Long-acting recombinant human granulocyte colony-stimulating factor and preparation method therefor and use thereof
A technology of colony-stimulating factor and granulocytes, applied in the field of long-acting recombinant human granulocyte colony-stimulating factor and its preparation, can solve the problems of low yield, increased protein molecular weight, high production cost, etc., and achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Construction of recombinant human granulocyte colony stimulating factor rhG-CSF and CTP fusion protein expression vector:
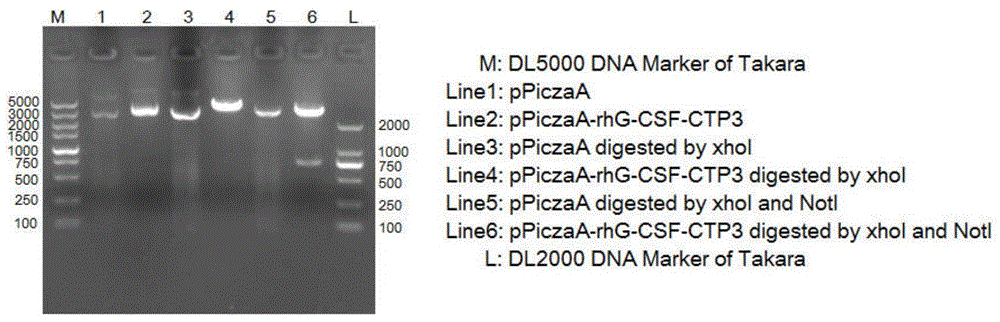
[0060] The recombinant human granulocyte colony stimulating factor rhG-CSF and CTP fusion protein coding gene sequence with restriction enzyme cut sites and signal peptides were artificially synthesized and cloned into pUC57simple plasmid vector to obtain a plasmid containing the fusion protein coding gene. (Completed by Nanjing KingScript Biotechnology Co., Ltd.). The 5'end of the plasmid fusion protein contains the XhoI restriction site and the signal peptide cleavage recognition site (i.e. 5’CTCGAGAAAAGA-); and the 3’ end contains the NotI restriction site (i.e. 3’GCGGCCGC-)
[0061] Take the pUC57simple-rhG-CSF-CTP3 plasmid and pPICZaA expression plasmid, and use XhoI and NotI restriction endonucleases for double digestion. The reaction system for double enzyme digestion is 20μL, in which plasmid 10μL, NotI1μL, XhoI1μL, 10XbufferH2μL, an...
Embodiment 2
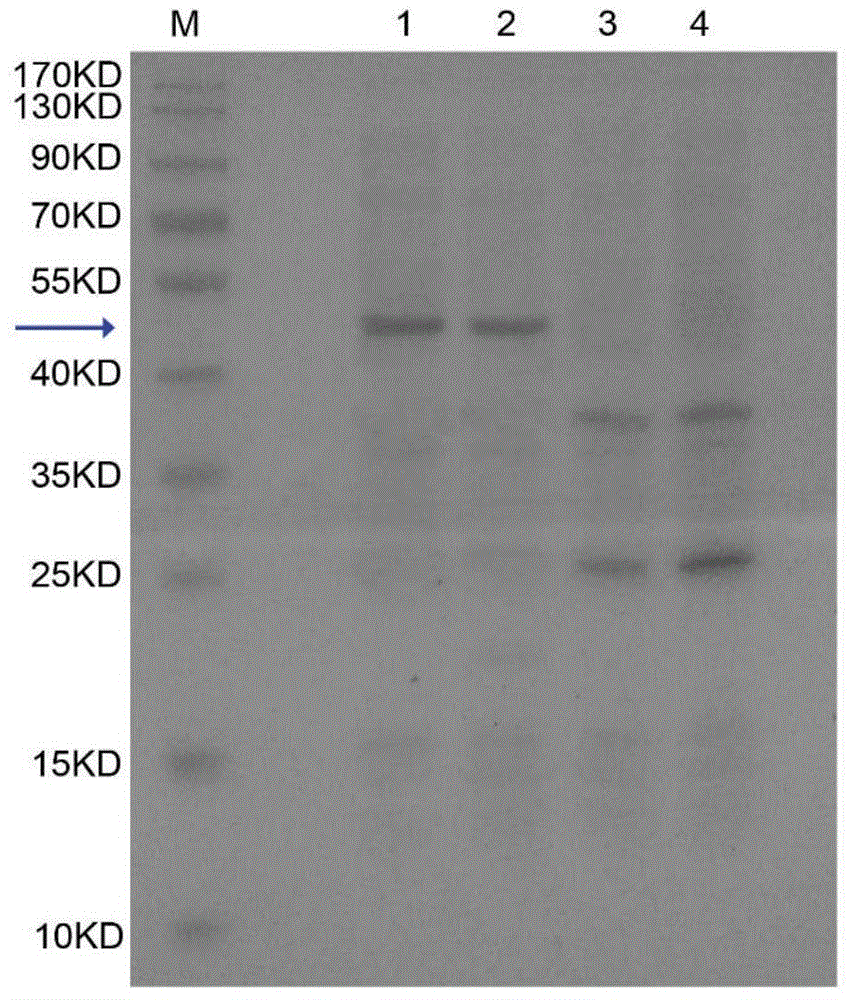
[0064] Example 2 Expression of recombinant human granulocyte colony stimulating factor rhG-CSF and CTP fusion protein
[0065] First, prepare competent Pichia pastoris GS115 cells. The specific steps are as follows: first pick out the GS115 monoclonal colony in 3mL YPD medium, and incubate for 48h at a constant temperature of 30°C at a rotation speed of 180rpm. Then, the cultured Pichia pastoris GS115 cells were transferred into 100 mL of YPD medium with an inoculum of 1:200, and cultured to O.D.600=1.2. Centrifuge at 4000 rpm / min 4°C for 5 minutes, discard the supernatant, collect the precipitate and resuspend it twice with 1mol / L sorbitol solution, centrifuge at 4000 rpm / min 4°C for 10 minutes, and resuspend the precipitate with 100 μL ice-cold sorbitol solution. Prepare competent Pichia pastoris GS115 cells.
[0066] Secondly, the recombinant human granulocyte colony stimulating factor rhG-CSF and CTP fusion protein expression vector pPICZaA-rhG-CSF-CTP3 was sequenced and linea...
Embodiment 3
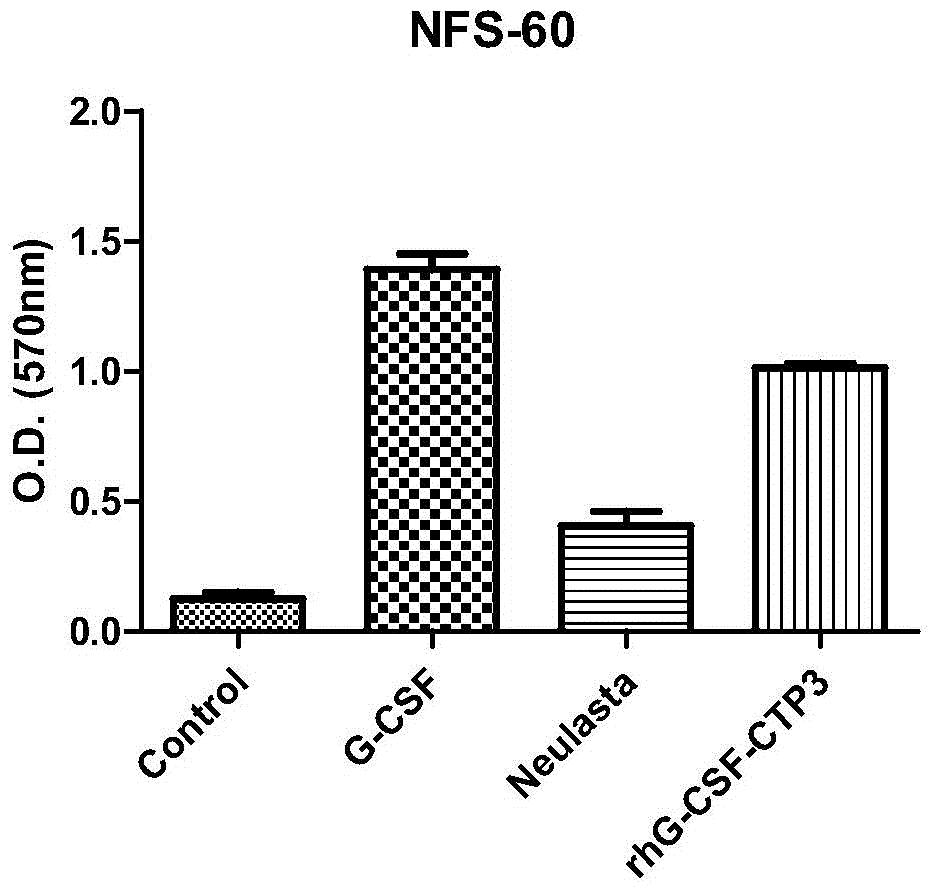
[0069] Example 3 In vitro activity determination of recombinant human granulocyte colony stimulating factor rhG-CSF and CTP fusion protein
[0070] In this example, in accordance with the Pharmacopoeia of the People's Republic of China (2010 Edition, Part III), the G-CSF-dependent cell line NFS60 was selected, and the biological activity was measured by the MTT method. The specific steps are as follows: FS60 cell line with complete culture medium at 37℃, 5% CO 2 Culture, control the cell concentration to 1*10 per milliliter 5 Cells are used for the assay. Take a sufficient amount of NFS60 cell culture, collect the cells by centrifugation, wash twice with RPMI1640 culture medium, and then resuspend in the basic culture medium (RPMI1640900ml + newborn calf serum 100ml) to make 2*10 per ml 5 Cell suspension of two cells. Add 50μL of cell suspension to each well of a 96-well plate with standard and test products, at 37℃, 5% CO 2 Cultivate for 72 hours. Add 20μL of MTT solution to ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com