Medicine-carring particulates composed of hydrophilic resin and hydrophobic resin and its preparation method
A hydrophilic resin and hydrophobic resin technology, which is applied in the field of drug-loaded particles and their preparation, can solve the problems of poor release rate and cycle controllability, large variation in drug release, and reduced drug content, so as to maintain stability and The effect of biological activity, high clinical application value, and broad promotion prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
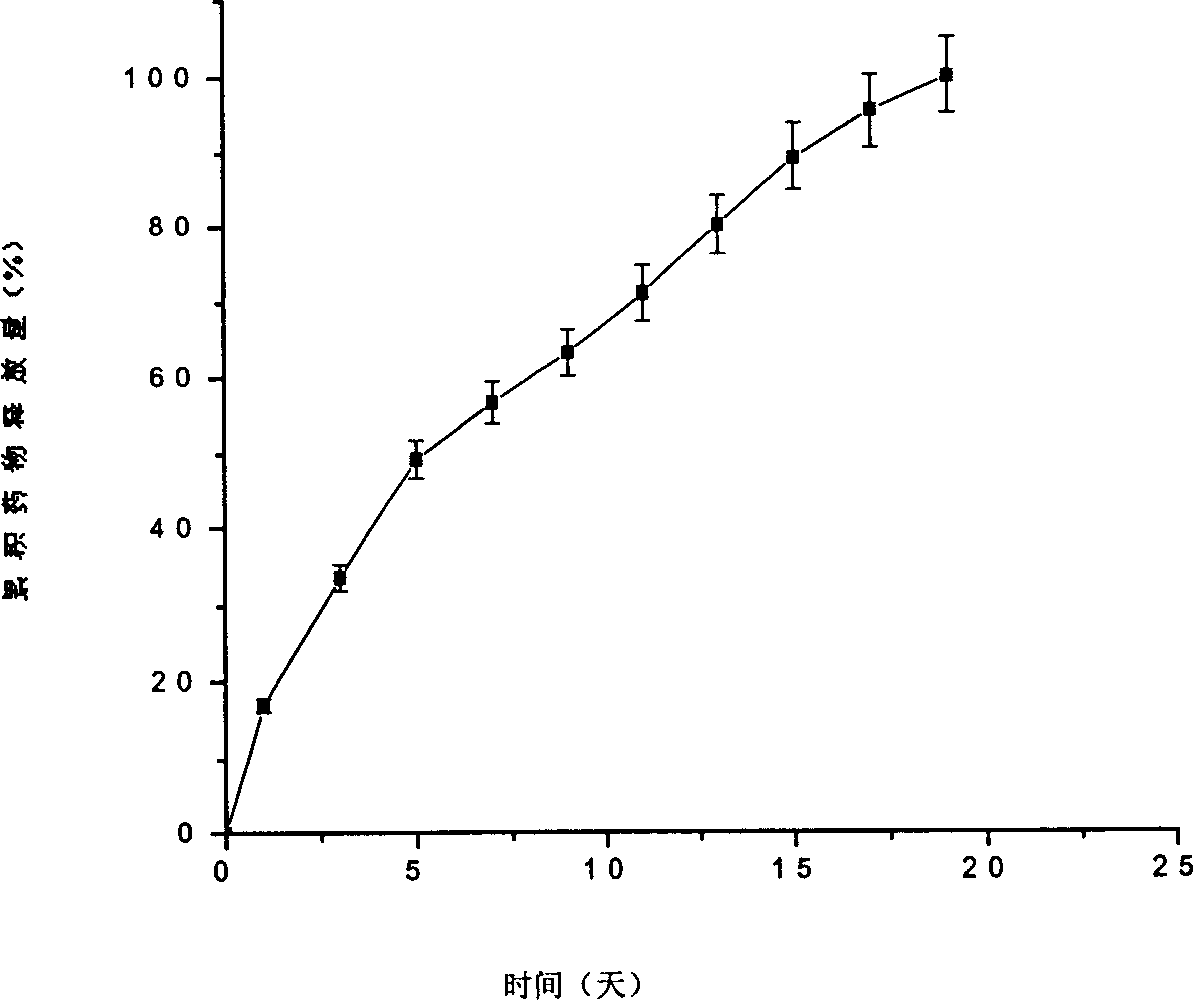
[0025] Implementation Example 1: Preparation of aspirin-loaded agarose-polylactic acid sustained-release particles by solvent evaporation method
[0026] Polylactic acid (weight average molecular weight M w 17,550) 30g was dissolved in 50mL dichloromethane to make an oil phase resin solution; 908.1mg agarose was dissolved in 10mL high-purity water to form an agarose aqueous solution, and 1.5g aspirin was suspended in the agarose solution under magnetic stirring at a speed of 200rpm The resulting solution is mixed with the oil-phase polymer solution, and the ultrasonic generator is used to ultrasonic the mixture 5 times, each ultrasonic time is 60s, and the ultrasonic output power is 20W to form a water-in-oil emulsion; the gained emulsion is cooled to 4 in the refrigerator ℃, then add 500mL of 0.5% polyvinyl alcohol aqueous solution, filter the suspension with a 90-mesh sieve, transfer the filtrate into a centrifuge tube, and centrifuge for 5min (centrifugal force 400×g force...
Embodiment 2
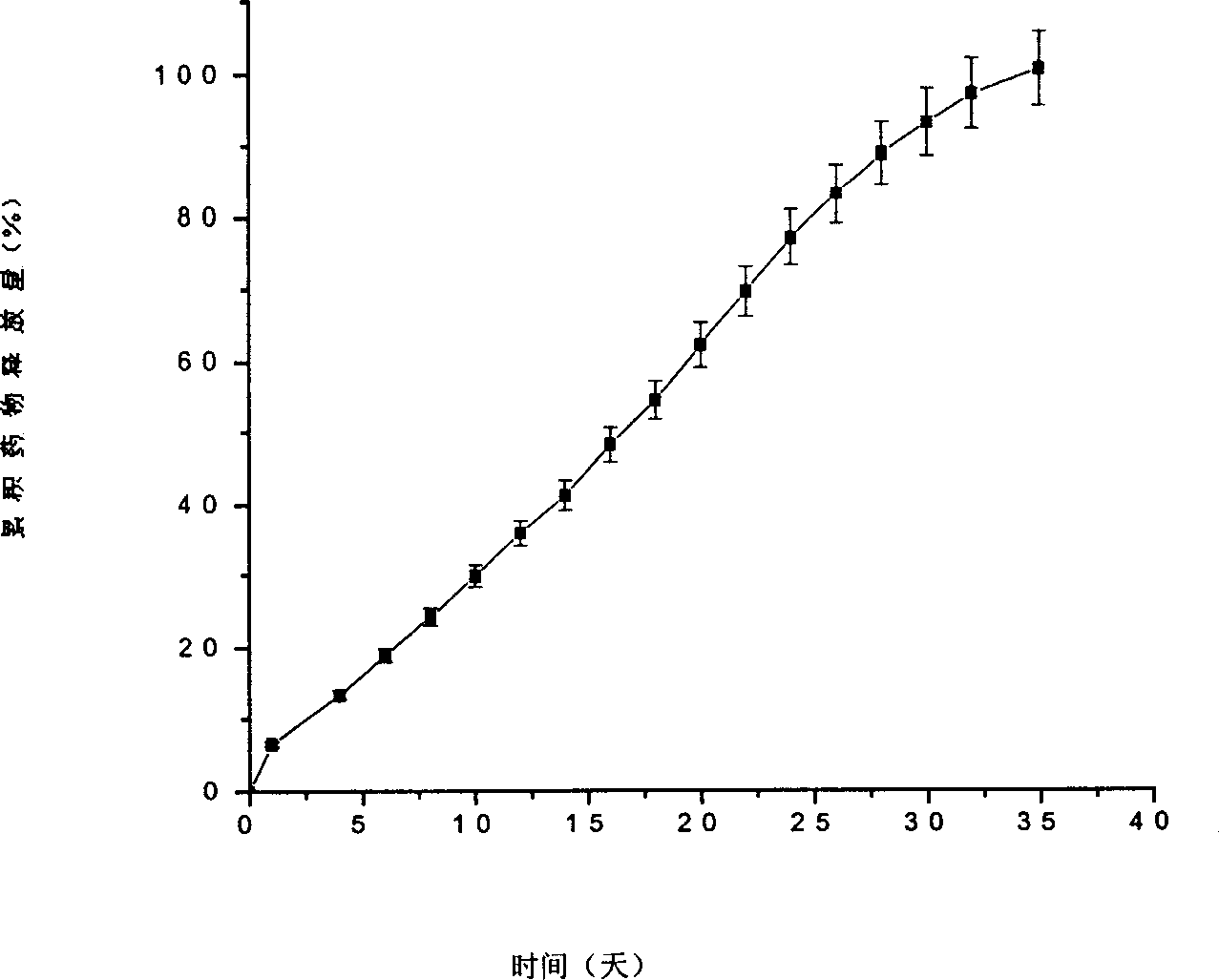
[0039] Implementation Example 2: Preparation of Gelatin-Lactic-Glycolic Acid Copolymer Resin (PLGA) Drug-loaded Microparticles by Solvent Evaporation
[0040] Weigh two kinds of lactic acid glycolic acid copolymer PLGA75 / 25 (M w 10,091)23.69g, PLGA75 / 25(M w 1,715) 7.14g was dissolved in 50mL dichloromethane to make an oil phase resin solution; 908.1mg type B gelatin was dissolved in 10mL high-purity water to form a gelatin aqueous solution, and 1g bovine serum albumin was dissolved in the gelatin solution; the resulting solution was mixed with the oil phase The polymer solution was mixed, and an ultrasonic generator was used to ultrasonicate the mixture 5 times, each ultrasonic time was 60s, and the ultrasonic output power was 20W to form a water-in-oil emulsion; the obtained emulsion was cooled to about 4°C in a refrigerator, and then 500mL 0.5 % polyvinyl alcohol aqueous solution, the resulting mixture was homogenized with a needle homogenizer at a speed of 10,000rpm for 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com