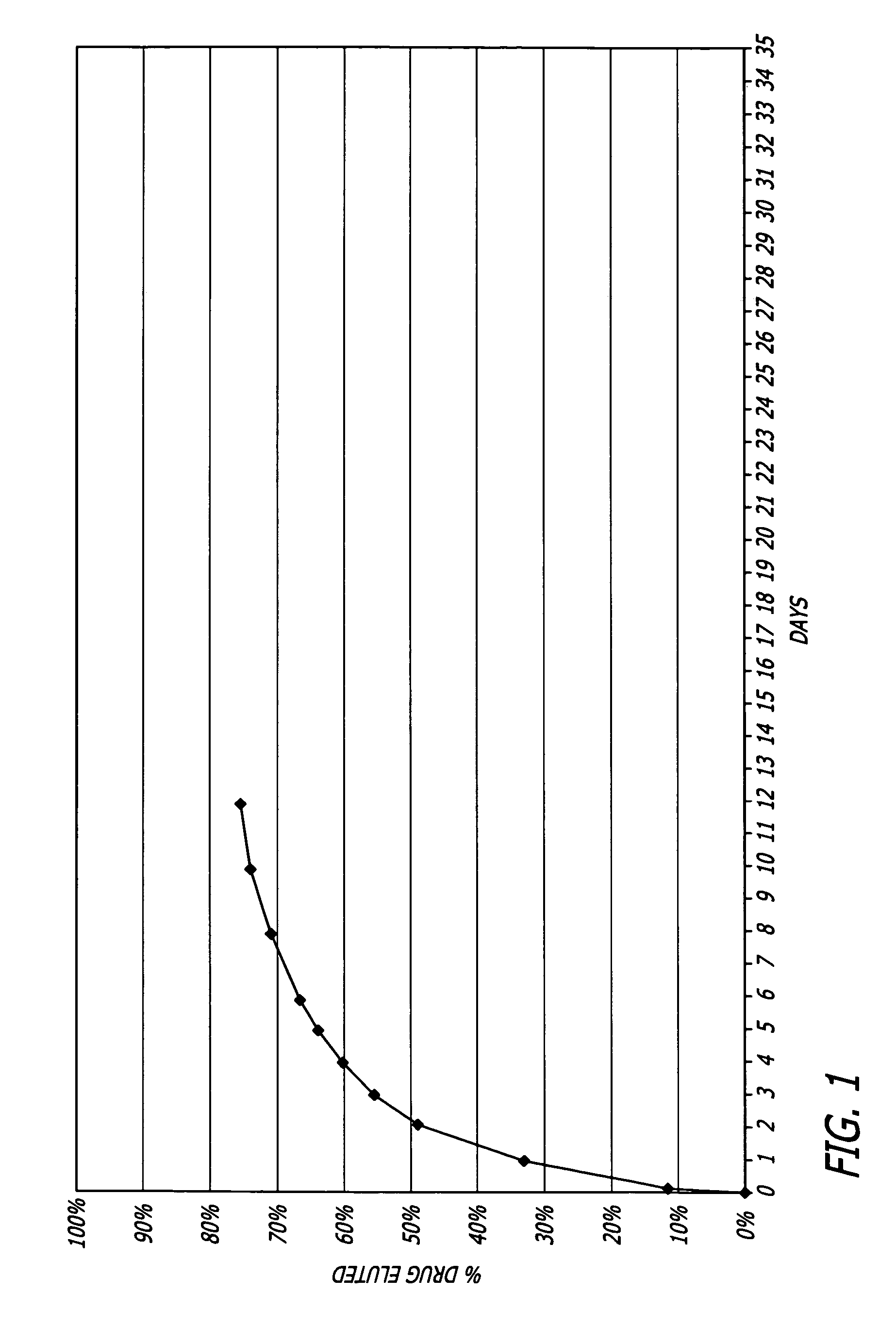
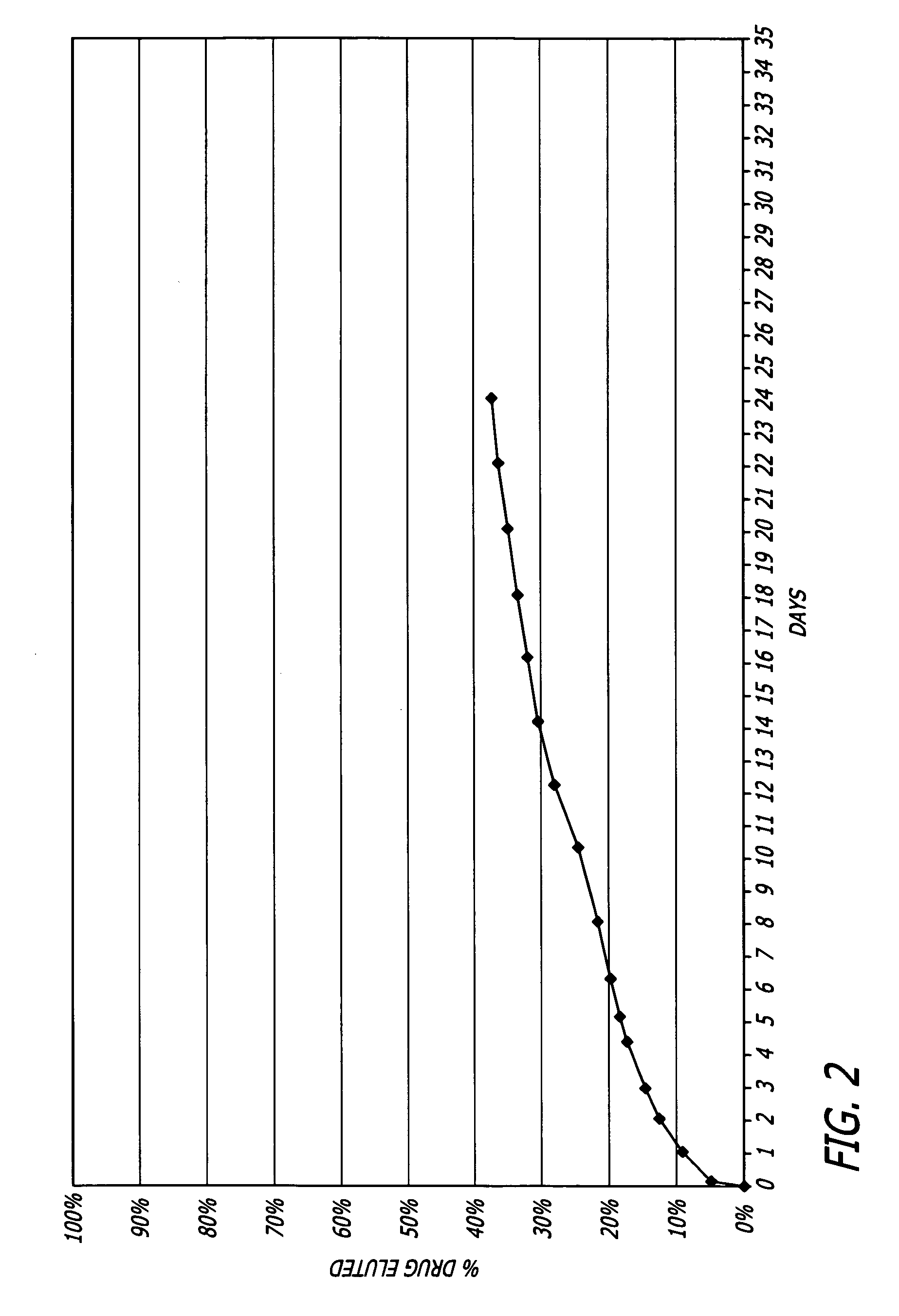
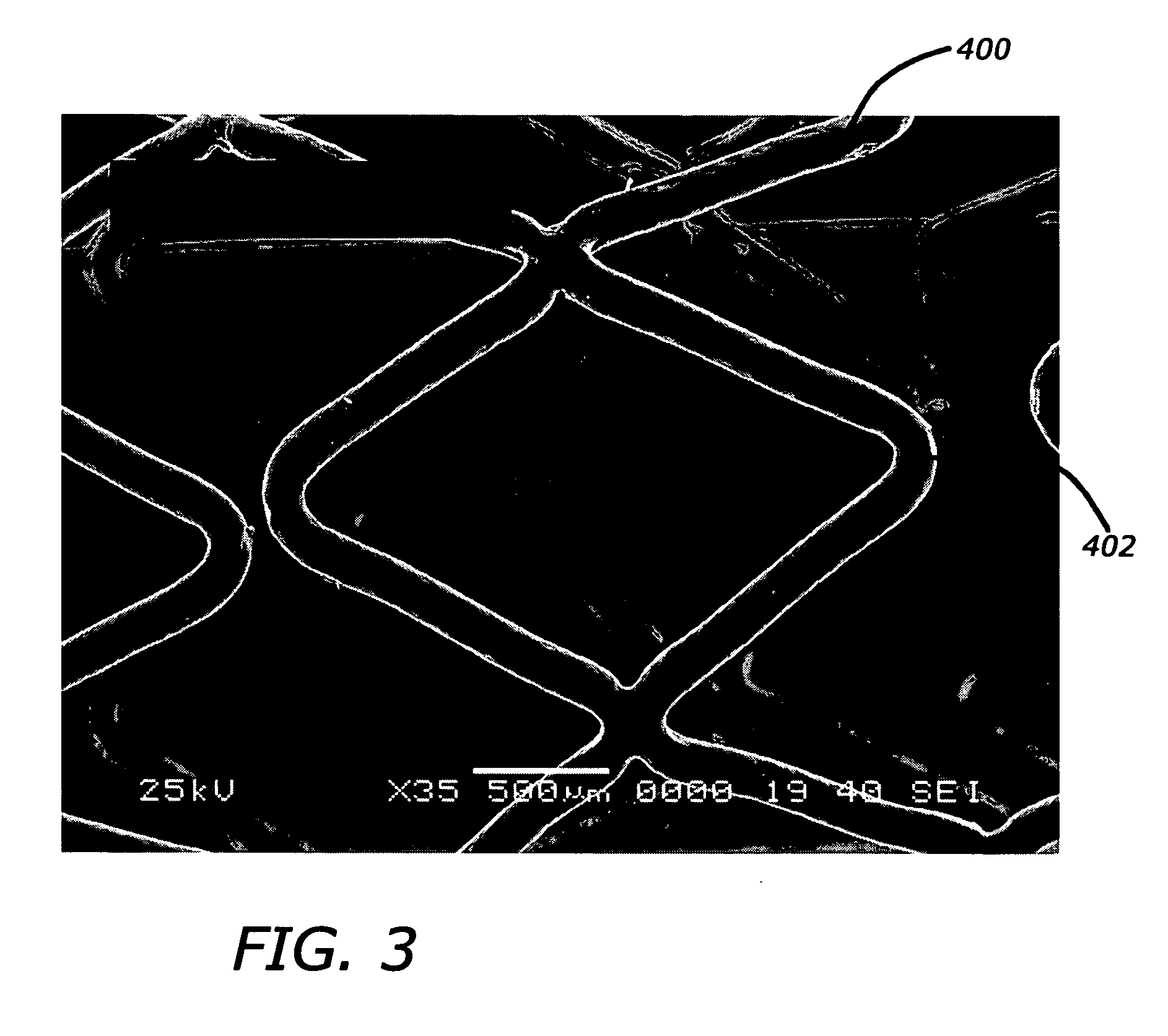
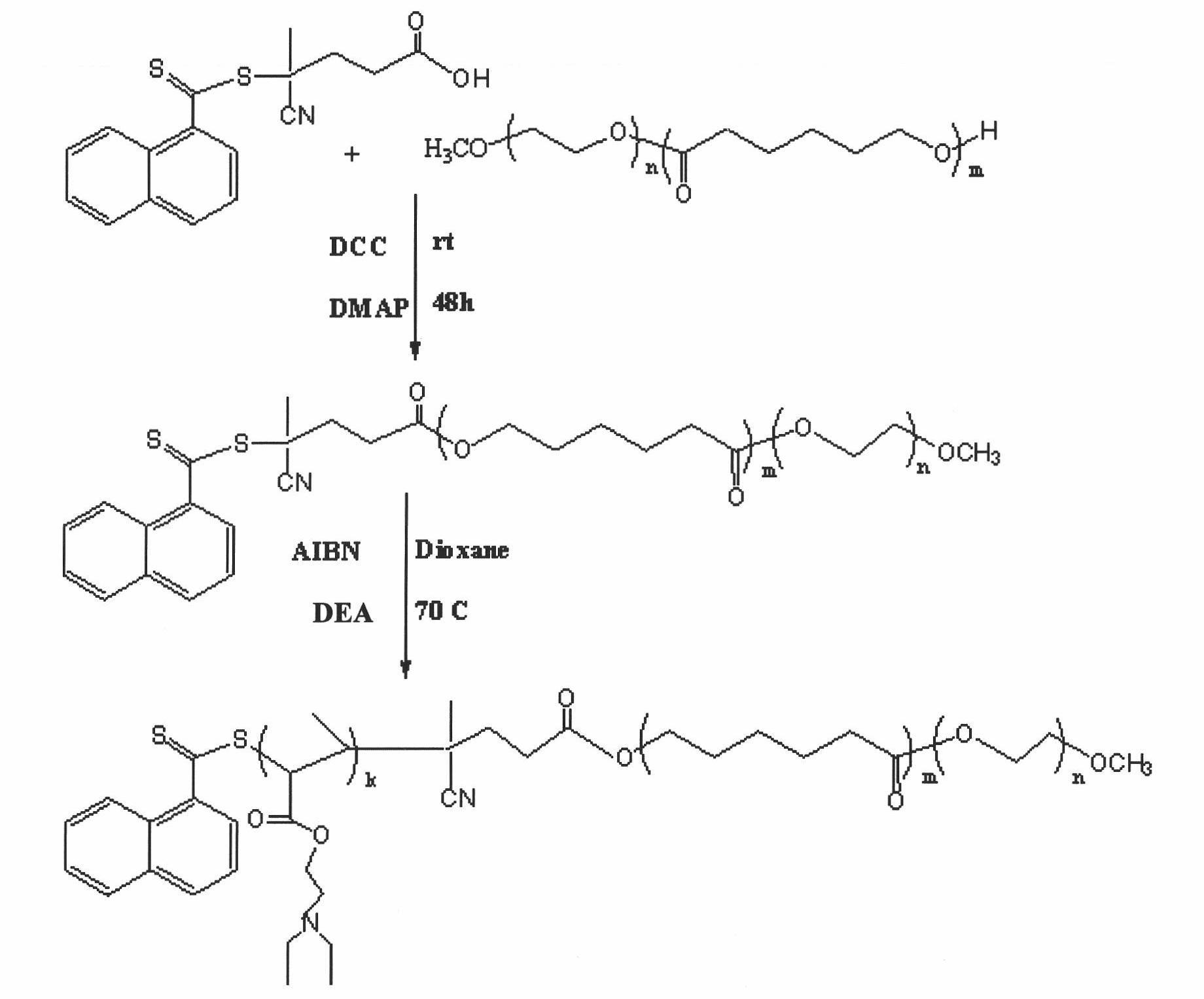
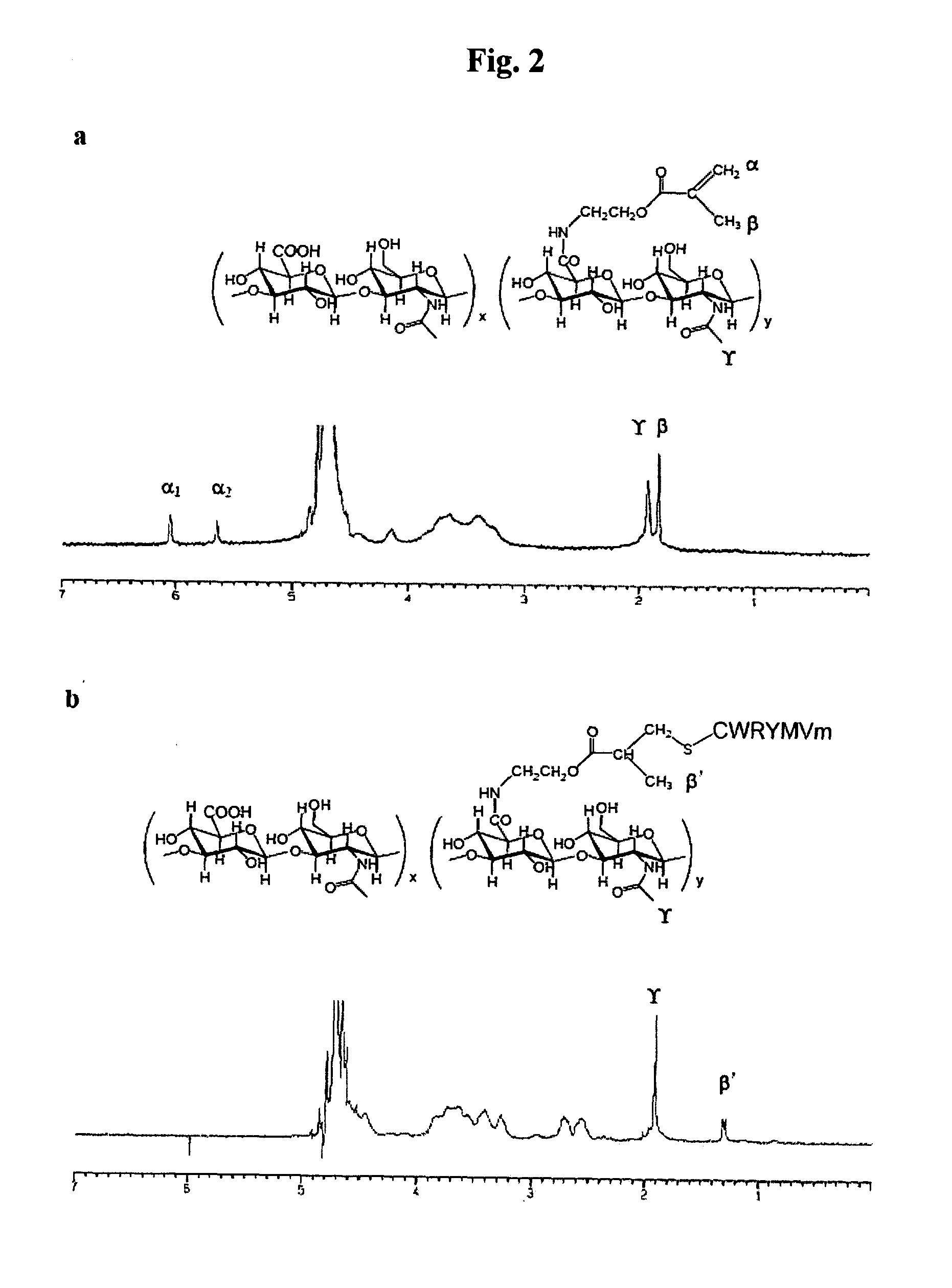
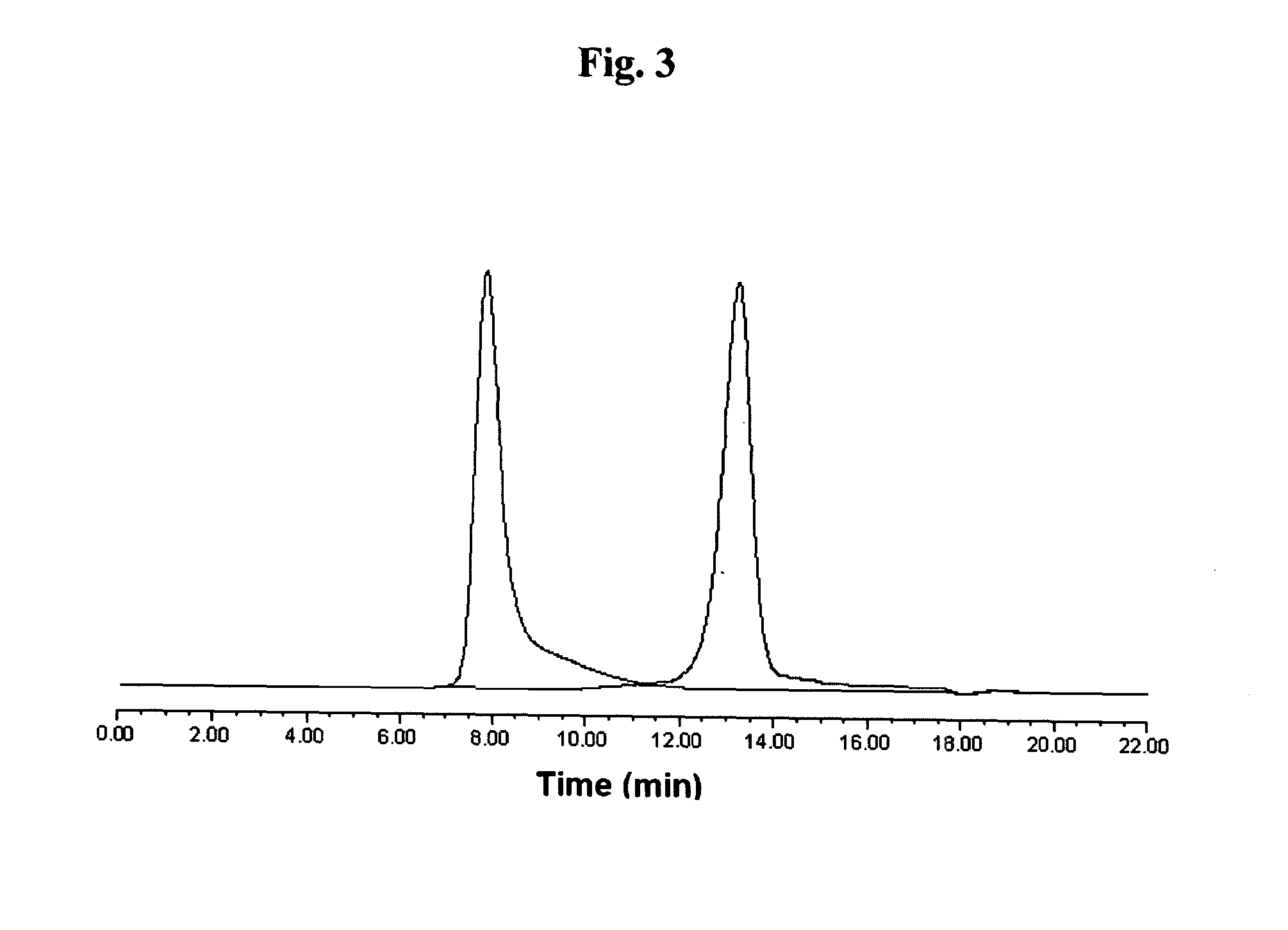
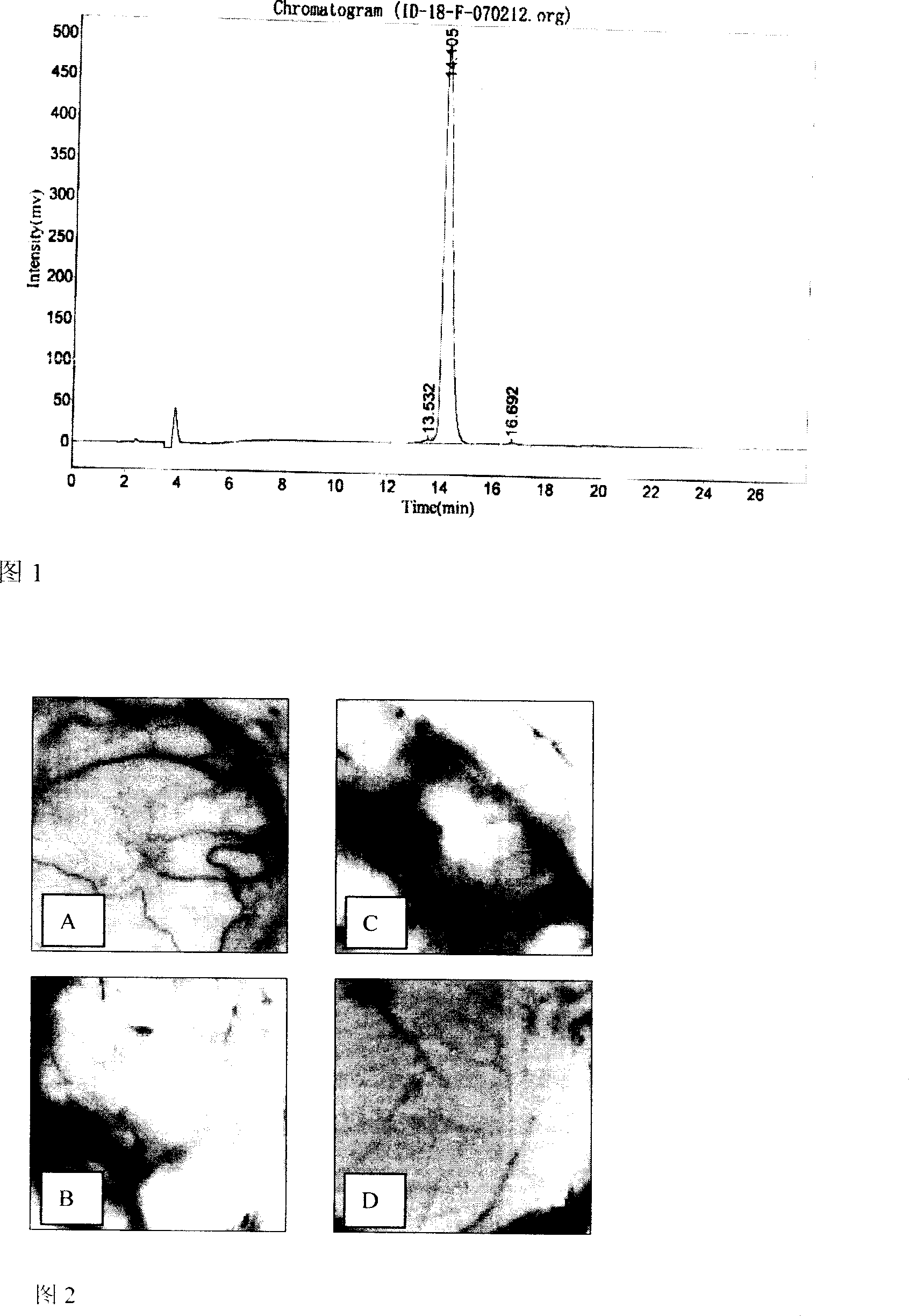
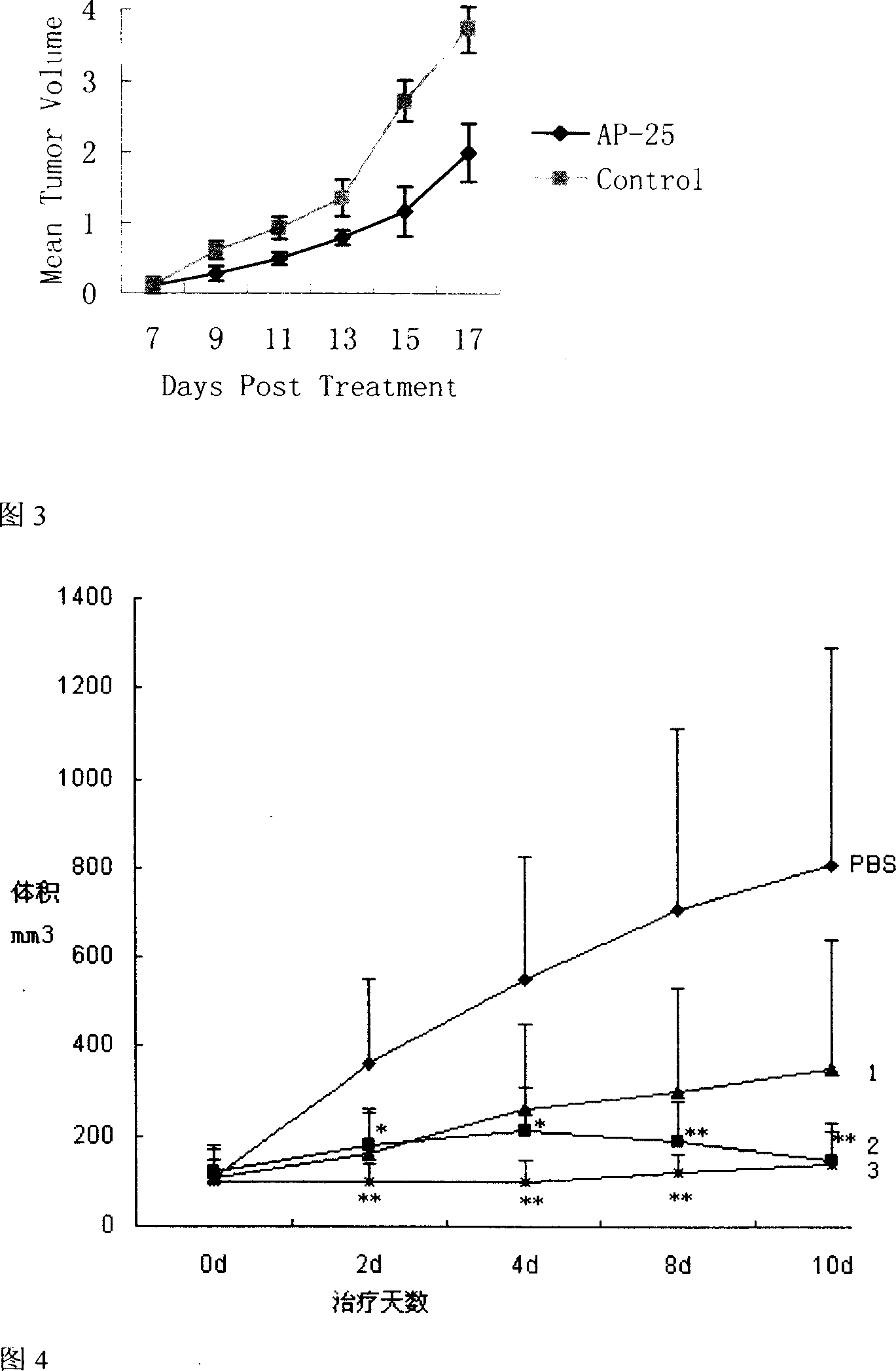
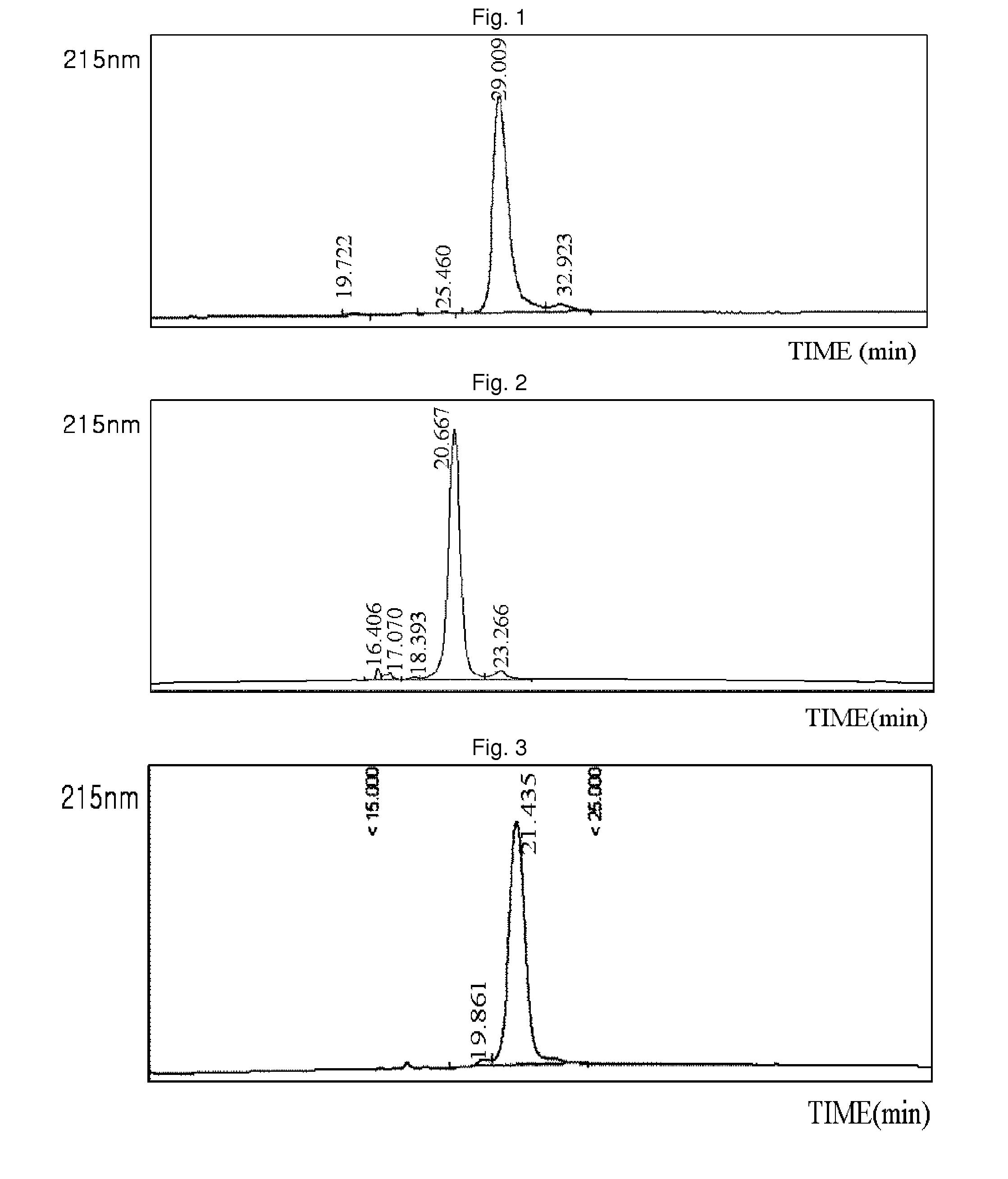
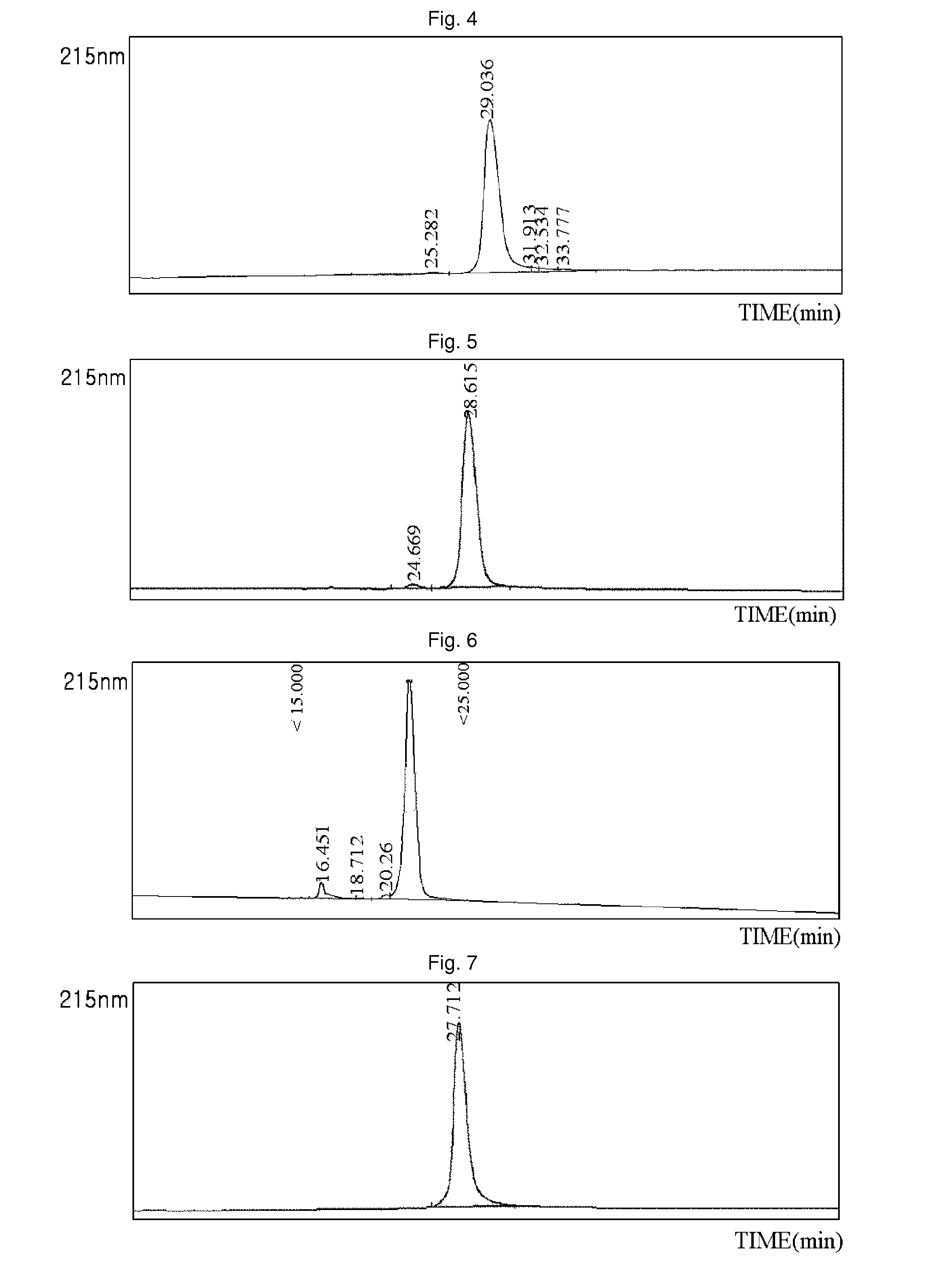
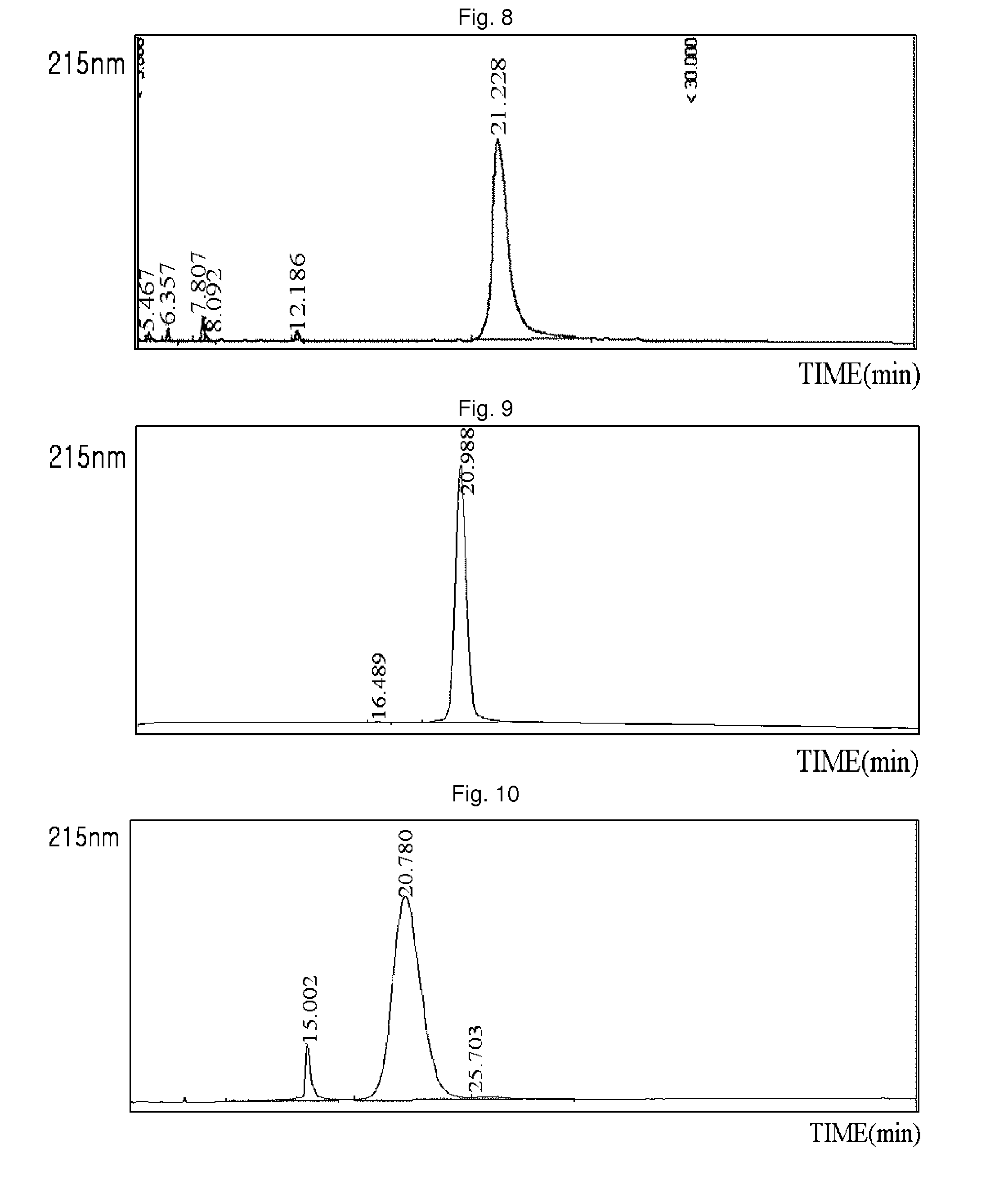
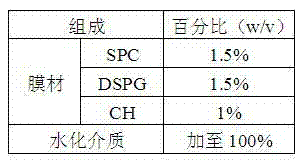
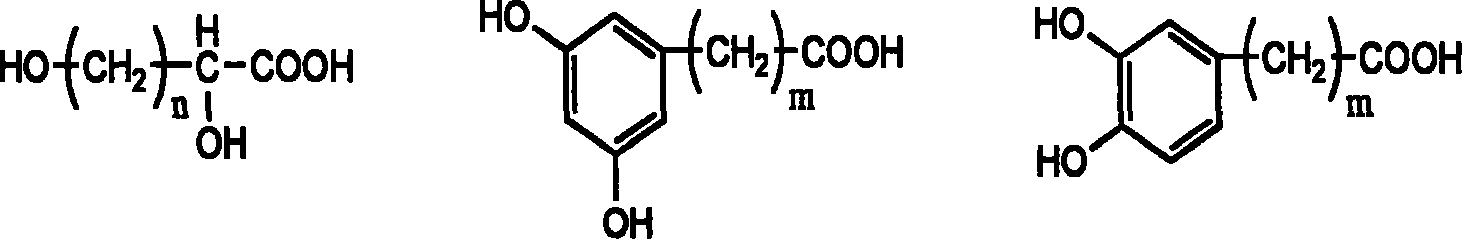
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
188 results about "Peptide drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptide drug refers to a peptide having a specific therapeutic effect extracted by chemical synthesis, genetic recombination or animal or plant, and is a specific application of the peptide in the field of medicine.
Peptide for high performance inhibition of angiogenesis and method for preparing same and use thereof
ActiveCN1699408AImprove and enhance growthImprove and enhance the anti-tumor effectPeptide/protein ingredientsSkeletal disorderEscherichia coliInclusion bodies
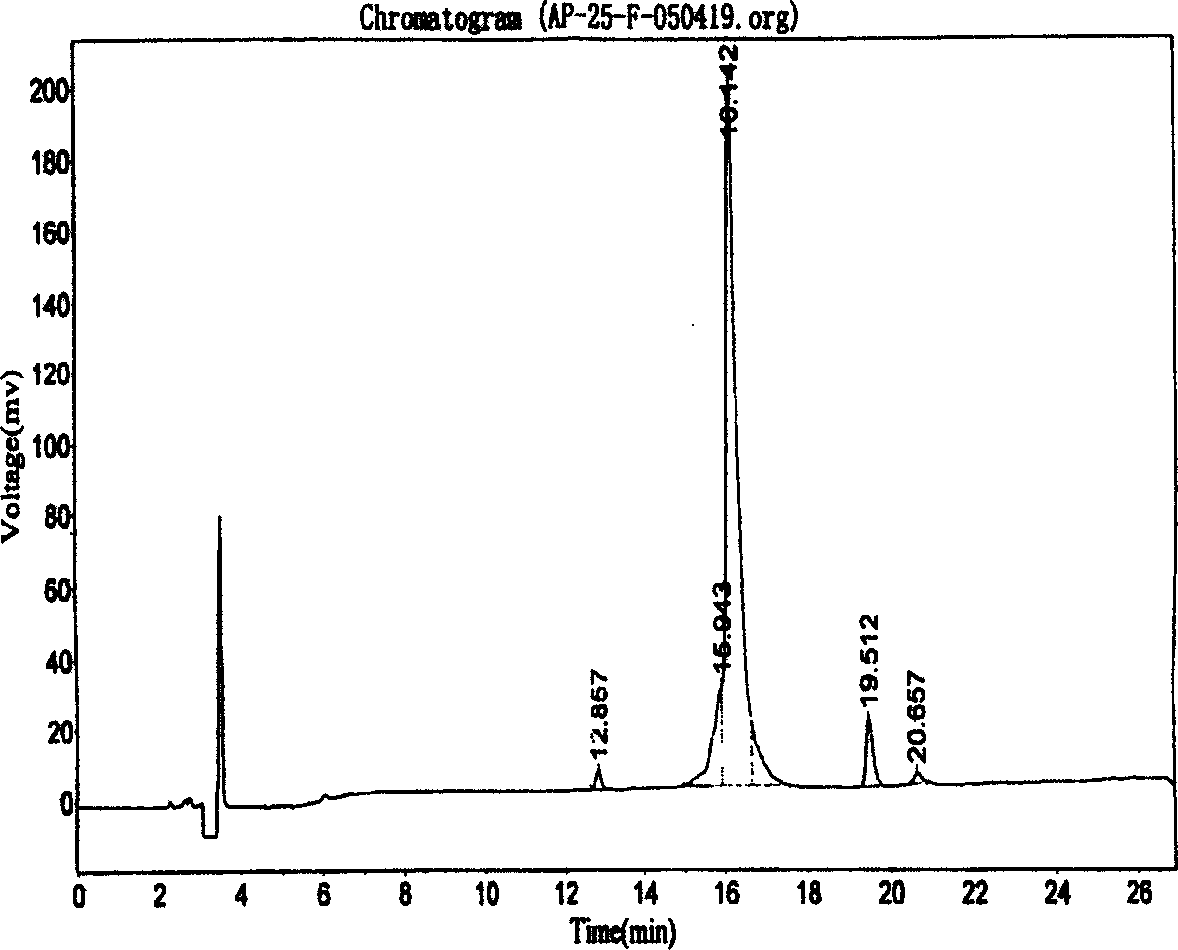
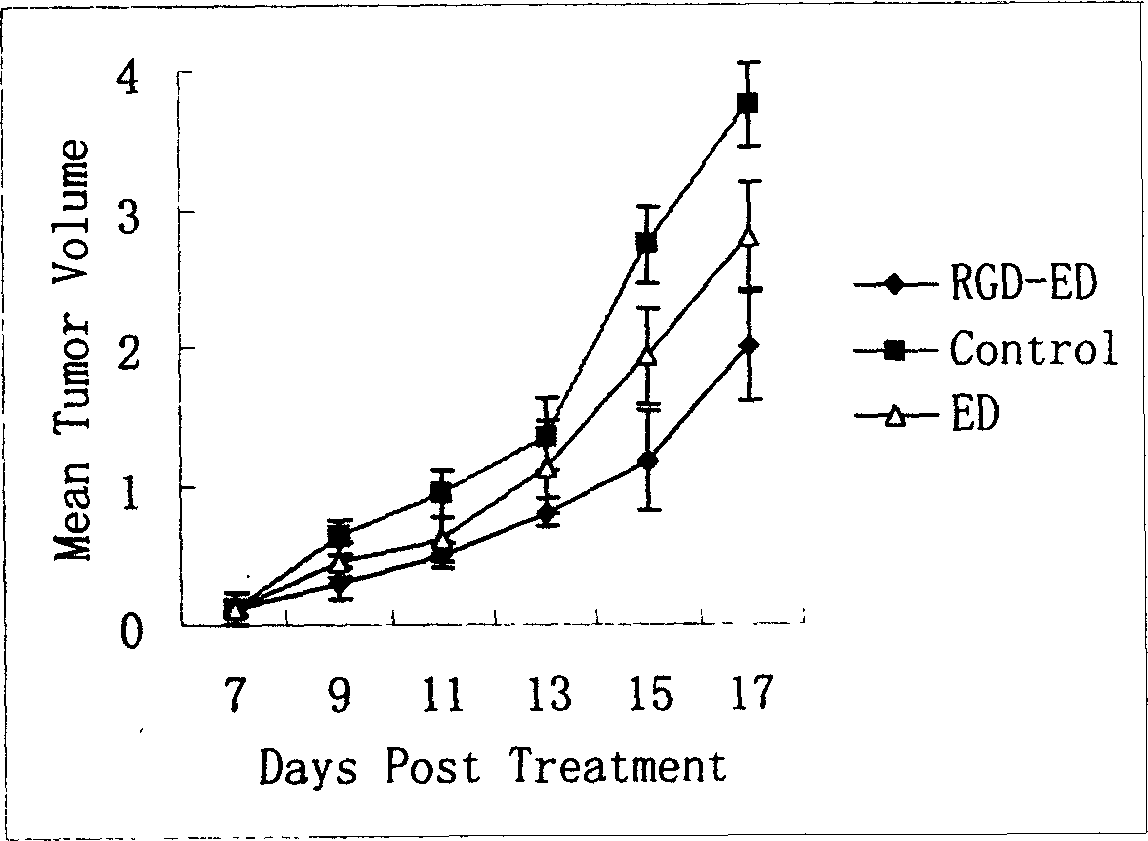
The invention relates to a peptide for high performance inhibition of angiogenesis and method for preparing same and use, wherein high performance blood vessel production inhibiting agent RGD-ED with integration compatibility is designed, the inhibiting agent comprises polypeptide polypeptide-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-praline, its one or two ends are connected with polypeptides containing arginine-glycine-aspartic acid sequence. The RGD-ED provided by the invention can be synthesized. The invention also discloses the expression of one RGD-ED in bacillus coli through gene engineering method, wherein the RGD-ED is prepared through the steps of inclusion body protein segregation, dissolution and renaturation, and ion-exchange chromatography segregation and purification.
Owner:CHINA PHARM UNIV
Fine Particle and Pharmaceutical Preparation
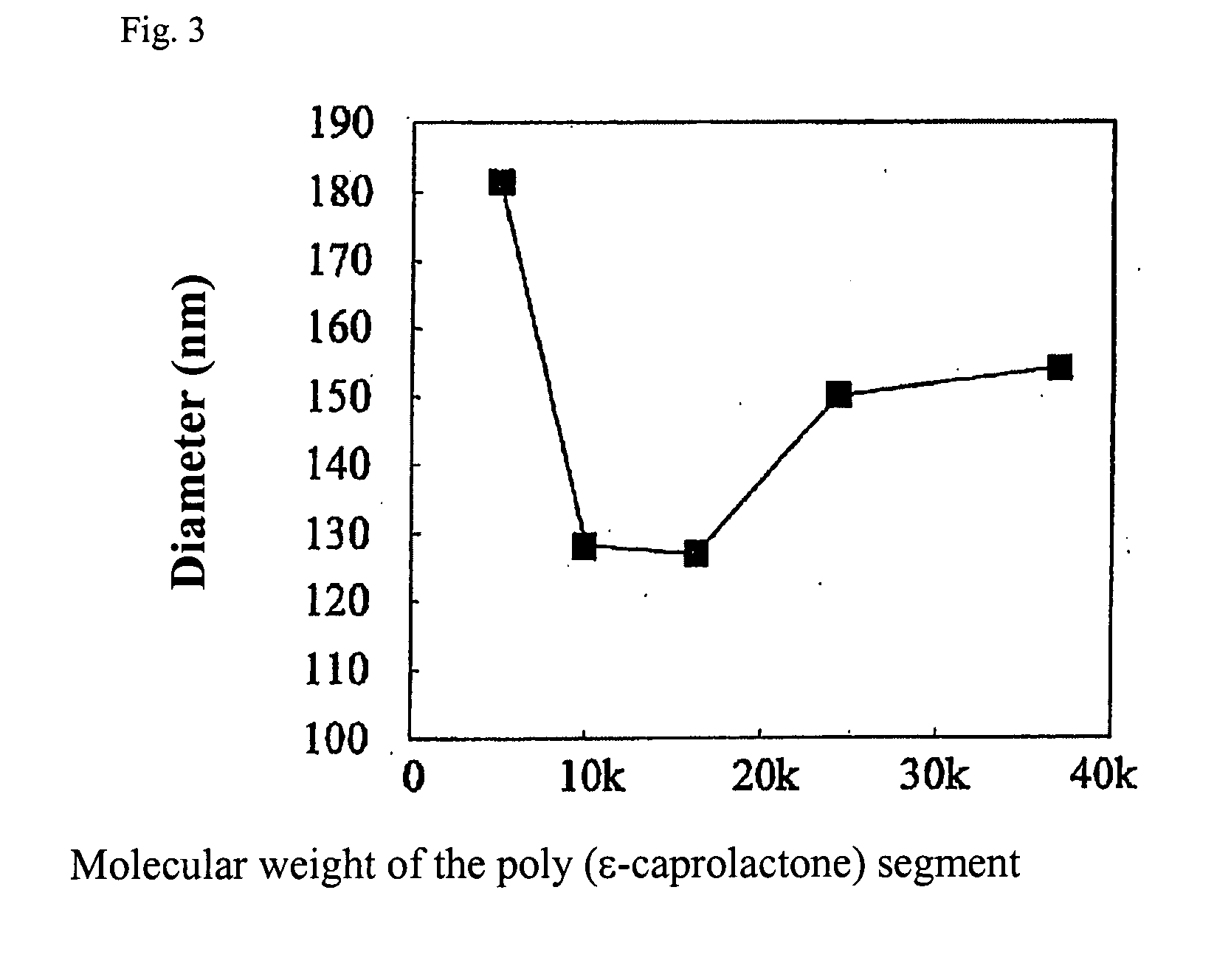
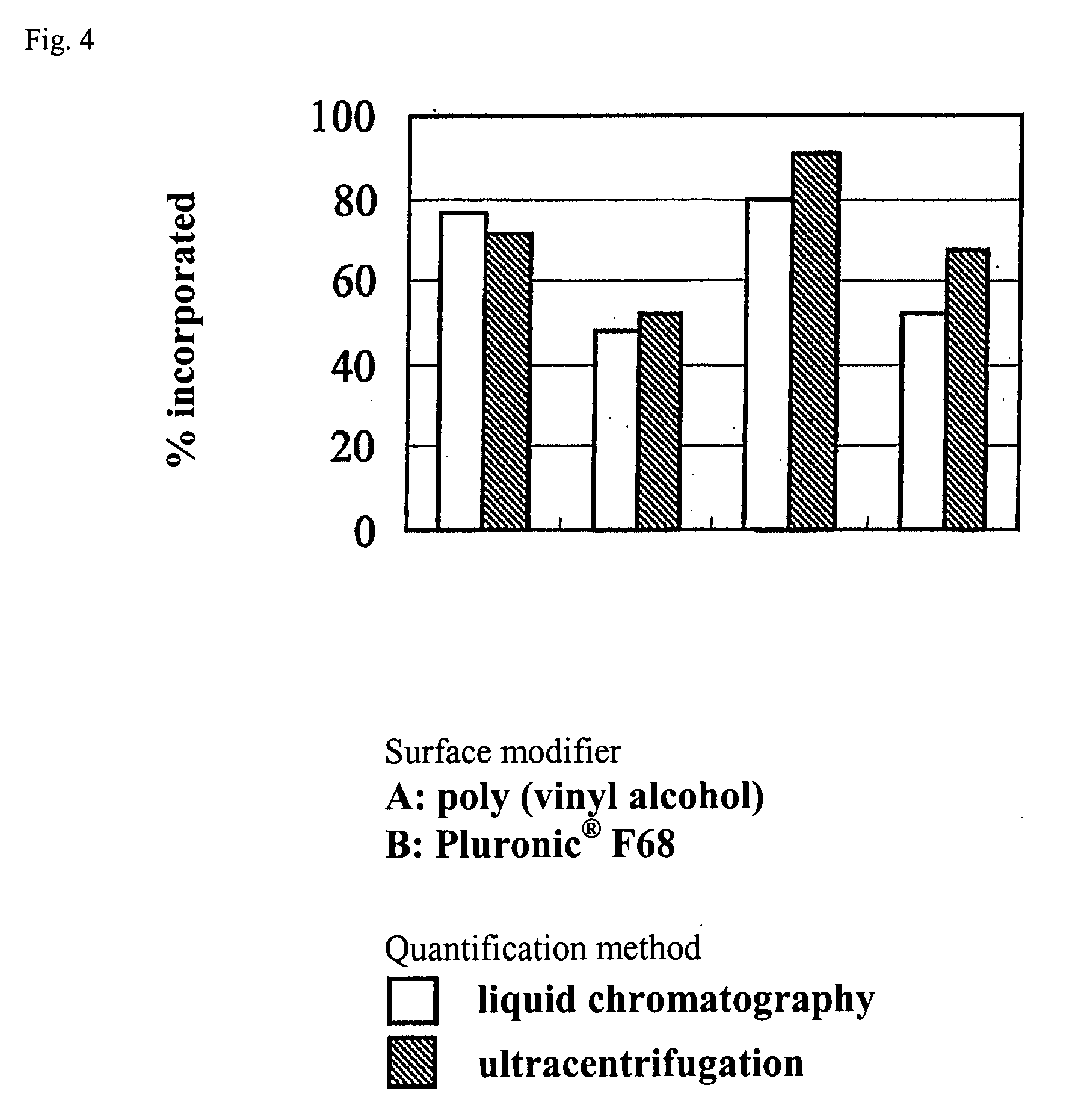
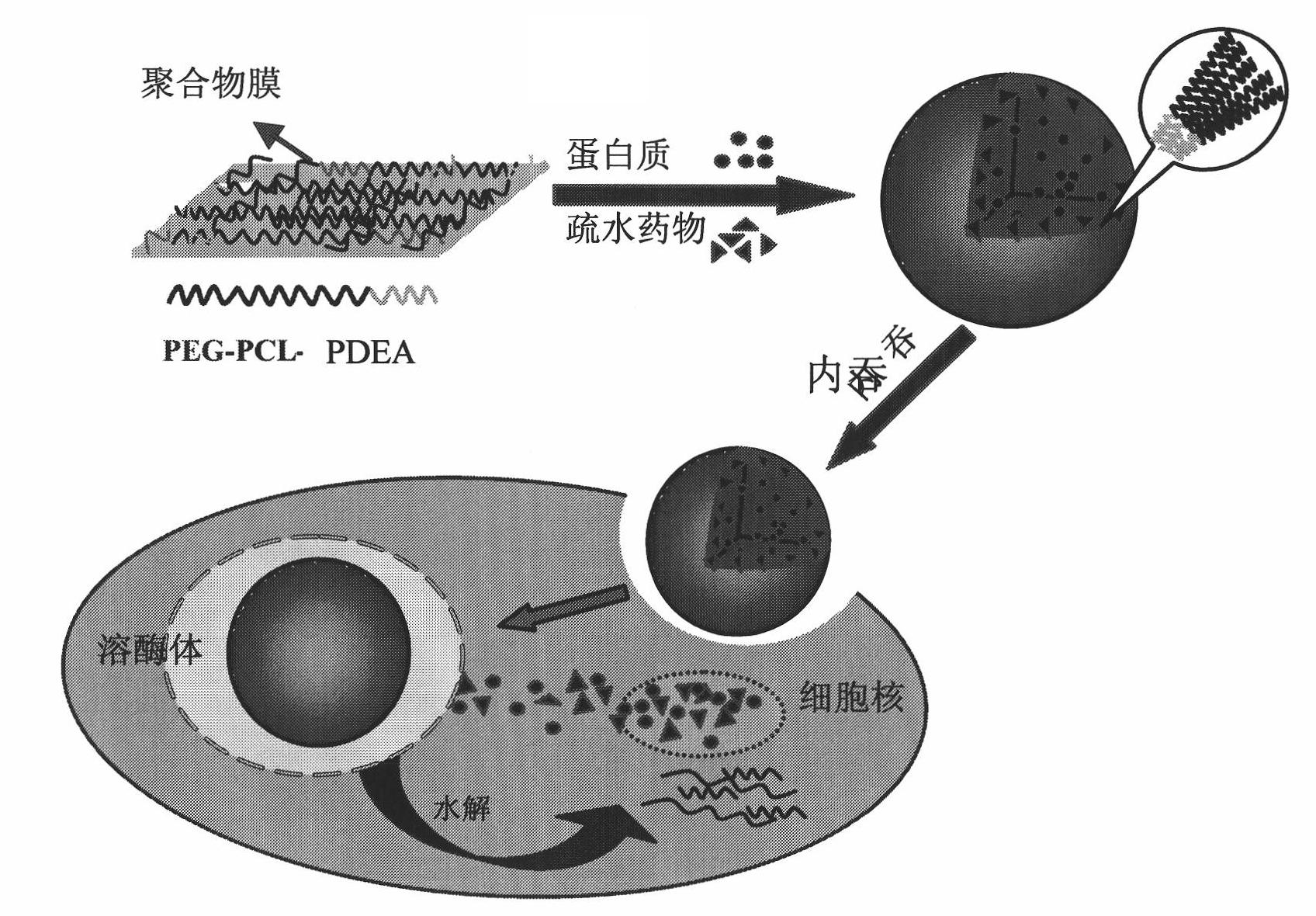
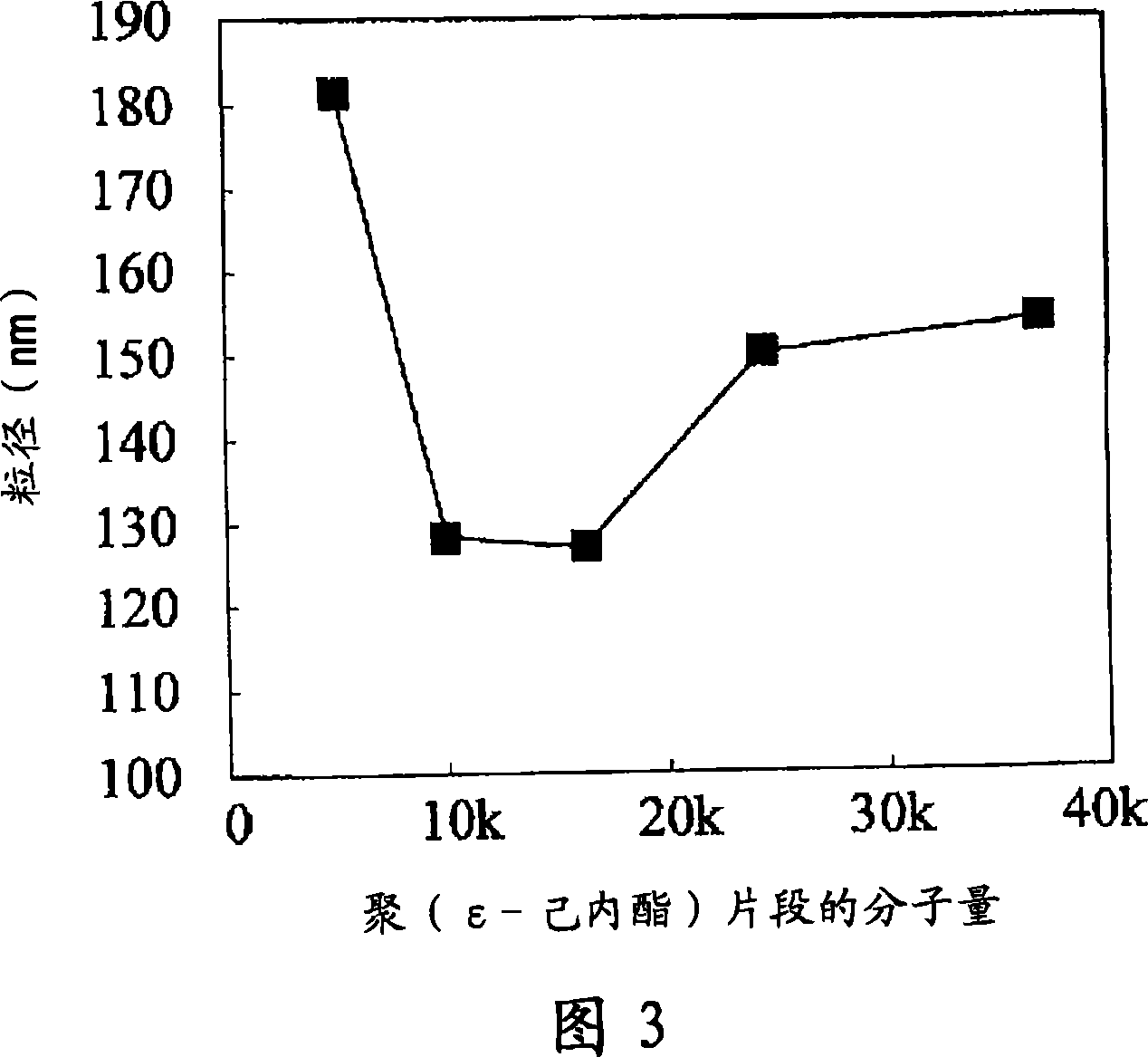
A fine particle comprising an amphiphilic polymer, further comprising an inner nucleus of hydrophilic segment of amphiphilic polymer, and a hydrophobic outer layer of hydrophobic segment of amphiphilic polymer, and having a surface modifier bonded to the hydrophobic outer layer.A fine particle of the invention effectively enclose protein, peptide drug, nucleic acid medicine of hydrophilic property and large molecular weight in the inner nucleus of hydrophilic segment of amphiphilic polymer, and are preferable for stabilizing in the body and promoting absorption.
Owner:TORAY IND INC
An insulinotropic complex using an immunoglobulin fragment
ActiveUS20100105877A1Improve efficacyProlong lifePeptide/protein ingredientsVasoactive intestinal peptidePeptide drugHalf-life
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and an immunoglobulin Fc region, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI SCI CO LTD
Stable formulations for parenteral injection of peptide drugs
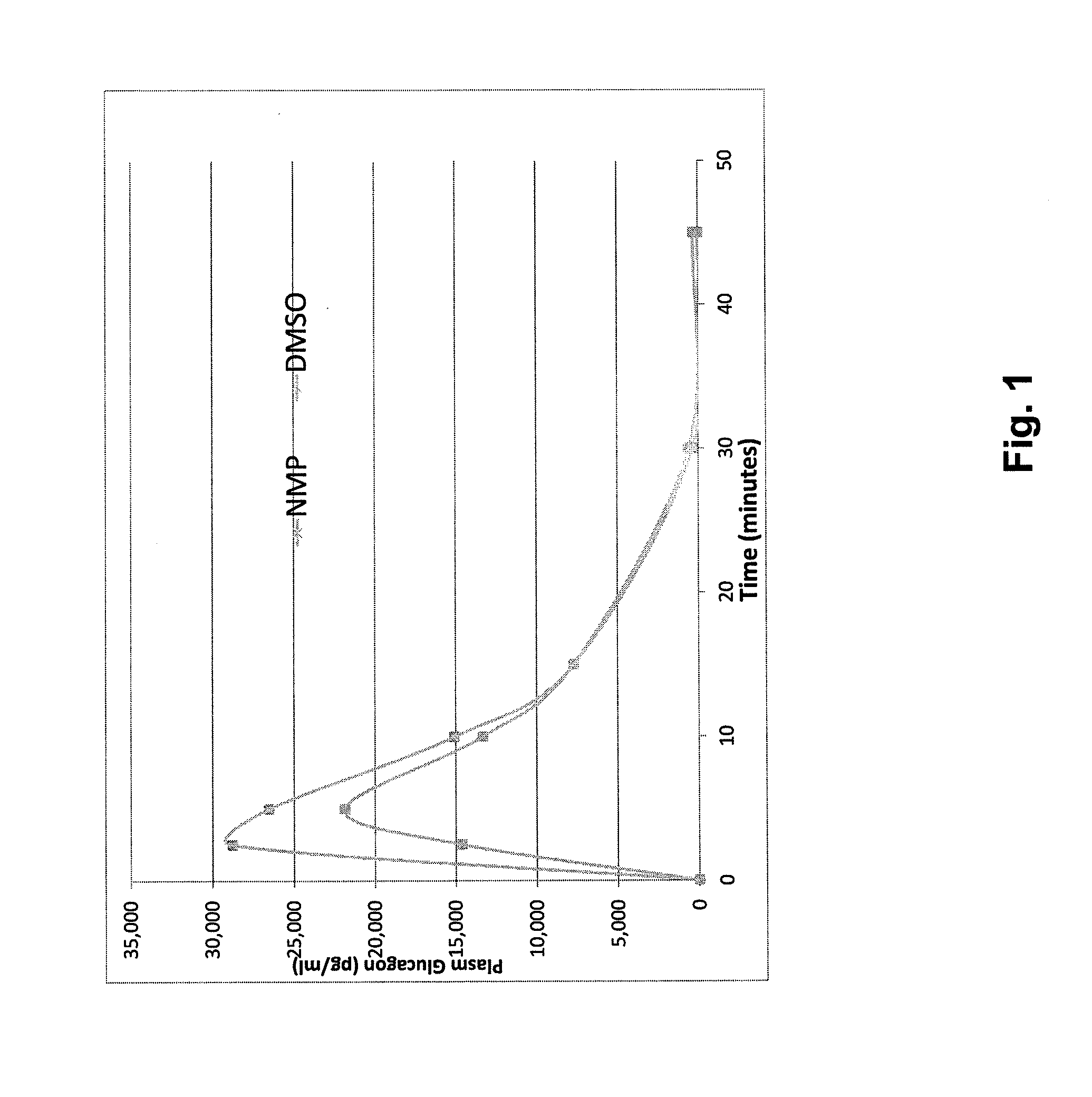
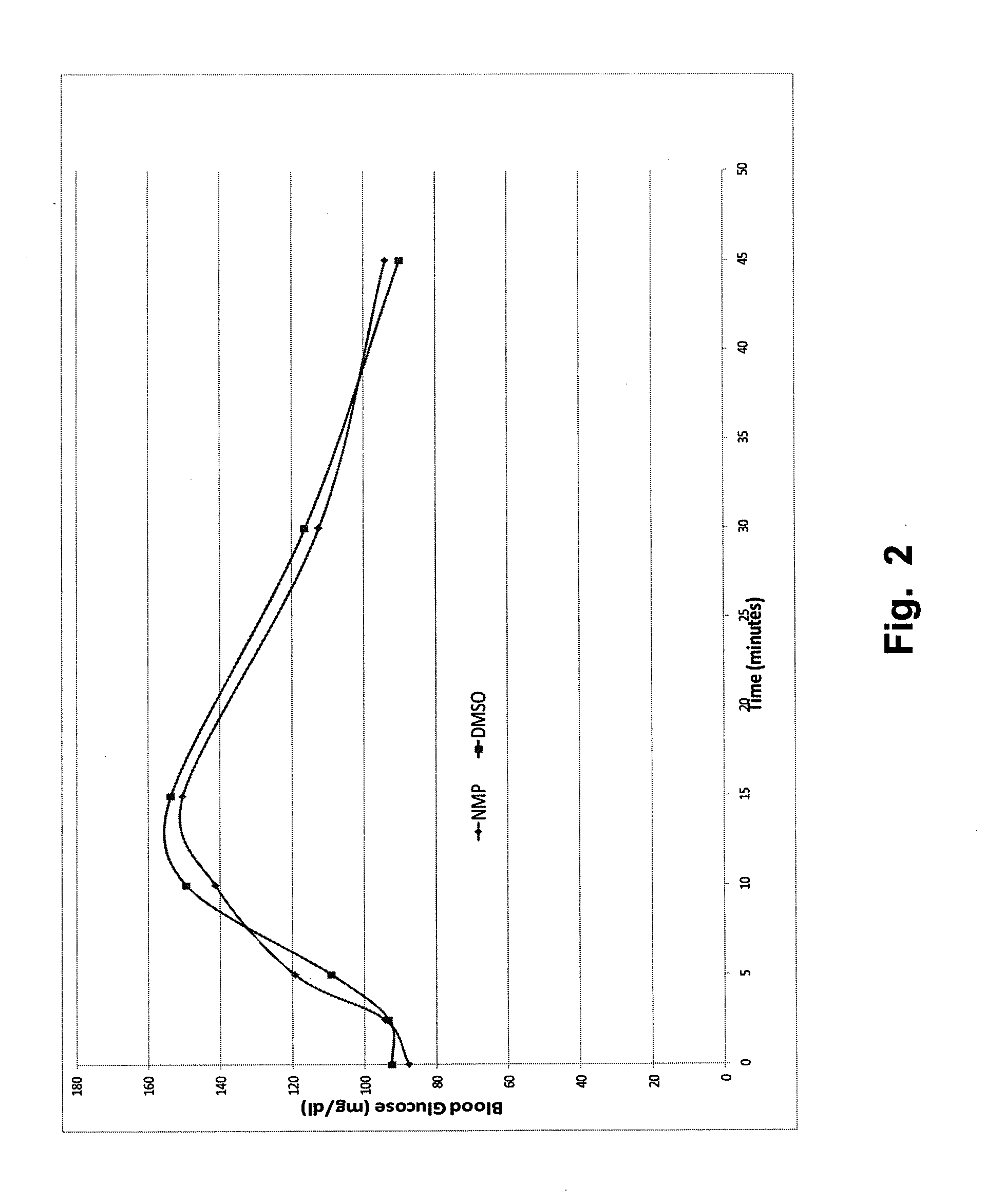
Stable formulations for parenteral injection of peptide drugs and methods of using such stable formulations are provided. In particular, the present invention provides stable formulations for parenteral injection of glucagon and methods of using such glucagon formulations to treat hypoglycemia, especially severe hypoglycemia in emergency situations.
Owner:XERIS PHARMA
Lipophilic-coated microparticle containing a protein drug and formulation comprising same
InactiveUS20050158392A1Enhanced release propertiesBiocideOrganic active ingredientsInorganic saltsPeptide drug
A solid lipophilic microparticle having an average particle size ranging from 0.1 to 200 μm, comprising a lipophilic substance, hyaluronic acid or an inorganic salt thereof and an active ingredient selected from the group consisting of a protein or peptide drug, retains the full activity of the active ingredient, and when formulated in the form of an oil dispersion or oil-in-water emulsion, it releases in an in vivo environment the active ingredient in a controlled manner over a long period.
Owner:LG CHEM LTD
Physiologically active polypeptide conjugate having prolonged in vivo half-life
InactiveUS20050176108A1Reduce decreaseProlong half-life in vivoHybrid immunoglobulinsAntibody ingredientsPeptide drugHalf-life
A protein conjugate having a prolonged in vivo half-life of a physiological activity, comprising i) a physiologically active polypeptide, ii) a biocompatible non-peptidic polymer, and iii) an immunoglobulin, is useful for the development of a peptide drug due to the enhanced in vivo stability and prolonged half-life in blood, while reducing the possibility of inducing an immune response.
Owner:HANMI PHARMA
Insulinotropic peptide conjugate using an immunoglobulin fc
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and a carrier substance, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI PHARMA
Biocompatible and hemocompatible polymer compositions
InactiveUS20060088571A1Good biocompatibilityImproved hemocompatibilitySurgeryMedical devicesBiocompatible coatingPercent Diameter Stenosis
Biocompatible coatings for medical devices are disclosed. Specifically, polymer coatings designed to control the release of bioactive agents from medical devices in vivo are disclosed. The present application also discloses providing vascular stents with controlled release coatings and related methods for making these coatings. Additional embodiments of the present invention include stents coated with the disclosed copolymer(s) and peptide drugs. Methods for treating or inhibiting post-stent implantation restenosis in patients are also provided.
Owner:MEDTRONIC VASCULAR INC
Biodegradable polymer vesicles and preparation and application thereof
InactiveCN101792516AEfficient packagingEliminate drug resistancePharmaceutical non-active ingredientsPolymer sciencePolyethylene glycol
The invention relates to a medicament carrier and a preparation method thereof, in particular to a medicament delivery system comprising biodegradable polymer vesicles with asymmetric membranes. The invention discloses the biodegradable polymer vesicles and preparation and application thereof. The biodegradable polymer vesicles are prepared from A-B-C type block polymer, wherein a block A is polyethylene glycol (PEG) distributed on outer surfaces of the vesicles; a block B is hydrophobic biodegradable polymer to form the nucleuses of the vesicles; and a block C is polyelectrolyte distributed on inner walls of the vesicular membranes and used for efficiently loading medicaments with opposite electric charge. The biodegradable polymer vesicles are formed in aqueous solution directly, can efficiently load protein and polypeptide medicaments, nucleic acid medicaments and micro-molecular medicaments, and are expected to be applied to the protein therapy and the combination therapy of cancers.
Owner:SUZHOU UNIV
Membrane translocating peptide drug delivery system
InactiveUS7268214B2Facilitated DiffusionImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsLipid formationCell layer
The present invention relates to a novel membrane translocating full-length peptide sequence, fragment, motif, derivative, analog or peptidomimetic thereof (MTLPs), to nucleotide sequences coding therefor, and to compositions comprising a MTLP-active agent complex and a MTLP-active particle complex. The MTLP or the nucleotide sequence coding therefor enhance movement of the active agent or of the active particle across a lipid membrane. More particularly, the present invention relates to a MTLP-active agent complex and a MTLP-active particle complex, wherein the MTLP enhances uptake of the active agent into a cell, into or out of an intracellular compartment and across a cell layer. Methods of making and methods of using MTLPs also are included.
Owner:MERRION RES I
Long acting hyaluronic acid - peptide conjugate
The invention relates to a novel bioconjugation protocol for peptide suitable for in vivo applications. Bioconjugation of the peptide to HA derivative increases its half life in circulation contributing for a high efficacy. More over, conjugate of HA derivative and peptide which is treated with hyaluronidase shows increased bioactivity. And also, in contrast to PEGylation, HA derivative can be conjugated with many numbers of peptide molecules per single HA derivative chain, which enables multiple action of peptide drugs.
Owner:POSTECH ACAD IND FOUND +1
Highly effective polypeptide for inhibiting angiogenesis, physical chemistry modifying method and application thereof
InactiveCN101143894APrevent proliferationLittle side effectsPeptide/protein ingredientsPeptidesIon exchangeGenetic engineering
The invention relates to a high-performance angiogenesis inhibitor and a production method, which belongs to the field of the biological engineering pharmaceutical technology or protein polypeptide drugs. The invention designs a high-performance angiogenesis inhibitor RGD-ED with integrin compatibility, the inhibitor contains angiogenesis inhibition polypeptide isoleucine-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-proline, and one or two ends of the inhibitor are respectively connected with polypeptides containing arginine-glycin-aspartate sequence. The RGD-ED of the invention can be synthesized. By the method of genetic engineering, the invention also expresses one of RGD-Eds in escherichia coli or other eukaryotic cells, and the RGD-ED is obtained by carrying out the separation, dissolution and renaturation of inclusion body protein and separation and purification by ion exchange chromatography. All the polypeptide sequences of the invention are modified by Polyethylene Glycol (PEG), heparin, dextran, polyvinylpyrrolidone (PVP), polyglycol-poly (amino acid) copolymer, palmitic acid, colominic acid and liposome, which includes liposome (REV), drying liposomal (DRV) and multivesicular liposome (Mvl). In vivo and in vitro experiments, the synthetic polypeptide sequence, product of genetic engineering and modified product of the invention can notably increase the effects of inhibiting the growth of endothelial cells, inhibiting angiogenesis and resisting tumor of the present angiogenesis inhibitors, and moreover, the high-performance angiogenesis inhibitor can be used as a drug curing solid tumors and rheumatoid arthritis.
Owner:CHINA PHARM UNIV
In vivo half life increased fusion protein or peptide maintained by sustained in vivo release, and method for increasng in vivo half-life using same
ActiveUS20120094356A1Prolong half-life in vivoLoss minimizationPeptide/protein ingredientsPeptide preparation methodsPeptide drugHalf-life
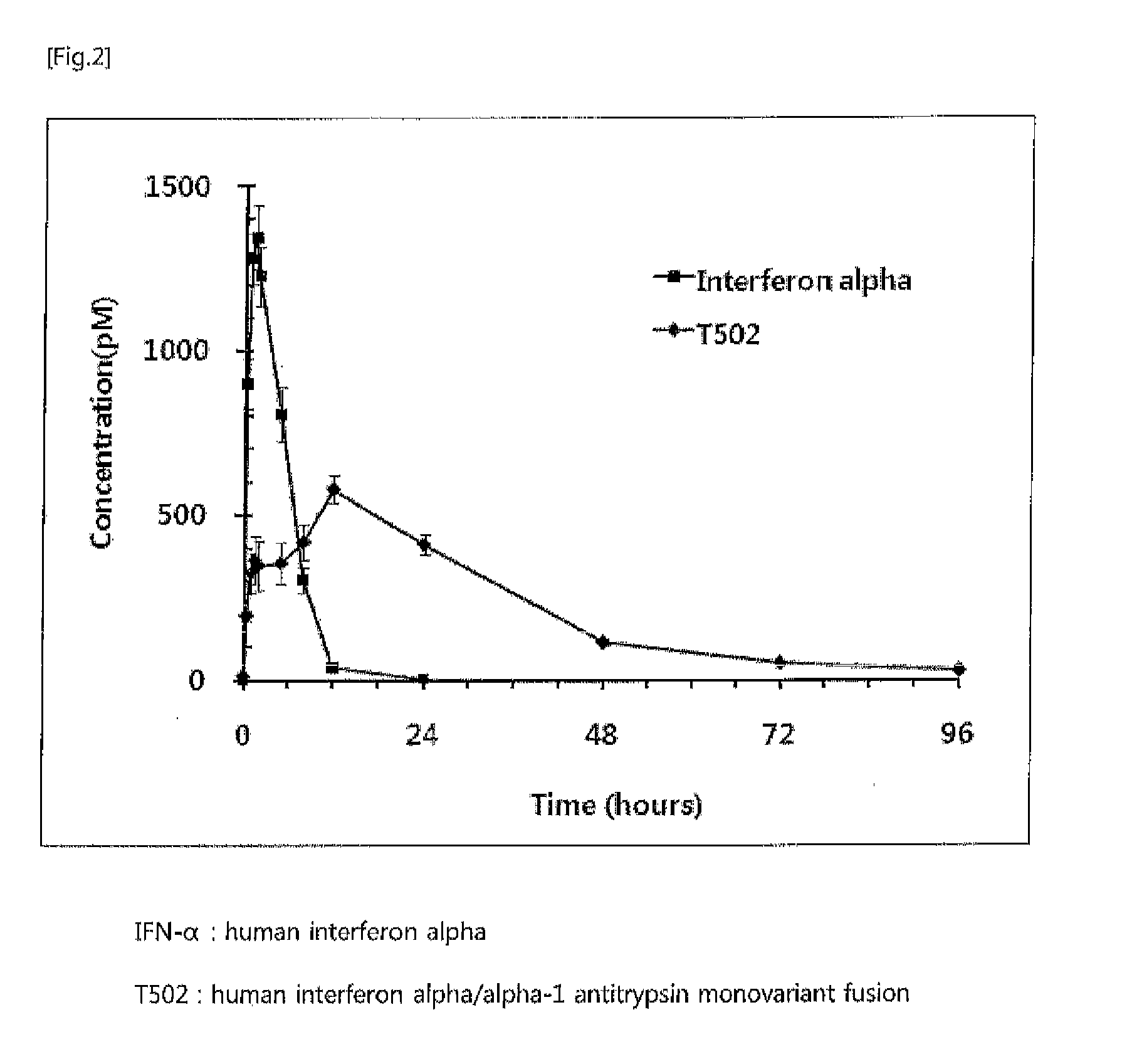
The present invention relates to a fusion protein or peptide, the in vivo half-life of which is increased by maintaining in vivo sustained release, and to a method for increasing in vivo half-life using same. A fusion protein or peptide according to the present invention has excellent in vivo stability by binding a physiologically active protein or physiologically active peptide to an alpha-1 antitrypsin or alpha-1 antitrypsin mutant with one or more amino acids mutated to maintain the in vivo sustained release and to significantly increase the half-life thereof in blood (T1 / 2) compared to an inherent physiologically active protein or physiologically active peptide. Thus, a fusion protein or peptide according to the present invention can be useful in developing a sustained-release preparation of a protein or peptide drug.
Owner:ALTEOGEN
Application of low-concentration vesicular phospholipid gel as slow release carrier for small-molecule peptide drug
ActiveCN102125517AHigh encapsulation efficiencyReduce the impact of stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMultiple formsPeptide drug
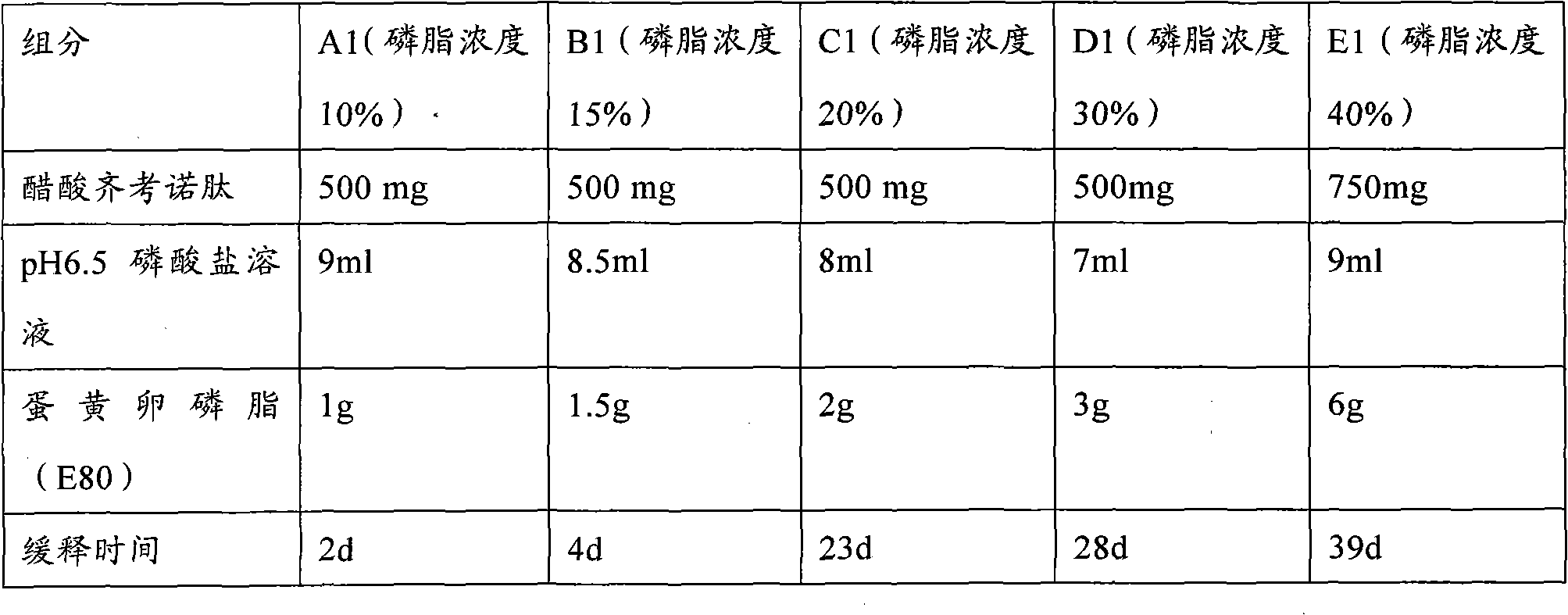
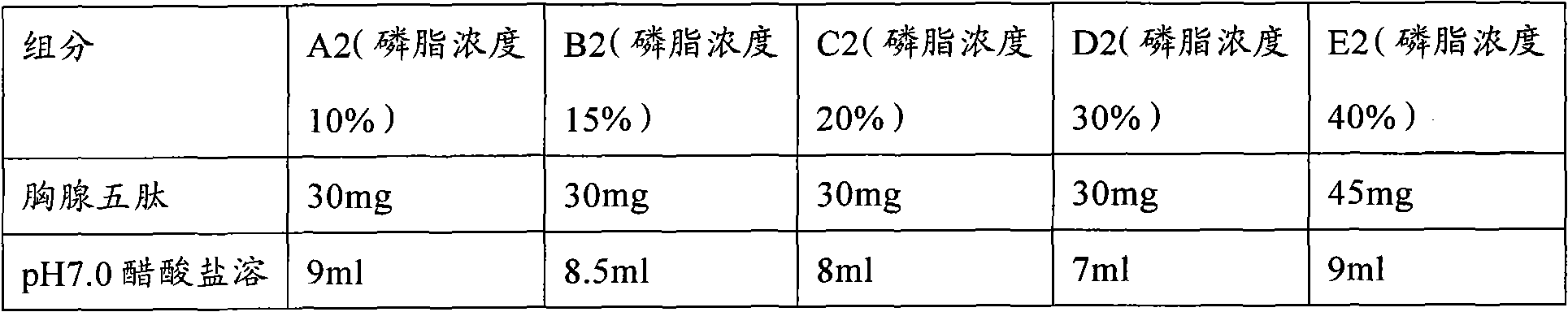
The invention provides a low-concentration vesicular phospholipid gel formulation of a small-molecule peptide drug and also provides a preparation method of the vesicular phospholipid gel formulation of the small-molecule peptide drug. In the low-concentration vesicular gel formulation, the content of phospholipids ranges from 20% to 40%, and the small-molecule peptide drug with a molecular weight ranging from about 300 D to about 3300 D is encapsulated in the phospholipids. The low-concentration vesicular phospholipid gel formulation is available in various drug administration forms suitable for injection drug administration, external drug administration and the like, has good biocompatibility, high drug-carrying capacity and good stability, and particularly has a long-acting slow release effect.
Owner:成都师创生物医药科技有限公司
Sustained-release composition of drugs encapsulated in microparticles of hyaluronic acid
InactiveUS20080063727A1Simple compositionPowder deliveryPeptide/protein ingredientsPeptide drugInorganic salts
A sustained-release drug composition consisting essentially of microparticles of hyaluronic acid having a high molecular weight or an inorganic salt thereof and a protein or peptide drug encased in said microparticles, wherein the average size of said microparticles ranges from 0.1 to 40 μm.
Owner:LG LIFE SCI LTD
Insulinotropic complex using an immunoglobulin fragment
ActiveUS8476230B2Improve efficacyProlong lifePeptide/protein ingredientsVasoactive intestinal peptidePeptide drugHalf-life
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and an immunoglobulin Fc region, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI SCI CO LTD
Targets for tumor growth inhibition
InactiveUS20070003519A1Reduce decreaseGrowth inhibitionGenetic material ingredientsScreening processPeptide drugMFGE8
The present invention relates to methods for treating cancers by manipulating a target gene expression by up-regulation, silencing and / or down-regulation of the gene, such as EGFR-RP, TRA1, MFGE8, TNFSF13 and ZFP236, respectively. The methods are useful in treating cancers and / or inhibiting tumor growth by enhancing expression of a gene that is validated as a target such as ICT1030, for protein, peptide drug and gene therapy modalities; or by RNA interference to silence and / or down-regulate targets such as ICT1024, ICT1025 and ICT1031 and ICB1003 that are validated for antibody, small molecule and other inhibitor drug modalities.
Owner:INTRADIGM CORP
Octreotide acetate preparation and preparation method thereof
InactiveCN102525927AFull appearanceNo significant change in encapsulation efficiencyPeptide/protein ingredientsDigestive systemOctreotide preparationFreeze-drying
The invention belongs to the field of medicament preparations, and discloses an octreotide acetate lipidosome precursor and a preparation method thereof. The precursor lipidosome contains octreotide acetate, negatively-charged phospholipid and a cryoprotectant, and can contain an appropriate quantity of other lipids including phosphatidylchline and cholesterol; components such as an antioxidant, a pH regulating agent and the like can be added as required; the molar ratio of the octreotide acetate to the negatively-charged phospholipid is smaller than 1:1; and the mass ratio of the negatively-charged phospholipid to the cryoprotectant is 1:1-1:10. In the invention, a tert-butyl alcohol-water cosolvent freeze-drying method is adopted. The entrapment rate of an octreotide acetate lipidosome / micelle obtained by hydrating a freeze-dried product can be over 50 percent, an octreotide acetate lipidosome / micelle obtained by hydrating the freeze-dried product has high stability, and the problem of difficulty in entrapping a protein polypeptide medicament during preparation of a lipidosome / micelle preparation is solved. The preparation method is simple and practicable, and is suitable for industrial mass production.
Owner:SHENYANG PHARMA UNIVERSITY
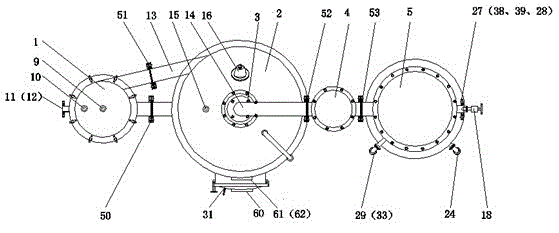
Single-effect external-cycle concentrator and application thereof
The invention discloses a single-effect external-cycle concentrator and an application thereof. The single-effect external-cycle concentrator comprises a heating chamber 1, an evaporating chamber 2, a separator I 4, a condenser 5, a cooler 6, a separator II 7 and a liquid receiver 8. The heating chamber, the evaporating chamber and the separator I are equipped with online cleaning devices respectively. The single-effect external-cycle concentrator has the advantages of a simple structure, convenience in mounting and dismounting, high evaporating efficiency, capacity of realizing online cleaning and the like, is safe and reliable in a using process, and capable of removing an organic solvent in a bone peptide crude drug liquid better. External-cycle heating is adopted, so that the heated liquid is sprayed after entering the evaporating chamber through a steam vent, and therefore, the evaporating area is increased, the solvent is quickly evaporated, and the concentration time can be shortened. Alcohol residues of the bone peptide drug liquid produced by the single-effect external-cycle concentrator are less than 10mg / ml. The single-effect external-cycle concentrator has the characteristics that a process for producing a bone peptide crude drug is easy to control, the quality of the produced bone peptide crude drug is stable, and the organic solvent residues are less.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
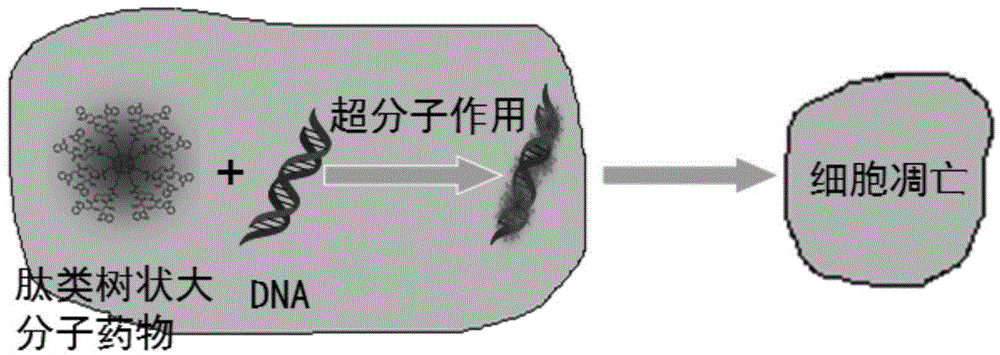
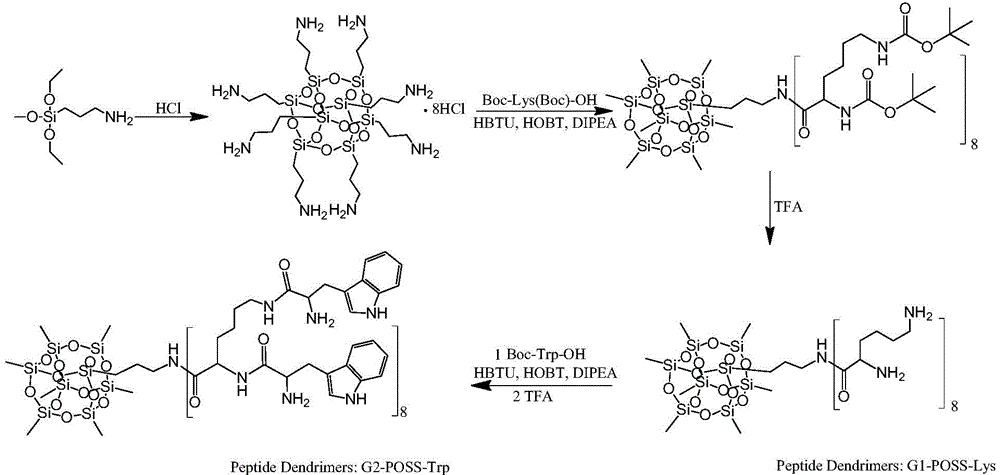
Peptide dentritic macromolecular drug and preparation method and application thereof
InactiveCN104650194AGood water solubilitySolve permeabilityPeptide/protein ingredientsDepsipeptidesSolubilityEnd-group
The invention discloses a peptide dentritic macromolecular drug and a preparation method and application thereof, and belongs to the field of biomedicine. The drug comprises a peptide dentritic macromolecular skeleton, and terminal-group functionalized groups. The biological activity of the drug is ensured through the terminal-group functionalized groups; and the periphery of the peptide dentritic macromolecular drug can be grafted with a lot of terminal-group functionalized groups, so that the biological effect of the terminal-group functionalized groups is effectively amplified by the amplification effect of dendritic macromolecules. The drug has an accurate molecular structure and abundant surface functional groups, is good in water solubility, and also has the biological characteristics of being similar to globulin, easy to be taken in by cells and the like; the biological effect of the peptide dentritic macromolecules is enriched; and the development of peptide drugs is promoted. The preparation method is simple in technology; and the structure of the peptide dentritic macromolecular drug is accurately controlled by controlling preparation of various raw materials.
Owner:SICHUAN UNIV
Multiple-arm polyethylene glycol with functional group at long chain end, its preparation method and uses
InactiveCN1995094AHigh selectivityExtension of timePharmaceutical non-active ingredientsSolubilityPeptide drug
The invention discloses a new multiple-arm carbowax and making method and application in the macromolecular material technical domain, which is characterized by the following: possessing active functional group on one end of arm; fitting for decorating micromolecular drug, protein and peptide drug; modifying the solubility, stability and immunity of drug; lengthening the half-life of drug.
Owner:FUDAN UNIV
Oral delivery of proteins and peptides
InactiveUS20100303901A1Improve bioavailabilityRapid drug releasePeptide/protein ingredientsCalcitoninsPeptide drugFast release
Enteric coated capsules or tablets for oral delivery of a protein, polypeptide or peptide drug, in particular for oral delivery of insulin, are provided, comprising microparticles of the protein, polypeptide or peptide drug, microparticles of a protease inhibitor and, optionally, microparticles of an absorption enhancer. The protease inhibitor and the absorption enhancer may be together in the same microparticles. The microparticles of each component are embedded in an enteric polymer matrix. The enteric coated tablet or capsule of the invention enables fast release of the protein, polypeptide or peptide drug at different times at desired loci in the gastrointestinal tract
Owner:TECHNION RES & DEV FOUND LTD
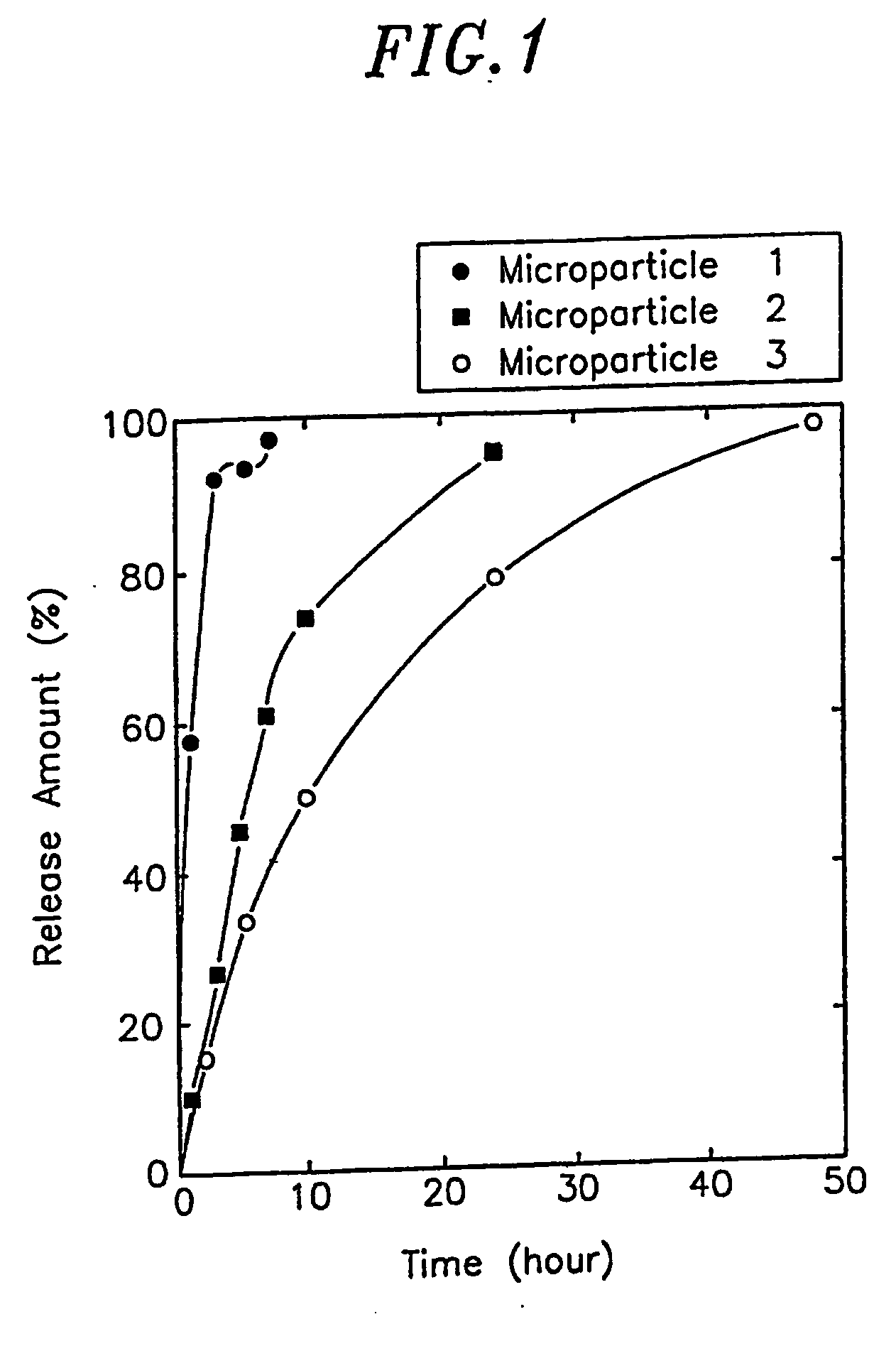
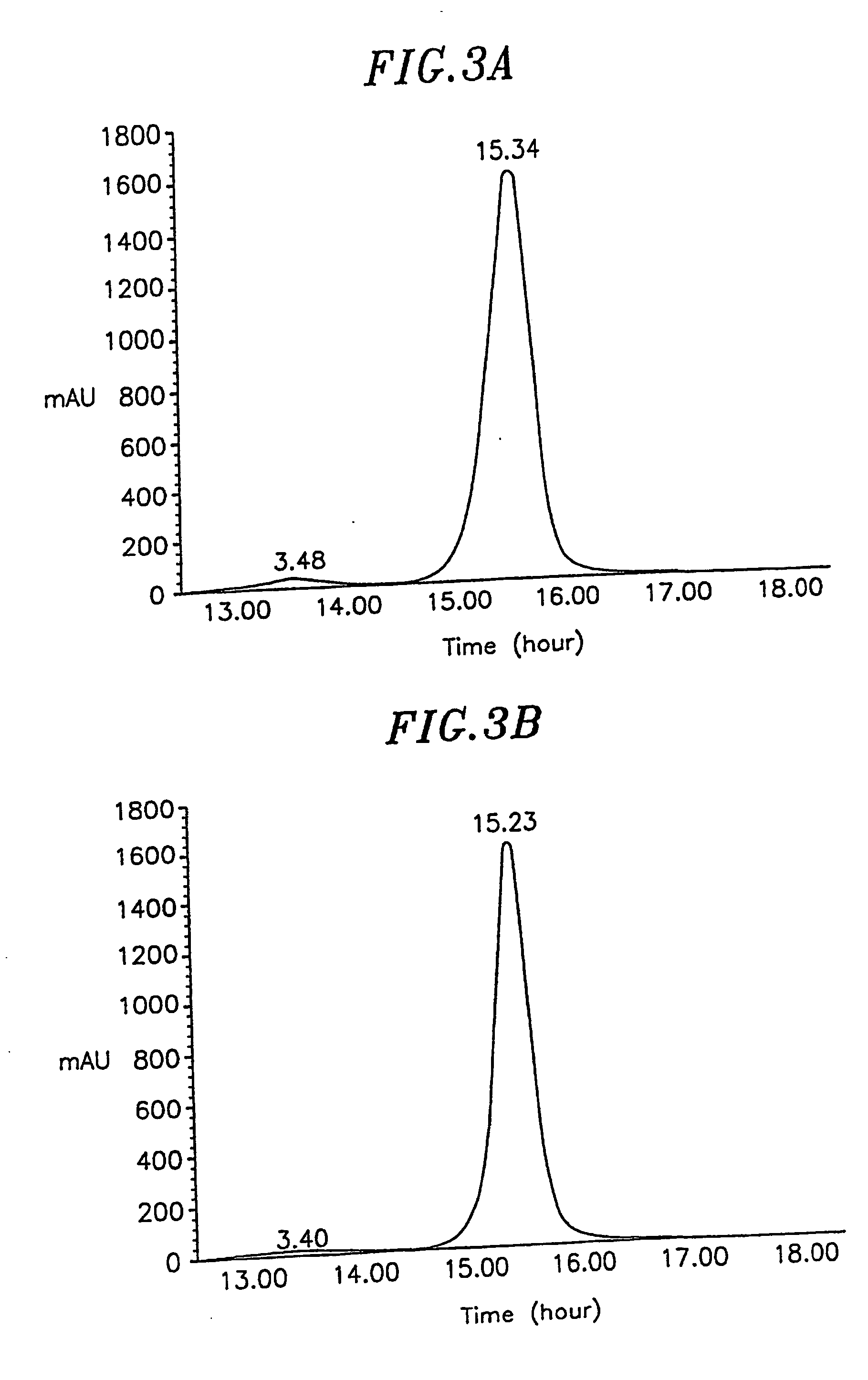
Slow-release polylactic acid microsphere capable of maintaining protein and polypeptide drug activity and preparation method thereof
InactiveCN103585635AHigh activityImprove stabilityPowder deliveryPeptide/protein ingredientsMicrosphereBioactive glass
The invention discloses a slow-release polylactic acid microsphere capable of maintaining protein and polypeptide drug activity and a preparation method thereof. The slow-release polylactic acid microsphere comprises 5-30 parts of a protein and polypeptide drug, 30-90 parts of a polylactic acid biodegradable material and 5-50 parts of nano bioactive glass. The slow-release polylactic acid microsphere is prepared by an electrospinning technique, and mainly comprises the polylactic acid biodegradable material (PLGA (polylactic acid-glycolic acid copolymer) or PLA (polylactic acid)), the bioactive glass and a main drug. The slow-release polylactic acid microsphere can greatly promote activity retention of the protein and polypeptide drug in a preparation process, and can improve stability of the protein and polypeptide drug in microsphere storage and release processes.
Owner:JIANGSU UNIV
Microparticle and pharmaceutical composition
A fine particle comprising an amphiphilic polymer, further comprising an inner nucleus of hydrophilic segment of amphiphilic polymer, and a hydrophobic outer layer of hydrophobic segment of amphiphilic polymer, and having a surface modifier bonded to the hydrophobic outer layer. A fine particle of the invention effectively enclose protein, peptide drug, nucleic acid medicine of hydrophilic property and large molecular weight in the inner nucleus of hydrophilic segment of amphiphilic polymer, and are preferable for stabilizing in the body and promoting absorption.
Owner:TORAY IND INC
Peptide drugs for chronic lymphocytic leukemia (CLL) and other cancers
InactiveUS20060198832A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsPeptide drugChronic lymphocytic leukemia
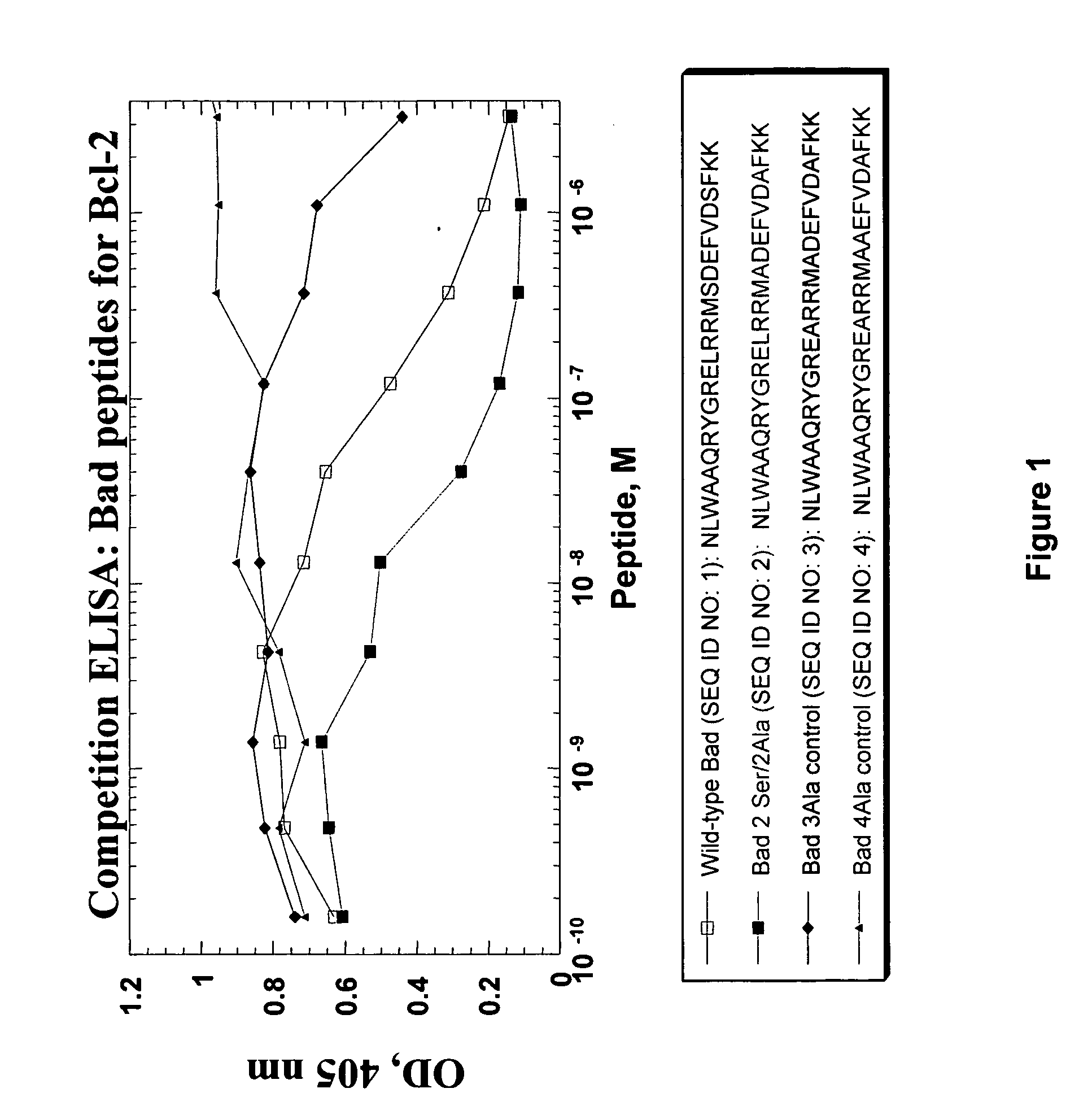
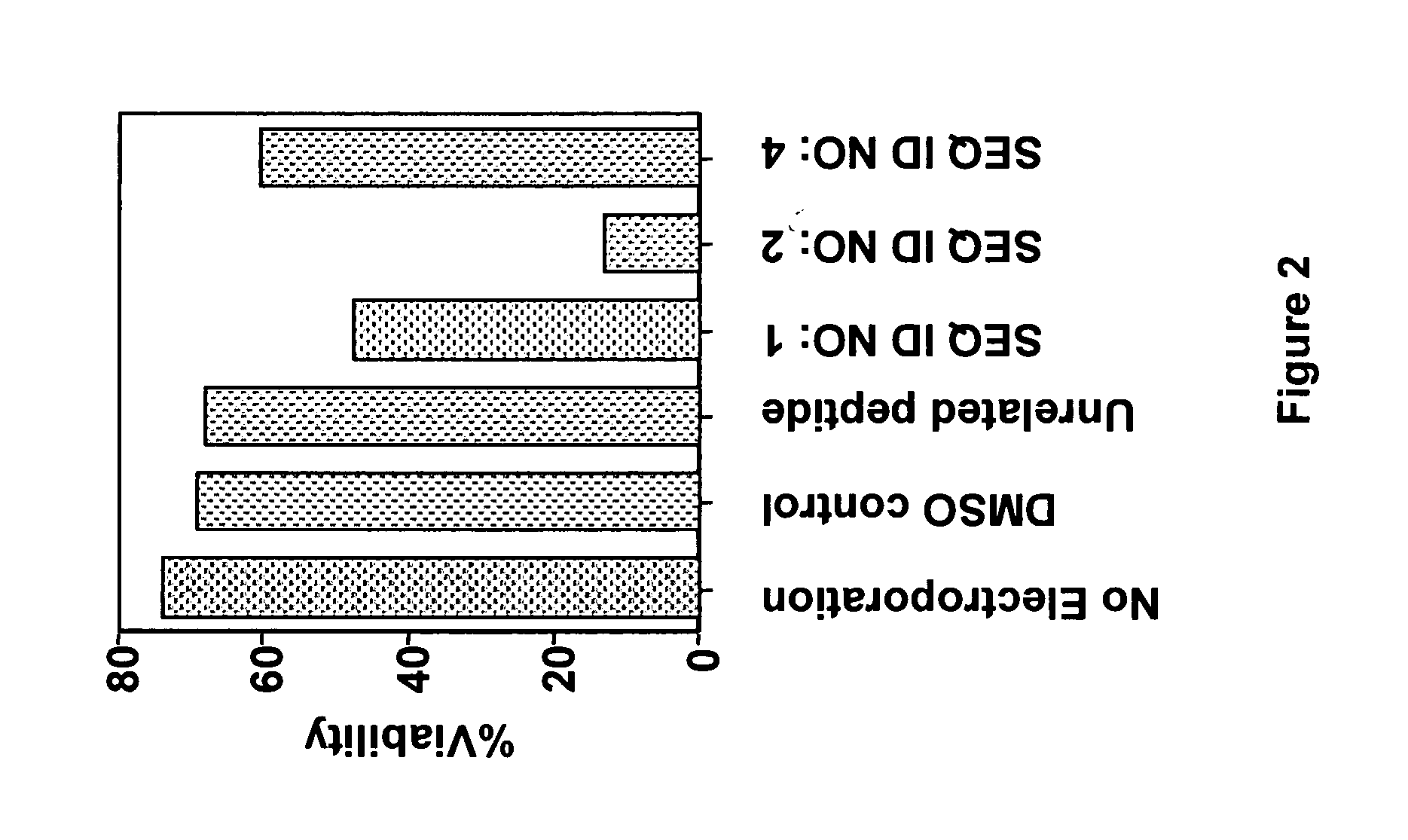
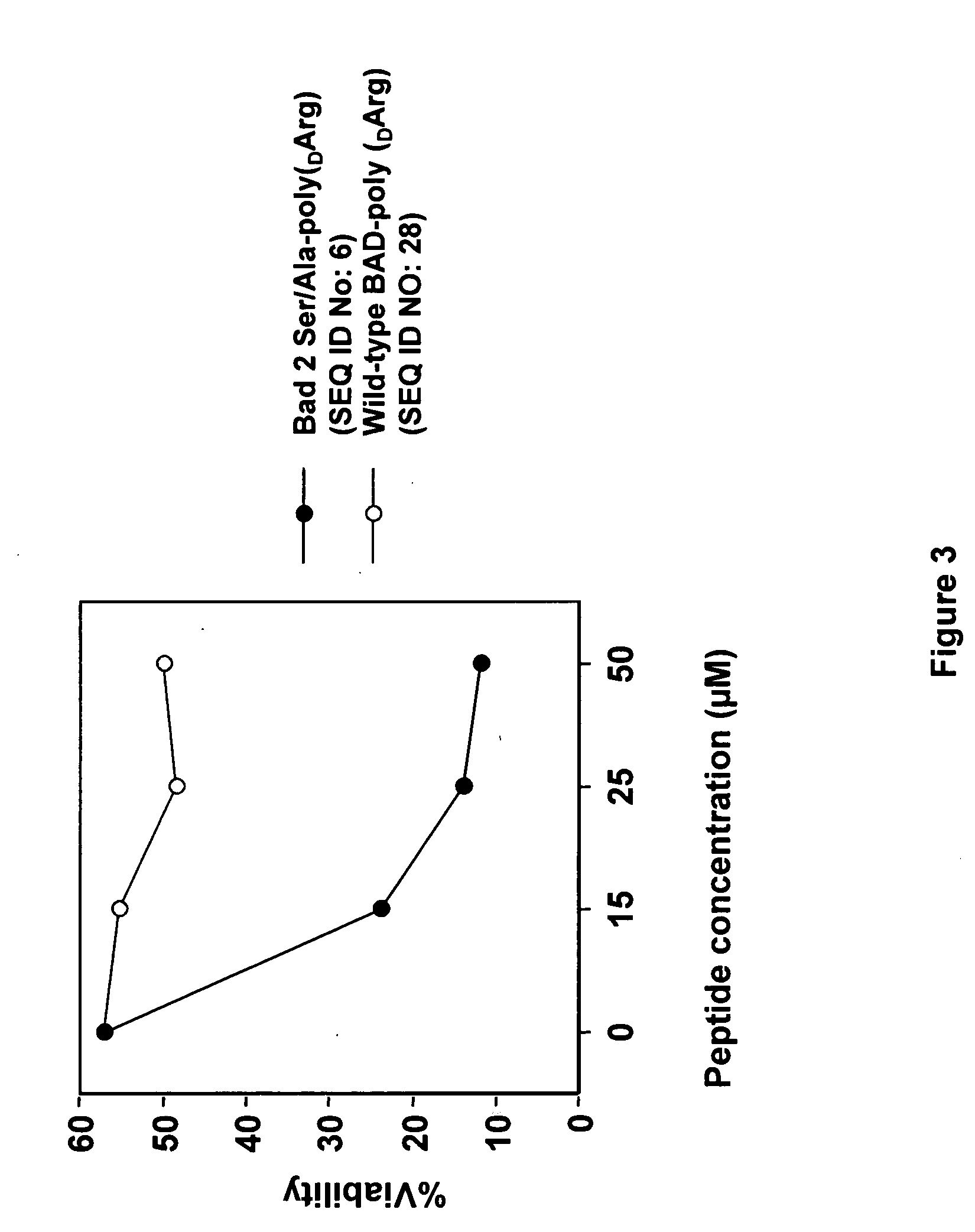
The present invention provides a modified BAD peptide or peptidomimetic which includes an amino acid sequence having at least 60% amino acid identity with SEQ ID NO: 1, where the modified BAD peptide or peptidomimetic has enhanced affinity for Bcl-2 as compared to wild type BAD peptide (SEQ ID NO: 1). Further provided herein is a composition containing a delivery agent and a modified BAD peptide or peptidomimetic which includes an amino acid sequence having at least 60% amino acid identity with SEQ ID NO: 1, where the modified BAD peptide or peptidomimetic has enhanced affinity for Bcl-2 as compared to wild type BAD peptide (SEQ ID NO: 1).
Owner:THE BURNHAM INST
Oral administration of a calcitonin
InactiveUS20100210526A1Promote oral absorptionIncreases systemic bioavailabilityBiocideOrganic active ingredientsWhole bodyBuccal administration
The invention is directed to a method of administering pharmaceutical compositions comprising peptide drugs such as a calcitonin in combination with one or more oral delivery agents, together with an amount of a liquid, and method of treatment of disorders responsive to the action of peptide drugs such as a calcitonin employing such method of administration so as to enhance the oral bioavailability of a calcitonin. The methods of the invention increase the oral absorption and systemic bioavailability of peptide drugs, such as a calcitonin.
Owner:NOVARTIS AG
Full-automatic polypeptide synthetic instrument
InactiveCN1966515AImprove completenessImprove reaction efficiencyPeptide preparation methodsChemical recyclingOrganic synthesisSoftware system
This invention involves a full-automatic peptide synthesizer, including a CPU system, a GMP software operating system, a liquid control system, a gas control system, a materials input system, a reactor system, a limitless speed-regulating stirring system, a personal safety system, a reaction efficiency automatic feedback system, a fault alarm system, and a solution circulatory system. The invention has the advantages of higher synthesis efficiency of peptide, better product quality, shorter development cycles, lower production cost, and more convenient and safe operation. The invention is also consistent with the strict requirements of FDA on GMP production, meets a variety of synthetic peptide research and production requirements on the whole, and can be widely applied to basic research, peptide drugs, health, beauty, food, polymer materials, fine chemicals, organic synthesis, etc.
Owner:天津赛瑞多肽科技有限公司
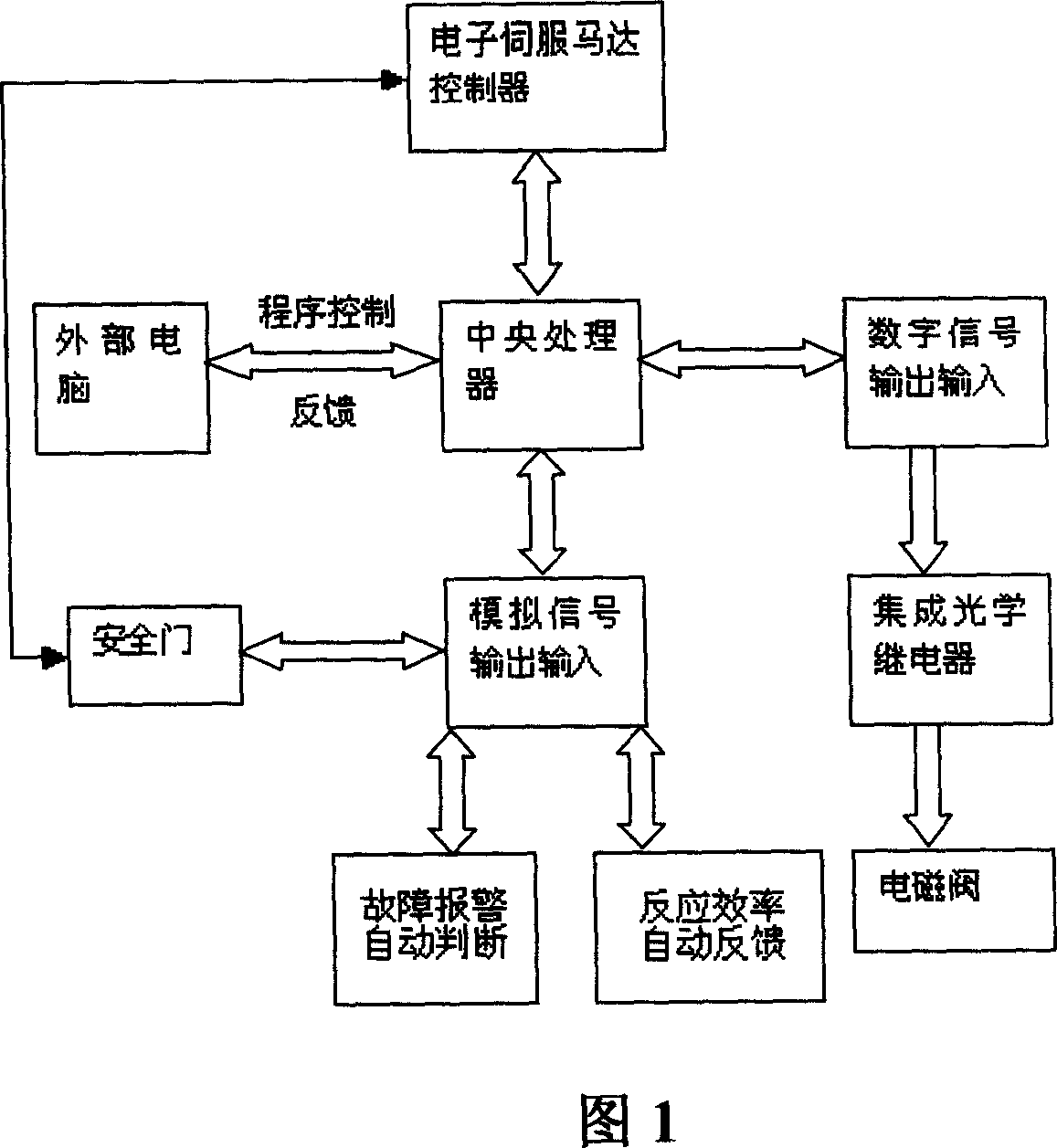
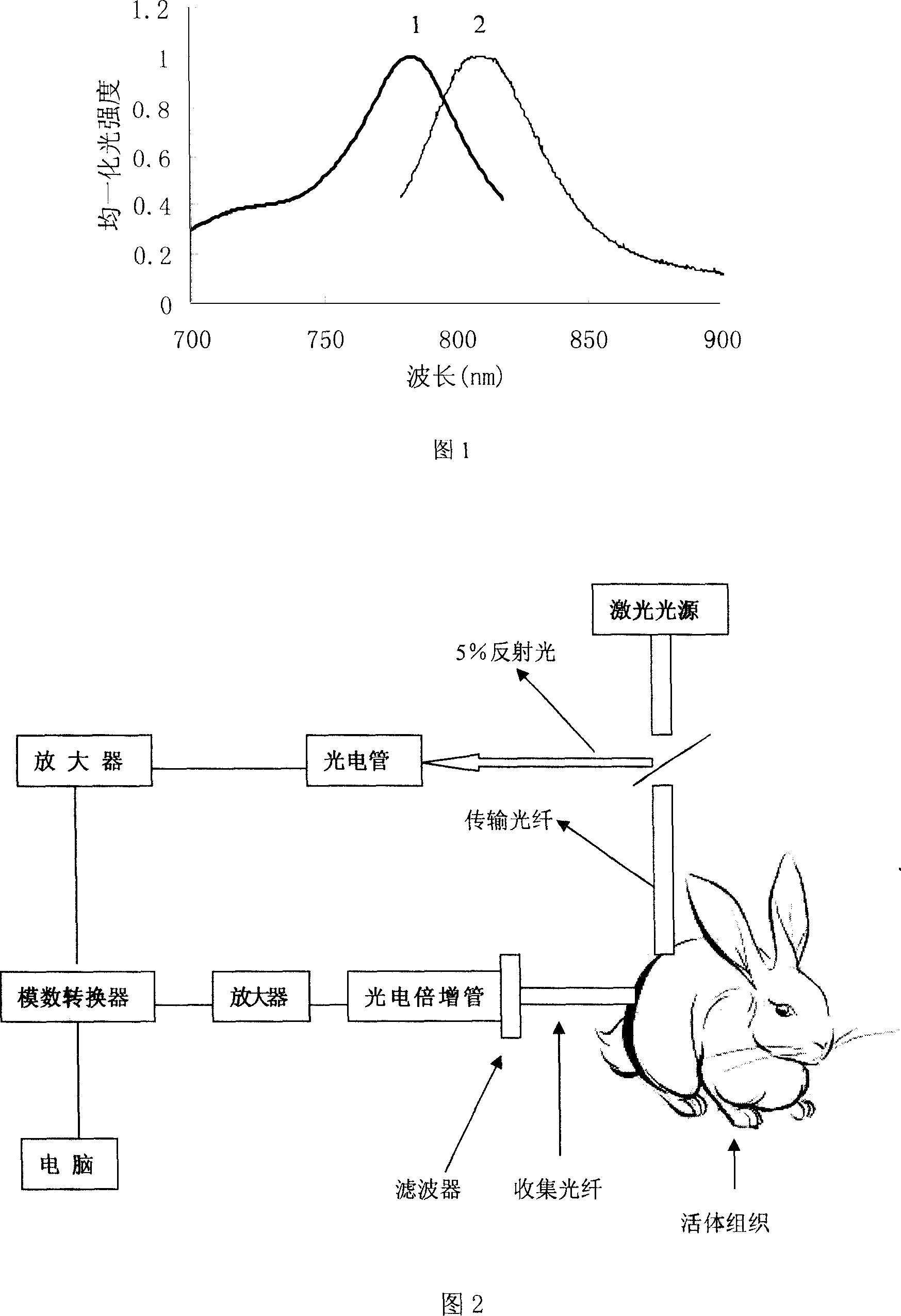
Pharmaceutical in vivo dynamics characteristic-nondestructive in situ monitoring system and monitoring method
InactiveCN101011237AReduced post-processingNo radioactivityDiagnostic recording/measuringSensorsLight filterLight source
The invention relates to a system used in the internal dynamic nondestructive online checking of protein polypeptide drug, and relative checking method. The inventive system comprises a fluorescence probe and a detecting system, while the detecting system is formed by a near-infrared light source, a transmission optical fiber, a receiving optical fiber, a near-infrared high-pass filter, and a near-infrared detector. The laser of light source and the fluorescence light received by the detector can be transmitted by the optical fiber; the near-infrared band-pass or high-pass filter is at the front of detector. The invention can avoid sampling timely in the test, avoid killing animals in the organism distribution test, to and avoid the following sample treatment.
Owner:CHINA PHARM UNIV
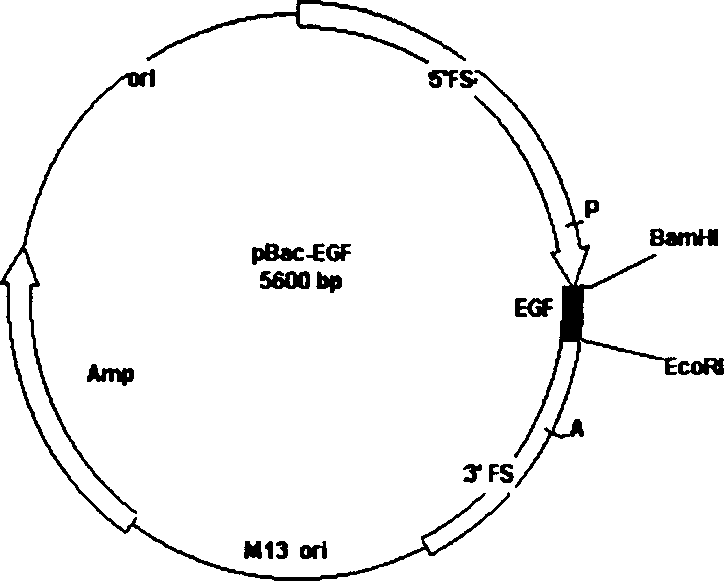
Method for producing medicine using silkworm expressed numan epidermal growth factor
InactiveCN1405310ADetermine therapeutic functionPeptide/protein ingredientsMicroencapsulation basedDiseaseCuticle
The invention belongs to the technique field of producing multi-peptide drugs by gene engineering. It clones human EGF gene into the silkworm bacilliform virus (Bombyx mori Nuclear polyhedrosis Virus) transferring carrier pBacPAK8 and makes them carry through homologous reformation in the silkworm cells to obtain the reformed virus BmBacEGF with human-coat growing gene. Use the reformed virus to to inocualte the silkworm grub and chrysalis by acupuncture in order to make the human-coat growing gene efficient expressed. Then make the grub and chrysalis into oral drugs which has remarkable action on the digestible ulcer by examination on animal.
Owner:正源堂(天津)生物科技有限公司
Medicine-carring particulates composed of hydrophilic resin and hydrophobic resin and its preparation method
InactiveCN1481783AHigh encapsulation efficiencyImprove controllabilityPowder deliveryPharmaceutical non-active ingredientsControlled releaseParticulates
The present invention belongs to medicine carrying resin particle and its preparation technology. The medicine carrying particle has matrix of hydrophobic resin and hydrophilic resin particles, which is dispersed inside the matrix, has medicine carried and is capable of forming aquogel. The preparation process of the medicine carrying particle includes preparing medicine carrying grain with aquogel of hydrophilic resin, preparing medicine carrying particle with matrix of hydrophobic resin and aquogel grain of hydrophilic resin, and freeze drying to form solid medicine carrying particle. The present invention has the advantages of high hydrophilic medicine enclosing rate up to 95 %, controlled and stable medicine release with period as long as half a month to one year, and well maintenance of the stability and bioactivity of protein and peptide during particle preparation, storage and medicine throwing.
Owner:天津麦凯泰生物制品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com