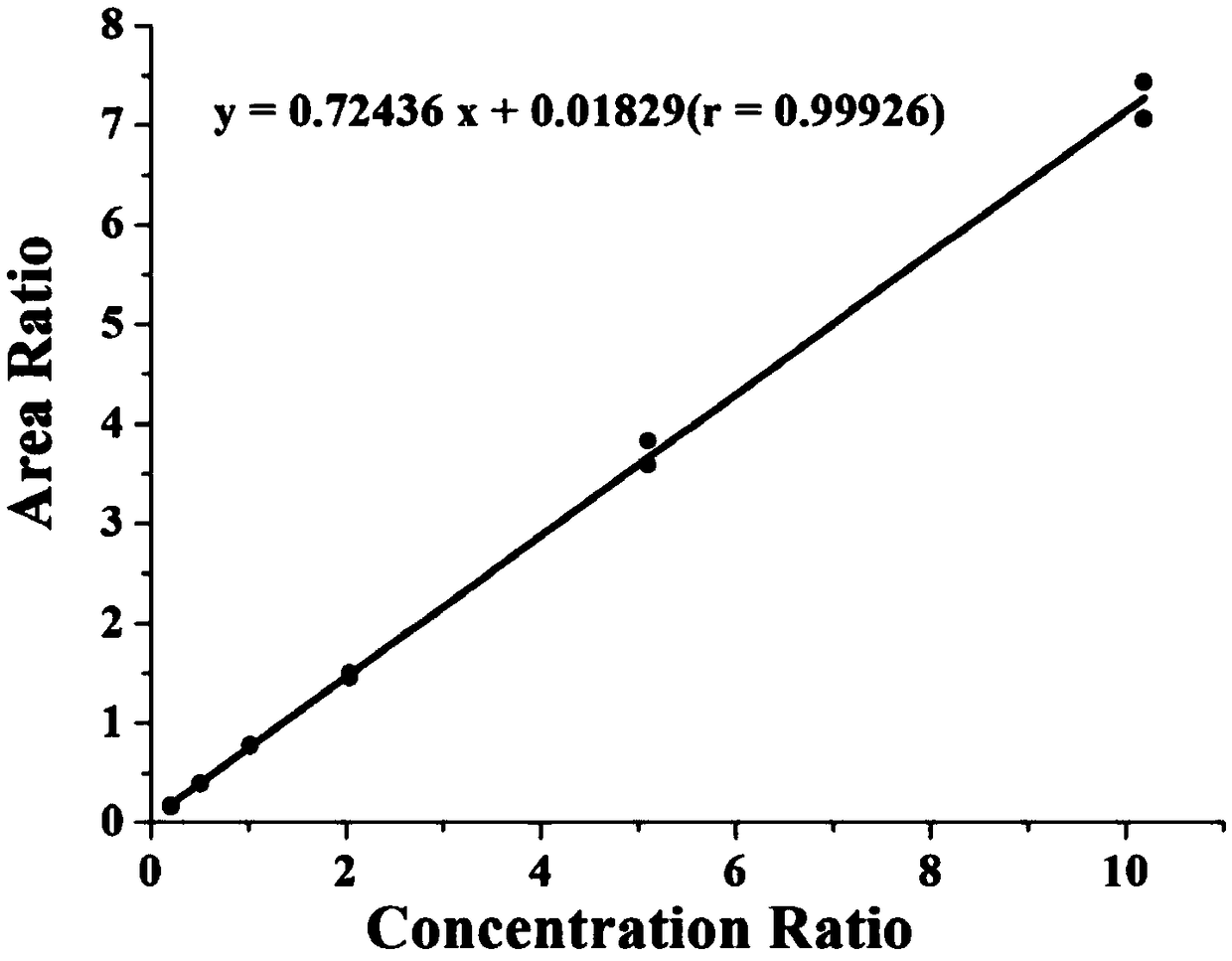
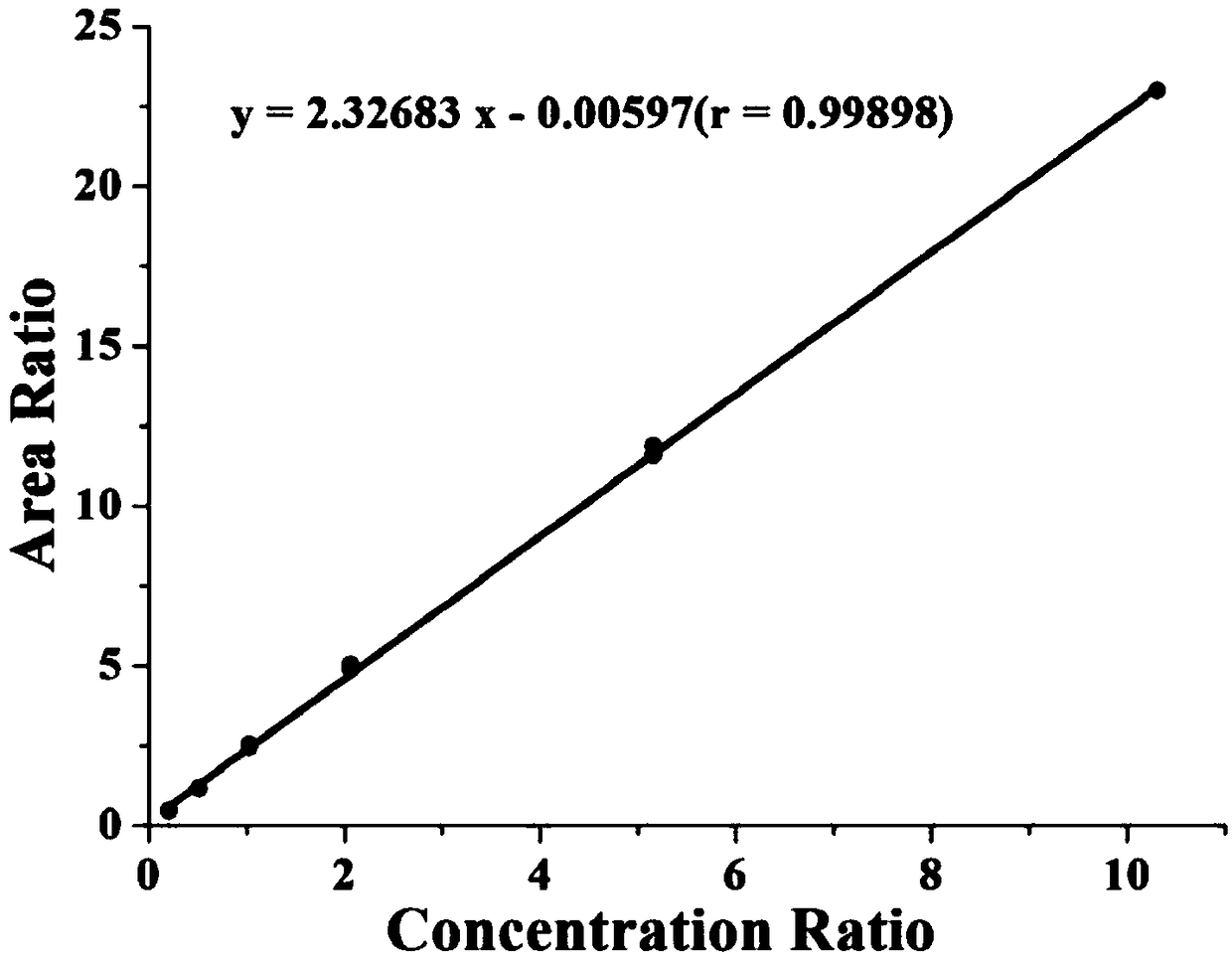
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
175 results about "Everolimus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
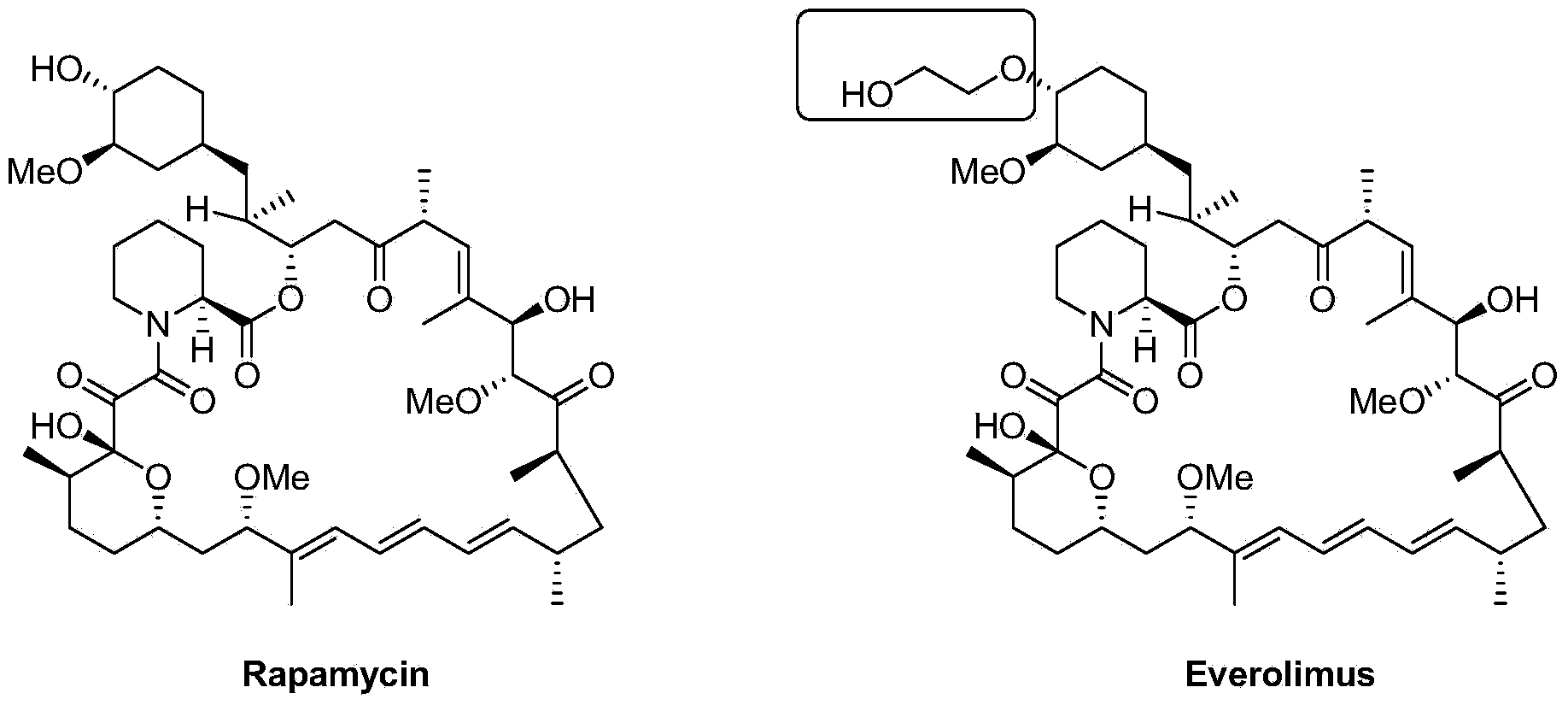
Everolimus is used to treat a certain type of benign (non-cancerous) brain tumor in people with a certain genetic disorder (tuberous sclerosis complex).
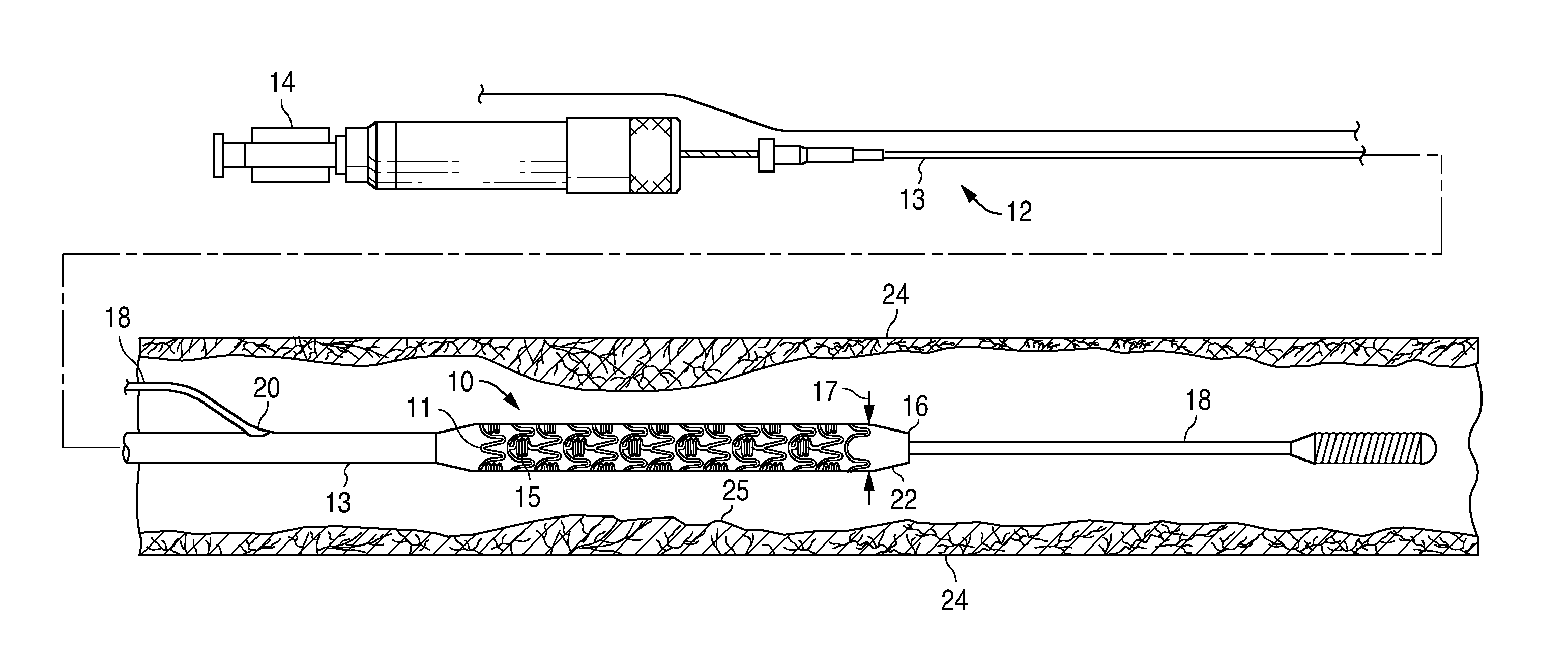
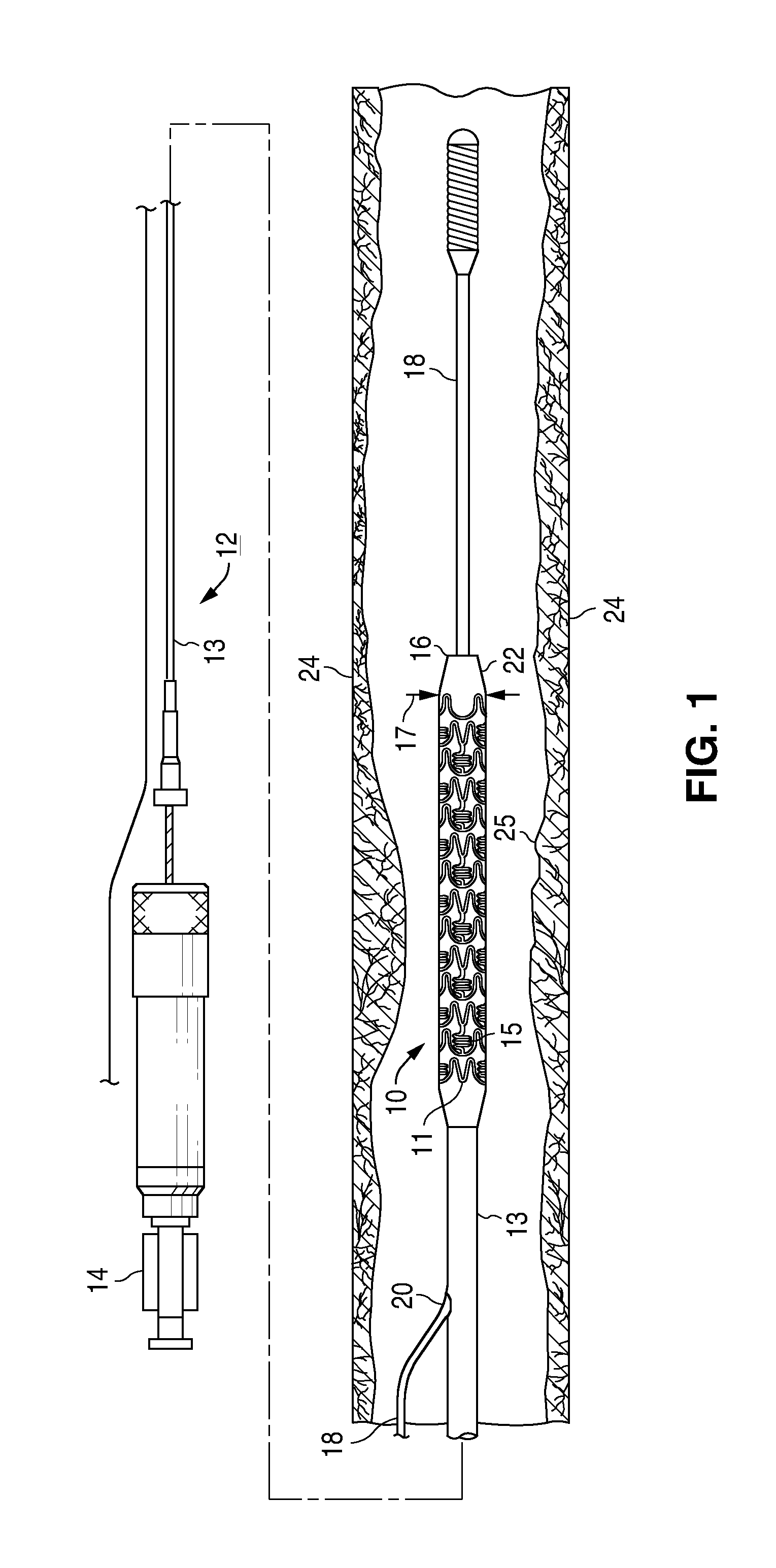
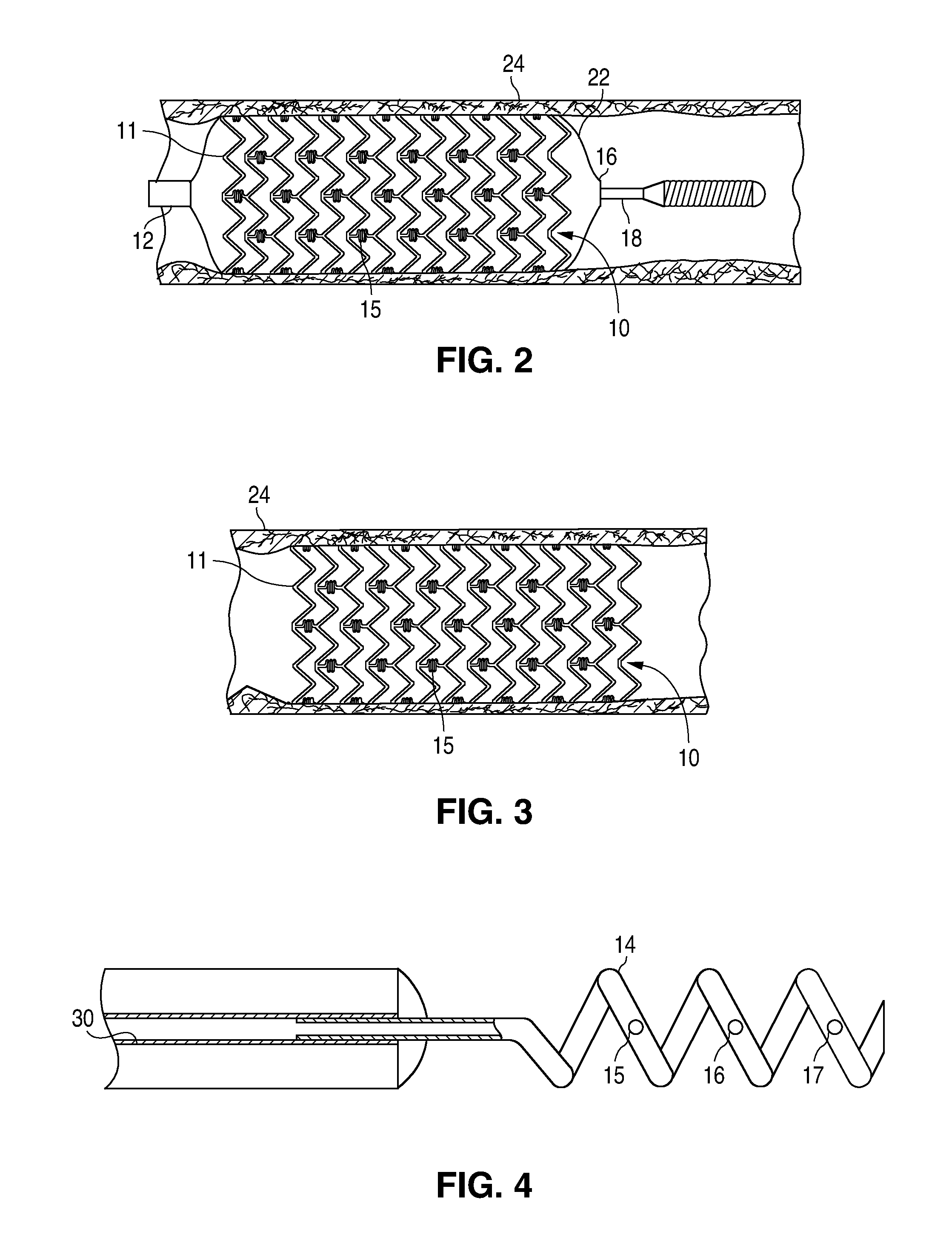
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Drug-delivery stent formulations for restenosis and vulnerable plaque
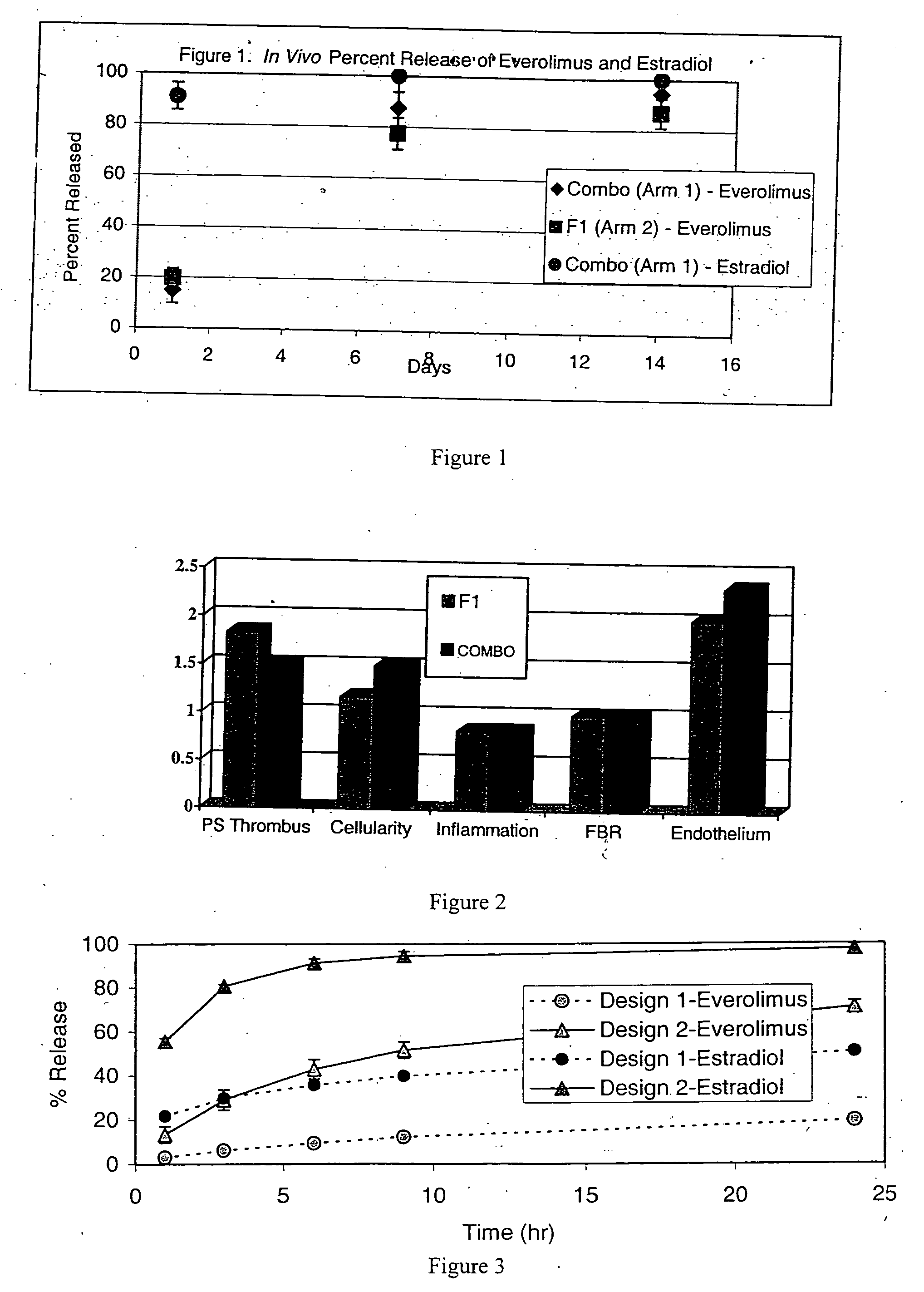
Drug-delivery stents capable of providing release of two or more drugs such as everolimus and estradiol are provided. The stents can be used for treating a disease such as restenosis and vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Composition
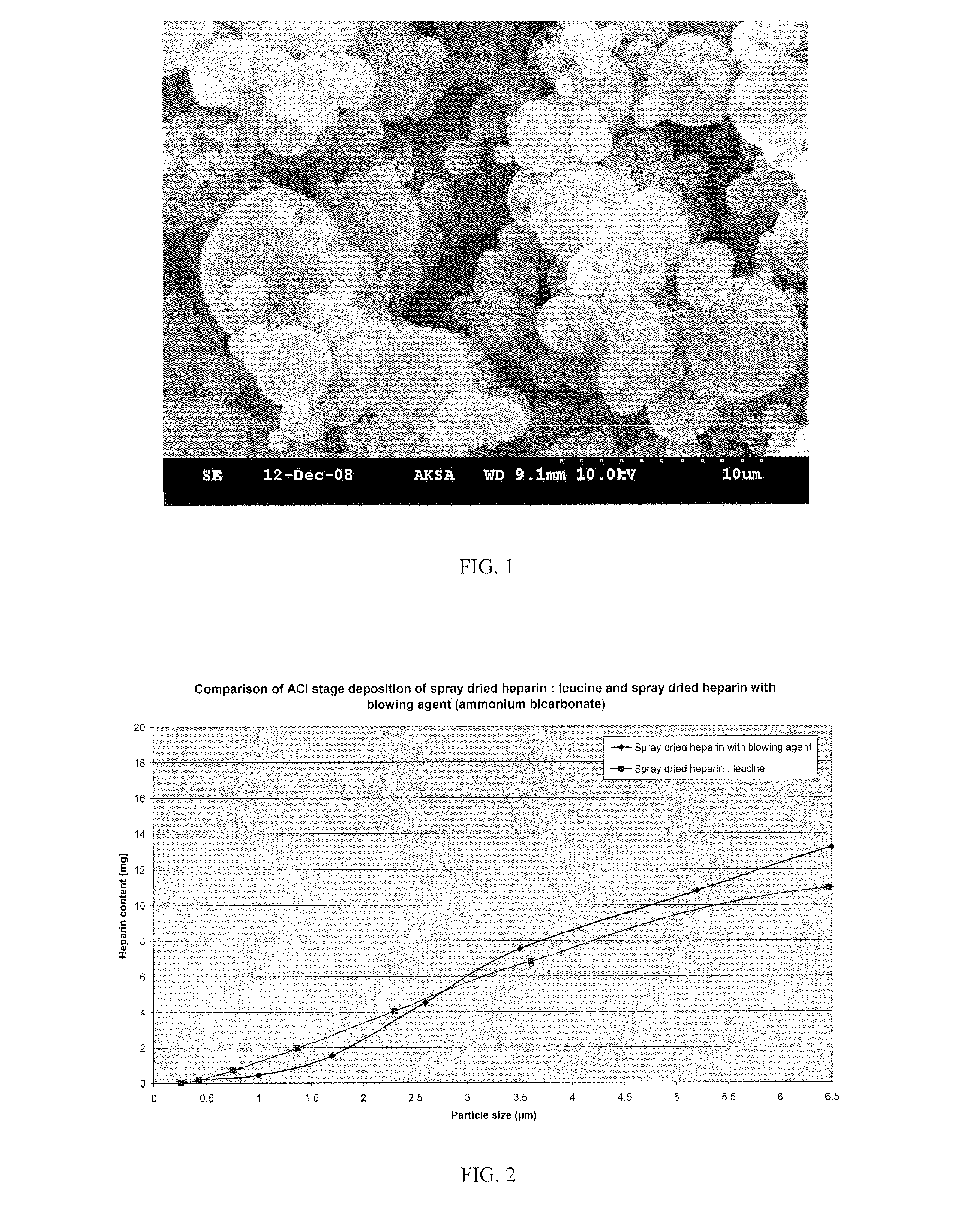
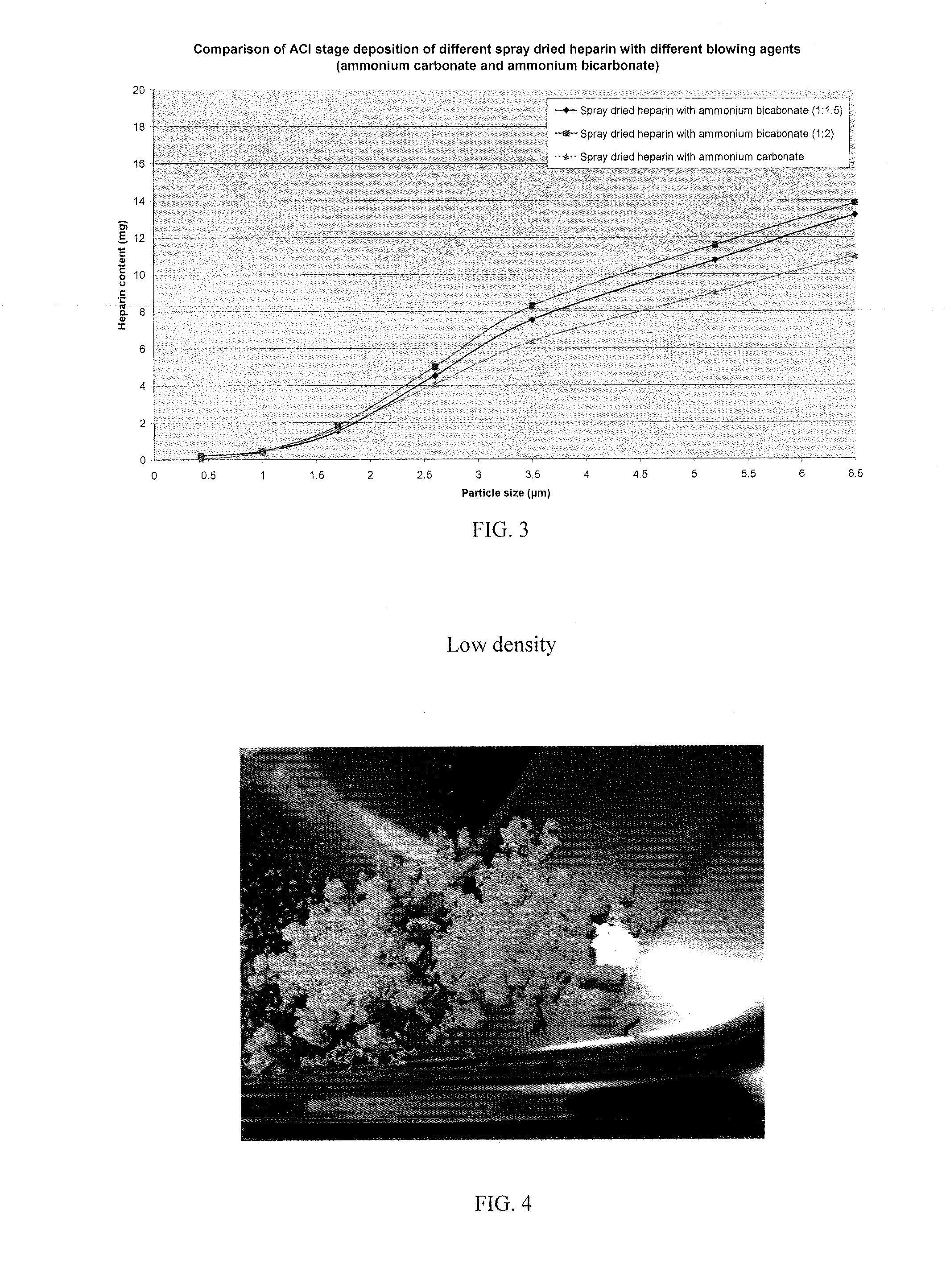
The invention provides microparticles comprising an immunosuppressant, such as tacrolimus, sirolimus, pimecrolimus, ciclosporin, everolimus or a derivative thereof, and optionally a pharmaceutically acceptable excipient or carrier, such as a saccharide, amino acid, a sugar alcohol or a mixture thereof, and having a median geometric diameter of less than, or equal to, about 10 μm and which have a tap density of less than or equal to about 0.3 g / cm3.
Owner:INNOVATA LTD
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders
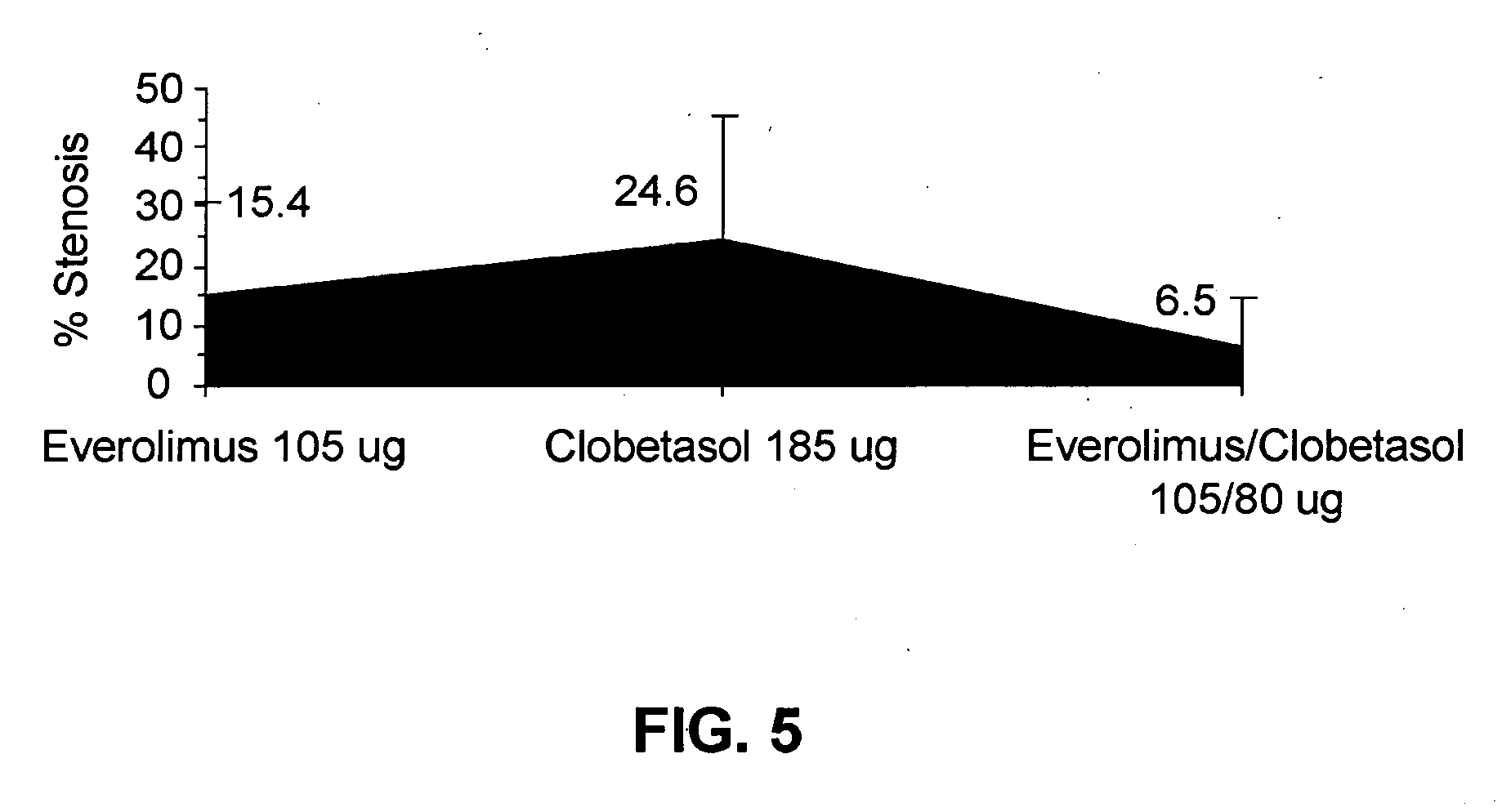
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Ocular solutions
InactiveUS20050063996A1Reduce turbidityReduce inflammationAntibacterial agentsBiocideEverolimusCell migration
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS20060228394A1Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:MINU
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Biosoluble coating comprising Anti-proliferative and Anti-inflammatory agent combination for treatment of vascular disorders
InactiveUS20090297578A1Less neointima thicknessPromote healingBiocideSurgeryEverolimusPercent Diameter Stenosis
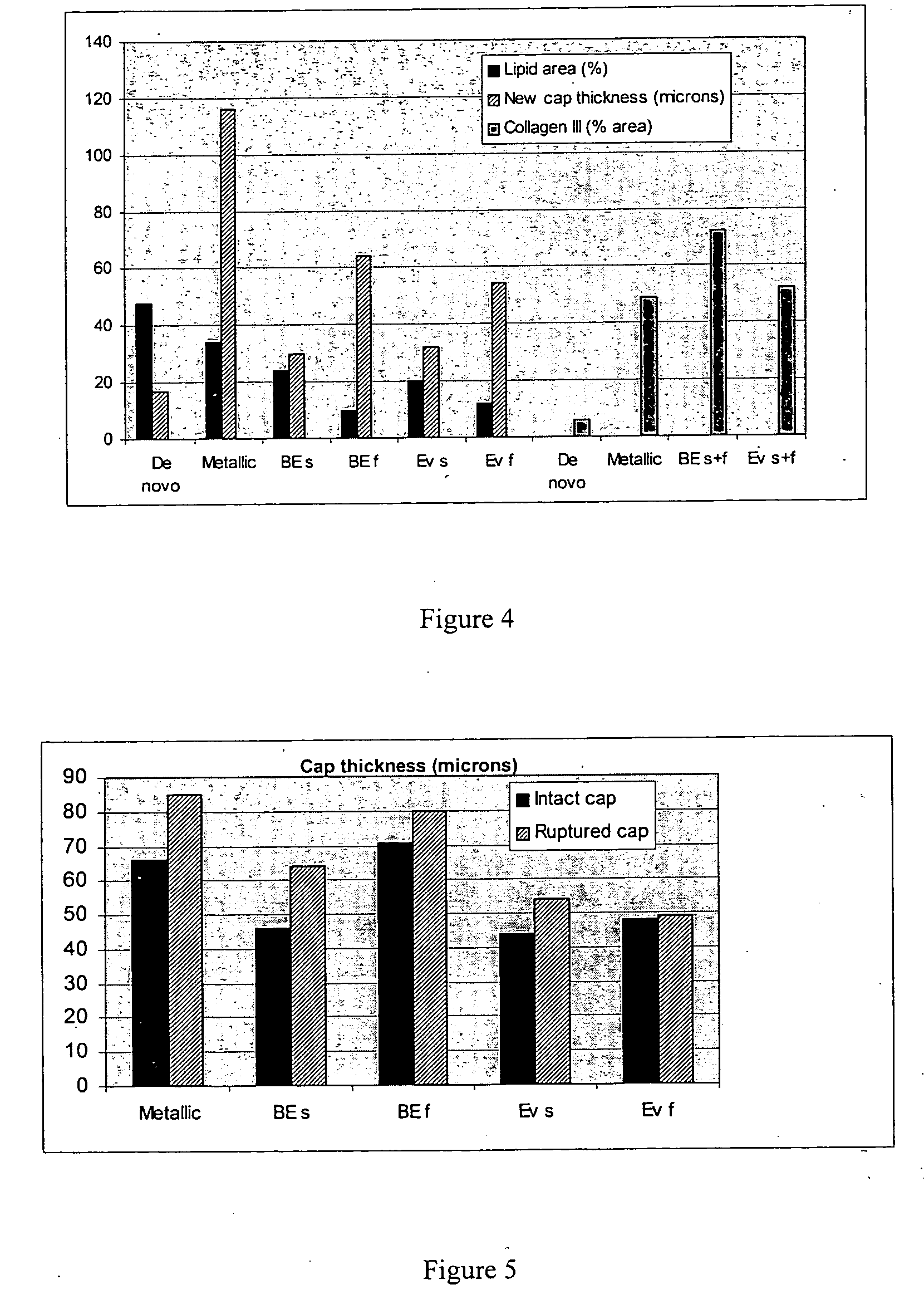
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque
Owner:ABBOTT CARDIOVASCULAR
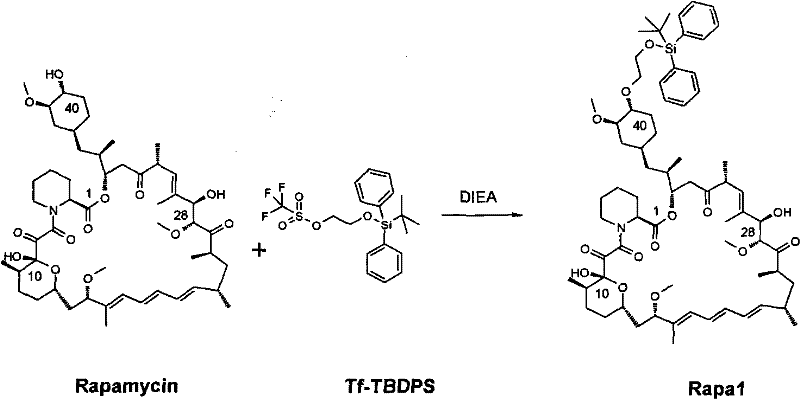
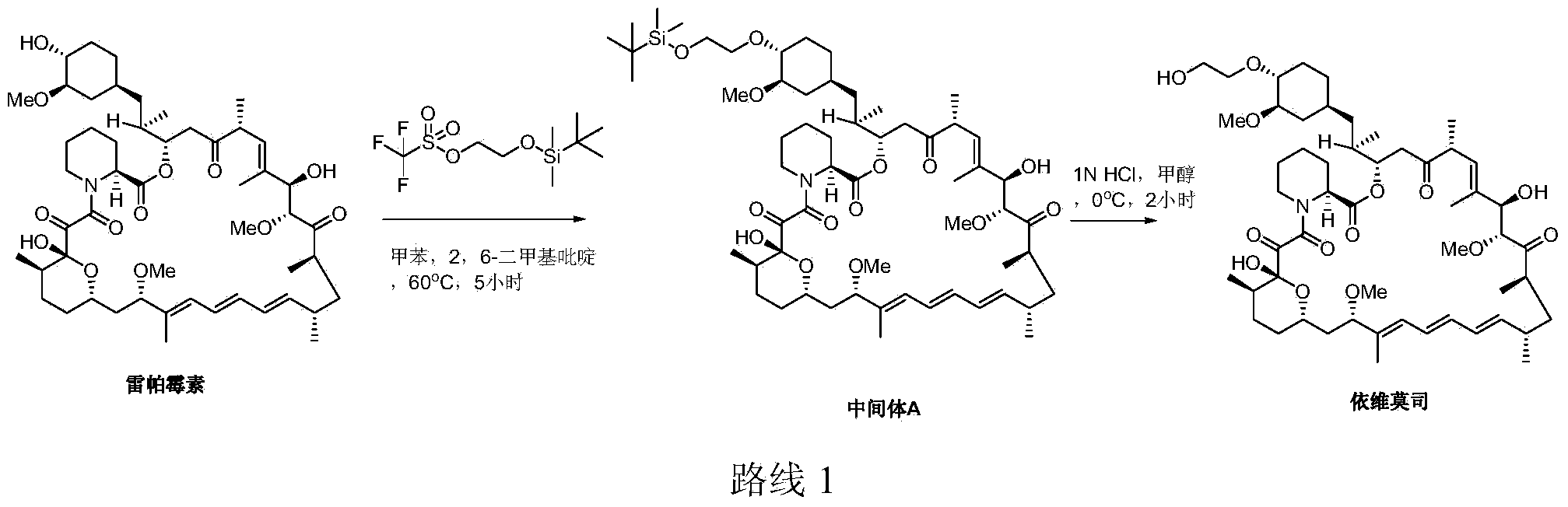
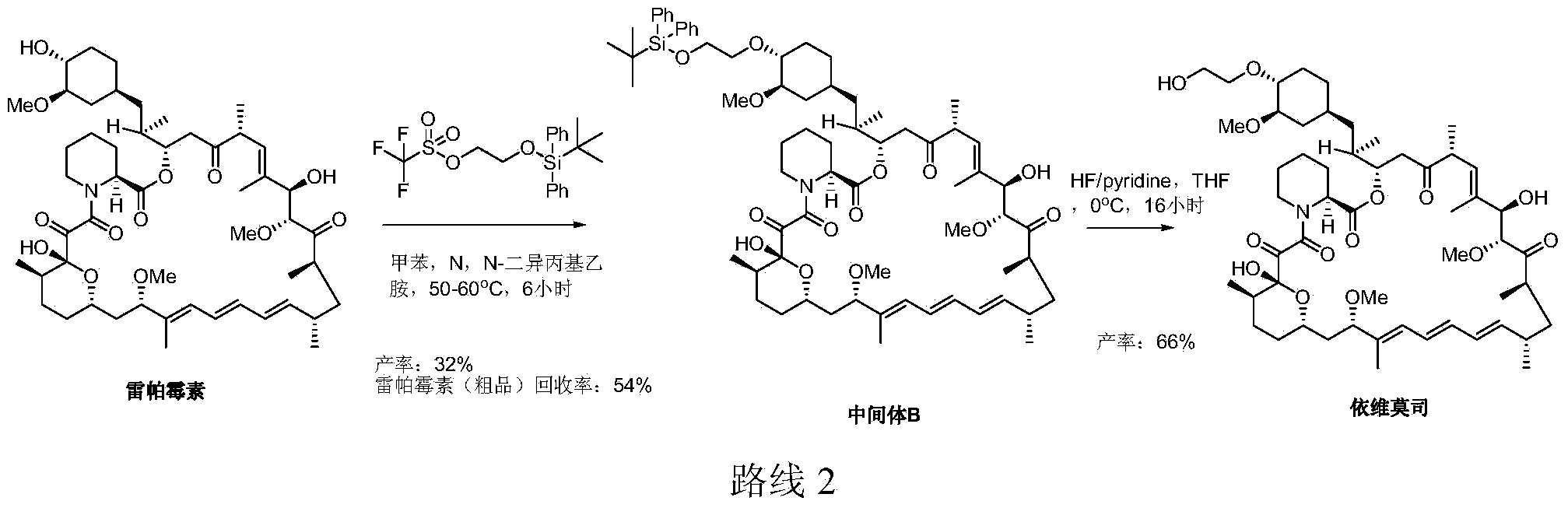
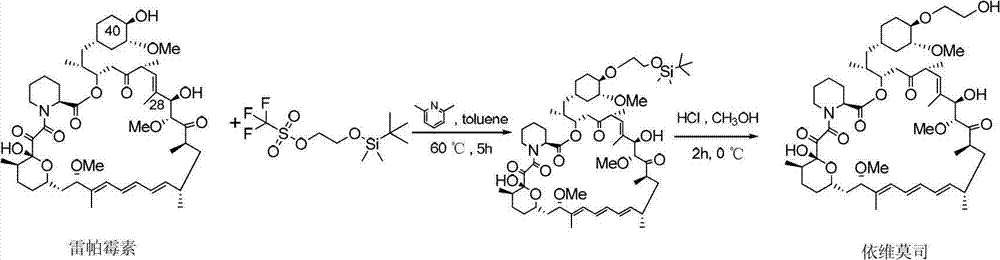
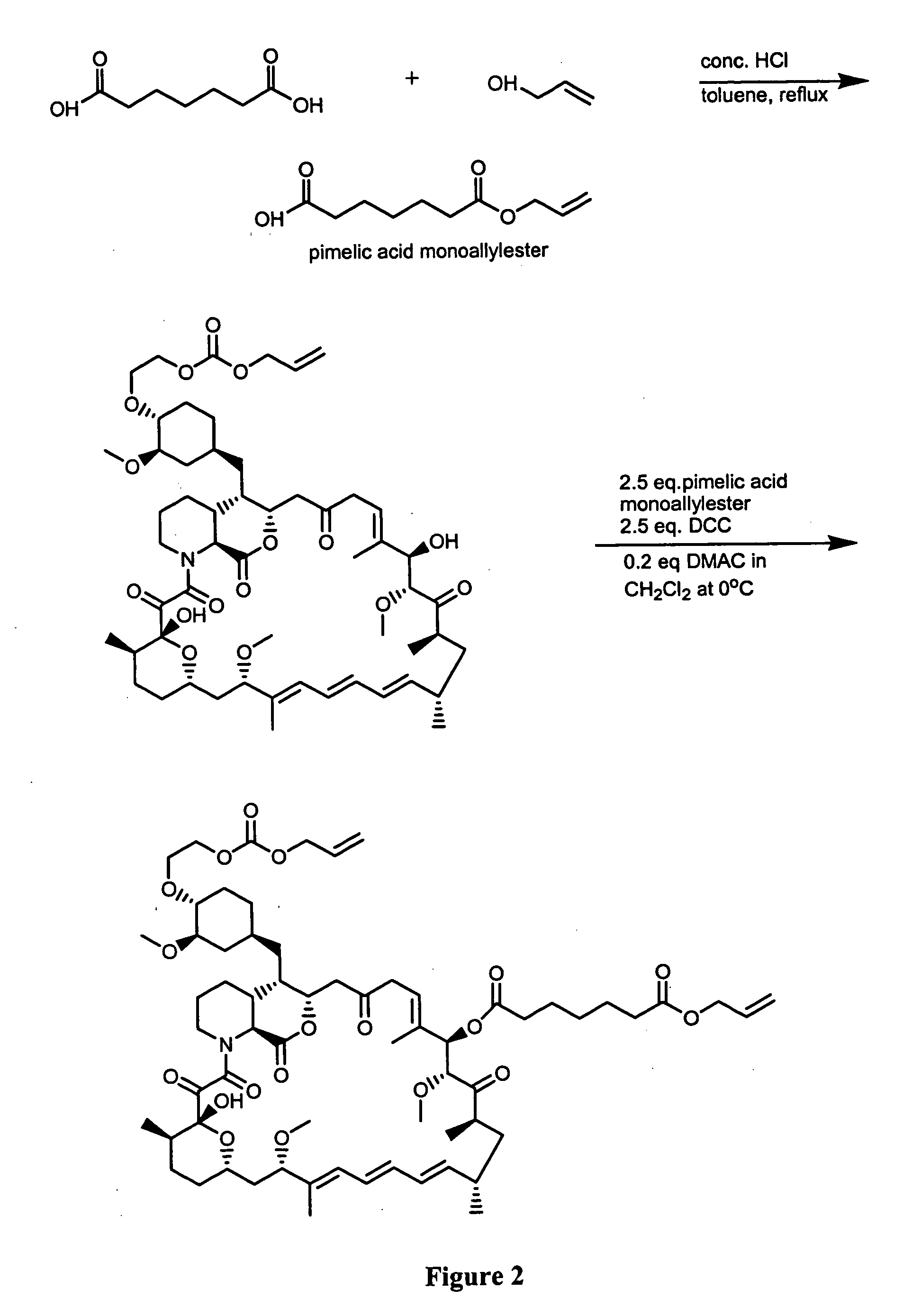
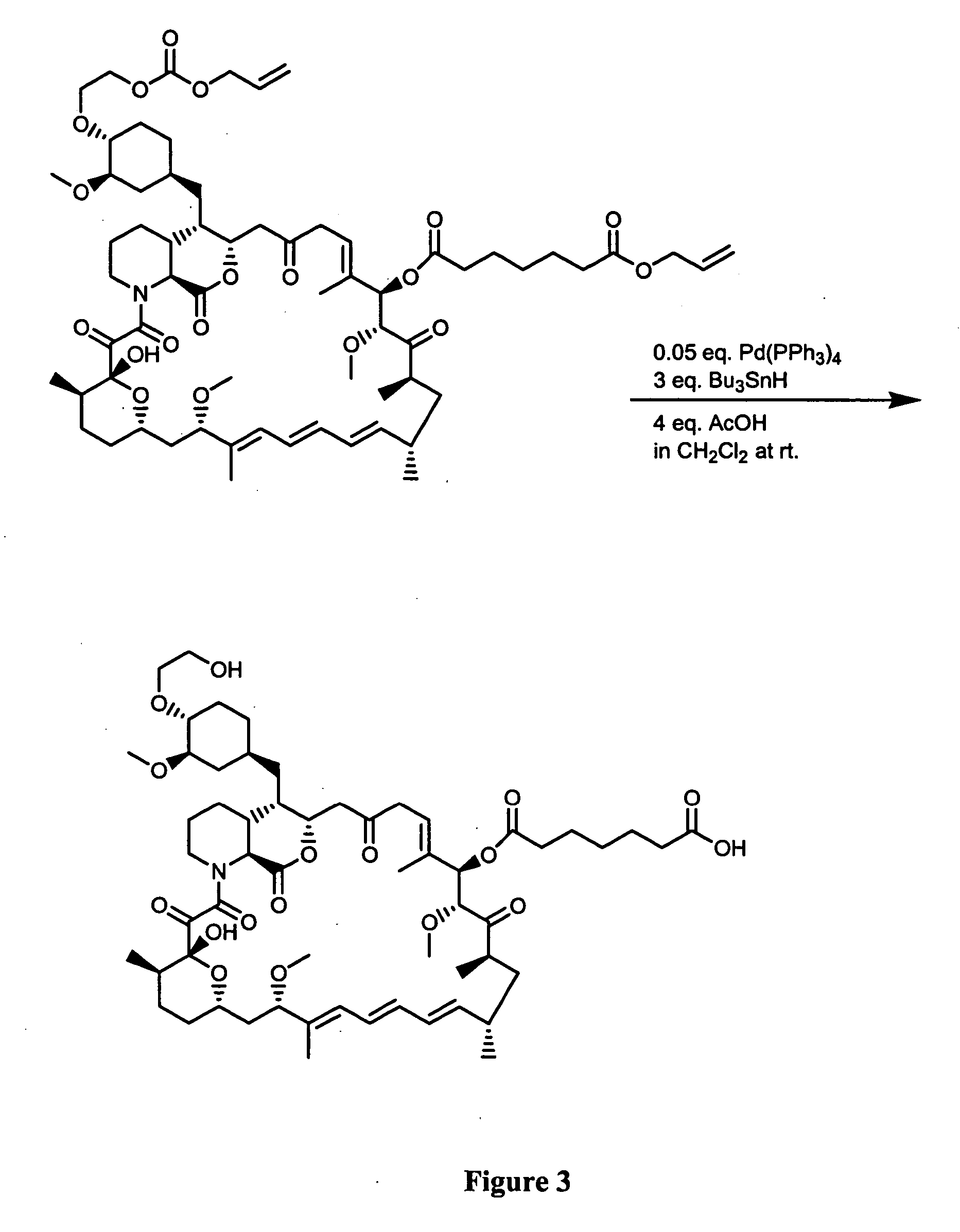
Preparation of Everolimus
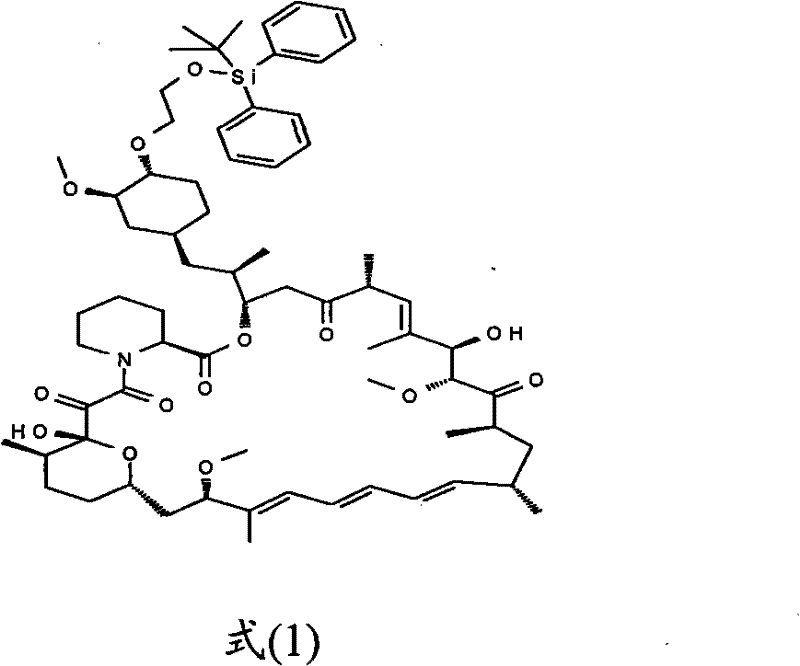
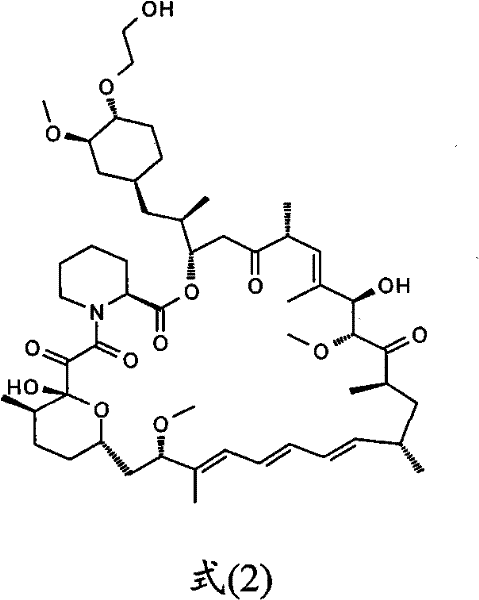
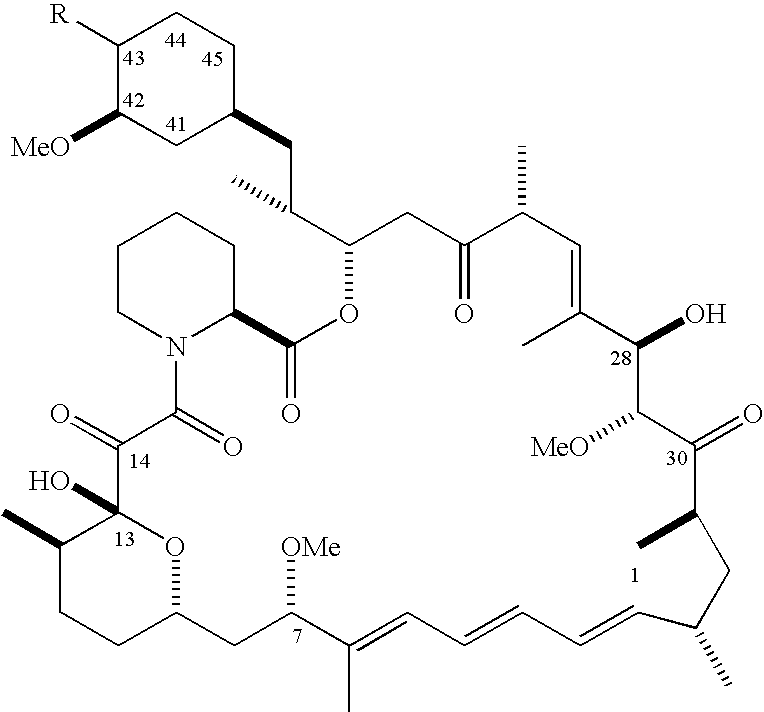
The invention relates to an effective preparation method of a medicine, namely, Everolimus. The preparation method comprises: reacting rapamycin, diisopropylethylamine and 2-(tert-butyldiphenylsilyl)ethoxytrifluoromethanesulfonate at 50 to 60 DEG C in toluene, and obtaining the intermediate represented by a formula (1) by separation by column chromatography; and reacting the intermediate with hydrogen fluoride-pyridine at 0 DEG C in tetrahydrofuran, reacting at room temperature and obtaining Everolimus represented by the formula (2) by separation by column chromatography. The preparation method has the advantages that: the yield is high; and the part of rapamycin serving as an initiative raw material can be recycled.
Owner:SOUTHEAST UNIV
Intravenous pacemaker electrode
InactiveUS7643885B2Low change over timeGuaranteed long-term use effectElectrotherapyEverolimusTransvenous pacemakers
Owner:SIEMENS AG
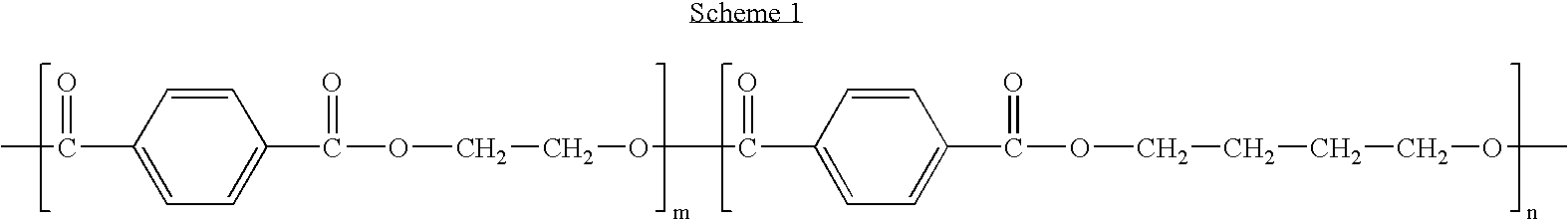
Polyethylene glycol/poly(butylene terephthalate) copolymer coated devices including EVEROLIMUS
Owner:ABBOTT CARDIOVASCULAR
Anti-Proliferative and Anti-Inflammatory Agent Combination for Treatment of Vascular Disorders with an Implantable Medical Device
A drug-delivery system is provided including at least 100 μg of everolimus and clobetasol, such that the ratio of everolimus to clobetasol is at least 10:1 (w / w) or the amount of everolimus by weight is at least 10 times more than clobetasol. The system can be a stent. Also provided a method of treating restenosis or vulnerable plaque of a blood vessel, the method includes locally administering to a patient a first drug selected from a group consisting of rapamycin (sirolimus), Biolimus A9, deforolimus, AP23572, tacrolimus, temsirolimus, pimecrolimus, zotarolimus (ABT-578), 40-O-(2-hydroxy)ethylrapamycin (everolimus), 40-O-(3-hydroxy)propylrapamycin, 40-O-[2-(2-hydroxy)ethoxy]ethylrapamycin, 40-O-tetrazolylrapamycin and 40-epi-(N1-tetrazolyl)rapamycin, and locally administering to a patient a second drug consisting of clobetasol, wherein the minimum amount of the first drug that is locally administered is 100 μg, and wherein the ratio of the first drug to the second drug is, for example, 10:1 to 100:1 (w / w).
Owner:ABBOTT CARDIOVASCULAR
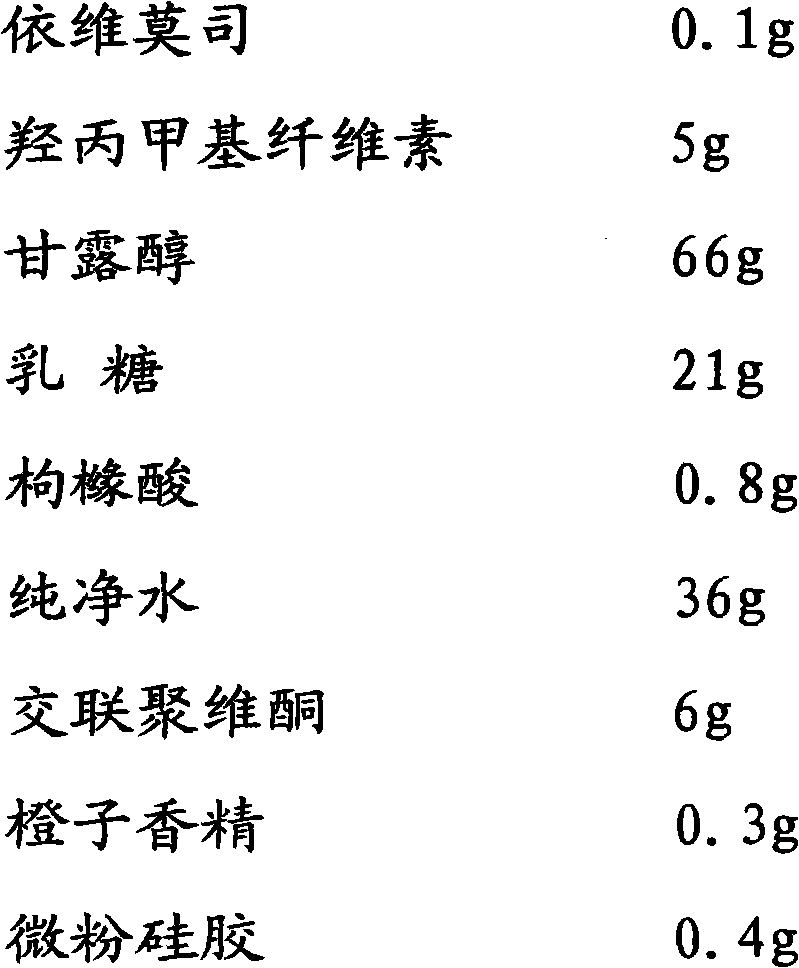
Everolimus solid oral medicinal composition
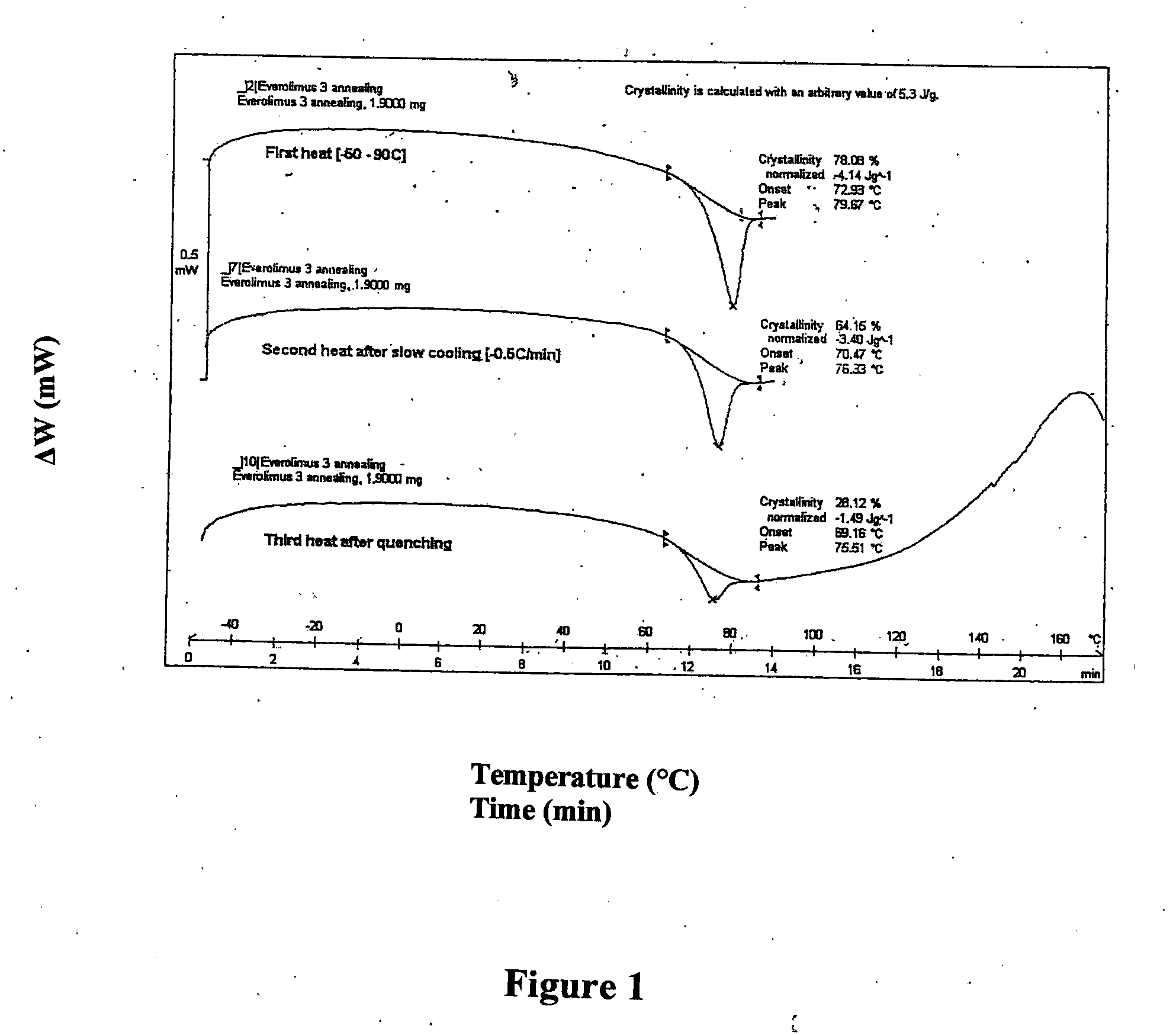
ActiveCN102138903AImprove solubilityImprove stabilityOrganic active ingredientsPowder deliverySolubilityEverolimus
The invention discloses an everolimus solid oral medicinal composition, which comprises a composition consisting of everolimus or a derivative thereof and excipient, wherein the pH value of aqueous solution of the composition is 5 to 6; and the everolimus or the derivative thereof accounts for 0.05 to 5 percent based on the weight of the composition. The composition can be prepared into a high-bioavailability and non-micronized preparation which overcomes the defects of low dissolubility and stability by the regulation of the pH value of the everolimus or the derivative thereof and the excipient, adoption of the reasonable formula ratio, and uniform dispersion of main ingredients by employing the fluidized bed coating technology.
Owner:苏州特瑞药业股份有限公司
Drug eluting coatings for medical implants
InactiveUS7438925B2Control releaseMinimize any pathologies associatedSuture equipmentsStentsEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Preparation technology for everolimus
The invention provides a preparation technology for everolimus. The preparation method comprises the two steps of: 1) reacting sirolimus with 2-(tert-butyldiMethylsilyloxy) ethyl trifluoromethane sulfonate in the presence of proper solvent and organic base, to obtain an intermediate A; 2) reacting the intermediate A with inorganic acid in an organic solvent to obtain everolimus, wherein the organic base used in the step 1) is selected from large-steric hindrance or non-nucleophilic bases such as triethylamine, N,N-diisopropylethylamine, 1,8-diazabicycloundec-7-ene or N-methylmorpholine, acid used in the step 2) is hydrochloric acid, sulfuric acid or phosphoric acid. According to the technology, the total yield in the two steps and the purity of a final product are greatly improved as compared with those reported by the existing literature, the process route is short, the reaction conditions are mild, and the reaction result is also stable and reliable.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Ocular solutions
InactiveUS20050063997A1Reduce turbidityReduce inflammationBiocideSenses disorderEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:PEYMAN GHOLAM A DR
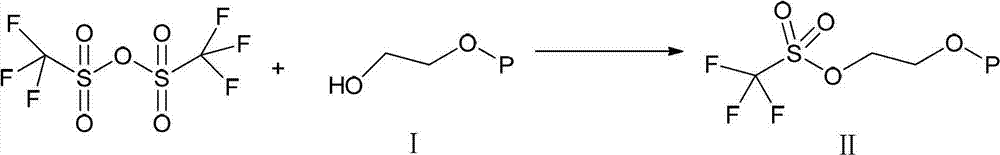
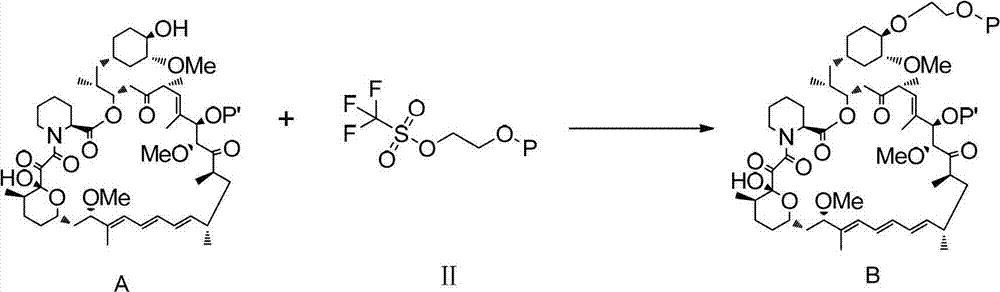
A kind of synthetic method of everolimus
ActiveCN102268015AFully convertedThorough responseOrganic chemistryTrifluoromethanesulfonic anhydrideEverolimus
The invention discloses a synthesis method of everolimus. The synthesis method of the everolimus comprises the following steps: based on rapamycin or a rapamycin derivative the 31-hydroxy of which is protected as a raw material, firstly carrying out a reaction on the raw material with triflic anhydride so as to obtain an intermediate 02; then carrying out a reaction on the intermediate 02 with mono-protected glycol so as to obtain an intermediate 03; and de-protecting the intermediate 03 so as to obtain the everolimus. In the process in the invention, the raw material can be fully converted into the intermediate 02 through reacting with triflic anhydride, and the intermediate 02 can be fully converted into the intermediate 03 through reacting with mono-protected glycol; and each step reaction can be fully carried out, and the total yield is greatly improved to above 50%.
Owner:四川摩尔生物制药有限公司
Preparation method of everolimus
InactiveCN102786534AHigh yieldEasy to operateOrganic chemistryBulk chemical productionTrifluoromethanesulfonic anhydrideCompound a
The invention belongs to the technical field of the preparation method of everolimus. The preparation method of everolimus provided by the invention comprises the steps as follows: 1) adding Trifluoromethanesulfonic anhydride into an alkaline organic solution of tert butyl dimethyl hydroxyl ethoxy silane under the protection of nitrogen to obtain a compound II through a reaction; 2) adding the compound II into an alkaline organic solution of a compound A to obtain a compound B through a reaction; and 3) reacting the compound B in a solvent with an acid to obtain the everolimus. The synthesis method provided by the invention has advantages of short synthetic route, high yield, simple operation, stable reaction and low cost, and is suitable for application to industrialized production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
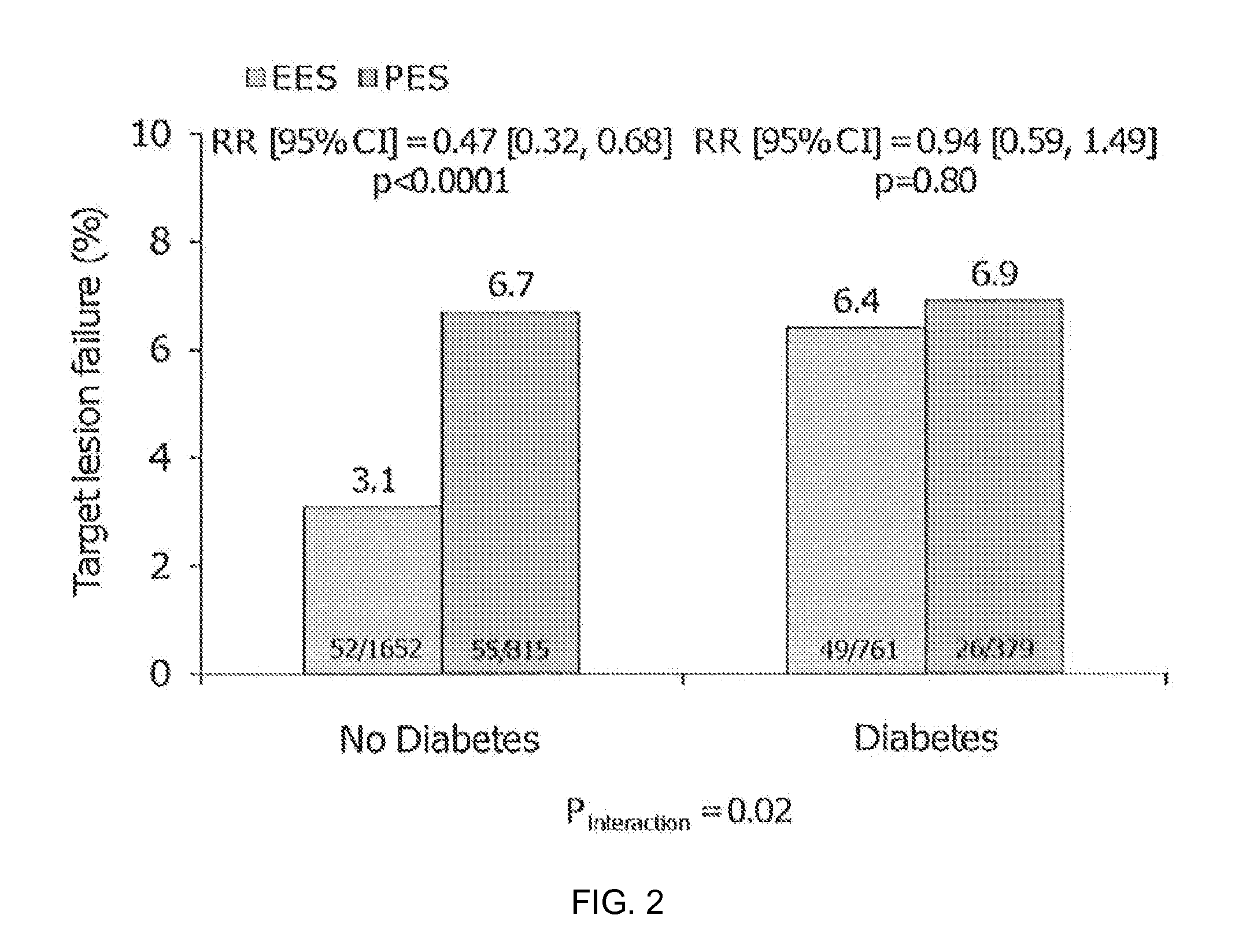
Bioresorbable Polymer Scaffold Treatment of Coronary and Peripheral Artery Disease in Diabetic Patients
Methods of treating coronary and peripheral artery disease in diabetic patients with bioresorbable polymer stents are described. The stents may include everolimus.
Owner:ABBOTT CARDIOVASCULAR
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-flammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
Therapeutic methods
InactiveUS20070185150A1Great cumulative exposureImprove compromiseBiocideAnimal repellantsEverolimusMedicine
Disclosed are methods for treating a patient with an mTOR inhibitor such as AP23573, sirolimus, temsirolimus, everolimus, etc.
Owner:ARIAD PHARMA INC
Anti-proliferative and anti-inflammatory agent combination for treatment of vascular disorders with an implantable medical device
Drug-delivery systems such as drug-delivery stents having an anti-proliferative agent such as everolimus and an anti-inflammatory agent such as clobetasol are provided. Also disclosed are methods of treating a vascular impairment such as restenosis or vulnerable plaque
Owner:ABBOTT CARDIOVASCULAR
Treatment Of Diabetic Patients With Stent And Locally Administered Adjunctive Therapy
Embodiments of the present invention include methods of treating, preventing, and / or ameliorating a vascular disease and / or disorder in a diabetic or pre-diabetic patient. The methods include implanting a stent in a vascular region in a diabetic or pre-diabetic patient, and prior to and / or during the implantation procedure, delivering a lubricant formulation to the vascular region. The stent may be a bare metal stent, or a drug eluting stent, such as a metal stent with a coating including a drug, such as everolimus or sirolimus.
Owner:ABBOTT CARDIOVASCULAR
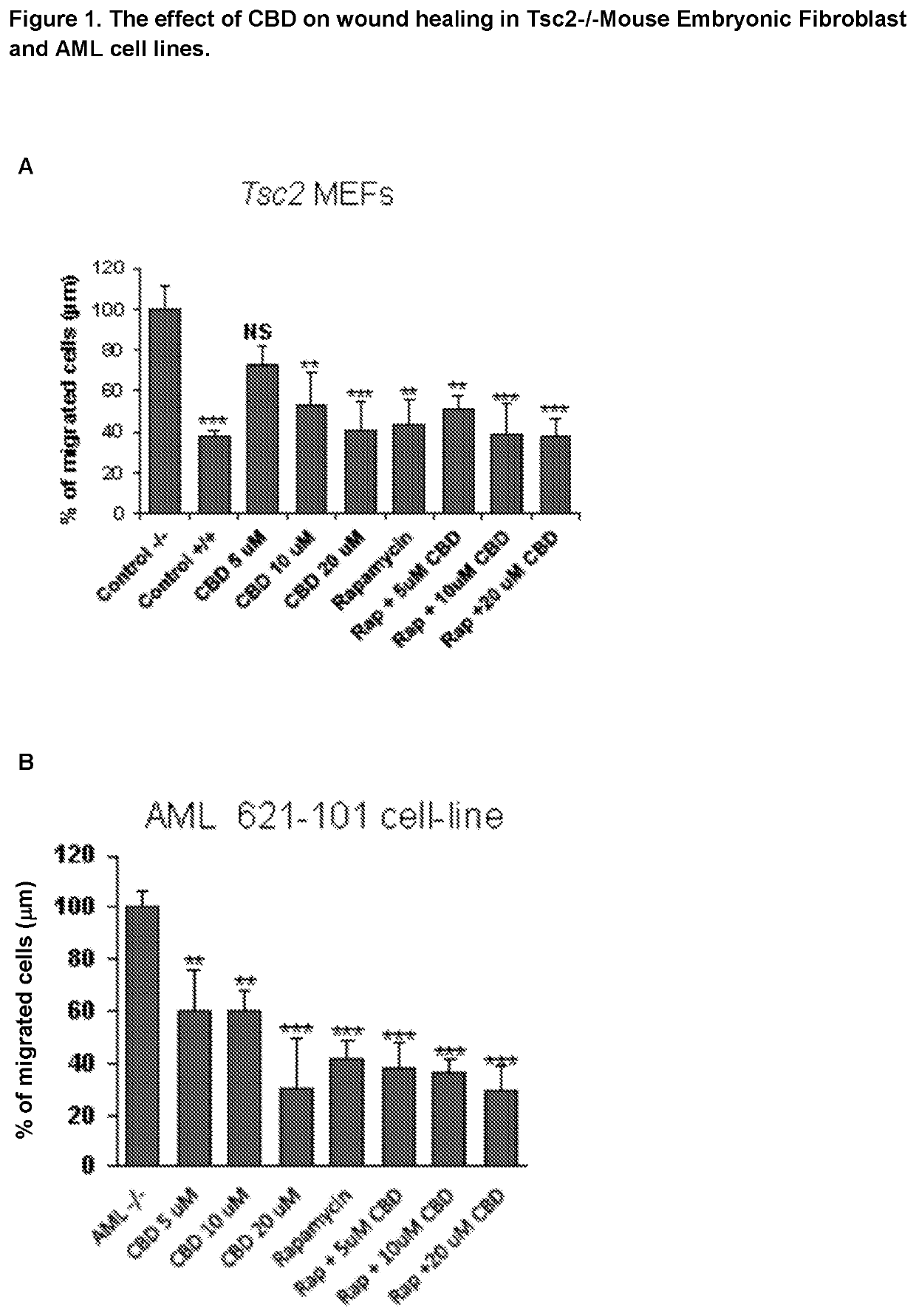
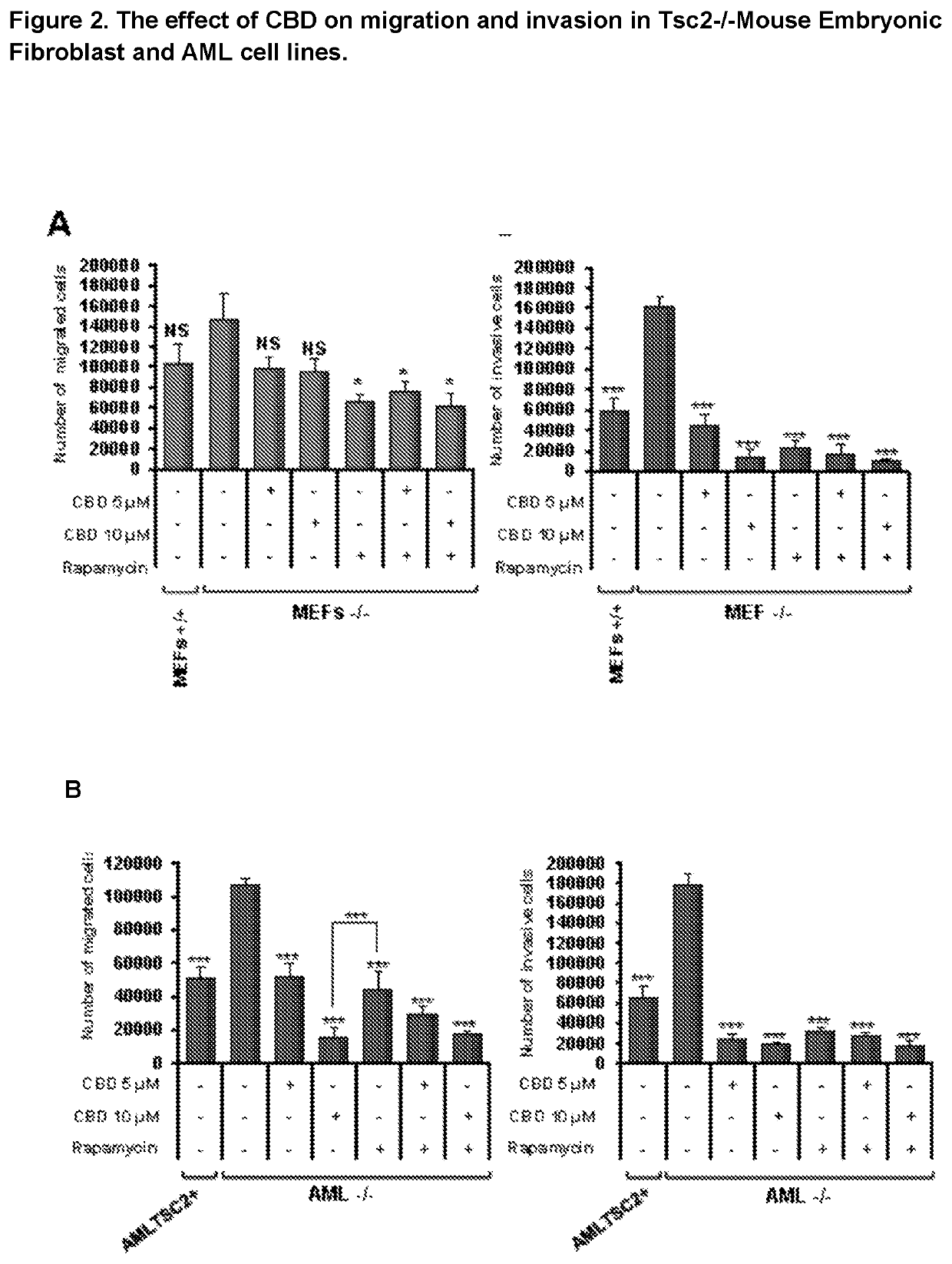
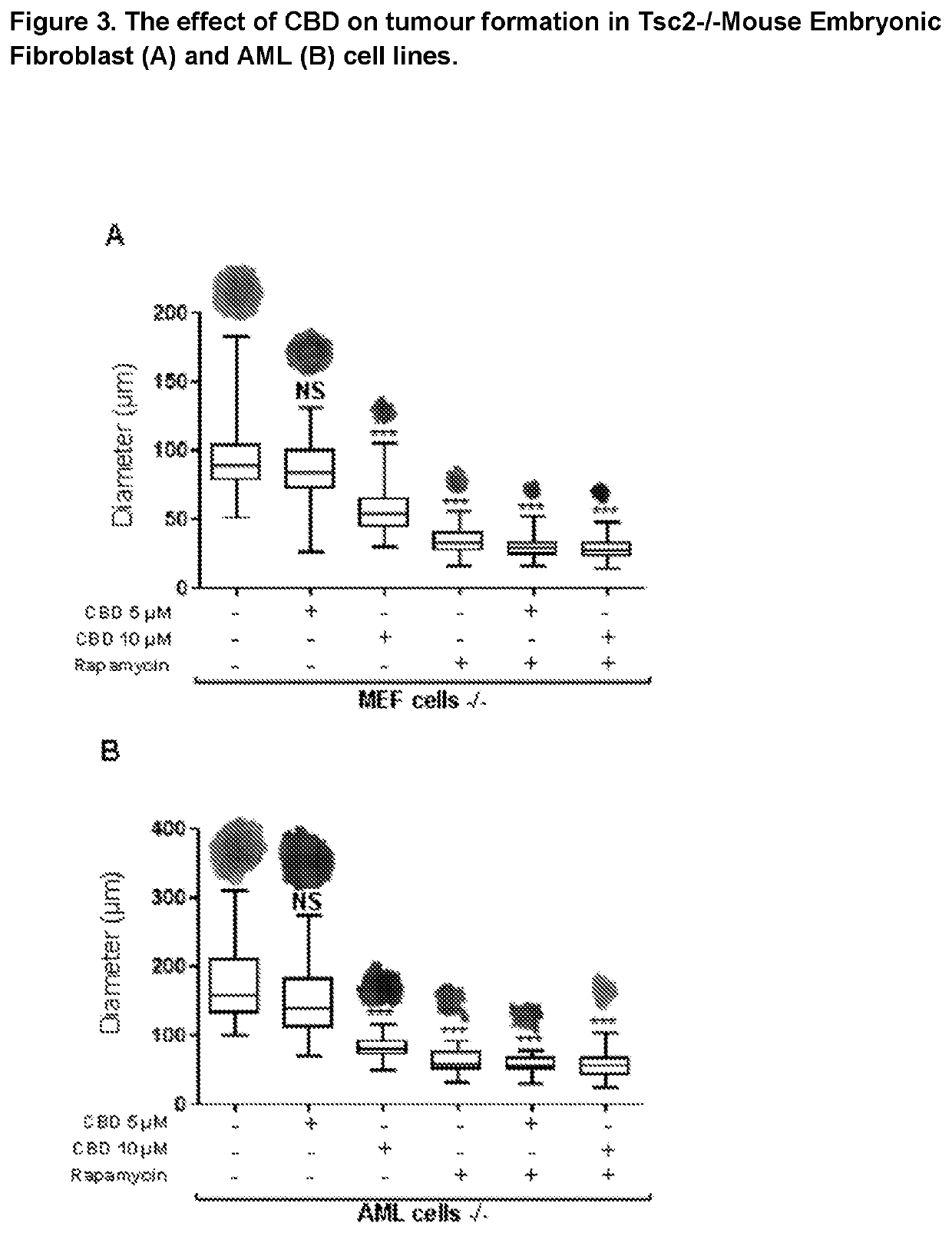
Use of cannabidiol in the treatment of tuberous sclerosis complex
InactiveUS20200206153A1Prevent and reduce tumourReduce doseHydroxy compound active ingredientsAntineoplastic agentsBenign tumoursDisease
The present invention relates to the use of cannabidiol (CBD) for the treatment of tumours associated with Tuberous Sclerosis Complex (TSC). In particular the CBD was able to decrease the number and size of marker cells, pS6, in a zebrafish model of TSC. This is5 suggestive of a disease modifying effect whereby treatment with CBD could result in the reduction or prevention of the benign tumours that occur in TSC patients. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially 10 removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD. In use the CBD is given concomitantly with one or more other drugs used in the treatment of TSC. Such drugs may include rapamycin and / or everolimus.
Owner:GW RES LTD
Immunoassays for everolimus
Immunoassays for the detection of everolimus are provided. Compounds for producing antibodies for everolimus, as well as antibodies produced therefrom, are also provided.
Owner:ROBERTS MARK +7
Immunoassays for everolimus
Owner:SERADYN INC
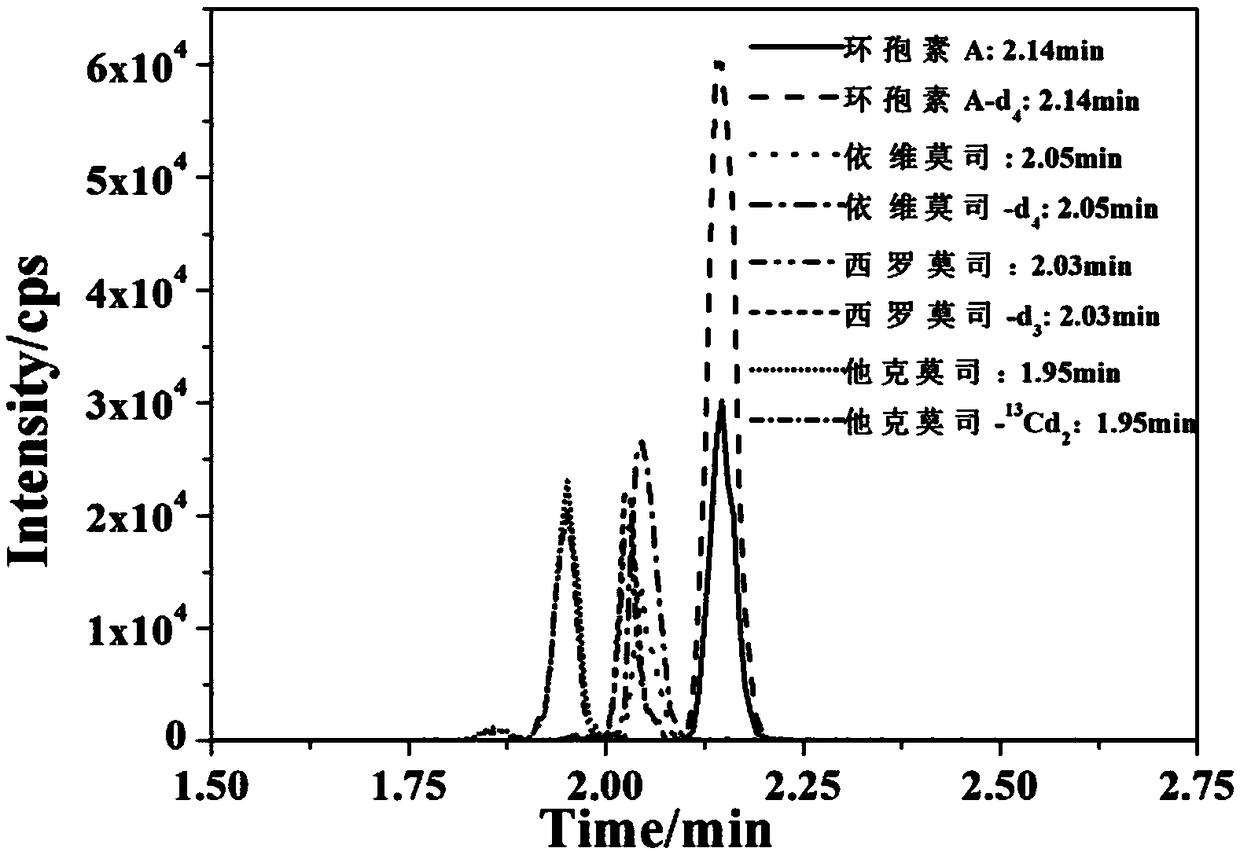
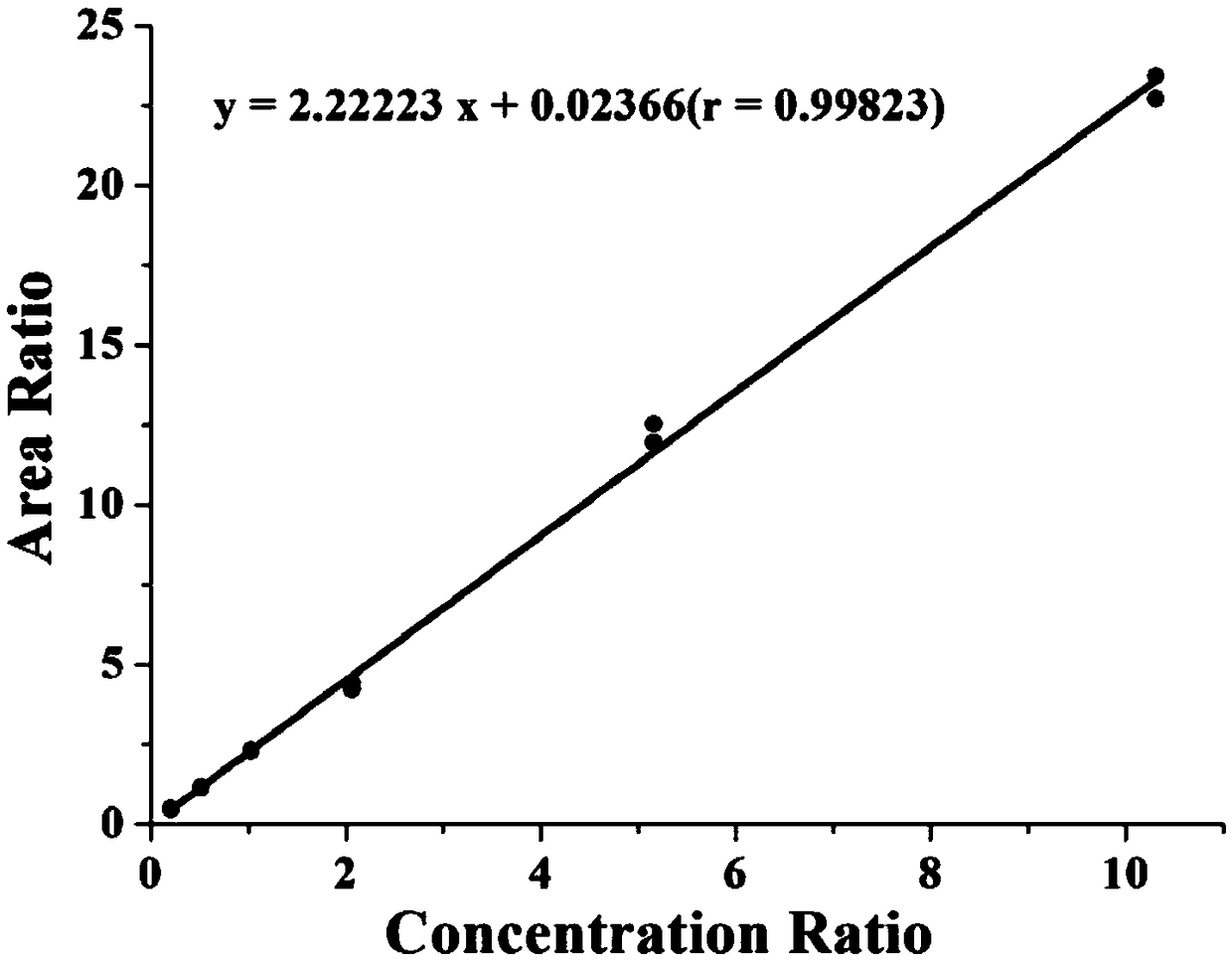
Kit for accurately determining concentration of four kinds of immunosuppressant type medicine in human whole blood and detecting method
InactiveCN109406650ASmall sample sizeSmall detection specificityComponent separationEverolimusPretreatment method
The invention provides a kit for accurately determining the concentration of four kinds of immunosuppressant type medicine in human whole blood. The kit comprises Tacrolimus (TAC), sirolimus (SIR), everolimus (EVR) and cyclosporine A (CsA) calibrators, Tacrolimus-<13>Cd2, sirolimus-d3, everolimus-d4 and cyclosporine A-d4 interior labels, quality control products of Tacrolimus, sirolimus, everolimus and cyclosporine A, sample treatment liquid, extraction liquid, flowing phases and redissolution solution. The kit is used for treating whole blood samples; by a simple protein precipitation-liquidliquid extraction pretreatment method, clean treatment liquid can be obtained without complicated purification and refinement steps; the required sample quantity is small; the kit can be used by beingmatched with a liquid chromatograph tandom mass spectrometer; meanwhile, the kit can be used for simultaneously detecting four kinds of different immunosuppressant type medicine; the single sample multi-index synchronous detection can be realized; the detection specificity is high; the sensitivity is high; the whole detection flow process time is short; the flux is great; the cost is low.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Kit and detecting method for accurately measuring concentration of four immunosuppressant drugs in human whole blood
InactiveCN109187839ASmall sample sizeHigh detection specificityComponent separationEverolimusPretreatment method
The invention provides a kit for accurately measuring the concentration of four immunosuppressant drugs in human whole blood. The kit comprises the calibrators of tacrolimus, sirolimus, everolimus andcyclosporine A, the internal standards of the tacrolimus-13Cd2, the sirolimus-d3, the everolimus-d4 and the cyclosporine A, the quality control serums of the tacrolimus, the sirolimus, the everolimusand the cyclosporine A, sample treatment liquid, a 96-well plate assembly, and a mobile phase. According to the kit and detecting method for accurately measuring the concentration of four immunosuppressant drugs in human whole blood, a clean treatment liquid can be obtained through a simple 96-well plate protein precipitation pretreatment method without complex purification steps, and the required sample amount is small and can be used together with a liquid chromatography-tandem mass spectrometry; and four different immunosuppressant drugs can be detected at the same time, that is, the single-sample multi-index synchronous detection can be realized, the detection specificity is good, the sensitivity is high, the whole detection time is short, the throughput is high, and the cost is low.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com