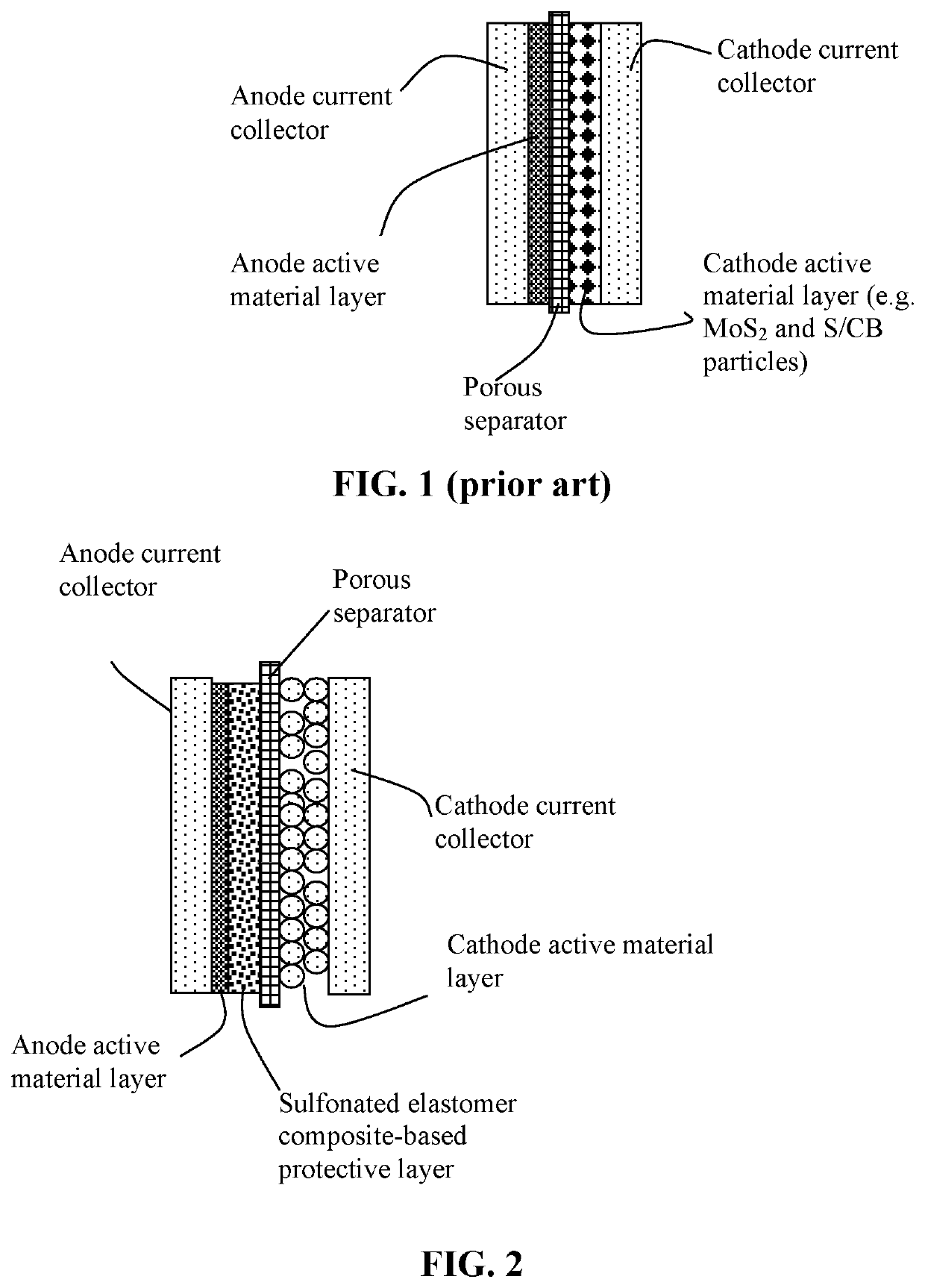
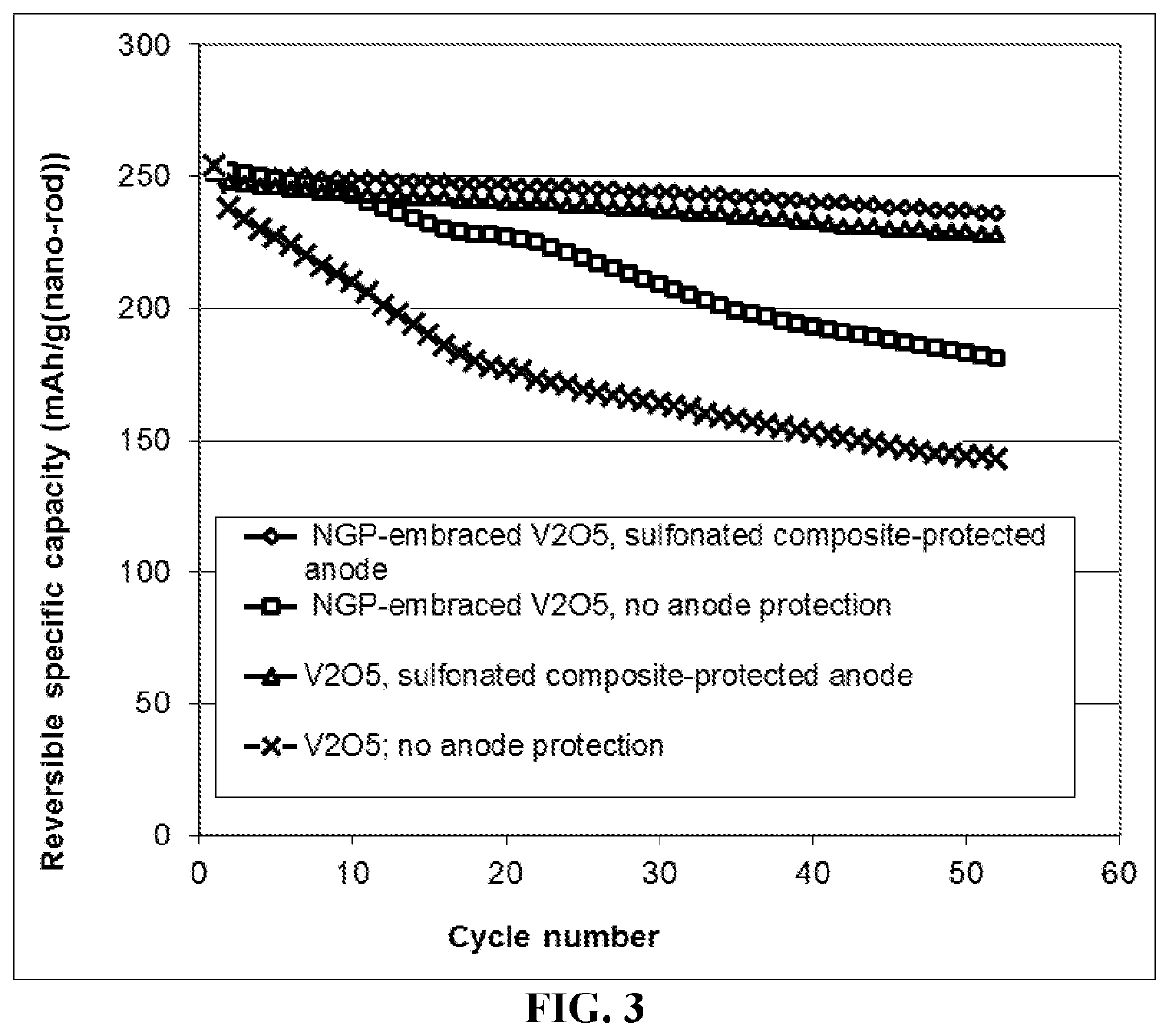
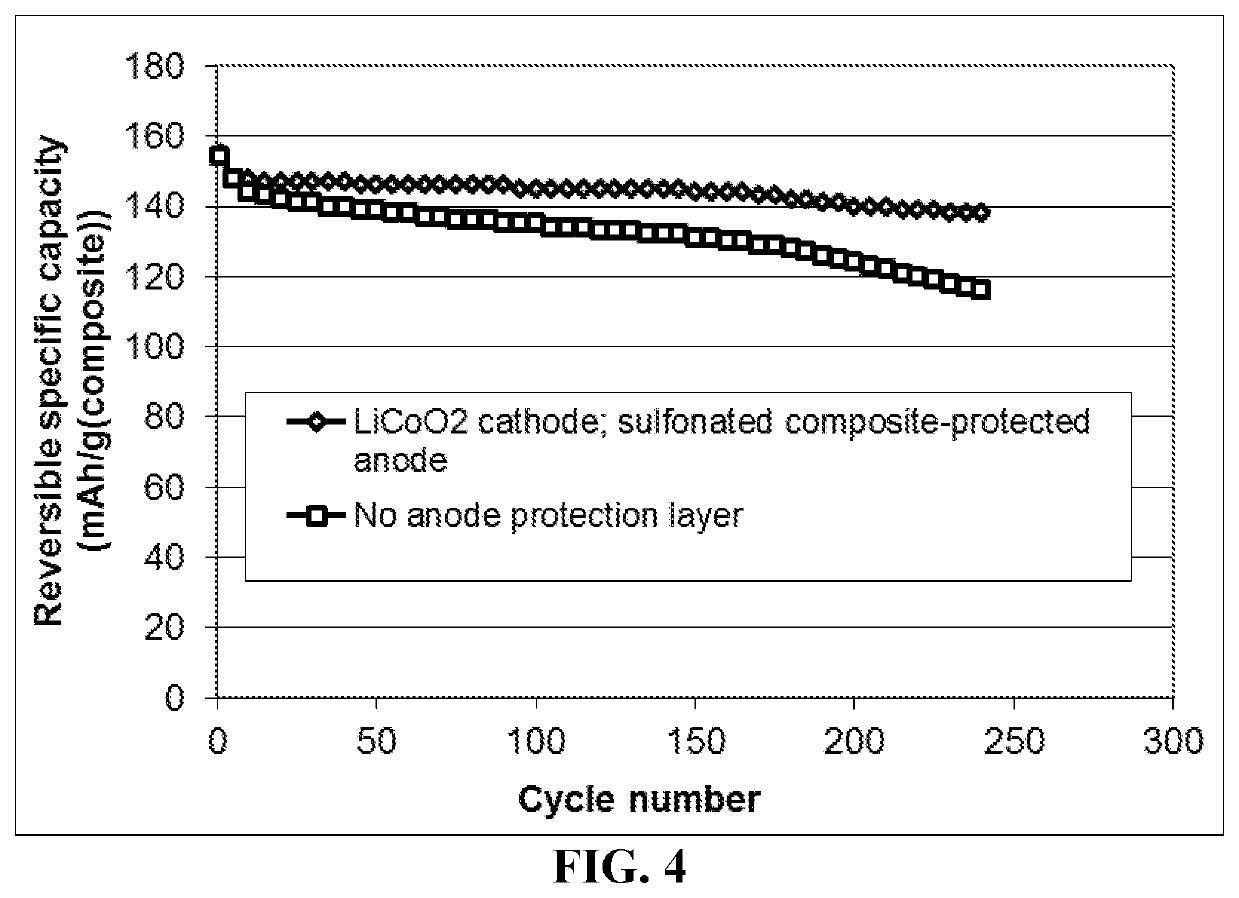
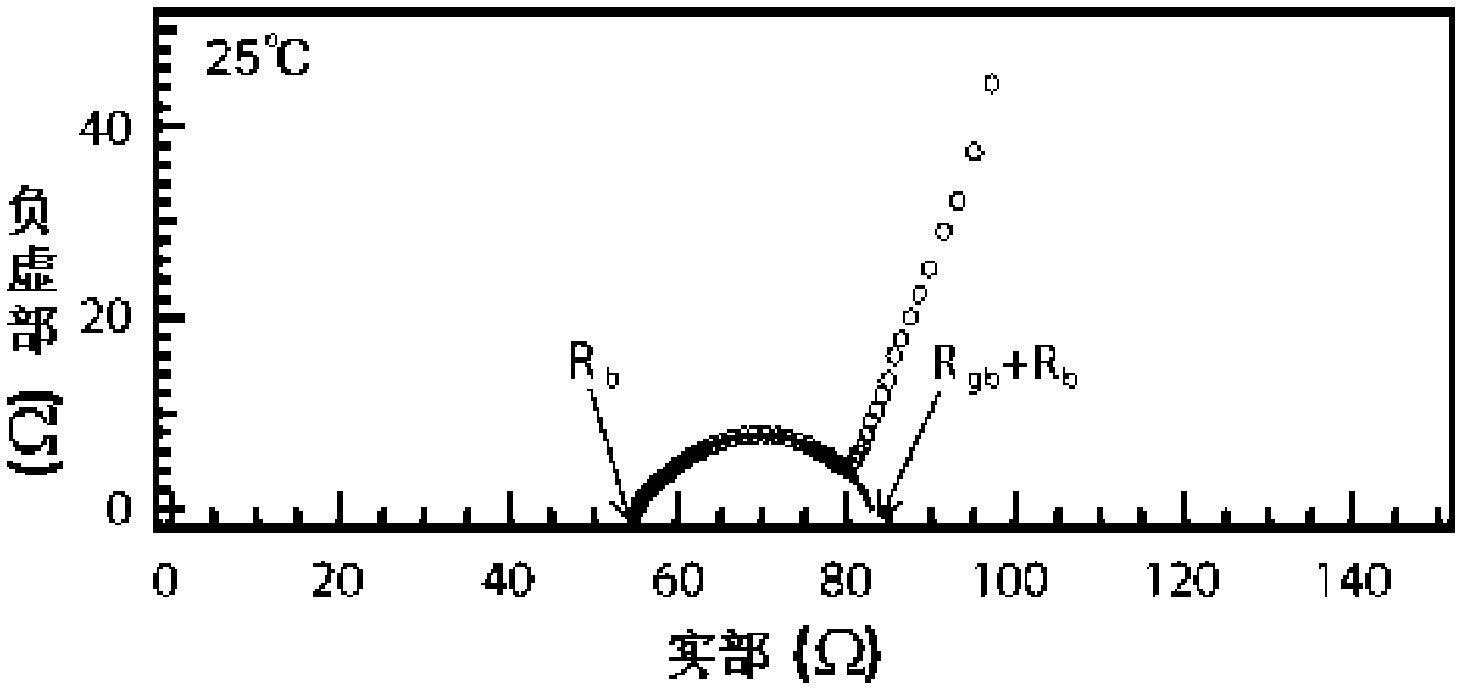
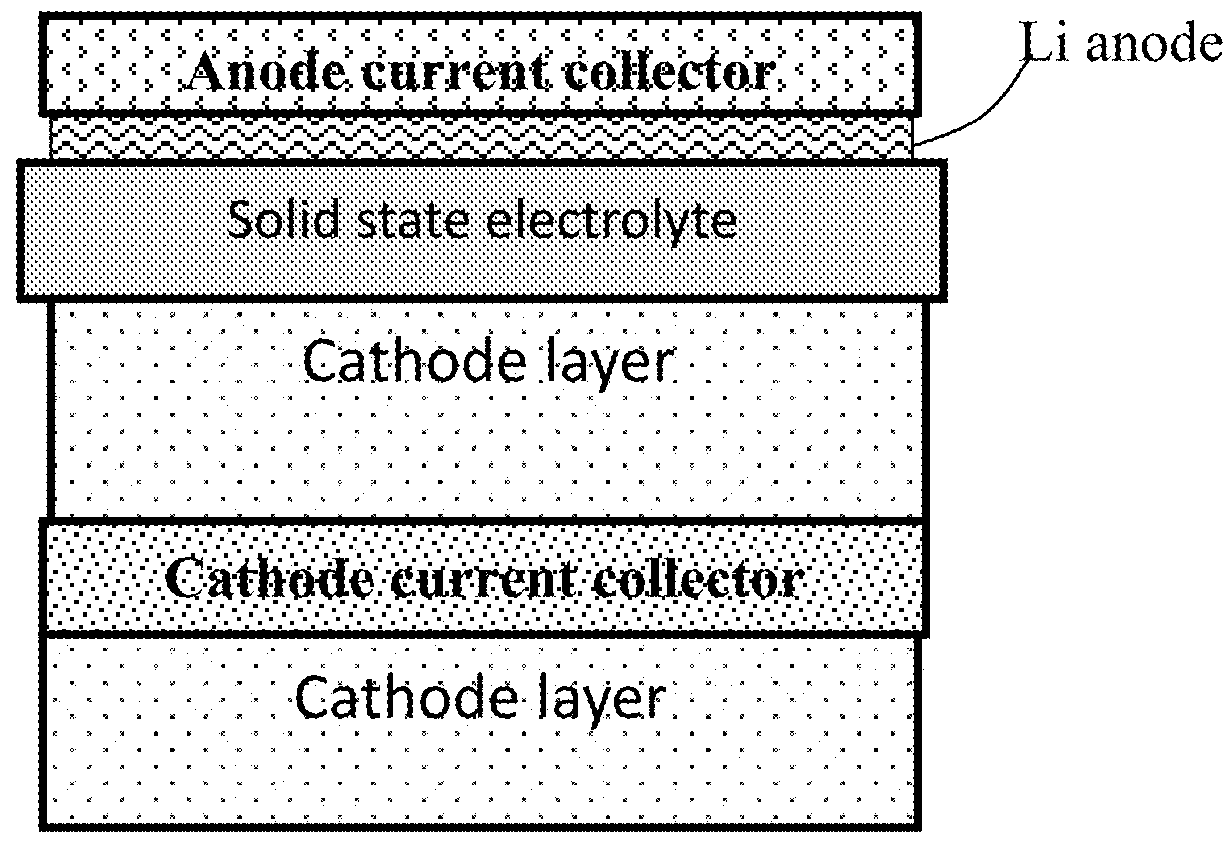
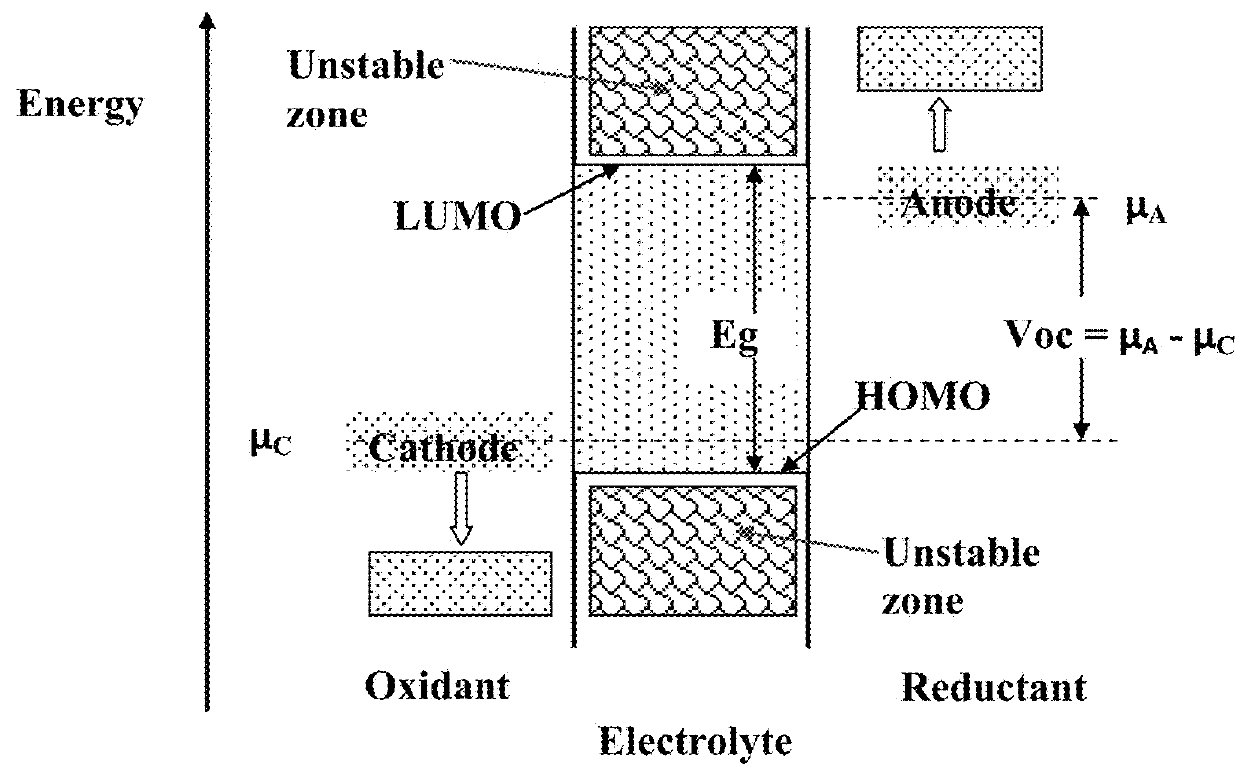
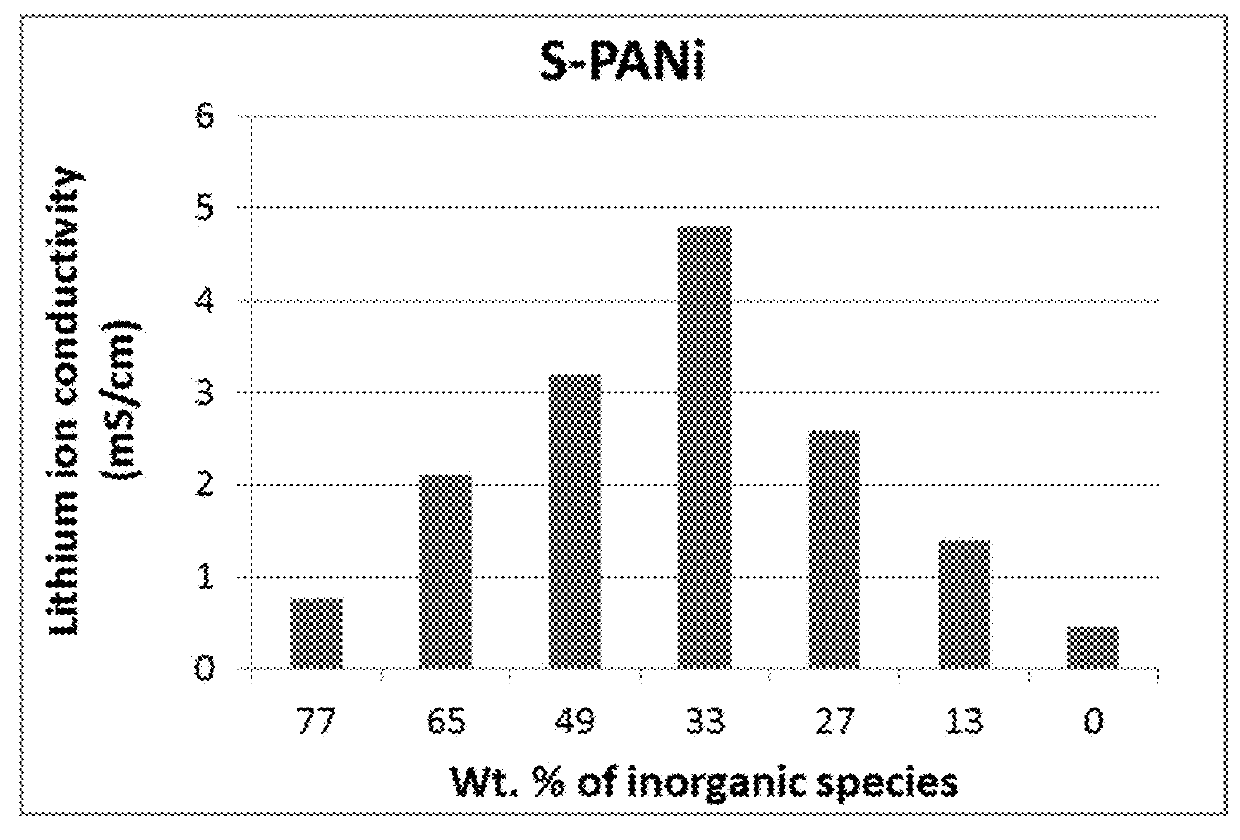
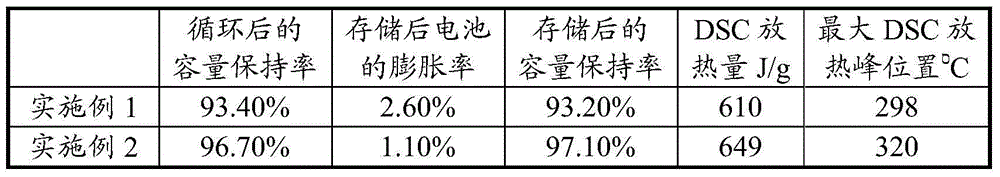
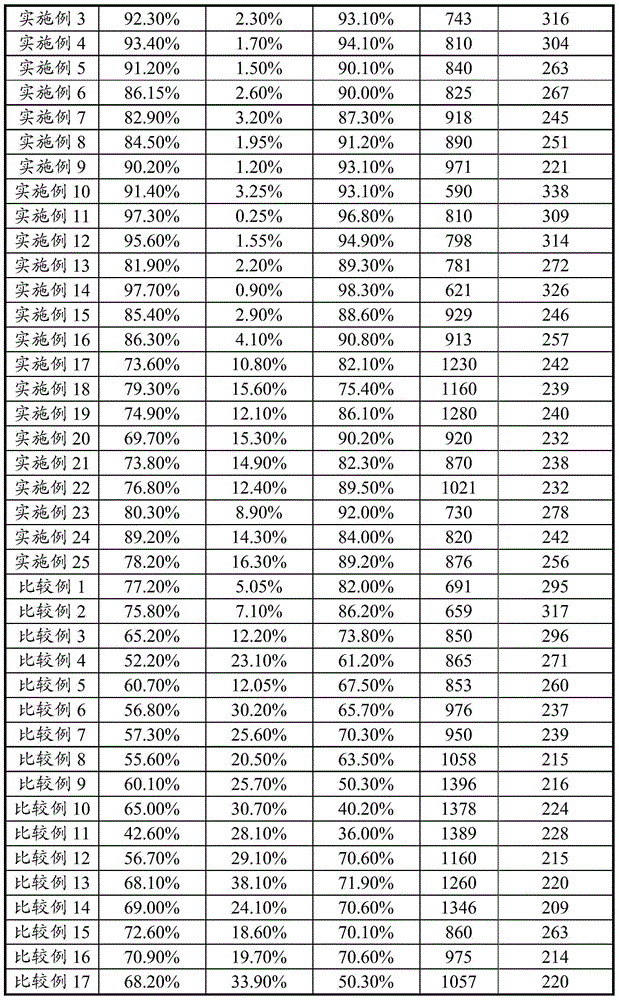
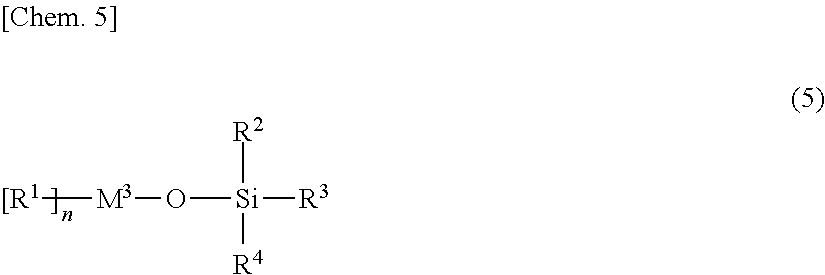Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
511results about How to "Improve lithium ion conductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
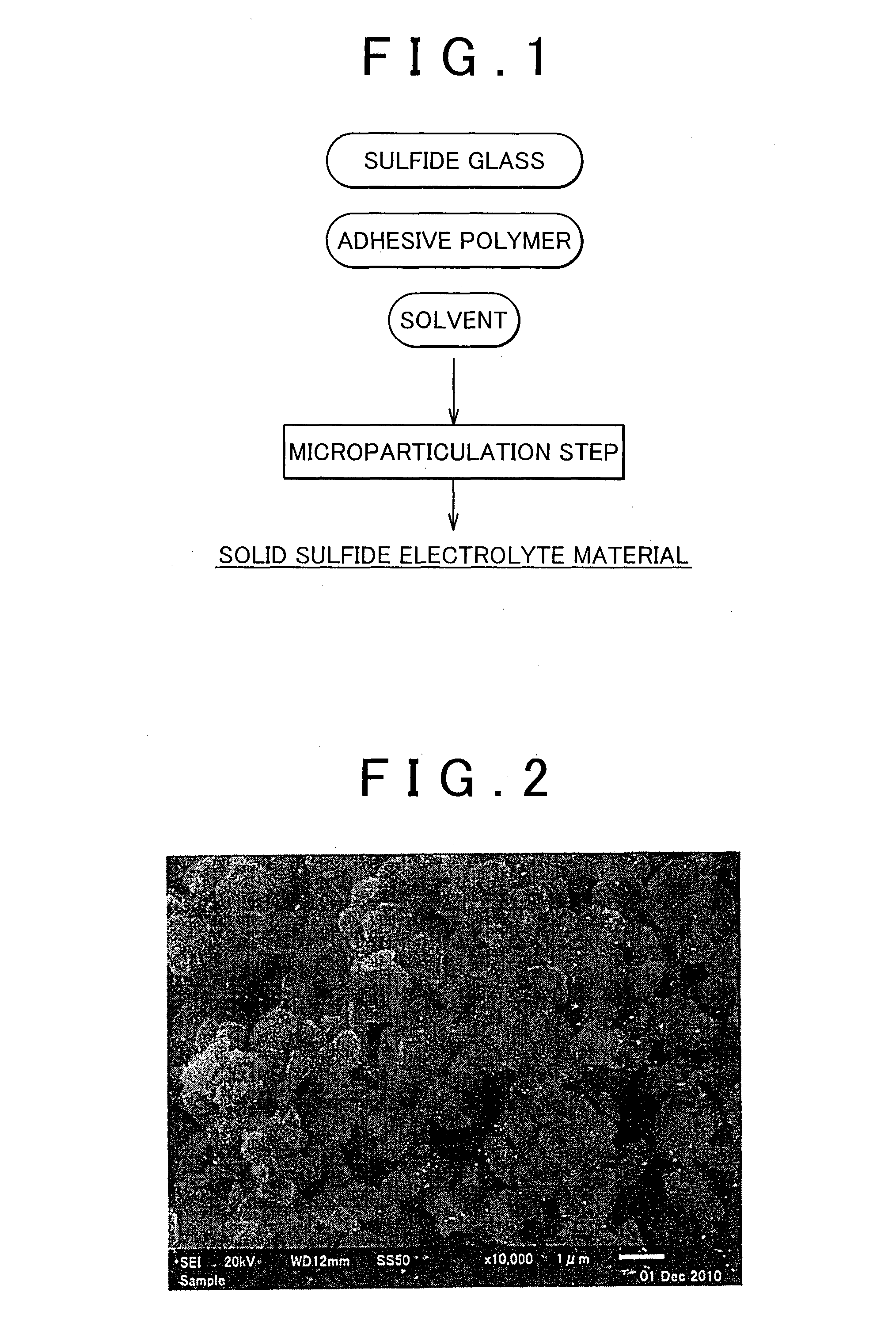
Method of producing solid sulfide electrolyte material and solid sulfide electrolyte material
InactiveUS20140093785A1Improve lithium ion conductivityHigh yieldSolid electrolyte cellsElectrolytesElectrolytePolymer
Owner:TOYOTA JIDOSHA KK
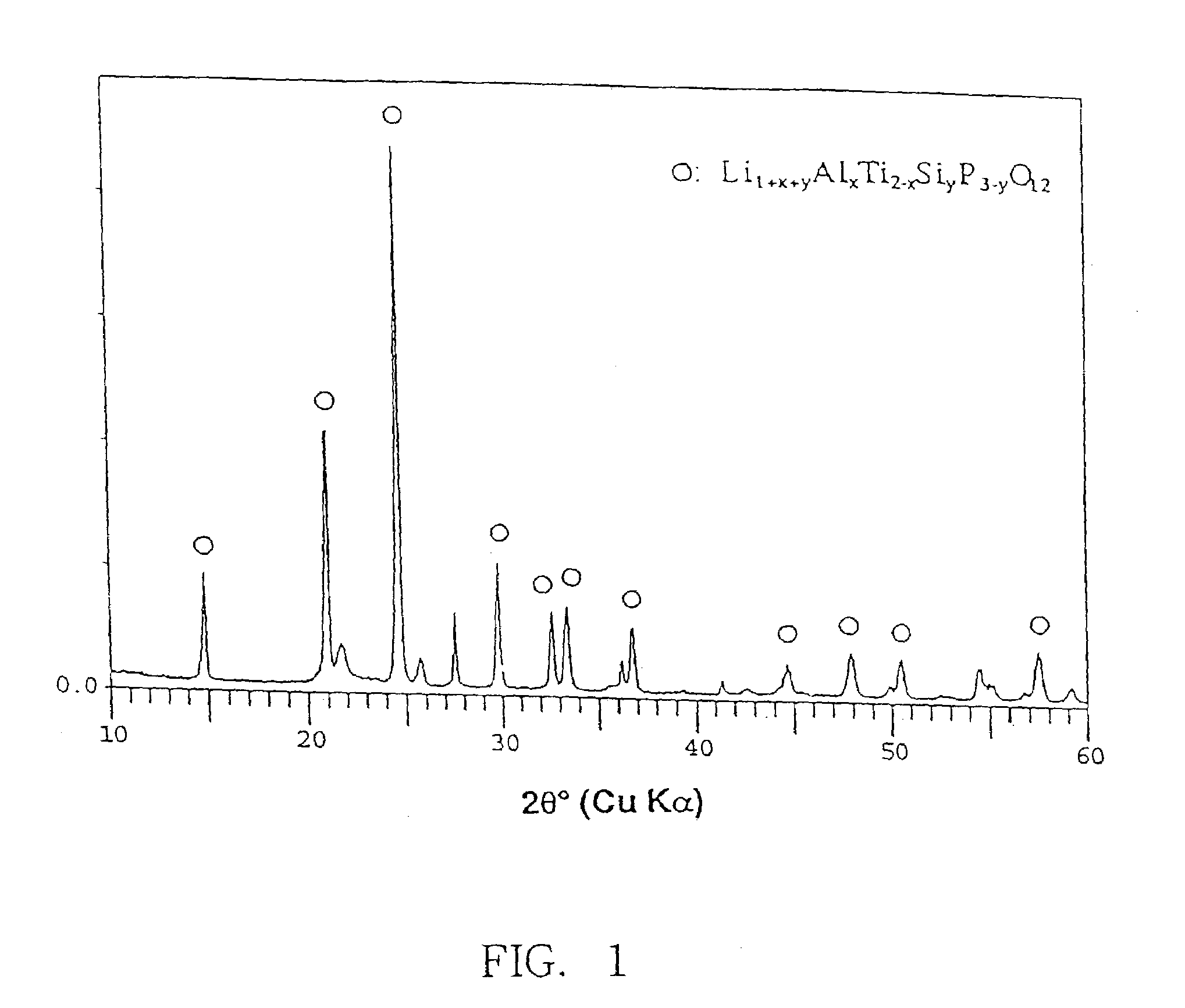
Alkali ion conductive glass-ceramics and electric cells and gas sensors using the same
InactiveUS7211532B2Improve performanceImprove conductivityMaterial analysis by electric/magnetic meansSecondary cellsLithiumAlkali ions
There are provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O538–40%TiO225–45%M2O3 (where M is Al or Ga) 5–15%Li2O10–20%and contain Li1+X(Al, Ga)XTi2−X(PO4)3 (where 0<X<0.8) as a main crystal phases. There are also provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O5 26–40%SiO20.5–12%TiO2 30–45%M2O3 (where M is Al or Ga) 5–10%Li2O 10–18%and contain Li1+X+YMXTi2−XSiYP3−YO12 (where 0<X≦0.4 and 0<Y≦0.6) as a main crystal phase. There are also provided solid electrolytes for an electric cell and a gas sensor using alkali ion conductive glass-ceramics, and a solid electric cell and a gas sensor using alkali ion conductive glass-ceramics as a solid electrolyte.
Owner:OHARA
Graphene-modified lithium iron phosphate positive electrode active material, preparation of the same and lithium-ion secondary cell
InactiveUS20120315550A1Excellent electrical conductivityGood lithium ion conductivityMaterial nanotechnologySolid waste disposalCell basedCyclic stability
The invention relates to a graphene-modified lithium iron phosphate positive electrode active material and a method for preparing the same, as well as a lithium-ion secondary cell based on this positive electrode active material. The positive electrode active material is prepared by a method in which graphene or graphene oxide and lithium iron phosphate are dispersed in an aqueous solution, agitated and ultrasonicated to mix homogeneously and for a mixture, dried to obtain a lithium iron phosphate material compounded with graphene or graphene oxide, and annealed at high temperature to obtain finally a graphene-modified lithium iron phosphate positive electrode active material. When compared with conventional modified lithium cells coated with carbon or doped with conductive polymers, the lithium-ion secondary cell based on this positive electrode active material features high cell capacity, good cycling performance of charge and discharge, long life and high cycle stability, and has great utility value.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +1
Lithium ion battery and anode strip thereof and stabilization lithium metal powder
ActiveCN102642024AImprove lithiation efficiencyImprove electronic conductivitySecondary cellsNon-aqueous electrolyte accumulator electrodesElectrical conductorElectrical battery
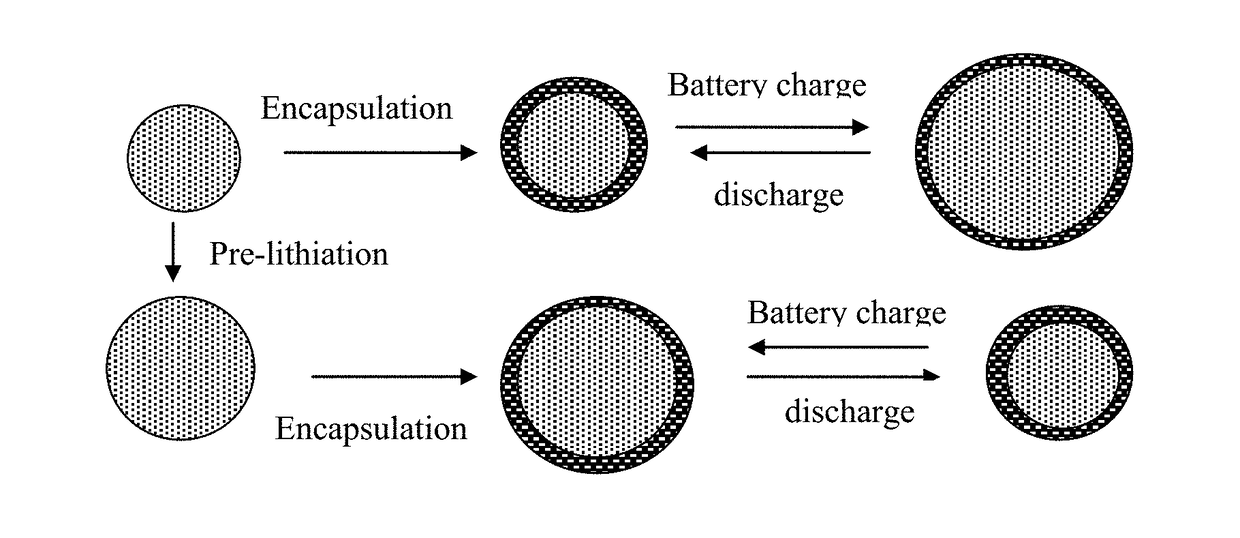
The invention belongs to the technical field of a lithium ion battery, and particularly relates to stabilization lithium metal powder. The stabilization lithium metal powder has a core shell structure; and the core layer is formed by lithium metal and is a composition consisting of an electron good conductor and a lithium ion good conductor. Compared with the prior art, the stabilization lithium metal powder provided by the invention has the advantages that: in the process of performing pre-lithiation of the anode-active material by use of the stabilization lithium metal powder, no limitation is imposed on the pressure of the cold pressing process, the 'dead lithium' disabling lithiation reaction is not produced, and the lithiation efficiency of the lithium metal powder is improved; and moreover, since the shell layer left on the electrode surface has good electron and lithium ion conductivity after the pre-lithiation, the electron and ion conductivity of the anode can be effectively improved so as to improve the electrochemical performance of the battery. In addition, the invention also discloses an anode strip performing pre-lithiation by use of the stabilization lithium metal powder, and a lithium ion battery comprising the anode strip.
Owner:NINGDE AMPEREX TECH +1
Separation membrane for lithium sulfur batteries
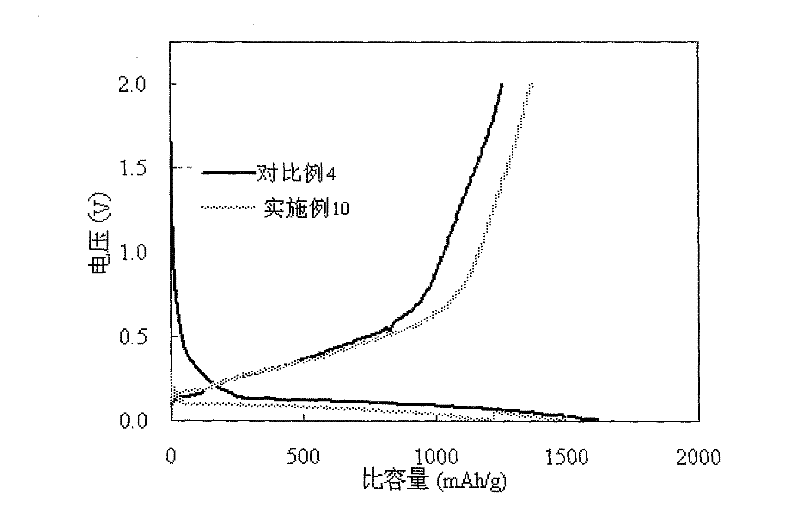
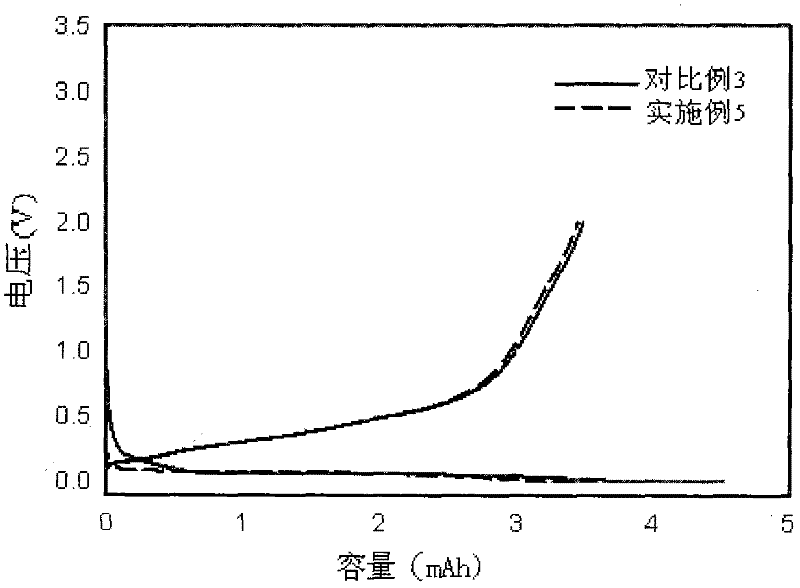
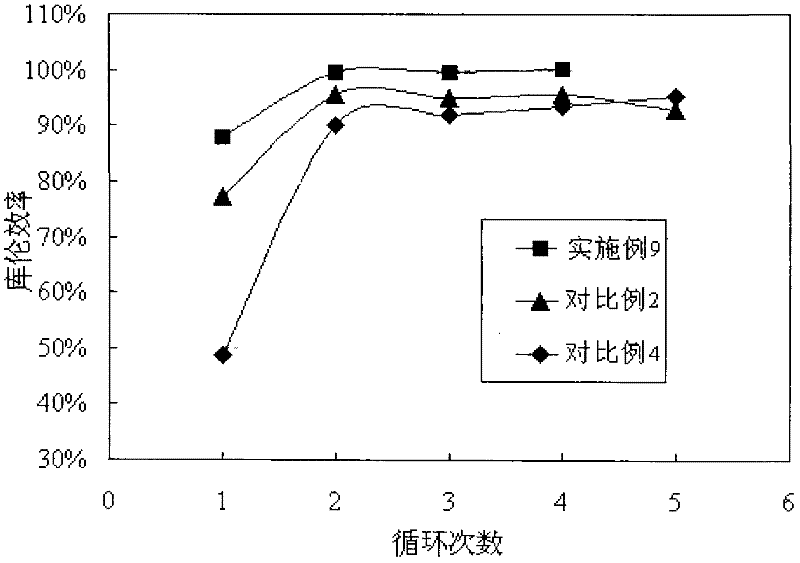
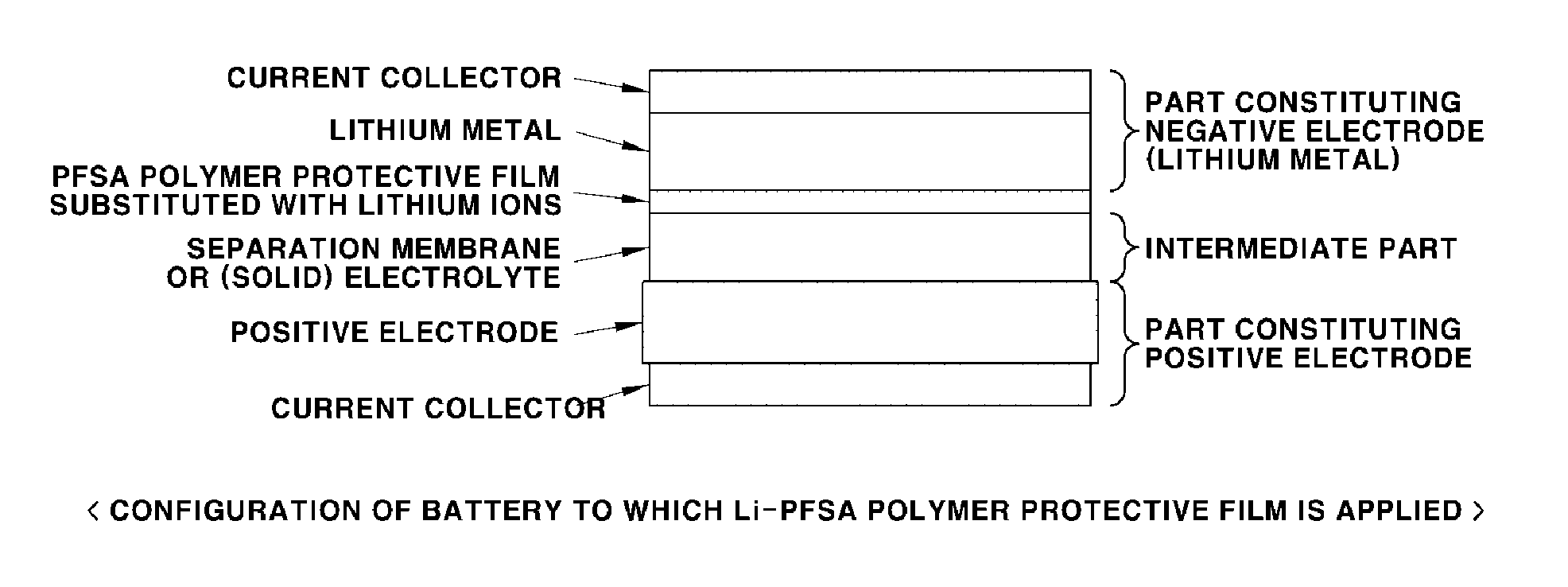
InactiveUS20150255782A1Improve lithium ion conductivityImprove stabilityFuel and secondary cellsElectrode thermal treatmentLithium–sulfur batteryLithium metal
Disclosed is a material which enhances stability for lithium in all the batteries, which use the lithium metal as an electrode material, by using and applying a lithium-substituted perfluoro sulfonic acid (PFSA) material in the form of a membrane or a powder to a lithium anode. Methods of manufacturing the material are also enclosed.
Owner:HYUNDAI MOTOR CO LTD
Lithium ion-conducting garnet-like compounds
ActiveUS20140295287A1Loss can be compensatedImprove stabilityTantalum compoundsZirconium compoundsLithiumCompound a
A lithium ion-conducting compound, having a garnet-like crystal structure, and having the general formula: Lin[A(3-a′-a″)A′(a′)A″(a″)][B(2-b′-b″)B′(b′)B″(b″)][C′(c′)C″(c″)]O12, where A, A′, A″ stand for a dodecahedral position of the crystal structure, where A stands for La, Y, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm and / or Yb, A′ stands for Ca, Sr and / or Ba, A″ stands for Na and / or K, 0<a′<2 and 0<a″<1, where B, B′, B″ stand for an octahedral position of the crystal structure, where B stands for Zr, Hf and / or Sn, B′ stands for Ta, Nb, Sb and / or Bi, B″ stands for at least one element selected from the group including Te, W and Mo, 0<b′<2 and 0<b″<2, where C and C″ stand for a tetrahedral position of the crystal structure, where C stands for Al and Ga, C″ stands for Si and / or Ge, 0<c′<0.5 and 0<c″<0.4, and where n=7+a′+2·a″−b′−2·b″−3·c′−4·c″ and 5.5<n<6.875.
Owner:ROBERT BOSCH GMBH
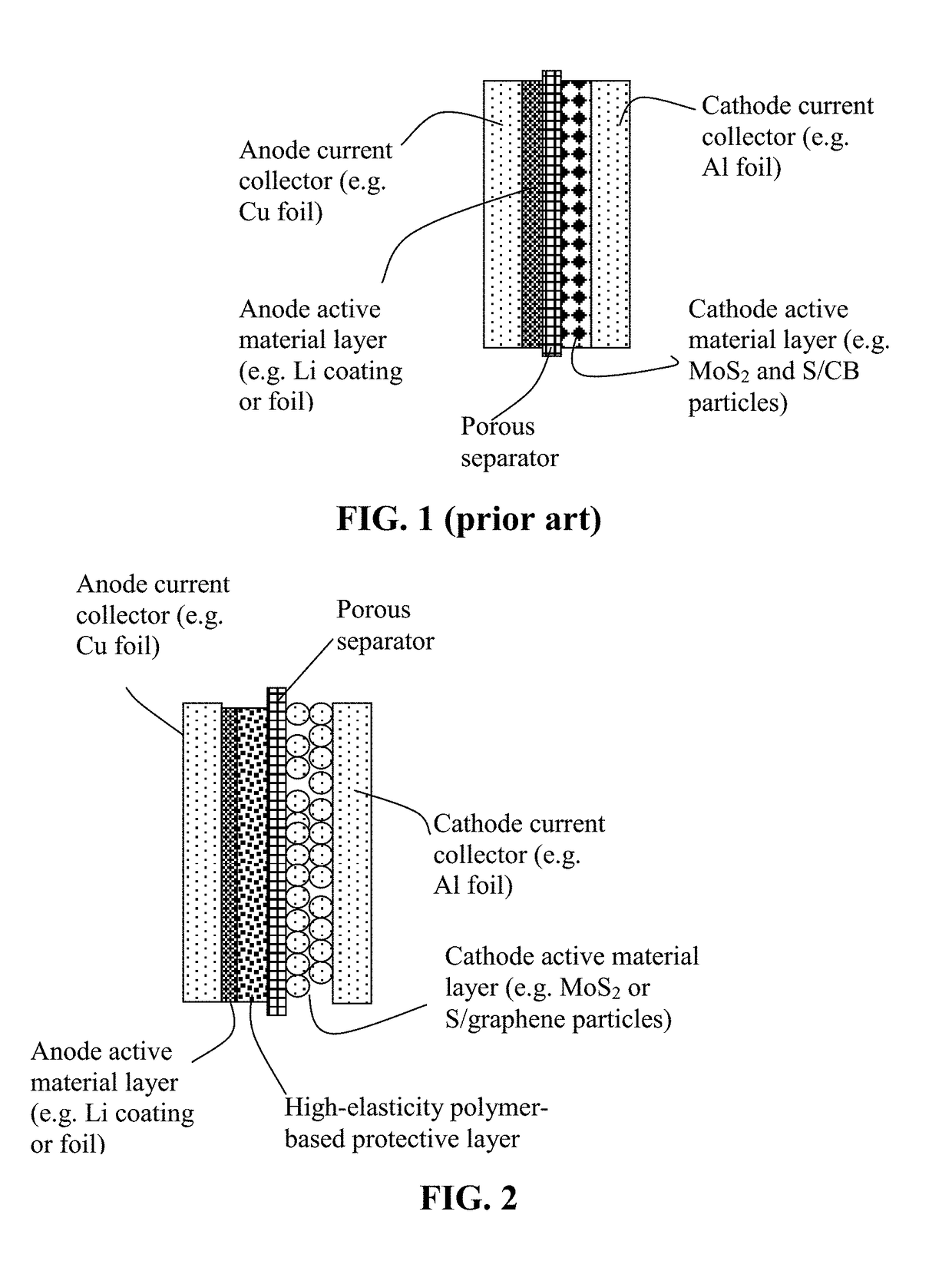
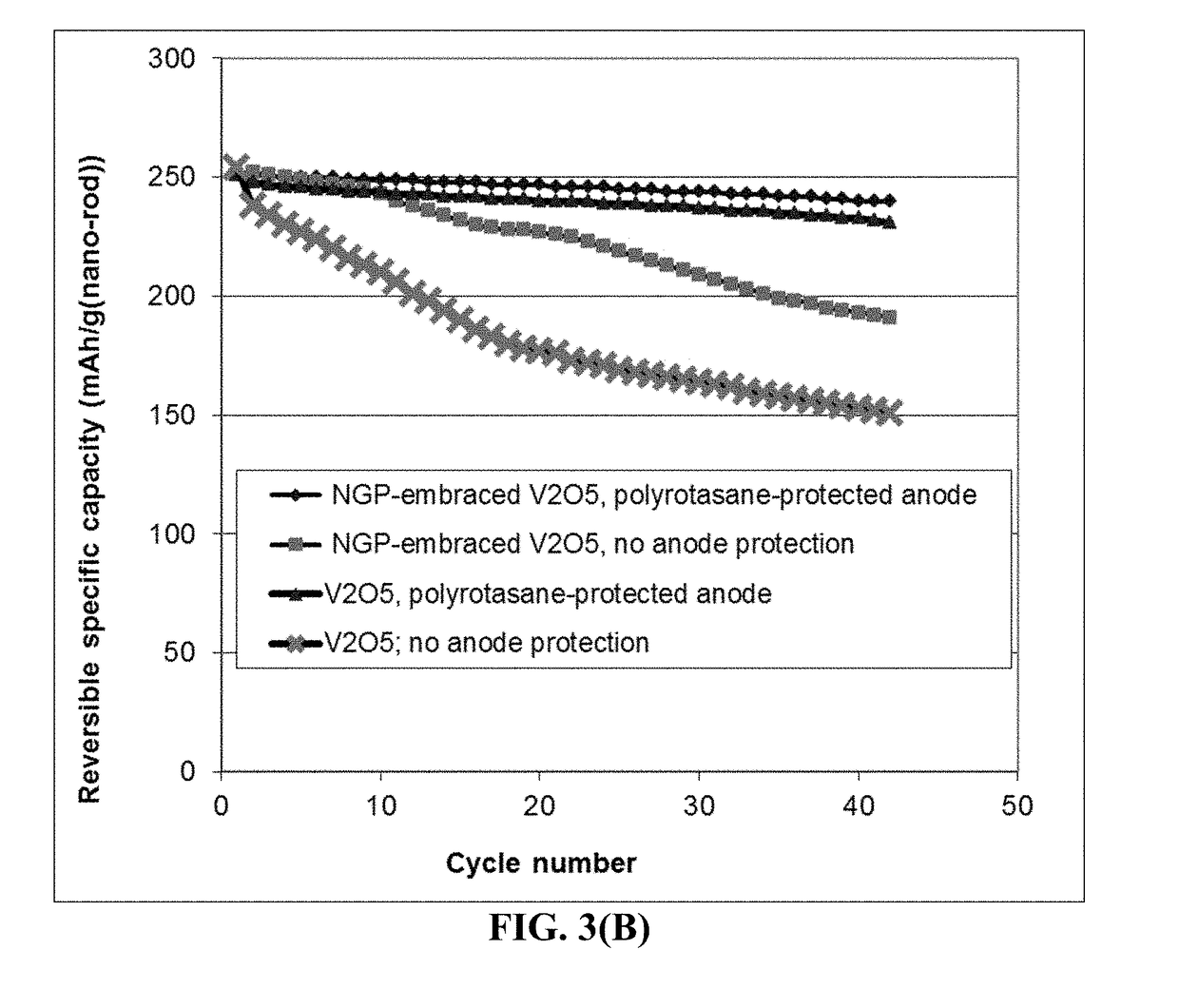
Lithium Secondary Batteries Containing Protected Particles of Anode Active Materials and Method of Manufacturing
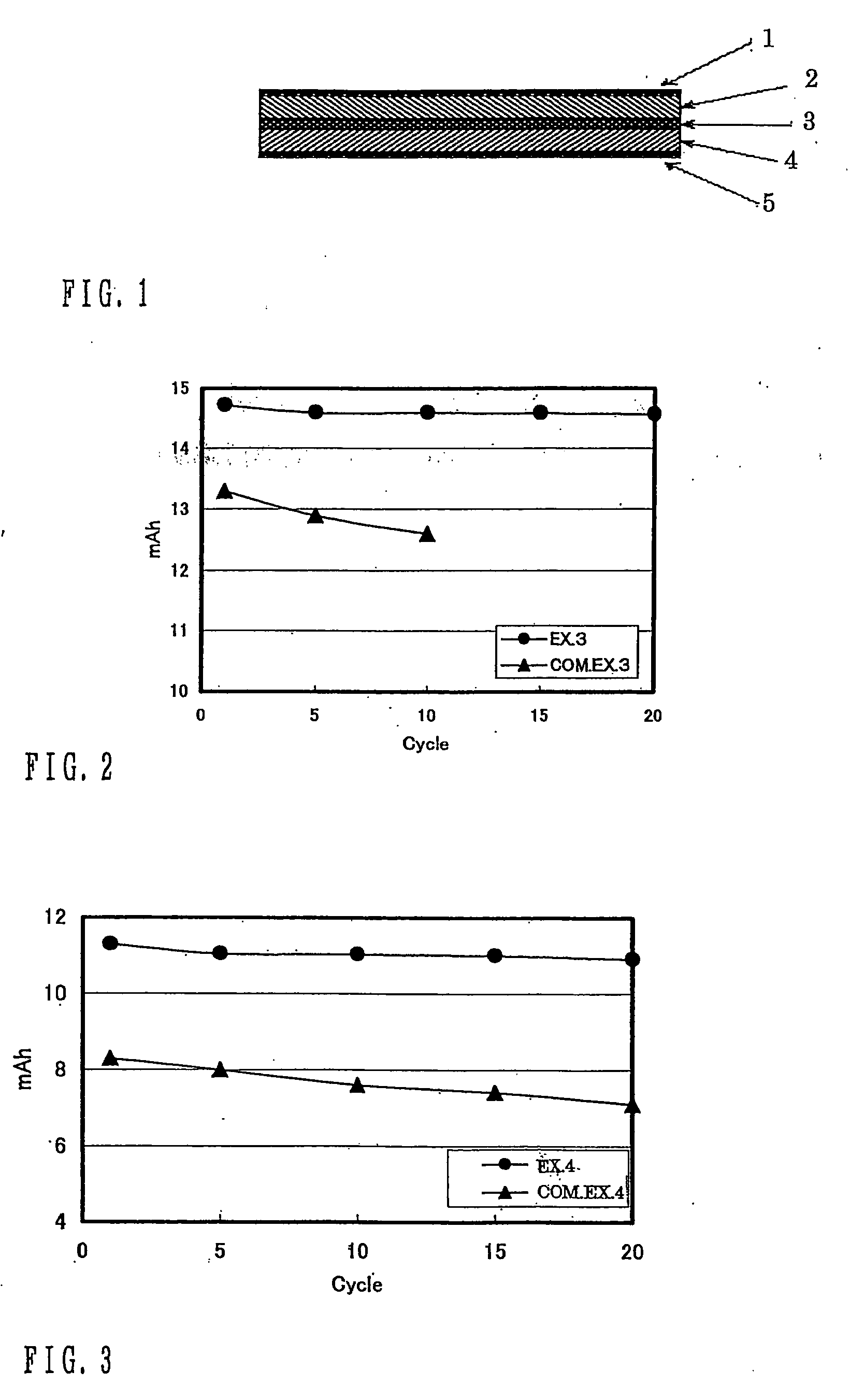
ActiveUS20180241032A1Improve lithium ion conductivityIncrease elasticityFuel and secondary cellsSecondary cellsParticulatesTensile strain
Provided is anode active material layer for a lithium battery, comprising multiple particulates of an anode active material, wherein a particulate is composed of one or a plurality of particles of a high-capacity anode active material being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 10% when measured without an additive or reinforcement, a lithium ion conductivity no less than 10-5 S / cm at room temperature, and a thickness from 0.5 nm (or a molecular monolayer) to 10 μm (preferably less than 100 nm), and wherein the high-capacity anode active material has a specific lithium storage capacity greater than 372 mAh / g (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.).
Owner:GLOBAL GRAPHENE GRP INC
Encapsulated Anode Active Material Particles, Lithium Secondary Batteries Containing Same, and Method of Manufacturing
ActiveUS20180287142A1Improve lithium ion conductivitySolid electrolytesNegative electrodesParticulatesPolyethylene oxide
Provided is particulate of an anode active material for a lithium battery, comprising one or a plurality of anode active material particles being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 5%, a lithium ion conductivity no less than 10−6 S / cm at room temperature, and a thickness from 0.5 nm to 10 μm, wherein the polymer contains an ultrahigh molecular weight (UHMW) polymer having a molecular weight from 0.5×106 to 9×106 grams / mole. The UHMW polymer is preferably selected from polyacrylonitrile, polyethylene oxide, polypropylene oxide, polyethylene glycol, polyvinyl alcohol, polyacrylamide, poly(methyl methacrylate), poly(methyl ether acrylate), a copolymer thereof, a sulfonated derivative thereof, a chemical derivative thereof, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Lithium Ion Secondary Battery and a Solid Electrolyte Therefof
InactiveUS20080268346A1Improve lithium ion conductivityImprove charge and discharge cycle characteristicsNon-metal conductorsHybrid capacitor electrolytesElectrolyteLithium
A solid electrolyte comprising powder of an inorganic substance comprising a lithium ion conductive crystal or powder of a lithium ion conductive glass-ceramic and an organic polymer added with an inorganic or organic lithium salt, and being free of an electrolytic solution. The organic polymer is a copolymer, a bridge structure or a mixture thereof of polyethylene oxide and other organic polymer or polymers. A lithium ion secondary battery comprises this solid electrolyte.
Owner:OHARA
Anode-Protecting Layer for a Lithium Metal Secondary Battery and Manufacturing Method
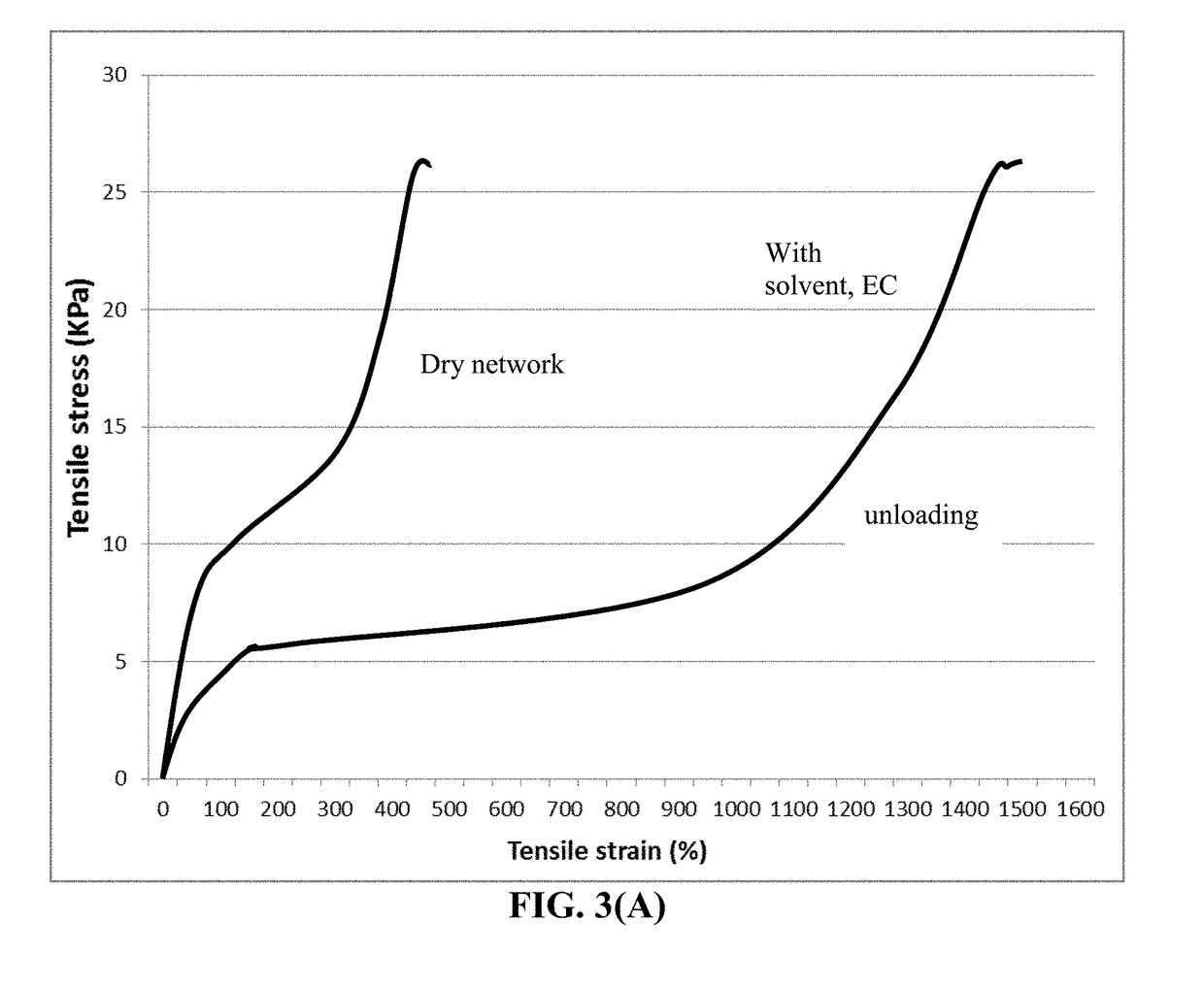
ActiveUS20190051905A1Improve lithium ion conductivityReduce thicknessSolid electrolytesCell electrodesTensile strainPolyrotaxane
Provided is a lithium secondary battery, comprising a cathode, an anode, and a porous separator or electrolyte disposed between the cathode and the anode, wherein the anode comprises: (a) an anode active layer containing a layer of lithium or lithium alloy, in a form of a foil, coating, or multiple particles aggregated together, as an anode active material; and (b) a thin layer of a high-elasticity polymer, disposed between the anode active layer and the porous separator or electrolyte; the polymer having a recoverable tensile strain from 2% to 1,500%, a lithium ion conductivity no less than 10−6 S / cm (typically up to 5×10−2 S / cm) at room temperature, and a thickness from 1 nm to 10 μm, wherein the high-elasticity polymer contains a polyrotaxane network having a rotaxane structure or a polyrotaxane structure at a crosslink point of the polyrotaxane network.
Owner:GLOBAL GRAPHENE GRP INC
Polymer Binder for Lithium Battery and Method of Manufacturing
ActiveUS20180248173A1Increase elasticityIncrease strainSolid electrolytesFuel and secondary cellsTensile strainRoom temperature
Provided is an anode active material layer for a lithium battery. The anode active material layer comprises multiple anode active material particles and an optional conductive additive that are bonded together by a binder comprising a high-elasticity polymer having a recoverable or elastic tensile strain no less than 10% when measured without an additive or reinforcement in the polymer and a lithium ion conductivity no less than 10−5 S / cm at room temperature. The anode active material preferably has a specific lithium storage capacity greater than 372 mAh / g (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.).
Owner:GLOBAL GRAPHENE GRP INC
Anode active material for lithium secondary battery and lithium secondary battery comprising the same
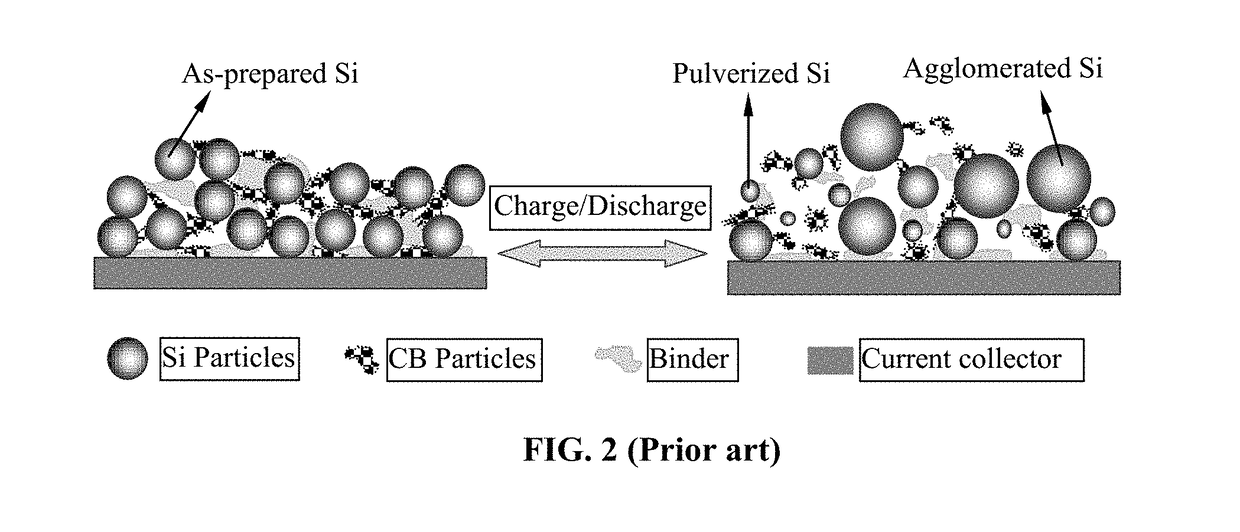
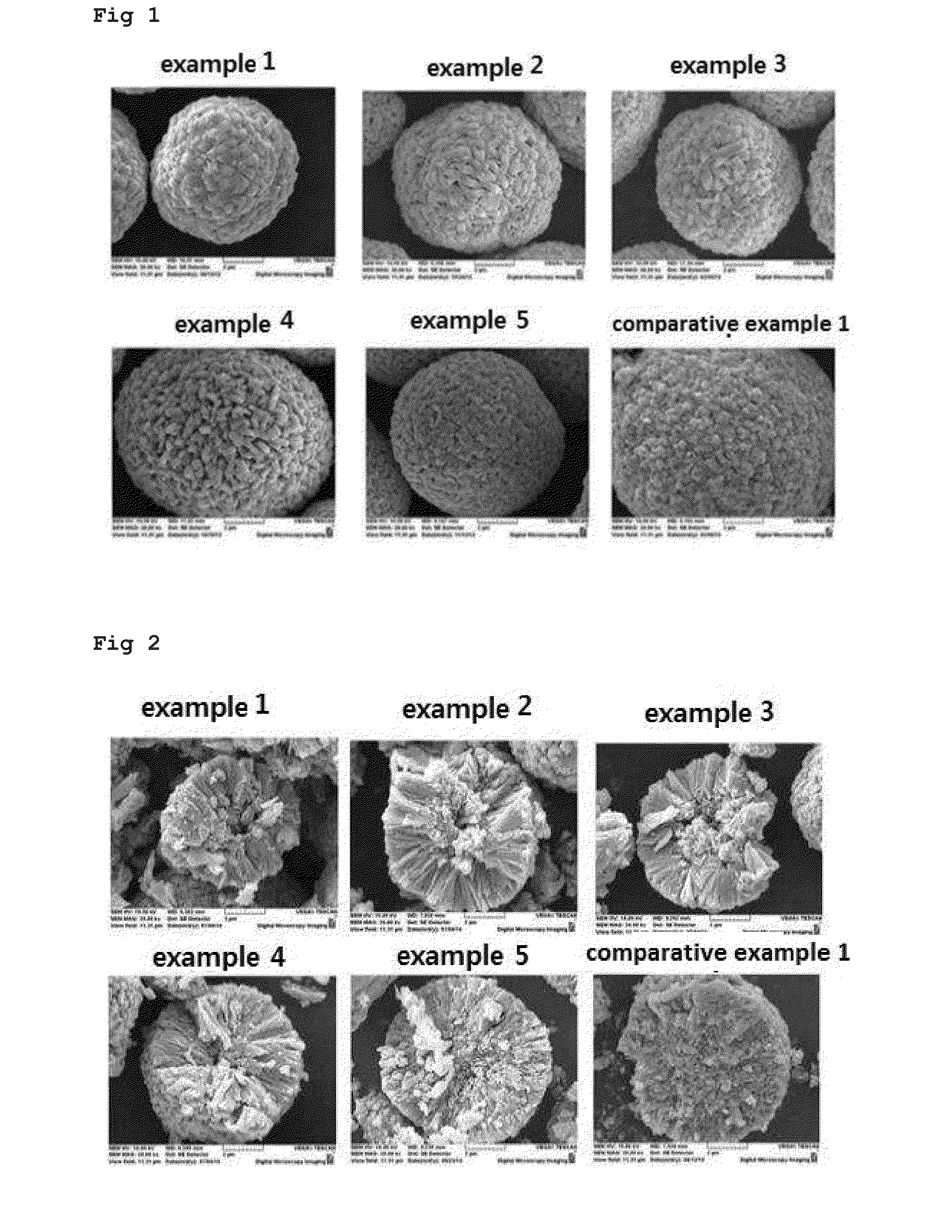
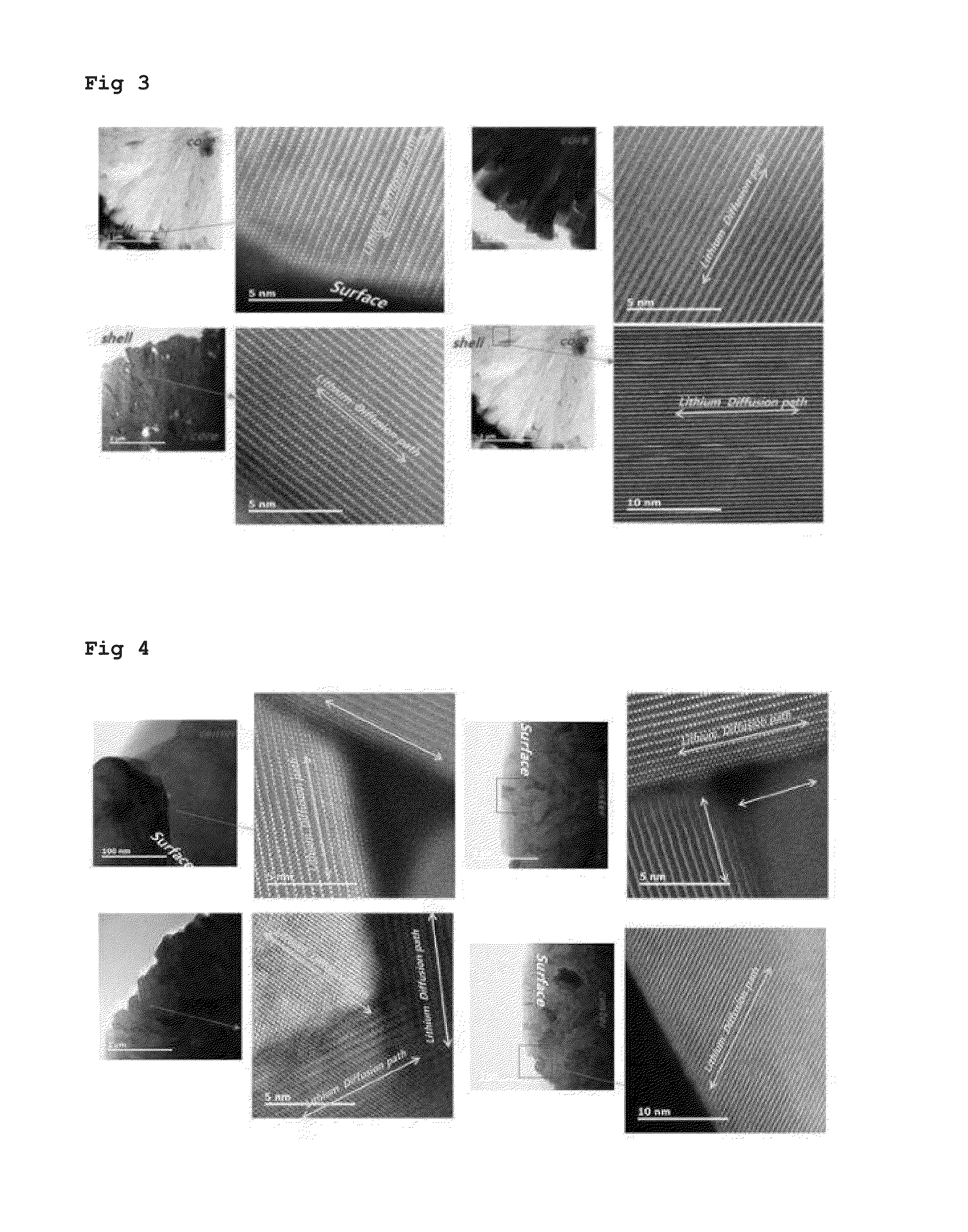
ActiveUS20160181597A1Facilitated releaseEasy to storeNegative electrodesNon-aqueous electrolyte accumulator electrodesLithiumPhysical chemistry
The present invention relates to an anode active material for lithium secondary battery and a lithium secondary battery including the same, and more specifically it relates to an anode active material for lithium secondary battery in which the lithium ion diffusion path in the primary particles is formed to exhibit directivity in the center direction of the particles, and a lithium secondary battery including the same.The anode active material for lithium secondary battery of the present invention has a lithium ion diffusion path exhibiting specific directivity in the primary particles and the secondary particles, and thus not only the conduction velocity of the lithium ion is fast and the lithium ion conductivity is high but also cycle characteristics are improved as the crystal structure hardly collapses despite repeated charging and discharging.
Owner:ECOPRO BM CO LTD
Sulfide-based lithium-ion-conducting solid electrolyte glass, all-solid lithium secondary battery, and method for manufacturing all-solid lithium secondary battery
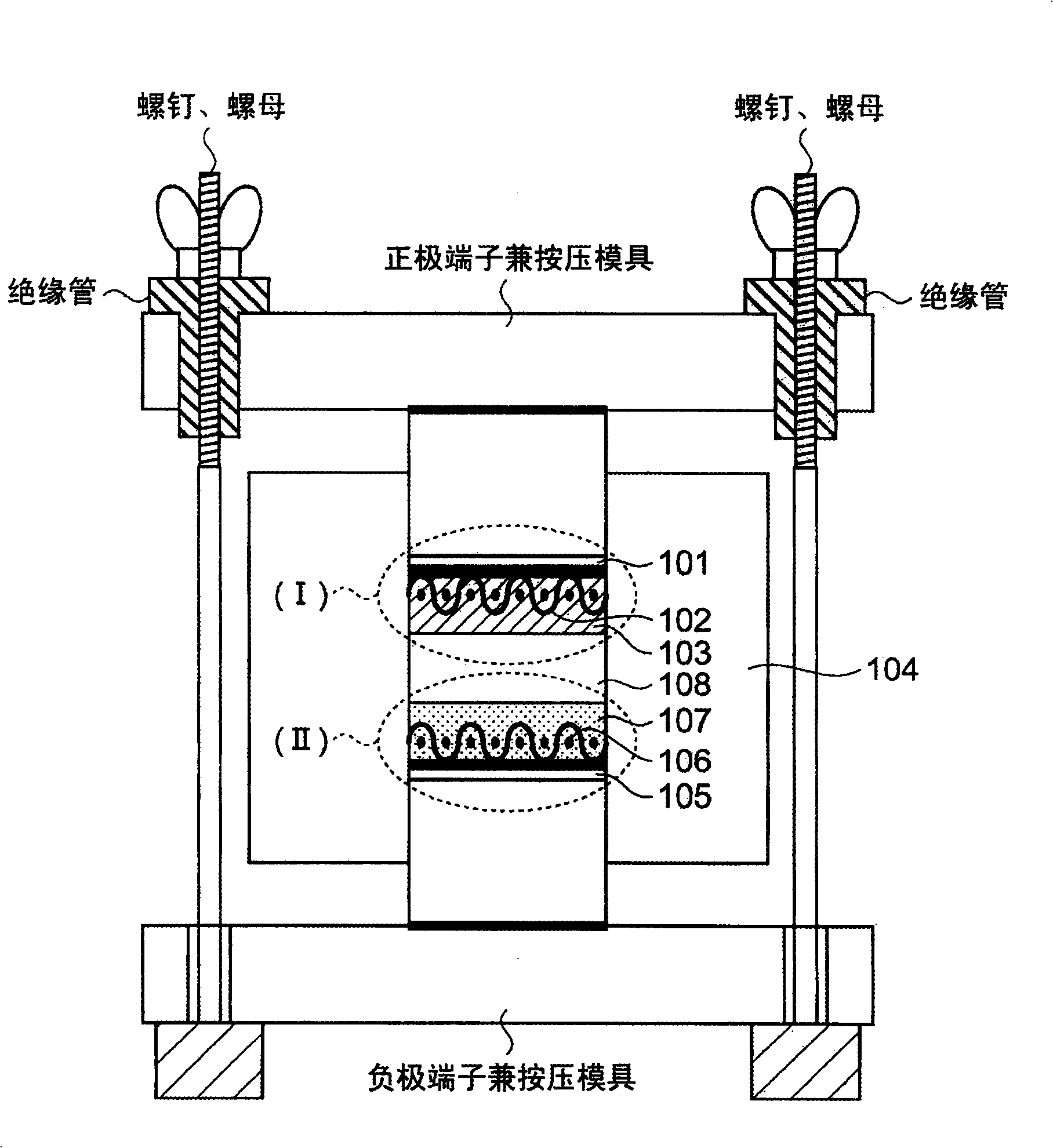
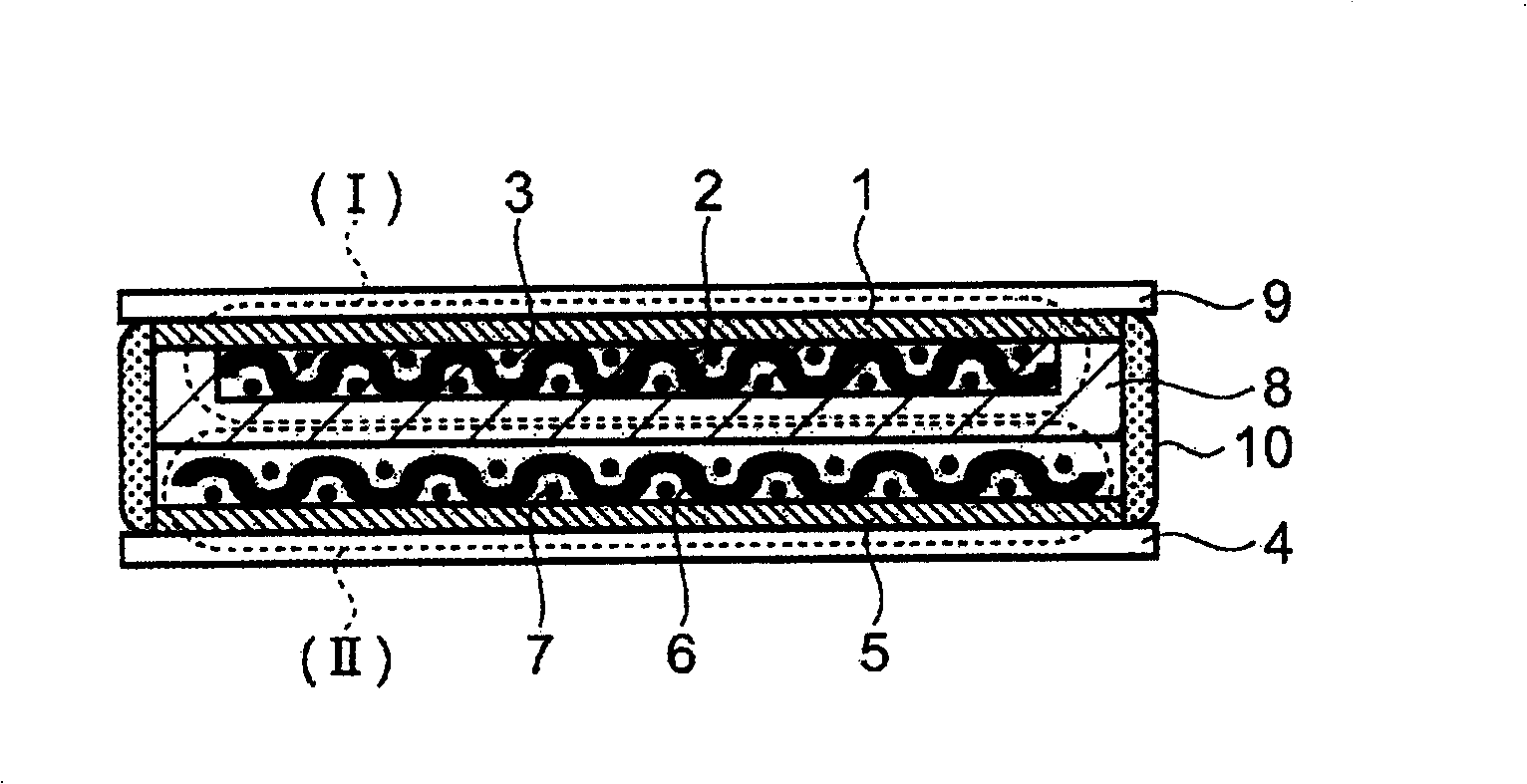
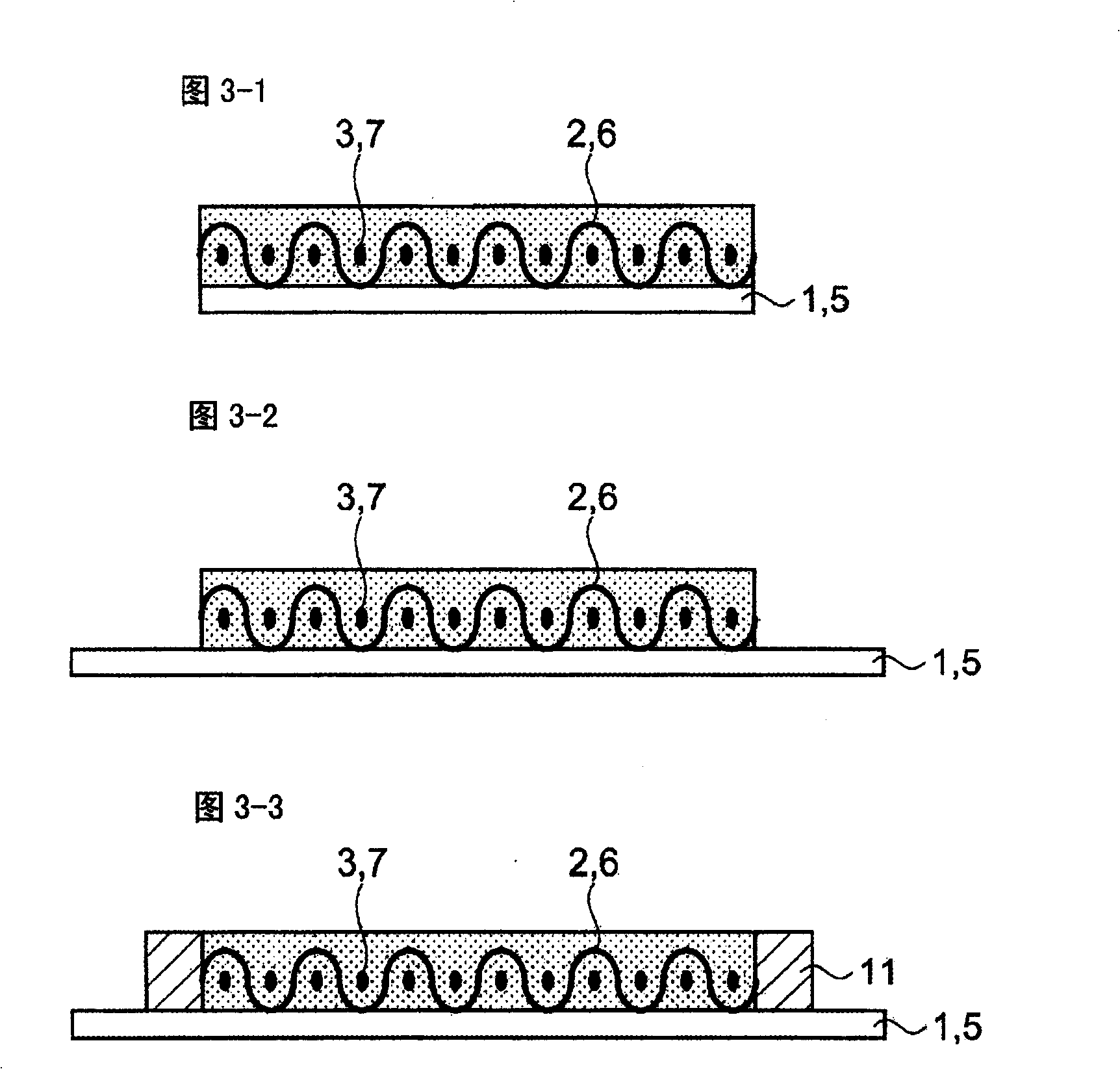
InactiveCN101494299APrevent precipitationPromote precipitationNon-metal conductorsCell electrodesVitrificationLithium
The present invention discloses a all-solid lithium secondary battery that has large chare and discharge output current denseness and excellent charge and discharge cycle life which can be easily manufactured at low cost. In the manufacturing process of the all-solid lithium secondary battery, a new lithium ion conductor with ionic conduction improved can be obtained through vitrification to mixed electrolytes by mixing Alpha-oxide of alumina in various sulfide system lithium ion conductivity solid electrolyte. The electrolytes layer 8 used the lithium ion conductor, and positive and negative electrode (I), (II) are formed by positive and negative electrode material 3, 7 are constituted. Subsequently, cascading at least 1 layer in positive and negative electrode (I), (II) with electrolytes layer, and producing battery while electrolytes not crystallizes by heating and compressing as a whole.
Owner:SEIKO EPSON CORP
Non-aqueous electrolyte for secondary battery and non-aqueous electrolyte secondary battery employing the same
ActiveUS20130330610A1Improve lithium ion conductivityIncreased durabilityCell electrodesSecondary cellsSolventNon aqueous electrolytes
Provided are: a non-aqueous electrolyte solution for a secondary battery that exhibits both excellent low-temperature discharge characteristics and excellent cycle characteristics on a long-term basis; and a secondary battery.A non-aqueous electrolyte solution for a non-aqueous electrolyte secondary battery that has a positive electrode and a negative electrode capable of the absorbing and releasing of a metal ion, and a separator,the non-aqueous electrolyte solution comprising, in addition to an electrolyte and a non-aqueous solvent, 0.01 mass % or more to less than 3 mass % of a compound having one or more partial structure represented by the following general formula (1) and two or more isocyanate groups in the molecule:(In the general formula (1), R represents hydrogen or a C1-C12 organic group that may contain an isocyanate group and is constituted of atoms selected from the group consisting of hydrogen atom, carbon atom, nitrogen atom, oxygen atom, sulfur atom, phosphorus atom, and halogen atom).
Owner:MU IONIC SOLUTIONS CORP +1
Lithium metal secondary battery featuring an anode-protecting layer
ActiveUS20190393543A1Reduce eliminatePromotes uniform depositionCell seperators/membranes/diaphragms/spacersElectrode carriers/collectorsTensile strainLithium metal
Provided is a lithium secondary battery, comprising a cathode, an anode, and a porous separator or electrolyte disposed between the cathode and the anode, wherein the anode comprises: (a) an anode active layer containing a layer of lithium or lithium alloy, in a form of a foil, coating, or multiple particles aggregated together, as an anode active material; and (b) an anode-protecting layer of a conductive sulfonated elastomer composite, disposed between the anode active layer and the separator / electrolyte; wherein the composite has from 0.01% to 50% by weight of a conductive reinforcement material dispersed in a sulfonated elastomeric matrix material and the protecting layer has a thickness from 1 nm to 100 μm, a fully recoverable tensile strain from 2% to 500%, a lithium ion conductivity from 10−7 S / cm to 5×10−2 S / cm, and an electrical conductivity from 10−7 S / cm to 100 S / cm.
Owner:GLOBAL GRAPHENE GRP INC
Fully solid-state lithium secondary battery electrolyte material, preparation method thereof and fully solid-state lithium secondary battery
The invention provides a fully solid-state lithium secondary battery electrolyte material, which contains Li2S, a first sulfide, a second sulfide and a dopant, wherein the first sulfide is GeS and / or GeS2; the second sulfide is one or more of FeS, FeS2, SiS2, P2S5, B2S3, CeS2 and Al2S3; and the dopant is a lithium salt or an oxide capable of forming raw acid. According to the fully solid-state lithium secondary battery electrolyte material, the lithium ion conductivity of the fully solid-state lithium secondary battery electrolyte material at room temperature is increased by using the first sulfide, the second sulfide and the dopant. Experiments show that the lithium ion conductivity of the fully solid-state lithium secondary battery electrolyte material reaches up to 10<-3> S.cm<-1> at the room temperature, the conductivity is better, and the application is facilitated. In addition, the lithium ion conductivity of the fully solid-state lithium secondary battery electrolyte material has better electrochemical stability. The invention provides a preparation method of the lithium ion conductivity of the fully solid-state lithium secondary battery electrolyte material and a fully solid-state lithium secondary battery.
Owner:ZHEJIANG FUNLITHIUM NEW ENERGY TECH CO LTD
Method of Manufacturing a Lithium Secondary Battery Having a Protected High-Capacity Anode Active Material
ActiveUS20180233736A1Improve lithium ion conductivityReduce breakageFinal product manufactureNegative electrodesParticulatesElastomer
Provided is an anode active material layer for a lithium battery. This layer comprises multiple particulates of an anode active material, wherein at least a particulate is composed of one or a plurality of particles of a high-capacity anode active material being encapsulated by a thin layer of elastomeric material that has a lithium ion conductivity no less than 10−7 S / cm (preferably no less than 10−5 S / cm) at room temperature and an encapsulating shell thickness from 1 nm to 10 μm, and wherein the high-capacity anode active material (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.) has a specific capacity of lithium storage greater than 372 mAh / g (the theoretical lithium storage limit of graphite).
Owner:GLOBAL GRAPHENE GRP INC
Method for preparing lithium phosphate/carbon-coated lithium iron phosphate composite material
ActiveCN103730657AImprove conductivityImprove performanceCell electrodesSecondary cellsLithium iron phosphateLITHIUM PHOSPHATE
The invention discloses a method for preparing a high-performance lithium phosphate / carbon-coated lithium iron phosphate composite material. The method mainly comprises the processes of preparing lithium iron phosphate not subjected to carbon coating and preparing the lithium phosphate / carbon-coated lithium iron phosphate composite material. The method comprises the following steps: performing primary sintering to prepare lithium iron phosphate not subjected to carbon coating, and adding a certain amount of lithium phosphate and carbon source in the secondary mixing process to finally prepare the lithium phosphate / carbon-coated lithium iron phosphate composite material. The lithium phosphate is a fast ionic conductor, the aim of improving the electrical conductivity of lithium iron phosphate is achieved, and the aim of improving the overall performance of the lithium iron phosphate is also achieved.
Owner:QINGHAI TAIFENG XIANXING LITHIUM ENERGY TECH CO LTD
Hybrid Solid State Electrolyte for Lithium Secondary Battery
ActiveUS20180166759A1Reduce electrical conductivityLow ionic conductivityFuel and primary cellsSolid electrolytesSolid state electrolyteElectrolyte composition
Provided is a solid state electrolyte for a rechargeable lithium battery, comprising a lithium ion-conducting polymer matrix or binder and a lithium ion-conducting inorganic species dispersed in or chemically bonded by the polymer matrix or binder, wherein the lithium ion-conducting inorganic species is selected from a mixture of a sodium-conducting species or sodium salt and a lithium-conducting species or lithium salt selected from Li2CO3, Li2O, Li2C2O4, LiOH, LiX, ROCO2Li, HCOLi, ROLi, (ROCO2Li)2, (CH2OCO2Li)2, Li2S, LixSOy, or a combination thereof, wherein X=F, Cl, I, or Br, R=a hydrocarbon group, x=0-1, y=1-4; and wherein the polymer matrix or binder is in an amount from 1% to 99% by volume of the electrolyte composition. Also provided are a process for producing this solid state electrolyte and a lithium secondary battery containing such a solid state electrolyte.
Owner:GLOBAL GRAPHENE GRP INC
Composite solid electrolyte and preparation method thereof
InactiveCN109786816AReduce interface contact resistanceImprove lithium ion conductivityLi-accumulatorsGap fillingPorosity
The present invention discloses a composite solid electrolyte and a preparation method thereof. The composite solid electrolyte comprises an inorganic solid electrolyte and a polymer electrolyte. Theinorganic solid electrolyte is of a porous structure; the porosity of the inorganic solid electrolyte is less than 30%; and the inside of the inorganic solid electrolyte is provided with a continuouslithium ion channel composed of particles communicating with each other. The polymer electrolyte is arranged between the inorganic solid electrolytes for filling gaps, and account for less than 30% ofthe composite solid electrolyte. The porous inorganic solid electrolyte of the invention constitutes a main frame structure, which has a porosity below 30% and is the main bearer of lithium ion transportation. The polymer electrolyte is a gap filling material, which is mainly used for improving the overall flexibility of the composite solid electrolyte and reducing the impedance of the solid-solid contact interface between the positive electrode plate and negative electrode plate.
Owner:杭州阳名新能源设备科技有限公司
Non-aqueous electrolyte secondary battery and method of manufacturing the same
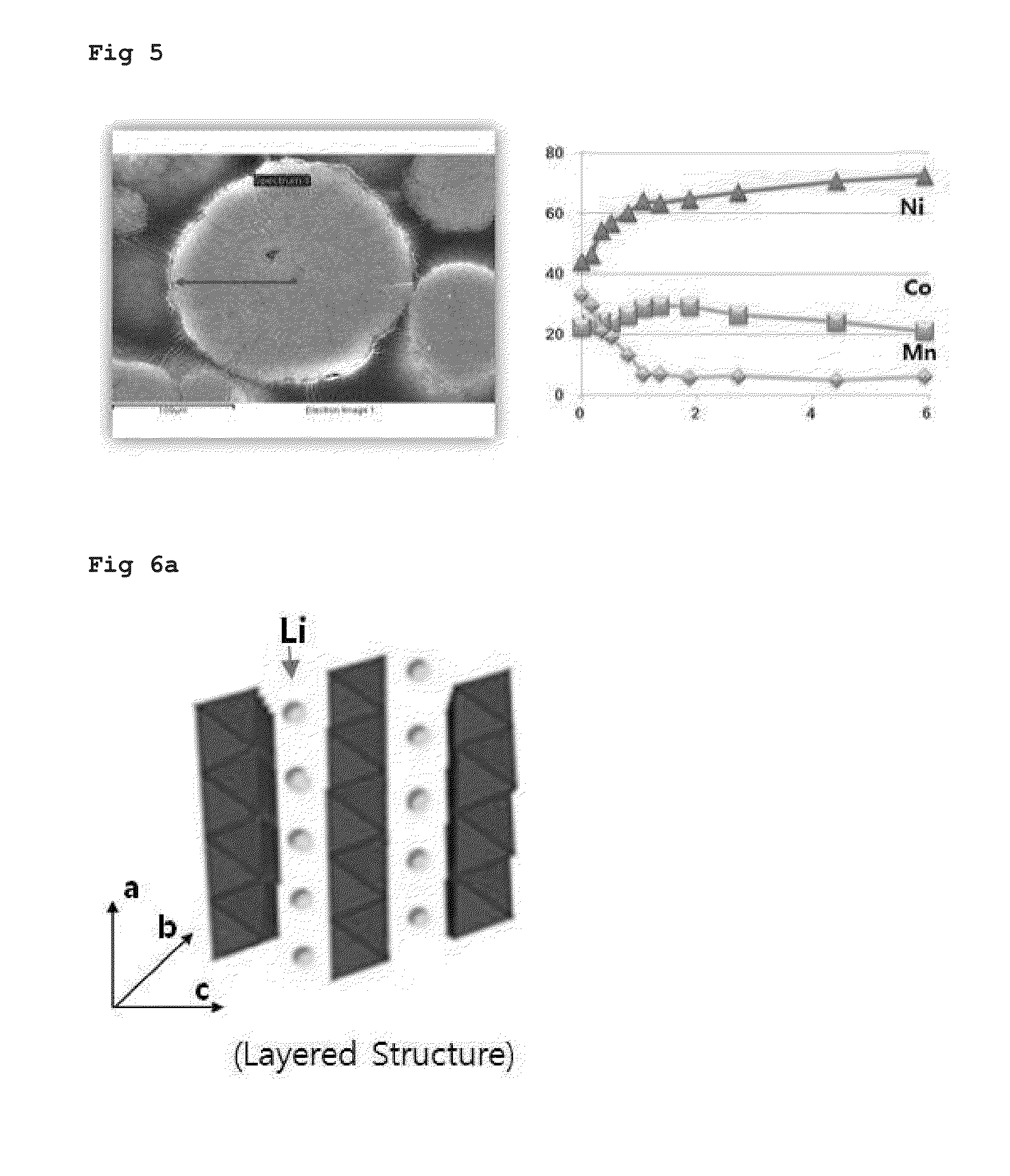
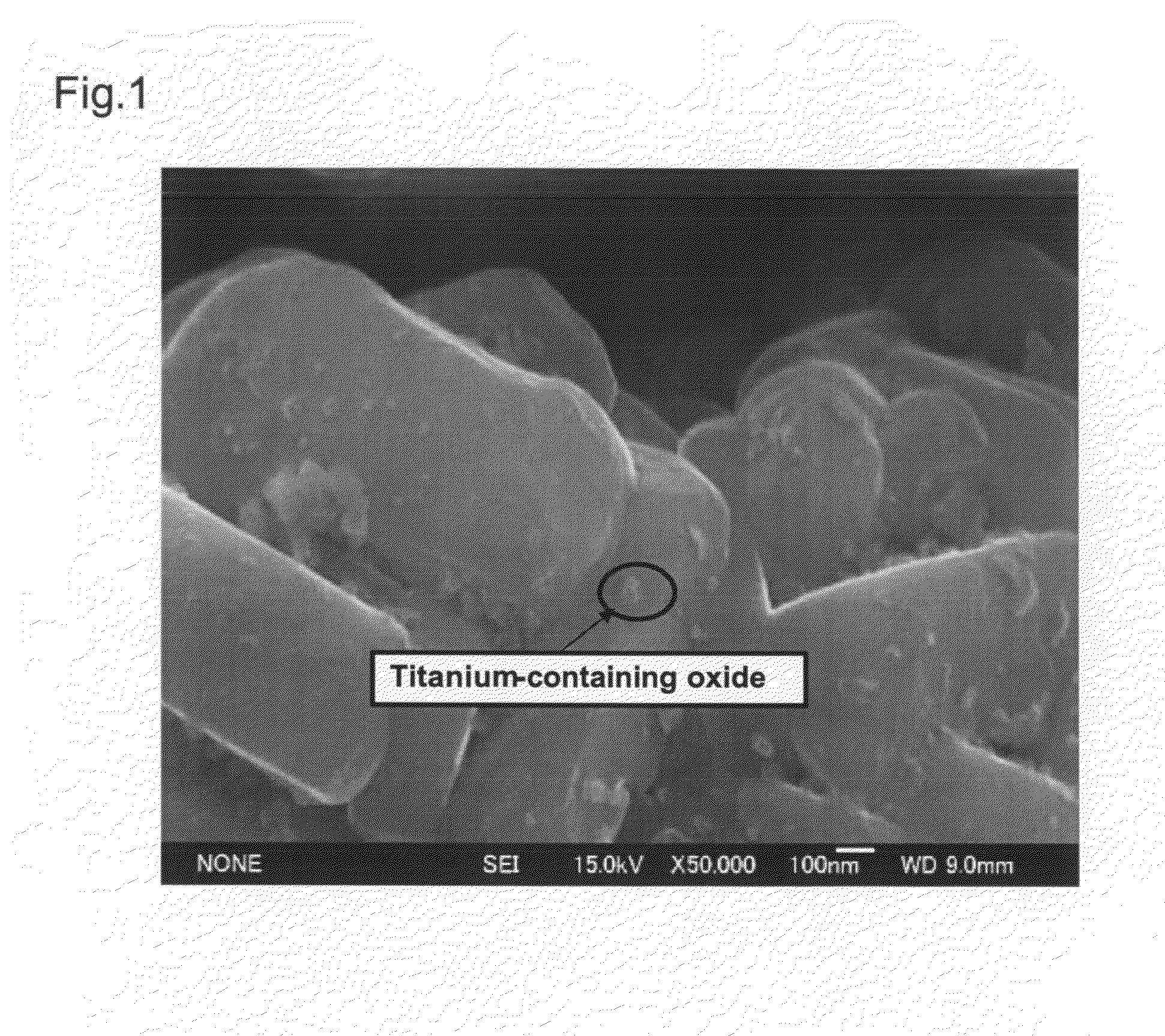
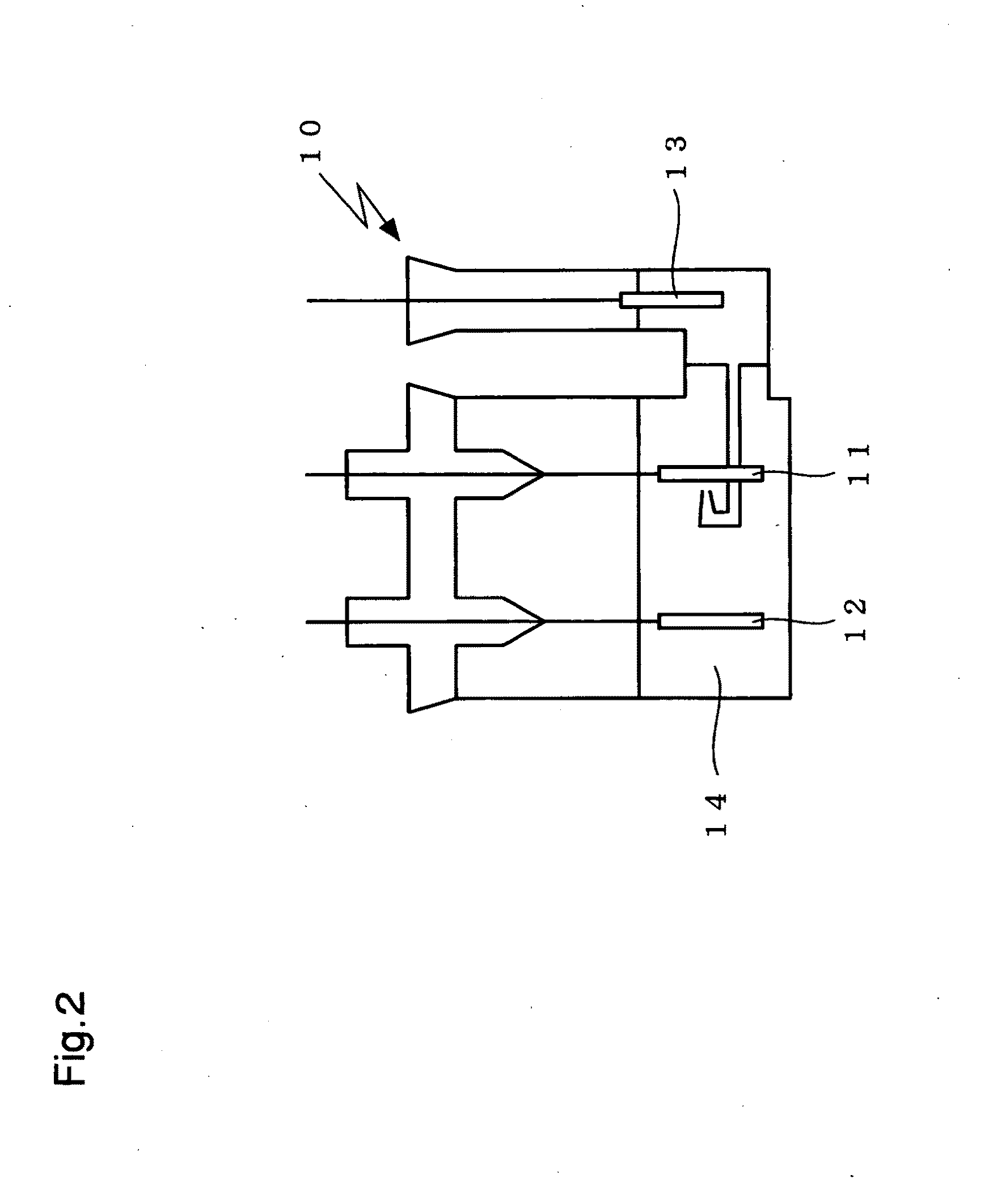
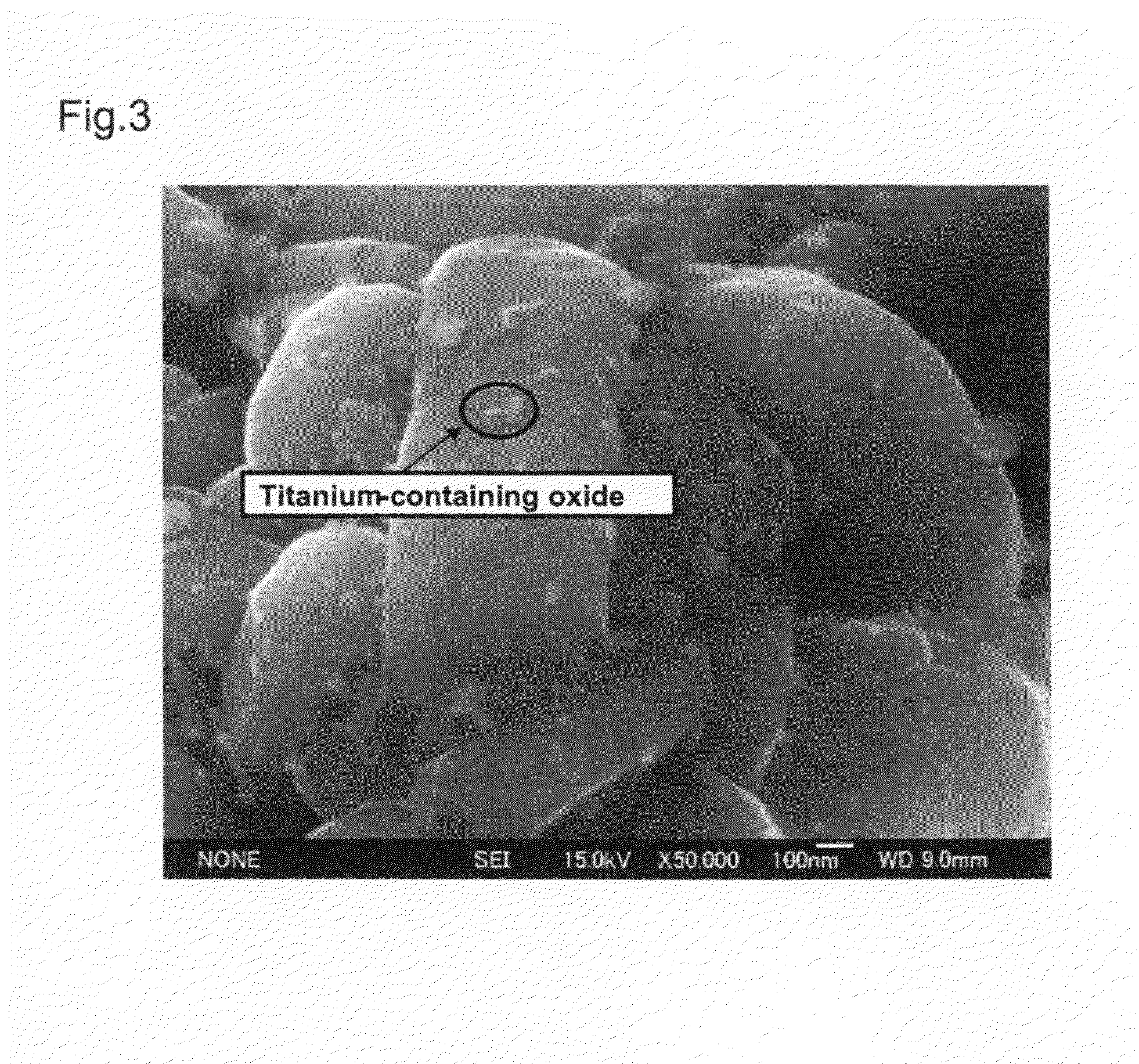
InactiveUS20090305136A1Stable crystal structureImproving high-rate charge-discharge capabilityMaterial nanotechnologyElectrode thermal treatmentPhysical chemistryTitanium
A non-aqueous electrolyte secondary battery has a positive electrode (11) containing a positive electrode active material, a negative electrode (12) containing a negative electrode active material, and a non-aqueous electrolyte solution (14) in which a solute is dissolved in a non-aqueous solvent. The positive electrode active material is obtained by sintering a titanium-containing oxide on a surface of a layered lithium-containing transition metal oxide represented by the general formula Li1+xNiaMnbCocO2+d, where x, a, b, c, and d satisfy the conditions x+a+b+c=1, 0.7≦a+b, 0≦x≦0.1, 0≦c / (a+b)<0.35, 0.7≦a / b≦2.0, and −0.1≦d≦0.1.
Owner:SANYO ELECTRIC CO LTD
Organic/inorganic composite electrolyte and preparation method thereof
InactiveCN103515649AHigh lithium ion conductivityImprove mechanical propertiesFinal product manufactureElectrolytesPolymer electrolytesComposite electrolyte
The invention discloses an organic / inorganic composite electrolyte and a preparation method thereof. The organic / inorganic composite electrolyte is obtained by dispersing a lithium salt and a modified inorganic solid electrolyte into a polymer in a mixing manner, wherein the polymer contains an ethylene oxide repeating unit. The modification of the inorganic solid electrolyte is carried out for the first time; a polymer electrolyte and the inorganic electrolyte are effectively and evenly composited, so that the organic / inorganic composite electrolyte material is obtained. The dispersion of the inorganic solid electrolyte in the polymer is improved by the modification of the inorganic solid electrolyte, so that the adverse effect that the inorganic solid electrolyte is automatically gathered is avoided. The organic / inorganic composite electrolyte material obtained according to the preparation method has the advantages of the polymer electrolyte and the inorganic electrolyte, so that the comprehensive performance of the organic / inorganic composite electrolyte material is obviously improved. The organic / inorganic composite electrolyte material has practical value and can be popularized in lithium ion secondary batteries.
Owner:TORAY ADVANCED MATERIALS RES LAB CHINA
Lithium ion secondary battery and a method for manufacturing the same
InactiveUS20060234130A1Improve lithium ion conductivityImprove performanceSolid electrolytesFinal product manufactureElectrolyteLithium
A lithium ion secondary battery includes a positive electrode, a negative electrode and a thin film solid electrolyte including lithium ion conductive inorganic substance. The thin film solid electrolyte has thickness of 20 μm or below and is formed directly on an electrode material or materials for the positive electrode and / or the negative electrode. The thin film solid electrolyte has lithium ion conductivity of 10−5Scm−1 or over and contains lithium ion conductive inorganic substance powder in an amount of 40 weight % or over in a polymer medium. The average particle diameter of the inorganic substance powder is 0.5 μm or below. According to a method for manufacturing the lithium ion secondary battery, the thin film solid electrolyte is formed by coating the lithium ion conductive inorganic substance directly on the electrode material or materials for the positive electrode and / or the negative electrode.
Owner:OHARA
Three-layer core-shell structure positive electrode material, preparation method thereof and lithium ion battery
ActiveCN108598400AImprove ionic conductivityAvoid erosionCell electrodesSecondary cellsElectrical conductorLithium-ion battery
The invention relates to a three-layer core-shell structure positive electrode material. The three-layer core-shell structure positive electrode material comprises a three-layer structure, namely, a ternary positive electrode material inner core, an aluminum oxide layer and a rapid ion conductor layer, wherein the aluminum oxide layer wraps the ternary positive electrode material inner core, and the rapid ion conductor layer wraps the aluminum oxide layer. The invention also comprises a preparation method of the three-layer core-shell structure positive electrode material. Since the rapid ionconductor wrapping an outer layer of the three-layer core-shell structure positive electrode material has super strong ionic conductivity, the integral ionic conductivity of the Al2O3-coated positiveelectrode material can be improved, the electric energy loss is reduced, and the cycle property of a battery is improved; moreover, the aluminum oxide layer and the ternary positive electrode materialinner core form a Li-Al-Co-O co-melting body by high-temperature calcination during the preparation process, the ionic conductivity of the material can be further improved, and the electrical conductivity and the microstructure stability of the composite material are improved; and by the three-layer core-shell structure, the corrosion of an electrolyte to the ternary positive electrode material inner core can be reduced, and the battery safety is improved.
Owner:SOUNDON NEW ENERGY TECH CO LTD
Secondary lithium battery and positive electrode material thereof, and positive electrode material preparation method
ActiveCN105098177AImprove lithium ion conductivityGood chemical stabilityElectrode thermal treatmentPositive electrodesPhosphateSodium-ion battery
The present invention provides a positive active material for use in a lithium ion battery, a method for preparing the positive active material and a lithium ion battery containing the positive active material. The positive active material includes a core of lithium containing transition metal oxide represented by Formula LixMyN1-yO2-αAβ and a coating layer of lithium containing transition metal phosphate represented by Formula LiaMbN′1-bPO4-λBζ in situ formed on the core, wherein 0.9≰x≰1.2, 0.6≰y≰1.0, 0.9≰a≰1.1, 0.6≰b≰1.0, 0≰α≰0.2, 0≰β≰0.4, 0≰λ≰0.5, 0≰ζ≰0.5. The positive active material for use in a lithium ion battery according to the present invention has high capacity, desirable cycling performance and safety performance, as well as desirable thermal stability.
Owner:CONTEMPORARY AMPEREX TECH CO
Lithium Secondary Batteries With Enhanced Safety And Performance
InactiveUS20080131781A1Minimize degradationImprove battery safetyPrimary cellsActive material electrodesInorganic particleLithium intercalation
Disclosed is an electrode obtained from electrode slurry comprising: (a) an electrode active material capable of lithium intercalation / deintercalation; and (b) inorganic particles having lithium ion conductivity. An electrochemical device comprising the same electrode is also disclosed. The electrochemical device, using such inorganic particles having lithium ion conductivity added to electrode slurry, can show improved safety, while minimizing degradation in the quality caused by the use of additives.
Owner:LG CHEM LTD
Anode for lithium air battery and preparation method thereof
ActiveUS20180053978A1Long lastingImprove battery efficiencyElectrode rolling/calenderingFuel and primary cellsLithium–air batteryLithium metal
Provided herein is an anode for a lithium air battery with a long lifetime and high lithium ion conductivity and a method for preparing thereof. The anode includes a lithium metal and a protective layer disposed on one surface of the lithium metal, in which the protective layer includes an inorganic material-based solid electrolyte powder dispersed in a polymer matrix.
Owner:HYUNDAI MOTOR CO LTD
Positive Electrode for Lithium Ion Battery and Lithium Ion Battery Using Same
ActiveUS20070292759A1Prevent degradationReduce conductivityActive material electrodesNon-aqueous electrolyte accumulator electrodesManganeseCobalt
A positive electrode for a lithium ion battery has a conductive collector, a positive electrode active material layer being contact with the collector, and a cover layer disposed on at least part of the surface of the positive electrode active material layer. The positive electrode active material layer has a compound containing at least one kind selected from a group of cobalt, nickel, and manganese as a component. The cover layer is made of a compound with lithium ion conductivity that is expressed by general formula LixPTyOz or LiaMObNc and has high moisture resistance.
Owner:PANASONIC CORP
Lithium ion battery diaphragm, preparation method of the lithium ion battery diaphragm, and lithium ion battery containing the lithium ion battery diaphragm
InactiveCN103137929AHigh lithium ion conductivityReduced risk of short circuitsSecondary cellsCell component detailsPhysicsLithium electrode
The invention provides a lithium ion battery diaphragm. The lithium ion battery diaphragm comprises a diaphragm base material and a solid electrolyte layer arranged on at least one surface of the diaphragm base material. The invention also provides a preparation method of the lithium ion battery diaphragm, a device adopted by the preparation method and a lithium ion battery containing the lithium ion battery diaphragm. A high rate of lithium ion transmission between positive and negative poles of the lithium ion battery containing the lithium ion battery diaphragm is kept, a risk of short circuit between the positive and negative poles is reduced, and a diaphragm heat shrinkage problem and battery safety are improved.
Owner:BYD CO LTD
Ceramic solid electrolyte and preparation method thereof
InactiveCN105406118AEnable mass manufacturingUniform size distributionSecondary cellsElectrolytesPerovskite (structure)Spray dried
The invention discloses a ceramic solid electrolyte and a preparation method thereof. The ceramic solid electrolyte comprises at least one of NASICON structure type (LiM<2>(PO<4>)<3>, M=Zr, Ge, Mg, Al), oxide (Li<3x>La<2 / 3-x>TiO<3>) of perovskite structure and oxide (Li<5>La<3>M<2>O<12>) of garnet structure. The preparation method of the ceramic solid electrolyte comprises the following steps of: a) weighing raw materials according to molar ratio of elements in chemical formula of the ceramic solid electrolyte, dissolving the raw materials in solvent, and obtaining mixed solution; b) preparing ceramic solid electrolyte precursor powder from the mixed solution through a spray drying process; c) sintering the precursor powder obtained by spray drying in the air, and finally obtaining the ceramic solid electrolyte with relatively high ionic conductivity and relatively low electronic conductivity. In the method provided by the invention, the spray drying process is used for preparing the ceramic solid electrolyte, spray drying has the advantages that the drying procedure is fast, the mixed solution is directly dried into powder and particle size distribution of the powder is uniform, and thus, large-scale preparation of the ceramic solid electrolyte is expected to be realized, and the method has practical value.
Owner:HARBIN INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com