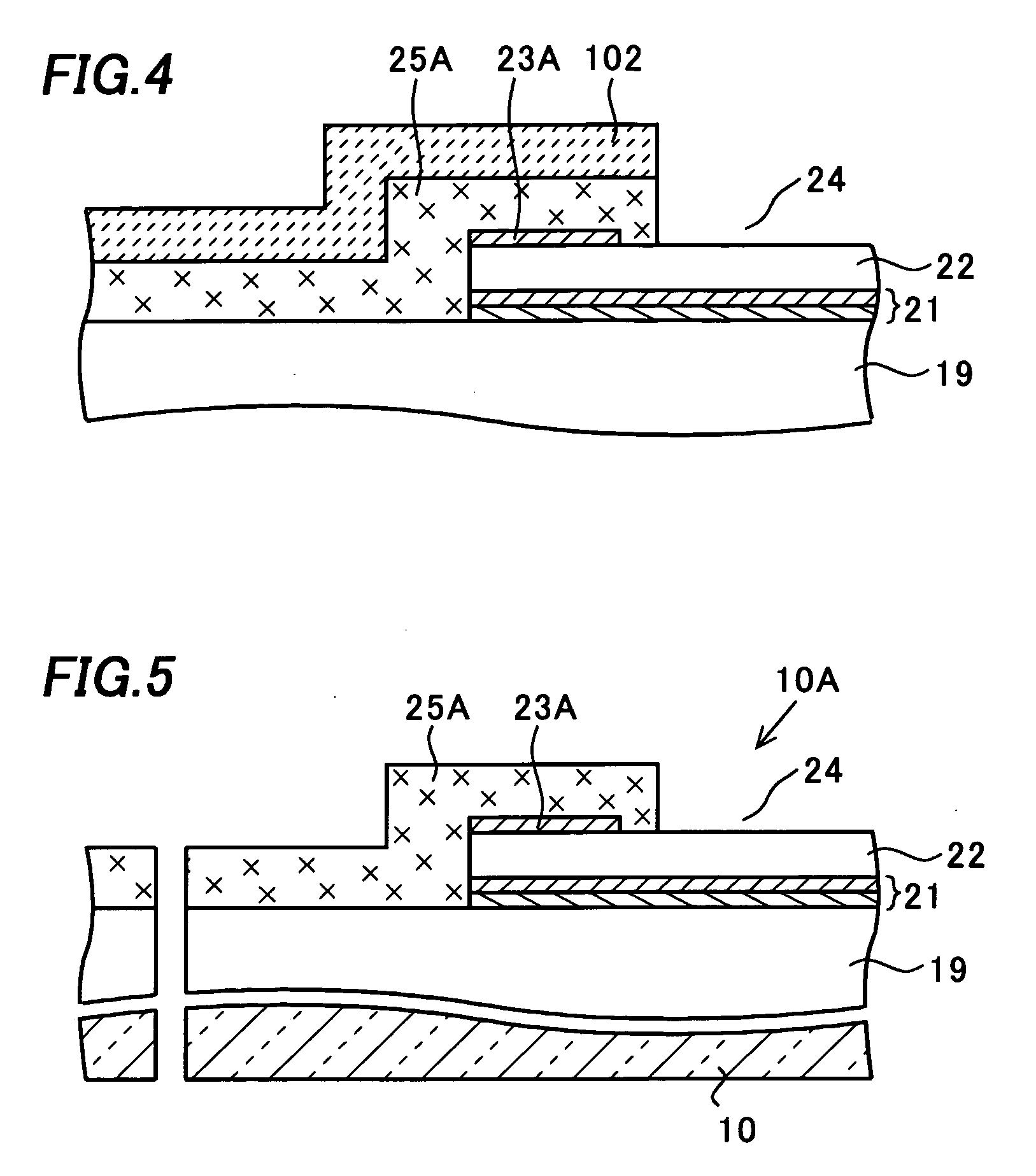
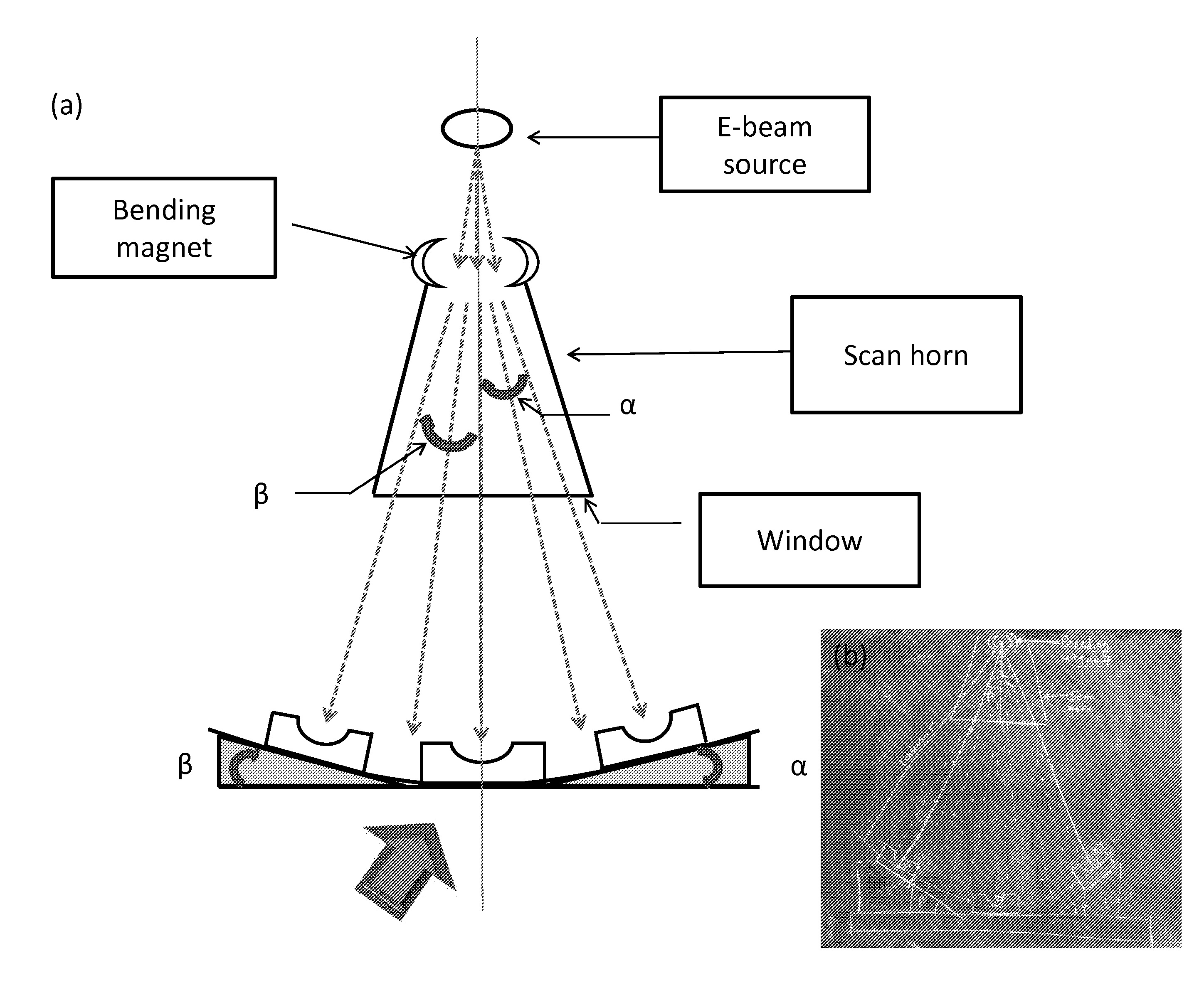
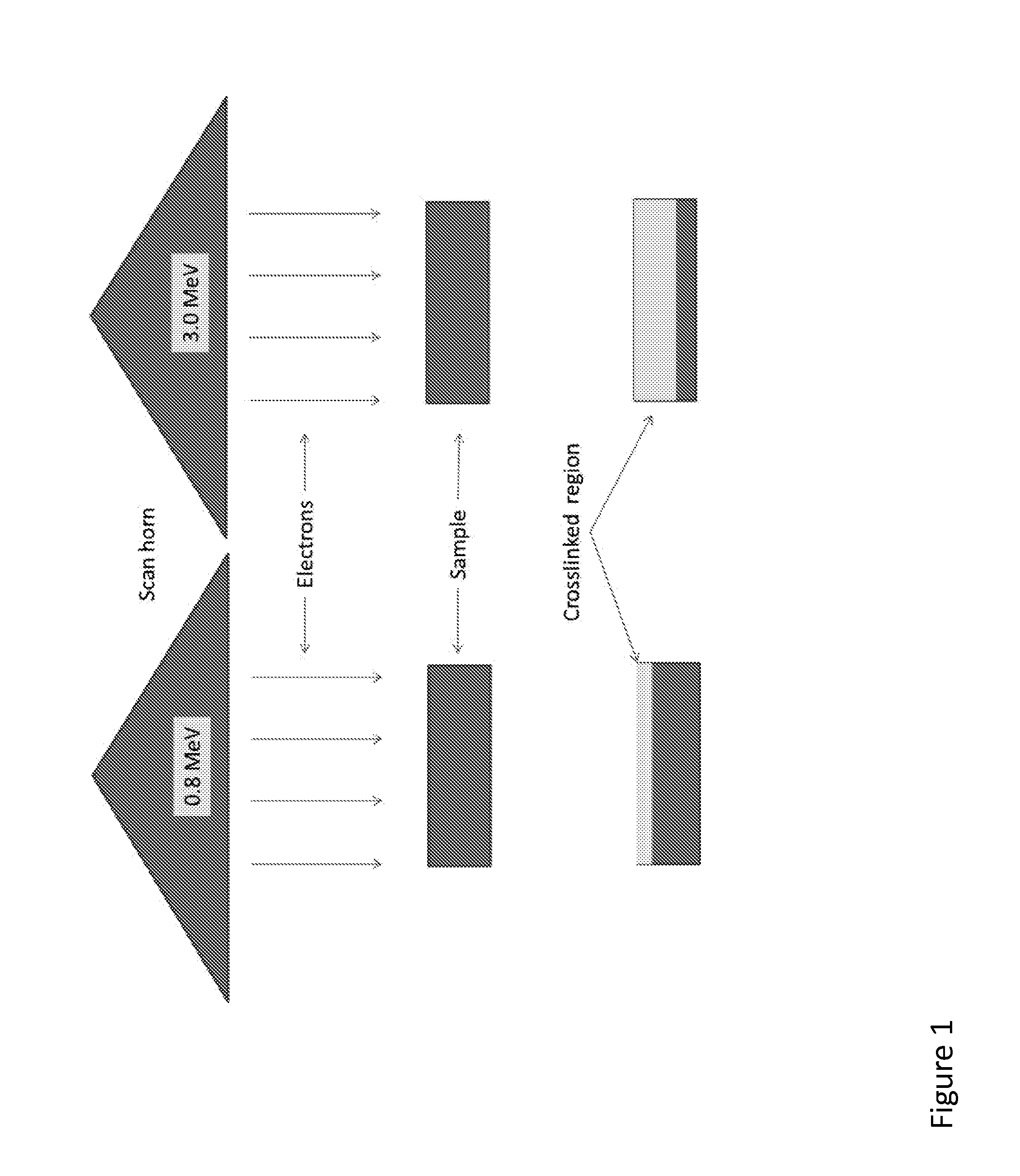
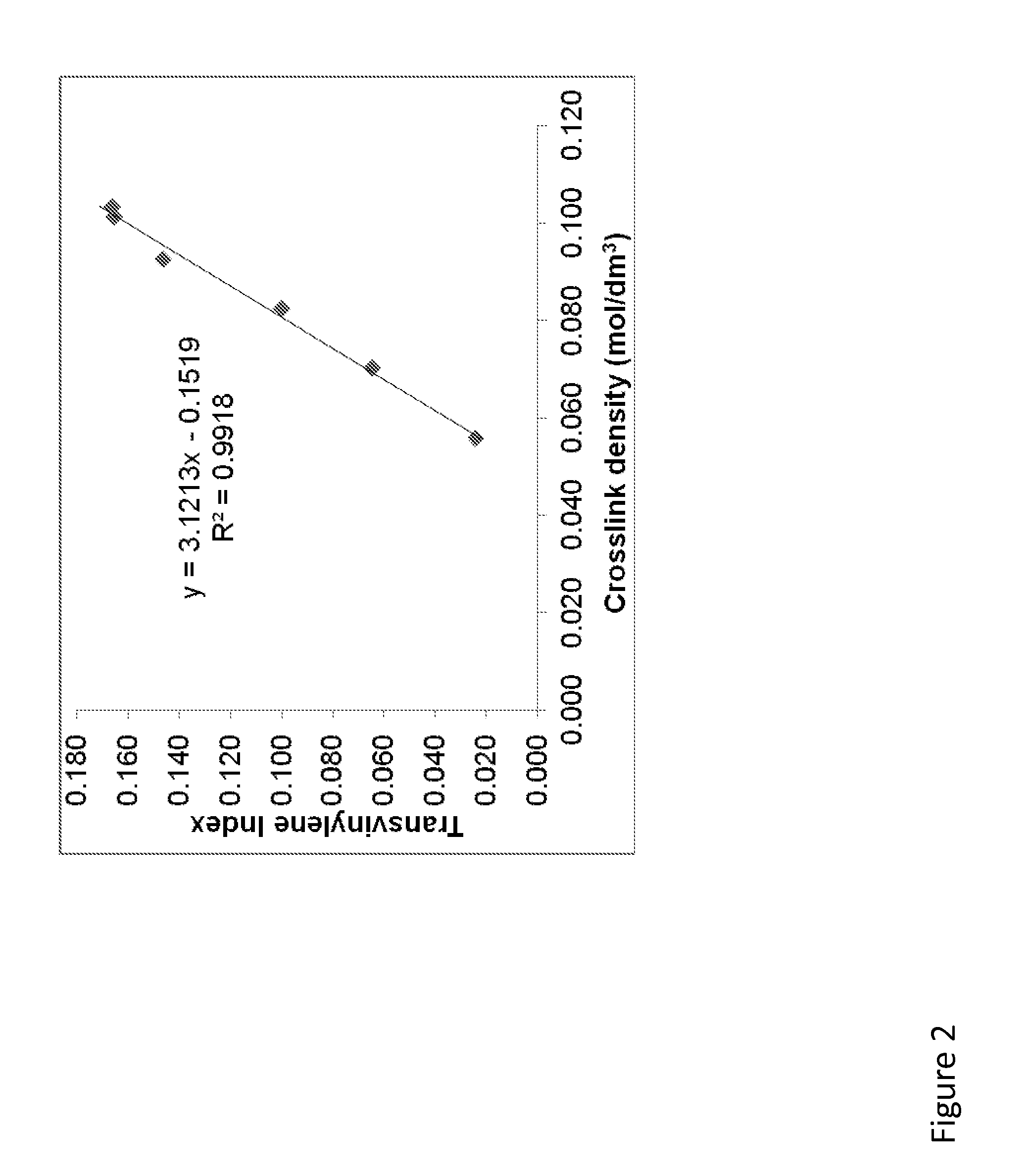
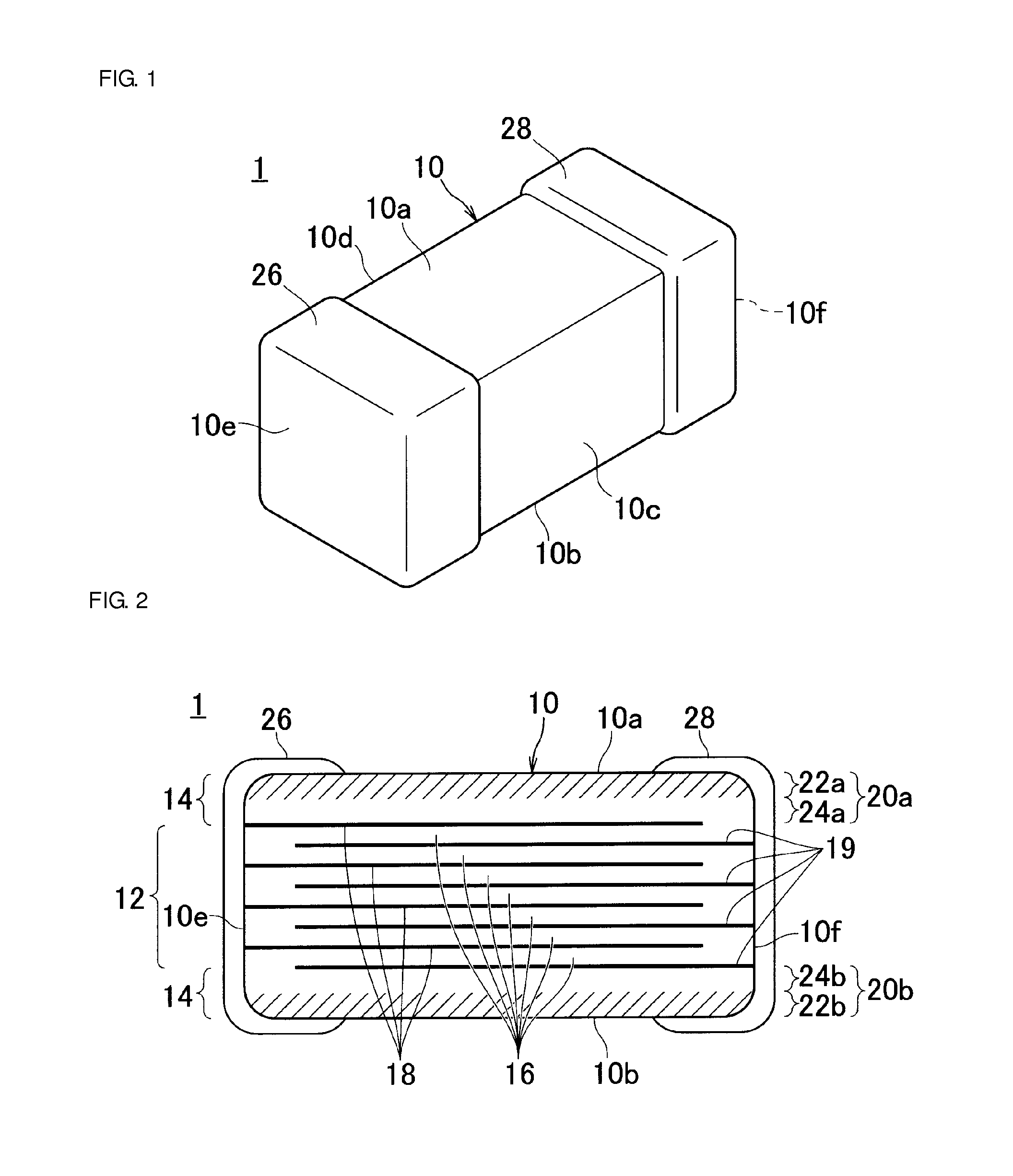
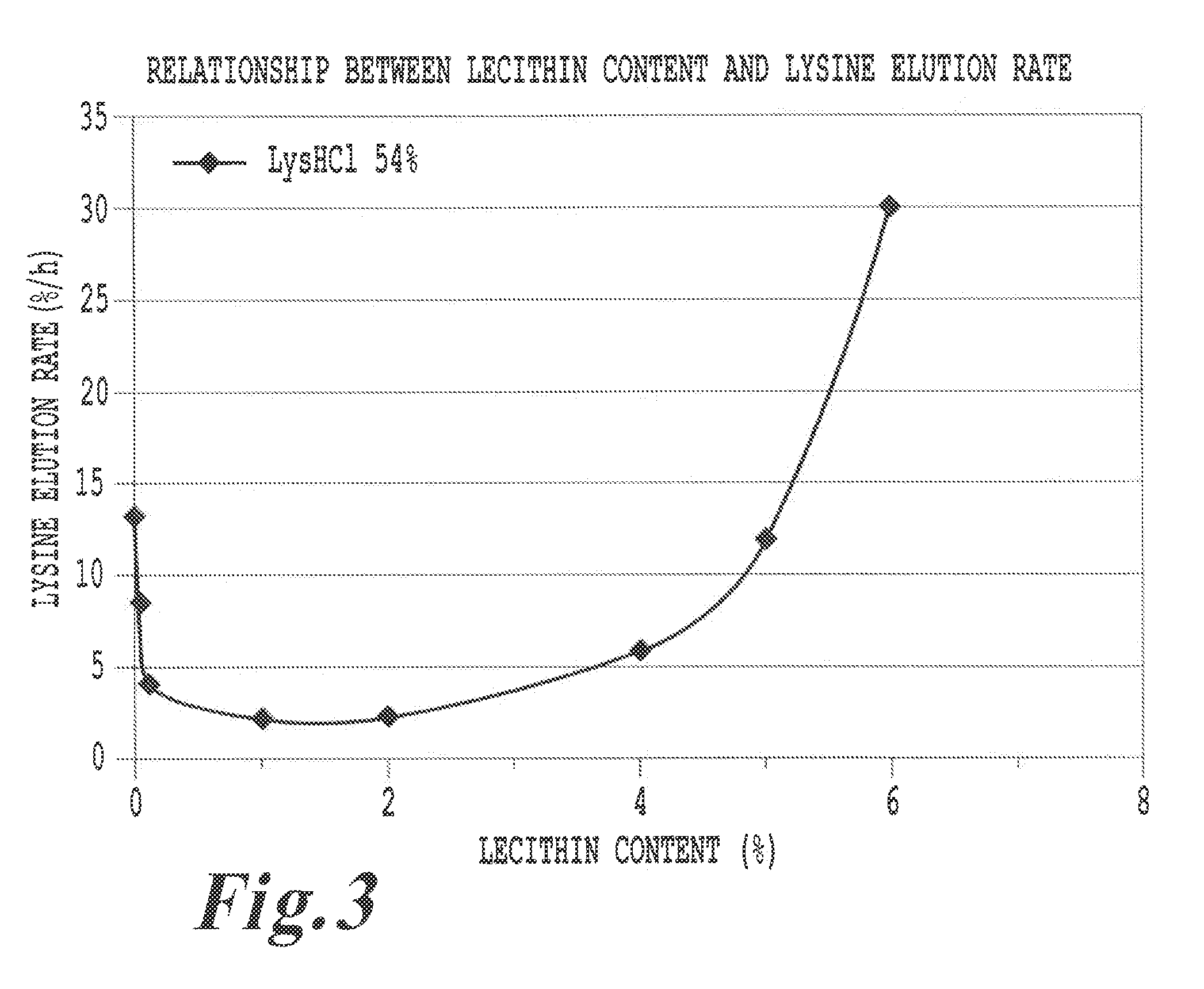Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
277results about How to "Prevent elution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
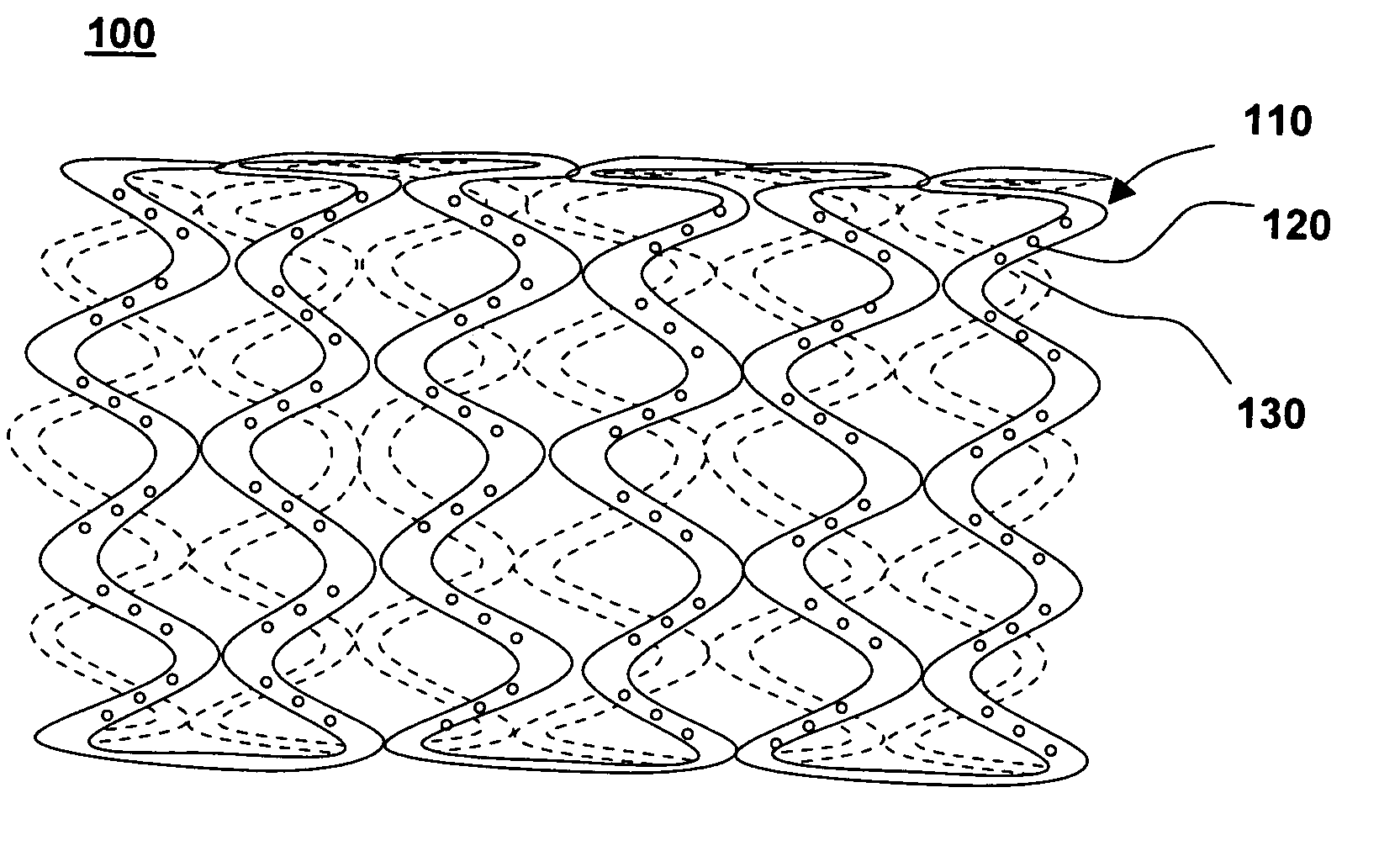
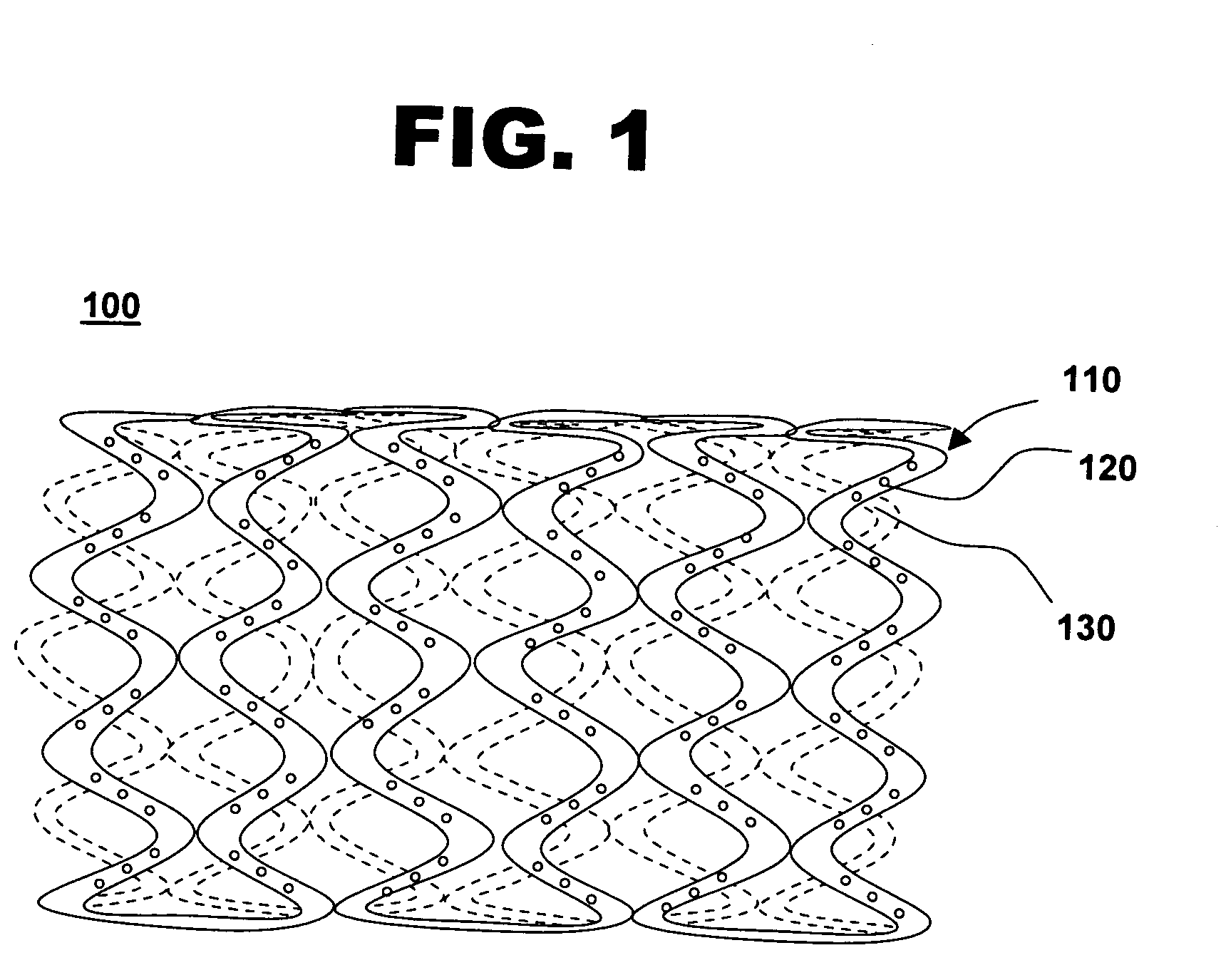
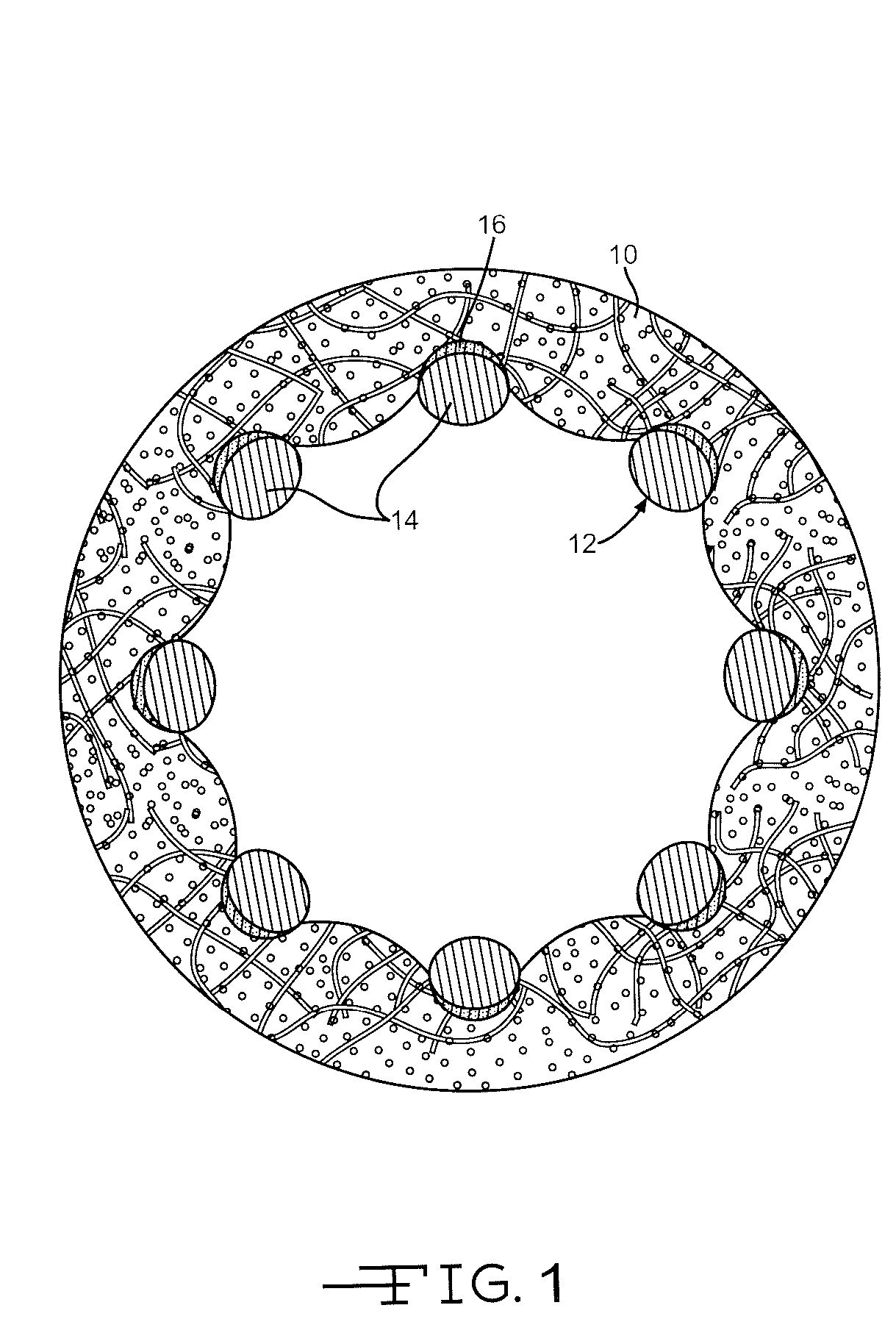
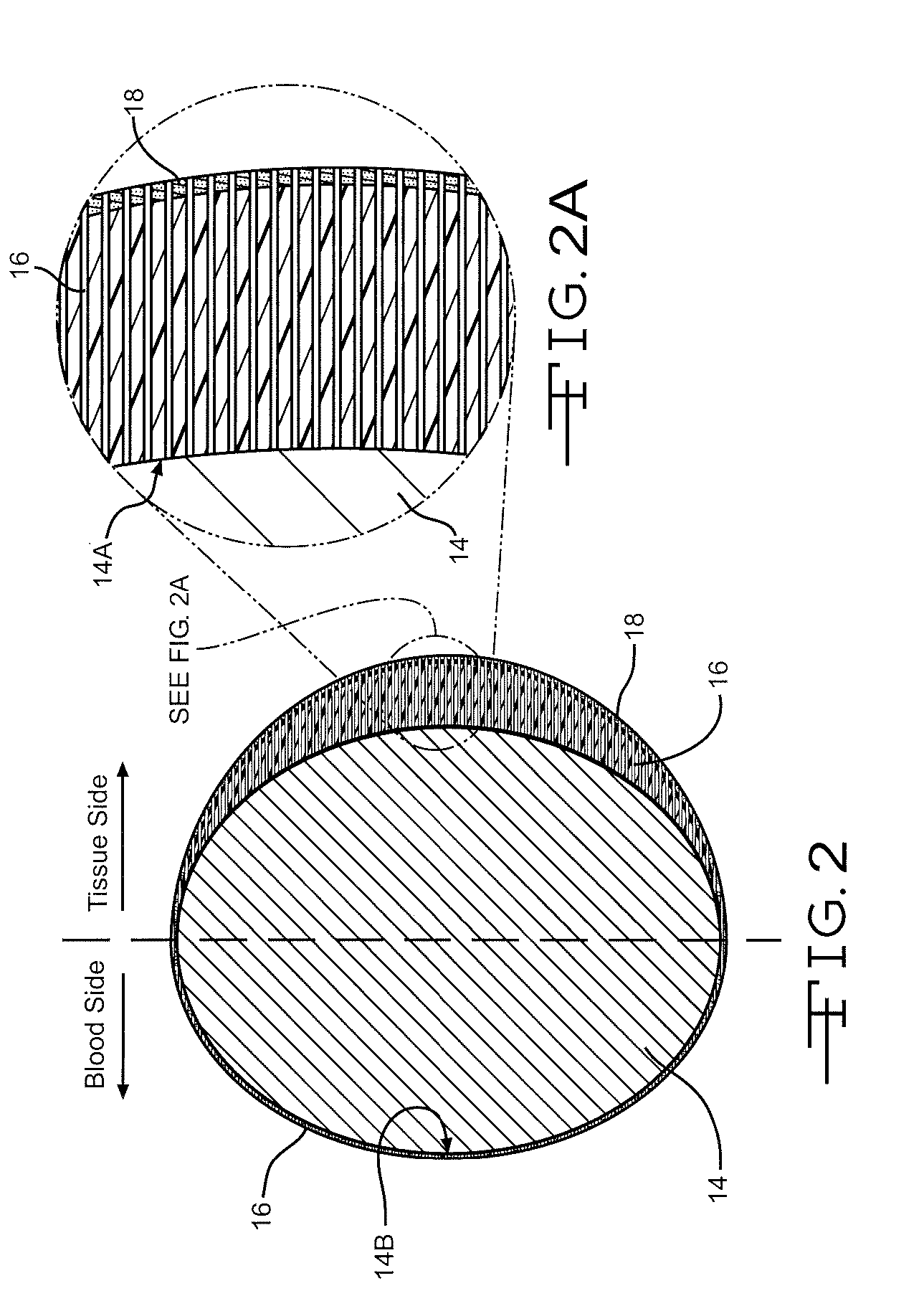
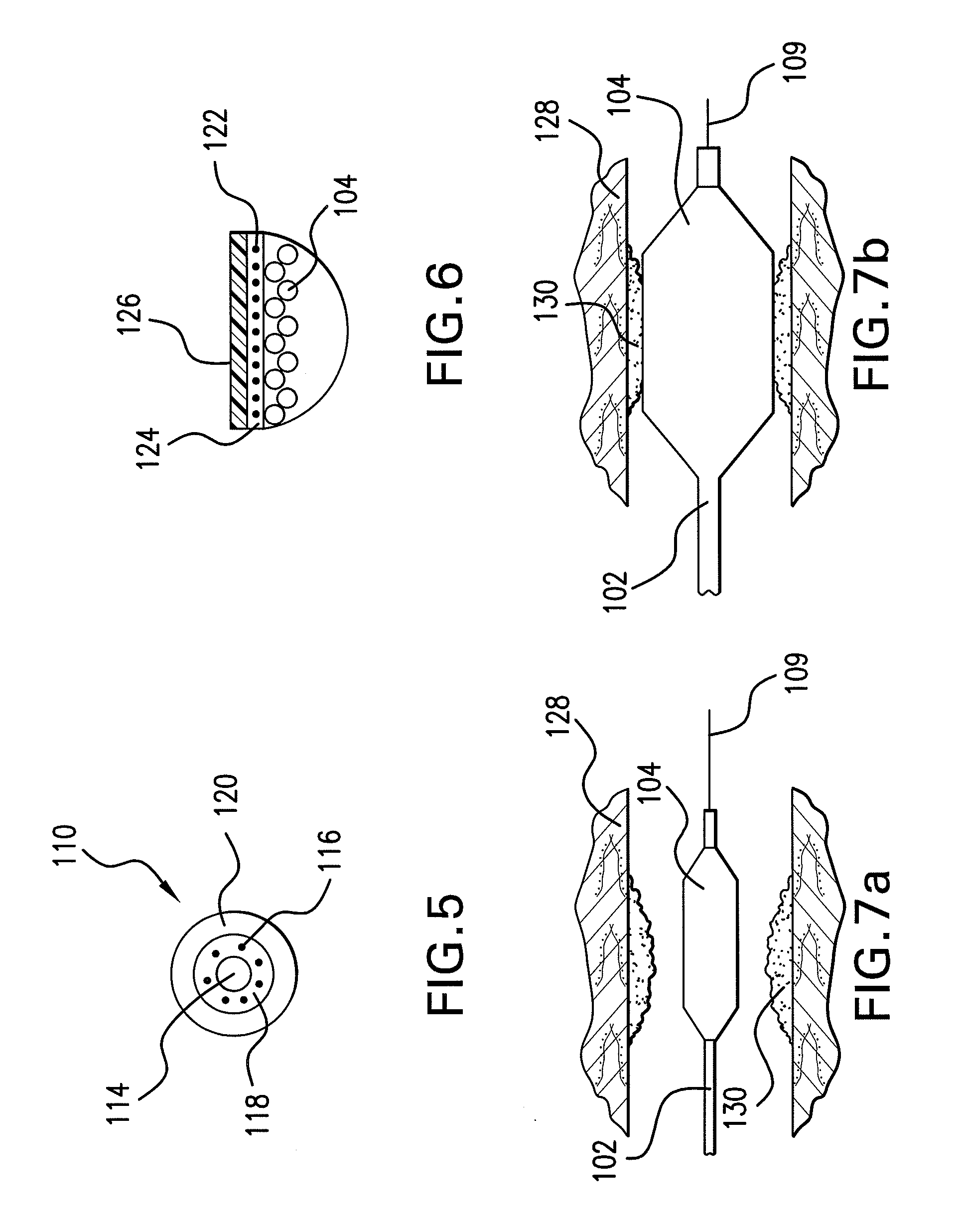
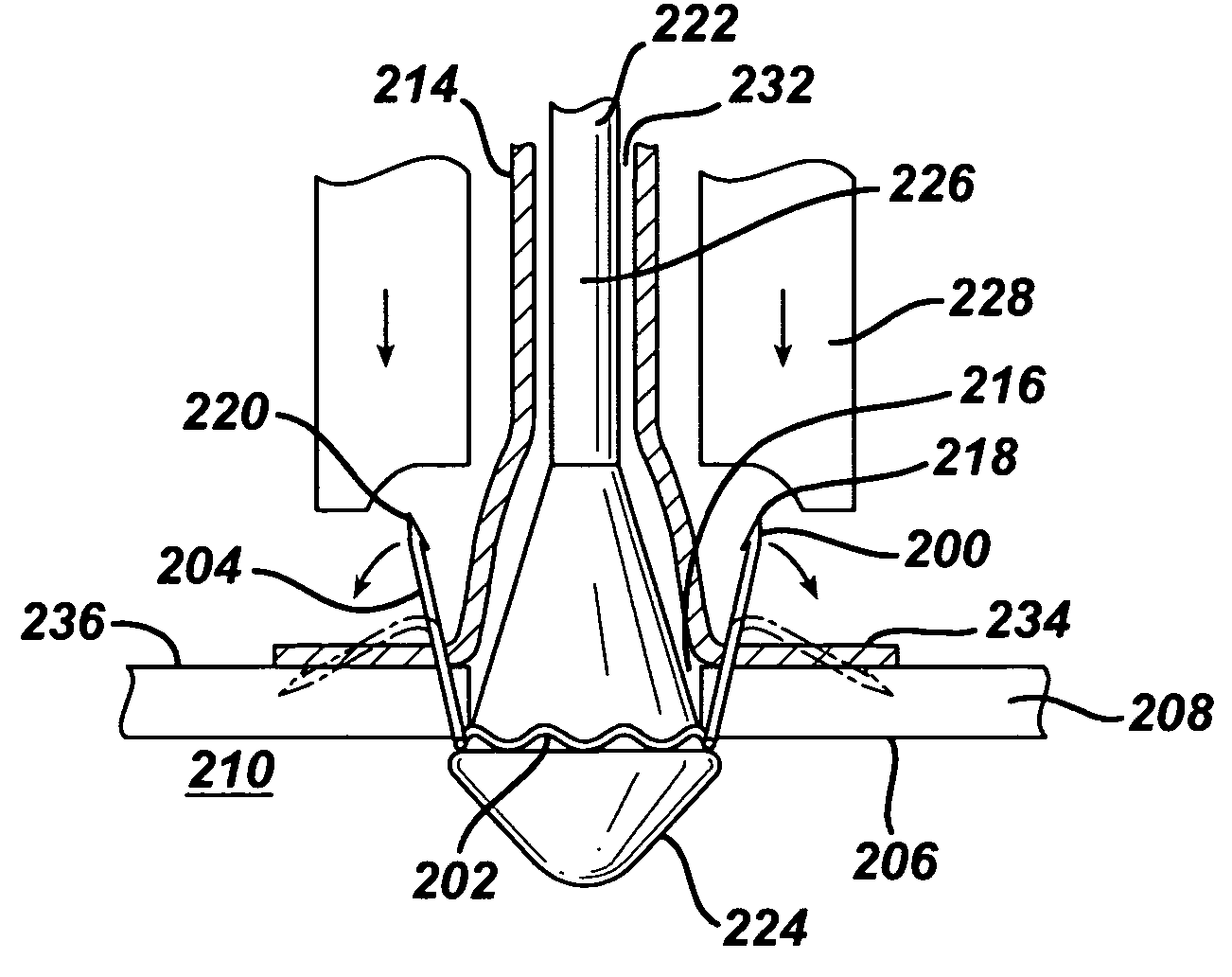
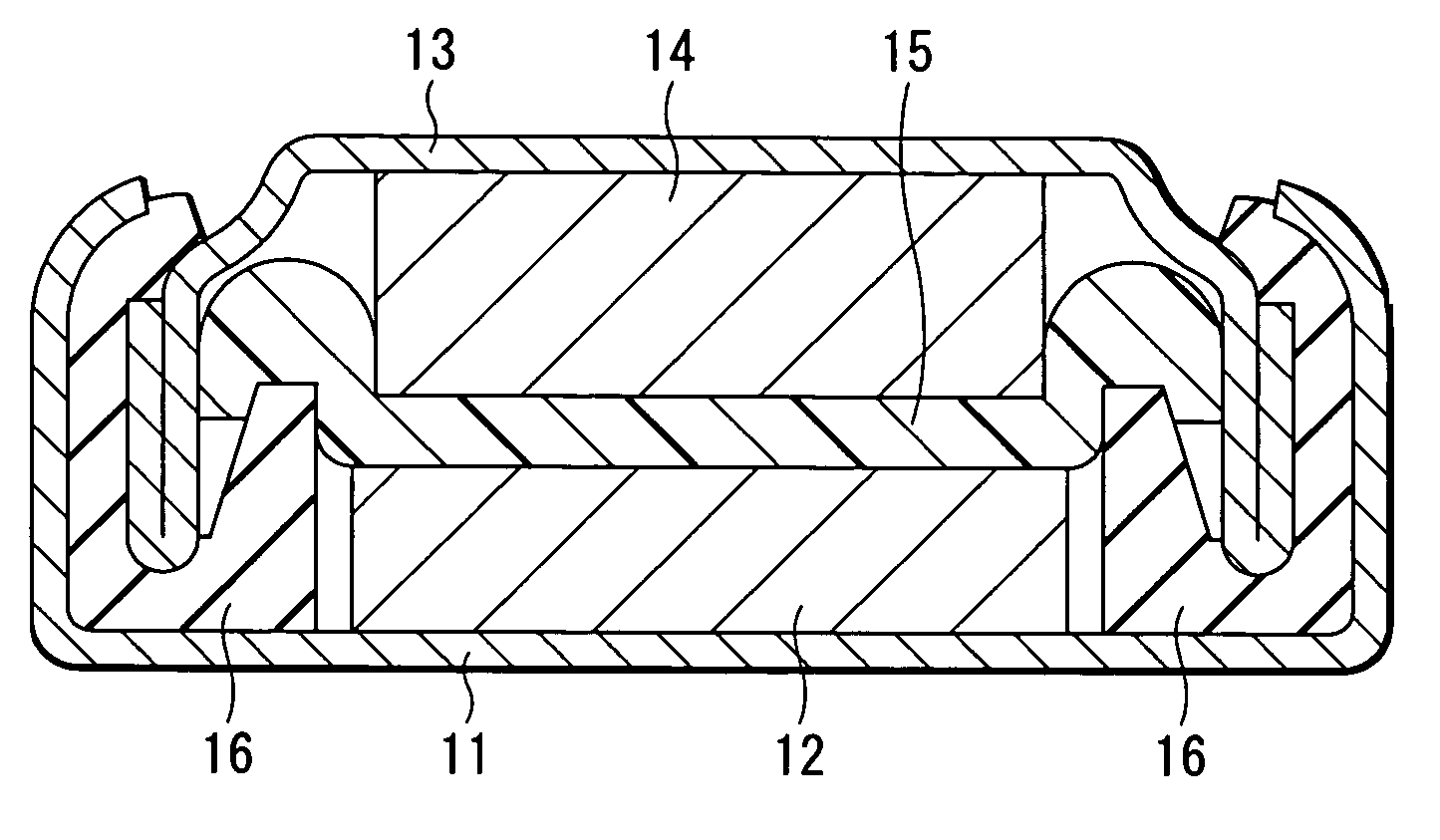
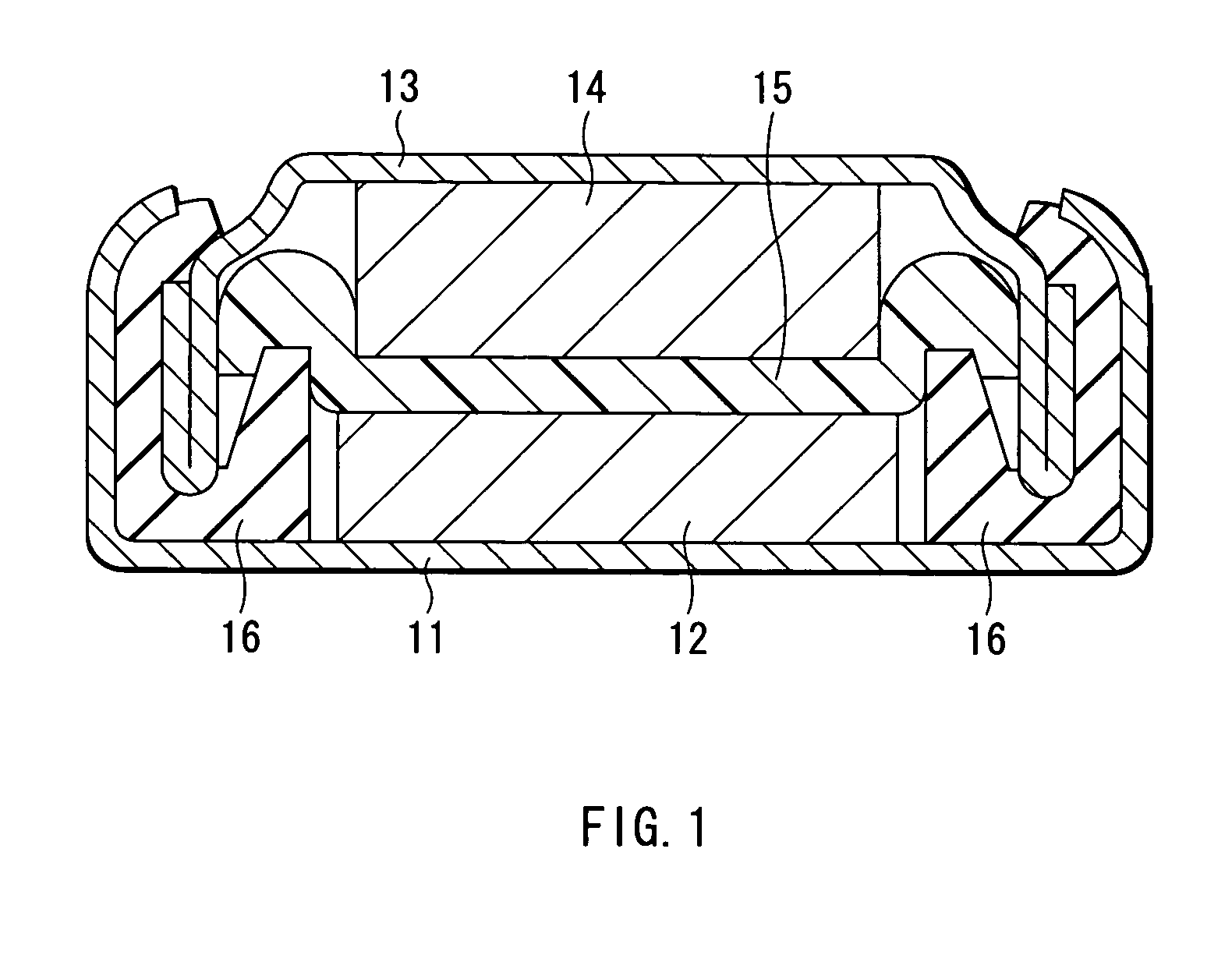
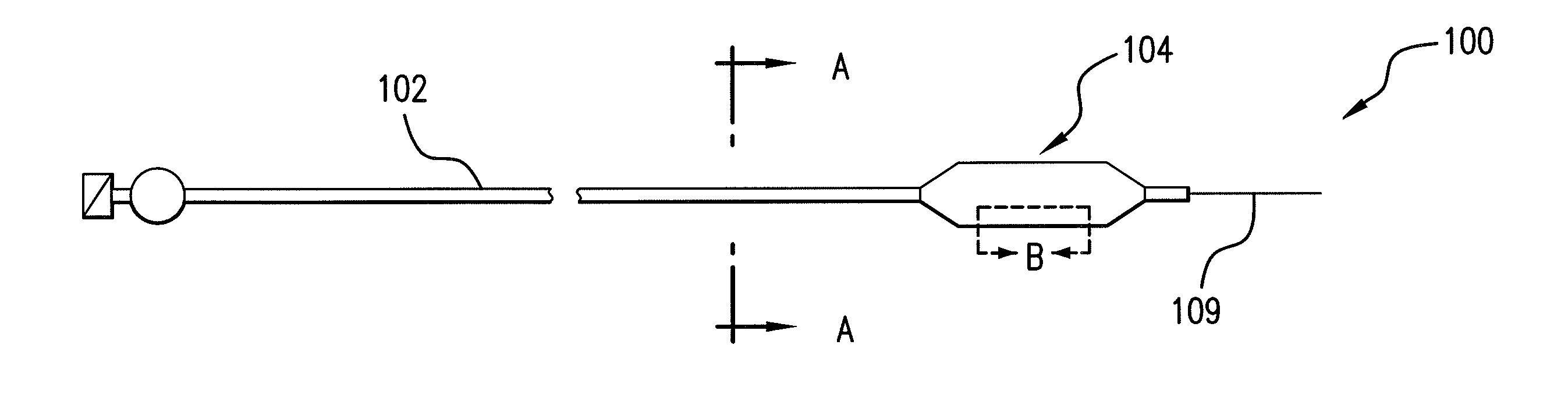
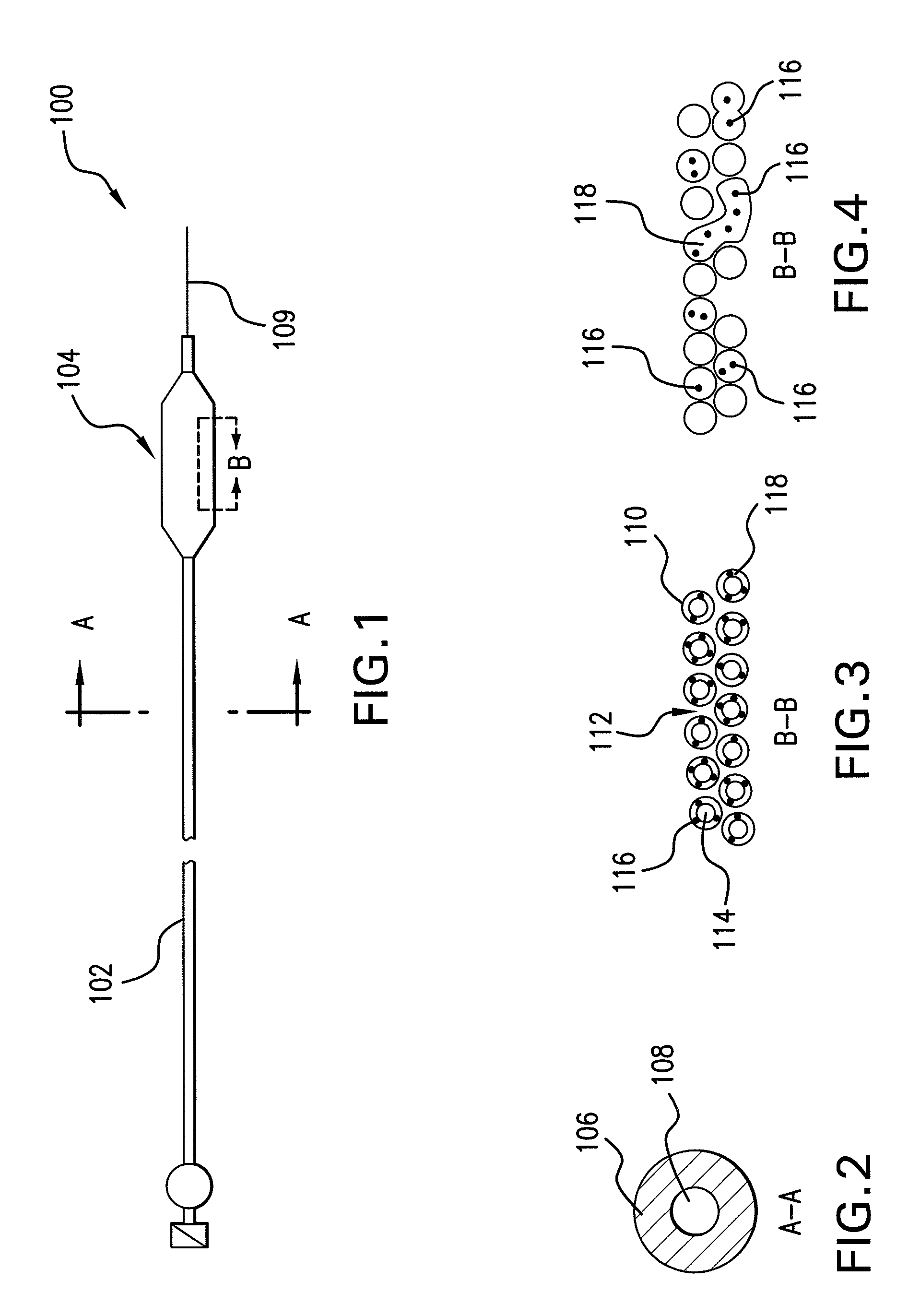
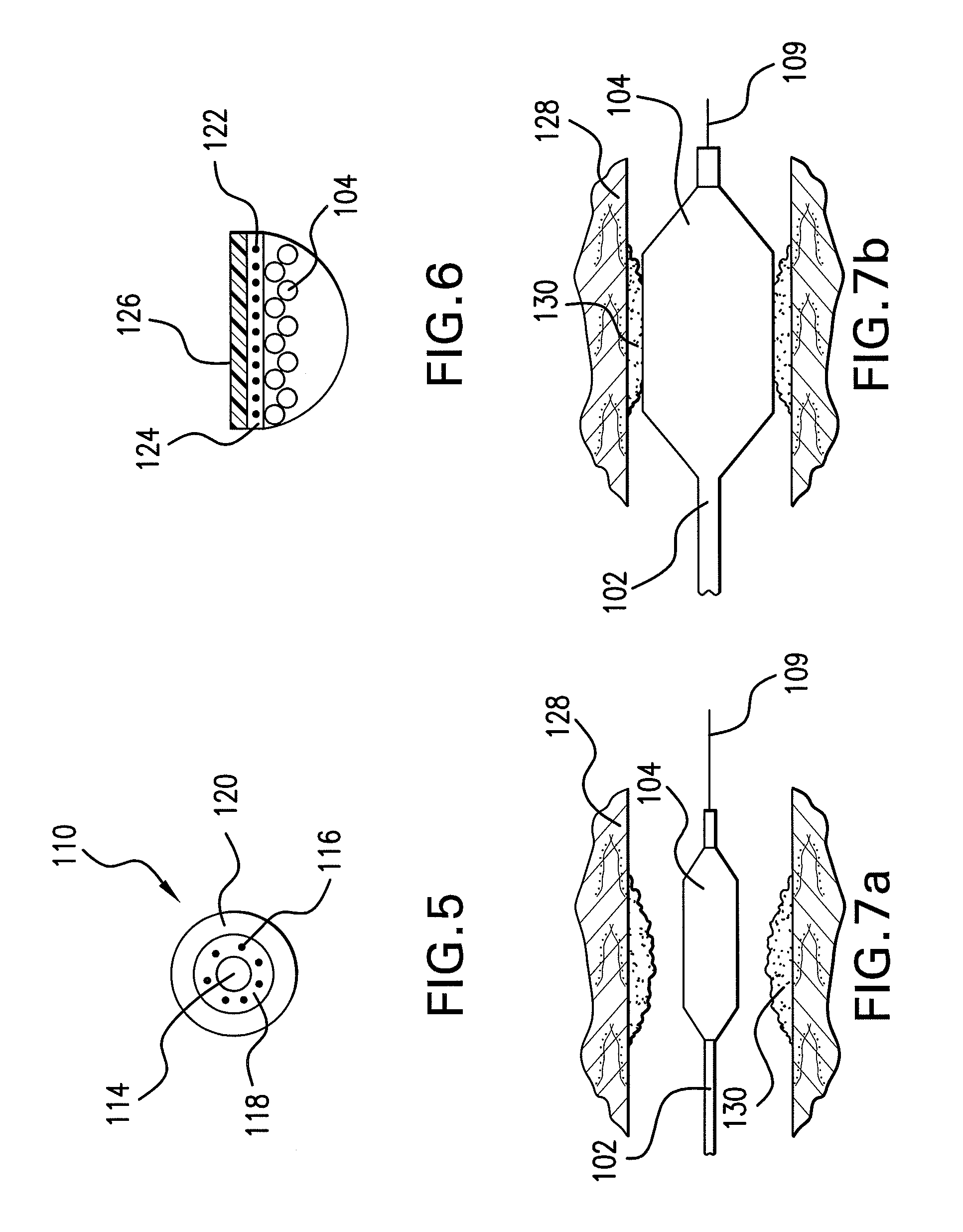
Drug-eluting stent for controlled drug delivery
The present invention provides a stent for delivering drugs to a vessel in a body, including a stent framework with a plurality of micropore reservoirs formed therein using a femtosecond laser, a drug polymer positioned in the reservoirs, and a polymer layer positioned on the drug polymer. The present invention also provides a method of manufacturing a drug-polymer stent and a method of treating a vascular condition using the drug-polymer stent.
Owner:MEDTRONIC VASCULAR INC
Stent Coating For Eluting Medication
InactiveUS20060200231A1Good biocompatibilityPrevention and therapyStentsSurgeryPorosityDiamond-like carbon
A vascular stent comprising a drug-eluting outer layer of a porous sputtered columnar metal having each column capped with a biocompatible carbon-containing material is described. This is done by placing the stent over a close-fitting mandrel and rotating the assembly in a sputter flux. The result is a coating that is evenly distributed over the outward-facing side of the stent's wire mesh while preventing the sputtered columnar coating from reaching the inward facing side where a smooth hemocompatible surface is required. The stent is then removed from the mandrel, exposing all surfaces, and finally coated with a layer of carbon such as amorphous carbon or diamond-like carbon. The carbonaceous coating enhances biocompatibility without preventing elutriation of a therapeutic drug provided in the porosity formed between the columnar structures. The result is a stent that is adapted to both the hemodynamic and the immune response requirements of its vascular environment.
Owner:WILSON GREATBATCH LTD
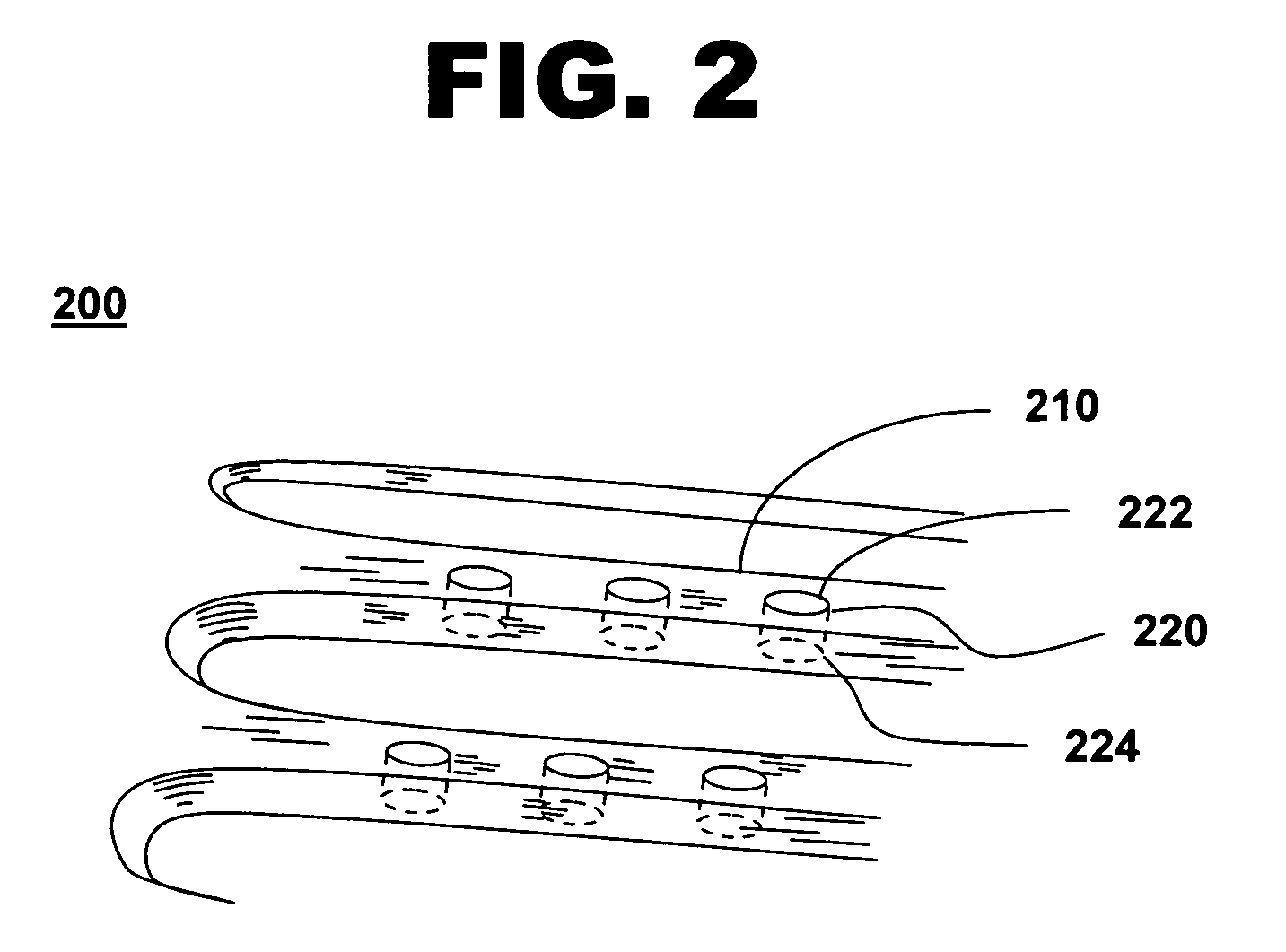
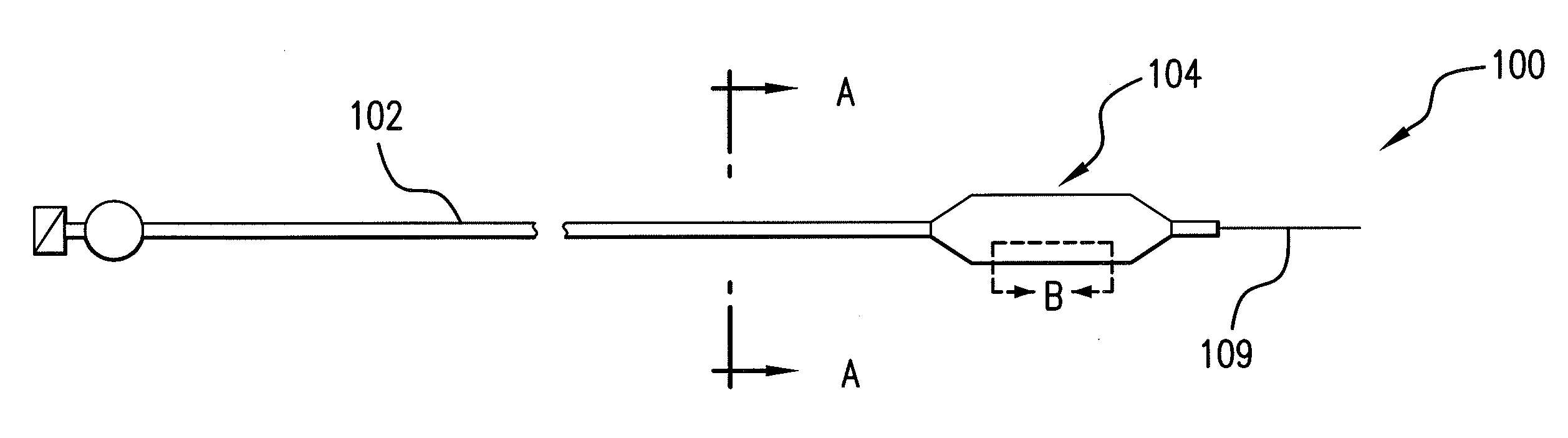
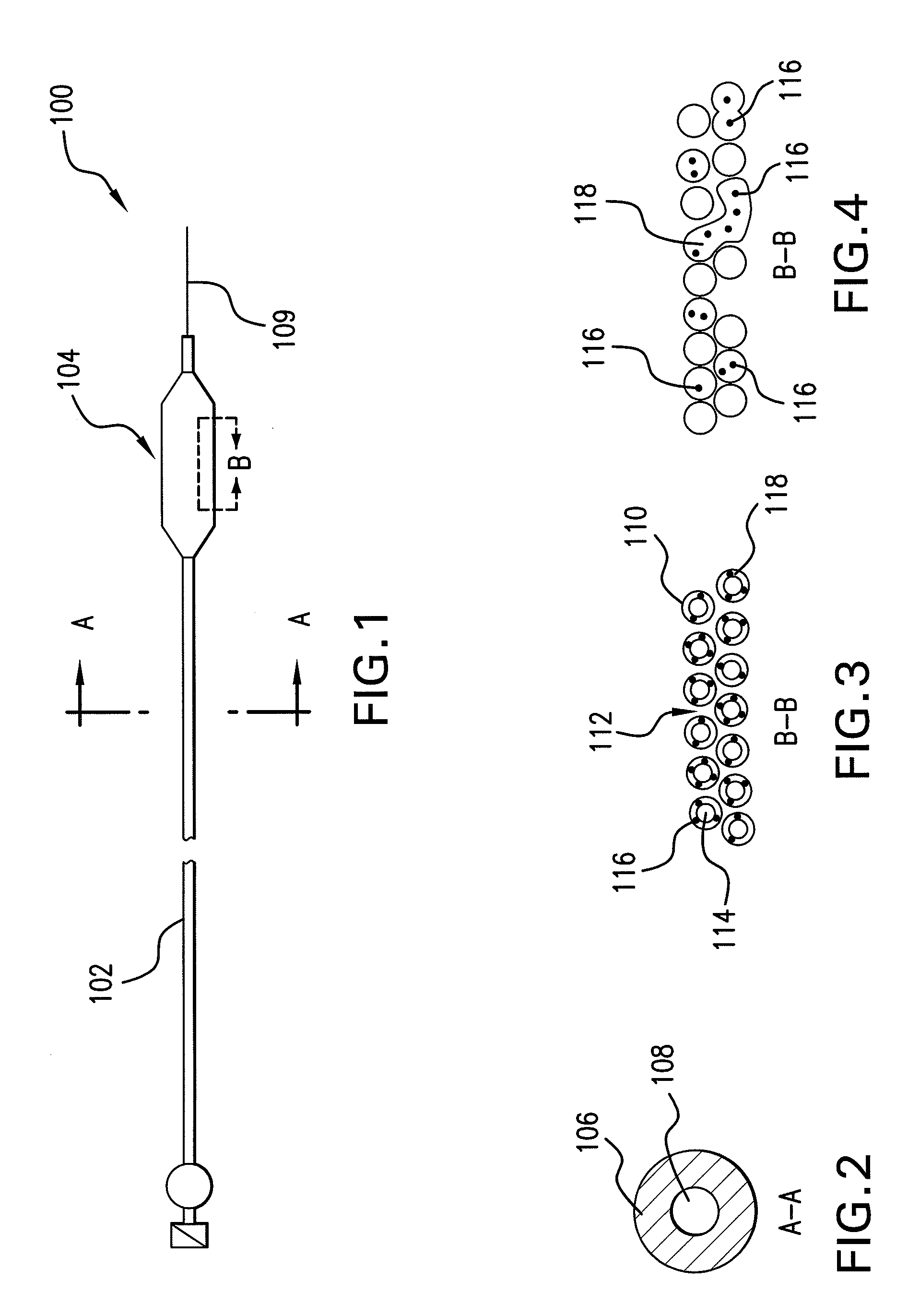
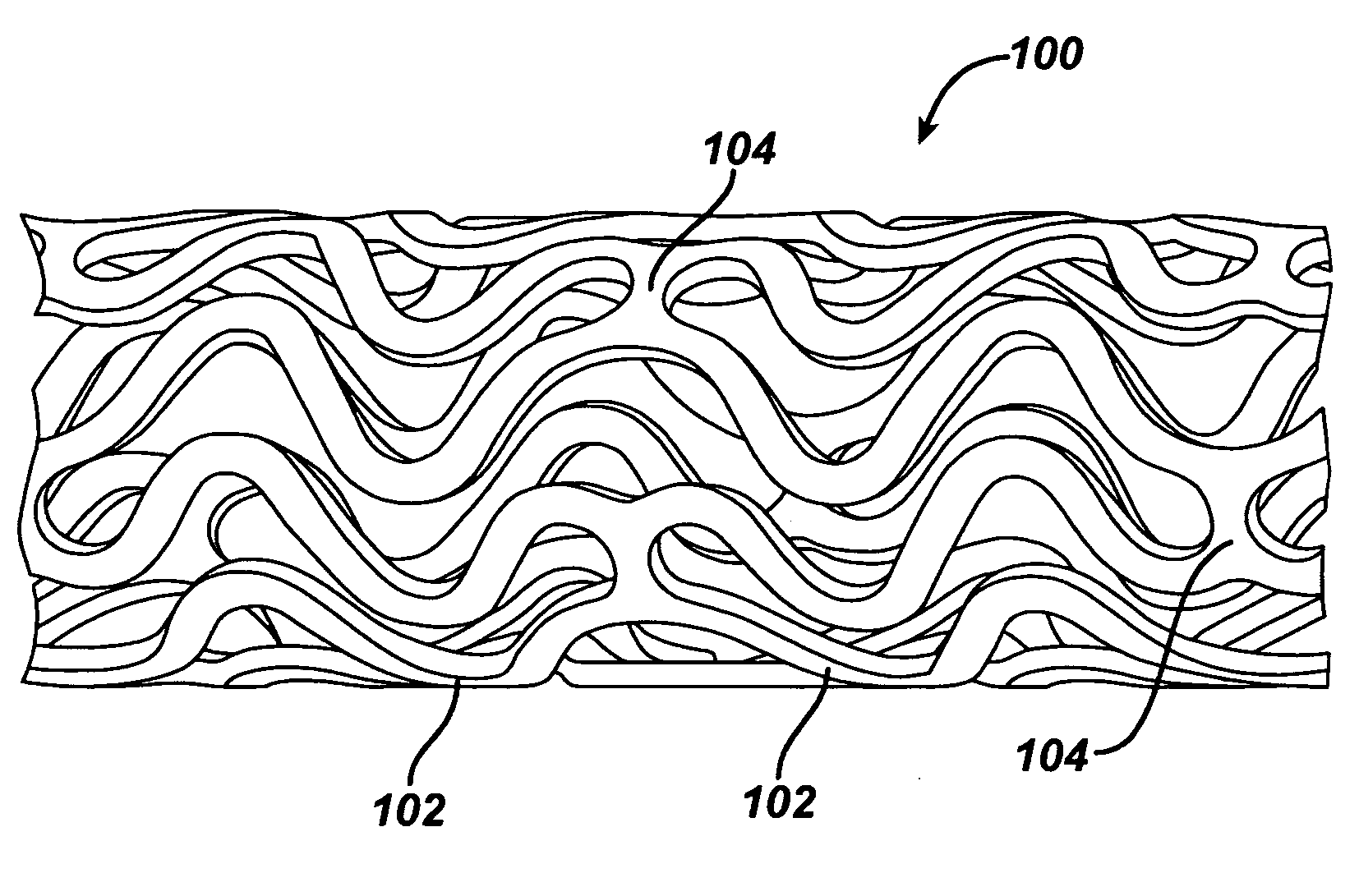
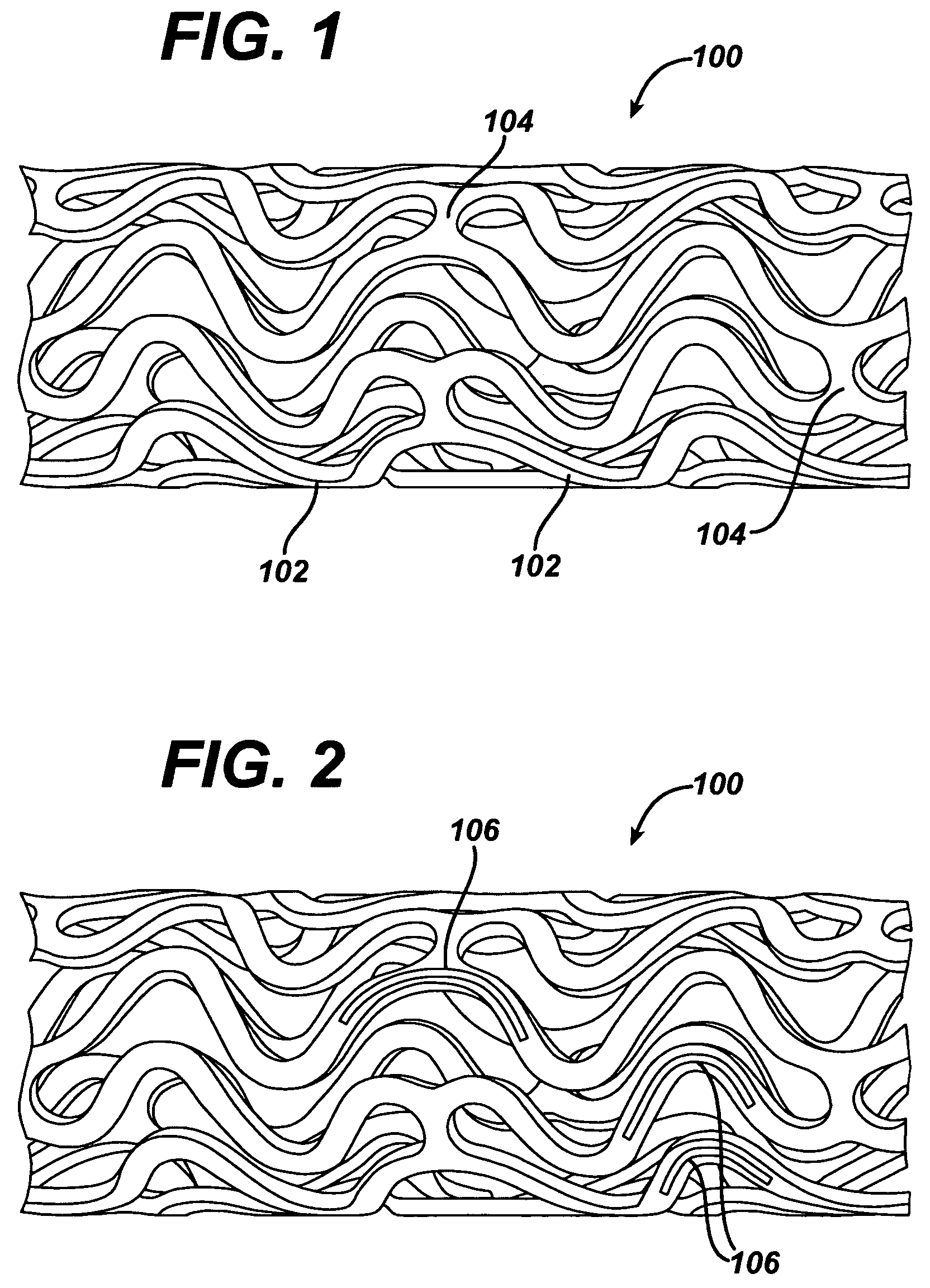
Expandable Member Formed Of A Fibrous Matrix Having Hydrogel Polymer For Intraluminal Drug Delivery
An intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent incorporated therein. The hydrogel polymer can be coated on the fiber elements in a co-axial configuration. The fiber elements may also have a second coating including a protective substance surrounding the hydrogel polymer having a therapeutic agent therein. The matrix of fiber elements can be formed by electrospinning. A process of delivering a therapeutic agent to a target site includes providing an intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent dispersed therein, and advancing the catheter device at a desired treatment site. Once at the desired treatment site, fluid is introduced into the inflation lumen to expand the expandable member from a first profile to a second profile, and the therapeutic agent is released from the hydrogel polymer and delivered to the desired treatment site.
Owner:ABBOTT CARDIOVASCULAR
Ophthalmic lenses capable of sustained drug release and preservative solutions therefor
ActiveUS20060187410A1Prevent elutionStay in shapePowder deliveryEye treatmentHydrophilic monomerMethacrylate
An object of the present invention is to provide a practical ophthalmic lens which has an effect of effectively retaining and sustainedly releasing a drug and has form stability before and after release of the drug, wherein the ionic polymer gel having sustained drug releasability can regulate the amount of the drug included therein, depending on the efficacy of the drug used, and storing solution for a practical ophthalmic lens. The present invention relates to a drug delivery ophthalmic lens comprising a cationic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer having a hydroxyl group in its molecule, at least one member selected from specific phosphate group-containing methacrylates a monomer having a nitrogen atom in its side chain, and a monomer copolymerizable with these components, and also relates to a drug delivery ophthalmic lens comprising an anionic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer, cationic and anionic monomers, and a monomer copolymerizable with these components, wherein the copolymer contains the anionic monomer in a ratio of 30 to 90 mol % to the cationic monomer, and also relates to storing solution for a practical ophthalmic lens.
Owner:VIEWDLE INC
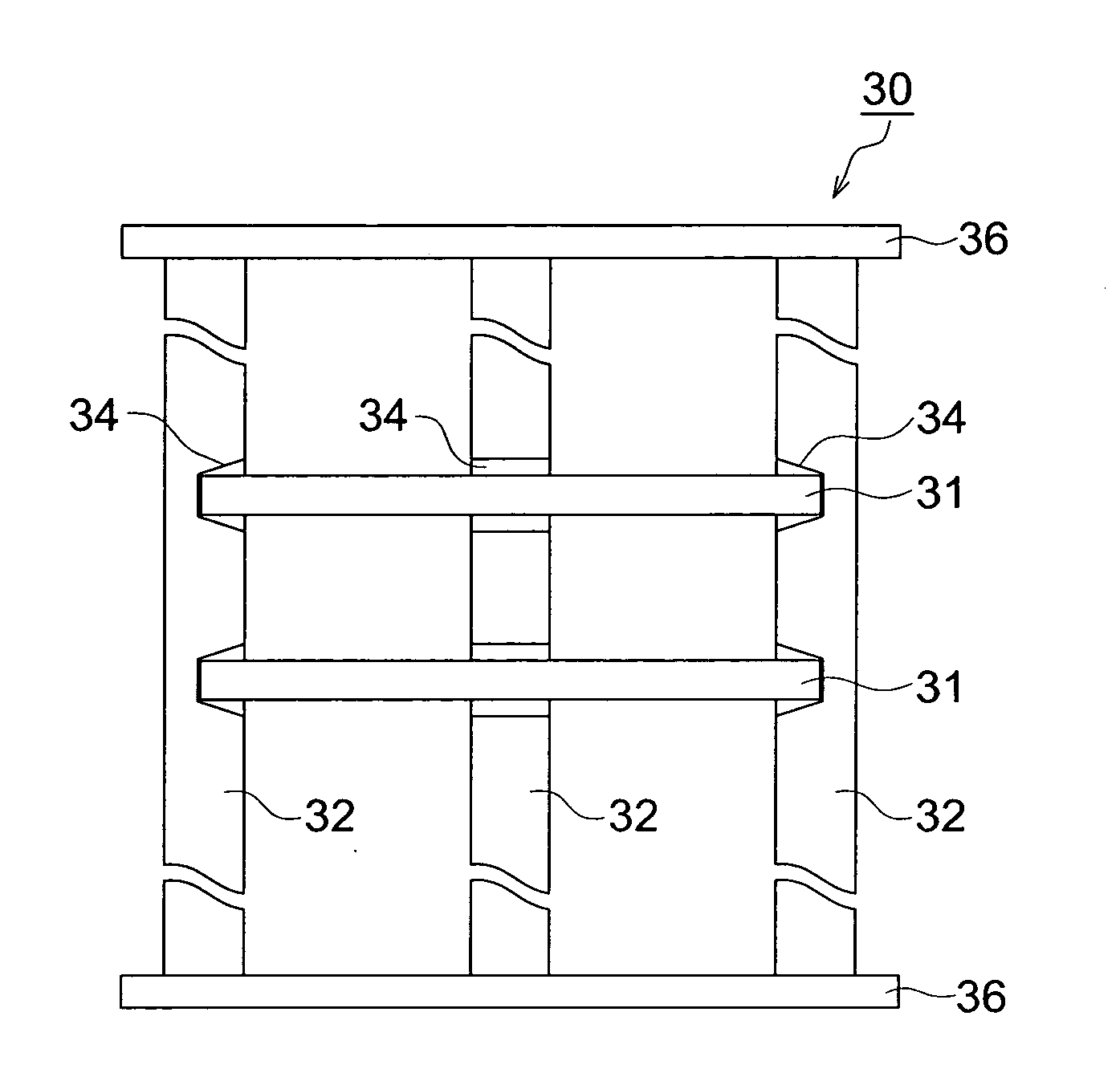
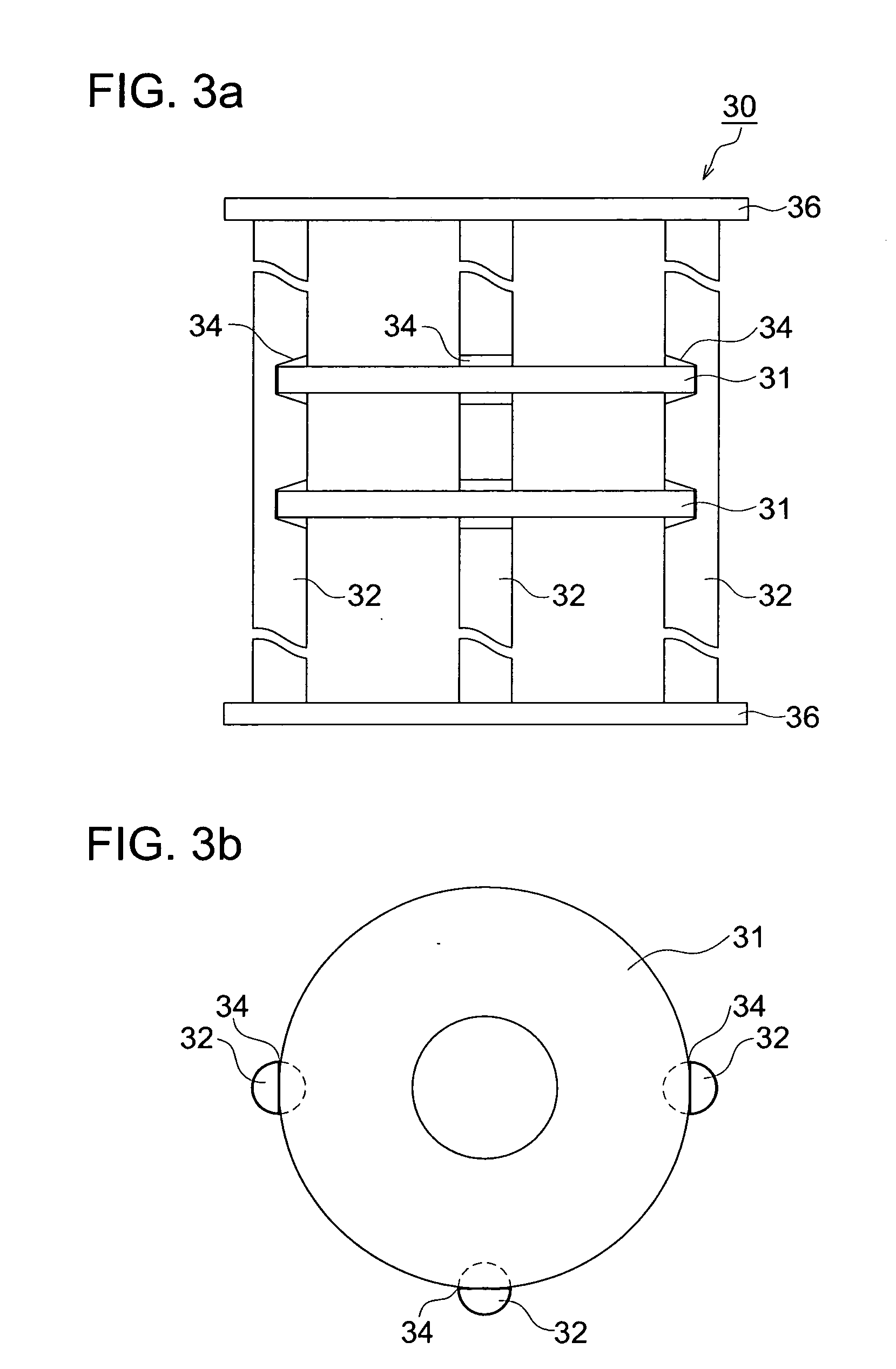
Method of manufacturing glass substrate for recording medium, glass substrate for recording medium, recording medium and holding jig
ActiveUS20100062284A1Suppress generationIncreased durabilityMagnetic materials for record carriersRecord information storageForeign matterAlkali metal
Provided is a method of manufacturing a glass substrate efficiently via prevention of foreign matter adhesion to a glass substrate as to chemical strengthening. Disclosed is a method of manufacturing a glass substrate for a recording medium possessing the step of conducting a chemical strengthening process by which a glass substrate held by a holding jig and the holding jig are immersed in a chemical strengthening solution, and 1st alkali metal ion on a surface of the glass substrate is substituted by 2nd alkali metal ion having a larger ion diameter than that of 1st alkali metal ion contained in the chemical strengthening solution, wherein the holding jig possesses a member of material made of a metal comprising an alkali metal element, or a metal film comprising an alkali metal element to cover a surface of the holding jig from the very beginning of the chemical strengthening process.
Owner:HOYA CORP
Photoelectric conversion device fabrication method, photoelectric conversion device, electronic apparatus manufacturing method, electronic apparatus, metal film formation method and layer structure, and semiconductor fine particle layer and layer structure
InactiveUS20050016578A1Improve adhesionProtective function is improvedDomestic stoves or rangesLight-sensitive devicesSemiconductor electrodeElectron
With respect to a photoelectric conversion device comprising a semiconductor electrode composed of semiconductor fine particles and a metal film to be an opposite electrode, a polyethylene dioxythiophene (PEDOT) / polystyrenesulfonic acid (PSS) film is formed by spin coating on a transparent electrode of a metal oxide such as ITO to remarkably improve the adhesion property of the metal film to the metal oxide film and the pollution by the different type metal of the metal film to be the opposite electrode can be prevented in the photoelectric conversion device. Further, a semiconductor electrode composed of semiconductor fine particles can be formed well on the metal oxide film by low temperature process while elution of the metal oxide film is prevented to obtain the photoelectric conversion device.
Owner:SONY CORP
Ink jet recording head and recording apparatus
A highly reliable, small-sized, and inexpensive ink jet recording head includes an electrical wiring member that is joined with a back surface of a liquid discharge substrate and includes a liquid supply port communicating with a liquid supply port of the liquid discharge substrate and an electrical connection portion connected to the electrode; and a holding member that holds the liquid discharge substrate through the electrical wiring member and includes a liquid supply port for supplying the liquid to the liquid supply port of the liquid discharge substrate. The liquid supply ports of the electrical wiring member, the liquid discharge substrate, and the holding member communicate with one another. A sealing agent for sealing the electrical connection portion is filled up between the liquid discharge substrate and the electrical wiring member, and a side surface of the liquid supply port of the electrical wiring member is covered with the sealing agent.
Owner:CANON KK
Manufacturing method of laminated body, manufacturing organic device and organic thin-film solar cell using same, and organic device and organic thin-film solar cell
InactiveUS20070082140A1Prevent elutionGood effectLiquid surface applicatorsFinal product manufactureSolventPolymer
A main object of the invention is to provide a manufacturing method of a laminated body which can make the following matters possible: when two or more layers are formed to be laminated by coating, constituents of an underlying layer are restrained from eluting into a solvent in a coating-solution for forming an upper layer; and the plural layers are laminated without restricting the kind of the solvent used in the upper layer forming coating-solution or constituent materials of the upper layer. To resolve the object, the present invention provides a manufacturing method of a laminated body, comprising an underlying layer forming step of coating an underlying layer forming coating-solution comprising a polymer material, thereby forming an underlying layer, and an upper layer forming step of coating an upper layer forming coating-solution on the underlying layer.
Owner:DAI NIPPON PRINTING CO LTD
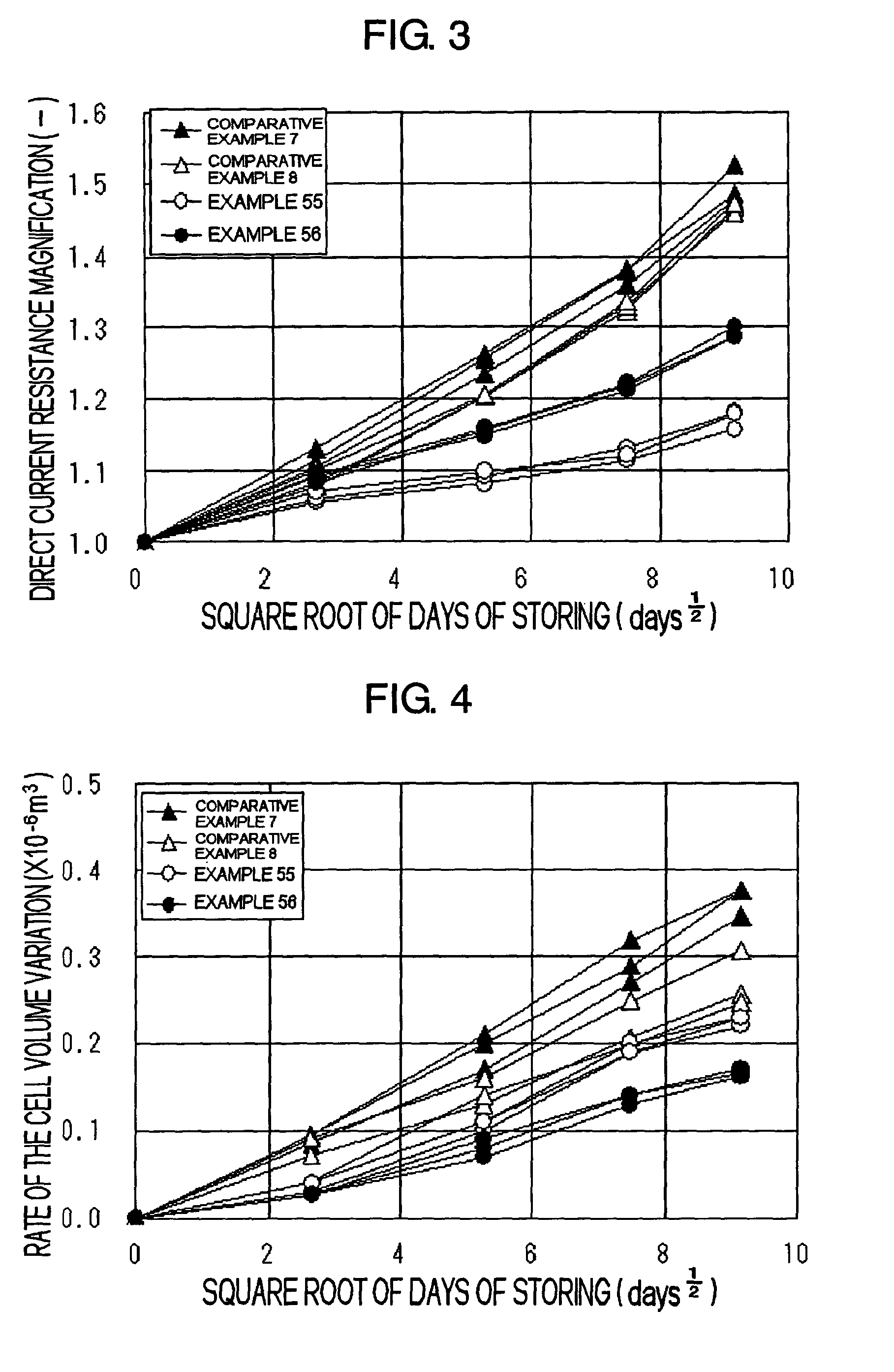
Electrolyte solution for secondary battery and secondary battery using the same
ActiveUS7163768B2Prevent elutionPrevent adhesionElectrolytic capacitorsOrganic electrolyte cellsSolventDecomposition
The present invention provides a technology of inhibiting the decomposition of the solvent of the electrolyte solution for the secondary battery. Further, the present invention provides a technology of prohibiting the resistance increase of the secondary battery and improving the storage properties such as improving the capacity retention ratio. The electrolyte solution 15 comprising non-proton solvent and cyclic sulfonic ester including at least two sulfonyl groups may be used.
Owner:NEC CORP
Ion eluting unit and device loaded with same
ActiveUS20060164093A1Eluted stablyPrevent precipitationSpecific water treatment objectivesWater/sewage treatment by electrochemical methodsElutionElectrical polarity
In an ion elution unit, a drive circuit applies a voltage between electrodes to elute metal ions from the electrodes. Polarities of the electrodes are reversed cyclically with a voltage application halt period placed in-between. A current detection circuit detects the current flowing between the electrodes. A check of operation of the current detection circuit is carried out before the application of a voltage to the electrodes is started. The operation of the current detection circuit is started when a predetermined period of time passes after the application of a voltage to the electrodes is started.
Owner:SHARP KK
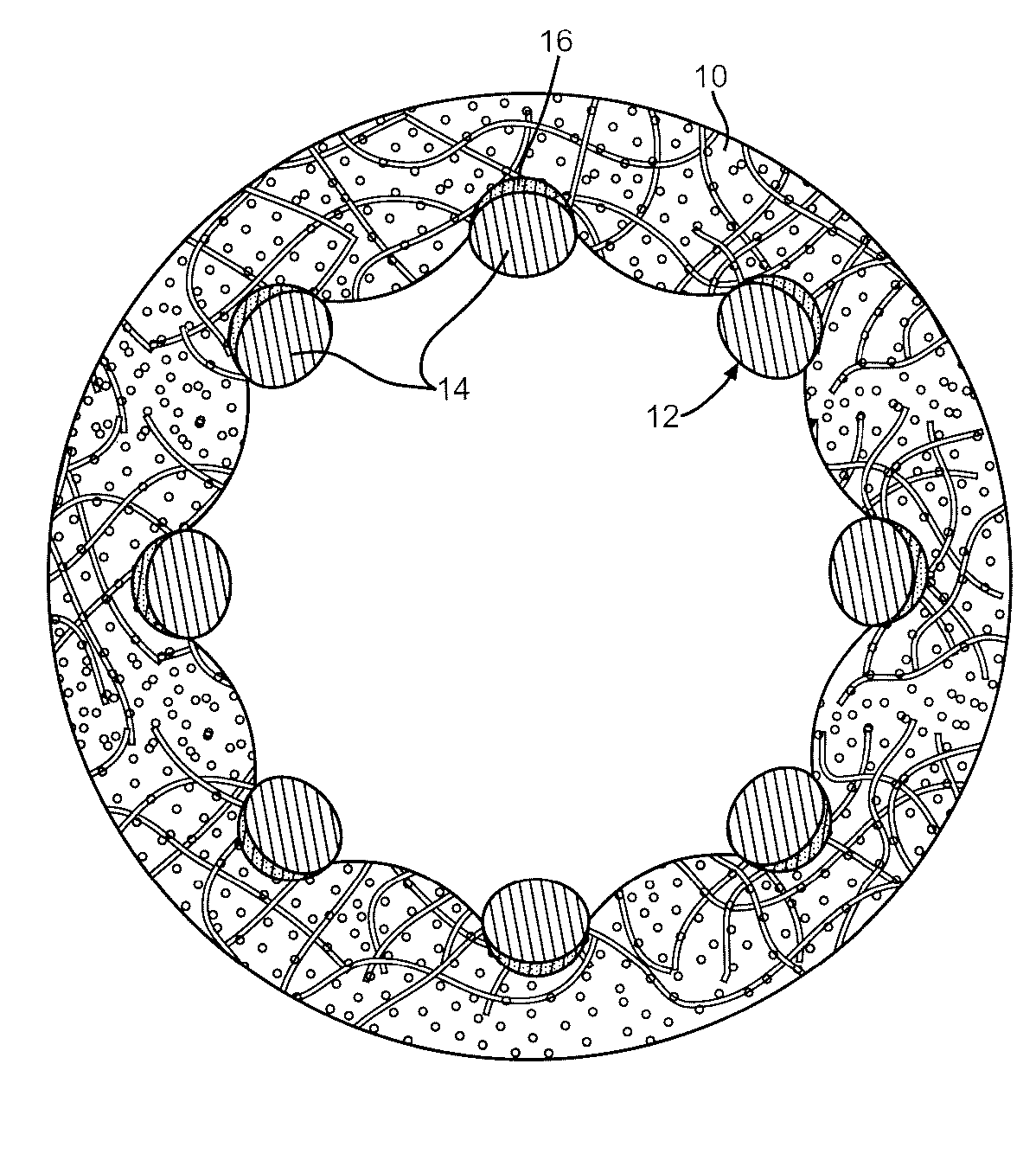
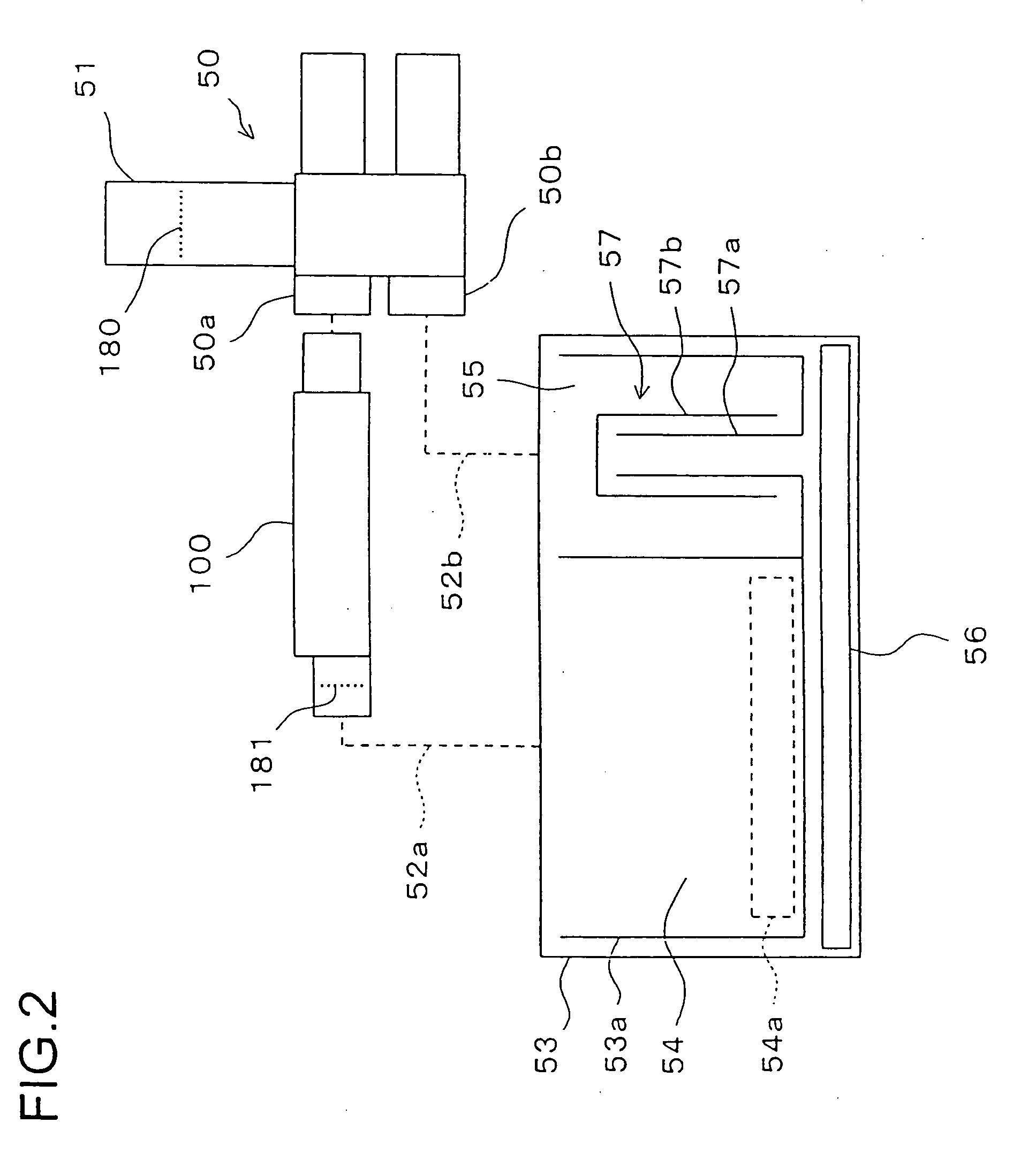
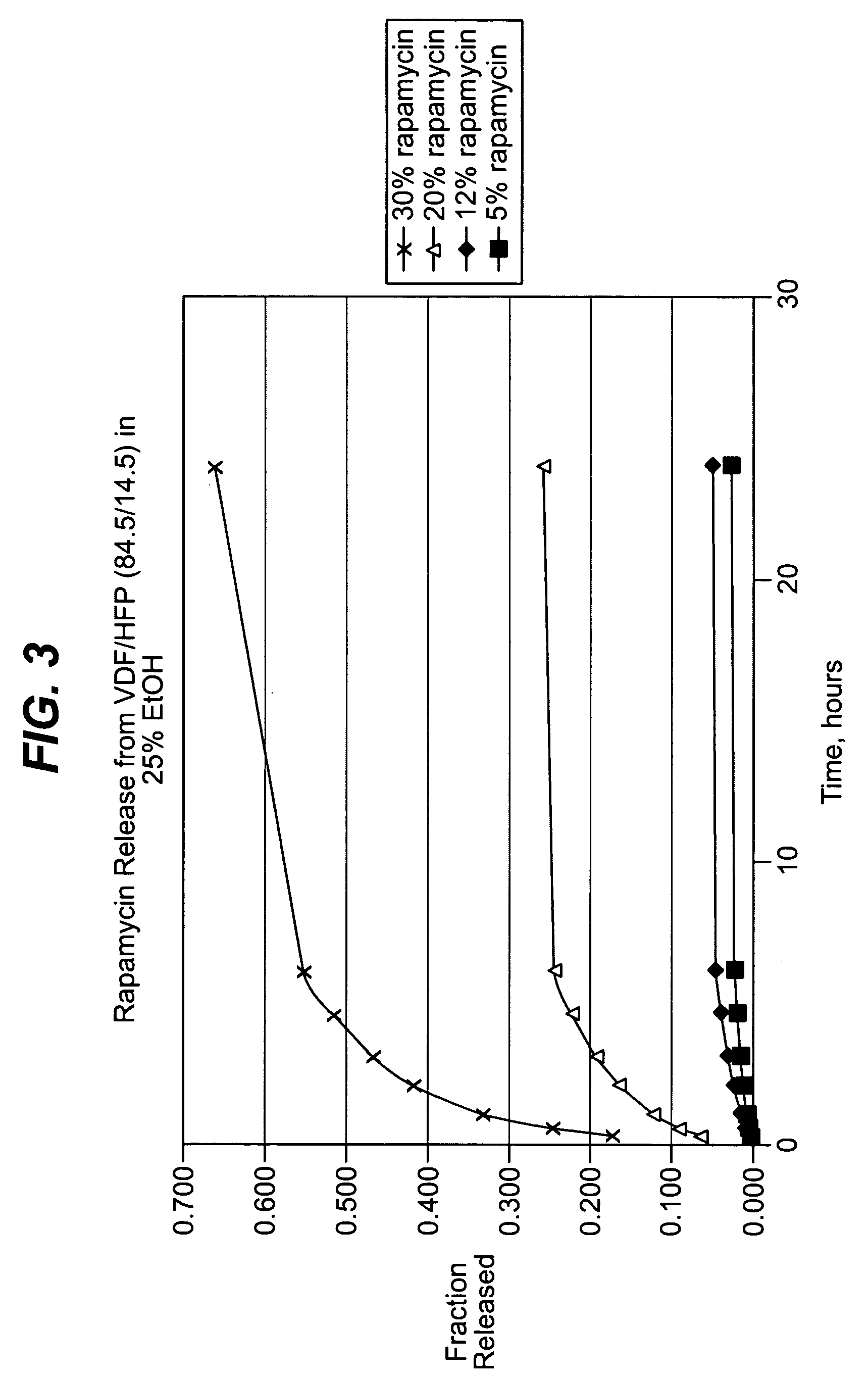
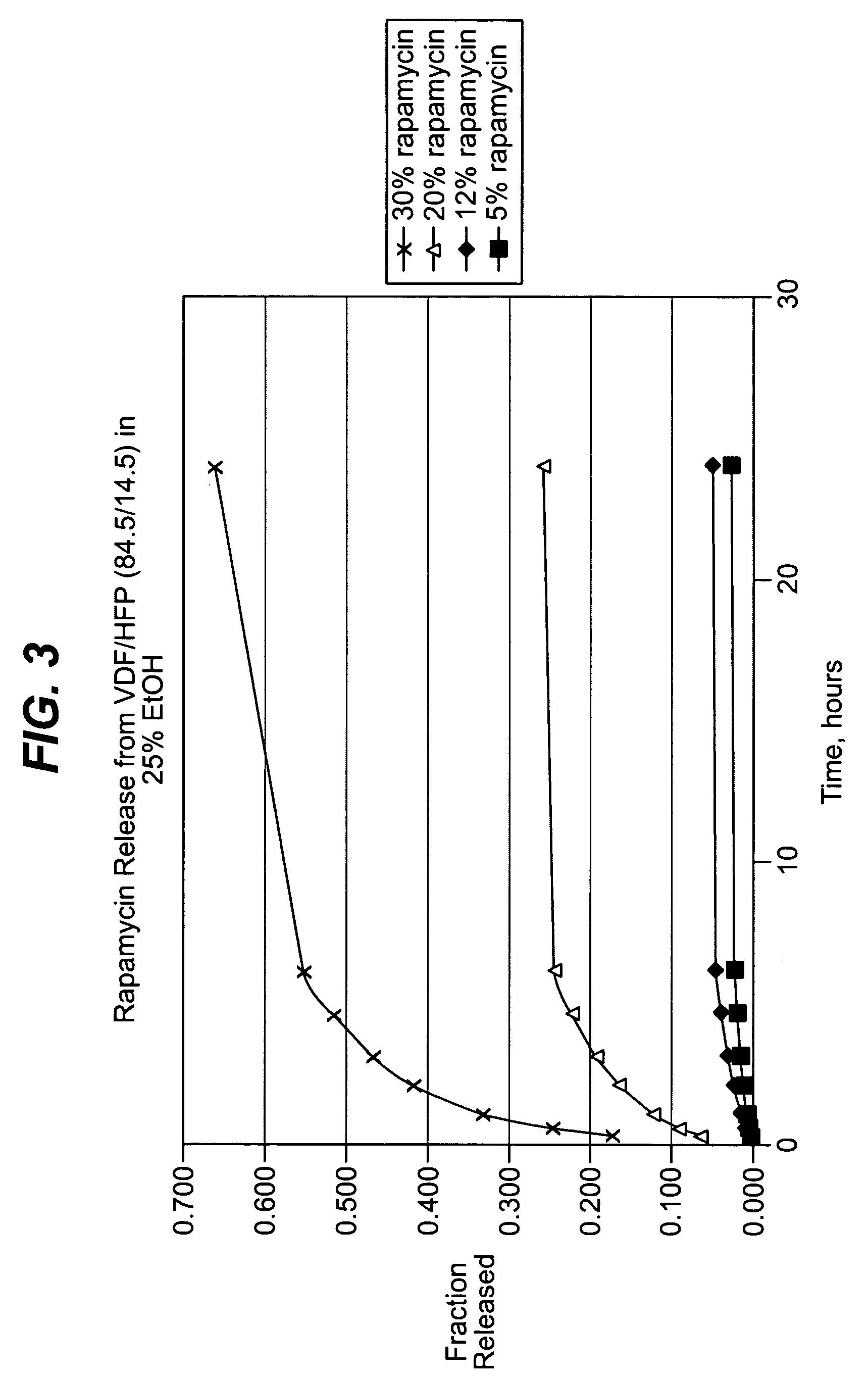
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050158360A1Minimize potential risk of damageReduce frictionStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
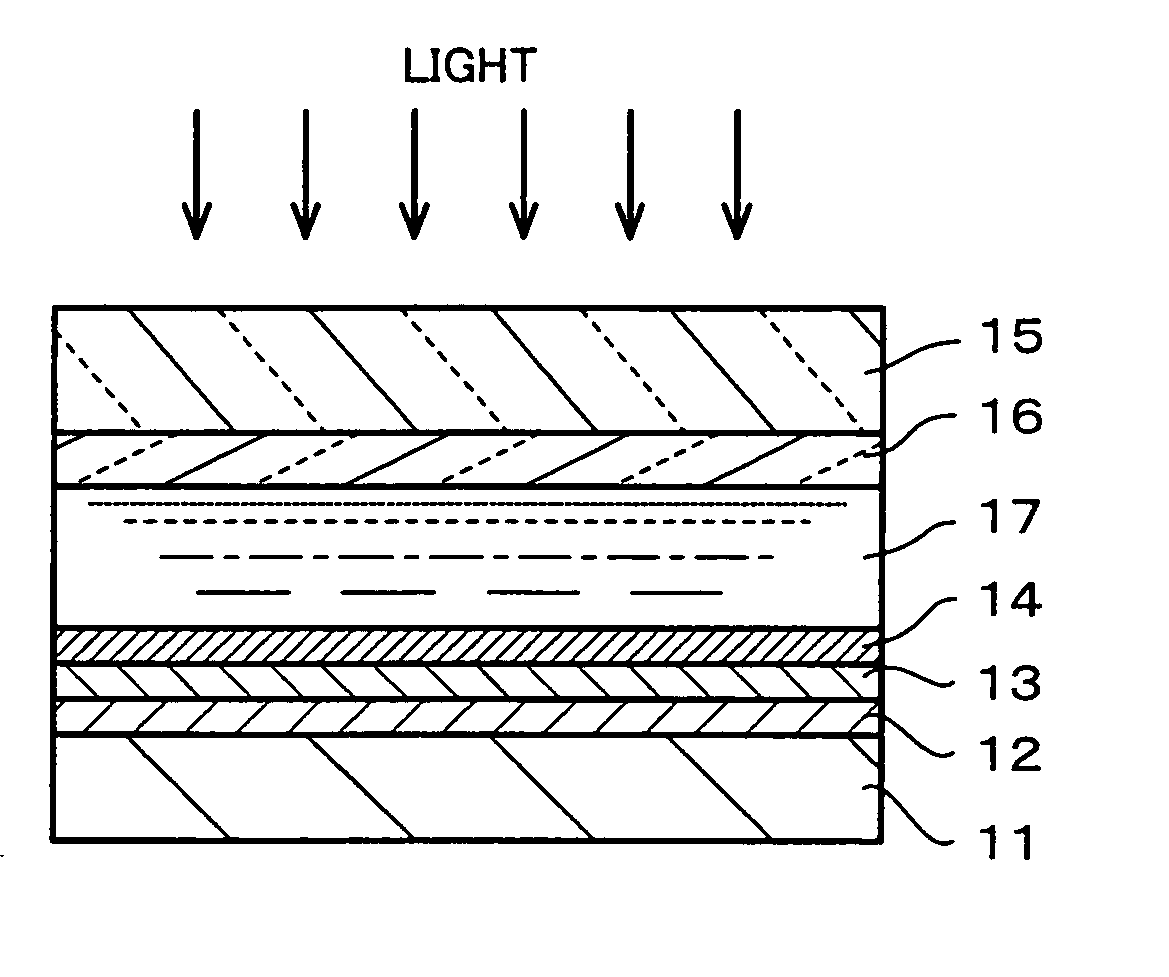
Battery
A battery with a high battery voltage at charging and improved energy density is provided. A cathode (12) and an anode (14) are laminated with a separator (15) sandwiched therebetween which is impregnated with an electrolyte. The cathode (12) has a cathode active material including a lithium composite oxide which contains lithium, at least either cobalt or nickel, and oxygen. The battery voltage at charging is 4.25 V or more. The total amount of lithium carbonate and lithium sulphate in the cathode (12) to the cathode active material is 1.0 wt % or less, a concentration of protic impurities in the electrolyte, which is converted to a mass ratio of protons to the electrolyte, is 20 ppm or less, or moisture content in the electrolyte is 20 ppm mass ratio or less to the electrolyte. This inhibits metal eluting from the lithium composite oxide even at high voltages.
Owner:SONY CORP
Local vascular delivery of mycophenolic acid in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS7303758B2Prevent elutionProvide controlStentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Owner:WYETH LLC
Wax dispersant for toner and toner
ActiveUS9969834B2Prevent elutionSufficient charging performanceDevelopersAcrylic resinAlicyclic compound
Owner:CANON KK
Resin for hydrophobitizing resist surface, method for manufacturing the resin, and positive resist composition containing the resin
ActiveUS8080363B2Prevent elutionSuppression of development defectPhotosensitive materialsRadiation applicationsResistSolid content
Owner:FUJIFILM CORP
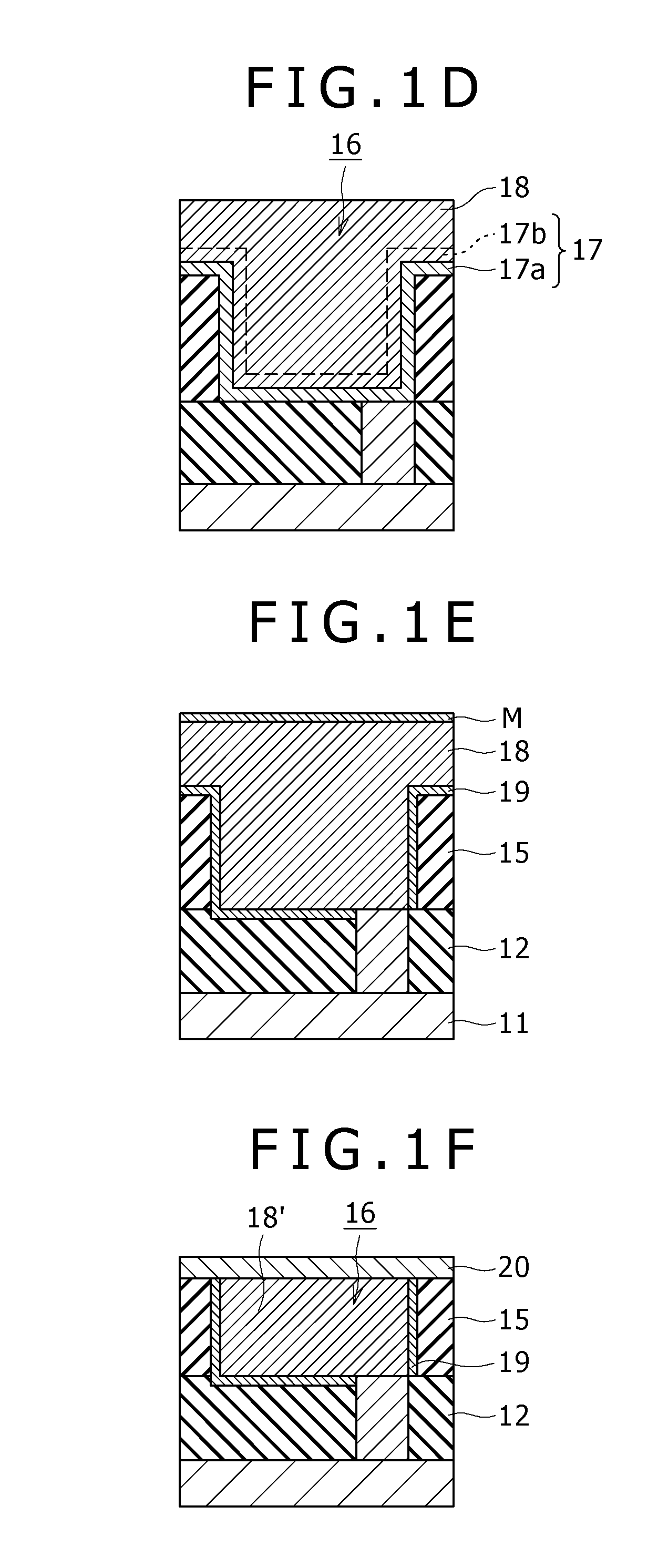
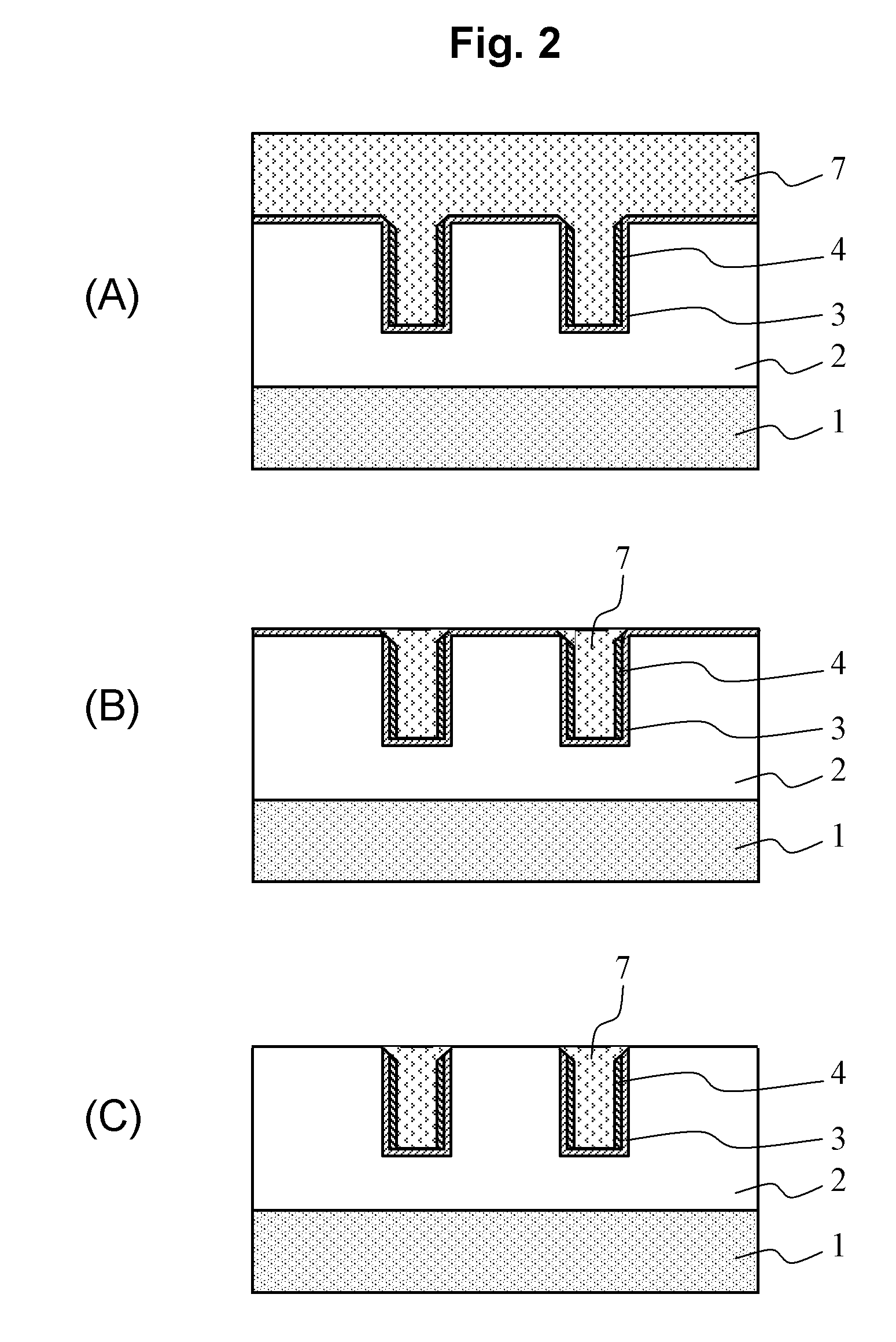
Semiconductor device and manufacturing method of the same
ActiveUS20060249845A1Increase productionPrevent elutionPipe supportsSemiconductor/solid-state device detailsDevice materialEngineering
The invention provides a semiconductor device with a bonding pad made of a wiring layer including aluminum and its manufacturing method that enhance the yield of the semiconductor device. The method of manufacturing the semiconductor device of the invention includes removing a portion of an antireflection layer (e.g. made of a titanium alloy) formed on an uppermost second wiring layer (e.g. made of aluminum) on a semiconductor substrate by etching, forming a passivation layer covering the antireflection layer and a portion of the second wiring layer where the antireflection layer is not formed and having an opening exposing the other portion of the second wiring layer, and dividing the semiconductor substrate into a plurality of semiconductor dice by dicing. These processes can prevent the antireflection layer from being exposed in the opening, and this can prevent a component of the second wiring layer from being eluted due to cell reaction between the second wiring layer and the antireflection layer as has been seen in the conventional art.
Owner:SEMICON COMPONENTS IND LLC
Antioxidant-stabilized joint implants
ActiveUS20150151866A1Improve wear resistanceImprove mechanical propertiesElectric discharge tubesPackage sterilisationMedicineAntioxidant
Owner:THE GENERAL HOSPITAL CORP
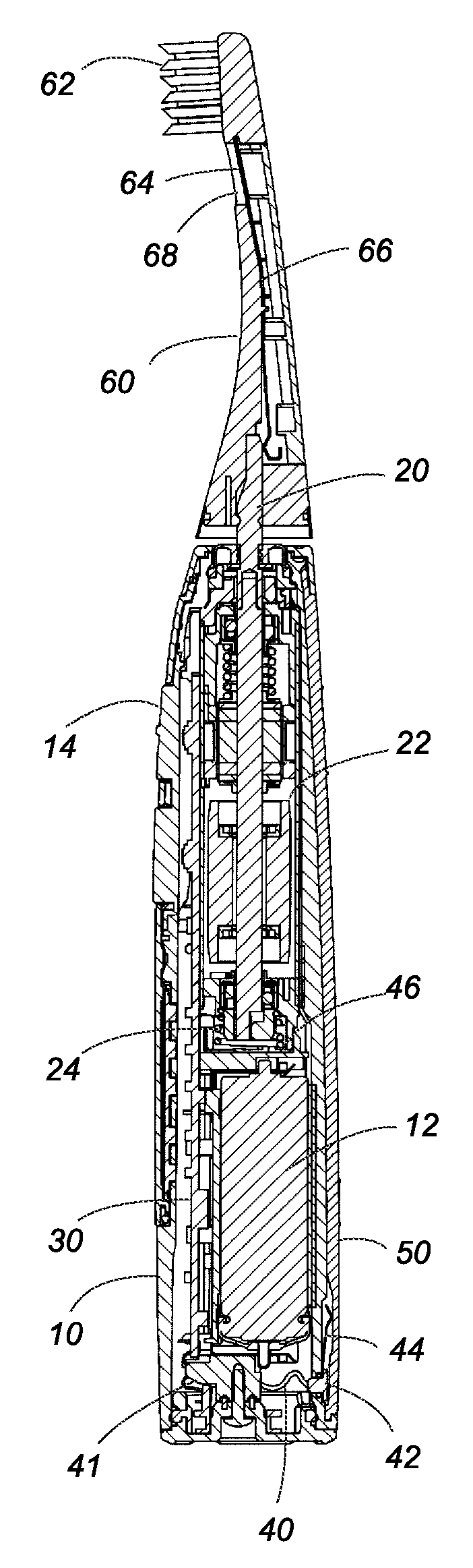
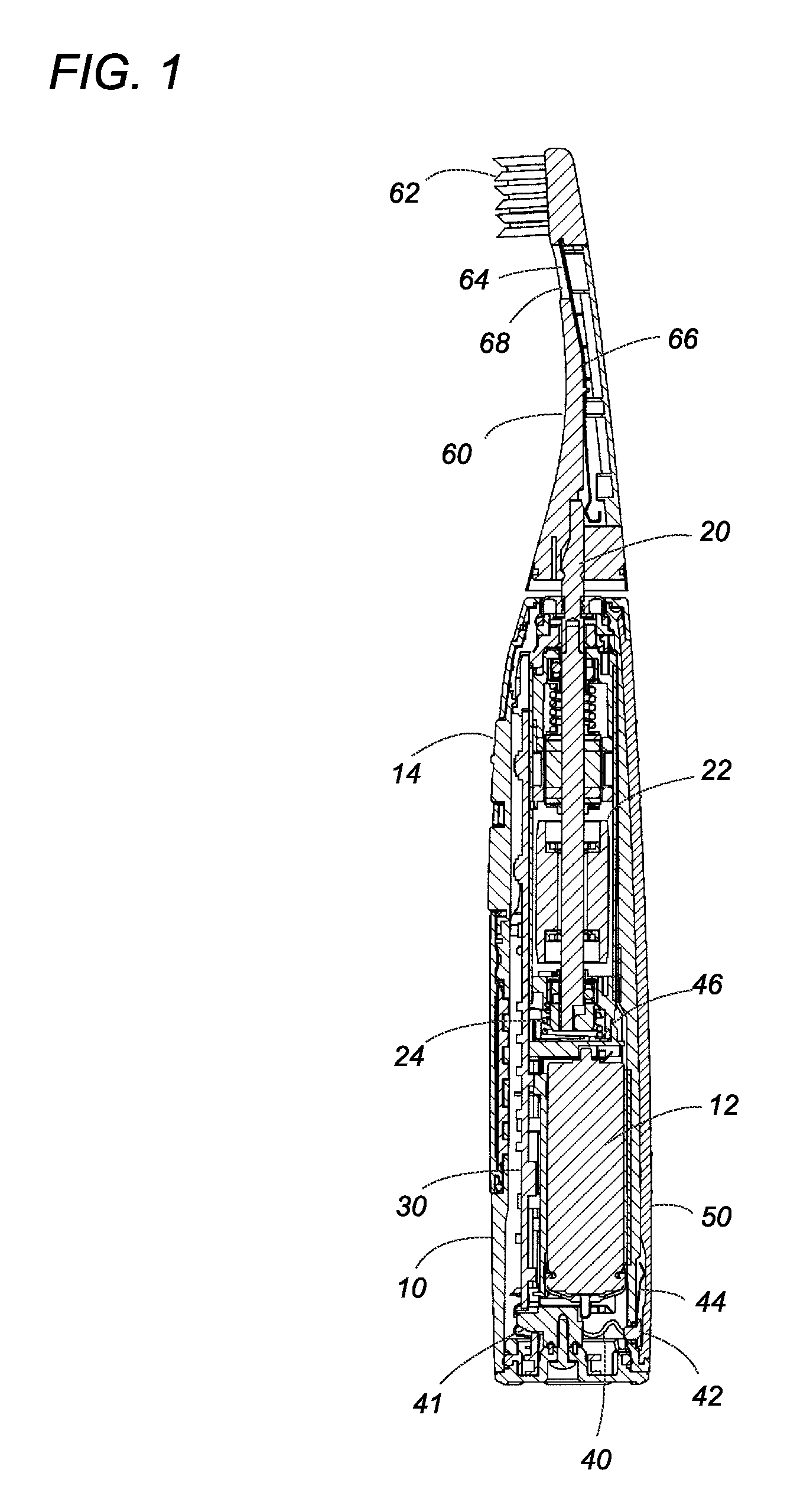
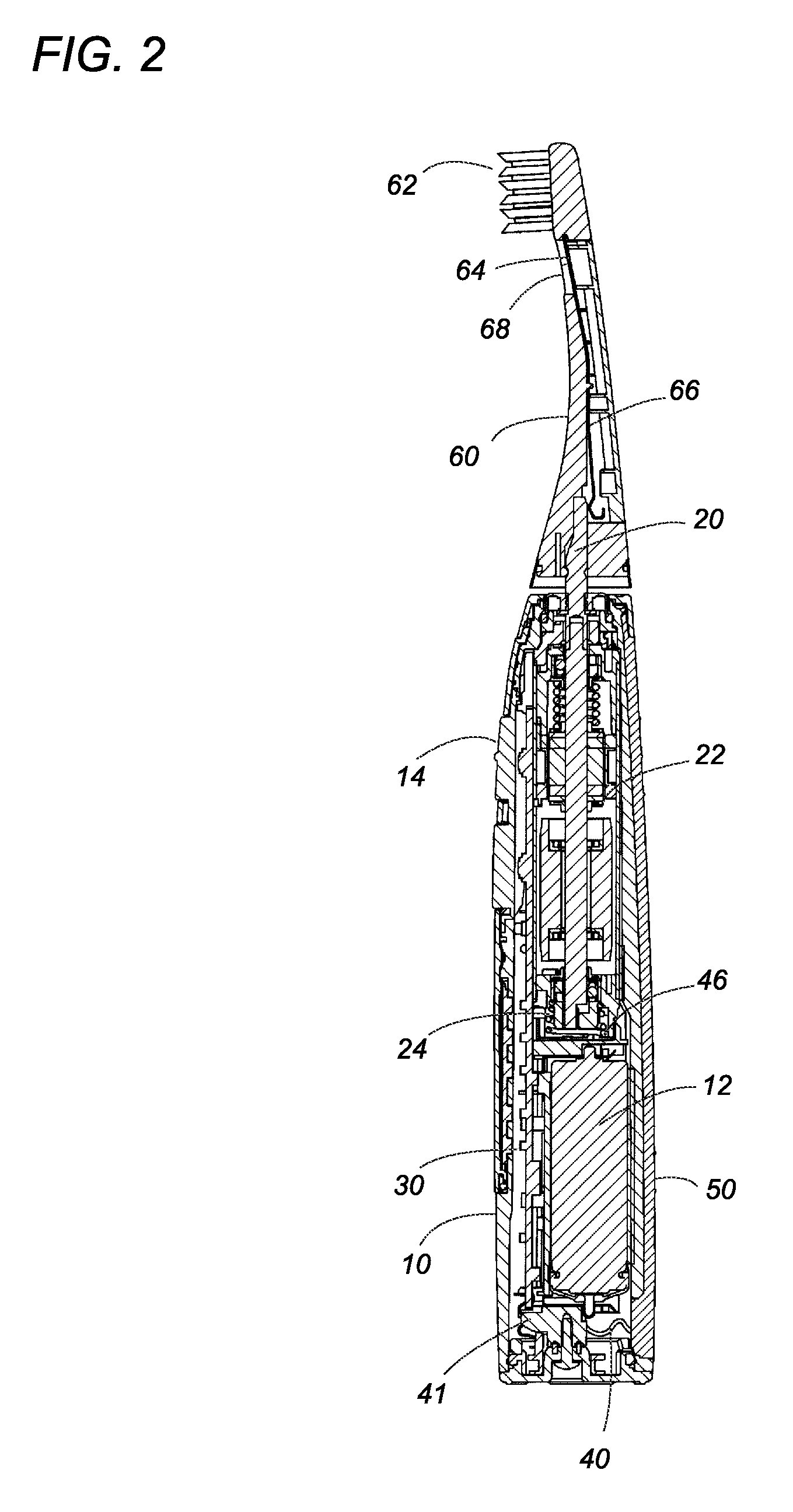
Oral care device
InactiveUS20080183249A1Without deterioration of appearance and performanceEasy to useElectrotherapyCarpet cleanersMouth careMetallic materials
An oral care device has a handle with a touch electrode and a brush handle with a brush electrode for flowing a minute current into an oral cavity of a user by way of the user's body for removing plaque. The touch electrode is made of a non-metal material filled with carbon filaments, and is free from any metal elution or corrosion while it is in contact with the user's hand, which assures to keep the oral care device in good appearance and performance over a prolonged use.
Owner:MATSUSHITA ELECTRIC WORKS LTD
Multilayer ceramic capacitor
ActiveUS20150049413A1Excellent resistance to elutionPrevent elutionFixed capacitor electrodesFixed capacitor dielectricRare-earth elementPerovskite
Owner:MURATA MFG CO LTD
Method for preparing heteropoly acid/ordered mesic porous silicon oxide catalyst, and its application
InactiveCN1947840APrevent elutionGood dispersionMetal/metal-oxides/metal-hydroxide catalystsRefining to eliminate hetero atomsAlcoholHeteropoly acid
A heteropoly acid / ordered meso-porous silicon oxide catalyst used for removing the S-contained benzothiophenium compounds from the fractional oil of petroleum is prepared through such steps as dissolving heteropoly acid in the mixture of absolute alcohol and distilled water, adding ethyl n-silicate, magnetic stirring to obtain solution A, dissolving the polyoxyethene-polyoxypropene-polyoxyethene triblock copolymer in the mixture of absolute alcohol and distilled water to obtain solution B, slowly adding B to A, magnetic stirring to obtain sol, culturing it at 38-42 deg.C for 6-8 days, pulverizing, and calcining in N2.
Owner:WUHAN UNIV OF TECH
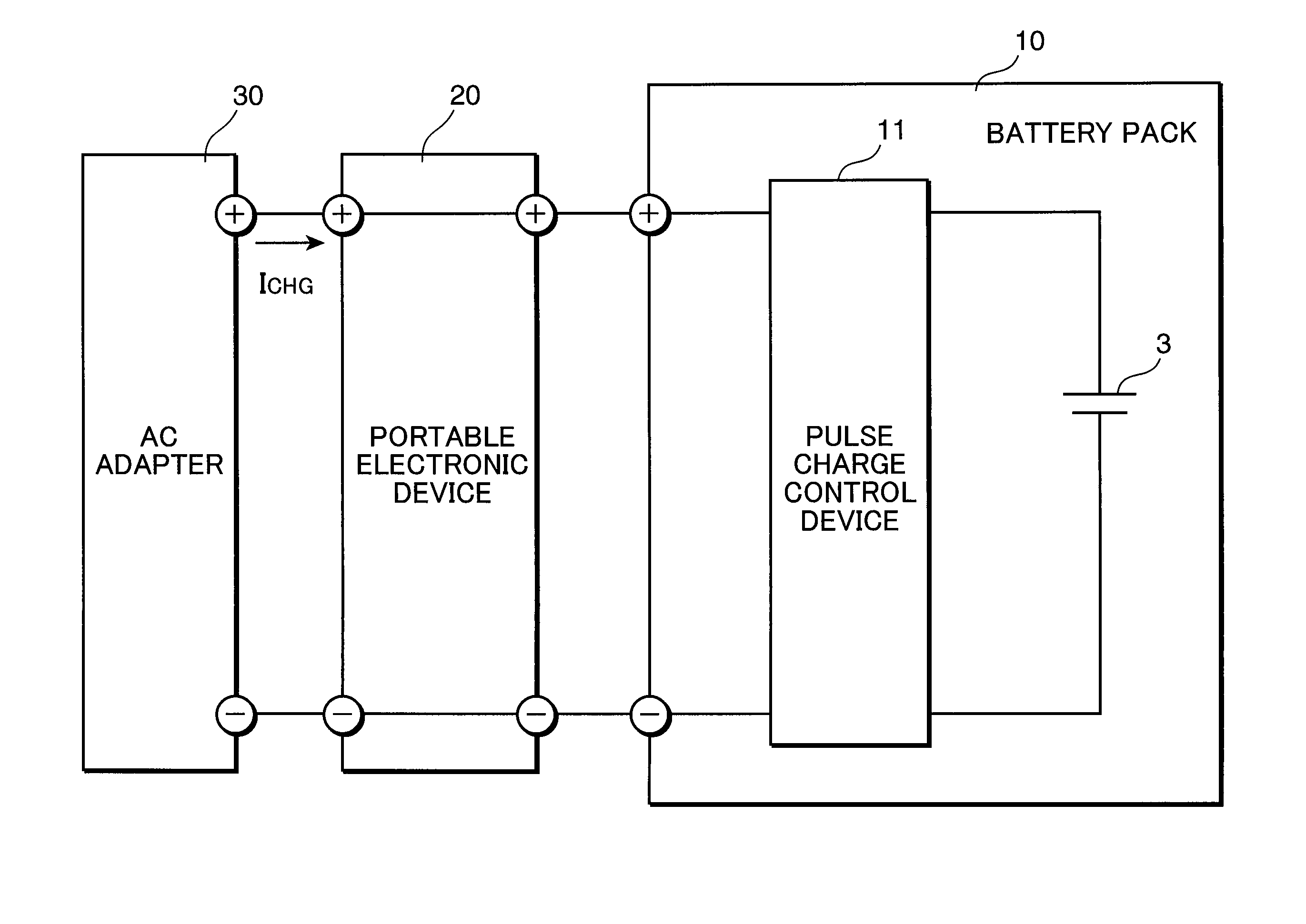
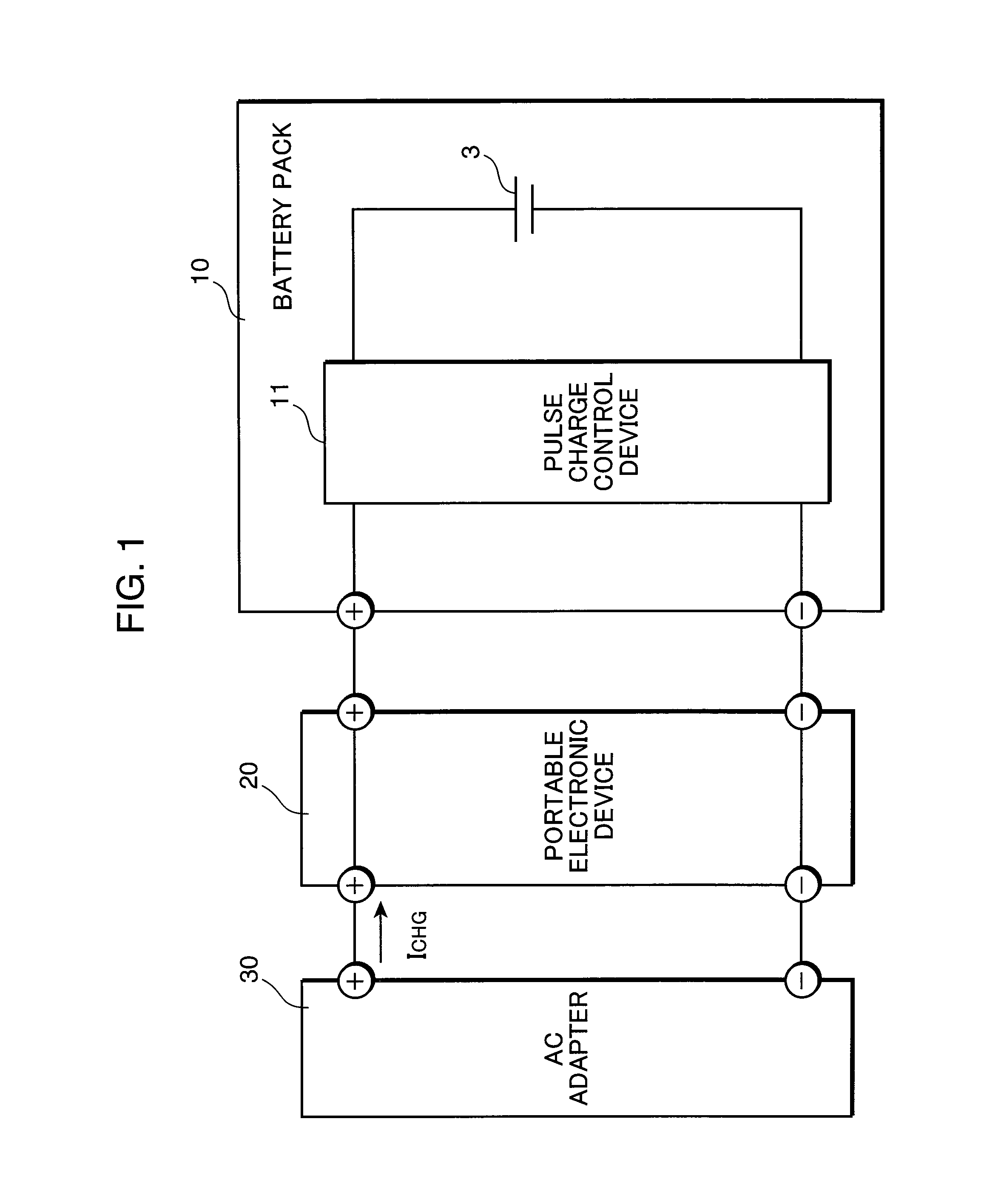
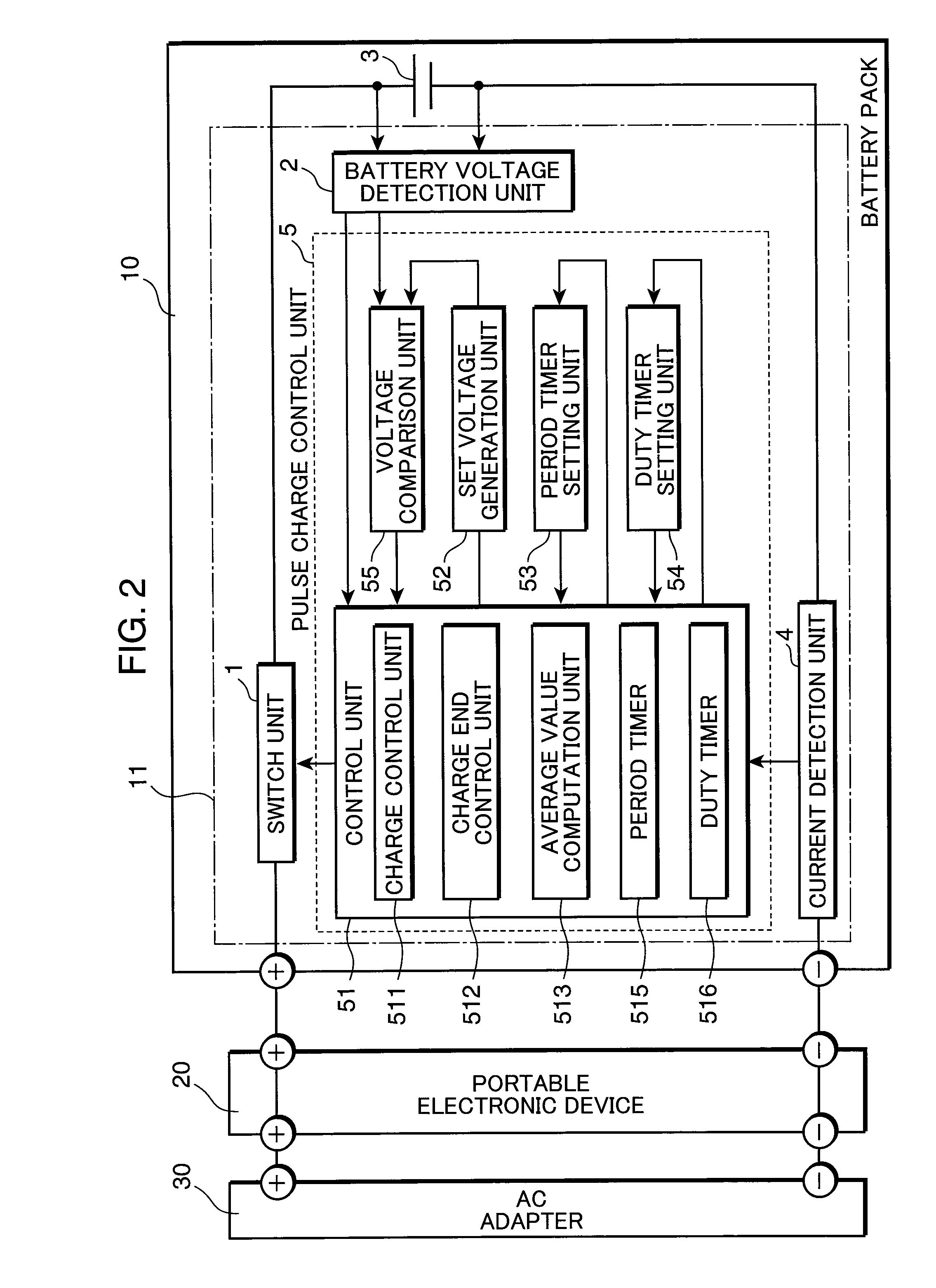
Pulse charge method for nonaqueous electrolyte secondary battery and pulse charge control device
InactiveUS20100219795A1Increase in battery voltage is preventedPrevent elutionBatteries circuit arrangementsSecondary cells charging/dischargingCell voltageElectrolyte
A pulse charge method for a nonaqueous electrolyte secondary battery, includes: a charge control step of pulse charging by supplying a pulsed current periodically to the nonaqueous electrolyte secondary battery; a battery voltage detection step of measuring a battery voltage of the nonaqueous electrolyte secondary battery; a comparison step of comparing the battery voltage, measured in the battery voltage detection step when the pulsed current is supplied, with a predetermined set voltage; and a charge end control step of ending the pulse charging when a comparison result shows that the measured battery voltage is equal to or higher than the set voltage.
Owner:PANASONIC CORP
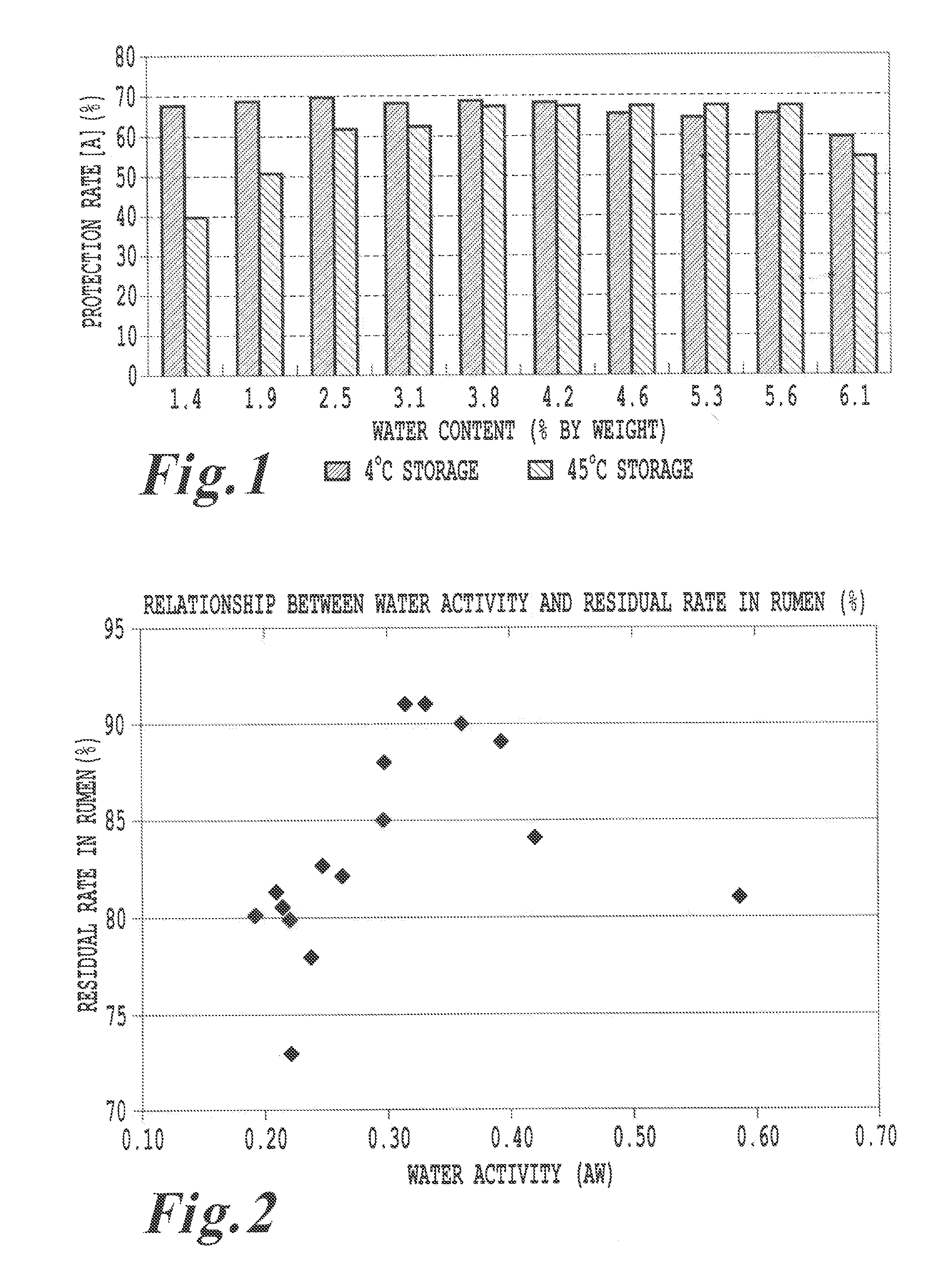
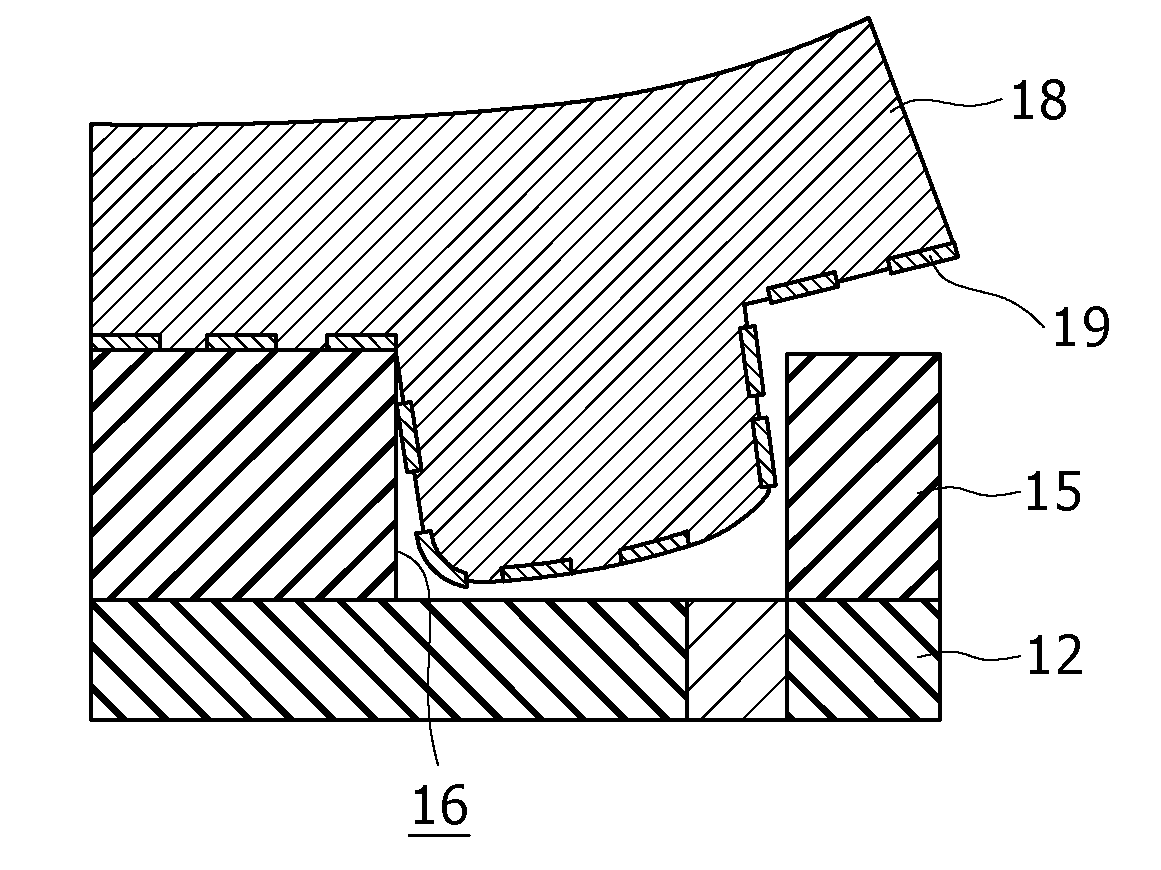
Feed additive composition for ruminants and method of producing the same
ActiveUS20110081444A1Promote milk productionImprove efficiencyAnimal feeding stuffAccessory food factorsFeed additiveProtective Agents
A feed additive composition includes a protective agent, lecithin in an amount of 0.05 to 6% by weight relative to a total weight of the composition, a basic amino acid in an amount of at least 40% by weight and less than 65% by weight relative to the total weight of the composition, and water. A method of producing a feed additive composition includes preparing a molten mixture of at least one protective agent, lecithin and at least one basic amino acid, and solidifying the molten mixture by immersing the molten mixture in water or an aqueous liquid. The protective agent includes hydrogenated vegetable oils and / or hydrogenated animal oils having melting points of greater than 50° C. and less than 90° C.
Owner:AJINOMOTO CO INC
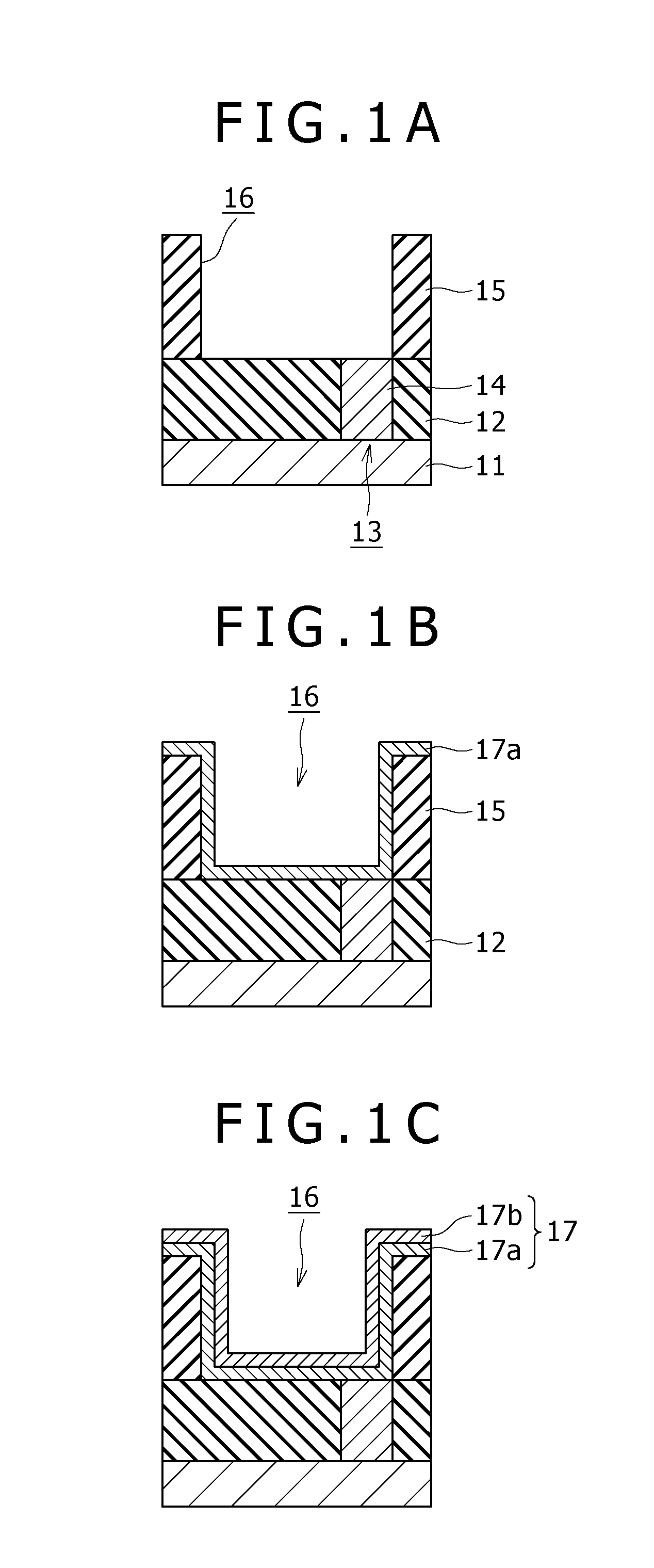
Method of manufacturing semiconductor device
InactiveUS20080173547A1Avoid separationImprove uniformitySemiconductor/solid-state device detailsSolid-state devicesAlloyCopper
Disclosed herein is a method for manufacturing a semiconductor device, the method including the steps of: forming a recess in an insulating film provided over a substrate; forming a plating seed layer in such a way that an inner wall of the recess is covered, the plating seed layer arising from sequential deposition of an alloy layer composed of copper and a metal other than copper and a conductive layer composed mainly of copper; burying a conductive layer composed mainly of copper by plating in the recess on which the plating seed layer is provided; and carrying out heat treatment to cause the metal in the alloy layer to react with a constituent in the insulating film, to thereby form a barrier film composed of a metal compound having a copper diffusion barrier function at an interface between the alloy layer and the insulating film.
Owner:SONY CORP
Method of making active material and electrode
ActiveUS20100123096A1Improve adhesionMany of characteristicSecondary cellsNon-aqueous electrolyte accumulator electrodesLithiumAqueous solution
There is provided a method of making an active material with satisfactory cycle characteristics. The method of making an active material according to the invention comprises contacting an aqueous solution containing a metal-fluoro complex and lithium salt with lithium-containing metal oxide particles.
Owner:TDK CORPARATION
Expandable member formed of a fibrous matrix having hydrogel polymer for intraluminal drug delivery
An intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent incorporated therein. The hydrogel polymer can be coated on the fiber elements in a co-axial configuration. The fiber elements may also have a second coating including a protective substance surrounding the hydrogel polymer having a therapeutic agent therein. The matrix of fiber elements can be formed by electrospinning. A process of delivering a therapeutic agent to a target site includes providing an intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent dispersed therein, and advancing the catheter device at a desired treatment site. Once at the desired treatment site, fluid is introduced into the inflation lumen to expand the expandable member from a first profile to a second profile, and the therapeutic agent is released from the hydrogel polymer and delivered to the desired treatment site.
Owner:ABBOTT CARDIOVASCULAR
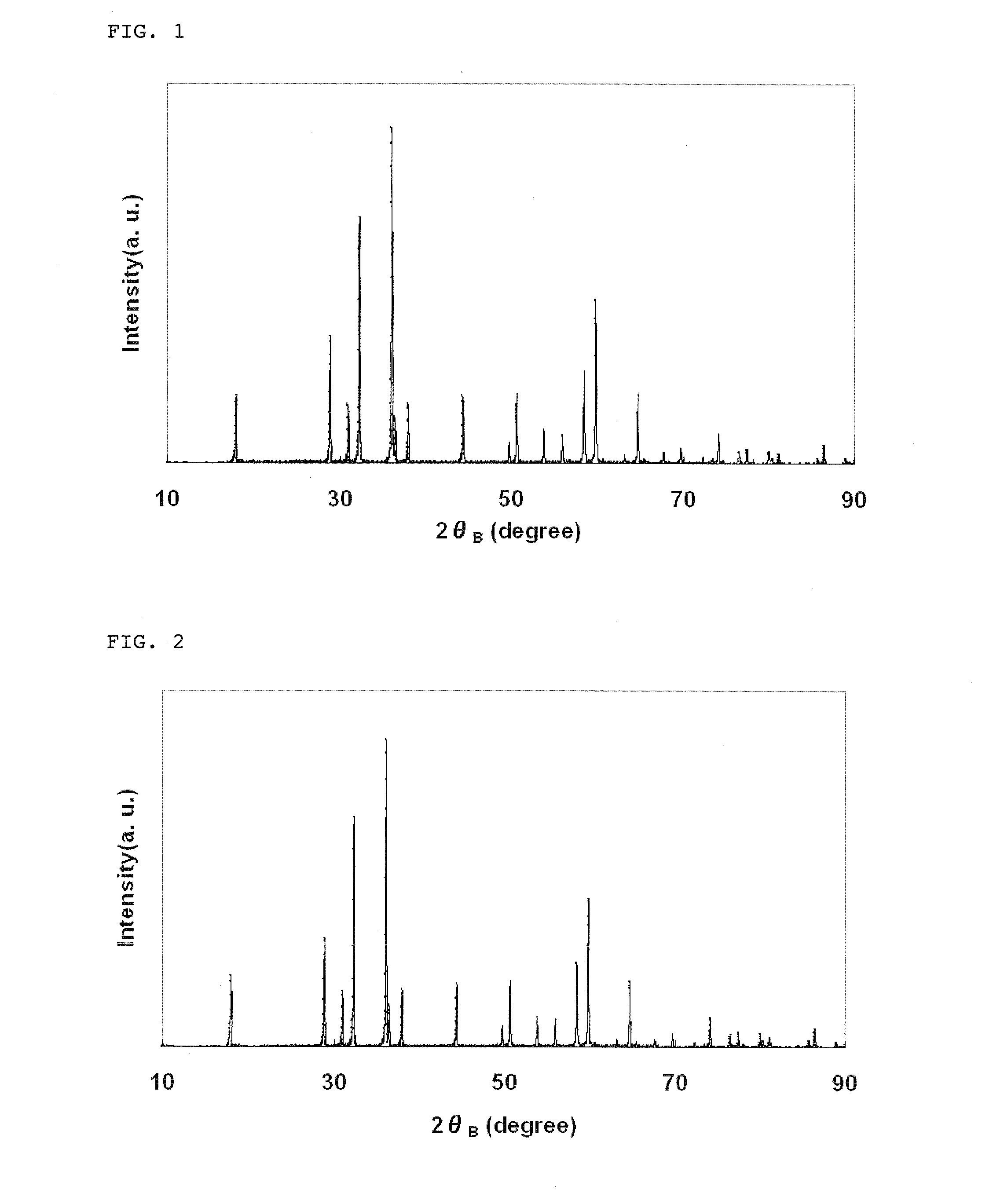
Lithium manganate for non-aqueous electrolyte secondary battery, process for producing the same, and non-aqueous electrolyte secondary battery
ActiveUS20100327221A1Increase output powerImprove high temperature stabilityCell electrodesIron compoundsElectrical batteryManganate
The present invention relates to lithium manganate particles having a primary particle diameter of 1 to 8 μm and forming substantially single-phase particles, which have a composition represented by the following chemical formula:Li1+xMn2−x−yY1yO4+Y2in which Y1 is at least one element selected from the group consisting of Ni, Co, Mg, Fe, Al, Cr and Ti; Y2 is P and is present in an amount of 0.01 to 0.6 mol % based on Mn; and x and y satisfy 0.03≦x≦0.15 and 0.05≦y≦0.20, respectively, andwhich lithium manganate particles have a specific surface area of the lithium manganate particles of 0.3 to 0.9 m2 / g (as measured by BET method); and have an average particle diameter (D50) of the lithium manganate particles of 3 to 10 μm. A positive electrode active substance of a lithium ion secondary battery using the lithium manganate particles of the present invention has a high output and is excellent in high-temperature stability.
Owner:TODA IND
Process for production of electroluminescent element and electroluminescent element
InactiveUS20060008742A1Improve production efficiencyPrevent elutionSolid-state devicesPhotomechanical apparatusLuminescenceElectroluminescence
There is provided a production process of an electroluminescent element, which, even when a buffer layer patterned by a photolithographic process is formed, luminescence failure derived from cross contamination or a variation in film thickness does not take place and can realize high production efficiency. The production process comprises repeating at least twice the step of forming an electroluminescent layer comprising a buffer layer and a luminescent layer by patterning using a photolithographic process, thereby producing an electroluminescent element comprising a patterned electroluminescent layer, and comprises the steps of forming a first pattern part comprising a first buffer layer as the lowermost layer; and coating a solution for second buffer layer formation in a region including said first pattern part, the first buffer layer being immiscible with said solution for second buffer layer formation.
Owner:DAI NIPPON PRINTING CO LTD
Non-aqueous electrolyte and non-aqueous electrolyte secondary battery using the same
ActiveUS20090253049A1Avoid decompositionPrevent elutionElectrolytic capacitorsOrganic electrolyte cellsSolventDecomposition
A non-aqueous electrolyte can suppress decomposition of a solvent, improve the cycle life of a secondary battery, suppress the rise of resistance of a secondary battery and improve the capacity maintenance ratio of a secondary battery A non-aqueous electrolyte secondary battery formed by using such a non-aqueous electrolyte includes a non-aqueous electrolyte containing an aprotic solvent and a disulfonic acid ester as expressed by chemical formula 1 shown below, a positive electrode and a negative electrode:
Owner:NEC ENERGY DEVICES LTD
Semiconductor device and method for manufacturing the same
InactiveUS20100295182A1Reliable preventionPrevent elutionSemiconductor/solid-state device detailsSolid-state devicesElutionSlurry
Provided is a method for forming a Cu wiring that does not cause Cu elution during CMP when a Ru material is used as a barrier metal film for the Cu wiring. The method has a step (d) of removing a second barrier metal film (Ru film) formed on a first barrier metal film on an upper surface of an interlayer insulating film, and a step (e) of depositing a seed copper (Cu) film on the first and the second barrier metal films after the step (d). By removing the second barrier metal film on the upper surface before the seed copper film is formed, copper is prevented from eluding into a slurry due to a battery effect of the second barrier metal film and copper.
Owner:PANASONIC CORP
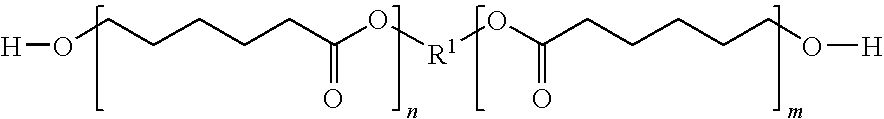
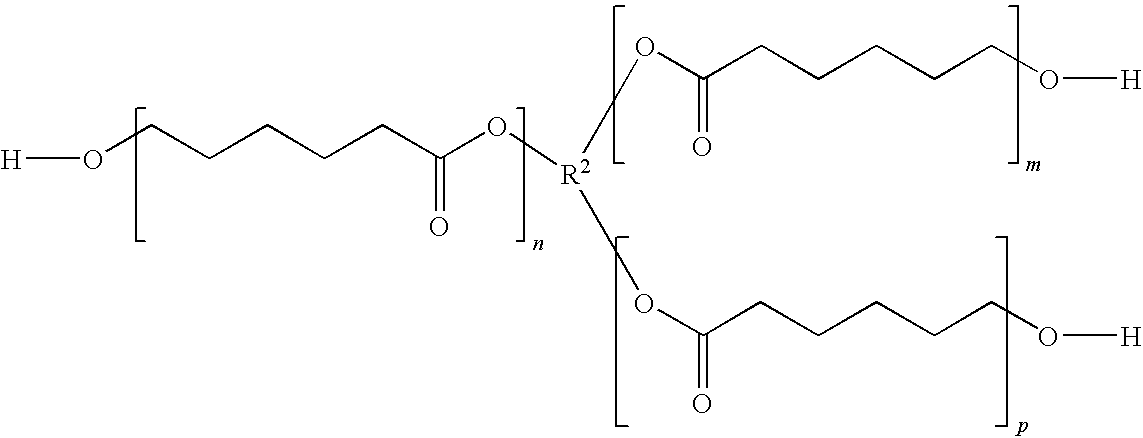
Granule coated with urethane resin
ActiveUS20100196431A1Excellent elution controllabilityEasily degradable in soilBiocideNitrogenous fertilisersPolyesterPolyol
A coated granule is obtained by coating a bioactive substance-containing granule with a urethane resin obtained by reaction of an aromatic diisocyanate with a polyol mixture containing a polyesterpolyol and a C2-C8 polymethylene glycol, wherein the molar ratio of the polyesterpolyol to the polymethylene glycol is 1:20 to 20:1. The granule is capable of controlling elution of the bioactive substance appropriately, and the urethane resin forming the coating film shows degradability in soil.
Owner:SUMITOMO CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com