formimino-(6S)-tetrahydrofolic acid, pharmaceutically acceptable salts of these tetrahydrofolic acids and glutamyl derivatives of these tetrahydrofolic acids; and at least 0.1 mg vitamin B 12. Other aspects of the present invention relate to a hormonal contraceptive method and a method of hormone replacement therapy comprising the at least once daily oral administration of one or more steroid containing dosage units to a mammalian female, wherein the dosage units additionally contain at least 0.1 mg of one or more of the aforementioned tetrahydrofolate components and at least 0.1 mg vitamin B 12.
Owner:PANTARHEI BIOSCI
Method of preventing or treating benign gynaecological disorders
ActiveUS8071576B2Few side-effectsLow recurrence rateBiocideOrganic active ingredientsDiseaseGynecological disorders
The present invention relates to a method of preventing or treating benign estrogen sensitive gynecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
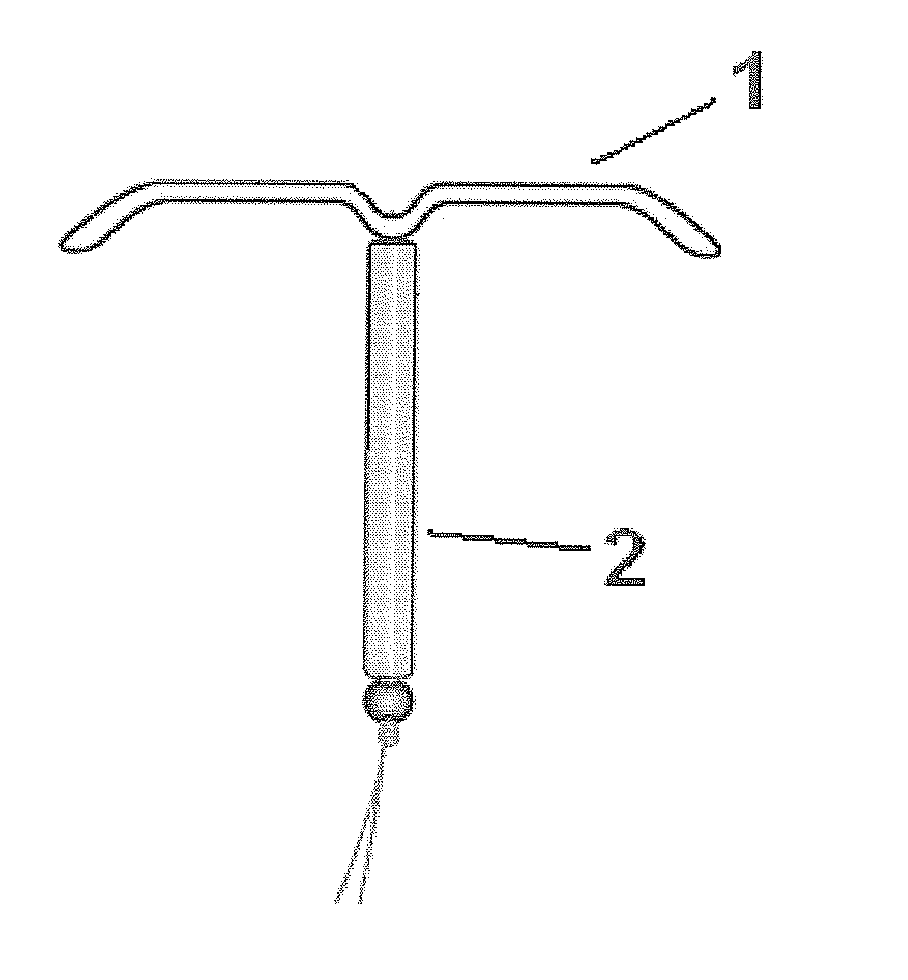
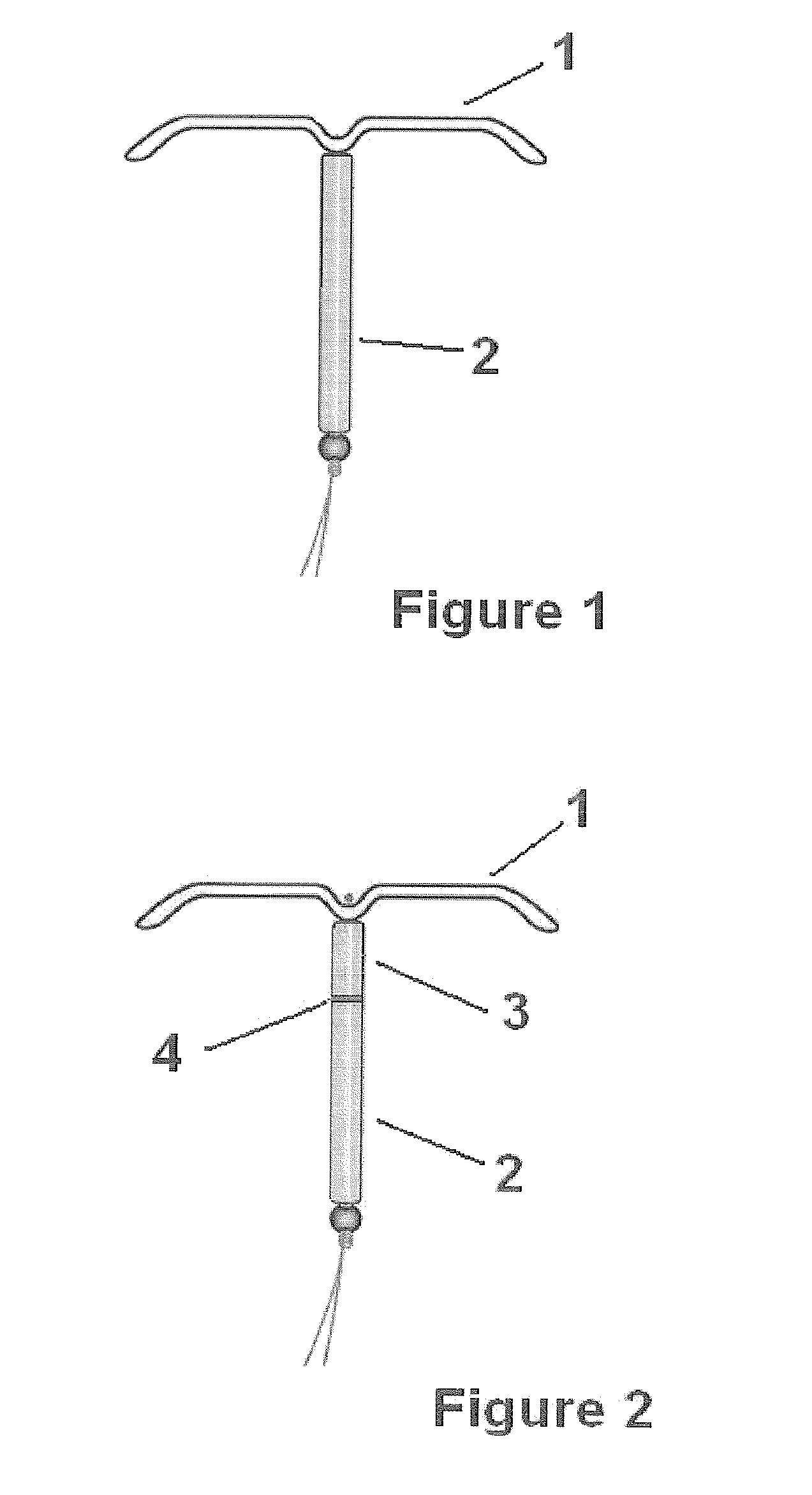
Intrauterine delivery system for contraception
ActiveUS20140127280A1High success rateMinimal to no side-effects or related complicationsBiocideOrganic active ingredientsControl releaseEndometrium
The invention relates to a method for contraception and for reducing menstrual problems and inducing amenorrhea, wherein an intrauterine delivery device is used for the controlled release of a combination of progestogen or a drug having a progestogenic activity and at least one therapeutically active substance capable of preventing or suppressing abnormal and / or irregular endometrial bleeding over a prolonged period of time.
Owner:BAYER OY
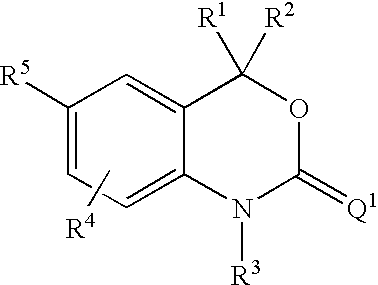
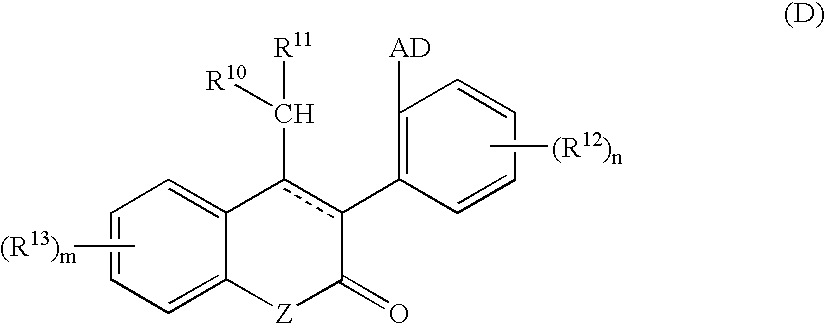
6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators
InactiveUS20070225281A1Organic active ingredientsOrganic chemistryArylProgesterone receptor modulators
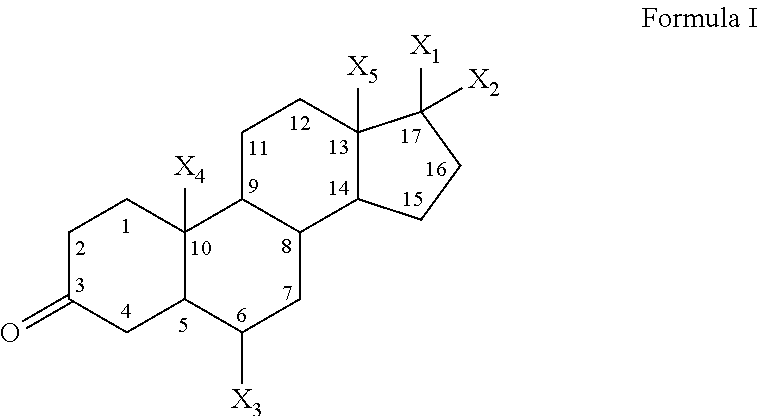
Compounds having the structure of formula I are provided. In formula I, R1 is H, OH, substituted or unsubstituted C1 to C3 alkyl, C1 to C3 perfluoroalkyl, or COR6; R6 is H, substituted or unsubstituted C1 to C4 alkyl, aryl, substituted or unsubstituted C1 to C4 alkoxy, substituted or unsubstituted C1 to C3 aminoalkyl; R2 and R3 are H, substituted or unsubstituted C1 to C6 alkyl, C1 to C6 perfluoroalkyl, substituted or unsubstituted C2 to C6 alkenyl, substituted or unsubstituted C2 to C6 alkynyl, substituted or unsubstituted C3 to C6 cycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heterocyclic; or R2 and R3 are fused to form spirocyclic rings; R4 is NHR7, OR7, NHSO2R7, or OSO2R7; Q is O, S, NR8, or CR9R10; or a pharmaceutically acceptable salt, ester, or prodrug thereof. Such compounds are useful as progesterone receptor modulators and for treating progesterone receptor related conditions.
Owner:WYETH LLC
External patch containing estrogen and/or progestogen
InactiveUS8486442B2Stable drug releaseLess irritatingBiocideOrganic active ingredientsIrritationProlonged release
An external patch capable of stable prolonged release and transdermal absorption of active ingredient hormones (estrogens and / or progestogens) contained in a pressure sensitive adhesive layer, which external patch ensures low irritation on the skin. In particular, an external patch comprising a support and, superimposed thereon, a pressure sensitive adhesive layer, characterized in that the pressure sensitive adhesive layer comprises, as indispensable components, 5 to 50 wt. % of styrene / isoprene / styrene block copolymer, 20 to 70 wt. % of tackifier resin, 10 to 60 wt. % of softener and 1 to 20 wt. % of polyvinylpyrrolidone and contains, as an active ingredient, estrogen and / or progestogen.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
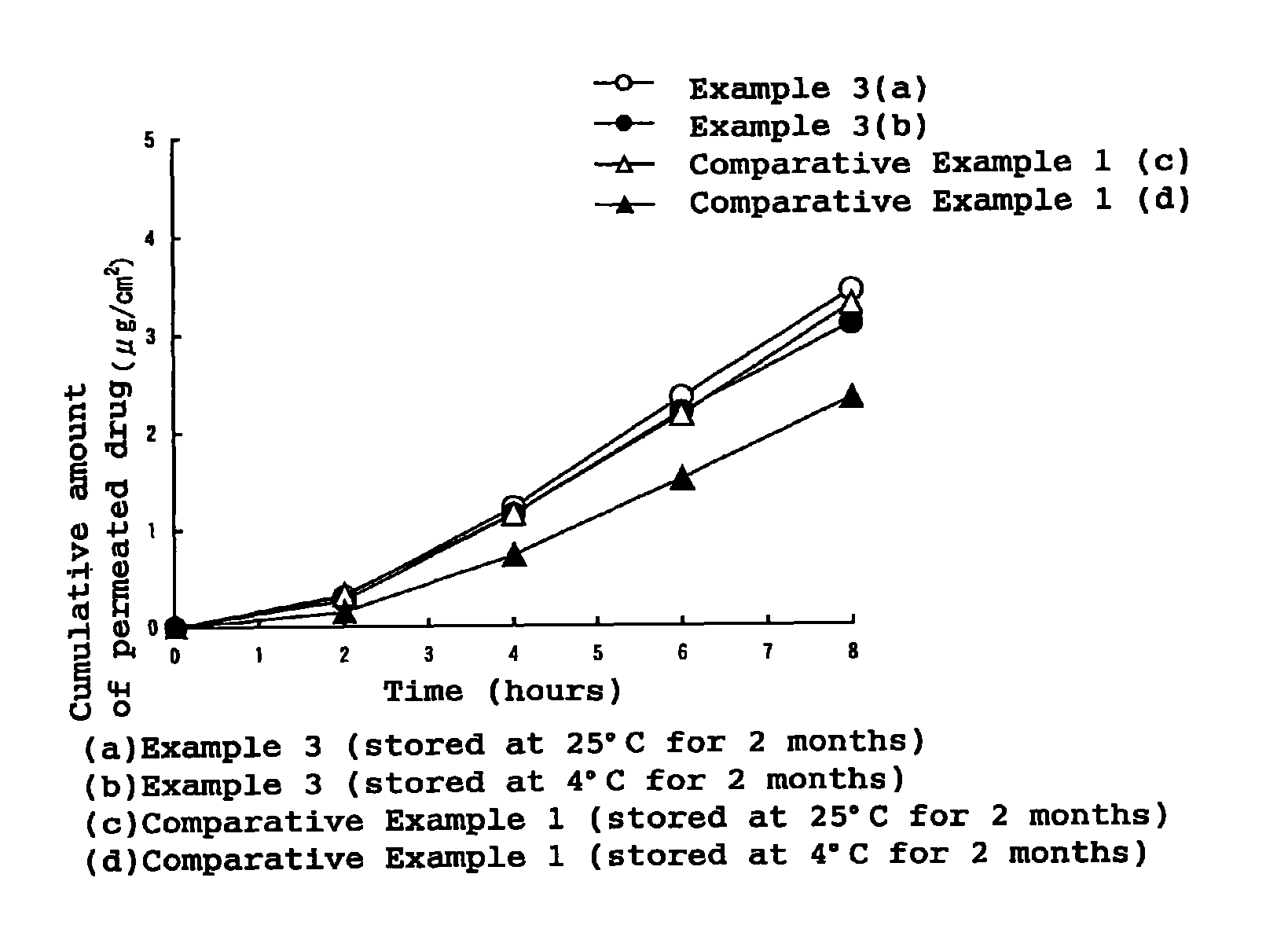
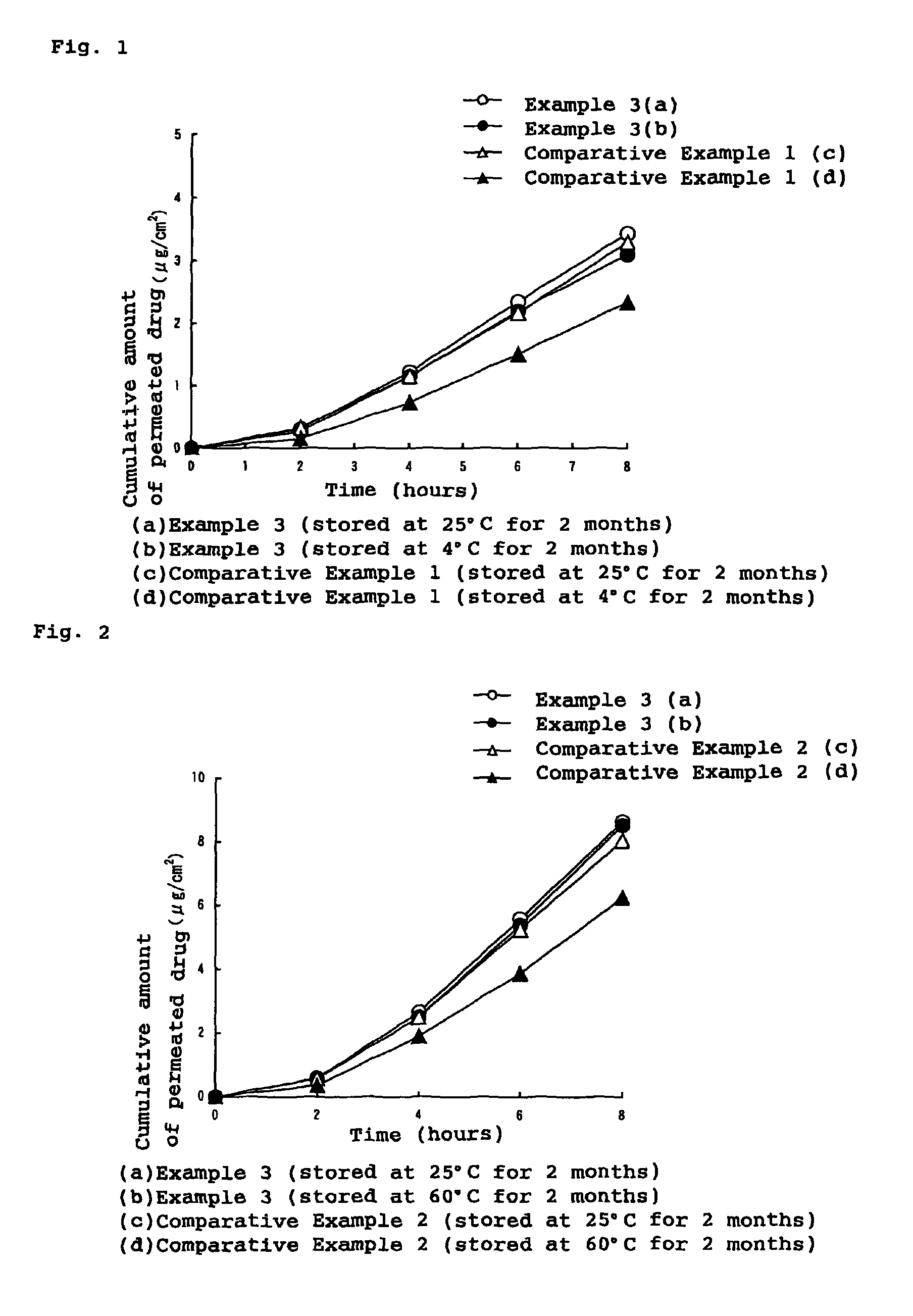
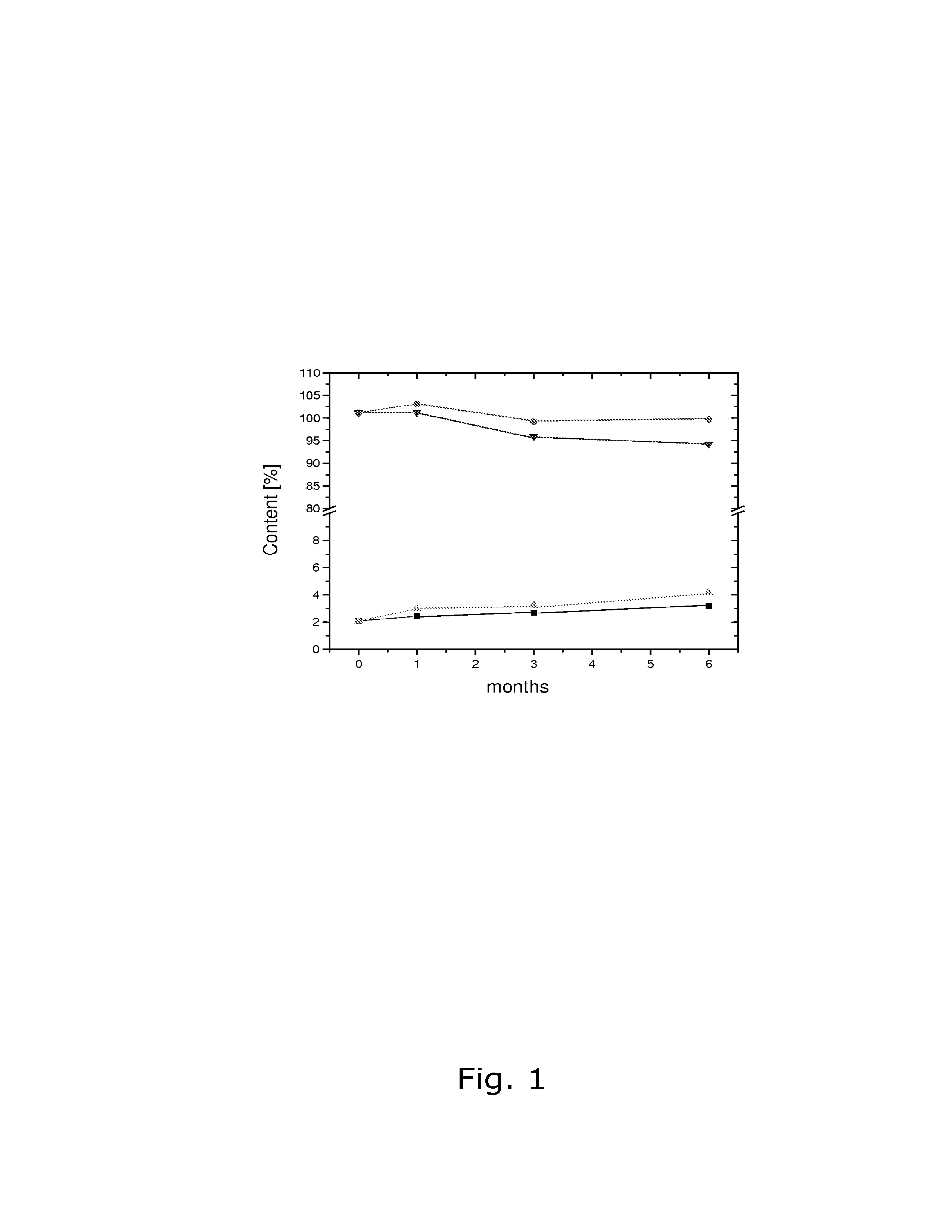
Stability of progestogen formulations
InactiveUS20060257472A1Extended shelf lifeImprove stabilityOrganic active ingredientsBiocideCellulosePhysiology
In a preparation for hormone replacement therapy having a low content of progestogen, the stability of the progestogen component can be enhanced by using a cellulosic binder, for example hydroxypropylcellulose, in stead of a non-cellulosic binder.
Owner:NOVO NORDISK AS
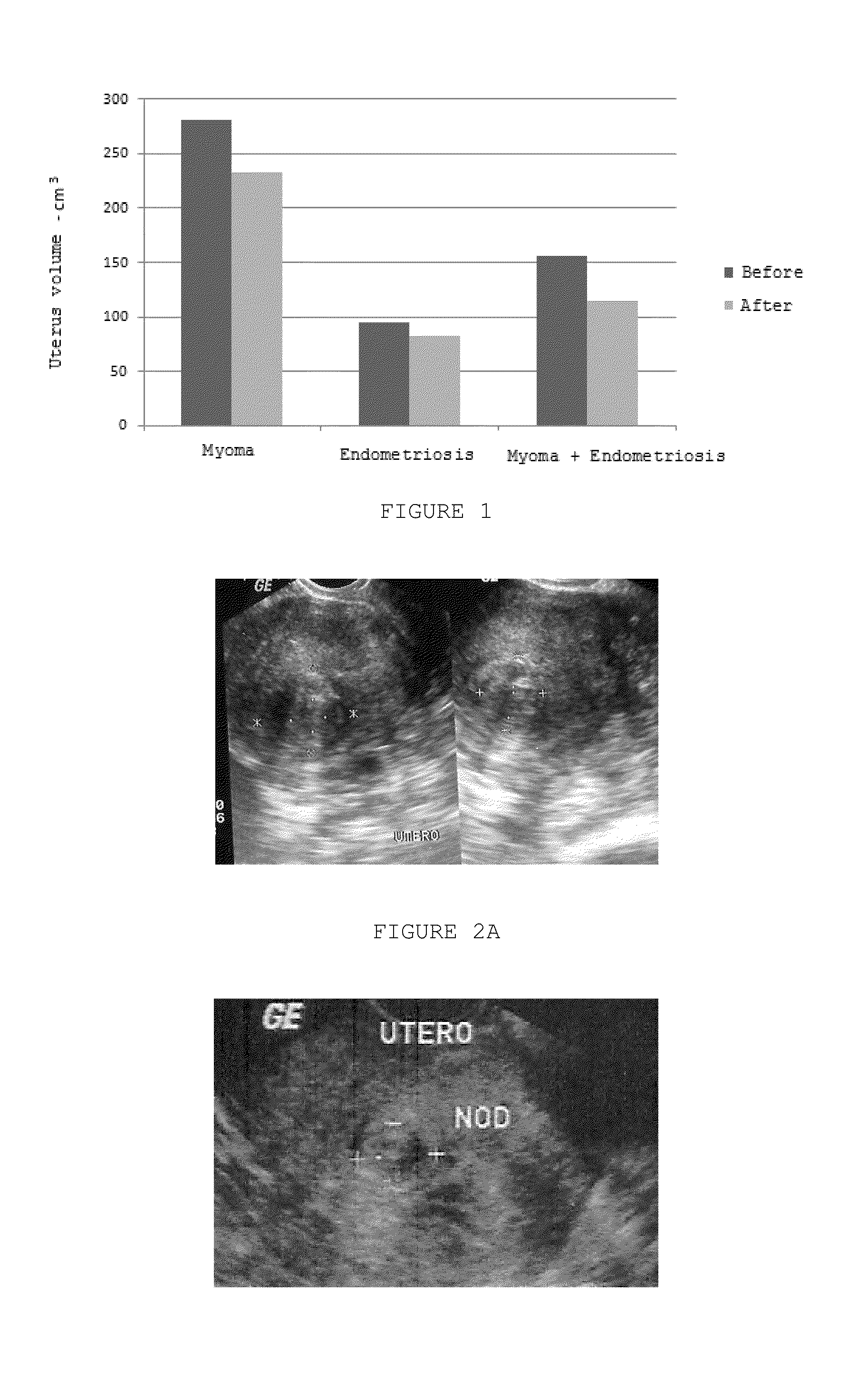
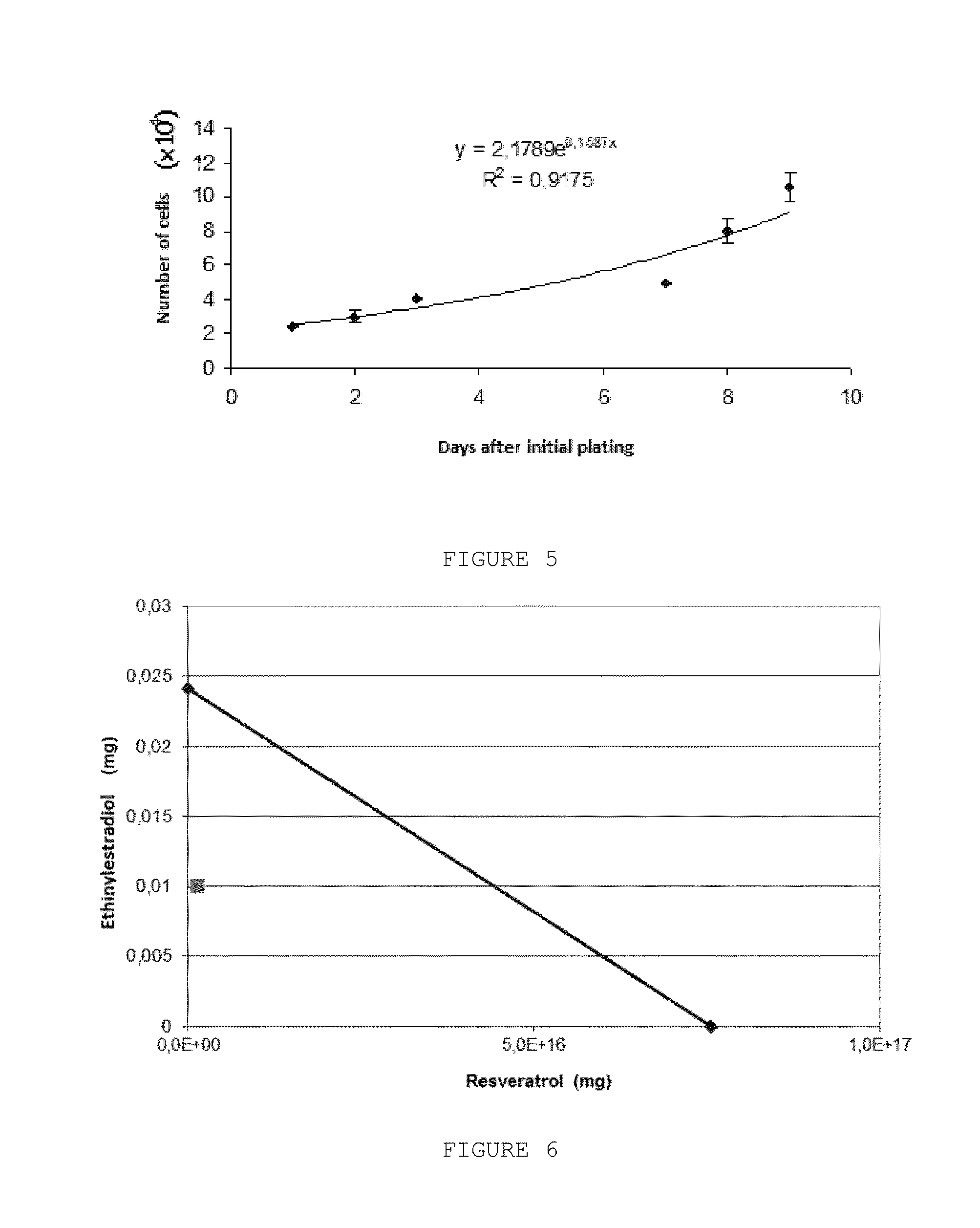
Pharmaceutical Combination of Resveratrol and Progestin to Treat and/or Prevent Myoma and/or Endometriosis
The present invention relates to a combination of resveratrol with progestogen, and medicaments and pharmaceutical compositions containing the same, which are useful in the treatment and / or prevention of myoma and / or endometriosis, providing their size reduction and the control of related symptoms. Said association may additionally comprise an estrogen component. A treatment method and a kit are also objects of this invention.
Owner:LIBBS FARM
Pharmaceutical composition containing a tetrahydrofolic acid
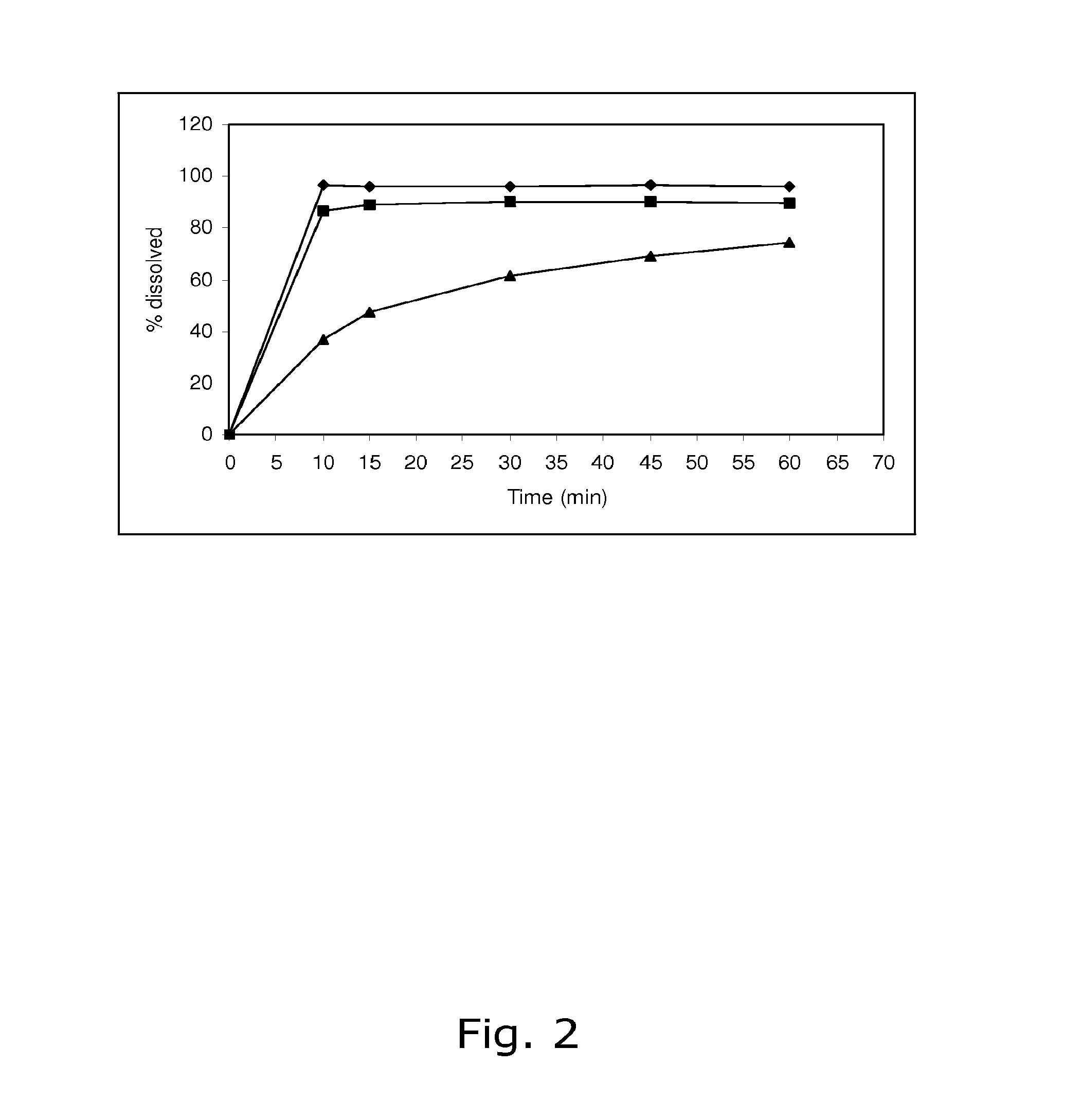
The present invention relates to solid pharmaceutical compositions, in particular to oral contraceptives, comprising a progestogen, such as drospirenone; an estrogen, such as ethinylestradiol; a tetrahydrofolic acid or a pharmaceutically acceptable salt thereof, such as calcium 5-methyl-(6S)-tetrahydrofolate; and at least one pharmaceutical acceptable excipient or carrier. The compositions of the invention provide good stability of the tetrahydrofolic acid upon storage while still ensuring a fast and reliable release of the estrogen and the progestogen present in the composition.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
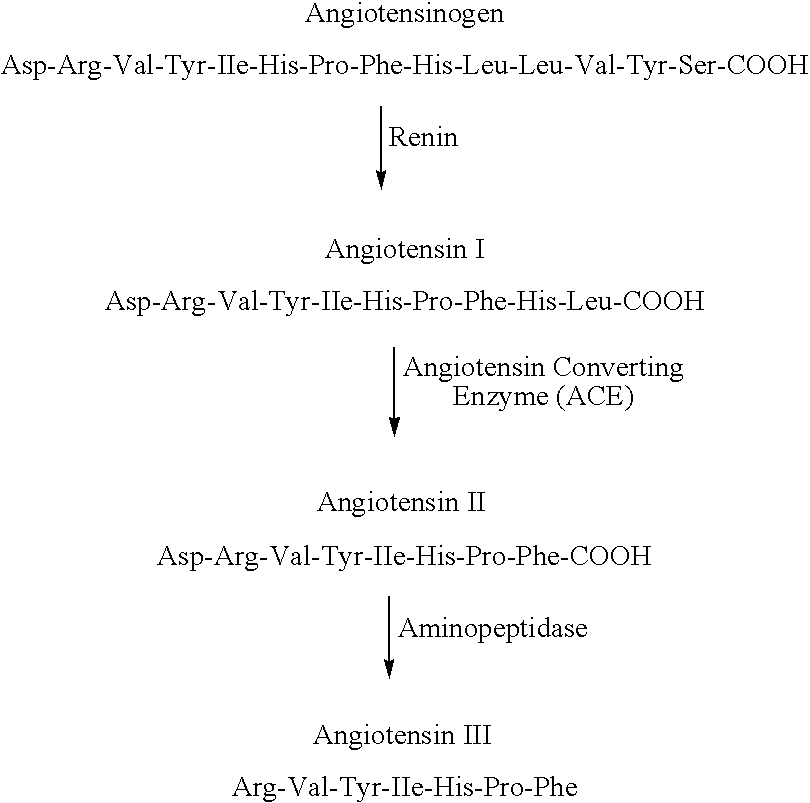
Treatment of Prevention of Unscheduled Bleeding in Women on Progestogen Containing Medication
InactiveUS20090197843A1Increased chronotropismIncreased inotropismBiocidePeptide/protein ingredientsPhysiologyProgestogen
The present invention relates to a method of treating or preventing unscheduled bleeding in women, the unscheduled bleeding being the result of repeated administration of a hormonal composition that contains a progestogen, wherein the method includes the administration of an effective amount of Renin Angiotensin System (RAS) suppressor selected from angiotensin converting enzyme inhibitors; angiotensin II receptor antagonists; renin inhibitors and combinations thereof. Other aspects of the invention relate to a pharmaceutical composition containing a RAS suppressor and a progestogen and to a pharmaceutical kit having a plurality of dosage units, wherein at least one dosage unit contains a progestogen; at least one dosage unit contains an estrogen; and at least one dosage unit contains a RAS suppressor.
Owner:PANTARHEI BIOSCI
Heteroatom containing tetracyclic derivatives as selective estrogen receptor modulators
The present invention is directed to novel heteroatom containing tetracyclic derivatives, pharmaceutical compositions containing them, their use in the treatment and / or prevention of disorders mediated by one or more estrogen receptors and processes for their preparation. The compounds of the invention are useful in the treatment and / or prevention of disorders associated with the depletion of estrogen such as hot flashes, vaginal dryness, osteopenia and osteoporosis; hormone sensitive cancers and hyperplasia of the breast, endometrium, cervix and prostate; endometriosis, uterine fibroids, osteoarthritis and as contraceptive agents, alone or in combination with a progestogen or progestogen antagonist.
Owner:ORTHO MCNEIL PHARM INC
Hormone replacement therapy method and hormone dispenser
InactiveUS6083528AAvoid possibilityPrevent projectionOrganic active ingredientsClosuresHormone replacementPhysiology
Varying the daily dose of either or both of the estrogen and the progestogen administered for hormone replacement therapy (HRT) is readily and inexpensively accomplished, without the necessity of the physician prescribing a new product each time the daily dose of the estrogen or progestogen is changed, by administering preferably transdermally the estrogen and the progestogen contained in separate extrudable pharmaceutical compositions from a dispenser which contains means, preferably adjustable only by the attending physician or dispensing pharmacist, for varying the volume of either or both of the respective compositions which is dispensed as a single dose from the dispenser in response to a defined digital dispensing manipulation of the dispenser thereby facilitating optimal compliance to a combination of HRT with individually adjusted dosages of the estrogen and progestogen.
Owner:BAYER SCHERING PHARMA AG
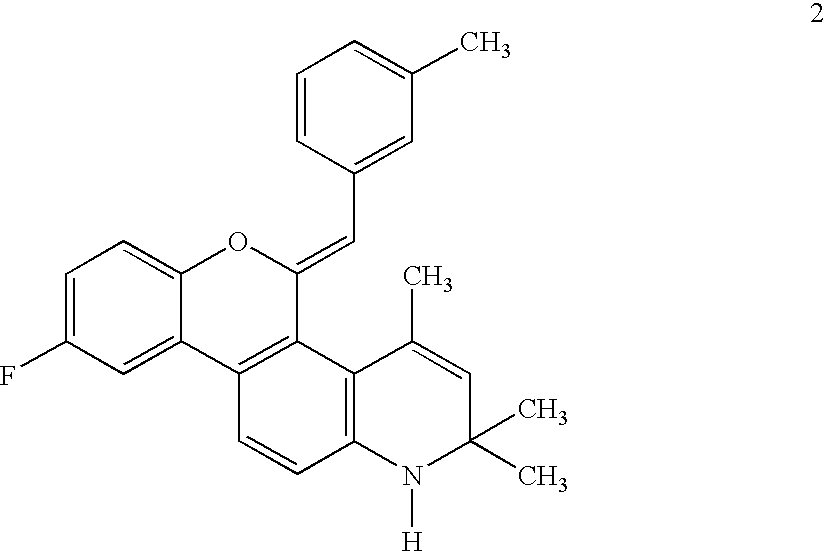
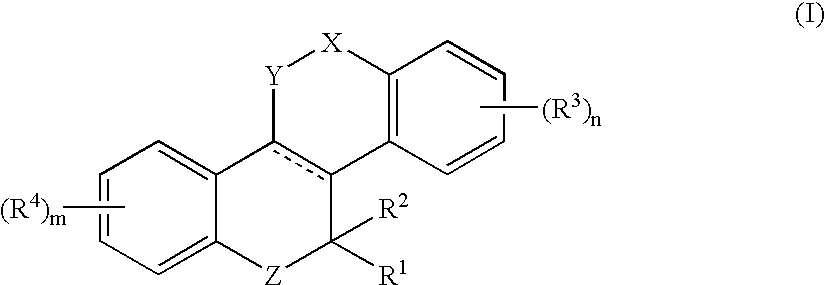
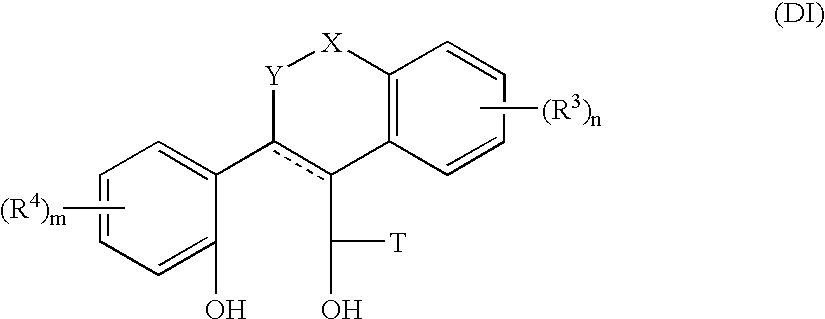
Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors
The present invention is directed to novel heterocyclic benzo[c]chromene derivatives, pharmaceutical compositions containing them and their use in the treatment of disorders mediated by one or more estrogen receptors. The compounds of the invention are useful in the treatment of disorders associated with the depletion of estrogen such as hot flashes, vaginal dryness, osteopenia and osteoporosis; hormone sensitive cancers and hyperplasia of the breast, endometrium, cervix and prostate; endometriosis, uterine fibroids, osteoarthritis and as contraceptive agents, alone or in combination with a progestogen or progestogen antagonist.
Owner:JANSSEN PHARMA NV
Benzonitryl and nitrobenzyl derivatives that modulate androgen receptors
This invention relates to benzonitryl and nitrobenzyl derivatives that are modulators of androgen, glucocorticoid, mineralocorticoid, and progesterone receptors, and also to the methods for the making and use of such compounds. These compounds are useful, for example, in the treatment or prophylaxis of conditions or disorders that respond to selective androgen receptor modulation.
Owner:SMITHKLINE BECKMAN CORP
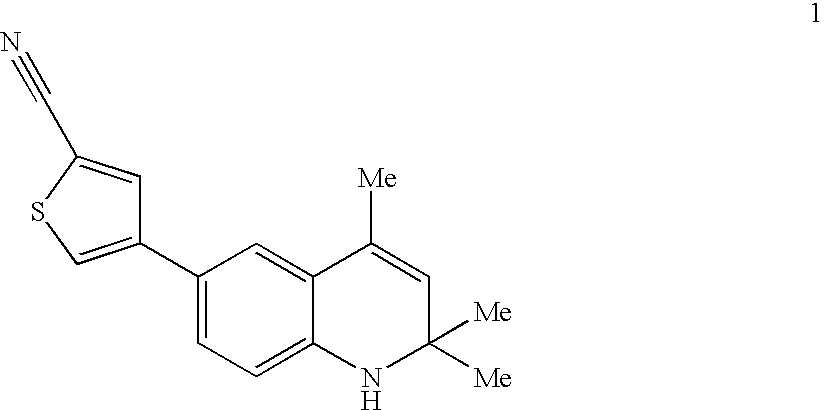
5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-5H-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds
ActiveUS7084151B2Improve fertilityReduced fertilityBiocideOrganic chemistryPR - Progesterone receptorQuinoline
The present invention is directed to compounds, pharmaceutical compositions, and methods for modulating processes mediated by Progesterone Receptor. Also provided are methods of making such compounds and pharmaceutical compositions.
Owner:LIGAND PHARMA INC
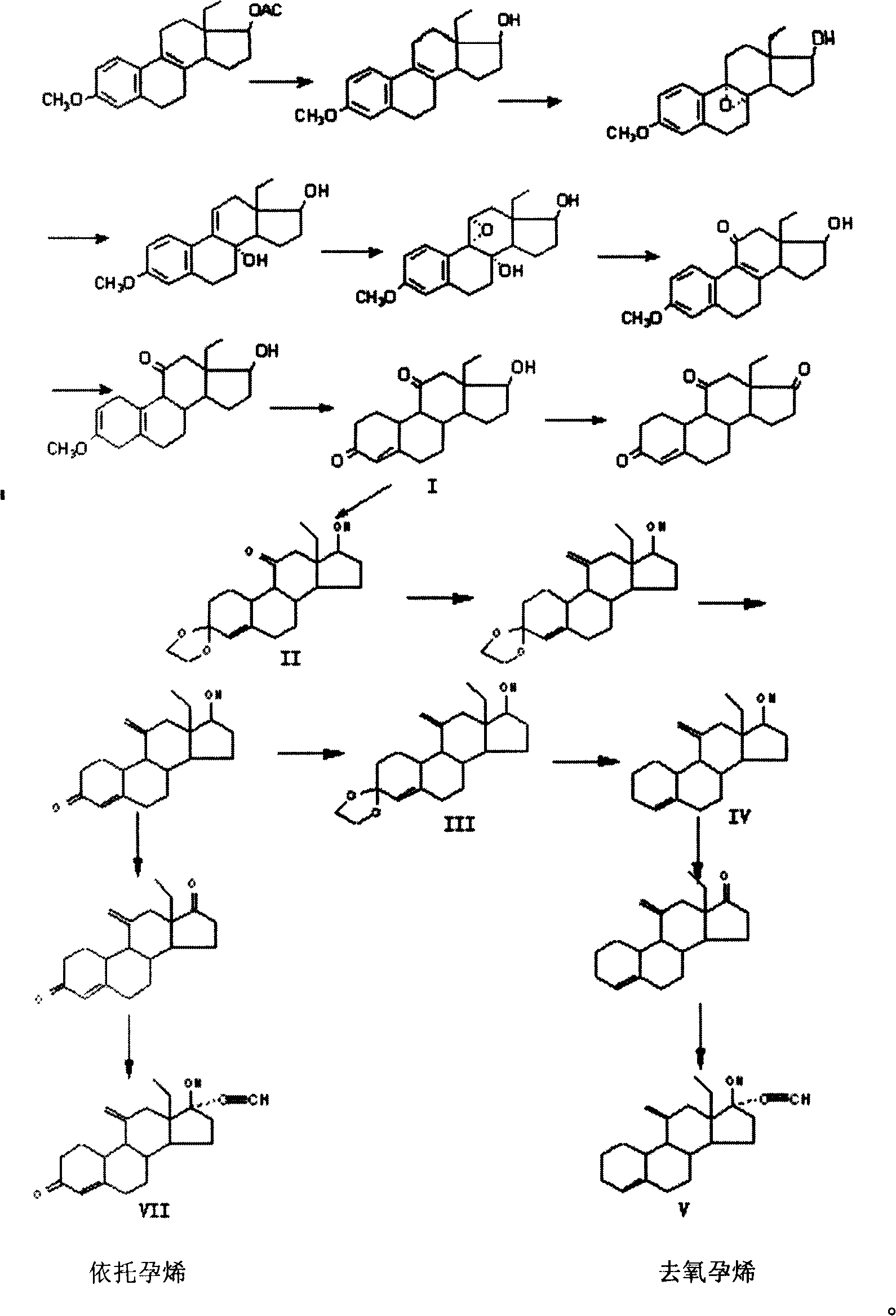
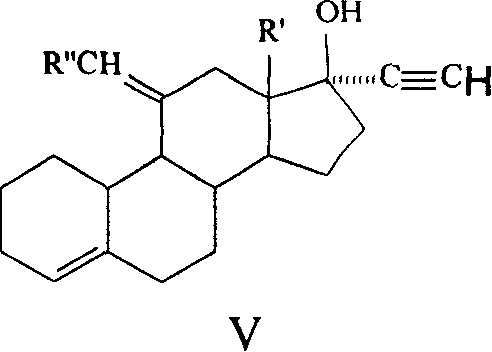
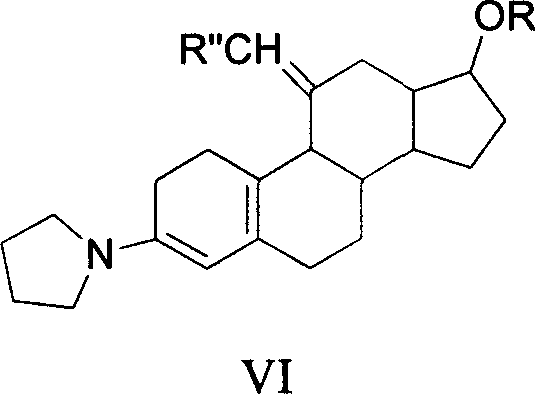
Method for synthesizing steroid progestogen
The invention discloses a synthetic method of steroidal gestagen and key medium I, II, III, IV in the drug chemical technological domain, which adopts 13 ethyl- 1,3,5(10),8(9)- estrange tetraenes-17-alcohol as raw material to synthesize deoxidation pregnancy and depending pregnancy.
Owner:JIANGSU CHUANGUO PHARMA CO LTD
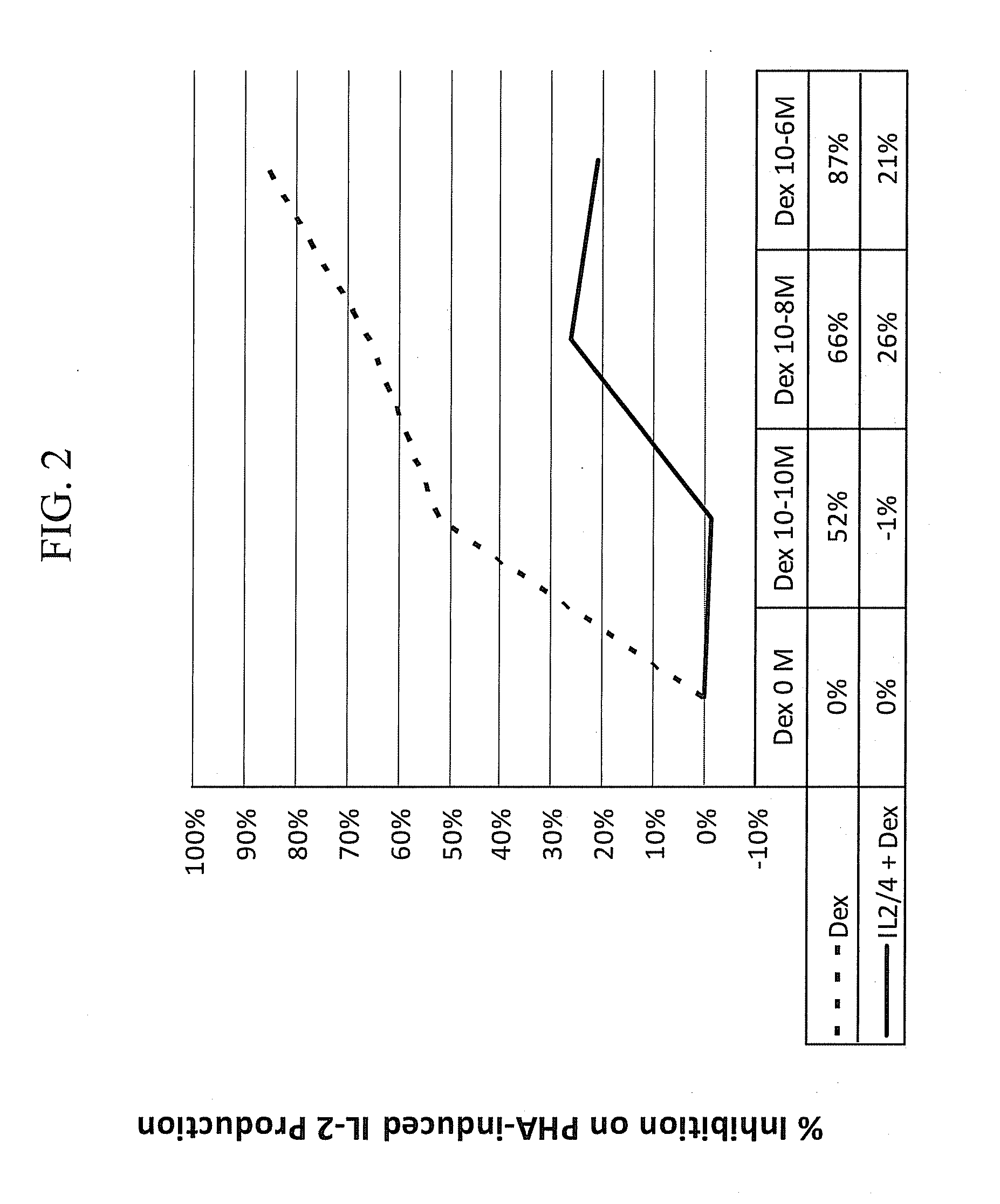
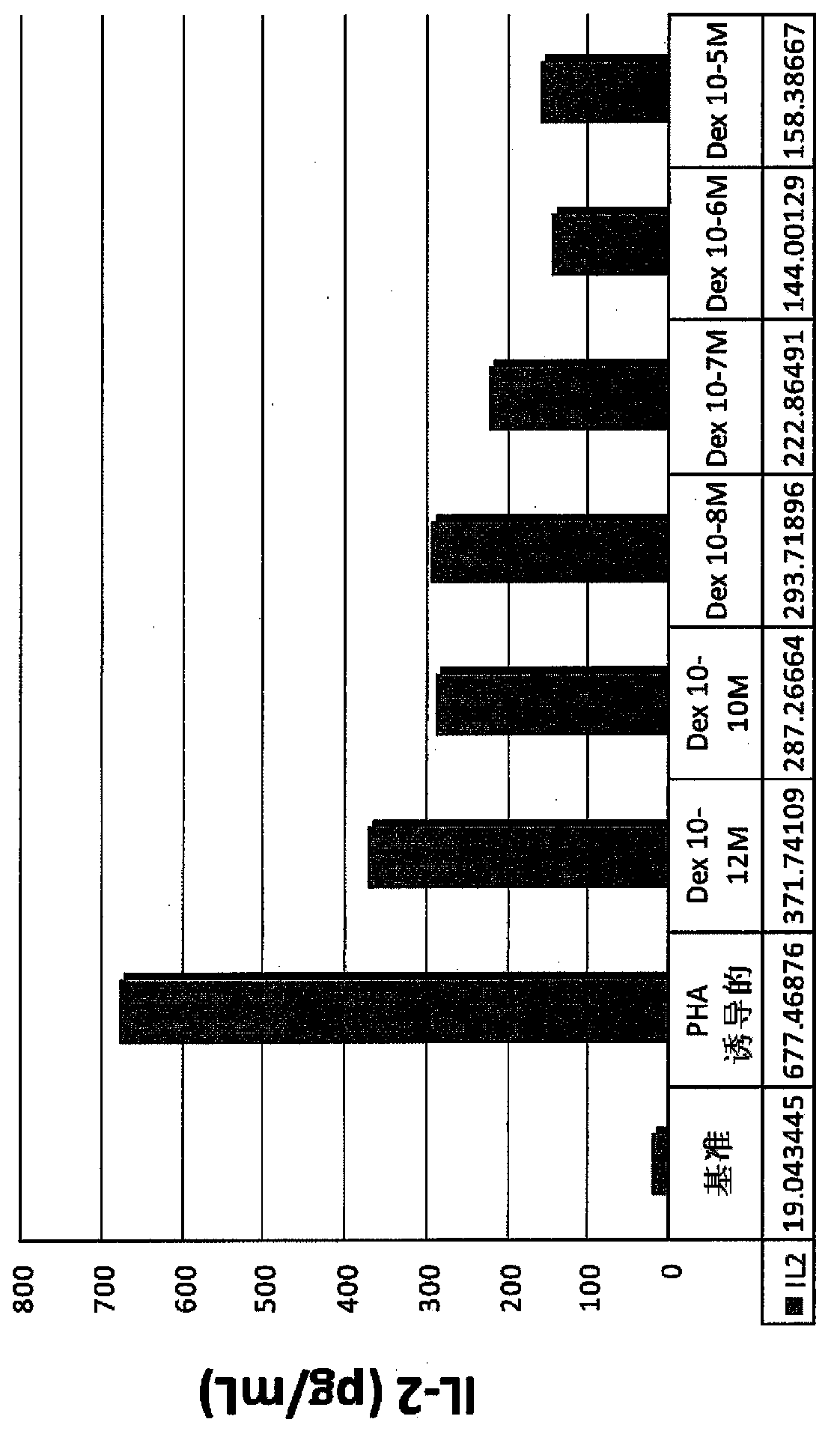
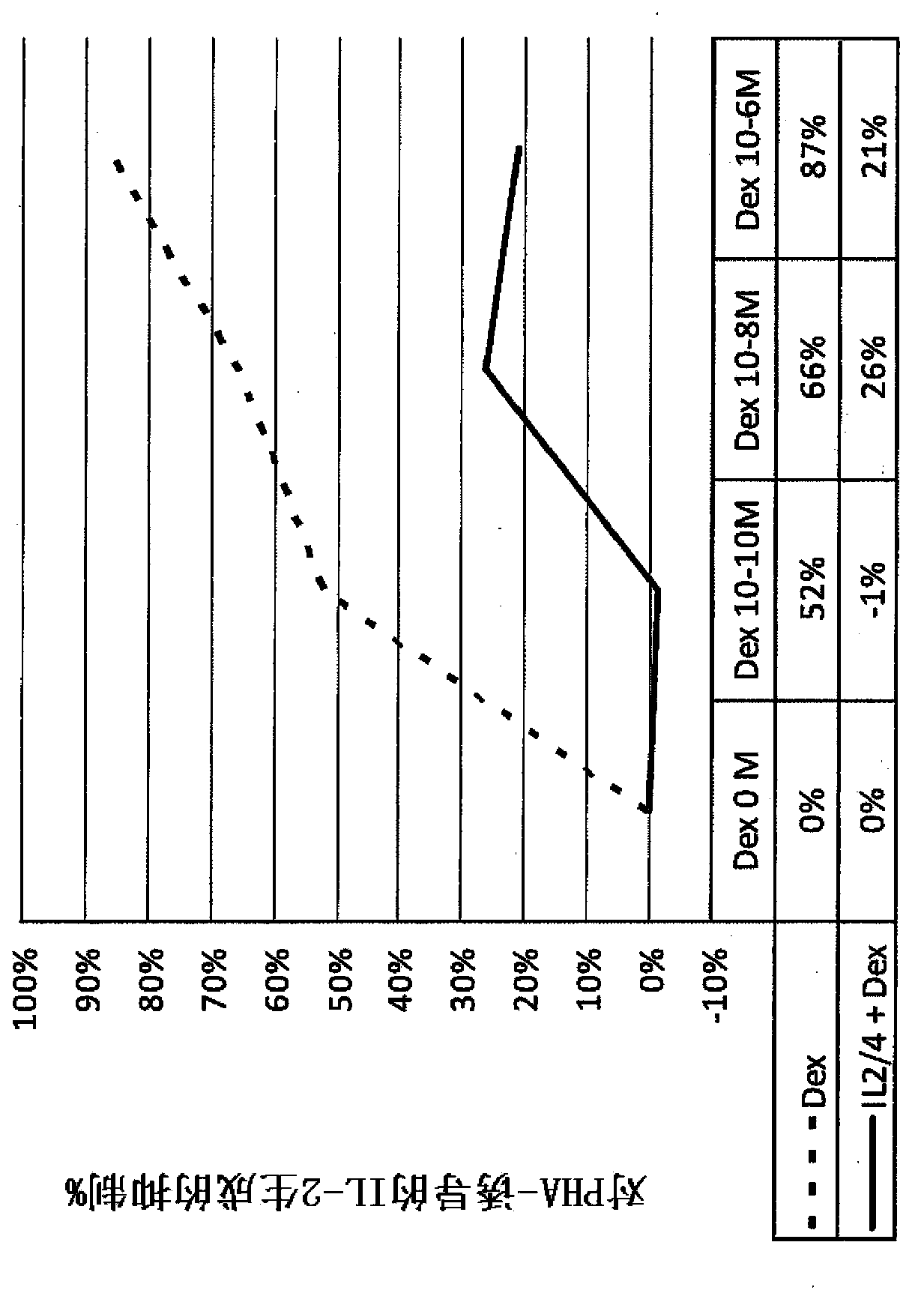
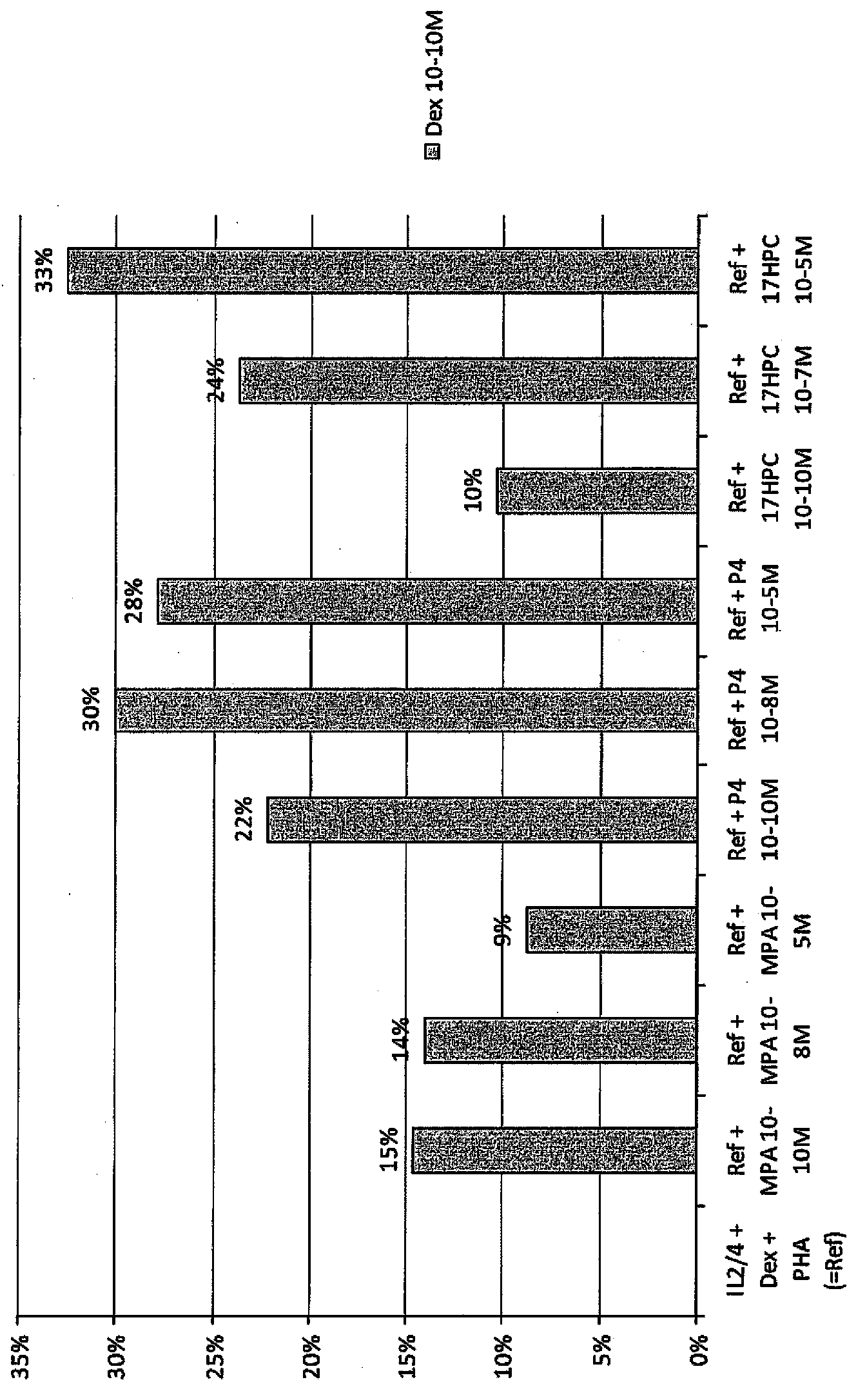
Pulmonary delivery of 17-hydroxyprogesterone caproate (17-hpc)
InactiveUS20110262502A1Improve responsivenessImprove toleranceAntibacterial agentsOrganic active ingredientsPowder mixtureInhalation
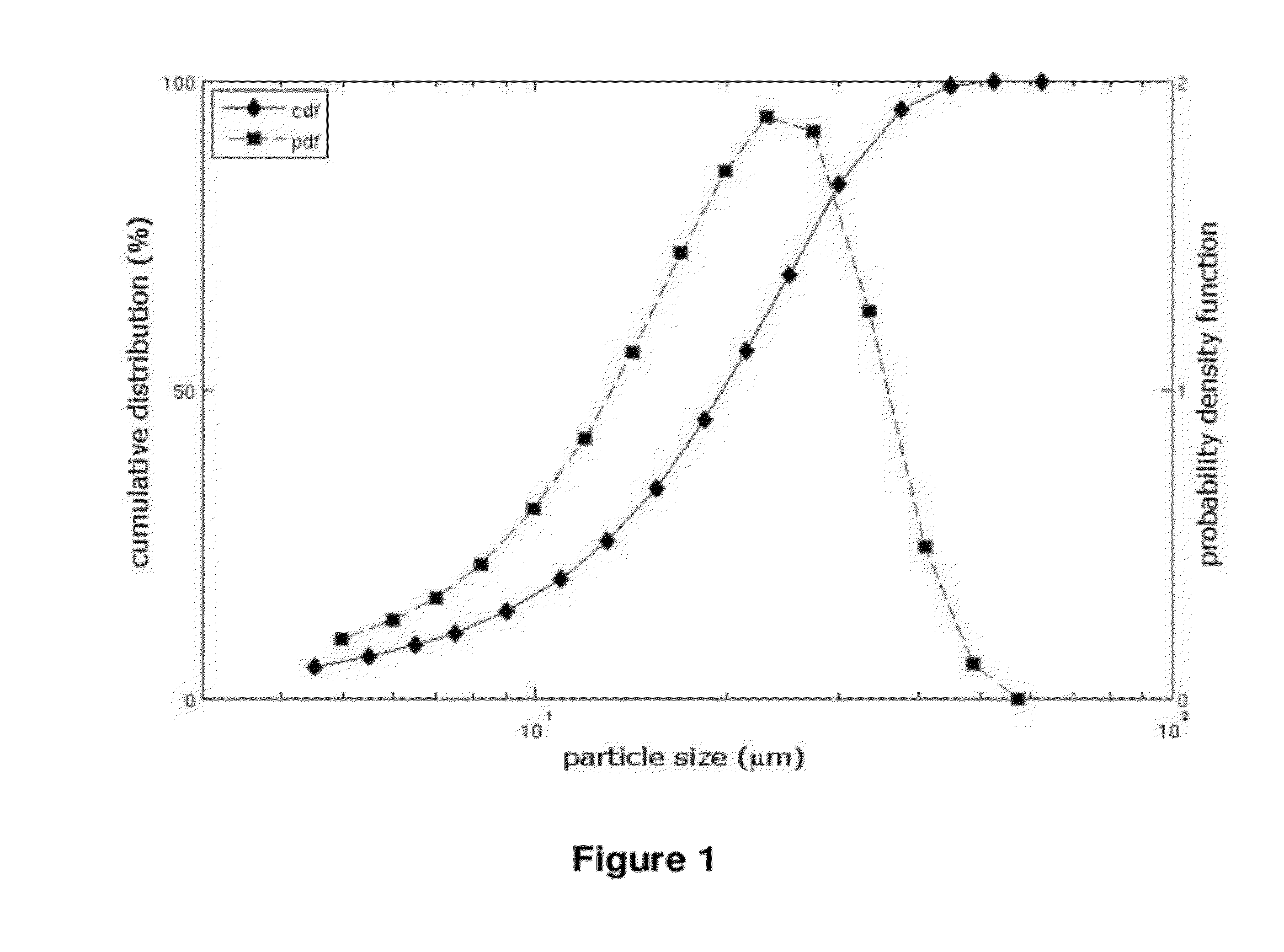
The invention relates to 17-HPC pulmonary formulations for administration by inhalation comprising 17-HPC and a pharmaceutically acceptable excipient. Particle size reduction of 17-HPC is required for the pulmonary delivery, and can be achieved with a surfactant or water without the surfactant. Preferred pulmonary formulations include a powder blend comprising a therapeutically effective amount of at least one steroid hormone (progestogen) as a glucocorticoid sensitizer, and at least one pharmaceutically acceptable excipient, wherein the at least one steroid hormone (progestogen) has a particle size distribution profile ranging from about one nanometer to about ten microns in the powder blend.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Method of controlled ovarian hyperstimulation and pharmaceutical kit for use in such method
InactiveUS20060217315A1High implantation ratePremature LH-surgesBiocideOrganic active ingredientsMeiosisProgesterones
Owner:PANTARHEI BIOSCI
Pulmonary delivery of 17-hydroxyprogesterone caproate (17-HPC)
The invention relates to 17-HPC pulmonary formulations for administration by inhalation comprising 17-HPC and a pharmaceutically acceptable excipient. Particle size reduction of 17-HPC is required for the pulmonary delivery, and can be achieved with a surfactant or water without the surfactant. Preferred pulmonary formulations include a powder blend comprising a therapeutically effective amount of at least one steroid hormone (progestogen) as a glucocorticoid sensitizer, and at least one pharmaceutically acceptable excipient, wherein the at least one steroid hormone (progestogen) has a particle size distribution profile ranging from about one nanometer to about ten microns in the powder blend.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Nasal spray formulation and method
InactiveUS20060008420A1Prevent signAvoid symptomsOrganic active ingredientsPeptide/protein ingredientsCyclodextrinNasal spray
A nasal spray formulation for use in female contraception or in the treatment of benign gynecological disorders is described. The nasal preparation is comprised of a GnRH compound and an estrogenic compound in the form of a water-soluble complex with a water-soluble cyclodextrin. The preparation effectively suppresses ovarian estrogen and progesterone production, and prevents signs and symptoms of estrogen deficiency, without a significant increase in the risk of endometrial hyperplasia.
Owner:BALANCE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)
![6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators 6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f34d082a-8763-413a-ac02-c77660029595/US20070225281A1-20070927-C00001.png)
![6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators 6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f34d082a-8763-413a-ac02-c77660029595/US20070225281A1-20070927-C00002.png)
![6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators 6-Amino-1,4-dihydro-benzo[d][1,3]oxazin-2-ones and analogs useful as progesterone receptor modulators](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f34d082a-8763-413a-ac02-c77660029595/US20070225281A1-20070927-C00003.png)
![Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b1a46e-06bc-4b13-9c75-4a9a0b69d2de/US07399767-20080715-C00001.png)
![Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b1a46e-06bc-4b13-9c75-4a9a0b69d2de/US07399767-20080715-C00002.png)
![Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors Heterocyclic benzo[c]chromene derivatives useful as modulators of the estrogen receptors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/48b1a46e-06bc-4b13-9c75-4a9a0b69d2de/US07399767-20080715-C00003.png)



![5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds 5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c08410d8-05dc-45d9-92b5-f7dcb5b56077/US07084151-20060801-C00001.png)
![5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds 5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c08410d8-05dc-45d9-92b5-f7dcb5b56077/US07084151-20060801-C00002.png)
![5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds 5-(1',1'-cycloalkyl/alkenyl)methylidene 1,2-dihydro-<i>5H</i>-chromeno[3,4-ƒ]quinolines as selective progesterone receptor modulator compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c08410d8-05dc-45d9-92b5-f7dcb5b56077/US07084151-20060801-C00003.png)