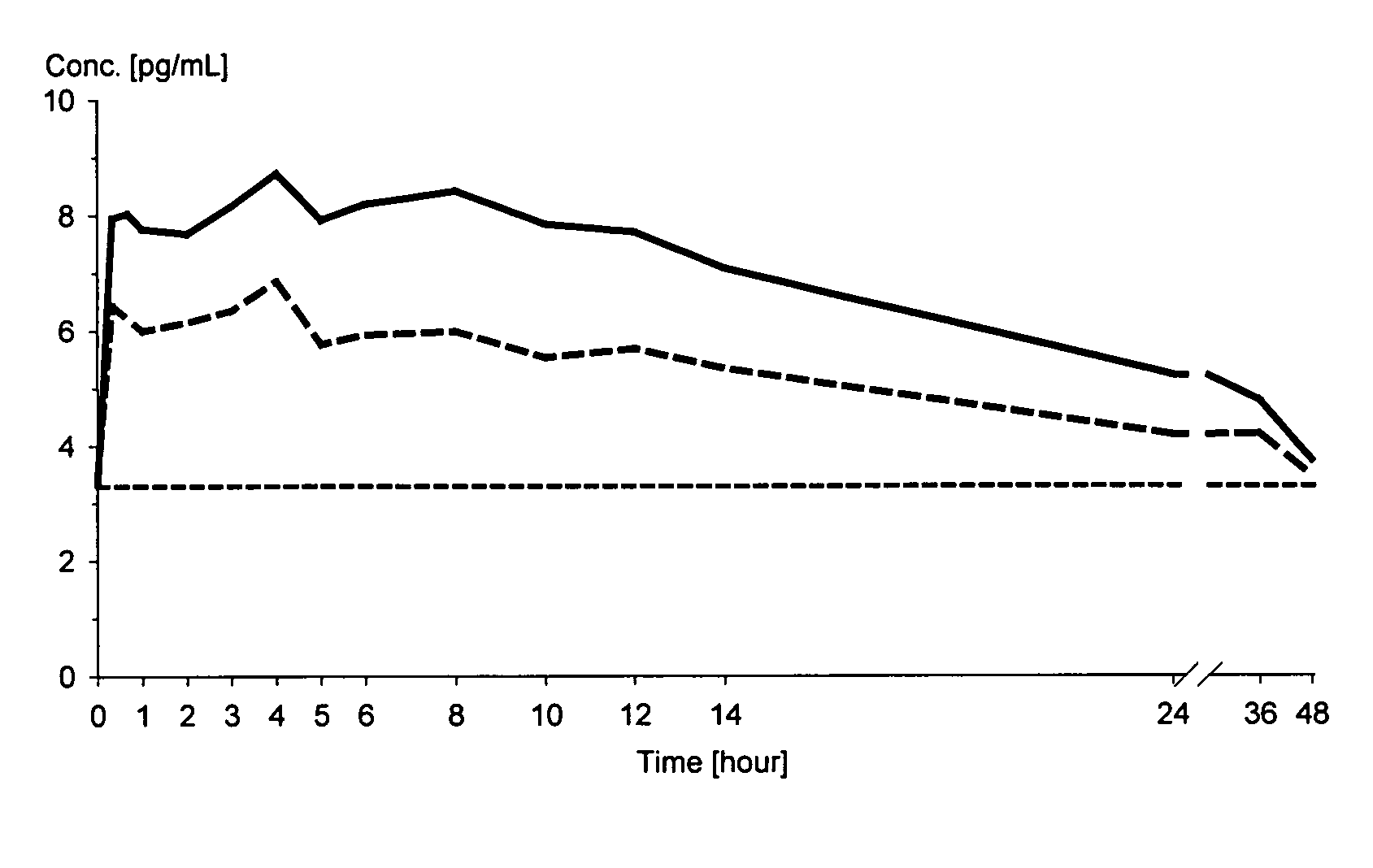
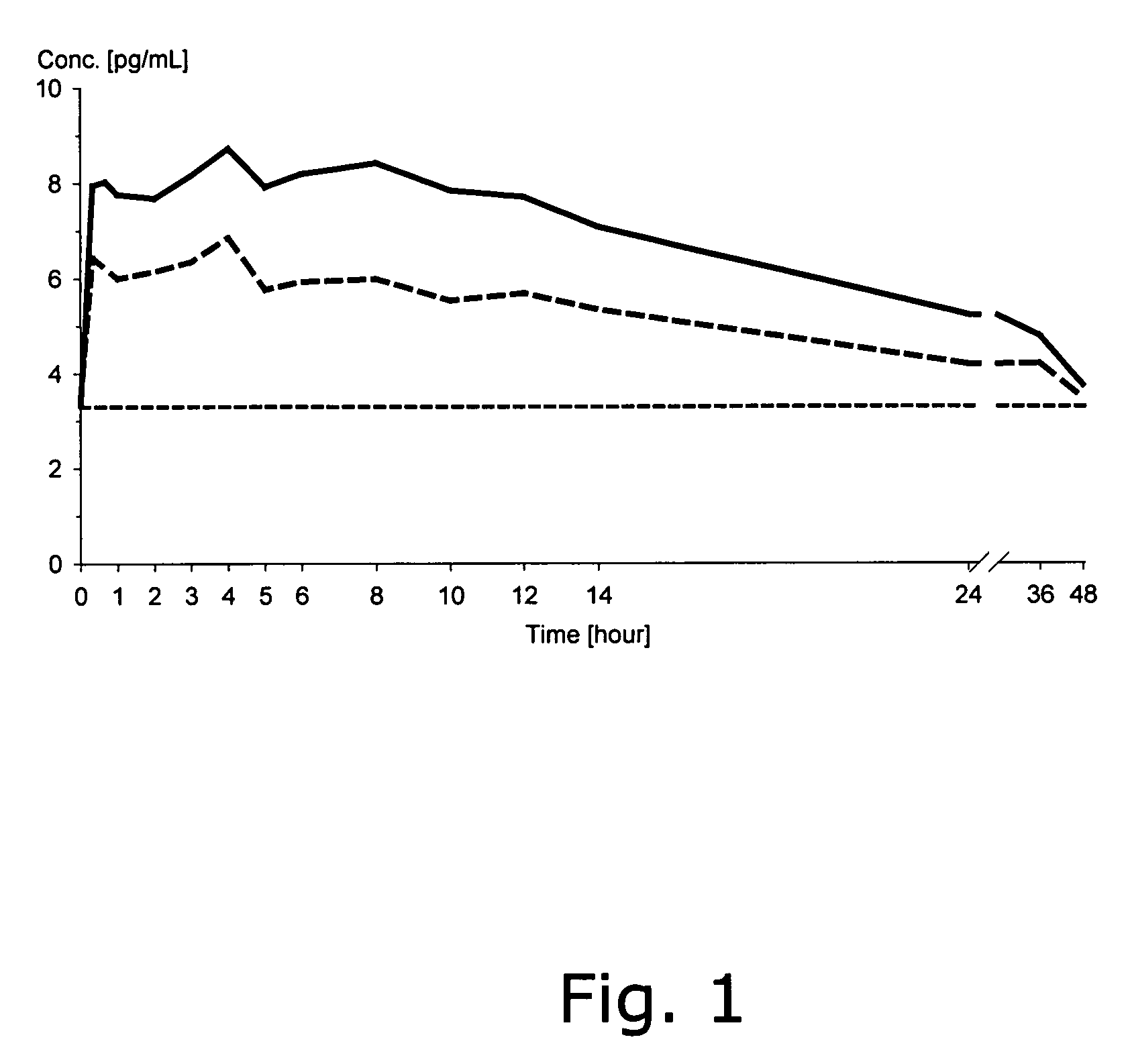
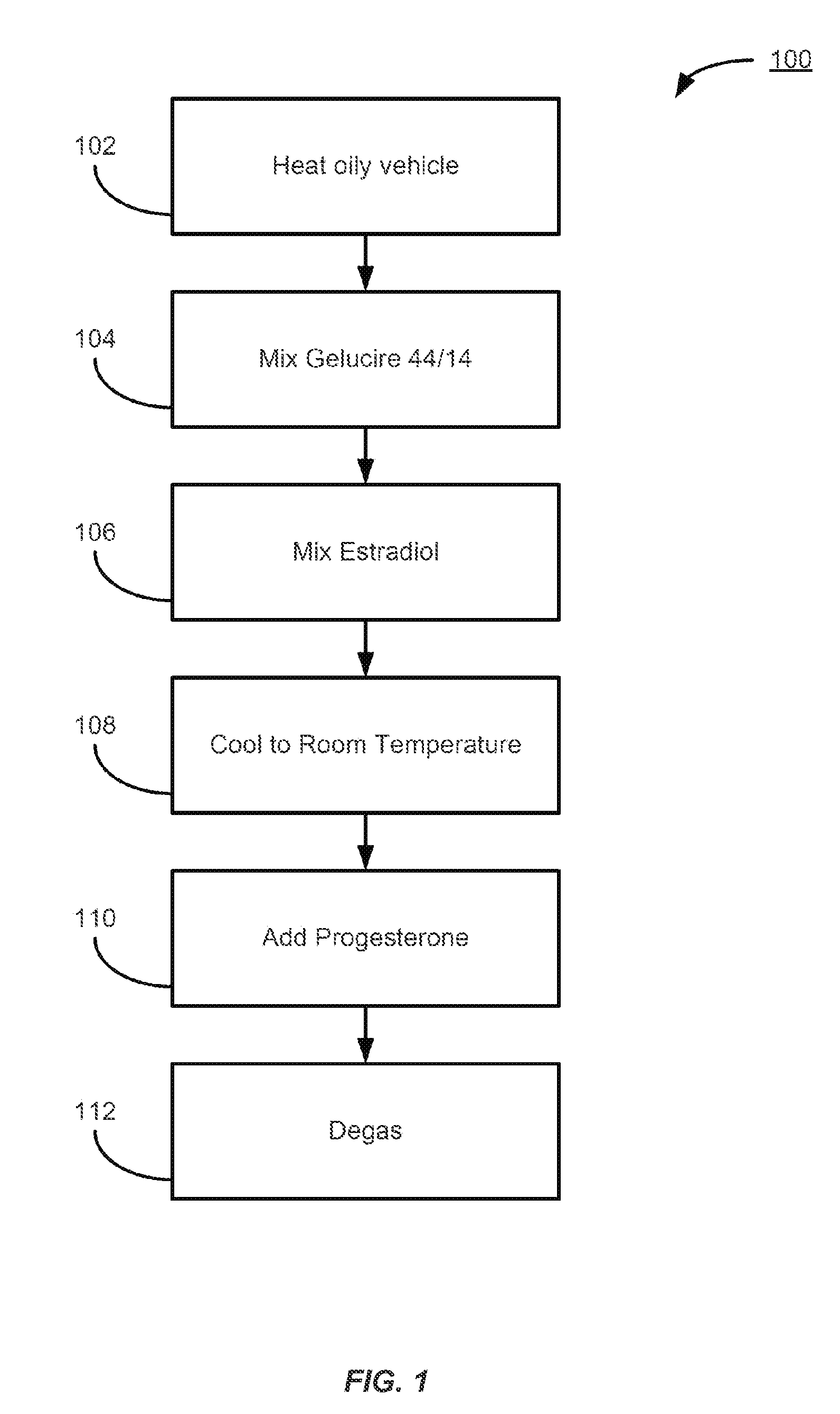
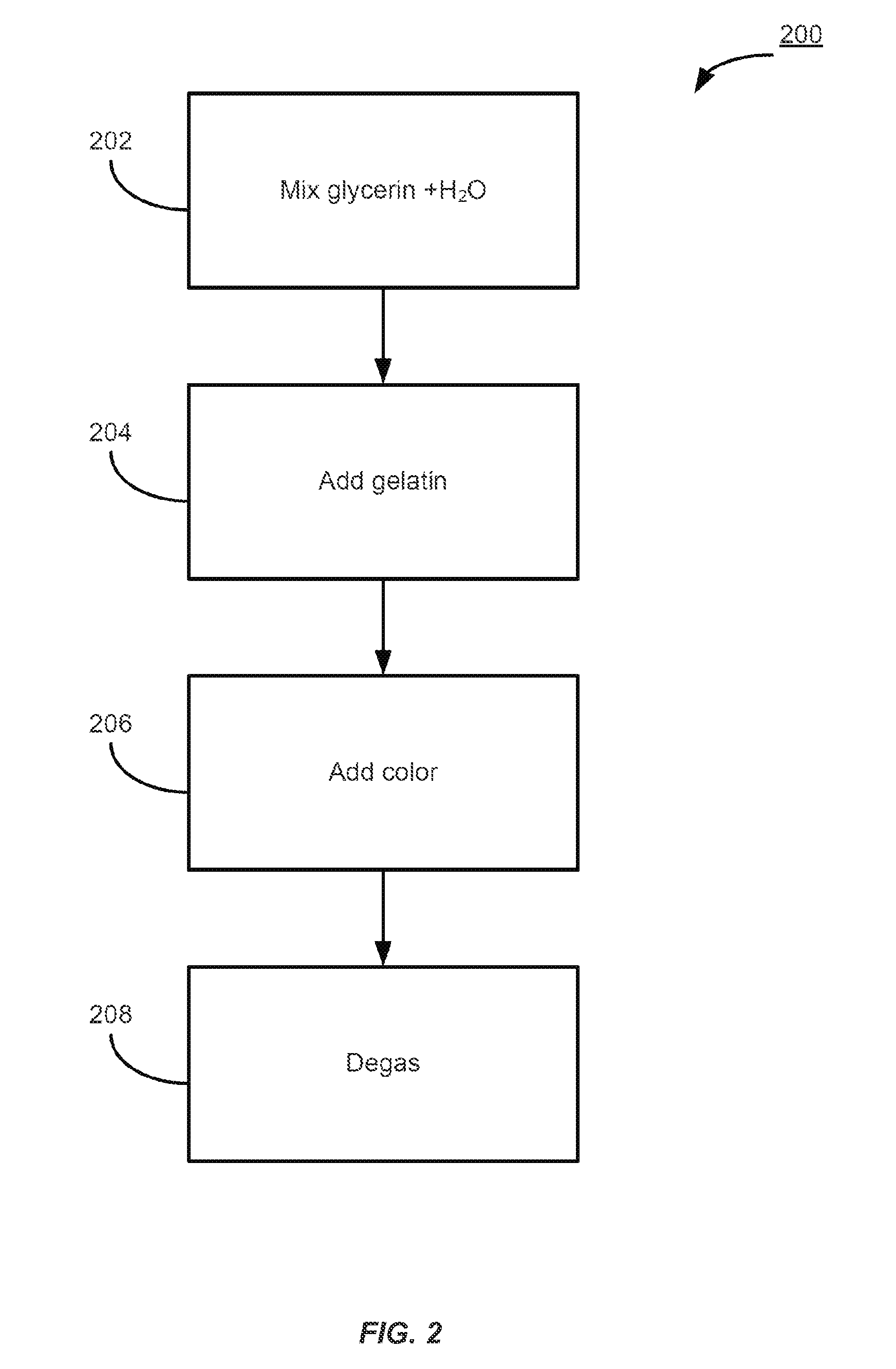
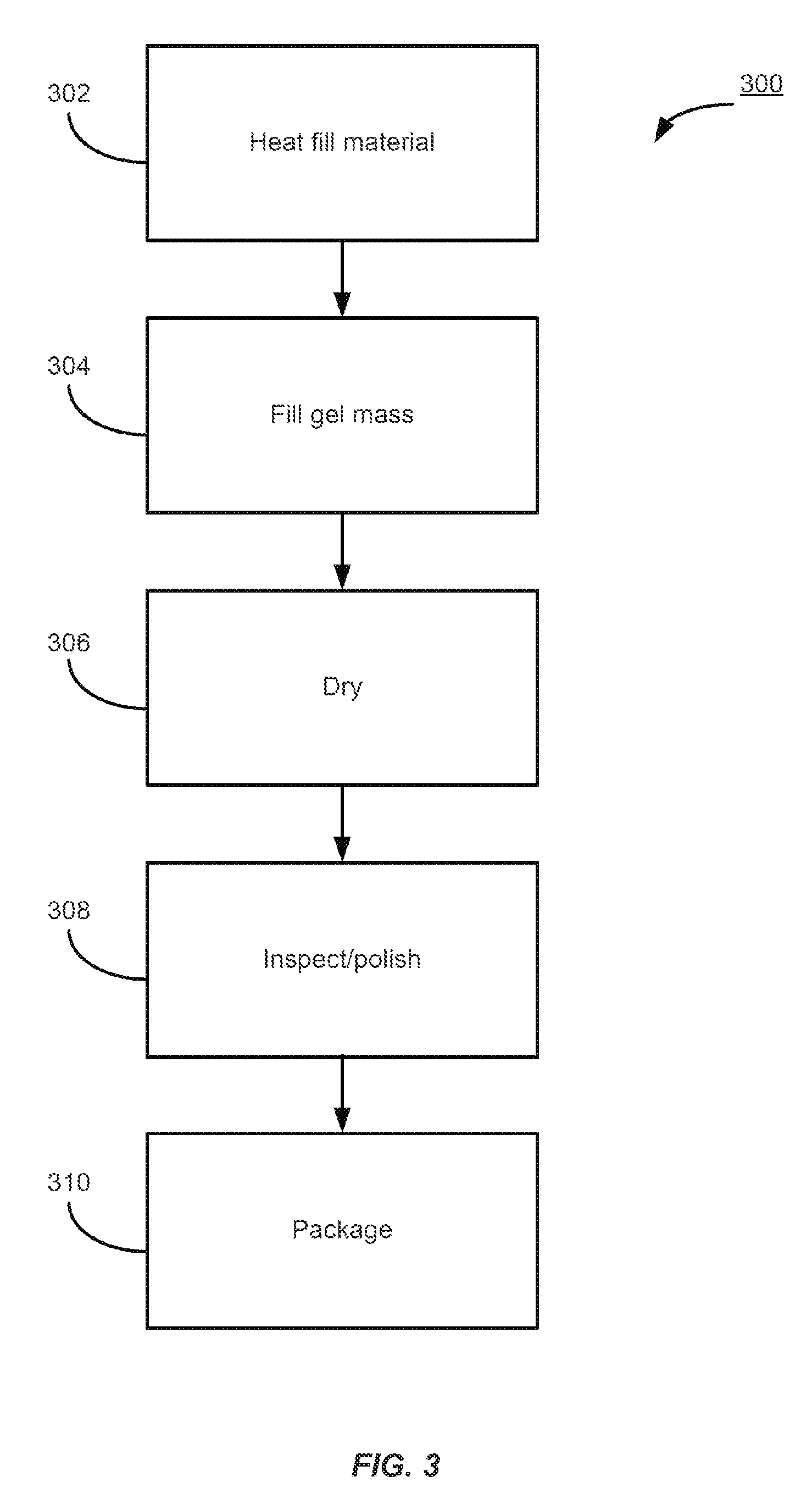
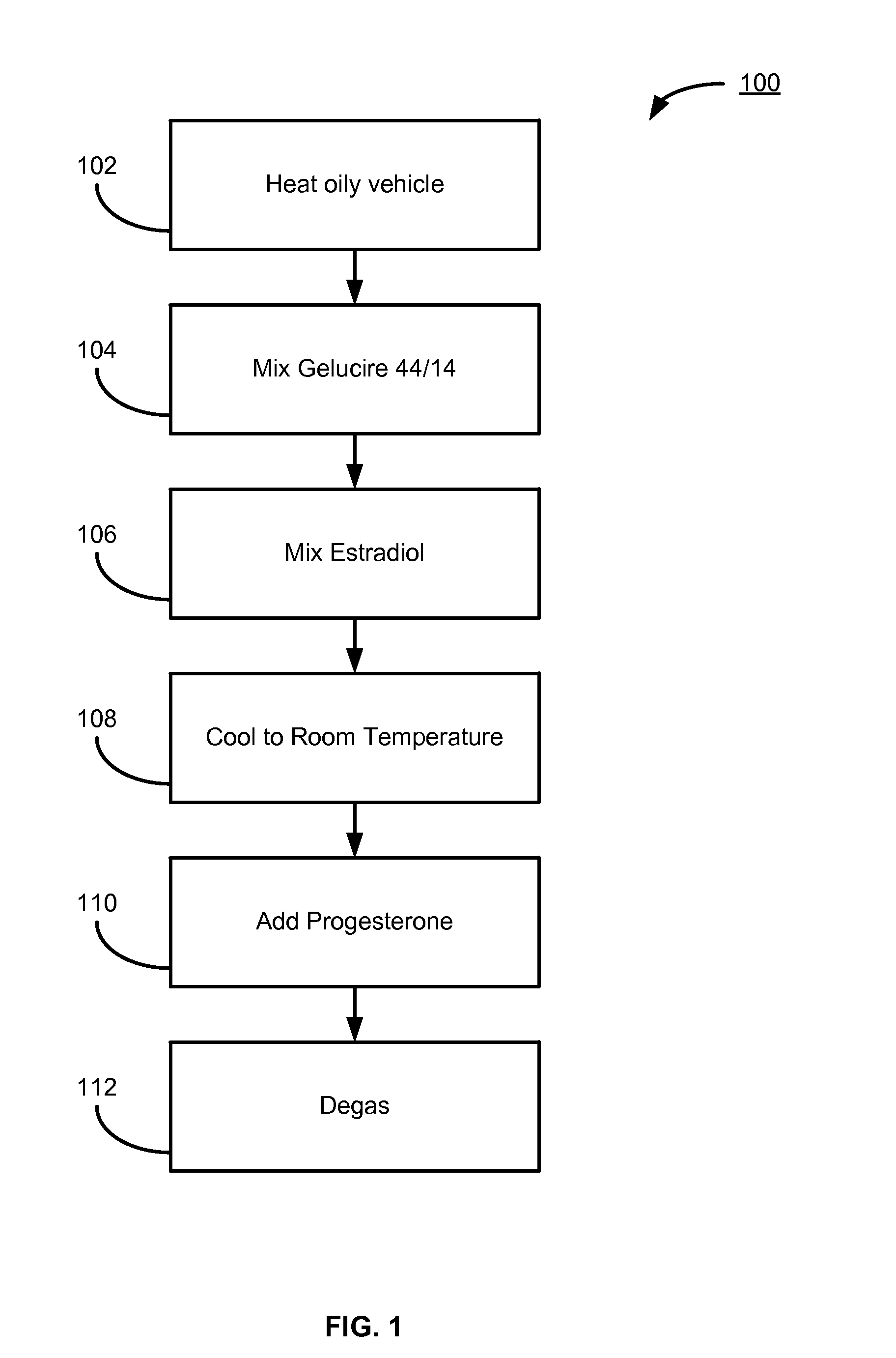
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
545 results about "Estradiols" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A steroid sex hormone
Drospirenone for hormone replacement therapy
InactiveUS20020132801A1Dissolve fastImprove bioavailabilityOrganic active ingredientsBiocideDrospirenoneBULK ACTIVE INGREDIENT
Drospirenone for Hormone Replacement Therapy A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Molecular dispersions of drospirenone
InactiveUS20050220825A1Improve bioavailabilityGood chemical stabilityPowder deliveryPill deliveryParticulatesDrospirenone
Described are pharmaceutical compositions comprising at least one steroidal drug such as a progestin (e.g. drospirenone, progesterone, eplerenone, etonogestrel) and / or an estrogen (estradiol and esters thereof) in molecularly dispersed form. The composition comprises a steroidal drug, preferably drospirenone, which is present in the composition in a non-particulate form. Preferably, the drug is present in a dissolved state in the excipient. The molecularly dispersed drug will be released very fast as dissolution takes place instantly when the dosage unit has disintegrated. Also described are methods for preparing the pharmaceutical compositions and methods of using the compositions.
Owner:BAYER SCHERING PHARMA AG
Transdermal estradiol/progestogen agent patch and its production
PCT No. PCT / EP96 / 05759 Sec. 371 Date Sep. 14, 1998 Sec. 102(e) Date Sep. 14, 1998 PCT Filed Dec. 20, 1996 PCT Pub. No. WO97 / 23227 PCT Pub. Date Jul. 3, 1997The invention concerns a transdermal patch for the release through the skin of estradiol and a progestogen agent and a process for its production.
Owner:ROTTA RES
Pharmaceutical compositions of conjugated estrogens and methods of analyzing mixtures containing estrogenic compounds
A composition of matter is provided having a mixture of active estrogenic compounds. The mixture is present in chemically pure form. The mixture includes salts of conjugated estrone, conjugated equilin, conjugated Δ8,9-dehydroestrone, conjugated 17α-estradiol, conjugated 17α-dihydroequilin, conjugated 17β-dihydroequilin, conjugated 17β-estradiol, conjugated equilenin, conjugated 17α-dihydroequilenin, and conjugated 17β-dihydroequilenin. The mixture also contains the same essential estrogenic compounds present in naturally derived equine conjugated estrogens. Drug products including the composition of matter are also provided, as are methods of using these drug products to treat mammals in need of treatment. Methods of analyzing mixtures containing conjugated estrogens are also provided.
Owner:DURAMED PHARMA
Pharmaceutical composition based on estrogen and progesterone
InactiveUS6165491AReduce riskEffective protectionOrganic active ingredientsFemale contraceptivesProgesteronesEstradiols
Owner:EFFIK BAT
Drug-delivery stent formulations for restenosis and vulnerable plaque
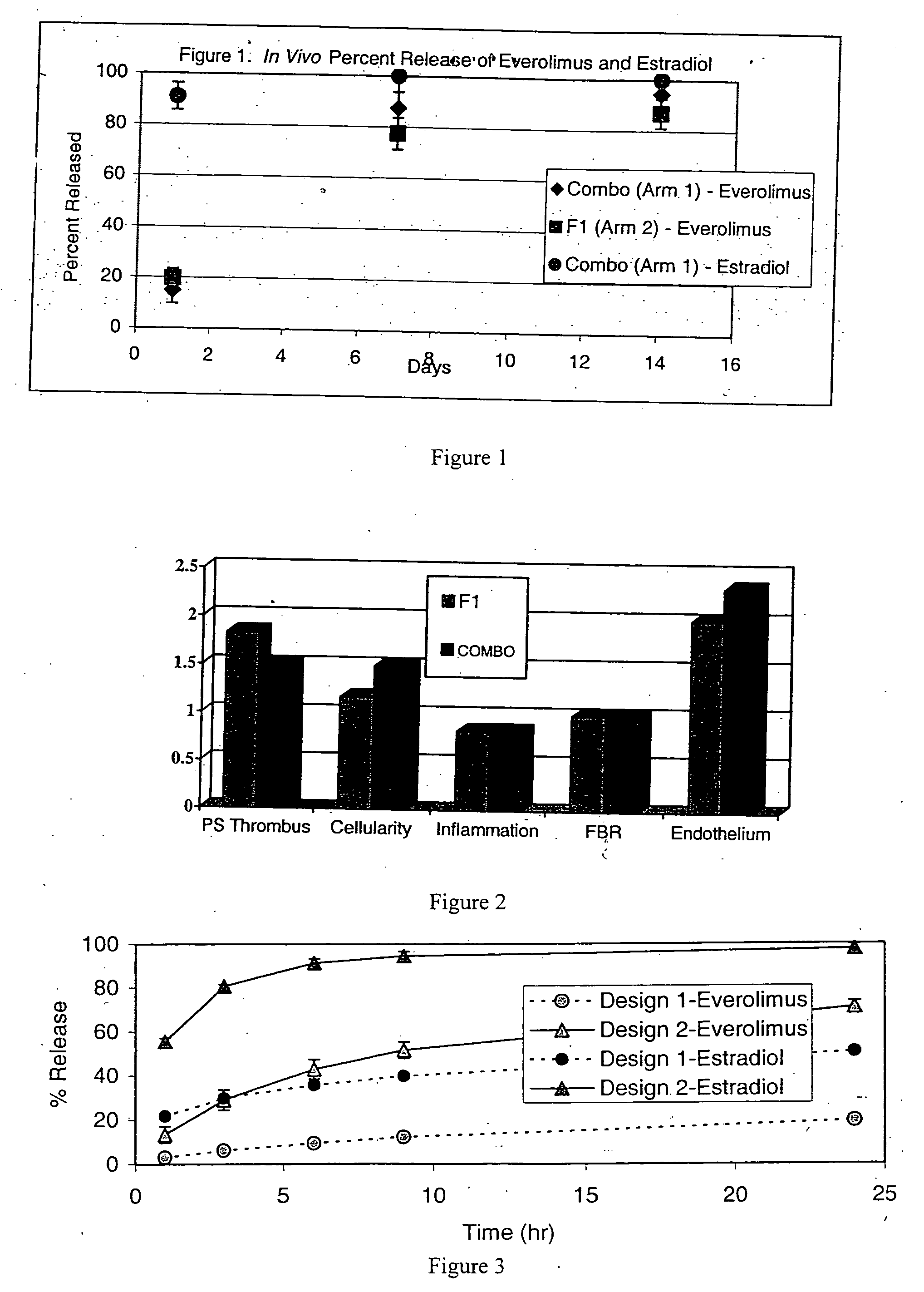
Drug-delivery stents capable of providing release of two or more drugs such as everolimus and estradiol are provided. The stents can be used for treating a disease such as restenosis and vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Methods to administer ethinyl estradiol and prodrugs thereof with improved bioavailability
InactiveUS20070286819A1Improve bioavailabilityReducing potential hormonal side effectOrganic active ingredientsPill deliveryHormone replacementBioavailability
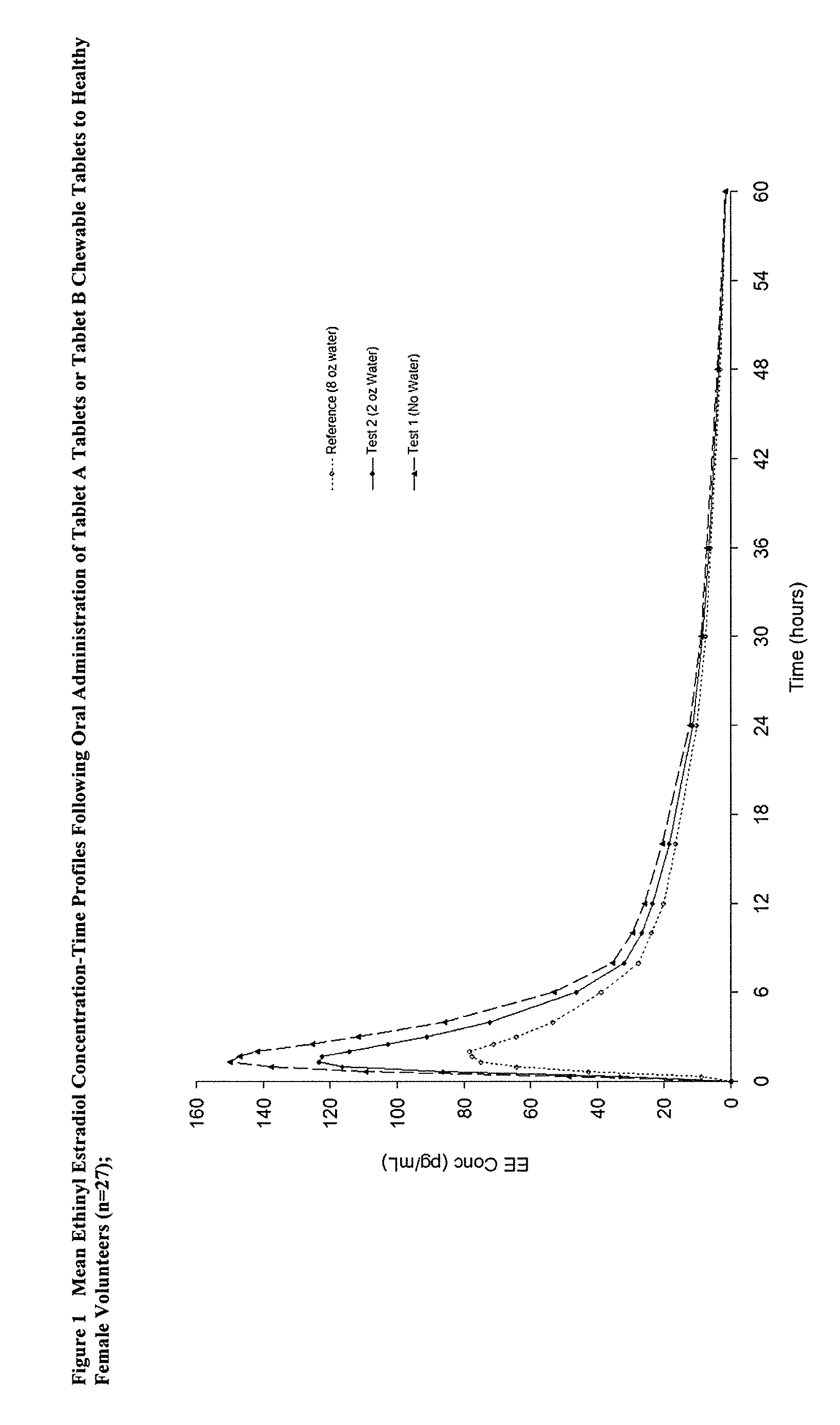
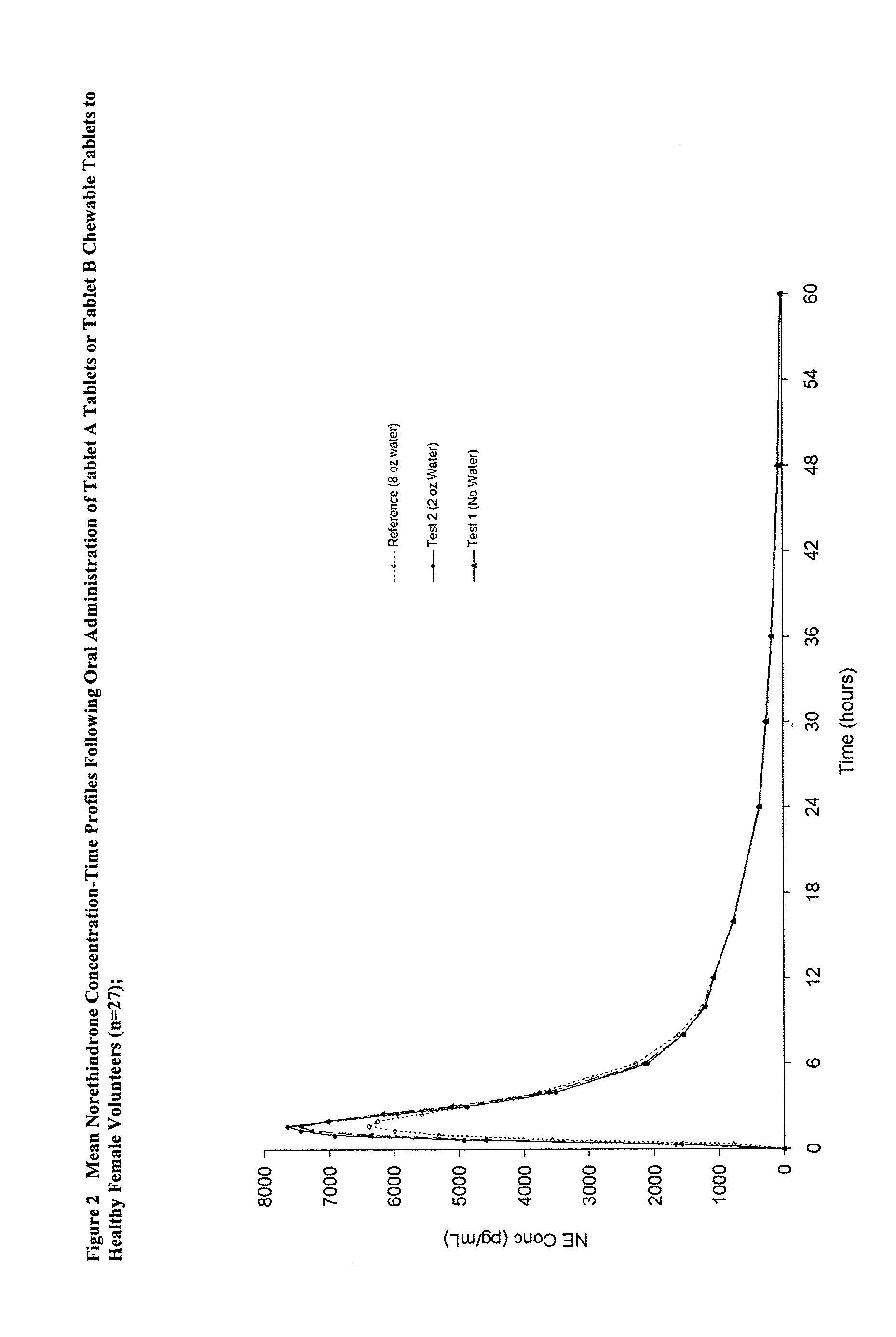
Methods of improving the bioavailability of ethinyl estradiol by orally administering to a patient a solid dosage form containing ethinyl estradiol or prodrug thereof where that dosage form releases at least some of the ethinyl estradiol or prodrug thereof in the oral cavity for absorption through the oral mucosa to treat the patient for a predetermined indication such as, for example, hormone replacement therapy or contraception. The solid dosage forms may be selected from, among others, chewable tablets, fast melt tablets, films, dissolving films, mucoadhesive tablets, lozenges, and chewing gum.
Owner:WARNER CHILCOTT CO LLC
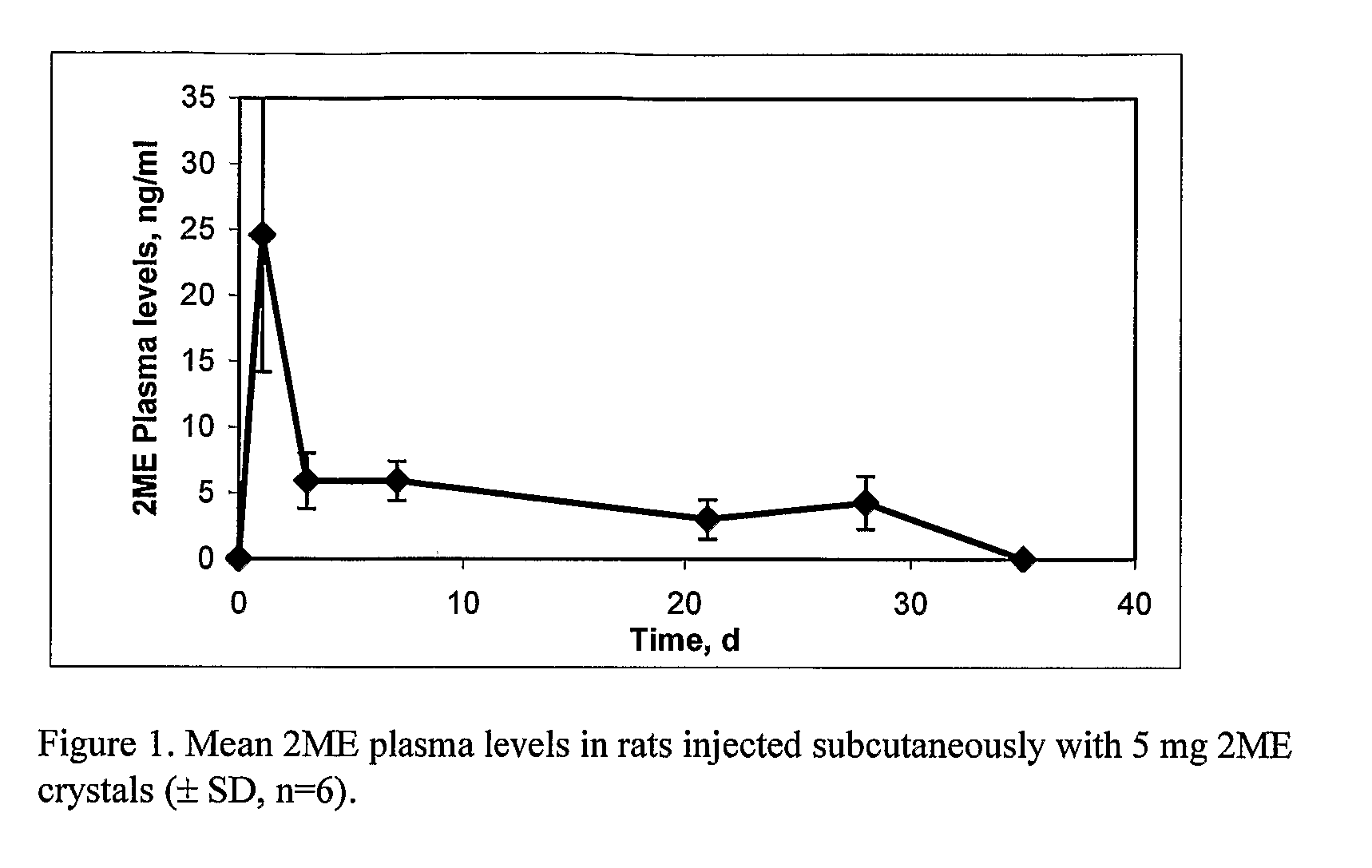
Long Acting Injectable Crystal Formulations of Estradiol Metabolites and Methods of Using Same
InactiveUS20080220069A1Simpler and less-expensive to manufactureEasy to managePowder deliveryOrganic active ingredientsDiseaseMetabolite
The present invention provides sustained release formulations of estradiol metabolites whereby the in vivo pharmacokinetics are manipulated by a method selected from the group consisting of chemical modification, crystal packing formation, particle size or a combination thereof. Such compositions are useful in the long-term treatment of a wide variety of diseases.
Owner:PR PHARMA
Drospirenone for hormone replacement therapy
InactiveUS20030144258A1Dissolve fastImprove bioavailabilityBiocideOrganic active ingredientsDrospirenoneBULK ACTIVE INGREDIENT
A pharmaceutical composition comprising as a first active ingredient an estrogen, such as estradiol or estradiol valerate, in sufficient amounts to treat disorders and symptoms associated with deficient endogenous levels of estrogen in women, and as a second active ingredient 6beta,7beta; 15beta; 16beta-dimethylene-3-oxo-17alpha-preg-4-ene-21,17-carbolactone (drospirenone, DRSP) in sufficient amounts to protect the endometrium from the adverse effects of estrogen is useful for, amongst others, treating peri-menopausal, menopausal and post-menopausal women. This composition may be used for hormone replacement therapy and may be administered as a multi-phased pharmaceutical preparation. This combination therapy may comprise continuous, sequential or interrupted administration, or combinations thereof, of DRSP and estrogen, each optionally in micronized form.
Owner:SCHERING AG
Topical management of ocular and periocular conditions
Chronic glaucoma, cataract, ocular and periocular aging are treated and prevented by the administration of agents that affect metabolic subsystems such as (i) mitochondrial bioenergetics, (ii) free radical moderation and glutathione maintenance, (iii) constitutive nitric oxide / endothelin-1 balance, and (iv) calcium wave signaling and associated neuronal excito-toxicity. Included among the agents are tetrahydrobiopterin, R-alpha-lipoic acid, coenzyme Q10, 17 alpha-estradiol, and glutathione.
Owner:CHRONORX
Controlled release compositions of estradiol metabolites
The present invention provides improved sustained release formulations of estradiol metabolites, including 2-hydroxyestradiol, 2-methoxyestradiol, 4-hydroxyestradiol and 4-methoxyestradiol, useful for therapeutic treatments. The invention also provides methods of producing sustained release forms of estradiol metabolites. The compositions of the present invention include microparticles, nanoparticles, patches, crystals, gels, rods, stints, pallets, discs, lozenges, wafers, capsules, films, microcapsules nanocapsules, hydrogels, liposomes, implants and vaginal rings. Compositions also include formulations for transdermal and intravenous delivery of estradiol metabolites. The present invention provides numerous improvements over previous forms of estradiol metabolites, such advantages including the sustained release of normally short half-life compounds to maintain therapeutic blood levels.
Owner:PR PHARMA
Natural combination hormone replacement formulations and therapies
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD INC
Oral pharmaceutical products containing 17 beta-estradiol-3-lower alkanoate, method of administering the same and process of preparation
InactiveUS6962908B2Improve bioavailabilityIncrease intakePowder deliveryBiocideAcetic acidOral medication
A pharmaceutical dosage unit for oral administration to a human female comprising a therapeutically effective amount of 17β-estradiol-3-lower alkanoate, most preferably 17β-estradiol-3-acetate, and a pharmaceutically acceptable carrier is disclosed. Also disclosed is a method for treating a human female in need of 17β-estradiol and a contraceptive method by oral administration of the pharmaceutical dosage unit and a method of preparing a pharmaceutical composition that may be used to form the pharmaceutical dosage unit of the invention.
Owner:ALLERGAN THERAPEUTICS LLC
Trace elements
The invention discloses a trace element solution, which comprises at least one metal selected from the group comprising selenium, copper, zinc, manganese and chromium; and at least one component selected from the group comprising a vitamin, a vaccine, a growth stimulant, a dewormer, iron dextran, an antibiotic and a synchronization preparation. The synchronization preparation is a combination of injectable hormonal preparations, inplantable hormonal preparations, intravaginal hormonal preparation and other slow release hormonal preparation. The antibiotics include oral, injectable and implantable theurapeutic remedies. The vaccine includes antigens and a combination of antigens and adjuvents. The growth stimulants include zeranol, estradiol, testosterone, progesterone and trenbolone acetate. The dewormer includes macrocydic lactones, leramizoles, benzimidazoles and salicylanilides. The macrocydic lactones include doramectin, ivermectin, abamectin and moxidectin.
Owner:WARBURTON TECH
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Therapeutic methods of using estrogen compositions
A method for preventing or treating a catamenial migrainous disorder in a female subject comprises administering to a vulvovaginal surface of the subject a pharmaceutical composition that is bioadhesive thereto and comprises at least one estrogenic compound in an amount of about 50 μg to about 1000 μg estradiol equivalent per unit dose of the composition. A related method comprises administering to a vulvovaginal surface of the subject a pharmaceutical composition comprising at least one estrogenic compound, wherein upon administration of the composition to the vulvovaginal surface, a decline in serum estradiol concentration during a luteal phase of a menstrual cycle is moderated.
Owner:DRAGTEK CORP
Oral solid dosage forms containing a low dose of estradiol
The present invention relates to oral solid dosage forms containing a very low dose of estradiol. The dosage forms are formulated in a manner so as to avoid degradation of the estradiol and to minimise the content of polyvinylpyrrolidone, while still achieving similar fast dissolution of the estradiol. The dosage forms are useful in preventing or treating a physical condition in a woman caused by insufficient endogenous levels of estradiol.
Owner:BAYER SCHERING PHARMA AG
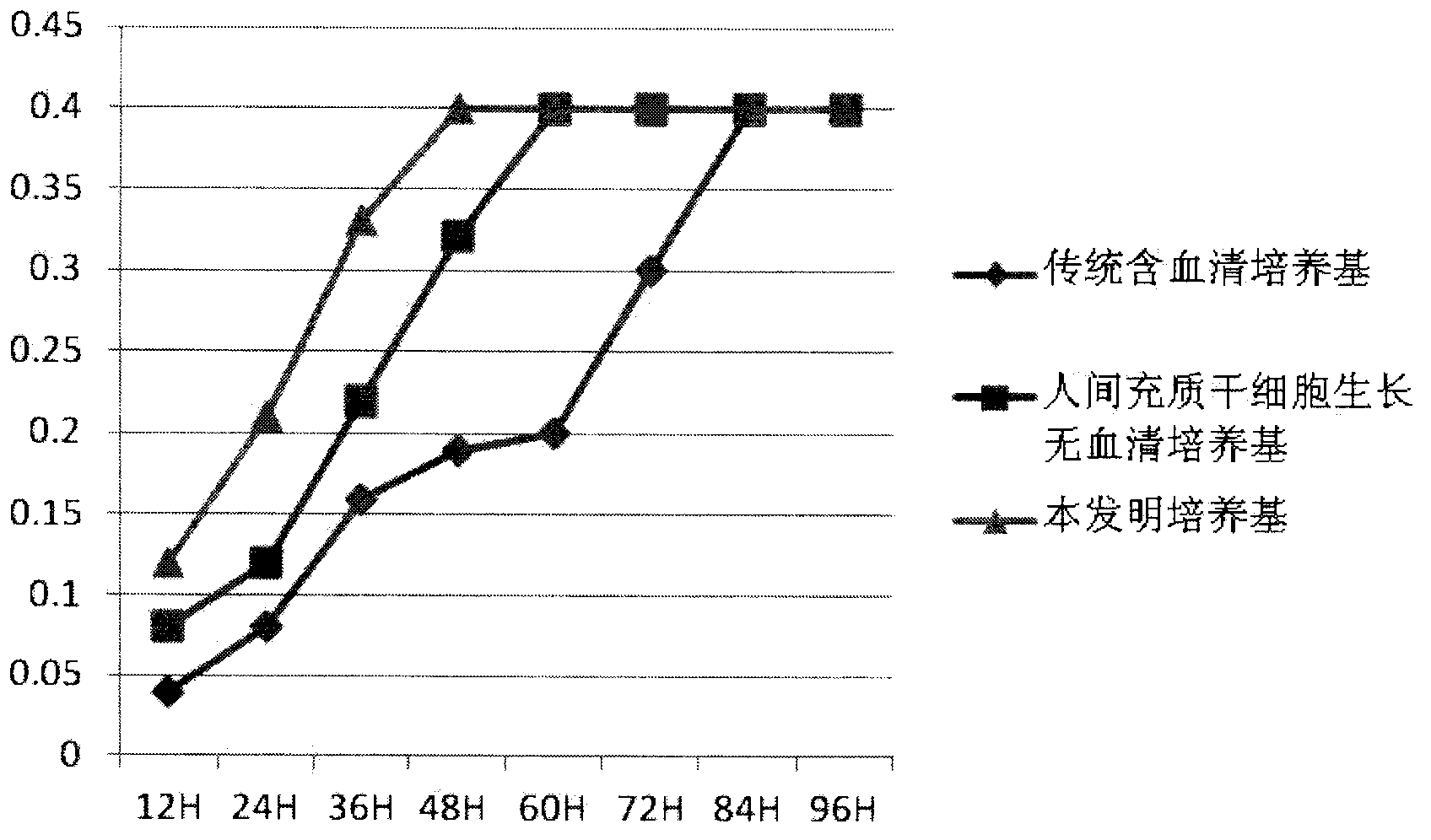
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
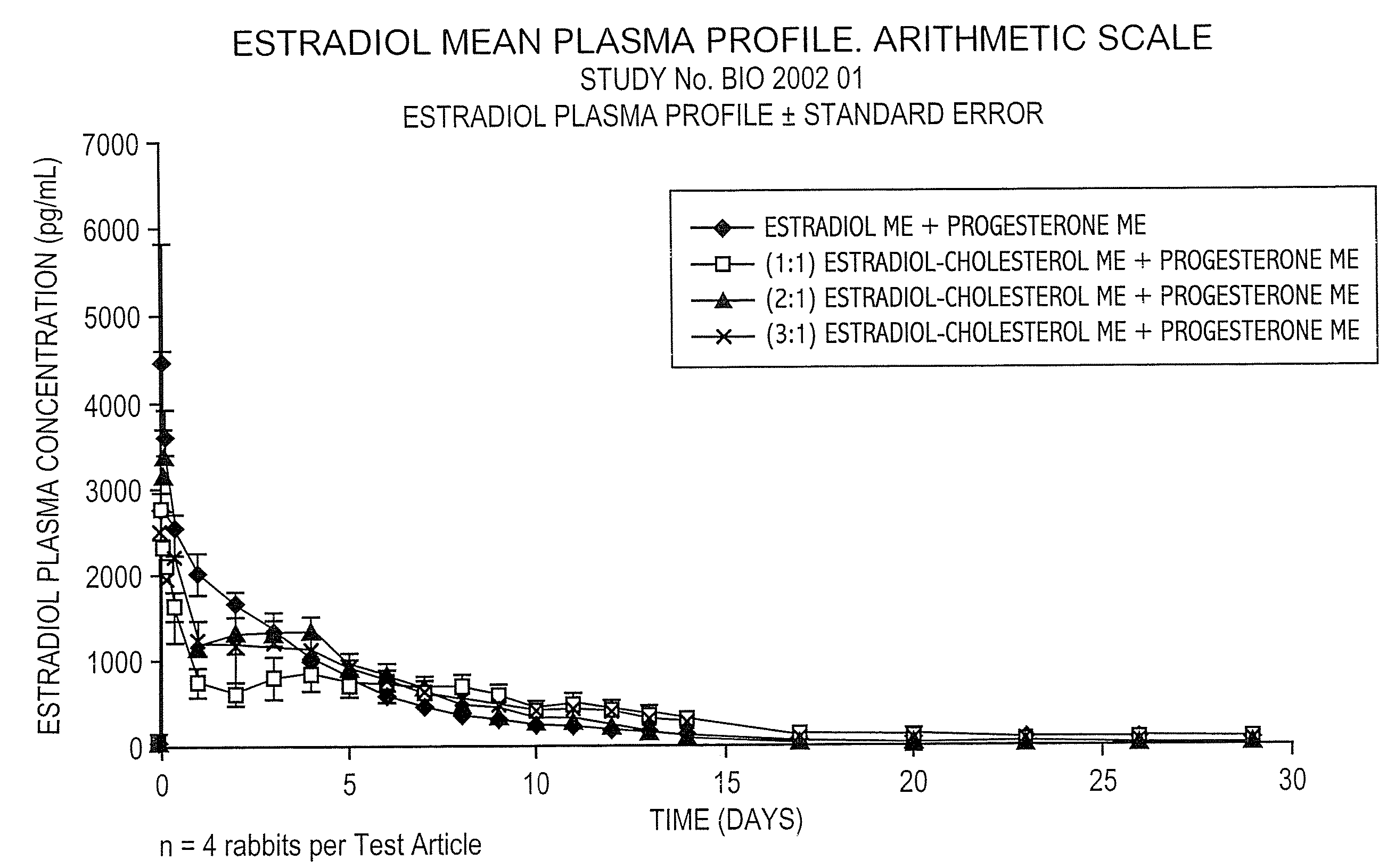
Pharmaceutical formulation for contraception and hormone-replacement therapy
InactiveUS20090081303A1Extended dwell timeAvoid disadvantagesPowder deliveryOrganic active ingredientsRegimenCholesterol
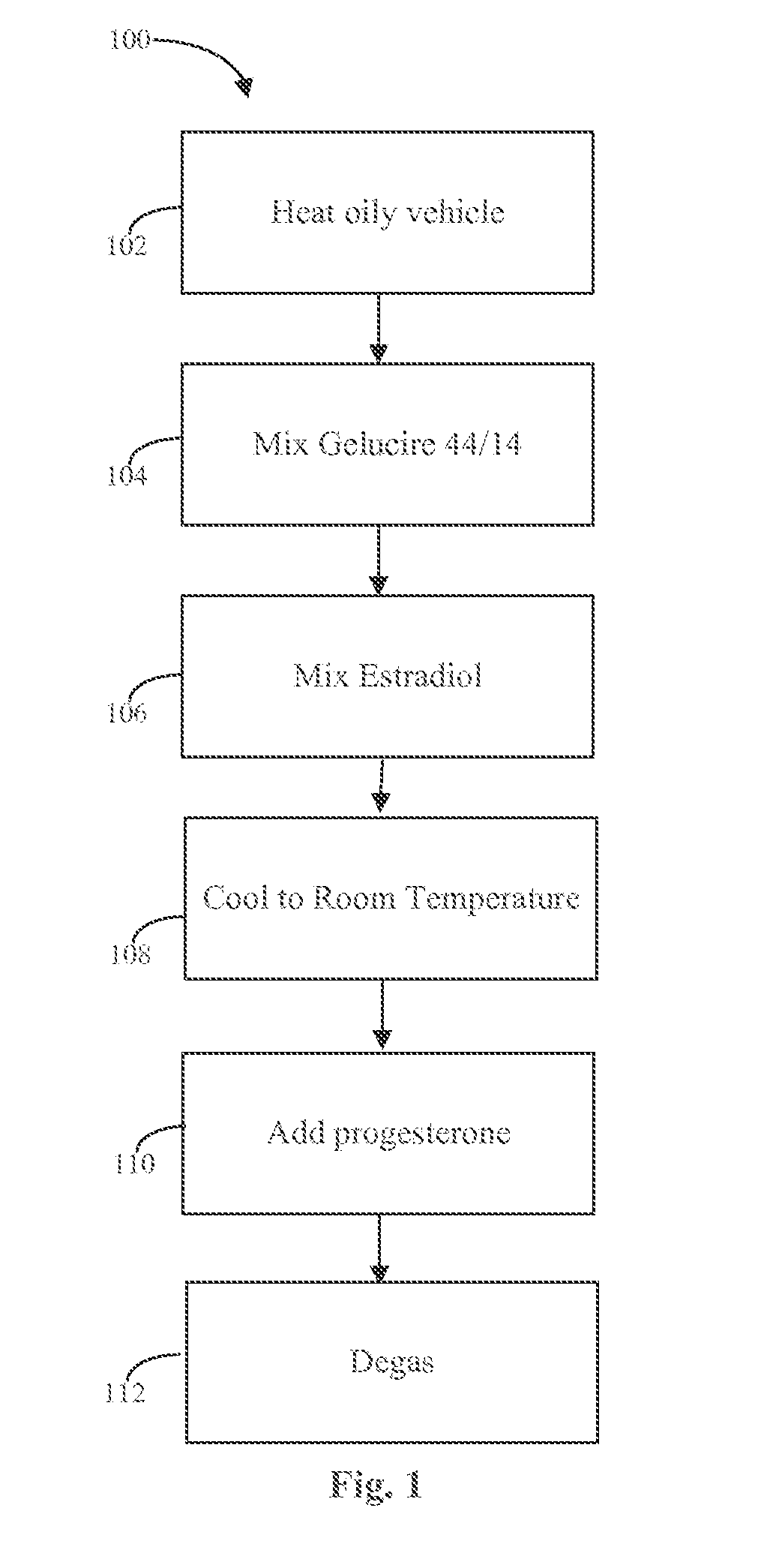
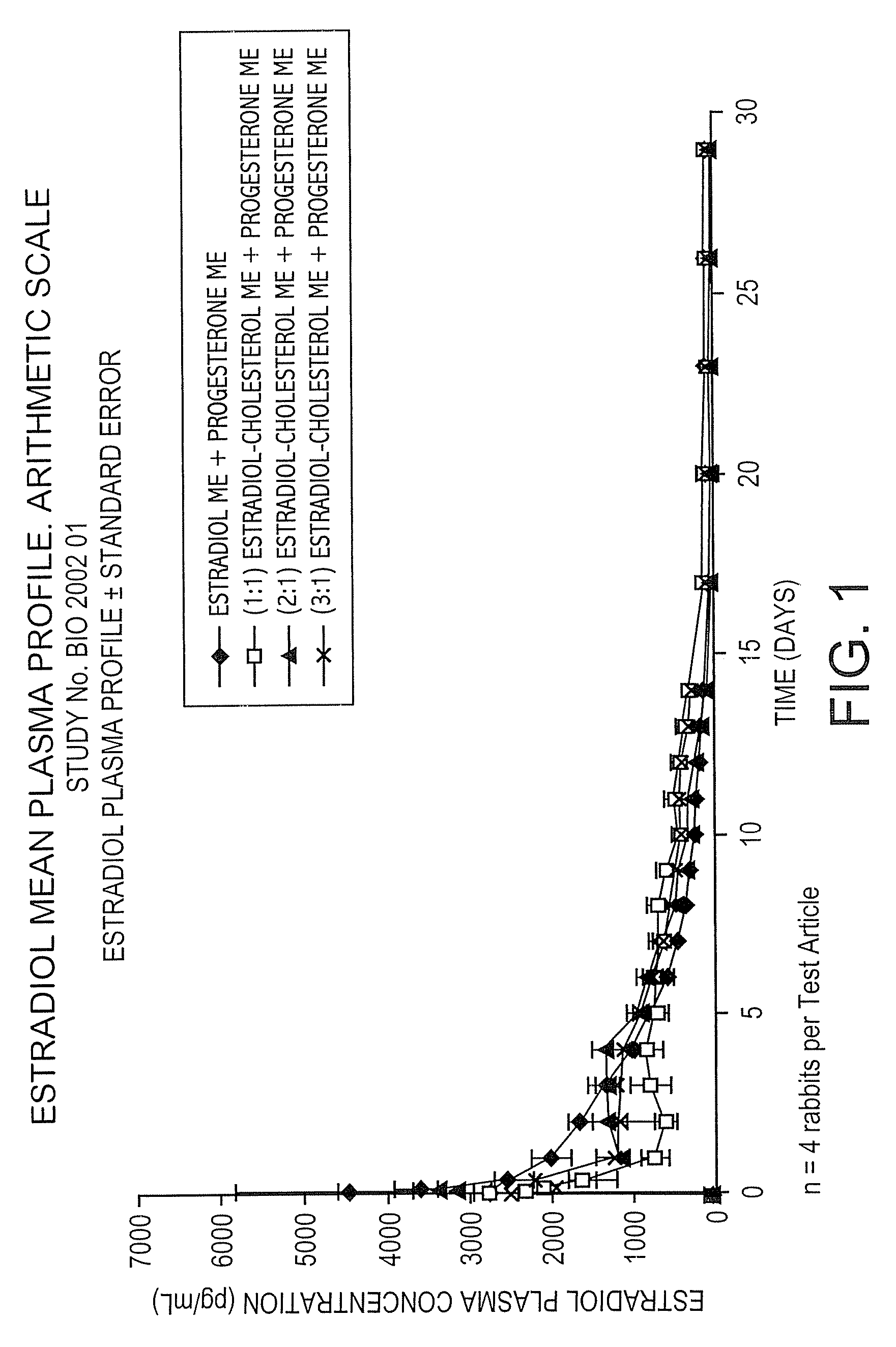
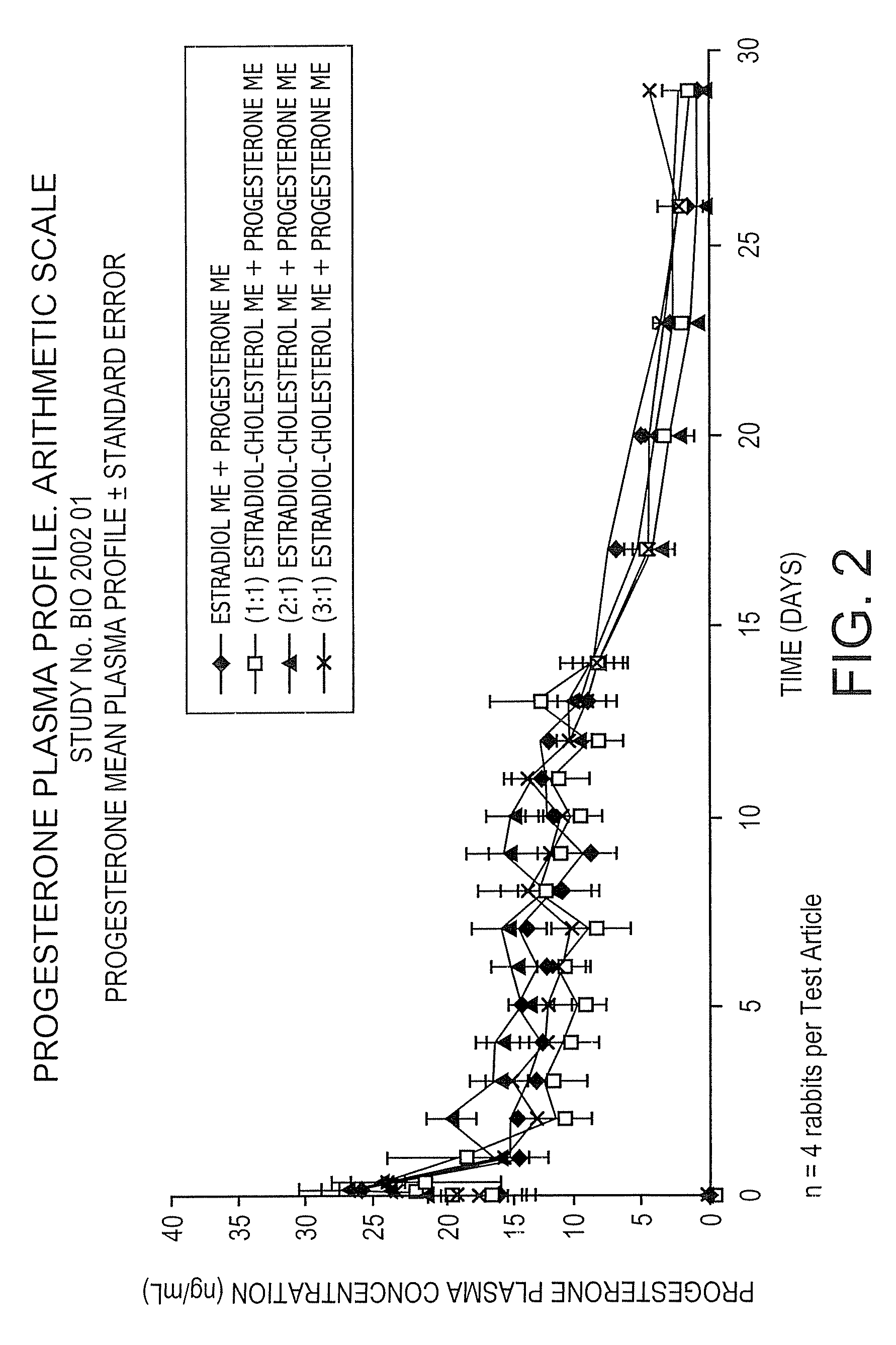
The present invention provides slow release estradiol-progesterone formulations that can be used in either contraception or hormone replacement therapies. The formulations comprise shaped particles of estradiol that is in a hemicrystalline form that exhibits especially low dissolution rates. The shaped particles comprise estradiol compounded in a 1:1 molar ratio with cholesterol, and are administered in combination with progesterone. The slow release formulations of the present invention afford the dual advantages of a low dose estradiol formulation with a low frequency administration regimen. The formulations can be parenterally administered once a month or less often.
Owner:SKENDI FINANCE
Estrogen replacement regimen
The present invention provides an improved method to deliver estrogen to menopausal women comprising administering ultra-low dose estradiol alternating with standard-dose estradiol.
Owner:CASPER ROBERT +2
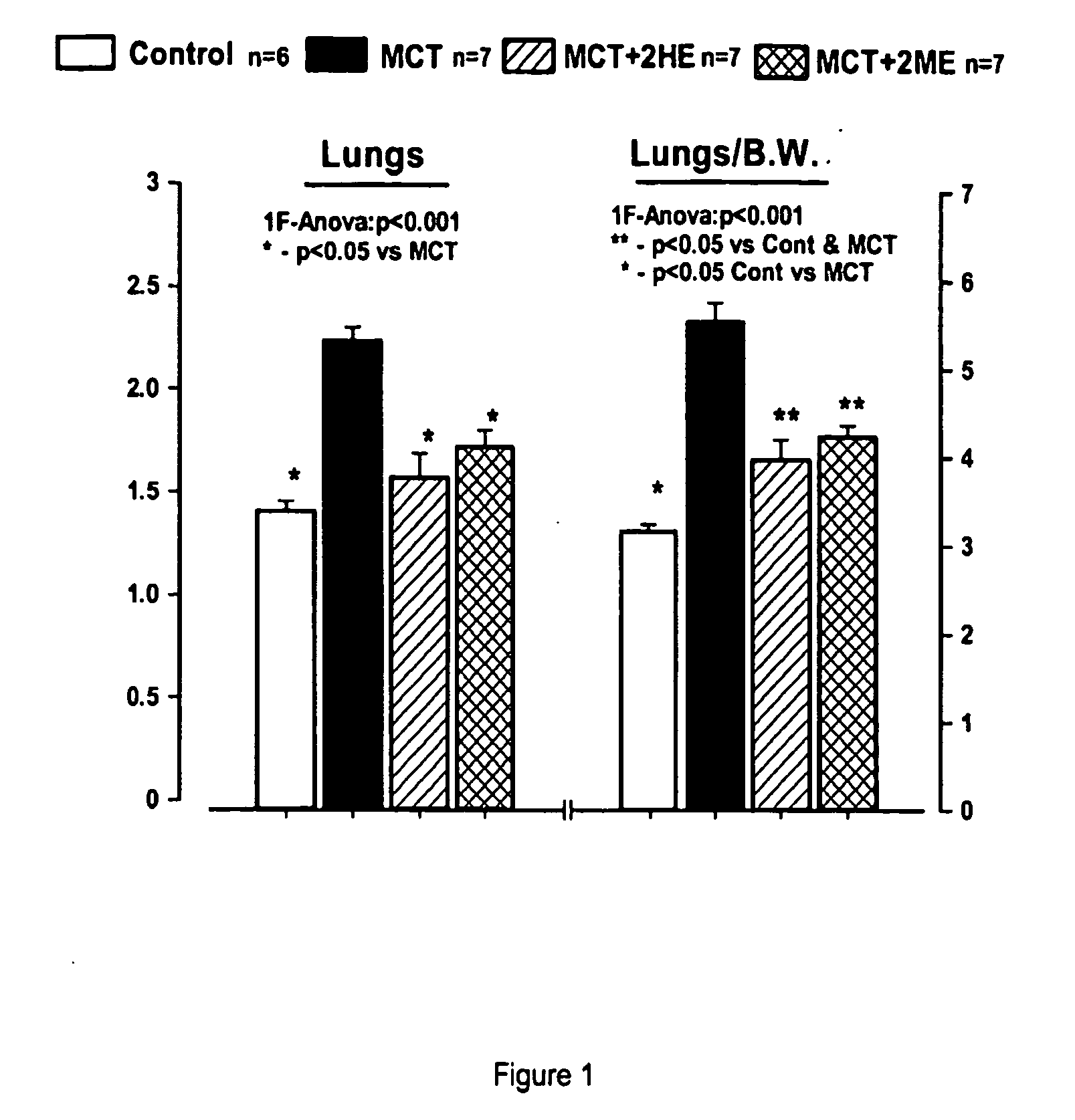
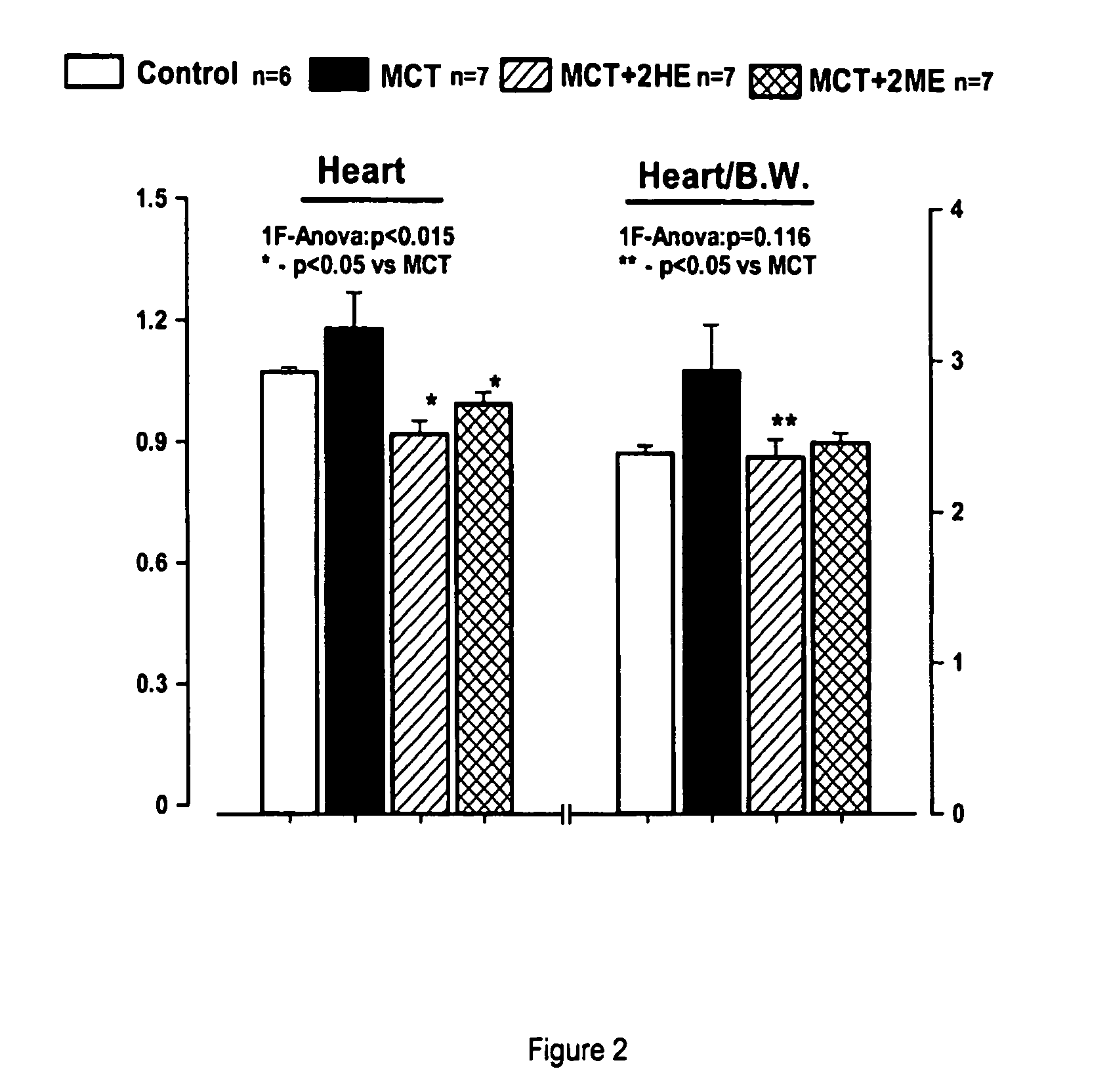
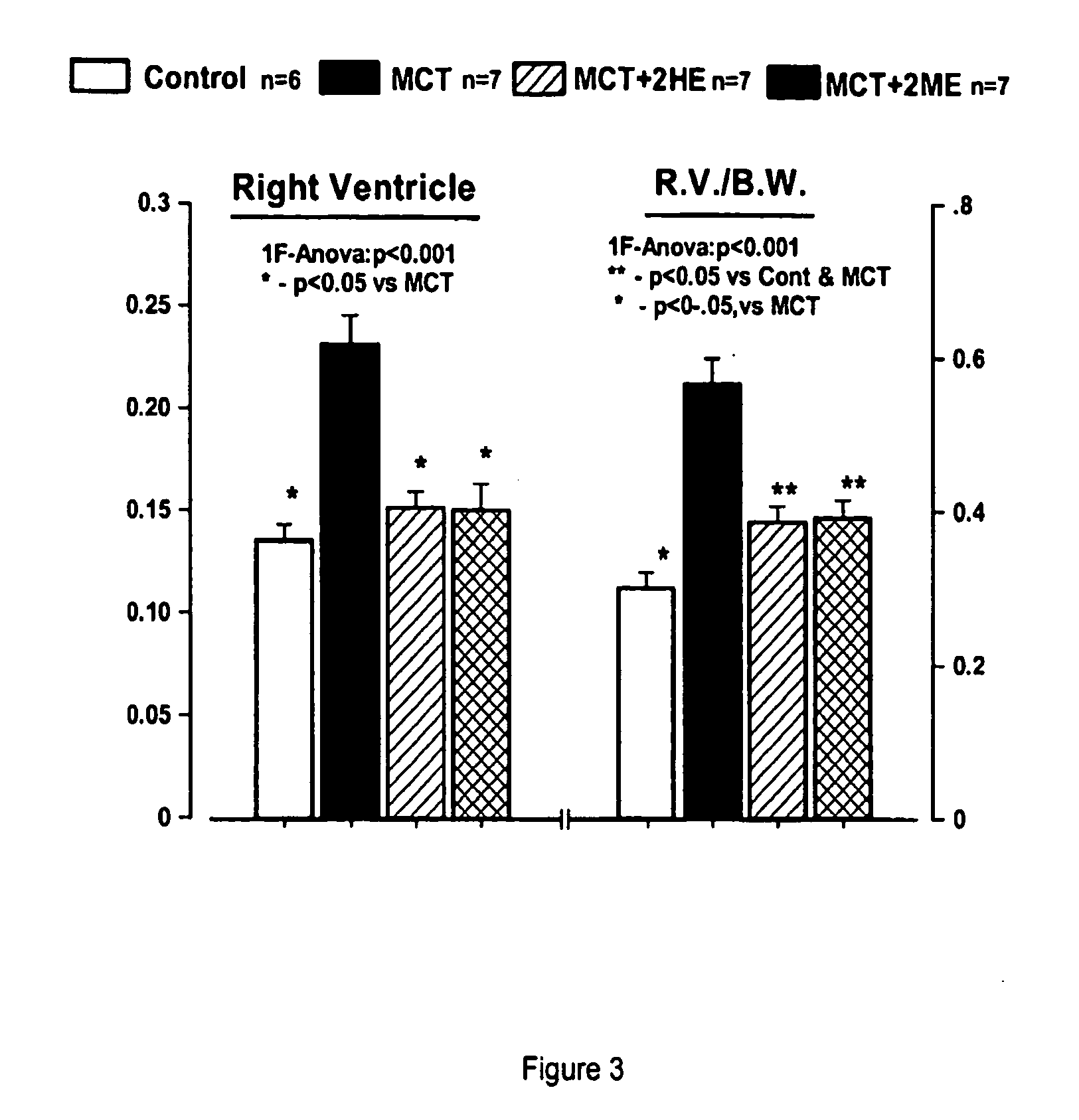
Estradiol metabolites for the treatment of pulmonary hypertension
InactiveUS20060194775A1Decrease lung weightDecrease right ventricular hypertrophyOrganic active ingredientsPharmaceutical delivery mechanismDisease4-Methoxyestradiol
Methods are provided for the treatment of pulmonary hypertension and other conditions associated therewith. In particular, the methods include treatment of pulmonary hypertension with an estradiol metabolite or alkoxy analogue of an estradiol metabolite. The estradiol metabolite or alkoxy analogue thereof may be associated with biodegradable microparticles or nanoparticles alone or in combination with other therapeutic agents. Preferred estradiol metabolites include 2-methoxyestradiol, 4-methoxyestradiol, 2-hydroxyestradiol, and 4-hydroxyestradiol and preferred alkoxy analogues of estradiol metabolites include 2-ethoxyestradiol, and / or to synthetic derivatives and analogues thereof or prodrugs thereof. The compositions may also be in the form of a controlled release formulation.
Owner:UNIVERSITY OF PITTSBURGH
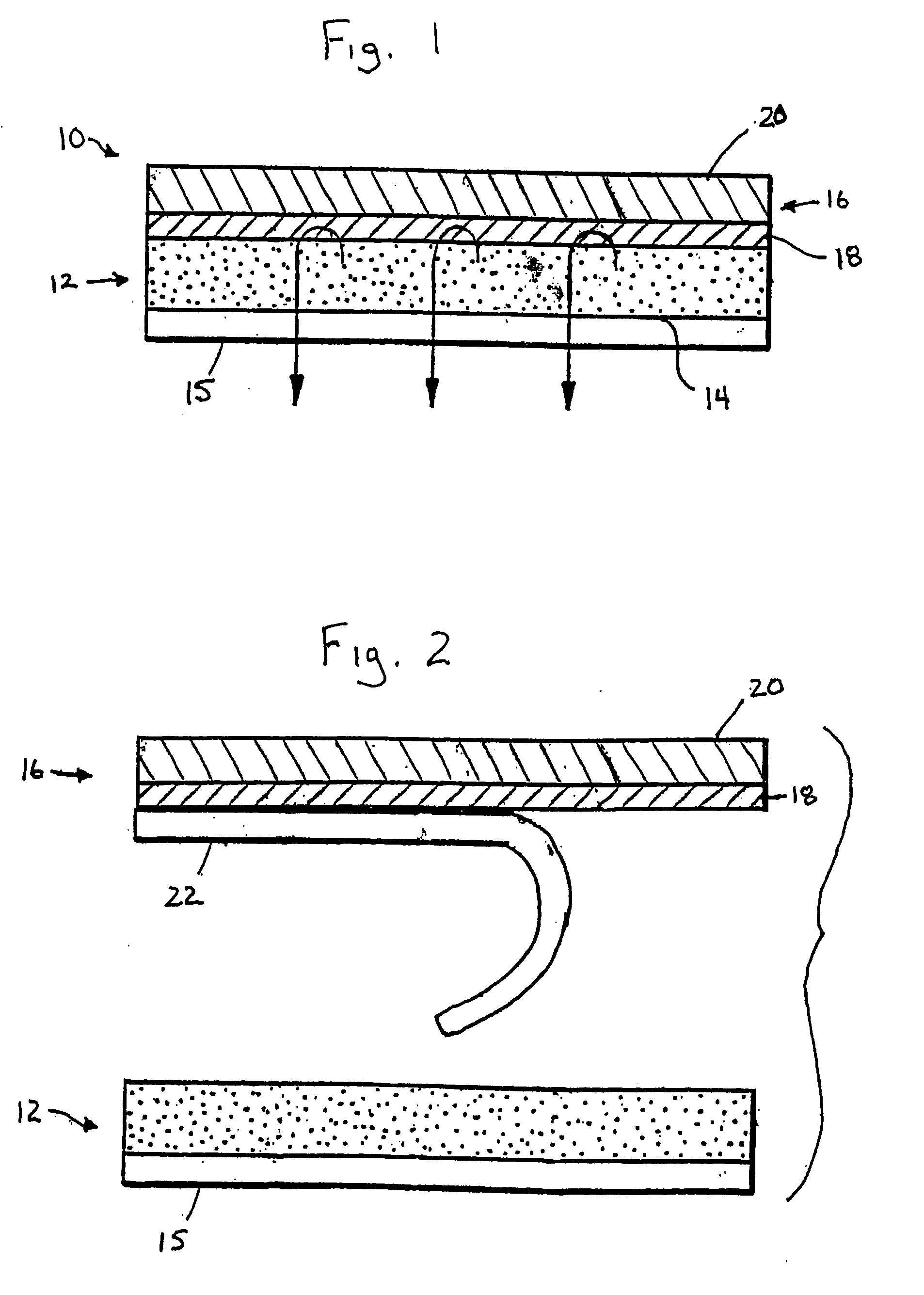
Compositions and methods for delivering estradiol in transdermal drug delivery systems
ActiveUS20060078601A1Simple and inexpensive to manufactureLoss in componentAdhesive dressingsAbsorbent padsHuman skinPharmaceutical drug
A blend of at least two polymers in combination with a drug provides a pressure-sensitive adhesive composition for a transdermal drug delivery system in which the drug is delivered from the pressure-sensitive adhesive composition and through dermis when the pressure-sensitive adhesive composition is in contact with human skin.
Owner:NOVEN PHARMA
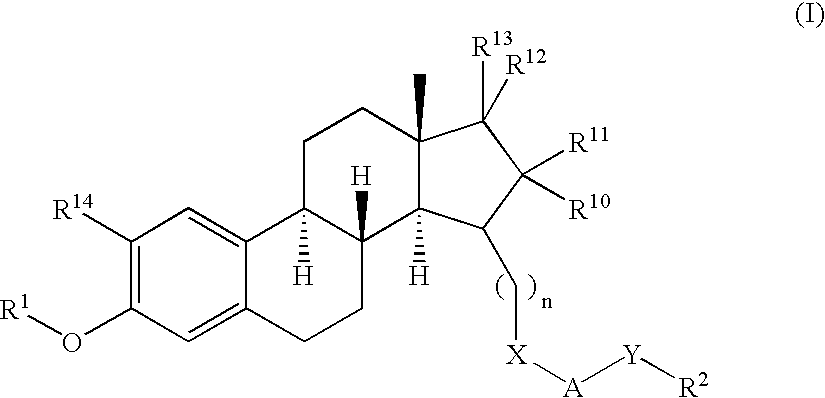
17SS-HSD1 and STS inhibitors
The present invention relates to novel substituted steroid derivatives which represent selectiv inhibitors of the 17β-hydroxysteroid dehydrogenase type I (17β-HSD1) and, in addition, which may represent inhibitors of the steroid sulphatase, as well as to their salts, to pharmaceutical preparations containing these compounds and to processes for the preparation of these compounds. Furthermore, the invention concerns the therapeutic use of said novel substituted steroid derivatives, particularly their use in the treatment, inhibition, prophylaxis or prevention of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of 17β-hydroxysteroid dehydrogenase type I and / or steroid sulphatase enzymes and / or requiring the lowering of the endogenous 17β-estradiol concentration.
Owner:ABBVIE PHARMA GMBH
Natural combination hormone replacement formulations and therapies
Owner:THERAPEUTICSMD
Vaginal inserted estradiol pharmaceutical compositions and methods
ActiveUS20150133421A1Reduce in quantityIncrease the number ofOrganic active ingredientsPharmaceutical non-active ingredientsGynecologyVaginal atrophy
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD INC
Natural combination hormone replacement formulations and therapies
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD INC
Making method and application of CdS sensitized TiO2 environmental estrogen photoelectrochemical sensor
ActiveCN104297305AImprove photoelectric conversion efficiencySave raw materialsMaterial electrochemical variablesAntigenAntigen capture
The invention relates to a making method and an application of a CdS sensitized TiO2 environmental estrogen photoelectrochemical sensor. The method uses TiO2 as an antigen capture substrate material, a CdS photoelectric active material is in situ generated on the surface of a Cd2<+> functional TiP nanomaterial maker modified electrode through a direct Na2S dropping technology, and CdS is irritated by an LED lamp of visible light wavelength to be converted to a photoelectric current signal. The substrate material TiO2 can be well matched with CdS energy band, so the photocurrent conversion signal of CdS is further improved, thereby the competitive photoelectrochemical immunosensor for ultra sensitive detection of estradiol, estriol, diethylstilbestrol, bisphenol A, nonylphenol, oestrone and other environmental pollutants is made.
Owner:UNIV OF JINAN
Estradiol formulations and therapies
InactiveUS20140370084A1Improve complianceOrganic active ingredientsCapsule deliveryEstradiol preparationEstradiol.free
Estrogen and progesterone replacement therapies are provided herein. Among others, the following formulations are provided herein: solubilized estradiol without progesterone; micronized progesterone without estradiol; micronized progesterone with partially solubilized progesterone; solubilized estradiol with micronized progesterone; solubilized estradiol with micronized progesterone in combination with partially solubilized progesterone; and solubilized estradiol with solubilized progesterone.
Owner:THERAPEUTICSMD
Vaginal inserted estradiol pharmaceutical compositons and methods
ActiveUS20150045335A1Reduce in quantityIncrease the number ofOrganic active ingredientsOintment deliveryVaginal atrophyVagina
According to various embodiments of this disclosure, pharmaceutical compositions comprising solubilized estradiol are provided. In various embodiments, such compositions are encapsulated in soft capsules which may be vaginally inserted for the treatment of vulvovaginal atrophy.
Owner:THERAPEUTICSMD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com