Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
A composition, the technology of mycophenolic acid, applied in the field of modified release pharmaceutical composition, can solve the problems of increasing toxicity and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
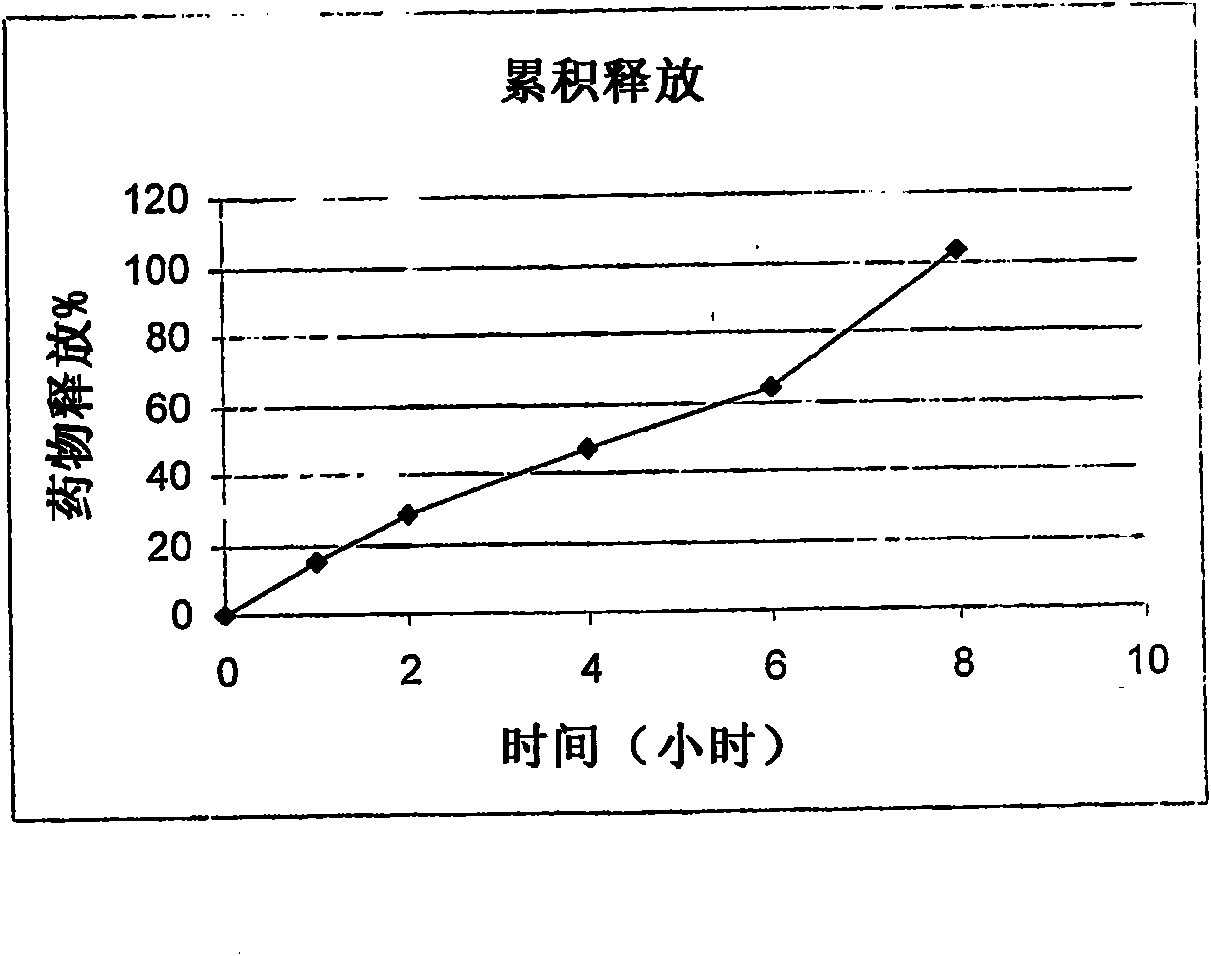
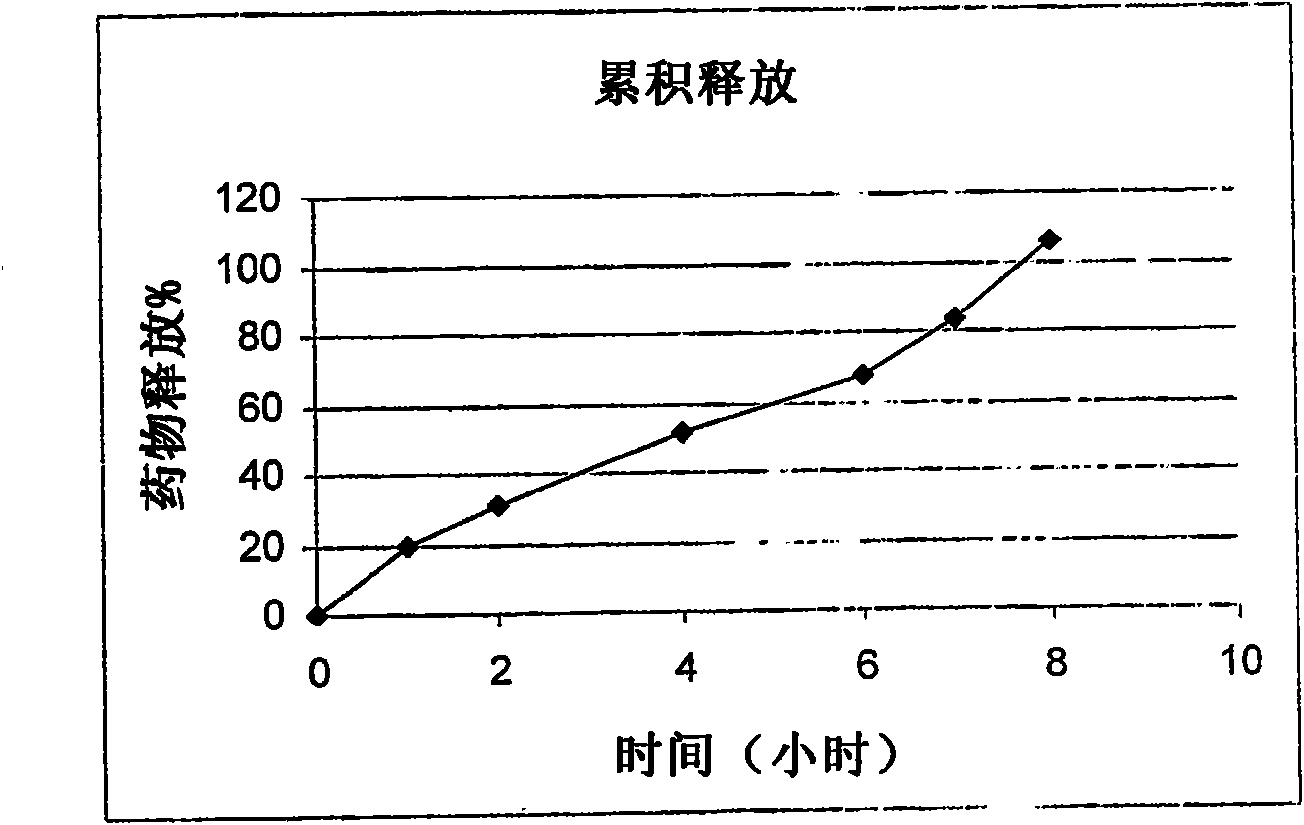
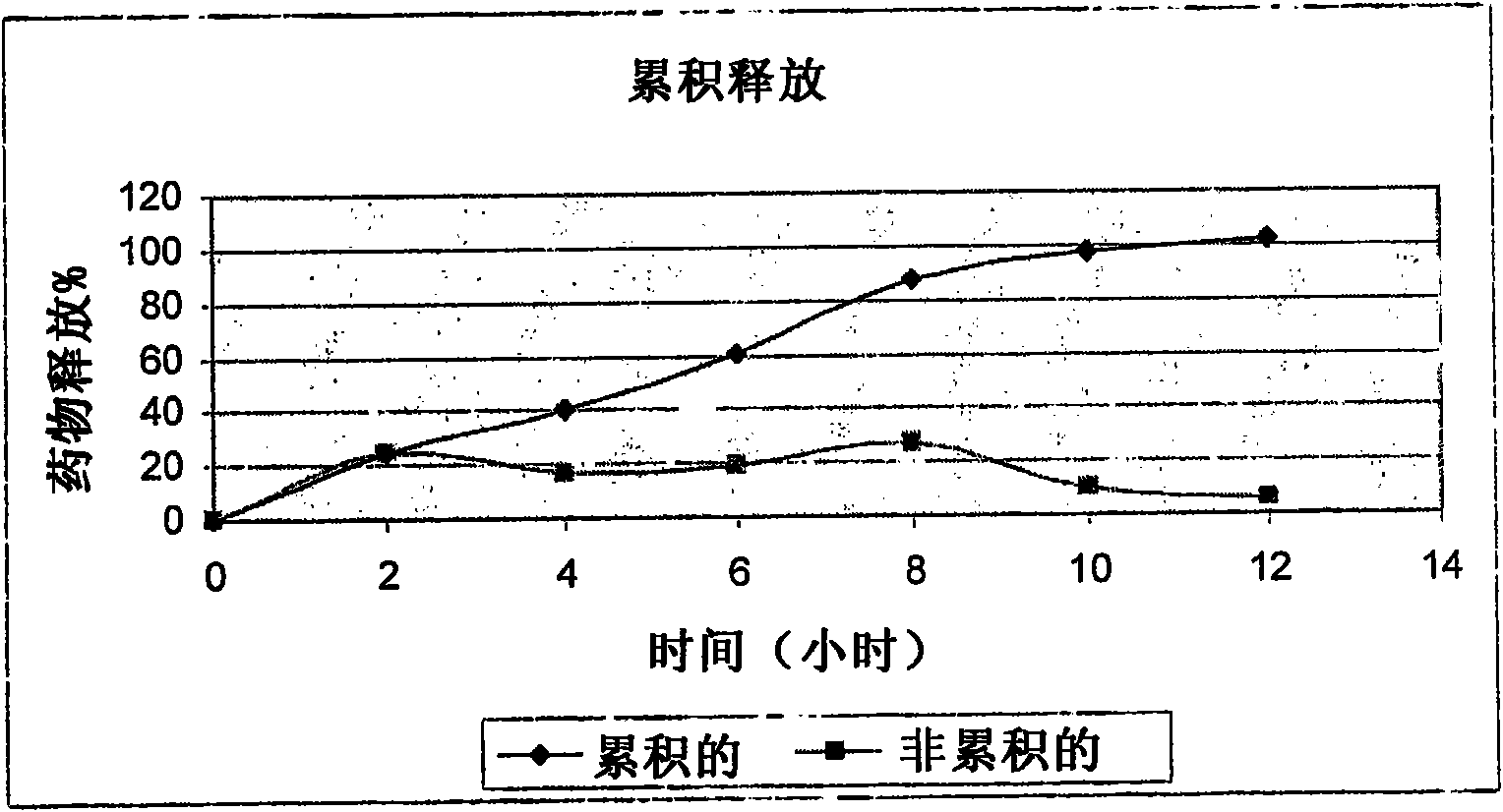
Examples
Embodiment 3
[0088] 0 0.000
[0089] 0.33 5.202
[0090] 0.5 7.198
[0091] 0.67 6.736
[0092] 0.833 7.106
[0093] 1 6.619
[0094] 1.25 5.111
[0095] 1.5 4.941
[0096] 2 4.137
[0097] 2.5 3.761
[0098] 3 3.030
[0099] 3.5 2.649
[0100] 4 2.312
[0101] 4.5 3.109
[0102] 5 4.166
[0103]5.5 3.740
[0104] 6 2.816
[0105] 6.5 2.140
[0106] 7 1.753
[0107] 7.5 1.556
[0108] 8 1.585
[0109] 9 1.445
[0110] 10 1.283
[0111] 11 1.444
[0112] 12 1.413
[0113] 14 1.237
[0114] 16 1.102
[0115] 18 0.817
[0116] 22 0.972
[0117] 24 0.764
[0118] AUC of the compositions mentioned in Example 3 below 0-12 with AUC 12-24 The result of the ratio is as follows:
[0119] AUC 0-12 AUC 12-24 Ratio (AUC 0-12 : AUC 12-24 )
[0120] Average 26.51 11.671 2.27:1
Embodiment 1
[0121] Example 1: (80:20)SR:IR
[0122] Substance No. Composition Amount / tablet (mg)
[0123] Sustained Release (SR) layer
[0124] 1. Sodium mycophenolate mofetil (equivalent to 576mg mycophenolic acid) 630.98
[0125] 2. Lactose DCL21 5.5
[0126] 3. Aerosil 200 150.02
[0127] 4. Polyvinylpyrrolidone (PVP K-90) 55
[0128] 5. Hypromellose 55
[0129] 6. Polyethylene oxide (Polyox WSR 301) 110
[0130] 7. Polyvinylpyrrolidone (PVP K-30) 27.5
[0131] 8. Sufficient amount of isopropanol (lost during processing)
[0132] 9. Magnesium stearate 10.5
[0133] Immediate Release (IR) layer
[0134] 10. Sodium mycophenolate mofetil (equivalent to 148mg mycophenolate mofetil) 158.3
[0135] 11. Microcrystalline cellulose 44
[0136] 12. Polyvinylpyrrolidone (PVP K-30) 2.2
[0137] 13. Sufficient amount of isopropanol (lost during processing)
[0138] 14. Magnesium stearate 2.2
[0139] step:
[0140] i) Sodium mycophenolate mofetil, anhydrous lactose, colloidal silic...
Embodiment 2
[0157] Example 2: (80:20)SR:IR
[0158] Substance No. Composition Amount / tablet (mg)
[0159] Sustained Release (SR) layer
[0160] 1. Sodium mycophenolate mofetil (equivalent to 576mg mycophenolic acid) 630.98
[0161] 2. Lactose DCL21 5.5
[0162] 3. Aerosil 200 150.02
[0163] 4. Polyvinylpyrrolidone (PVP K-90) 55
[0164] 5. Hypromellose 55
[0165] 6. Polyethylene oxide (Polyox WSR 301) 110
[0166] 7. Polyvinylpyrrolidone (PVP K-30) 27.5
[0167] 8. Sufficient amount of isopropanol (lost during processing)
[0168] 9. Magnesium stearate 10.5
[0169] Immediate Release (IR) layer
[0170] 10. Sodium mycophenolate mofetil (equivalent to 148mg mycophenolate mofetil) 158.3
[0171] 11. Starch 1500 42
[0172] 12. Succinic acid 22
[0173] 13. Polyvinylpyrrolidone (PVP K-30) 2.5
[0174] 14. Sufficient amount of isopropanol (lost during processing)
[0175] 15. Magnesium stearate 2.5
[0176] step:
[0177] i) Sodium mycophenolate mofetil, anhydrous lactose, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com