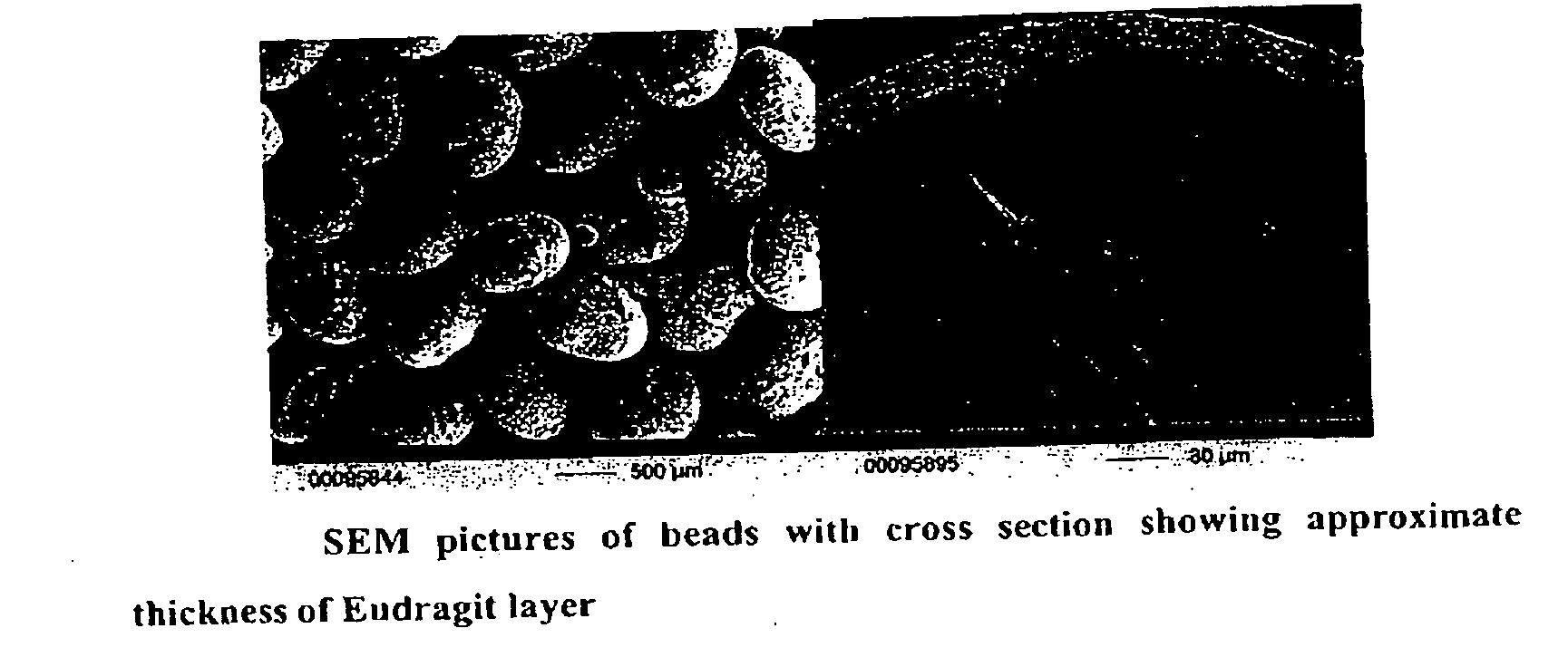
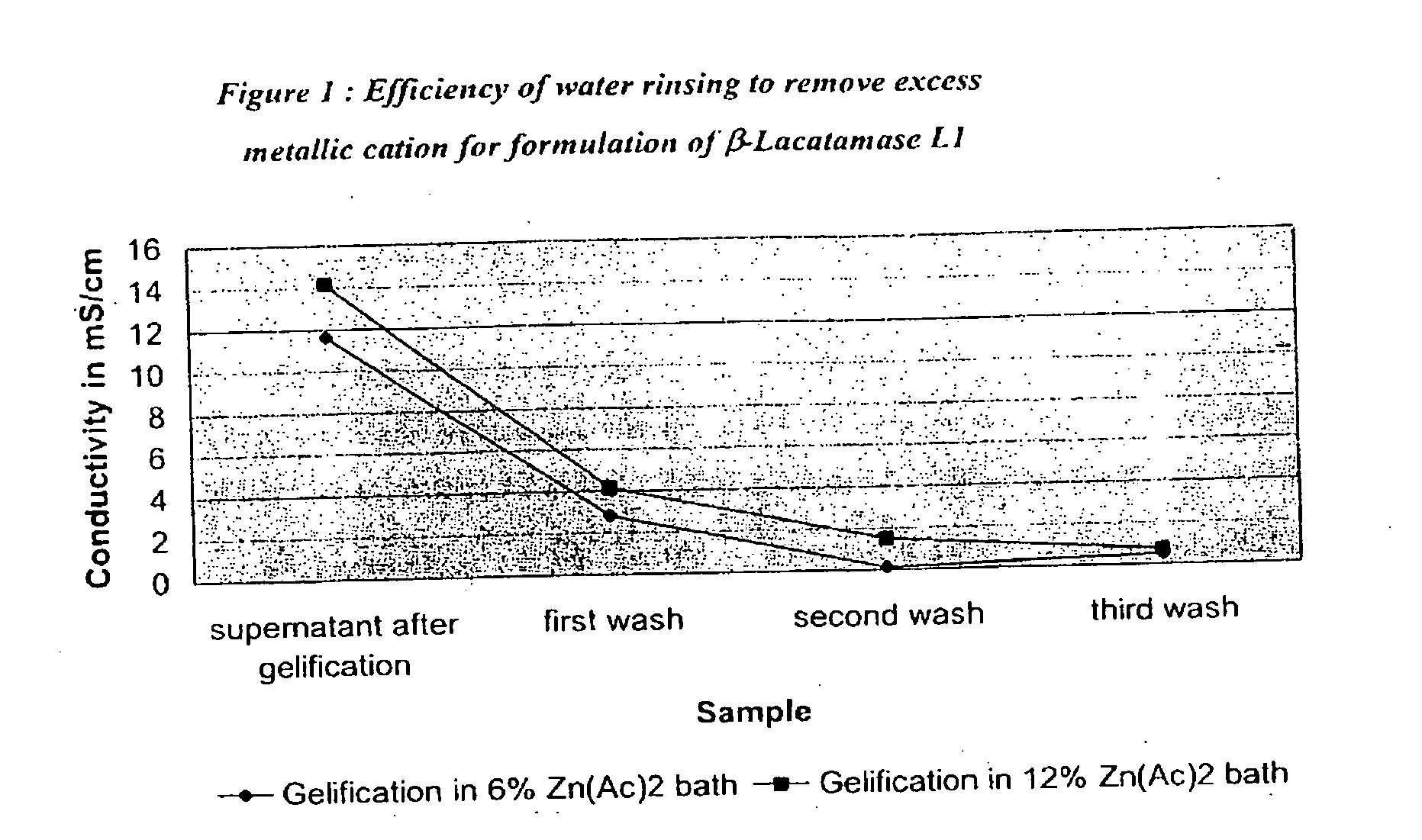
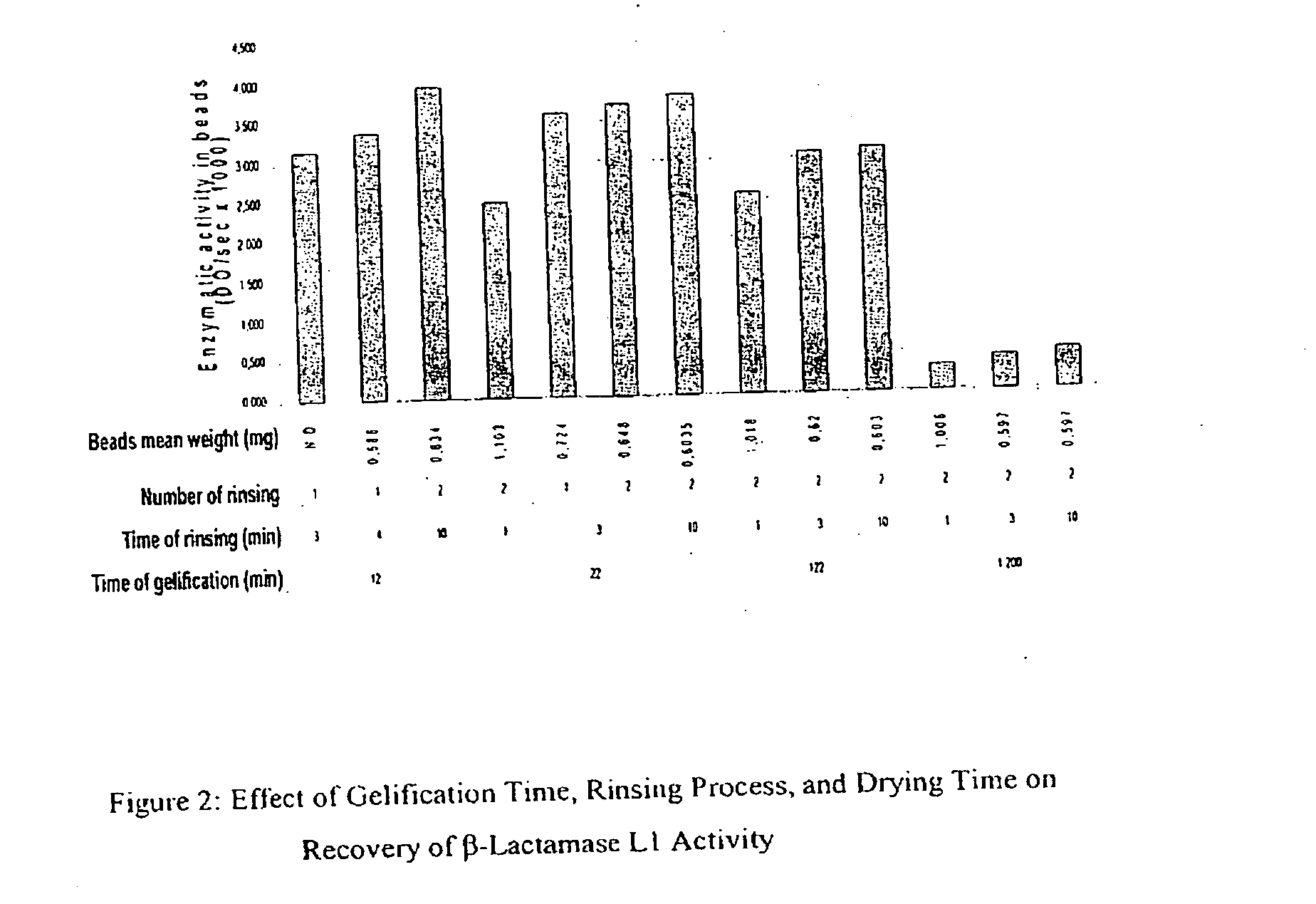
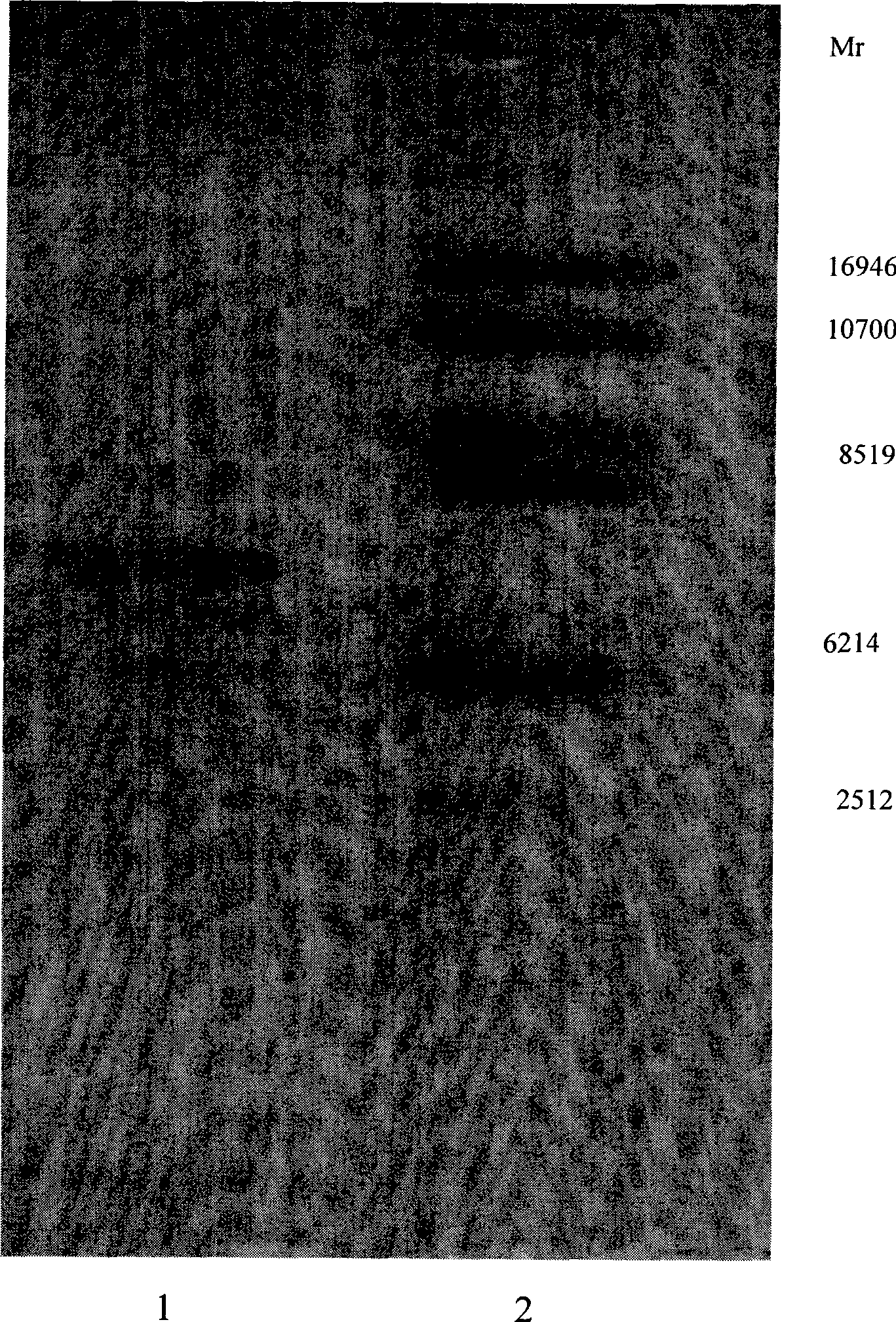
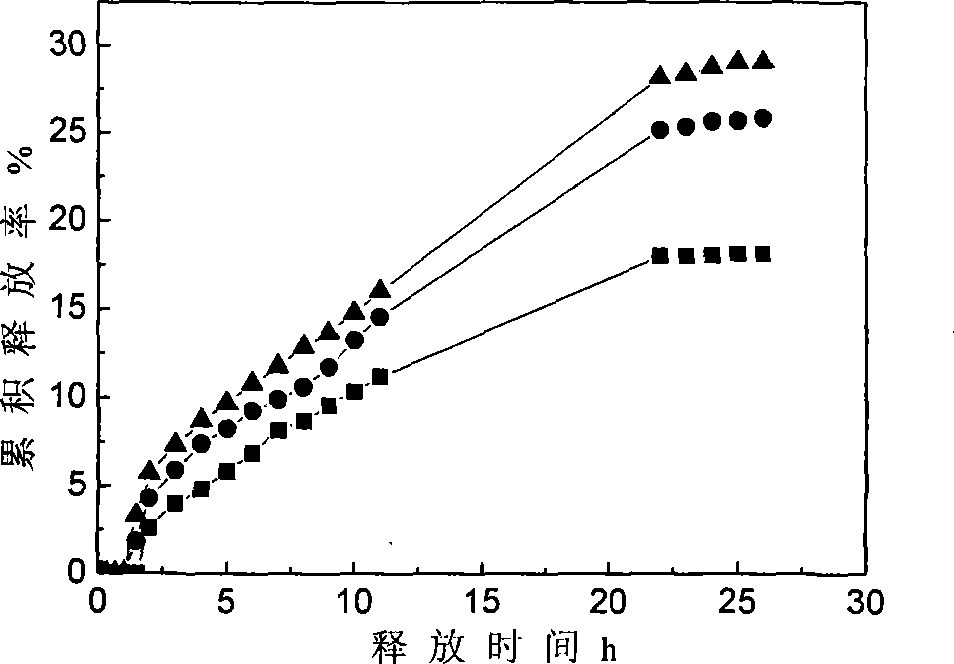
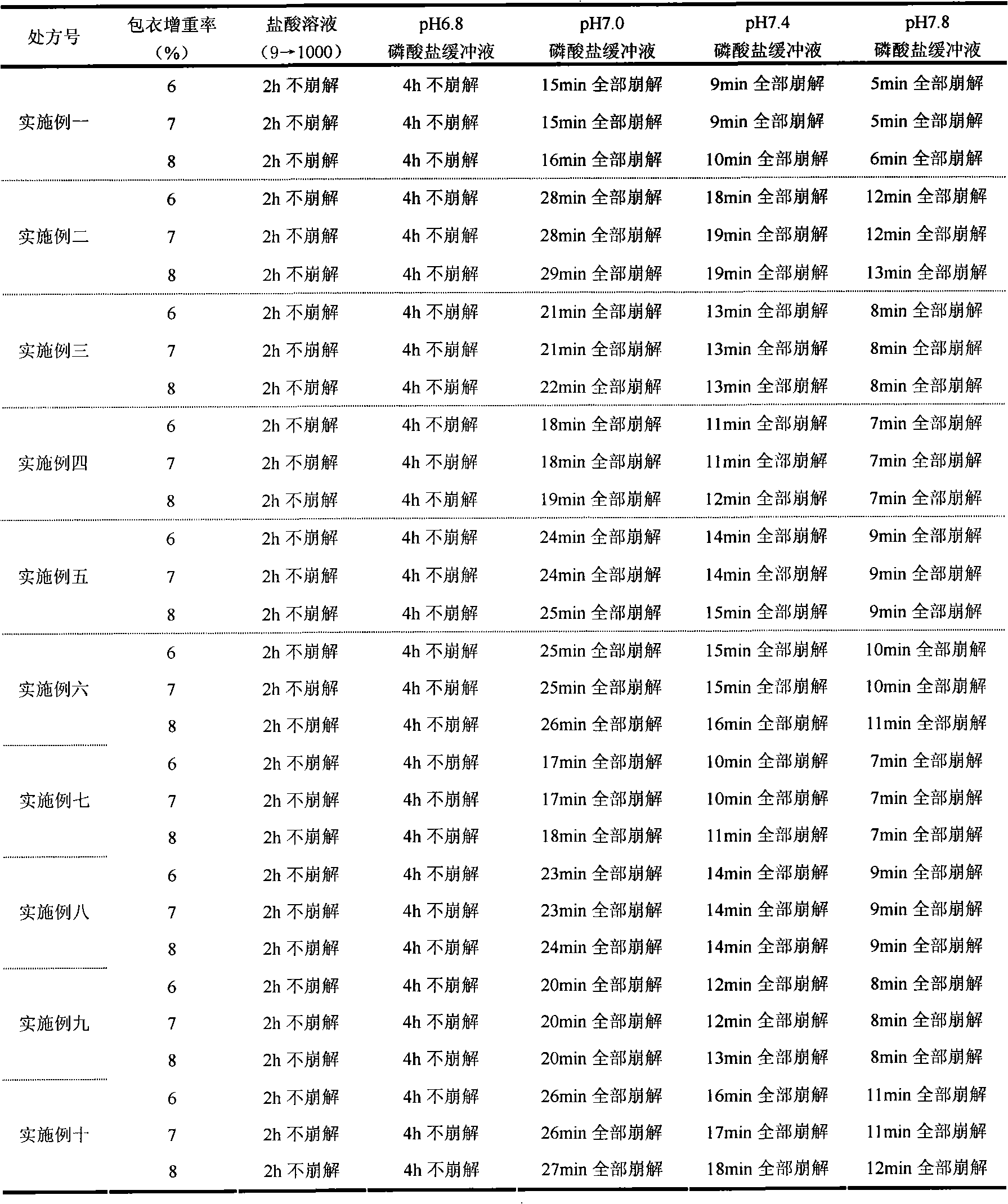
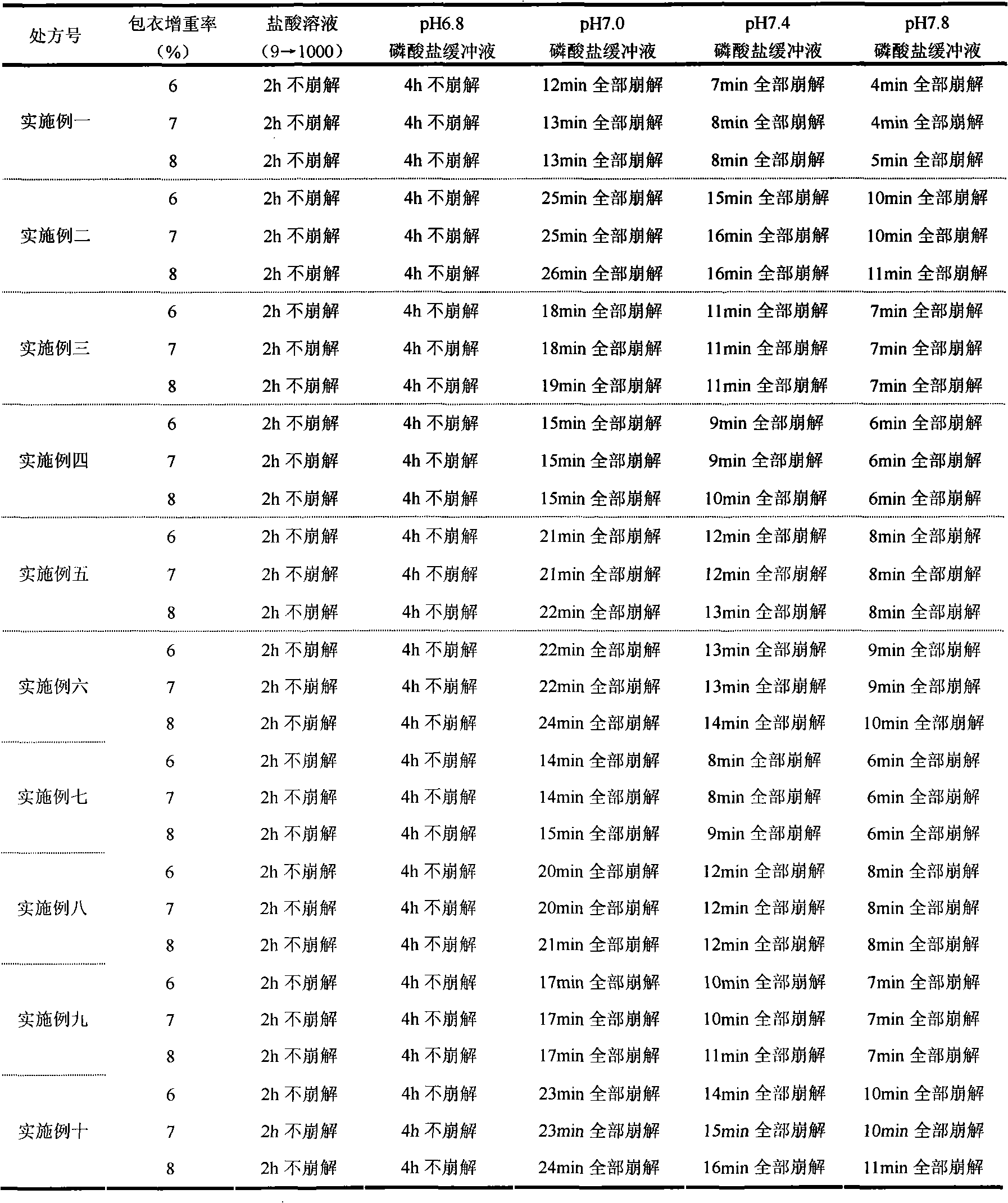
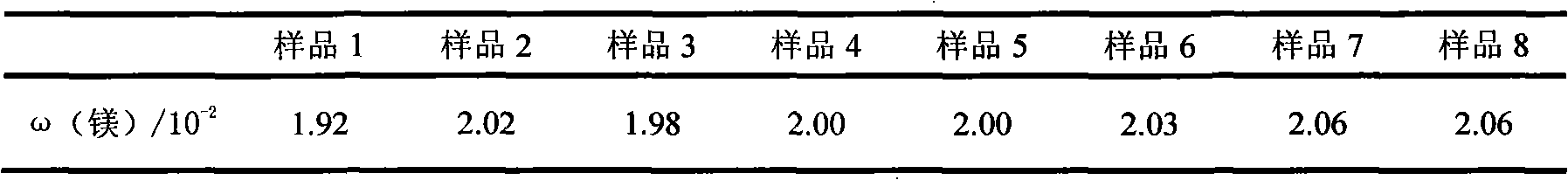
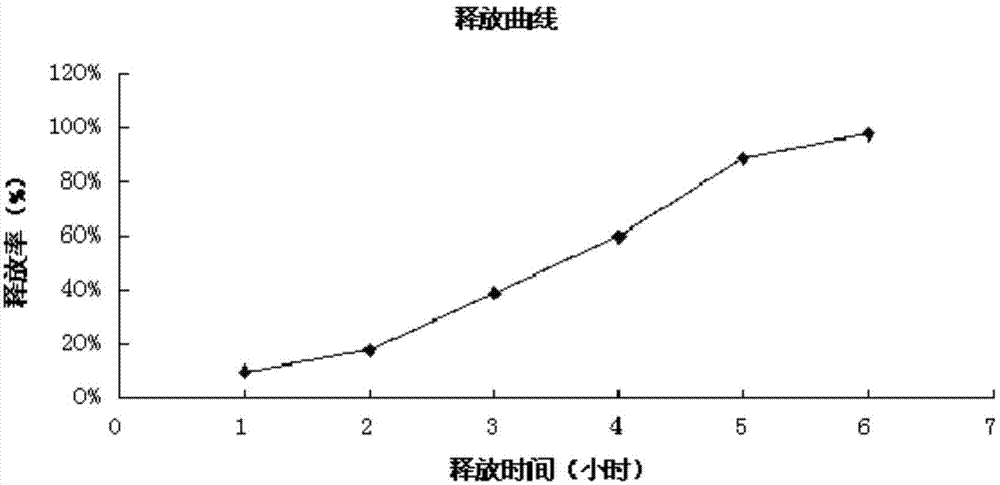
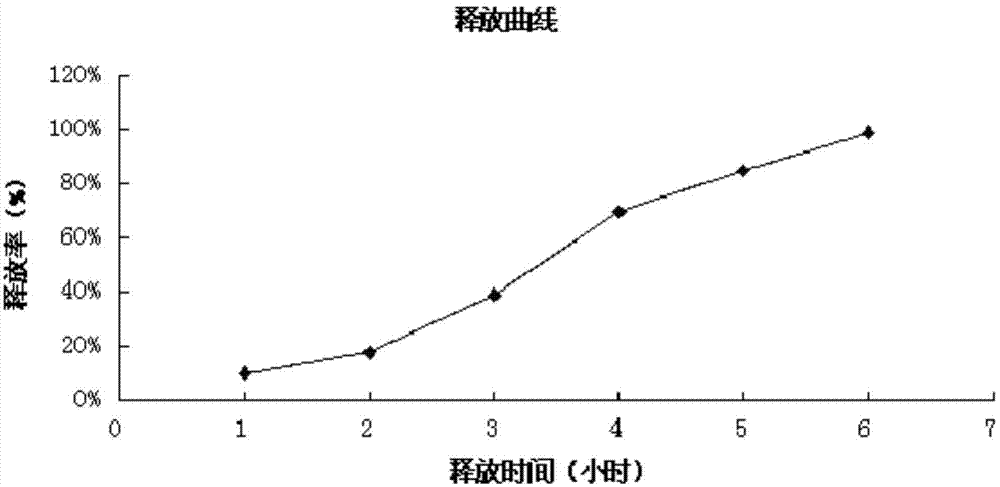
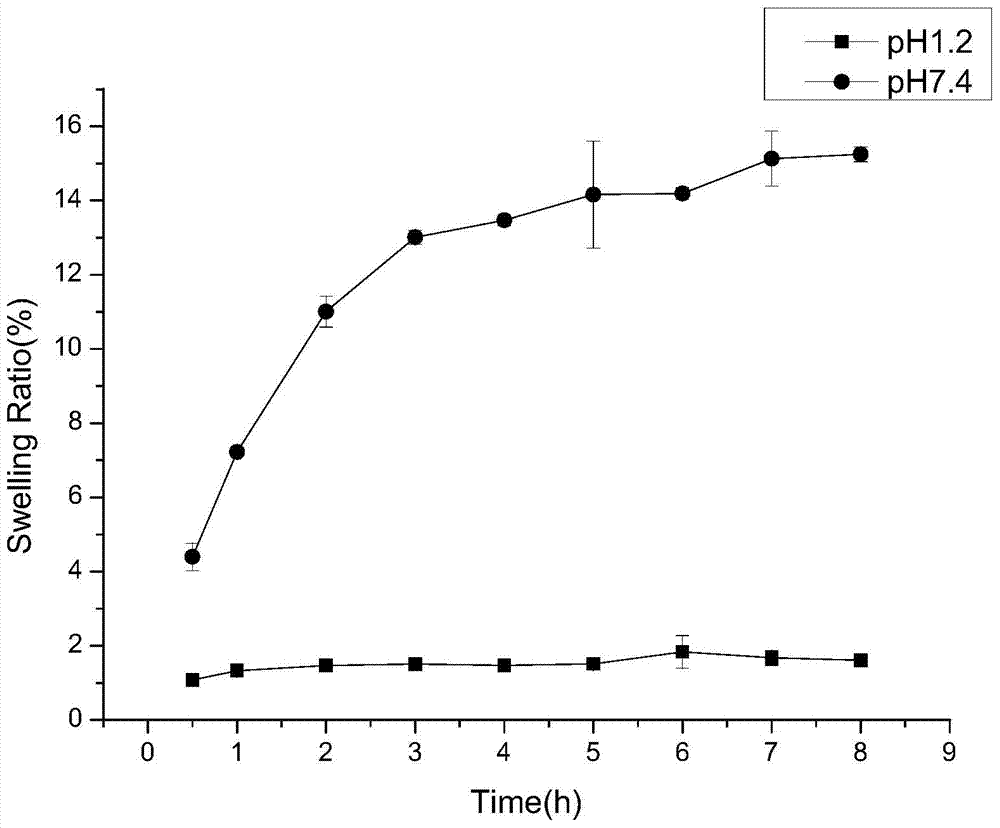
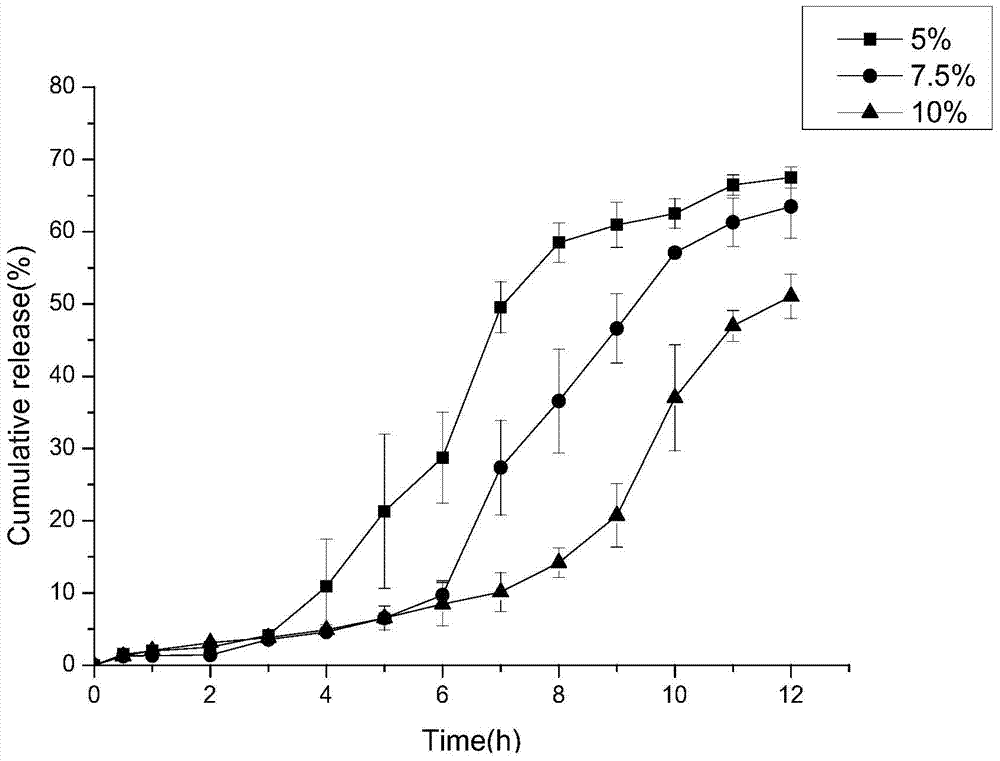
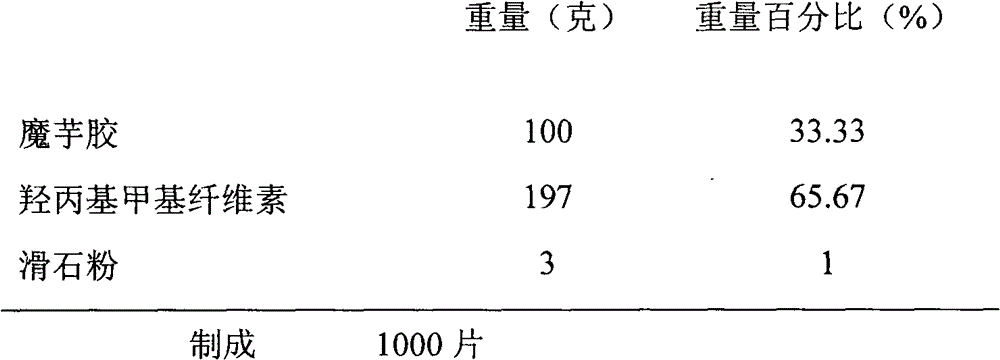
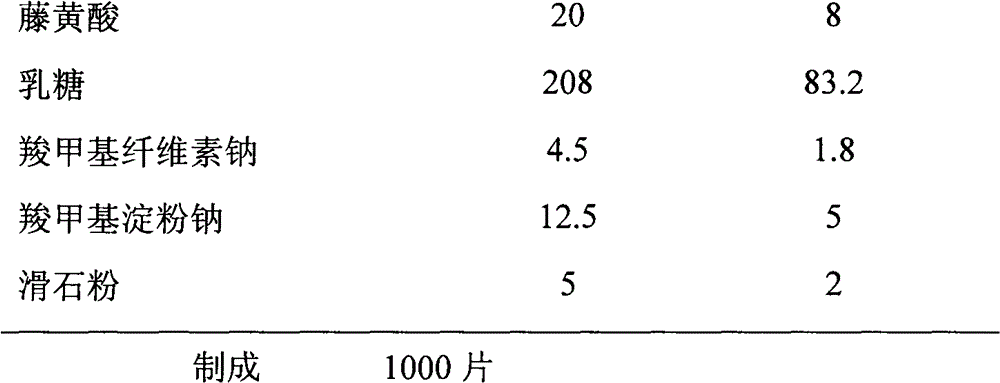Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Colon specific" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral pharmaceutical preparation for colon-specific delivery
InactiveUS20100209520A1Sufficient therapeutic effectPrecision releaseBiocideNervous disorderInter layerAnionic polymers
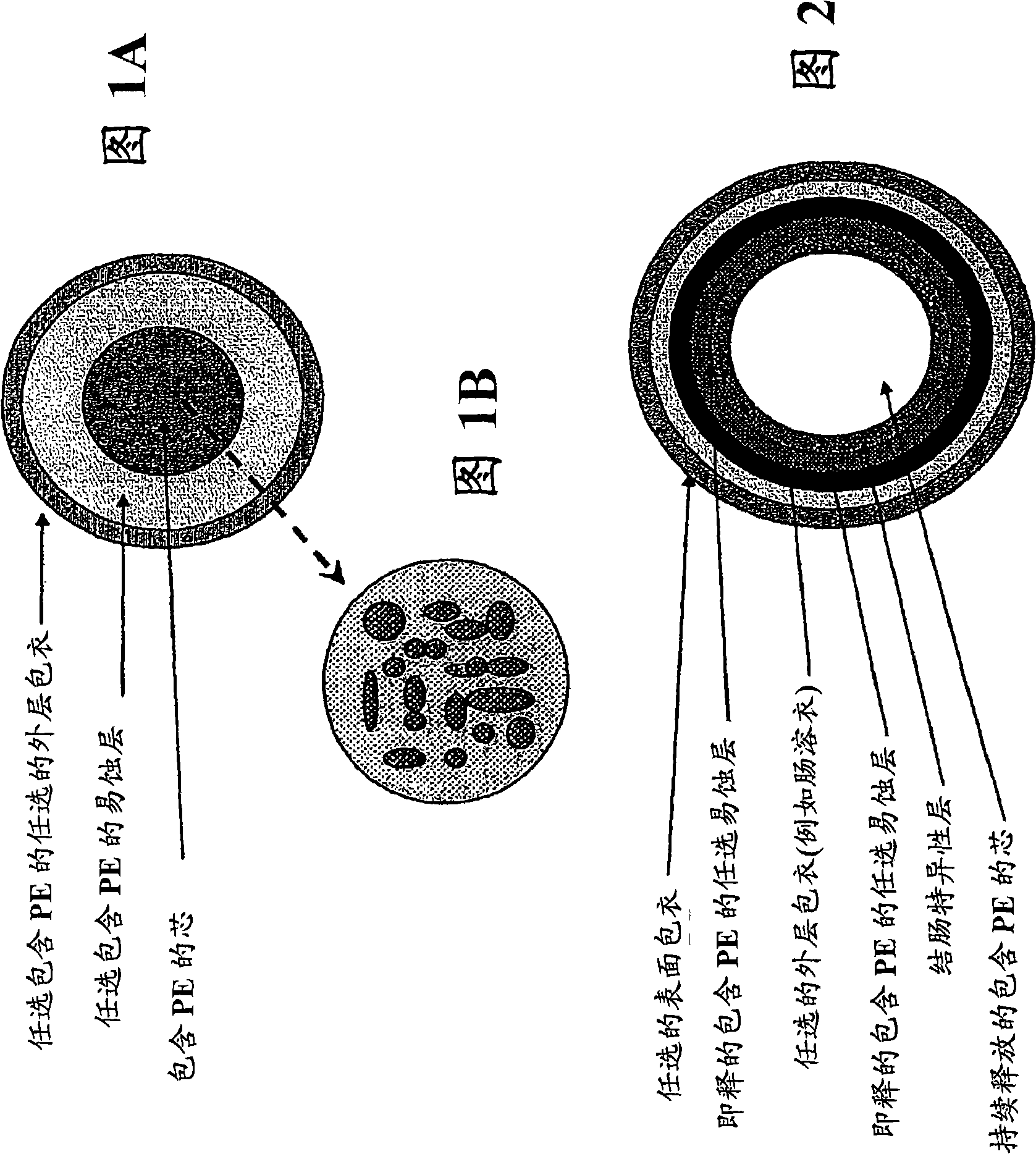
The present invention relates to an oral pharmaceutical preparation having an excellent capability of delivering a drug to colon, more specifically an oral pharmaceutical preparation for delivering a drug to colon and comprising a core comprising at least a pharmaceutically acceptable vehicle, an inner layer covering said core and comprising said drug, an intermediate layer covering said inner layer and comprising a cationic polymer soluble or swellable at a pH of not more than 6.6, and an outer layer covering said intermediate layer and comprising an anionic polymer soluble at a pH of not less than 7.0.
Owner:AMATERASPHARMA INC
Colonic delivery using Zn/pectin beads with a Eudragit coating
InactiveUS20080124279A1Quality improvementGood effectPowder deliveryDigestive systemActive agentInfective disorder
Drug delivery systems that can deliver therapeutic and / or diagnostic agents to the colon are disclosed. The systems include pectin beads crosslinked with zinc or any divalent cation of interest, which beads are then coated with Eudragit®-type polymers. The drug delivery systems are orally administrable, but can deliver the active agents to the colon. In some embodiments, they can administer the agents to various positions in the gastro-intestinal tract, including the colon. The agent can be a small molecule, peptide, protein, nucleic acid, or complex structures of natural, recombinant or synthetic origin. In still other embodiments, the agent is a diagnostic agent. The agents can be used to diagnose, treat or investigate humans and animals for a variety of conditions, including infectious diseases, inflammatory diseases, cancers and the like. Colon-specific delivery is obtained by formulating a prophylactic, therapeutic, and / or diagnostic agent with specific polymers that degrade in the colon, such as pectin. The pectin is gelled / crosslinked with a cation such as a zinc cation. The formulation, typically in the form of ionically crosslinked pectin beads, is subsequently coated with a specific polymer such as a Eudragit® polymer. Processes for obtaining such beads are also disclosed.
Owner:DA VOLTERRA FACULTE DE MEDECINE BICHAT
Cobratide extraction method, cobratide extracted thereby and formulation containing cobratide
ActiveCN101381408AHigh puritySimple methodNervous disorderPeptide/protein ingredientsFreeze-dryingUltrafiltration
The invention relates to a method for extracting cobratide, a medicine used to treat chronic pain, the cobratide extracted by the method and a preparation containing the cobratide. The method for extracting the cobratide comprises the following steps: firstly, a stock solution of snake poison of a cobra is pretreated with ammonium sulphate to remove partial other compositions in the snake poison,is subjected to desalination and concentration through ultrafiltration, dialysis or gel chromatography, and after the concentration, is purified by a cation column, and finally is subject to freeze-drying preservation after the concentration. The cobratide preparation comprises an enteric preparation, a rectal administration preparation, a colon-specific administration preparation and so on. Withcobratide extraction and purification processes provided by the method, the purity of the extracted cobratide is more than 95 percent, and the yield reaches 70 percent; and the method is simple and reliable, and saves cost and time.
Owner:BEIJING SAISHENG PHARMA
Oral mesalazine colon-specific adhesive pellet
InactiveCN103211780AHas absorptionBioadhesiveOrganic active ingredientsDigestive systemTolerabilityPatient compliance
The invention discloses an oral mesalazine colon-specific adhesive preparation with a high drug-loading rate, which can be used for effectively treating ulcerative colitis and segmental ileitis, as well as a preparation method of the oral mesalazine colon-specific adhesive preparation. The preparation is composed of a pill-containing core and an enteric coating layer, wherein the pill-containing core consists of mesalazine, excipient, adhesive and boning agent. Compared with the other oral preparations, the oral mesalazine colon-specific adhesive pellet has the effects of improving the curative effect and lowering adverse reactions when the dosage is the same. Compared with enema and suppository, the pellet has better tolerance. The preparation method can be used for effectively controlling the temperature in the production process and avoiding an overheating phenomenon in the preparation process, and is simple in preparation process and high in drug-loading rate. Besides, the oral mesalazine colon-specific adhesive pellet is applicable to the large-dose dosing characteristic of mesalazine, thereby reducing the medicine taking inconvenience of a patient and improving the dependence of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Sustained release pharmaceutical formulation comprising phenylephrine
The invention discloses a pharmaceutical composition comprising phenylephrine or a pharmaceutically acceptable salt thereof and an erodible layer which is for oral administration wherein the composition delivers phenylephrine or a pharmaceutically acceptable salt thereof via absorption in the colon. The pharmaceutical composition comprises a core comprising phenylephrine or a pharmaceutically acceptable salt thereof and an erodible layer which is in a time-dependent, pH-dependent, or colon-specific enzyme-dependent erodible layer that degrades to expose the core to release phenylephrine in the colon. In one preferred embodiment, the erodible layer encases the core. The composition optionally further comprises phenylephrine in the erodible layer or other additional layer(s). The pharmaceutical composition can further comprise one or more additional therapeutically active agents selected from one or more of the group consisting of antihistamines, analgesics, anti-pyretics, and non-steroidal anti-inflammatory agents. The invention also discloses methods of administering phenylephrine via the colon, thereby increasing the bioavailable amount of therapeutically active unconjugated phenylephrine relative to the total phenylephrine in the plasma.
Owner:BAYER CONSUMER CARE
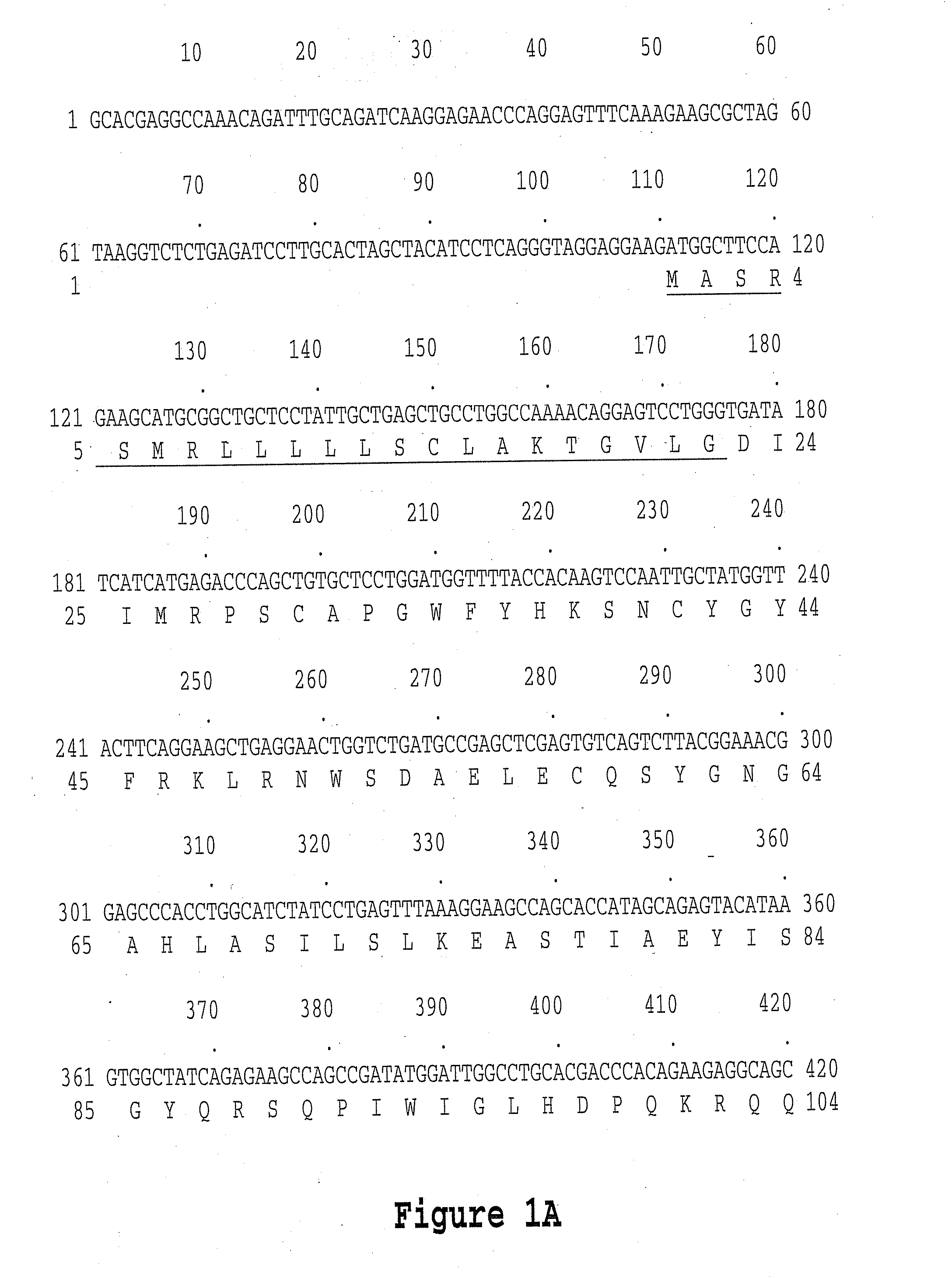
Colon Specific Gene and Protein and Cancer
InactiveUS20070178108A1Facilitated DiffusionPeptide/protein ingredientsMicrobiological testing/measurementCancer cellNucleotide
Human Colon Specific Polynucleotides (DNA and RNA), Polypeptides, and Antibodies, as well as methods for using and producing such polynucleotides, polypeptides, and antibodies are disclosed. More particularly, methods are disclosed for utilizing such polynucleotides, polypeptides, and antibodies to detect, diagnose, prevent, treat, and / or ameliorate cancer (particularly gastrointestinal tract cancers such as colon and pancreatic cancer). Also disclosed are compositions and methods for targeting and destroying cancer cells (particularly gastrointestinal tract cancers such as colon and pancreatic cancer) via the Colon Specific Protein and / or via the Colon Specific Protein Receptor. Moreover, methods of screening for antagonists and binding partners of the Colon Specific Protein and therapeutic uses of such antagonists and binding partners are also disclosed.
Owner:HUMAN GENOME SCI INC
Rheum officinale anthraquinone oral colon targeted drug delivery composition and application thereof
InactiveCN103565941ADoes not diminish laxative effectSolve the problem of drug source toxicityDigestive systemPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention relates to an oral colon targeted drug delivery composition capable of reducing toxic and side effects of rheum officinale anthraquinone and application of the oral colon targeted drug delivery composition. The oral colon targeted drug delivery composition is composed of rheum officinale anthraquinone subjected to freeze drying and auxiliary materials, wherein the auxiliary materials are selected from a colon specificity enzymatic degradable material, a material soluble in medium with pH more than or equal to 6.8, a filler and a bonding agent. The oral colon targeted drug delivery composition can not weaken purgation effect of rheum officinale and also can be used for effectively preventing toxicity of rheum officinale from being played after colon-targeted drug delivery and is basically non-toxic after being taken for a long time, so that the problem of pharmaceutical toxicity of rheum officinale is solved.
Owner:承德医学院中药研究所
Segmented intestine targeted drug feeding preparation of brain protein polypeptide and method of preparing the same
InactiveCN101130062AAvoid painSimple production processNervous disorderPeptide/protein ingredientsIntestinal structurePeptide drug
The invention discloses a colon specific targeting drug-delivery preparation of cerebroprotein polypeptides and its preparing process, wherein the preparation is prepared through coating the drug-containing micro-pellets made from medicinal powder containing cerebroprotein polypeptides components with colon targeting coating layer, or directly loading the drug-containing micro-pellets into the shells of the colon targeting capsule. The process for preparing the colon targeting drug-delivery preparation of the cerebroprotein polypeptides consists of producing drug-containing micro-pellets containing cerebroprotein polypeptides components, then preparing the colon targeting coating liquid, finally coating the drug-containing micro-pellets with the colon targeting coating liquid. The preparation provided by the invention can make the colon release drugs enter blood circulation through colon, thereby avoiding metabolism destruction of the conventional oral preparation peptides drugs on the stomach, as a result, the therapeutic action of the drugs can be sufficiently exploited.
Owner:李立
Cation-modified konjac glucomannan gellan gum microsphere as well as preparation method and application
InactiveCN104225625AIncreased efficiency of entry into colon tissueReduce the possibility of being intercepted by the stomach and small intestineGenetic material ingredientsDigestive systemGellan gumMicrosphere
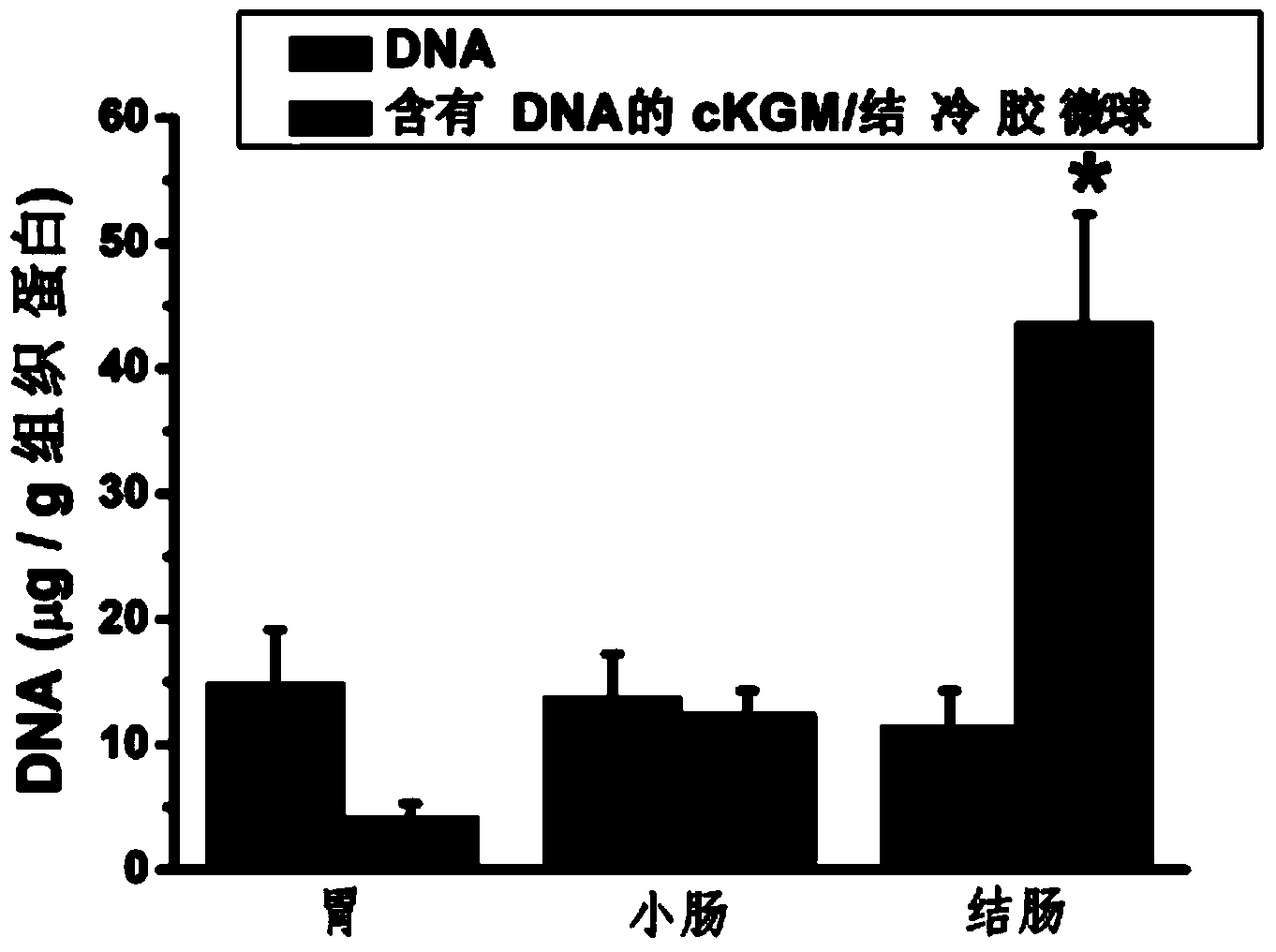
The invention belongs to the technical field of the biological medicine, and particularly relates to a cation-modified konjac glucomannan gellan gum microsphere as well as a preparation method and an application. The cation-modified konjac glucomannan gellan gum microsphere is prepared from a nucleic acid drug and a drug delivery carrier, wherein the nucleic acid drug is ribonucleic acid or desoxyribonucleic acid, and a drug delivery system comprises the components as follows: 20-30 parts of cKGM (cation konjac glucomannan), 1-9 parts of gellan gum and 0.01-6 parts of the nucleic acid drug. According to the microsphere, the requirement for colon-specific drug delivery of the oral microsphere is met, and the microsphere is simple in preparation technology and easy to industrialize and has very broad development and application prospects.
Owner:NANJING UNIV
Segmented intestine targeted drug feeding preparation of myocardium protein polypeptide and method of producing the same
InactiveCN101130061AAvoid painSimple production processPeptide/protein ingredientsHydroxy compound active ingredientsIntestinal structureAdditive ingredient
The invention discloses a colon specific targeting drug-delivery preparation of myocardial protein polypeptides and its preparing process, wherein the preparation is prepared through coating the drug-containing micro-pellets made from medicinal powder containing myocardial protein polypeptides components with colon targeting coating layer liquid, or directly loading the drug-containing micro-pellets into the shells of the colon targeting capsule. The process for preparing the colon targeting drug-delivery preparation of the myocardial protein polypeptides consists of producing drug-containing micro-pellets containing myocardial protein polypeptides components, then preparing the colon targeting coating liquid, finally coating the drug-containing micro-pellets with the colon targeting coating liquid. The preparation provided by the invention can make the colon release drugs enter blood circulation through colon, thereby avoiding metabolism destruction of the conventional oral preparation peptides drugs on the stomach, as a result, the therapeutic action of the drugs can be sufficiently exploited.
Owner:李立
Novel capsule colon-specific drug delivery system (CSDDS) and preparation method thereof
InactiveCN102133207AThe principle of controlled release is clearPrecision releaseCapsule deliveryOil/fats/waxes non-active ingredientsFluidized bedColon parts
The invention relates to a novel colon-specific drug delivery system (CSDDS) and preparation method. The capsule CSDDS has the advantages that the release control principle is clear, the preparation process is simple, the drug release is precise, the scope of suitable drugs is wide, and the drug release adjustment is convenient. The capsule CSDDS provided by the invention comprises an insoluble capsule, an enzymolysis capsule, a soluble capsule cap and an enteric coating, can be used for filling chemical medicaments, traditional Chinese medicines, biological product medicines, and the like, and can be used for controlling the release of the medicaments on a colon part. The insoluble capsule of the CSDDS can be prepared by using a conventional capsule shell preparation method; the enzymolysis capsule can be prepared by using a conventional tabletting method; the enteric coating can be prepared by using a fluidized bed method; and a release test shows that the capsule shell has the advantage of controlling the medicaments to release at the colon part.
Owner:WENZHOU MEDICAL UNIV
Novel pH-dependent colon-specific drug delivery system
InactiveCN101209246AIncrease concentrationImprove efficacyPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineColon parts
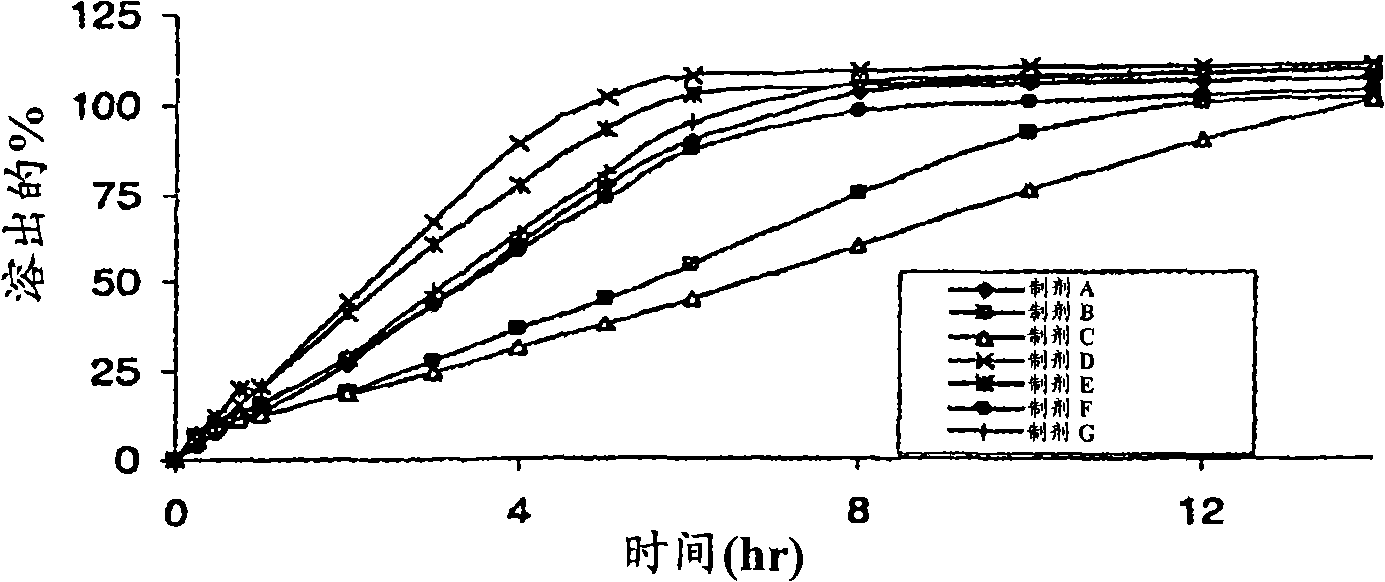
The invention relates to a novel pH-dependent colon-specific drug delivery system, which adopts pH-dependent colon-specific drug delivered polymer thin film coating material as the main coating material, a tablet core forms a hydrophilic gel frame structure, the coating film can be dissolved at specific parts (part is dissolved at the tail end of small intestine and the most is dissolved at the colon part) according to the condition of high pH (pH is more than 7.2) of the tail end of small intestine and the colon part, so as to play the role of colon-specific drug delivery. The invention is based on the principles of chrono-pharmacology and human physiology, which can ensure the pH-dependent colon-specific drug delivered polymer thin film coating material to be released slowly and for 12 hours in a sustained way after the dissolution at least, so as to achieve the oral colon-specific slow release effect.
Owner:TIANJIN MEDICAL UNIV
Colon targeting drug delivery soft capsule and preparation method thereof
ActiveCN101167765AMake up for the gap in development and applicationEasy to prepareDigestive systemCapsule deliveryCellulose acetateMedicine
The invention discloses a colonic targeting drug-delivery soft capsule, which comprises a brucea javanica oil soft capsule and high polymer coating film, wherein the high polymer coating film is pH-sensitive enteric coated cover which contains component of ethyl cellulose or acetyl cellulose. The invention provides accurate composition of high polymer coating film, realizes indisintegration of soft capsule after being coated in the buffer solution with pH value less than seven and disintegration in the buffer solution with pH value more than seven, and achieves requirements of ideal colon-specific drug delivery. The invention with simple process for preparation and mature process condition compensates the blank that no soft capsule for colon drug delivering is developed and applied.
Owner:惠州市九惠药业有限公司
Ph-dependent composite bone peptide preparation, preparing method and use thereof
ActiveCN101015580AHigh densityHigh strengthPeptide/protein ingredientsSkeletal disorderRheumatismHepatic first pass effect
This invention relates to a pH dependent compound bone peptide preparation and its preparation method and application. The preparation method comprises carrying out pretreatment of animal bones of limbs powder and cucumber seed powder, extracting compound bone peptide, concentrating, clathrating with beta-cyclodextrin, and making into colon-specific slow release capsule or tablet in order to avoid gastric juice hydrolysis and hepatic first pass effect to destroy its bioactivity. The compound bone peptide has a molecular weight below 5000 Daitor, and can be used for preventing fracture and osteoporosis, treating ostalgia, rheumatism and rheumatoid diseases.
Owner:GUANGDONG MEDICAL UNIV
Method for preparing colon positioning compression-coated tablets
InactiveCN101433525AColon-localized drug release effect is obviousRegulated drug release processPharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarrageenanAdhesive
The invention discloses a method for preparing a colon-specific pressed coated tablet. The method comprises the following steps: (1) mixing Konjac Glucomannan, xanthan gum or carrageenan and a diluting agent; adding an adhesive to the mixture, pelletizing the mixture by a wet method, drying the mixture until constant weight, finishing pellets, and adding a lubricating agent to the mixture to evenly mix, so as to obtain a coating material of the colon-specific pressed coated tablet; (2) evenly mixing bulk drugs and a disintegrant, adding an adhesive to the mixture, pelletizing the mixture by the wet method, drying the mixture until constant weight, finishing pellets, and adding the lubricating agent to the mixture to evenly mix, so as to obtain a drug-contained tablet core; and (3) coating the coating material of the colon-specific pressed coated tablet outside the drug-contained tablet core to be prepared into the colon-specific pressed coated tablet. The colon-specific pressed coated tablet prepared by the method has the characteristic of obvious colon-specific release effect, can conveniently regulate the release process of the tablet through changing the thickness of a coating outer layer, the proportion of blending amylose and the content of the diluting agent. The method has the advantages of wide drug loading system, wide raw material source, low price and simple preparation process.
Owner:TIANJIN UNIV
Production of mesalazine colon-specific drug administration sustained-release tablet
The invention discloses a production of a mesalazine colon-specific drug administration sustained-release tablet. The mesalazine (5-ASA) is widely applied clinically, and becomes a priority drug for treating inflammatory bowel diseases. The mesalazine is ineffective when being orally taken, because the mesalazine is quickly absorbed in the small intestine, then acetylated and discharged along with urine, thus being not able to reach the colon part. 5-ASA can only play the treatment function when reaches the colon lesion part in a prototype, and is produced into a pH-dependent colon-specific drug administration sustained-release tablet, so that the 5-ASA drug which reaches the colon has enough concentration to have an anti-inflammatory effect. Eudragit L100 and Eudragit S100 can be used to produce a film coating which meets the colon-specific release requirement according to a certain proportion. The mesalazine is produced into a coating sustained-release tablet, so that the 5-ASA drug which reaches the colon has enough concentration to achieve the anti-inflammatory effect.
Owner:JILIN INST OF CHEM TECH
Oral colon-specific release film coating premixed accessory of pH dependent type and preparation method thereof
ActiveCN101934079AMeet disintegration requirementsImprove quality and stabilityInorganic non-active ingredientsPharmaceutical delivery mechanismPlasticizerALLYL SUCROSE
The invention relates to an oral colon-specific release film coating premixed accessory of a pH dependent type, characterized by mainly comprising the following components in percentage by weight: 40-70 percent of polyacrylic acid resin III, 15-23 percent of plasticizer and the balance of accessory materials, wherein the polyacrylic acid resin III has the viscosity number of 20-35 mPa*s and the acid number of 210-220. The technical scheme of the invention has the effects of low cost, favorable disintegration release performance and less and non-sensitive coating weight increase.
Owner:浙江瓯伦包衣技术有限公司
Controlled-release enteric acrylic resin latex and preparation method thereof
ActiveCN102432737ATo achieve the effect of sustained releaseGood water penetration resistancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsPotassium persulfateAcrylic resin
The invention discloses a controlled-release enteric acrylic resin latex which is characterized by being prepared from the following raw materials in percentage by weight: 100 percent of methacrylic acid, 79-105 percent of methyl acrylate, 95-128 percent of methyl methacrylate, 638-789 percent of purified water, 1.23-2.03 percent of potassium persulfate or ammonium persulfate, 0.85-1.76 percent of lauryl sodium sulfate and 2.41-4.10 percent of polysorbate 80. The invention also relates to a preparation method of the controlled-release enteric acrylic resin latex. The controlled-release enteric acrylic resin latex disclosed by the invention can be used as a colon-specific delivery coating material or can be used as a controlled-release skeleton material of a solid preparation, is convenient in use and good in controlled-release effect.
Owner:连云港万泰医药辅料技术有限公司
Crosslinking wrapped core slice in vivo for
InactiveCN101112363AReduce usageGood delayed release effectOrganic active ingredientsDigestive systemDiseaseCross-link
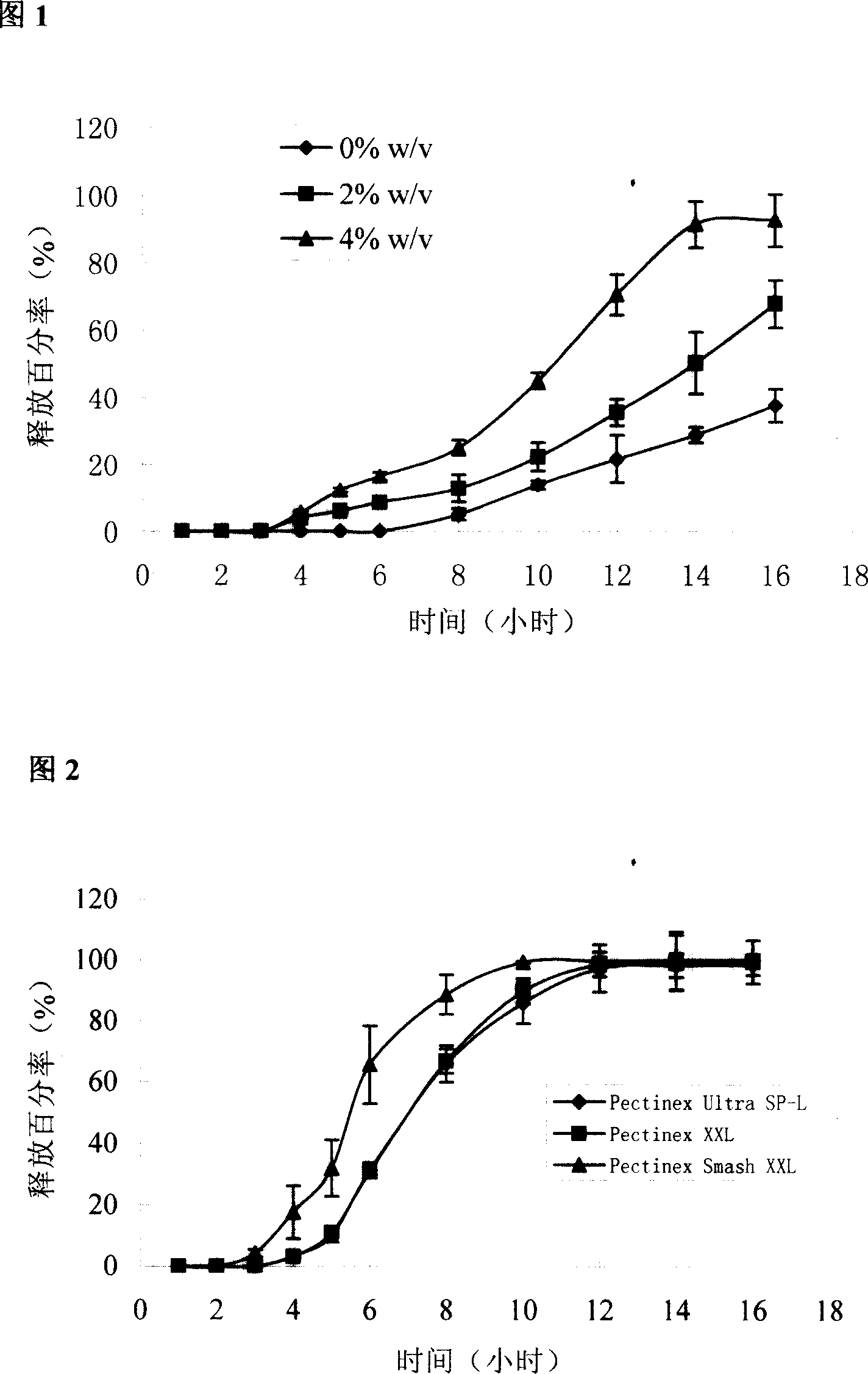
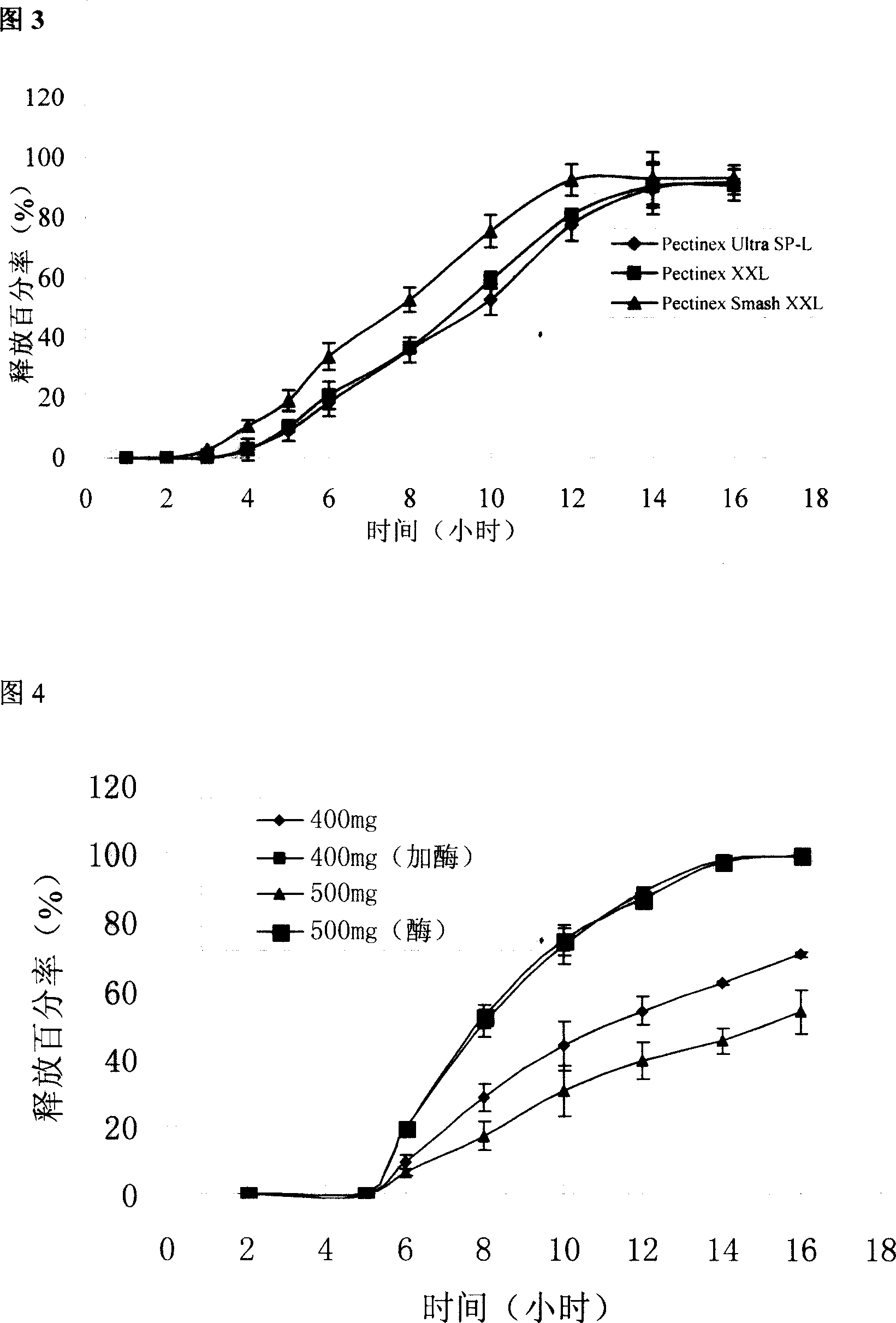
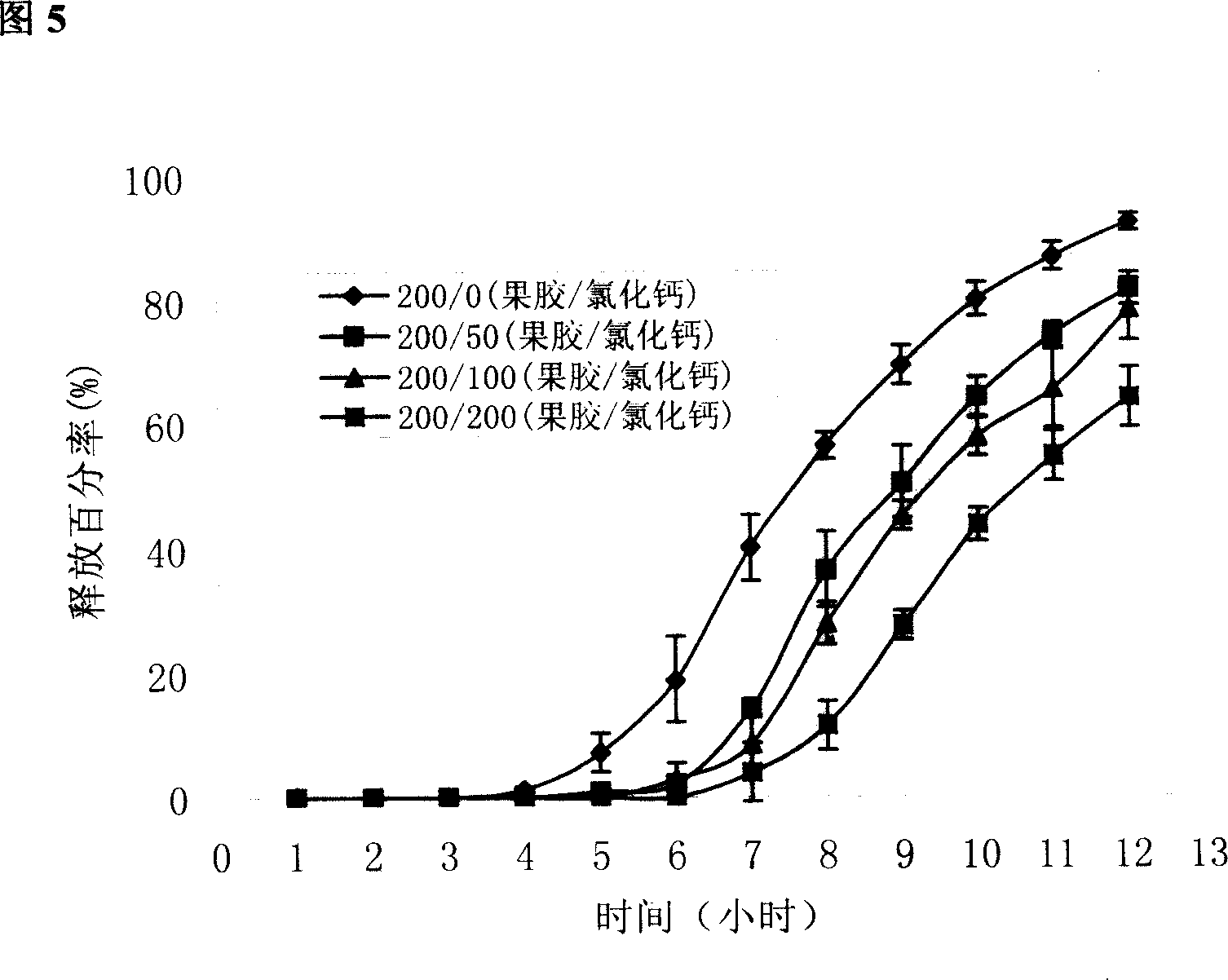
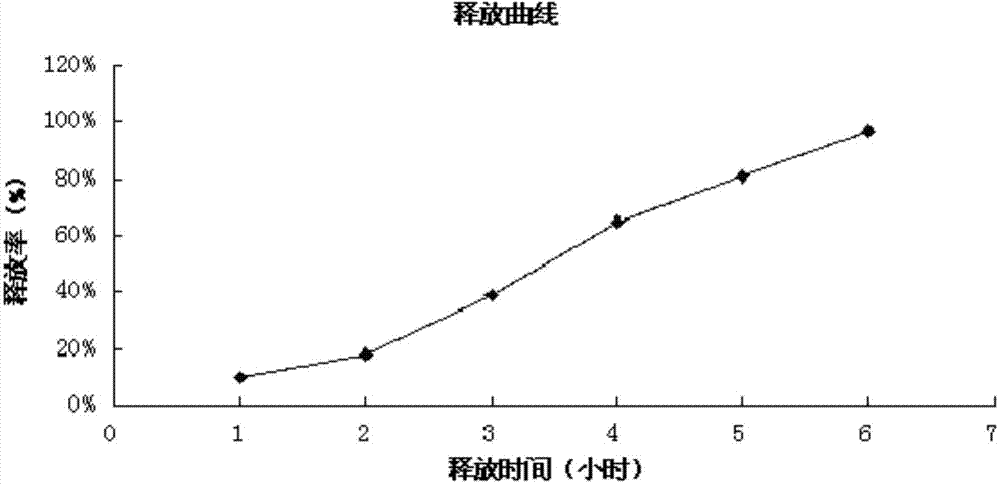
The invention pertains to the field of pharmaceutical preparation. The invention relates to a cross-linked covered tablet which is used for colon-specific drug delivery. The invention takes a drug-containing tablet as the core, the periphery of which is wrapped with a coating material, and the outer layer coating retarding material adopts the mixture of pectin of the calcium salts / calcium salts with the special amount and high parts by weight; the retarding release effect of the pectin is strengthened through the cross-linked process, so the invention is applicable to the colon-specific drug delivery, in particular to the insoluble drugs. The experimental results show that the invention can retard the release of the drug, so as to achieve the ideal colon-specific drug delivery, at the same time, the invention does not need to use more coating materials, which can significantly save the using amount of the pectin materials. The invention is applicable to the treatments of the diseases with the need of colon-specific drug delivery, such as colitis, colon cancer and other colon diseases.
Owner:FUDAN UNIV
Cefdinir colon-positioning enteric tablet
InactiveCN101468022APrecise transportationGood treatment effectAntibacterial agentsOrganic active ingredientsSide effectMedicine
The invention discloses cefdinir colon-specific enteric-coated tablets, comprising tablet cores and coating layers. The tablet cores are composed of 10-90 wt.% of cefdinir, 2-70 wt.% of diluting auxiliary materials, 1-20 wt.% of disintegrating auxiliary materials, 1-20 wt.% of adhering auxiliary materials and 0.1-5 wt.% of lubricant auxiliary materials. The cefdinir colon-specific enteric-coated tablets can transmit medicament to colons, improve curative effect of colonitis, have low side effect and improve compliance of patients.
Owner:BEIJING HOPE HUGE PHARM SCI
Segmented intestine targeted preparation of animal skin glue collagen polypeptide and method of preparing the same
InactiveCN101130066AAvoid destructionInhibit metabolismOrganic active ingredientsAnthropod material medical ingredientsIntestinal structurePeptide drug
The invention provides a colon specific targeting preparation of animal skin collagen polypeptides and its preparing process, wherein the preparation is prepared through coating the drug-containing micro-pellets containing animal skin collagen polypeptides components with colon targeting coating liquid, or directly loading the drug-containing micro-pellets into the shells of the colon targeting capsule. The preparation provided by the invention can make the colon release drugs enter blood circulation through colon, thereby avoiding metabolism destruction of the conventional oral preparation peptides drugs on the stomach, as a result, the therapeutic action of the drugs can be sufficiently exploited.
Owner:李立
Microencapsulated capsicin and preparation method
ActiveCN103494791AReduce stimulationImprove solubilityOrganic active ingredientsMetabolism disorderWater bathsSolubility
The invention discloses a preparation method of microencapsulated capsicin. The preparation method includes the steps: (1) melting capsicin in water bath by heating, and adding an emulsifier to prepare a core emulsion; (2) adding chitosan, pectin and calcium chloride into water to prepare a wall material solution; (3) adding the core emulsion into the wall material solution, mixing, adding a curing agent prior to mixing, and spraying and drying to obtain the microencapsulated capsicin. The microencapsulated capsicin has the advantages that stimulation of the capsicin on digestive tract mucosae is reduced, application range of the capsicin is enlarged, and the capsicin can not only be used in condiments but also applied to health care products; the microencapsulated capsicin is good in solubility and high in stability; a barrier function of drug delivery on upper segments of the gastrointestinal tracts can be enhanced, and colon-specific drug delivery of preparations is facilitated; the method is simple to operate and easy for industrial production, and a feasible technical route is provided for development of the capsicin.
Owner:晨光生物科技集团天津有限公司
Colon-specific pH sensitive hydrogel, preparation method and application thereof
ActiveCN103919716AInhibition releaseReduce early releaseOrganic active ingredientsAerosol deliveryCooking & bakingCalcification
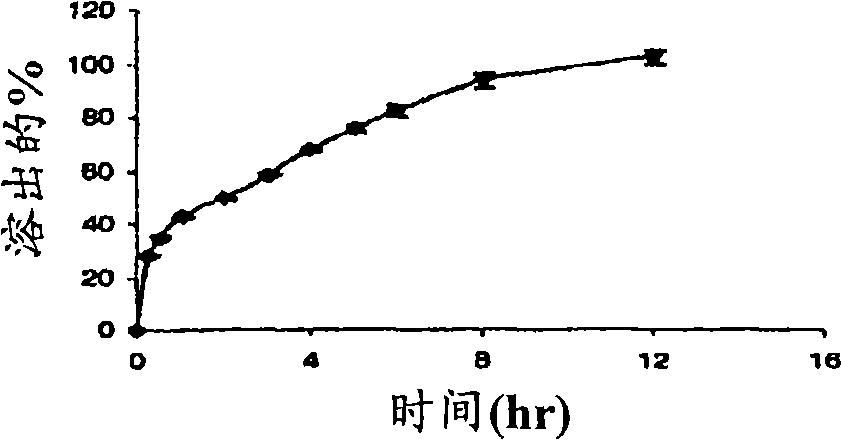
The invention discloses a colon-specific pH sensitive hydrogel, a preparation method and an application thereof. The hydrogel is prepared by mixing glycerol monostearate serving as a delayed-release material and medicines, uniformly coating the surface of the mixture with sodium alginate with a certain concentration, and baking. The in vitro drug release behavior is taken as an index to investigate the concentrations of sodium alginate and calcium chloride, the calcification time and the drug-release behavior of drug loading capacity on medicines in artificial gastric juice for 2 hours, artificial intestinal juice for 4 hours and artificial colonic juice for 4 hours, and the optimal hydrogel for the in vitro drug release behavior is screened. According to the colon-specific pH sensitive hydrogel, release of a medicine in a pH 6.8 environment can be effectively controlled by using the glycerol monostearate as a delayed-release material, and the targeting property can be further improved after the natural polysaccharide material sodium monostearate is coated, so that the glycerol monostearate is not released or little released in pH1.2, and over 90 percent of the medicine can reach the colon part.
Owner:吉林省派瑞斯生物技术有限公司
Starch based segmented intestine targeting specific adhesion material, preparation and application thereof
ActiveCN101366946AGood biocompatibilityImprove colon targetingPeptide/protein ingredientsDigestive systemDiseaseIntestinal structure
The invention discloses a starch based colon idiosyncratic adhesion material for coupling concanavalin A and a method for preparing the same. The anti-digestion starch undergoes the activating treatment by glutaraldehyde, couples with the concanavalin A and is subjected to centrifuge washing by phosphate buffer and inactivation by ethanolamine to obtain the starch based colon idiosyncratic adhesion material. The invention also discloses application of the starch based colon targeting idiosyncratic adhesion material in preparing biomacromolecule medicines of polypeptide protein or medicines of treating colon diseases. For the first time, the invention utilizes the characteristics of the idiosyncratic adsorption between the concanavalin A and the colon epithelial cell, successfully prepares the carrier material of the starch based colon idiosyncratic adhesion material by coupling the concanavalin A with the anti-digestion starch molecule by the glutaraldehyde and uses the starch based colon idiosyncratic adhesion material as an oral taking colon targeting control-release carrier material, so that starch based colon idiosyncratic adhesion material and the method have the characteristics of cell adsorption, enzymolysis triggering targeting, good biocompatibility, safety and non-toxin, good targeting slow-release effect, and the like.
Owner:SOUTH CHINA UNIV OF TECH
Folate receptor-mediated colon-localized release of curcumin from microemulsions
InactiveCN102266287AImprove solubilityImprove oral absorption and availabilityAntipyreticDigestive systemSolubilityOil phase
The invention discloses a folate-receptor-mediated curcumin self-microemulsion colon-specific delivery preparation, which is composed of the following components in parts by weight: 0.05-7.5 parts of curcumin, 10-40 parts of oil phase, 30-60 parts of surfactant, 30-60 parts of cosurfactant and a folic acid lipid material the amount of which accounts for 0.01-3% the weight of the surfactant. In the invention, curcumin is processed into a self-microemulsion preparation, which is composed of a pharmaceutically common oil phase, a surfactant and a cosurfactant, and the solubility of curcumin can be increased by the oil phase, surfactant and cosurfactant; when the oil phase, surfactant and cosurfactant are mixed in an appropriate ratio, an O / W microemulsion can be formed in water by self-microemulsification, so that the solubility of curcumin is up to 30-70 mg / mL and the solubility is increased by more than 40000 times; and the solubility of turmeric is effectively improved, the oral absorption availability of the preparation is improved, and the preparation has the advantages of strong targeting, simple preparation method, low cost, etc.
Owner:SHANDONG UNIV
Gambogic acid colon-specific controlled release tablet and preparation method thereof
InactiveCN102871983ATargetedHigh efficiency and low toxicityOrganic active ingredientsDigestive systemTreatment effectSide effect
The invention relates to a gambogic acid colon-specific controlled release tablet and a preparation method thereof. The colon-specific controlled release tablet comprises a tablet core and a coating layer; the tablet core comprises the following material in percentage by weight: 1 to 60 percent of gambogic acid, 2 to 95 percent of diluent adjuvant, 2 to 20 percent of disintegration adjuvant, 1 to 20 percent of adhesion adjuvant, and 0.1 to 5 percent of lubricating adjuvant; and the coating layer comprises the following material in percentage by weight: 15 to 80 percent of polysaccharide compound, 15 to 80 percent of adhesion adjuvant, and 0.2 to 5 percent of lubricating adjuvant. The polysaccharide compound of the coating layer preferably adopts one of pectin and konjac glucomannan. The preparation method comprises the steps of tablet core preparation and coating layer preparation. The gambogic acid colon-specific controlled release tablet can accurately transfer drug to colon, improves the treatment effect of colon cancer, reduces the side effect, and improves the compliance of patients.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Oral colon-targeted lycopene nano-liposome and preparation method thereof
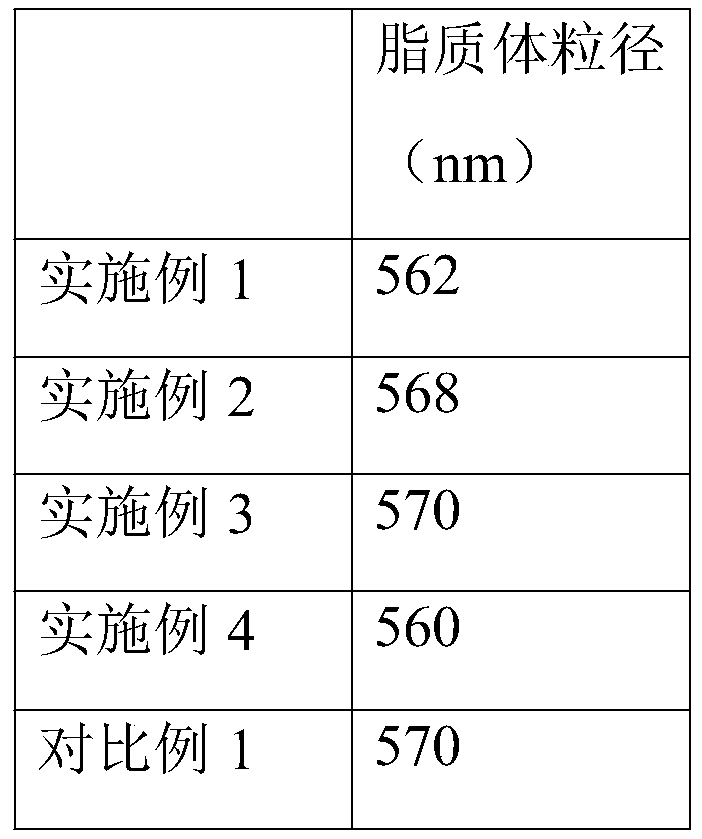
InactiveCN111317827ASmall particle sizeCore-shell structureHydrocarbon active ingredientsAntipyreticLycoperseneColon parts
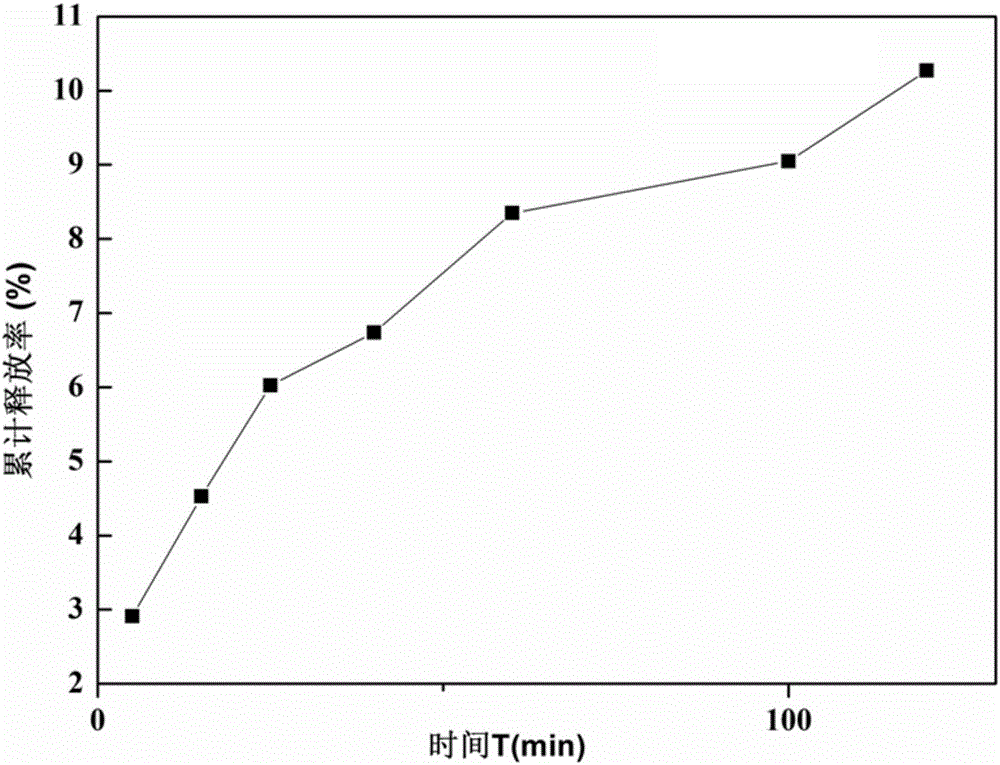
The invention relates to the technical field of pharmaceutics, and specifically relates to an oral colon-targeted lycopene nano-liposome and a preparation method thereof. The nano-liposome consists ofa liposome core and a modified layer shell; the liposome core is the lycopene-loaded liposome with negative charge prepared by adopting an ether injection method; and the modified layer shell is formed by a colon-specific degradation material. Compared with conventional lipids, the stability of the obtained nano-liposome in gastrointestinal tracts can be greatly enhanced; and the nano-liposome has obvious colon-targeted release effects and can obviously enhance the repair efficiency of colon parts, and the average particle size is about 560 nm.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Icariin controlled release microsphere, preparation method thereof and application thereof
InactiveCN106309380AGood pH responsivenessReduce releaseOrganic active ingredientsDigestive systemDiseaseMicrosphere
The invention discloses an icariin controlled release microsphere, a preparation method thereof and application thereof. The controlled release microsphere is used for pH response controlled release microsphere for treating colon disease, high-molecular polymers including chitosan, sodium alginate and like are used as carrier materials, the icariin is used as the active ingredient to construct an oral colon specific drug delivery system with biological controlled release targeting ability. The biological controlled release targeting microsphere disclosed by the invention has good pH responsiveness, the drug release amount under stomach low pH environment is low, the drug release can be promoted under the colon high pH environment, and the colon residence time of the drug is prolonged; the drug absorption is increased, and the local bioavailability of the oral drug for treating the colon disease is improved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Colon-specific bioadhesive tablet containing monosialotetrahexosyl ganglioside sodium
ActiveCN106361718AImprove medicinal effectMucoadhesiveOrganic active ingredientsNervous disorderAdditive ingredientBioadhesive
Owner:赛隆药业集团股份有限公司
Oral galenic form, polymer production method and use of same
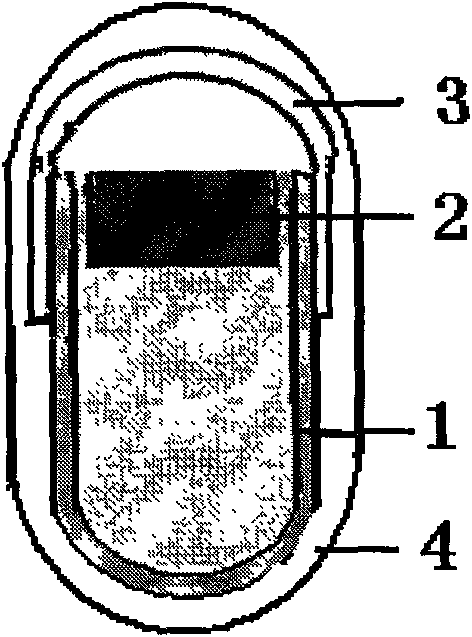
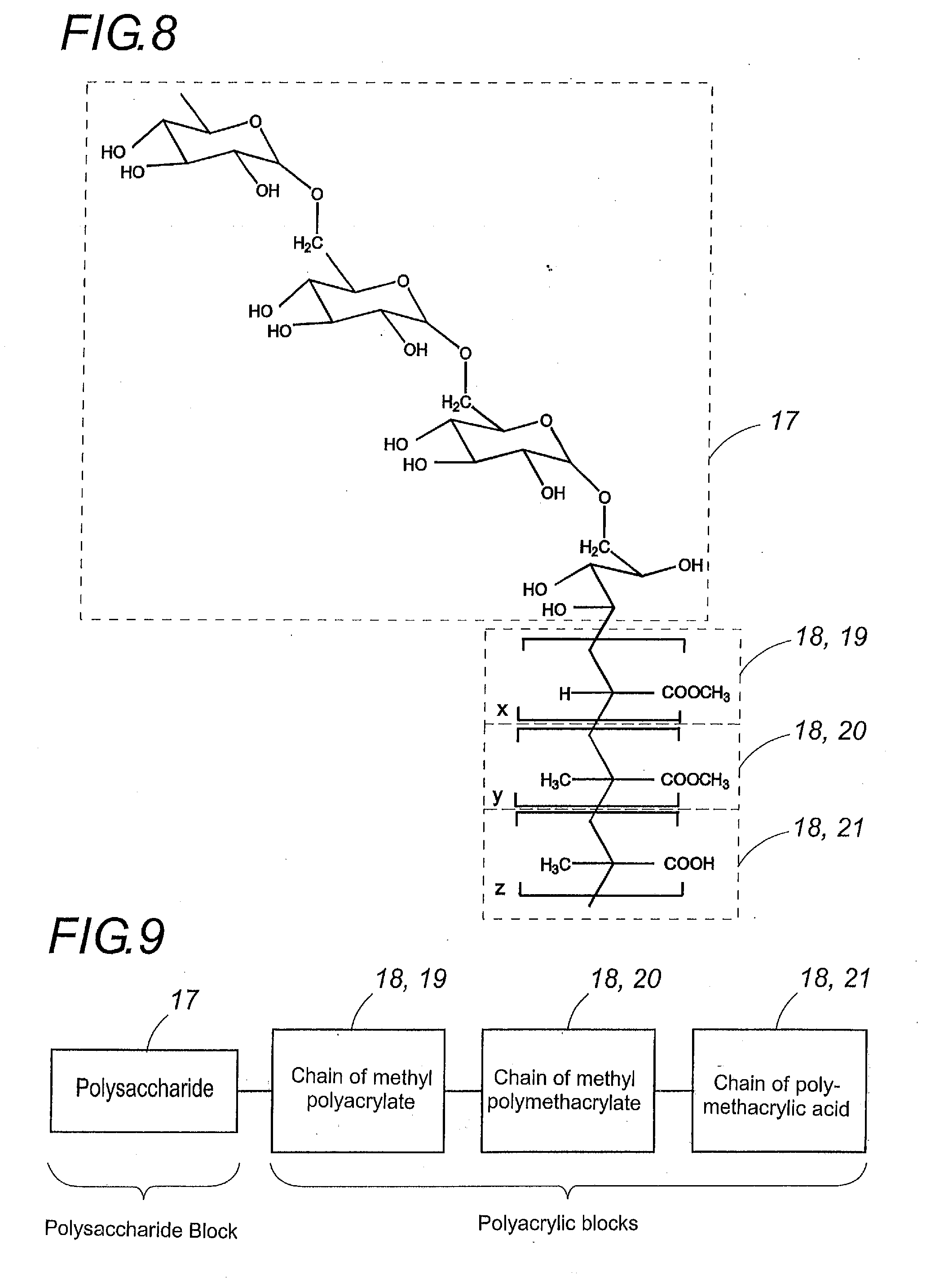
InactiveUS20110189242A1No harmDisperse fastDigestive systemPharmaceutical non-active ingredientsPharmacyPolymer science
The polymers, according to the invention, comprise a polysaccharide block (17) and several hydrophobic polyacrylic blocks (18) enabling the polymer to remain intact until the polymer reaches the colon. The polysaccharide block is degraded by the colonic microflora irrespective of the pH, while the polyacrylic blocks are solublilized at neutral pH. Colon-specific release of the active ingredients is thus provided in all cases. These polymers may serve as a coating in particular for tablets, gel capsules, granules or microgranules, or serve as matrix agents in the preparation of such pharmaceutical forms. This invention concerns the fields of medicine, pharmacy and dietetics.
Owner:UNIVERSITY OF STRASBOURG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com