Oral colon-targeted lycopene nano-liposome and preparation method thereof
A technology of nano-liposome and lycopene, which is applied in the field of pharmacy, can solve the problems of unsatisfactory colon-targeted drug release, poor mechanical properties, and structural damage, and achieve obvious colon-targeted release effect and stability Improve and reduce the damage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
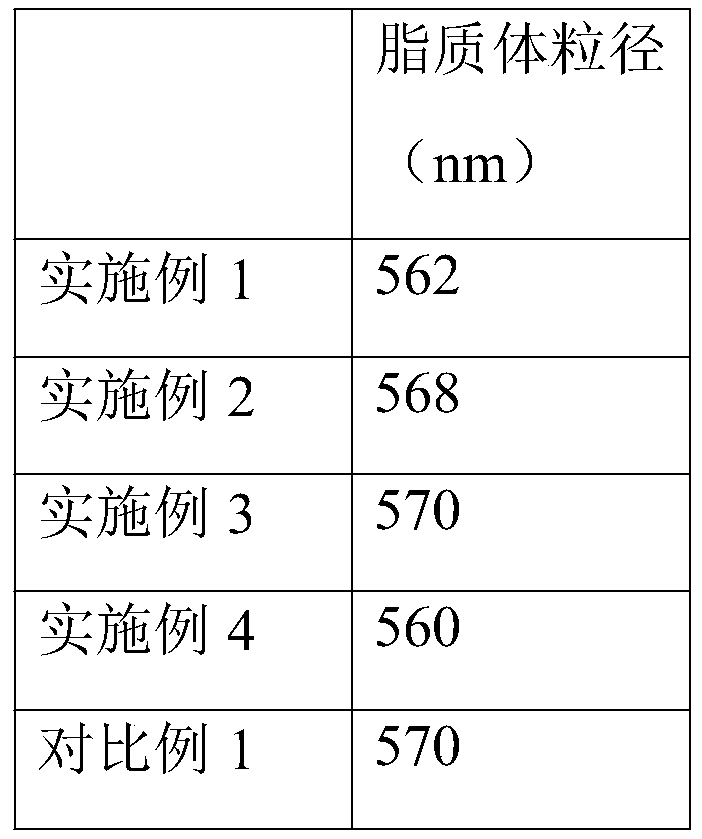
[0028] Weigh 0.5g egg yolk lecithin, 0.1g cholesterol and completely dissolve in 50mL of anhydrous ether, another 20mg of lycopene is completely dissolved in 10mL of dichloromethane, mix the above two solutions, slowly inject into 200mL, pH7 .0, in a phosphate buffer solution with a concentration of 0.02M / L, magnetically stirred at 45°C for 20min, then transferred to a round-bottomed flask, removed anhydrous ether and dichloromethane by vacuum rotary evaporation, and heated at 150MPa Circulate the homogeneous treatment twice under pressure to obtain lycopene conventional liposomes; inject the lycopene conventional liposome solution into 200mL, 0.25% chitosan acetic acid solution (0.2% concentration of acetic acid solution), and stir to react After 20 minutes, continuous ultrasonic treatment was performed for 20 minutes to obtain chitosan-modified liposomes. Then add chitosan-modified nano-liposomes into 600mL, 0.35% pectin phosphate solution drop by drop while stirring, after ...
Embodiment 2
[0030] Weigh 0.31g of lecithin, 0.03g of cholesterol and completely dissolve in 40mL of anhydrous ether, another 10mg of lycopene is completely dissolved in 5mL of dichloromethane, mix the above two solutions, slowly inject into 300ml of pH6.8 , concentration of 0.02M phosphate buffer solution, magnetically stirred at 45°C for 20min, then transferred to a round-bottom flask, vacuum rotary evaporation to remove anhydrous ether and dichloromethane, and intermittent ultrasonication at 250w for 5min to obtain Lycopene conventional liposomes; the lycopene conventional liposome solution was added dropwise to 300mL, 0.3% chitosan acetic acid solution (0.1% concentration of acetic acid solution), stirred and reacted for 20min, and then ultrasonically treated continuously for 20min, Chitosan-modified liposomes were obtained. Add the chitosan-modified liposome dropwise to 400mL, 0.4% pectin phosphate solution (phosphate solution pH 6.8, concentration 0.02M) while stirring, stir for 20mi...
Embodiment 3
[0032] Weigh 10g of lecithin, 4.16g of cholesterol and completely dissolve in 300mL of anhydrous ether, another 100mg of lycopene is completely dissolved in 50mL of dichloromethane, mix the above two solutions, slowly inject into 350mL of pH6.8, concentration In a 0.2M phosphate buffer solution, stir magnetically at 45°C for 20min, then transfer it to a round-bottom flask, remove anhydrous ether and dichloromethane by vacuum rotary evaporation, and then circulate and homogenize at 150MPa for 3 The conventional liposome of lycopene was obtained; the conventional liposome solution of lycopene was added dropwise to 300mL, 4% chitosan acetic acid solution (0.1% concentration of acetic acid solution), and after stirring for 20min, continuous ultrasonic Treated for 20 minutes to obtain chitosan-modified liposomes. Add the chitosan-modified liposome dropwise to 350mL, 2.2% pectin phosphate solution (phosphate solution pH 6.8, concentration 0.2M) while stirring, stir for 20min, and ci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com