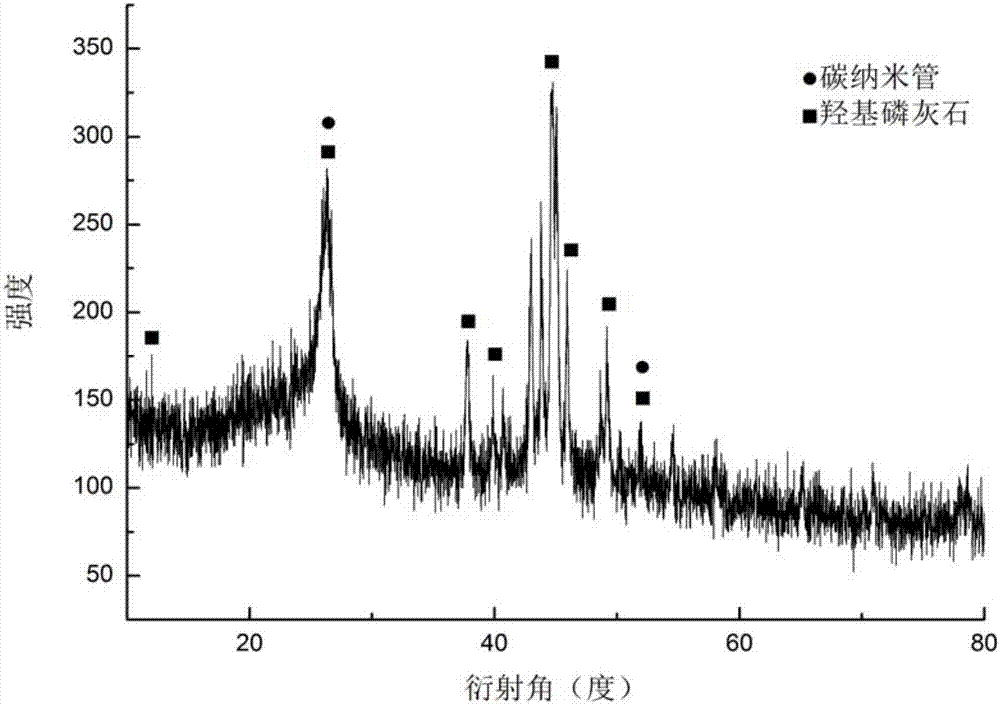
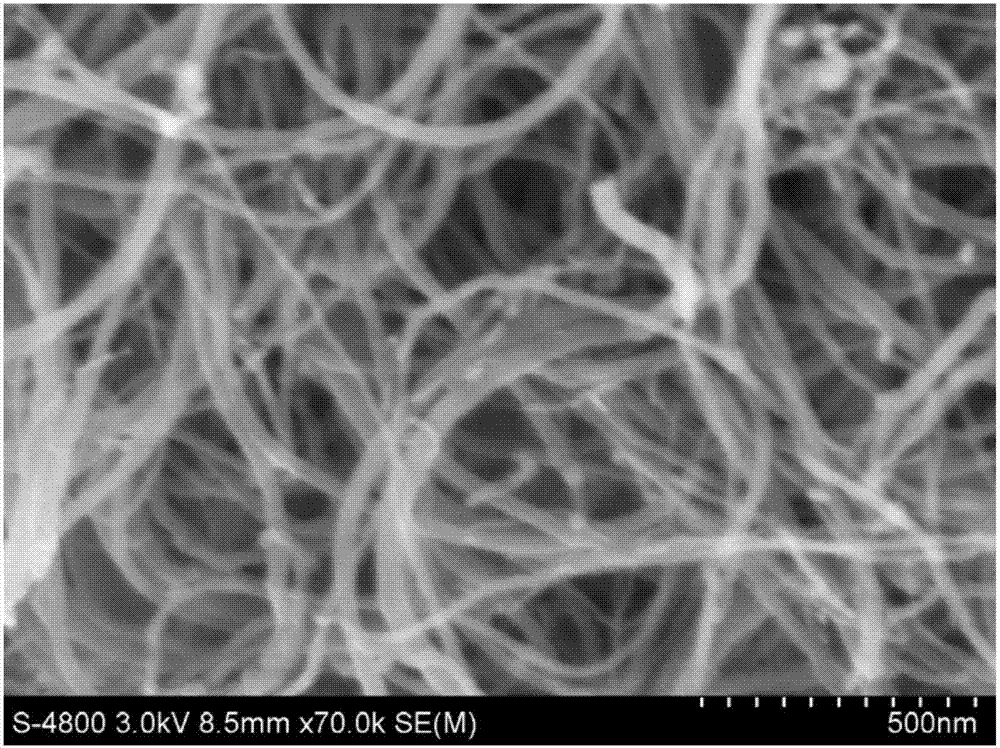
Preparation method of collagen cladded carbon nano-tube composite material
A technology of composite materials and carbon nanotubes, which is applied in the direction of carbon nanotubes, nanocarbons, carbon compounds, etc., can solve problems such as difficulty in achieving uniform dispersion of components, poor drug loading and drug release capabilities, and poor biocompatibility. High dosage, avoid agglomeration phenomenon, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0029] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 10nm at a mass ratio of 0.55:1, and mix the weighed The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.01mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 2h, ferric chloride is uniformly impregnated in hydroxyapatite to obtain suspension I, suspension I: 25% (mass percentage) ammonia water=100:1 in volume ratio, add 25% (mass percentage) to above suspension I percentage) of ammonia water, and continue to stir for 1 hour to obtain suspension II, place the formed suspension II in an ultrasonic disperser, and disperse ultrasonically at a frequency of 20 kHz for 40 min to fully react ferric chloride and ammonia water to form Fe(OH...
Embodiment 2
[0039] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0040] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 40nm in a mass ratio of 1.2:1, and mix the weighed ferric chloride hexahydrate with a mechanical stirrer at a speed of 250r / min. The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.15mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 3h, ferric chloride is uniformly impregnated in the hydroxyapatite to obtain the suspension I, the suspension I: 25% (mass percentage) ammonia water=60:1 in the volume ratio, add 25% (mass percentage) to the above suspension I percentage) of ammonia water, and continue to stir for 2.5 hours to obtain suspension II. Place the formed suspension II in an ultrasonic disperser, and ultrasonically disperse...
Embodiment 3
[0046] The first step is to prepare carbon nanotube-hydroxyapatite composite powder:
[0047] Weigh the required mass of ferric chloride hexahydrate and hydroxyapatite particles with a particle size of 60nm according to the ratio of 1.75:1 in mass ratio, and under the condition of stirring at a speed of 400r / min with a mechanical stirrer, the weighed The hydroxyapatite particles were added to deionized water to form a hydroxyapatite suspension with a molar concentration of 0.3mol / L, and then the weighed ferric chloride hexahydrate was added to the above-mentioned hydroxyapatite suspension, and stirred 4h, ferric chloride is uniformly impregnated in hydroxyapatite to obtain suspension I, suspension I: 25% (mass percentage) ammonia water=20:1 in volume ratio, add 25% (mass percentage) to above-mentioned suspension I percentage) of ammonia water, and continue to stir for 4 hours to obtain suspension II, place the formed suspension II in an ultrasonic disperser, and disperse it by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com