A drug carrier for slow-release medicine
A drug, carrier technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
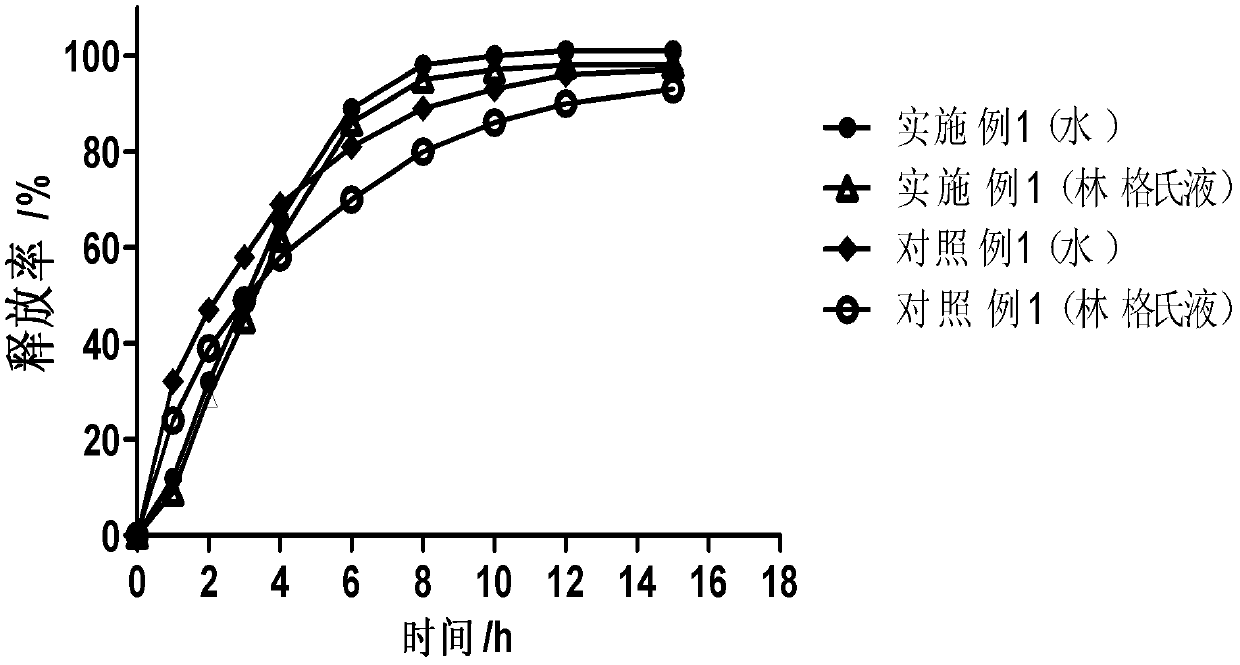
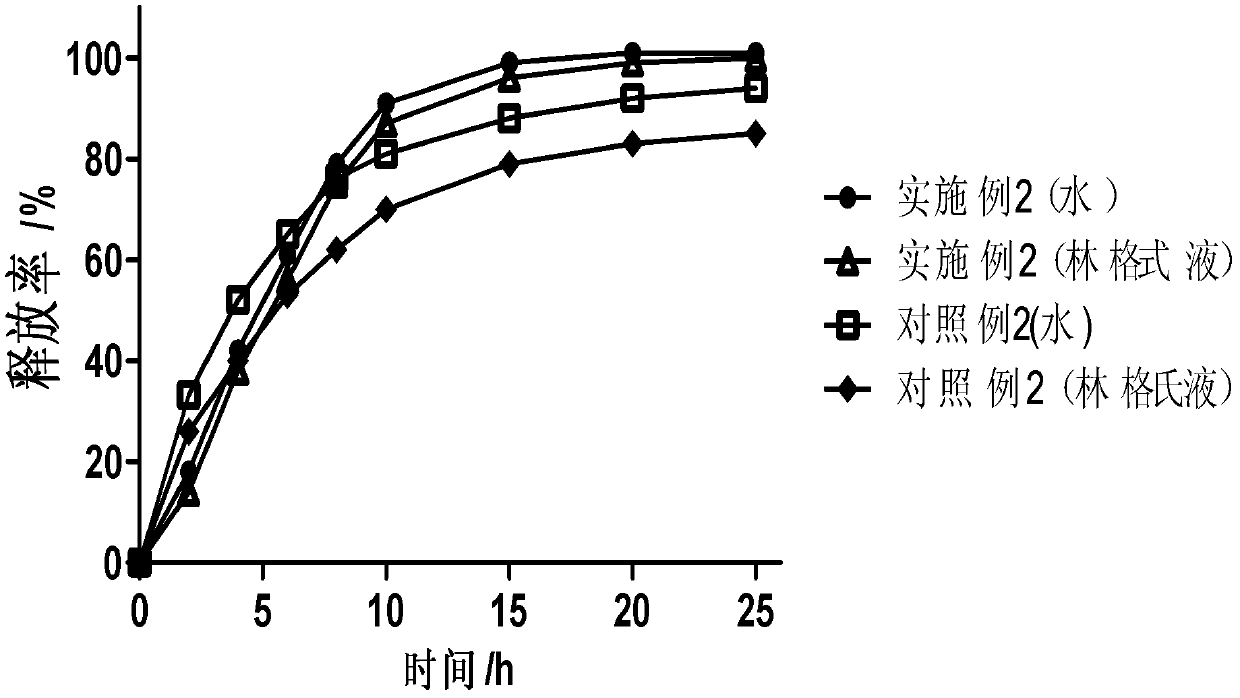
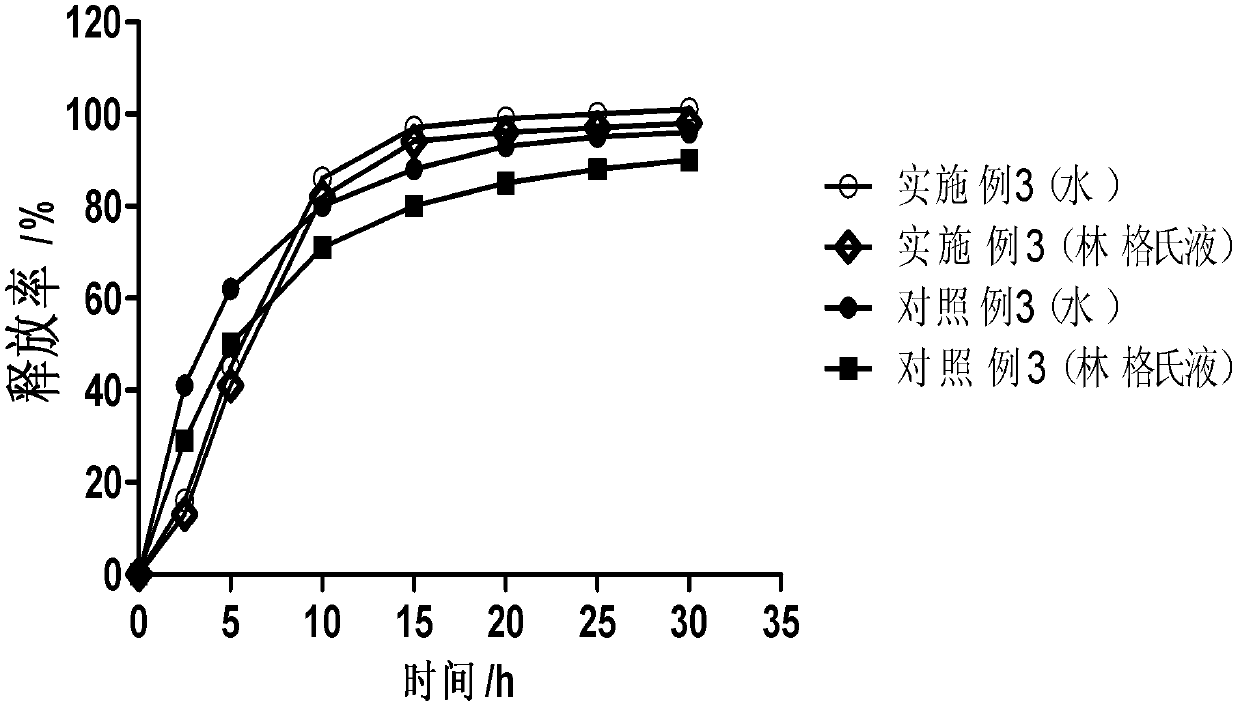
[0030] The following is a detailed description of the above-mentioned drug carrier.
[0031] The matrix of the drug carrier and the material for controlling the release of the drug involved in the present invention are pharmaceutically acceptable aliphatic additives with a melting point higher than 37°C and a melting point higher than 37°C when in contact with body fluids in the body cavity, preferably Aliphatic additives with a melting point of 40°C to 90°C, more preferably 40°C to 70°C, more preferably 40°C to 50°C, the aliphatic additives include but are not limited to melting point Animal and vegetable oils, semi-synthetic oils, fatty acid glycerides, waxes, higher fatty acids, higher fatty alcohols, higher fatty acid esters, higher Aliphatic hydrocarbons and their mixtures.
[0032]Among them, fatty acid glycerides whose melting point is higher than the temperature of 37°C and whose melting point is also higher than the temperature of 37°C when in contact with body fluid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com