Ceramic solid electrolyte and preparation method thereof
A solid-state electrolyte and solid-state electrolysis technology, which is applied in electrolytes, circuits, electrical components, etc., can solve the problems of large-scale preparation difficulties, low electrolyte conductivity, and low sintering temperature, and achieve rapid drying process, high conductivity, and high sintering temperature. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] First take 600ml of deionized water, add 5.53g of citric acid to dissolve, then press Li 7 La 3 Zr 2 o 12 The stoichiometric ratio weighs 4.45g of lithium nitrate, 4.68g of lanthanum nitrate, and 1.12g of zirconium oxynitrate into the above-mentioned citric acid solution, wherein the excess of lithium salt is 10%, stirs the mixed solution for 12h, and then sprays and dries it. The spray feed rate was set at 2r / min, the outlet temperature of the nozzle was 180°C, and the blast pressure was 0.2MPa. The obtained spray material is heated up to 800°C at 1°C / min for sintering, and the sintering time is 6h.
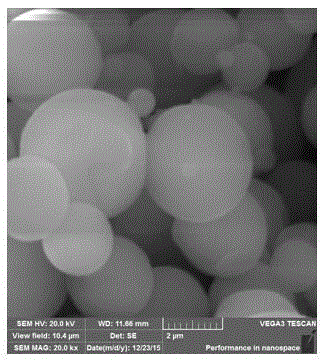
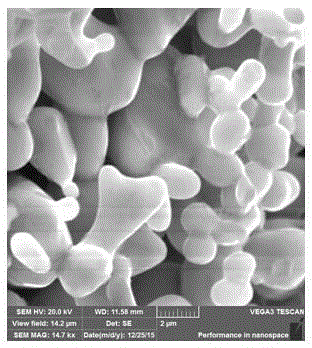
[0032] Morphological characterization of the obtained solid electrolyte and its precursor, such as figure 1 , 2 As shown, it can be seen that the precursor material is spherical, and the morphology changes significantly after sintering, which is due to the increase in crystallinity of the electrolyte at high temperature.
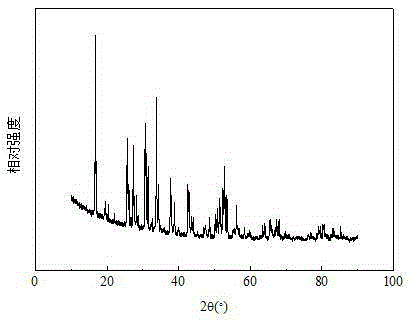
[0033] Carry out XRD test to gained electro...
Embodiment 2
[0036] First take 600ml of deionized water, add 5.53g of citric acid to dissolve, then press Li 7 La 3 Zr 2 o 12 The stoichiometric ratio weighs 4.45g of lithium nitrate, 4.68g of lanthanum nitrate, and 1.12g of zirconium oxynitrate into the above-mentioned citric acid solution, wherein the excess of lithium salt is 10%, stirs the mixed solution for 12h, and then sprays and dries it. The spray feed rate was set at 2r / min, the outlet temperature of the nozzle was 180°C, and the blast pressure was 0.2MPa. The obtained sprayed material is heated up to 800°C at 5°C / min for sintering, and the sintering time is 6h.
Embodiment 3
[0038] First take 600ml of deionized water, add 1.38g of citric acid to dissolve, then press Li 7 La 3 Zr 2 o 12 The stoichiometric ratio weighs 4.45g of lithium nitrate, 4.68g of lanthanum nitrate, and 1.12g of zirconium oxynitrate into the above-mentioned citric acid solution, wherein the excess of lithium salt is 10%, stirs the mixed solution for 12h, and then sprays and dries it. The spray feed rate was set at 2r / min, the outlet temperature of the nozzle was 180°C, and the blast pressure was 0.2MPa. The obtained spray material is heated up to 800°C at 1°C / min for sintering, and the sintering time is 6h.
[0039] Morphological characterization of the obtained solid electrolyte precursor, such as Figure 5As shown, it can be seen that there is a certain difference in the morphology of the precursor material of Example 2 and the precursor of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com