Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3846results about "Electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
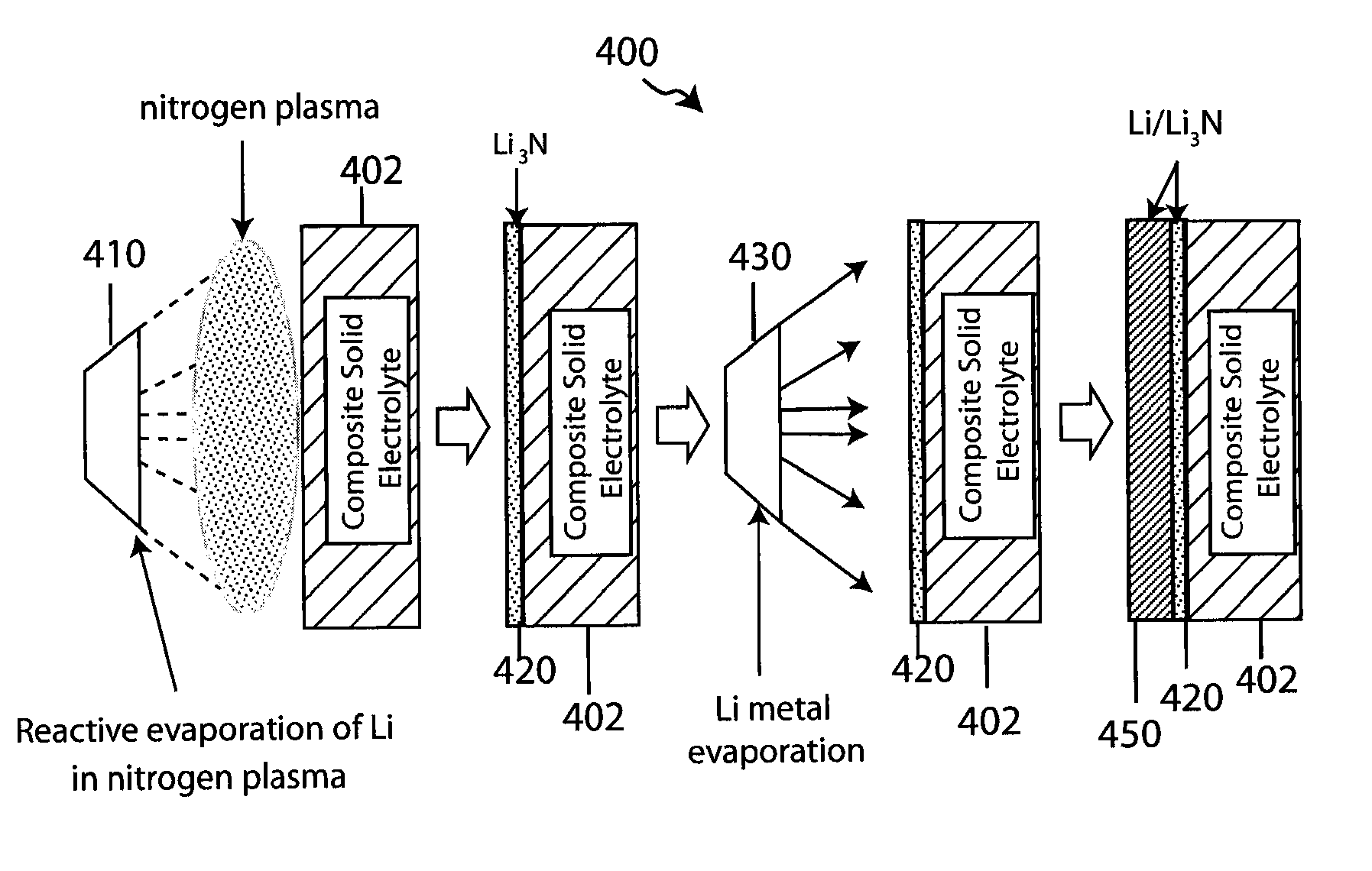
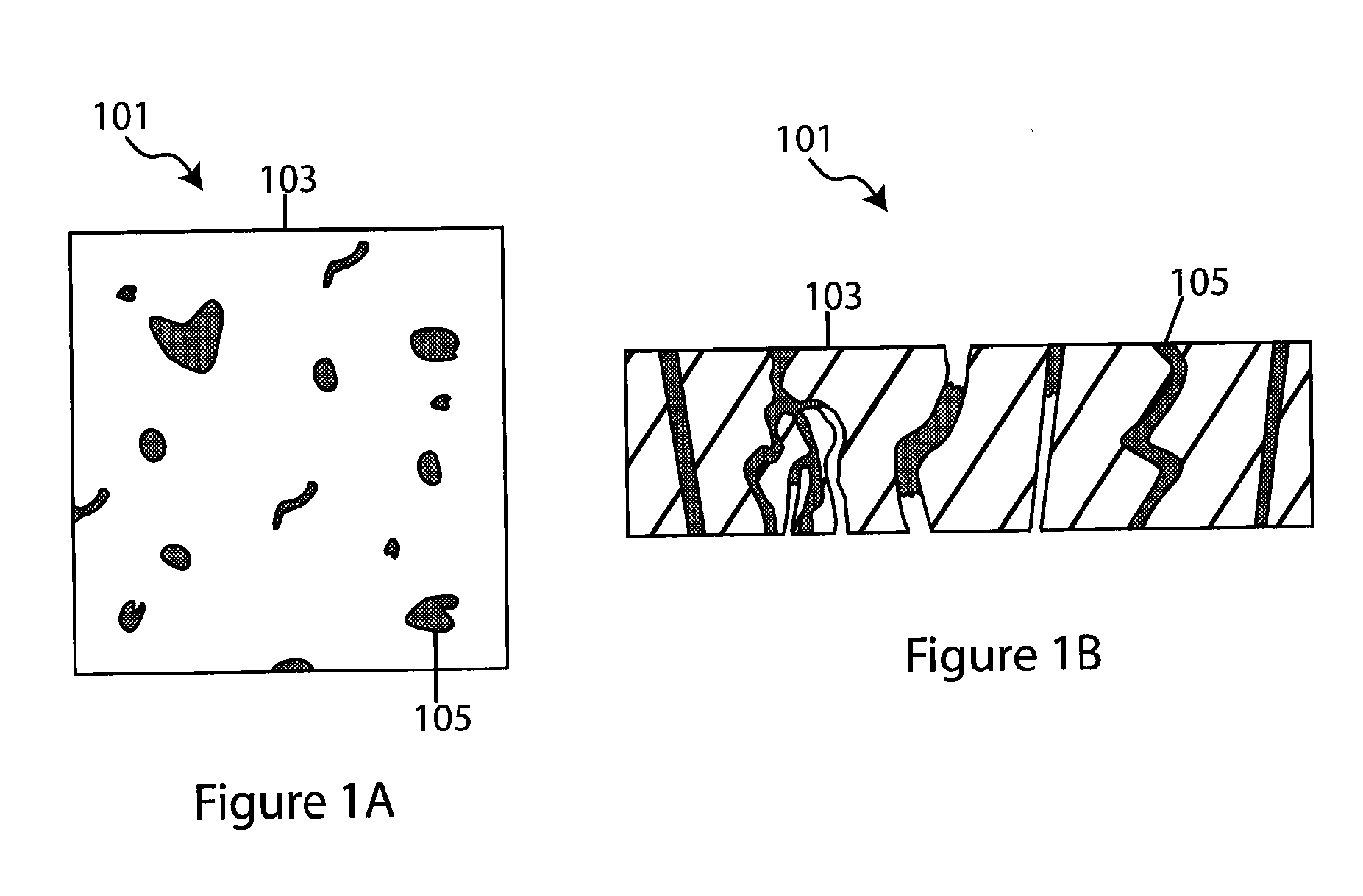
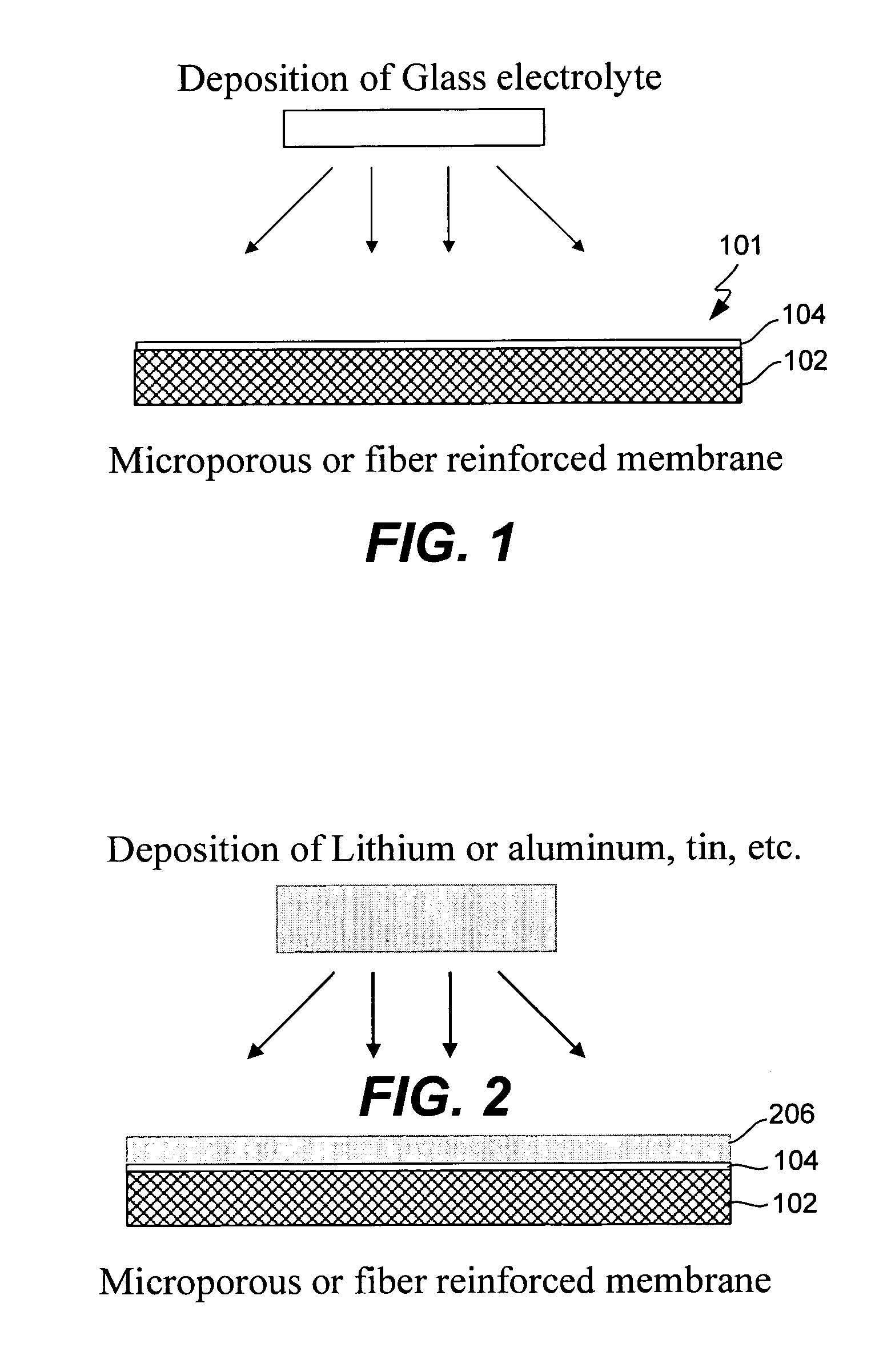
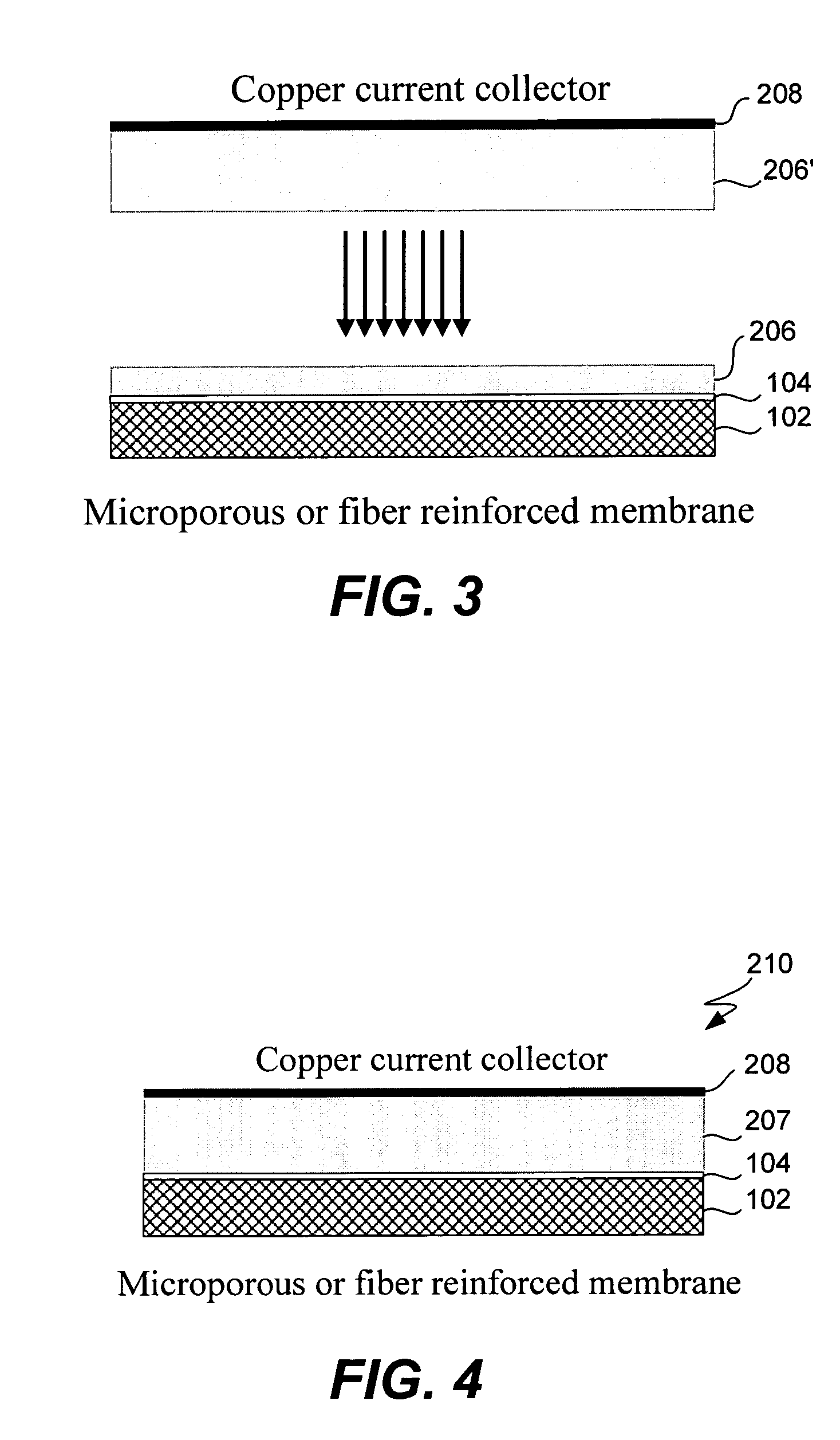
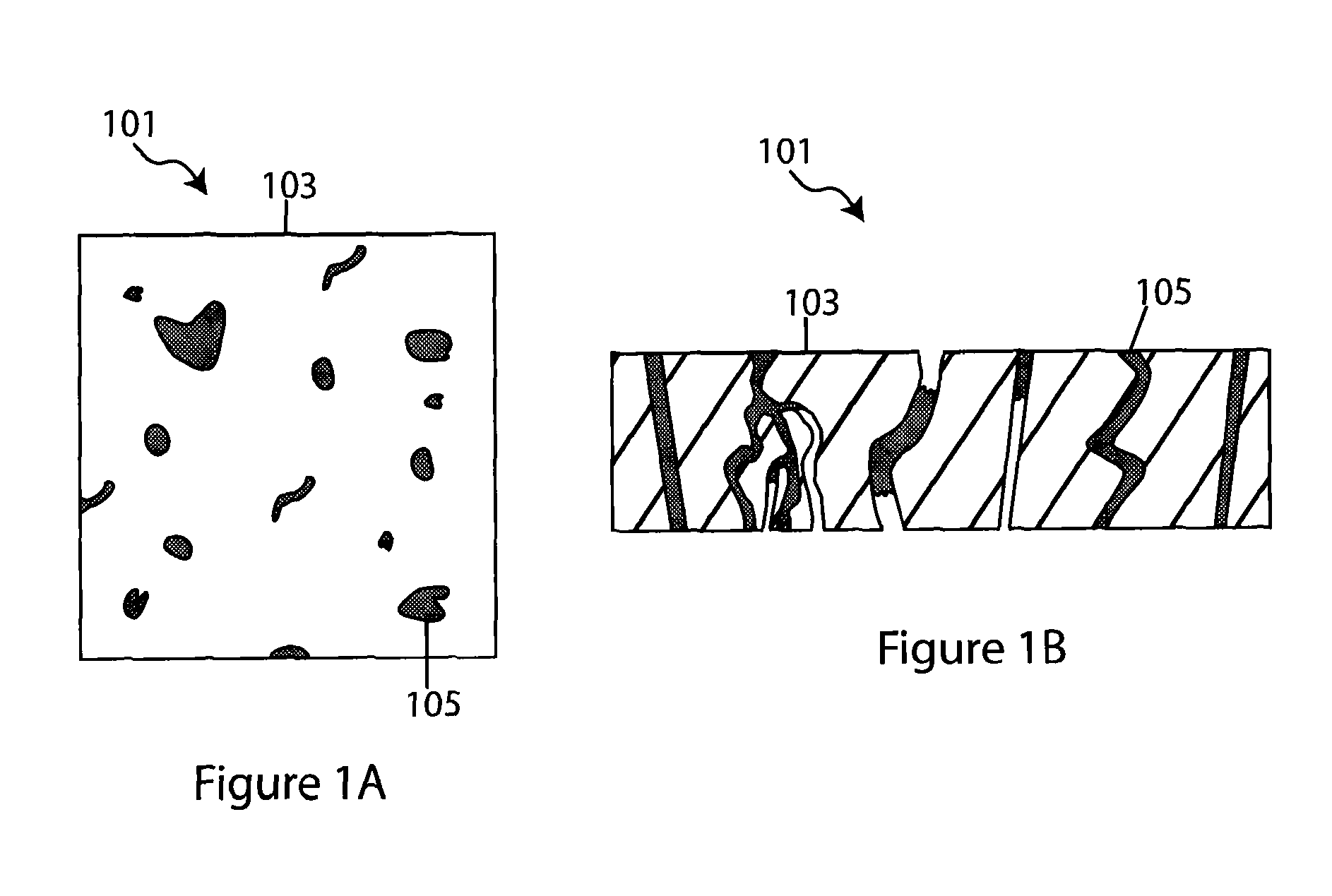
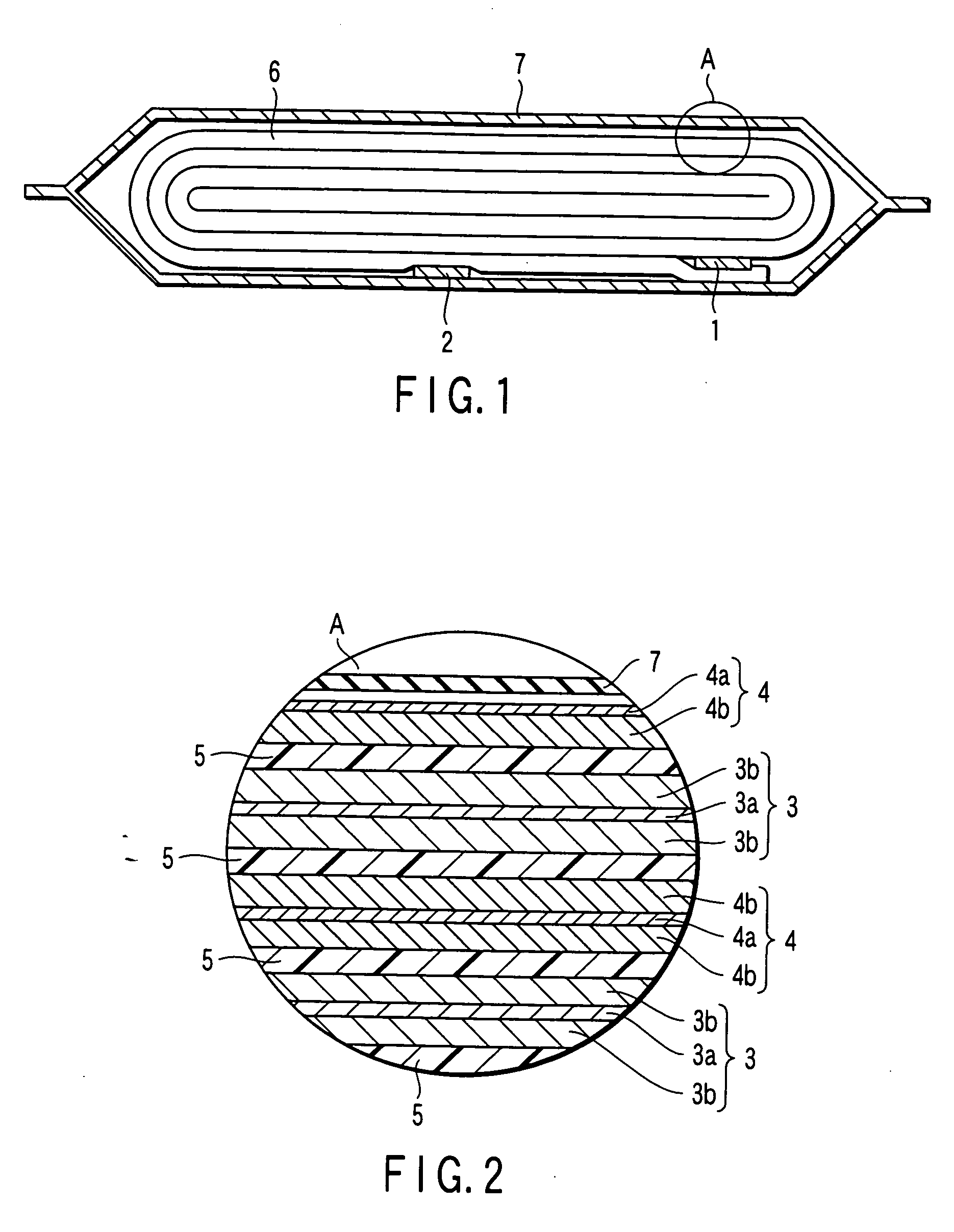
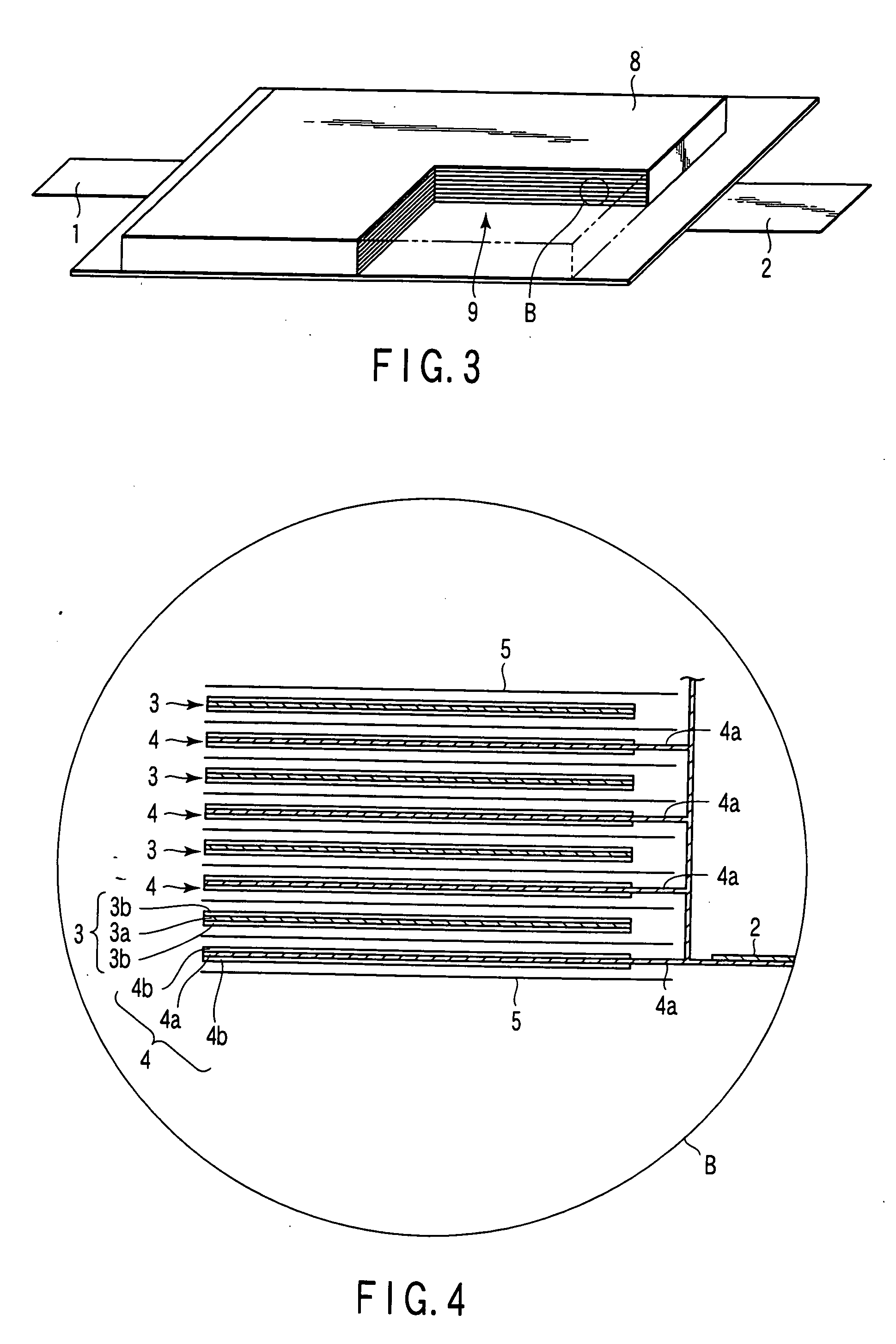
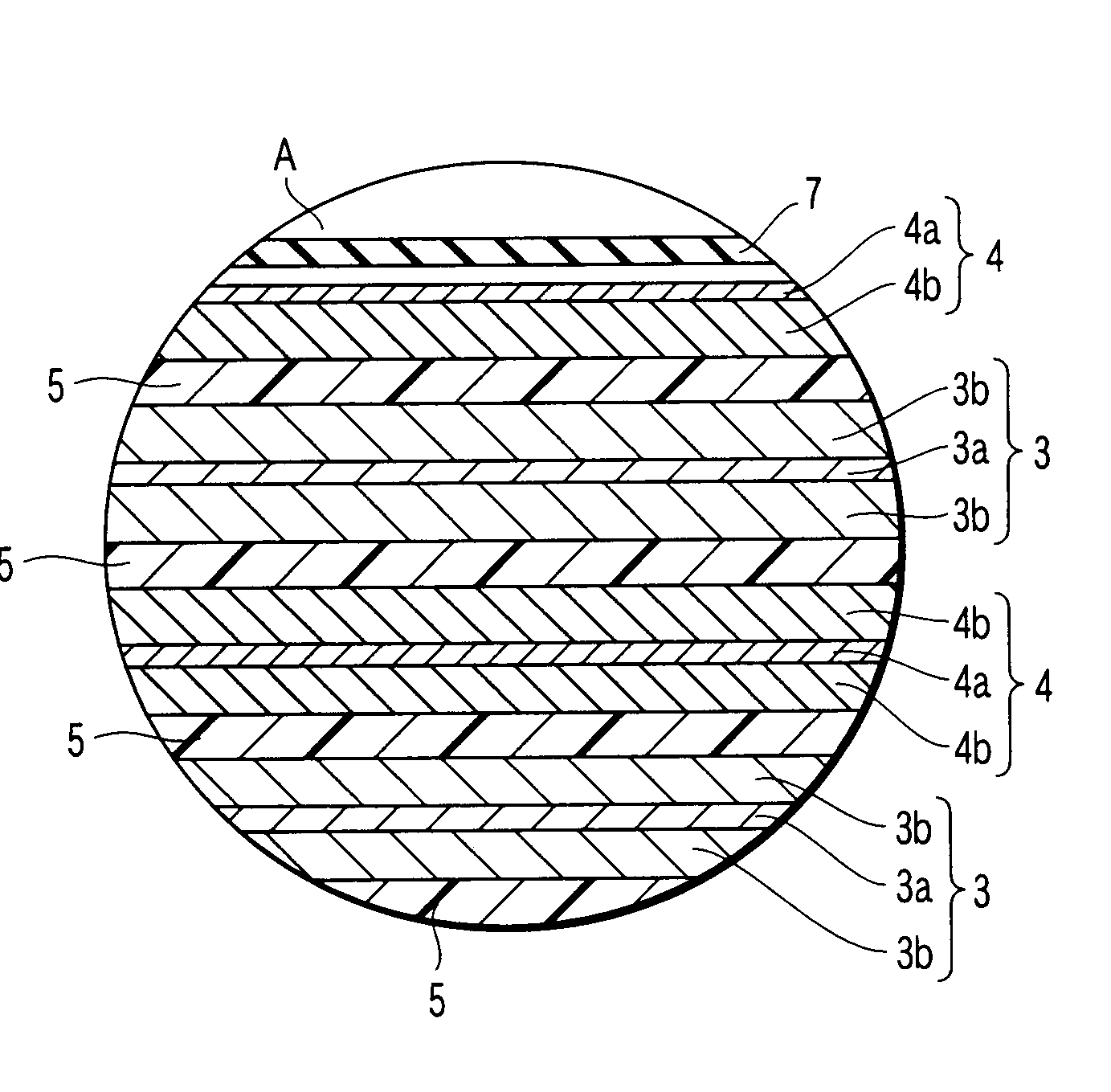
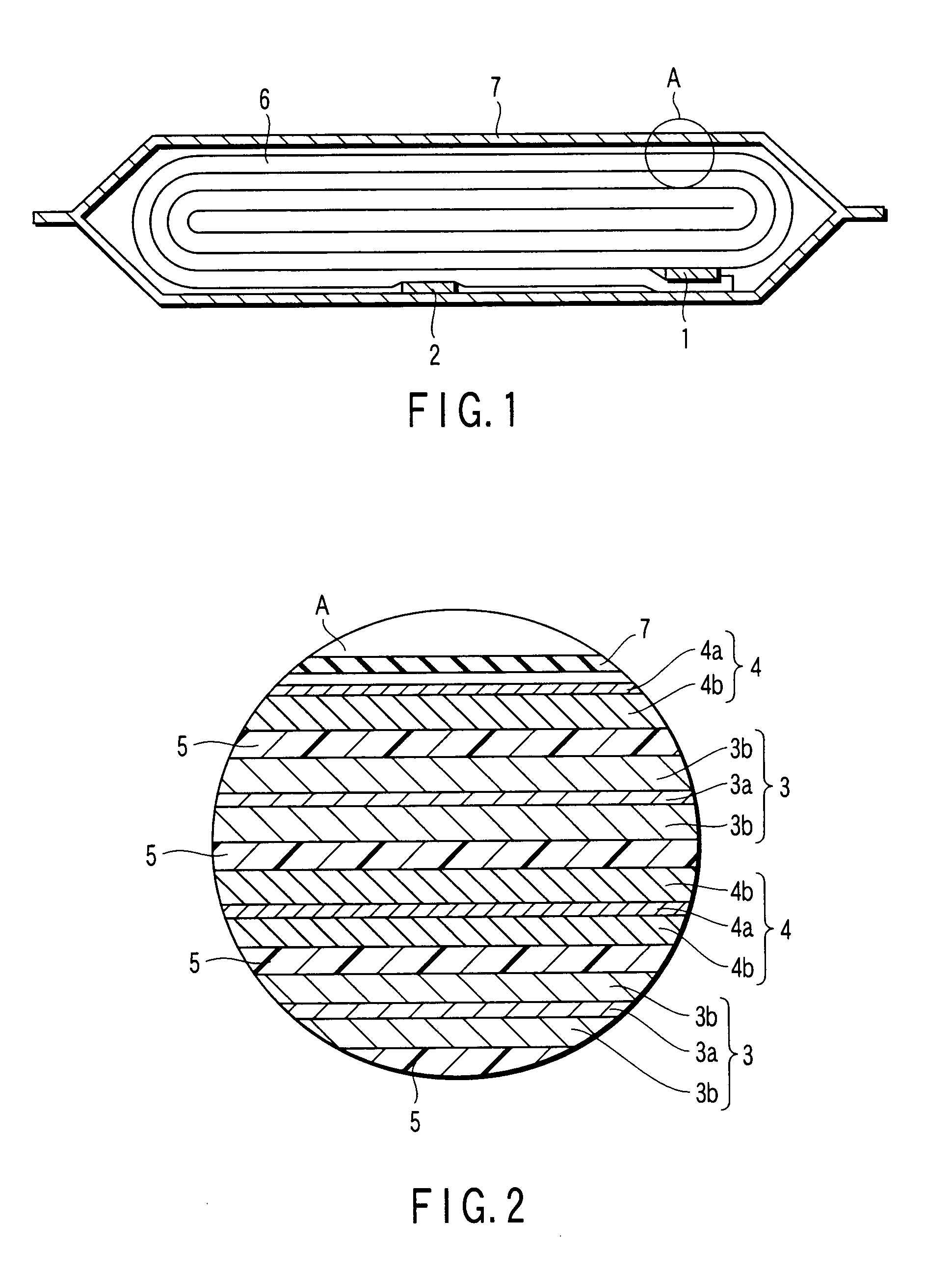
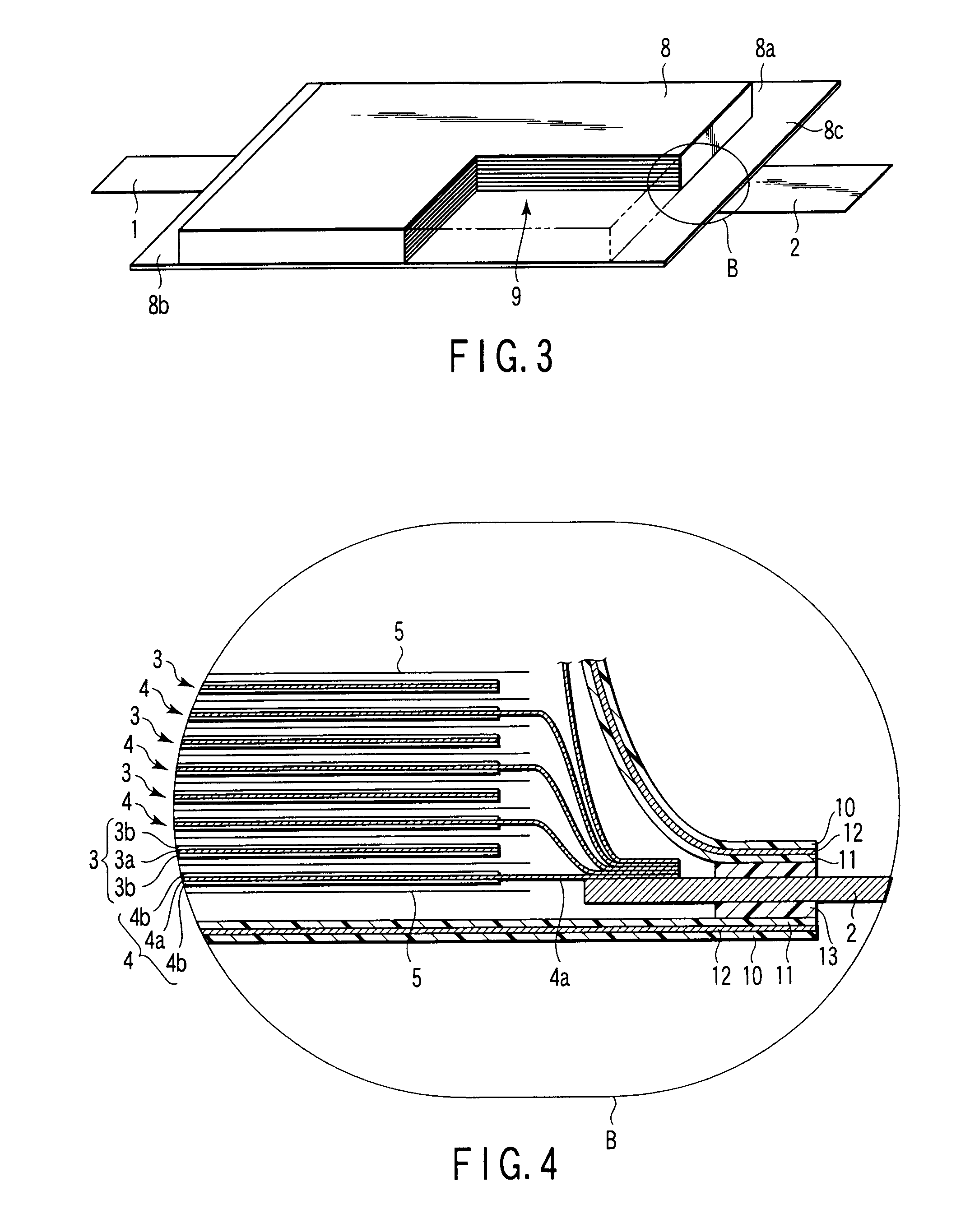
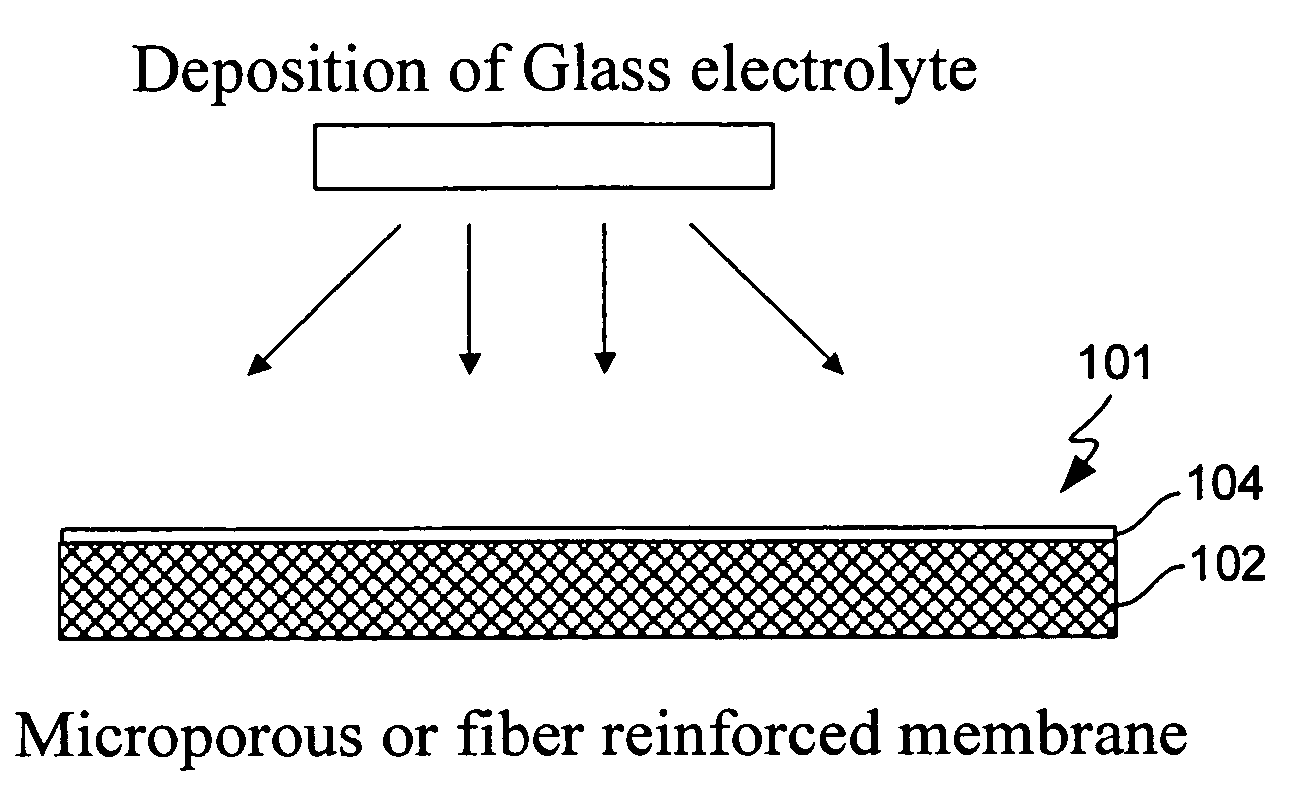
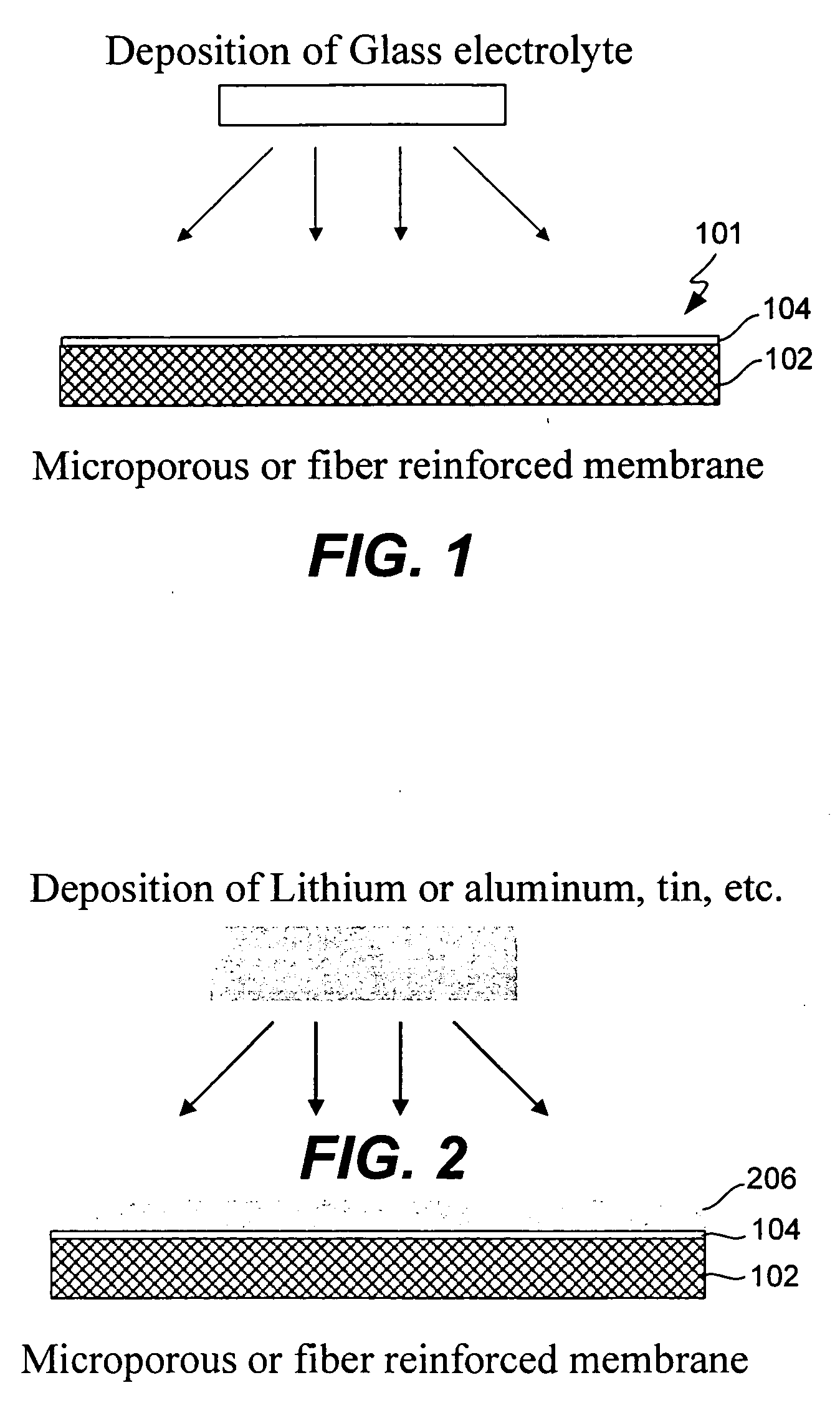
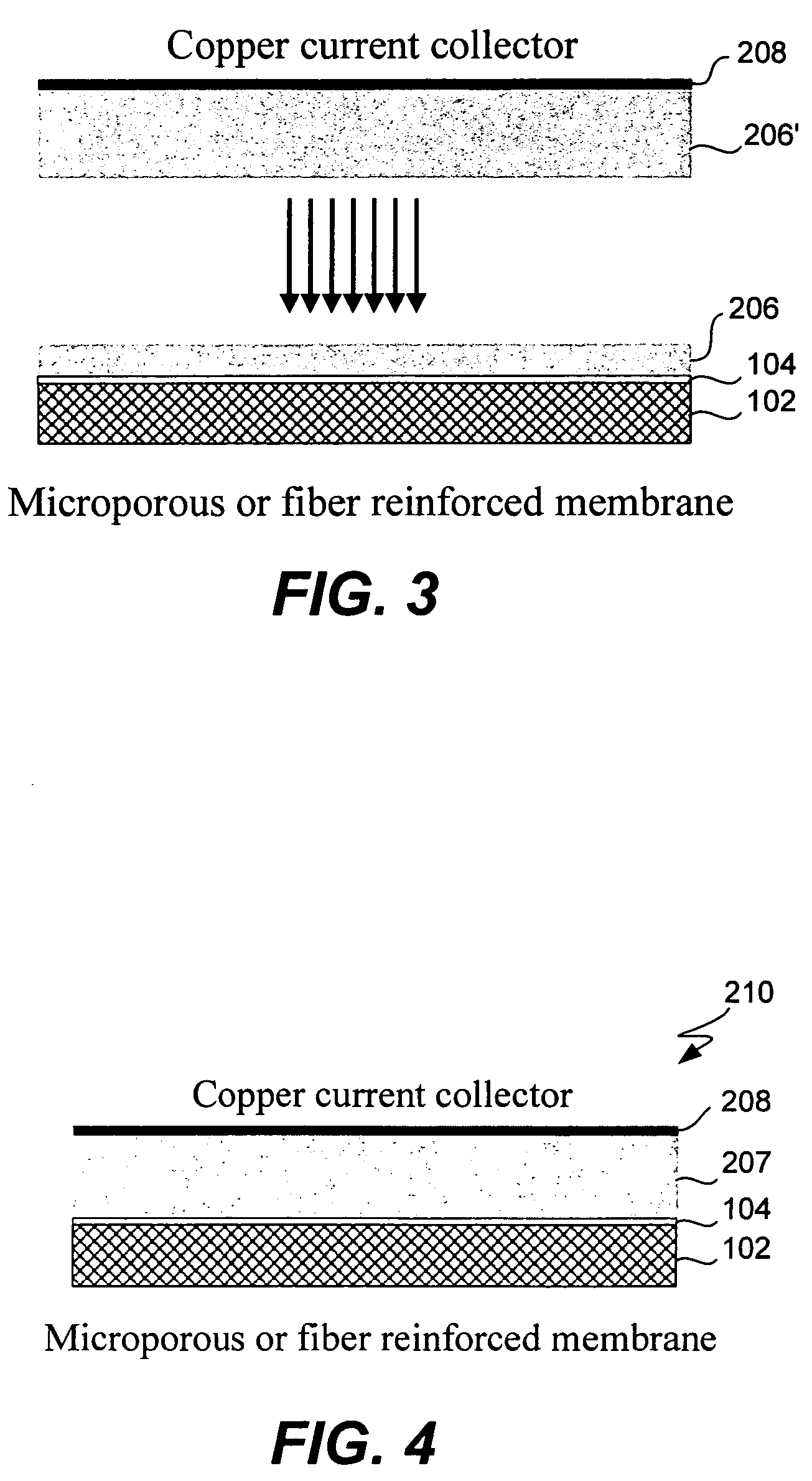
Composite solid electrolyte for protection of active metal anodes
ActiveUS20070172739A1Eliminate through-porosityHigh metal ion conductivityCell electrodesPrimary cellsPorosityTectorial membrane
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
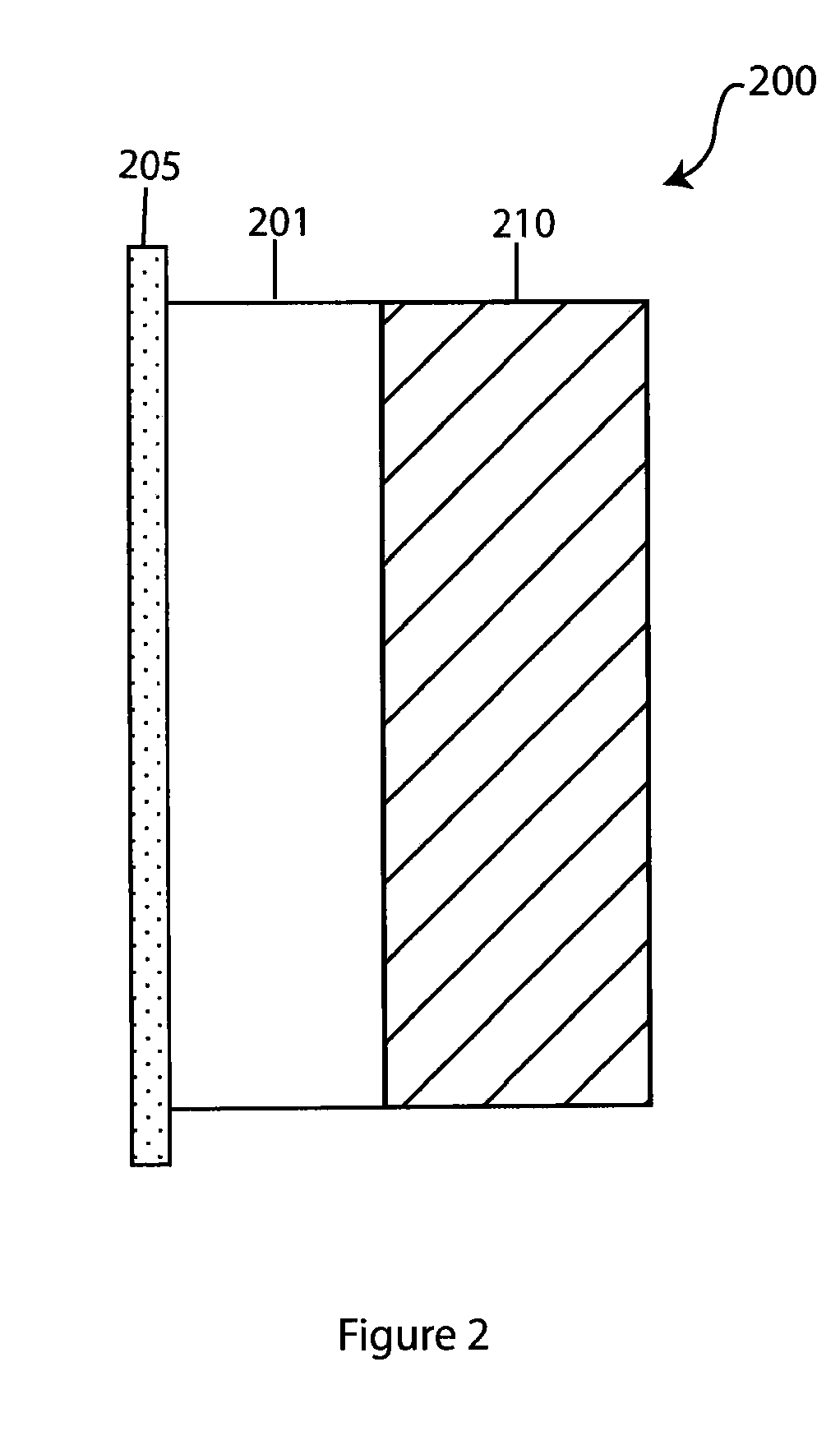
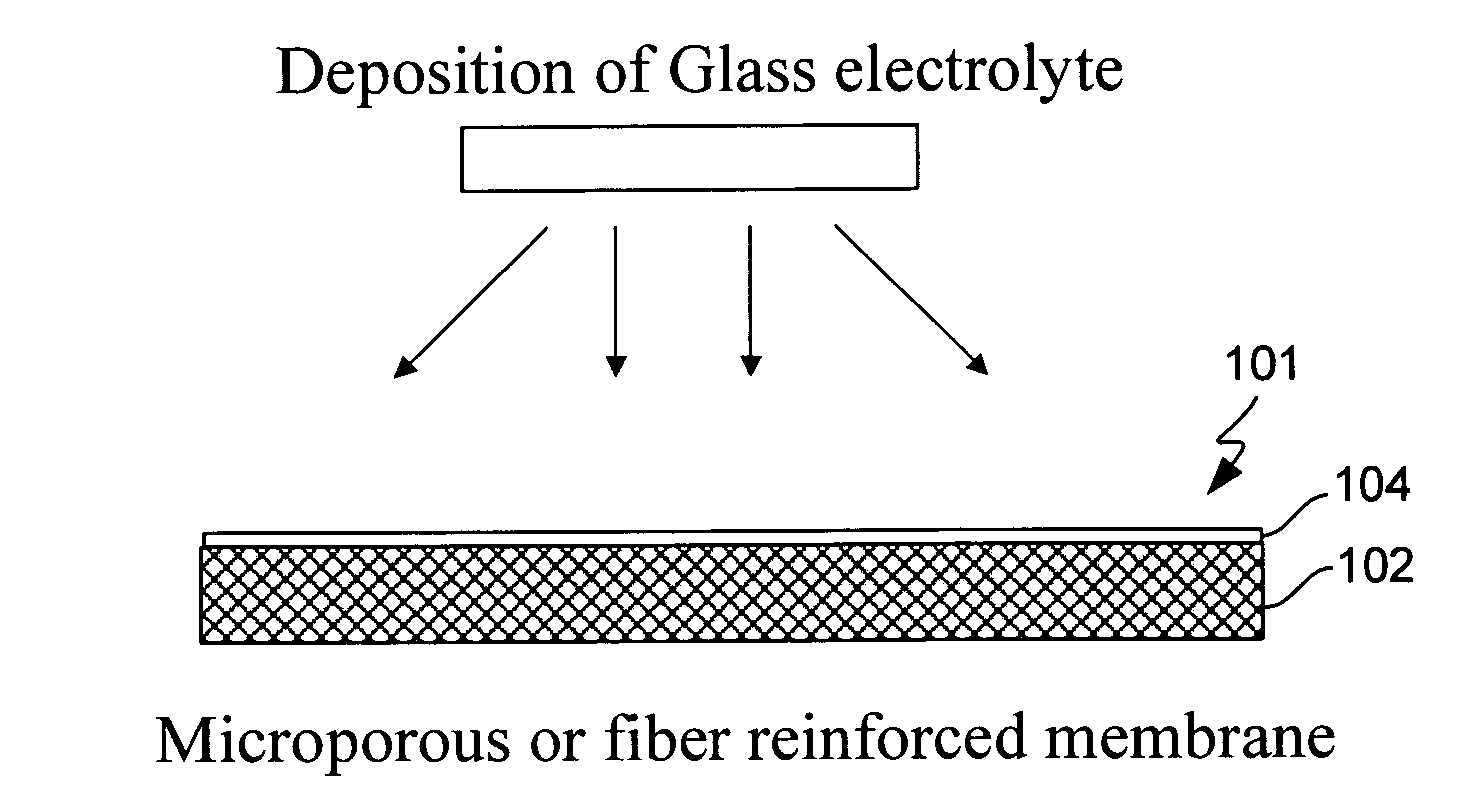
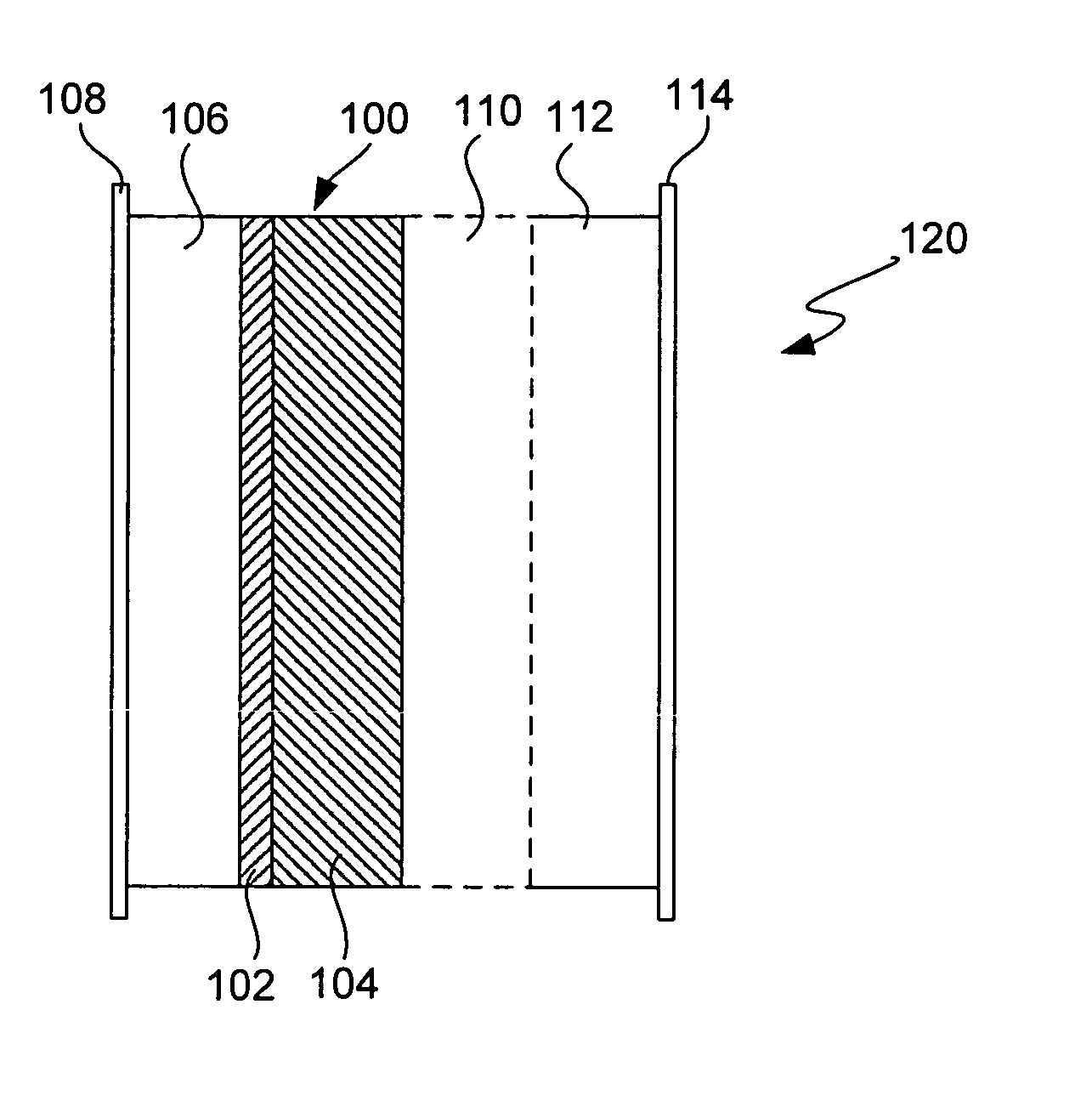
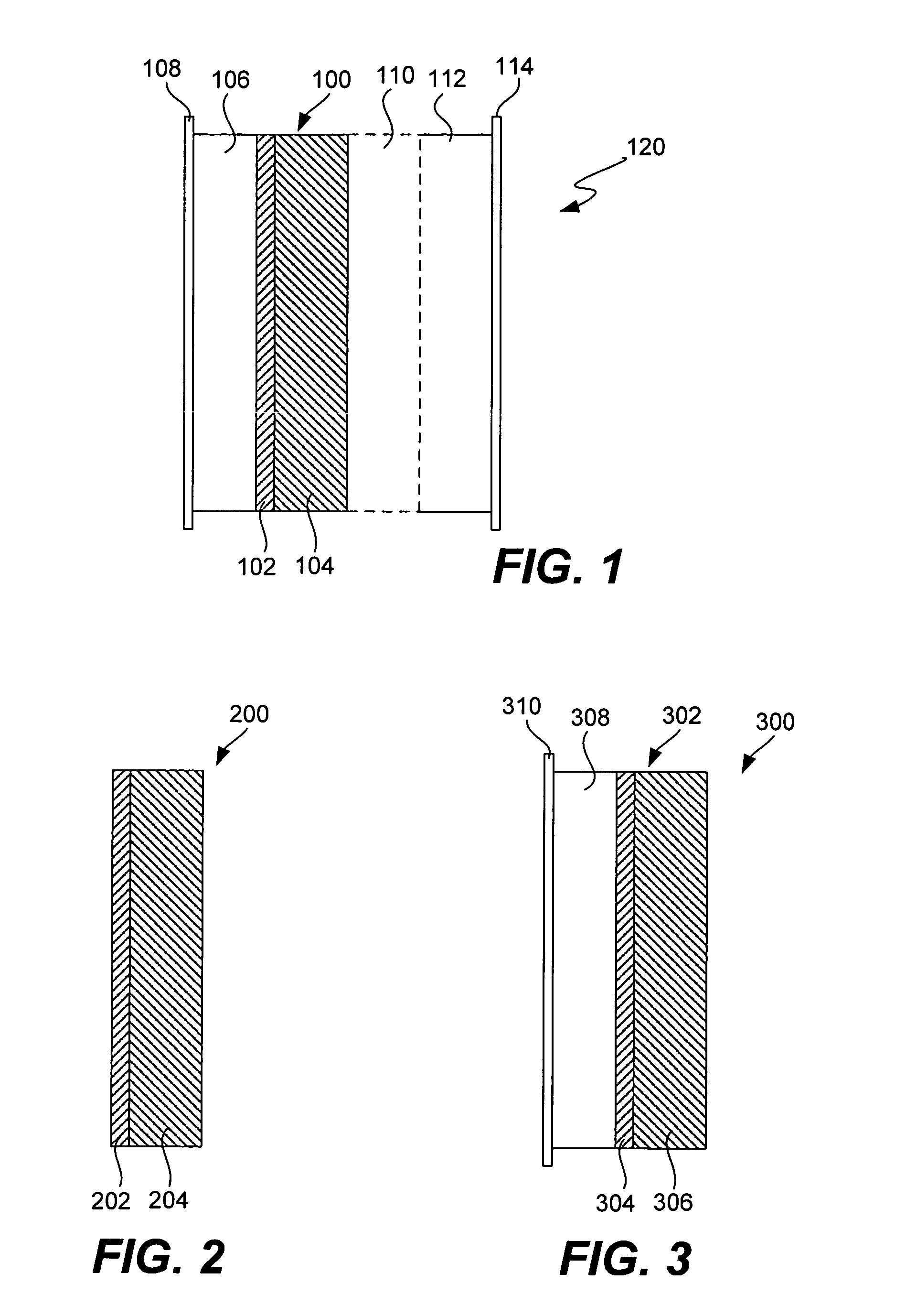
Electrochemical device separator structures with barrier layer on non-swelling membrane
InactiveUS7070632B1Avoid harmful reactionsElectrode carriers/collectorsSolid electrolyte cellsElectrochemistryFluid electrolytes
Disclosed are electrochemical device separator structures which include a substantially impervious active metal ion conducting barrier layer material, such as an ion conducting glass, is formed on an active metal ion conducting membrane in which elongation due to swelling on contact with liquid electrolyte is constrained in at least two of three orthogonal dimensions of the membrane. The non-swelling character of the membrane prevents elongation in the x-y (or lateral, relative to the layers of the composite) orthogonal dimensions of the membrane when it is contacted with liquid electrolyte that would otherwise cause the barrier layer to rupture. Substantial swelling of the membrane, if any, is limited to the z (or vertical, relative to the layers of the composite) dimension.
Owner:POLYPLUS BATTERY CO INC
Ionically conductive composites for protection of active metal anodes
ActiveUS7282296B2Easy to manufactureImprove battery performanceFinal product manufactureElectrode carriers/collectorsChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
Lithium ion conductive solid electrolyte and production process thereof
InactiveUS20070231704A1Increase battery capacitySimple and convenient manufactureSecondary cellsSolid electrolyte cellsPorosityLithium metal
A lithium ion conductive solid electrolyte formed by sintering a molding product containing an inorganic powder and having a porosity of 10 vol % or less, which is obtained by preparing a molding product comprising an inorganic powder as a main ingredient and sintering the molding product after pressing and / or sintering the same while pressing, the lithium ion conductive solid electrolyte providing a solid electrolyte having high battery capacity without using a liquid electrolyte, usable stably for a long time and simple and convenient in manufacture and handling also in industrial manufacture in the application use of secondary lithium ion battery or primary lithium battery, a solid electrolyte having good charge / discharge cyclic characteristic in the application use of the secondary lithium ion battery a solid electrolyte with less water permeation and being safe when used for lithium metal-air battery in the application use of primary lithium battery, a manufacturing method of the solid electrolyte, and a secondary lithium ion battery and a primary lithium battery using the solid electrolyte.
Owner:OHARA
Garnet-type lithium ion-conducting oxide and all-solid-state lithium ion secondary battery containing the same
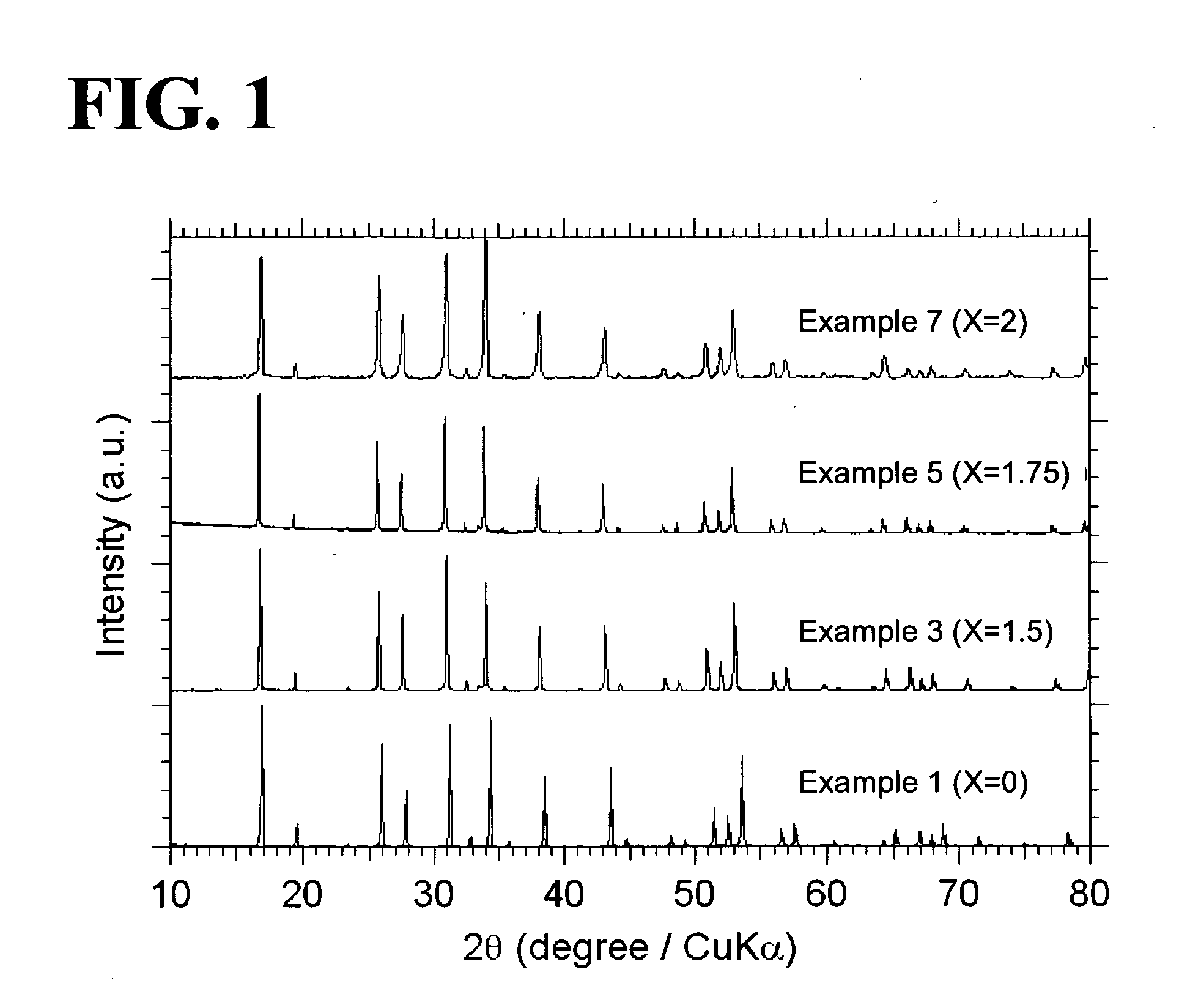
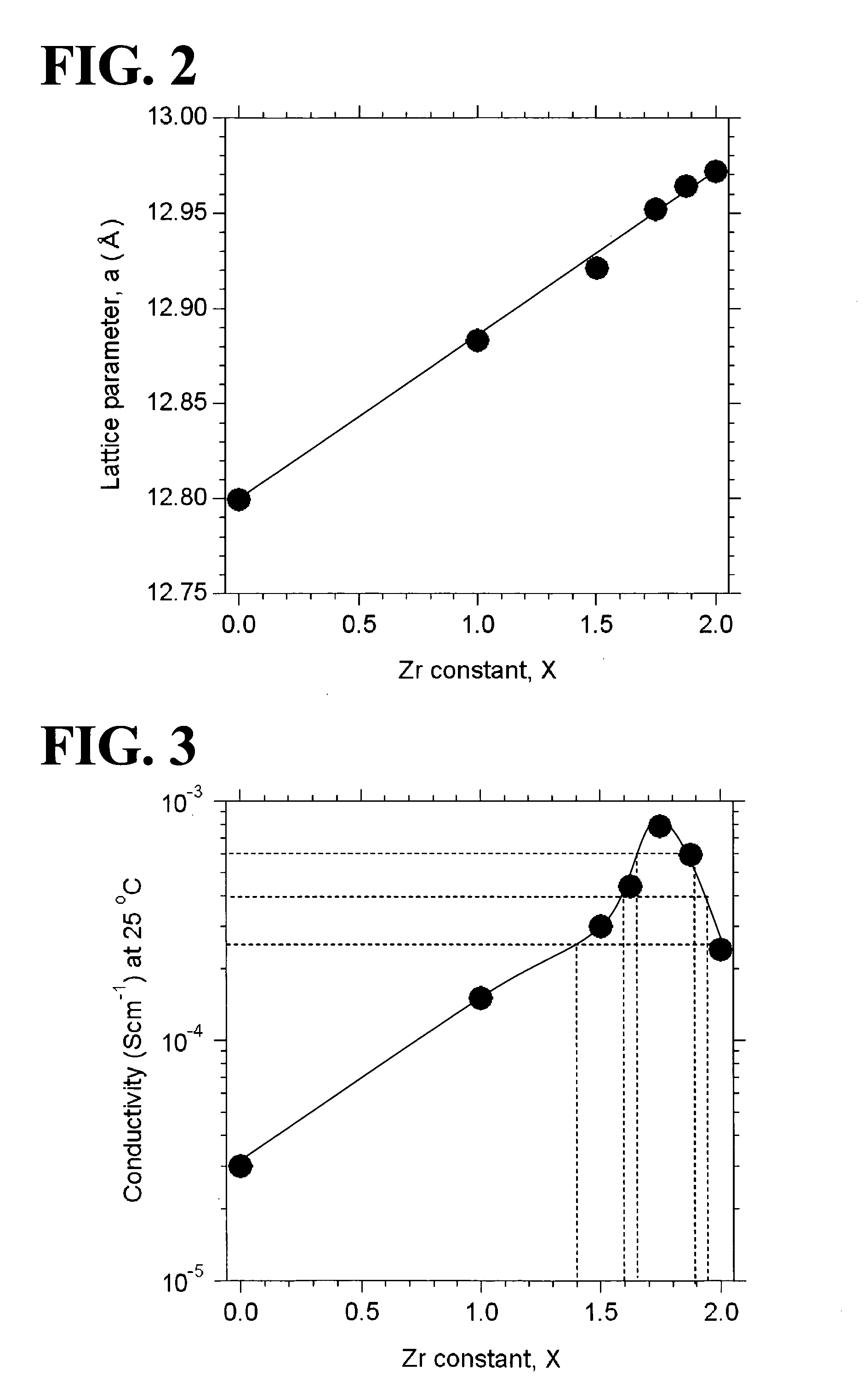
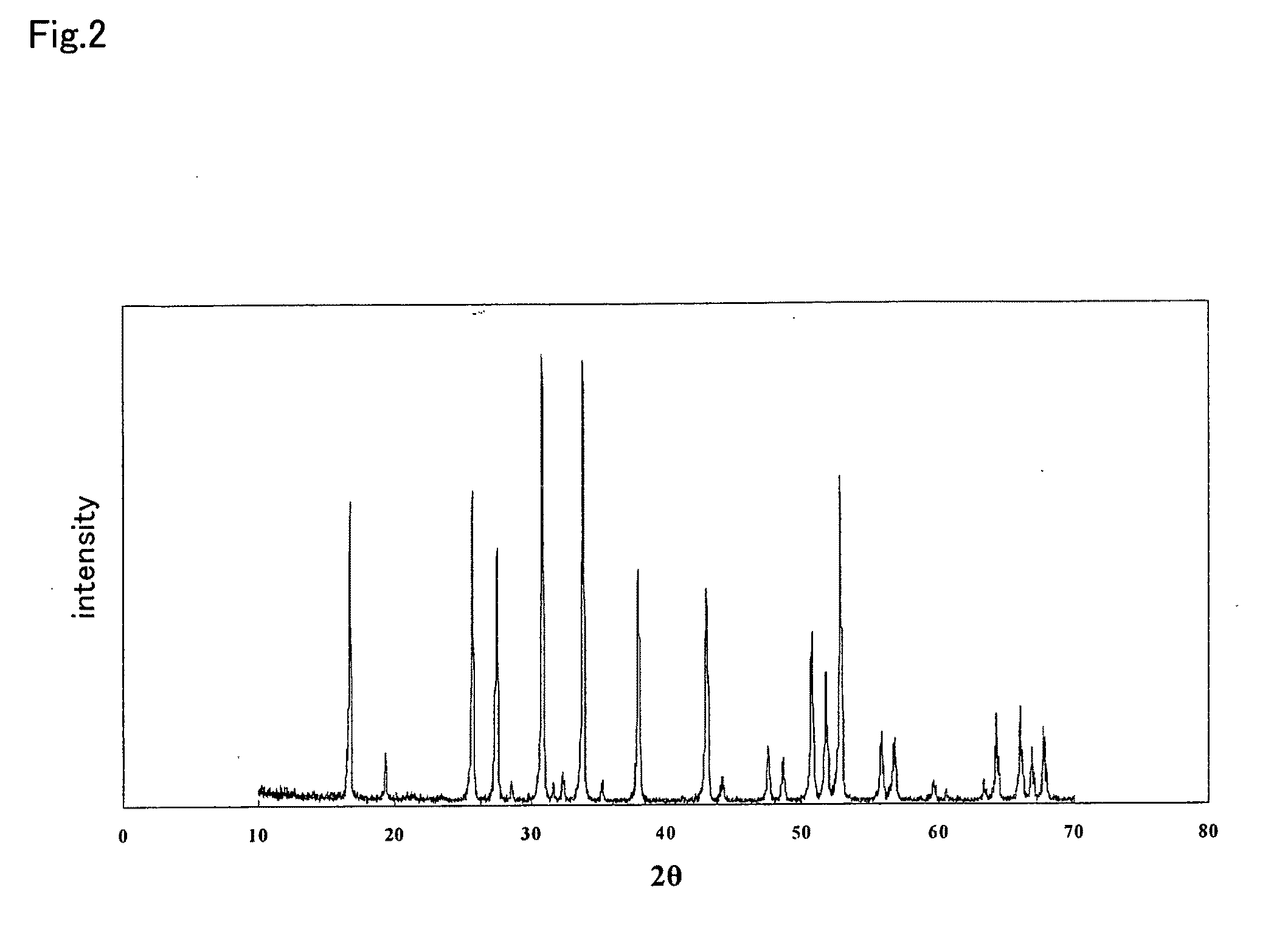
ActiveUS20110244337A1Improve lithium ion conductivitySmall rate of changeSolid electrolyte cellsElectrolytesAll solid stateIntensity normalization
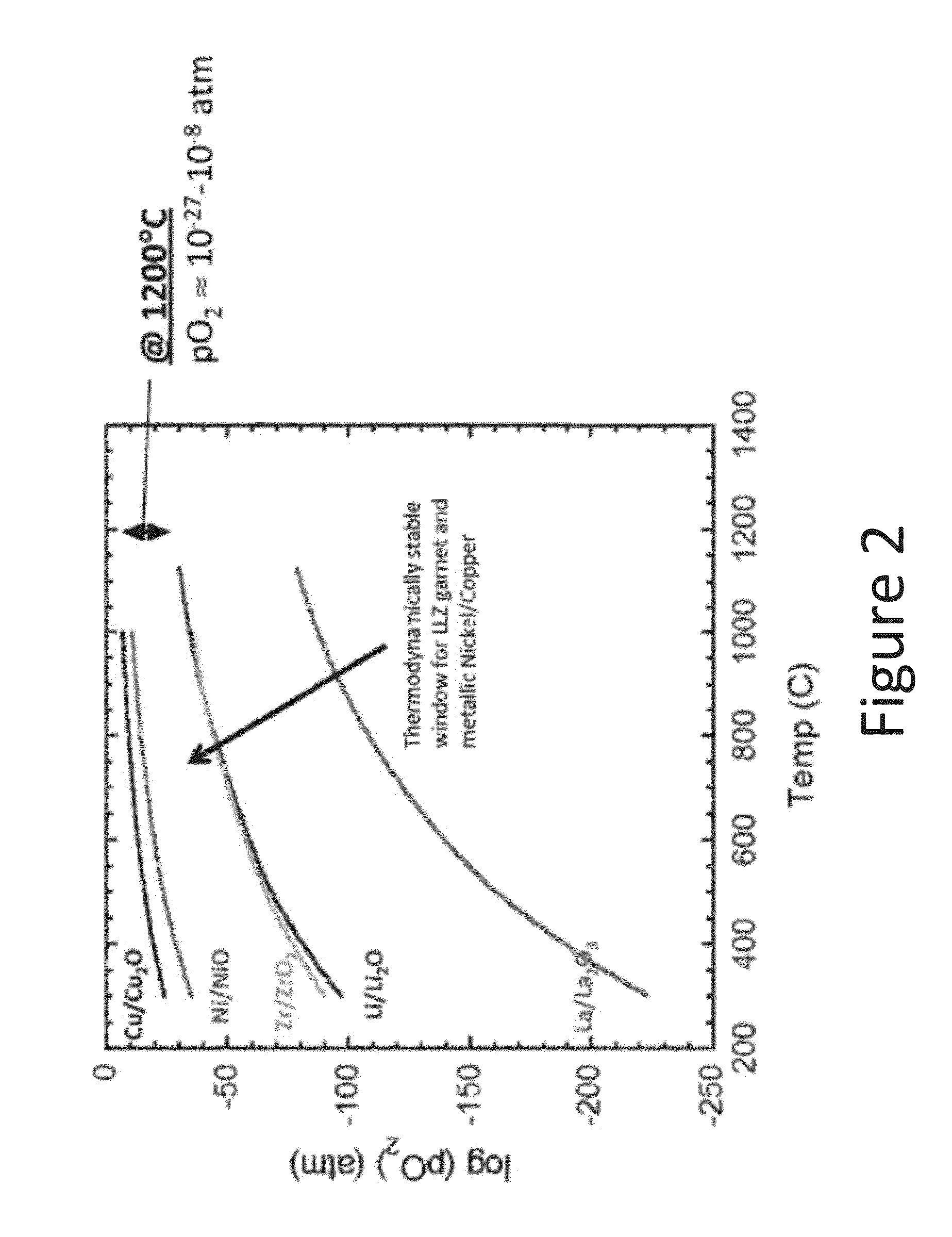
An all-solid-state lithium ion secondary battery containing a novel garnet-type oxide serving as a solid electrolyte. The garnet-type lithium ion-conducting oxide is one represented by the formula Li5+XLa3(ZrX, A2-X)O12, wherein A is at least one selected from the group consisting of Sc, Ti, V, Y, Nb, Hf, Ta, Al, Si, Ga, Ge, and Sn and X satisfies the inequality 1.4≦X<2, or is one obtained by substituting an element having an ionic radius different from that of Zr for Zr sites in an garnet-type lithium ion-conducting oxide represented by the formula Li7La3Zr2O12, wherein the normalized intensity of an X-ray diffraction (XRD) pattern with a diffraction peak, as normalized on the basis of the intensity of a diffraction peak, is 9.2 or more.
Owner:TOYOTA CENT RES & DEV LAB INC
Liquid Composite Compositions Using Non-Volatile Liquids and Nanoparticles and Uses Thereof
InactiveUS20080209876A1Reduce CTEImprove performanceElectrolytic capacitorsCell electrodesOrganic solventNanoparticle
A solvent composition comprising an organic solvent; dispersed nanoparticles; and a non-volatile electrolyte.
Owner:ESIONIC
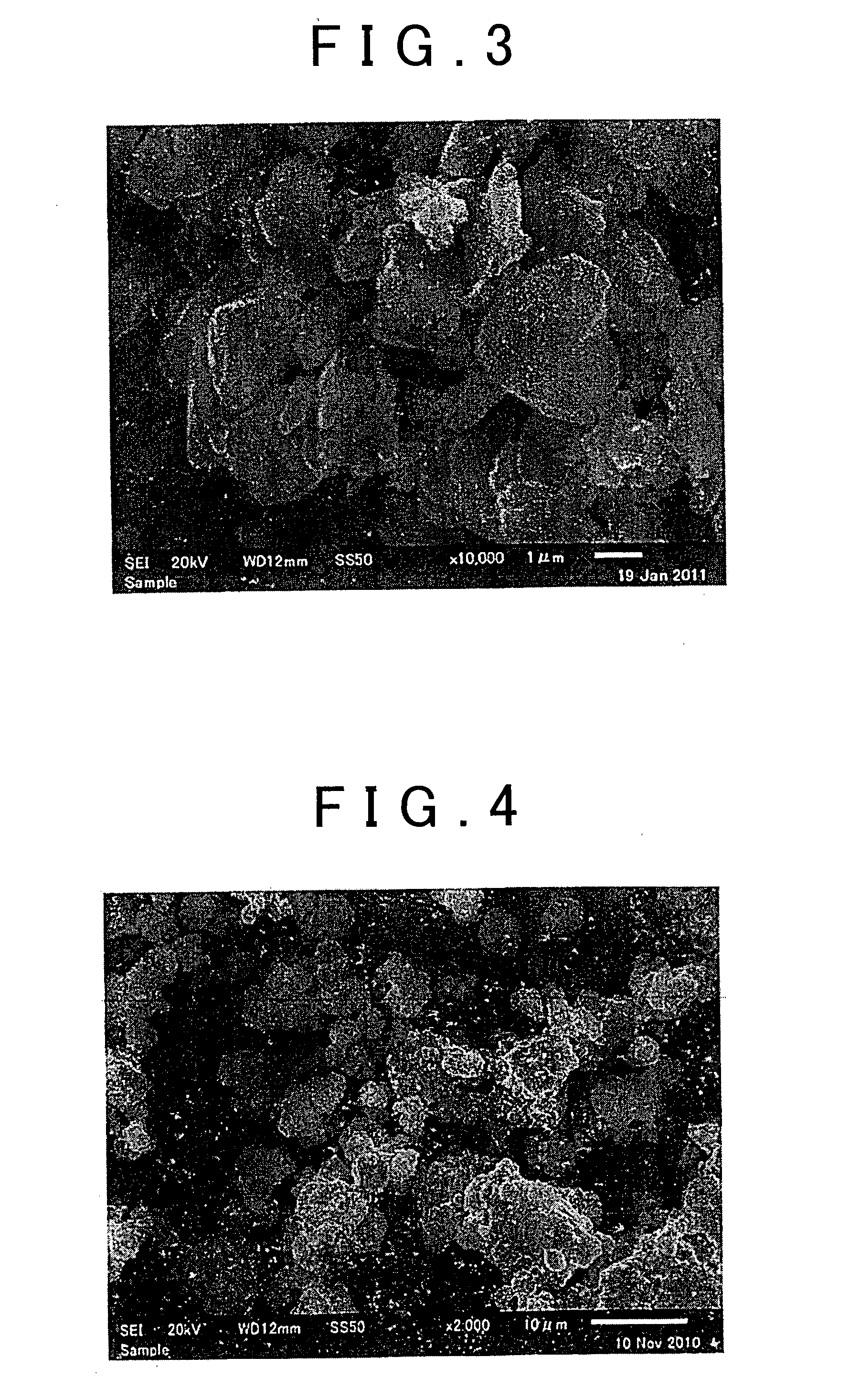
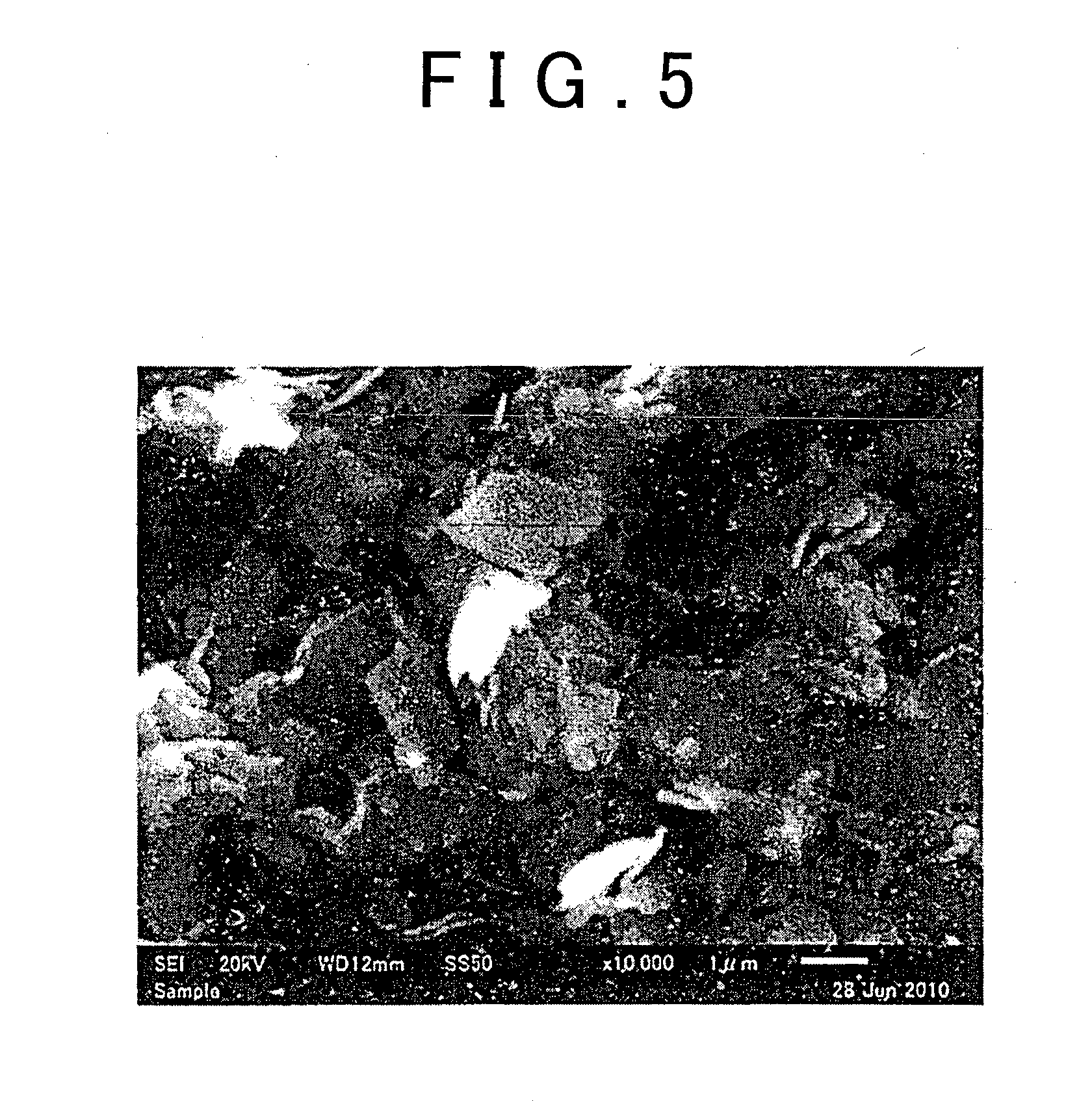
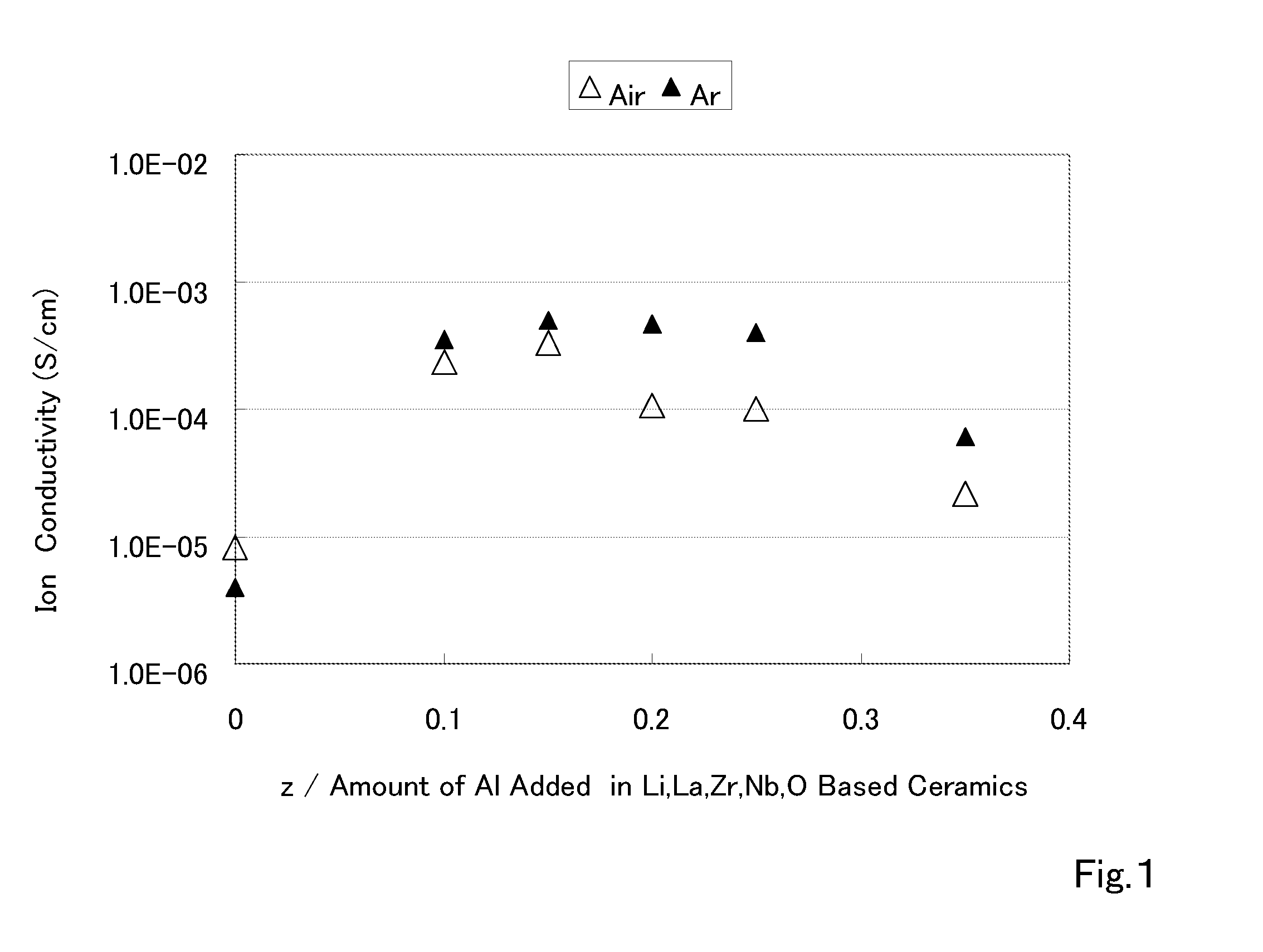
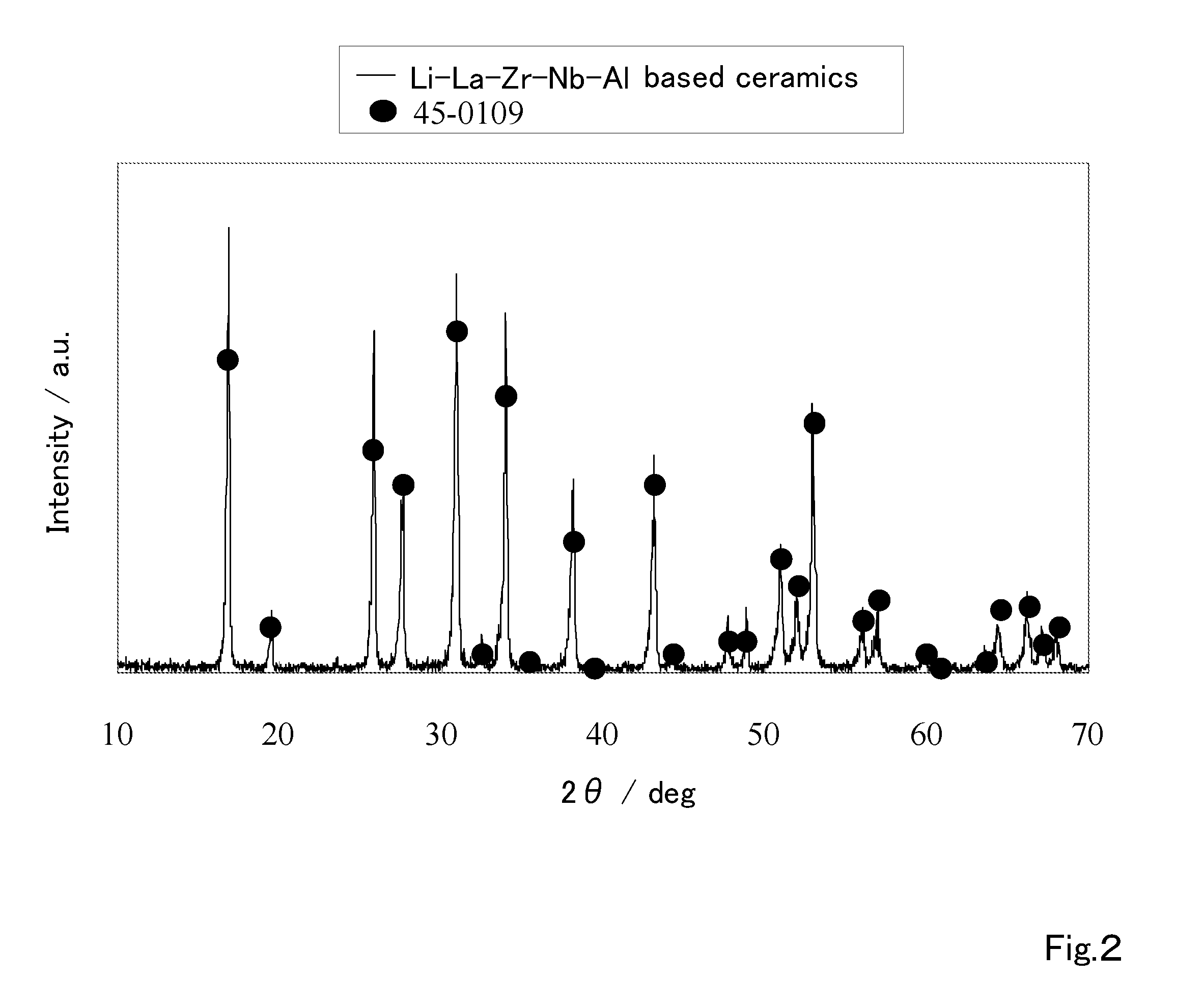
Ceramic material and process for producing the same
ActiveUS20100047696A1Compactness sufficientOvercome lack of conductivitySolid electrolyte cellsCapacitor electrolytes/absorbentsLithiumCrystal structure
A ceramic material that can exhibit sufficient compactness and lithium (Li) conductivity to enable the use thereof as a solid electrolyte material for a lithium secondary battery and the like is provided. The ceramic material contains aluminum (Al) and has a garnet-type crystal structure or a garnet-like crystal structure containing lithium (Li), lanthanum (La), zirconium (Zr) and oxygen (O).
Owner:NGK INSULATORS LTD
Power generation system and method
InactiveUS6915869B2Internal combustion piston enginesElectric propulsion mountingInternal combustion engineFuel supply
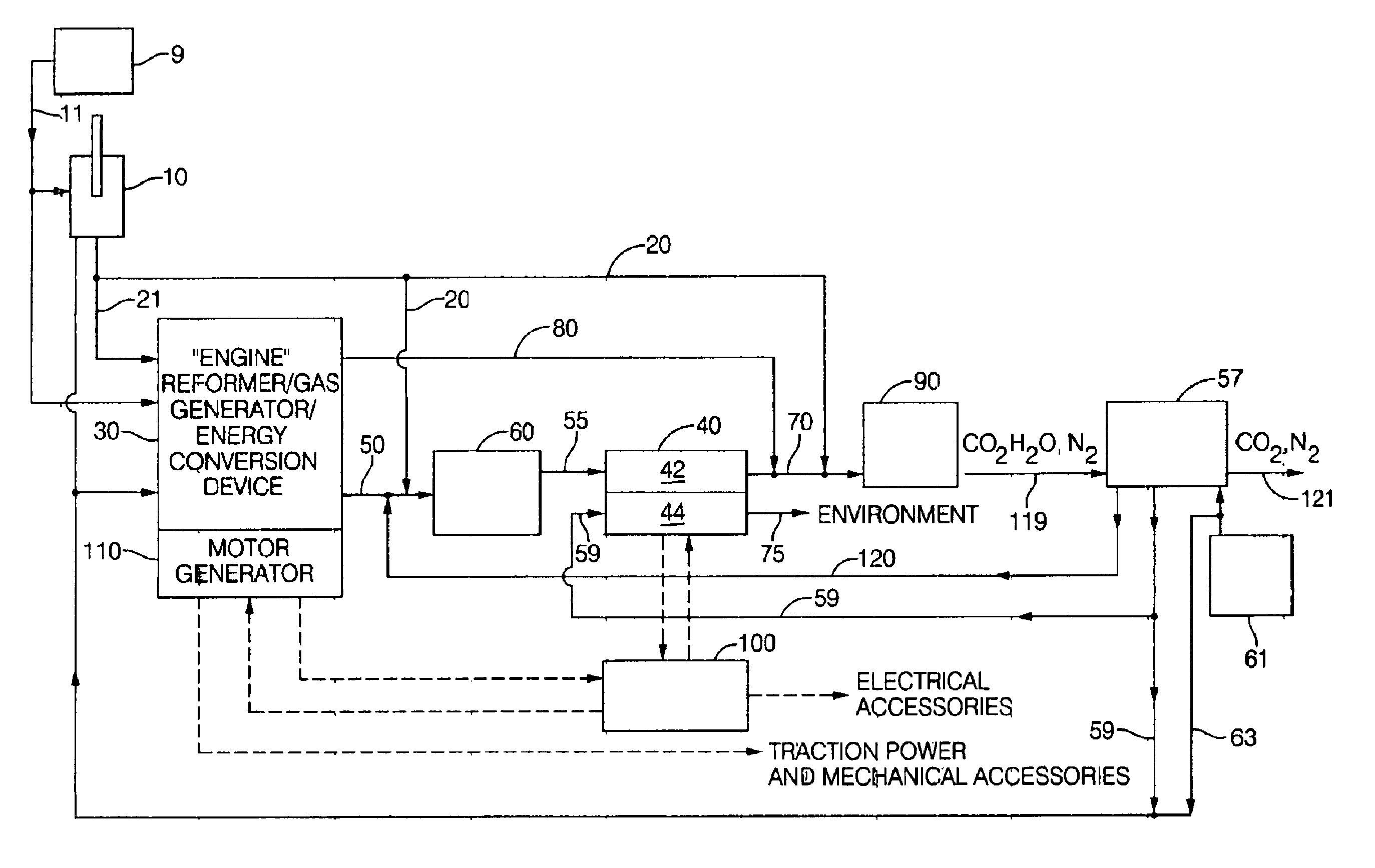
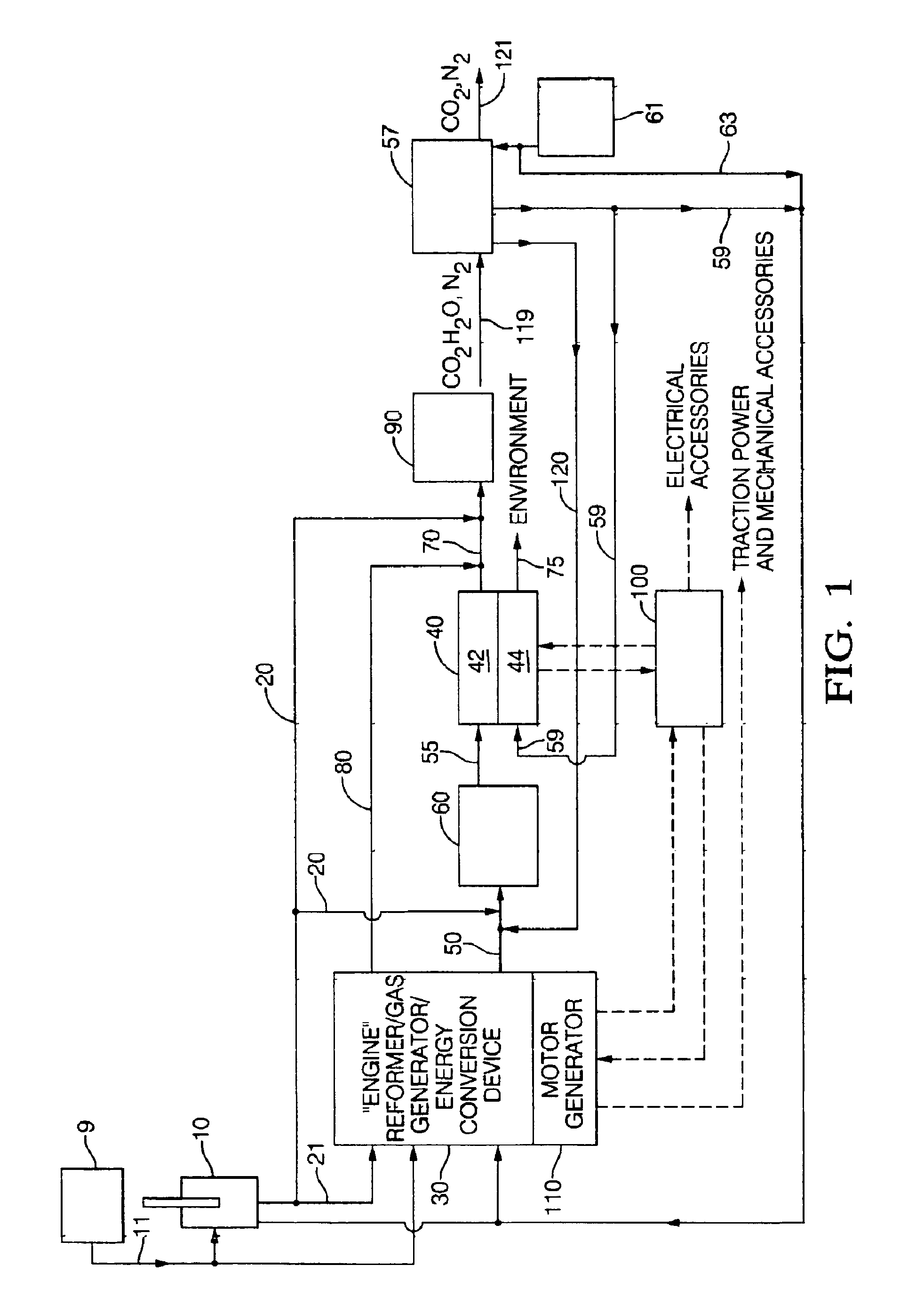
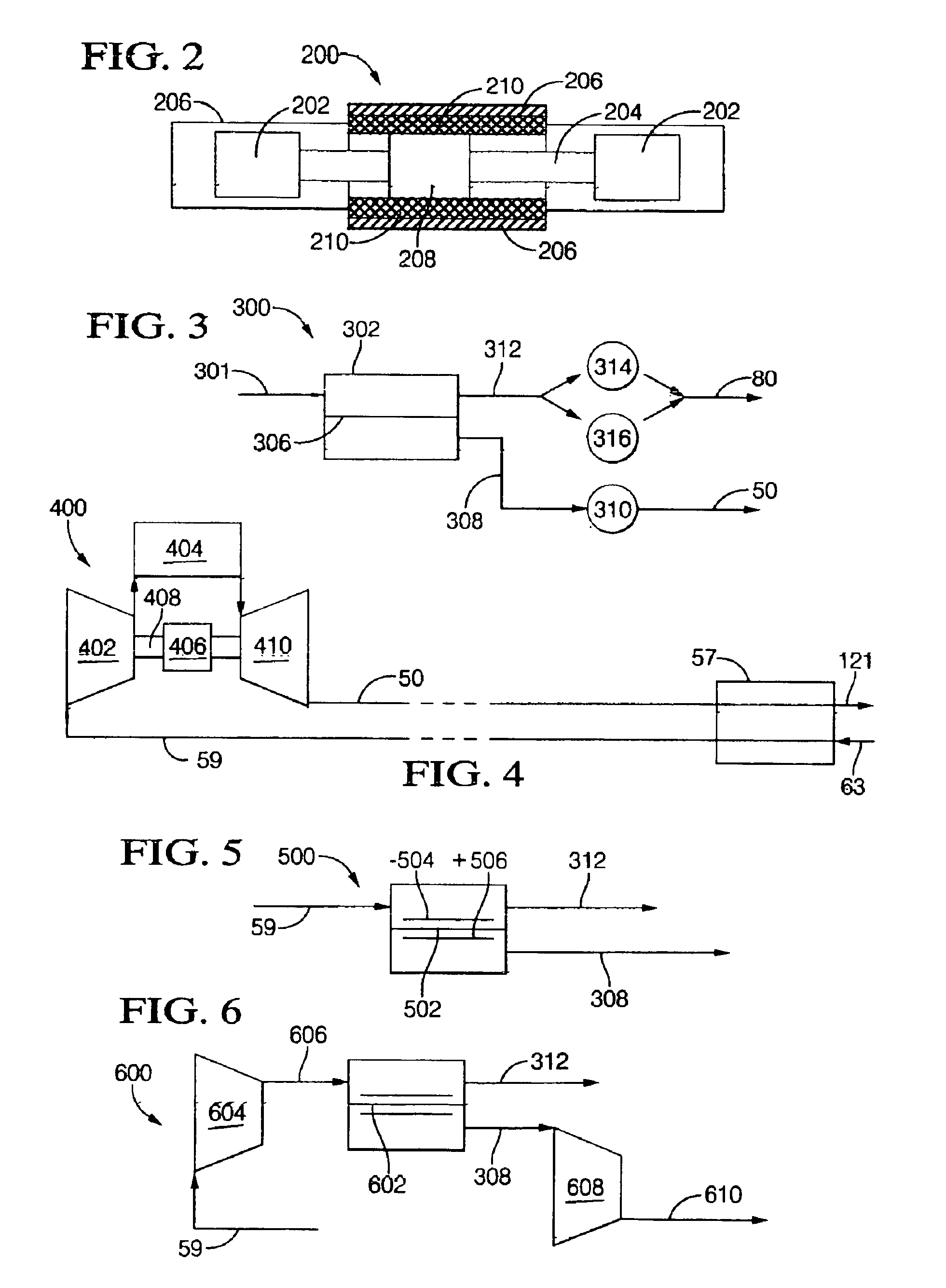
A power generation system and method providing an engine configured to produce hydrogen rich reformate to feed a solid oxide fuel cell includes an engine having an intake and an exhaust; an air supply in fluid communication with the engine intake; a fuel supply in fluid communication with the engine intake; at least one solid oxide fuel cell having an air intake in fluid communication with an air supply, a fuel intake in fluid communication with the engine exhaust, a solid oxide fuel cell effluent and an air effluent. Engines include a free piston gas generator with rich homogenous charge compression, a rich internal combustion engine cylinder system with an oxygen generator, and a rich inlet turbo-generator system with exhaust heat recovery. Oxygen enrichment devices to enhance production of hydrogen rich engine exhaust include pressure swing absorption with oxygen selective materials, and oxygen separators such as an solid oxide fuel cell oxygen separator and a ceramic membrane oxygen separator.
Owner:DELPHI TECH INC
Ion conducting batteries with solid state electrolyte materials
ActiveUS20140287305A1Final product manufactureActive material electrodesSolid state electrolytePorous layer
Solid-state, ion-conducting batteries with an ion-conducting, solid-state electrolyte. The solid-state electrolyte has at least one porous region (e.g., porous layer) and a dense region (e.g., dense layer). The batteries are, for example, lithium-ion, sodium-ion, or magnesium-ion conducting solid-state batteries. The ion-conducting, solid-state electrolyte is, for example, a lithium-garnet material.
Owner:UNIV OF MARYLAND
Solid state activity-activated battery device and method
InactiveUS6906436B2Sufficient energy storageBatteries circuit arrangementsFinal product manufactureEngineeringElectron
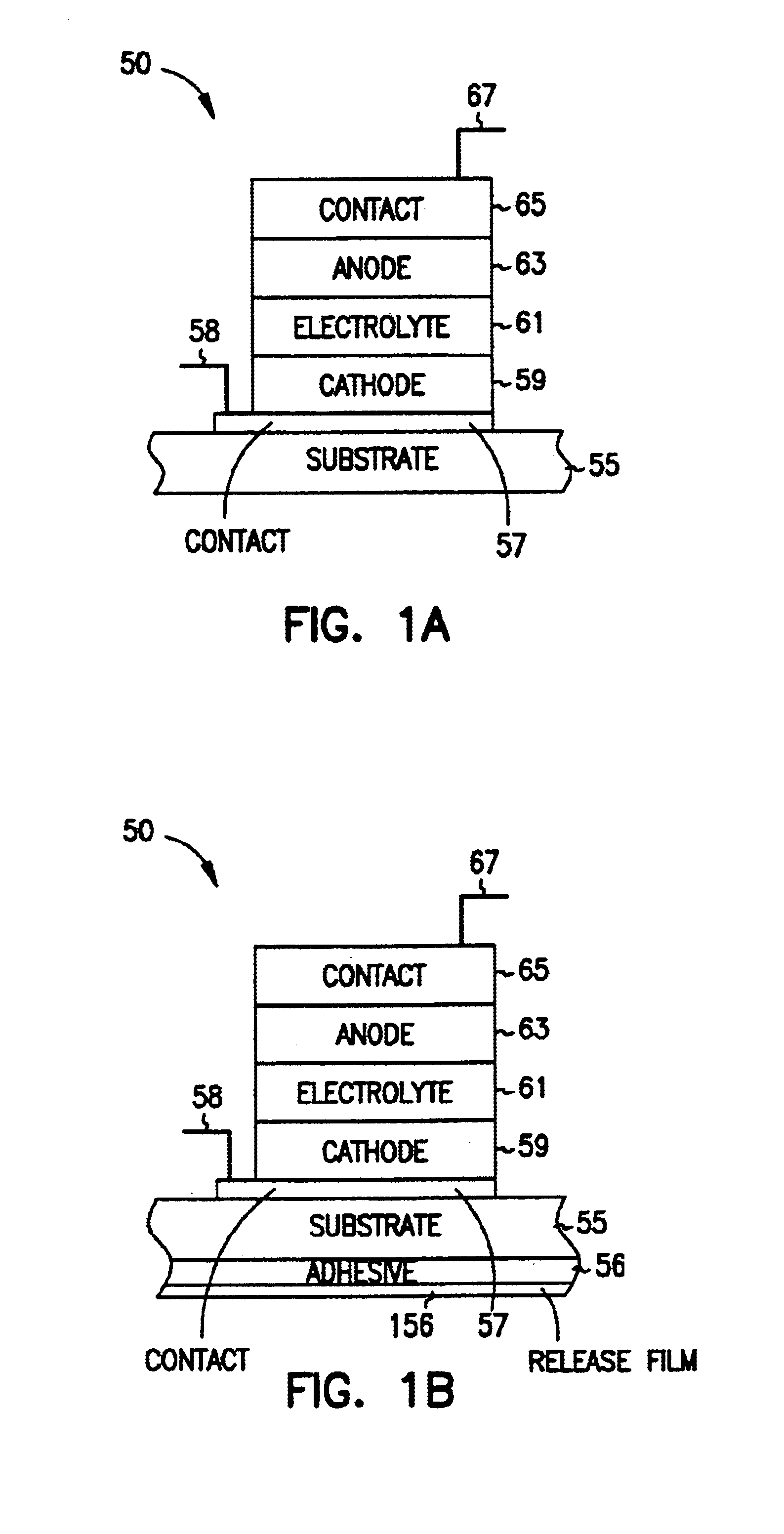
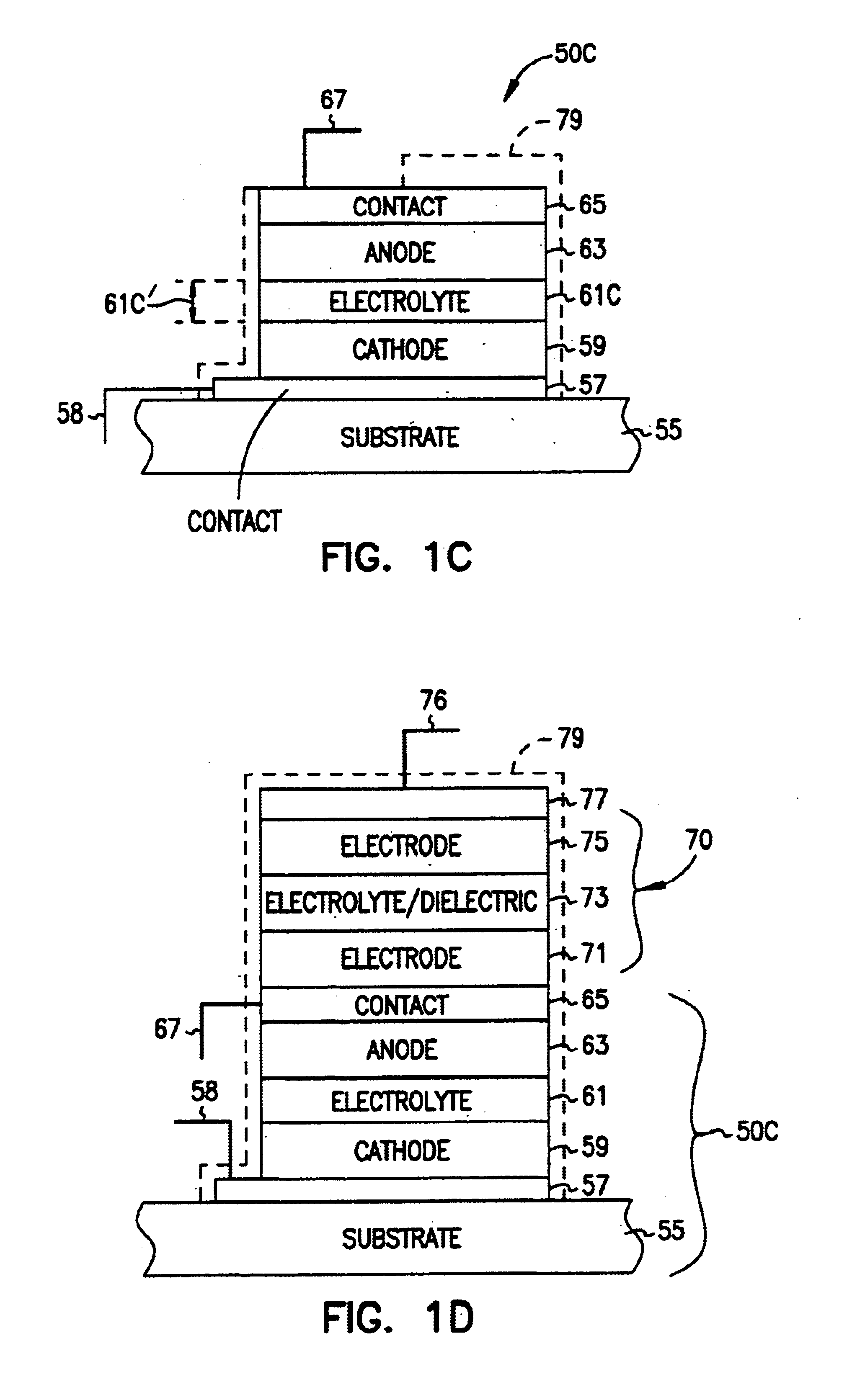
A system includes a thin-film battery and an activity-activated switch. The system is placed on a substrate with an adhesive backing. In some embodiments, the substrate is flexible. Also formed on the substrate is an electrical circuit that includes electronics. The activity-activated switch places the thin-film battery in electrical communication with the circuit and electronics. The battery and the circuit are formed on the substrate and may be comprised of one or a plurality of deposited layers.
Owner:CYMBET CORP
THIN-FILM BATTERIES WITH POLYMER AND LiPON ELECTROLYTE LAYERS AND METHOD
InactiveUS20070015061A1Improve environmental resistanceInhibition formationFinal product manufactureElectrode carriers/collectorsLithium metalPolymer gel
A method and apparatus for making thin-film batteries having composite multi-layered electrolytes with soft electrolyte between hard electrolyte covering the negative and / or positive electrode, and the resulting batteries. In some embodiments, foil-core cathode sheets each having a cathode material (e.g., LiCoO2) covered by a hard electrolyte on both sides, and foil-core anode sheets having an anode material (e.g., lithium metal) covered by a hard electrolyte on both sides, are laminated using a soft (e.g., polymer gel) electrolyte sandwiched between alternating cathode and anode sheets. A hard glass-like electrolyte layer obtains a smooth hard positive-electrode lithium-metal layer upon charging, but when very thin, have randomly spaced pinholes / defects. When the hard layers are formed on both the positive and negative electrodes, one electrode's dendrite-short-causing defects on are not aligned with the other electrode's defects. The soft electrolyte layer both conducts ions across the gap between hard electrolyte layers and fills pinholes.
Owner:CYMBET CORP
Plating technique for electrode
ActiveUS20130017441A1Electrode manufacturing processesFinal product manufactureElectrical batteryEngineering
Articles and methods for forming protected electrodes for use in electrochemical cells, including those for use in rechargeable lithium batteries, are provided. In some embodiments, the articles and methods involve an electrode that does not include an electroactive layer, but includes a current collector and a protective structure positioned directly adjacent the current collector, or separated from the current collector by one or more thin layers. Lithium ions may be transported across the protective structure to form an electroactive layer between the current collector and the protective structure. In some embodiments, an anisotropic force may be applied to the electrode to facilitate formation of the electroactive layer.
Owner:SION POWER CORP
Garnet materials for li secondary batteries and methods of making and using garnet materials
Set forth herein are garnet material compositions, e.g., lithium-stuffed garnets and lithium-stuffed garnets doped with alumina, which are suitable for use as electrolytes and catholytes in solid state battery applications. Also set forth herein are lithium-stuffed garnet thin films having fine grains therein. Disclosed herein are novel and inventive methods of making and using lithium-stuffed garnets as catholytes, electrolytes and / or anolytes for all solid state lithium rechargeable batteries. Also disclosed herein are novel electrochemical devices which incorporate these garnet catholytes, electrolytes and / or anolytes. Also set forth herein are methods for preparing novel structures, including dense thin (<50 um) free standing membranes of an ionically conducting material for use as a catholyte, electrolyte, and, or, anolyte, in an electrochemical device, a battery component (positive or negative electrode materials), or a complete solid state electrochemical energy storage device. Also, the methods set forth herein disclose novel sintering techniques, e.g., for heating and / or field assisted (FAST) sintering, for solid state energy storage devices and the components thereof.
Owner:QUANTUMSCAPE BATTERY INC
Solid electrolytes based on lithium hafnium phosphate for active metal anode protection
InactiveUS20060078790A1Avoid harmful reactionsCell electrodesNon-aqueous electrolyte cellsPhosphateOrganic liquids
Active metal electrochemical structure, in particular an active metal negative electrode (anode) protected with an ionically conductive protective architecture incorporating a glassy, ceramic or glass-ceramic solid electrolyte material based on lithium hafnium phosphate, and associated electrochemical devices and methods, provides advantages over conventional structures. The protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air or organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
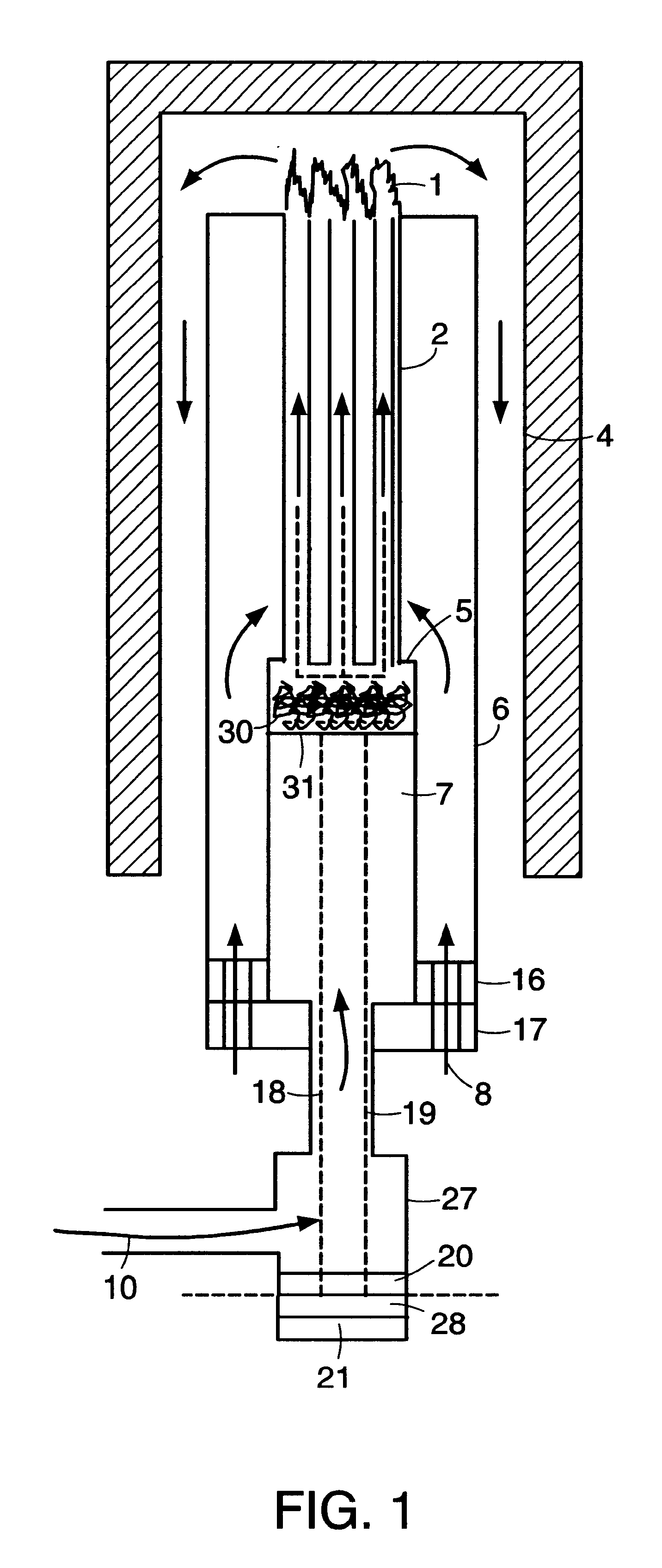
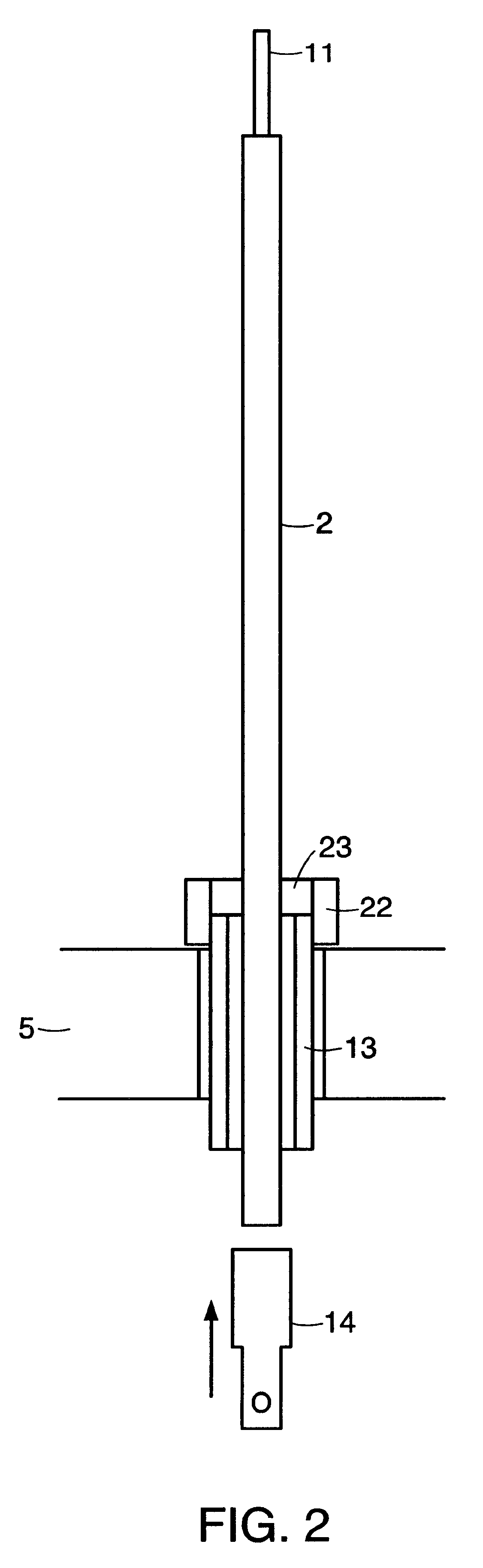
Integrated solid oxide fuel cell and reformer
A disclosed apparatus for generating electrical power has, according to one embodiment of the invention, a plurality of tubular solid oxide fuel cells contained in a reaction chamber. The fuel cells are secured at one end thereof in a manifold block, the other ends thereof passing freely through apertures in a baffle plate to reside in a combustion chamber. Reaction gases are supplied to the insides of the tubular fuel cells from a plenum chamber below the manifold block and to the reaction chamber surrounding the outsides of the fuel cells through an annular inlet path, which may include a reformation catalyst. The gases inlet path to the plenum chamber, and the annular inlet path surround the reaction chamber, are both in heat conductive relation with the reaction chamber and the combustion chamber, and raise the gases to formation and reaction temperatures as appropriate.
Owner:ACUMENTRICS
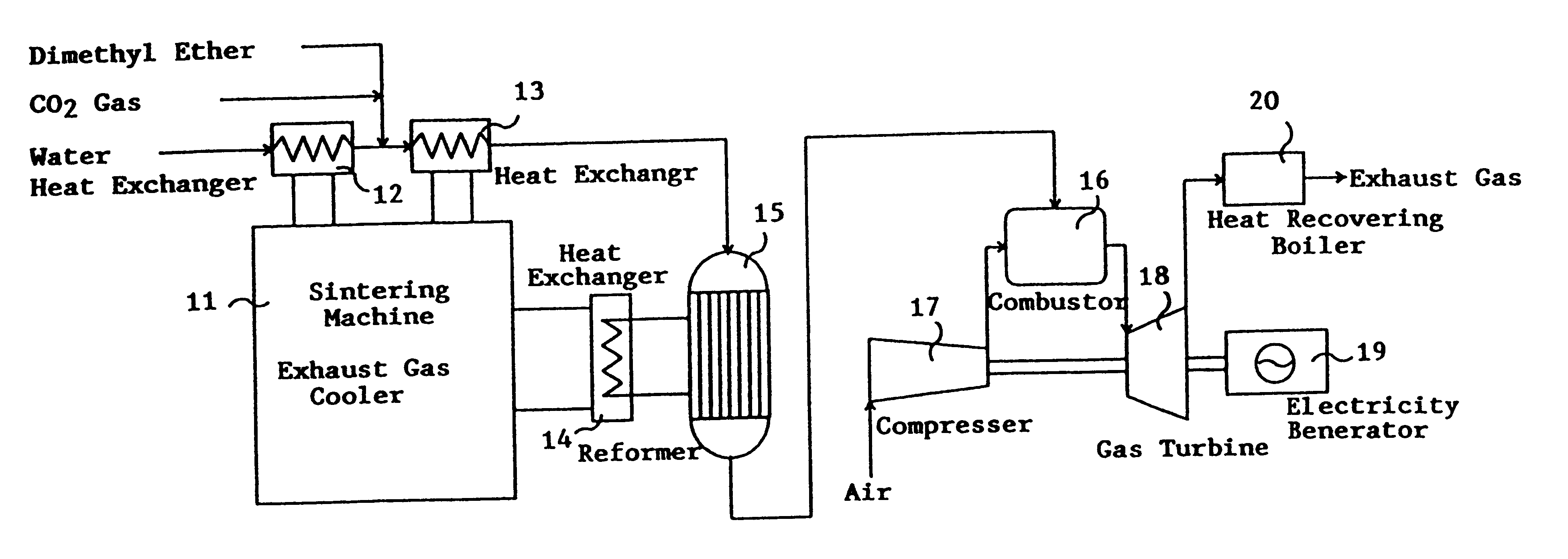
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Ultrathin porous nanoscale membranes, methods of making, and uses thereof
ActiveUS20060278580A1Flat surfaceNot time-consumePaper/cardboard articlesCell electrodesFuel cellsSemiconductor materials
A process for forming a porous nanoscale membrane is described. The process involves applying a nanoscale film to one side of a substrate, where the nanoscale film includes a semiconductor material; masking an opposite side of the substrate; etching the substrate, beginning from the masked opposite side of the substrate and continuing until a passage is formed through the substrate, thereby exposing the film on both sides thereof to form a membrane; and then simultaneously forming a plurality of randomly spaced pores in the membrane. The resulting porous nanoscale membranes, characterized by substantially smooth surfaces, high pore densities, and high aspect ratio dimensions, can be used in filtration devices, microfluidic devices, fuel cell membranes, and as electron microscopy substrates.
Owner:UNIVERSITY OF ROCHESTER
Planar solid oxide fuel cell stack with metallic foil interconnect
A solid oxide fuel cell stack having a plurality of integral component fuel cell units, each integral component fuel cell unit having a porous anode layer, a porous cathode layer, and a dense electrolyte layer disposed between the porous anode layer and the porous cathode layer. The porous anode layer forms a plurality of substantially parallel fuel gas channels on its surface facing away from the dense electrolyte layer and extending from one side to the opposite side of the anode layer, and the porous cathode layer forms a plurality of substantially parallel oxidant gas channels on its surface facing away from the dense electrolyte layer and extending from one side to the opposite side of the cathode. A flexible metallic foil interconnect is provided between the porous anode and porous cathode of adjacent integral component fuel cell units.
Owner:VERSA POWER SYST
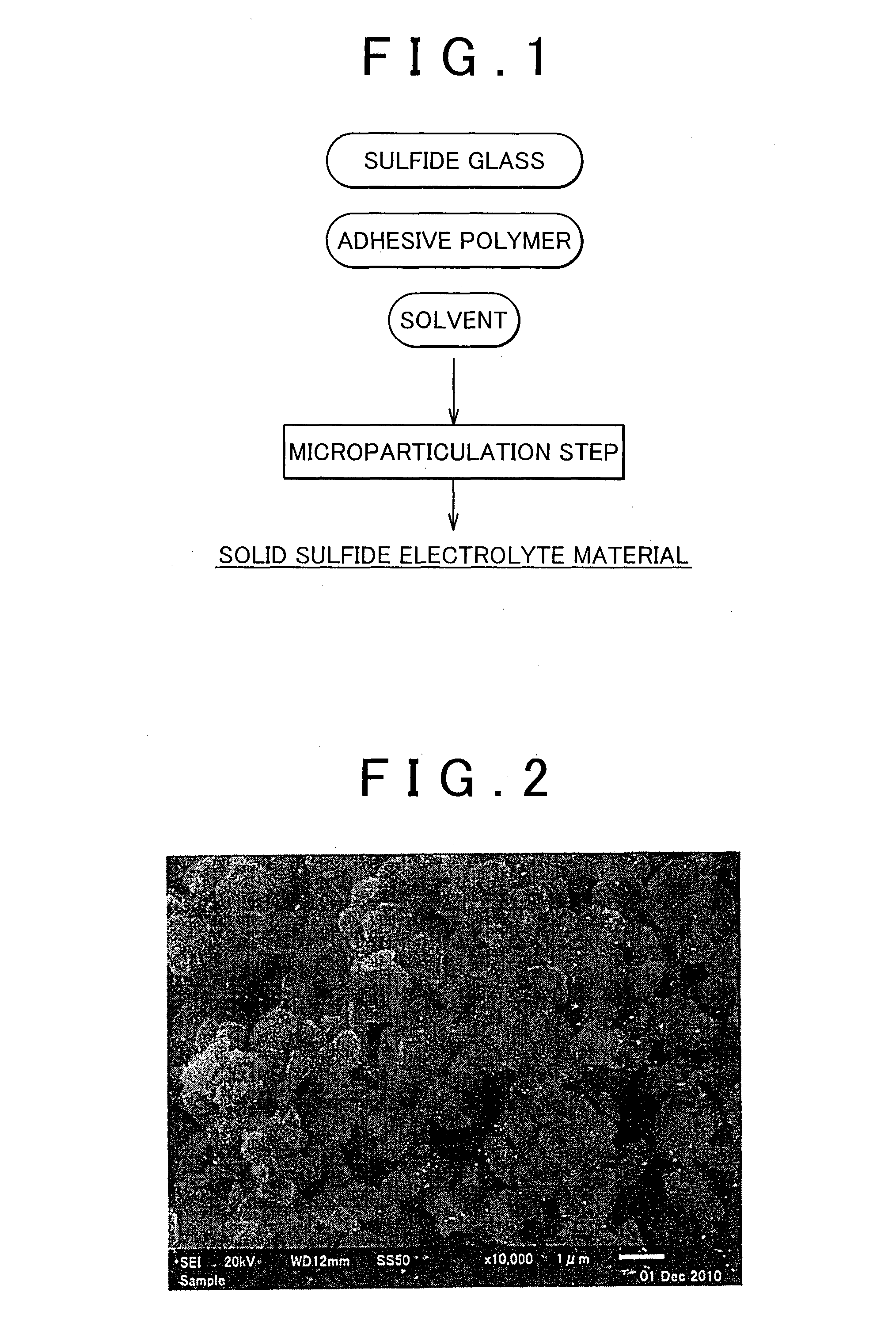
Method of producing solid sulfide electrolyte material and solid sulfide electrolyte material
InactiveUS20140093785A1Improve lithium ion conductivityHigh yieldSolid electrolyte cellsElectrolytesElectrolytePolymer
Owner:TOYOTA JIDOSHA KK
Ceramic material and preparation method therefor
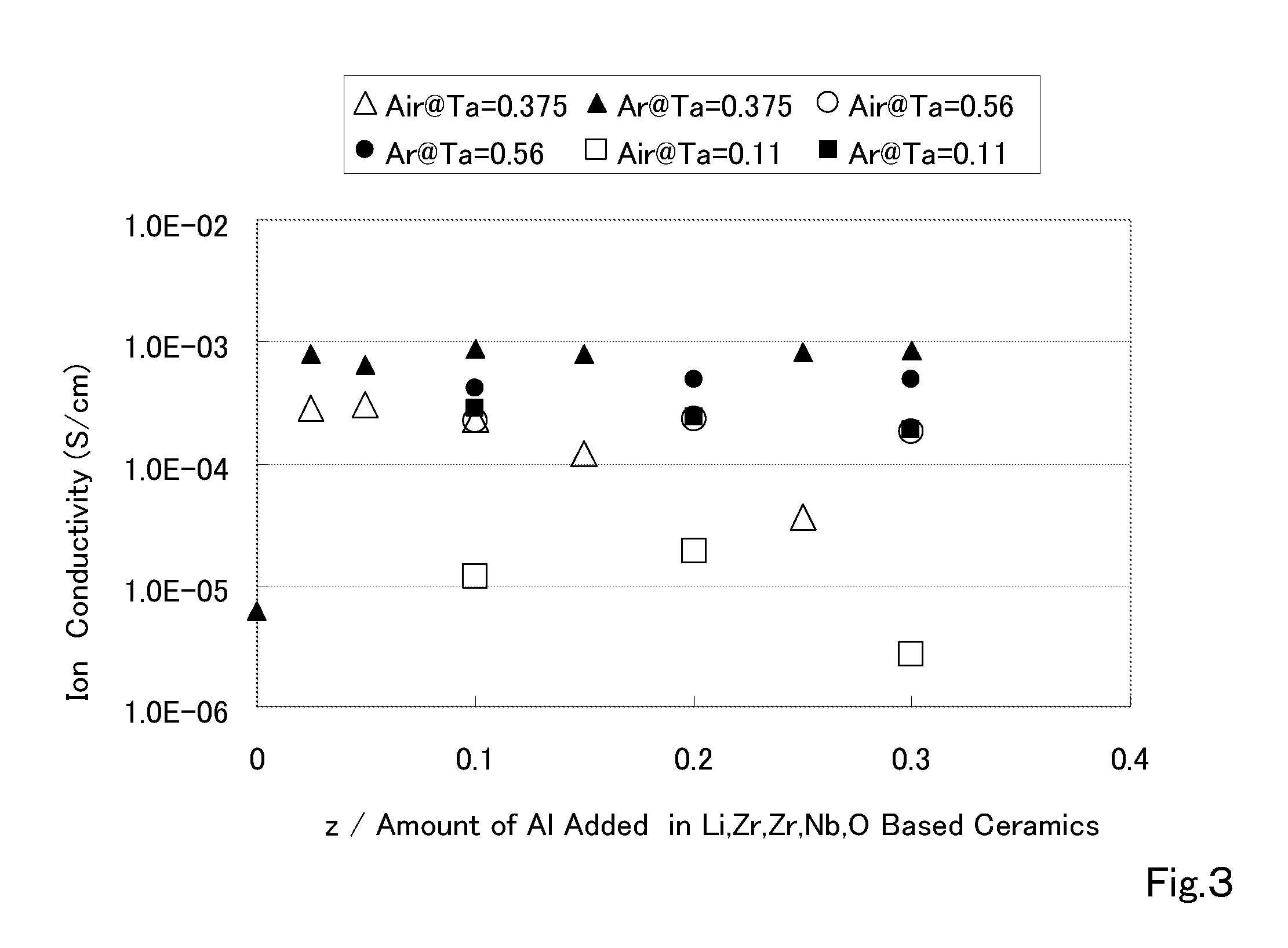
ActiveUS20110053002A1Demonstrating compactnessDemonstrating conductivityFinal product manufactureTantalum compoundsSolid state electrolyteMetallurgy
The present invention provides a ceramic material capable of demonstrating compactness and Li ion conductivity to an extent that enables the use of the ceramic material as a solid-state electrolyte material for a lithium secondary battery, or the like. A ceramic material containing Li, La, Zr, Nb and / or Ta, as well as O and having a garnet-type or garnet-like crystal structure is used.
Owner:NGK INSULATORS LTD
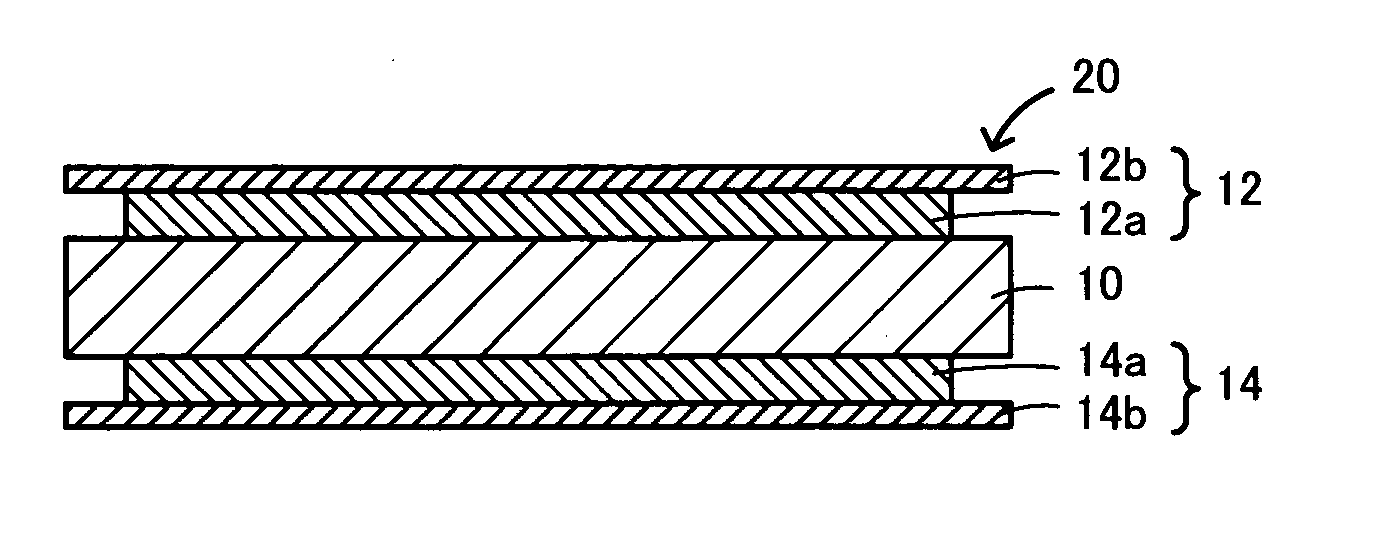
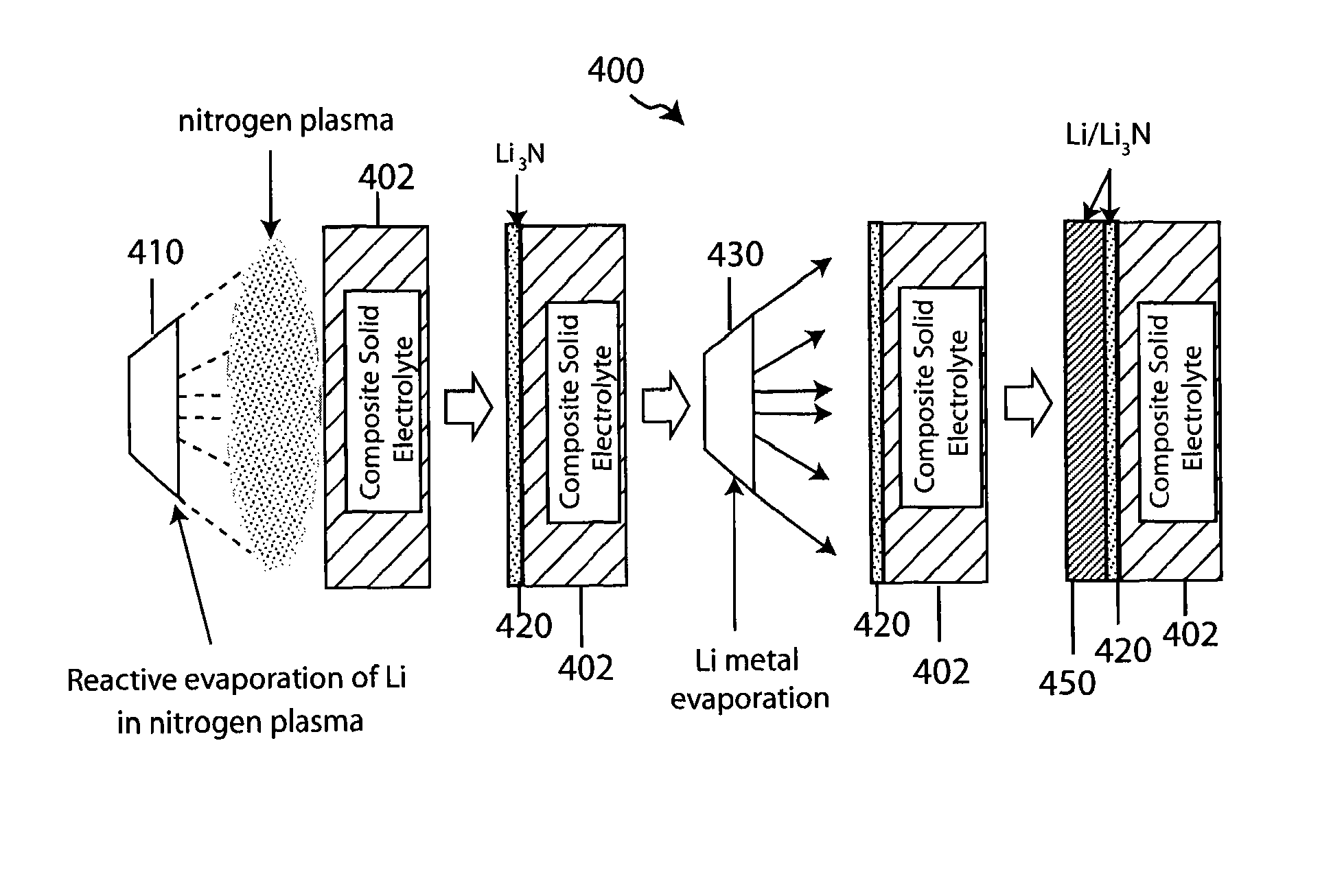
Composite solid electrolyte for protection of active metal anodes
ActiveUS8182943B2Eliminate through-porosityImprove ionic conductivityCell electrodesPrimary cellsPorosityLithium
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Layered Lithium Nickel Manganese Cobalt Composite Oxide Powder For Material Of Positive Electrode Of Lithium Secondary Battery, Process For Producing The Same, Positive Electrode Of Lithium Secondary Battery Therefrom, And Lithium Secondary Battery
ActiveUS20070202405A1Improve securityImprove battery performanceNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsManganeseCobalt
A powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is provided which, when used as a positive-electrode material for lithium secondary battery, enables a cost reduction and higher safety to be reconciled with improved battery performances. The powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is composed of secondary particles formed by the aggregation of primary particles. It has a composition represented by the following formula (I), has a volume resistivity of 5×105 Ω·cm or lower in the state of being compacted at a pressure of 40 MPa, and has a value of C / S, wherein C is the concentration of carbon contained therein (% by weight) and S is the BET specific surface area thereof (m2 / g), of 0.025 or smaller. The powder has been regulated so as to have a volume resistivity not higher than the specified value and a considerably reduced carbon content while having a composition in a limited range, whereby a cost reduction and higher safety can be reconciled with improved battery performances. Li1+zNixMnyCo1−x−yOδ (I) (0<z≦0.91, 0.1≦x≦0.55, 0.20≦y≦0.90, 0.50≦x+y≦1, 1.9≦δ≦3)
Owner:MITSUBISHI CHEM CORP
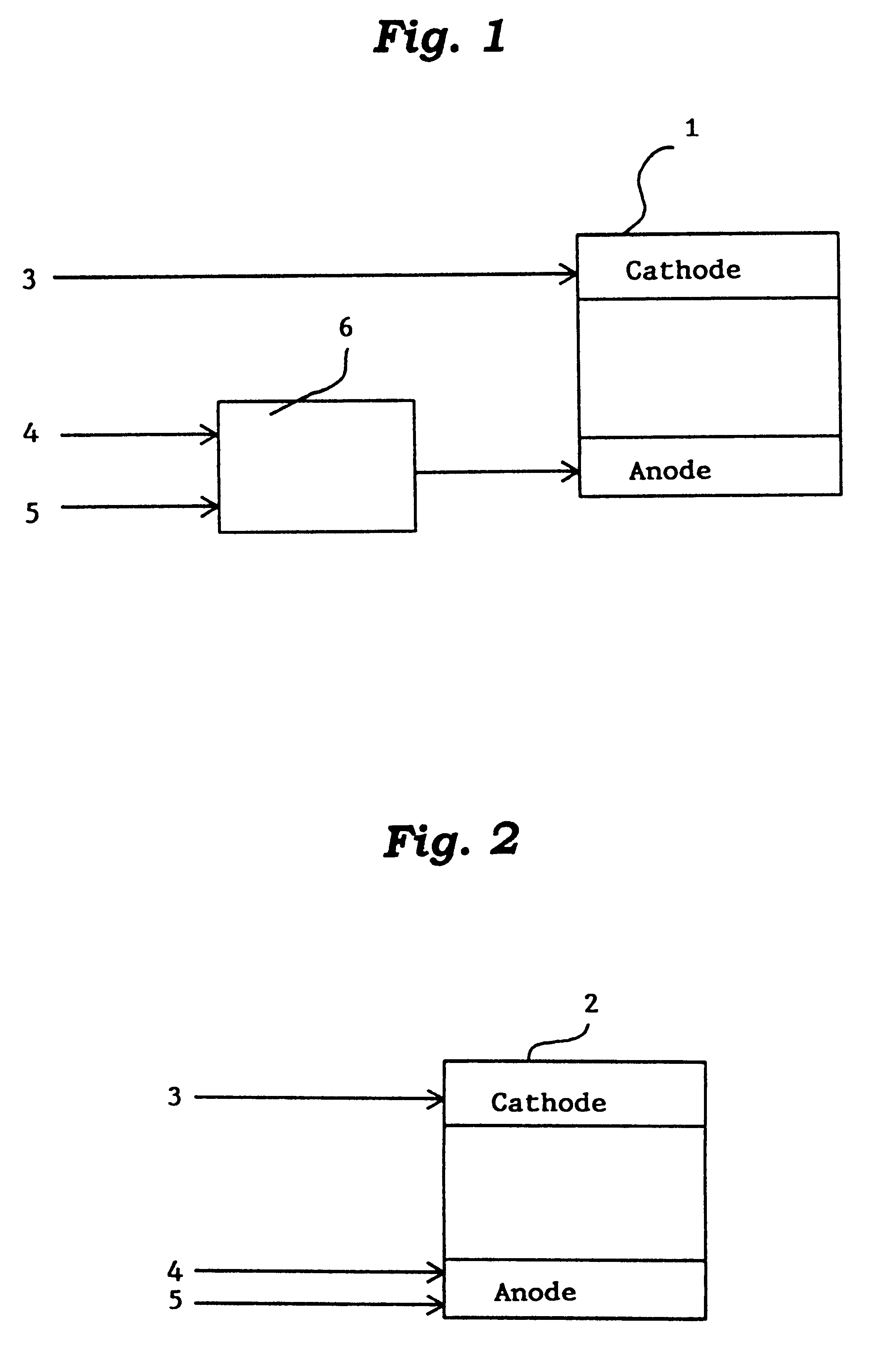
Active metal/aqueous electrochemical cells and systems
InactiveUS7645543B2Degree of flexibilityWithout performanceFuel and primary cellsAlkaline accumulatorsElectrochemical cellBattery cell
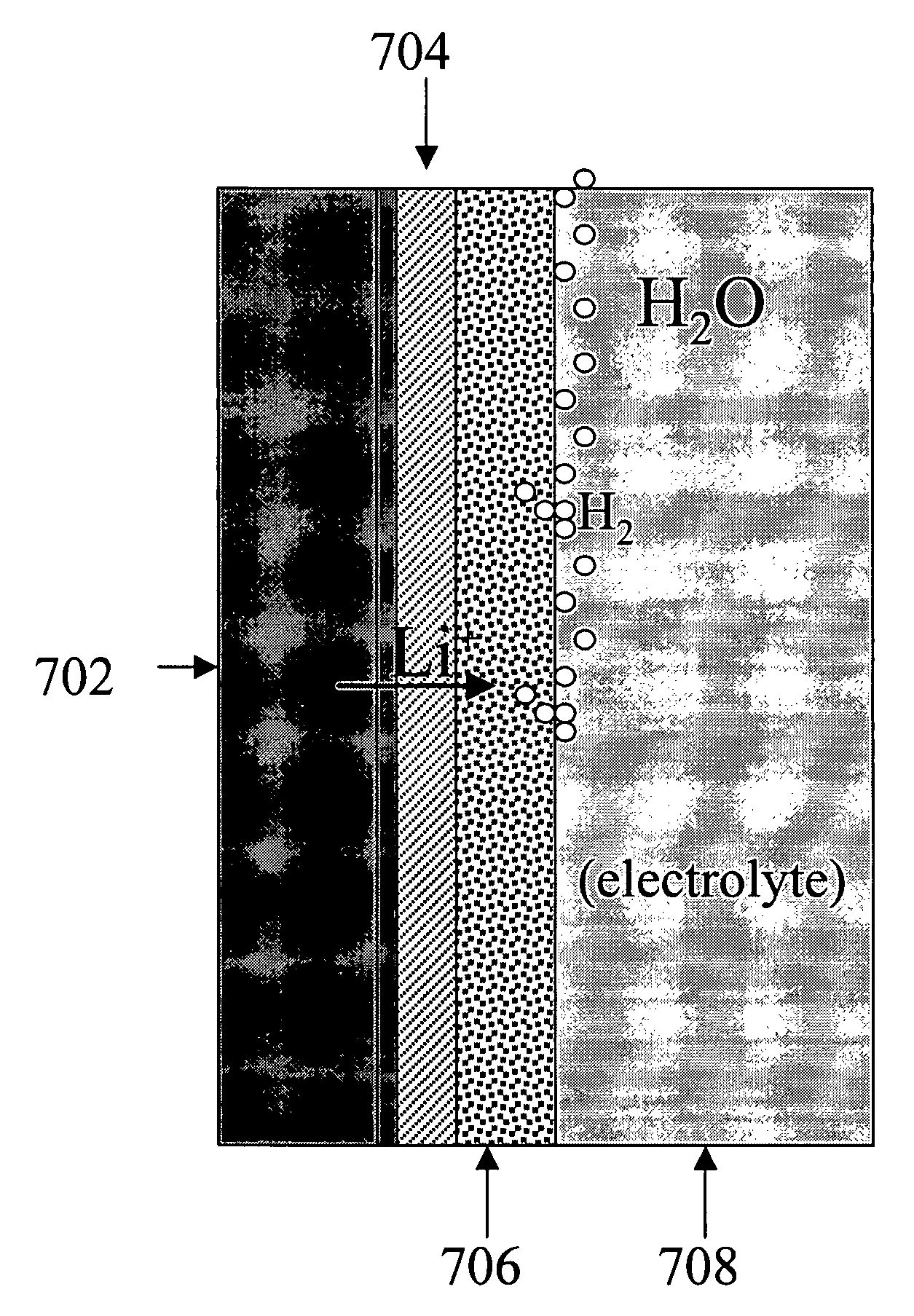
Alkali (or other active) metal battery and other electrochemical cells incorporating active metal anodes together with aqueous cathode / electrolyte systems. The battery cells have a highly ionically conductive protective membrane adjacent to the alkali metal anode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the anode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or cathode material in conjunction with the anode. Also, optimization of electrolytes or cathode-side solvent systems may be done without impacting anode stability or performance. In particular, Li / water, Li / air and Li / metal hydride cells, components, configurations and fabrication techniques are provided.
Owner:POLYPLUS BATTERY CO INC
Nonaqueous electrolyte battery, battery pack and positive electrode active material
InactiveUS20060134520A1Improve storage characteristicsOrganic electrolyte cellsPositive electrodesLithium oxideLithium hydroxide
A nonaqueous electrolyte battery includes a case, a positive electrode housed in the case and including a positive electrode active material containing a lithium-nickel composite oxide and at least one of lithium hydroxide and lithium oxide, the sum of lithium hydroxide and lithium oxide falling within not less than 0.1% to not more than 0.5% by weight based on the total amount of the positive electrode active material, a negative electrode housed in the case and capable of lithium intercalation-deintercalation, and a separator sandwiched between the positive electrode and the negative electrode and impregnated with a nonaqueous electrolyte containing γ-butyrolactone.
Owner:KK TOSHIBA
Nonaqueous electrolyte battery, battery pack and vehicle
ActiveUS20080166637A1Final product manufactureElectric propulsion mountingUnsaturated hydrocarbonTitanium
A nonaqueous electrolyte battery includes a positive electrode, a negative electrode and a nonaqueous electrolyte. The negative electrode contains a titanium-containing oxide. The nonaqueous electrolyte contains a compound having a functional group represented by the formula (1) below and a sultone having an unsaturated hydrocarbon group.[Chem.]
Owner:KK TOSHIBA
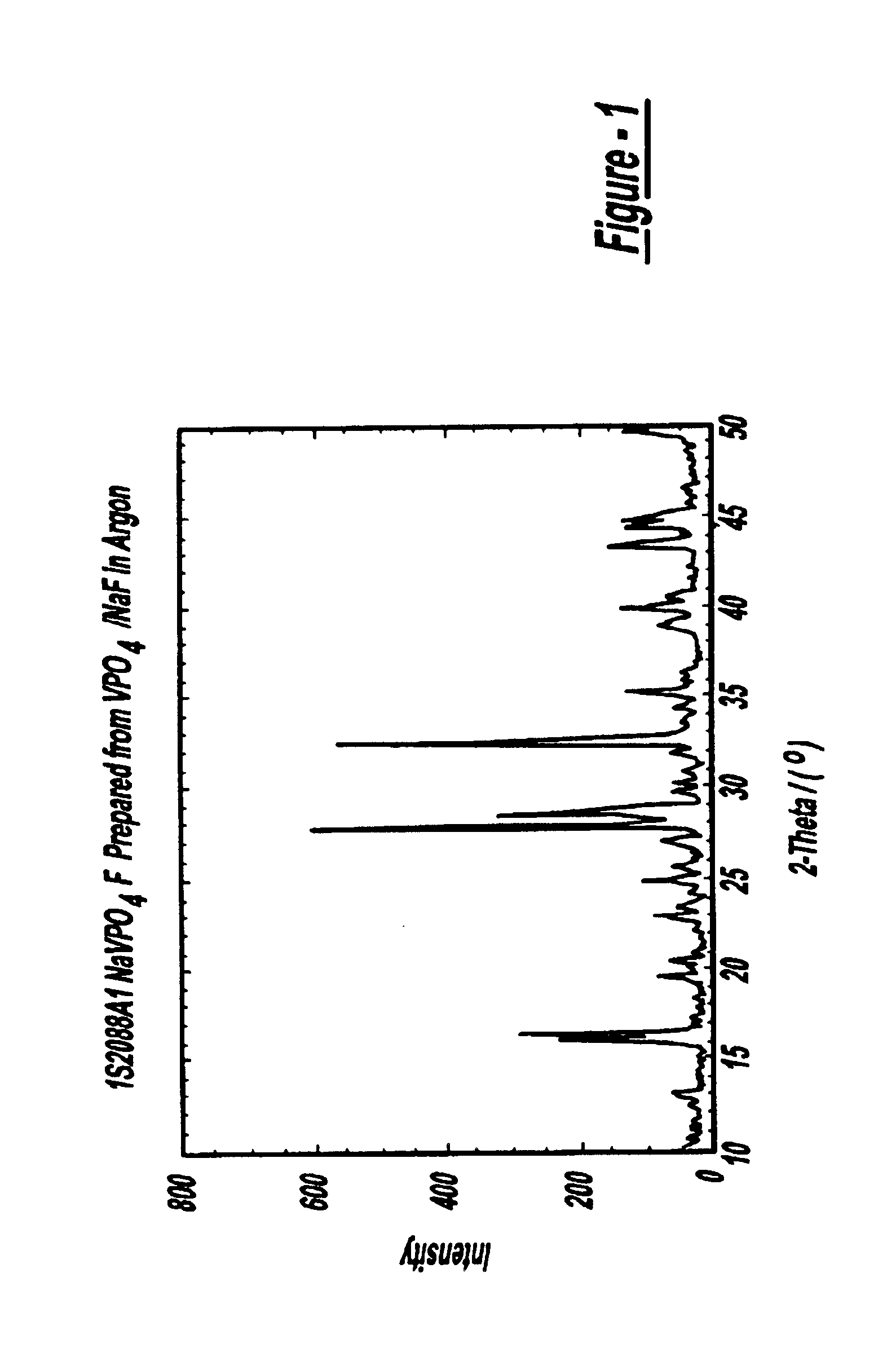
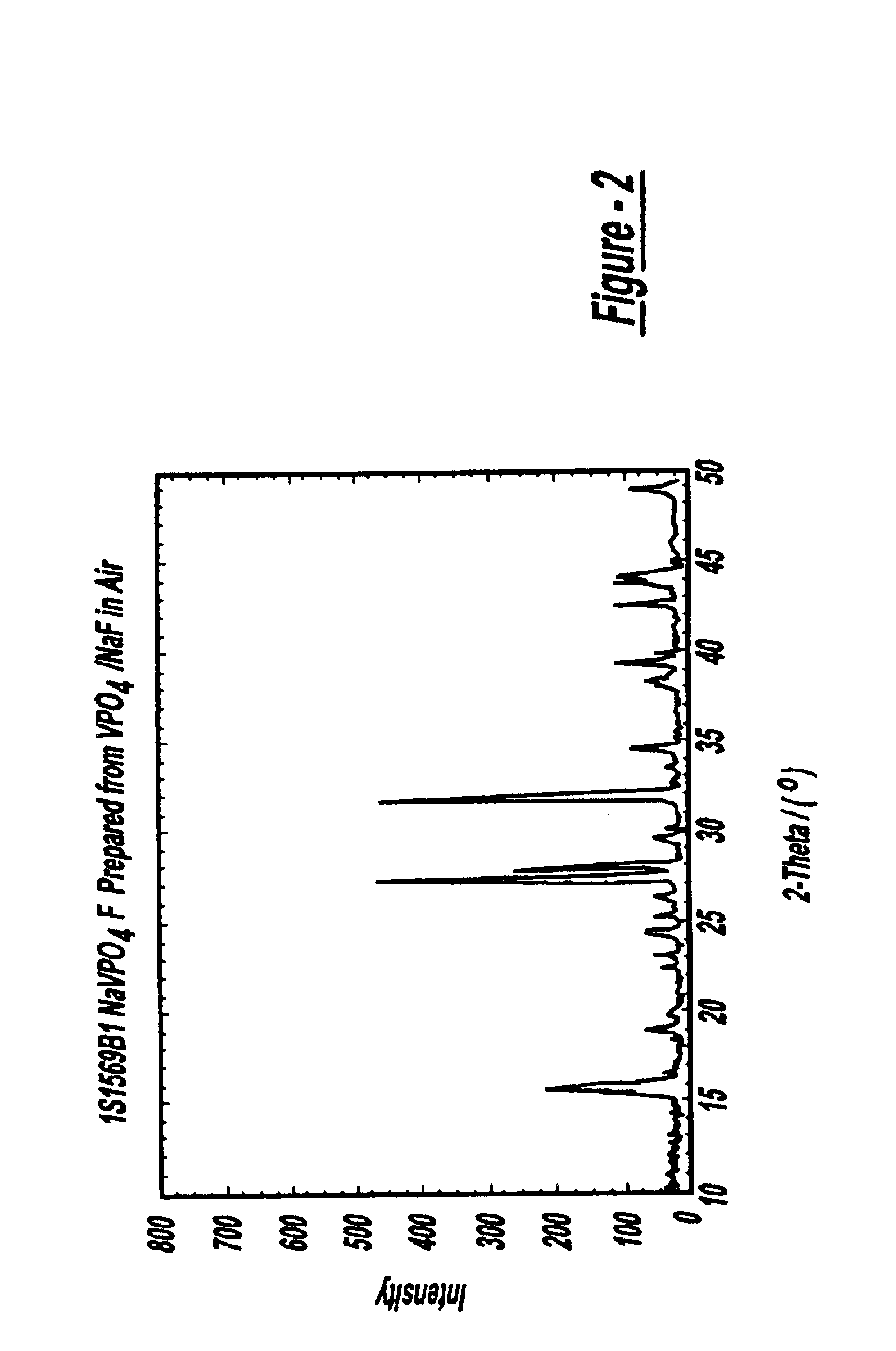
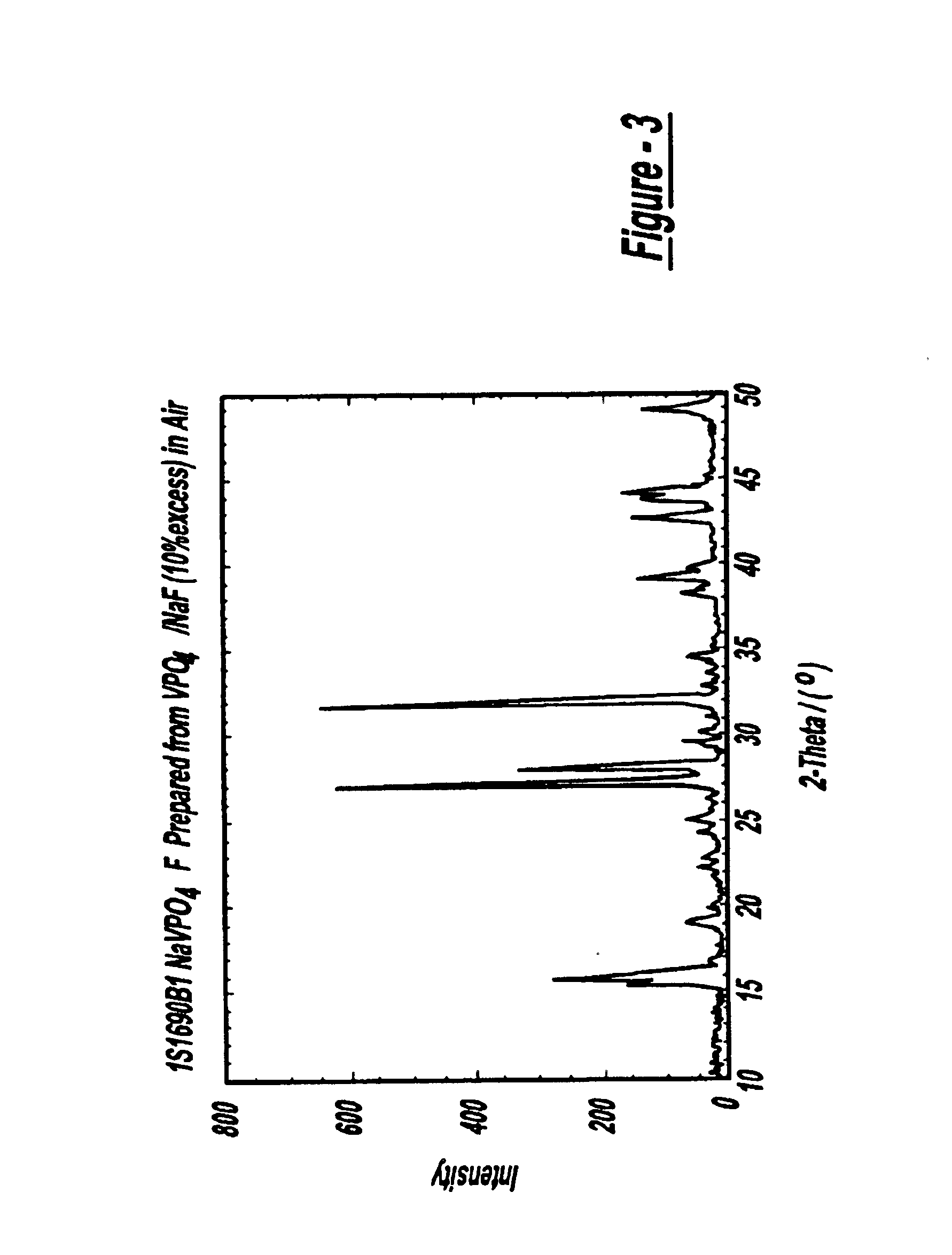
Sodium ion batteries
InactiveUS6872492B2High operation potentialHigh specific capacityOrganic electrolyte cellsSecondary cellsPhosphateSodium-ion battery
Sodium ion batteries are based on sodium based active materials selected among compounds of the general formula:AaMb(XY4)cZd,wherein A comprises sodium, M comprises one or more metals, comprising at least one metal which is capable of undergoing oxidation to a higher valence state, Z is OH or halogen, and XY4 represents phosphate or a similar group. The anode of the battery includes a carbon material that is capable of inserting sodium ions. The carbon anode cycles reversibly at a specific capacity greater than 100 mAh / g.
Owner:VALENCE TECH INC
Battery cell with barrier layer on non-swelling membrane
InactiveUS20060177732A1Avoid harmful reactionsCell electrodesSolid electrolyte cellsFluid electrolytesMembrane configuration
Owner:POLYPLUS BATTERY CO INC
Garnet materials for li secondary batteries and methods of making and using garnet materials
Set forth herein are garnet material compositions, e.g., lithium-stuffed garnets and lithium-stuffed garnets doped with alumina, which are suitable for use as electrolytes and catholytes in solid state battery applications. Also set forth herein are lithium-stuffed garnet thin films having fine grains therein. Disclosed herein are novel and inventive methods of making and using lithium-stuffed garnets as catholytes, electrolytes and / or anolytes for all solid state lithium rechargeable batteries. Also disclosed herein are novel electrochemical devices which incorporate these garnet catholytes, electrolytes and / or anolytes. Also set forth herein are methods for preparing novel structures, including dense thin (<50 um) free standing membranes of an ionically conducting material for use as a catholyte, electrolyte, and, or, anolyte, in an electrochemical device, a battery component (positive or negative electrode materials), or a complete solid state electrochemical energy storage device. Also, the methods set forth herein disclose novel sintering techniques, e.g., for heating and / or field assisted (FAST) sintering, for solid state energy storage devices and the components thereof.
Owner:QUANTUMSCAPE BATTERY INC
Electrolyte compositon, photoelectric converter and dye-sensitized solar cell using same
ActiveUS20060174932A1Improve conductivityDecrease in flowabilityMaterial nanotechnologyLight-sensitive devicesElectrolyte compositionPhotoelectric conversion
An electrolyte composition containing an ionic liquid and conductive particles as main components, an electrolyte composition containing an ionic liquid, and oxide semiconductor particles or oxide semiconductor particles, and conductive particles, and an electrolyte composition containing an ionic liquid and insulating particles are provided. Furthermore, a photoelectric conversion element comprising: a working electrode, the working electrode comprising an electrode substrate and an oxide semiconductor porous film formed on the electrode substrate and sensitized with a dye; a counter electrode disposed opposing the working electrode; and an electrolyte layer made of these electrolyte compositions is provided.
Owner:THE FUJIKURA CABLE WORKS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com