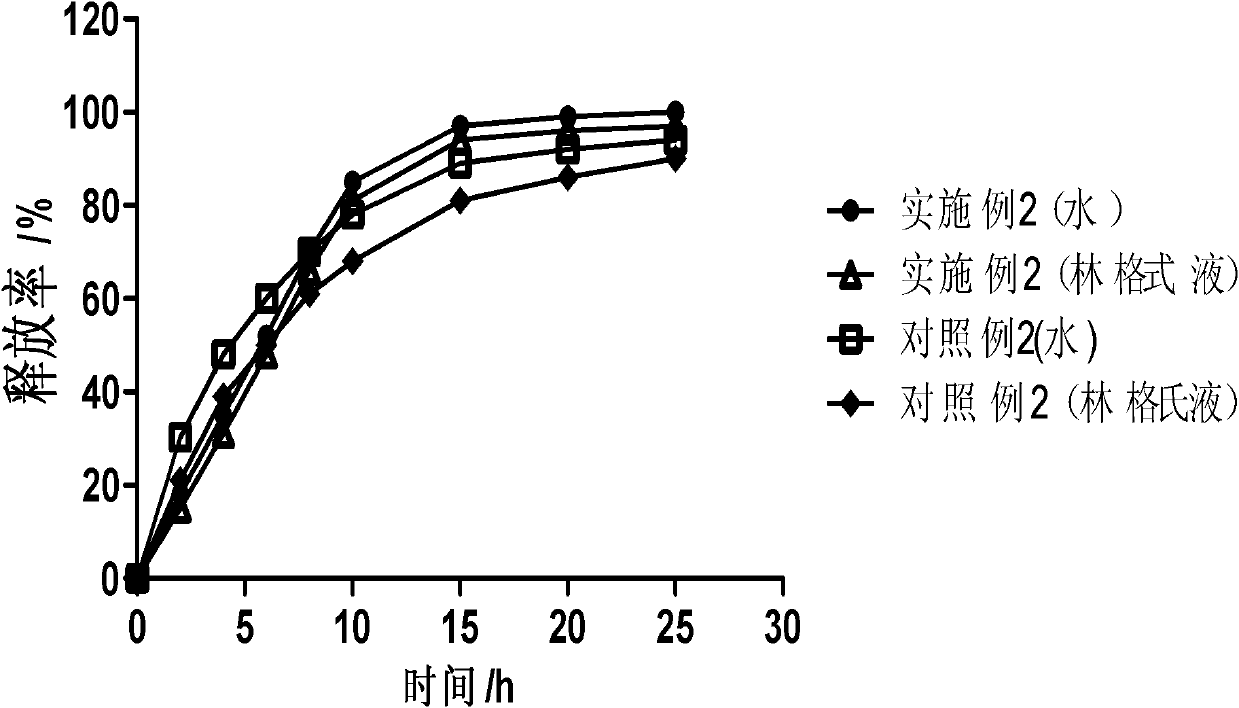
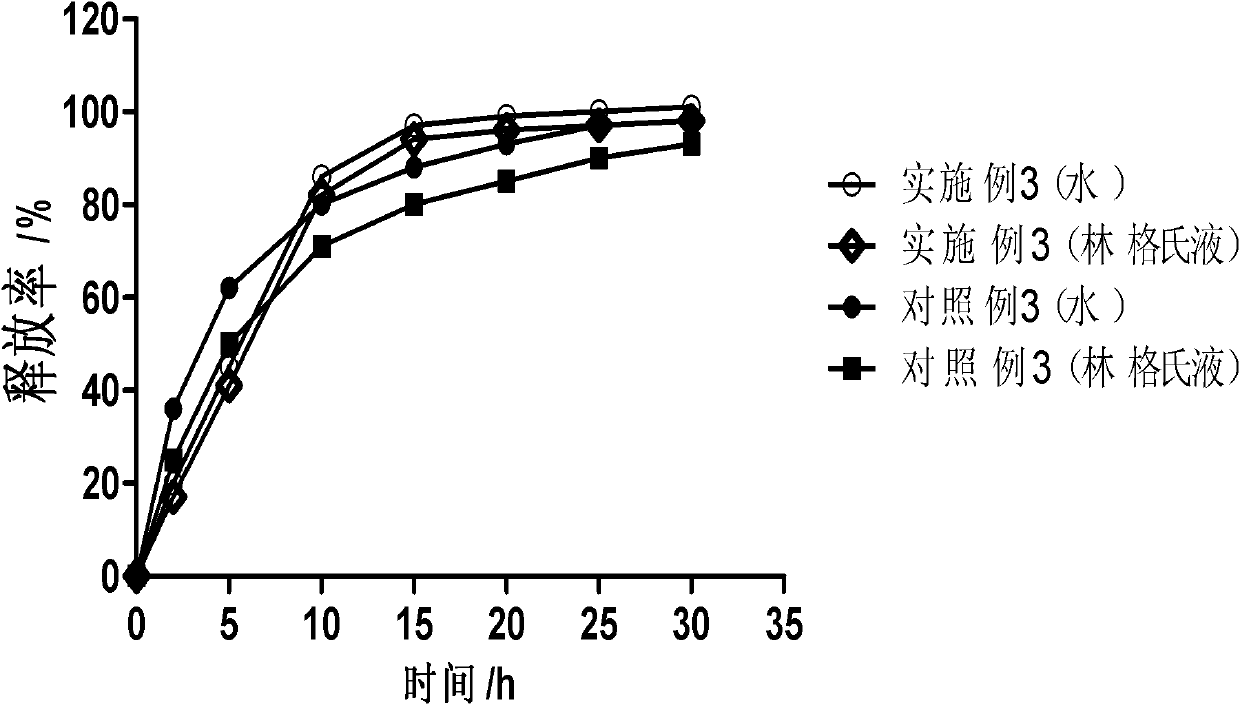
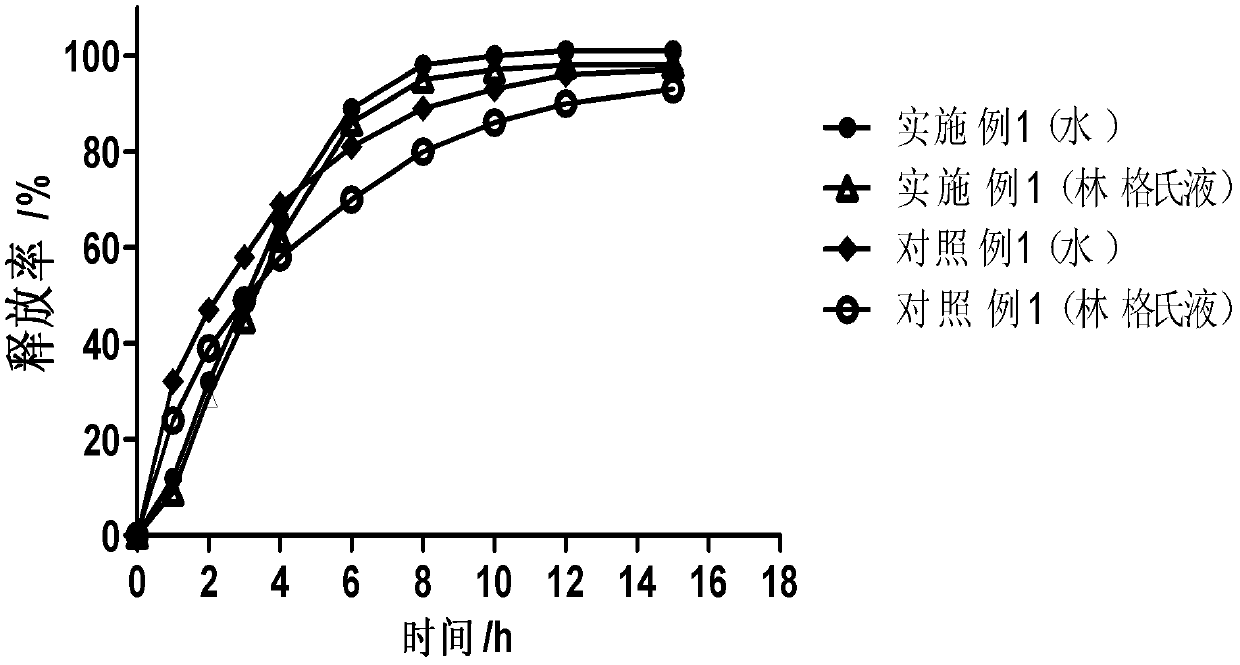
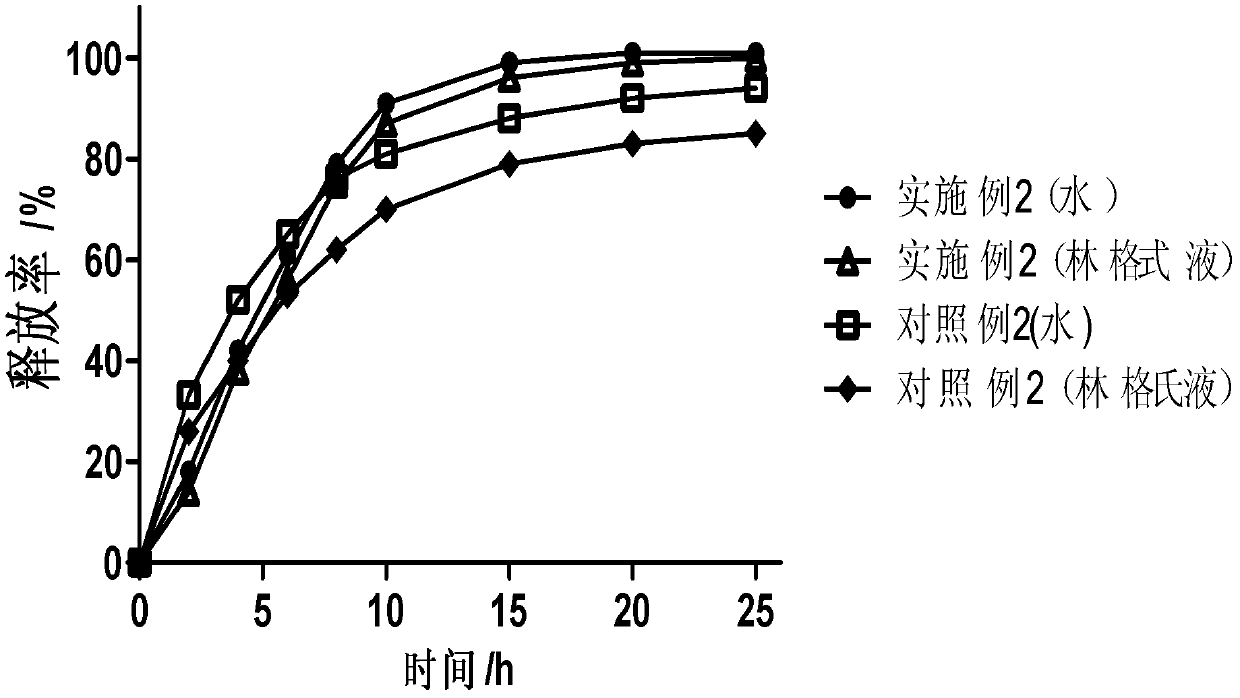
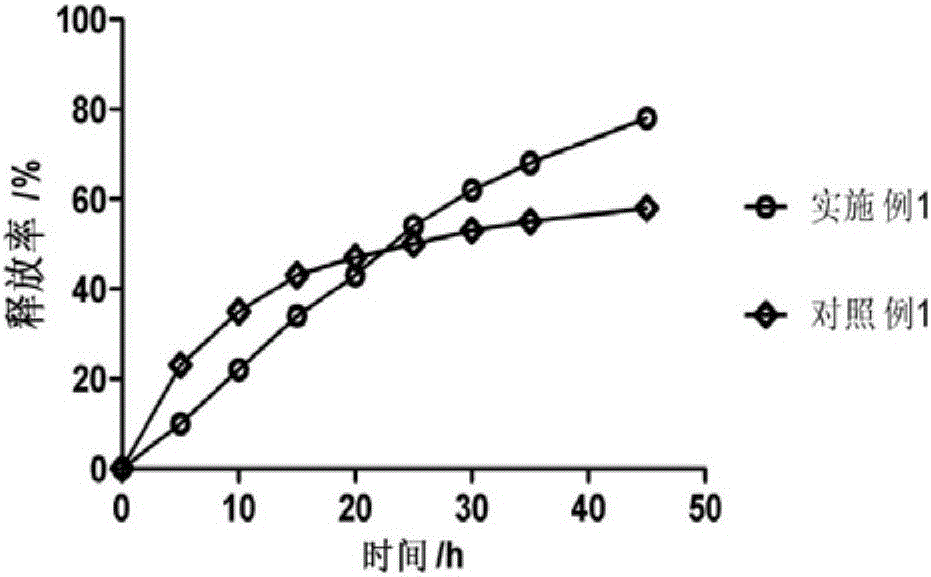
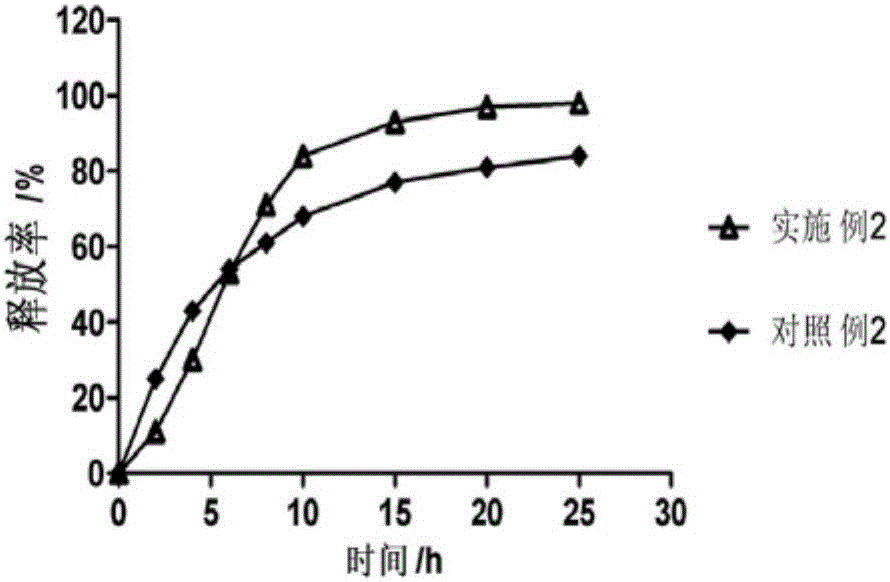
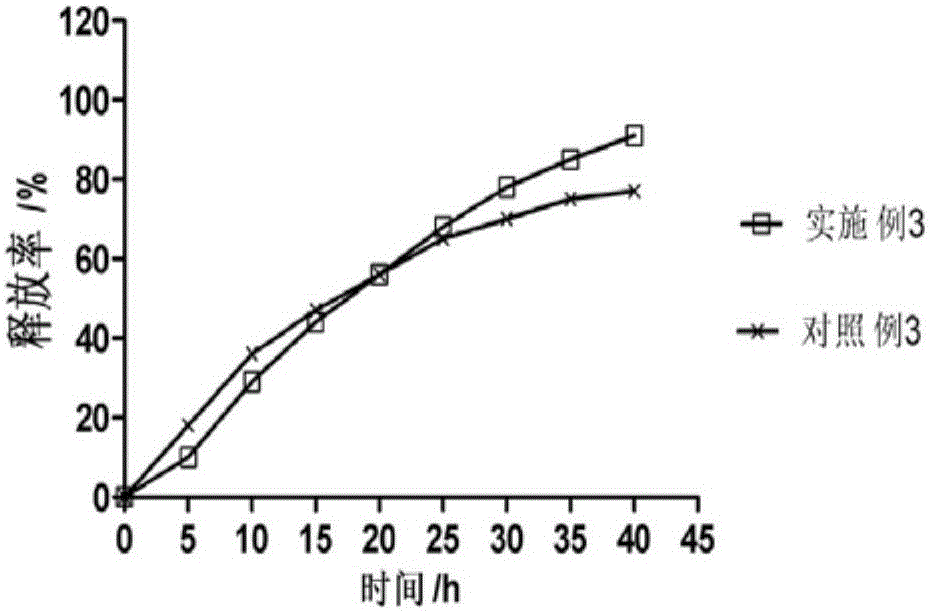
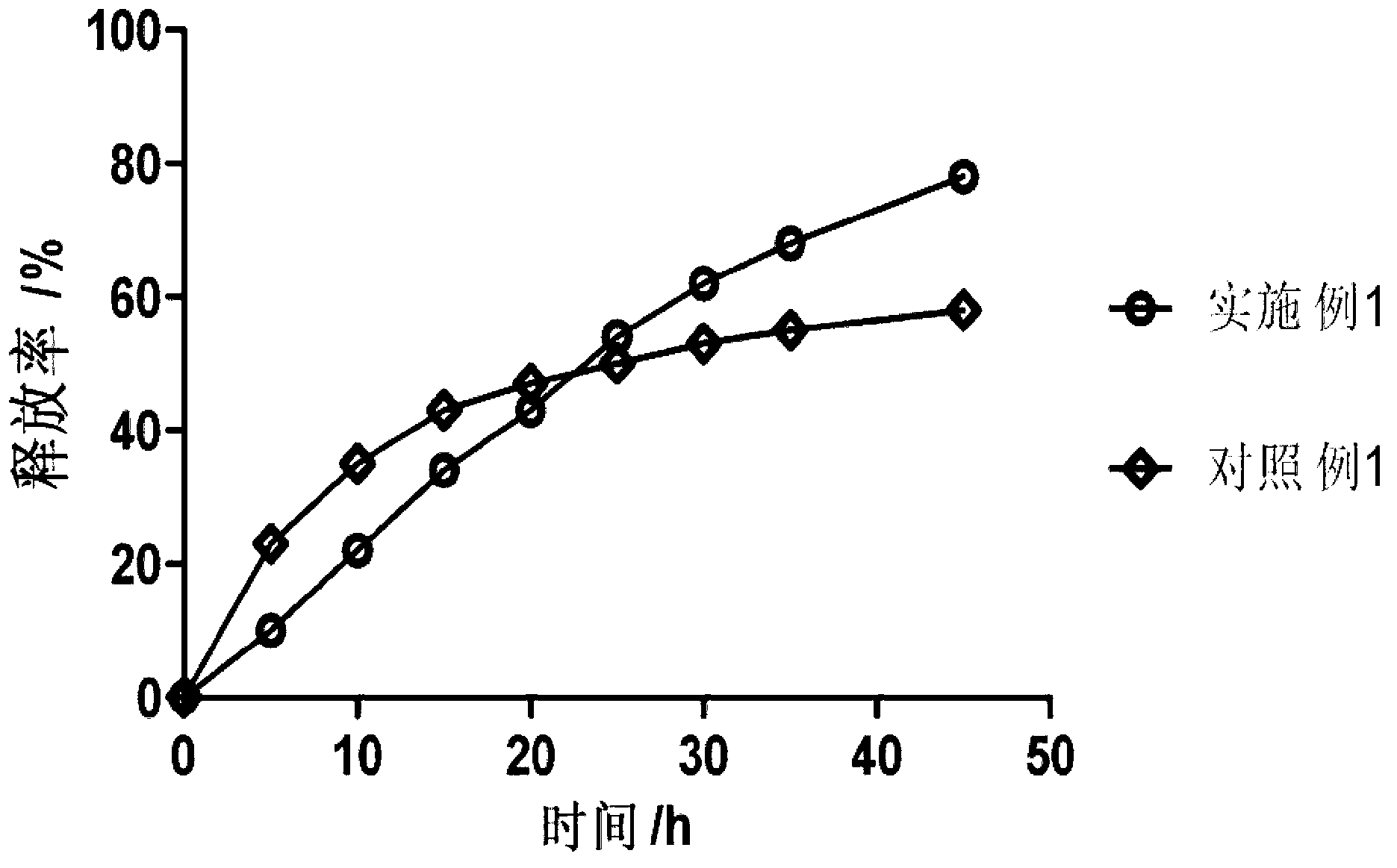
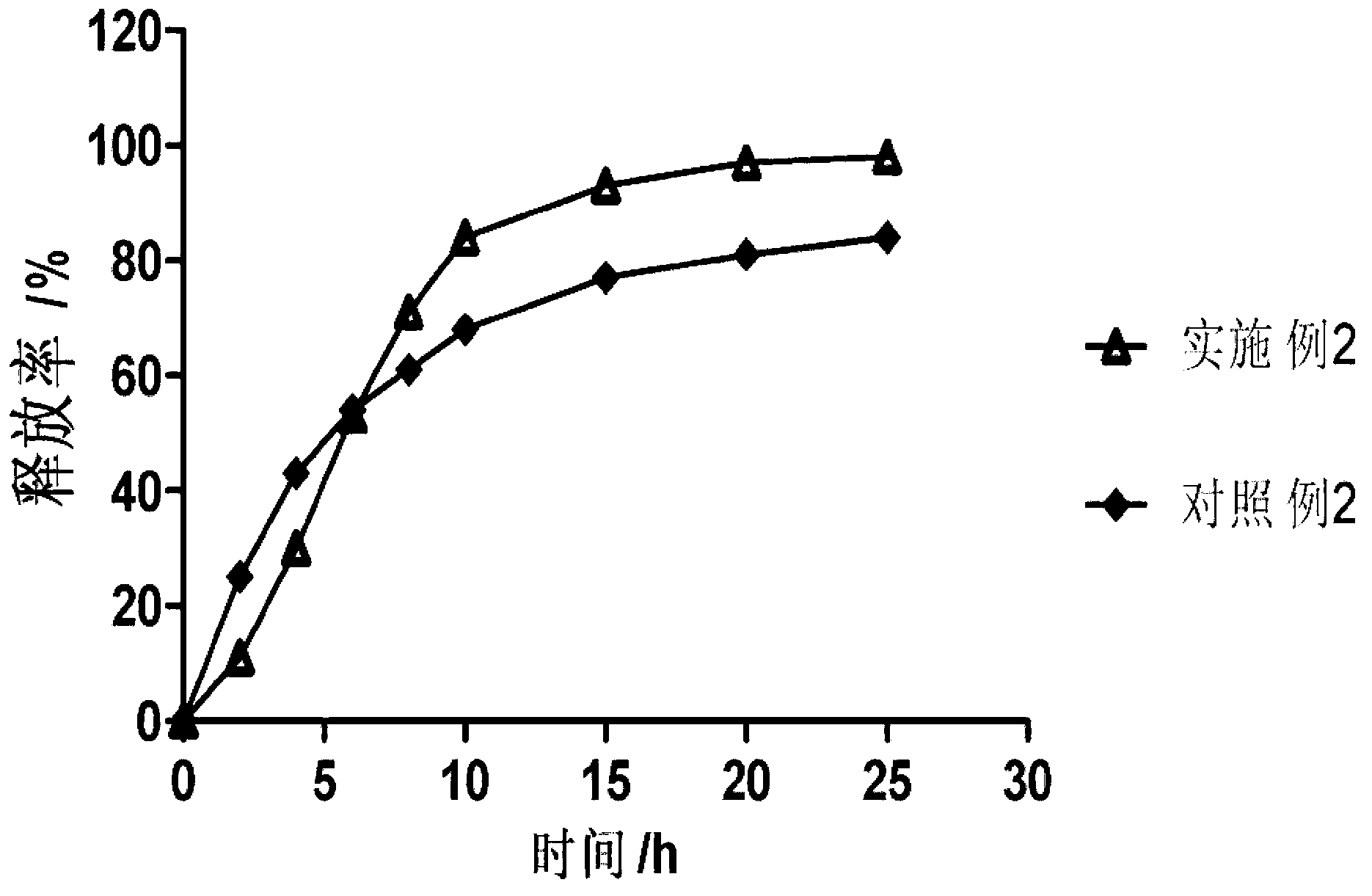
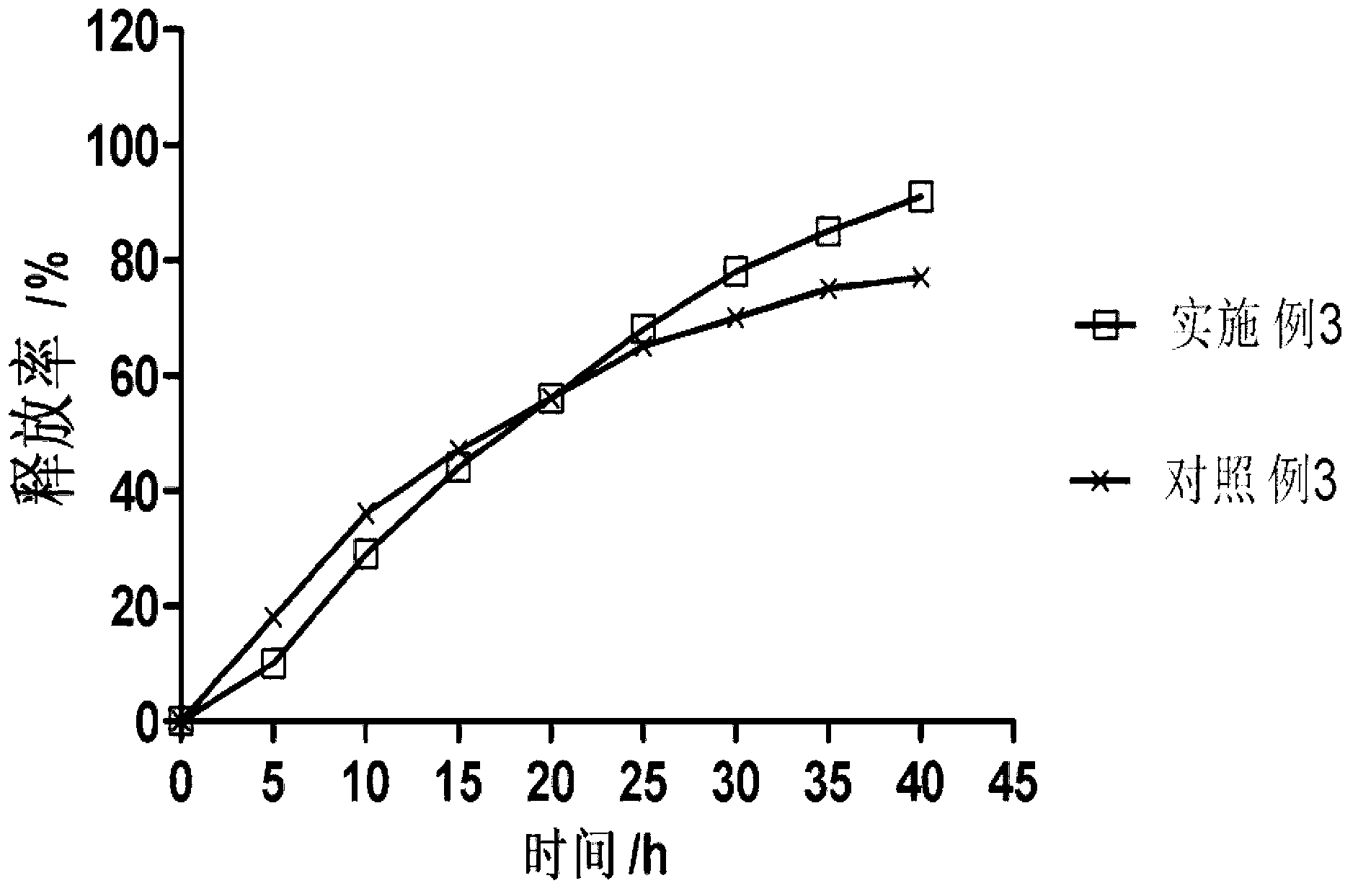
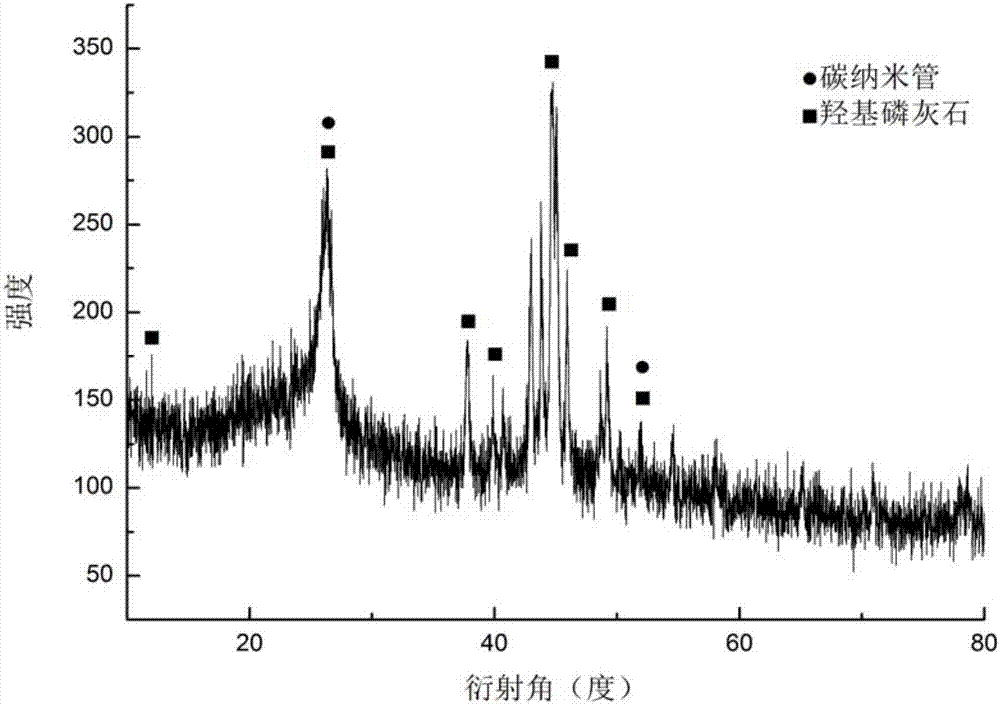
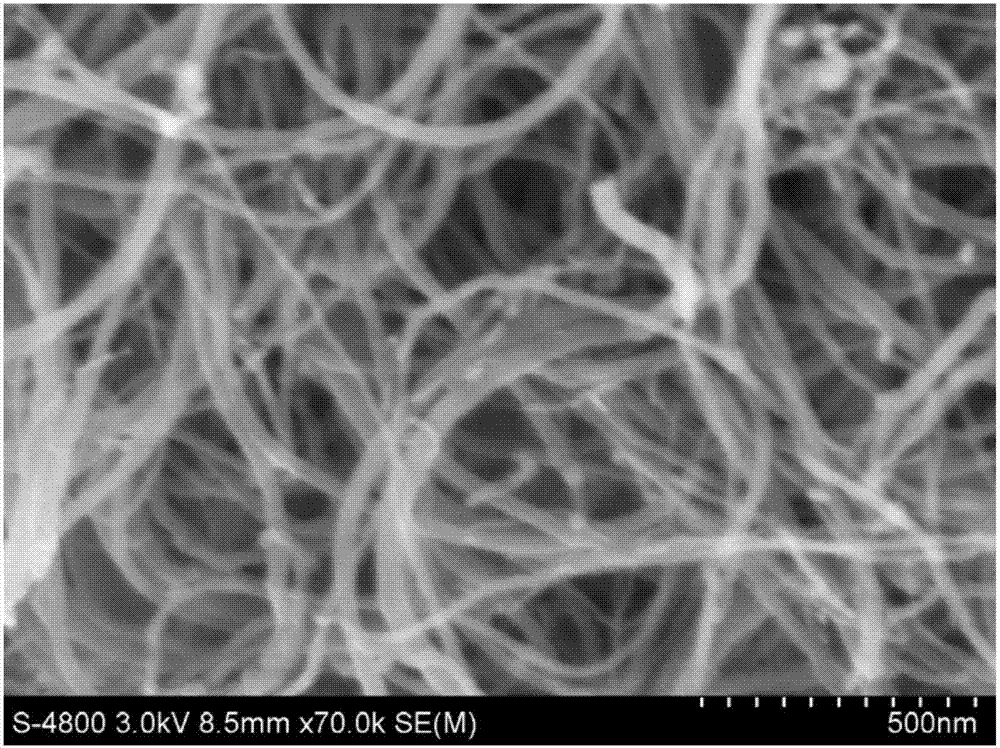
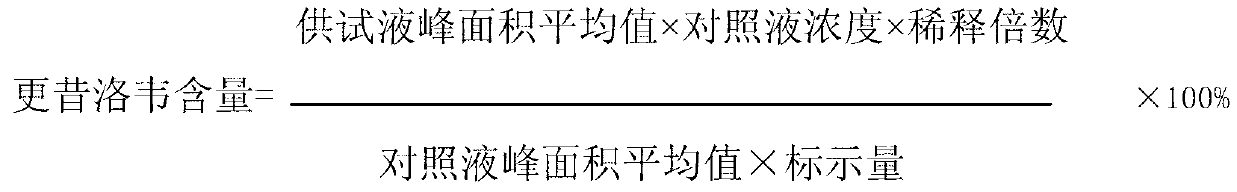
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108results about How to "Good drug release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mesoporous nano silicon ball compound targeting drug delivery system as well as preparation method and application thereof
InactiveCN104474555AOvercoming multidrug resistanceSignificant effectOrganic active ingredientsPowder deliveryMicrosphereFluorescence
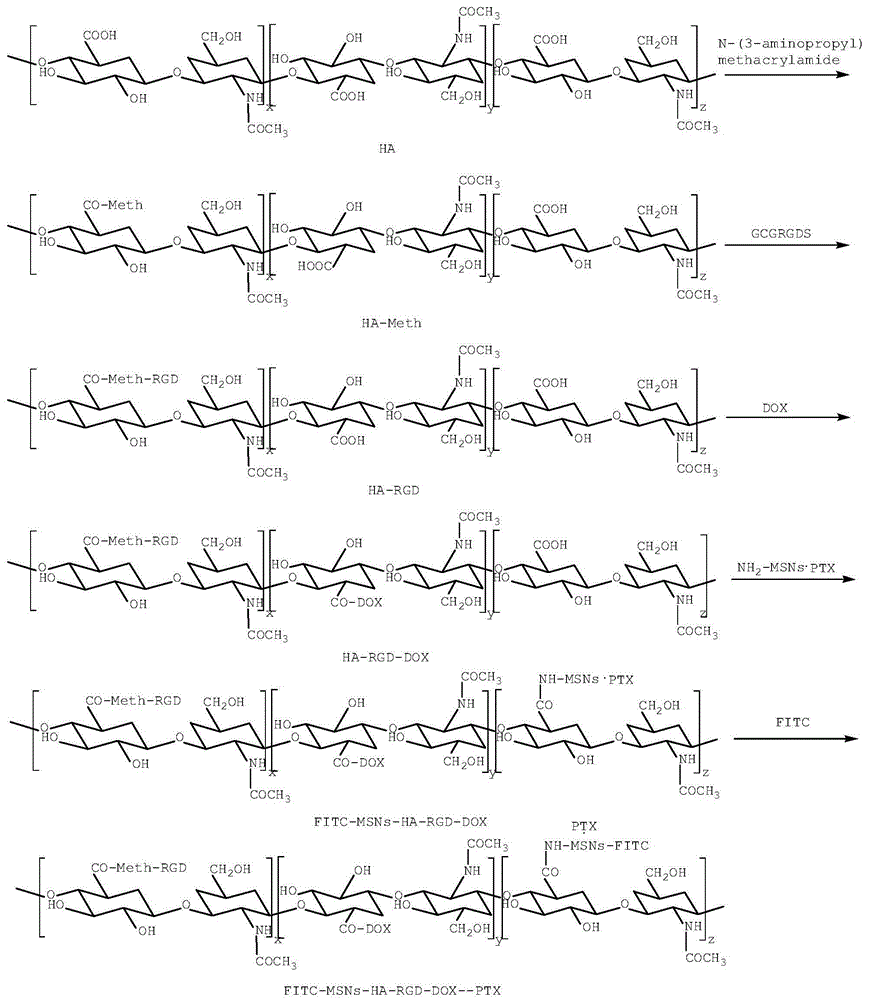
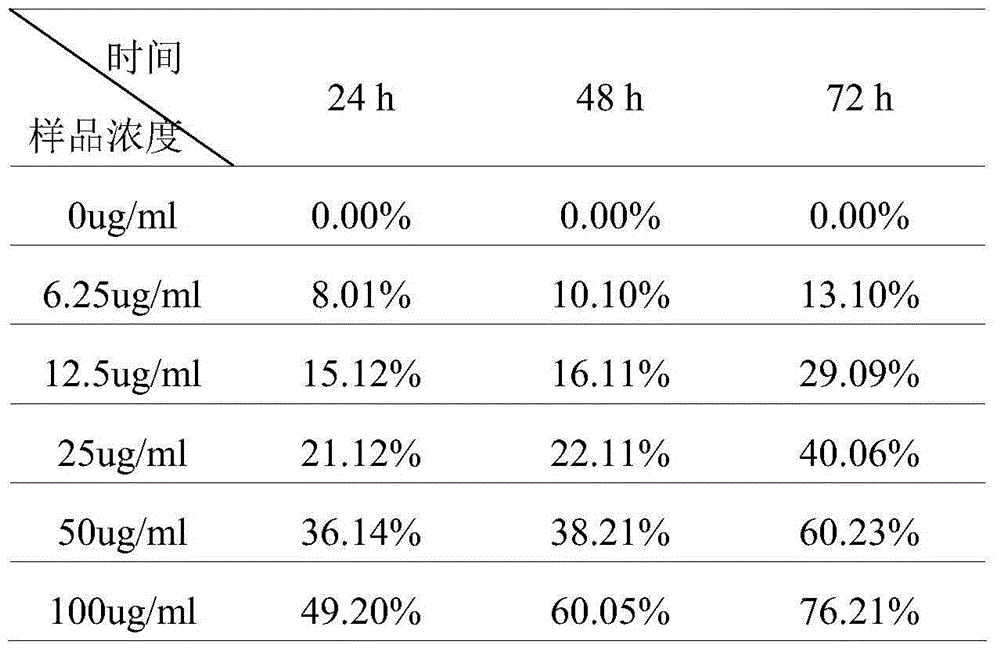
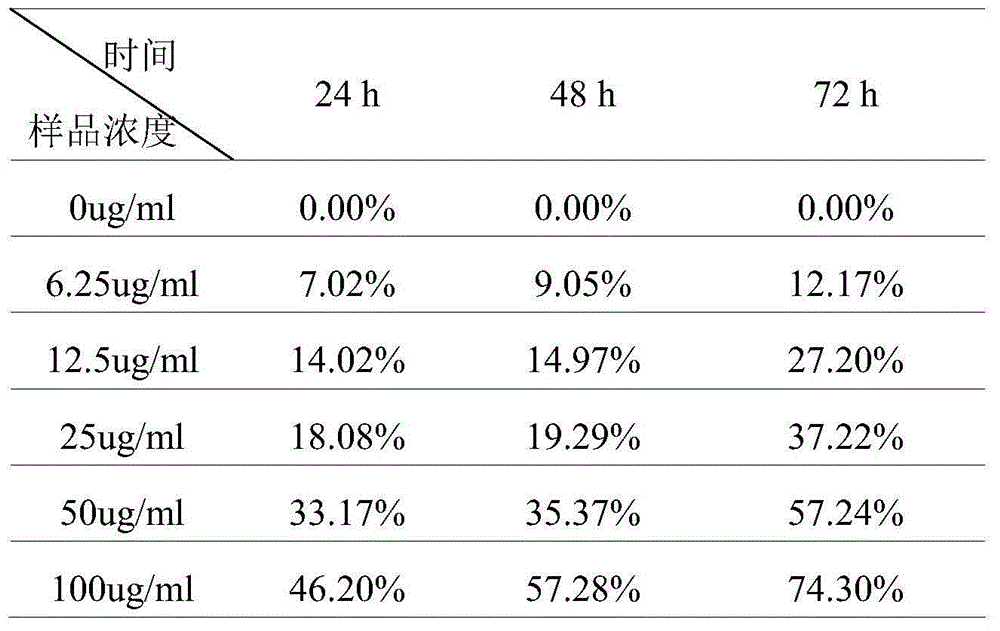
The invention relates to a mesoporous nano silicon ball compound targeting drug delivery system as well as a preparation method and application thereof. The preparation method of the mesoporous nano silicon ball compound targeting drug delivery system comprises the following steps: 1) preparing amino-functionalized drug loading mesoporous silicon dioxide microspheres; 2) preparing hyaluronic acid-hydrosulphonyl polypeptide-adriamycin (HA-RGD-DOX); 3) preparing mesoporous microsphere-hyaluronic acid-hydrosulphonyl polypeptide-adriamycin--paclitaxel (MSNs-HA-RGD-DOX_PTX); and 4) preparing fluorescent marker modified mesoporous microsphere-hyaluronic acid-hydrosulphonyl polypeptide-adriamycin--paclitaxel compound (MSNs-HA-RGD-DOX-PTX). The mesoporous nano silicon ball compound targeting drug delivery system has the beneficial effects that firstly multi-targeting synergistic drug delivery is realized, multiple tumour cells and tissues can be killed, and reversal drug resistance is good; secondly, blood stability is excellent; thirdly, invisibility, drug release degree and controlled release properties are good; fourthly, in vivo tracing function is good; and fifthly, the mesoporous nano silicon ball compound targeting drug delivery system has good general applicability.
Owner:WUHAN UNIV OF TECH
Improved-performance tablet and preparation method thereof
InactiveCN103432091AMaintain integrityPrevent or reduce cracksPill deliveryPorosityWeather resistance
The invention discloses an improved-performance tablet which comprises an active component A1, a hydrophilic diluent B1 and a meltable solid dispersion and / or a solid coating C1, wherein the diluent B1 and / or the active component A1 are / is bonded and bridged by the solidified melt of the meltable solid dispersion and / or solid coating C1; and / or the tablet comprises a hydrophilic diluent B2 and a meltable solid dispersion containing an active component A2 and / or a solid coating C2, wherein the diluent B2 is bonded and bridged by the solidified melt of the meltable solid dispersion and / or solid coating C2. The invention also discloses a preparation method of the tablet. The tablet has stronger mechanical performance and / or better weather resistance and / or better hydrophilcity or better disintegration property or better medicine dissolubility and higher porosity.
Owner:钟术光
Suppository composition
InactiveCN101919807AStrong retention matrix”Low retention variabilitySuppositories deliveryPharmaceutical non-active ingredientsBiocompatibility TestingPolyethylene glycol
The invention discloses a suppository composition with improved performance. The suppository composition comprises an aliphatic suppository substrate, mono-capryl-based glycerin, mono-lauroyl-based glycerin, polyoxyethylene, a suppository substrate of which the molecular structure contains polyethylene glycol and alkyl having 8 to 24 carbon atoms and a suppository medicament. The suppository composition has the advantages of improving production repeatability and retention value otherness, enhancing the function of a cavity administration retention substrate, improving stability, namely, changing melting performance, decreasing medicament releasing rate and solving or relieving problems such as scumming and the like, improving medicament releasing performance, increasing liquefaction or gelling speed and achieving stronger salt resistance and acid resistance and higher biocompatibility and the like.
Owner:钟术光
Lansoprazole nano-particle frozen preparation for injection and preparation method thereof
ActiveCN102198106APromote absorptionImprove bioavailabilityPowder deliveryOrganic active ingredientsLansoprazoleSulfite salt
The invention discloses a lansoprazole nano-particle frozen preparation for injection capable of simultaneously improving the stability and dissolubility, and a preparation method thereof. The preparation comprises the following components in parts by weight: 20-40 parts of lansoprazole, 5-50 parts of dextran, 5-40 parts of sodium sulfite, 5-60 parts of solubilizer, 10-100 parts of nano-carrier material and 10-100 parts of freeze drying excipient. The preparation method comprises the steps of: adding the dextran, the solubilizer and the sodium sulfite into a liquid preparation tank, adding water for injection and stirring until dissolved, regulating the pH value, adding the lansoprazole and the nano-carrier material, continuing to stir evenly, adding the freeze drying excipient and stirring until dissolved, supplementing the water for injection to the full dose, decoloring, finely filtering, subpackaging and freeze drying to obtain the frozen preparation.
Owner:WUHAN PUSHENG PHARMA
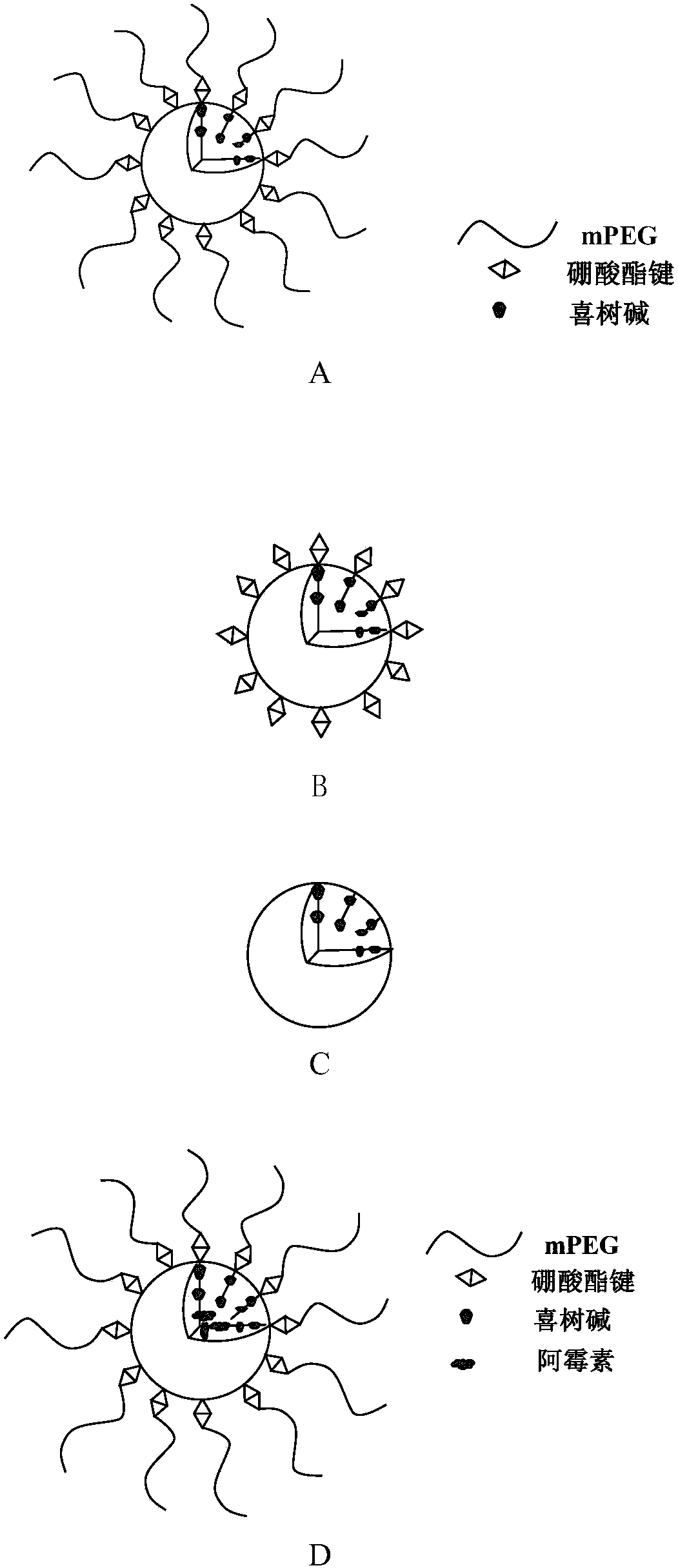
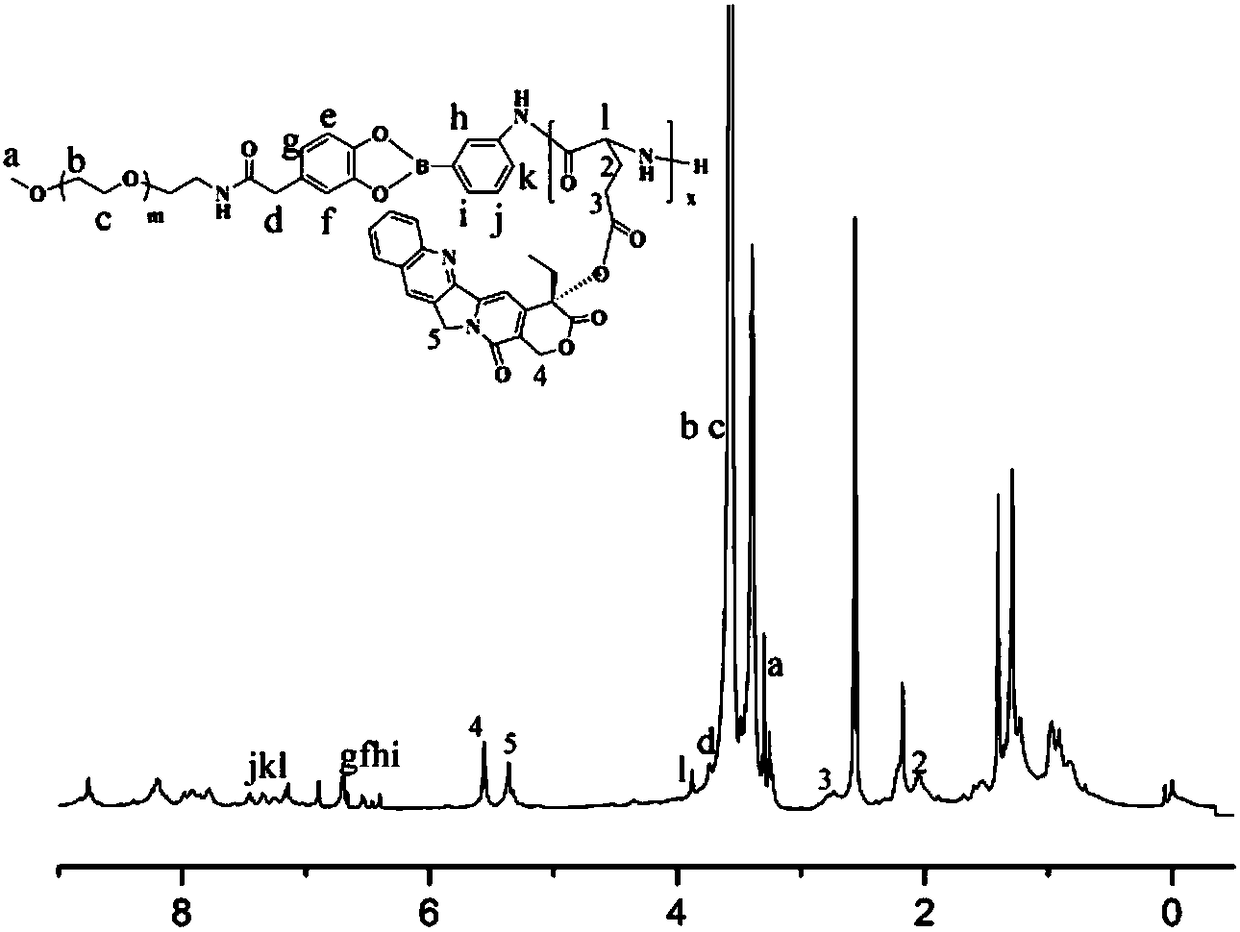
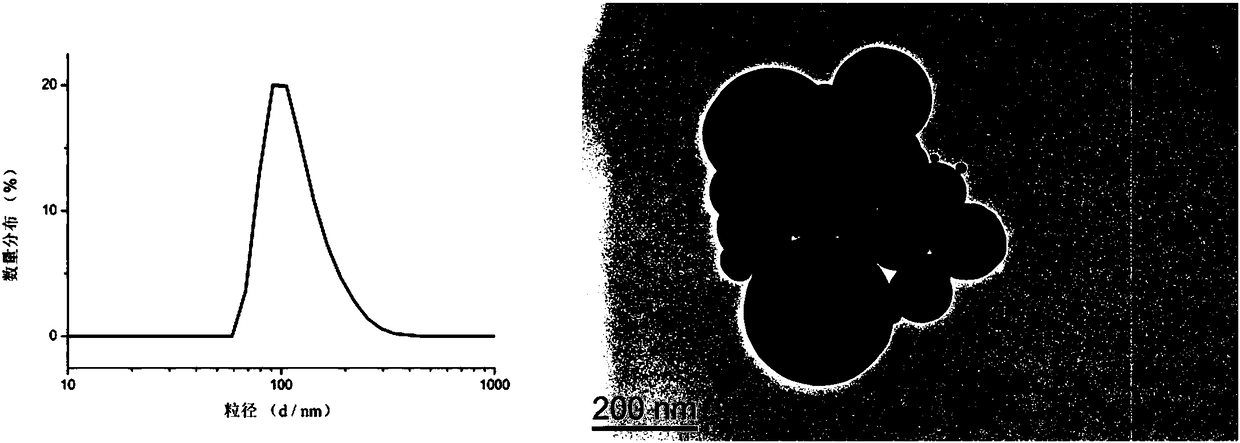
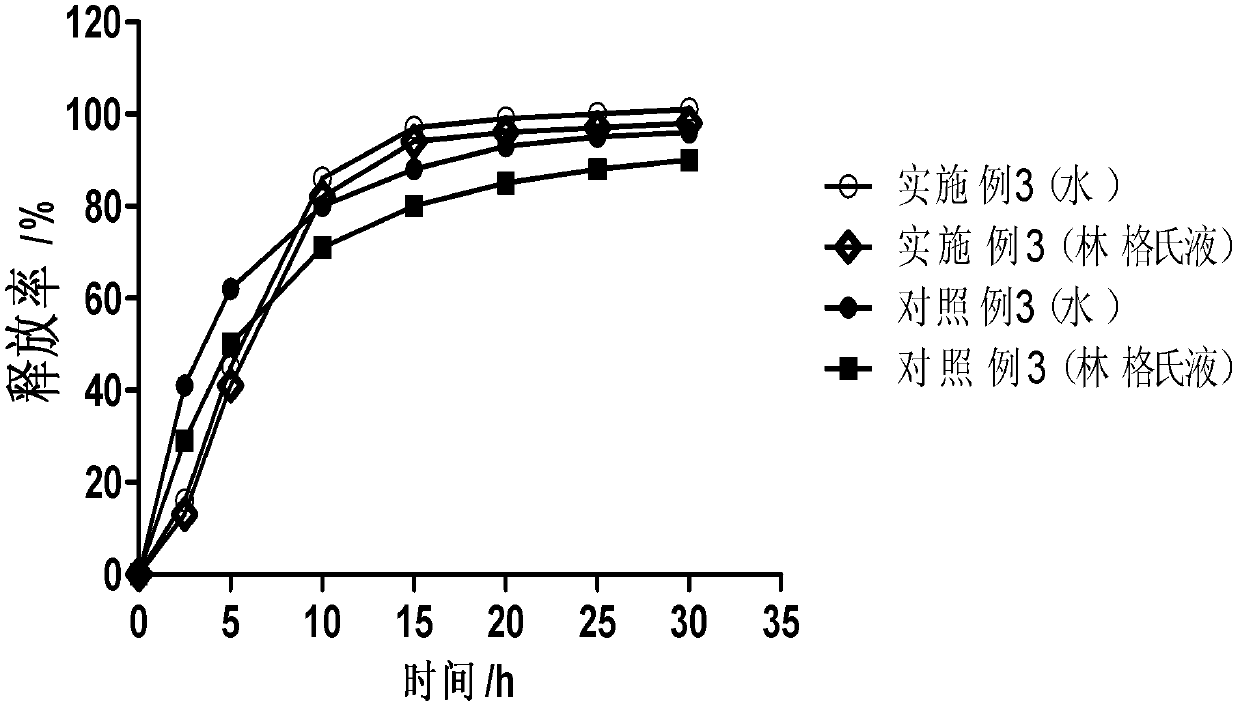
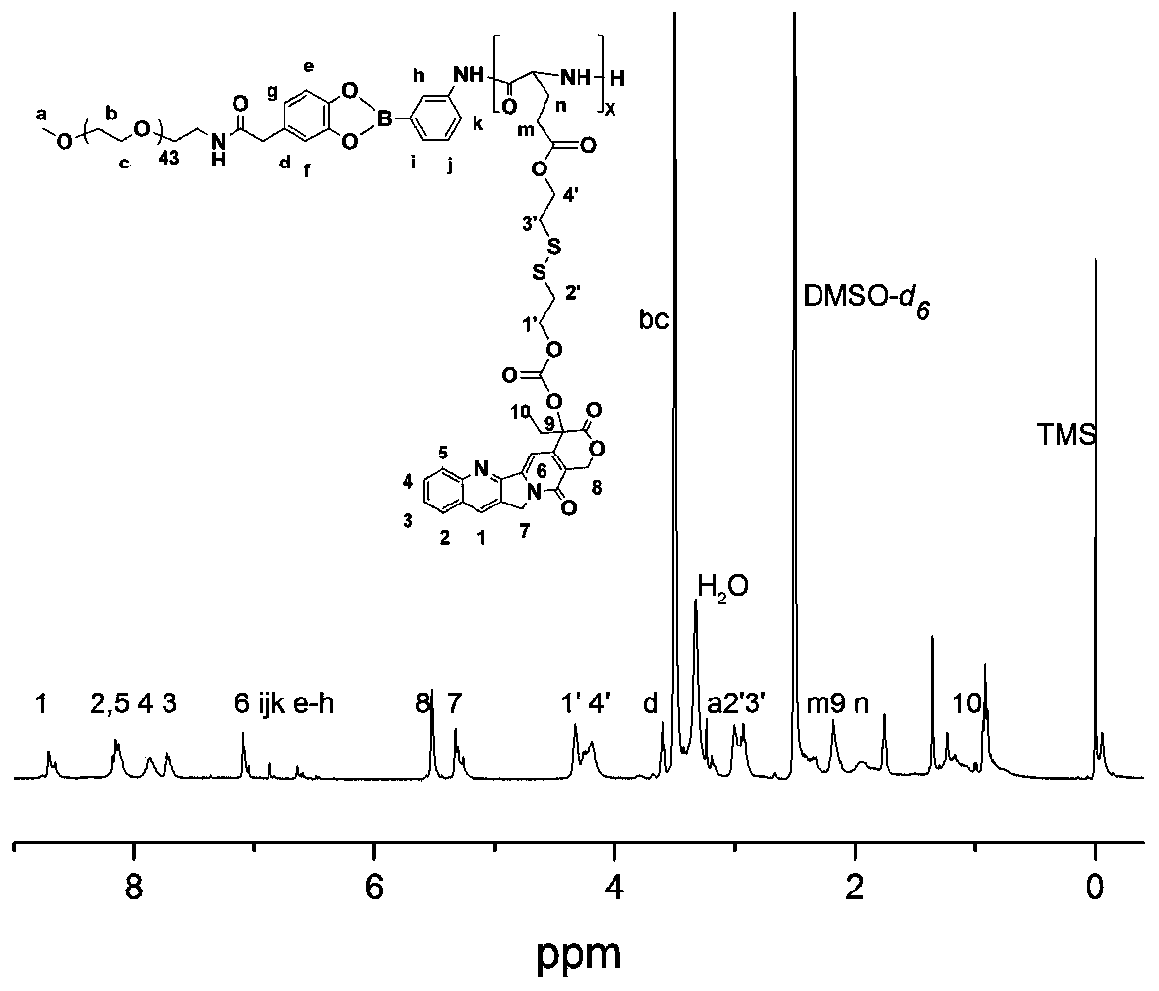
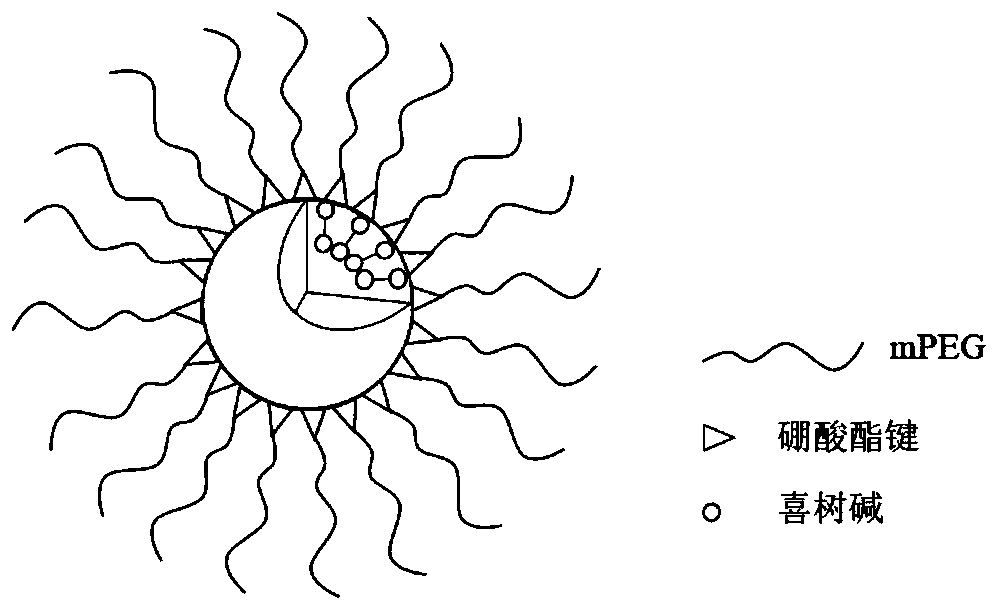
Amphiphilic camptothecin polymer prodrug taking phenylboronic acid ester as connecting unit, as well as preparation method and application thereof
InactiveCN108727581AImprove solubilityGood curative effectOrganic active ingredientsEmulsion deliverySolubilityPolymer science
The invention discloses an amphiphilic camptothecin polymer prodrug taking phenylboronic acid ester as a connecting unit and a co-delivery micelle system thereof. A polyethylene glycol-polyglutamate camptothecin two-block polymer (mPEG-BC-PGluCPT) is synthesized by taking catechol phenylborate (BC) as a connecting unit, and then a doxorubicin loaded micelle (mPEG-BC@PGluCPT.Dox) of the polymer isconstructed. Aiming at the poor water solubility of camptothecin, a polymer prodrug using camptothecin as a hydrophobic end by modifying 20 sites of hydroxyl groups of camptothecin is synthesized, which can effectively promote the assembly of the two-block polymer into a micelle. The solubility of camptothecin is improved, the stability of a camptothecin lactone ring is increased, and the curativeeffect and bioavailability are improved in order to overcome limitations of clinical treatment of camptothecin. The amphiphilic camptothecin polymer prodrug prepared by the method provided by the invention can be used for constructing a nano drug common delivery system, and has good drug release property, strong cell inhibition rate and good cell phagocytosis.
Owner:EAST CHINA NORMAL UNIV
Curcumin-carried pH-response color-changing antibacterial fiber and preparation method thereof
InactiveCN104762684AUniform colorGood level dyeingMonocomponent synthetic polymer artificial filamentDye addition to spinning solutionColor changesSolvent
The invention provides a curcumin-carried pH-response color-changing antibacterial fiber and a preparation method thereof. The preparation method comprises the following steps: step 1, drying and dewatering polyacrylonitrile powder, and filtering for separating by utilizing a screen mesh; step 2, mixing curcumin, the polyacrylonitrile powder obtained in the step 1 and a solvent, wherein the mass concentration of the polyacrylonitrile is 18-22%, and the consumption of the curcumin is 0.5-5% of the polyacrylonitrile powder in dry weight; and step 3, stirring a mixed liquid obtained in the step 2 to puff and dissolve polyacrylonitrile to obtain a spinning solution, performing deaeration on the spinning solution, performing wet spinning to obtain a nascent fiber, performing drying densification on the obtained nascent fiber, and performing heat setting treatment, so as to obtain the curcumin-carried pH-response color-changing antibacterial fiber. According to the curcumin-carried pH-response color-changing antibacterial fiber and the preparation method thereof, the fiber is colored uniformly, the color fastness is excellent, and the fiber has the weaving mechanical property and the pH-response color-changing performance; the spinning temperature is low, and the medicine activity of curcumin can be still retained, so that the fiber has the anti-bacterial function.
Owner:DONGHUA UNIV
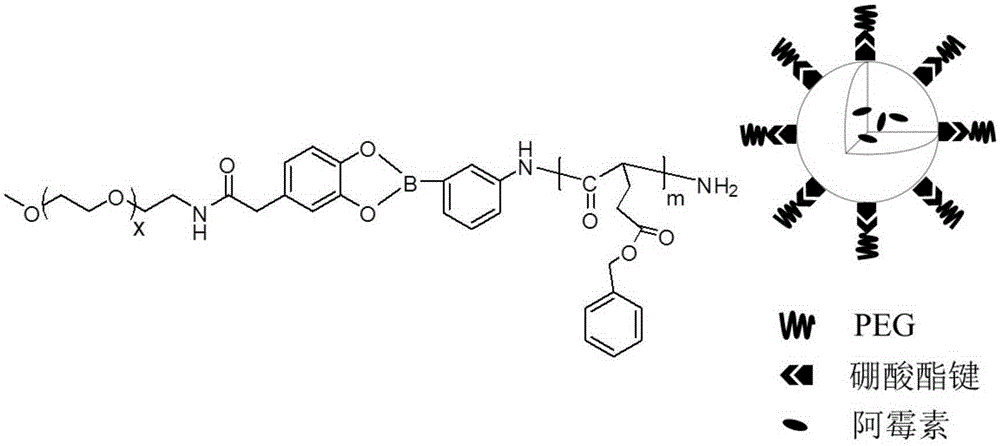
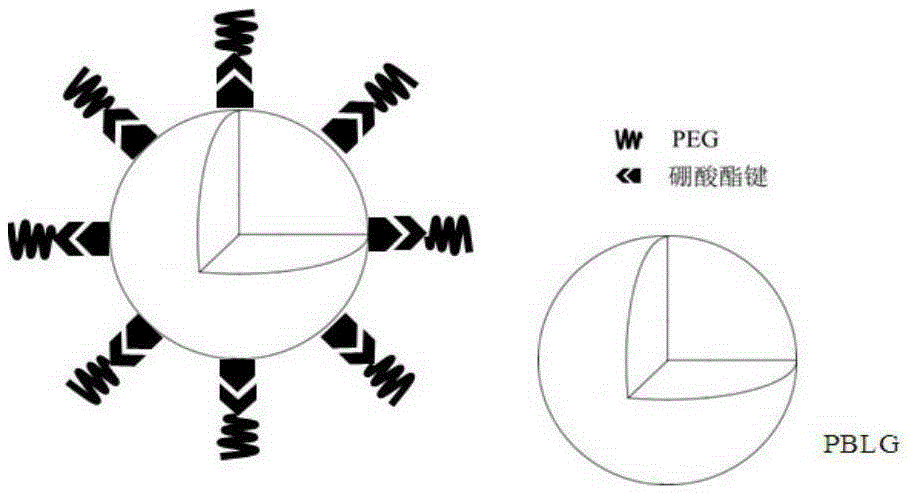
Block polymer with benzeneboronic acid ester as connecting unit, synthesis method and application thereof
InactiveCN105273205AGood drug releasePromote phagocytosisOrganic active ingredientsPharmaceutical non-active ingredientsPolymer scienceSynthesis methods
The invention discloses a diblock polymer with benzeneboronic acid ester as the connecting unit. Benzeneboronic acid catechol ester (BC) is adopted as the connecting unit to synthesize PEG-BC-PBLG diblock polymer so as to construct doxorubicin loaded micelle (PEG-BC@PBLG.Dox). The invention also discloses a preparation method of the diblock polymer, and the method includes: (1) preparing the dopa derivative PEG-3, 4-DA of PEG-NH2; (2) subjecting PEG-3, 4-DA and 3-aminophenylboronic acid to dehydration condensation so as to synthesize the benzeneboronic acid ester derivative PEG-BC of PEG; and (3) using PEG-BC to perform ring opening on 5-benzyl ester-L-glutamic acid-N-carboxyanhydride (BLG-NCA), thus obtaining the diblock polymer PEG-BC-PBLG with benzeneboronic acid ester as the connecting unit. The diblock polymer prepared by the method provided by the invention can be used for construction of a nano-micelle, and has the advantages of good drug release, low cytotoxicity and good cell phagocytosis.
Owner:EAST CHINA NORMAL UNIV
Tablet with improved combination properties and preparation method thereof
InactiveCN101919822AMaintain integrityAvoid crackingPill deliveryOil/fats/waxes non-active ingredientsPolymer scienceAdhesive
The invention discloses a tablet with improved combination properties. The tablet comprises an active constituent, a water soluble crystalline granular or powdered diluent, a melting adhesive, a melting mechanical property improver and / or does not comprise a pharmaceutically acceptable additive, wherein the diluent and / or the active constituent are / is adhered and bridged by the cured smelt of theadhesive and the mechanical property improver; the ratio of the mechanical property improver to the summation of the adhesive and the mechanical property improver is 0.25-0.70, and the ratio of the diluent to the summation of the adhesive, the mechanical property improver and the diluent is 0.40-0.93; the content of the diluent is not lower than 25 percent; and the contents of the adhesive and the mechanical property improver are not lower than 5 percent. The invention also discloses a preparation method of the tablet. The tablet has the advantages of stronger mechanical property, better weather resistance, better hydrophilcity, higher porosity and better disintegration property.
Owner:钟术光
Slow-release medicine carrier
InactiveCN101983723AGood drug releaseGood drug release propertiesSuppositories deliveryMacromolecular non-active ingredientsIrritationIn vivo
The invention discloses a slow-release medicine carrier with improved performance, which includes (a) fatty glyceride whose melting point in vivo and vitro is more than 37 DEG C; (b) additive particles which are soluble in water, has low viscosity and no irritation; (c) an acidic (or alkalic) polymer with water swelling ability and water insolubility; (d ) an alkalic (or acidic) surfactant; and (e) a medicament. The medicament carrier has better medicament-release characteristic, high salt poisoning resistance, low irritation to mucous membrane, better biological compatibility and better medicament-release homogeneity and better relieves or solves the problem of end release; and meanwhile it is not easy to generate medicament safety problems such as burst release or dose dumping of medicament.
Owner:钟术光
Medical cold-compressing plaster and preparation method thereof
InactiveCN109394737AEasy to useDoes not affect movementPeptide/protein ingredientsAntipyreticSide effectBiocompatibility Testing
The invention provides a medical cold-compressing paster which comprises a back lining layer, a gel layer and a covering layer. The gel layer is prepared from raw materials, the raw materials include,by mass, 2%-15% of a polymer substance, 2%-10% of an epidermal growth factor, 2%-8% of ceramide, 3%-8% of a moisturizer, 3%-10% of a radix bupleuri extract, 2%-8% of a dandelion extract, 3%-12% of agolden cypress extract, 5%-10% of an aloe extract, 2%-10% of a honeysuckle flower extract and the balance purified water. The invention also provides a preparation method of the medical cold-compressing paster. The medical cold-compressing paster has the advantages that the medical cold-compressing paster is convenient to use; the medical cold-compressing paster achieves both cold-compressing cooling and pain relieving, also promotes organization healing; the biocompatibility is good, and the medical cold-compressing paster does not have sensitization and irritation; the drug release performance is good, and the application time is long; the medical cold-compressing paster provides different shapes, and the different demands are met; the preparation method is easy to operate, the raw materials are wide in source, the preparation cost is low, no toxic-side effect is produced, and the medical cold-compressing paster is safe and reliable.
Owner:SHANDONG ZHUSHI PHARMA GRP CO LTD
Receptor-mediated quantum dot tracing targeted drug delivery system, preparation method thereof and application
InactiveCN102743762AIncrease drug concentrationAchieving passive targetingOrganic active ingredientsGenetic material ingredientsPolyethylene glycolMelphalan
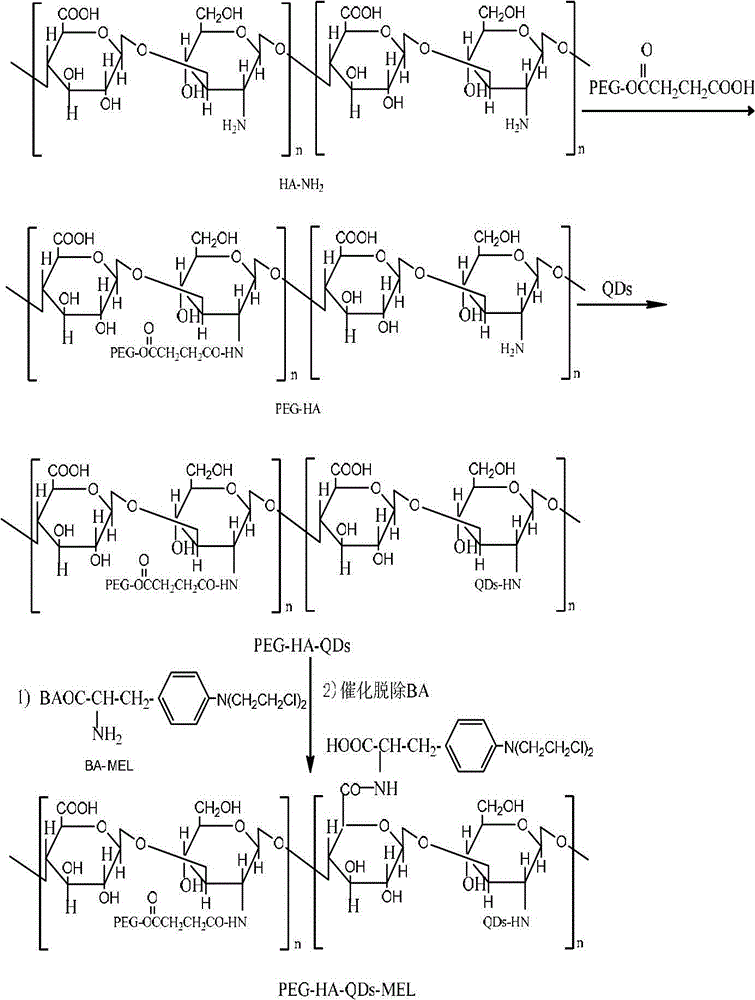
Disclosed are a receptor-mediated quantum dot tracing targeted drug delivery system, a preparation method thereof and application. The targeted drug delivery system comprises polyethylene glycol, hyaluronic acid, quantum dots and melphalan complex which are abbreviated as PEG-HA-QDs-MEL. The PEG is connected with HA amide in a dewatered and condensed manner, the water-soluble CdTe / CdS quantum dots are connected into the HA, and finally, the PEG, the HA and the QDs are prepared with the MEL complex to obtain the targeted drug delivery system. The component content of the PEG-HA-QDs-MEL includes, by weight, 30-100 parts of the MEL, 600-1500 parts of the PEG, 200-500 parts of the HA, 5-50 parts of the CdTe / CdS quantum dots, 20-50 parts of DCC (dicyclohexylcarbodiimide), 1-10 parts of DMAP (dimethylamino-pyridine), 100-500 parts of succinic anhydride, 20-50 parts of NHS, 20-50 parts of EDC (carbodiimide) and 10-100 parts of sodium bisulfite. The targeted drug delivery system is applied to preparing antitumor drugs.
Owner:WUHAN UNIV OF TECH
Beneficiation method for copper-lead sulphide ore
ActiveCN108405189APromote separationImprove recovery rate and gradeFlotationCitrate sodiumCalcium hypochlorite
The invention discloses a beneficiation method for copper-lead sulphide ore, and belongs to the technical field of beneficiation. The method comprises raw ore grinding, copper-lead mixing flotation and copper-lead separation. Copper-lead mixing flotation comprises primary copper-lead roughing, tertiary copper-lead fine selecting and secondary copper-lead scavenging. Copper-lead separation comprises primary copper roughing, tertiary copper fine selecting and secondary copper scavenging. Lime, calcium hypochlorite, zinc sulfate, sodium sulfite, sodium sulphide, dimethyl dicarbonate, sodium pyrophosphate, sodium citrate, ethionine ester, ethyl thio carbamate, sodium n-butylxanthate, aniline aerofloat, diphenyl amino phosphorodithioic acid and sodium carbonate are added in the copper-lead roughing and copper-lead scavenging processes. Lime, water glass, sodium phosphate starch, carboxymethyl starch, phthalic acid, thiosalicylic acid, ethionine ester and sodium ethylxanthate are added in the copper roughing and copper scavenging processes. The beneficiation method solves the problem that in the process of beneficiation of the copper-lead sulphide ore through a traditional flotation method, the copper and lead recycling rate is low.
Owner:广西华洋矿源材料有限公司
Temperature-sensitive hydrogel containing exendin-4 and injection thereof
ActiveCN102370611AImprove complianceGood drug releasePeptide/protein ingredientsMetabolism disorderPolyesterLarge dose
The invention relates to a temperature-sensitive hydrogel containing exendin-4, comprising exendin-4 as an effective ingredient and an amphiphilic copolymer as a medicine slow release carrier, wherein, the amphiphilic copolymer comprises polyester and polyethylene glycol, preferred PLGA-PEG-PLGA. The temperature-sensitive hydrogel is a liquid at room temperature, and is a solid water insoluble gel at the temperature of 37 DEG C, can be used for treating diabetes, and has good drug release property, environmental protection, little irritation, convenient application, and obvious effect. Compared with preparations with a large dose of exendin-4, the hydrogel disclosed herein has the advantages of in vivo formation of gel, easy operation, and continuous release property, and improves bioavailability, reduces administration times, and enhances patient compliance.
Owner:BEIJING GENETECH PHARML
Hydrocolloid patch containing panax notoginseng saponins and preparation method thereof
ActiveCN104274859AImprove adhesionNot easy to fall offAbsorbent padsDermatological disorderPANAX NOTOGINSENG ROOTHydrophilic polymers
The invention discloses a hydrocolloid patch containing panax notoginseng saponins and a preparation method thereof. The hydrocolloid patch is composed of a lining layer, a medicine-containing colloid layer and an anti-adhesion layer, and the hydrocolloid patch is prepared in the following manner: adding styrene-isoprene-styrene block copolymer, polyisobutene, mineral oil and an antioxidant into a reaction kettle, smelting at 150-160 DEG C, stirring uniformly, adding hydrogenated rosin resin, uniformly stirring at 110-140 DEG C to obtain an adhesive, transferring the adhesive into an internal mixer, adding a premix of a hydrophilic polymer and the panax notoginseng saponins, internally mixing at 110-140 DEG C for 30-40 min under N2 protection to obtain the medicine-containing colloid layer, transferring the medicine-containing colloid layer into a coating machine for coating, compounding and cutting, and adhering the medicine-containing colloid layer with the lining layer and the anti-adhesion layer to obtain the hydrocolloid patch. The hydrocolloid patch disclosed by the invention has excellent adhesion properties, drug release performance and high soakage, and can significantly improve the wound healing speed.
Owner:云南白药集团无锡药业有限公司 +1
Drug carrier capable of realizing sustained release of drug
InactiveCN102000339AGood drug releaseGood drug release propertiesSuppositories deliveryMacromolecular non-active ingredientsHigh resistanceIrritation
The invention discloses a drug carrier capable of realizing sustained release of a drug, which improves the performances and contains (a) an aliphatic additive, wherein the in vivo melting point and the in vitro melting point are higher than the temperature of 37 DEG C; (b) water-soluble additive particles with low viscosity and no irritation; (c) a water-insoluble acidic (or alkaline) polymer with water swelling property; (d) an alkaline (or acidic) surfactant; and (e) the drug. The drug carrier has better drug release property, higher resistance to salt poisoning, lower mucosa irritation, better biocompatibility and better drug release uniformity, can better ease or solve the problem of end release, and is difficult to cause initial burst release of the drug or dose dumping and other drug safety problems.
Owner:钟术光
A drug carrier for slow-release medicine
InactiveCN102258785AGood drug releaseGood drug release propertiesSuppositories deliveryPharmaceutical non-active ingredientsIrritationWater insoluble
The invention discloses a slow-release drug carrier with improved performance. The drug carrier contains (a) an aliphatic additive in vivo-in vitro fusing points of which is higher than 37 DEG C, (b) low-viscosity water-soluble nonirritant additive particles, (c) a water-swelling and water-insoluble acidic (or alkaline) polymer, (d) an alkaline (or acidic) surfactant, and (e) a medicament. The drug carrier has the advantages of better drug release character, higher 'salt poisoning' resistance effect, lower mucosal irritation, better biocompatibility and better drug release uniformity, thus better relieving or solving 'terminal release' problem and avoiding medication safety problems such as burst drug release or dose dumping and the like.
Owner:钟术光
Two-block double-sensitive camptothecin polymer prodrug taking benzeneboronic ester as connecting unit and preparation method of two-block double-sensitive camptothecin polymer prodrug
InactiveCN109985007AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityPolyethylene glycol
The invention discloses a two-block double-sensitive camptothecin polymer prodrug taking benzeneboronic ester as a connecting unit and a preparation method of the two-block double-sensitive camptothecin polymer prodrug. The two-block double-sensitive camptothecin polymer prodrug takes benzeneboronic catechol ester (BC) as the connecting unit, polyethylene glycol-polyglutamate disulfide ethanol camptothecin two-block polymer (PEG-BC-PGlu-ss-CPT) is synthesized, thereby constructing a self-assembly prodrug nano micelle, namely PEG-BC@PGlu-ss-CPT; PEG-BC is taken as a macroinitiator an initiatorto perform an open-loop polymerization reaction on a reduction-sensitive camptothecin monomer to obtain the two-block double-sensitive camptothecin polymer prodrug PEG-BC-PGlu-ss-CPT taking the benzeneboronic ester as the connecting unit. The prepared prodrug micelle can improve the solubility of camptothecin and improve the stability of a camptothecin lactone ring so as to overcome the limitationof camptothecin clinical treatment, and the prodrug micelle has a good drug release property and dual sensitive properties of acid sensitivity and reduction sensitivity.
Owner:EAST CHINA NORMAL UNIVERSITY
High-viscoelasticity Pickering emulsion and preparing method and application thereof
ActiveCN107140651AEasy to operateEasy to industrializeBiocideCosmetic preparationsSilica particleBiocompatibility Testing
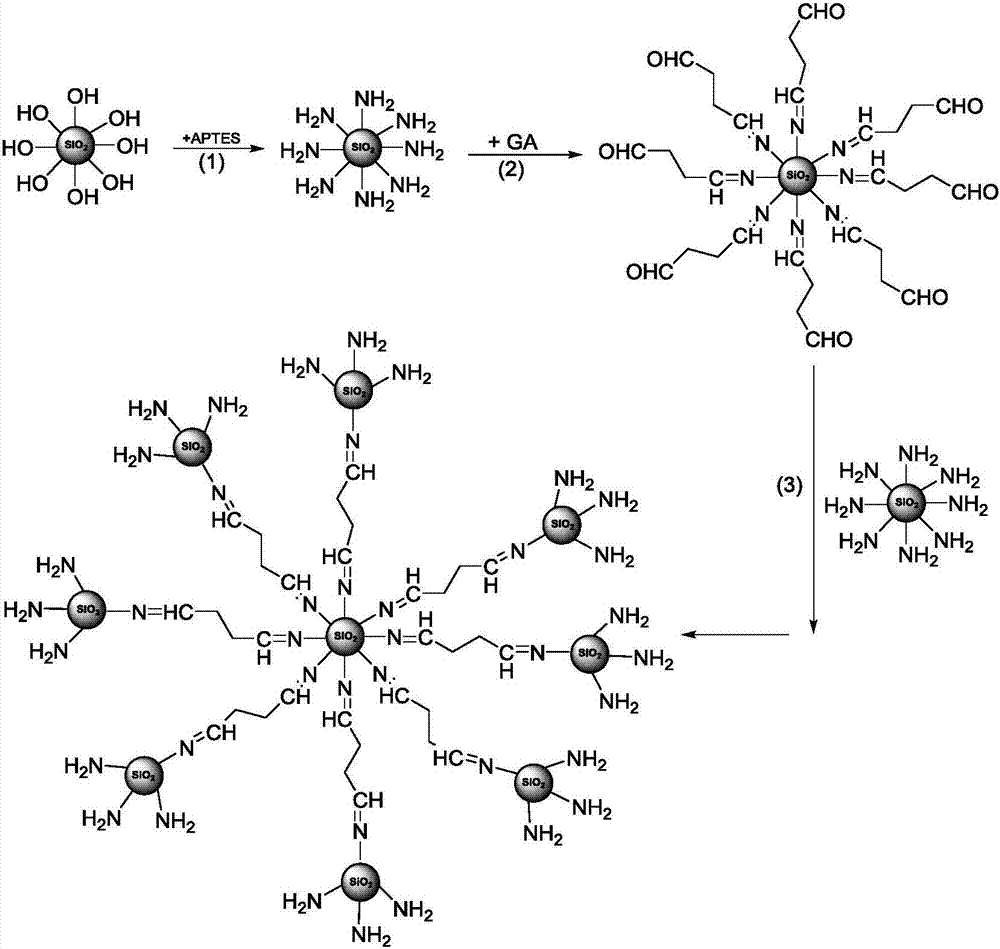
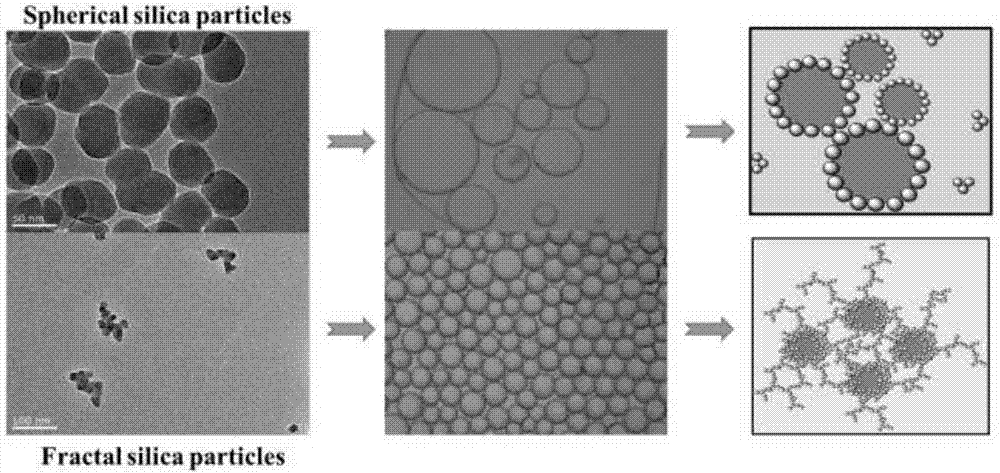
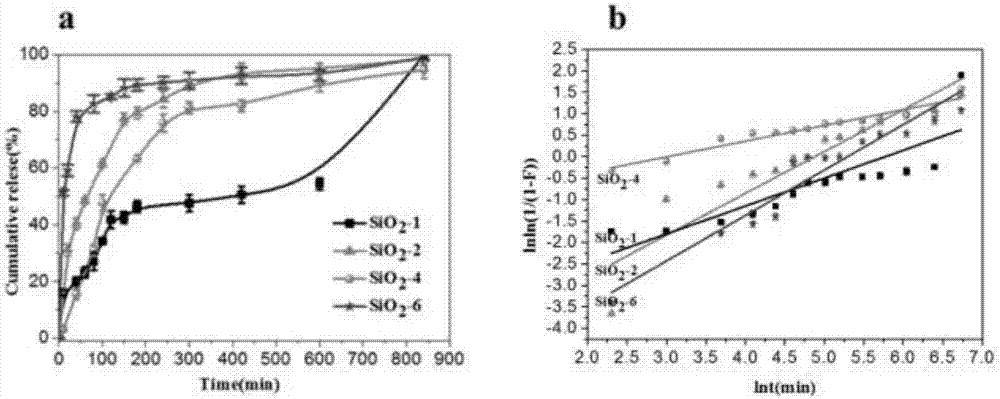
The invention provides multi-layer grafted silicon dioxide nanoparticles, layer-by-layer molecular imprints are used for covalently assembling amino-terminated silicon dioxide and aldehyde-terminated silicon dioxide, and the imprint process is repeated according to needs until the needed silicon dioxide layer number is reached. The invention further provides high-viscoelasticity Pickering emulsion prepared from the nanoparticles. The fractal silicon dioxide is synthesized through the layer-by layer molecular imprint technology and can be used for preparing the high-viscoelasticity Pickering emulsion, the obtained emulsion is high in viscoelasticity, high in stability and good in drug release degree. In addition, the used raw material silicon dioxide particles are low in price and easy to obtain, and the high-viscoelasticity Pickering emulsion is low in production cost and good in biocompatibility and therefore not limited when applied to the fields of food, cosmetics and medicine. The layer-by layer molecular imprint technology is easy to operate and industrialize.
Owner:HAINAN UNIVERSITY
Dexibuprofen sustained-release pellet and preparation method thereof
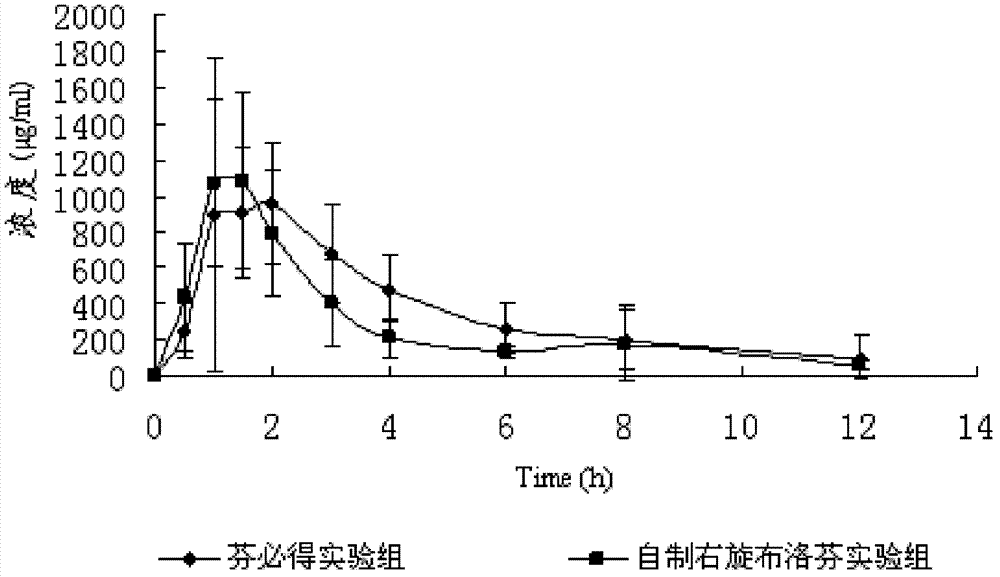
ActiveCN102228441BReduce or eliminate irritationWidely distributedOrganic active ingredientsAntipyreticSustained release pelletsBioavailability
The invention discloses a dexibuprofen sustained-release pellet and a preparation method thereof. The dexibuprofen sustained-release pellet comprises a celphere, a drug layer and an external coating. In the terms of the total weight of the pellet, the celphere accounts for 15 % to 30 %. The drug layer consists of dexibuprofen, a binding agent and talcum powder, wherein the dexibuprofen accounts for 70-85 %, the binding agent accounts for 1-10 %, and the talcum powder accounts for 0.1-3 %. The external coating consists of a coating material and the talcum powder, the coating material accounts for 0.5-3 % and the talcum powder accounts for 0.1-3 %. The invention further provides the preparation method of the dexibuprofen sustained-release pellet. In the invention, the dexibuprofen sustained-release pellet has the advantages of good stability, high bioavailability, good mobility and wide application prospect and is beneficial to subpackage of preparations or is further pressed in a form of a tablet.
Owner:湖北舒邦药业有限公司
New drug delivery system of gynaecologic panchrest plaster
InactiveCN102218129AConsistent color on one sideCut hole levelAntipyreticAnalgesicsLanolinSkin irritant
The invention relates to a new drug delivery system of gynaecologic panchrest plaster. The system consists of 0.16-0.36kg of dimethyl sulfone, 0.13-0.33kg of eucalyptus oil, 5.68-7.68kg of rubber, 0.6-1.5kg of vaseline, 0.30-1.00kg of lanolin, 7.8-9.8kg of zinc oxide, 0.28-0.48kg of liquid paraffin, 5.0-7.0kg of rosin and 5.33kg of traditional Chinese medicine extract. With the drug delivery system of the above prescription for drug loading, the gynaecologic panchrest plaster produced has consistent color, smooth cut opening and good drug delivery effect, with the incidence rate of skin irritation and anaphylaxis reduced to less than 0.8%.
Owner:LIAONING YUHUANG PHARMA
Preparation method of porous nano-hydroxyapatite sustained-release gel
ActiveCN110538346AUniform size distributionLarge size distributionOintment deliveryPharmaceutical non-active ingredientsPorosityApatite
The invention provides a preparation method of porous nano-hydroxyapatite sustained-release gel. Hydroxyapatite composite microspheres prepared by the method provided by the invention have uniform size distribution, a particle size distribution range of 10-100 mu m, and a central particle size of 20-60 mu m. Increase of an organic phase in a mixing process can effectively enhance the suspension stability of a suspension; the prepared microspheres have uniform size distribution and a larger particle size than the microspheres prepared by a traditional spray-drying method, and can be used as aninternal support material of injections; and the microspheres have a good spherical shape and high porosity, can enhance the injectability of a material, help to accelerate the metabolism time of drugs, maintain a stable and effective drug concentration in the body, and have good drug-release properties.
Owner:SHANGHAI MOYANG BIOTECHNOLOGY CO LTD
Fusion protein and encoding gene and preparation method of fusion protein as well as pharmaceutical composition and preparation method of pharmaceutical composition
ActiveCN103880962AHigh drug loading efficiencyGood drug releaseMacromolecular non-active ingredientsHybrid peptidesNucleotideBiocompatibility Testing
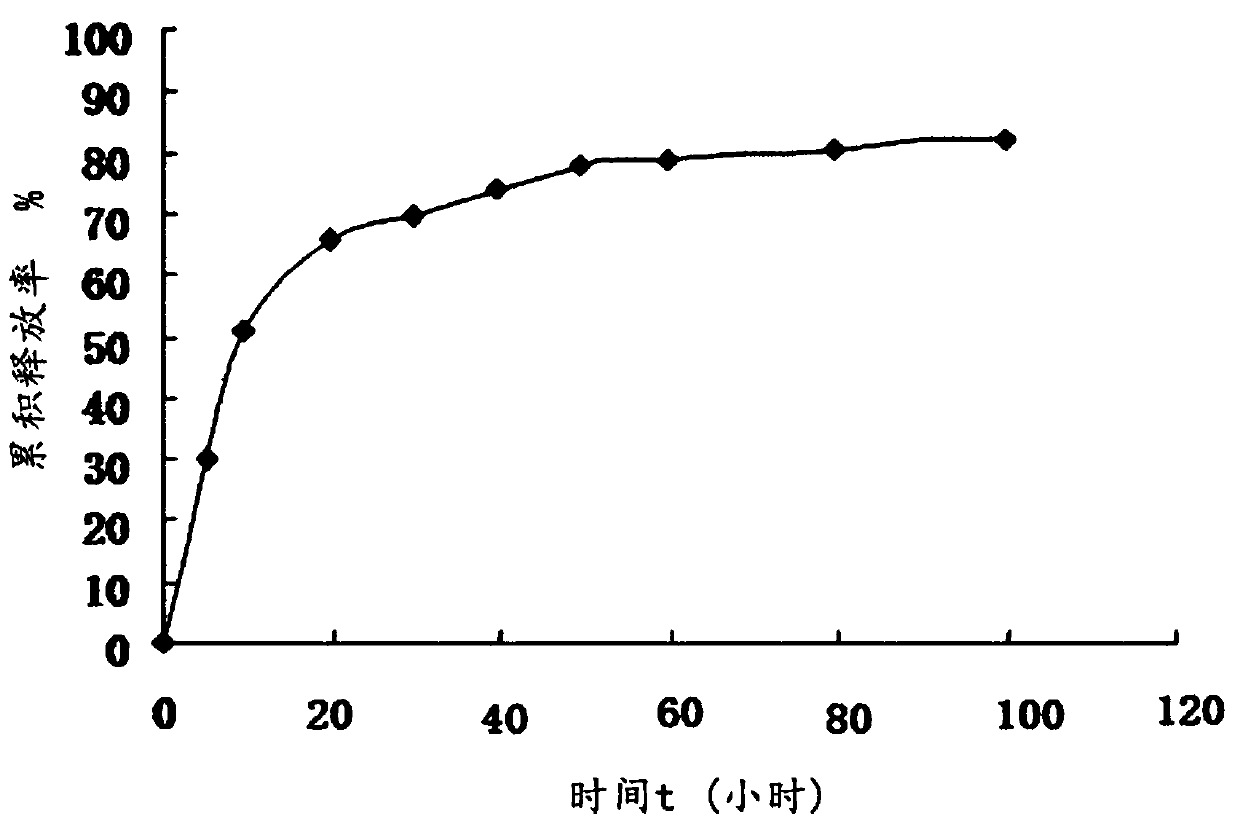
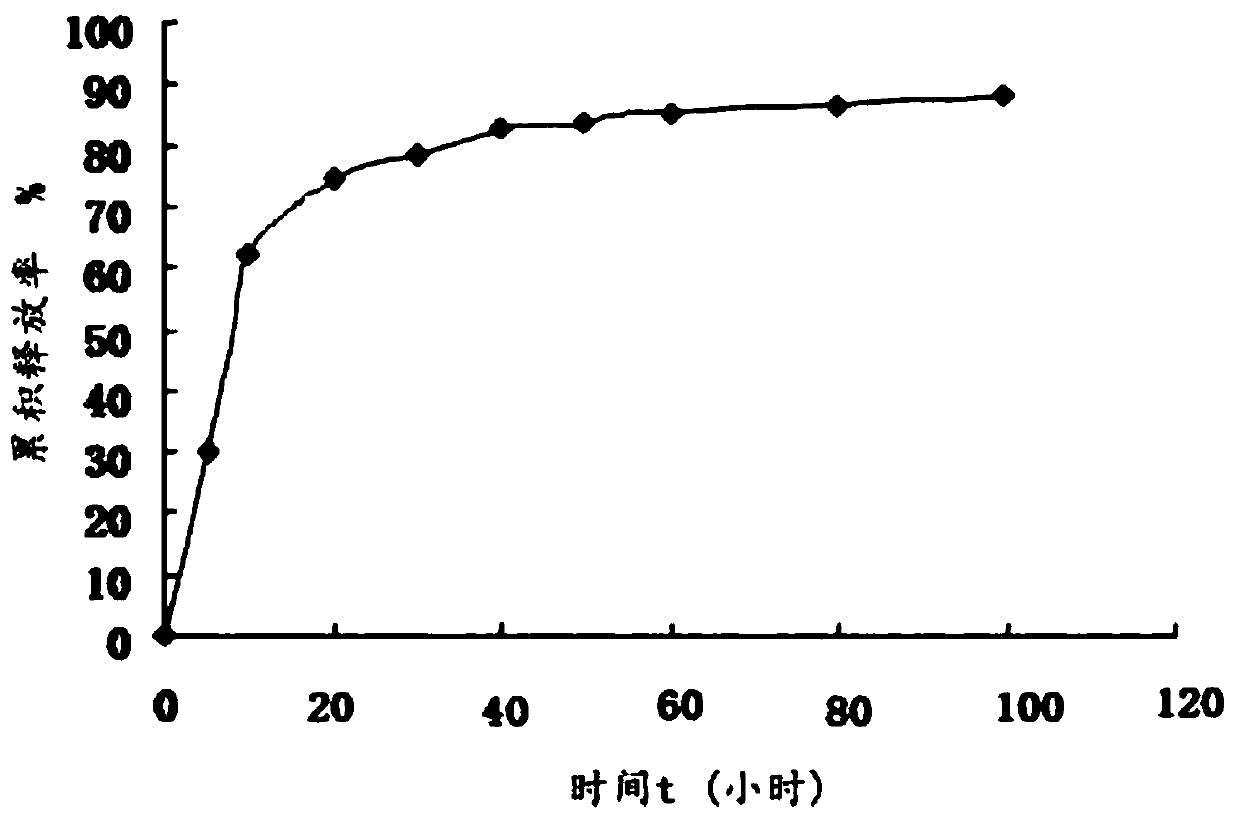
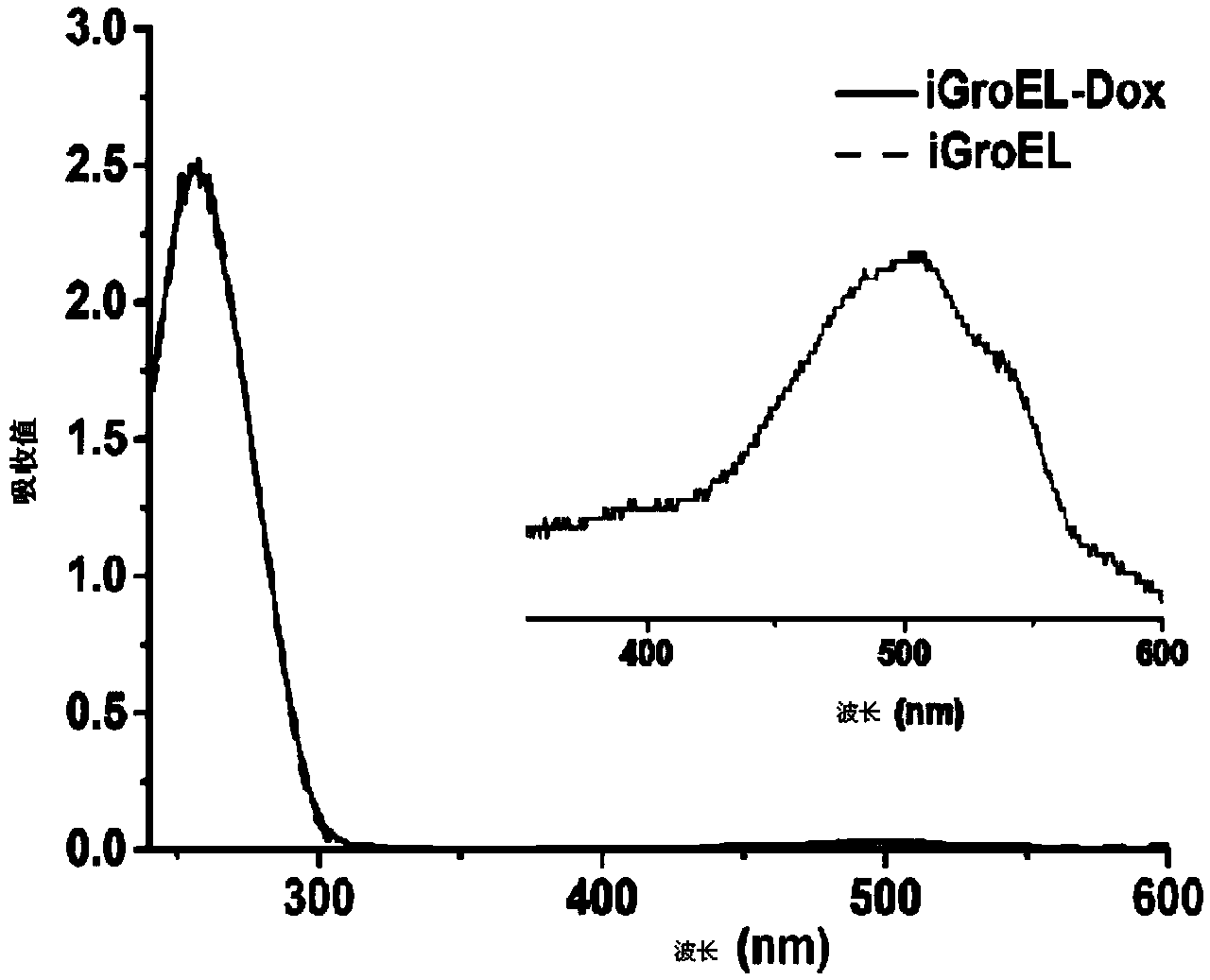
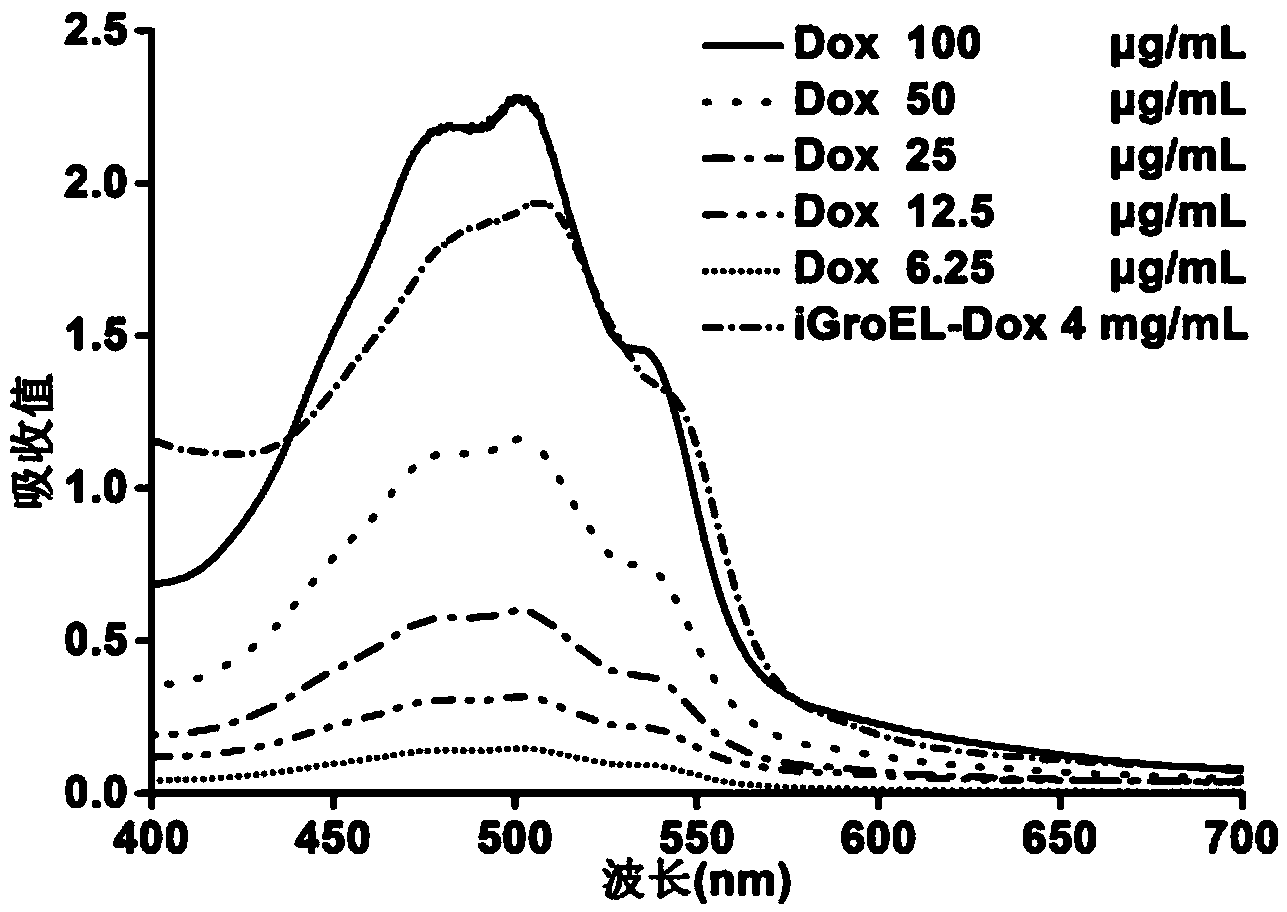
The invention discloses a fusion protein, the amino acid sequence of which contains an iRGD peptide sequence shown as SEQ ID No:1 and a molecular chaperone GroEL sequence shown as SEQ ID No:2. The invention further discloses an encoding gene of the fusion protein, the sequence of which is a nucleotide sequence capable of encoding the fusion protein. The invention further discloses a preparation method of the fusion protein. The preparation method comprises the following step of expressing the encoding gene in a bacterial strain to obtain the fusion protein. A method of preparing a pharmaceutical composition comprises the following steps of contacting the pharmaceutical compound with the fusion protein disclosed by the invention to obtain a contacted material in the presence of a solvent. The invention further discloses the pharmaceutical composition prepared by the method of preparing the pharmaceutical composition. The fusion protein provided by the invention can stably load a hydrophobic drug, and is high in drug-carrying efficiency and good in drug release effect. The pharmaceutical composition prepared by the fusion protein provided by the invention has the advantages of good biocompatibility and good drug release effect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Rapamycin slow-release dosage form as well as preparation method, rapamycin slow-release injection and application
InactiveCN108771656ALong-term therapeutic effectHigh drug contentOrganic active ingredientsAntipyreticControlled releaseDrug content
The invention provides a rapamycin slow-release dosage form as well as a preparation method, a rapamycin slow-release injection and application, which relate to the technical field of medicines. The rapamycin slow-release dosage form comprises rapamycin and a slow-release material in a mass ratio of 1: (10 to 50). The rapamycin slow-release dosage form is high in drug content, capable of realizinga slow-release and controlled-release effect of a drug, and capable of effectively alleviating and treating osteoarthritis. The preparation method of the rapamycin slow-release dosage form provided by the invention is simple to operate, and the prepared rapamycin slow-release dosage form is good in stability, long in retention time in a human body, high in bioavailability, and favorable for the popularization and application of industrialized production. The rapamycin slow-release injection can be directly injected without using an organic solvent, can reduce the adverse reaction and safety accidents caused by the organic solvent, is long in retention time in the human body, does not need to be repeatedly injected for multiple times, can reduce the cost of administrating the drug for multiple times, and can alleviate the pain of a patient and the irritation.
Owner:白晓春
Preparation method of porous nano-hydroxyapatite sustained release gel
InactiveCN109529110AUniform size distributionLarge size distributionOintment deliveryPharmaceutical non-active ingredientsPorosityApatite
The invention discloses a preparation method of porous nano-hydroxyapatite sustained release gel. Hydroxyapatite composite microspheres prepared by the preparation method disclosed by the invention are uniform in size distribution, and have a granularity distribution range of 10-100 microns as well as a central particle size of 20-60 microns. By adding organic phase in mixing process, suspension stability of a suspension can be effectively enhanced; so that, the prepared microspheres can be used as an internal support material used for injection for the microspheres are uniform in size distribution and larger in particle size compared with microspheres prepared by conventional spray-drying methods. Moreover, the microspheres are relatively good in shape and high in porosity; so that, the microspheres are capable of enhancing injectability of materials, promoting shortening of drug metabolism time, maintaining stable and effective drug concentration in vivo, as well as ensuring good drug release properties.
Owner:SHANGHAI MOYANG BIOTECHNOLOGY CO LTD
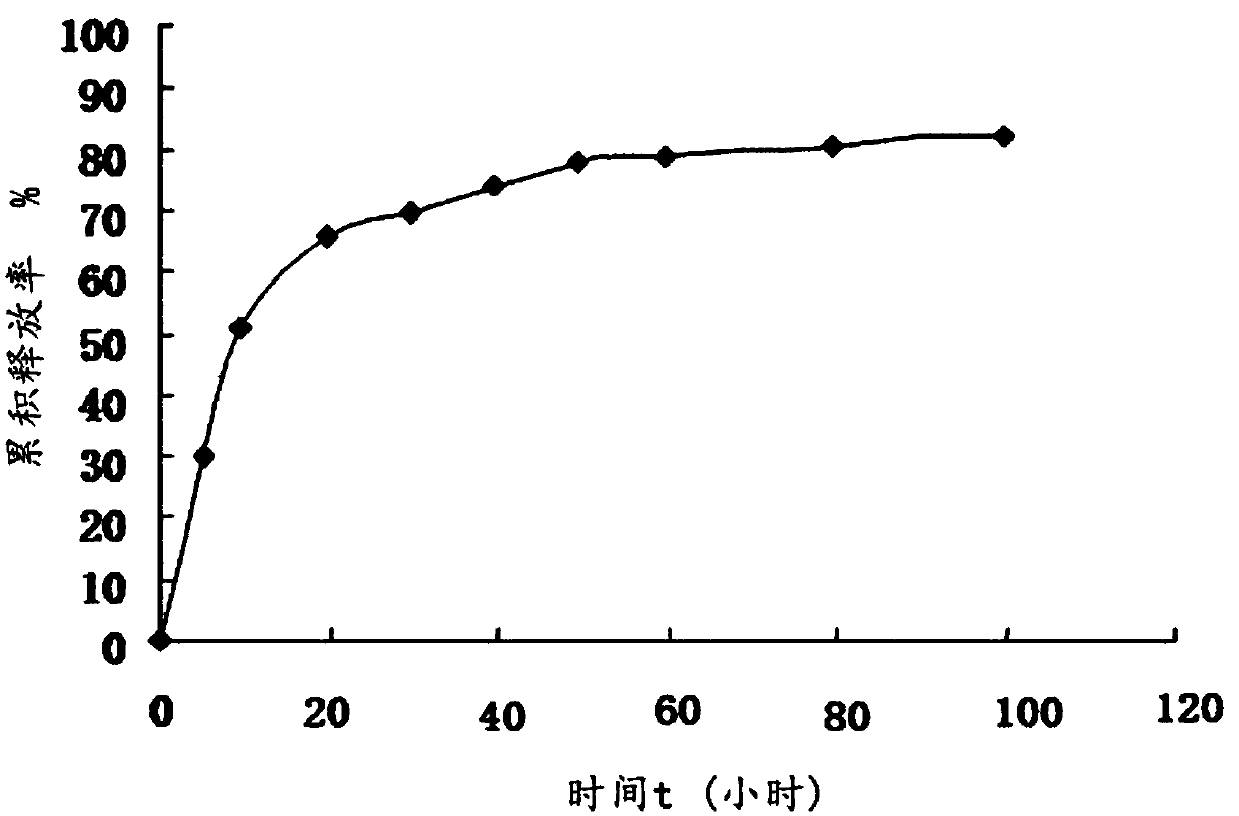
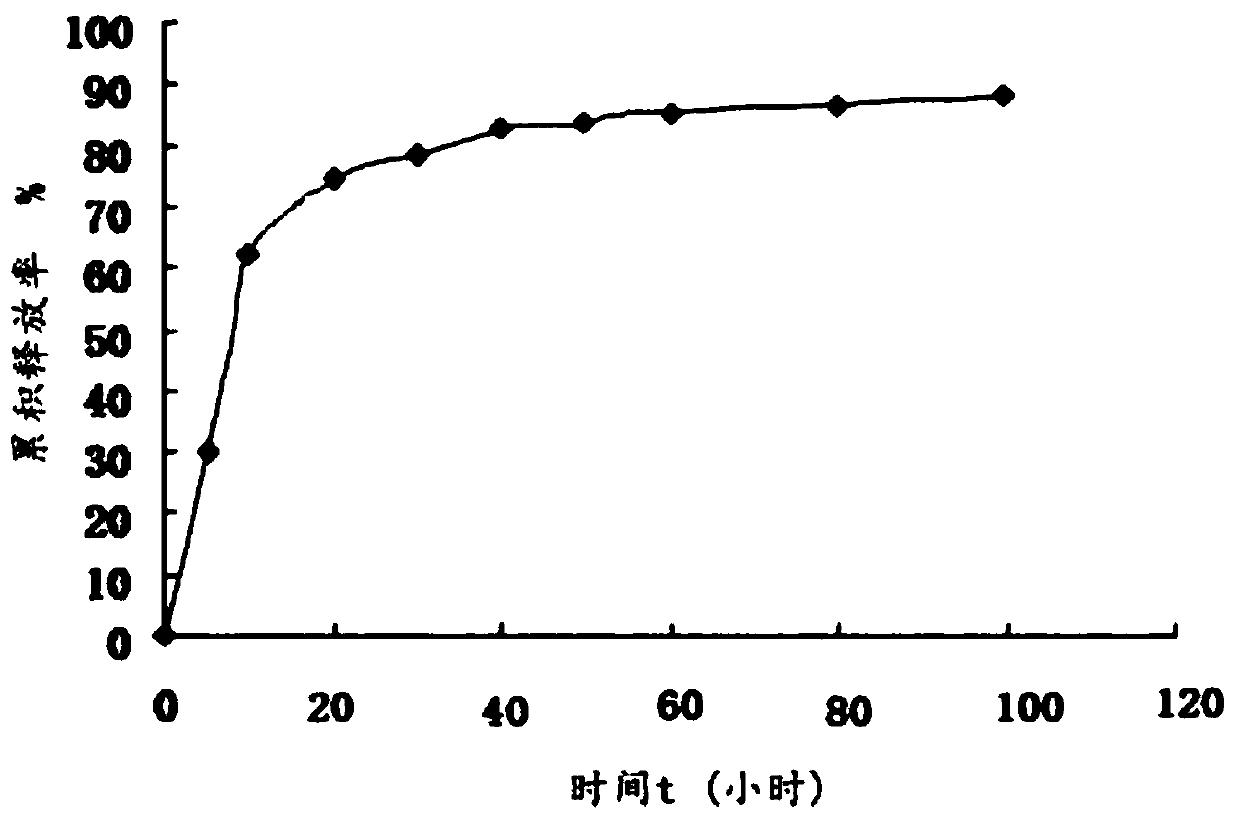
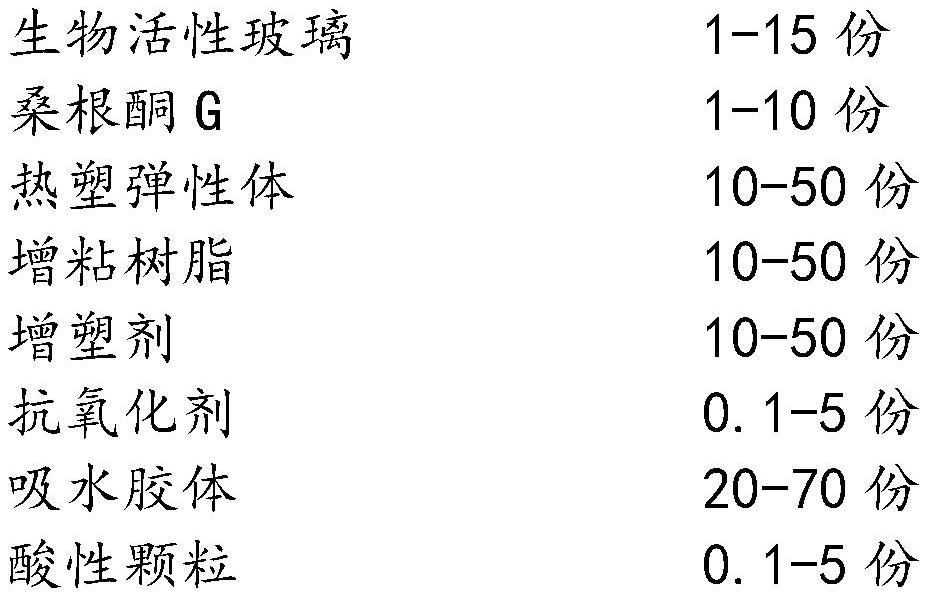
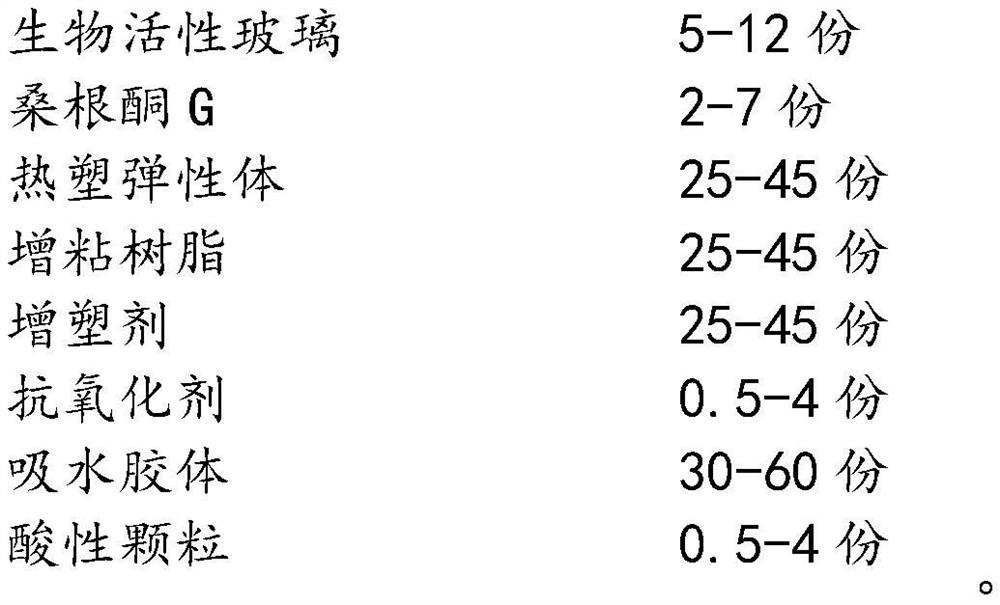
Bioactive glass hydrocolloid dressing for promoting wound healing
ActiveCN112807153AImprove water absorptionComplete structureAdhesive dressingsAbsorbent padsElastomerDiabetic ulcer
The invention relates to a bioactive glass hydrocolloid dressing for promoting wound healing and a preparation method and application thereof. The hydrocolloid dressing consists of a backing layer, a drug-containing colloid layer and an anti-sticking layer; the drug-containing colloid layer consists of a thermoplastic elastomer, tackifying resin, a plasticizer, an antioxidant, a water-absorbent colloid, acidic particles, bioactive glass and sanggenone G; and the bioactive glass consists of Na2O, CaO, P2O5 and SiO2, and the particle size is 40-60 [mu]m. The drug-containing bioactive glass hydrocolloid dressing of the invention has excellent adhesion performance, drug release performance and high water absorption capacity; the function of promoting wound healing and wound repair of the hydrocolloid dressing can be greatly enhanced; and the dressing is convenient to use, high in stability, low in irritation, and is especially suitable for treating chronic ulcerative wounds such as diabetic ulcers, diabetic feet and bedsores.
Owner:温州医科大学慈溪生物医药研究院
Drug carrier releasing drug slowly
InactiveCN106139155AGood drug releaseGood drug release propertiesOrganic non-active ingredientsSaccharide peptide ingredientsWater insolubleIrritation
The invention discloses a drug carrier releasing a drug slowly; the drug carrier includes: (a), an aliphatic additive melting in vivo and in vitro at a temperature of 37 DEG C and above; (b), water-soluble, low-viscosity and nonirritating additive particles; (c), a water-swellable and water-insoluble acidic (or alkaline) polymer; (d), an alkaline (or acidic) substance; (e), a water-soluble surfactant; (f) a drug. The drug carrier has better drug release characteristics, higher anti-'salt-poisoning' effect and better drug release uniformity, and better alleviates or solves the 'end release' problem; the drug carrier has low mucosal irritation and better biocompatibility; the drug carrier rarely presents usage problems such as sudden drug release or dosage decline.
Owner:钟术光
Medicament carrier for releasing medicament in sustained-release way
InactiveCN102698277AGood drug releaseGood drug release propertiesSuppositories deliveryOrganic non-active ingredientsWater insolubleIrritation
The invention discloses a medicament carrier for releasing a medicament in a sustained-release way. The medicament carrier comprises (a) an aliphatic additive of which the melting points in vivo and vitro are higher than 37 DEG C, (b) water-soluble, low-viscosity and non-irritating additive particles, (c) a water-swellable and water-insoluble acidic (or alkaline) polymer, (d) an alkaline (or acidic) substance, (e) a water-soluble surfactant and (f) a medicament. The medicament carrier has high medicament release properties, a good anti-salt poisoning effect and high medicament release uniformity, so that the medicament can be released in a sustained way or the problem about end-release is solved; the medicament carrier has low mucous membrane irritation and high biocompatibility; and the medicament safety problems such as medicament burst release and dose pour release are not easily caused.
Owner:钟术光
Preparation method of collagen cladded carbon nano-tube composite material
InactiveCN107158394AHighlight substantive featuresImprove adhesionInorganic non-active ingredientsCarbon nanotubesBiocompatibility TestingDrug carrier
The invention discloses a preparation method of a collagen cladded carbon nano-tube composite material and relates to a carbon nano-tube material. The preparation method is a preparation method for preparing the collagen cladded carbon nano-tube composite material by in-situ cladding a collagen layer on the surface of a functionalized carbon nano-tube by combing a magnetic liquid phase stirring method and a hydrogel method. The preparation method comprises the following steps of preparing carbon nano-tube-hydroxyapatite composite powder; preparing the functionalized carbon nano-tube; and preparing the collagen cladded carbon nano-tube composite material by the technical method of combing the magnetic liquid phase stirring method and a hydrogel method. The defects that the carbon nano-tube is easily agglomerated, uniform dispersion of components is difficult and production efficiency is low in the preparation method of a collagen-carbon nano-tube composite material in the prior art are overcome; and when the prepared collagen-carbon nano-tube composite material is used as a medicine carrying material, the detects that the biocompatibility is still relatively poor, medicine-carrying and medicine-releasing abilities are poor, and hidden danger of toxin is not thoroughly eliminated generally are overcome.
Owner:HEBEI UNIV OF TECH
Nano ganciclovir freeze-drying preparation for injection and preparation method thereof
ActiveCN103340830AAvoid local irritation side effectsImprove tolerancePowder deliveryAntiviralsAlkalinityActivated carbon
The invention discloses a nano ganciclovir freeze-drying preparation for injection and a preparation method thereof. The nano ganciclovir freeze-drying preparation for injection comprises the following components in parts by weight: 100-400 parts of ganciclovir, 10-50 parts of dextran 40, 5-50 parts of solubilizer, 10-100 parts of nano carrier material and 10-80 parts of freeze-drying skeleton agent. The preparation method comprises the following steps of: sequentially adding dextran 40, solubilizer, ganciclovir, nano carrier material and freeze-drying skeleton agent into water for injection to be dissolved, filtering a solution step by step, and carrying out freeze-drying, thus a freeze-drying preparation is obtained. According to the preparation method, no activated carbon is introduced, thus a risk that damage is done to a human body when activated carbon particles are introduced into the preparation as the activated carbon is used is avoided; besides, pH of the nano ganciclovir freeze-drying preparation for injection is 6-8 and close to that of plasma, and local irritation produced to the human body owning to overhigh alkalinity is avoided.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Two-stage reagent removal method for ilmenite flotation concentrate
ActiveCN106563577ARemoval reachedSolve the problem of drug withdrawalFlotationSlurryIron(III) sulfate
The invention relates to a two-stage reagent removal method for ilmenite flotation concentrate and belongs to the field of mineral processing engineering. The two-stage reagent removal method for the ilmenite flotation concentrate includes the following specific steps of first-stage reagent removal, wherein after the slurry concentration of the ilmenite flotation concentrate is adjusted, sodium hydroxide is added, the pH value is adjusted to be 11 to 12, ferric sulfate is added, the materials are fully stirred for 15-20 min, filtering is carried out, and the ilmenite flotation concentrate obtained after first-stage reagent removal is obtained; and second-stage reagent removal, wherein after the slurry concentration of the ilmenite flotation concentrate obtained after first-stage reagent removal is adjusted, sodium hypochlorite and aluminum sulfate are sequentially added, the materials are fully stirred for 15-20 min, and then the final reagent-removed concentrate is obtained. The method aims at the ilmenite flotation concentrate obtained with sodium oleate and diesel oil as a collecting agent, the reagent removal effect is complete, the process is simple, and high adaptability is achieved.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com