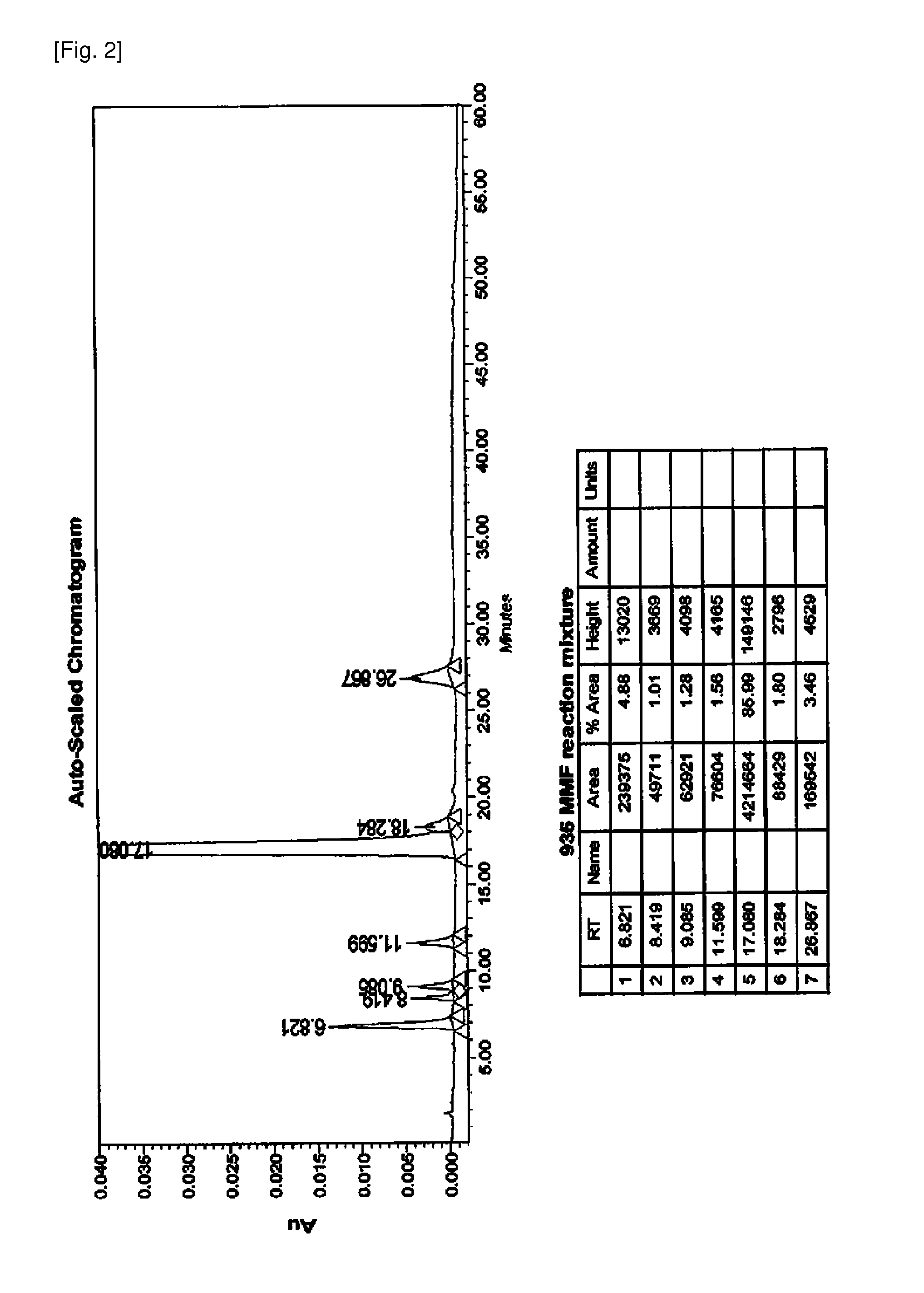
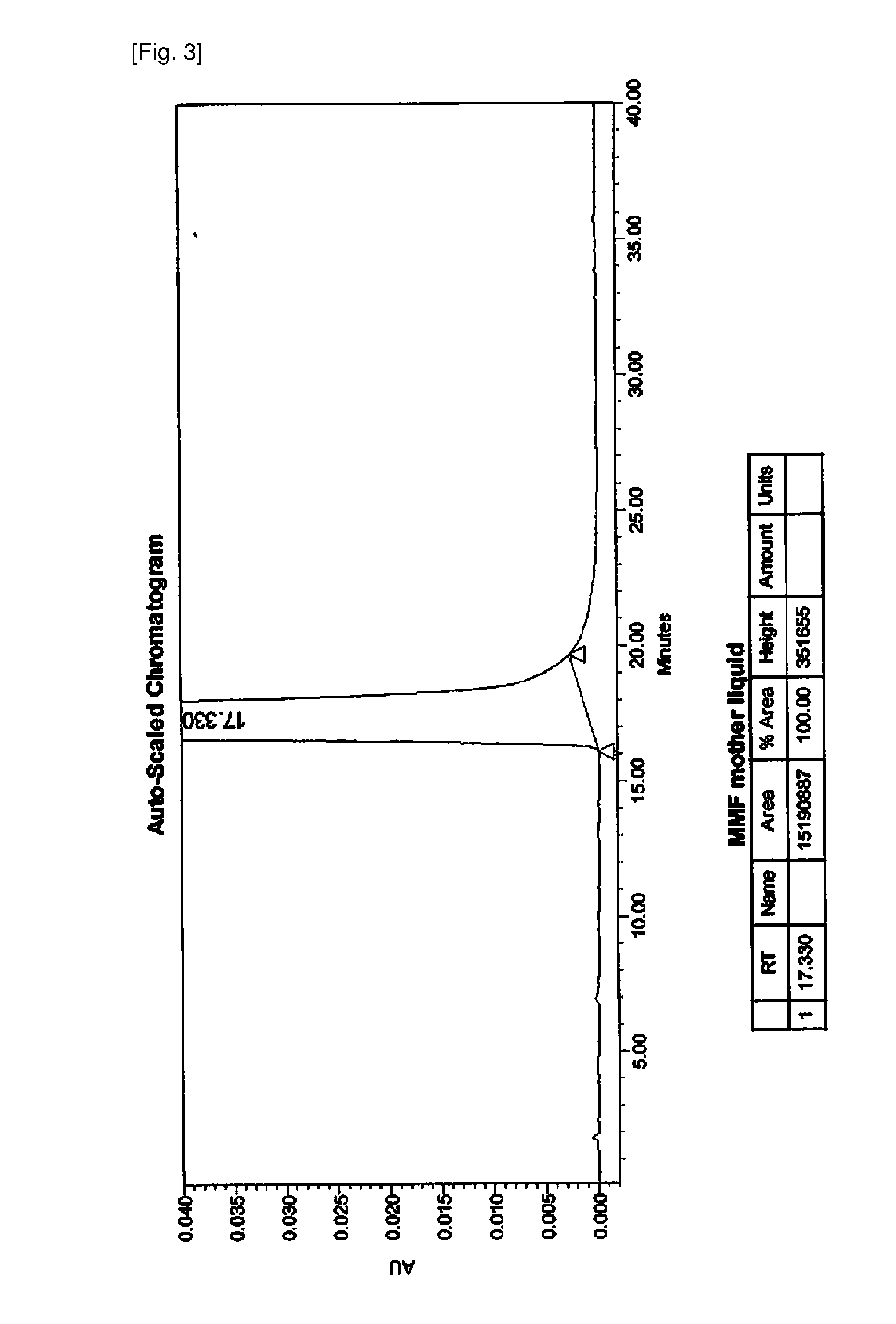
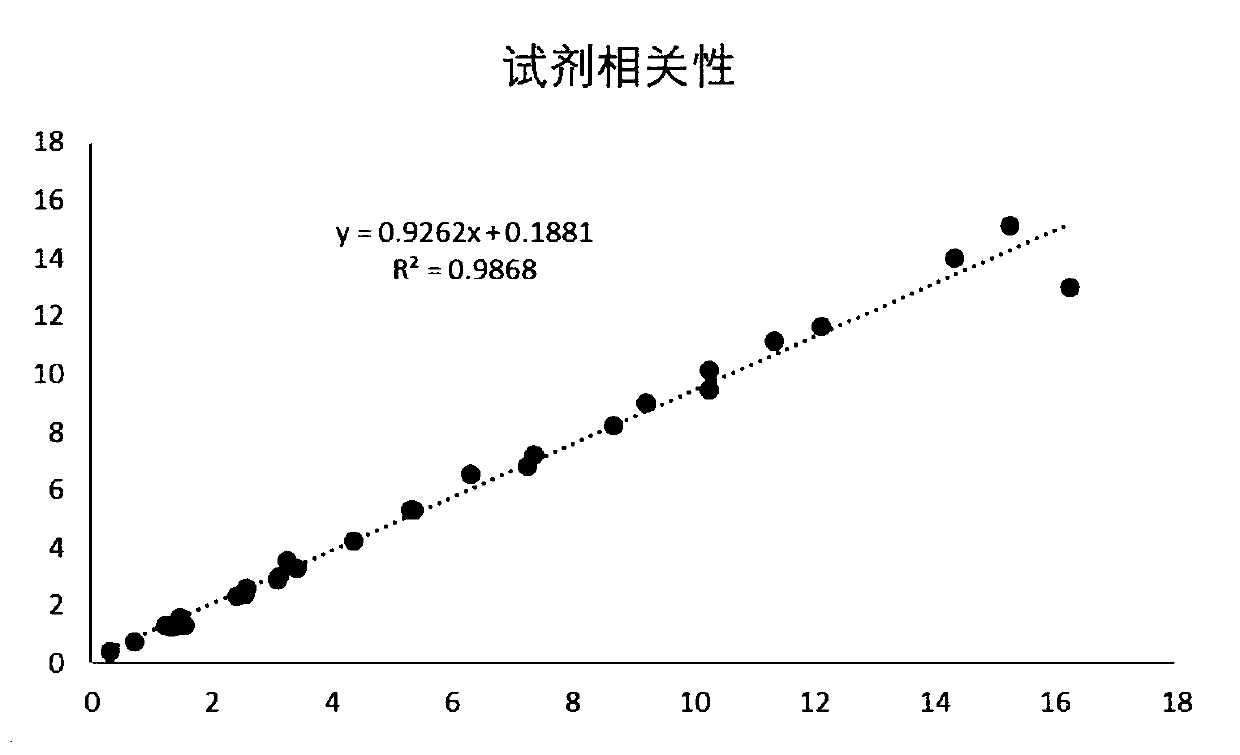
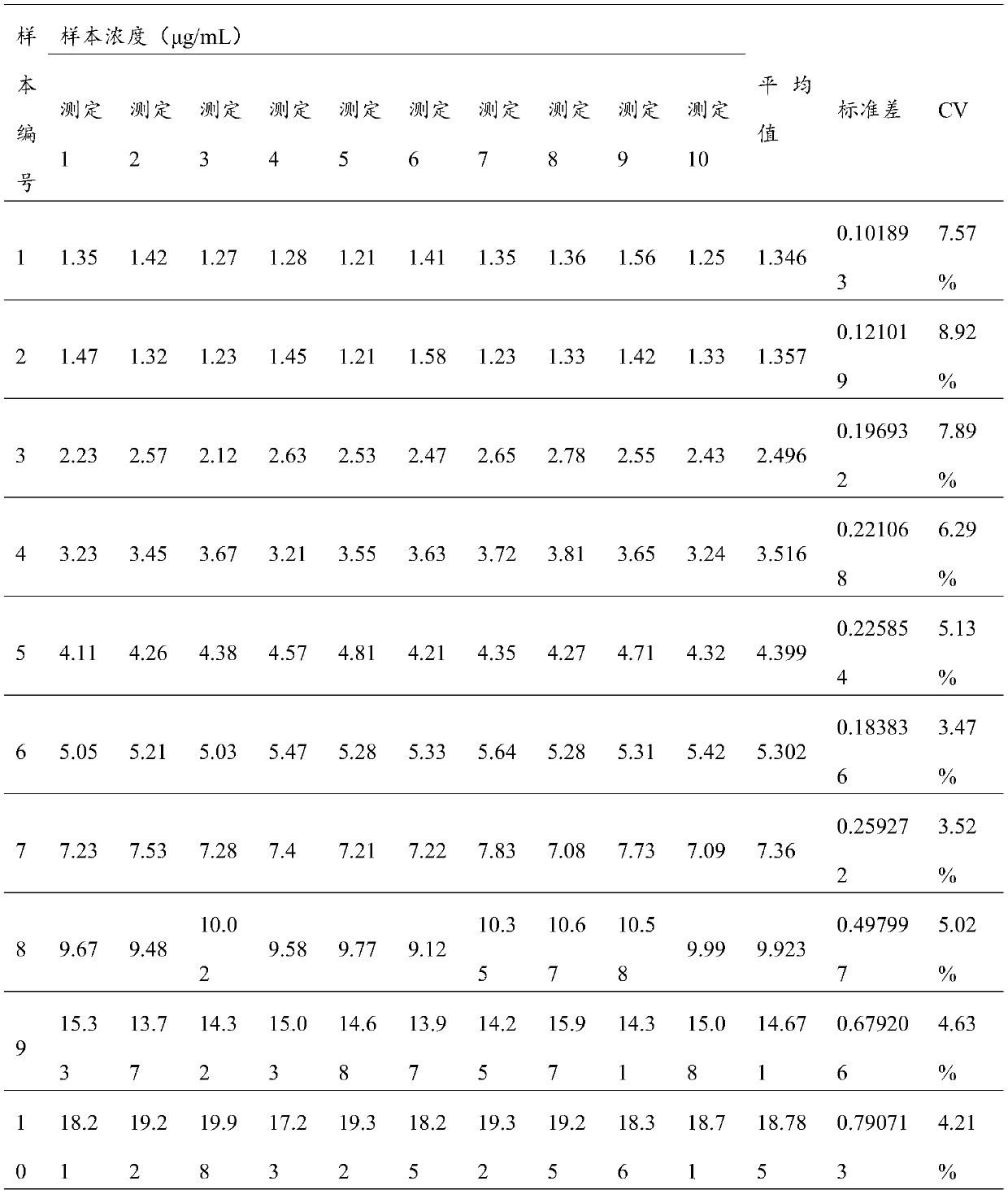
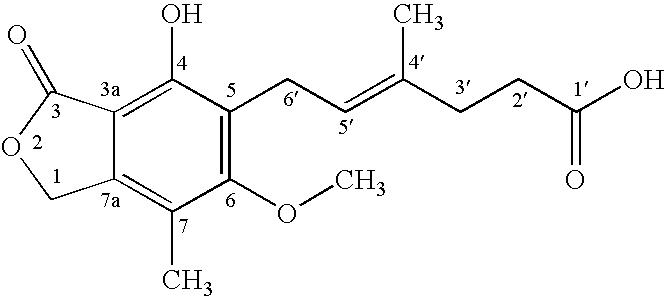
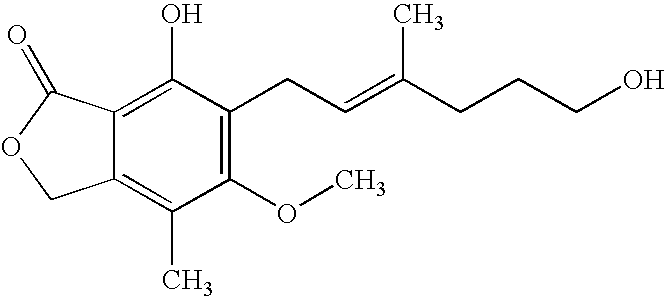
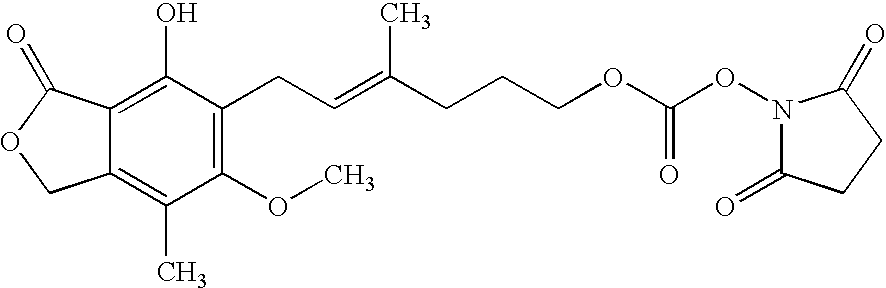
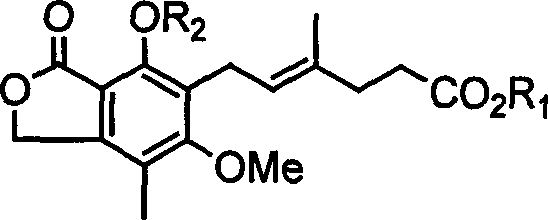
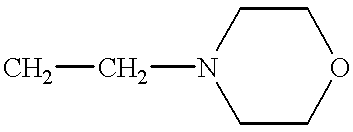Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Mycophenolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
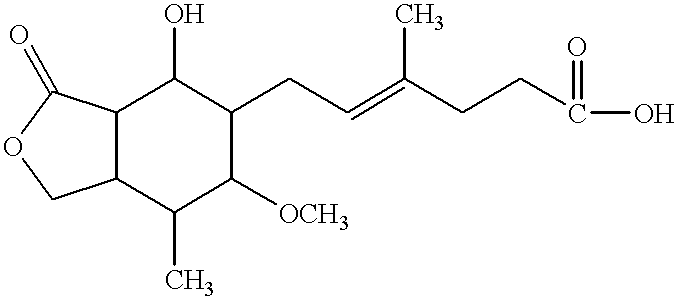
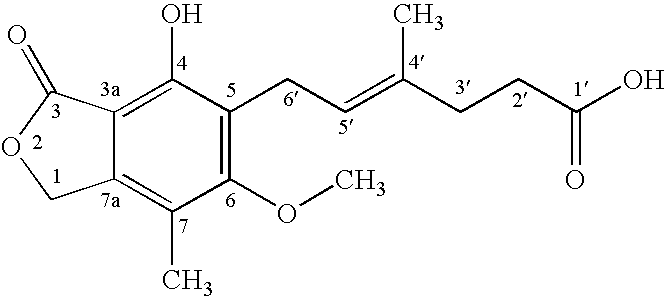
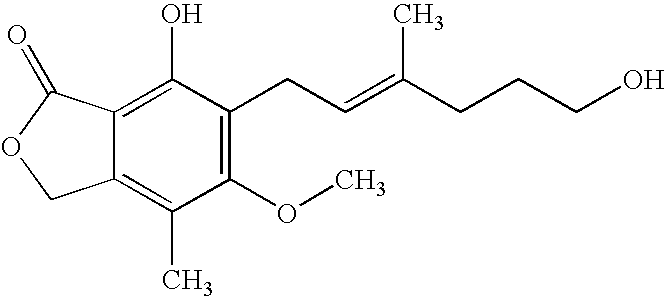
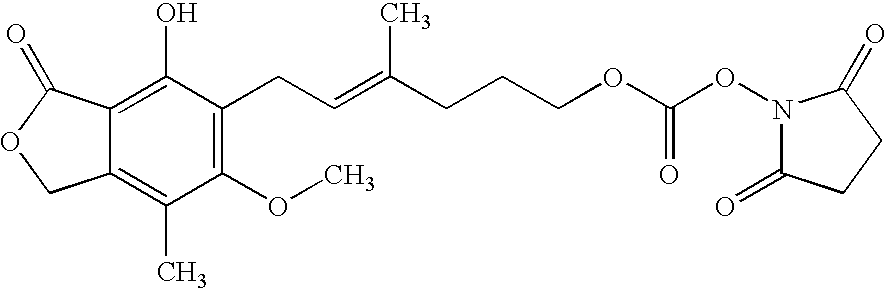
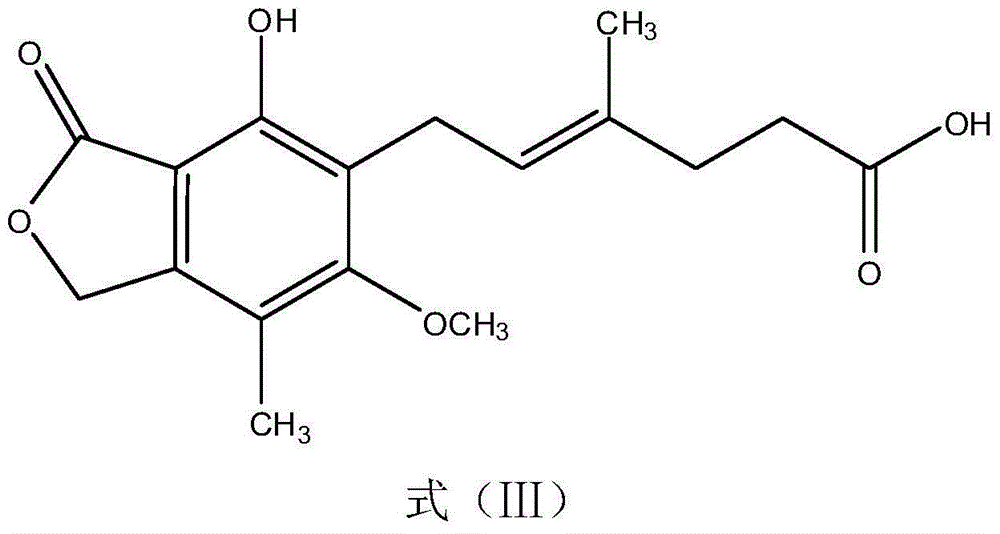
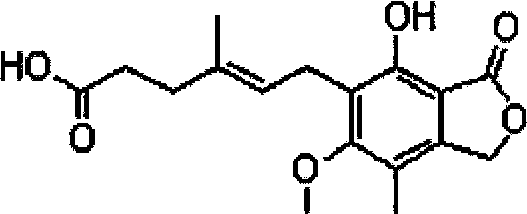
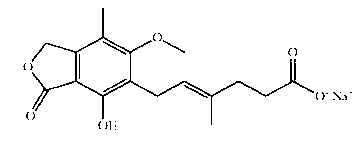
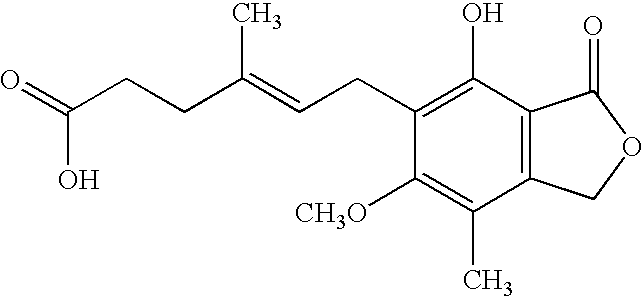
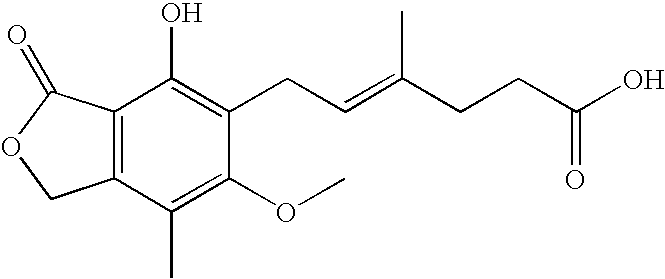
Mycophenolic acid, (MPA) is a non-competitive reversible inhibitor of the inosine monophosphate dehydrogenase (IMPDH), a key rate-limiting enzyme in the de novo purine synthesis pathway and also called mycophenolate, is an immunosuppressant drug used to prevent rejection in organ transplantation. It was initially introduced as the prodrug mycophenolate mofetil (MMF, trade name CellCept) to improve oral bioavailability. More recently, the salt mycophenolate sodium (trade name Myfortic) has also been introduced. Enteric-coated mycophenolate sodium is an alternative MPA formulation. However, the kinetics of EC-MPS differ in that the drug is enteric coated, dissolves at pH levels of ≥ 5,with the goal of delaying the delivery of MPA until it reaches the small intestine...
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS20050063996A1Reduce turbidityReduce inflammationAntibacterial agentsBiocideEverolimusCell migration
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS20060228394A1Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:MINU
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Intravascular delivery of mycophenolic acid
InactiveUS6858221B2Improve efficiencyImprove efficacyBiocideStentsSmooth musclePercent Diameter Stenosis
The present invention provides improved devices and methods for minimizing and / or inhibiting restenosis and hyperplasia after intravascular intervention. In particular, the present invention provides luminal prostheses which allow for programmed and controlled mycophenolic acid delivery with increased efficacy to selected locations within a patient's vasculature to inhibit restenosis. An intraluminal delivery prosthesis may comprise an expansible structure and means on or within the structure for releasing mycophenolic acid at a rate selected to inhibit smooth muscle cell proliferation.
Owner:ALTAI MEDICAL TECH
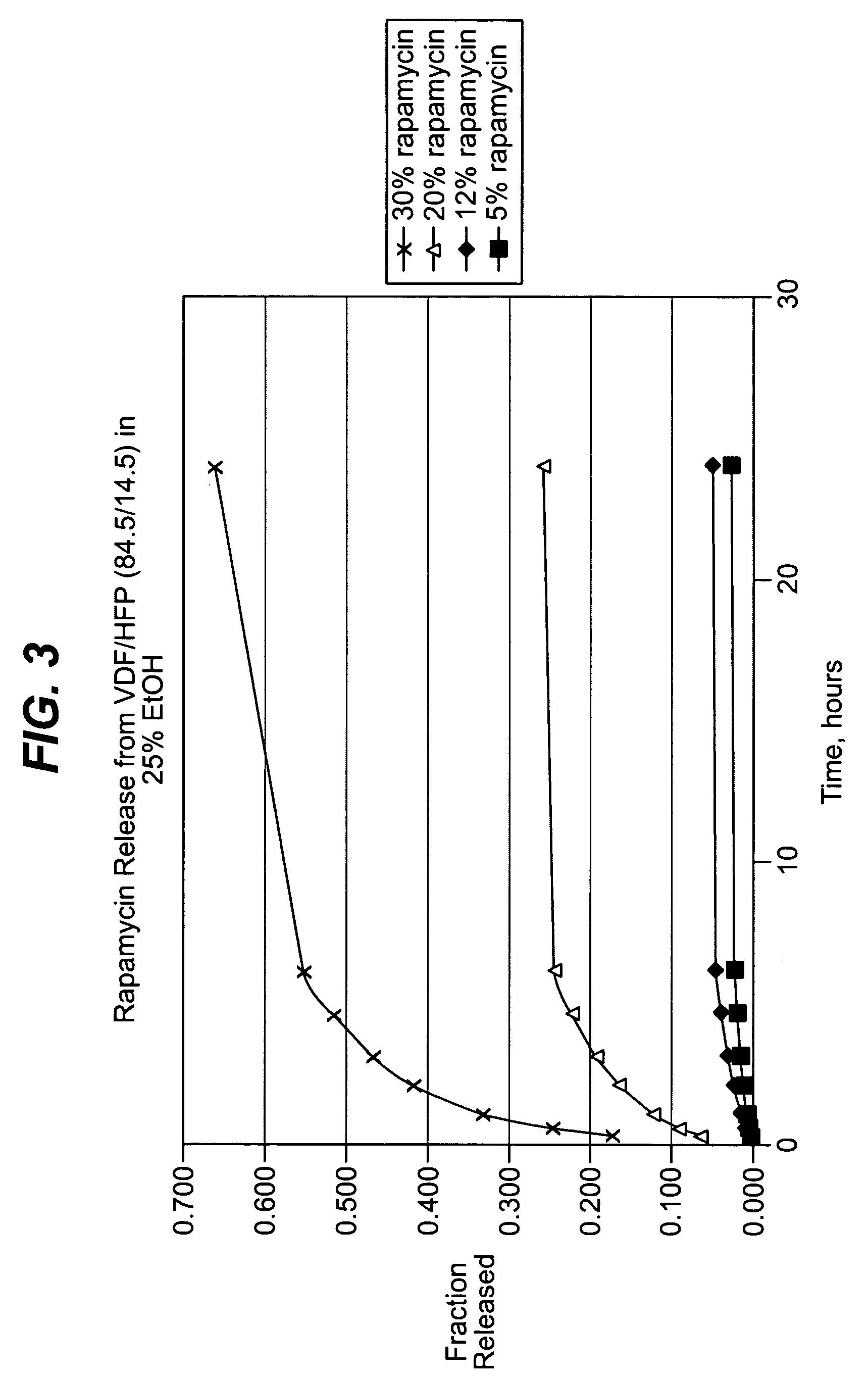
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050158360A1Minimize potential risk of damageReduce frictionStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
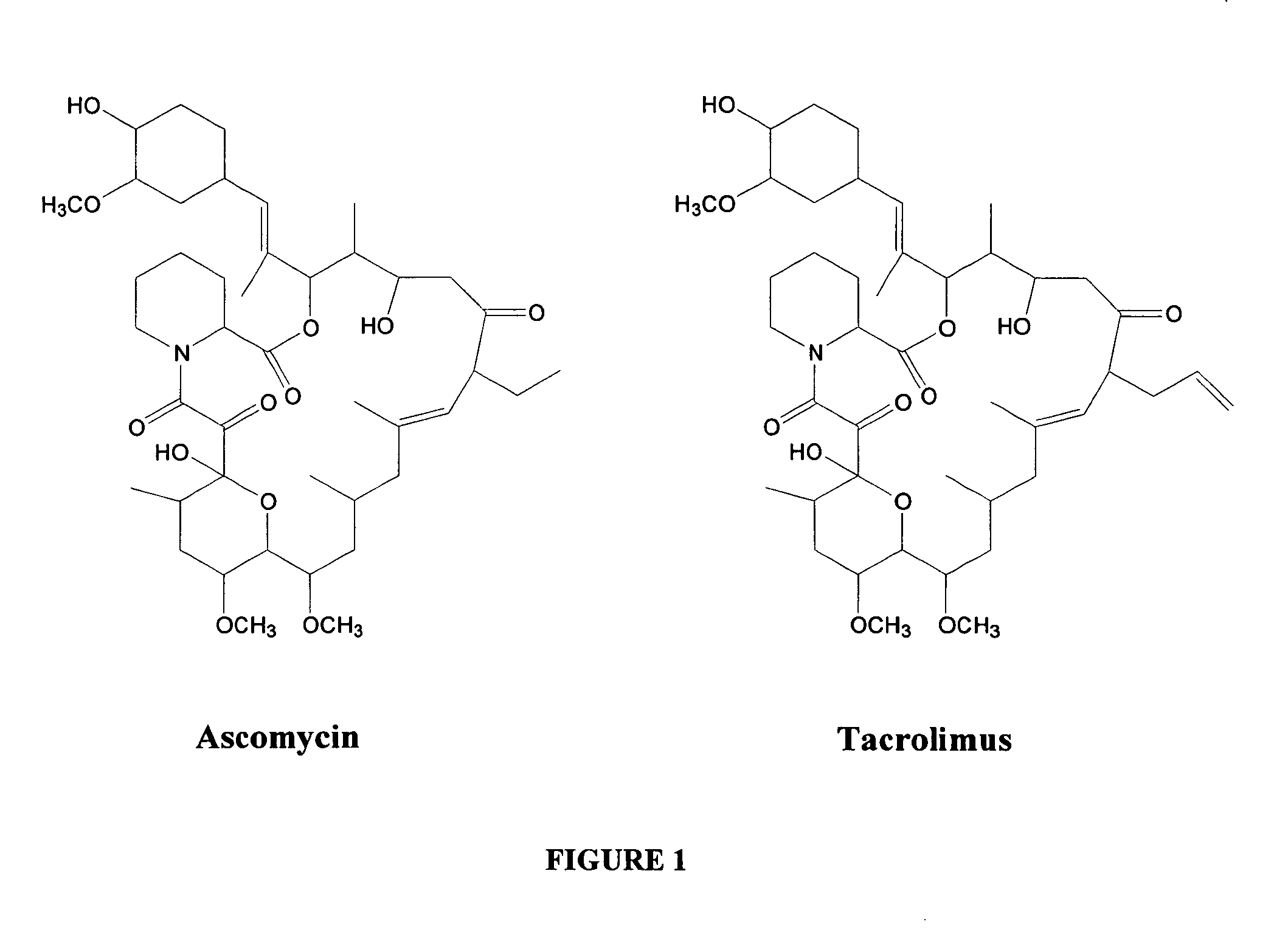
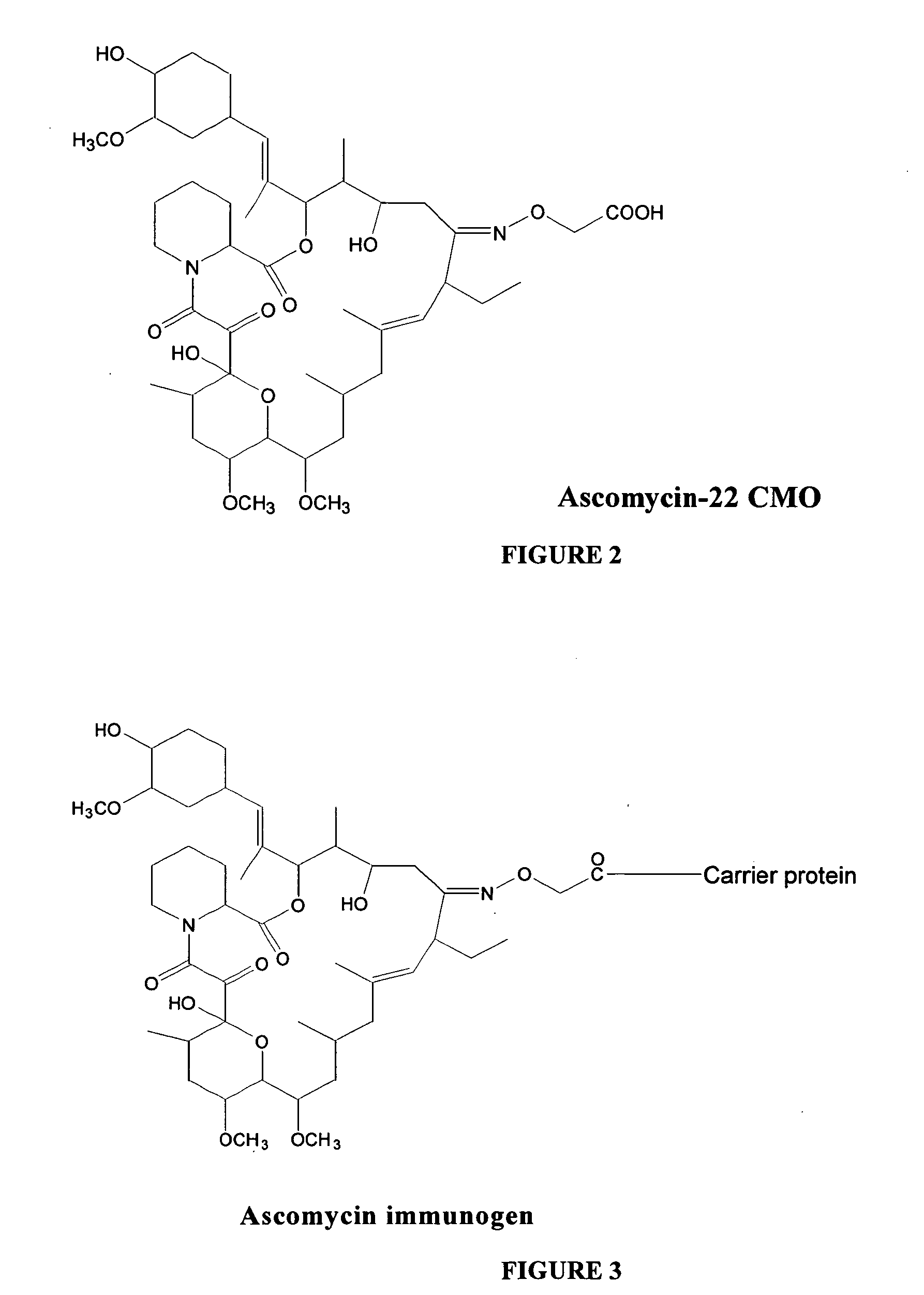
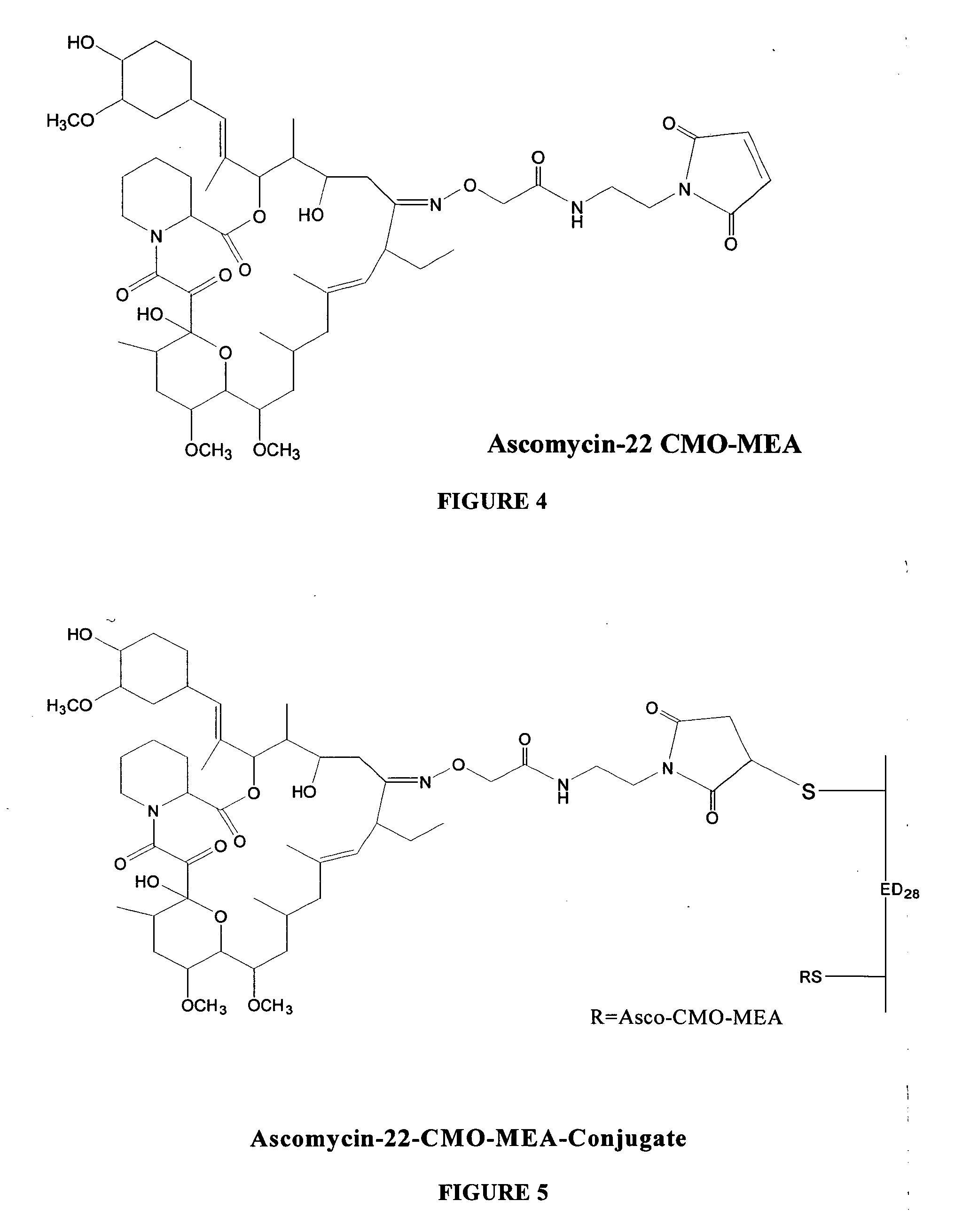
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS7303758B2Prevent elutionProvide controlStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Owner:WYETH LLC
Drug eluting coatings for medical implants
InactiveUS7438925B2Control releaseMinimize any pathologies associatedSuture equipmentsStentsEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Ocular solutions
InactiveUS20050063997A1Reduce turbidityReduce inflammationBiocideSenses disorderEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:PEYMAN GHOLAM A DR
Method for treating hepatitis C
A method of treating a patient infected with a hepatitis C virus to decrease the severity of the viral infection. The method comprises concomitantly administering over a given period of time to the patient a first component and a second component. The first component consists of a pharmaceutical composition containing as an active ingredient a pharmaceutically acceptable salt or prodrug of mycophenolic acid in a therapeutically effective amount to decrease the severity of the viral infection. The second component consists of an injection solution containing as an active ingredient interferon-alpha or pegylated interferon-alpha in a therapeutically effective amount to decrease the severity of the viral infection. The components are concomitantly administered over a period of time at least sufficient to reduce the amount of HCV-RNA present in the peripheral blood of said patient to less than 100 copies / ml at 24 weeks after the end of treatment.
Owner:GRAVES MARY +2
Reagents and methods for mycophenolic acid immunoassay
The invention teaches derivatives of mycophenolic alcohol and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of mycophenolic acid (MPA) and / or active metabolites of MPA in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to MPA with cross-reactivity of no more than 5% with inactive metabolites and commonly co-prescribed drugs. Further, immunoassays for measuring the concentration of MPA using such antibodies are taught.
Owner:MICROGENICS CORP
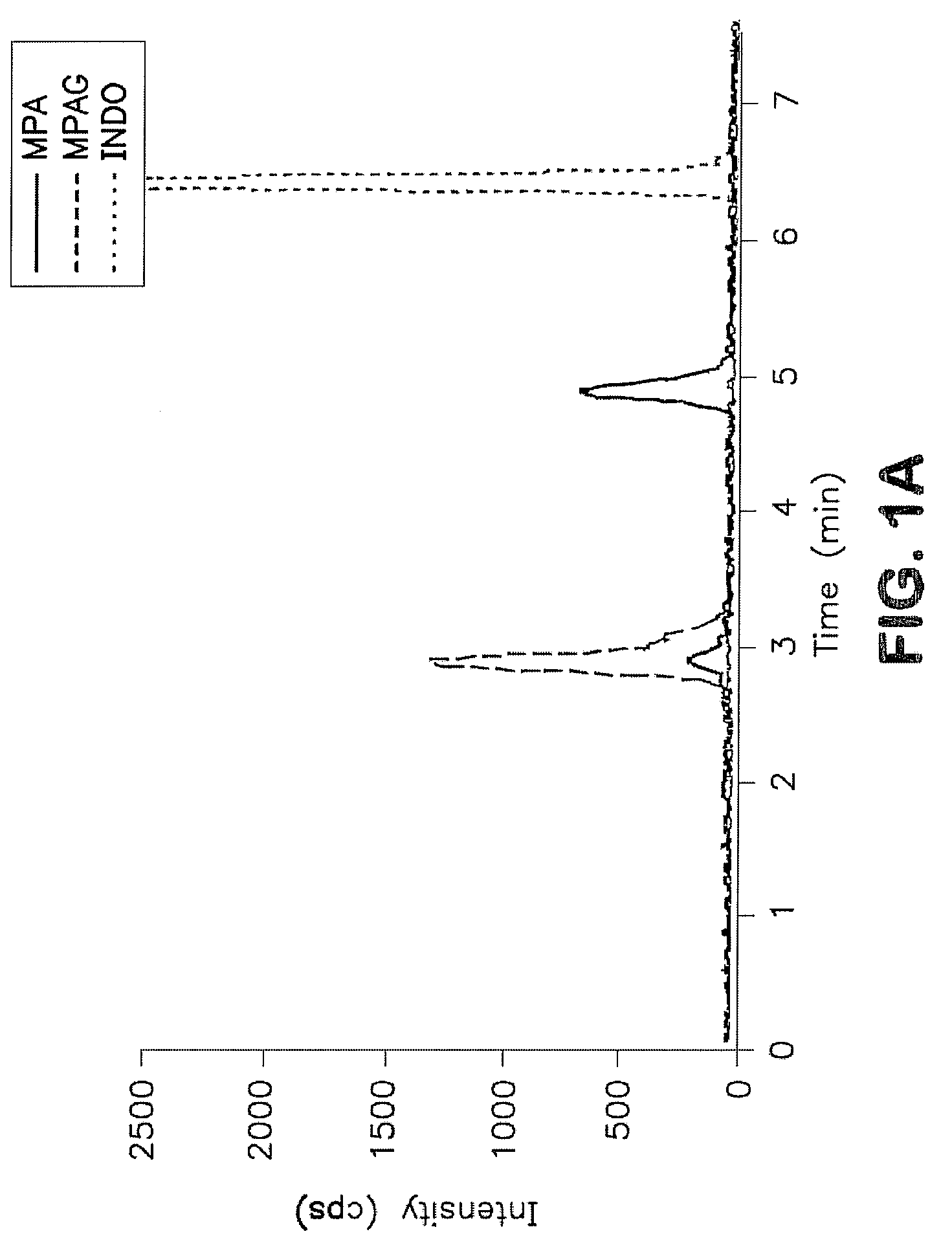
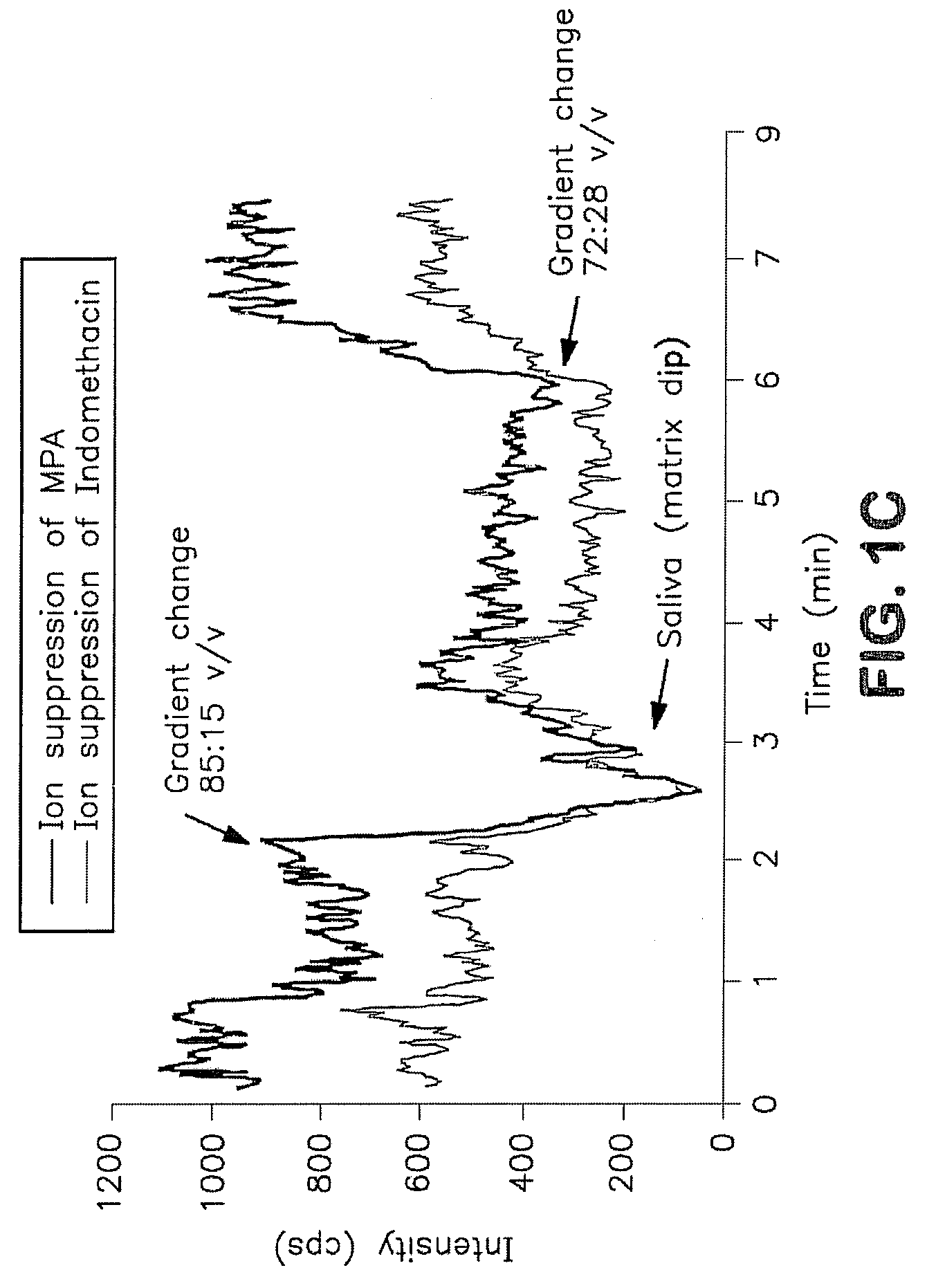
Analysis of mycophenolic acid in saliva using liquid chromatography tandem mass spectrometry
InactiveUS20080318322A1Component separationBiological testingSaliva sampleGas chromatography–mass spectrometry
A method for mass spectrometric analysis of a saliva sample possibly containing mycophenolic acid or its metabolites mycophenolic acid phenyl glucuronide (MPAG) or mycophenolic acid acyl-glucuronide (Acyl-MPAG), including the steps: (a) providing a saliva sample containing one or more drug or metabolites; (b) deproteinating the sample; (c) separating the one or more drug or metabolites from the saliva sample; and (d) analyzing the one or more drug or metabolites using a mass spectrometer. The sample containing one or more MPA or metabolites is obtained from in an oral fluid based biological samples i.e. whole saliva or saliva obtained by chemical or mechanical stimulation or from specific salivary glands. The size of the sample contains one or more MPA or metabolites is at least about 100 microL. A kit for use in mass spectrometric analysis of a sample may contain one or more MPA or metabolites from saliva samples, comprising: (a) reagents for deproteinating of the saliva sample, including internal standards; (b) reagents for separating the one or more MPA or metabolites from the saliva sample; (c) reagents for analyzing the one or MPA or metabolites using a mass spectrometer; (d) a solution of one or more MPA or metabolites in saliva samples; and (e) instructions for analyzing the one or more MPA or saliva using a mass spectrometer. The kit includes (a) mobile phase solutions; (b) a chromatography column; and (c) a quality control specimen.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
Carbohydrate supplementing method in fermentation process of mycophenolic acid
ActiveCN101671706AImprove utilizationEasy to operateMicroorganism based processesFermentationCulture mediumsBasal medium
The invention belongs to the field of biological fermentation, more particularly relates to a method for realizing the purpose of producing high-density mycophenolic acid through continuously or intermittently supplementing high-density solution of monosaccharide, disaccharide or polysaccharide into a fermentation liquor to culture penicillium brevicompactum. In the method, high-density solution of monosaccharide, disaccharide or polysaccharide is supplemented as a supplementary carbon source during the fermentation process of mycophenolic acid in a basic culture medium which takes glucose asa carbon source. The carbohydrate supplementing method in the fermentation process provided by the invention is easy to be operated and implemented, can improve the fermentation titer by 80-100 percent, and is very applicable to the demands of industrial production.
Owner:SHANDONG NEWTIME PHARMA
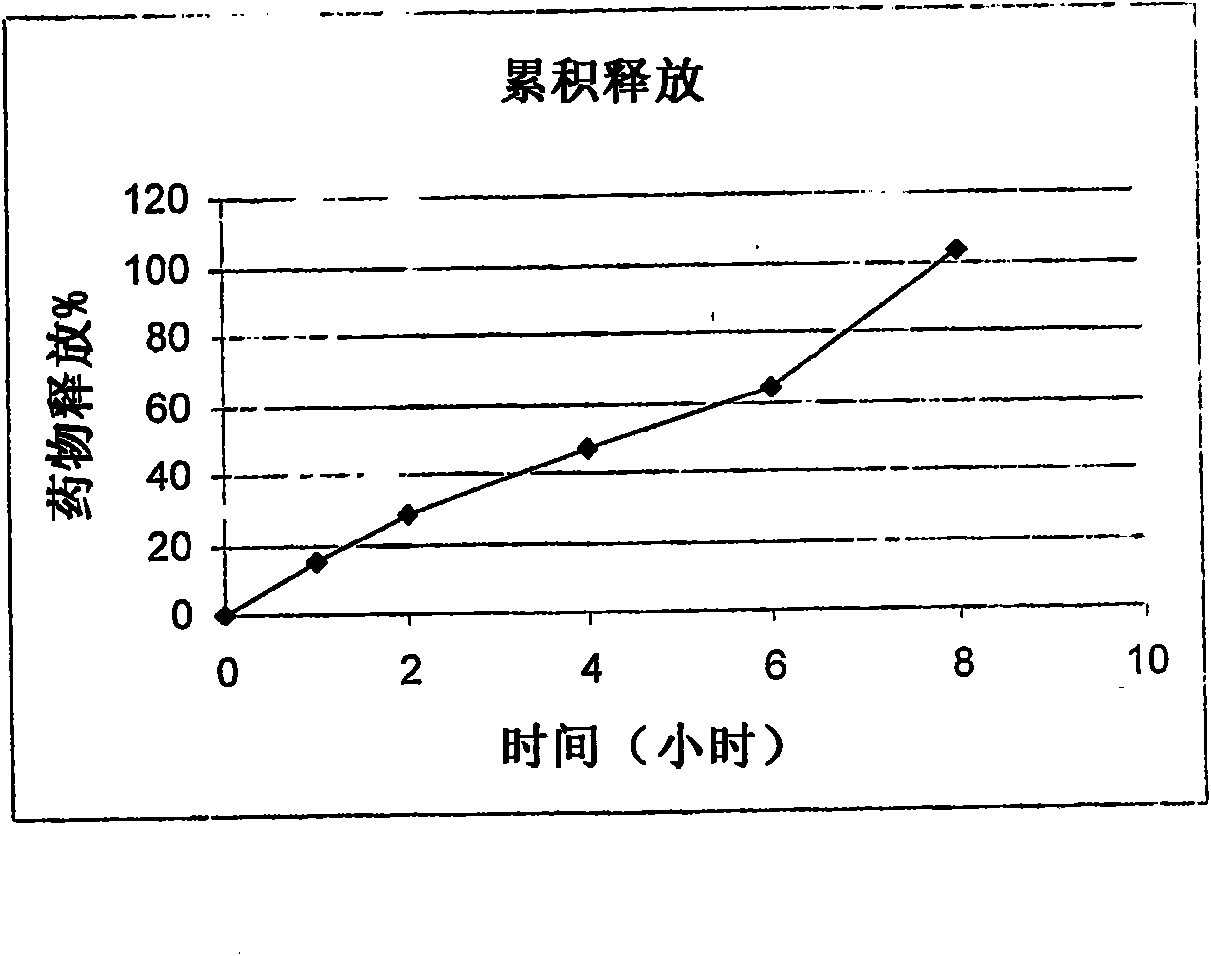
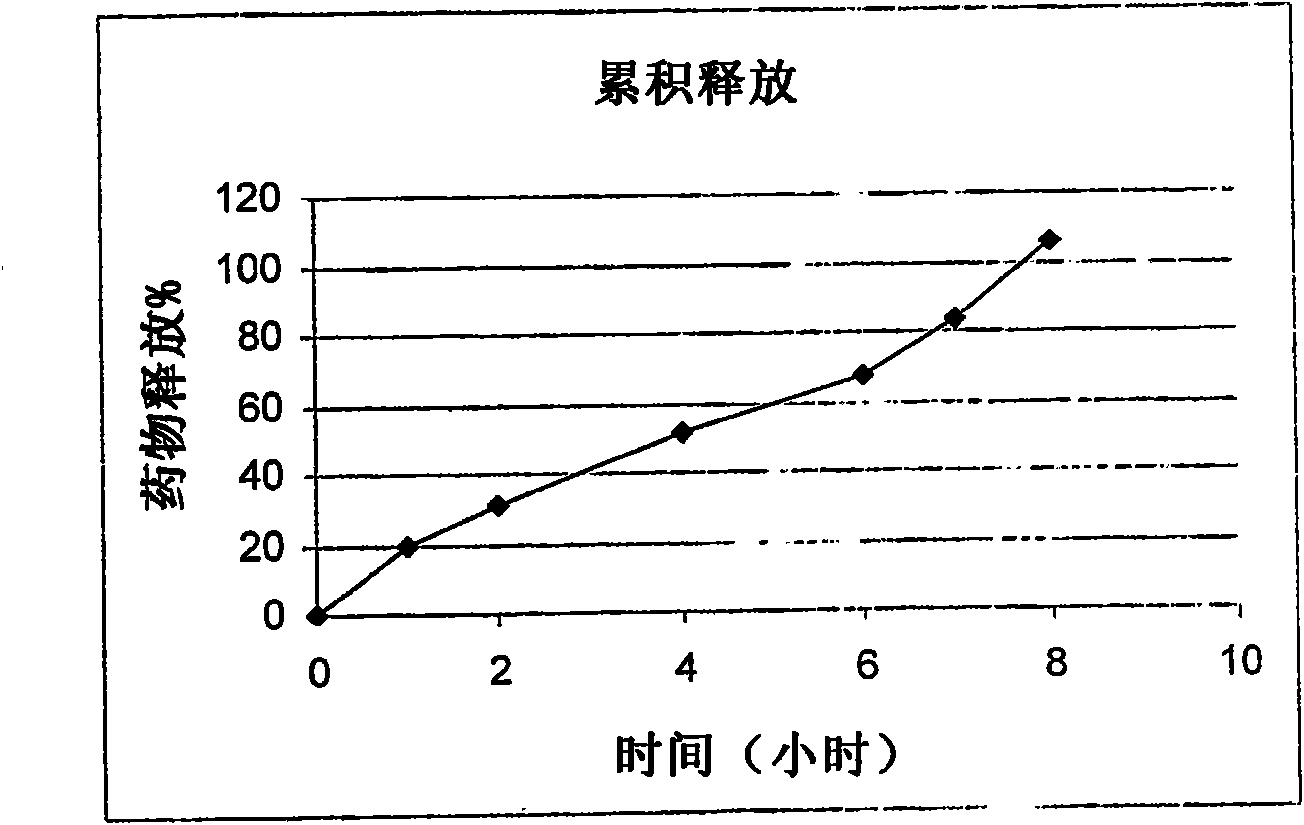
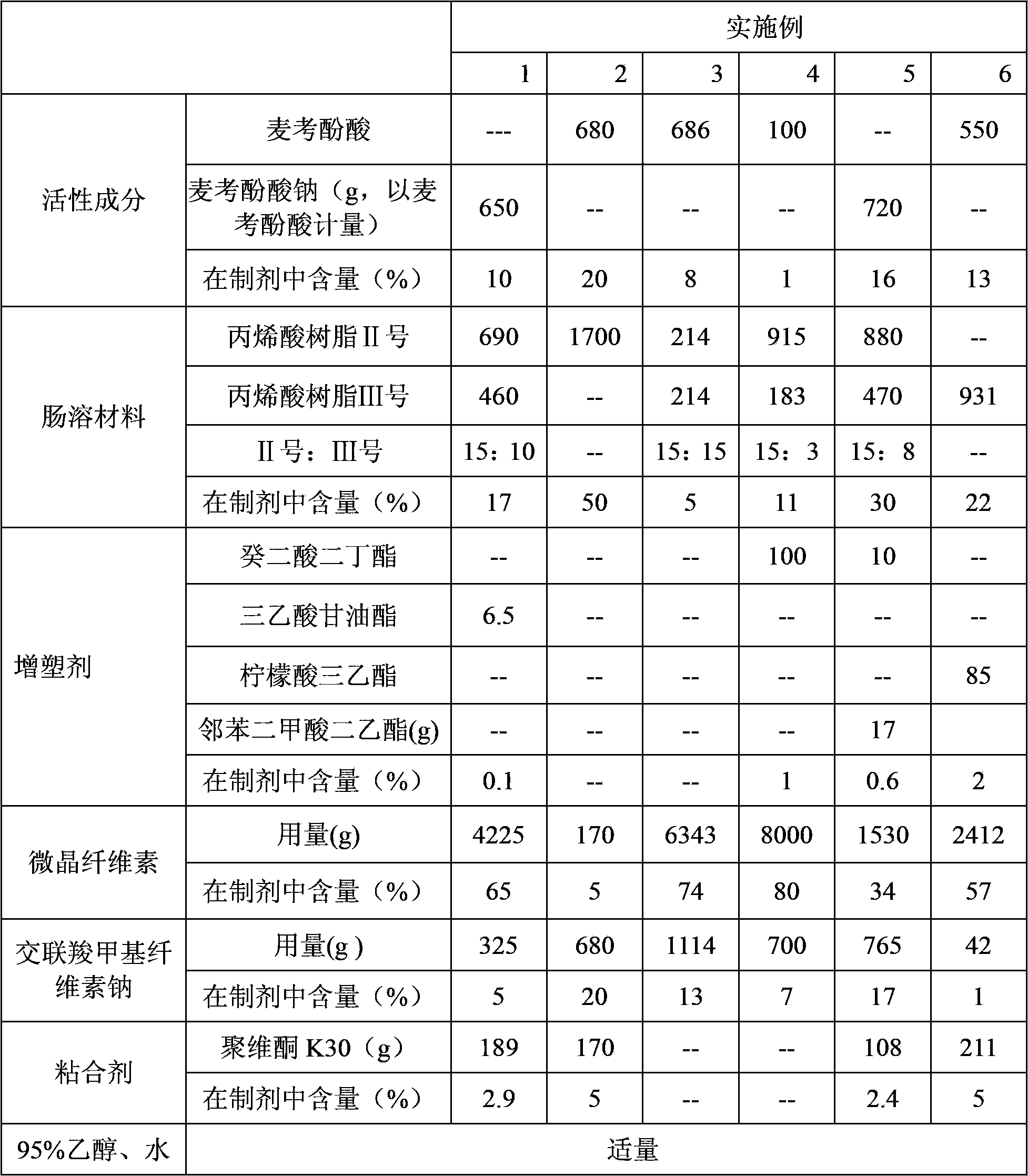
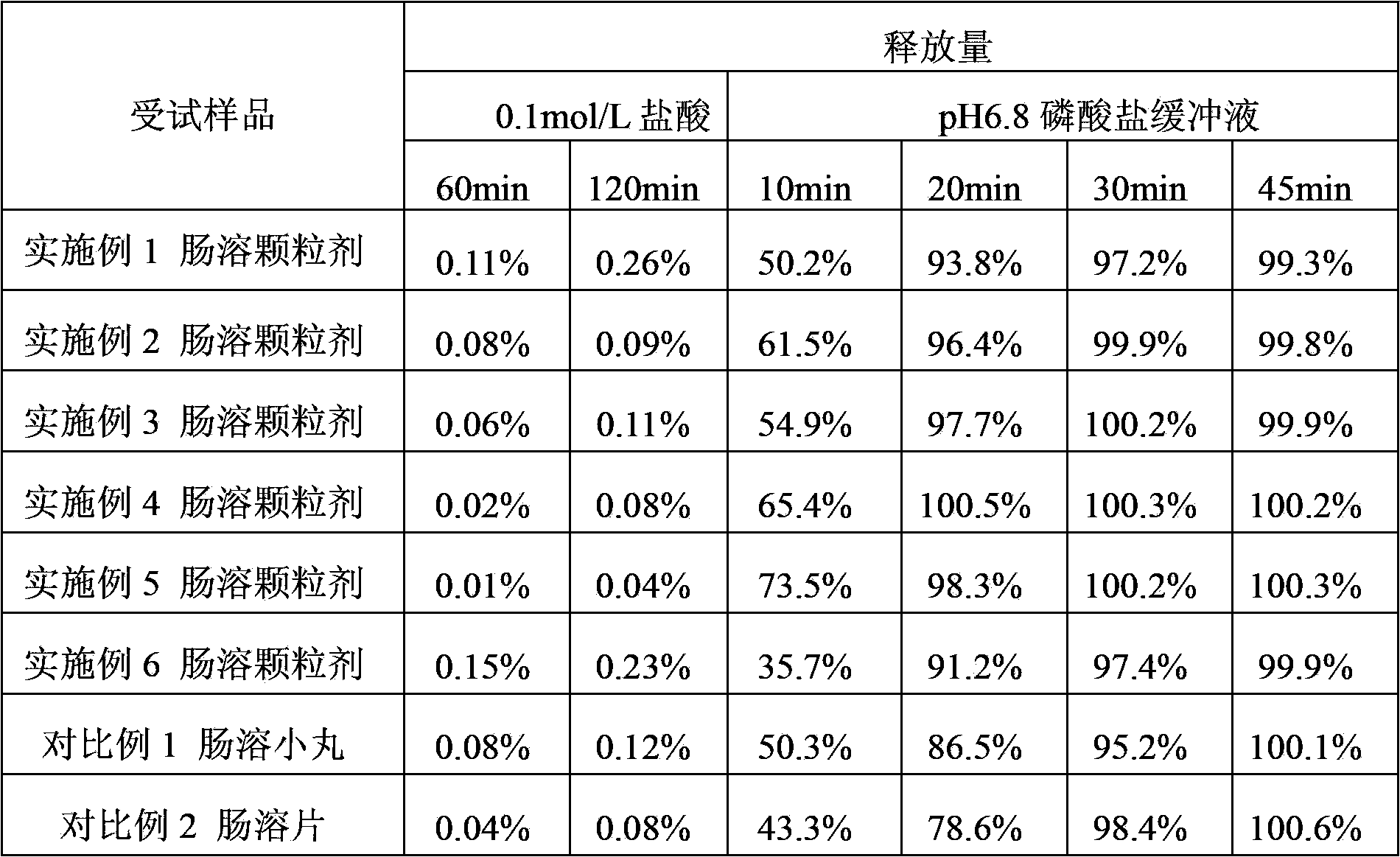
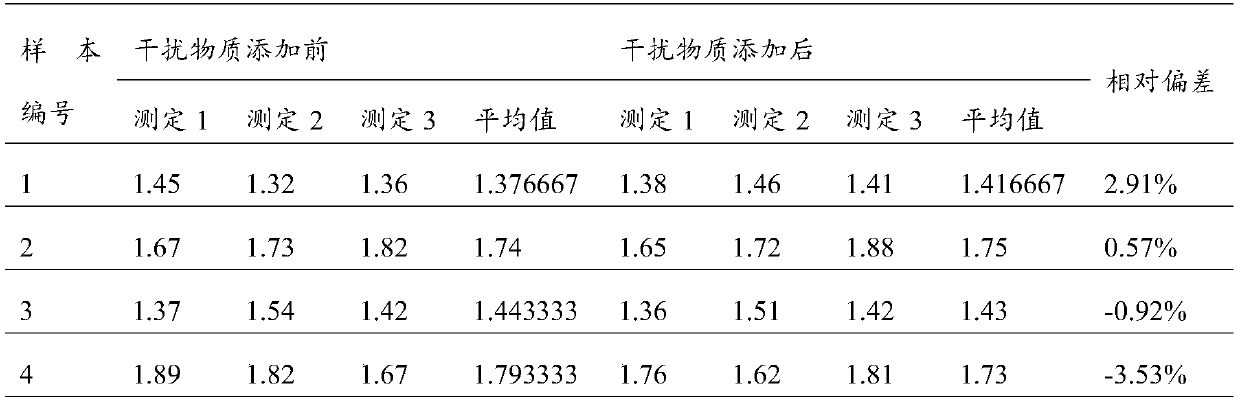
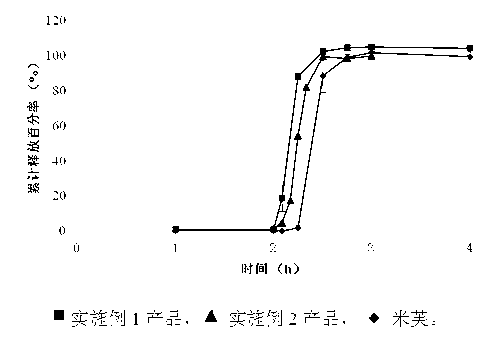
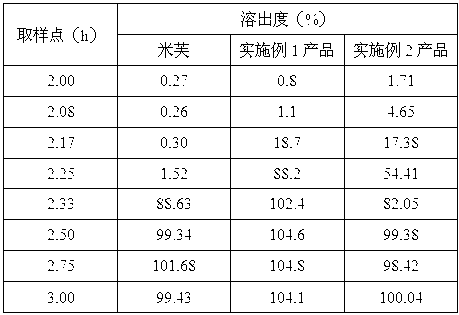
Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
InactiveCN101969931AMaintain therapeutic effectEasy to solveOrganic active ingredientsPill deliveryDrugs levelsActive agent
Modified release pharmaceutical compositions comprising mycophenolate as the active agent or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof, wherein the said composition exhibits a biphasic release profile when subjected to in- vitro dissolution and / or upon administration in- vivo are provided. The composition provides a drug release in a manner such that the drug levels are maintained above the therapeutically effective concentration (EC) constantly for an extended duration of time. Further, the difference between the maximum plasma concentration of the drug (Cmax) and the minimum plasma concentration of the drug (Cmjn), and in turn the flux defined as ((Cmax - Cmjn) / Cavg) is minimal. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such compositions.
Owner:PANACEA BIOTEC
Mycophenolic acid-xenopus laevis glucagon like peptide-1 conjugated peptide and application thereof
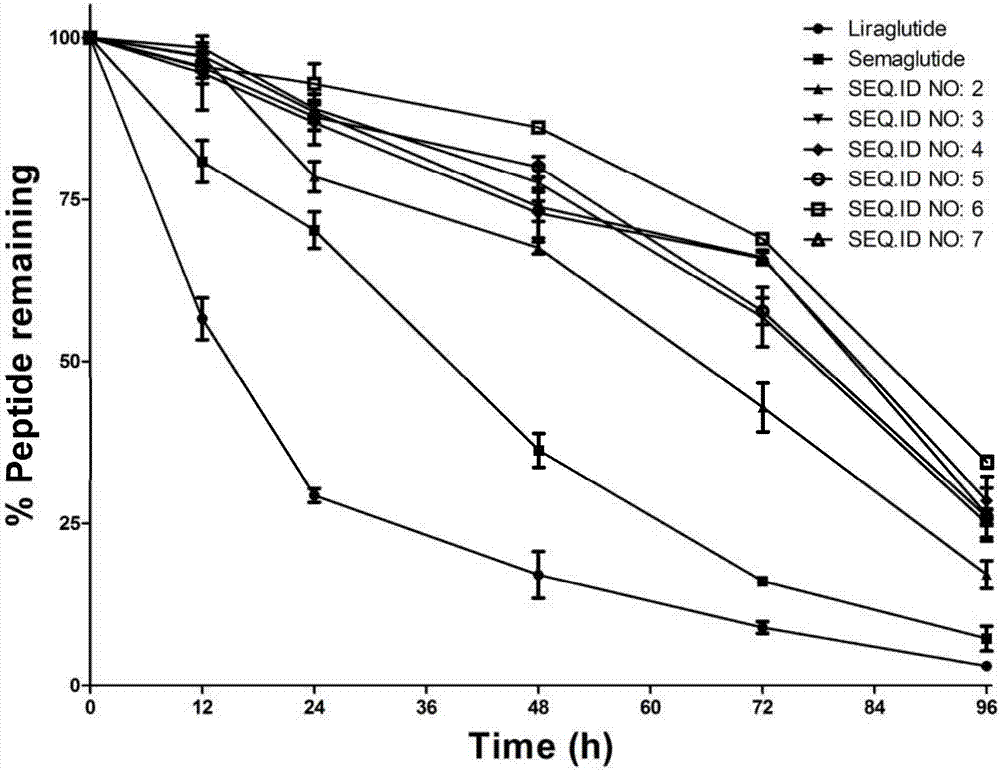
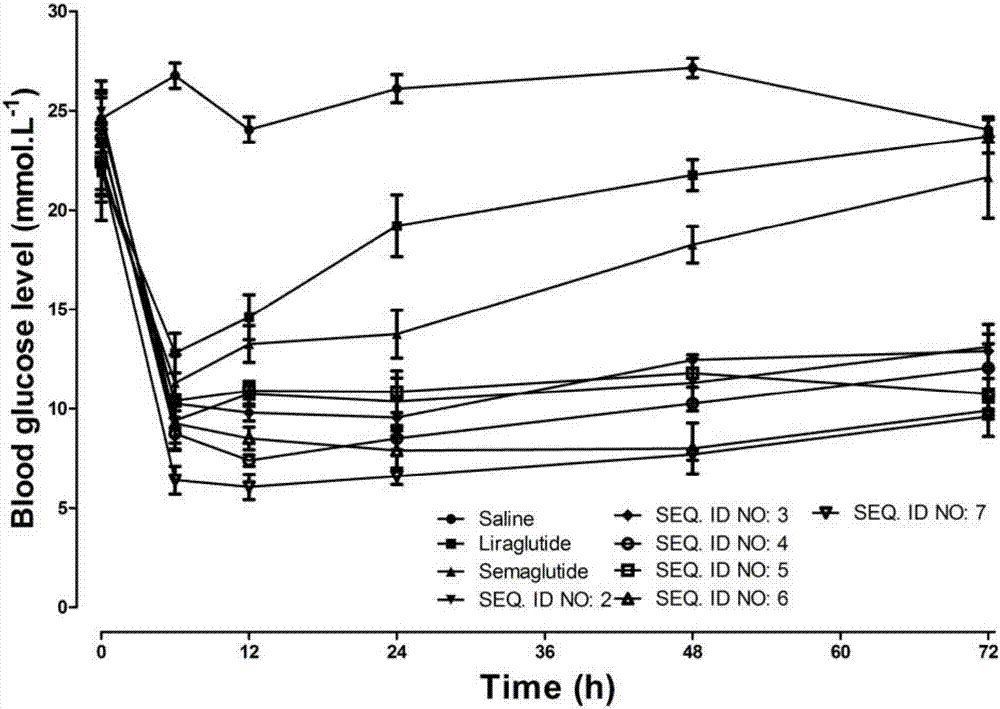
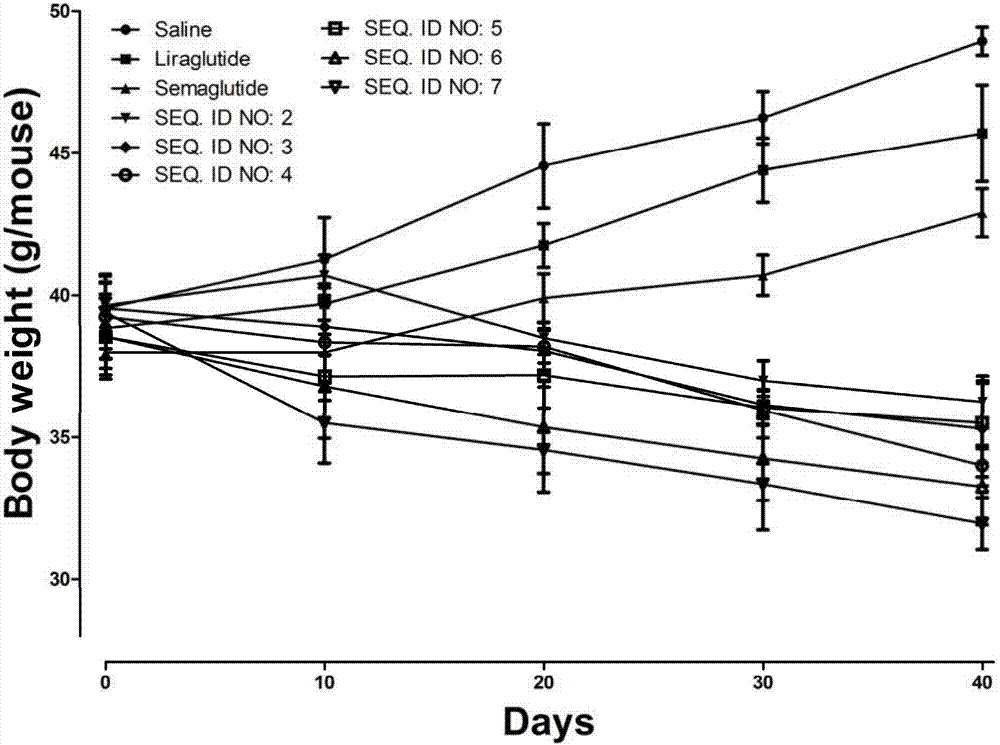
InactiveCN107987152AExtensive treatmentImprove in vivo stabilityPeptide/protein ingredientsMetabolism disorderSide chainGlucagon-like peptide-1
The invention relates to a mycophenolic acid-xenopus laevis glucagon like peptide-1 (XenGLP-1) conjugated peptide and application thereof. The preparation method comprises the following steps: performing structural modification on side chains of XenGLP-1, and introducing mycophenolic acid small-molecule derivatives with a high binding rate of serum protein, thereby obtaining the XenGLP-1 conjugated peptide with high hypoglycemic activity and long pharmacological action time. The XenGLP-1 conjugated peptide disclosed by the invention has the effect of obviously improving the biological activity, and has excellent effects of reducing blood glucose and losing weight for a long time.
Owner:XUZHOU NORMAL UNIVERSITY
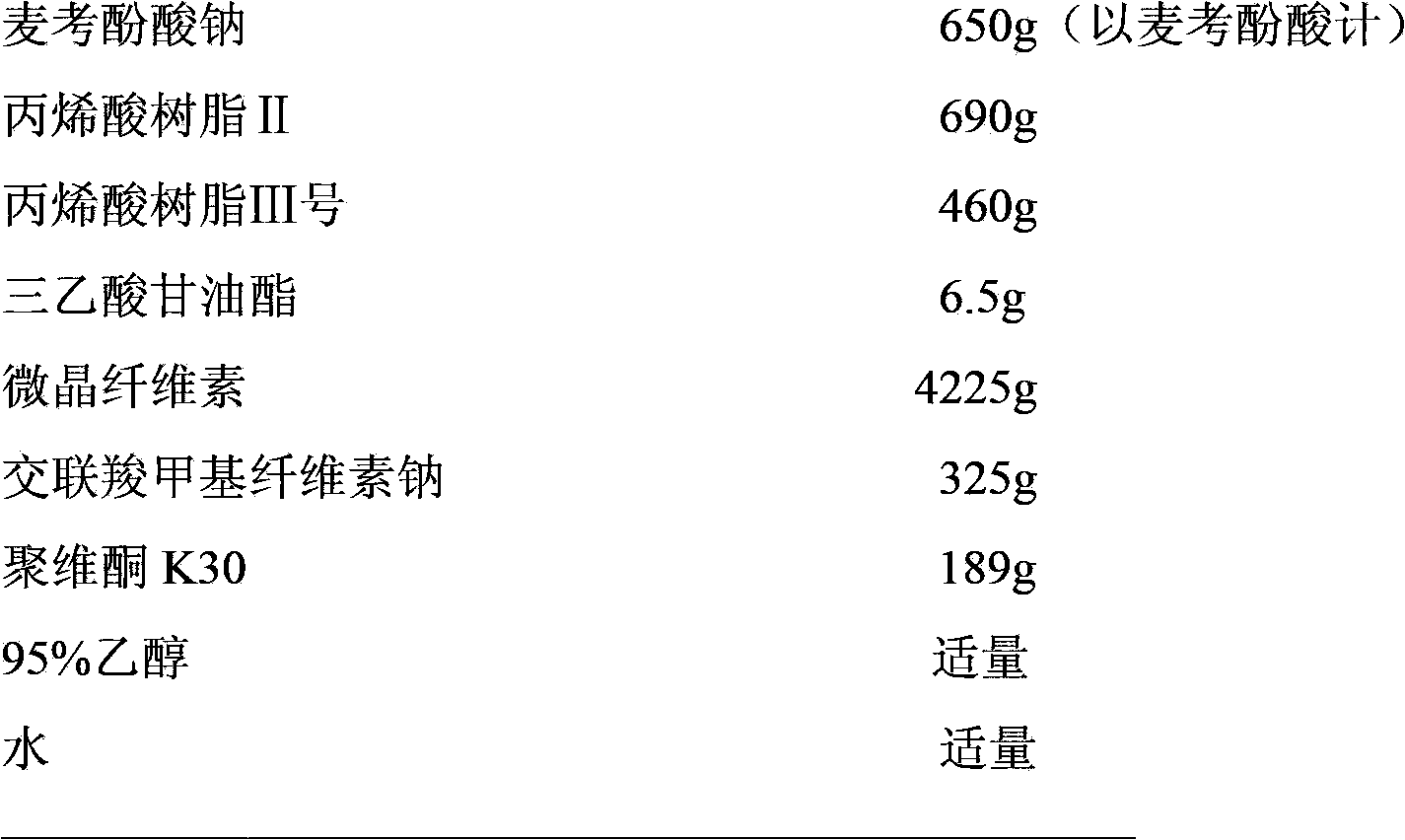
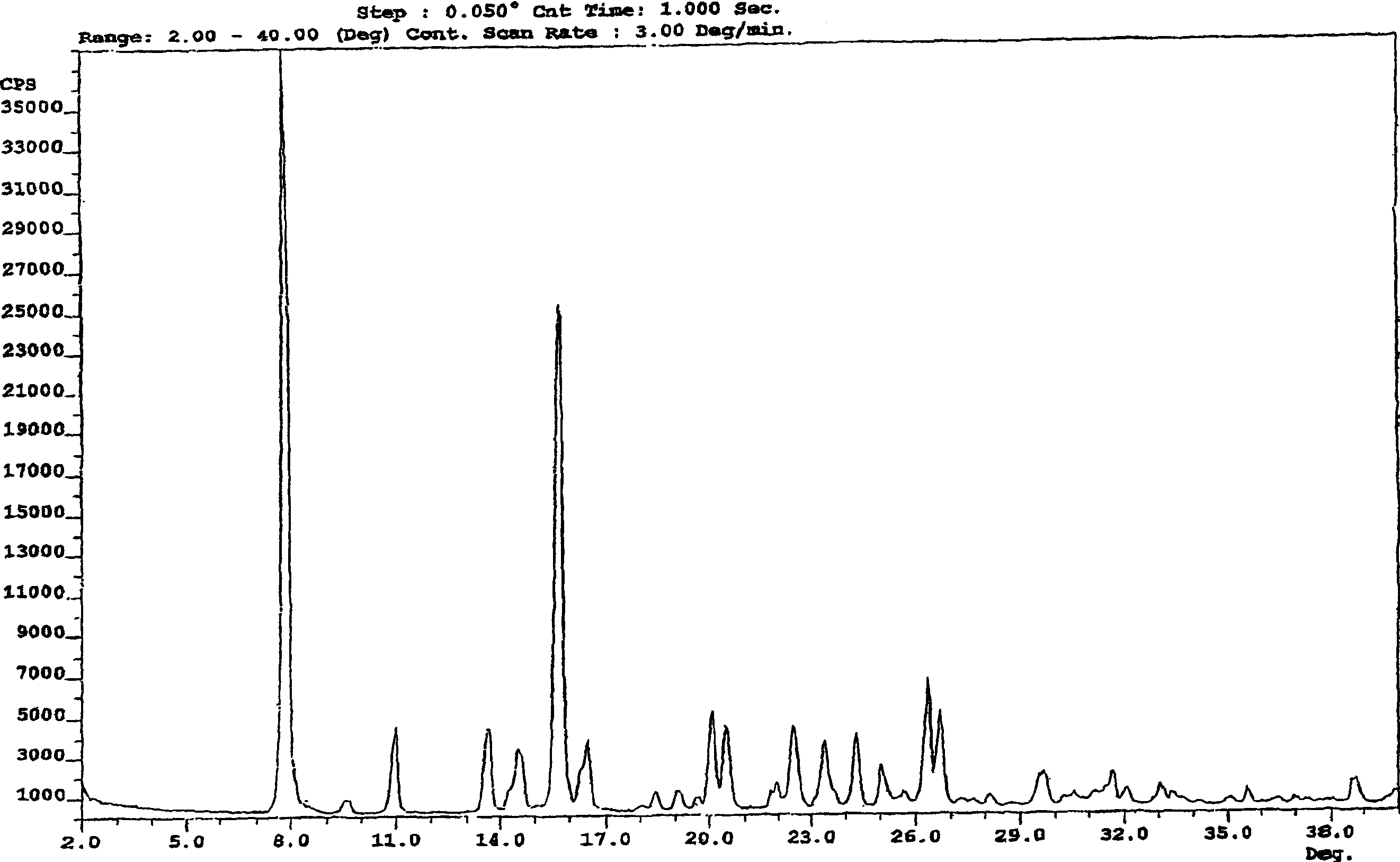
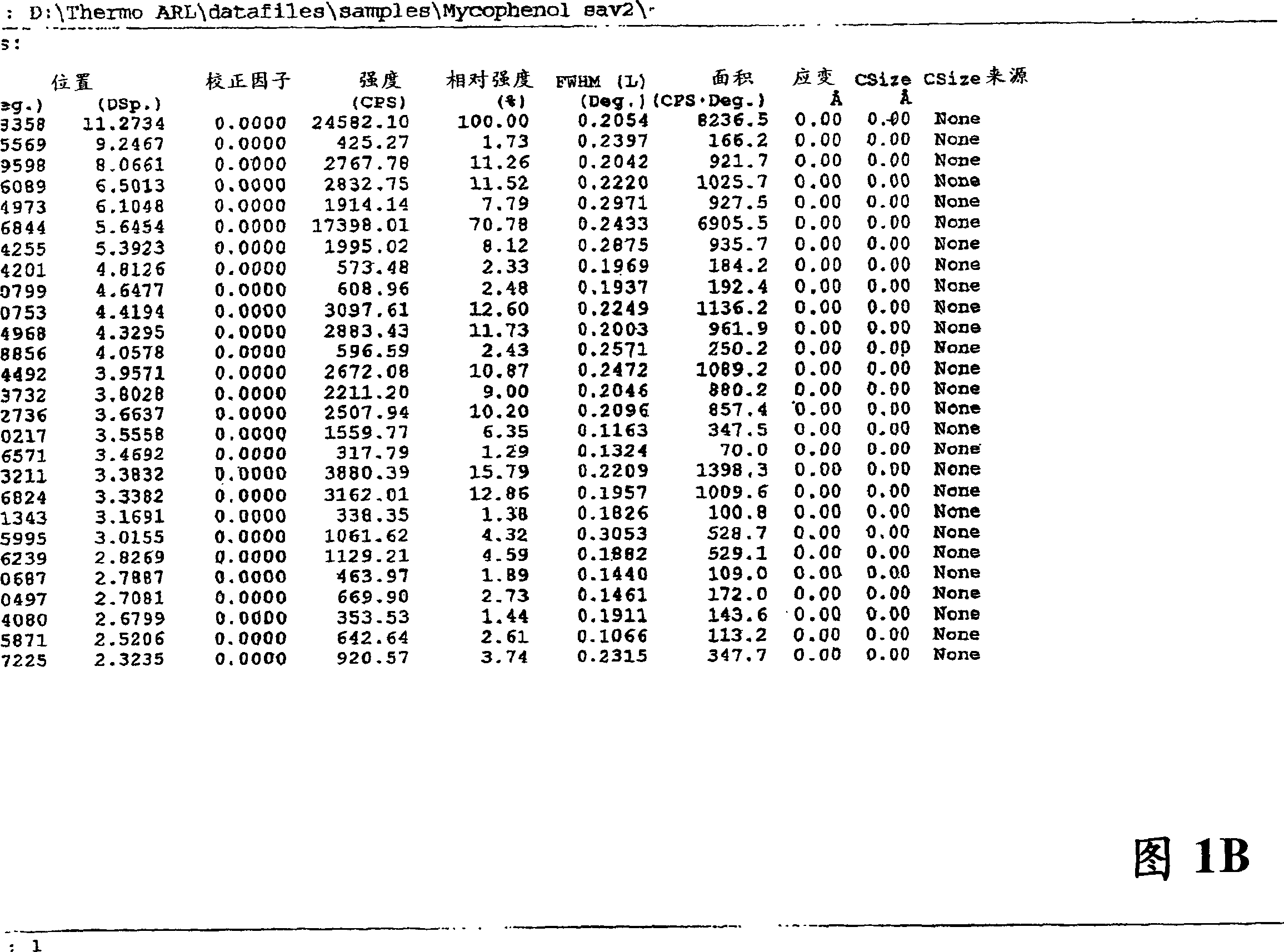
Enteric-coated preparation containing mycophenolic acid and salts thereof, and preparing method thereof
InactiveCN103845323ASimple processGood enteric propertiesOrganic active ingredientsAntipyreticAcrylic resinBULK ACTIVE INGREDIENT
A preparing method of an enteric-coated preparation containing mycophenolic acid and salts thereof is disclosed. The method is characterized in that: the method includes directly mixing an enteric coating material and active components, adding a wetting agent suitable for medicinal preparations, uniformly mixing and granulating; and the enteric coating material is acrylic resin II and / or acrylic resin III. The method simplifies processes for preparing the enteric-coated preparation, and an independent coating process is not needed. Experiments show that: the preparation has better enteric properties than enteric-coated preparations in the prior art, release of the active components is not influenced by gastric emptying and foods, and the preparation provided by the invention has faster releasing speed. The preparation is stable in medicine effect, is in a form of granules and is convenient to take.
Owner:CHONGQING HUAPONT PHARMA
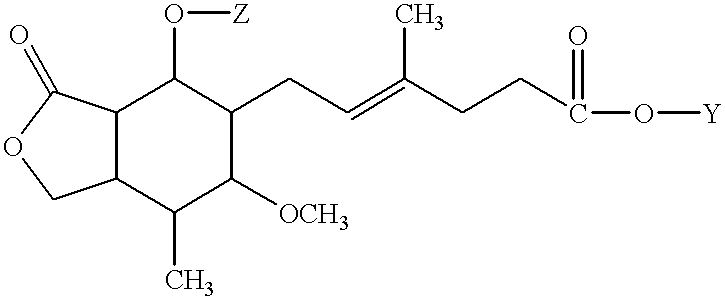
Process for preparation of mycophenolic acid and ester derivatives thereof
Owner:TEVA GYOGYSZERGYAR RESVENYTARSASAG
Phosphonate Derivatives of Mycophenolic Acid
Phosphorus substituted mycophenolate oxime derivatives with anti-cancer, anti-viral, anti-inflammatory anti-tissue / organ transplant rejection properties having use as therapeutics and for other industrial purposes are disclosed. The compositions inhibit tumor growth, viral growth, inflammation, and tissue / organ transplant rejection and / or are useful therapeutically for the treatment or prevention of cancer, viral infection, inflammation and tissue / organ transplant rejection, as well as in assays for the detection of cancer, viral infection, inflammation and tissue / organ transplant rejection.
Owner:GILEAD SCI INC
Mycophenolic acid homogeneous phase enzyme immunological detection reagent as well as preparation and detection methods thereof
ActiveCN104597238AStrong immunogen specificityStrong specificityImmunoglobulinsMaterial analysisMycophenolic acidImmunogenicity
The invention relates to a mycophenolic acid detection reagent as well as preparation and detection methods thereof and particularly relates to a mycophenolic acid homogeneous phase enzyme immunological detection reagent as well as preparation and detection methods thereof. The mycophenolic acid homogeneous phase enzyme immunological detection reagent comprises an anti-mycophenolic acid specific antibody, and an indication reagent for detecting an anti-mycophenolic acid specific antibody-mycophenolic acid compound; and the anti-mycophenolic acid specific antibody is prepared from mycophenolic acid immunogen immunological animals. The mycophenolic acid detection reagent has the beneficial effects that mycophenolic acid immunogen has strong specificity and high immunogenicity; the prepared anti-mycophenolic acid specific antibody has strong specificity and high valence and has no crossed reaction with common 62 types of medicines; the homogeneous phase enzyme immunological detection reagent containing the anti-mycophenolic acid specific antibody can be used for conveniently, rapidly and accurately determining the content of mycophenolic acid in a sample, and determining a plurality of samples on a full-automatic biochemical analyzer at the same time, so that the high-flux rapid determination for the mycophenolic acid is realized; and the accuracy is high, the specificity is strong, and the accuracy and the detection efficiency can be relatively greatly improved.
Owner:苏州博源医疗科技有限公司
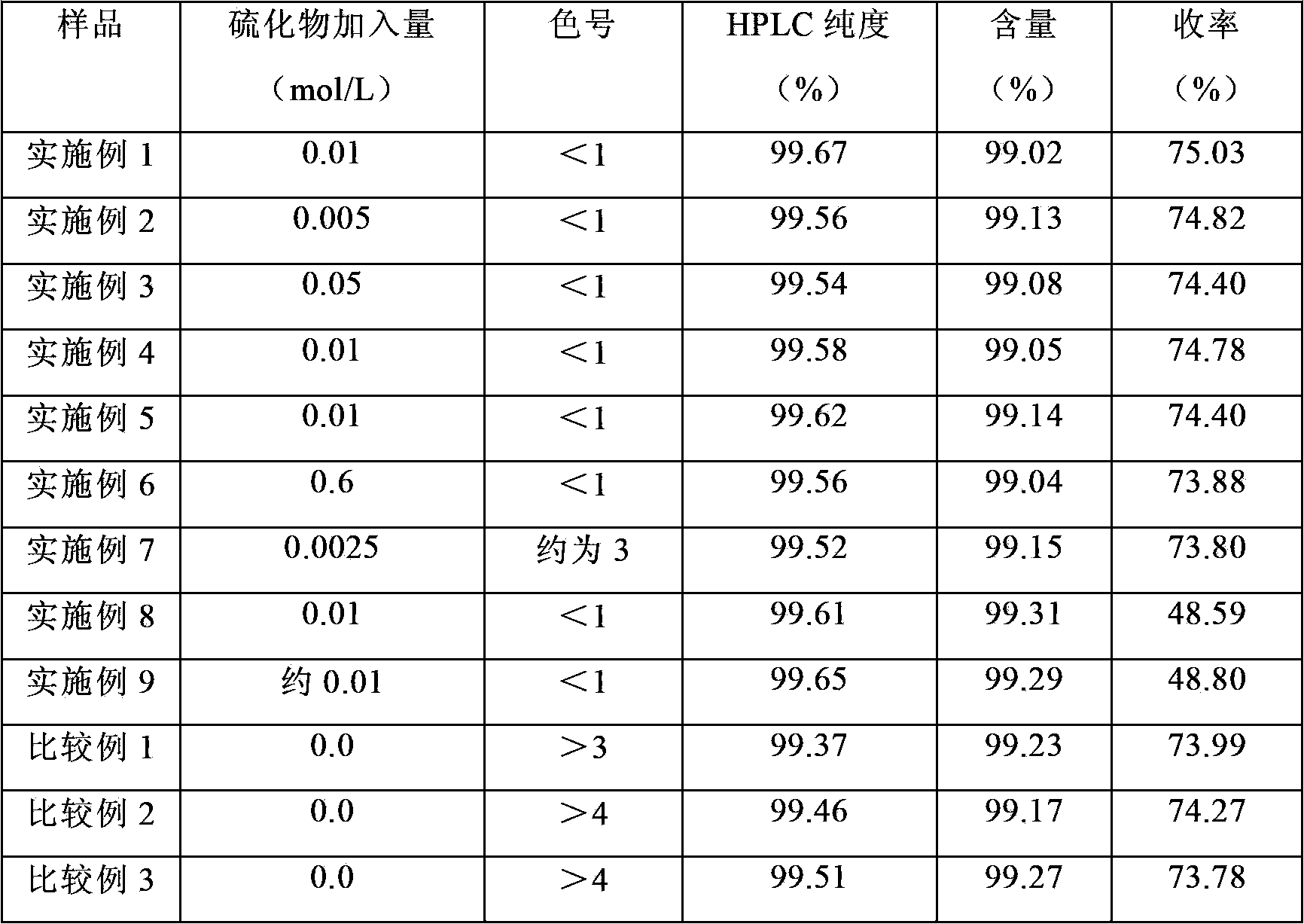
Method for extracting mycophenolic acid
ActiveCN103570655AQuality improvementNice appearanceOrganic chemistryMycophenolic acidFermentation broth
The invention relates to a method for extracting mycophenolic acid. The method comprises the step of extracting mycophenolic acid from a mycophenolic acid fermentation broth, wherein sulfide is used for inhibiting oxidation and / or complexing of the mycophenolic acid. By the method provided by the invention, the quality and condition of the mycophenolic acid product can be improved.
Owner:NEW FOUNDER HLDG DEV LLC +2
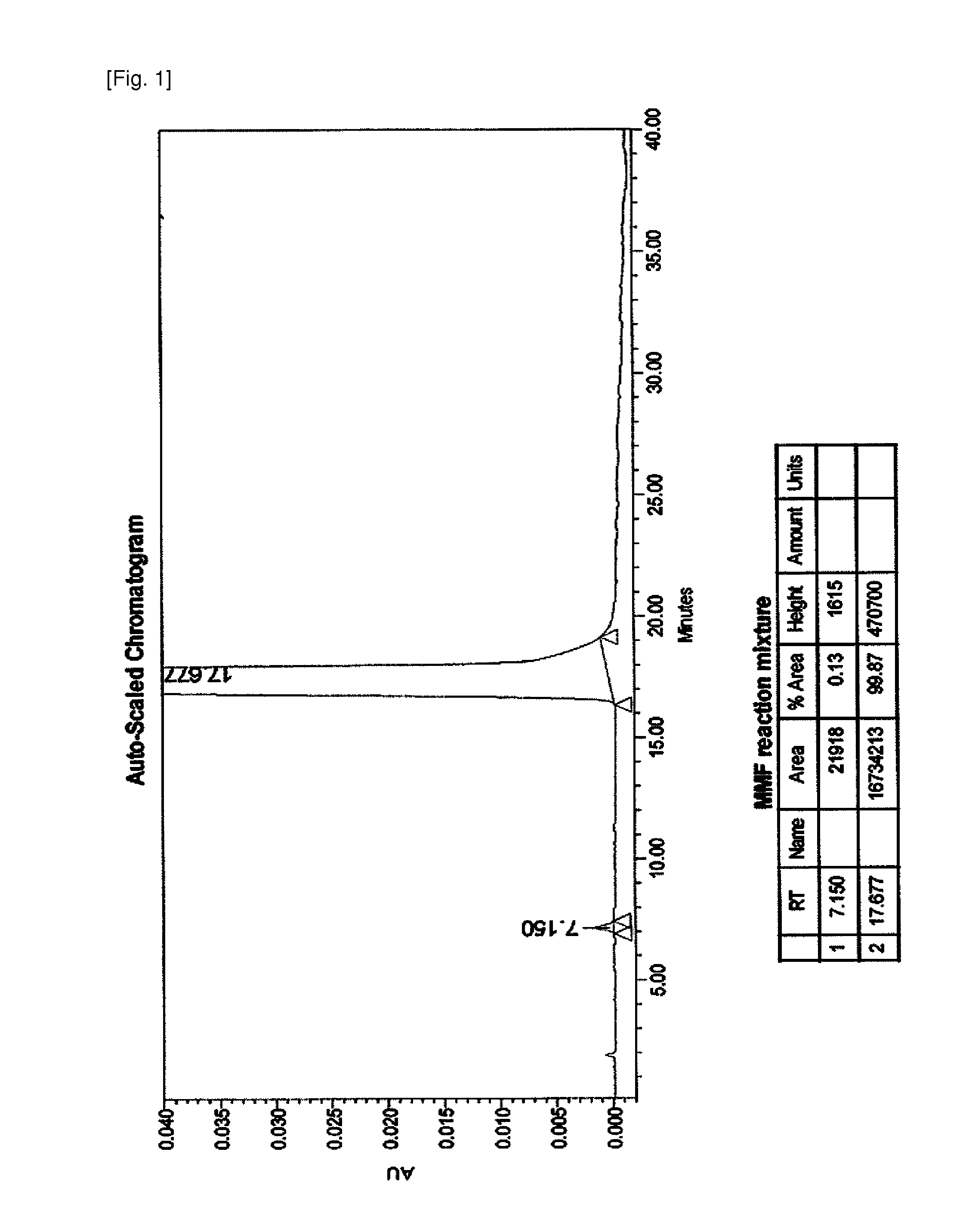
Process for preparing mycophenolate mofetil
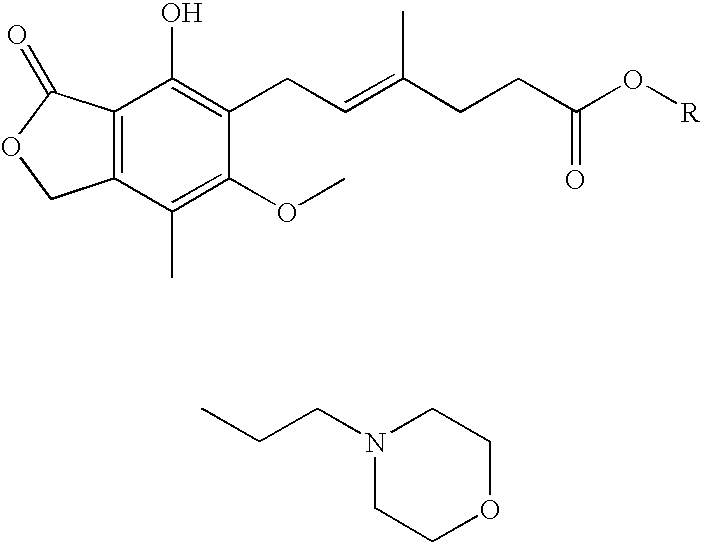
The present invention relates to an improved method of manufacturing mycophenolate mofetil. More particularly, the present invention relates to a method of manufacturing mycophenolate mofetil with high purity comprising : a) converting mycophenolate to an amine salt by reacting with an amine base; and b) reacting the resultant with a halogenating agent and 2-morpholinoethanol continuously.
Owner:CHONGKUNDANG BIO
Solid-state fermentation method of mycophenolic acid
InactiveCN101348810AWon't collapse and hardenIncrease productionMicroorganism based processesFermentationBiotechnologyDistilled water
The invention discloses a method for solid state fermentation of mycophenolic acid, comprising the following steps: firstly, subcultured penicillium is inoculated into a seed culture medium for culture and then inoculated into a secondary seed culture medium for culture, so as to obtain secondary seed solution; and secondly, 0 to 2 portions of non-inert vector and 0.2 to 3 portions of inert vector are added into a container in mass portion and uniformly mixed, and then added with distilled water so as to make the relative humidity in the container be between 65 and 95 percent; and the mixture is sterilized for 0.5 to 1.5 hours, cooled until the temperature is between 22 and 28 DEG C, inoculated with 0.2 to 2 mass portions of the secondary seed solution and cultured for 90 to 360 hours at a temperature of between 24 and 26 DEG C and with the relative humidity between 70 and 95 DEG C. The method has low energy consumption, low investment and low pollution. Moreover, the culture medium in the method can not be shrunk and hardened, thereby the method is favorable for mass transfer and heat transfer; and the inert vector can be reused, thereby reducing the discharge amount of solid waste residue and being favorable for environmental protection.
Owner:天津北洋百川生物技术有限公司
Test strip and kit for detecting mycophenolic acid, and preparation method of test strip
ActiveCN110376386AImprove stabilityQuick checkBiological material analysisBiological testingGlass fiberCellulose
The invention provides a test strip and a kit for detecting mycophenolic acid, and a preparation method of the test strip, which belong to the technical field of mycophenolic acid detection. The teststrip comprises a bottom plate, and a sample pad, a glass fiber membrane, a nitrocellulose membrane and water absorbing paper which are sequentially in overlap joint with the surface of the bottom plate, wherein the sample pad is treated with a sample pad treatment liquid; the glass fiber membrane is treated with a glass fiber membrane treatment liquid; the glass fiber membrane is coated with a mycophenolic acid specific antibody conjugate; and a detection line and a quality control line are arranged on the nitrocellulose membrane, and a mycophenolic acid protein conjugate is sprayed on the detection line. The test strip provided by the invention can quickly detect the content of mycophenolic acid in a blood sample, and has the advantages of good stability, wide linearity range, low detection cost and simple operation.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
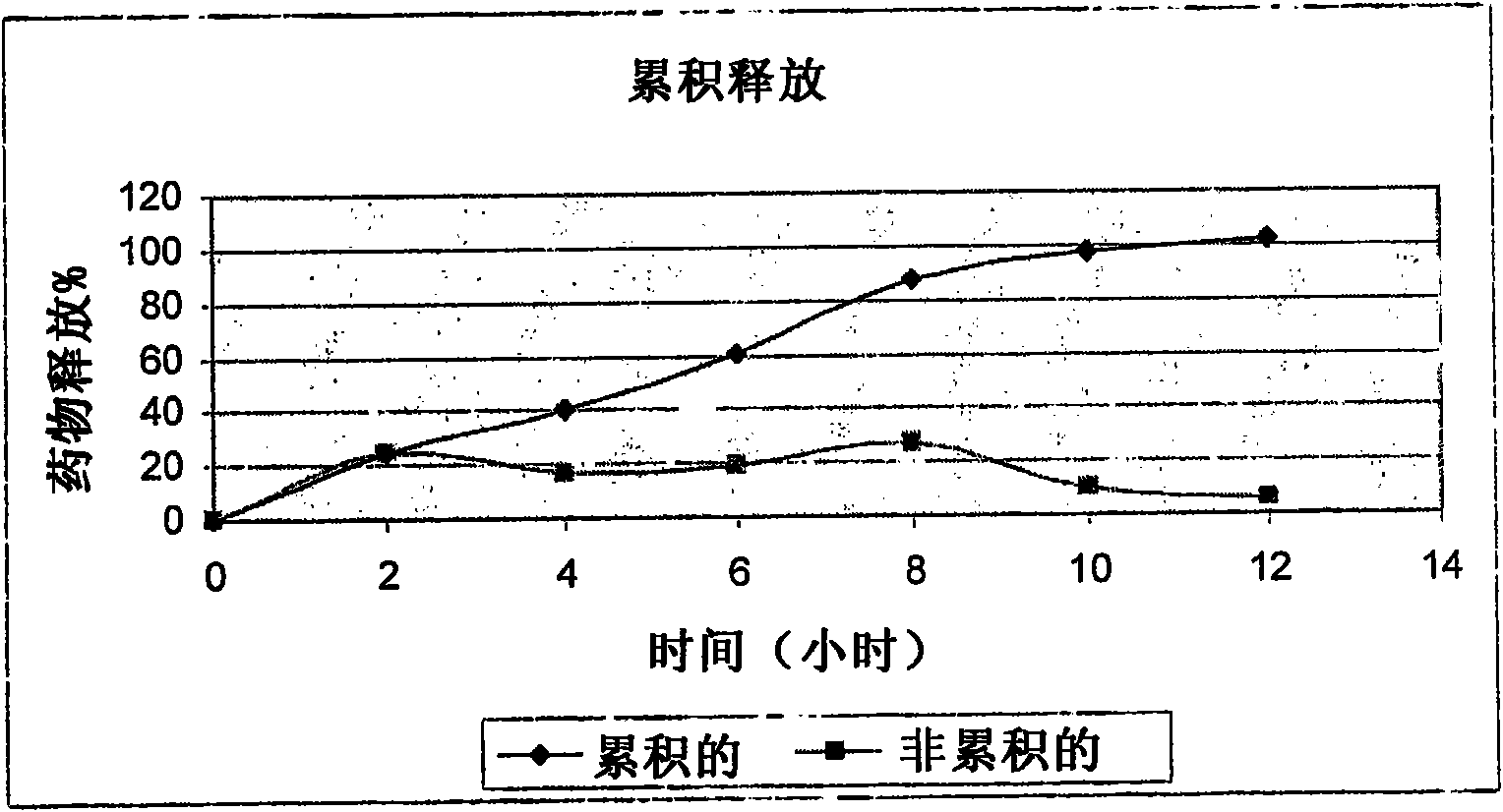
Matrix-type preparation containing mycophenolic acid or mycophenolic acid salt and coated tablet thereof
InactiveCN102793658AEfficient deliveryPrevent precipitationOrganic active ingredientsPharmaceutical delivery mechanismCoated tabletsAdhesive
The invention discloses a matrix-type preparation containing mycophenolic acid or a mycophenolic acid salt and a coated tablet thereof, and belongs to the technical field of medicinal preparations. The matrix-type preparation and the coated tablet thereof contain mycophenolic acid or a mycophenolic acid salt, and one or more pharmaceutically acceptable enteric polymers, one or more filling agents, one or more disintegrating agents and one or more lubricants and / or one or more adhesives. The matrix-type preparation containing mycophenolic acid or a mycophenolic acid salt and the coated tablet thereof can convey a drug to an upper end of the intestinal tract and release the drug so that precipitation of the drug in the stomach is avoided and bioavailability is improved.
Owner:WUXI FORTUNE PHARMA
Reagents and methods for mycophenolic acid immunoassay
ActiveUS20060035297A1Accurate methodImmunoglobulins against animals/humansDepsipeptidesAlcoholMycophenolic acid
The invention teaches derivatives of mycophenolic alcohol and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of mycophenolic acid (MPA) and / or active metabolites of MPA in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to MPA with cross-reactivity of no more than 5% with inactive metabolites and commonly co-prescribed drugs. Further, immunoassays for measuring the concentration of MPA using such antibodies are taught.
Owner:MICROGENICS CORP
Prepn process of mofe-til mycophenolate
The preparation process of mofetil mycophenolate includes the following successive steps: 1. dissolving mycophenolic acid and 2-morpyolinyl ethanol in the mixed solvent of toluene and xylene via stirring; 2. throwing in catalyst C1-6 fatty acid; 3. heating, stirring and refluxing for direct estification; 4. throwing in decolorizing agent active carbon; 5. throwing in ethyl acetate and decolorizing agent vitamin C, and washing with 10 % concentration sodium carbonate solution and water successively; and 6. heating, cooling to separate solid, washing with ethyl acetate and final vacuum drying to obtain the product. The preparation process of the present invention has short reaction time, low preparation cost, complete product decolorizing, yield up to 83 % and product purity over 99.6 %.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Manufacture and purification of mycophenolic acid
InactiveUS6927047B1Moisture loss because of passage of sterile air is significantly reducedPromote growthMicroorganism based processesFermentationMycophenolic acidSolid substrate
The present invention provides an improved method for the manufacture of Mycophenolic acid by solid substrate fermentation of Pencillium brevi-campactum, in a contained bioreactor under optimal fermentation parameters with its subsequent purification steps.
Owner:BIOCON LTD
Process for preparation of mycophenolate mofetil and other esters of mycophenolic acid
InactiveUS20050250773A1BiocideOrganic compounds purification/separation/stabilisationMycophenolic acidMedicinal chemistry
Provided are processes for the preparation of mycophenolate mofetil and other esters of mycophenolic acid.
Owner:TEVA PHARM USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com