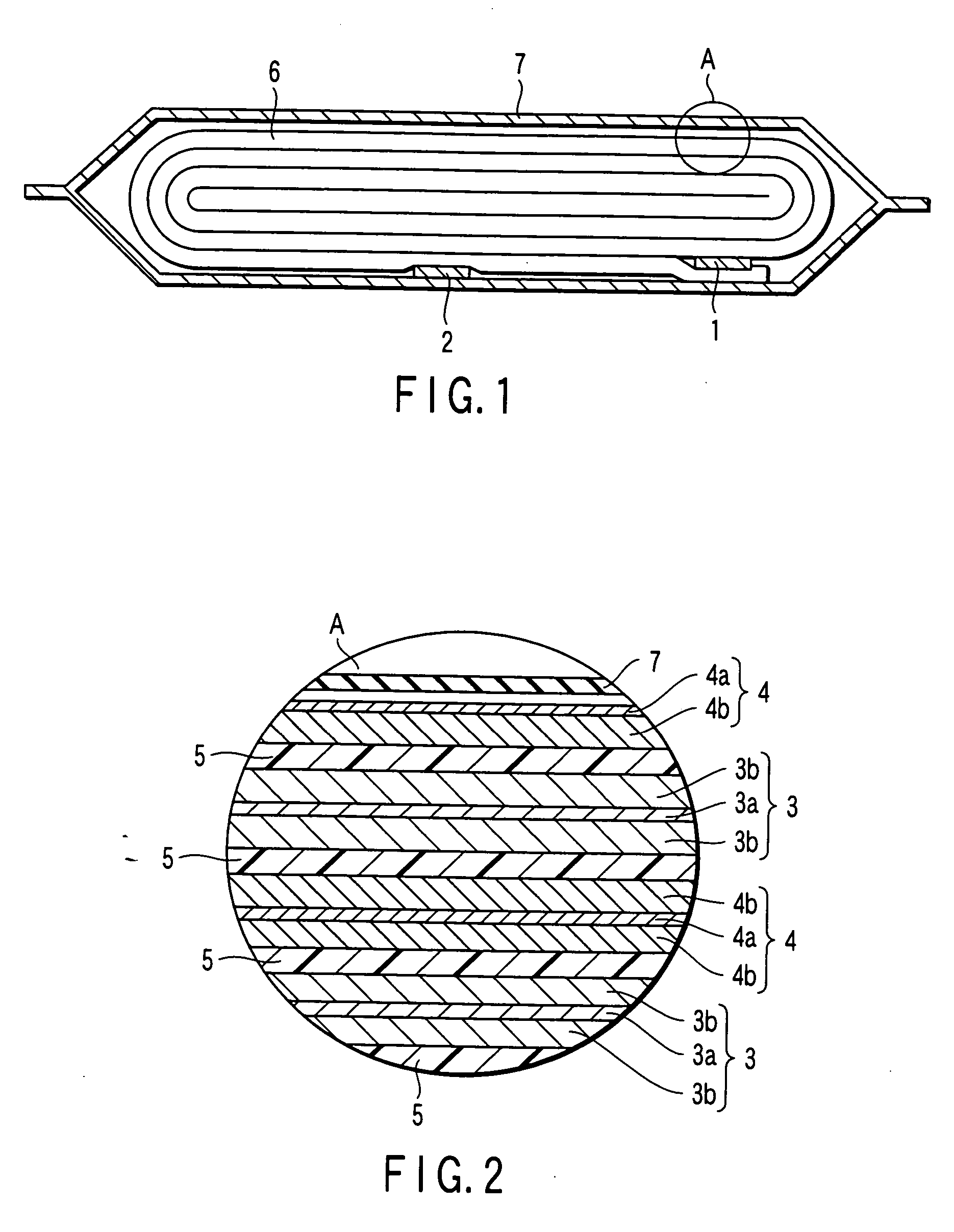
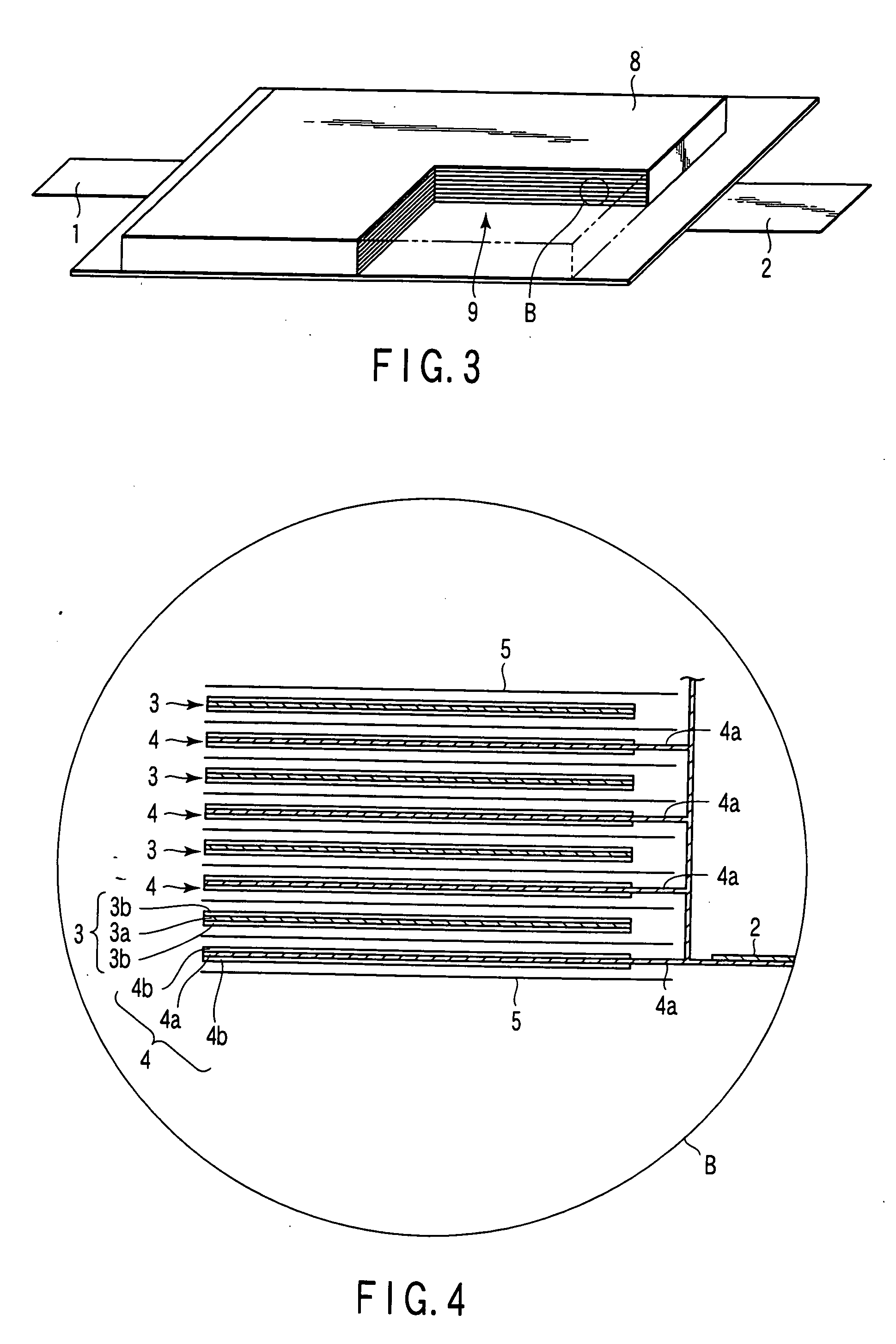
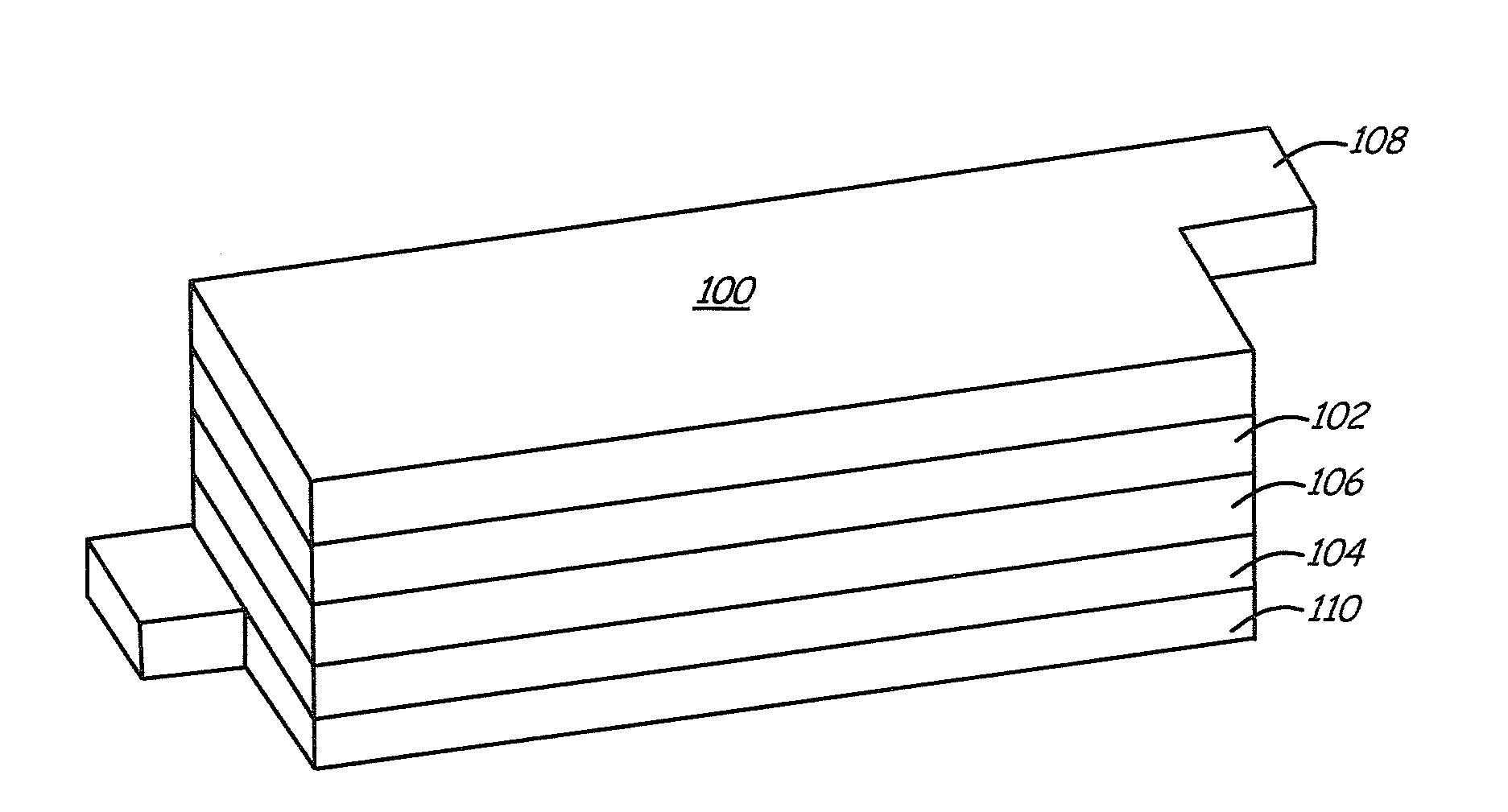
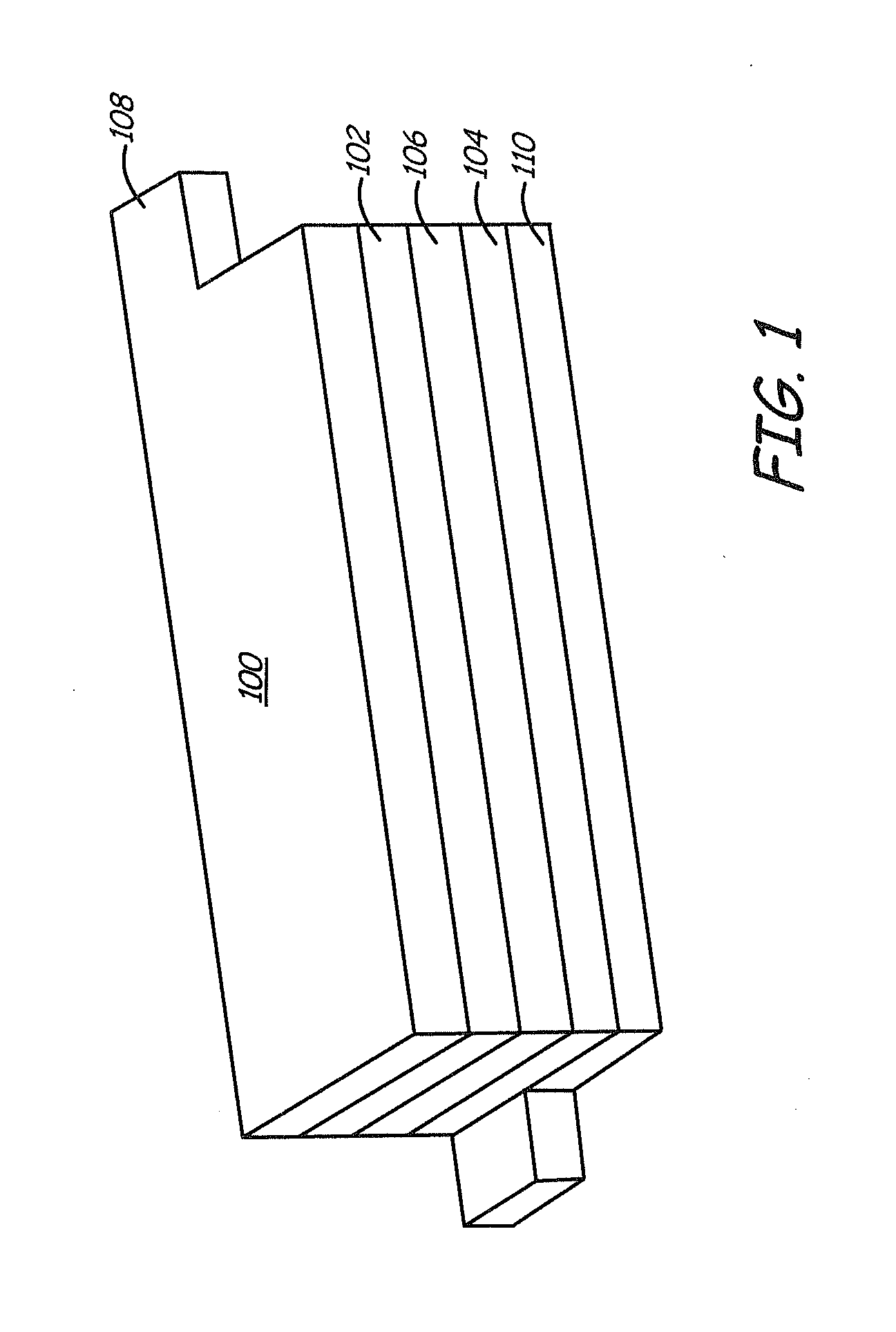
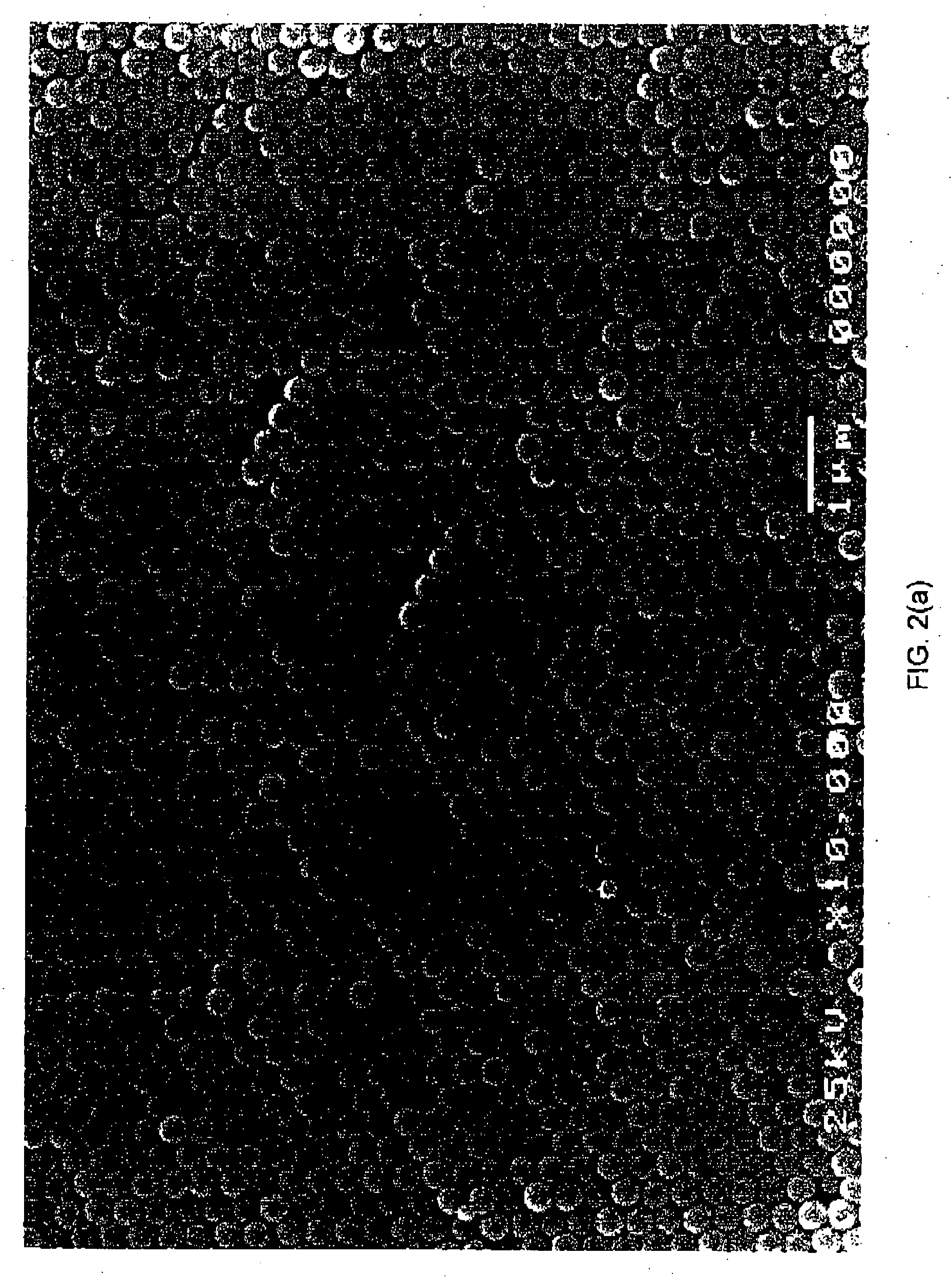
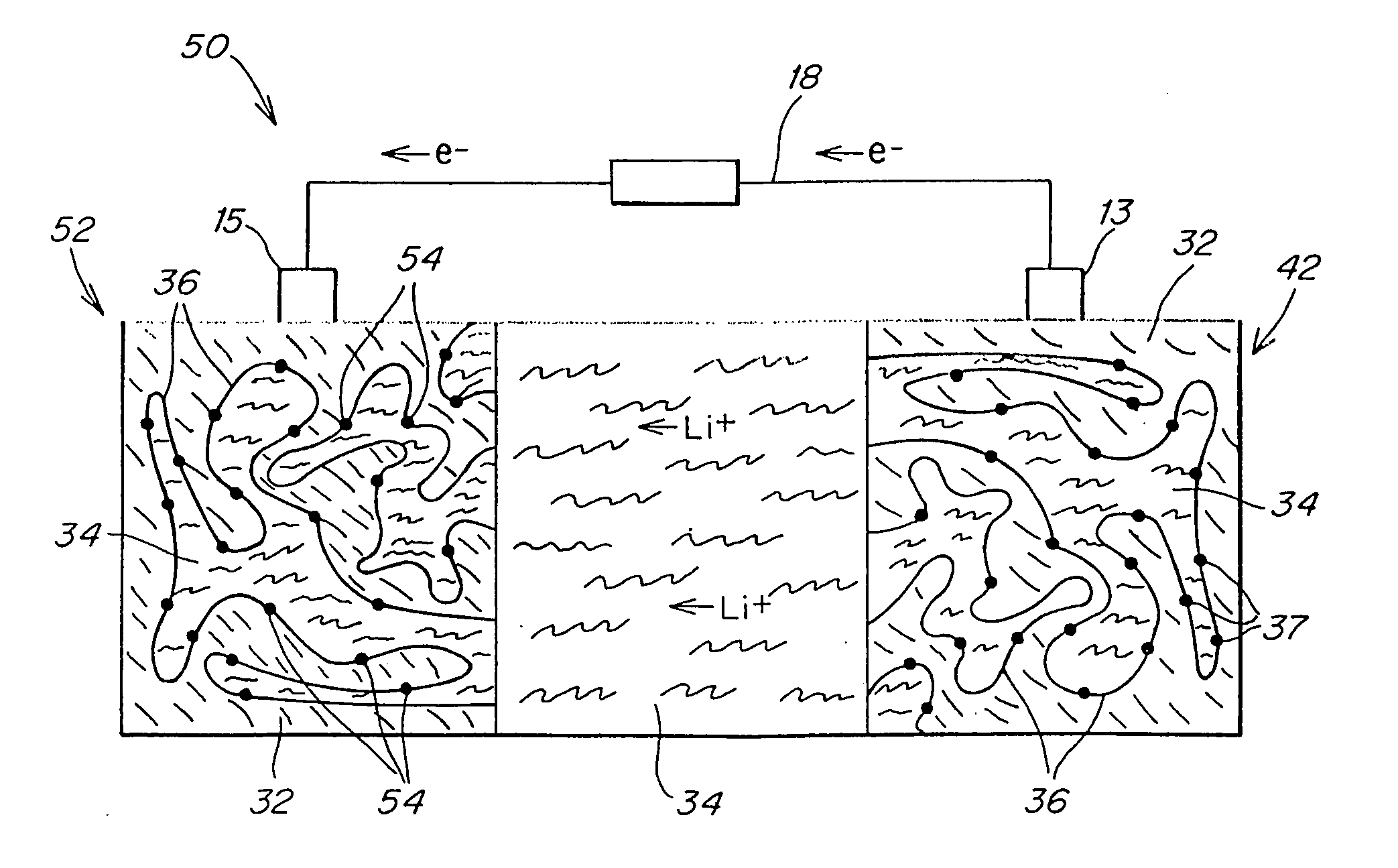
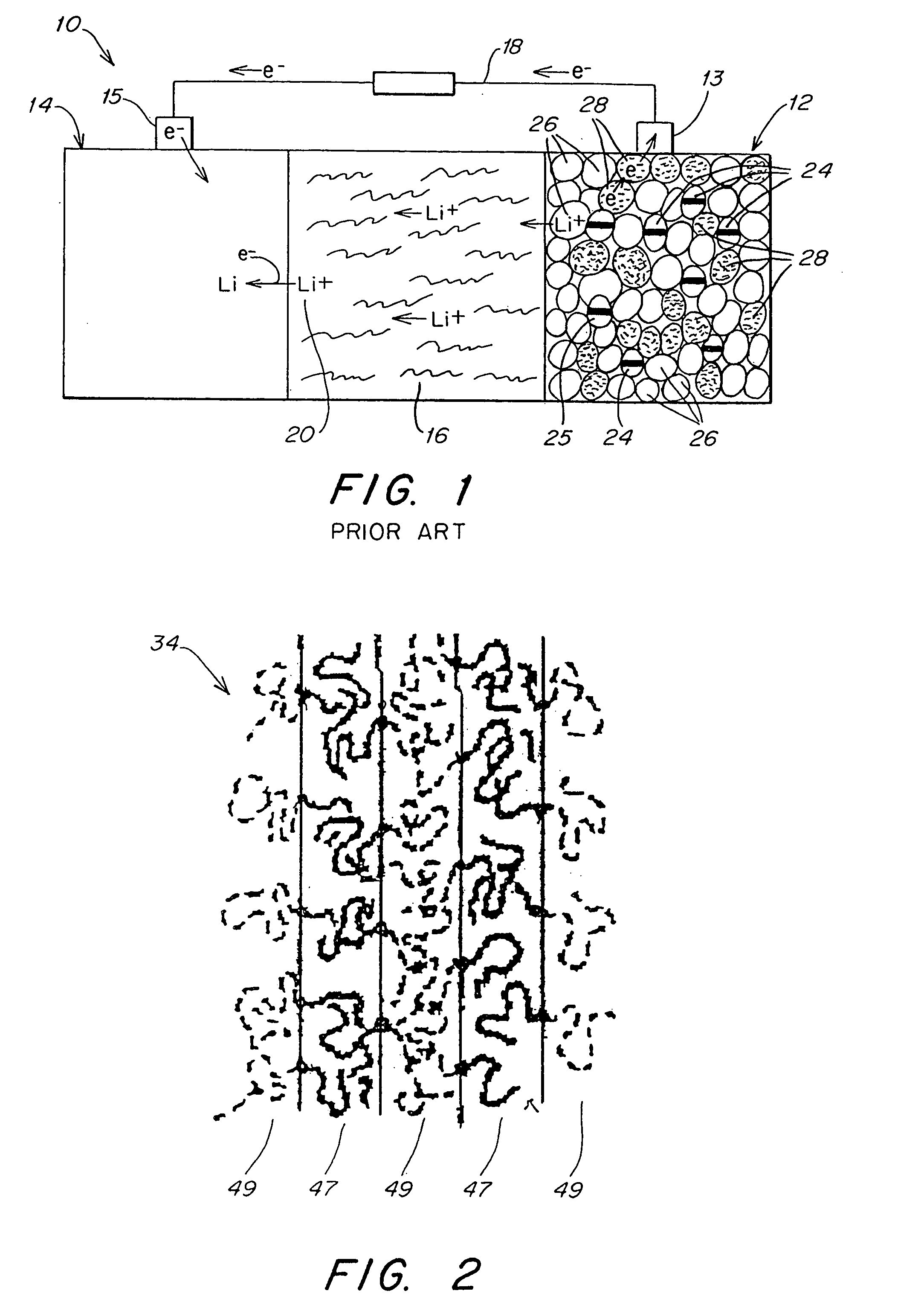
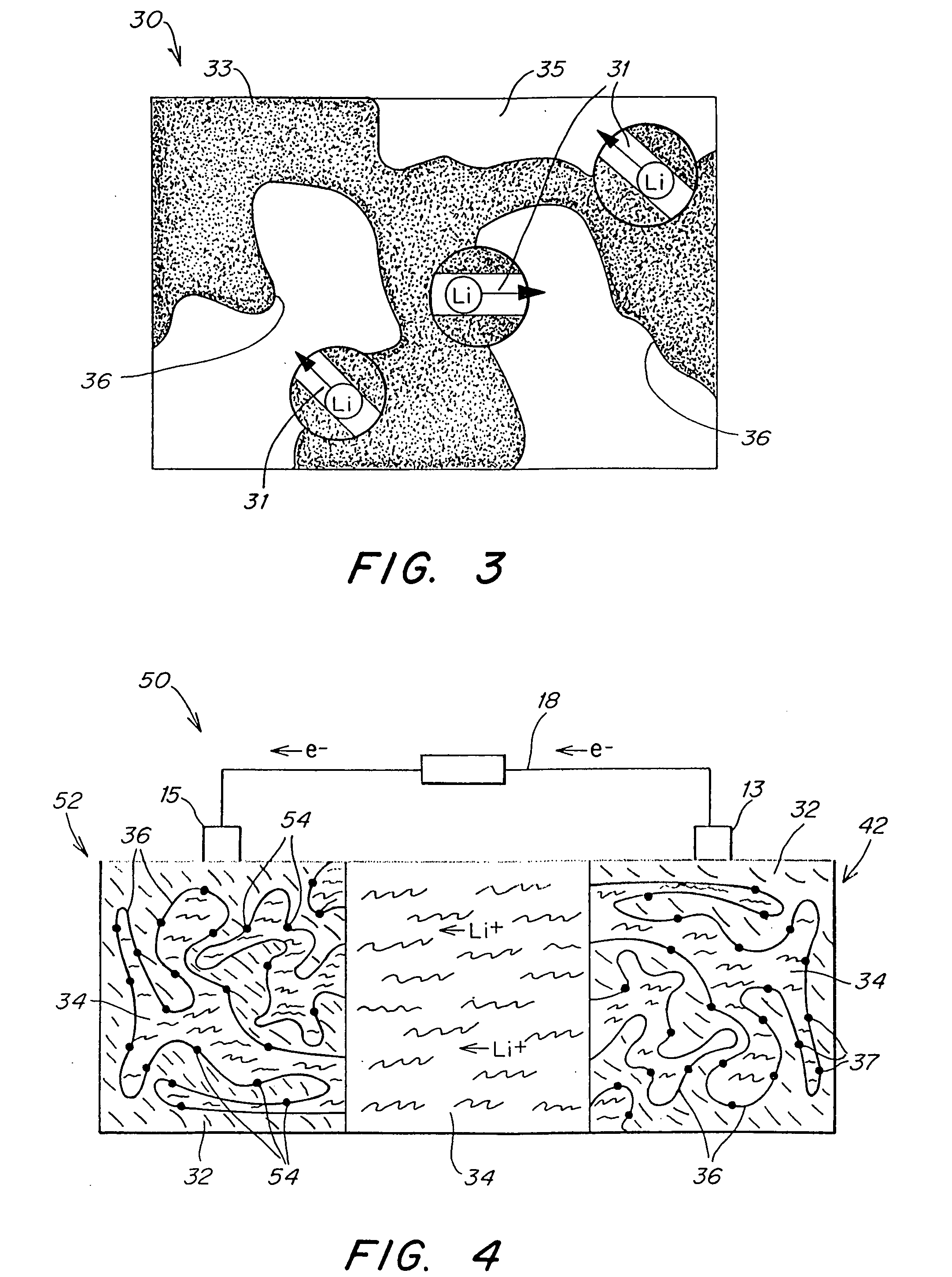
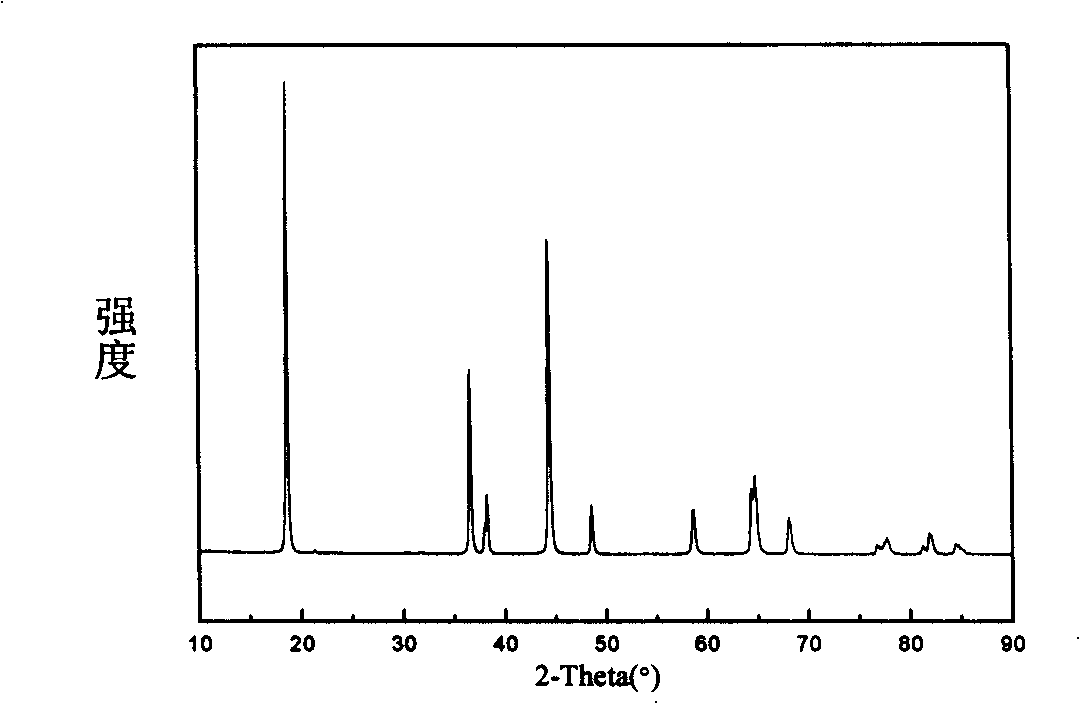
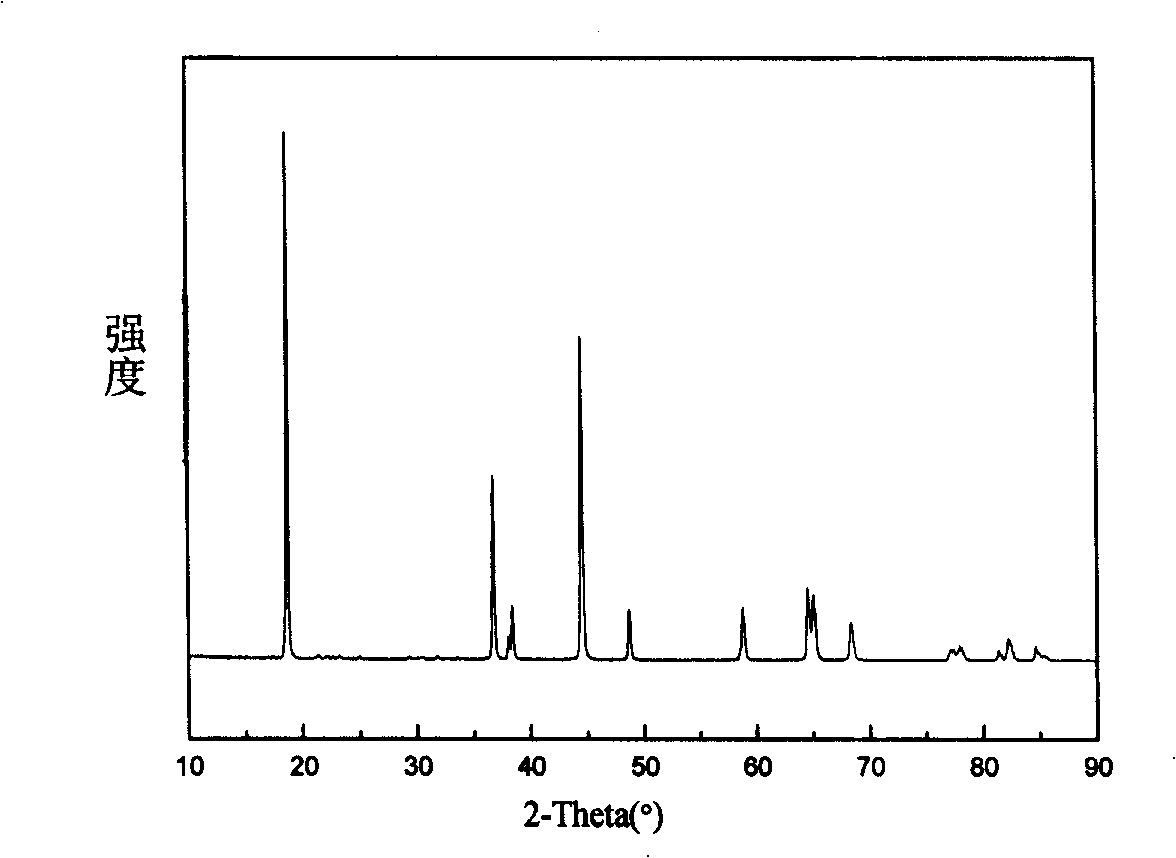
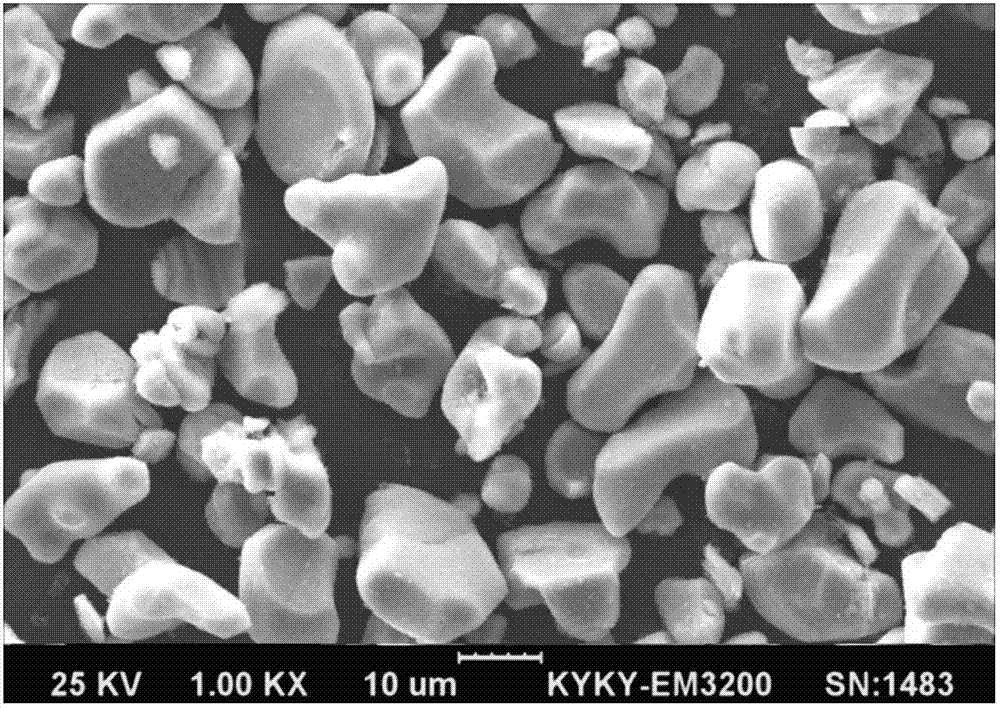
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
570 results about "Lithium intercalation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
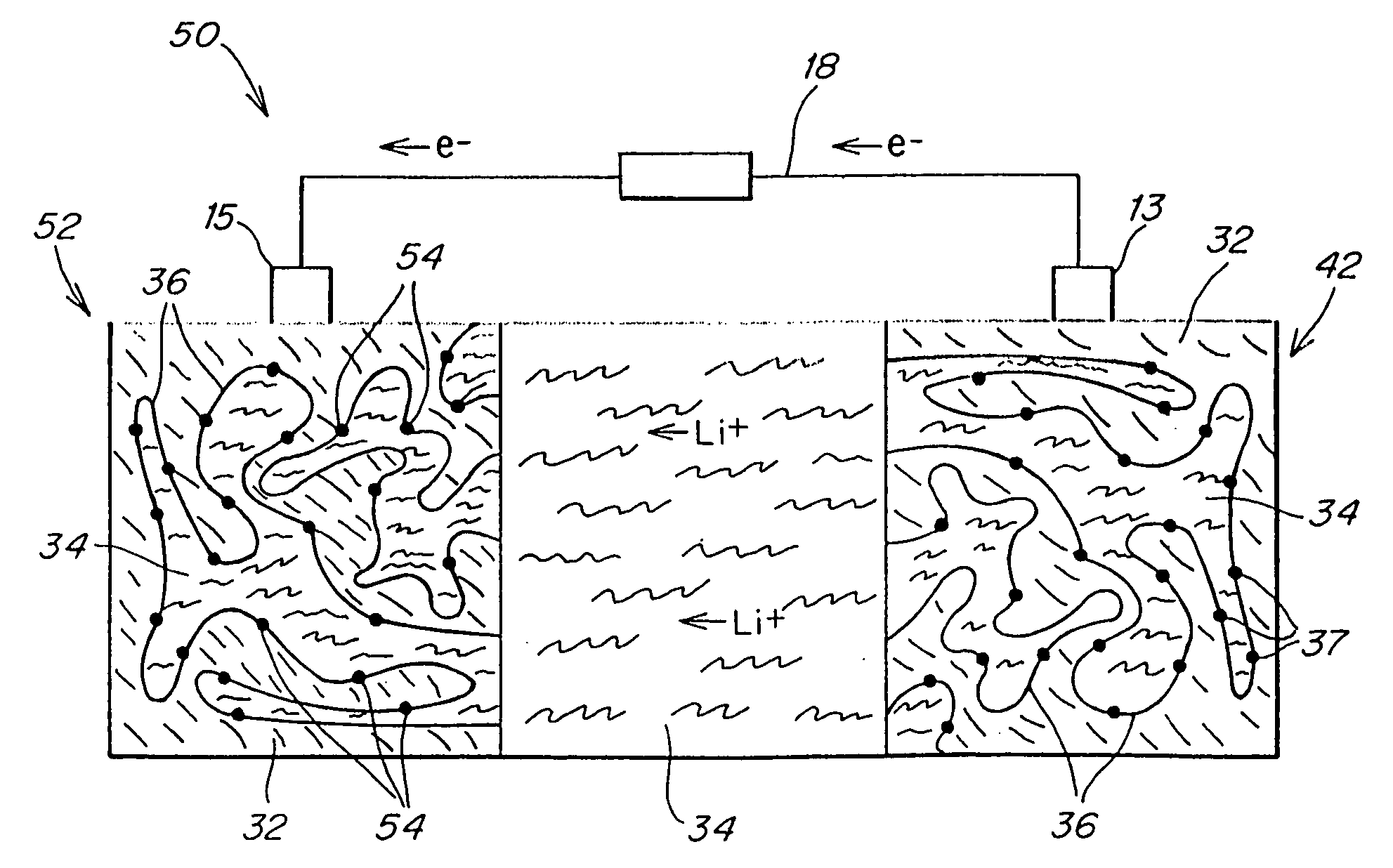
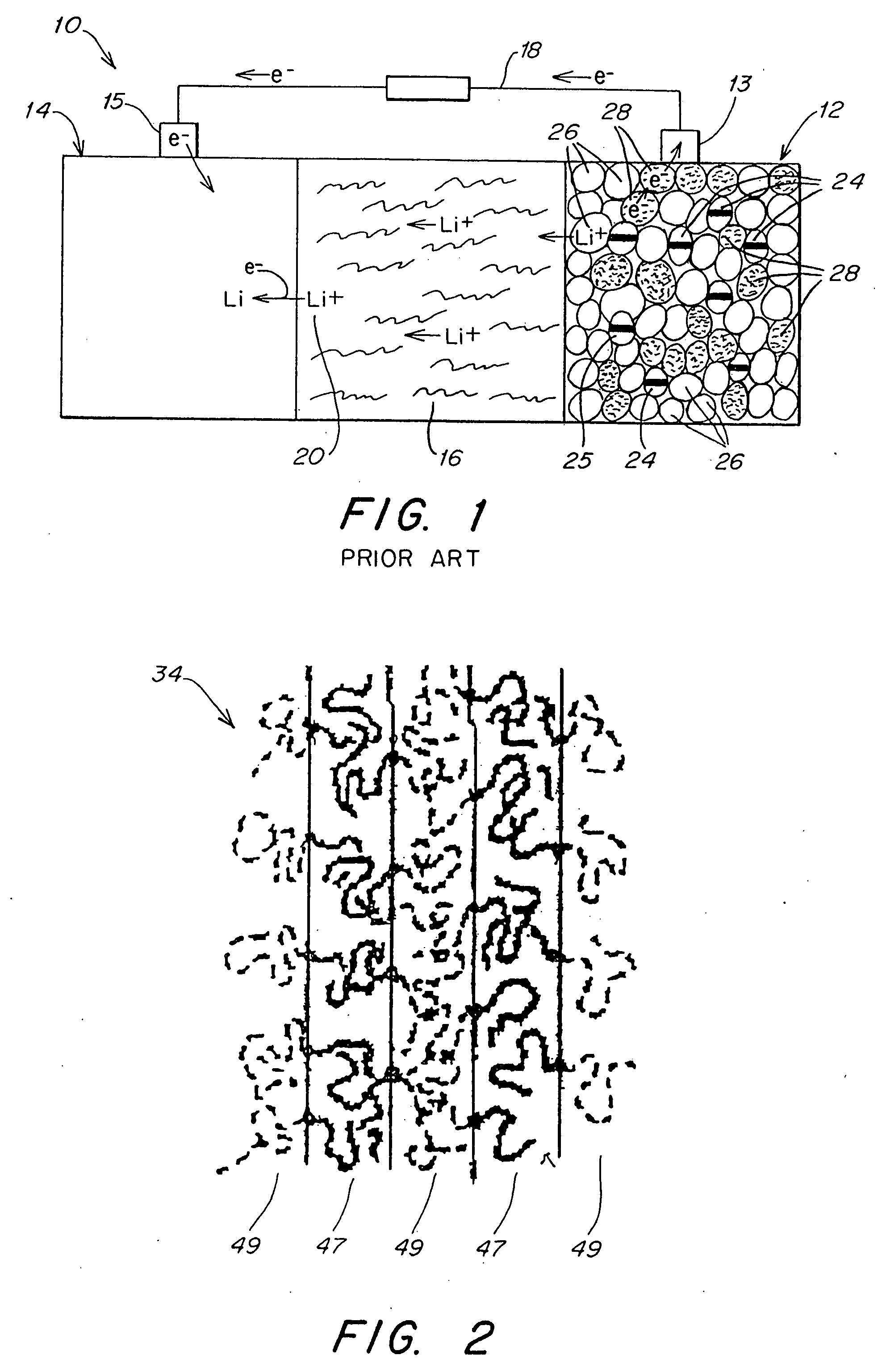
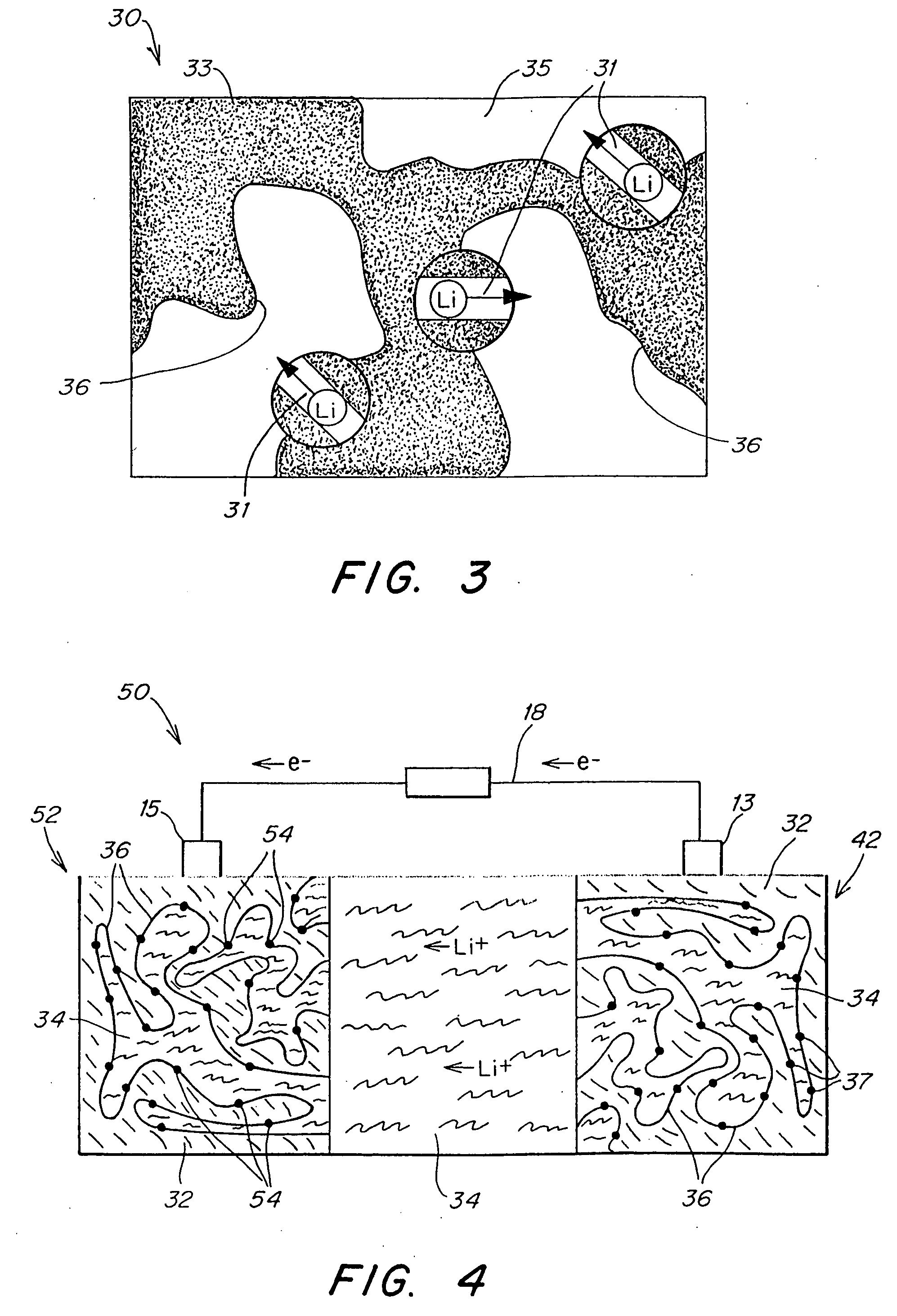
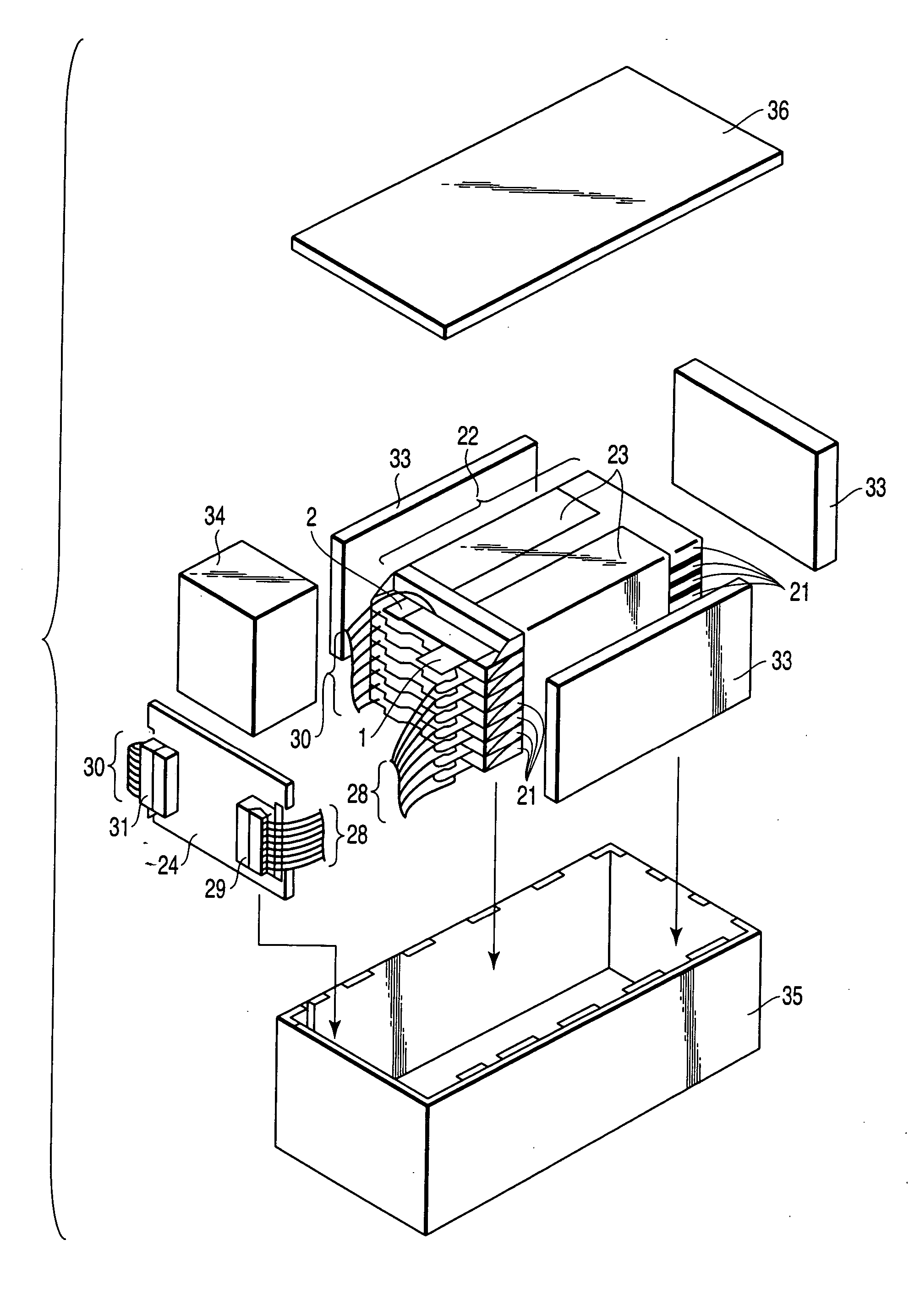
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4-x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Nonaqueous electrolyte battery, battery pack and positive electrode active material
InactiveUS20060134520A1Improve storage characteristicsOrganic electrolyte cellsPositive electrodesLithium oxideLithium hydroxide
A nonaqueous electrolyte battery includes a case, a positive electrode housed in the case and including a positive electrode active material containing a lithium-nickel composite oxide and at least one of lithium hydroxide and lithium oxide, the sum of lithium hydroxide and lithium oxide falling within not less than 0.1% to not more than 0.5% by weight based on the total amount of the positive electrode active material, a negative electrode housed in the case and capable of lithium intercalation-deintercalation, and a separator sandwiched between the positive electrode and the negative electrode and impregnated with a nonaqueous electrolyte containing γ-butyrolactone.
Owner:KK TOSHIBA
Lithium ion batteries with long cycling performance
ActiveUS20110017528A1Maintain discharge capacitySmall-sized cells cases/jacketsElectric propulsion mountingHigh energyEngineering
Batteries with high energy and high capacity are described that have a long cycle life upon cycling at a moderate discharge rate. Specifically, the batteries may have a room temperature fifth cycle discharge specific energy of at least about 175 Wh / kg discharged at a C / 3 discharge rate from 4.2V to 2.5V. Additionally, the batteries can maintain at least about 70% discharge capacity at 1000 cycles relative to the fifth cycle, with the battery being discharged from 4.2V to 2.5V at a C / 2 rate from the fifth cycle through the 1000th cycle. In some embodiment, the positive electrode of the battery comprises a lithium intercalation composition with optional metal fluoride coating. Stabilizing additive maybe added to the electrolyte of the battery to further improve the battery performance. The batteries are particularly suitable for use in electric vehicles.
Owner:IONBLOX INC
Method of producing prelithiated anodes for secondary lithium ion batteries
ActiveUS20100120179A1High specific capacityLong charge-discharge cycle lifeElectrode manufacturing processesActive material electrodesAverage sizeMaterials science
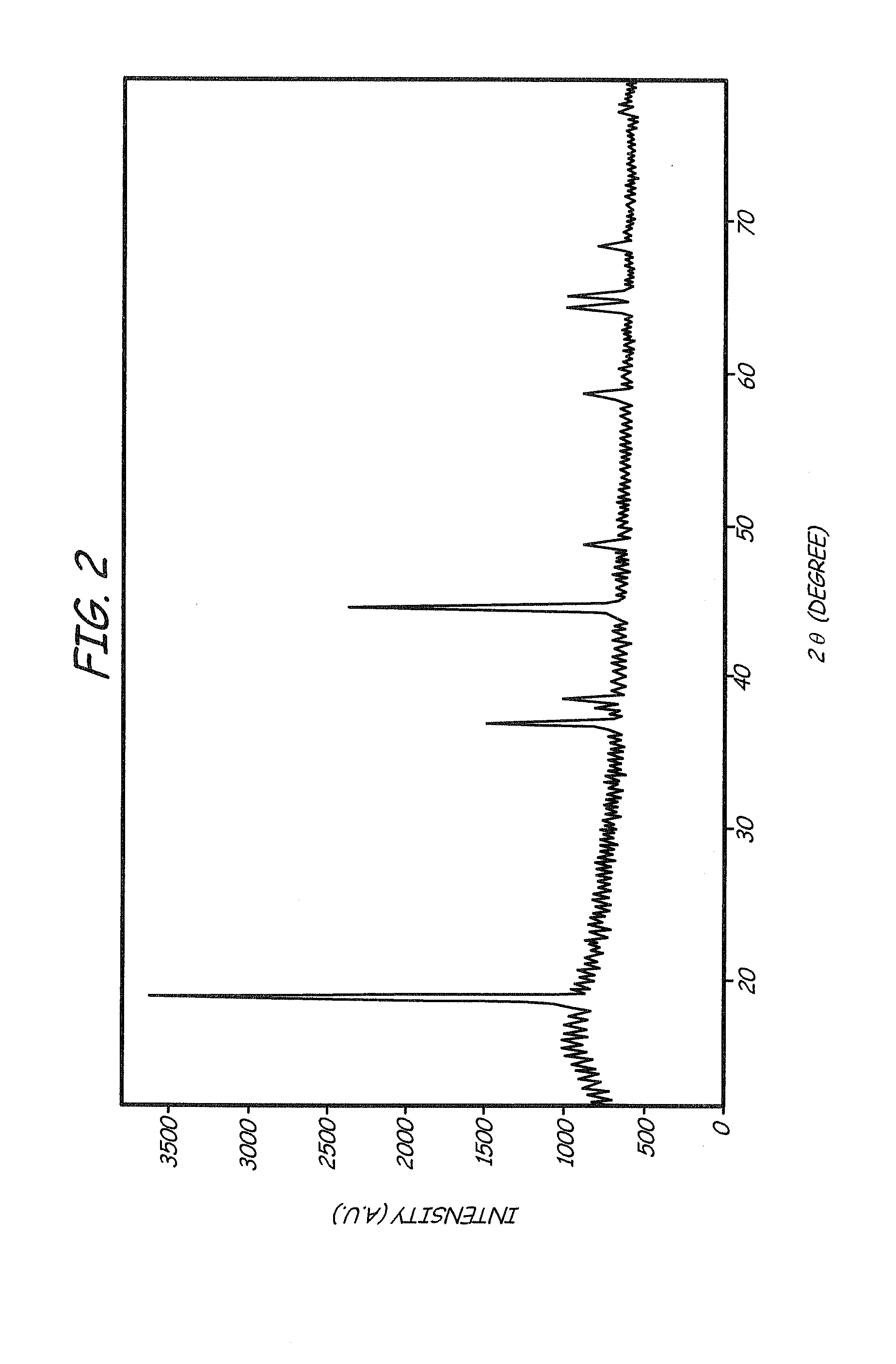
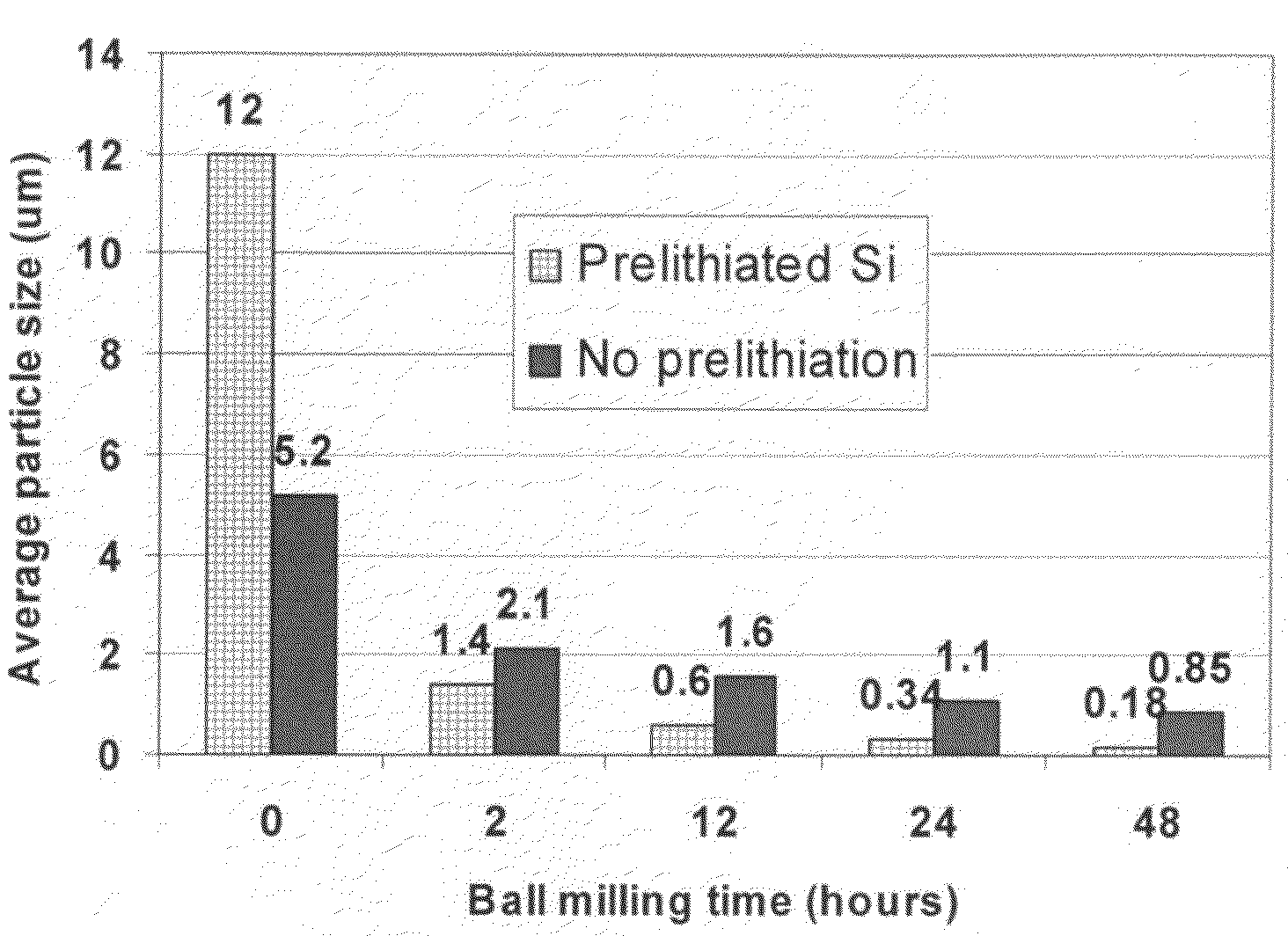
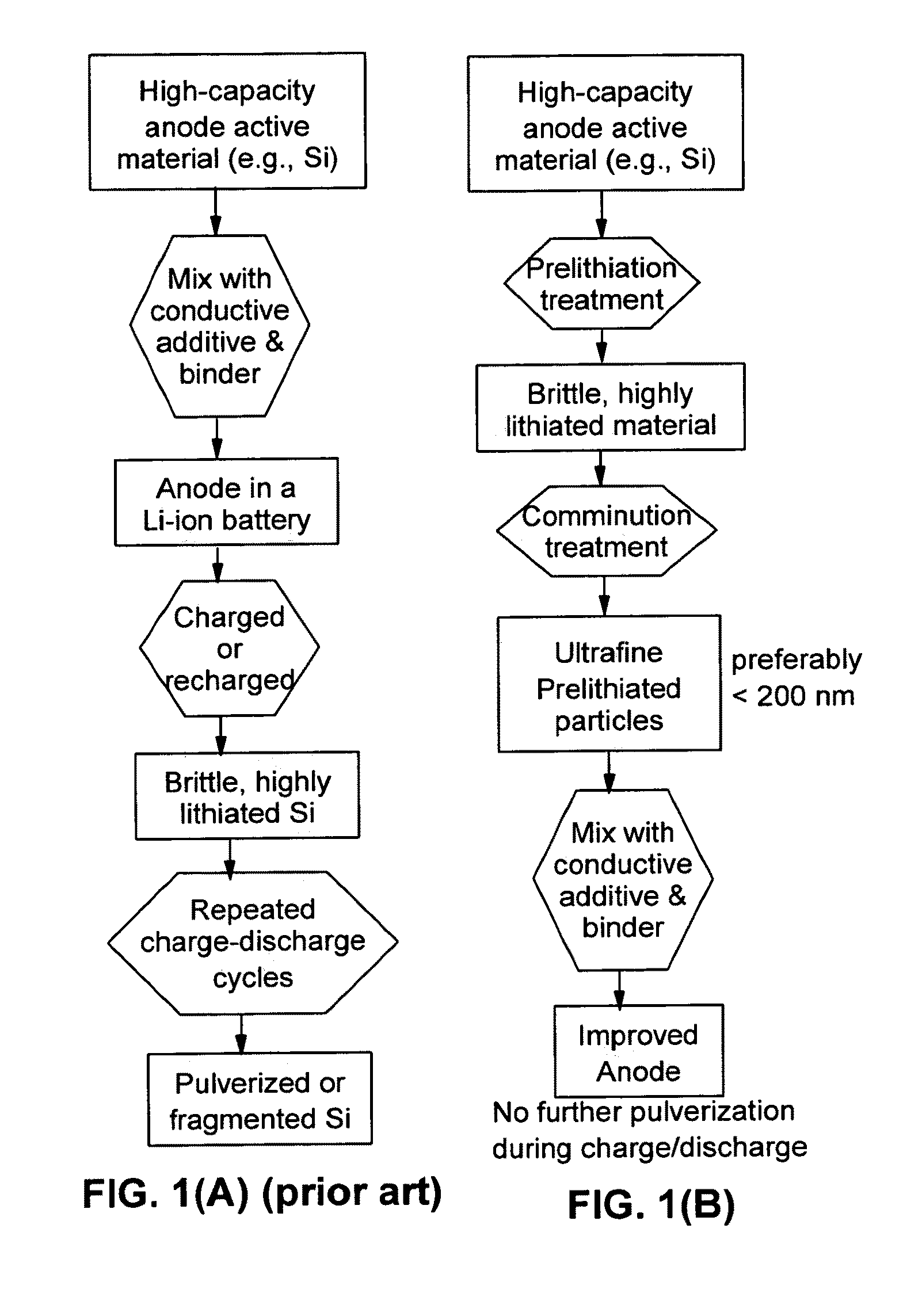
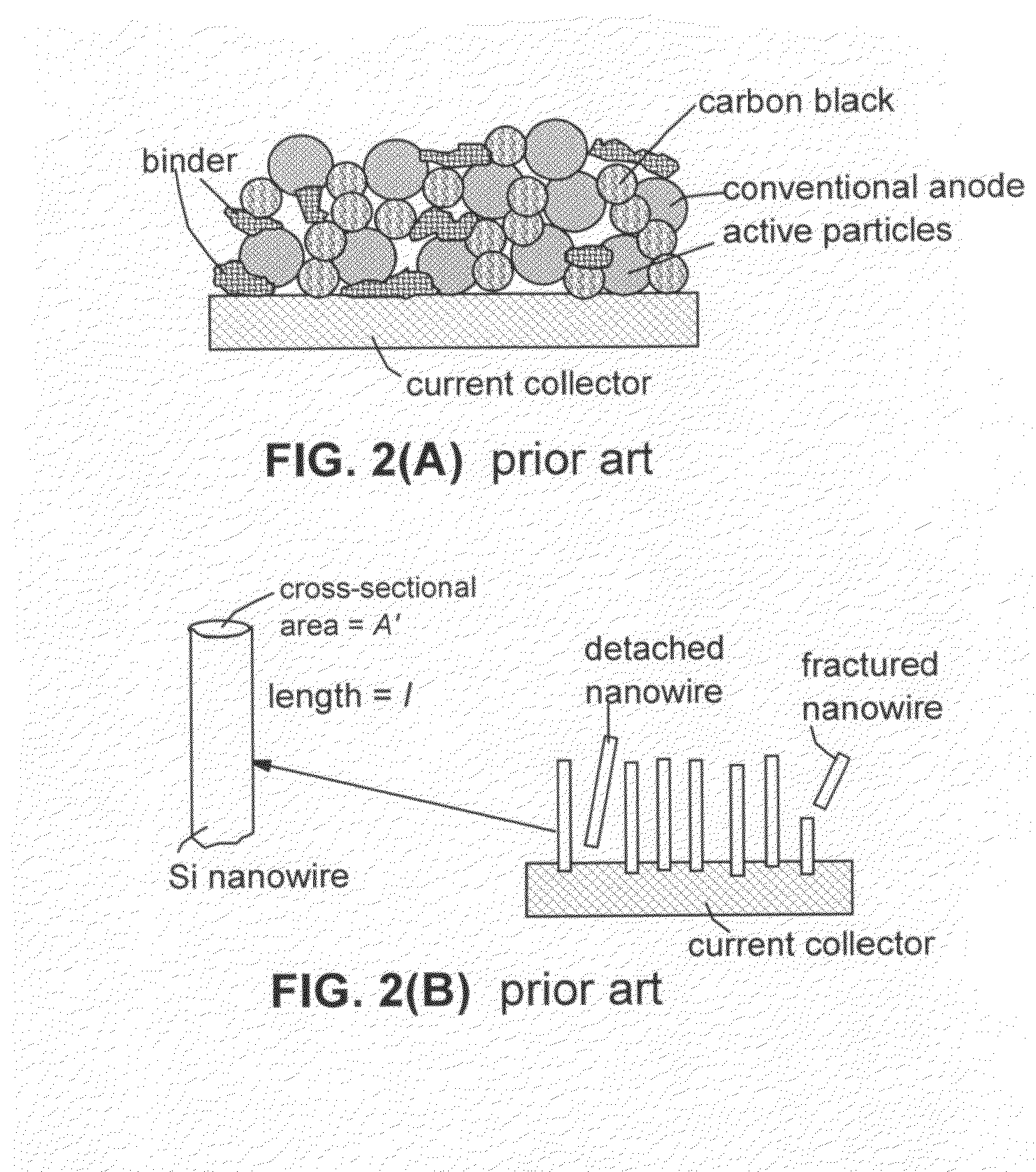
A method of producing a lithium-ion battery anode comprising: (a) providing an anode active material; (b) intercalating or absorbing a desired amount of lithium into this anode active material to produce a prelithiated anode active material; (c) comminuting the prelithiated anode active material into fine particles with an average size less than 10 μm (preferably sub-micron and more preferably <200 nm); and (d) combining multiple fine particles of prelithiated anode active material with a conductive additive and / or a binder material to form the anode. The battery featuring such an anode exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Hard carbon material for power and energy-storage battery and preparation method thereof
ActiveCN101916845AExcellent intercalation and delithiation abilityImprove cycle stabilityCell electrodesHigh rateCharge discharge
The invention discloses a hard carbon material for a power and energy-storage battery and a preparation method thereof, and aims to solve the technical problem of improving high-rate charge-discharge performance of lithium-ion batteries. The material is provided with a hard carbon matrix, and a coating is coated outside the hard carbon matrix; and the surface of the hard carbon matrix has a honeycomb opening structure. The preparation method comprises the following steps of: dipping, washing, dewatering and drying, presintering at low temperature, crushing, pyrolyzing, crushing and coating. Compared with the prior art, the hard carbon material has the reversible specific capacity of more than 450mAh / g, the first cycle columbic efficiency of over 81 percent, 0.2C 300-cycles capacity-retaining rate of over 97 percent at the temperature of 60 DEG C, and the 0.2C 300-cycles capacity-retaining rate of over 88 percent at the temperature of -30 DEG C, has the advantages of excellent lithium intercalation and deintercalation capability and cycling stability, and simple preparation process, and is applicable to the lithium ion battery cathode materials for lithium ion power batteries, various portable devices and electric tools.
Owner:深圳贝特瑞钠电新材料科技有限公司
Anode material for lithium secondary cell with high capacity
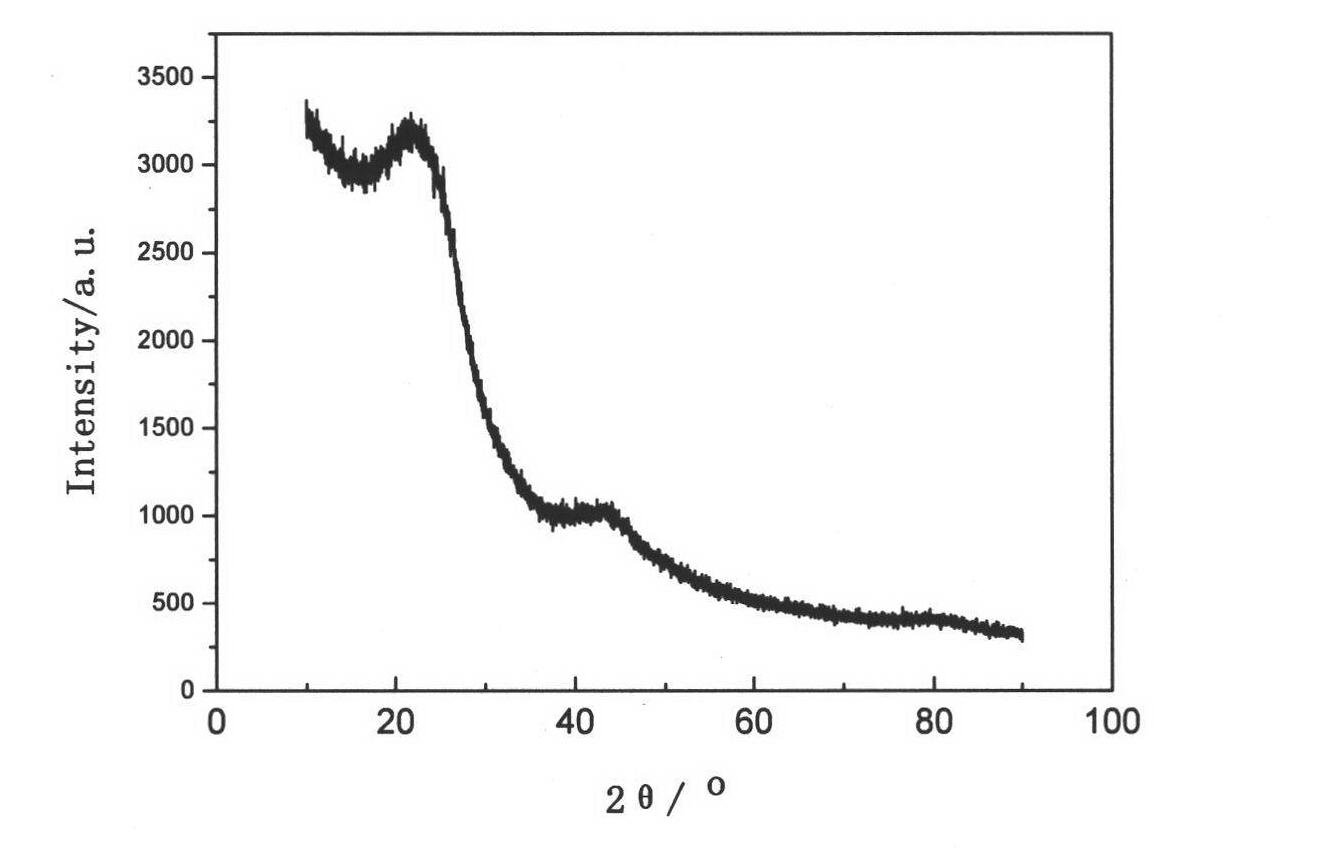
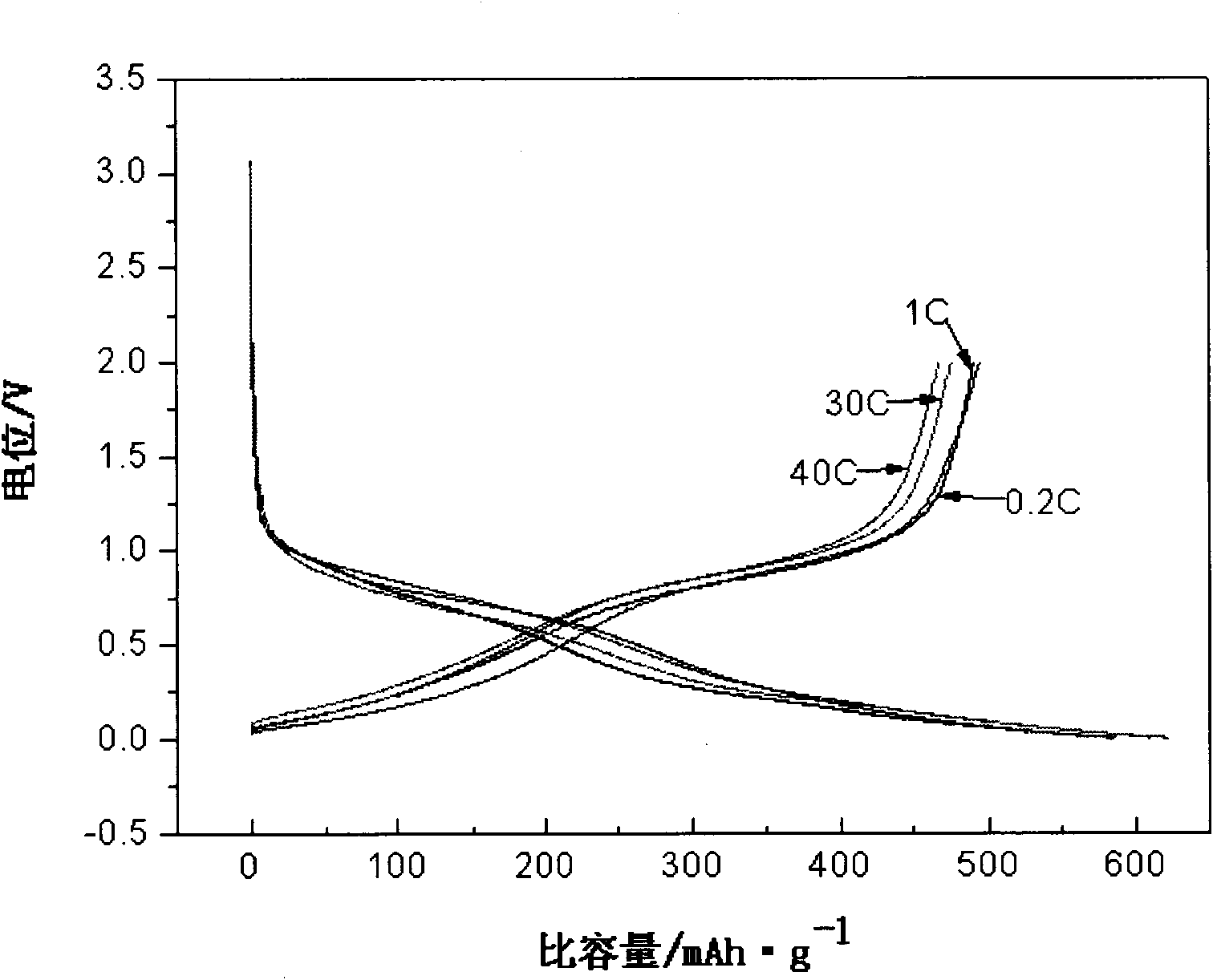
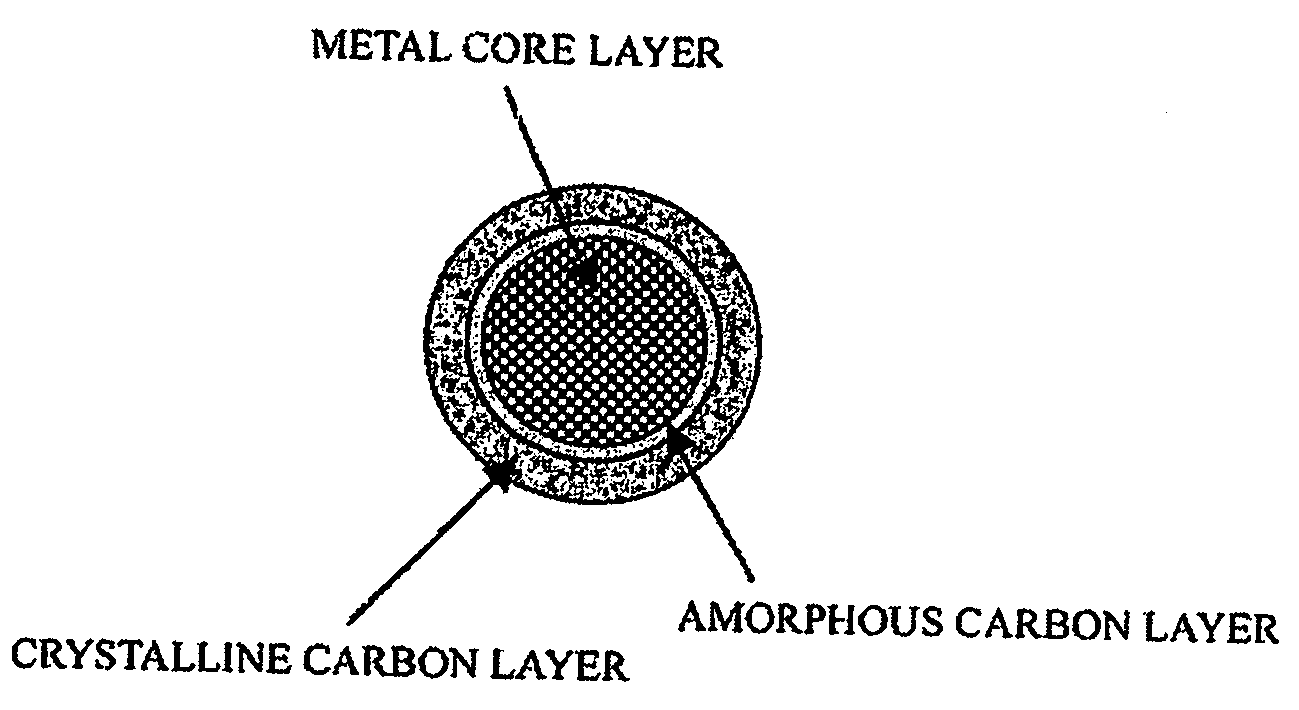
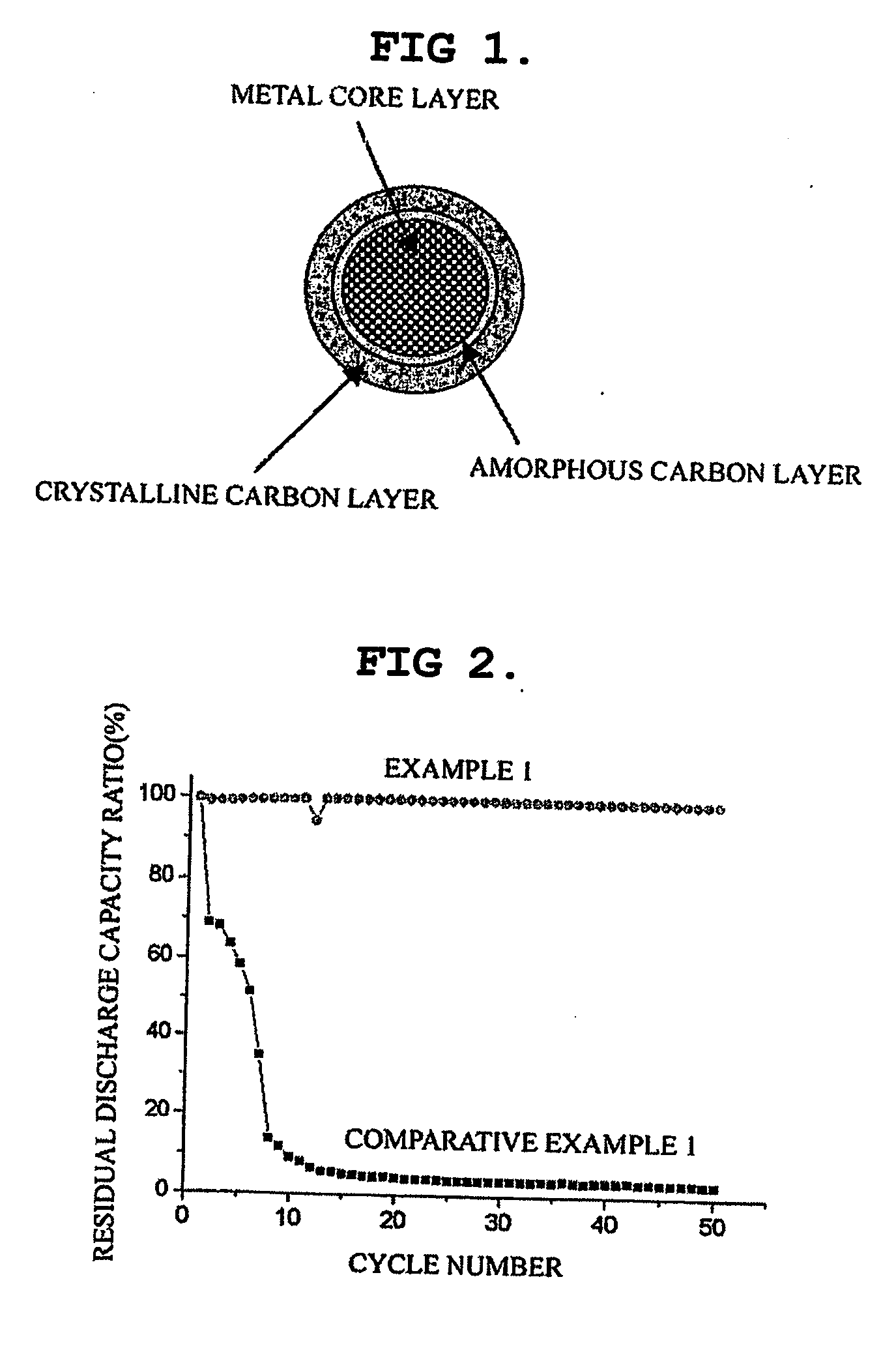
ActiveUS20060234127A1High charge and discharge capacityImproved cycle life characteristicsNegative electrodesLi-accumulatorsCarbon layerLithium intercalation
Disclosed is an anode material comprising a metal core layer capable of repetitive lithium intercalation / deintercalation; an amorphous carbon layer coated on the surface of the metal core layer, and a crystalline carbon layer coated on the amorphous carbon layer. The anode material not only maintains a high charge / discharge capacity, which is an advantage of a metal-based anode material, but also inhibits changes in the volume of a metal core layer caused by repetitive lithium intercalation / deintercalation in virtue of an amorphous carbon layer and a crystalline carbon layer, thereby improving the cycle life characteristics of cells.
Owner:LG ENERGY SOLUTION LTD
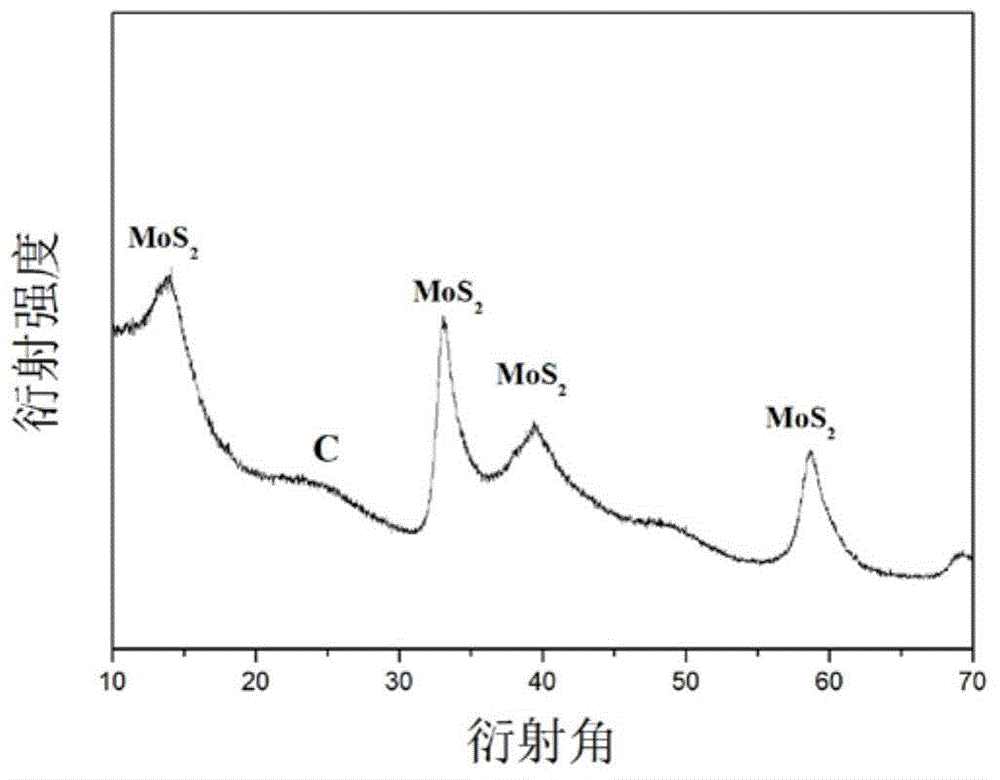
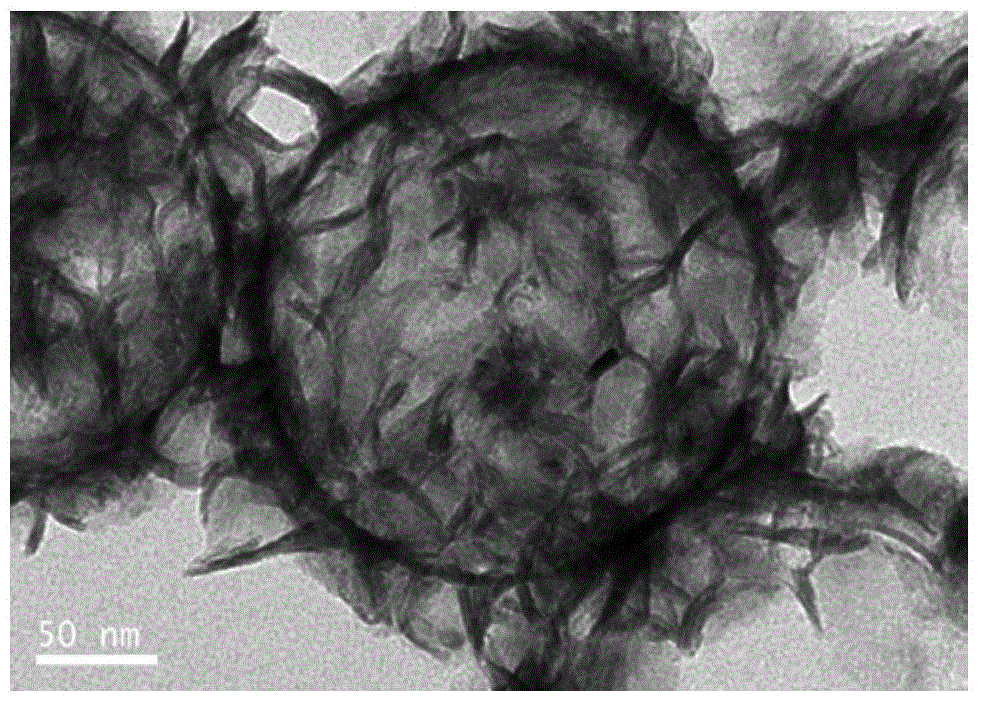
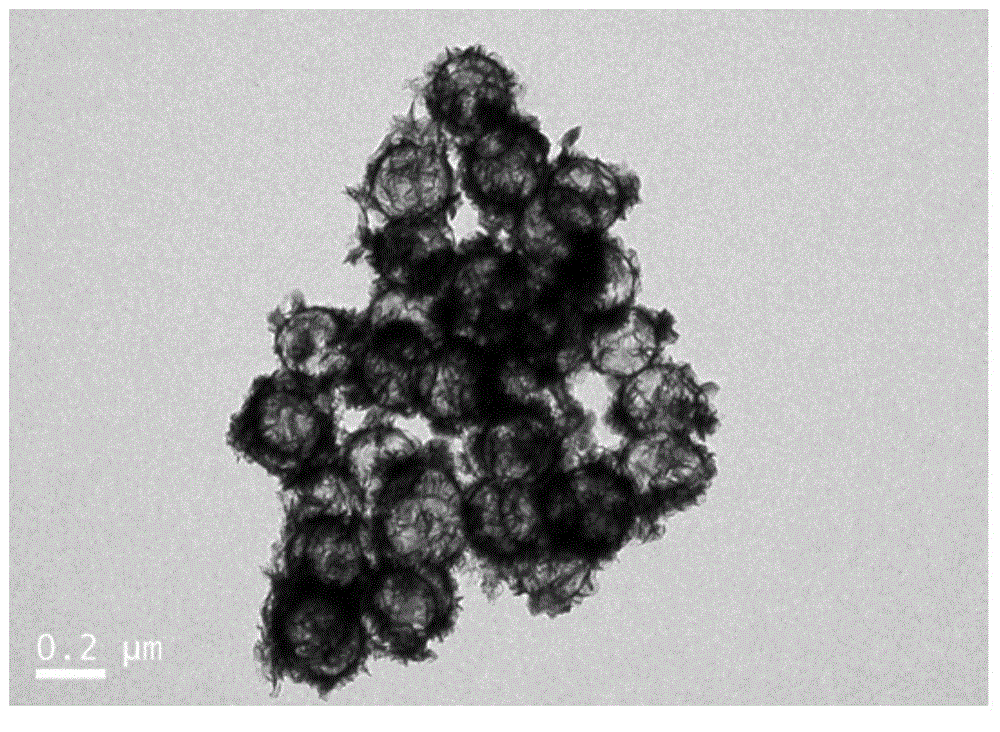
Molybdenum disulfide/carbon composite material and preparation method thereof
InactiveCN104934602AImprove poor conductivityNot easy to reuniteCell electrodesSecondary cellsCarbon compositesCarbonization
The invention discloses a molybdenum disulfide / carbon composite material. The molybdenum disulfide / carbon composite material comprises a molybdenum disulfide layer and a carbon hollow ball, wherein the molybdenum disulfide layer is positioned outside the carbon hollow ball; and the carbon hollow ball has a hollow structure. The invention also discloses a preparation method of the molybdenum disulfide / carbon composite material. The preparation method comprises the following steps of using amino modified silica spheres as a template; wrapping the template by pyrolyzation of an organic carbon source; performing thermal reaction on the template and ammonium tetrathiomolybdate through solvent; performing high-temperature carbonization in an inert atmosphere; and removing the silicon oxide template to obtain the molybdenum disulfide / carbon composite material. The lithium-intercalation capacity of the molybdenum disulfide / carbon composite material is 1467mAh / g for the first time, and the specific capacity of the molybdenum disulfide / carbon composite material can be kept at 733mAh / g after 30 times of repeated charging and discharging cycles.
Owner:SHANGHAI JIAO TONG UNIV
Lithium Battery Using an Aqueous Electrolyte
InactiveUS20090087742A1Controlling the riskInherent riskAlkaline accumulatorsSolid electrolyte cellsAqueous electrolyteLithium-ion battery
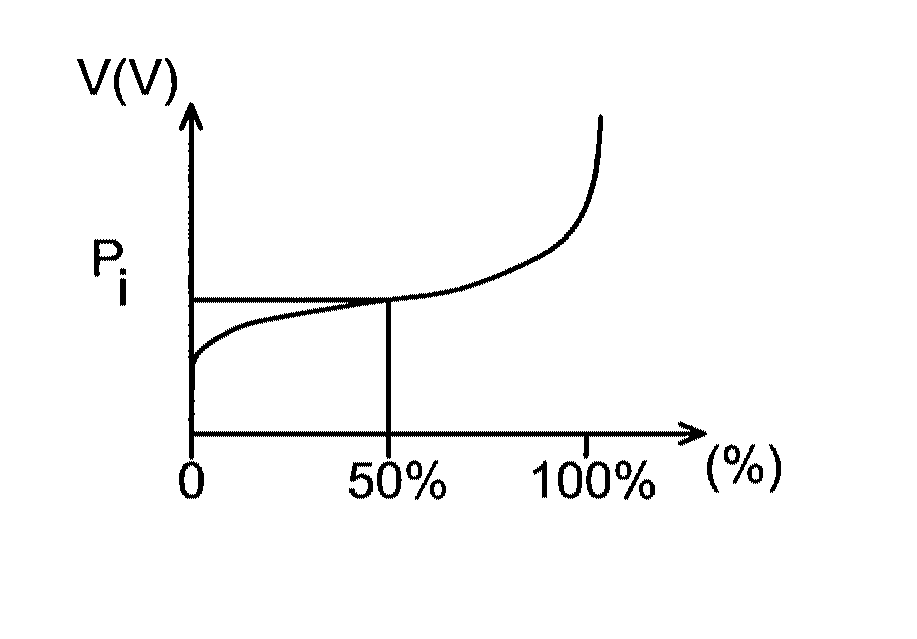
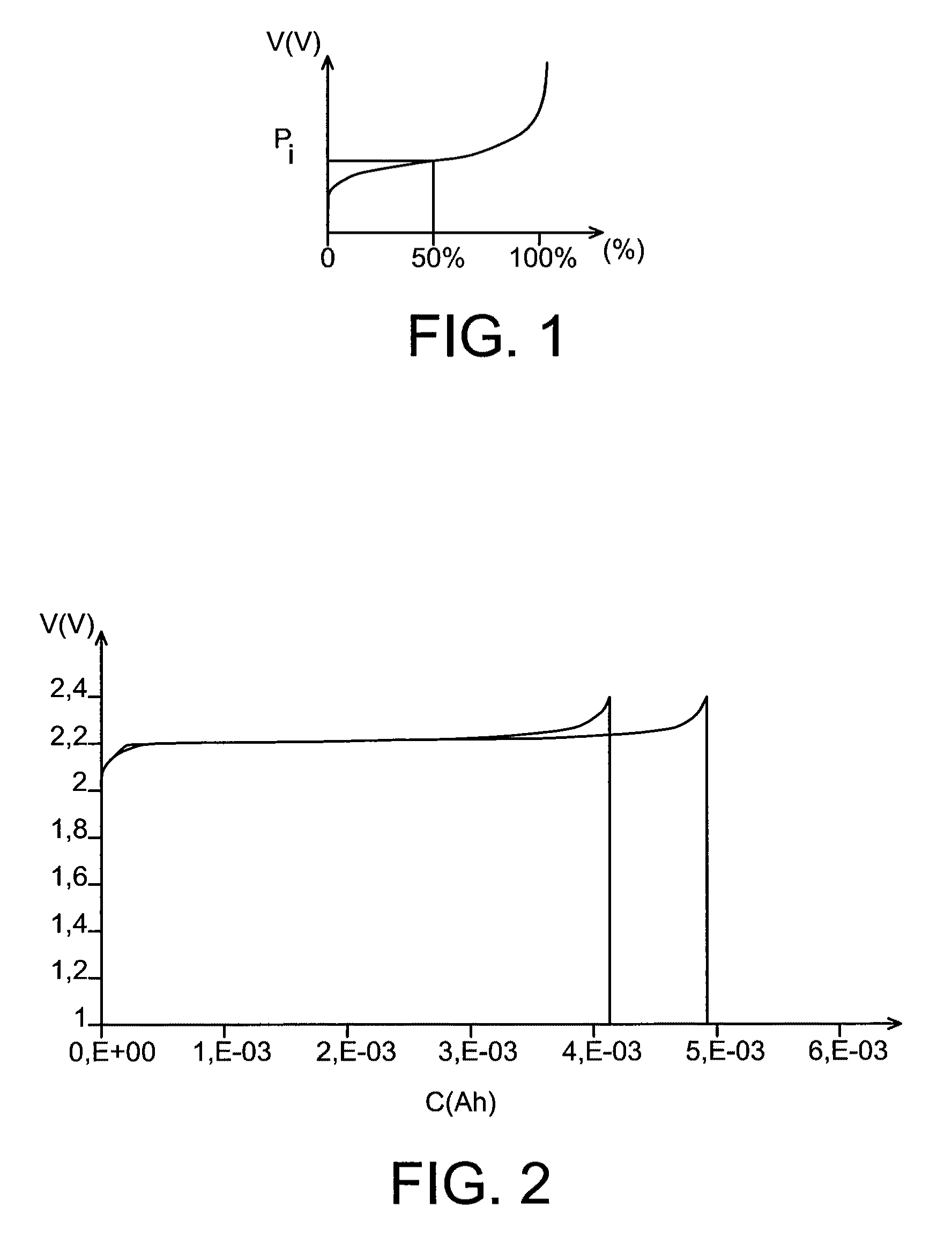
The invention relates to a lithium battery including a cell comprising:a positive electrode,a negative electrode, andan electrolyte consisting of an aqueous solution of a lithium salt,characterized in that the electrolyte has a pH of at least 14, the positive electrode has a lithium intercalation potential greater than 3.4 V, and the negative electrode has a lithium intercalation potential less than 2.2 V.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Pre-lithiation method of lithium ion battery negative electrode material
ActiveCN104538591AImprove the first Coulombic efficiencyIncrease capacityElectrode carriers/collectorsElectrolyte accumulators manufactureElectrical conductorReaction speed
The invention discloses a pre-lithiation method of a lithium ion battery negative electrode material. The method is characterized in that by adopting a method of coating or covering the surface of metal lithium with a lithium ion barrier layer and / or controlling a resistance value of a connection conductor, the pre-lithiated current magnitude is limited, and the reaction speed of a primary battery between metal lithium and negative electrode materials is regulated so as to regulate the lithium-intercalation speed of the negative electrode material and formation speed of surface SEI films, and the circulation performance of the negative electrode material is improved based on the improvement on coulombic efficiency for the first time of the negative electrode material; moreover, the method is simple in process and easy to operate, and suitable for pre-lithiation of the commercial lithium ion battery negative electrode material; lithium consumed by irreversible reaction is supplemented, so that the coulombic efficiency for the first time and the capacity of the battery are increased, and the circulation performance of the battery is improved.
Owner:TIANJIN B&M SCI & TECH
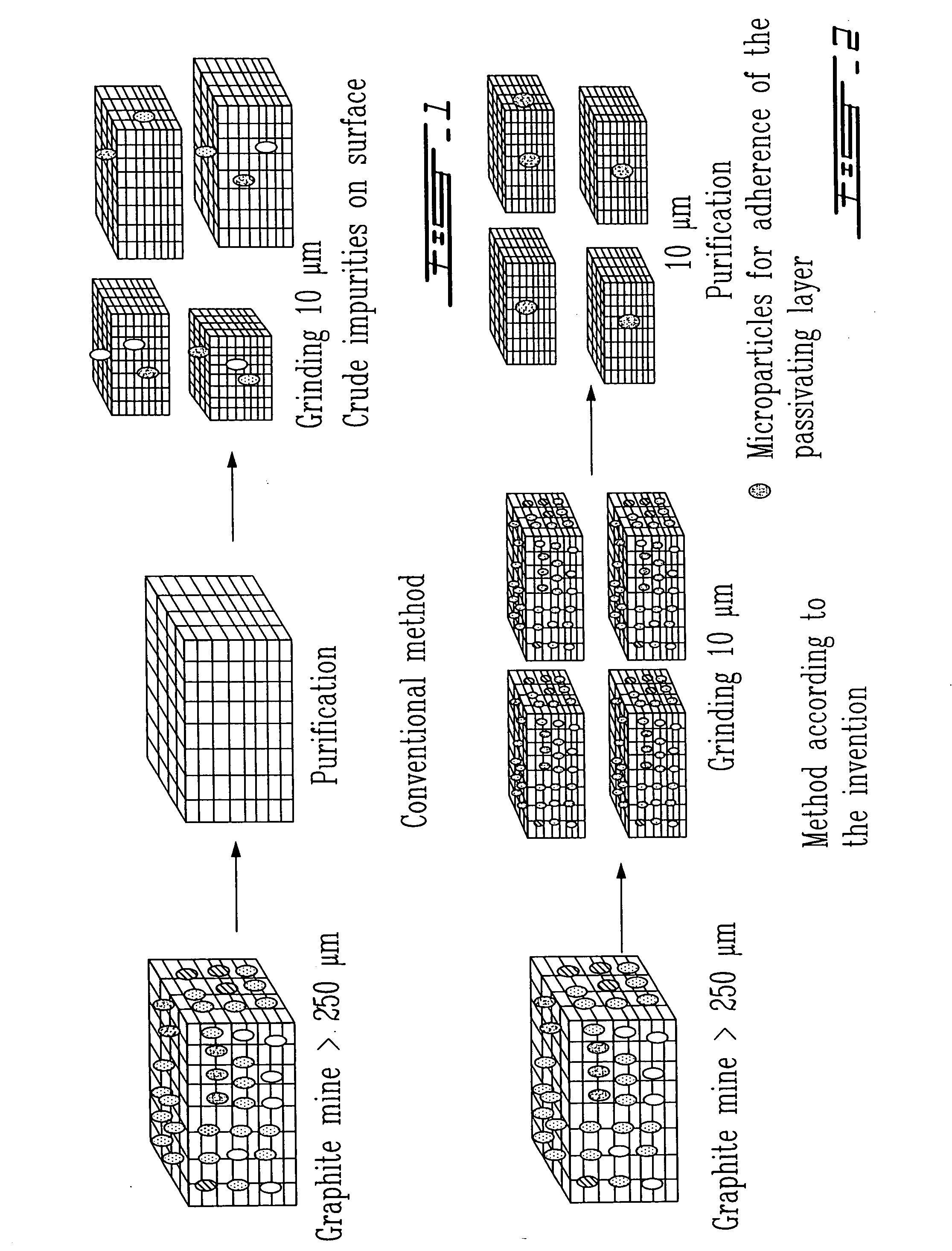
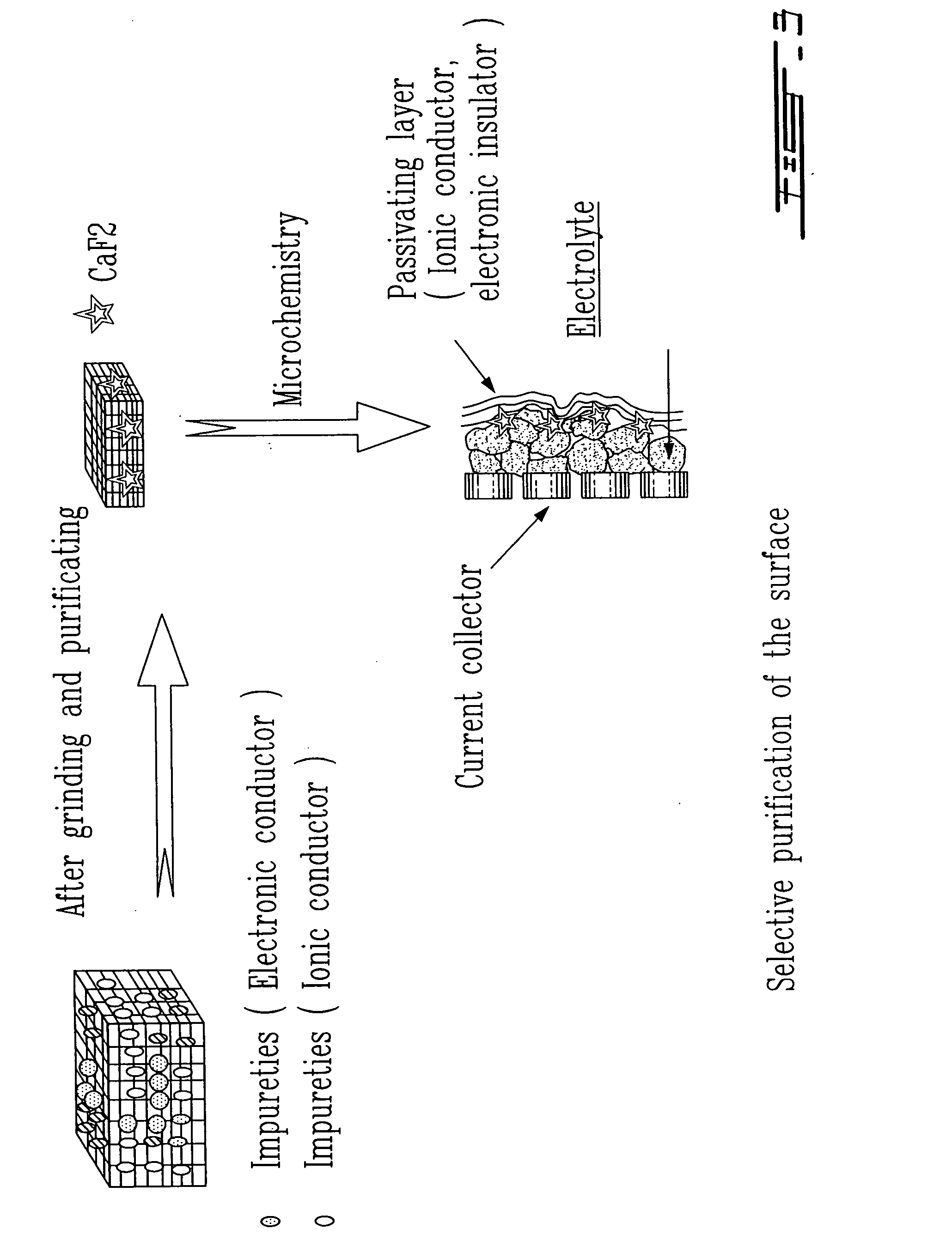
Surface preparation of natural graphite and the effect of impurities on grinding and the particle distribution
The present invention relates to the physical or chemical specific purification of natural mineral graphite. This purification is preferably applied to the surface of natural graphite in order to allow the formation of a passivation film during the first electrical discharge or the insertion of lithium in the graphite when the latter is used in a lithium-ion cell. The grinding to a small size before purification allows the optimization of the distribution of the particles, resulting in a more uniform electrode. This grinding is carried out in the presence of the natural impurities of the graphite that play the role of a micro-abrasive and result in a hardness of the graphite that increases its mechanical properties.
Owner:HYDRO QUEBEC CORP
Lithium battery using an aqueous electrolyte
InactiveUS8597828B2Inherent riskPromote resultsAlkaline accumulatorsSolid electrolyte cellsAqueous electrolyteLithium-ion battery
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Electrode active material with multi-element based oxide layers and preparation method thereof
ActiveUS20060083991A1Improve thermal safetyAvoid decompositionActive material electrodesElectrode collector coatingHalogenThermal safety
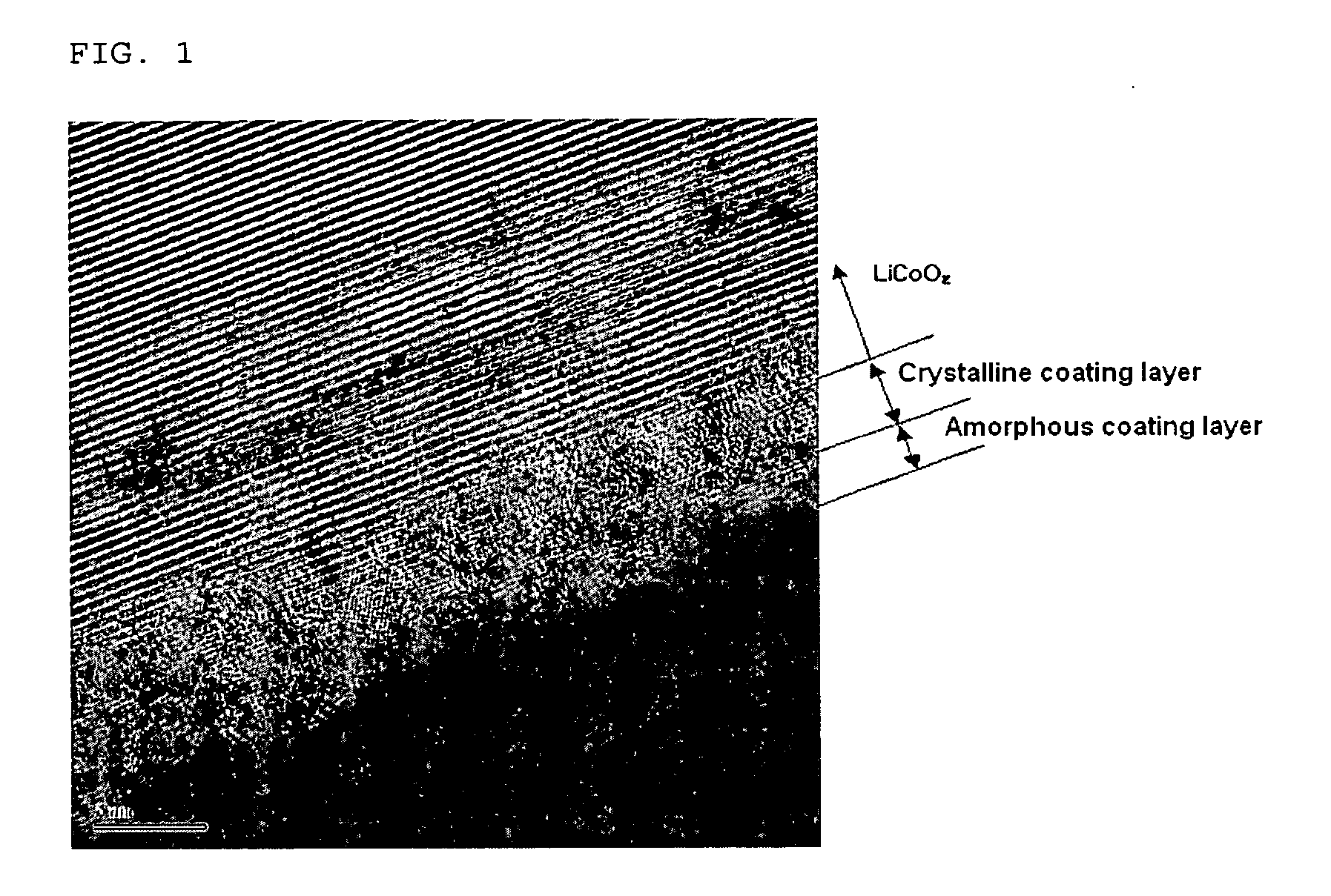
Disclosed is an electrode active material comprising: (a) electrode active material particles capable of lithium intercalation / deintercalation; and (b) a multinary oxide coating layer partially or totally formed on the surface of the electrode active material particles, the multinary oxide coating layer comprising Al, P and a halogen element. A method for preparing the electrode active material, an electrode using the electrode active material, and an electrochemical device comprising the electrode, preferably a lithium secondary battery, are also disclosed. The electrode active material comprising a multinary oxide coating layer has improved structural stability and thermal safety, and thus can provide an electrochemical device having high capacity, long service life and excellent safety.
Owner:LG ENERGY SOLUTION LTD
Production method of spherical graphite
The invention relates to a production method of spherical graphite, which is characterized in that spheroidizing equipment is used for processing the graphite into sphericity or potato shape with grain size of 10-45mu m; the spheroidizing equipment comprises n groups of devices; each group is formed by connecting a Roots blower, a dust collector, a cyclone separator and a pulverizer in turn; and a discharge outlet of the cyclone separator in the (n-1)th device is connected with a feed inlet of the pulverizer in the nth device. The method dispenses with personnel operation, is higher in yield and efficiency and lower in cost in unit time and realizes automatic closed production of the whole production line. Compared with the prior art, the method is characterized in that the spherical graphite produced by the method has better sphericity, higher tap density, large specific capacity being more than 350mAh / g, small specific area, excellent lithium intercalation and de-intercalation capabilities and cycle stability and 500-cycle capacity-keeping rate being more than 80%.
Owner:黑龙江省牡丹江农垦湠奥石墨烯深加工有限公司
Lithium ion battery silicon oxide and carbon composite negative pole material and preparation method thereof
ActiveCN106935836AEvenly dispersedGood dispersionMaterial nanotechnologyCell electrodesCarbon compositesHigh rate
The invention discloses a lithium ion battery silicon oxide and carbon composite negative pole material and a preparation method thereof. The technical purposes are improvement of first Coulomb effect, capacity and circulating properties and reduction of cost. According to the lithium ion battery silicon oxide and carbon composite negative pole material, a graphite-based silicon oxide composite material serves as a body, the body is wrapped with a pyrolytic carbon wrapping layer of an organic carbon source, and amorphous carbon in the graphite-based silicon oxide composite material is bonded with a silicon oxide and graphite with a nanoporous structure by means of Van der Waals force. The preparation method includes the steps of nano-silica sol preparation, ultrasonic dispersion, primary sintering, wrapping and secondary sintering. Compared with the prior art, stress generated by size change of the silicon oxide during high lithium intercalation and deintercalation is effectively reduced, and interfacial potential energy between materials is effectively reduced, so that the material has high specific capacity, and is suitable for high-rate charge and discharge, simple in preparation method, easy to control, low in cost and suitable for large-scale industrial production.
Owner:博尔特新材料(银川)有限公司
Preparation method for core-shell type carbon-coated iron nitride nano-composite particles and application of core-shell type carbon-coated iron nitride nano-composite particles
The invention discloses a preparation method for core-shell type carbon-coated iron nitride nano-composite particles and the application of the core-shell type carbon-coated iron nitride nano-composite particles, and belongs to the field of nano-material preparation technologies and application. The method is characterized by comprising the following steps of: automatically controlling direct current arc hydrogen plasma equipment to evaporate bulk iron raw materials, and simultaneously introducing methane and argon according to a certain proportion to obtain carbon-coated iron nano-particle precursors; and performing nitriding thermal treatment on the precursors in the ammonia atmosphere of 400 DEG C for 3 to 4 hours to obtain the carbon-coated iron nitride nano-composite particles. A lithium ion battery cathode prepared from the carbon-coated iron nitride nano-composite particles which serve as active substances has the first reversible specific capacity of 550mAh / g and high cycle stability. The method and the application have the advantages that: the carbon-coated iron nitride nano-composite particles prepared by the low-temperature nitriding of the in-situ synthesized carbon-coated iron nano-particle precursors have high lithium intercalation / de-intercalation capacity density and cycle stability; the raw materials are low in cost; a process is simple; the carbon-coated iron nitride nano-composite particles can be prepared in large scale; and industrial production requirements are met.
Owner:DALIAN UNIV OF TECH
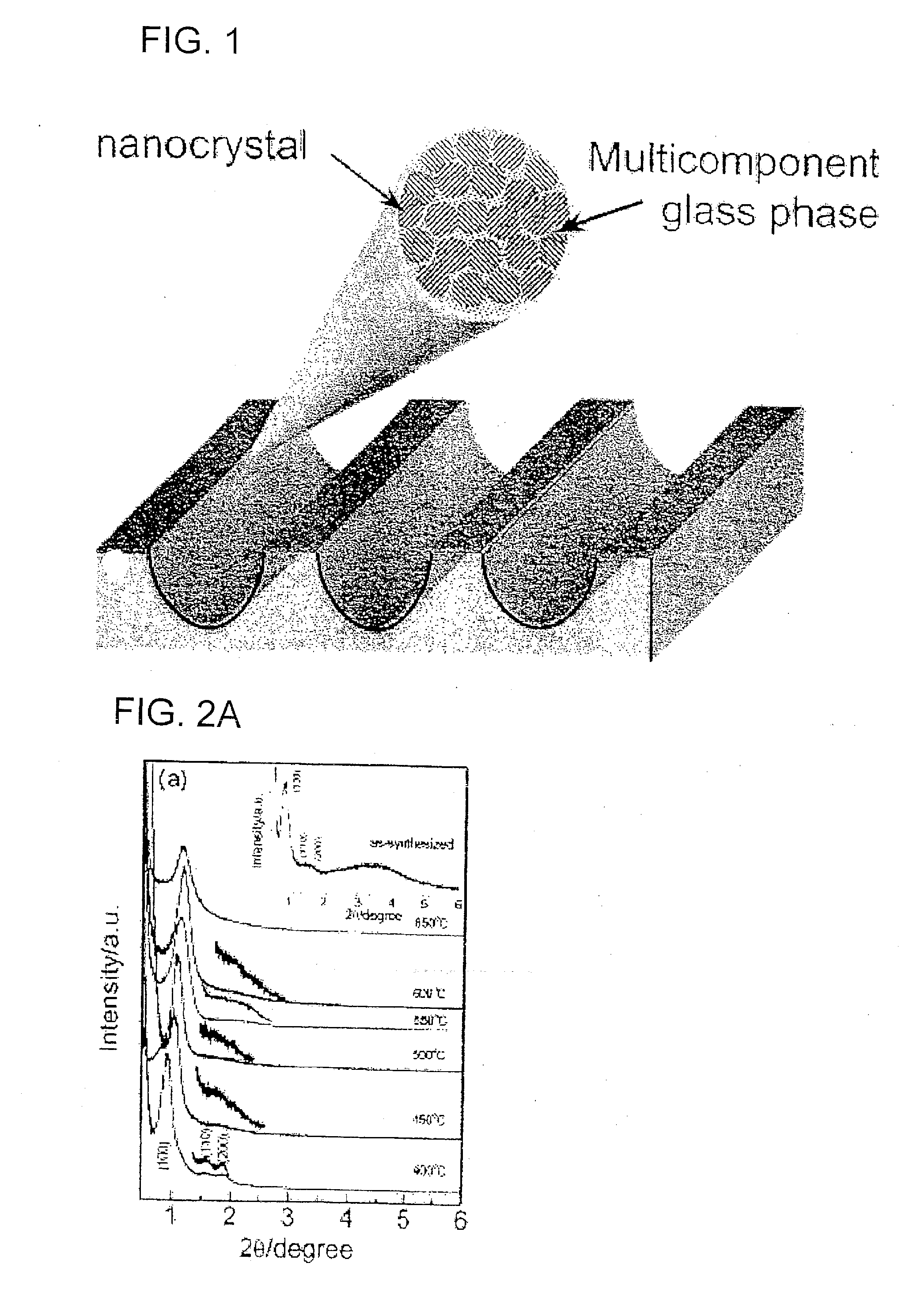
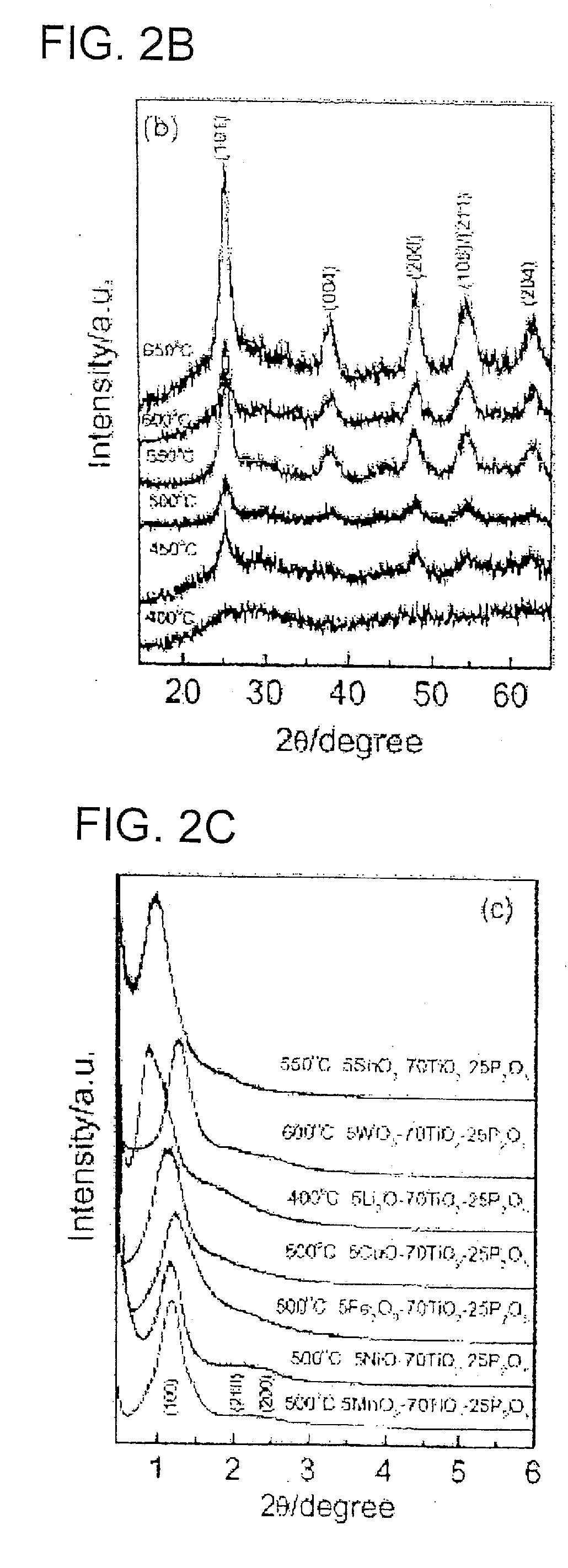
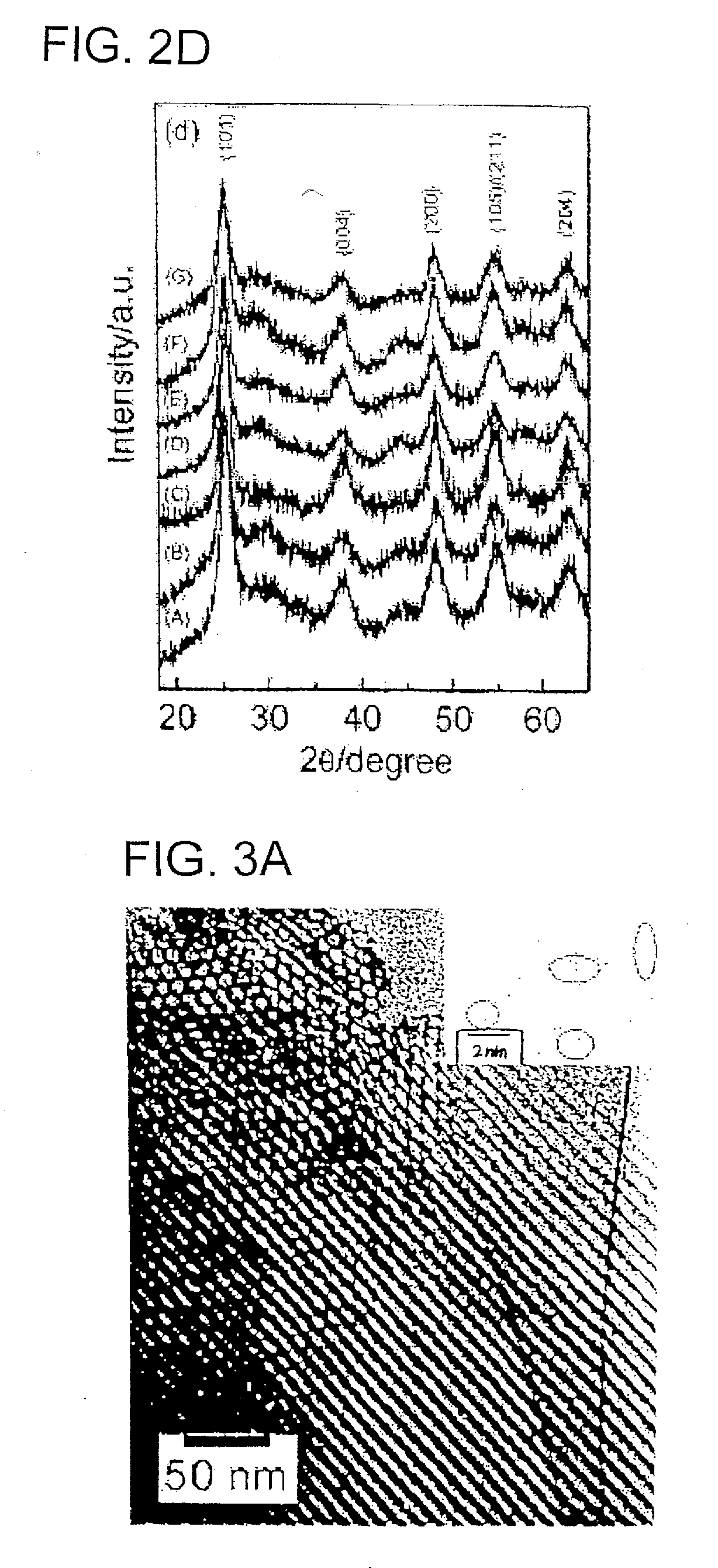
Nanocrystal oxide/glass composite mesoporous powder or thin film, process for producing the same, and utilizing the powder or thin film, various devices, secondary battery and lithium storing device
InactiveUS20070027015A1Simple methodLarge capacityPhosphorus oxidesMaterial nanotechnologyGlass compositesManufacturing technology
The present invention aims to realize (1) manufacture of a mesoporous composite powder or thin film composed of nanocrystalline metal oxide-glass having a three-dimensional structure with a large specific surface area, (2) construction of a porous structure framework with nanocrystalline metal oxide crystal and a slight amount of glass phase (SiO2 or P2O5, B2O3), (3) control of crystal growth of metal oxide with a slight amount of glass phase (SiO2 or P2O5, B2O3), (4) simplification of the manufacturing process, and (5) use thereof in manufacture of a lithium intercalation electric device, photocatalytic device, solar battery and energy storage device. Provided are a nanocrystal oxide-glass mesoporous composite powder or thin film having a three-dimensional structure with regularly arranged mesopores, and a secondary battery comprising the same.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Silicon-based electrode with adjustable pore structure and preparation method of silicon-based electrode
ActiveCN108767195AIntegrity guaranteedImprove distributionElectrode rolling/calenderingSecondary cellsPorosityCyclic process
The invention provides a silicon-based electrode with an adjustable pore structure; the porosity of the silicon-based electrode is 30%-60%; the pore structure of the silicon-based electrode is adjusted by controlling the compaction density of the electrode and adding a pore-forming additive, wherein the pore-forming additive is one or more of ammonium carbonate, ammonium hydrogen carbonate, ammonium acetate, ammonium nitrate and ammonium chloride. The invention further provides a preparation method of the silicon-based electrode. The porosity of the electrode is controlled by changing the compaction density, and the appropriate porosity can be consistent with the volume expansion rate of a high specific capacity silicon carbon negative electrode material in a lithium intercalation state, so that the integrity of the structure is kept in the circulation process of the electrode; and the volume change of silicon can be effectively buffered through the high-capacity silicon-based negativeelectrode with the variable pore structure, so that the diffusion speed of lithium ions and electrons is increased, the cycling stability of the electrode is obviously improved, and the large-currentdischarge performance of the electrode is improved.
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD
Chromogenically tunable photonic crystals
Owner:UNIVERSITE DE MONCTON
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4−x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Binder with good rate property and long cycleability for lithium secondary battery
ActiveUS20060257739A1Improve battery qualityMaterial analysis by electric/magnetic meansNon-aqueous electrolyte accumulator electrodesMethacrylateMeth-
Disclosed is a binder, which comprises polymer particles obtained by polymerization of: (a) 1˜80 parts by weight of a (meth)acrylic acid ester monomer; (b) 1˜20 parts by weight of an unsaturated carboxylic acid monomer; and (c) 0.001˜40 parts by weight of a vinyl monomer, based on 100 parts by weight of the binder polymer, and which allows electrode active material particles capable of lithium intercalation / deintercalation to be fixed and linked among themselves, and between the particles and a collector. An electrode comprising the binder, and a lithium secondary battery having the electrode are also disclosed. Further, a method for evaluating interrelation between wettability of a binder to an electrolyte and quality of a battery comprising the binder is disclosed. The binder shows excellent adhesion as well as excellent wettability to an electrolyte, and thus can improve rate characteristics and lifespan characteristics of a battery, when used in an electrode for a lithium secondary battery.
Owner:LG CHEM LTD
Preparation method of high-capacity silicon-based negative electrode material of lithium-ion battery
InactiveCN106025211AImprove the reunion effectAvoid performance degradationCell electrodesSecondary cellsDispersitySlurry
The invention discloses a preparation method of a high-capacity silicon-based negative electrode material of a lithium-ion battery. The method comprises the preparation processes of: mixing nano silicon, graphite and a pyrolytic carbon organic matter precursor to obtain composite material precursor slurry; and carrying out spray drying to obtain precursor powder; and finally carrying out roasting treatment in an inert atmosphere and then carrying out grinding to obtain an organic matter pyrolytic carbon-coated nano silicon / graphite composite material. According to the preparation method, the dispersity of the nano silicon in the silicon-carbon negative electrode material can be improved; the structure stability of the material in a lithium intercalation and deintercalation process is improved; the condition that the material has relatively high conductivity is ensured; a pyrolytic carbon coating layer effectively coats the surfaces of material particles; the interface characteristic of the material can be effectively improved; and the electrochemical properties of the silicon-carbon negative electrode material are improved.
Owner:田东
Novel lithium-pre-intercalated negative plate and preparation method thereof
ActiveCN105244472AExcellent charge and discharge characteristicsLarge amount of pre-intercalated lithiumCell electrodesComposite filmGraphite
The invention discloses a novel lithium-pre-intercalated negative plate and a preparation method thereof. The novel lithium-pre-intercalated negative plate comprises a porous current collector, an active material layer and a lithium pre-intercalation layer, wherein the lithium pre-intercalation layer is attached to the active material layer; the active material layer is prepared from the following components represented by mass percentage: 75%-90% of an active material, 5%-20% of a conductive agent and 5%-10% of a binder; the active material is a lithium-intercalated carbon material; the lithium pre-intercalation layer is a dense and uniform composite thin-film layer of which the thickness is 5-30 microns; the lithium pre-intercalation layer is prepared from the following components represented by mass percentage: 50%-70% of graphite and 30%-50% of a lithium salt; and the lithium pre-intercalation layer is obtained by magnetron sputtering coating on the active material layer. The novel lithium-pre-intercalated negative plate disclosed by the invention is high in lithium-pre-intercalated quantity, high in adhesion to the active material layer and uniform in lithium intercalation, is achieved through magnetron sputtering coating, and is safe and time-saving; and the lithium-pre-intercalated quantity can be controlled.
Owner:江西展枭新能源科技有限公司
Lithium supplementing method for energy storage device
InactiveCN106450467ADoes not reduce energy densityWide variety of sourcesFinal product manufactureSecondary cells servicing/maintenanceProduct gasEngineering
The invention relates to a lithium supplementing method for an energy storage device. The lithium supplementing method comprises the steps of adopting a device shell which consists of a cavity for placing a core pack, and m other cavities; putting the core pack and a lithium supplementing electrode into the core pack cavity and another cavity respectively, and injecting an electrolyte to enable the cavities where the core pack and the lithium supplementing electrode are located to be rich in electrolyte; performing formation treatment on the core pack, and then performing electrochemical lithium intercalation to obtain an A; or performing electrochemical lithium intercalation firstly, and then performing formation treatment on the core pack to obtain a B; after obtaining the A and B, performing n times of charge-discharge cycling treatment on the obtained A and B, next, extracting gas and redundant electrolyte from the core pack cavity, and sealing connection channels between the core pack cavity and other cavities to obtain a C; and finally, performing cavity-removing treatment on the obtained C to obtain a D, and performing machining and shaping on the D to obtain the finished energy storage device. The lithium supplementing method is simple and convenient to operate without greatly improving the production process of the existing lithium battery, and meanwhile, continuous production can be realized quite easily; and in addition, the lithium supplementing method is remarkable in effect and convenient to realize large-scale industrial application.
Owner:CENT SOUTH UNIV
Sol-gel preparation method of lithium vanadate negative electrode material of lithium ion battery
ActiveCN104241626ASmall grain sizeRich sourcesCell electrodesSecondary cellsGel preparationVanadium Compounds
The invention relates to a sol-gel preparation method of a lithium vanadate negative electrode material of a lithium ion battery. The sol-gel preparation method comprises the following steps: sequentially adding a precursor containing a vanadium compound and a precursor containing a lithium compound into water, and stirring fully; then adding a water soluble carbon material which is acted as a chelate and a carbon source, stirring the water solution until dry gel is formed, carrying out vacuum drying until the water content is dried completely, putting a gel body into a porcelain boat, pretreating in the reducing atmosphere or the inert atmosphere, and carrying out sintering reaction in the inert atmosphere or the reducing atmosphere so as to obtain the material. According to the method, the technology is simple, the operation is easy, and moreover, the structure of lithium vanadate and the valence state of vanadium cannot be changed by the existence of the carbon material and the reducing atmosphere. The carbon-coated lithium vanadate material synthesized by the method, which acts as the negative electrode material of the lithium ion battery, has excellent performance and low lithium intercalation potential, and is expected to be as the negative electrode material of the next generation of lithium ion batteries. The synthesis method is suitable for producing the negative electrode material of the high-performance lithium ion battery, namely lithium vanadate.
Owner:SOUTH CHINA UNIV OF TECH
Electrode with enhanced performance and electrochemical device comprising the same
Disclosed is an electrode slurry comprising: (a) an electrode active material capable of lithium intercalation / deintercalation; and (b) monomers capable of forming a polymer via polymerization. An electrode having a binder polymer formed by applying the electrode slurry onto a current collector and carrying out in situ polymerization of the monomers, and an electrochemical device comprising the electrode are also disclosed. The electrode uses monomers capable of forming a binder polymer via polymerization under heat or light upon drying of the electrode, instead of the conventional PVdF or SBR-based binders. Therefore, it is possible to simplify a process for manufacturing an electrode, to provide an eco-friendly electrode by virtue of the use of an aqueous solvent as a dispersion medium, to improve the ion conductivity of a binder by virtue of the use of a solvent for a battery electrolyte as a dispersion medium, and thus to improve the quality of an electrochemical device.
Owner:LG CHEM LTD
Lithium Secondary Batteries With Enhanced Safety And Performance
InactiveUS20080131781A1Minimize degradationImprove battery safetyPrimary cellsActive material electrodesInorganic particleLithium intercalation
Disclosed is an electrode obtained from electrode slurry comprising: (a) an electrode active material capable of lithium intercalation / deintercalation; and (b) inorganic particles having lithium ion conductivity. An electrochemical device comprising the same electrode is also disclosed. The electrochemical device, using such inorganic particles having lithium ion conductivity added to electrode slurry, can show improved safety, while minimizing degradation in the quality caused by the use of additives.
Owner:LG CHEM LTD
Anode material for super capacitor battery and preparing method thereof
InactiveCN101335346AHas a large capacityImprove power characteristicsElectrochemical generatorsElectrode manufacturing processesCapacitancePorous carbon
The invention discloses a super capacitor-battery used anode material and a preparation method thereof. Mechanical fusion granulation is carried out on compound material power which has the property of de-lithium-intercalation to obtain ball grains of 1 to 15 micro; porous carbon material with the weight percentage of 10 percent to 100 percent of the weight of the de-lithium-intercalation compound material is added and then the mechanical fusion granulation is carried out, thereby obtaining de-lithium-intercalation compound material coated with porous carbon material. The method of the invention has simple process, and the material obtained has both the large capacity property of a battery material and the high power of a capacitor material. The storage capacity of the anode material can reach 100mAh / g, and the discharge capacity of 10C can reach 90 percent of the discharge capacity of 0.5C. The anode material is the potential electrode material of the novel energy storage system which is applied to an electric driving automobile.
Owner:CENT SOUTH UNIV
Surface composite coated anode material, preparation method thereof and lithium ion battery
ActiveCN102956895AExcellent rate performanceExcellent high temperature cycle performanceCell electrodesSecondary cellsPhosphateMass ratio
The invention provides a surface composite coated lithium ion battery anode material, a preparation method thereof and a lithium ion battery. The anode material comprises a lithium intercalation compound basal body and an Al2O3-AlPO4 composite layer which is coated on the basal body. The mass ratio of Al elements to lithium intercalation compounds is 0.04-0.15 percent. The mass percent of Al2O3 in the composite layer is 10-90 percent. The preparation method comprises the following steps of: dissolving easily soluble aluminum salt in solvent to form solution A; dissolving easily soluble alkaline substances and easily soluble phosphate in solvent to form solution B; dispersing the lithium intercalation compounds in a dispersing medium to obtain a dispersion system M; enabling the solution B and the solution A to form sol substances, adding the sol substances into the dispersion system M and enabling sol particles to be evenly adsorbed on the surfaces of the lithium intercalation compounds; and finally conducting solid-liquid separation and subsequent treatment. By adopting the technical scheme, the defects of sole coating can be effectively overcome, and the lithium ion battery with excellent rate capability and high-temperature circulating performance can be prepared.
Owner:QINGHAI TAIFENG XIANXING LITHIUM ENERGY TECH CO LTD
Silicon-based composite negative electrode plate, preparation method, and lithium ion secondary battery
InactiveCN107799723AIncrease capacityExtended service lifeCell electrodesSecondary cellsHigh ratePorous carbon
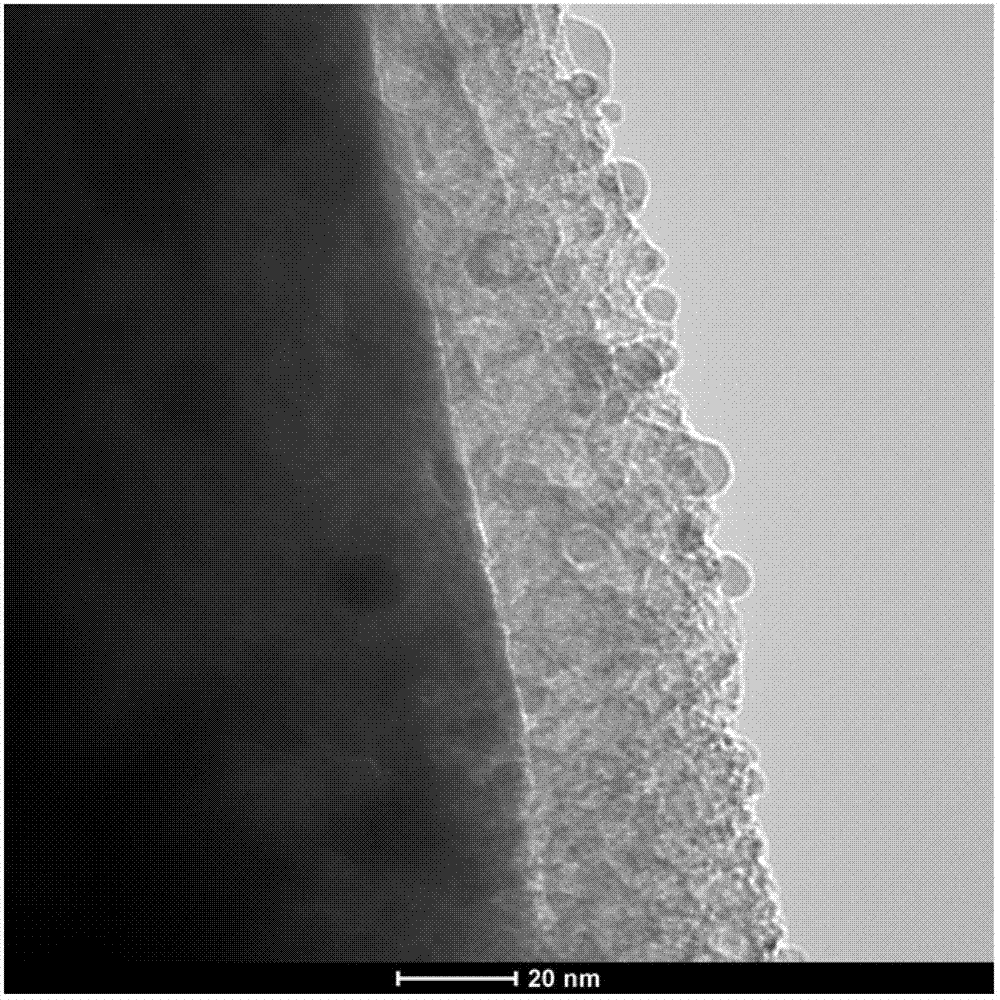
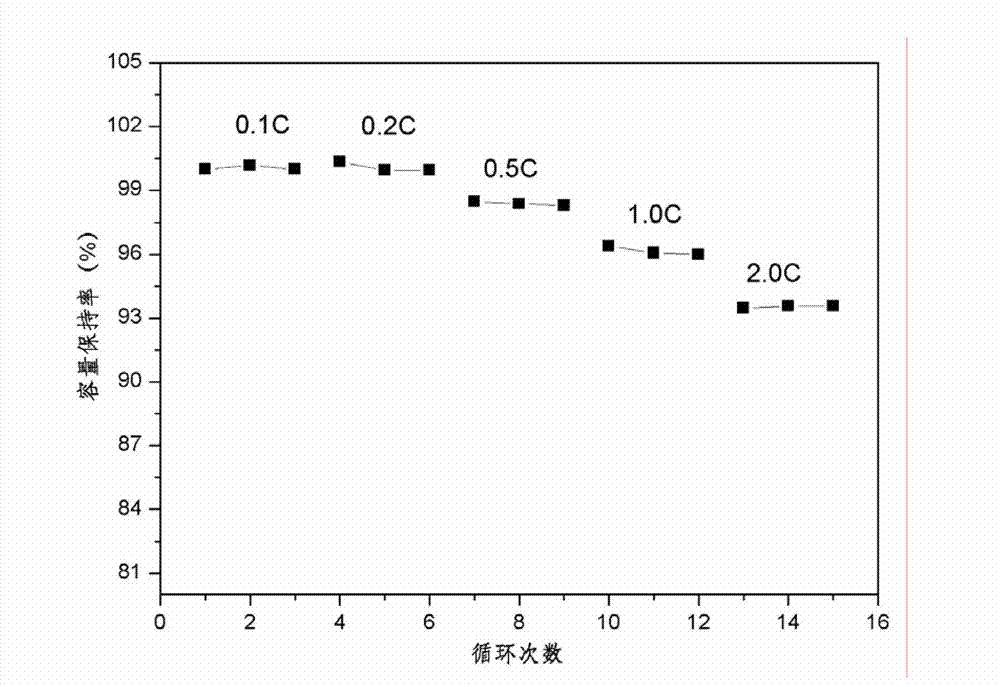
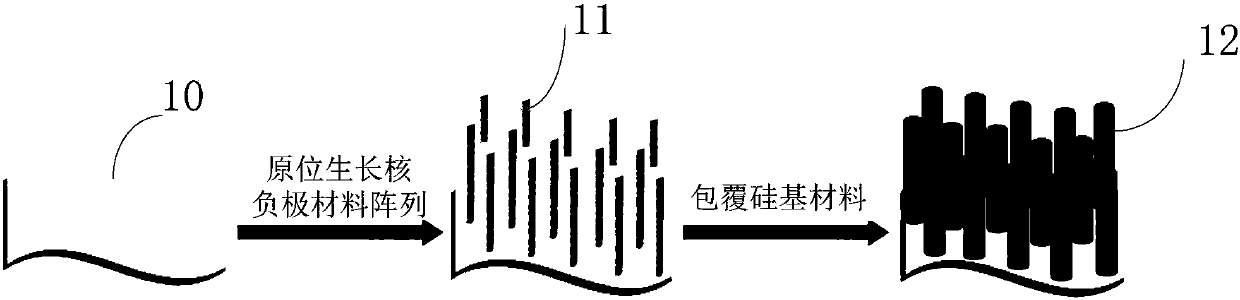
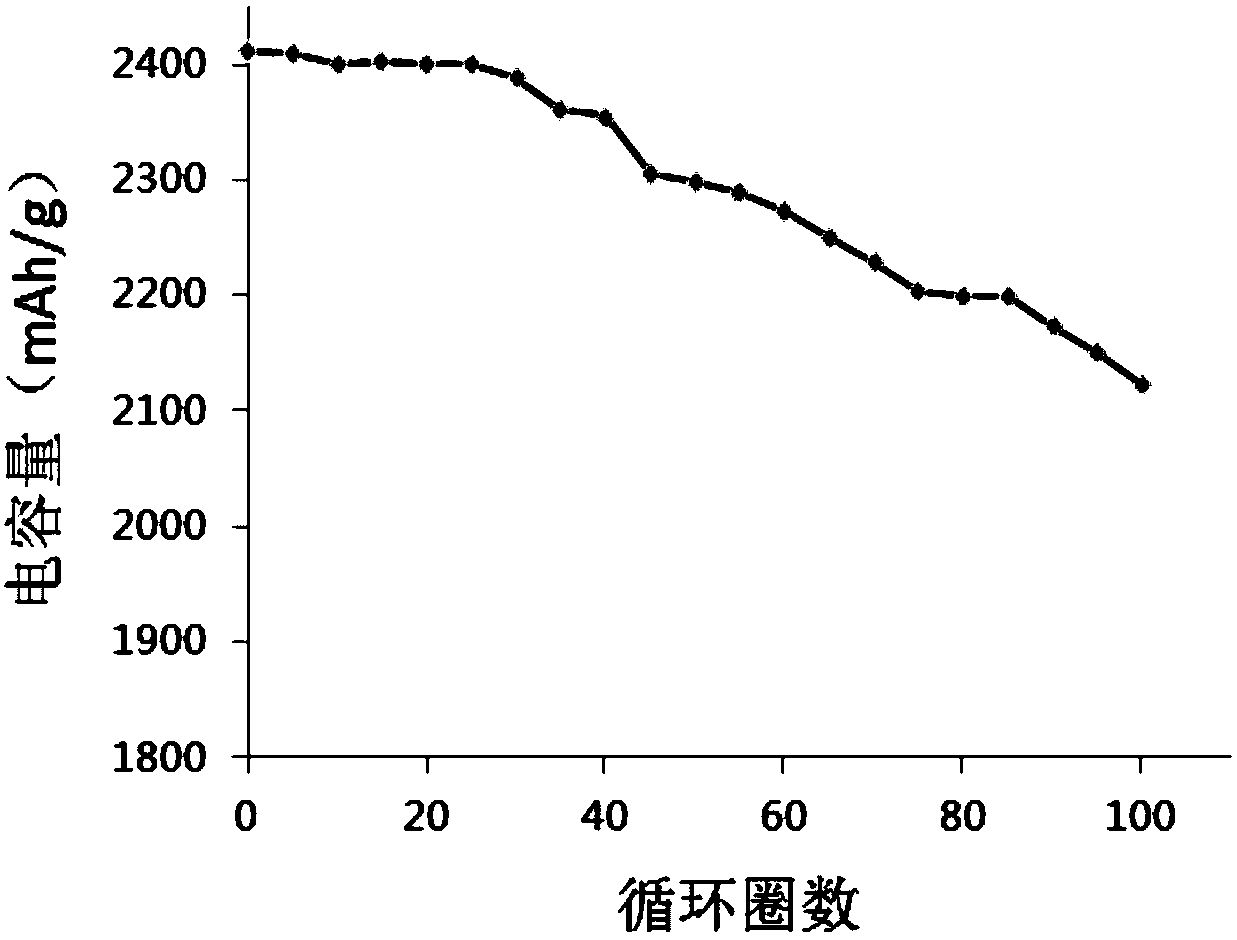
The invention provides a silicon-based composite negative electrode plate. The silicon-based composite negative electrode plate comprises a current collector, and a one-dimensional silicon-based shell-core composite structure array arranged on the current collector. According to the one-dimensional silicon-based shell-core composite structure array, a core negative electrode material formed on thecurrent collector via in-suit growth is taken as the core, and a silicon-based material is taken as a shell; the core negative electrode material is one or mixture of a plurality of ingredients selected from carbon nano tube, carbon nanofiber, porous carbon, graphene, lithium-intercalation metal and alloy, lithium titanate, a transition metal oxide, a bimetal oxide, a metal sulfide, a metal nitride, and a metal phosphide; and the core negative electrode material is arranged on the current collector in the manner of a one-dimensional vertical structure array. The silicon-based composite negative electrode plate possesses high-rate performance and high cycling stability, is capable of improving problems such as the low-rate performance of silicone and pulverization and polarization caused by swelling, increasing electrode capacity, and prolonging cycle life. The invention also provides a preparation method of the silicon-based composite negative electrode plate, and a lithium ion secondary battery containing the silicon-based composite negative electrode plate.
Owner:HUAWEI TECH CO LTD
Preparation method of nano silicon-based/carbon composite material
ActiveCN105261733AHigh specific capacityImprove permeabilityMaterial nanotechnologyCell electrodesCarbon layerCarbon composites
The invention provides a preparation method of a nano silicon-based / carbon composite material. The method comprises the following steps: firstly, coating a nano silicon surface with a micro-pore carbon layer by organic resin and a pore forming material in a liquid phase; and then adopting fermented starch as a carbon source, and carrying out coating and high-temperature carbonization to prepare the nano silicon-based / carbon composite material. The nano silicon-based / carbon composite material with pomegranate-type structure characteristics can be prepared by the method provided by the invention. When the nano silicon-based / carbon composite material is applied to a preparation of a lithium-ion battery anode material, the problems of a rapid volume expansion in a lithium intercalation process and grain breakage, pulverization and falling in a circulating process can be effectively solved; the specific capacity of the material can be up to 450-950mAh / g; and the capacity retention ratio is 85%-92% after circulating charge and discharge for 500 cycles.
Owner:HUNAN SHINZOOM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com