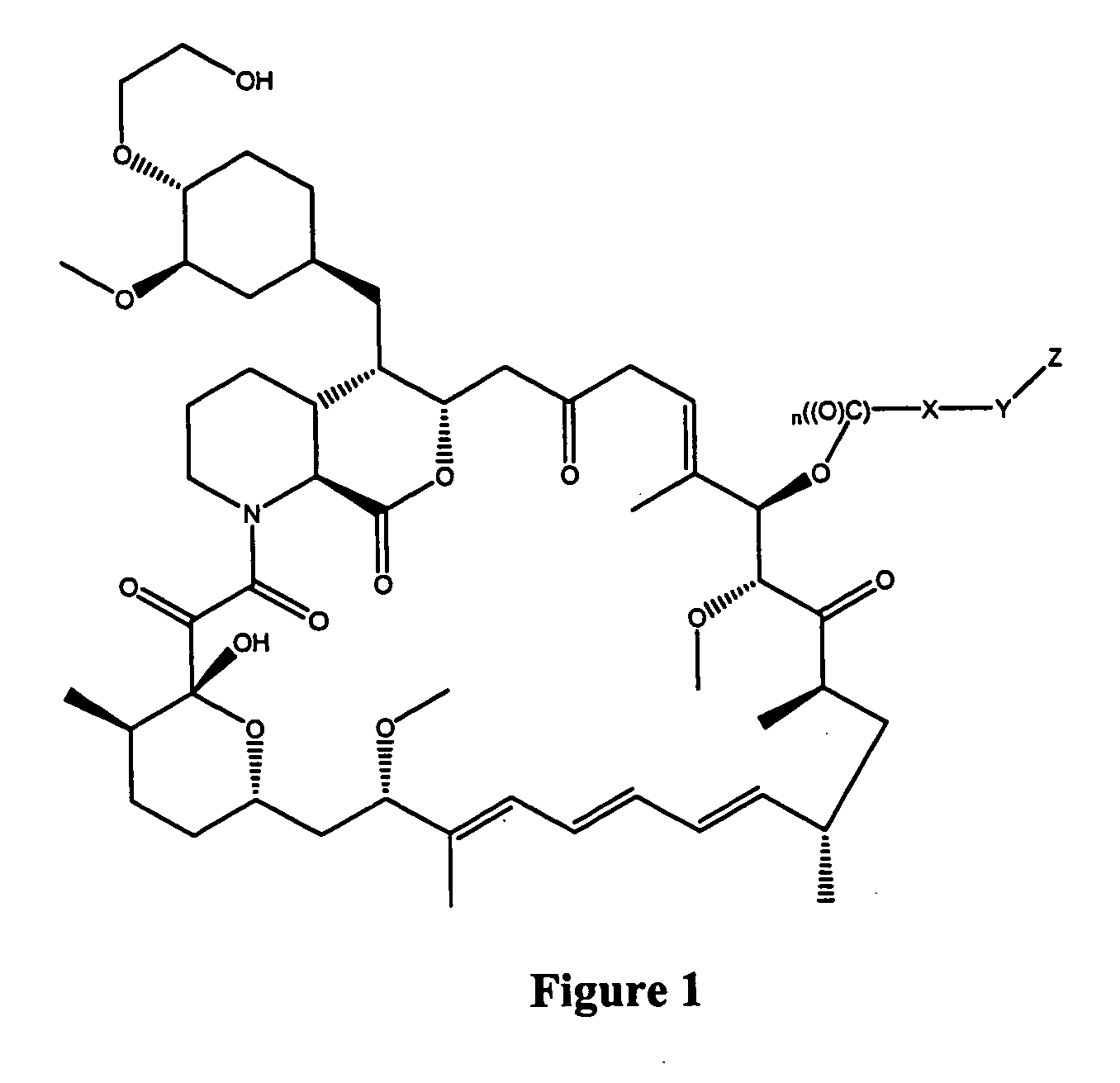
Immunoassays for everolimus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
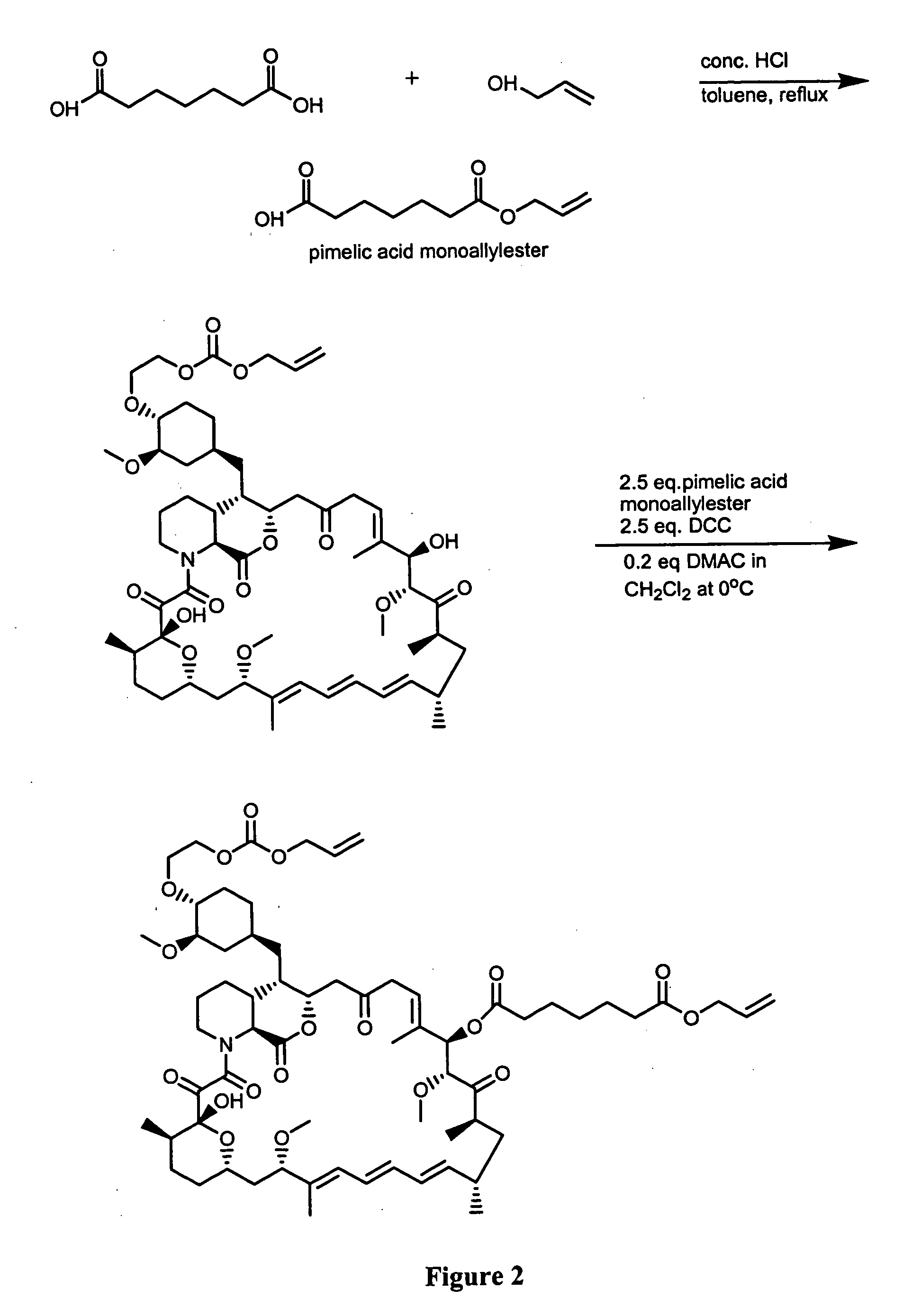
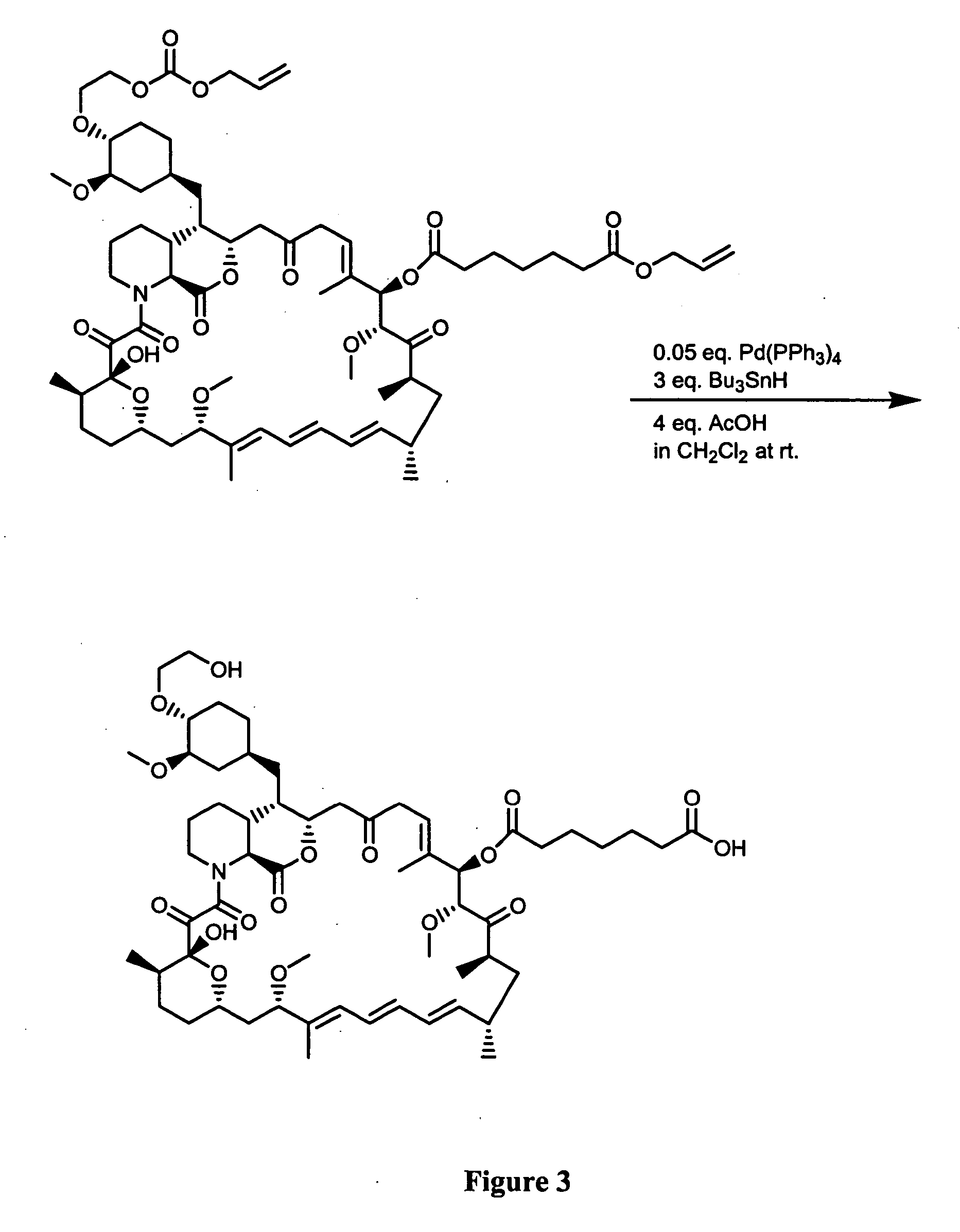
Synthesis of Immunogenic Compounds and Labeled Competitors Synthesis of RAD-mono-formate
[0077] 0.6 g of RAD was dissolved in 2 mL of dry methylene chloride in a 100 mL round bottom flask under an atmosphere of argon (Ar). An aliquot (˜10 μL) was saved for HPLC and TLC assays. The round bottom flask containing the reaction solution was placed in the ice / NaCl bath at about −20° C. The reaction flask was allowed to cool down for 3-5 min. Dry pyridine (0.25 mL) was added using a dry glass syringe with a metal needle (15 cm) all at once. 0.35 mL dry allyl chloroformate was added using a dry glass syringe with a metal needle (15 cm) within about ½-1 min. Shortly after addition, precipitation occurred. The stirring was allowed for one hour. The reaction was quenched by adding 5 mL of saturated NaHCO3. The quenched reaction was extracted with methylene chloride. The organic phase was combined, dried (Na2SO4) and filtered. The filtrate was transferred to a 250 ml bottom flask (100-250 mL) a...
example ii
Antibody Preparation
[0089] Polyclonal anti-everolimus antibodies can be prepared by conventional methods. Animals were immunized with everolimus immunogen (RAD 822 / BSA), as produced in Example I. The immunization program started with initial injection of 0.5 ml immunogen mixing with 0.5 complete Freunds adjuvant. Subsequent injections were performed with 0.5 ml immunogen mixing with 0.5 incomplete Freunds adjuvant. Animals were typically injected every two weeks. Sera were screened via FPIA using RAD 822: FAMCO-E tracer. Several bi-monthly production bleeds (˜20 mL per bleed) from three rabbits were pooled together. Before filter and dilution, the total pooled volume is about 500 mL. Anti-Sera from Rabbit were filtered with 0.2 um Cellulose Acetate Filter under vacuum and diluted with phosphate buffer with sodium azide and sodium chloride at pH 7.5. The final volume is about 1000 ml.
[0090] Monoclonal anti-everolimus antibodies can be prepared by immunization of mice. A mouse can b...
example iii
Fluorescence Polarization Immunoassay using RAD 822: FAMCO-E tracer Automated Fluorescence Polarization Immunoassay (FPIA)
[0091] This example describes an exemplary fluorescence polarization immunoassay (FPIA) for everolimus.
[0092] The fluorescence polarization immunoassay was performed using an automated TDx polarization analyzer (Abbott Laboratories, Irving, Tex.) using a competitive assay included anti-analyte antibody (the anti-everolimus antibody of Example II) or “A”, a fluorescein:everolimus analog conjugate (tracer or “T”), and a pretreatment buffer or “B.” The calibration of the automated assay described in the examples was achieved with a series of six calibrators that include specified concentrations of everolimus spiked into human serum. Patient samples (plasma) are placed in plastic sample cups in a circular carousel designed for the TDx instrument. The automated assay is described in detail in literature available from Abbott Laboratories, Irving, Tex. It is understo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com