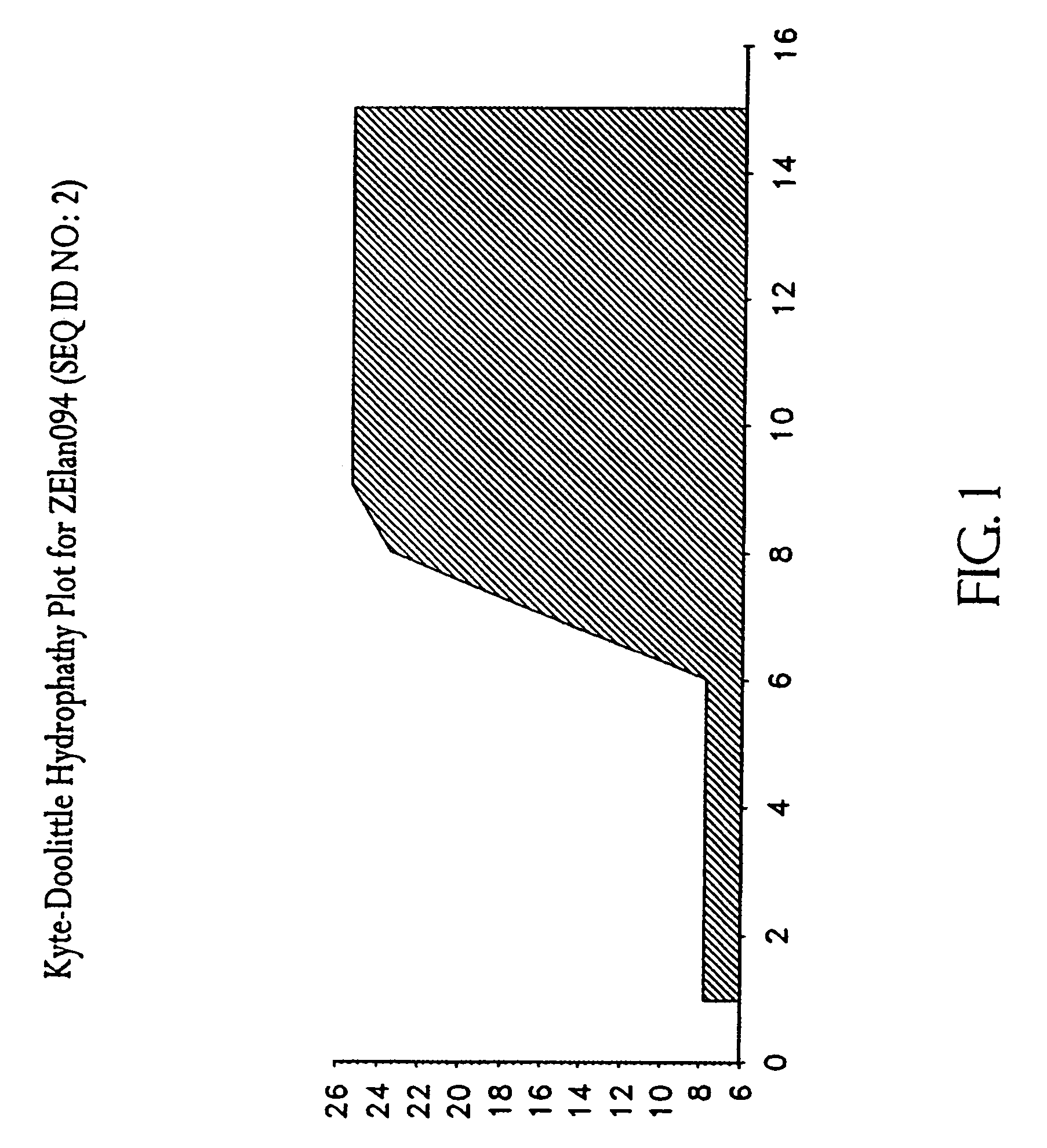
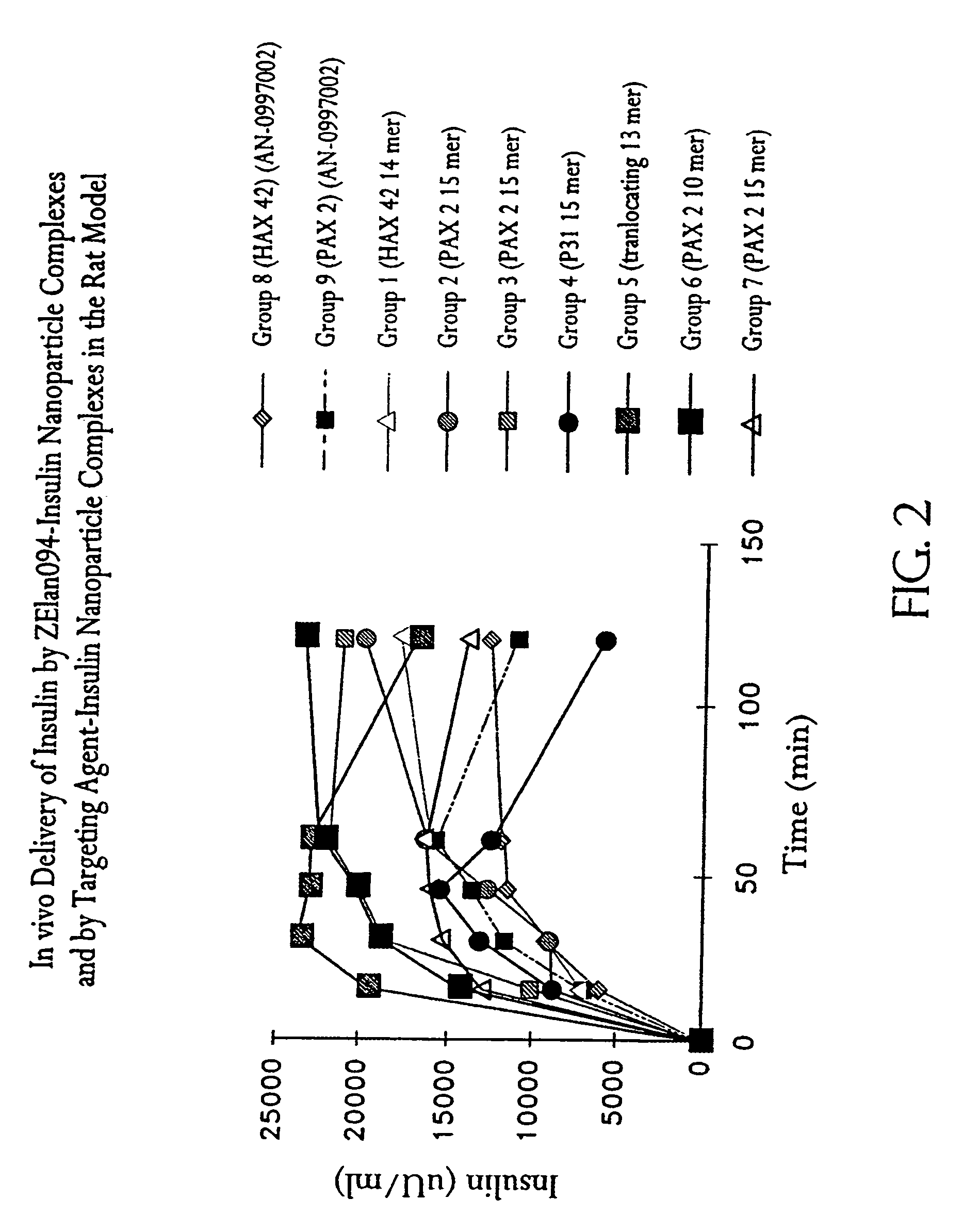
Membrane translocating peptide drug delivery system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptide Synthesis
[0096]The membrane translocating peptides ZElan094, 204N and 204 and the targeting peptides HAX42, PAX2, P31 and Sni34 (U.S. patent applications Ser. Nos. 09 / 079,819, 09 / 079,723 and 09 / 079,678) were synthesized chemically using a fmoc synthesis protocol (Anaspec, Inc., San Jose, Calif.). A dansyl group was added at the N-terminus of each sequence in order to enable the detection of the peptide with anti-dansyl antibody (Table 3).
[0097]
TABLE 3MTLPs and targeting peptide sequencesSEQUENCEPEPTIDEZELAN NO:RECEPTORSEQ ID NO:H2N-K(dns)KKAAAVLLPVLLAAPMTLP09448MTLP-amideAAVLLPVLLAAKKKRKAMTLP204N23KKKRKAAAAVLLPVLLAMTLP20424H2N-K(dns)SDHALGTNLRSDNAK-HAX42011HPT149EPGDYNCCGNGNSTGRKVFNRRRSAIPYH2N-K(dns)PGDYNCCGNGNSTGHAX42091HPT150(14 mer)H2N-K(dns)LSTPPSREAYSRPYSV-PAX2055HPT151DSDSDTNAKHSSHNRRLRTRSRPNH2N-K(dns)Lys-TrKSSrSNPrGrrHPGP3110152(15 mer cyclic D form)H2N-K(dns)rtrlrrnhsshkantPAX2144RPT153(15 mer D form retroinversion)H2N-K(dns)TNAKHSSHNRRLRTRPAX2129HPT154H2N-K(dns)Lys-...
example 2
Preparation of MTLP-Active Particle Complexes and of Targeting Peptide-Active Particle Complexes
[0100]Active particles are prepared from a polymer using a coacervation method. Preferably, particle size is between about 5 nm and 750 μm, more preferably between about 10 nm and 500 μm and most preferably between about 50 nm and 800 nm. MTLPs or targeting peptides are complexed to the particles using various methods known to those skilled in the art.
[0101]The following is a general method for preparation of coacervated particles.
Phase A
[0102]A polymer agent, a surface-active agent, a surface-stabilizing agent, a surface-modifying agent or a surfactant is dissolved in water (A). Preferably the agent is a polyvinyl alcohol (hereinafter “PVA”) or a derivative thereof having a % hydrolysis of about 50-100 and a molecular weight range of about 500-500,000 kDa. More preferably the agent is a PVA having a % hydrolysis of 80-100 and a molecular weight range of about 10,000-150,000 kDa. The mixt...
example 3
Bovine Insulin Loaded-MTLP Coated Nanoparticles—MTLP Added in the Final Wash
[0110]Fast acting bovine insulin (28.1 IU / mg) was incorporated into polylactide-co-glycolide (PLGA, Boehringer Ingelheim, Indianapolis, Ind.) at a theoretical loading of 300 IU of insulin / 210 mg of nanoparticles and the nanoparticles were coated with the dansylated ZElan094 (SEQ ID NO: 48).
[0111]
COMPONENTAMMOUNTPLGA RG504H (Lot # 250583) 2 gAcetone 45 mlsEthanol 5 mlsPVA (5% w / v) (13-23 kDa, 98% hydrolysis)400 mlsBovine Insulin (Lot # 86HO674)100 mgZElan094 (SEQ. ID NO: 48) 10 mg / 50 ml dH20
Preparation:[0112]1. Water was heated to near boiling, PVA was added to 5% w / v and the solution was stirred until cool (phase A).[0113]2. Acetone and ethanol were mixed to form the organic phase (phase B).[0114]3. PLGA was added to the acetone and ethanol (step 2) and dissolved by stirring (phase B).[0115]4. An IKA™ reactor vessel was set at 25° C. Phase A (step 1) was added into the reactor vessel and stirred at 400 rpm.[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com