Dexibuprofen sustained-release pellet and preparation method thereof
A technology of slow-release pellets and pellets, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Problems such as irritating odor and difficulty in sustained-release preparations, etc., to achieve the effects of improving bioavailability, good fluidity, and reducing interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] formula:
[0043]
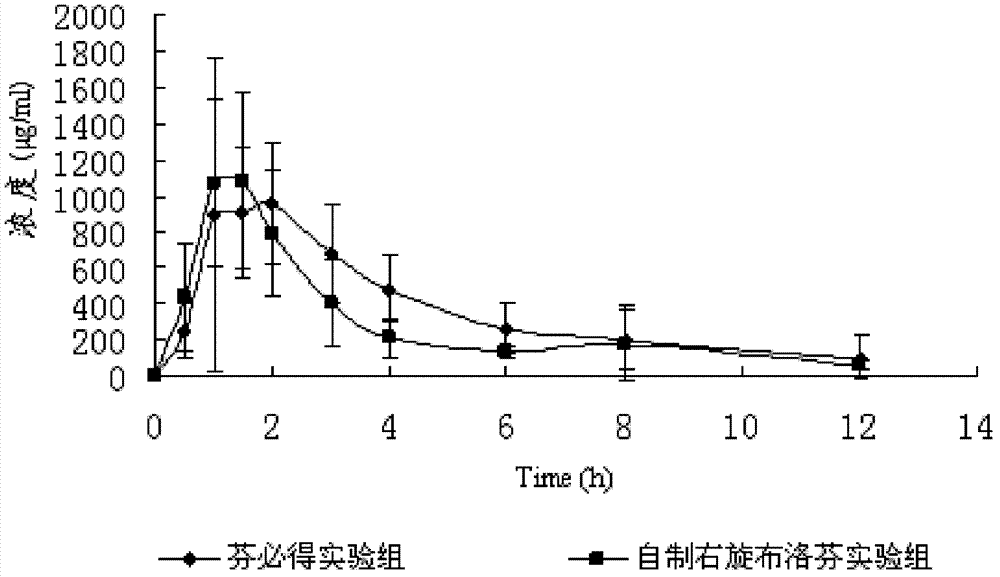
[0044] First prepare the drug solution and coating solution, in the fluidized bed, control the temperature of the material at 30°C, spray the drug at the bottom, and obtain 18-20 mesh uncoated pellets, take 18-20 mesh uncoated pellets For coating, stir while adding the solution to avoid sedimentation of the talcum powder. Capsules are filled according to the content to obtain 200mg specification Dexibuprofen sustained-release capsules. The preparation process of the micropills was smooth, the appearance was round and beautiful, and the micropills were stored without adhesion. The uncoated micropills released relatively fast in the in vitro release test, and passed the coating after coating.
Embodiment 2
[0046] formula:
[0047]
[0048] First prepare the drug solution and coating solution, in the fluidized bed, control the temperature of the material at 30°C, spray the drug at the bottom, and obtain 18-20 mesh uncoated pellets, take 18-20 mesh uncoated pellets For coating, stir while adding the solution to avoid talcum powder settling. During the preparation and storage of pellets, pellet adhesion may occur. Add appropriate amount of talc powder to prevent adhesion. The adhesion of pellets after coating is good. Fill the capsules according to the content to obtain 200mg Dexibuprofen sustained-release capsules. In vitro release test The uncoated pellets were synthesized, and the release of coated pellets was slow.
Embodiment 3
[0050] formula:
[0051]
[0052] First prepare the drug solution and coating solution, in the fluidized bed, control the temperature of the material at 30°C, spray the drug at the bottom, and obtain 18-20 mesh uncoated pellets, take 18-20 mesh uncoated pellets For coating, stir while adding the solution to avoid talcum powder settling. During the preparation and storage of pellets, pellet adhesion may occur. Add appropriate amount of talc powder to prevent adhesion. The adhesion of pellets after coating is good. Fill the capsules according to the content to obtain 200mg Dexibuprofen sustained-release capsules. In vitro release test The uncoated pellets were synthesized, and the release of coated pellets was slow.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com