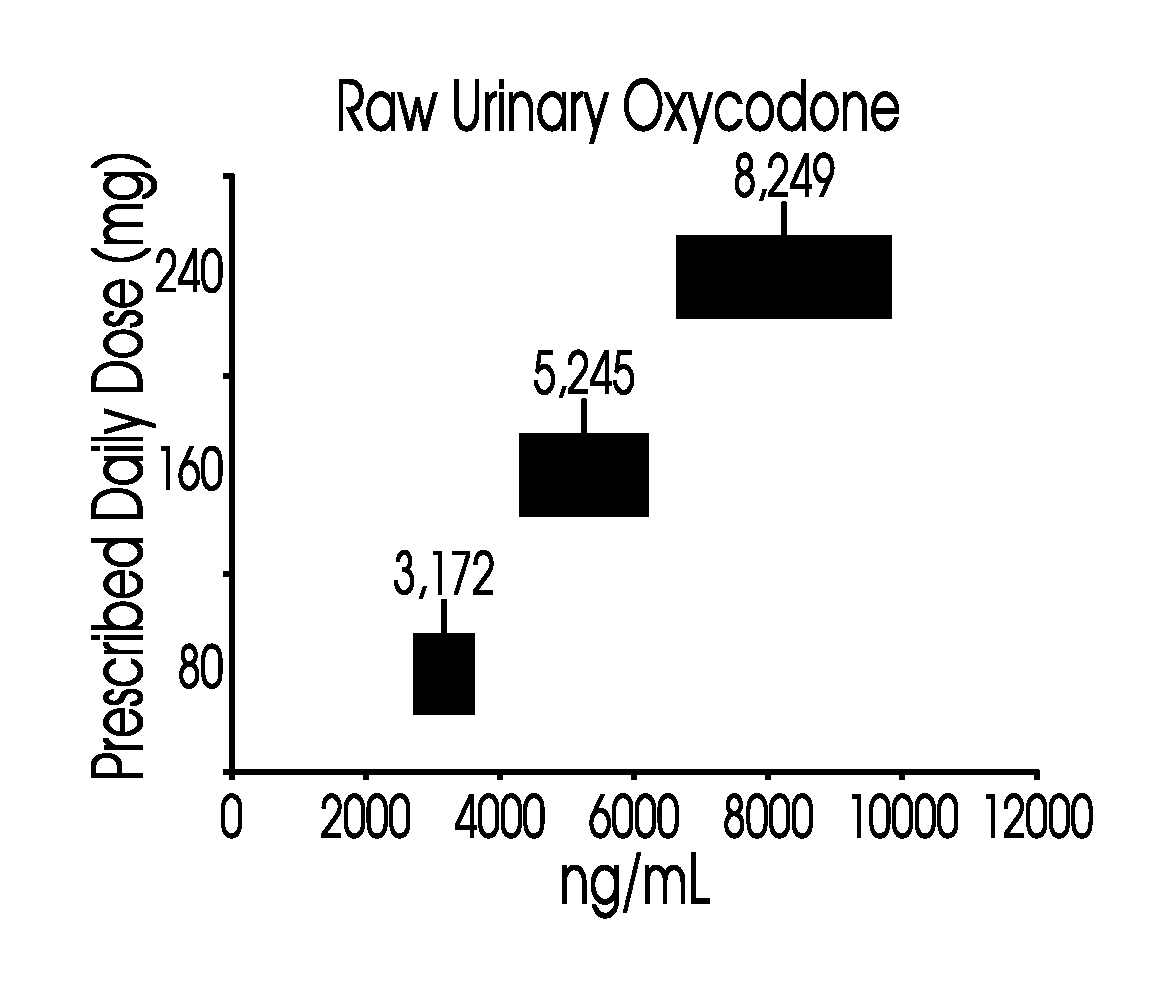
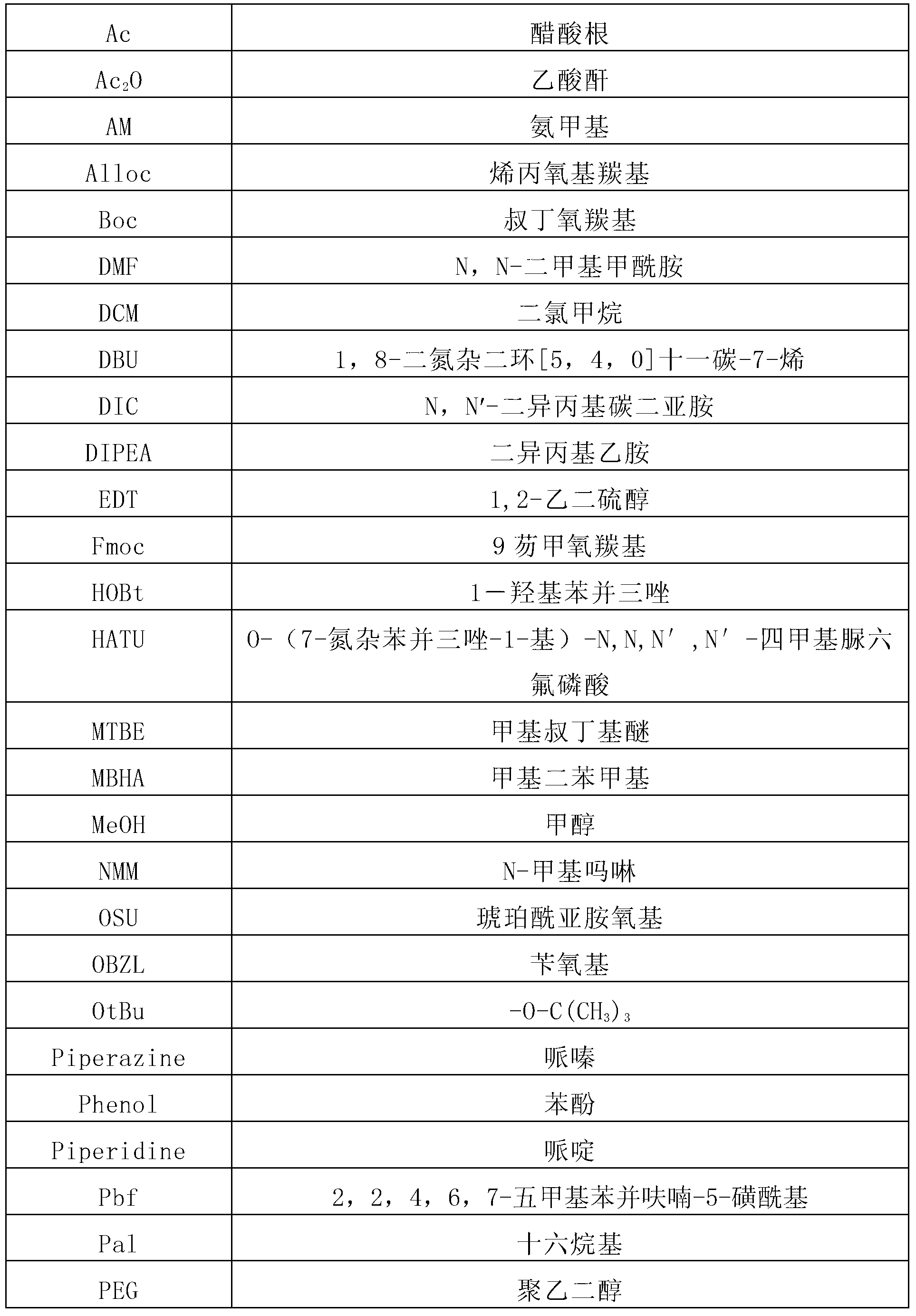
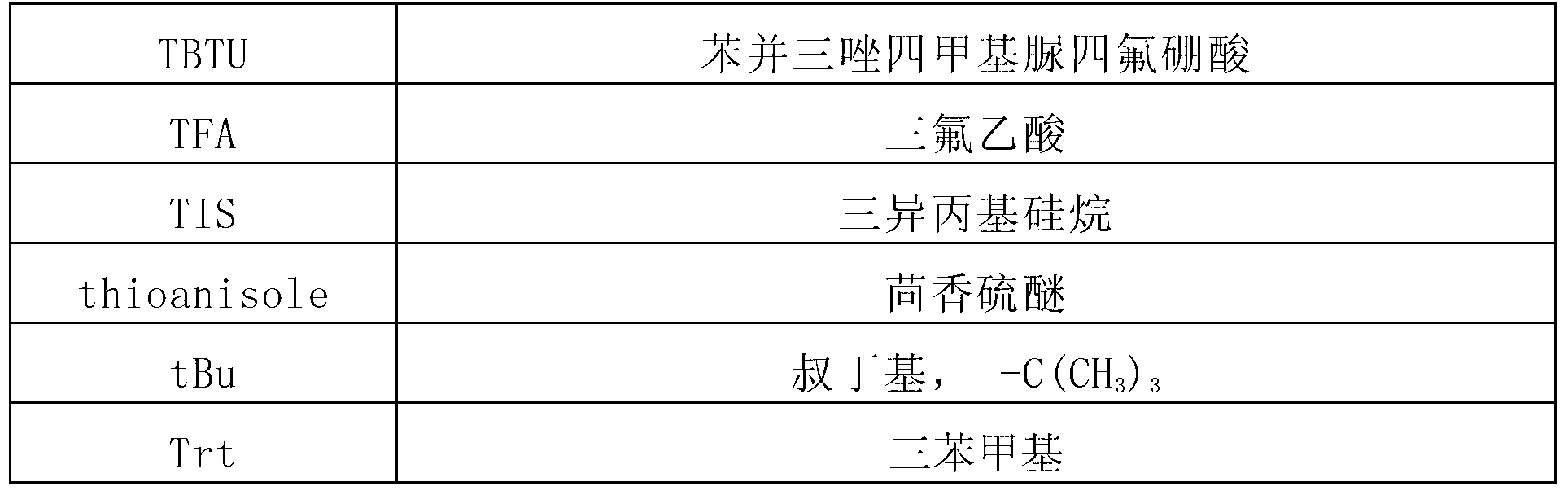
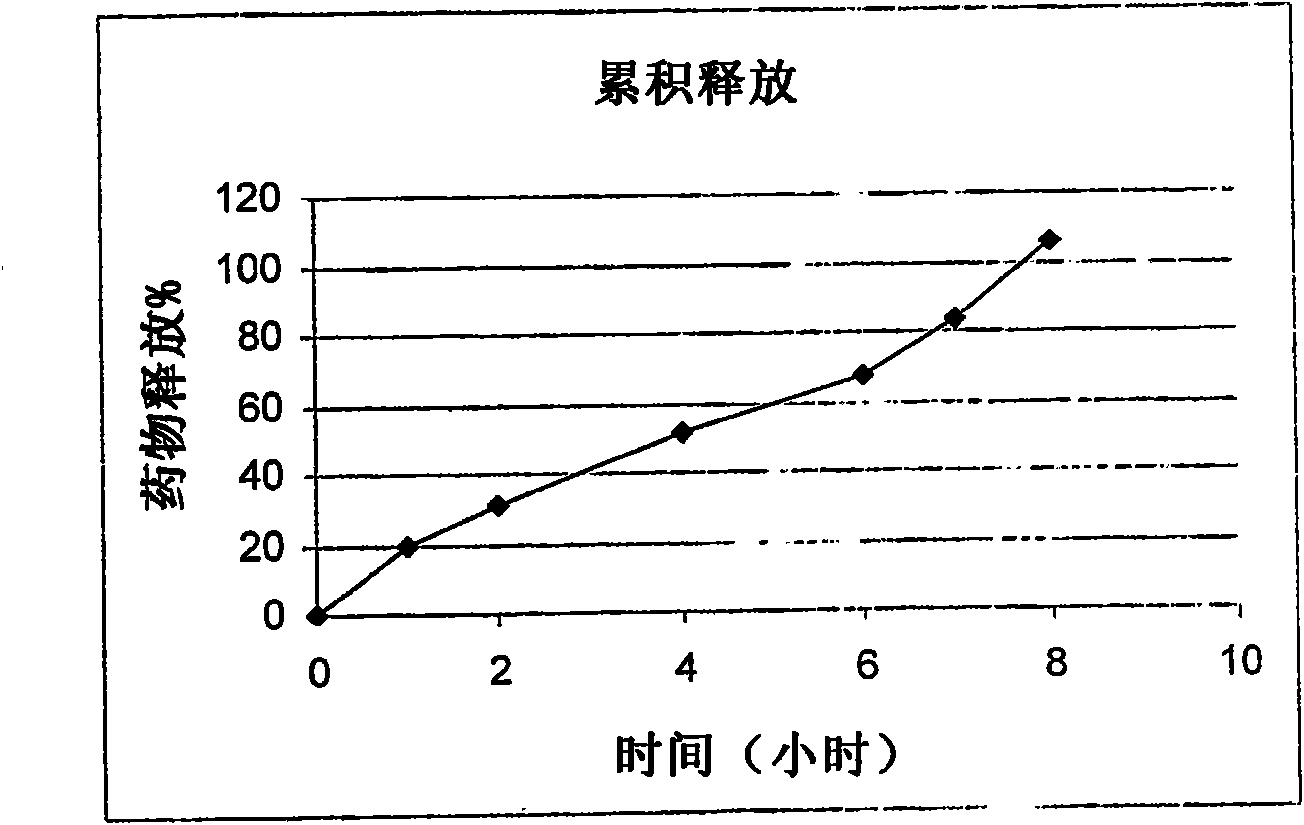
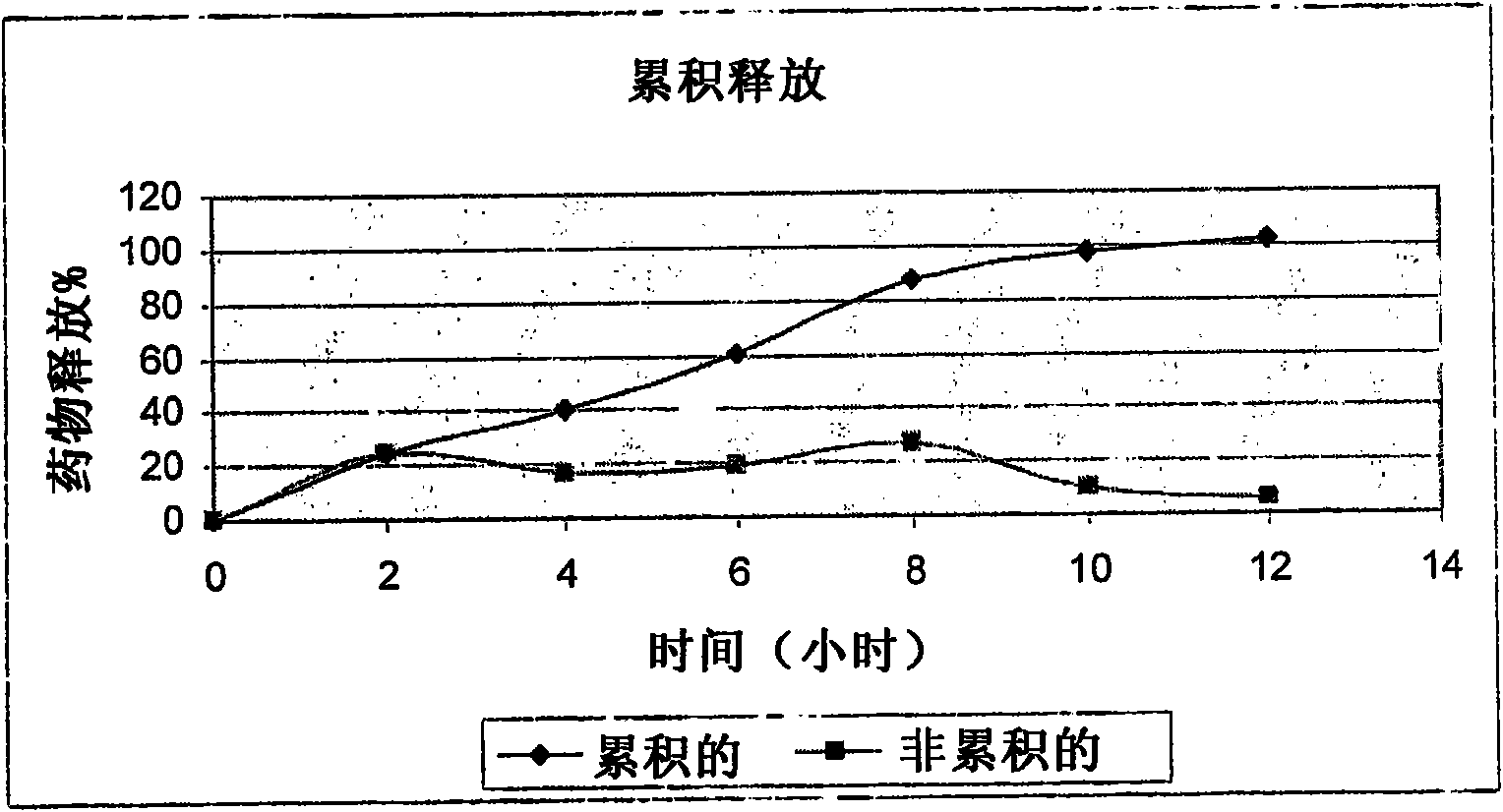
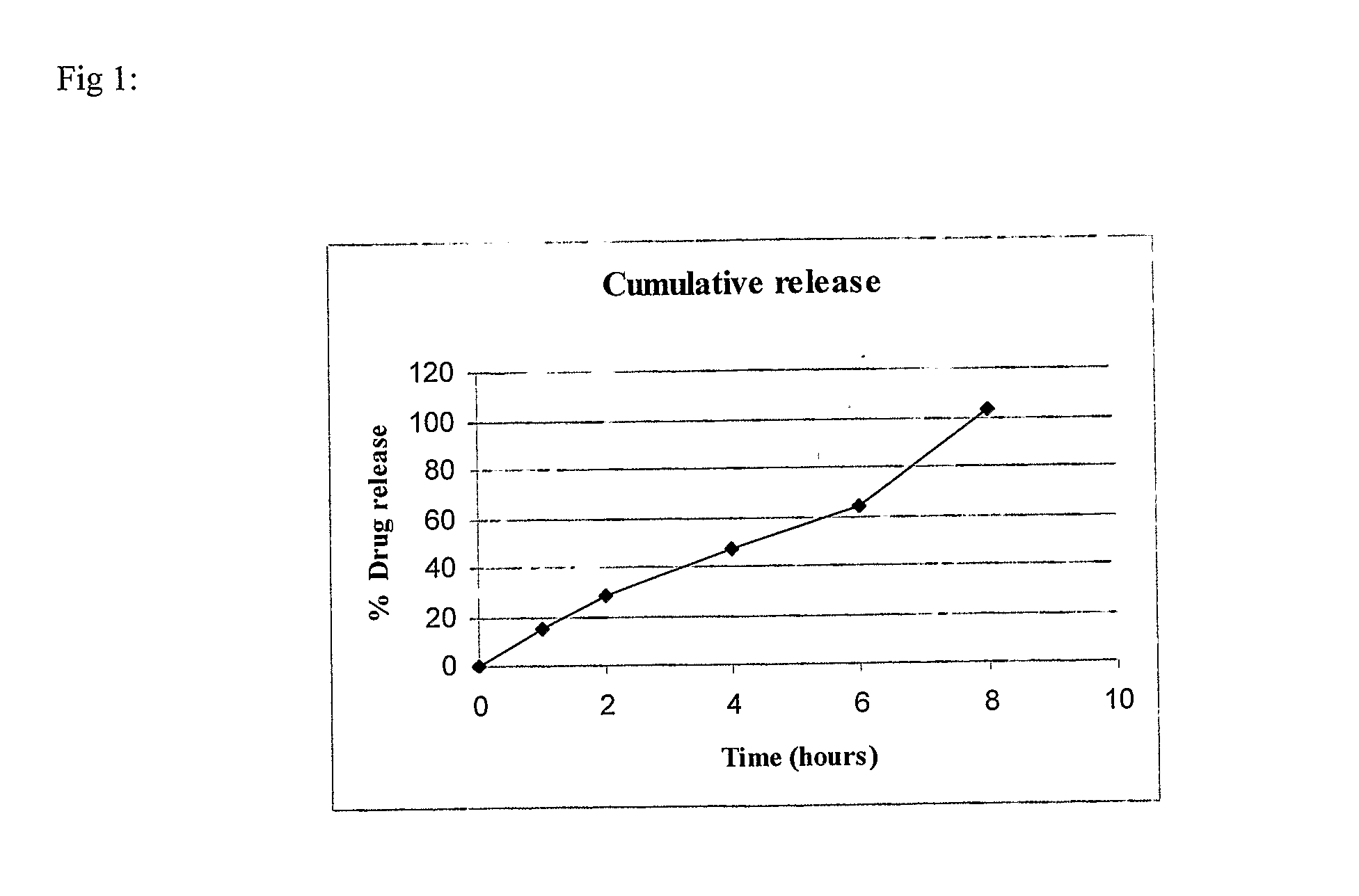
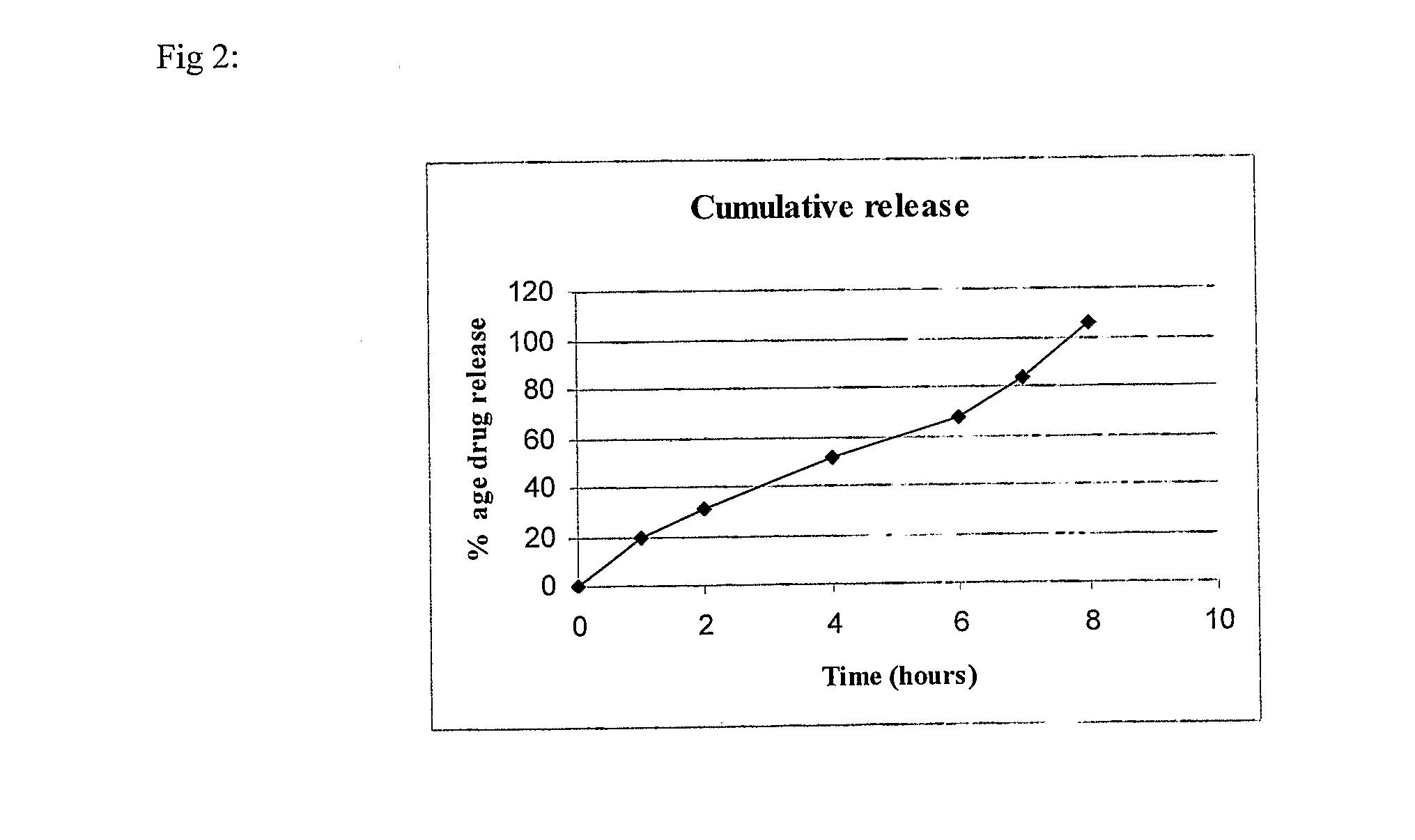
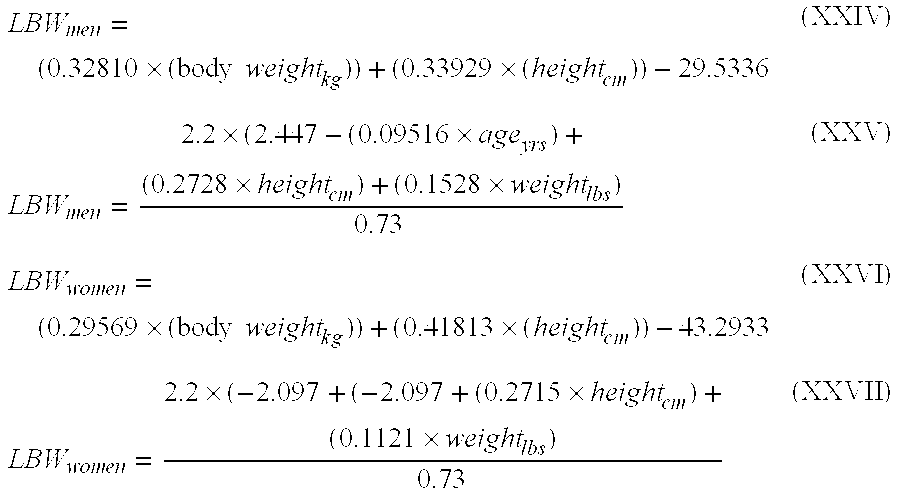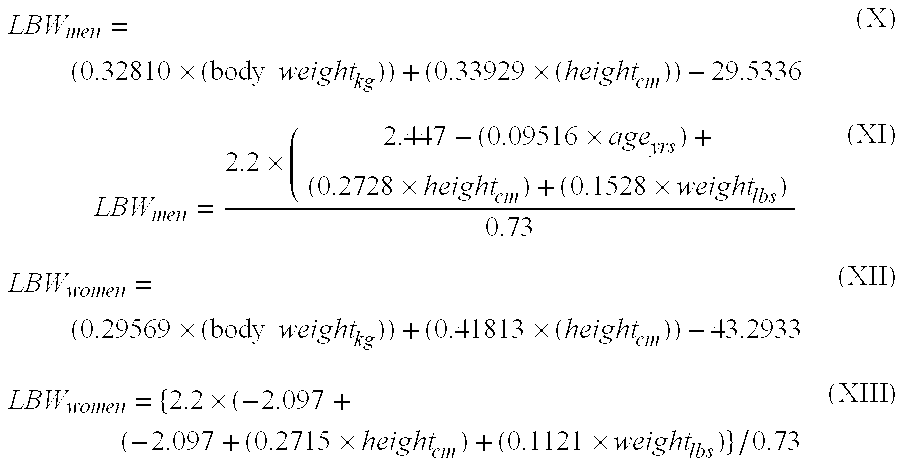Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Drugs levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of normalizing measured drug concentrations and testing for non-compliance with a drug treatment regimen
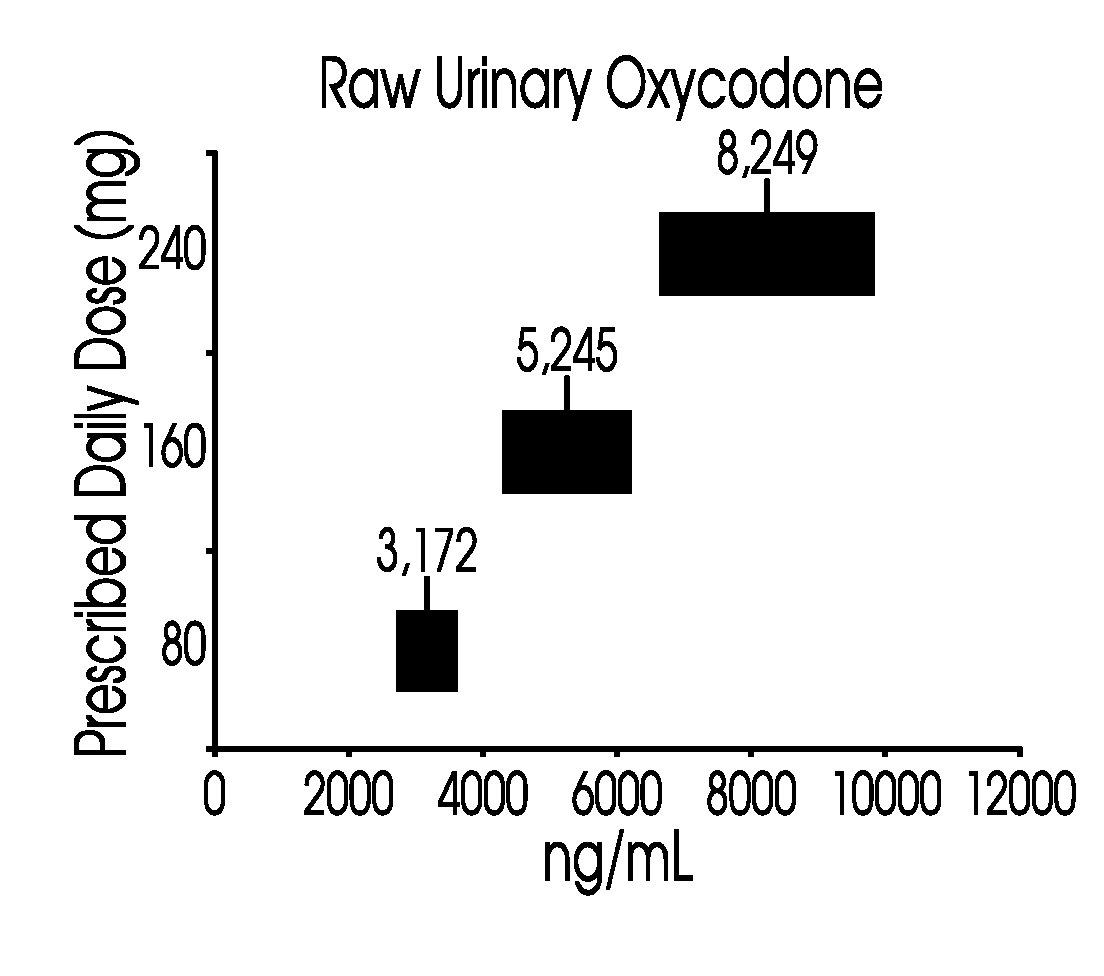
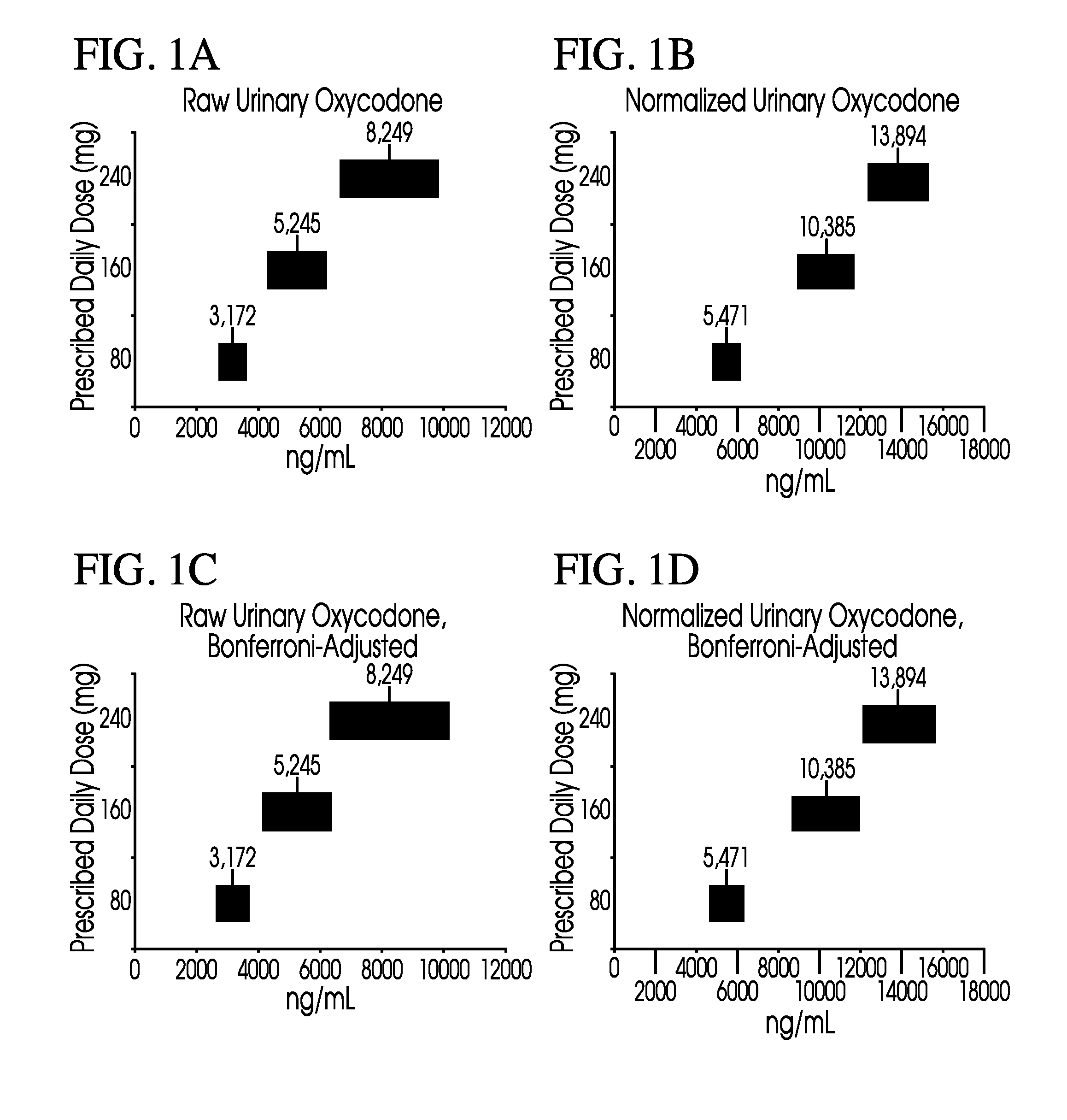
Methods for monitoring subject compliance with a prescribed treatment regimen are disclosed. In one embodiment, the method comprises measuring a drug level in fluid of a subject and normalizing said measured drug level as a function of one or more parameters associated with the subject. The normalized drug level is compared to a reference value and associated confidence intervals or to a concentration range. The reference value and associated confidence intervals and / or the concentration range may be normalized based on one or more parameters associated with subjects in a reference population.
Owner:AMERITOX LLC
Synthetic method of liraglutide
ActiveCN103304660AHigh selectivityHigh purityPeptide preparation methodsBulk chemical productionChemical synthesisDrugs levels
The invention discloses a full chemical synthetic method for hybridization of a solid phase and a liquid phase of liraglutide. The method comprises the following steps: chemically synthesizing a liraglutide precursor protected by N terminal and a cetyl derivative; de-protecting to remove tail end protection to obtain a target polypeptide. The liraglutide precursor semi-protected is obtained by polypeptide solid-phase synthesis, and the precursor purified to the drug level enters into the next chemical synthesis.
Owner:SHANGHAI AMBIOPHARM
Systems and methods for providing gastrointestinal pain management
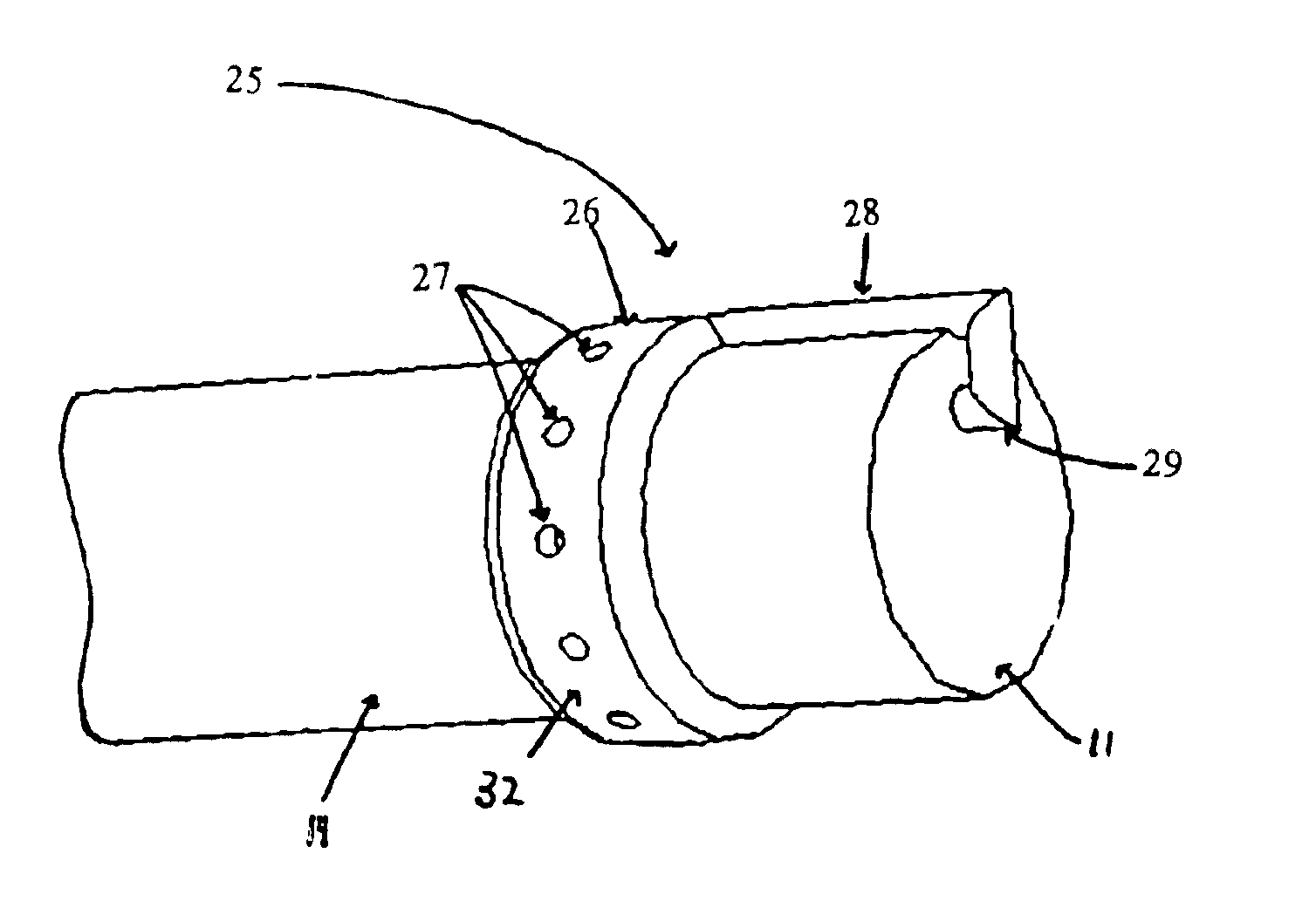
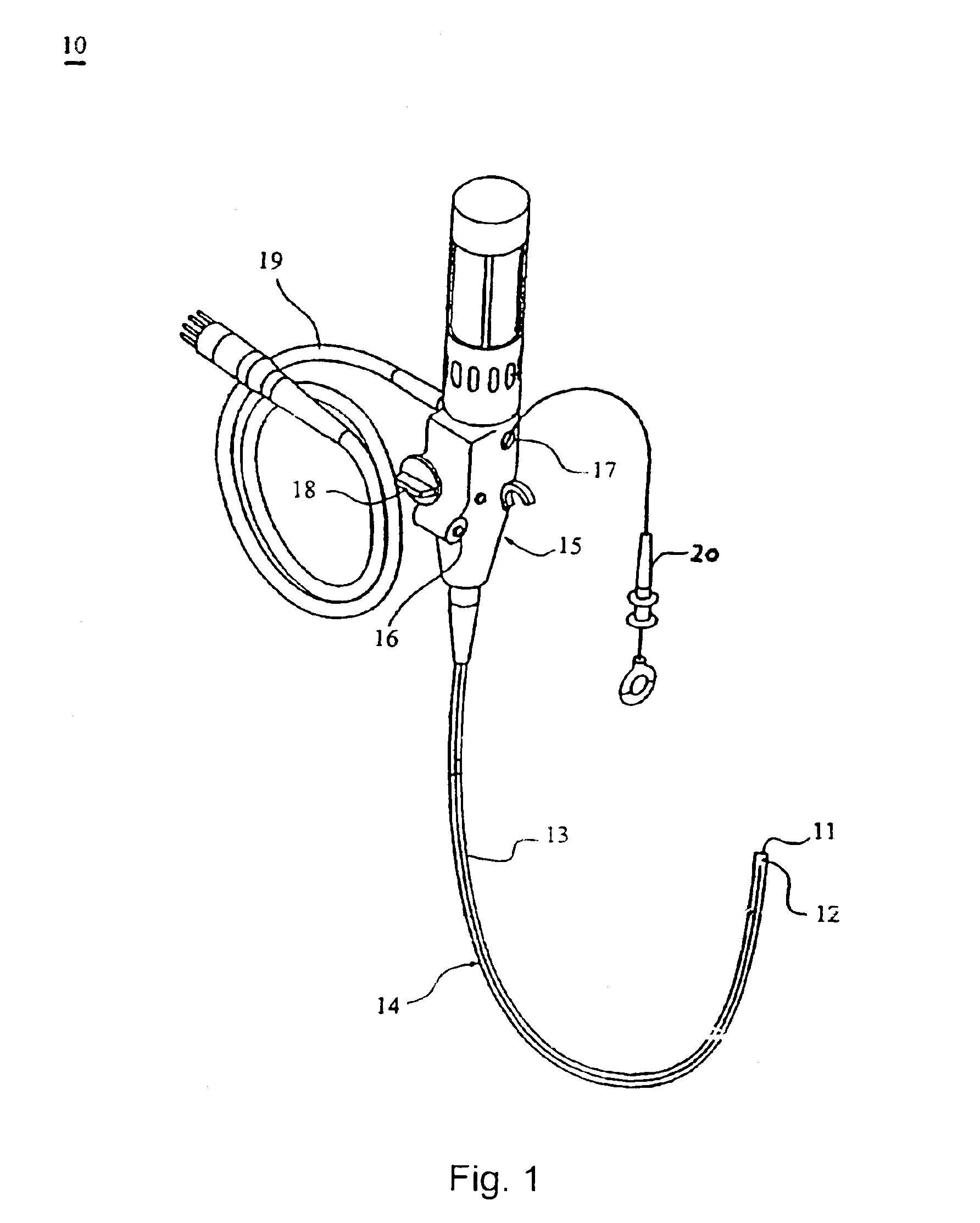
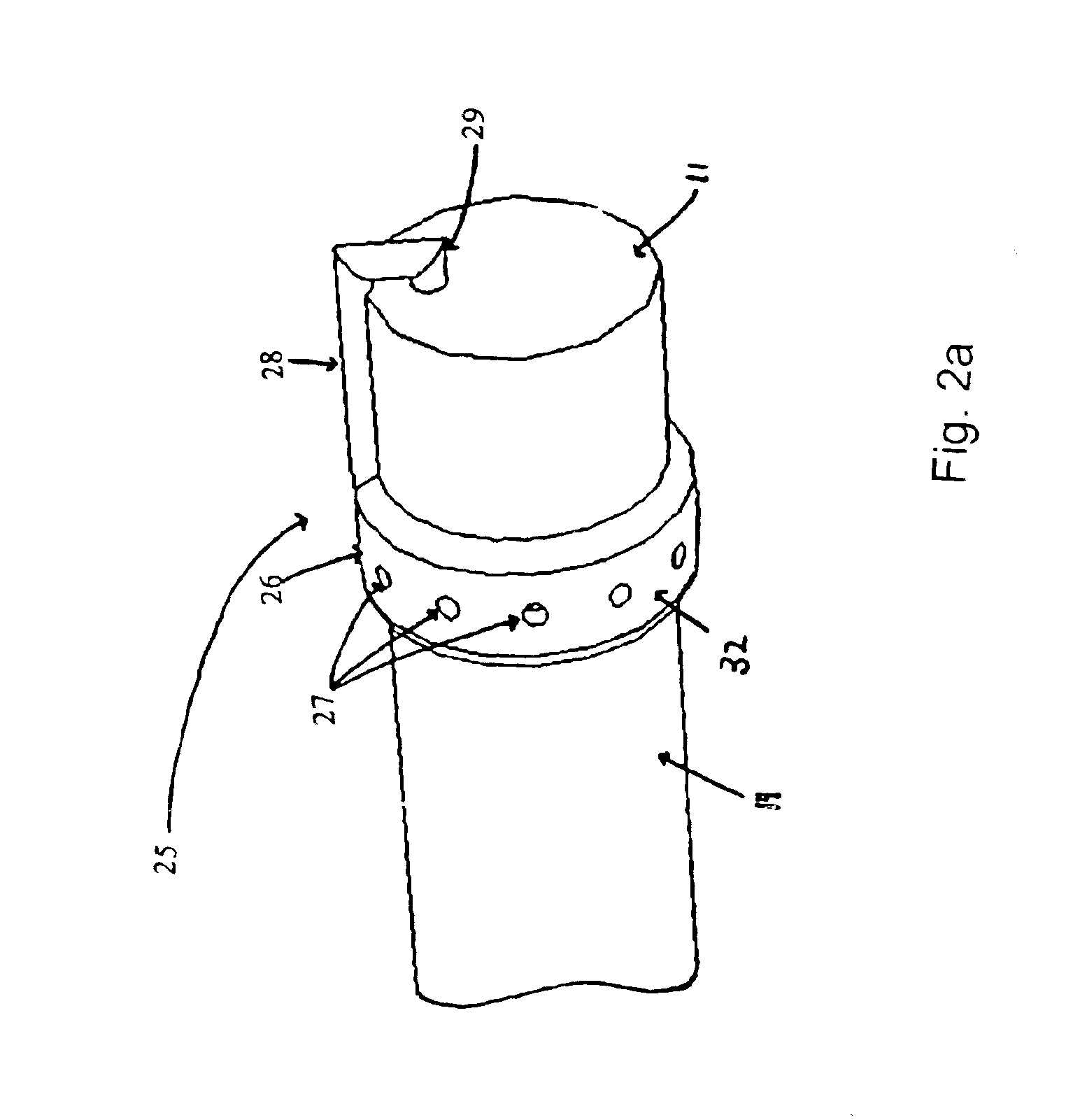
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
InactiveCN101969931AMaintain therapeutic effectEasy to solveOrganic active ingredientsPill deliveryDrugs levelsActive agent
Modified release pharmaceutical compositions comprising mycophenolate as the active agent or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof, wherein the said composition exhibits a biphasic release profile when subjected to in- vitro dissolution and / or upon administration in- vivo are provided. The composition provides a drug release in a manner such that the drug levels are maintained above the therapeutically effective concentration (EC) constantly for an extended duration of time. Further, the difference between the maximum plasma concentration of the drug (Cmax) and the minimum plasma concentration of the drug (Cmjn), and in turn the flux defined as ((Cmax - Cmjn) / Cavg) is minimal. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such compositions.
Owner:PANACEA BIOTEC
Modified release pharmaceutical compositions comprising mycophenolate and processes thereof
InactiveUS20110008426A1Easy and cost-effectiveImprove the level ofBiocidePowder deliveryDrugs levelsActive agent
Modified release pharmaceutical compositions comprising mycophenolate as the active agent or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof, wherein the said composition exhibits a biphasic release profile when subjected to in-vitro dissolution and / or upon administration in-vivo are provided. The composition provides a drug release in a manner such that the drug levels are maintained above the therapeutically effective concentration (EC) constantly for an extended duration of time. Further, the difference between the maximum plasma concentration of the drug (Cmax) and the minimum plasma concentration of the drug (Cmjn), and in turn the flux defined as ((Cmax−Cmjn) / Cavg) is minimal. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such compositions.
Owner:PANACEA BIOTEC
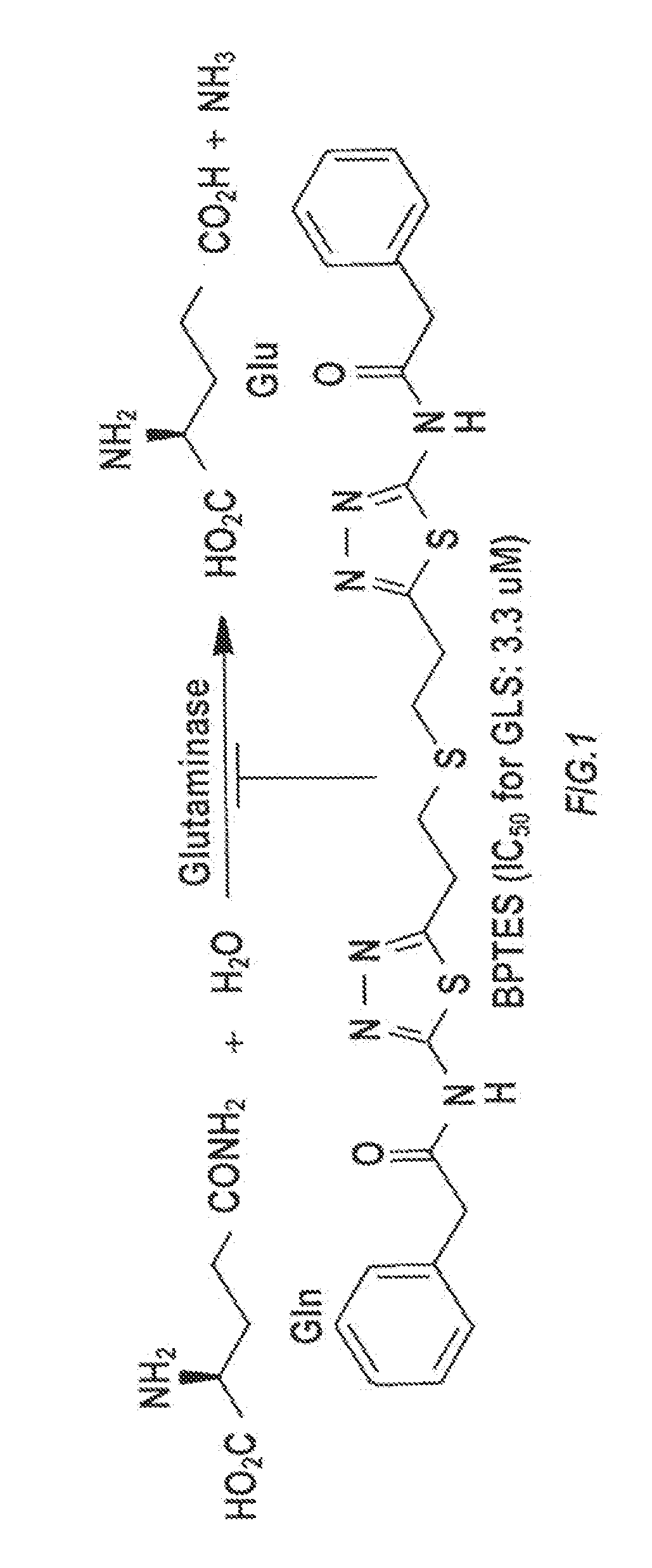
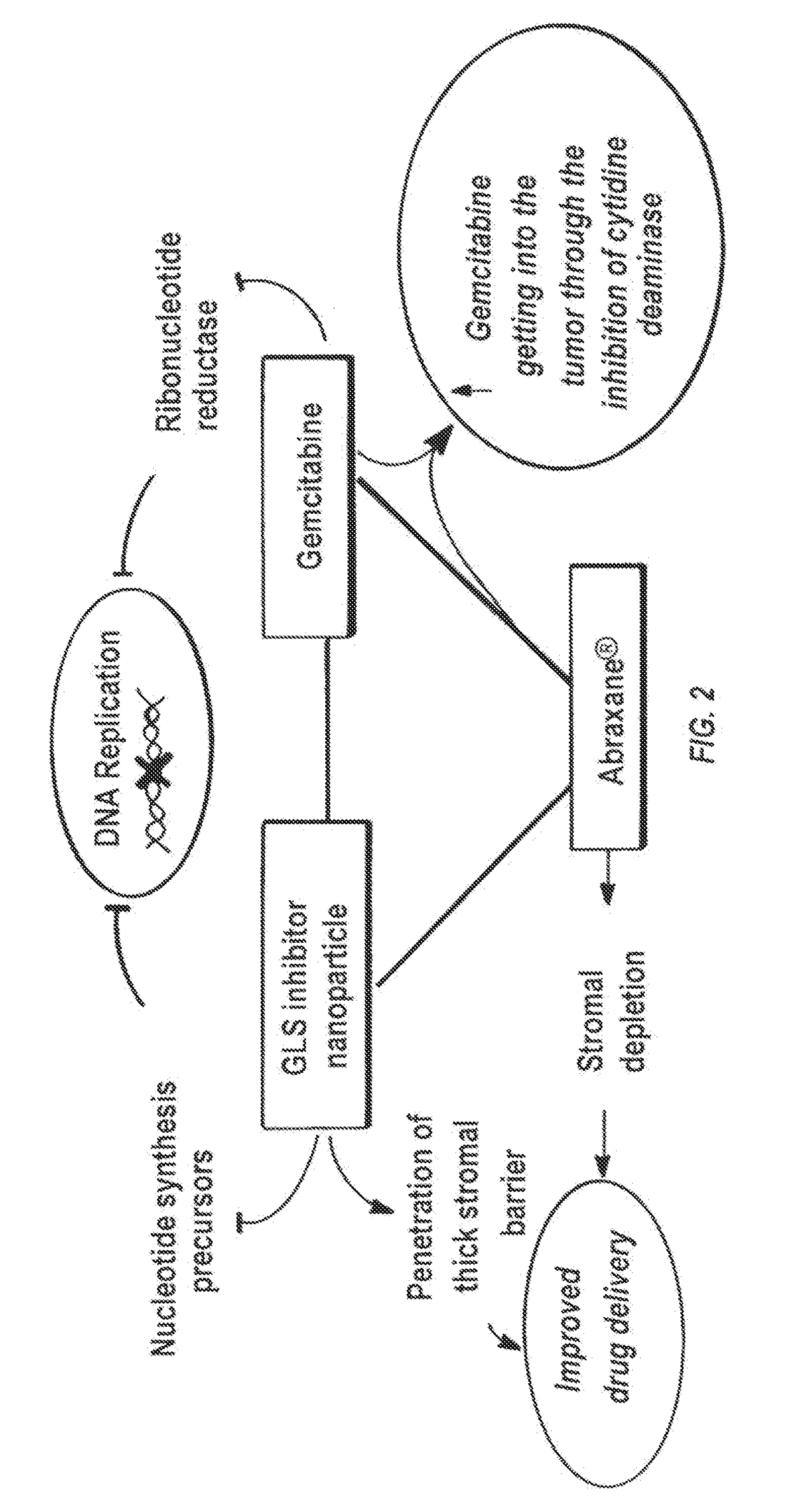
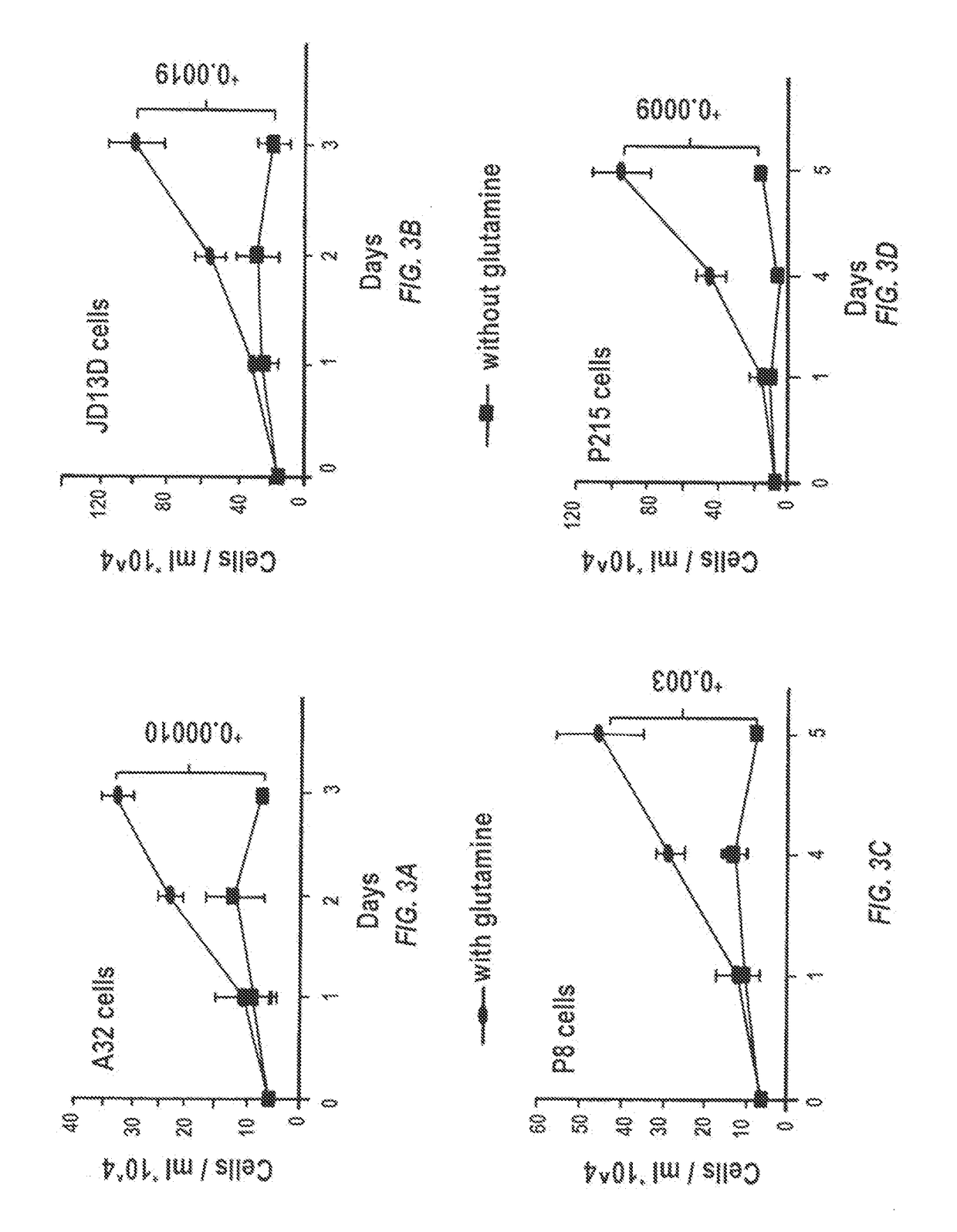
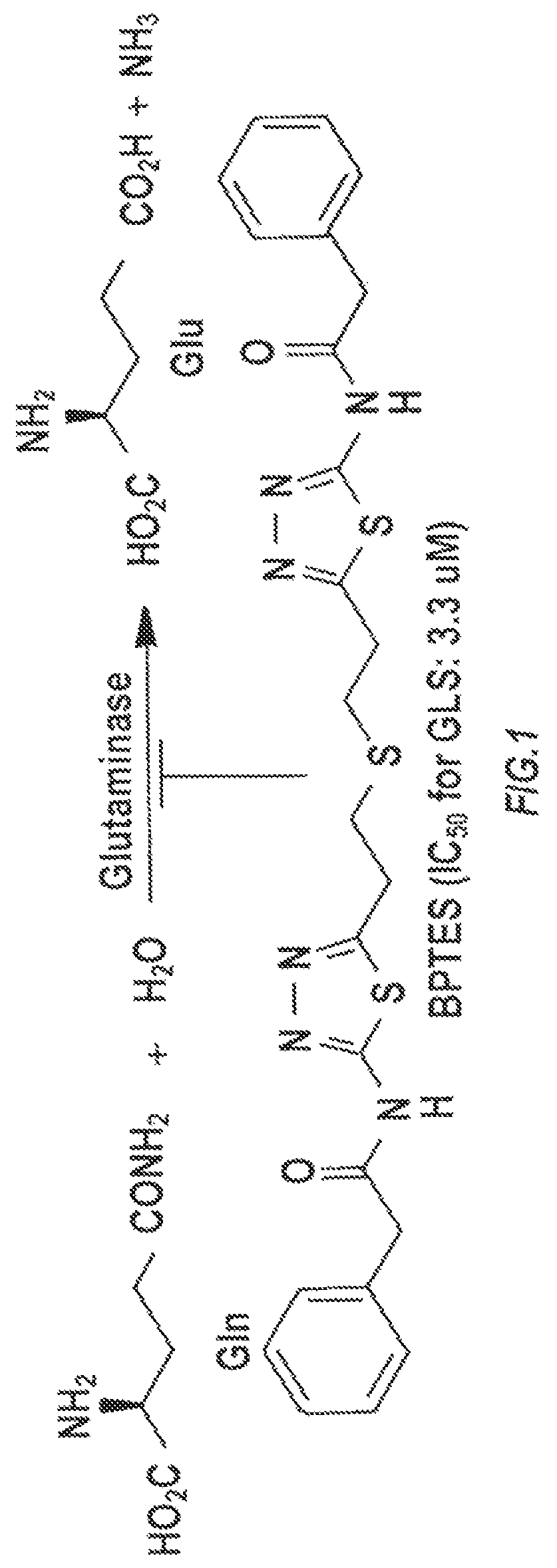
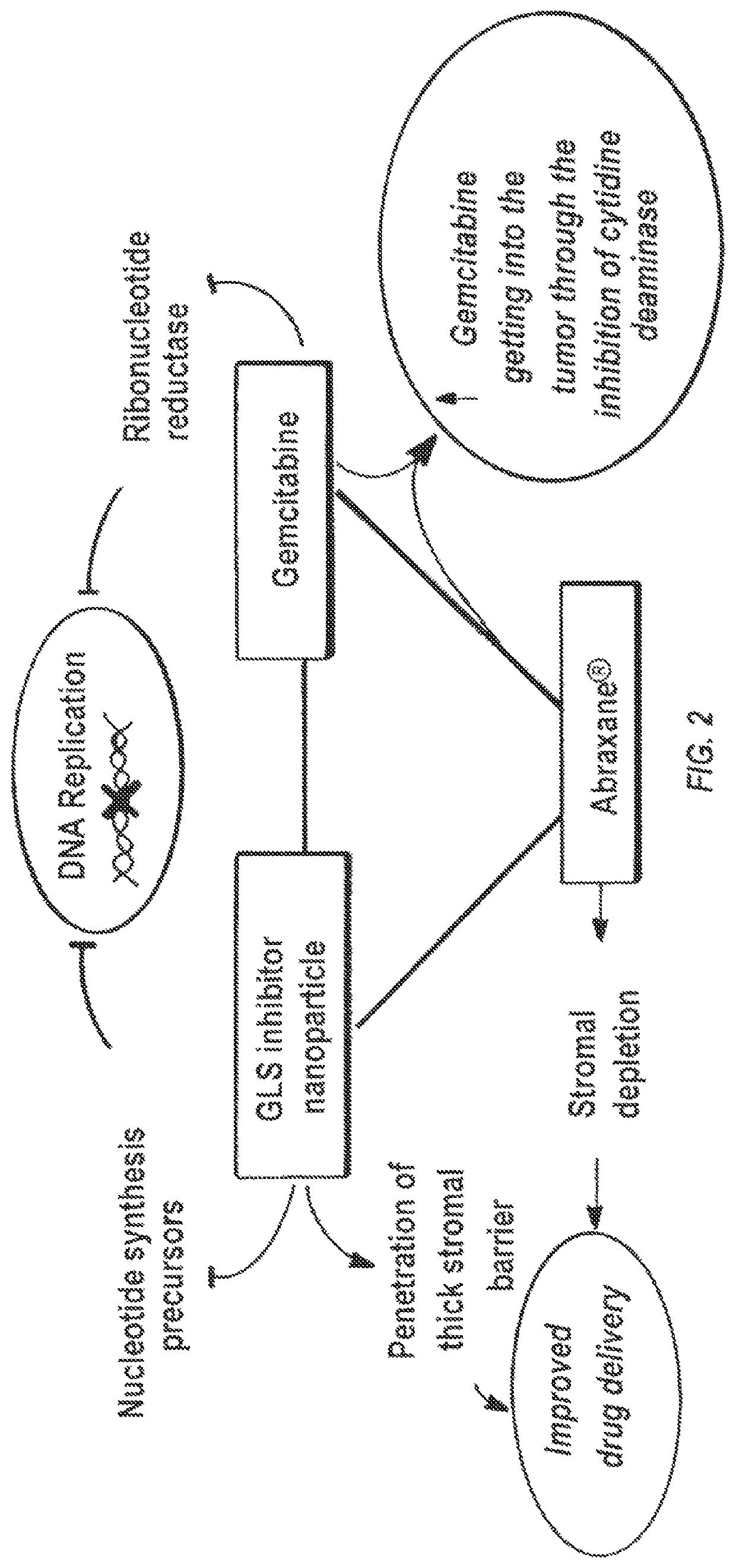
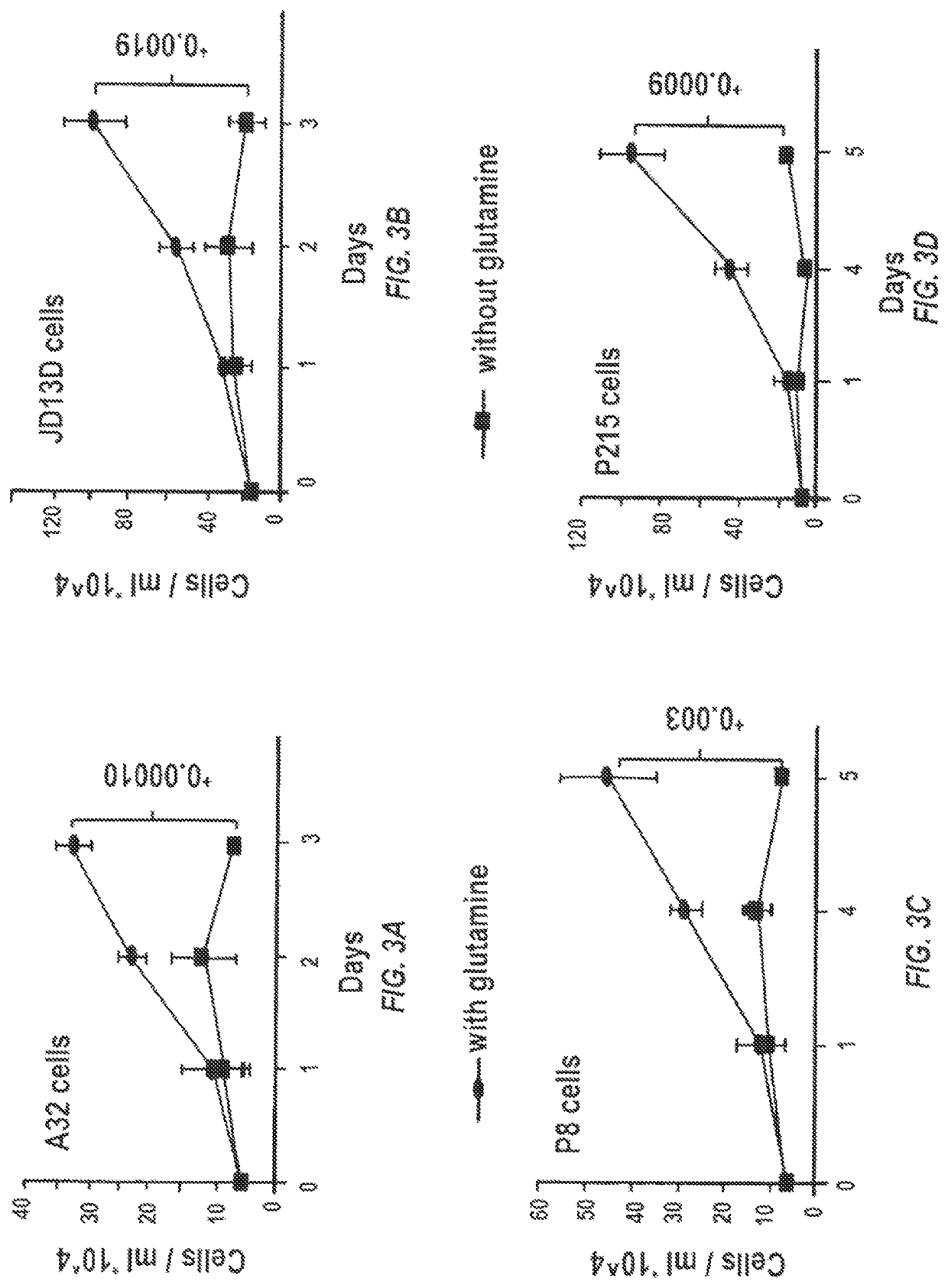
Glutaminase inhibitor discovery and nanoparticle-enhanced delivery for cancer therapy
ActiveUS20170209387A1Improve effectivenessEnhanced interactionOrganic active ingredientsOrganic chemistryHigh concentrationHigh doses
Currently available glutaminase inhibitors are generally poorly soluble, metabolically unstable, and / or require high doses, which together reduce their efficacy and therapeutic index. These can be formulated into nanoparticles and delivered safely and effectively for treatment of pancreatic cancer and other glutamine addicted cancers. Studies demonstrate that nanoparticle delivery of BPTES, relative to use of BPTES alone, can be safely administered and provides dramatically improved tumor drug exposure, resulting in greater efficacy. GLS inhibitors can be administered in higher concentrations with sub-100 nm nanoparticles, since the nanoparticles package the drug into “soluble” colloidal nanoparticles, and the nanoparticles deliver higher drug exposure selectively to the tumors due to the enhanced permeability and retention (EPR) effect. These factors result in sustained drug levels above the IC50 within the tumors for days, providing significantly enhanced efficacy compared to unencapsulated drug.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
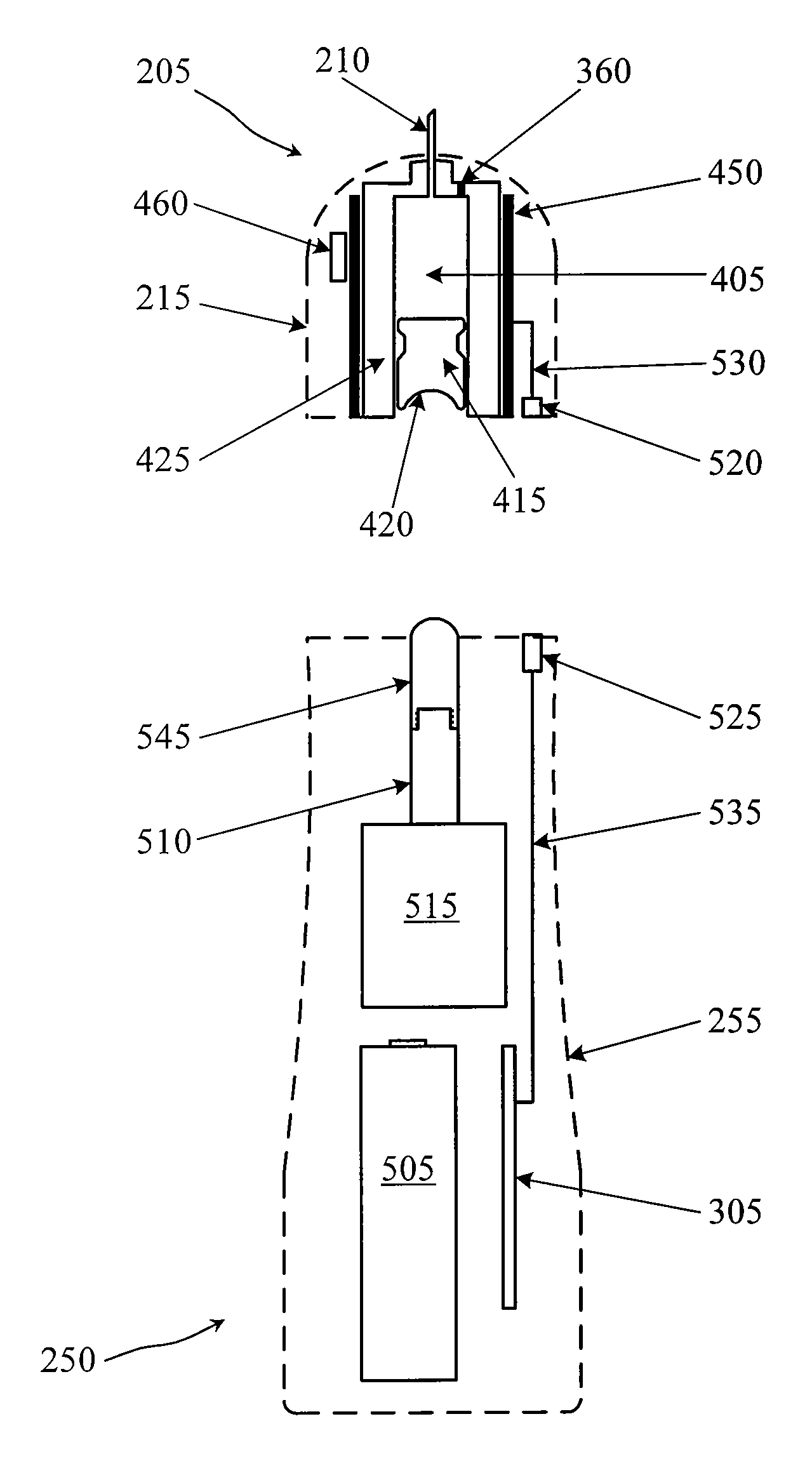
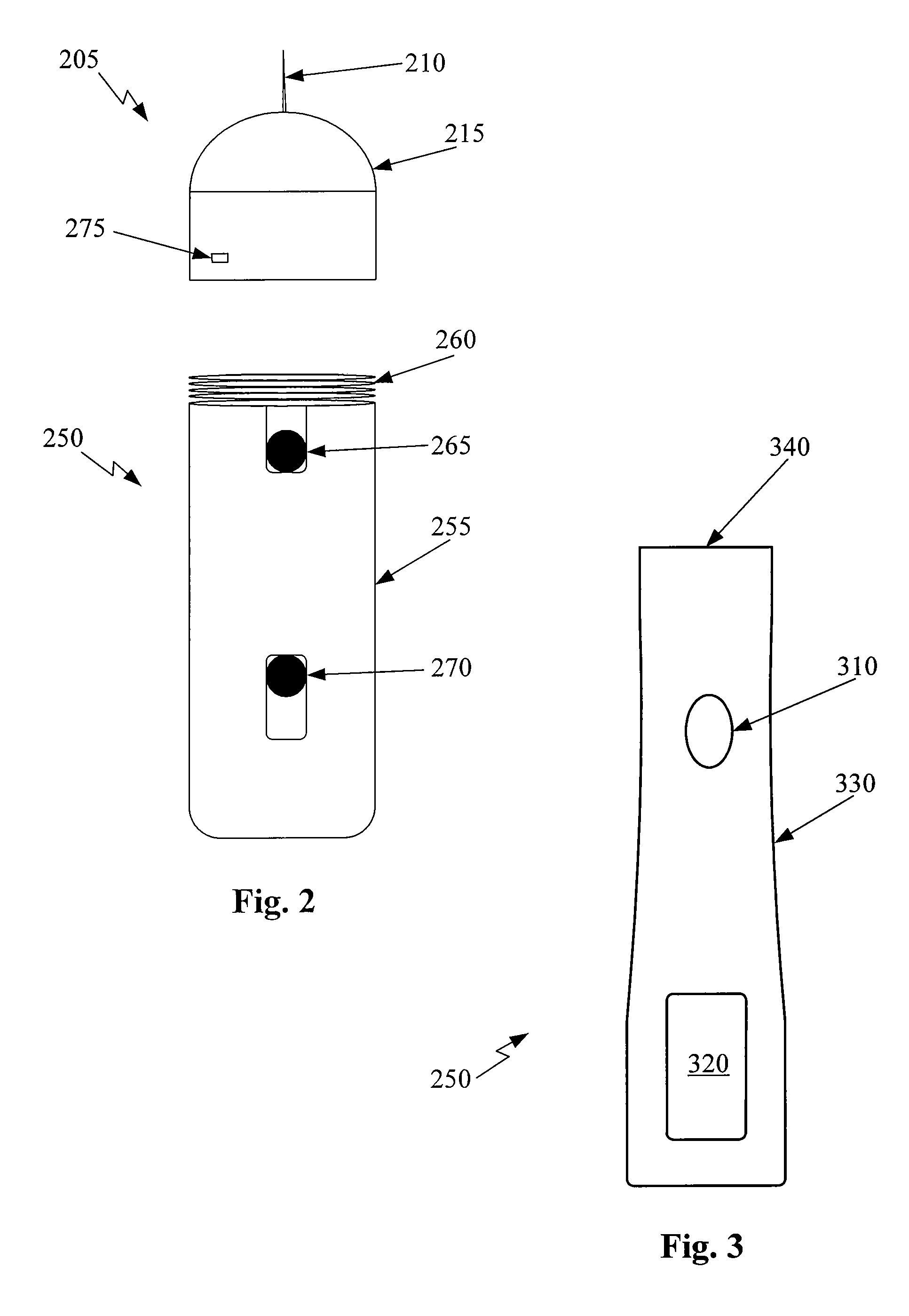
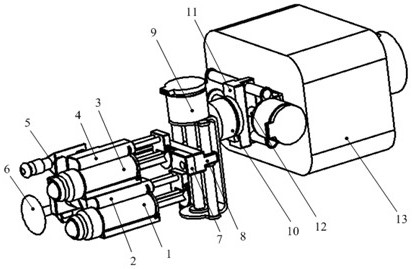
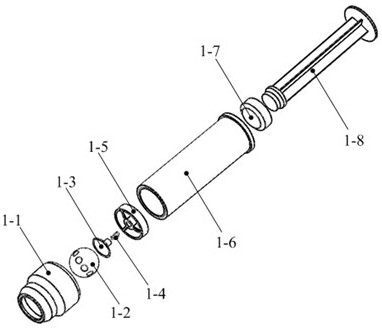
Drug Level Sensor for Injection Device
A dispensing chamber assembly has a dispensing chamber housing, a plunger, and a level sensor. The dispensing chamber housing at least partially surrounds a dispensing chamber that holds a quantity of a substance. The plunger is fluidly sealed to the inner surface of the dispensing chamber housing. The level sensor detects when the dispensing chamber is substantially full.
Owner:ALCON RES LTD
Preparation method of oleanolic acid slow-releasednano-microcapsule
ActiveCN110327311ASimple designGood choiceAntibacterial agentsOrganic active ingredientsSolubilityGastric juices
The invention provides a preparation method of an oleanolic acid slow-releasednano-microcapsule. The aim is to design and optimize oleanolic acid (OA) nanoparticles with poor water solubility to improve the oral bioavailability of the oleanolic acid (OA) nanoparticles and prolong the duration of the treatment drug level. Nanoparticle wall material is an amphiphilic polymer formed by hydrophilic chitosan oligosaccharide and hydrophobic deoxycholic acid. The particle size of nanoparticles with different wall materials is 200-400nm, and distribution is uniform. Releasing in vitro experiment is conducted in simulated gastrointestinal tract environment, results show that the oleanolic acid nanocapsule is released slowly in the simulated gastric juice and gradually released in the intestinal juice; explosive releasing is showed in early stage of releasing in the PBS solution, and then releasing is slowly; and the oleanolic acid nanocapsule is released significantly in each solution. The oleanolic acid controlled-release nanocapsule improves the bioavailability of oleanolic acid, has obvious in vitro controlled-release effect, and can be used as an effective oral preparation for future liver injury treatment.
Owner:HARBIN INST OF TECH
Alpha1-receptor antagonist slow-release pill preparation and its preparation method
InactiveCN101291657AUrinary disorderAmide active ingredientsSustained release pelletsSufficient time
A sustained-release pellet formulation comprising: a pellet core comprising an alpha1-receptor antagonist, a pellet-forming susbstance and a pharmaceutically acceptable excipient and a coating layer comprising an enteric coating substance and a water-insoluble polymer, which is coated on said pellet core maintains a therapeutically effective drug level in the blood for a sufficient time without an initial burst and sustains the release of the drug even in the small intestine due to the water-insoluble polymer in the coating layer.
Owner:AMOREPACIFIC CORP
Sustained-Released Pellet Formulation of Alpha1-Receptor Antagonist and Process For the Preparation Thereof
InactiveUS20080226738A1Continuous releaseUrinary disorderAmide active ingredientsSustained release pelletsDrugs levels
A sustained-release pellet formulation comprising: a pellet core comprising an α1-receptor antagonist, a pellet-forming substance and a pharmaceutically acceptable excipient and a coating layer comprising an enteric coating substance and a water-insoluble polymer, which is coated on said pellet core maintains a therapeutically effective drug level in the blood for a sufficient time without an initial burst and sustains the release of the drug even in the small intestine due to the water-insoluble polymer in the coating layer
Owner:AMOREPACIFIC CORP
Zaocys dhumnades oil product and preparation method thereof
InactiveCN104689296AAbundant raw materialsImprove stabilityPeptide/protein ingredientsUnknown materialsNeutral proteaseTrypsin
The invention relates to a product which is primarily based on zaocys dhumnades oil and a preparation method thereof. The preparation method comprises the following steps: by taking drug level zaocys dhumnades as a raw material, crushing the raw materials, adding acetone, extracting under nitrogen protection and deodorizing and decoloring through active carbon to obtain refined zaocys dhumnades oil; and adding neutral protease and trypsin into acetone extraction residues for enzymolysis, centrifugalizing after high-temperature treatment, adding ether into a supernate to be precipitated, wherein the precipitate is a small peptide dried object, and mixing with the refined zaocys dhumnades oil to obtain the zaocys dhumnades oil product. The method can effectively prevent damage of linoleic acid and is high in oil yield, shallow in luster, high in transparency, free of precipitate and suspended solids and relatively small in viscosity. The content of linoleic acid can be over 16% and the expiration date of the product is long.
Owner:向华
Integrated microfluidic organic electrochemical transistor biosensors for drug level detection
InactiveUS20190381503A1Solid-state devicesMaterial analysis by electric/magnetic meansAptamerDrugs levels
The present disclosure describes a systems and methods to rapidly detect a level of a drug present in a fluid sample. The systems and methods can be used to monitor drug levels in the blood of a patient to whom the drug has been prescribed. A system can include one or more organic electrochemical transistors that are functionalized with a coating that may include aptamers or antibodies. The coating can bind or otherwise interact with the drug of interest to change the transconductance of the organic electrochemical transistors. The system can detect a change in the transconductance of the organic electrochemical transistors and signal the presence of the drug.
Owner:CHARLES STARK DRAPER LABORATORY
Raltitrexed colon specific pellet and preparation method thereof
ActiveCN104490848AReduce volumeReduce the impactOrganic active ingredientsGranular deliveryCvd riskBULK ACTIVE INGREDIENT
The invention belongs to the field of pharmacy, relates to a medicine preparation using raltitrexed as an active ingredient, and particularly relates to a raltitrexed colon specific pellet and a preparation method thereof. The pellet sequentially comprises the following structural layers from inside to outside: a drug-loading body, a drug-containing layer, an isolating layer and a colon specific layer. The pellet has the characteristics of orally enteric coating and colon specific targeting, and is mainly dissolved and absorbed in the colon position after being taken orally. Compared with the prior art, the pellet has the advantages that the finally prepared multi-unit preparation has excellent gastrointestinal tract distribution and consistent passing time so as to achieve a consistent plasma drug level and reduce the risk of burst release of drugs. The pellet can be used for improving the bioavailability and has good safety performance.
Viral keratitis treating decoction drug and preparation method thereof
InactiveCN104857327AHeat-clearing and detoxifyingDisperses stagnation and detumescenceSenses disorderAntiviralsPeppermintsLicorice roots
The invention relates to a viral keratitis treating decoction drug and a preparation method thereof, wherein the drug comprises, by mass, 1-3 parts of forsythia suspensa, 1-3 parts of lonicera japonica, 1-3 parts of peppermint, 1-3 parts of platycodon grandiflorus, 1-3 parts of nepeta cataria, 1-3 parts of stir-fried mulberry bark, 1-3 parts of dandelion, 1-3 parts of bamboo leaf, 1-3 parts of licorice root, 1-3 parts of rhizoma phragmitis, 1-3 parts of mulberry leaf, 1-3 parts of chrysanthemum, 1-3 parts of caulis akebiae, 1-3 parts of gentiana scabra bunge, and 1-3 parts of scutellaria baicalensis. The preparation method comprises: selecting high-quality and clean grade-one forsythia suspensa, lonicera japonica, peppermint, platycodon grandiflorus, nepeta cataria, stir-fried mulberry bark, dandelion, bamboo leaf, licorice root, rhizoma phragmitis, mulberry leaf, chrysanthemum, caulis akebiae, gentiana scabra bunge, and scutellaria baicalensis, respectively removing impurities and dust, loading into a drug bag, bundling the opening for spare, placing the obtained drug bag into clean water, soaking for about half an hour, placing the soaked drug into a drug decocting machine by adopting the permeating of the water into the drug organization as the degree, injecting cold boiled water until the cold boiled water level 42 mm higher than the drug level, carrying out primary boiling for 35 min, filtering to take the drug liquid, injecting slightly-warm boiling water, carrying out secondary boiling for 30 min, and carrying out merged concentration to obtain 1665 ml of the 50% traditional Chinese medicine liquid.
Owner:四川金堂海纳生物医药技术研究所
Methods of normalizing measured drug concentrations and testing for non-compliance with a drug treatment regimen
Methods for monitoring subject compliance with a prescribed treatment regimen are disclosed. In one embodiment, the method comprises measuring a drug level in fluid of a subject and normalizing said measured drug level as a function of one or more parameters associated with the subject. The normalized drug level is compared to a reference value and associated confidence intervals or to a concentration range. The reference value and associated confidence intervals and / or the concentration range may be normalized based on one or more parameters associated with subjects in a reference population.
Owner:AMERITOX LLP
Methods of treating neurodegenerative diseases using indane acetic acid derivatives which penetrate the blood brain barrier
InactiveUS20180200230A1Organic active ingredientsNervous disorderHuntingtons choreaDementia with Lewy bodies
This invention describes the use of indane acetic acid derivatives which are dual PPAR delta / gamma agonists, and which penetrate the Blood Brain Barrier and achieve effective brain to plasma drug levels at non-toxic doses, for the treatment of neurodegenerative diseases including one or more of the following: Alzheimer's Disease (AD); Huntington's Disease (HD); Parkinson's Disease (PD); Amyotrophic Lateral Sclerosis (ALS); Frontal Temporal Dementia (FTD); Corticobasal Degeneration (CBD); Progressive Supranuclear Palsey (PSP); Dementia with Lewy Bodies (DLB); or Multiple Sclerosis (MS).
Owner:DARA BIOSCI
Method for preparing medicinal accessories sodium caprylate
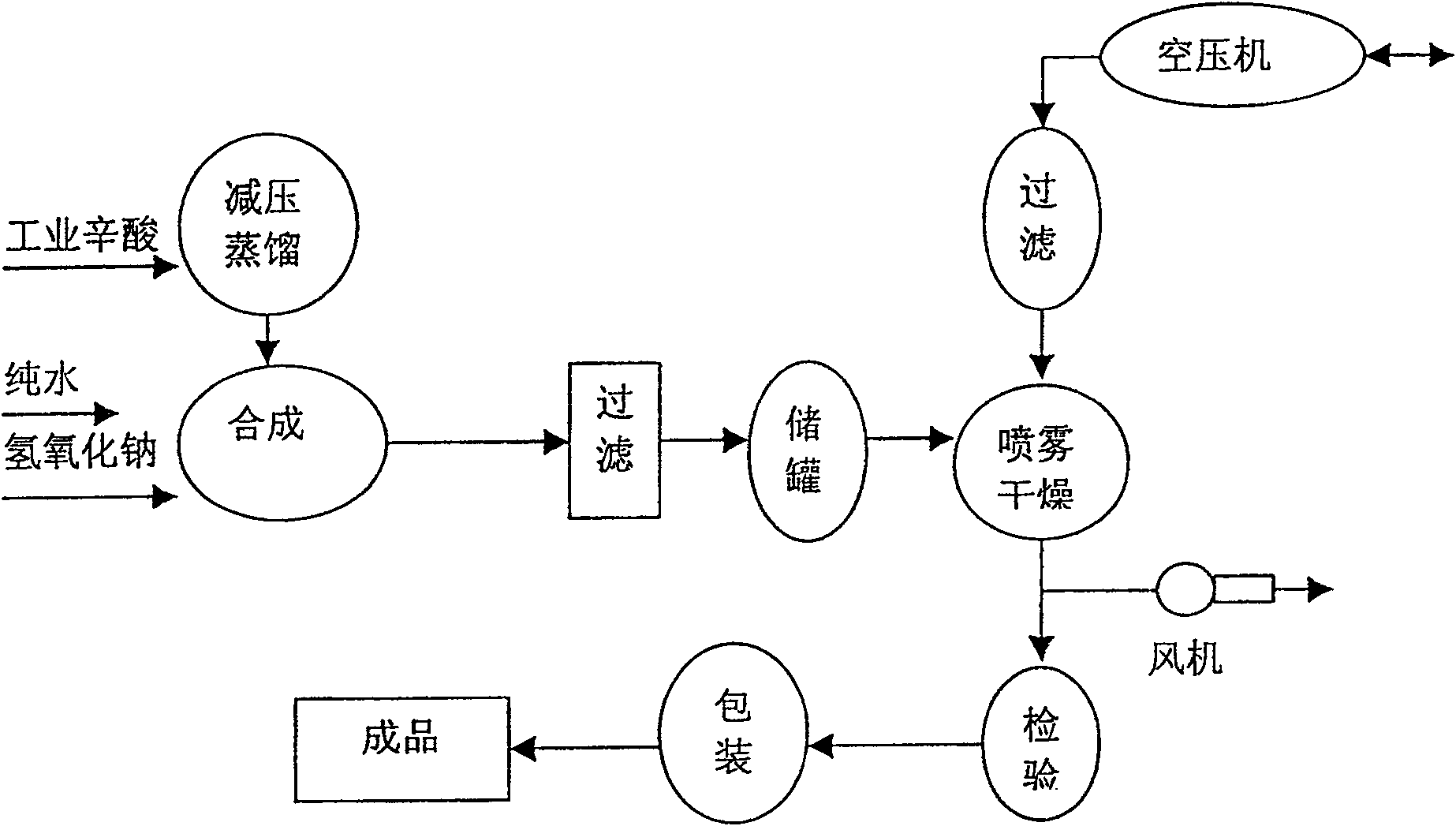
ActiveCN100546967CImprove stabilityHigh purityPharmaceutical non-active ingredientsCarboxylic acid salt preparationFiltrationDistillation
The invention relates to a preparation method of sodium octanoate as a medicinal auxiliary material. It includes the following steps: put industrial octanoic acid into an acid distillation tank, use a vacuum pump to reduce the pressure to 0.06-0.10MPa, and carry out distillation at a temperature of 130-150°C. After the acid vapor enters a condenser to cool, separate the purified Caprylic acid: put sodium hydroxide and pure water into the reaction tank at a ratio of 1:6~9, add octanoic acid in an equal amount while stirring, heat the temperature to 60~110°C, and generate sodium caprylate after fully reacting , the pH value is controlled within the range of 8 to 10.5; then the sodium caprylate solution is left to stand, filtered, and centrifugally sprayed and dried into a white crystalline powder; after passing the inspection, it is sealed and packaged to produce a finished product. The advantages of the present invention are: the sodium caprylate prepared by the present invention has good stability, high purity, is easily soluble in water, has good solution clarity, and the pyrogen quality is qualified and other quality control standards reach the level of the sodium caprylate pharmaceutical auxiliary material. It can be used in biological products and pharmaceutical industries to make finished drugs with high quality and good stability.
Owner:北京市师化精细化工科技开发有限责任公司
Long-acting recombinant human granulocyte colony-stimulating factor and preparation method therefor and use thereof
InactiveCN105461810ASimple preparation processMaintain biological activityPeptide/protein ingredientsPharmaceutical non-active ingredientsPichia pastorisHalf-life
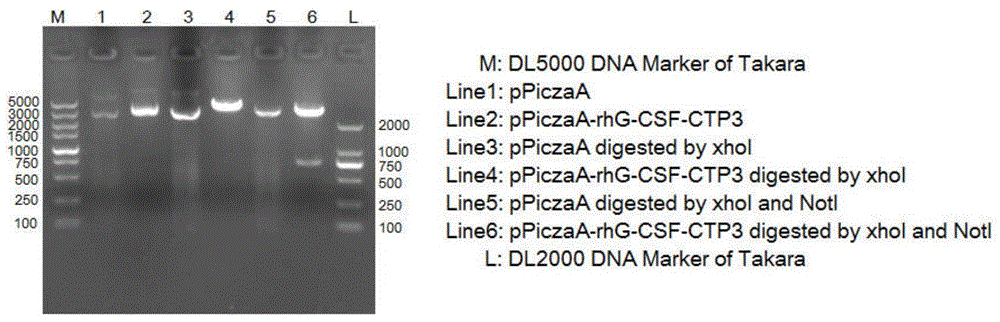
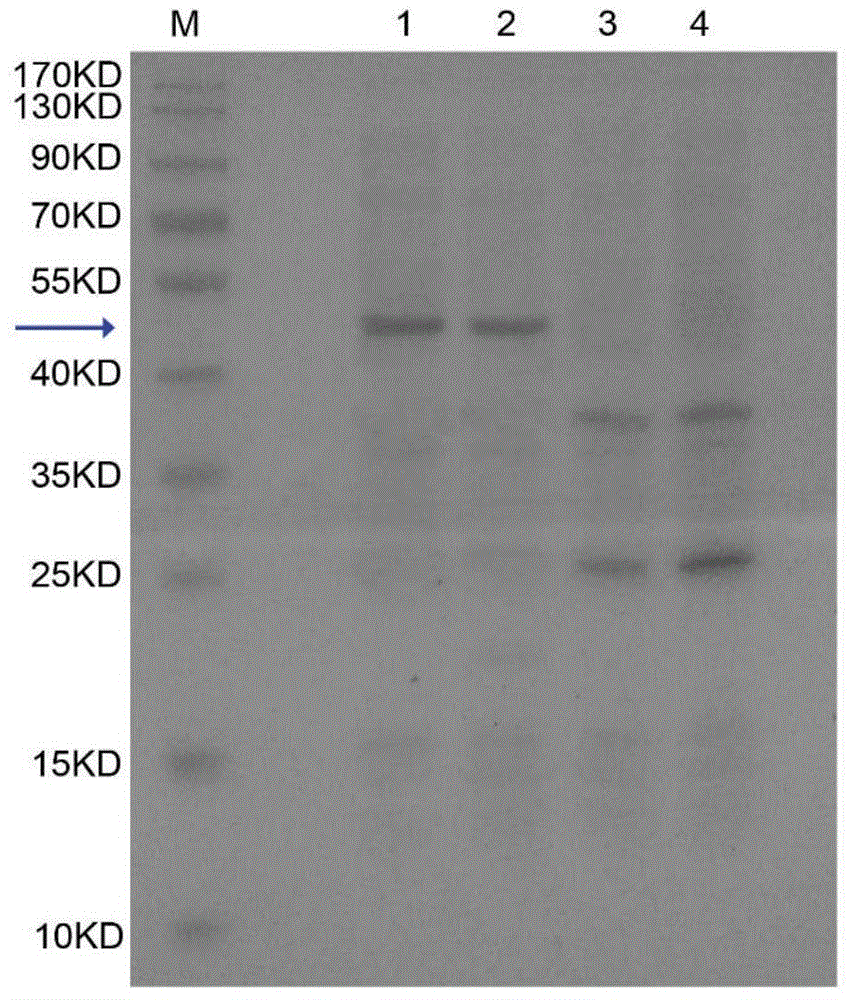
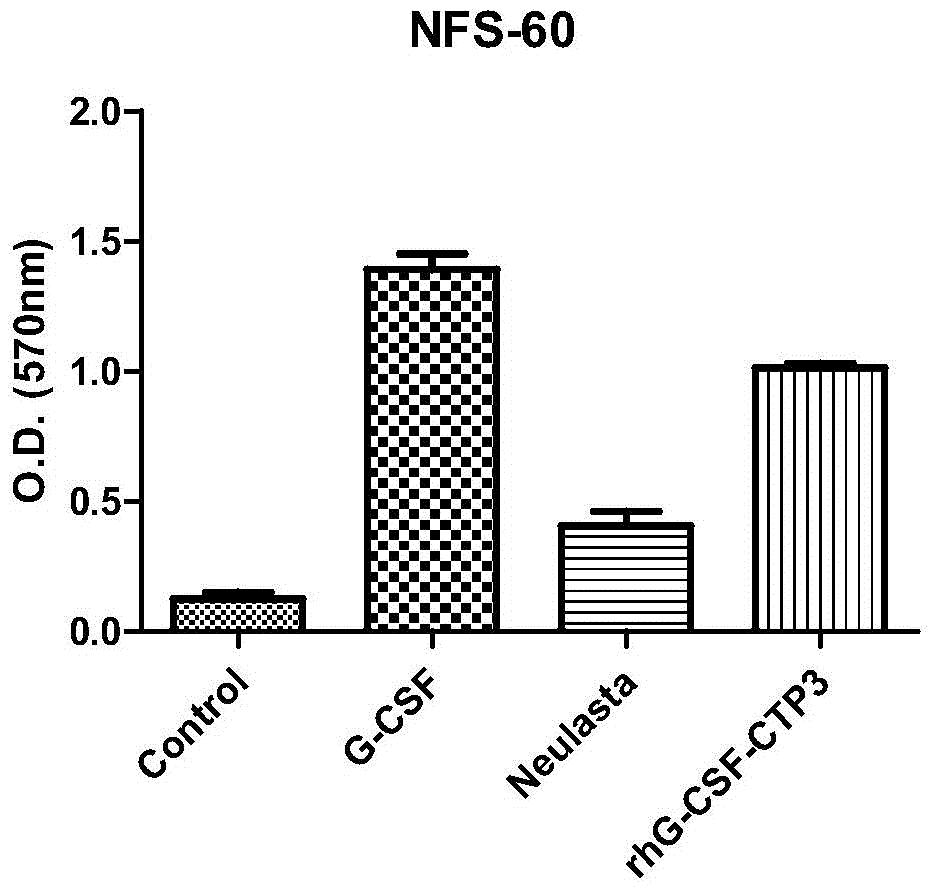
The invention belongs to the technical field of biological pharmacy and relates to a recombinant fusion protein of a human granulocyte colony-stimulating factor, particularly a long-acting recombinant human granulocyte colony-stimulating factor and a preparation method therefor and use thereof. A recombinant human granulocyte colony-stimulating factor and a short peptide (CTP) formed by amino acids from site 118 to site 145 of beta subunit end of one or more human chorionic gonadotropin (hCG) are expressed in a fusion manner to prepare a long-acting fusion protein; on the premise of maintaining the original activity of the human granulocyte colony-stimulating factor, the half-life of the protein in vivo is prolonged, so that the protein is further used for preparing drugs for resisting chemoradiotherapy or treating neutrophilic granulocytopenia and myelosuppression caused by other reasons. The fusion protein provided by the invention can be efficiently expressed in pichia pastoris, is simple in preparation process, and can be used for production and preparation of drug-level fusion proteins on a large scale.
Owner:FUDAN UNIV
Oral drug for treating hydrarthrosis and preparation method thereof
InactiveCN105055709ASolve the problem of getting back to health soonerPerfect compatibilitySkeletal disorderUnknown materialsFiltrationLicorice roots
The invention discloses an oral drug for treating hydrarthrosis and a preparation method thereof. The oral drug comprises, by mass, 1-3 parts of whole Chinese angelica, 1-3 parts of ligusticum wallichii, 1-3 parts of cyathula root, 1-3 parts of pubescent angelica root, 1-3 parts of poria cocos, 1-3 parts of rhizoma alismatis, 1-3 parts of root of three-nerved spicebush, 1-3 parts of safflower, 1-3 parts of pangolin, 1-3 parts of dried orange peel, 1-3 parts of bitter orange and 1-3 parts of licorice root. The preparation method comprises selecting high-quality and clean first order whole Chinese angelica, ligusticum wallichii, cyathula root, pubescent angelica root, poria cocos, rhizoma alismatis, root of three-nerved spicebush, safflower, pangolin, dried orange peel, bitter orange and licorice root according to the above mass part ratio, respectively removing impurities and dust, putting the materials into a cloth bag, tying the opening of the cloth bag, immersing the bag with the drugs in cold boiled water for about 30min until the water permeates the drug tissue, putting the immersed drugs and the clear liquid obtained by drug immersion into a decoction machine together, pouring cold boiled water into the decoction machine until the water level is 35mm higher than the drug level, carrying out primary decoction for 40min, carrying out filtration, collecting the filtrate, pouring tepor boiled water into the drugs until the water level is 30mm higher than the drug level, carrying out secondary decoction for 35min, mixing the decoctions obtained by the two decoction processes, and carrying out concentration to obtain 1620ml of traditional Chinese medicine soup with a concentration of 50%.
Owner:CHENGDU DRAGON WATER TREATMENT TECH RES INST
Anti-cancer medicine composition
InactiveCN1634585AIncreased sensitivityInhibit synthesisAntineoplastic agentsPharmaceutical active ingredientsTreatment effectWhole body
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and enwrapped anticancer effective constituent is disclosed. Wherein, the anticancer effective constituent is a composition of tumor resistant antibiotic and ToPo enzyme inhibiting agent, the ToPo enzyme inhibiting agent can inhibit the intracellular DNA repairing function and can lower the tolerance of tumor cell to the tumor resistant antibiotic, while the pharmaceutic adjuvant can mainly be biological compatible, degradable and absorbable macromolecule polymer, which can make the anticancer drug release slowly to the local region of tumor in the degradation and absorption process, therefore both lowers considerably the whole body toxic reaction and sustains effective drug level in local region of tumor.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
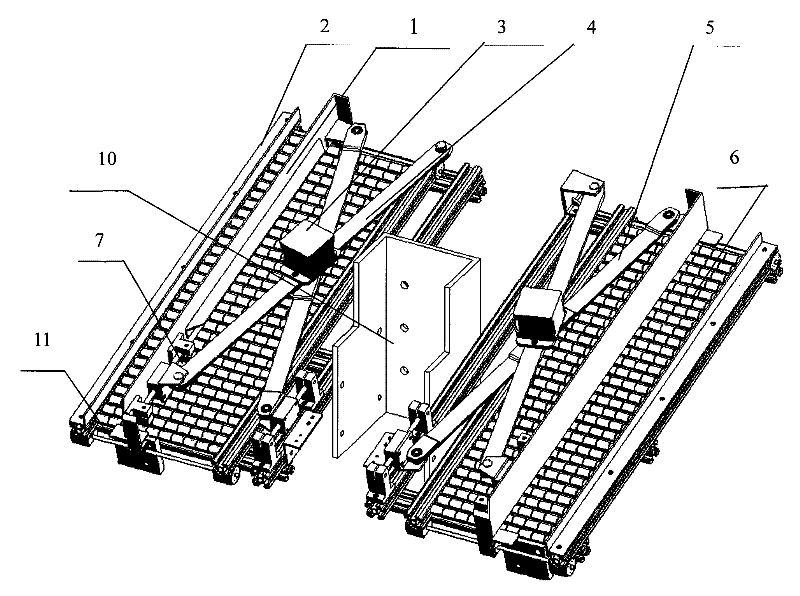
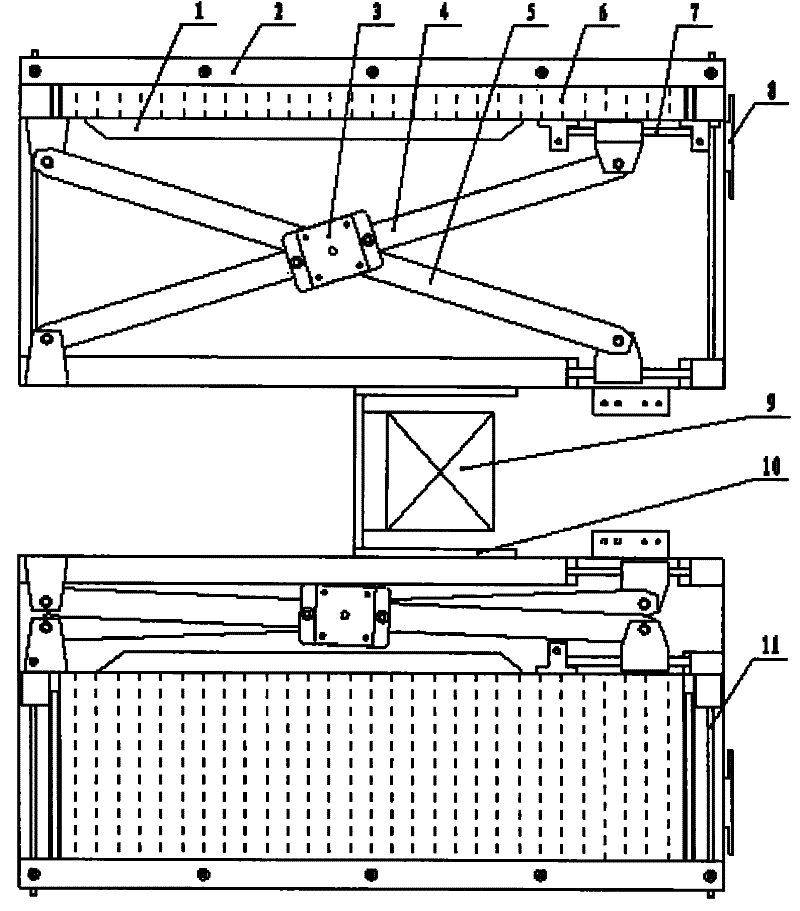
Scissor-fork type drug feeding mechanical hand
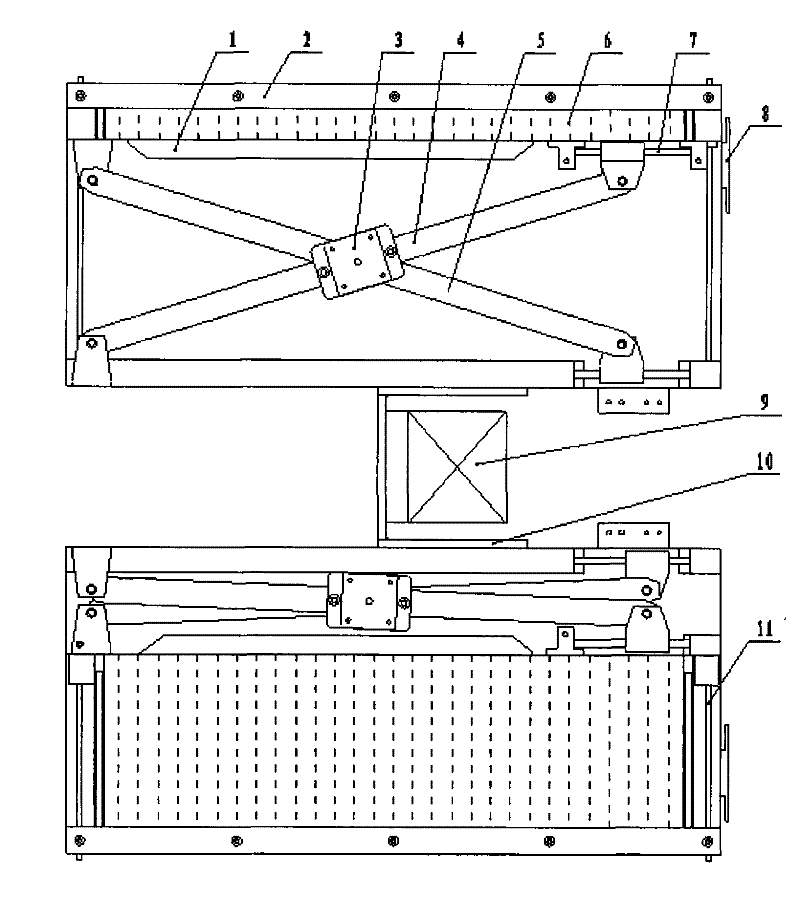
InactiveCN101758496BImplement stop and sendRealize automatic dosingProgramme-controlled manipulatorStorage devicesDrugs levelsDrug Storage
The invention provides a scissor-fork type drug feeding mechanical hand for realizing smooth, high-efficient and orderly automatic drug feeding, which comprises a scissor-fork mechanism, a power part, a frame body and a drug sending baffle plate mechanism, wherein the scissor-fork mechanism comprises an upper scissor-fork plate and a lower scissor-fork plate, the power part is connected with the scissor-fork mechanism and used for providing power for the scissor-fork mechanism, the scissor-fork mechanism is connected with a drug leveling moving plate, an X-guide shaft and a Y-guide shaft are fixedly mounted on the frame body, the X-guide shaft, the upper scissor-fork plate and the lower scissor-fork plate constitute a sliding pair, and the Y-guide shaft and the drug leveling moving plate constitute the sliding pair. A fluent strip on a machine frame receives and places drugs. The drug sending baffle plate mechanism is arranged at the end of an outlet of the fluent strip. The whole frame body forms a tilt angle of 10-30 degrees with the horizontal plane. The scissor-fork type drug feeding mechanical hand is particularly applicable to a drug storage mechanism with a tilted drug storage slot and can realize the smooth, high-efficient and orderly automatic drug feeding.
Owner:北京华康诚信医疗科技有限公司
A robot terminal drug applicator for skin disease treatment
InactiveCN109200455BRelieve painMeet dosage needsMedical devicesMedical applicatorsTactile sensationDisease patient
The invention discloses a drug applicator at the end of a robot for treating skin diseases. By installing a cleaning module, a temperature-regulating massage module, a drug applicator module I and a drug applicator module II on a drug applicator fixing frame, it can treat the affected part of a skin disease patient. Cleaning, heating massage and drug application treatment operations, the device has two drug application modules, which can apply different drugs according to the treatment needs, horizontal translation module, vertical translation module and rotating motor can make the drug application fixed frame move horizontally, vertically Moving and rotating movements, these movements can make the drug applicator simulate the multi-mode drug applicator actions of human hands on the patient's skin (one-line smear, spiral smear, etc.), according to different tactile sensations of patients (pain, itching, etc.), choose Different application modes, the drug application module and the drug delivery rack are detachable, which is convenient for adding and replacing drugs. By controlling the feed amount of the piston push rod, the patient's drug dosage can be controlled to avoid affecting the treatment due to too much or too little drug dosage Effect.
Owner:HARBIN UNIV OF SCI & TECH
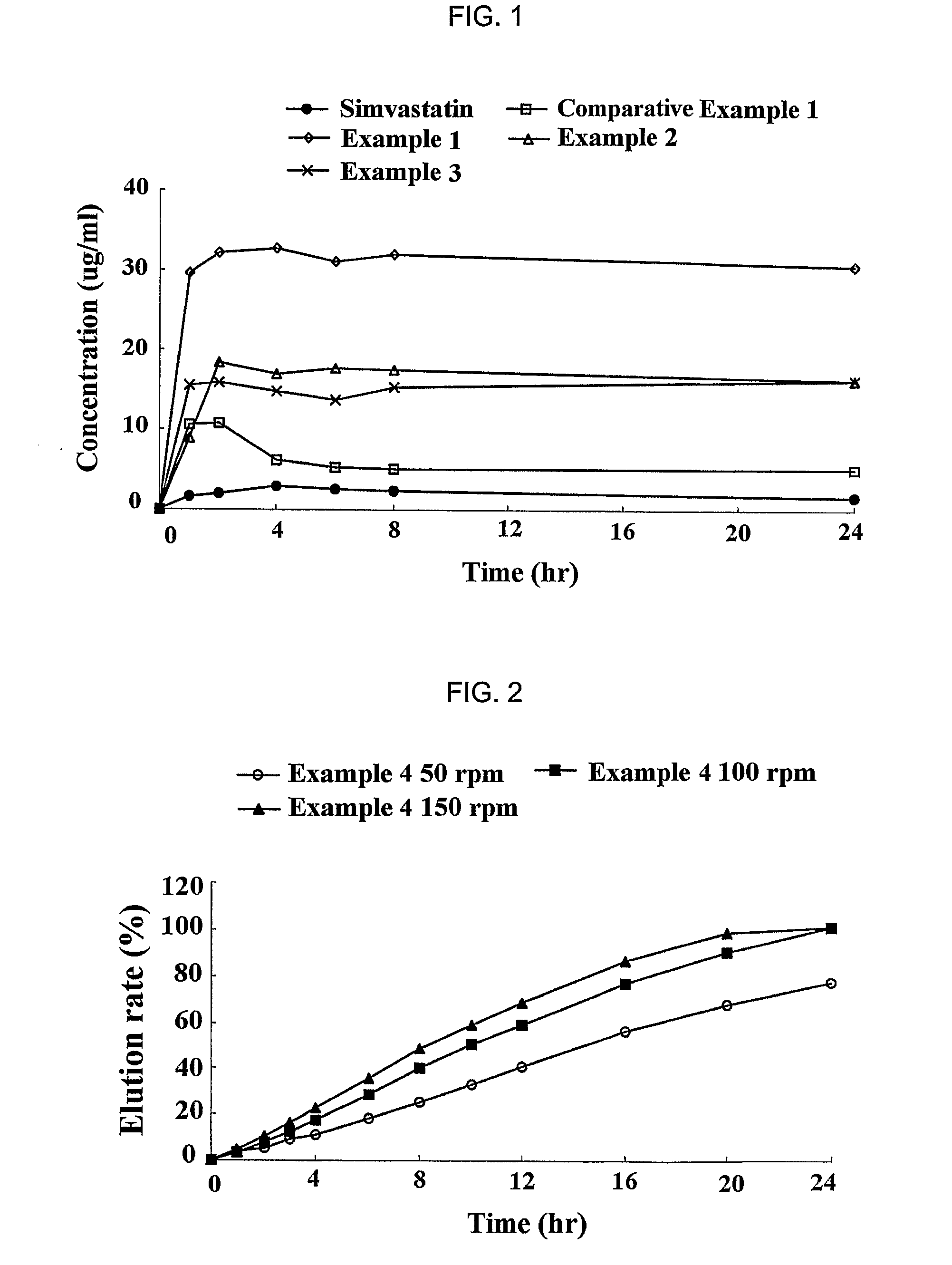
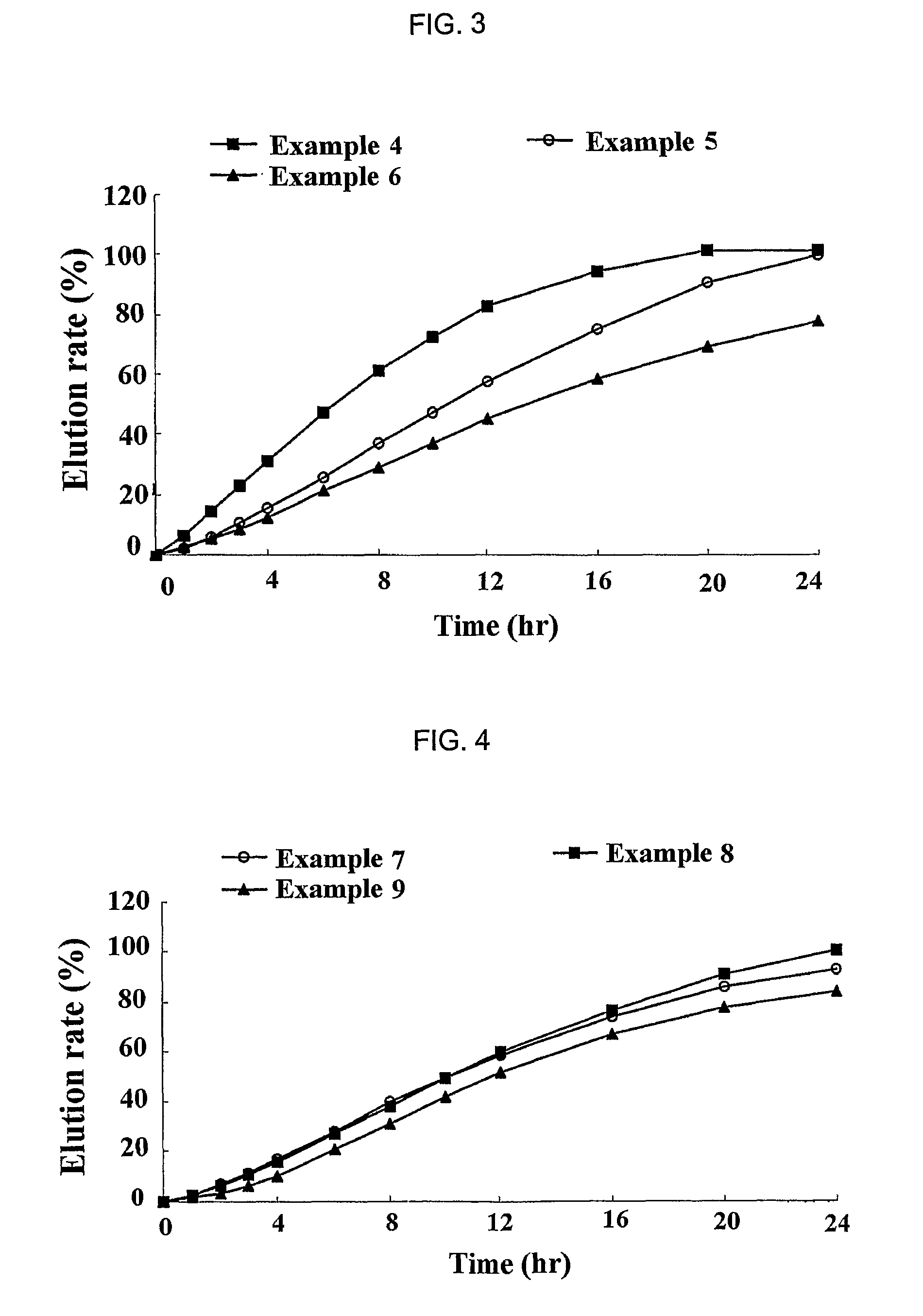
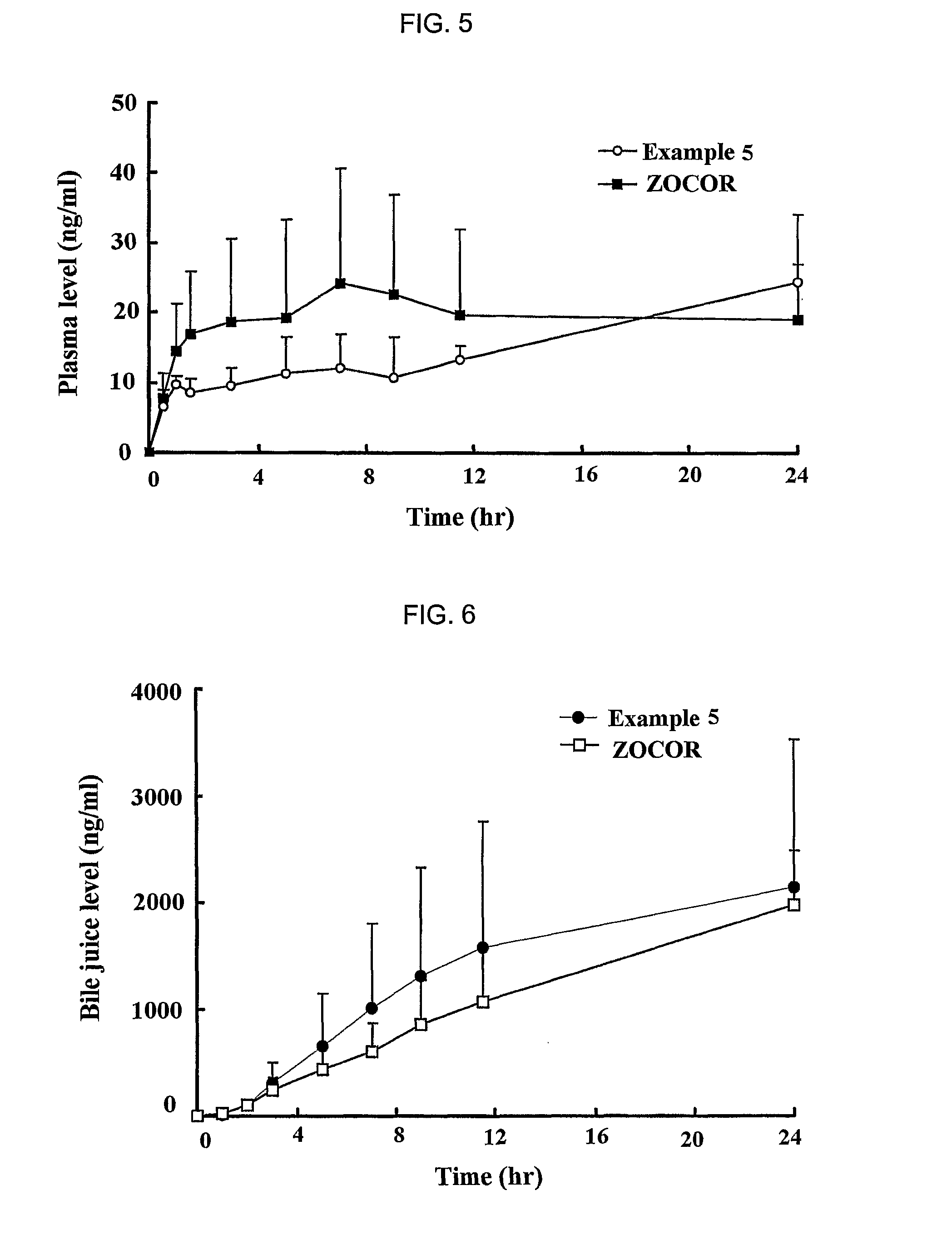
Sustained release formulation for oral administration of HMG-CoA reductase inhibitor and method for the preparation thereof
The sustained release formulation for oral administration of an HMG-CoA reductase inhibitor of the present invention can be easily and economically prepared and is capable of maintaining a constant drug level in blood by slowly releasing the HMG-CoA reductase inhibitor at a uniform rate for 24 hrs. Accordingly, the sustained release formulation of the present invention can be effectively used for lowering blood cholesterol and triglyceride levels.
Owner:HANMI SCI CO LTD
Sustained release composition for oral administration of drugs
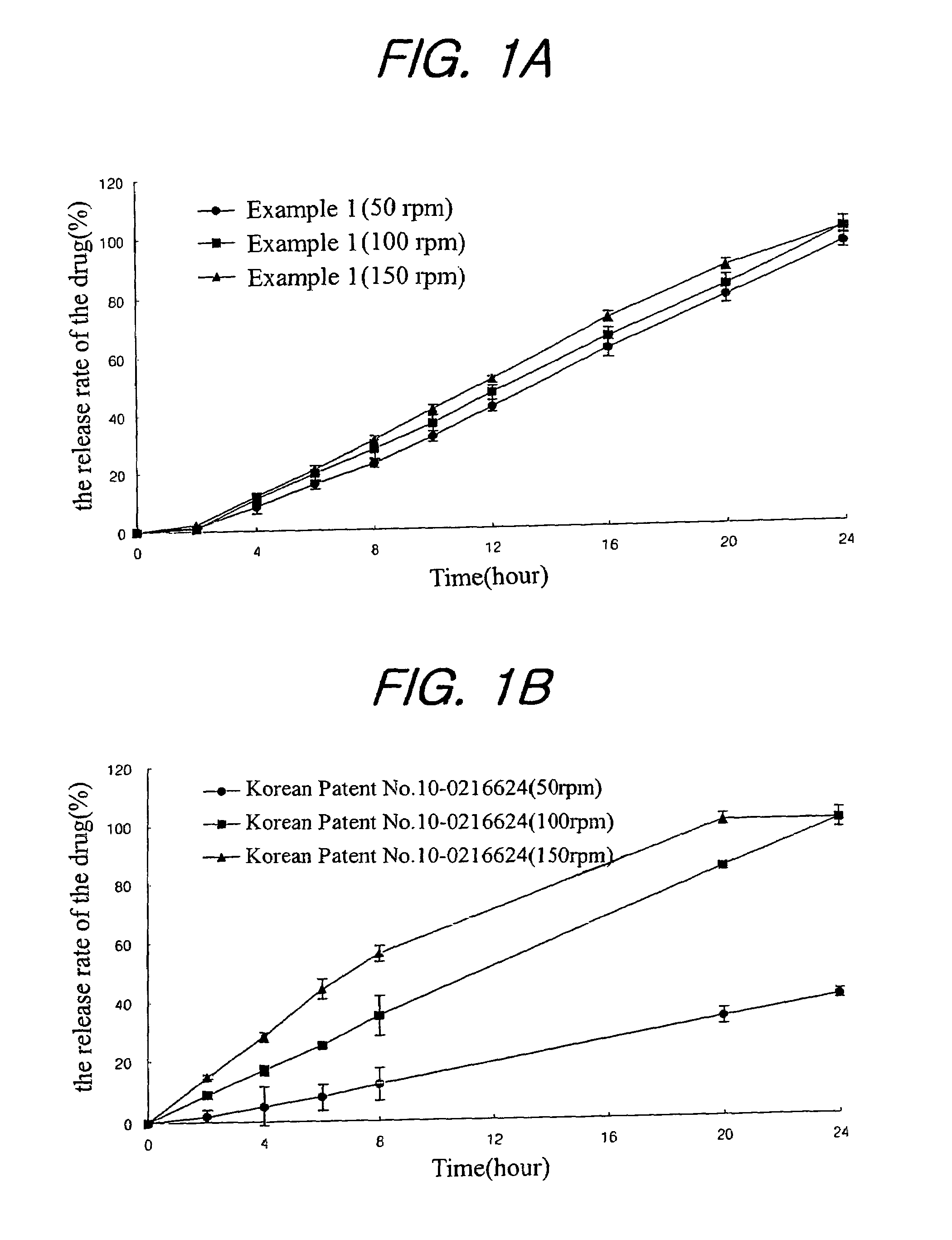
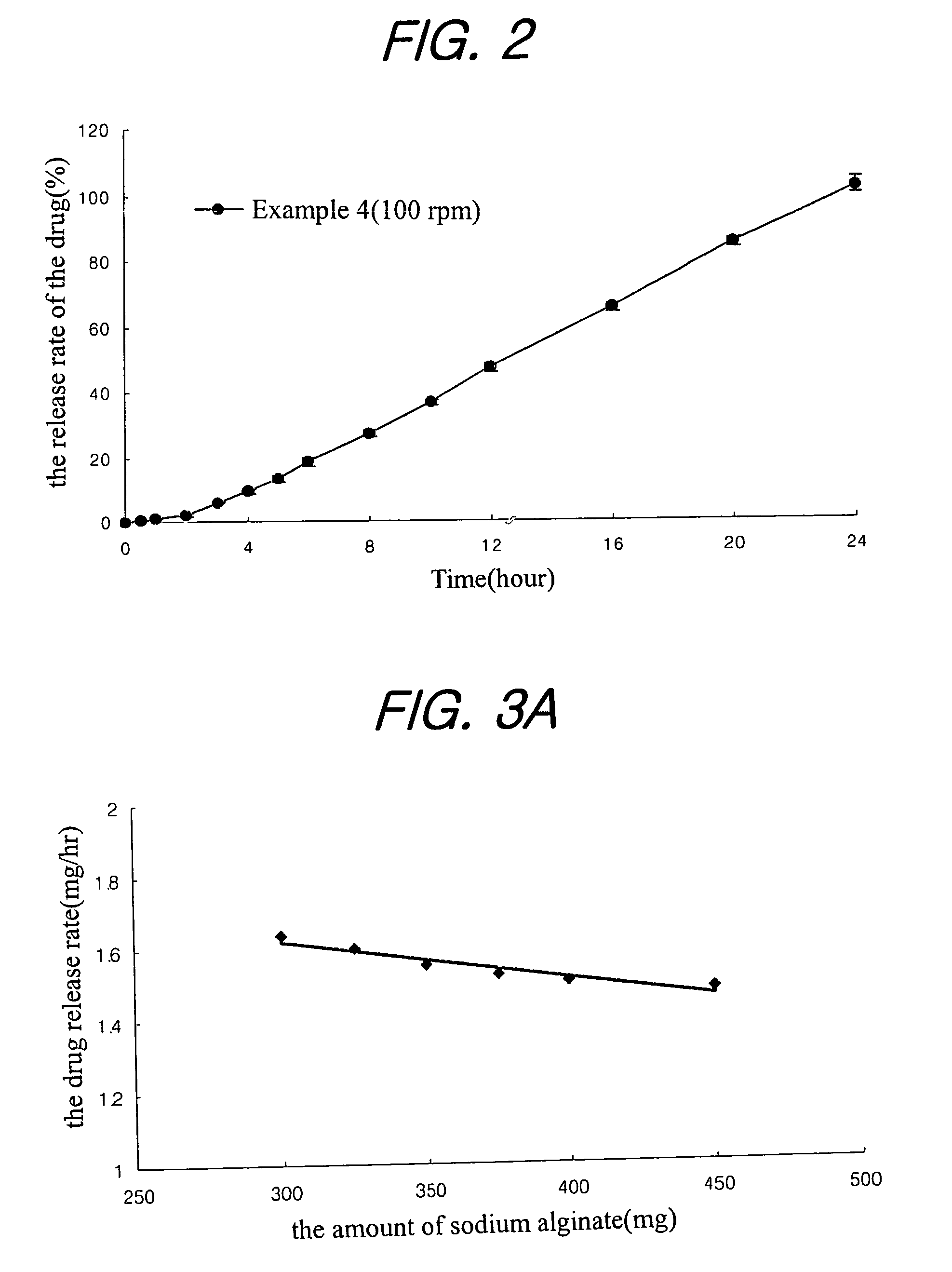
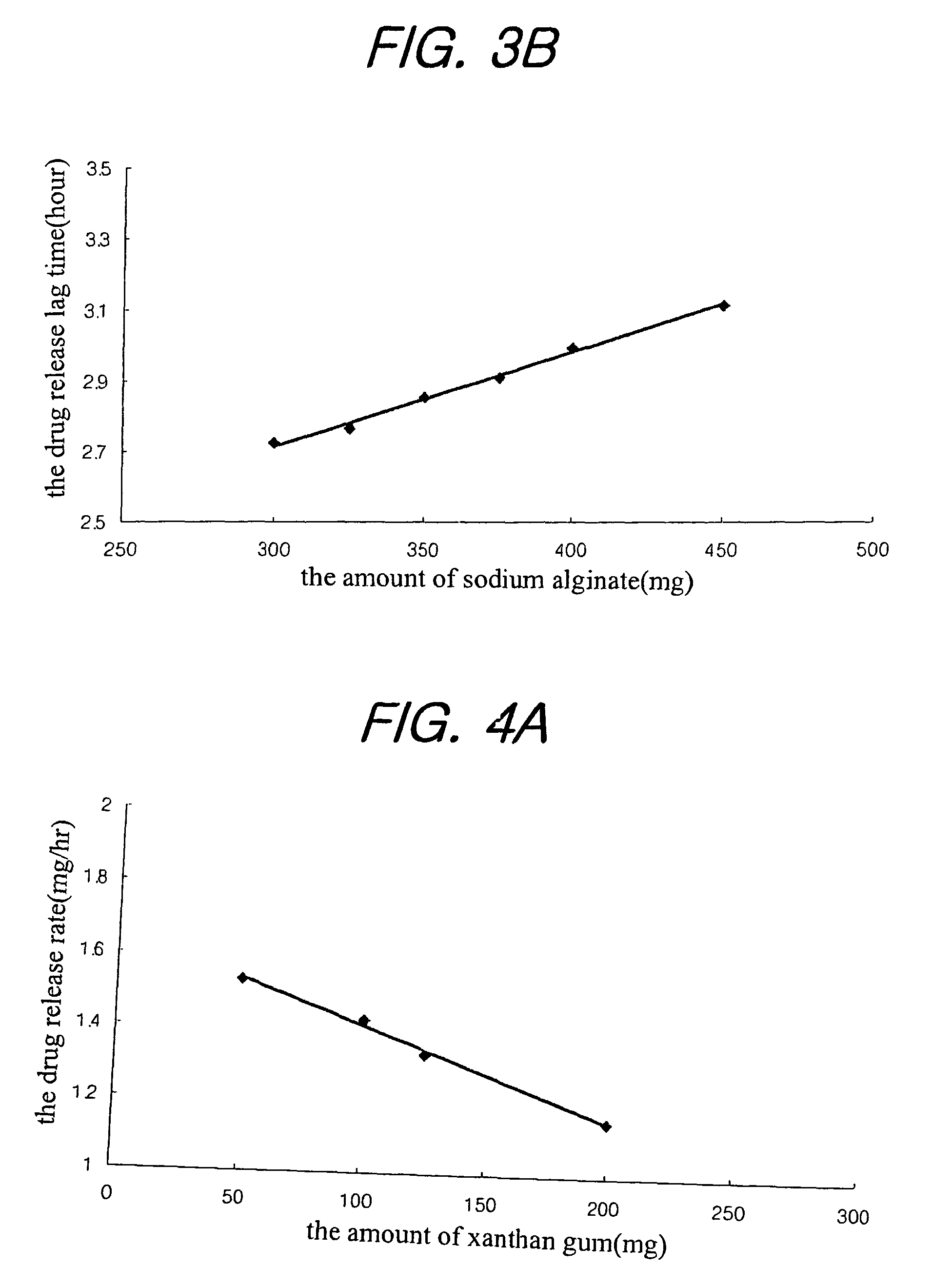
A sustained-release composition for oral administration of a drug, comprising the drug, a mixture of sodium alginate and xanthan gum as a carrier for sustained release and a mixture of hydroxypropyl methylcellulose and propylene glycol alginate as a gel hydration accelerator, which is capable of maintaining a constant drug level in blood for 24 hours or more. Due to rapid gel hydration without forming a non-gelated core, the drug release rate follows zero order kinetics and does not significantly vary with the degree of gastrointestinal motility.
Owner:HANMI PHARMA
Dissolution tank for drug level benzoic acid production and operation method
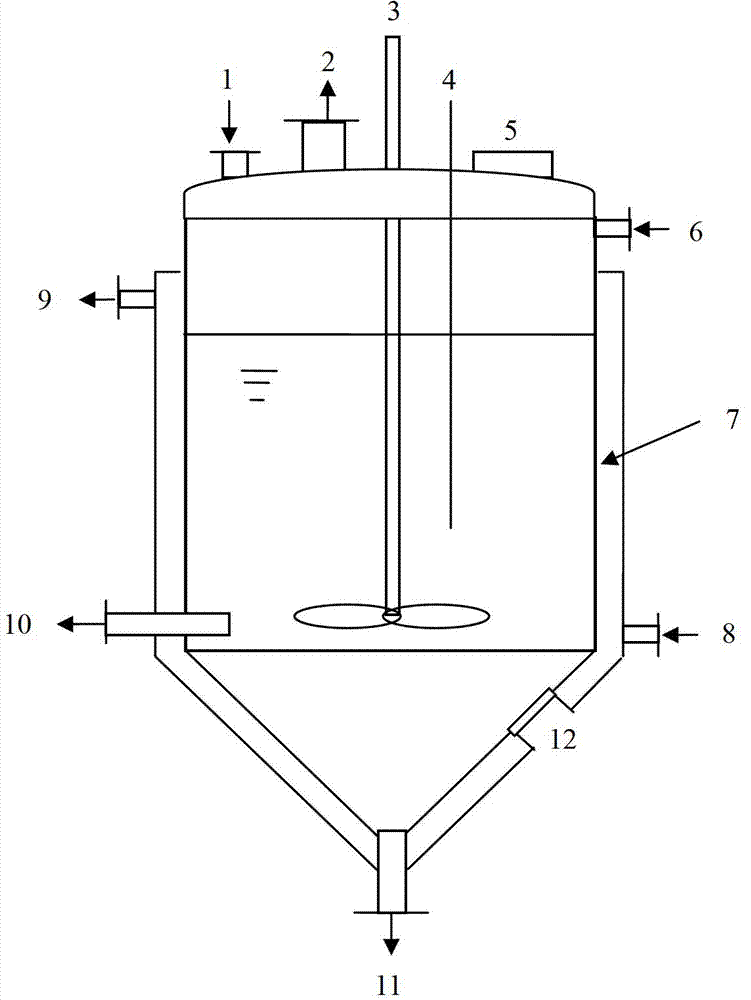
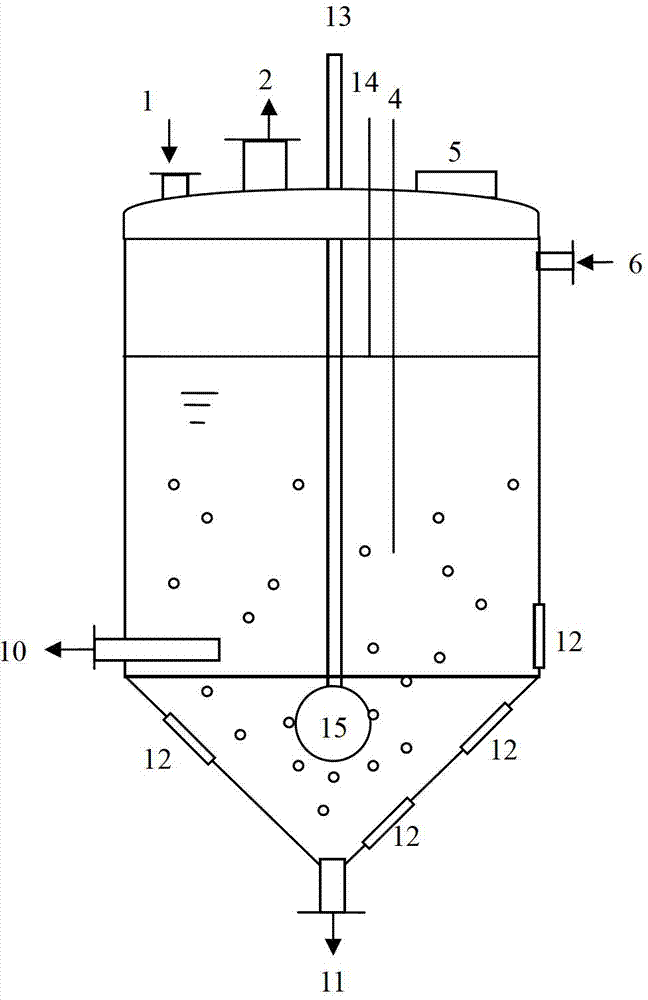
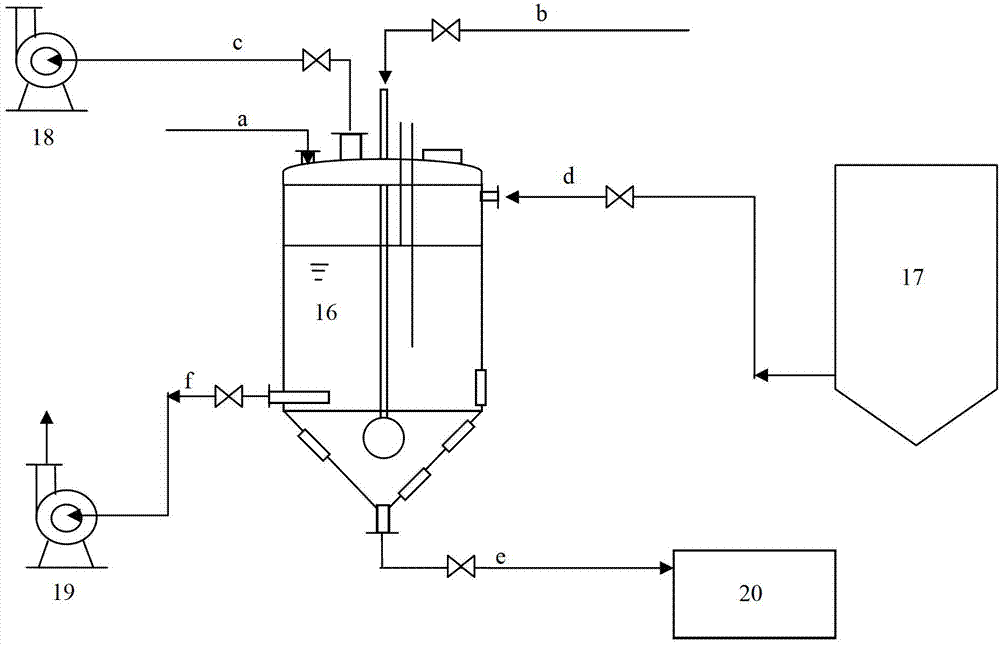
InactiveCN102961984BChange the heating and stirring methodImprove heat utilizationTransportation and packagingMixer accessoriesBenzoic acidDrugs levels
The invention provides a dissolution tank for drug level benzoic acid production and an operation method. The dissolution tank is divided into three parts including a butterfly-shaped sealing opening at the upper part, a straight cylinder at the middle part and a conical body at the lower part. The sealing opening at the upper part is provided with a steam inlet (13) in the middle; a steam inlet pipe is directly communicated with the conical body at the lower part; a steam distributor (15) is mounted at the tail end of a steam pipe; a raw material inlet (1), an air induction opening (2), a temperature sensor (4), a manhole (5) and a liquid level controller (14) are arranged at the top of the butterfly-shaped sealing opening; a mother liquor inlet (6) is arranged at the upper end of the straight cylinder; a discharging opening (10) and sight glass (12) are arranged at the lower end of the straight cylinder; a slag-out opening (11) is arranged at the bottom end of the conical body; two pairs of the sight glass are arranged at the upper part of the conical body; and one pair of sight glass is arranged at the lower part of the conical body. The dissolution tank for drug level benzoic acid production provided by the invention changes a heating and stirring manner of the dissolution tank, so that the heat energy use ratio is improved; the energy can be saved; the raw materials can be saved; the environment is protected; and the production efficiency is improved.
Owner:天津东大药业有限公司
Anti-cancer medicine composition
An anticancer pharmaceutical composition composed of pharmaceutic adjuvant and anticancer effective constituent is disclosed. The main anticancer constituents are anti-metabolism drug and DNA repair enzyme, wherein, the DNA repair enzyme inhibitor is chosen from poly(ADP ribose) polymerase inhibitor and / or DNA dependant protein kinase inhibitor, the DNA repair enzyme inhibitor can effectively destroy the DNA repairing function inside the tumor cell so as to lowers the tolerance of tumor cell to anti metabolism drug, while the main pharmaceutic adjuvant is biological compatible, degradable and absorbable macromolecule polymer, which can make the DNA repairing enzyme inhibitor release to the local region of tumor in the degradation and absorption process, therefore, it can both considerably lower the whole body toxic reaction and maintain the effective drug level of local region of tumor.
Owner:SHANDONG LANJIN PHARMA
Anti-cancer medicine composition
InactiveCN100438913CAntineoplastic agentsPharmaceutical active ingredientsWhole bodyTherapeutic effect
Owner:DASEN BIOLOGICAL PHARMA CO LTD
Externally-used traditional Chinese medicine for treating acne containing clam active ingredients
InactiveCN103877345BImprove removal effectGood curative effectDermatological disorderPlant ingredientsPimpleWhelk
The invention discloses an external traditional Chinese medicine containing effective components of astarte for treating acnes. The external traditional Chinese medicine is prepared by the following steps: putting striga asiatica, herba ardisiae japonicae, common achyranthes herb, inula flower, peperomia blanda, lance asiabell root, centipeda minima and semen allii tuberose into a decocting utensil; adding clean water according with the domestic drinking water standard; adding water to exceed the drug level 2-3cm, and soaking for half an hour; decocting by a slow fire for half an hour after boiling by a high fire; cleaning and mashing the clam meat and then adding to the decocting utensil to continue to decoct for half an hour by the slow fire; removing dregs and taking decoction to obtain the external traditional Chinese medicine. Compared with the prior art, the external traditional Chinese medicine is good in curative effect, short in course of treatment, small in side effect, convenient to use, and low in cost. The active components extracted from a natural animal and plant composition are developed by long-term exploration combined with civil experience. The external traditional Chinese medicine has a good removal effect on acnes, pimples and whelks, and is safe and reliable to use, convenient and sanitary.
Owner:ANHUI MUCHUNTANG NATURAL MEDICINE RES DEV CO LTD
A kind of synthetic method of liraglutide
ActiveCN103304660BHigh selectivityHigh purityPeptide preparation methodsBulk chemical productionChemical synthesisDrugs levels
The invention discloses a full chemical synthetic method for hybridization of a solid phase and a liquid phase of liraglutide. The method comprises the following steps: chemically synthesizing a liraglutide precursor protected by N terminal and a cetyl derivative; de-protecting to remove tail end protection to obtain a target polypeptide. The liraglutide precursor semi-protected is obtained by polypeptide solid-phase synthesis, and the precursor purified to the drug level enters into the next chemical synthesis.
Owner:SHANGHAI AMBIOPHARM
Glutaminase inhibitor discovery and nanoparticle-enhanced delivery for cancer therapy
ActiveUS10660861B2Poorly solubleIncrease heightOrganic active ingredientsOrganic chemistryPancreas CancersEfficacy
Currently available glutaminase inhibitors are generally poorly soluble, metabolically unstable, and / or require high doses, which together reduce their efficacy and therapeutic index. These can be formulated into nanoparticles and delivered safely and effectively for treatment of pancreatic cancer and other glutamine addicted cancers. Studies demonstrate that nanoparticle delivery of BPTES, relative to use of BPTES alone, can be safely administered and provides dramatically improved tumor drug exposure, resulting in greater efficacy. GLS inhibitors can be administered in higher concentrations with sub-100 nm nanoparticles, since the nanoparticles package the drug into “soluble” colloidal nanoparticles, and the nanoparticles deliver higher drug exposure selectively to the tumors due to the enhanced permeability and retention (EPR) effect. These factors result in sustained drug levels above the IC50 within the tumors for days, providing significantly enhanced efficacy compared to unencapsulated drug.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com