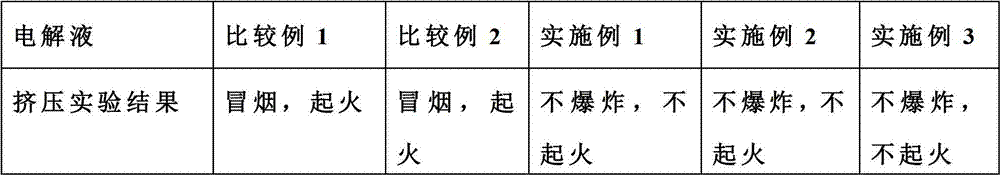
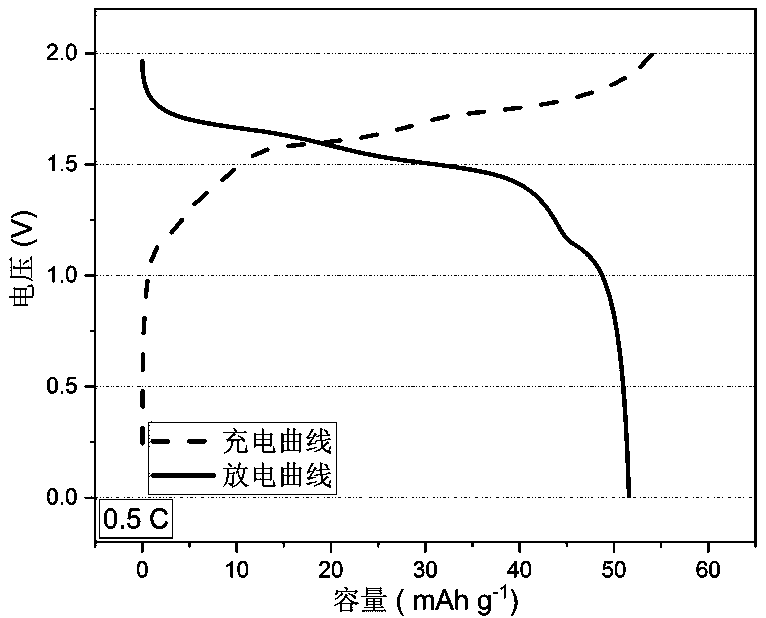
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37results about How to "Low ionic conductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
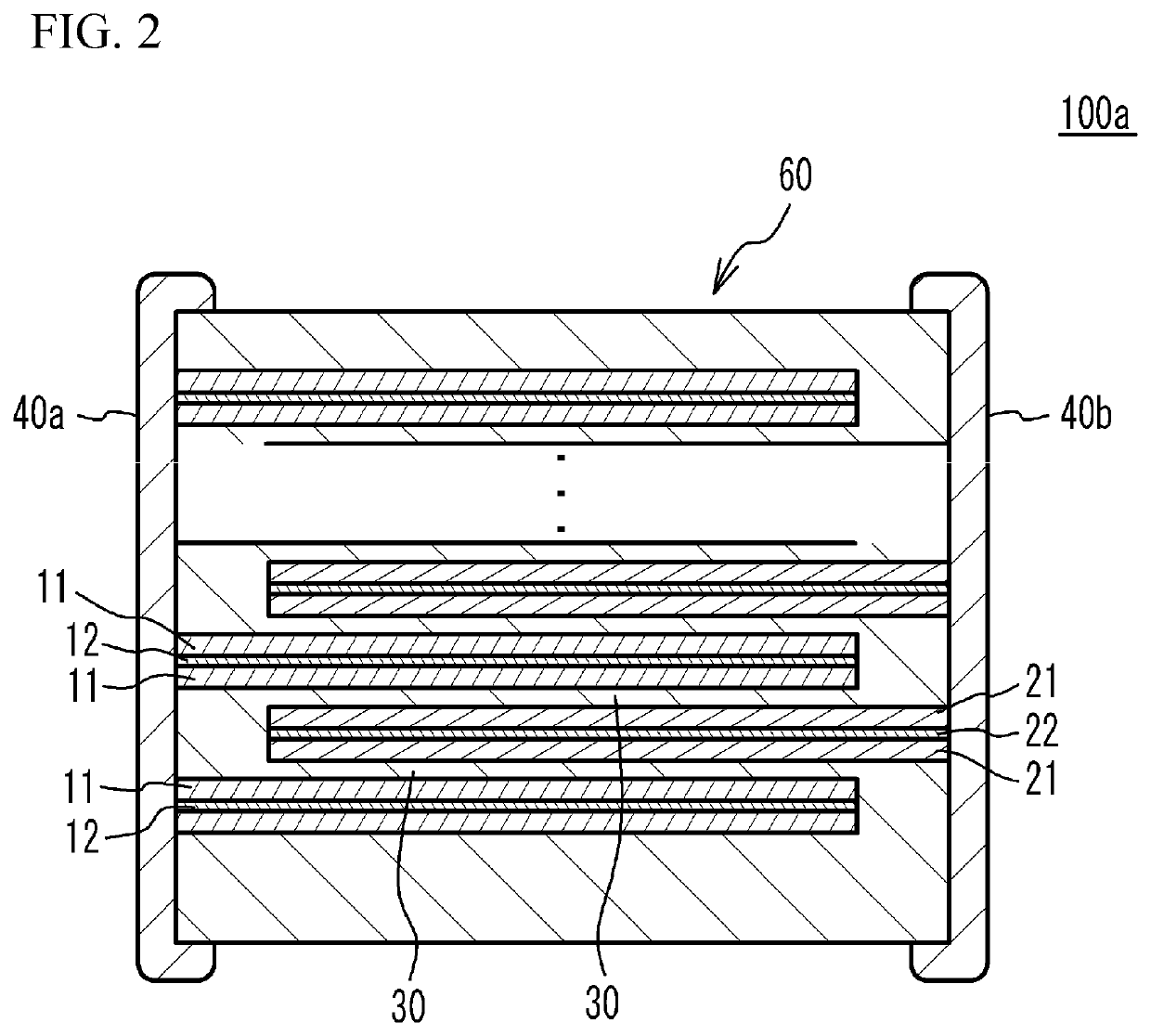
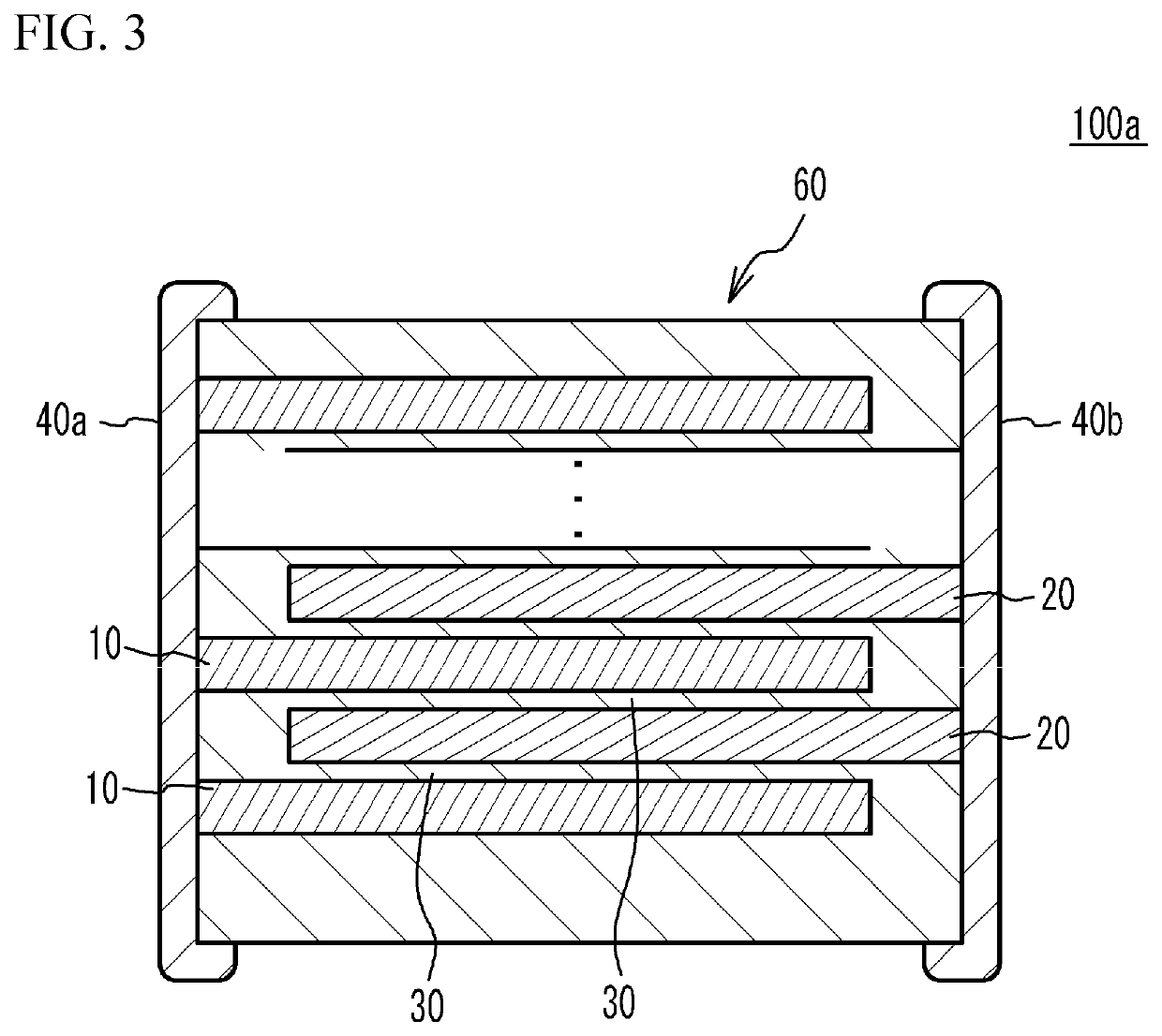
Lithium secondary batteries containing non-flammable quasi-solid electrolyte
ActiveUS20140363746A1Reduce electrical conductivityLow ionic conductivityFuel and secondary cellsSolid electrolyte cellsSolventVapor pressure
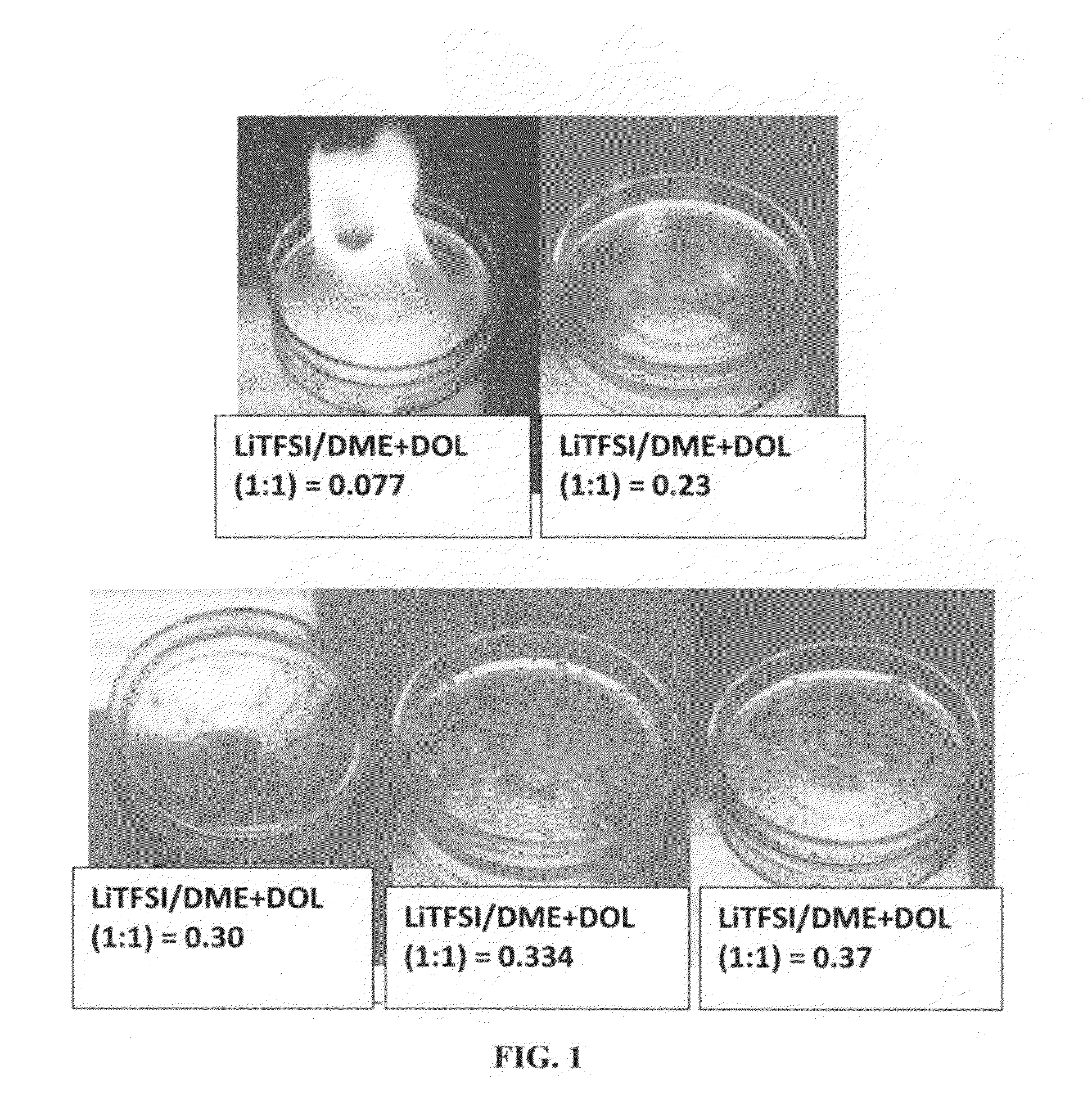
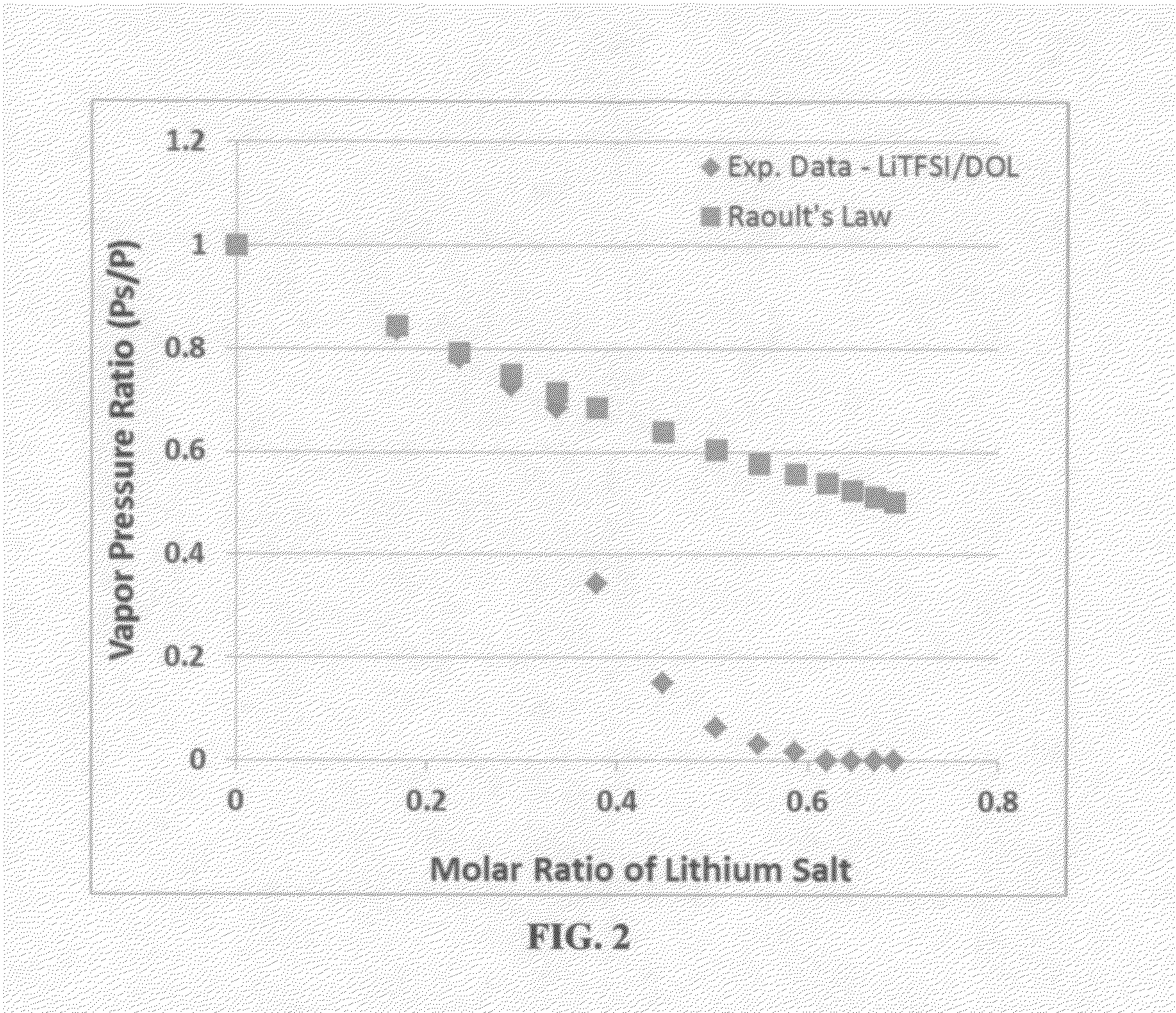
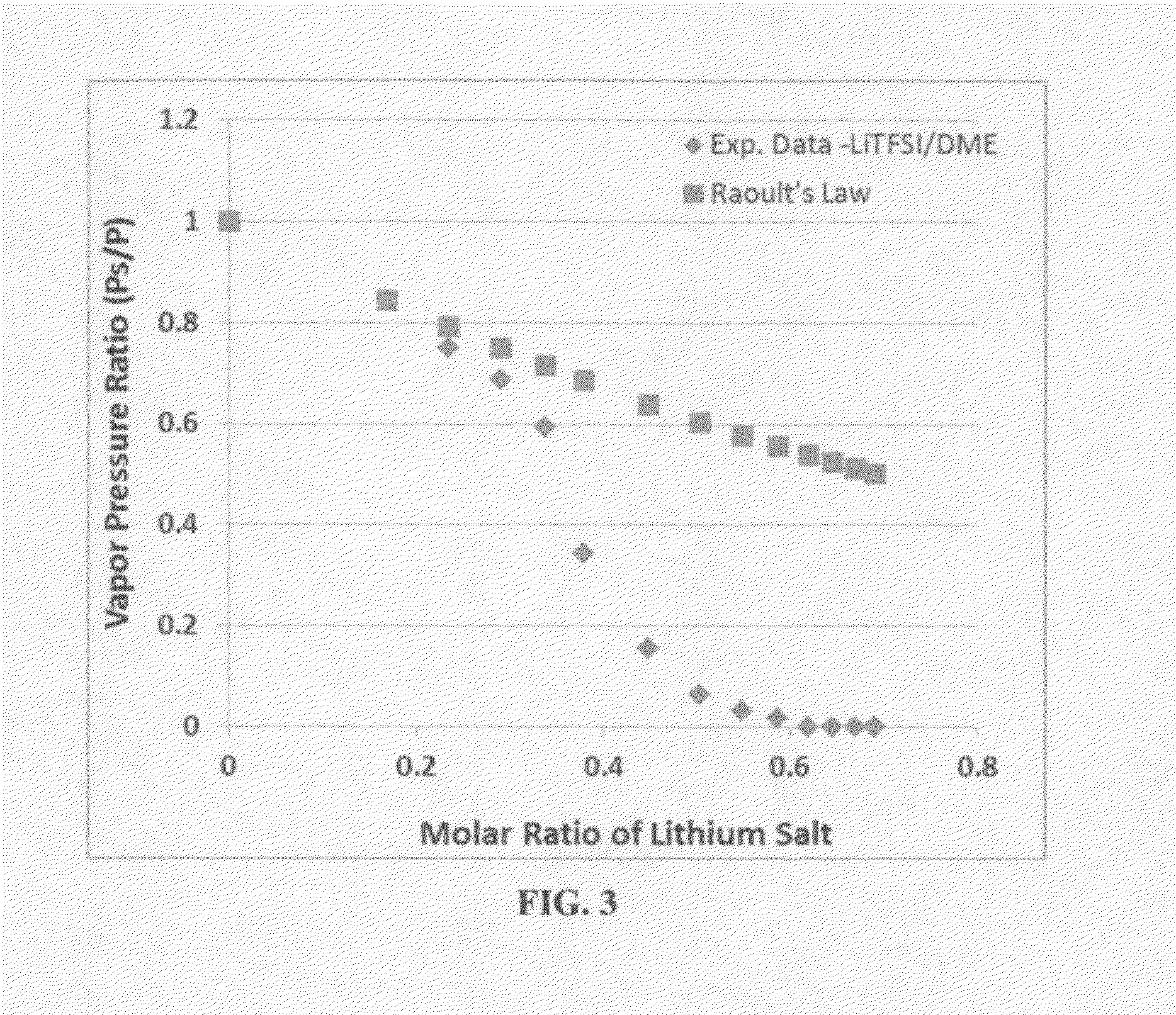
A rechargeable lithium cell comprising a cathode having a cathode active material, an anode having an anode active material, a porous separator electronically separating the anode and the cathode, a non-flammable quasi-solid electrolyte in contact with the cathode and the anode, wherein the electrolyte contains a lithium salt dissolved in a first organic liquid solvent with a concentration sufficiently high so that the electrolyte exhibits a vapor pressure less than 0.01 kPa when measured at 20° C., a flash point at least 20 degrees Celsius higher than the flash point of the first organic liquid solvent alone, a flash point higher than 150° C., or no flash point. This battery cell is non-flammable and safe, has a long cycle life, high capacity, and high energy density.
Owner:GLOBAL GRAPHENE GRP INC
Alkali metal-sulfur secondary battery containing a pre-sulfurized cathode and production process
ActiveUS20160240840A1Reduce electrical conductivityLow ionic conductivitySolid electrolytesNegative electrodesSolventElectrolyte
A method of producing a pre-sulfurized active cathode layer for a rechargeable alkali metal-sulfur cell; the method comprising: (a) Preparing an integral layer of meso-porous structure of a carbon, graphite, metal, or conductive polymer having a specific surface area greater than 100 m2 / g; (b) Preparing an electrolyte comprising a solvent and a sulfur source; (c) Preparing an anode; and (d) Bringing the integral layer and the anode in ionic contact with the electrolyte and imposing an electric current between the anode and the integral layer (serving as a cathode) to electrochemically deposit nano-scaled sulfur particles or coating on the graphene surfaces. The sulfur particles or coating have a thickness or diameter smaller than 20 nm (preferably <10 nm, more preferably <5 nm or even <3 nm) and occupy a weight fraction of at least 70% (preferably >90% or even >95%).
Owner:GLOBAL GRAPHENE GRP INC
Lithium-sulfur secondary battery containing gradient electrolyte
ActiveUS20140342209A1Reduce electrical conductivityLow ionic conductivityElectrode carriers/collectorsTwo electrolyte cellsLithium sulfurBattery cell
A rechargeable lithium-sulfur cell comprising a cathode, an anode, a separator electronically separating the two electrodes, a first electrolyte in contact with the cathode, and a second electrolyte in contact with the anode, wherein the first electrolyte contains a first concentration, C1, of a first lithium salt dissolved in a first solvent when the first electrolyte is brought in contact with the cathode, and the second electrolyte contains a second concentration, C2, of a second lithium salt dissolved in a second solvent when the second electrolyte is brought in contact with the anode, wherein C1 is less than C2. The cell exhibits an exceptionally high specific energy and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Flame retardant long-life electrolyte and lithium ion battery using same
InactiveCN102780040AImprove securityImprove cycle lifeSecondary cellsComposite filmPhysical chemistry
The invention discloses a multi-component electrolyte for lithium ion batteries. The multi-component electrolyte is good in ionic conductivity and is characterized by containing the following multiple components: (1) carbonic ester solvents, (2) flame retardant additives, (3) single or composite film-forming additives and (4) composite lithium salt. One of the film-forming additives (3) is selected from haloketones, the general formula of the haloketones is normally: XH2mCm-(C=O)-CnH2n+1, wherein both m and n are natural numbers, m is identical to or different from n, and X can be one of F, Cl, Br and I. The electrolyte can improve safety, magnification performance and cycle life of the lithium ion batteries.
Owner:LONG POWER SYST SUZHOU
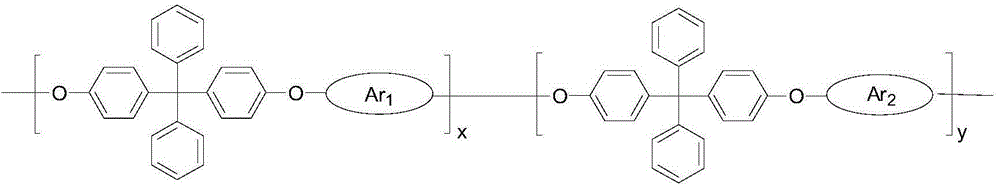
Strongly alkaline polyarylether ionomer and preparation and application thereof
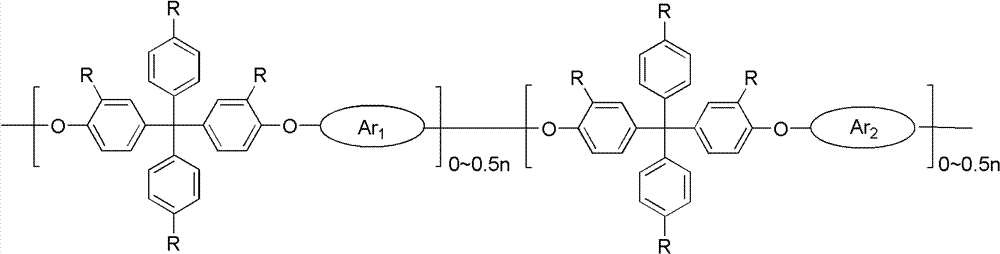
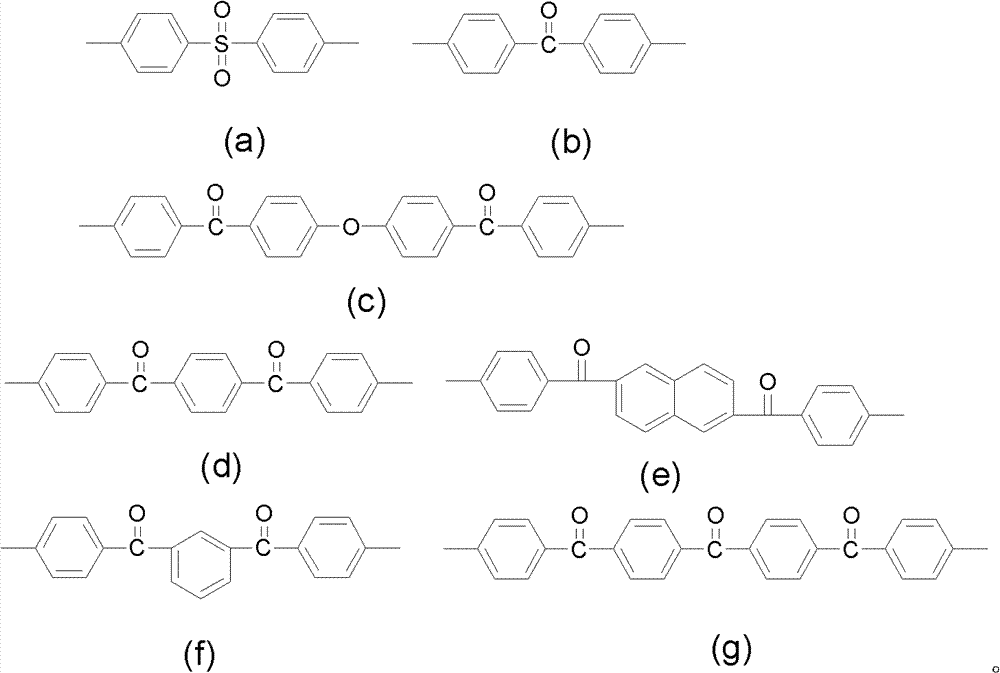
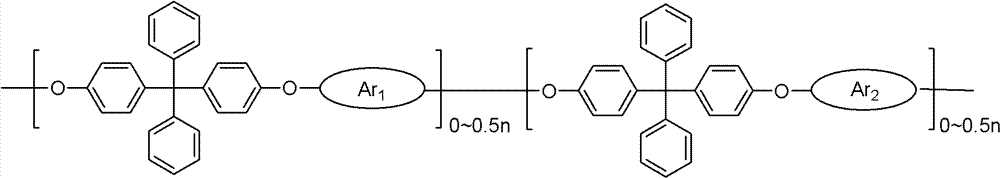
ActiveCN102702507AImprove ionic conductivityLow ionic conductivityCell component detailsFuel cell detailsDiphenylmethaneIonomer
The invention discloses strongly alkaline polyarylether ionomer and preparation and application thereof. The strongly alkaline polyarylether ionomer is prepared by polymerizing 2 (4, 4'-hydroxyphenyl) diphenylmethane and chlorine-containing or fluorine-containing aromatic monomer with Ar1 and Ar1 structure to obtain polyarylether, and subjecting the polyarylether to a series of functional processes such as chloromethylation, quaternization and alkalization. An alkaline anion exchange membrane is prepared by casting chloromethylation-based polyarylether solution into a membrane and subjecting the membrane to a series of functional processes such as quaternization and alkalization. The alkaline anion exchange membrane has fine barrier action on methyl alcohol, is fine in physicochemical property, high in thermal stability and fine in conductivity at high temperature, overcomes the defects of unstable property and low ionic conductivity of existing polyarylether anion exchange membranes, is applicable to fuel cell alkaline anion exchange membranes, and has broad application prospect.
Owner:SOUTH CHINA UNIV OF TECH
Battery diaphragm based on aramid fibre
InactiveCN101867030AOvercome the weakness of heat intoleranceProne to liquid-solid phase separationCell component detailsPorosityElectrical resistance and conductance
The invention discloses a battery diaphragm based on aramid fibre. The battery diaphragm is manufactured mainly from ultra-short aramid fibre in match with aramid fibre fibrid, and the specific requirements of air permeability and porosity of the battery diaphragm can be satisfied through adjusting fibre formula and molding process. The battery diaphragm can realize mechanical isolation function of the positive and negative poles of the battery, ensures low resistance and high ion electrical conductivity simultaneously, and has enough electrochemistry stability. Compared with traditional plastic diaphragm, the battery diaphragm in the invention has more excellent comprehensive properties, i.e. excellent heat resistance performance, high strength, fatigue resistance performance, low deformation, fire-resistant and flame retardant performance, chemical-corrosion resistance performance and the like. The battery diaphragm in the invention can be widely applied to the manufacturing of various ion batteries.
Owner:深圳昊天龙邦复合材料有限公司
Solid electrolyte of lithium ion battery and preparation method of solid electrolyte as well as lithium ion battery
ActiveCN107666010AImprove ionic conductivityReduce crystallinitySecondary cellsSolid state electrolyteAluminium-ion battery
The invention provides solid electrolyte of a lithium ion battery and a preparation method of the solid electrolyte as well as the lithium ion battery. The solid electrolyte of the lithium ion batterycomprises a core material and a shell material coating the outer surface of the core material; the core material comprises Li1+xMxTi2-x(PO4)3, wherein M is selected from at least one of Al, La, Cr, Ga, Y or In, and 0.05fx f0.4; the shell material comprises Li0.6+yB0.8SiyP1-yO4, wherein 0.01fy f0.5; the Li 0.6+yB0.8SiyP1-yO4 shell material makes full surface contact with the core material, so thatthe ability of resistance between grains of the core material is obviously reduced; in addition, the solid electrolyte of the lithium ion battery has lower electronic conductivity and a complete anddense electronic shielding layer is formed on the surface of the core material, so that the problem that Ti<4+> is reduced into Ti<3+> is better solved. The prepared solid electrolyte has the characteristics of a wide electrochemical window (the electrochemical window is greater than 5V), relatively-high ionic conductivity and low electronic conductivity.
Owner:BYD CO LTD
Lithium-air battery structure
ActiveCN104716405AAvoid erosionPromotes the electrochemical reduction reactionFuel and secondary cellsCell electrodesElectrochemical responseLithium–air battery
The invention relates to a lithium-air battery structure, including a lithium negative electrode, a porous diaphragm and a positive electrode which are sequentially laminated, an electrically conductive porous function layer is arranged between the lithium negative electrode and the porous diaphragm, the electrically conductive porous function layer is an electrically conductive porous carbon material layer or a composite layer of an electrically conductive porous carbon material and catalytic components, and mass ratio of electrically conductive porous carbon material to other functional components is 20:1 to 2:1. By the electrochemical reaction of the conductive porous functional layer and dissolved diffused oxygen or reactive oxygen species, the dissolved diffused oxygen or reactive oxygen species can be effectively consumed, the corrosion and damage to the lithium negative electrode can be reduced, and substantial improvement of the battery stability can be facilitated.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Solid thin film electrolyte material and preparation method thereof
ActiveCN103456983AHigh Li ion conductivityLow ionic conductivitySecondary cellsRadio frequency magnetron sputteringLithium-ion battery
The invention discloses a solid thin film electrolyte material and a preparation method thereof. The solid thin film electrolyte material has a structure as shown in the formula: Li-(Mn1-Xm1x)-(Til-yM2y)-O, wherein, 0<=x<=1, 0<=y<=1, M1 is selected from at least one of La, Sr, Na, Nd, Pr, Sm, Gd, Dy, Y, Eu, Tb and Ba, and M2 is selected from at least one of Mg, W, Al, Ge, Ru, Nb, Ni, Ta, Co, Fe, Zr, Hf, Fe, Cr and Ga. The solid thin film electrolyte material is obtained by using a sol-gel method or radio frequency magnetron sputtering method. The solid thin film electrolyte material has relatively high Li ion conductivity, relatively low electron conductivity and favorable thermodynamic stability, and is particularly suitable for producing solid lithium ion batteries.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Nonaqueous solvent, nonaqueous electrolyte, and power storage device
InactiveUS20140377644A1Improve conductivityLess likely to volatilizeOrganic electrolyte cellsSecondary cellsQuaternary ammonium cationSolvent
A nonaqueous solvent that includes an ionic liquid and has at least one of the following characteristics: high lithium ion conductivity, high lithium ion conductivity in a low temperature environment, high heat resistance, a wide available temperature range, a low freezing point (melting point), low viscosity, and the like. The nonaqueous solvent includes an ionic liquid and a fluorinated solvent. The ionic liquid contains an alicyclic quaternary ammonium cation which has a substituent and a counter anion to the alicyclic quaternary ammonium cation which has the substituent.
Owner:SEMICON ENERGY LAB CO LTD
Negative-pole active material and preparation method thereof as well as lithium ion battery using negative-pole active material
ActiveCN104752721AHigh specific capacityFast embeddingCell electrodesSecondary cellsLithium electrodeMaterials science
The invention discloses a negative-pole active material. The negative-pole active material comprises a core material and a cladding material formed outside the core material, wherein the cladding material is constituted by a first material and a second material cladding outside the first material, and the first material can form an alloy with lithium; ionic conductance of the second material is 10<-5>-10<-4>S / cm, electronic conductivity is lower than 10<-10>S / cm. In addition, the invention discloses a preparation method of the negative-pole active material and a lithium ion battery using the negative-pole active material. The negative-pole active material has the advantages that the cycle performance and charge-discharge efficiency of a lithium ion battery is improved, and the service life of the battery is prolonged.
Owner:BYD CO LTD
Practical carbon dioxide reduction film electrolyzer and preparation method thereof
ActiveCN111088504AHigh mechanical strengthImprove ionic conductivityCellsElectrode shape/formsPtru catalystElectrolysis
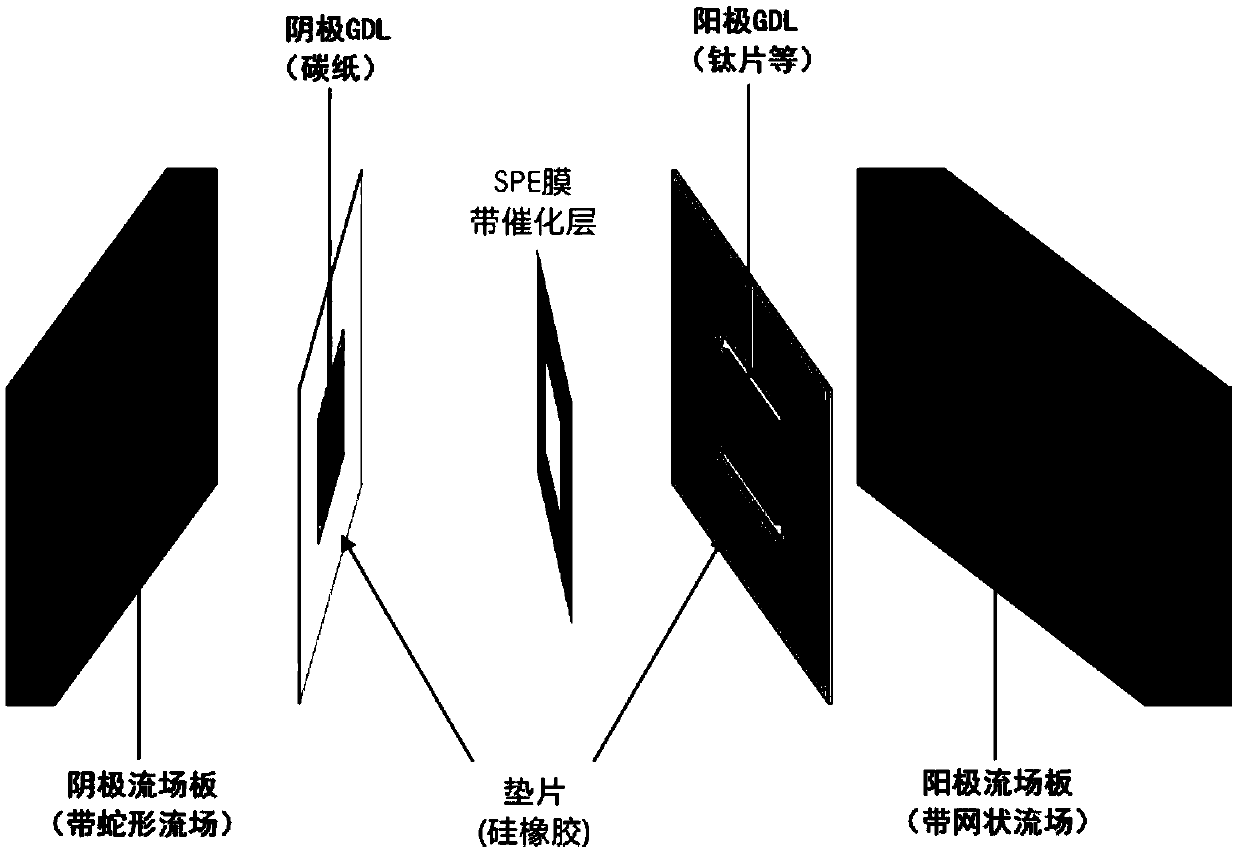
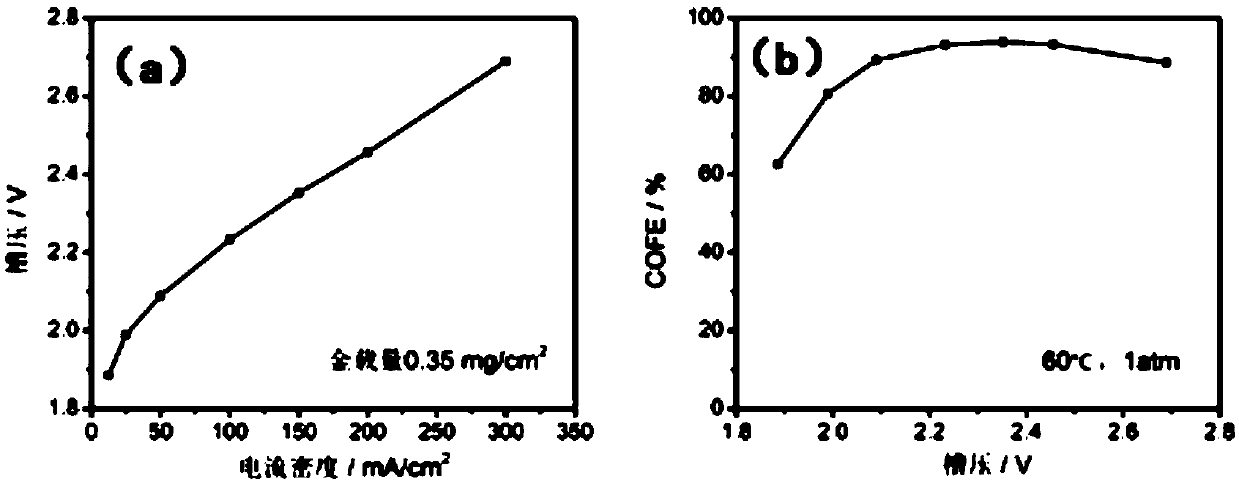
The invention discloses a practical carbon dioxide reduction film electrolyzer and a preparation method thereof, and belongs to the field of electrocatalytic carbon dioxide reduction. The film electrolyzer comprises a cathode flow field plate, a cathode gasket, a cathode diffusion layer, a membrane electrode, an anode diffusion layer, an anode gasket and an anode flow field plate which are assembled in sequence, wherein the membrane electrode comprises an SPE membrane, a cathode catalyst and an anode catalyst, the cathode catalyst and the anode catalyst are located on the two sides of the SPEmembrane respectively, the cathode flow field plate is smooth in a snake shape, and the anode flow field plate is a net-shaped flow field. The method is simple and safe to operate, the electrolyzer works efficiently and stably, the performance of the electrolyzer reaches a high level in the same field, for example, hundreds of milliamperes of current density can be achieved through a low cell voltage, the selectivity exceeds 90%, and the like, and the electrolyzer has novelty, practicability and wide prospects.
Owner:WUHAN UNIV
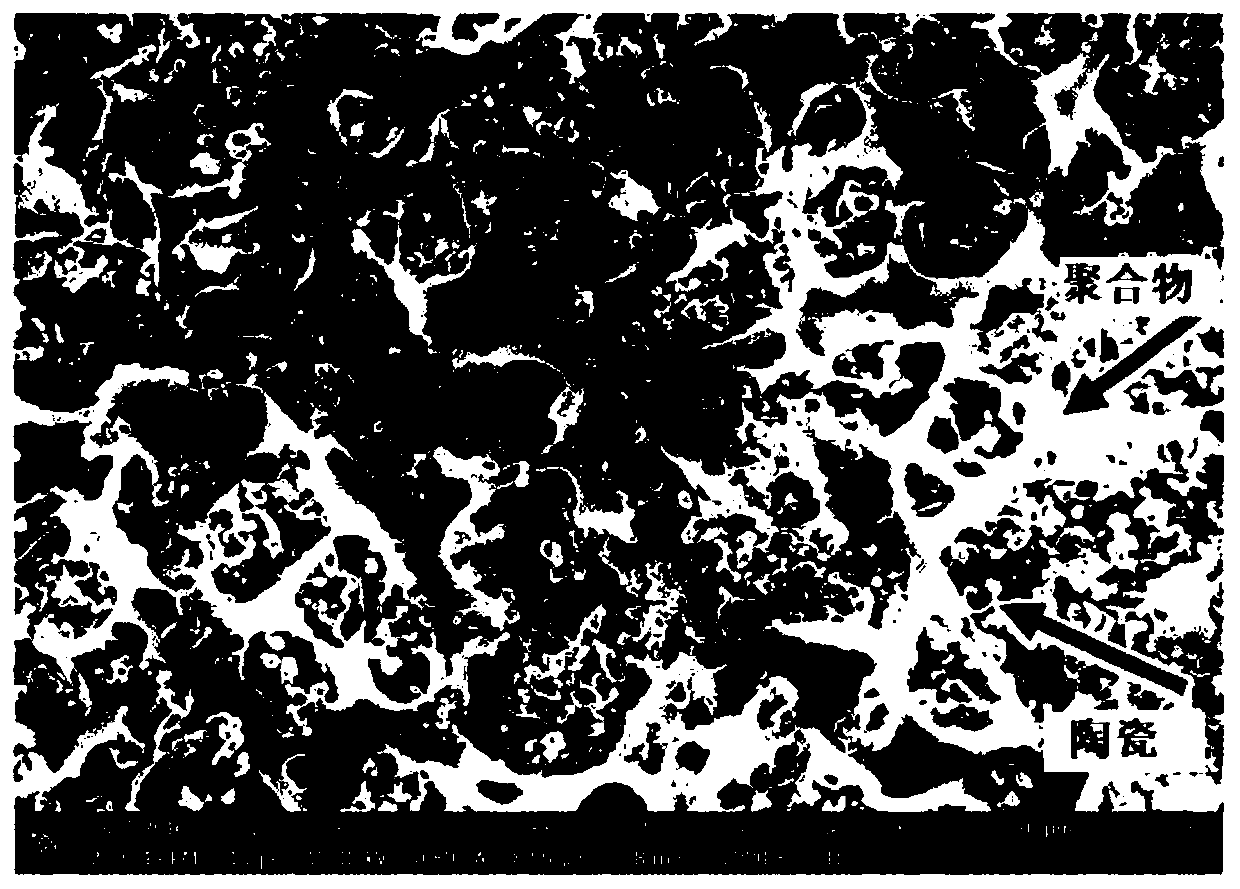
Multi-layer structured composite electrolyte and secondary battery using same
PendingCN110495037ALow ionic conductivityFix stability issuesSolid electrolytesLi-accumulatorsComposite electrolytePhysical chemistry
The present invention relates to a multi-layer structured composite electrolyte for a secondary battery and a secondary battery using the same. The multi-layer structured composite electrolyte of thepresent invention is prepared by laminating two or more layers of composite electrolyte containing a small amount of liquid electrolyte in addition to a mixture of a polymer and a ceramic material. The multi-layer structured composite electrolyte of the present invention has equal or superior electrochemical characteristics compared with a liquid electrolyte while having equal stability to the solid electrolyte. The multi-layer structured composite electrolyte of the present invention can also be used in wearable apparatuses since the multi-layer structured composite electrolyte can be arbitrarily folded.
Owner:SEVEN KING ENERGY
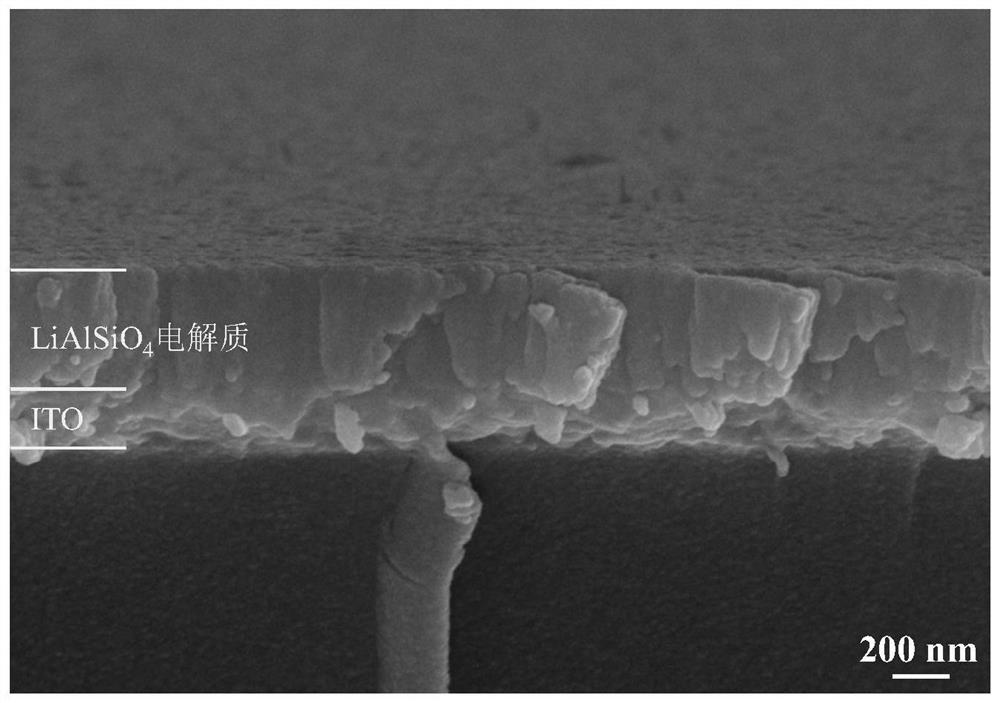
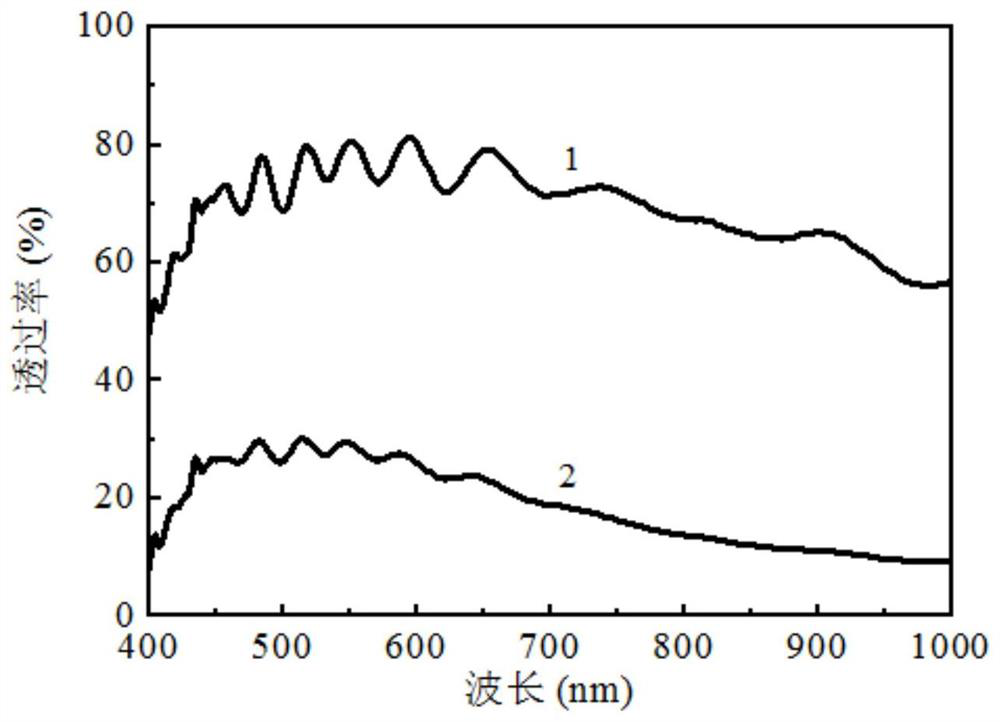
All-solid-state electrochromic device taking lithium aluminum silicate as electrolyte layer and preparation method of all-solid-state electrochromic device
PendingCN112285982AImprove stabilityImprove ionic conductivityNon-linear opticsAll solid stateAluminum silicate
The invention discloses an all-solid-state electrochromic device taking lithium aluminum silicate as an electrolyte layer and a preparation method of the all-solid-state electrochromic device, and relates to an electrochromic device and a preparation method thereof. The invention aims to solve the problems that the fading response speed of an existing all-solid-state electrochromic device is low,the transmittance modulation range of the device is small and the preparation cost is high. According to the method, the all-solid-state electrochromic device with the lithium aluminum silicate as theelectrolyte layer is composed of a substrate, a lower transparent conductive layer, an ion storage layer, a lower protective layer, the electrolyte layer, an upper protective layer, an electrochromiclayer and an upper transparent conductive layer which are sequentially arranged from bottom to top. The method comprises the steps: 1, depositing the lower transparent conductive layer, the ion storage layer and the lower protective layer by adopting a magnetron sputtering method; 2, depositing the electrolyte layer by adopting a magnetron sputtering method; and 3, depositing the upper protectivelayer, the electrochromic layer and the upper transparent conductive layer by adopting a magnetron sputtering method. According to the invention, the all-solid-state electrochromic device taking lithium aluminum silicate as the electrolyte layer can be obtained.
Owner:HARBIN INST OF TECH
Lithium-ion battery electrolyte and lithium-ion battery containing electrolyte
InactiveCN105428716AGood gas productionReduce gas productionSecondary cellsOrganic electrolytesOrganic solventPhosphate
The invention discloses lithium-ion battery electrolyte. The electrolyte comprises LiPF6, a non-aqueous organic solvent and additives, wherein the additives comprise fluoroalkenyl phosphate compounds and LiBOB, the structural general formula of the fluoroalkenyl phosphate compounds is the general formula I or the general formula II shown in the specification; according to the general formula, when R1 represents alkyl with 1-5 carbon atoms, R2 represents fluoroalkenyl, and when R1 represents fluoroalkyl, R2 represents any one of alkenyl with 2-5 carbon atoms and fluoroalkenyl with 2-5 carbon atoms. The flammability of the lithium-ion battery electrolyte is low, the gas production capacity of the lithium-ion battery prepared from the electrolyte is small in the charging and discharging processes, and the battery can be effectively prevented from expanding and has excellent high-temperature cycle performance.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
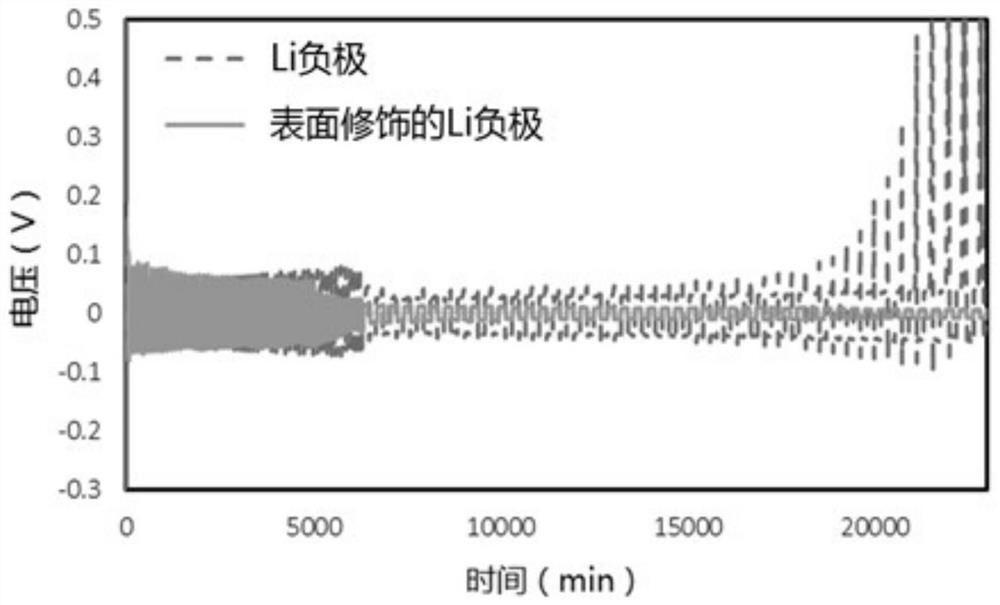
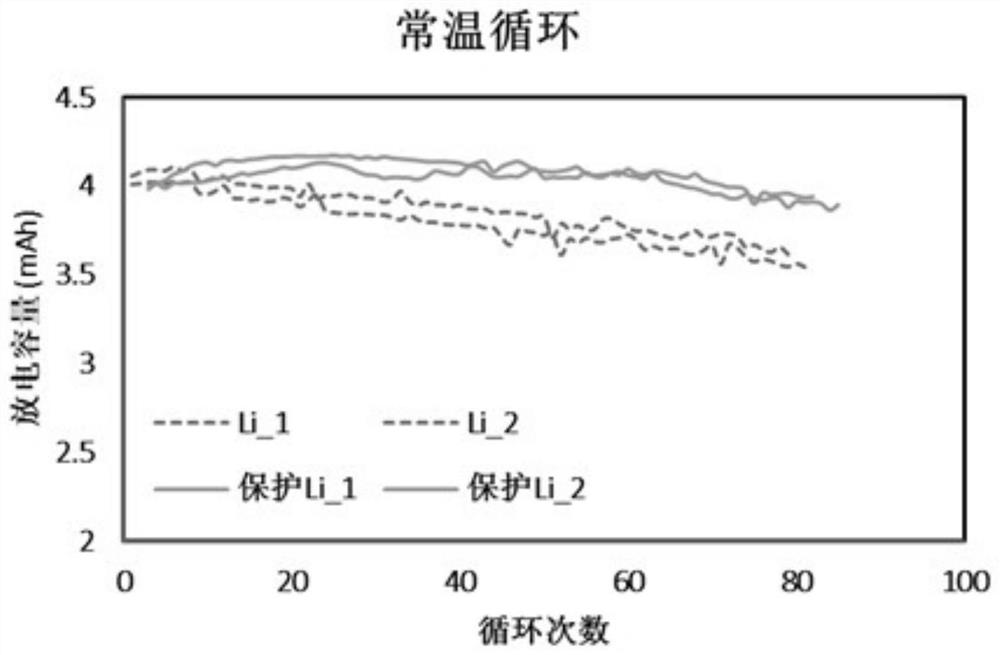
Negative electrode surface protection method suitable for solid-state lithium battery and secondary lithium battery
PendingCN113410437AReduced responseImprove thermal stabilityNegative electrodesSecondary cells servicing/maintenanceMetallic lithiumOrganic solvent
The invention provides a negative electrode surface protection method suitable for a solid-state lithium battery and a secondary lithium battery. The negative electrode surface protection method comprises the following steps: adding a polymer or a composite material into an organic solvent to prepare a thin film solution; coating the film solution on the surface of a substrate, and volatilizing and drying for a period of time to obtain a polymer film; transferring the polymer film to the surface of a metal lithium negative electrode; sequentially adding a lithium salt and an additive into an organic solvent according to a certain proportion to prepare an infiltration solution; and coating the metal lithium negative electrode of which the surface is coated with the polymer film with the infiltration solution, infiltrating for a period of time, removing the redundant infiltration solution on the metal lithium negative electrode, and drying for a period of time to obtain the protection layer. The invention has the beneficial effects that the interface side reaction can be effectively inhibited, the current density of the surface of the metal lithium negative electrode is uniform, the generation condition of lithium dendrites in the charging and discharging process is improved, and the cycling stability of the metal lithium negative electrode is improved.
Owner:天津中电新能源研究院有限公司 +1
Method for preparing Fe-element doped barium cerate solid electrolyte by using sol-gel self-combustion method
InactiveCN110218091AImprove conductivityGreat application valueFinal product manufactureFuel cellsMuffle furnaceIon
The invention discloses a method for preparing Fe-element doped barium cerate solid electrolyte by using a sol-gel self-combustion method and relates to the technical field of solid electrolyte material preparation. The method comprises the following steps: dissolving cerous nitrate, barium nitrate and ferric nitrate into distilled water according to a BaCe1-xFexO3-x metal element ratio, and conducting uniform stirring so as to obtain a mixed solution; adding citric acid and ethylene glycol into the mixed solution, weighing the components, and uniformly stirring the components in the mixed solution; adjusting the pH value of the system, conducting heating stirring so as to obtain foamed gel, and drying the gel so as to obtain dry gel; putting the dry gel into a muffle furnace, heating andpresintering the dry gel, and keeping the temperature so as to obtain presintered loosened powder; and grinding the presintered loosened powder into fine powder, adding an adhesive into the fine powder, conducting tabletting, and finally conducting sintering. A series of electrolyte materials prepared by using the method have good and low conductive activation energy and ion conductivity and havegood electrolyte material properties, and feasibility of a Fe-element doped barium cerate-based electrolyte material is proved.
Owner:HEFEI UNIV
Solid electrolyte material, and preparation method and application thereof
PendingCN113948763AImproved electrochemical oxidation potentialImprove stabilitySecondary cells servicing/maintenanceElectrolyte immobilisation/gelificationSolid state electrolyteRare-earth element
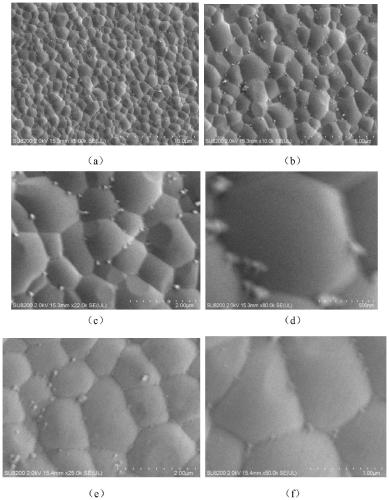
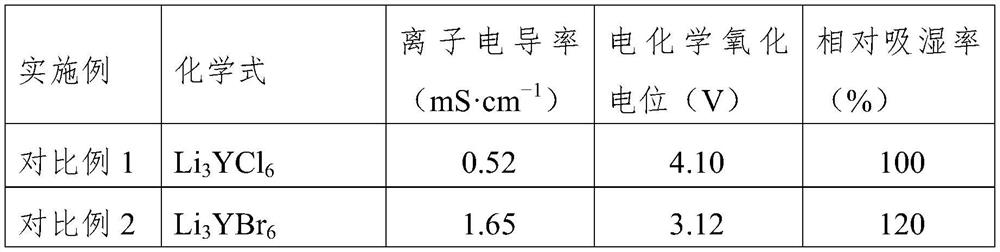
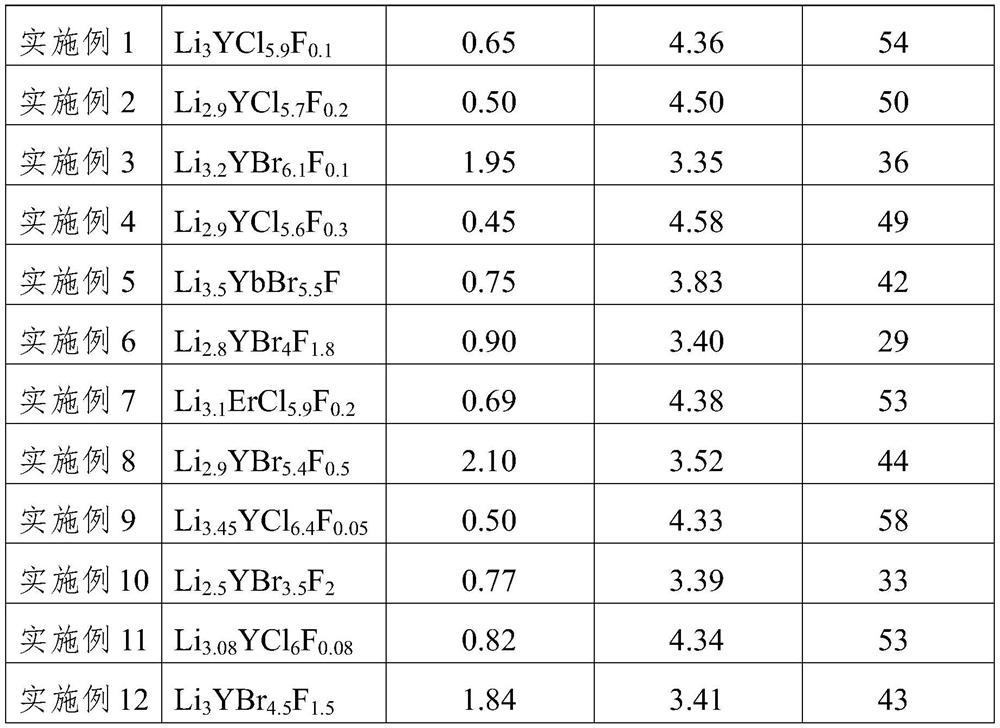
The invention relates to a solid electrolyte material, and a preparation method and application thereof. The chemical general formula of the material is LiaREXbFc, wherein RE is at least one of rare earth elements Y, Er and Yb, X is one or two of Cl and Br, 2.5 <= a <= 3.5, 3.5 <= b < 6.5, and 0 < c <= 2. The material improves the performance of the rare earth halide solid electrolyte material, and especially improves the electrochemical oxidation potential and air stability of the rare earth halide solid electrolyte material.
Owner:RARE EARTH FUNCTIONAL MATERIALS XIONG AN INNOVATION CENT CO LTD +1
Lithium-sulfur battery modified diaphragm and preparation method thereof
ActiveCN113725558AIncreased tortuosityImprove microporous structureLi-accumulatorsCell component detailsPtru catalystLithium–sulfur battery
The invention provides a lithium-sulfur battery modified diaphragm and a preparation method thereof, the modified diaphragm comprises a diaphragm matrix, the surface of the diaphragm matrix is coated with a conductive coating, and the conductive coating comprises a conductive skeleton and a polysulfide adsorbent and a catalyst loaded on the conductive skeleton; and the conductive skeleton is mainly prepared from a zero-dimensional conductive carbon material, a one-dimensional conductive carbon material and a two-dimensional conductive carbon material, and has a microporous structure. The preparation method comprises the following steps: uniformly mixing a polysulfide adsorbent, a zero-dimensional conductive carbon material, a one-dimensional conductive carbon material, a two-dimensional conductive carbon material, a catalyst, a high-molecular polymer, pure water and a polar organic solvent to obtain modified diaphragm slurry, coating a diaphragm substrate with the modified diaphragm slurry to obtain a coated diaphragm, transferring the coated diaphragm into pure water, and drying the coating diaphragm from which the solvent is removed. According to the modified diaphragm for the lithium-sulfur battery, the migration path of polysulfide can be prolonged, shuttling of the polysulfide in the diaphragm for the lithium-sulfur battery can be effectively inhibited, and the cycle performance of the lithium-sulfur battery is improved.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
Molten steel dearsenication fluxing agent, and preparation method and application method thereof
InactiveCN103642990AImproved stability and metallurgical effectImprove working environmentProcess efficiency improvementAluminiumMaterials science
The invention belongs to the field of metallurgy, and particularly relates to a molten steel dearsenication fluxing agent, and a preparation method and application method thereof. The molten steel dearsenication fluxing agent comprises the following components in percentage by mass: 88-95% of CaO, 5-12% of Li2O and the balance (0-5%) of impurity. After the molten steel is subjected to pre-deoxidation and desulfurization, [O] and [S] in the molten steel are respectively lowered to 0.001%; the dearsenication fluxing agent and aluminum-calcium alloy are added into a ladle together, wherein the addition amount of the dearsenication fluxing agent is calculated according to the principle that 10Kg of dearsenication fluxing agent can lower the arsenic content in every 100 ton of molten steel by 0.01%, and the addition amount of the aluminum-calcium alloy is 50 wt% of the dearsenication fluxing agent; and after dearsenication, the arsenic content in the molten steel is lowered to 0.001% below. Compared with the prior art, the dearsenication fluxing agent has the advantages of high dearsenication capacity, no pollution and high resource utilization ratio, and the dearsenication product does not pollute the molten steel, thereby solving the key problems in the existing dearsenication technique.
Owner:JIANGSU UNIV
Current collector modification method, and modified current collector and application thereof
ActiveCN113036071ALow cycle lifeImprove cycle lifeElectrode thermal treatmentElectrode carriers/collectorsEngineeringHeat conservation
The invention provides a current collector modification method, and a modified current collector and application thereof. The method comprises the following steps: mixing metal oxide powder with alkali metal amino salt, conducting heating to 280-350 DEG C under the condition of protective gas, and carrying out heat preservation for 3-6 hours to implement a nitridation reaction so as to obtain metal nitride; preparing turbid liquid from the metal nitride; and placing a foam metal current collector into the turbid liquid, conducting heating to 50-80 DEG C under the condition of protective gas or vacuum used by the nitridation reaction, and keeping the temperature for 10-20 minutes so as to allow the nitride to be loaded on the foam metal, thereby finishing the modification of the current collector. According to the nitride modified current collector provided by the invention, in a coulombic efficiency test, cycle life is 155-280 circles, fitting impedance is 21-33 ohm after ten circles of circulation, cycle life is long, the impedance is low, and good performance is obtained; and meanwhile, a relatively long service life of 660-1000 h can be obtained in a symmetrical battery test.
Owner:JIANGHAN UNIVERSITY
Preparation method of composite solid electrolyte
PendingCN114695951ALow electronic conductivityGood electrochemical stabilitySecondary cellsRadio frequencyRadio frequency plasma
The invention discloses a preparation method of a composite solid electrolyte for inhibiting growth of lithium dendrites, which comprises the following steps of: heating a solid electrolyte in a plasma enhanced chemical vapor deposition device, introducing a mixed gas of nitrogen, hydrogen and methane to start a radio frequency plasma source, stopping introducing hydrogen and methane after closing radio frequency plasma, and cooling to room temperature to obtain the composite solid electrolyte for inhibiting growth of lithium dendrites. The method comprises the following steps: introducing nitrogen, cooling, taking out a sample, selecting a pure copper target, pre-sputtering to clean the surface of the target, mixing nitrogen and argon by adopting a direct current sputtering mode, preparing the sample and metal lithium into a symmetrical battery after the growth reaction is finished, and reacting the sample with Li < + > to generate Li3N and copper nanoparticles in the charging and discharging process, the copper nanoparticles which are well dispersed form a uniform electric field at an interface, and graphene is used as a three-dimensional structure interface of the substrate, has a high specific surface area and a porous structure, and can also effectively reduce the current density and relieve the volume effect, so that metal lithium is uniformly deposited, and the growth of lithium dendrites is effectively inhibited.
Owner:WUHAN MARINE ELECTRIC PROPULSION RES INST CHINA SHIPBUILDING IND CORP NO 712 INST
Solid electrolyte, all solid battery, and manufacturing method of all solid battery
ActiveUS20210376376A1Improve ionic conductivityRaise the sintering temperatureSolid electrolytesFinal product manufactureSolid state electrolyteAll solid state
Solid electrolyte includes a first solid electrolyte that is a phosphate salt including Li and Ta, and a second solid electrolyte that is NASICON type solid electrolyte. In a cross section of the solid electrolyte, an area ratio of the first solid electrolyte is more than 10% and an area ratio of the second solid electrolyte is more than 10%.
Owner:TAIYO YUDEN KK
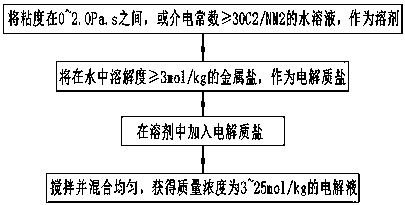
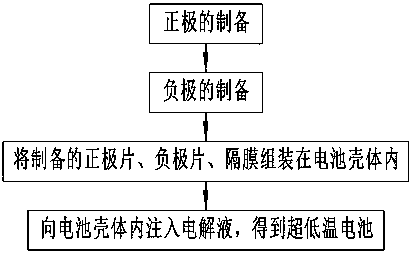
Ultralow-temperature electrolyte solution as well as preparation method thereof, battery using ultralow-temperature electrolyte solution and preparation method thereof
The invention belongs to the technical field of battery electrolyte solutions, and specifically relates to an ultralow-temperature electrolyte solution as well as a preparation method thereof, a battery using the ultralow-temperature electrolyte solution and a preparation method thereof. The invention aims to provide the ultralow-temperature electrolyte solution as well as the preparation method thereof, the battery using the ultralow-temperature electrolyte solution and the preparation method thereof, which can perform large-multiplying-power charging and discharging under an ultralow-temperature environment. According to the technical scheme adopted by the invention, the ultralow-temperature electrolyte solution is obtained as follows: electrolyte salt is added into a solvent to obtain the electrolyte solution with mass concentration of 3-25 mol / kg, wherein the solvent is an aqueous solution with viscosity of 0-2.0 Pa.s, or a dielectric constant being greater than or equal to 30 C<2> / MM<2>; and the electrolyte salt is metal salt with solubility being greater than or equal to 3 mol / kg.
Owner:闫博
Molten steel dearsenication fluxing agent, and preparation method and application method thereof
InactiveCN103642990BStrong arsenic removal abilityImproved ability to remove arsenicProcess efficiency improvementAlloyMolten steel
Owner:JIANGSU UNIV
A lithium-air battery structure
ActiveCN104716405BAvoid erosionPromotes the electrochemical reduction reactionFuel and secondary cellsCell electrodesElectrochemical responsePorous carbon
The invention relates to a lithium-air battery structure, comprising a lithium negative electrode, a porous diaphragm, and a positive electrode stacked in sequence, a conductive porous functional layer is arranged between the lithium negative electrode and the porous diaphragm, and the conductive porous functional layer is a conductive porous carbon material layer or In the composite layer of the conductive porous carbon material and the catalytic component, the mass ratio of the conductive porous carbon material to other functional components is 20:1-2:1. Through electrochemical reaction, the conductive porous functional layer can effectively consume dissolved and diffused oxygen or reactive oxygen species, reduce its corrosion and damage to the lithium anode, and greatly improve the battery stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Strong Basic Polyarylether Ionomer and Its Preparation and Application
ActiveCN102702507BImprove ionic conductivityLow ionic conductivityCell component detailsFuel cell detailsEthyl ChlorideMonomer
The invention discloses a strongly basic polyarylether ionomer, a preparation method and application thereof. This kind of strongly basic polyarylether ionomer is a kind of polymer based on the polymerization of bis(4,4'-hydroxyphenyl)diphenylmethane and chlorine-containing or fluorine-containing aromatic monomers with Ar1 and Ar2 structures. Aromatic ethers are obtained through a series of functionalization processes including chloromethylation, quaternary ammonium salination and alkalization. The basic anion exchange membrane is obtained by casting a chloromethylated polyarylether solution into a membrane and undergoing a series of functionalization processes of quaternization and alkalization. The obtained basic anion exchange membrane has a good barrier effect on methanol, good physical and chemical properties, high thermal stability, and good conductivity at high temperature, which overcomes the unstable properties of the existing polyarylether anion exchange membranes. The low ion conductivity is suitable for alkaline ion exchange membranes in fuel cells and has broad application prospects.
Owner:SOUTH CHINA UNIV OF TECH
Preparation technology of potassium aurous cyanide
InactiveCN110804741ALow ionic conductivitySlow electrolysisElectrolysis componentsElectrolytic organic productionPotassium aurocyanideElectrochemical dissolution
The invention belongs to the technical field of gold plating, and relates to a preparation technology of potassium aurous cyanide. The preparation technology comprises following steps: (1) washing a gold sheet: soaking a gold sheet in diluted nitric acid, washing the gold sheet by pure water, and drying; (2) carrying out electrochemical dissolution: carrying out electrolysis at a temperature of 65to 75 DEG C under a voltage of 6-7 V and a current density of 60-150 A / m2; (3) carrying out cooling and crystallization: carrying out twice cooling and crystallization on the electrolyte, filtering,washing obtained potassium aurous cyanide crystals by pure water until the pH reaches 8-10, and carrying out suction filtration in vacuum; and (4) drying: drying the crystals for 8 to 10 hours at a temperature of 80 to 100 DEG C. The provided preparation technology has the advantages that the electrolysis conditions are reasonable, a good environment is provided for gold electrolysis, the gold electrolytic rate is largely increased and can reach 85% or more; after crystallization, the gold mass percentage can reach 68.3% or more; the quality of prepared potassium aurous cyanide is stable; theproduction cost is controllable, and the economic benefit is good.
Owner:衡阳市晋宏精细化工有限公司
Thin film solid electrolyte material and preparation method thereof
ActiveCN103456983BHigh Li ion conductivityLow ionic conductivitySecondary cellsRadio frequency magnetron sputteringLithium-ion battery
Owner:GUILIN UNIV OF ELECTRONIC TECH
High-entropy alloy oxide coating and preparation method thereof
PendingCN114606457AImprove electronic conductivityLow ionic conductivityMolten spray coatingSolid state diffusion coatingHigh entropy alloysOxidation resistant
The invention discloses a high-entropy alloy oxide and a preparation method thereof, alloy elements are Cu, Mn, Co, Fe and Ni, and a high-entropy alloy oxide coating is divided into three layers, namely a (Mn, Cu) 3O4 spinel layer, a Fe oxide layer and a NiCo oxide layer in sequence from outside to inside; the preparation method of the high-entropy alloy oxide comprises the following steps: mechanically ball-milling and mixing pure metal powder, carrying out plasma spraying on a CuMnCoFeNi high-entropy alloy coating, controlling the thickness of the coating to be 15-30m, and carrying out high-temperature and high-oxygen-pressure thermal growth to form a composite conductive oxide coating. The high-entropy alloy oxide coating prepared through the method is beneficial for inhibiting external diffusion of the Cr element in a ferritic stainless steel matrix, and the high-temperature oxidation resistance of a metal connector is improved; the high-entropy alloy oxide coating with high-concentration CuMnCo elements is applied to the surface of the ferritic stainless steel, so that the coating composite connector has high electron conductivity and low ion conductivity, and the high-temperature application performance of the fuel cell metal connector is improved.
Owner:JIANGSU UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com