Temperature-sensitive amphiphilic cyclodextrin polymer as well as preparation method and application
A cyclodextrin polymer and amphiphilic technology, which is applied in the field of medicine, can solve the problems of limited hydrophobic space and limited inclusion effect, and achieve the effect of simple synthesis steps, improved encapsulation efficiency and drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of PCEC-β-CD copolymer
[0039] (1) Synthesis of PCEC 1000-6000-1000 :
[0040] Configure Sn(Oct) 2 Toluene solution 0.3g / ml. Weigh the PEG after dewatering and purification 600 0 9.0g, CL 3.0g, add Sn(Oct) 2 0.1ml of toluene solution was placed in a 100ml round bottom flask, protected by nitrogen, and reacted in an oil bath at 130°C for 24 hours. After the reaction was completed, the temperature of the reaction flask was lowered to room temperature, and the reaction product was dissolved in 6 ml of anhydrous dichloromethane, then magnetically stirred and precipitated in 200 ml of diethyl ether, filtered and sucked dry. The product was redissolved, filtered and drained, repeated twice, and placed in a vacuum oven overnight to evaporate the residual ether to obtain the final product PCEC.
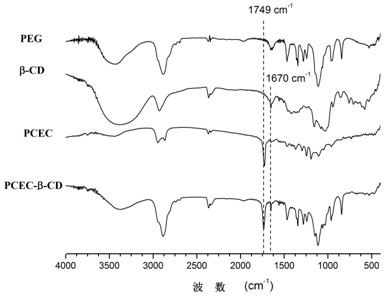
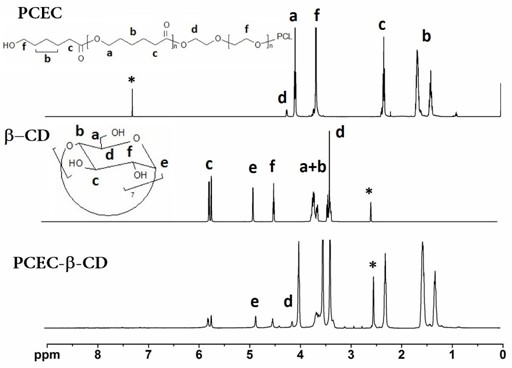
[0041] PCEC 1000-6000-1000 For NMR and IR spectra see figure 1 with figure 2 , the productive rate is 90.2%, the molecular weight calculated by N...
Embodiment 2
[0051] Example 2: Preparation of indomethacin PCEC-β-CD drug-loaded hydrogel and investigation of its release behavior
[0052] Weigh PCEC-β-CD1g, indomethacin 100mg, dissolve in 1ml DMF, dissolve evenly and transfer to the dialysis bag. Dialyze in 1L of deionized water at 80°C for 24 hours, changing the solution every two hours. After the dialysis is complete, transfer the gel formed in the dialysis bag to a 10ml graduated cylinder, add water to the 3ml mark, and mix well to obtain a drug-loaded hydrogel of a certain concentration. 0.5ml of the hydrogel was freeze-dried, reconstituted with DMF to measure the drug loading and encapsulation efficiency, and the rest of the indomethacin drug-loaded hydrogel was stored at 4°C.
[0053] PCEC-β-CD uses DMF as solvent, the obtained drug loading is 3.01%, and the encapsulation efficiency is 67.4%.
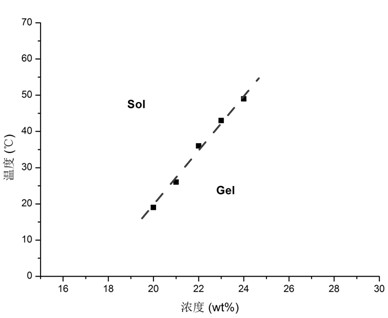
[0054] Separately weigh 500 mg of PCEC-β-CD, add 1.5 ml of deionized water to dissolve evenly, and form a hydrogel. Weigh 8.4 mg of in...
Embodiment 3
[0057] Example 3: Preparation of indomethacin PCEC-β-CD drug-loaded hydrogel
[0058] Weigh 500 mg of PCEC-β-CD and 50 mg of indomethacin, dissolve them in 500 μl DMSO, and transfer them to the dialysis bag after uniform dissolution. The dialysis method and the method for calculating the drug loading are the same as in Example 2. PCEC-β-CD uses DMSO as a solvent, the ratio of drug to material is 1:10, and the ratio of DMSO to material is 1:1 (ml / g). The content is 5.17%, and the encapsulation efficiency is 51.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com