A kind of dexlansoprazole sustained-release preparation and its preparation method and medicine
A technology of dexlansoprazole and sustained-release preparations, which is applied in the field of sustained-release drugs, and can solve the problem of drug effect and food delivery rhythm, large impact on gastric emptying, limited sustained-release ability of proton pump inhibitor drugs, and inability to take long-term onset To achieve the effects of drug efficacy and other issues, it is beneficial to the sub-packaging of the preparation, improving the bioavailability, and good reproducibility of the drug release pattern.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
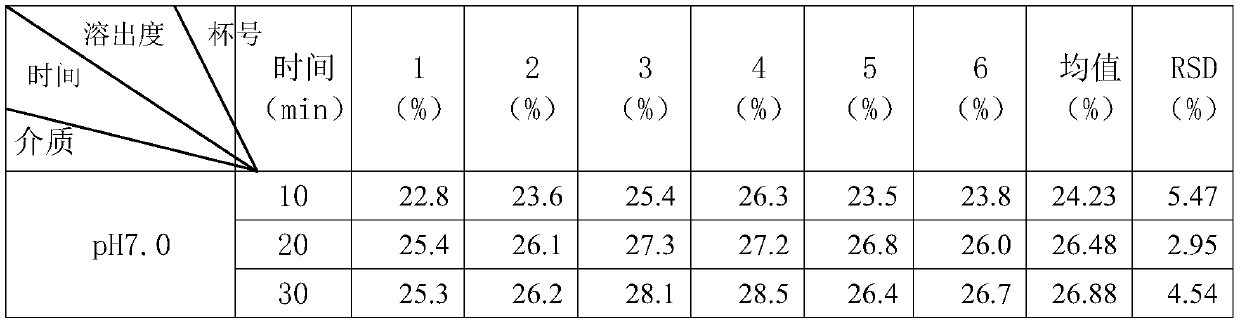
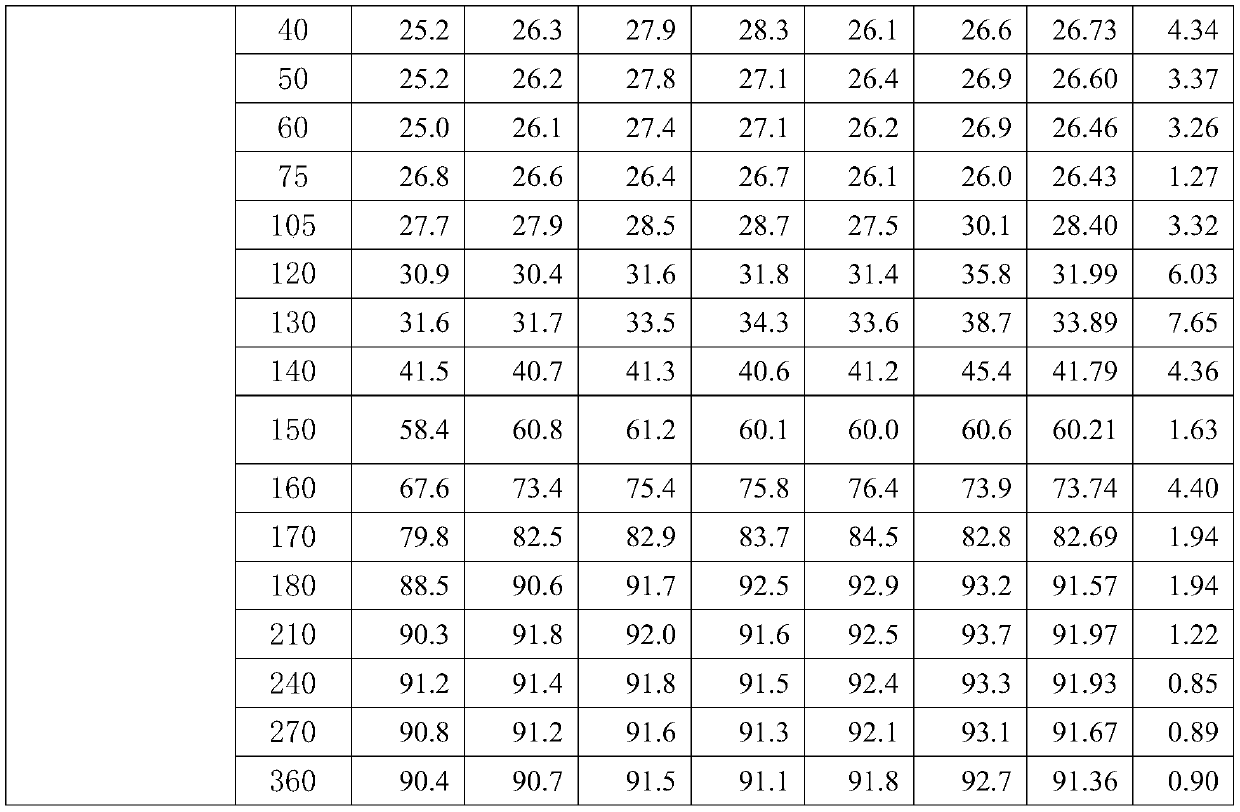
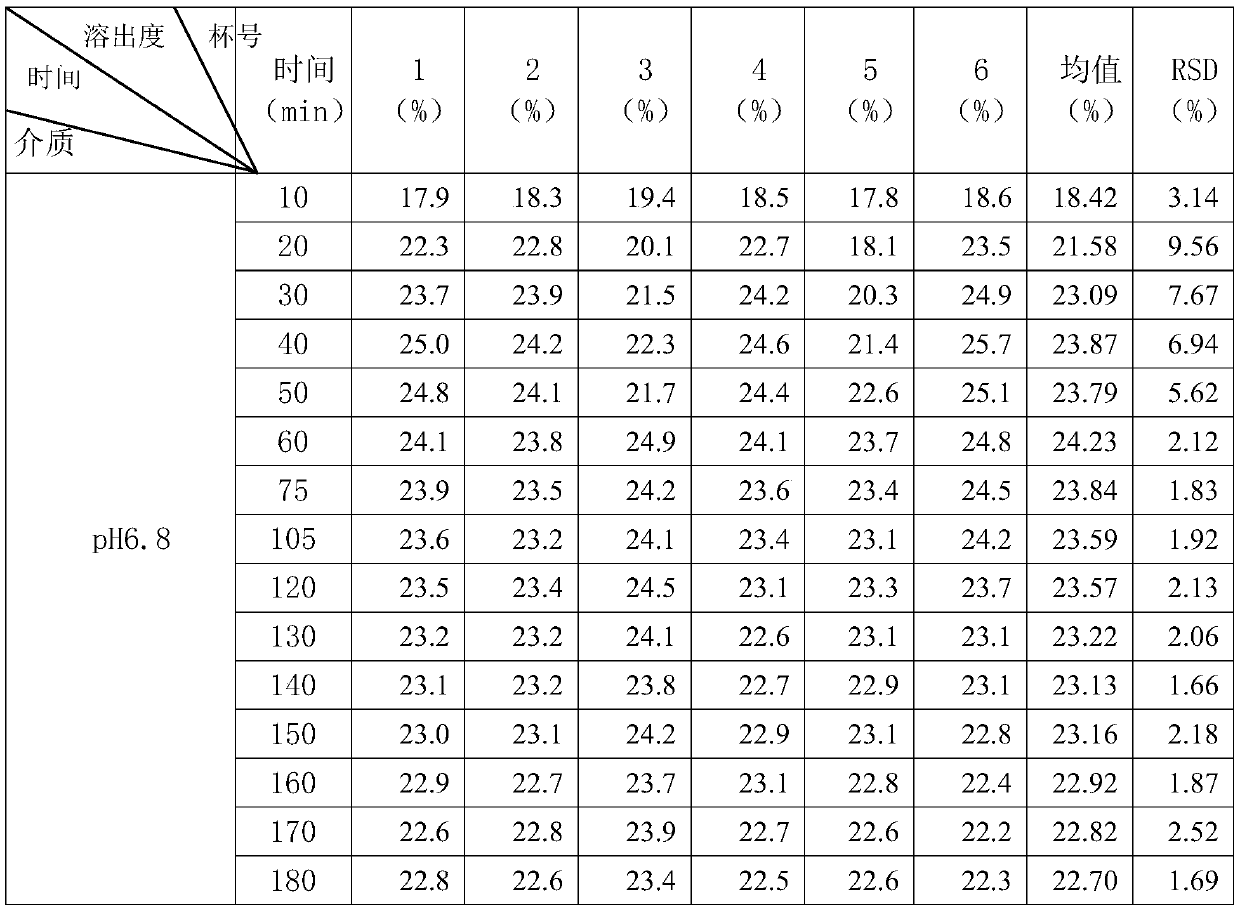
[0118] The preparation method of above-mentioned a kind of dexlansoprazole slow-release preparation, granulation after mixing base pellet raw material, coating isolation layer on the surface of gained base pellet, coating on the isolation layer surface of the base pellet coated with isolation layer The coating layer obtains a sustained-release preparation of dexlansoprazole.
[0119] The preparation method of the dexlansoprazole sustained-release preparation of the present invention has simple process, small variation factors, controllable quality and is suitable for large-scale production.
[0120] In a preferred embodiment of the present invention, in the granulation process, water or syrup is used as the liquid phase for granulation.
[0121] In a preferred embodiment of the present invention, the syrup is an aqueous sucrose solution.
[0122] Preferably, the mass fraction of the sucrose aqueous solution is 25%.
[0123] In a preferred embodiment of the present invention, a...
Embodiment 1
[0199] A kind of preparation method of the capsule drug that contains dexlansoprazole slow-release preparation, adopts above-mentioned preparation method to finish, and the quality ratio of each raw material is as follows:
[0200] Dexlansoprazole in base pill raw materials: sodium carboxymethylcellulose: sucrose: dextrin: starch: anhydrous sodium carbonate = 150:50:200:100:50;
[0201] Hypromellose: talc powder: titanium dioxide = 50:15:10 in the raw material of the isolation layer;
[0202] Coating layer A raw material methacrylic resin copolymer L100: polyethylene glycol 6000: Tween 80: triethyl citrate: titanium dioxide: talc = 50:2:10:30:4:15;
[0203] Polyacrylic acid resin S100: polyethylene glycol 6000: Tween 80: triethyl citrate: titanium dioxide: talcum powder in the coating layer B raw material = 150:6:30:100:12:60;
[0204] In the preparation coated pellet A, the mass ratio of the used dexlansoprazole, hypromellose and methacrylic resin copolymer L100 is dexlansop...
Embodiment 2
[0209] A kind of preparation method of the capsule drug that contains dexlansoprazole slow-release preparation, adopts above-mentioned preparation method to finish, and the quality ratio of each raw material is as follows:
[0210] Dexlansoprazole in base pill raw materials: sodium carboxymethylcellulose: sucrose: dextrin: starch: anhydrous sodium carbonate = 450: 150: 600: 200: 100;
[0211] Hypromellose in the raw material of the isolation layer: talc powder: titanium dioxide = 100:45:20;
[0212] The methacrylic resin copolymer L100 in the coating layer A raw material: polyethylene glycol 6000: Tween 80: triethyl citrate: titanium dioxide: talcum powder = 100: 6: 20: 70: 12: 45;
[0213] Polyacrylic acid resin S100: polyethylene glycol 6000: Tween 80: triethyl citrate: titanium dioxide: talcum powder in the coating layer B raw material = 300: 18: 60: 200: 36: 120;
[0214] In the preparation coated pellet A, the mass ratio of the used dexlansoprazole, hypromellose and meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com