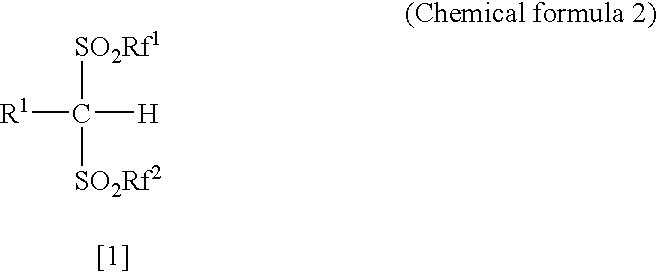Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
154 results about "Trifluoromethanesulfonic anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
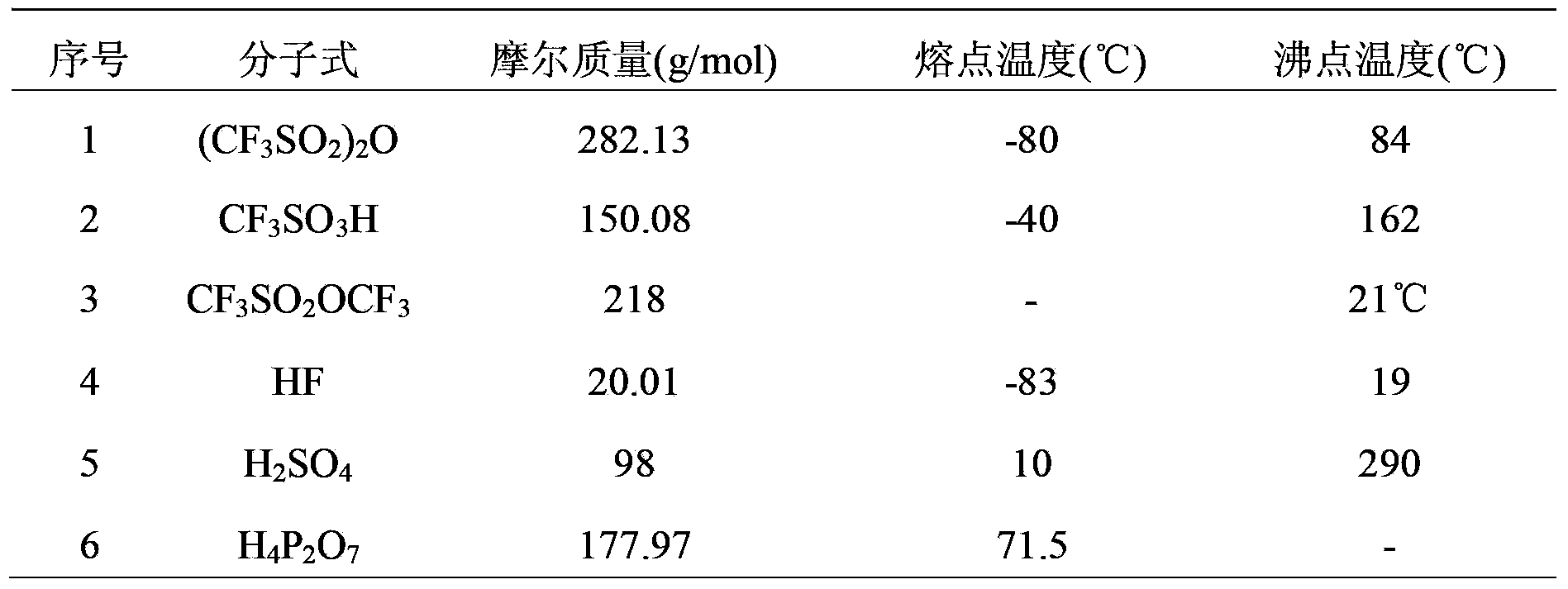
Triflic anhydride is the chemical compound with the formula (CF₃SO₂)₂O. It is the acid anhydride derived from triflic acid. This compound is a particularly strong electrophile, useful for introducing the triflyl group, CF₃SO₂. Abbreviated Tf₂O, triflic anhydride is the acid anhydride of the strong acid triflic acid, CF₃SO₂OH.
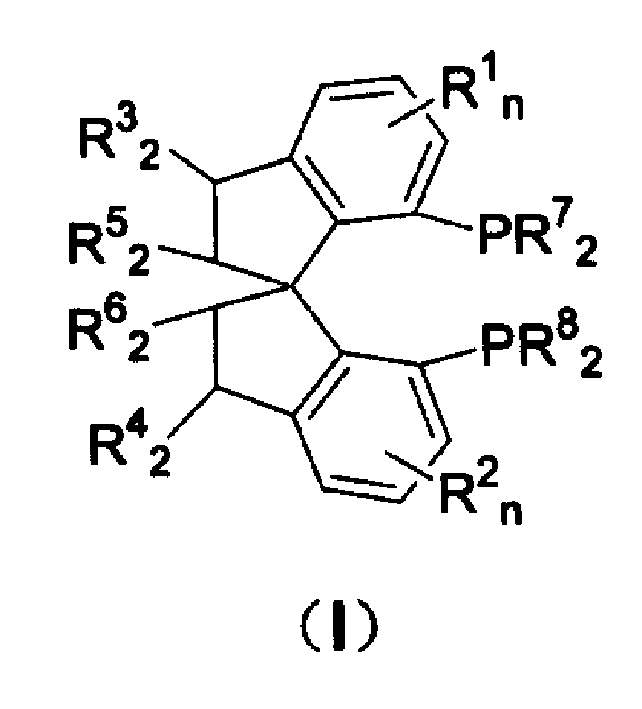
Spiro-diphosphine ligand
InactiveCN1439643AHigh stereoselectivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideDiphosphines
A spirocyclo-biphosphine ligand is prepared from spirocyclo-biphenol through esterifying by trifluoro methylsulfonic acid anhydride, coupling with diaryloxyphosphine under catalysis of Pd, and reduction reacting on trichlorosilane. It includes d-and levo-spricyclo-biphosphine ligands, whose mixture is dl-spirocyclo-biphosphine ligand. It can be used for asymmetrical catalytic hydrogenation reaction of latent chiral ketone with high stereo selectivity and e.e. value up to 99.5%.
Owner:NANKAI UNIV
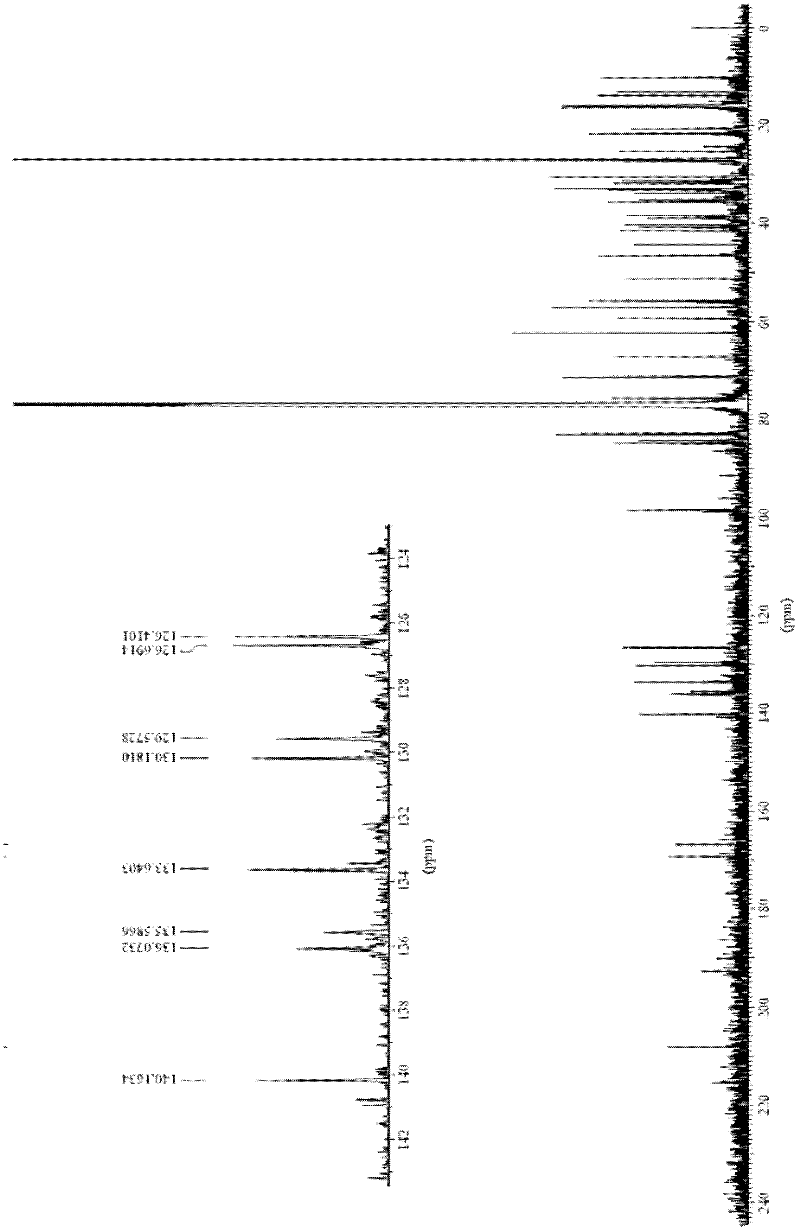
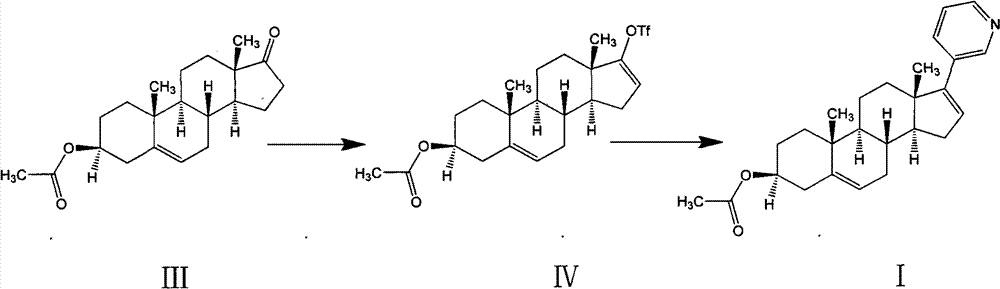
A kind of synthetic method of everolimus
ActiveCN102268015AFully convertedThorough responseOrganic chemistryTrifluoromethanesulfonic anhydrideEverolimus
The invention discloses a synthesis method of everolimus. The synthesis method of the everolimus comprises the following steps: based on rapamycin or a rapamycin derivative the 31-hydroxy of which is protected as a raw material, firstly carrying out a reaction on the raw material with triflic anhydride so as to obtain an intermediate 02; then carrying out a reaction on the intermediate 02 with mono-protected glycol so as to obtain an intermediate 03; and de-protecting the intermediate 03 so as to obtain the everolimus. In the process in the invention, the raw material can be fully converted into the intermediate 02 through reacting with triflic anhydride, and the intermediate 02 can be fully converted into the intermediate 03 through reacting with mono-protected glycol; and each step reaction can be fully carried out, and the total yield is greatly improved to above 50%.
Owner:四川摩尔生物制药有限公司
Preparation method of everolimus
InactiveCN102786534AHigh yieldEasy to operateOrganic chemistryBulk chemical productionTrifluoromethanesulfonic anhydrideCompound a
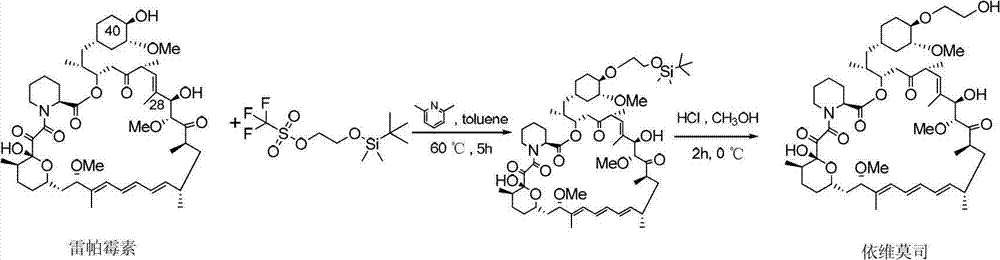
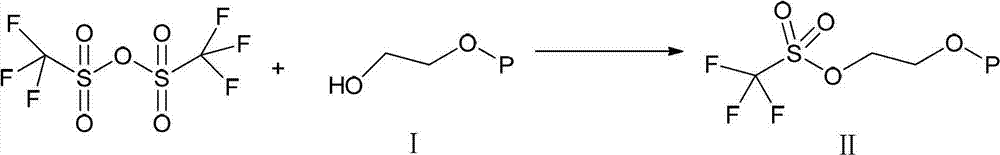
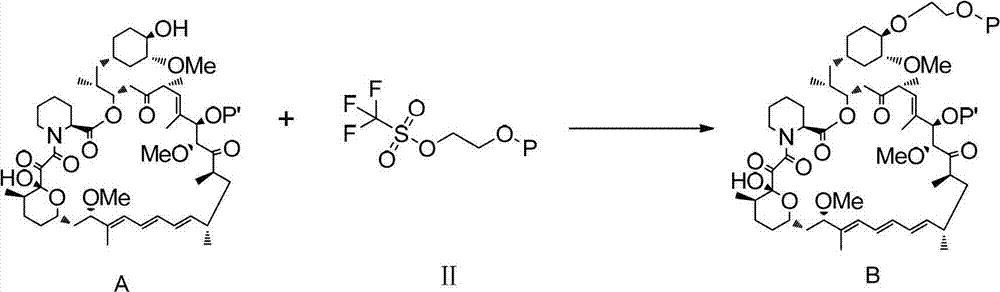
The invention belongs to the technical field of the preparation method of everolimus. The preparation method of everolimus provided by the invention comprises the steps as follows: 1) adding Trifluoromethanesulfonic anhydride into an alkaline organic solution of tert butyl dimethyl hydroxyl ethoxy silane under the protection of nitrogen to obtain a compound II through a reaction; 2) adding the compound II into an alkaline organic solution of a compound A to obtain a compound B through a reaction; and 3) reacting the compound B in a solvent with an acid to obtain the everolimus. The synthesis method provided by the invention has advantages of short synthetic route, high yield, simple operation, stable reaction and low cost, and is suitable for application to industrialized production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
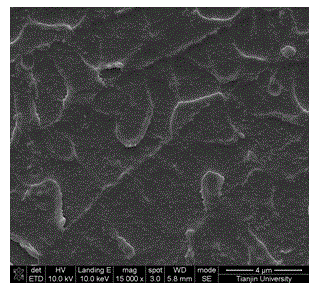
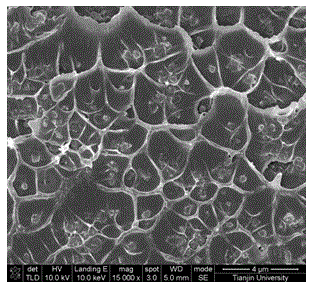
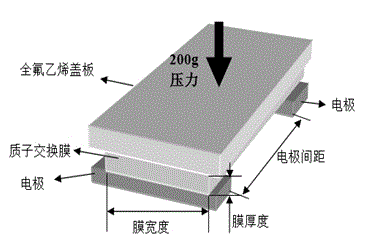
High polymer-modified metal organic framework material composite membrane, and preparation and application thereof
InactiveCN104037432AEasy to makeOptimal Control StructureSolid electrolyte fuel cellsFuel cell detailsTrifluoromethanesulfonic anhydrideHydrofluoric acid
The invention relates to a high polymer-modified metal organic framework material composite membrane and a preparation method and application thereof. A preparation method for a metal organic framework material comprises a step of subjecting terephthalic acid, chromium nitrate nonahydrate, hydrofluoric acid and water to a reaction and a step of carrying out modification by using trifluoromethanesulfonic anhydride and concentrated sulfuric acid. The prepared modified metal organic framework material is dispersed in a high polymer solution so as to obtain a membrane casting solution; the membrane casting solution is successively subjected to filtering, standing and defoaming and then is used to prepare a membrane through tape casting; and drying, quenching, acidifying with a sulfur acid solution, washing and drying are successively carried out so as to obtain the high polymer-modified metal organic framework material composite membrane. The composite membrane prepared in the invention has high proton conductivity and is directly applicable to a fuel cell.
Owner:TIANJIN UNIV
Preparation method of trifluoro methanesulfonic anhydride
ActiveCN102911086AHigh yieldReduce contentOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of trifluoro methanesulfonic anhydride. The method comprises the following steps of: firstly reacting trifluoro methanesulphonyl fluoride with alkali metal hydroxide to prepare trifluoro mesylate, purifying trifluoro mesylate by recrystallization by utilizing an organic solvent, reacting trifluoro methane sulfonyl chloride with trifluoro mesylate to generate a trifluoro methanesulfonic anhydride crude product, and finally purifying trifluoro methanesulfonic anhydride by atmospheric distillation. The preparation method of trifluoro methanesulfonic anhydride can be used for not only effectively simplifying reaction steps so that the operation process is simple and convenient and the operation is safe, but also avoiding byproducts generated in the process of the traditional method for producing trifluoro methanesulfonic anhydride, and effectively reducing the contents of F<-> and SO4<2-> in the product; by utilizing recrystallization, atmospheric distillation and other methods for purification, the product purity is up to 99.5%; and more importantly, the yield of anhydride is greatly increased and raised to 88% from original 60%.
Owner:JIANGXI GUOHUA IND CO LTD
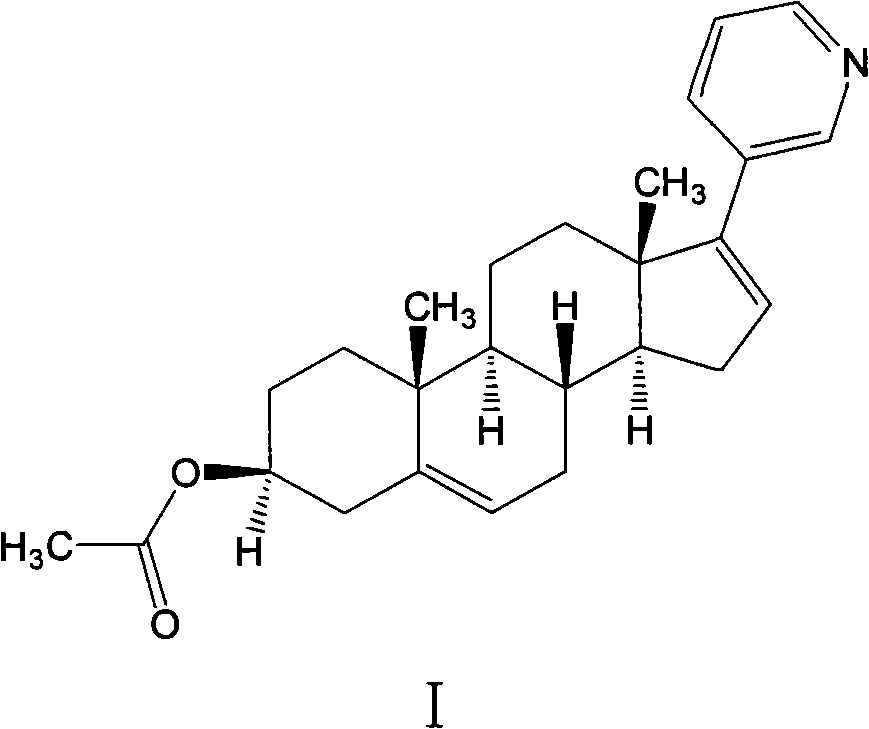
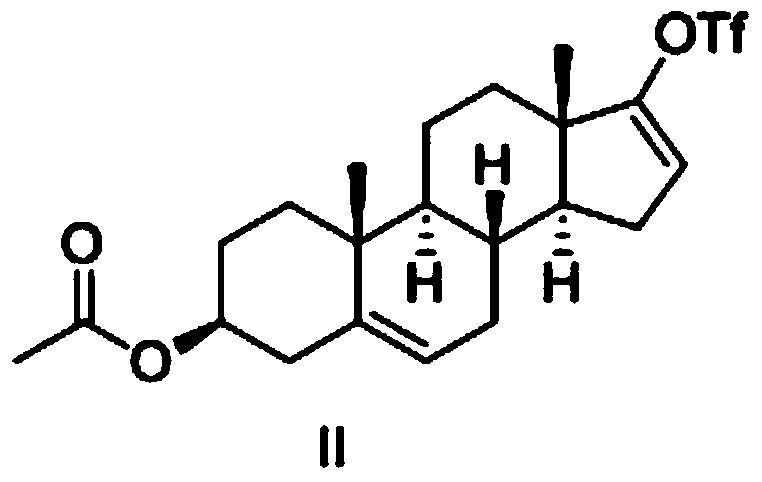
Preparation method of high-purity abiraterone acetate
ActiveCN103193849AHigh yieldHigh puritySteroidsTrifluoromethanesulfonic anhydrideDehydroepiandrosterone acetate
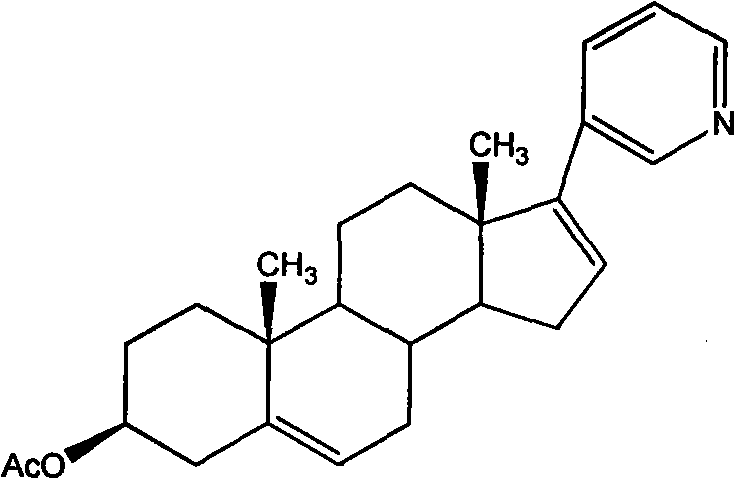
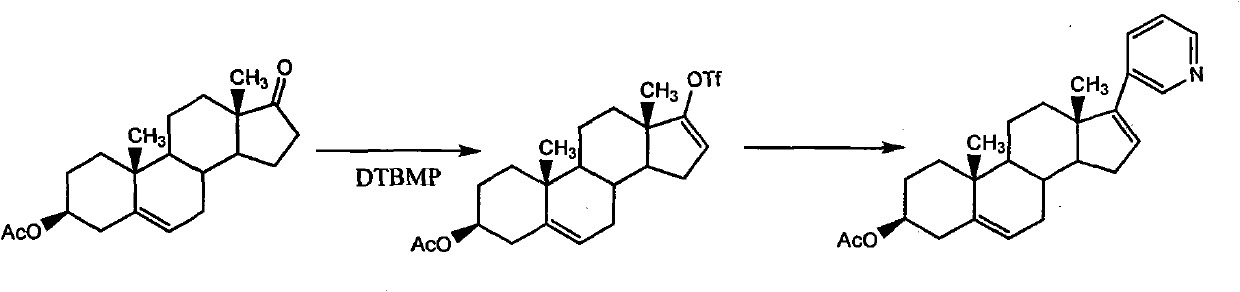
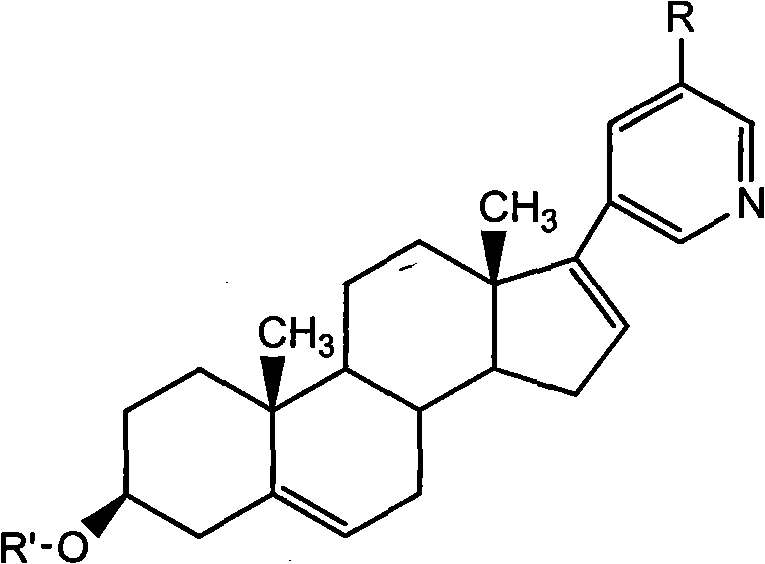
The invention relates to a preparation method of high-purity abiraterone acetate. The preparation method comprises that dehydroepiandrosterone acetate and trifluoromethanesulfonic anhydride undergo a sulfonylation reaction in the presence of an inorganic base. The preparation method obviously improves the yield of abiraterone acetate, greatly reduces by-products, has a low cost and no odor or strong smell, can be operated easily and is especially suitable for industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
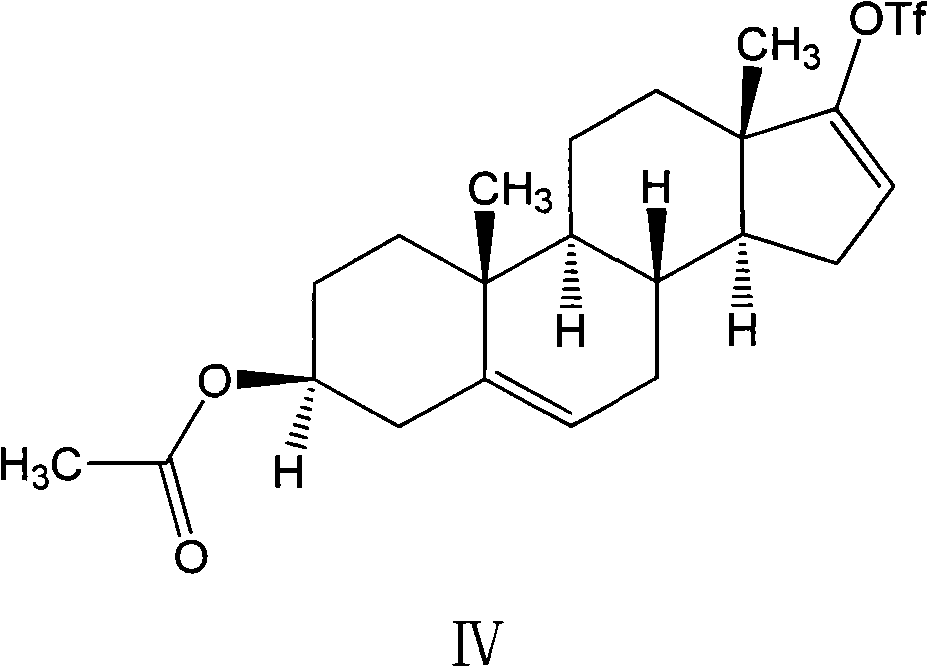
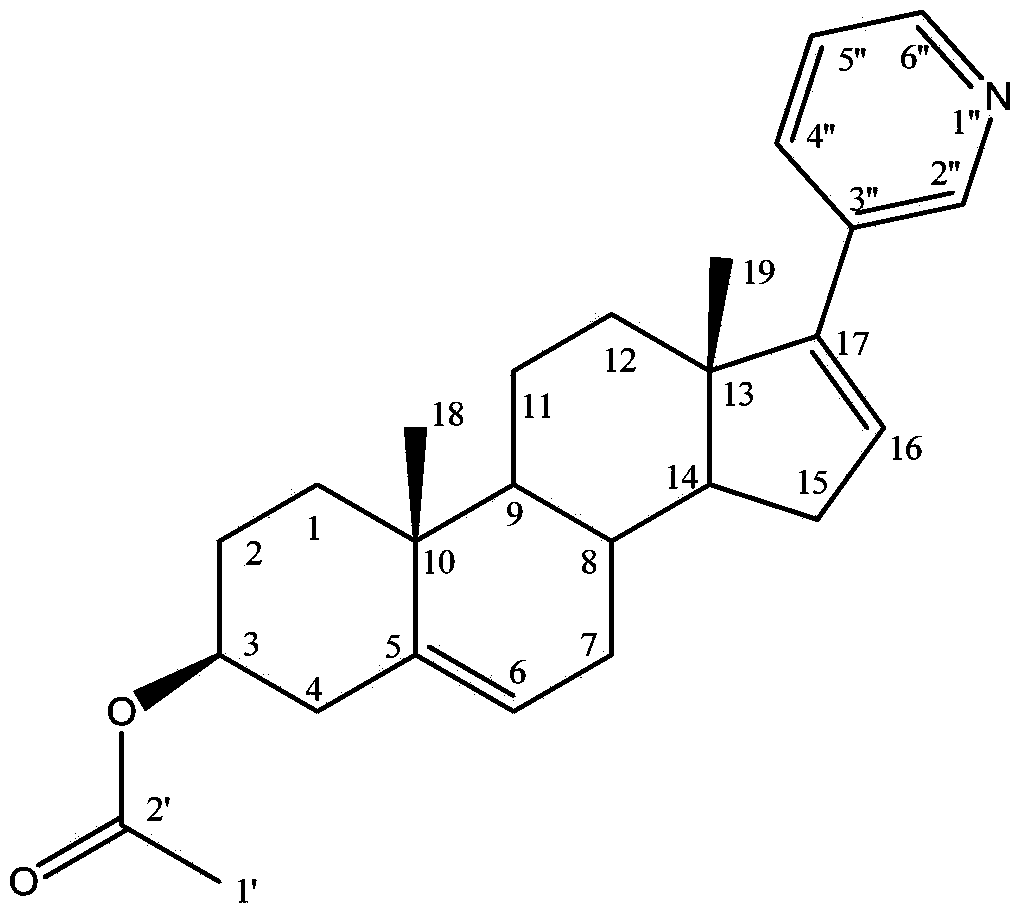
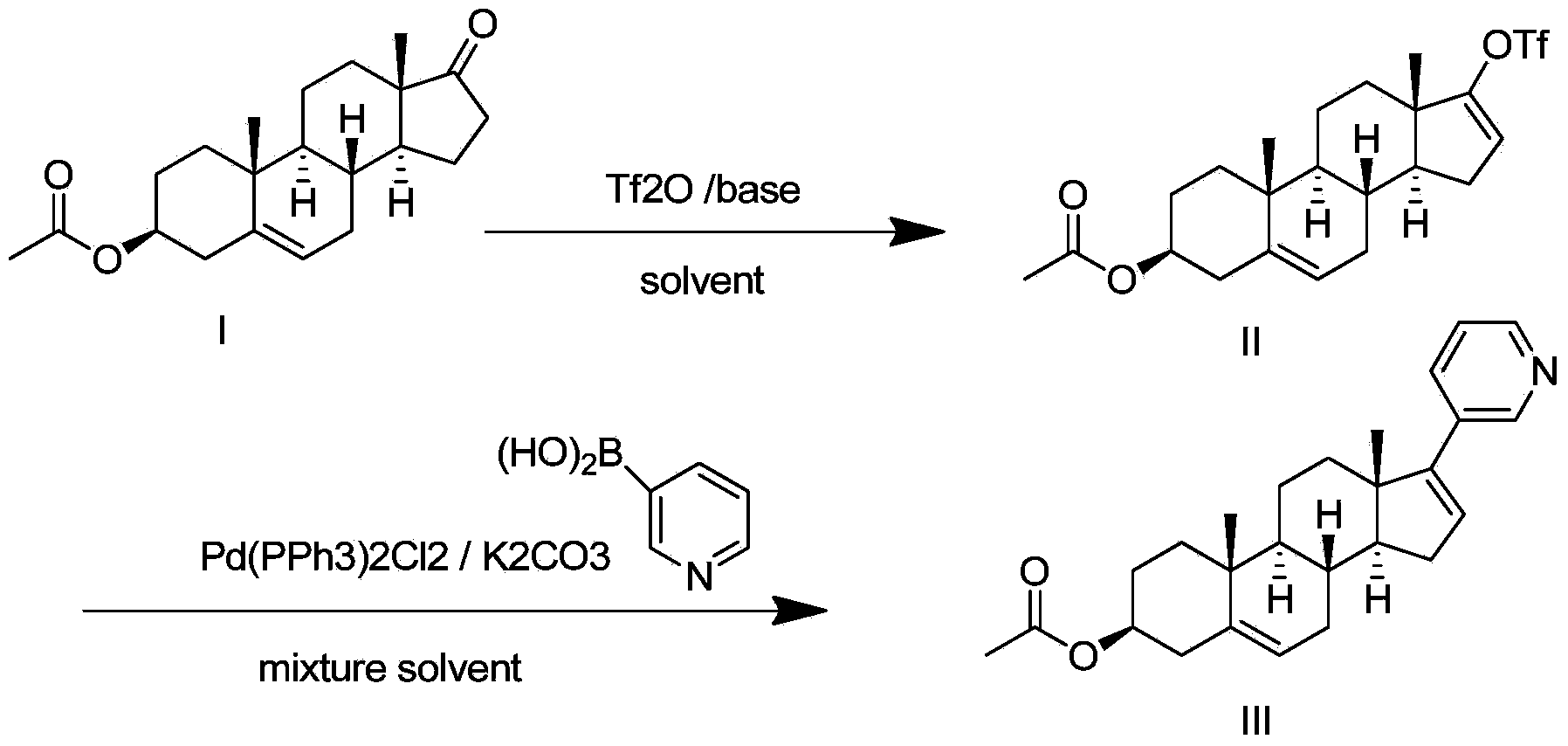
Preparation method for abiraterone acetate
ActiveCN103965282AMild reaction conditionsLow costSteroidsTrifluoromethanesulfonic anhydrideOrganic base
The invention discloses a preparation method for abiraterone acetate. The preparation method comprises the following steps: dehydroepiandrosterone acetate and trifluoromethanesulfonic anhydride are subjected to sulfonyl reaction at the presence of an organic base catalyst to obtain a compound expressed in the formula II shown in the Specification; the compound and 3-pyridine organoboron componud or 3-pyridine organosilicon compound react at the presence of triphenyl phosphine palladium dichloride catalyst to obtain crude product of abiraterone acetate; the crude product is recrystallized in a proton solvent or a non-proton solvent to obtain abiraterone acetate crystal; the crystal is put into a diffluent solvent again to heat for dissolution and then the dissolved crystal is dropwise added to a less diffluent solvent and stirred for crystallizing solids to obtain micro-powder abiraterone acetate; the diffluent solvent is a mixture of more than two of acetone, ethanol and water at will and the less diffluent solvent is water. According to the preparation method, the course is reasonable, the operation is simple and convenient, the product quality is good, the yield is high, no column flowing, salifying and refining are needed in the whole technology process, the requirement for industrial scale is met and abiraterone acetate with the approximate grain size of 10 Mu m is obtained simultaneously.
Owner:WUHAN BIOCAUSE PHARMA DEV +1
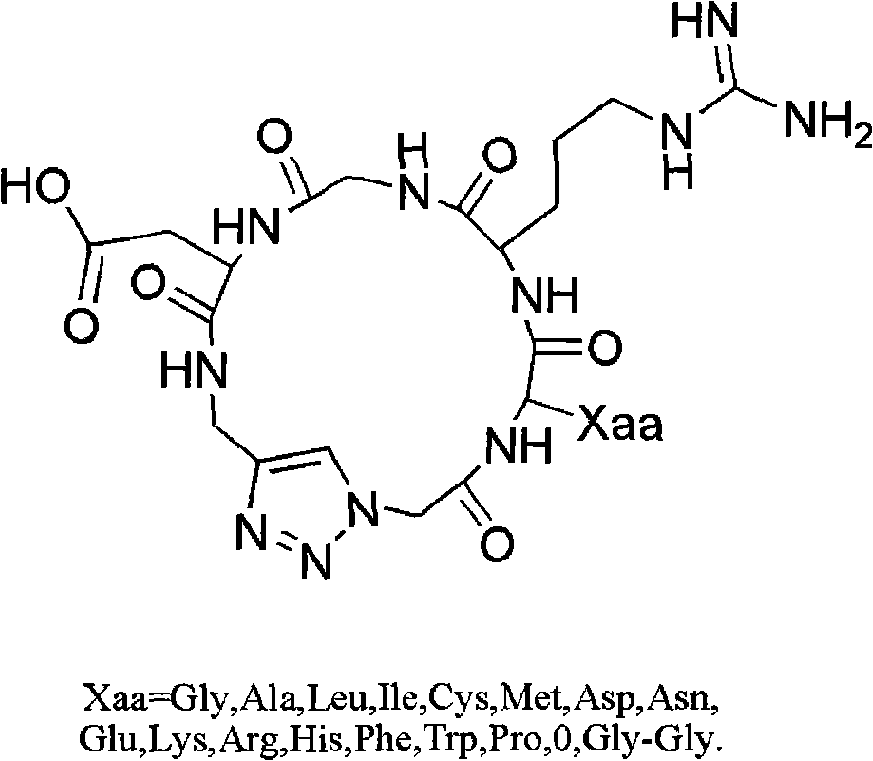
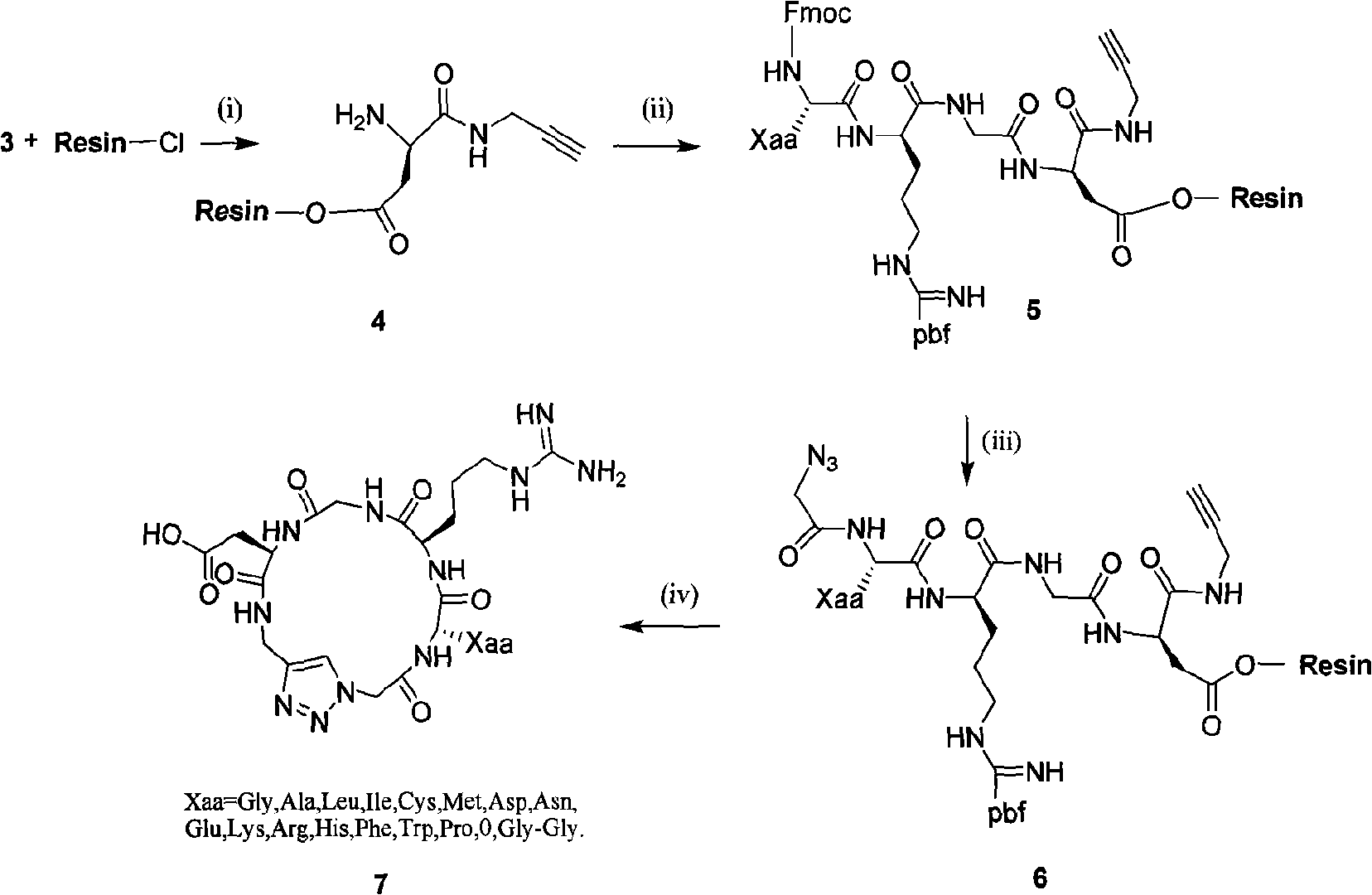
Integrin ligand cyclic peptide analogues and cyclization method
InactiveCN101492494AImprove biological activityThe cyclization method is simplePeptidesTrifluoromethanesulfonic anhydrideCyclic peptide
The invention relates to a cyclopeptide analog and a cyclization method thereof, which aims at providing an integrin ligand cyclopeptide analog and a cyclization method thereof. The cyclization method comprises the following steps: Azido-glycine is firstly generated by glycin with the effects of trifluoromethanesulfonic anhydride and Sodium azide; Fmoc-Asp-Propargyl is then generated by condensation with propargylamine in solution; after side-chain carboxyl of propargyl acyl aspartic acid is then hung on 2-CTC resin, linear tetrapeptide analogs are condensed in turn, and the linear tetrapeptide analogs are then cut down from the resin; and the cyclopeptide analog is synthesized in the condition of liquid phase (DCM is used as a solvent and CuBr / DBU is used as catalyst) after 4 to 6 hours of reaction at room temperature. The cyclopeptide analog and cyclization method has the following advantages: (1) the integrin ligand cyclopeptide analog in the invention is introduced with heterocyclic rings (triazole rings), thereby being capable of increasing biological activity; and (2) the cyclization method in the invention is more simple, convenient and high-efficient, and the condition is moderate.
Owner:ZHEJIANG UNIV
A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound
ActiveCN102276616AIncrease unsaturationMany substituentsOrganic chemistryFuranTrifluoromethanesulfonic anhydride
The invention relates to a method for synthesizing a furan[3,2-c] pyridine-4(5H) ketone compound, and belongs to the technical field of organic synthesis. The conventional synthetic method has the defects of complex synthetic steps, rigor reaction condition, high synthetic cost, low yield and few substituted groups and functional groups of the obtained products and the defect that the raw materials are not easily obtained. The method is characterized by comprising the following steps of: 1, mixing N,N-dimethylformamide (DMF) and triflate anhydride (Tf2O) at normal temperature in a molar ratioof (10-150):1, and stirring for 2 to 10 minutes; and 2, adding a cyclopropyl amide compound in an amount which is 0.3 to 1.0 molar time that of the triflate anhydride, heating to the temperature of between 70 and 120 DEG C, and stirring for 0.2 to 3.0 hours to generate the furan[3,2-c] pyridine-4(5H) ketone compound serving as a final product. The substituted groups and functional groups of the product are more, and the product can be used as an intermediate to participate in organic synthetic reaction further; and the synthetic cost is reduced substantially, and the yield is improved substantially.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
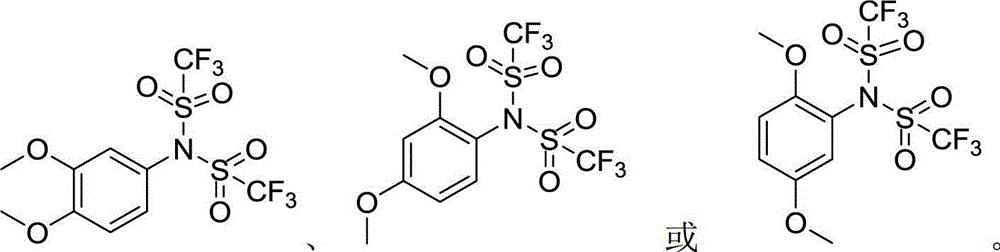
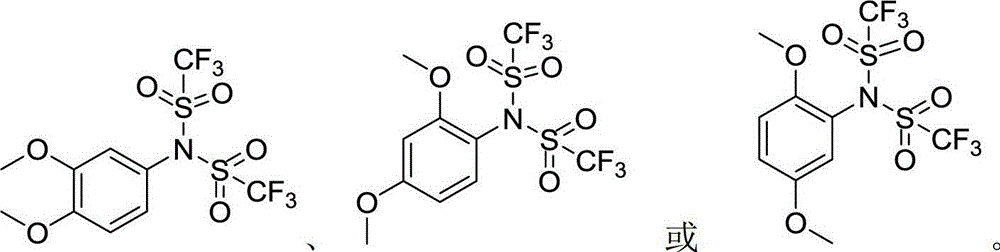
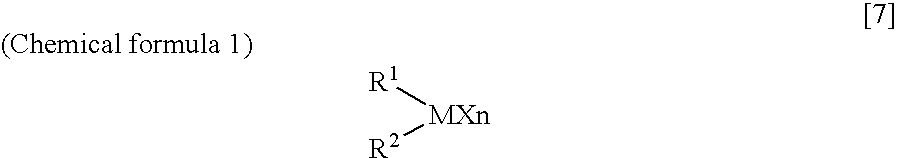
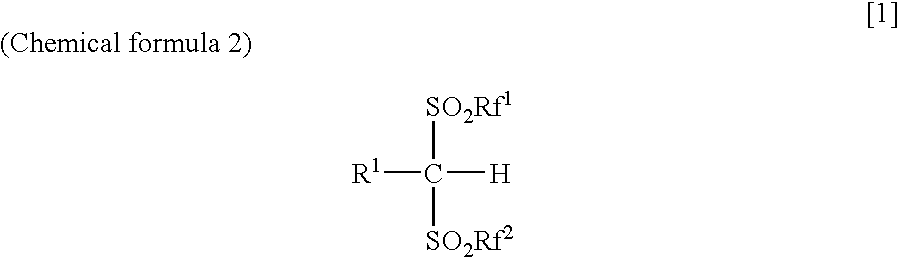
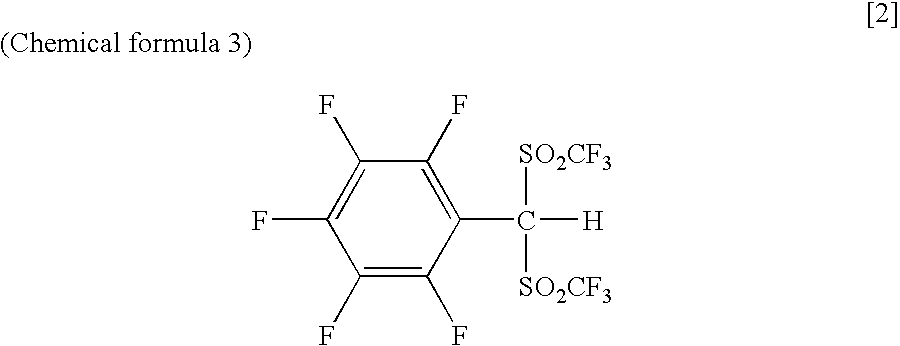
Arylbis(perfluoroalkylsulfonyl) methane and metallic salt thereof, and methods for producing the same
InactiveUS7193113B2Increase productionHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTrifluoromethanesulfonic anhydrideLithium
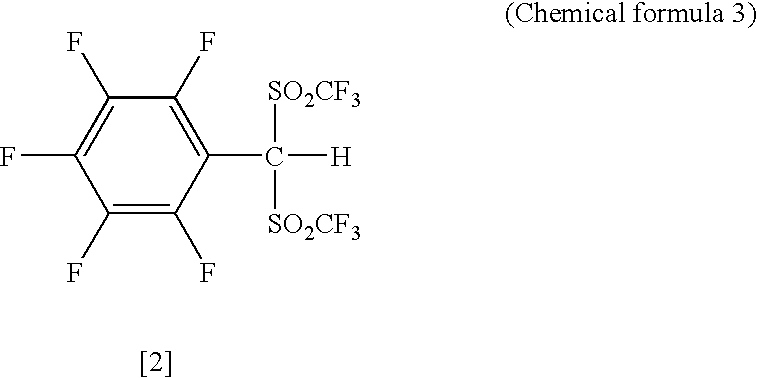
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis(trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
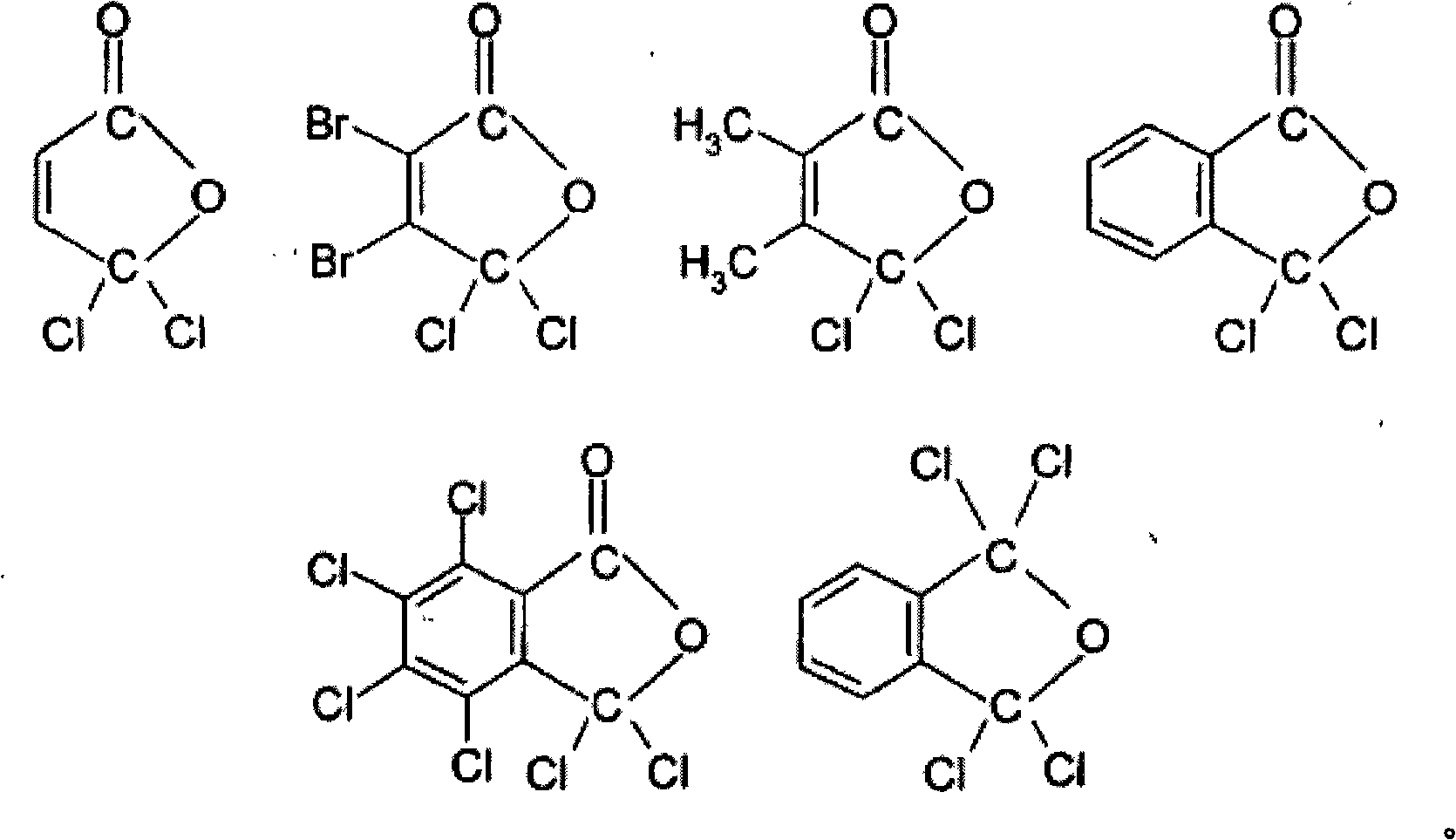
Process for preparing a sulphonic acid anhydride
ActiveCN101516836AOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideHalogen
The present invention relates to a process for preparing a sulphonic acid anhydride, and more particularly to the preparation of triflic anhydride. The process for preparing a sulphonic acid anhydride according to the invention is characterized in that it comprises reacting a sulphonic acid or a mixture of two sulphonic acids with a reactant exhibiting pseudo acid halide tautomerism with at least one carbon atom which is involved in the tautomerism bearing two halogen atoms.
Owner:RHODIA OPERATIONS SAS
Synthesis method of trifluoromethyl thioester compound
ActiveCN112358427AWide variety of sourcesLow priceOrganic chemistryTrifluoromethanesulfonic anhydrideSimple Organic Compounds
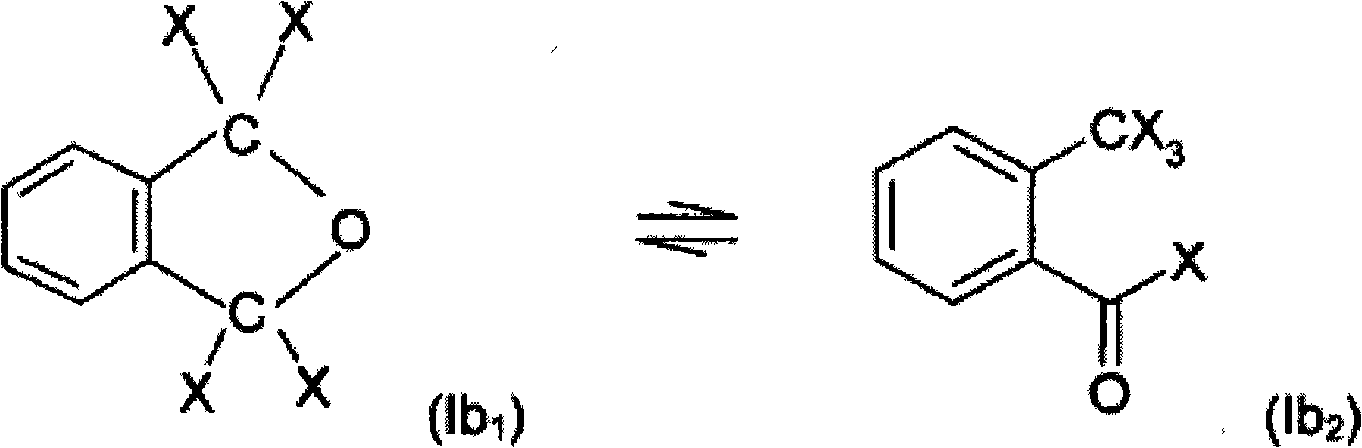
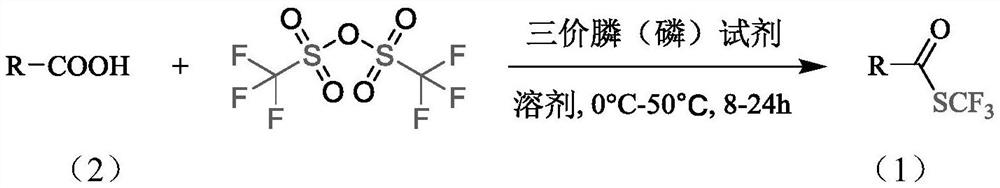
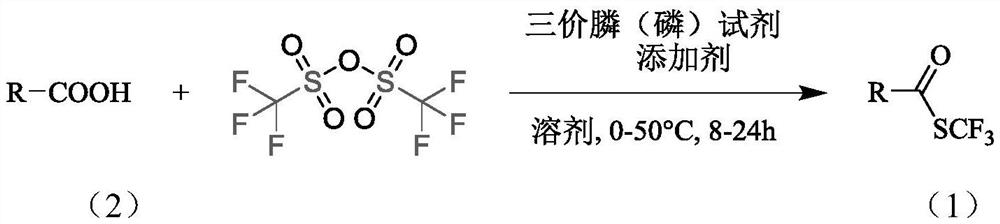
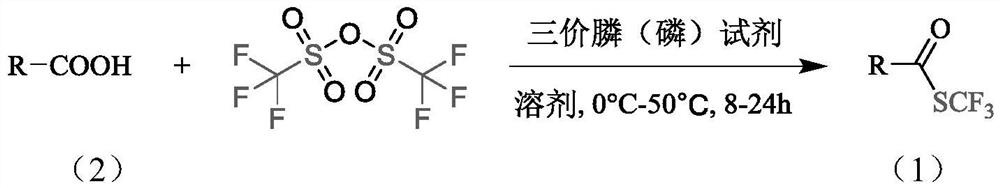
The invention belongs to the technical field of organic compound synthesis, and relates to a synthesis method of a trifluoromethyl thioester compound. The invention relates to the method for synthesizing a trifluoromethylthioester compound, which is used for synthesizing the trifluoromethylthioester compound by using trifluoromethanesulfonic anhydride as a trifluoromethylsulfur source. The methodcomprises the following steps: by taking a compound with carboxyl and trifluoromethanesulfonic anhydride as raw materials, constructing a carbon-sulfur bond under the activation of a trivalent phosphine reduction reagent, and separating and purifying to obtain the trifluoromethylthioester compound. According to the synthesis method of the trifluoromethyl thioester compound, the raw material sourceis wide, the price of a reaction reagent is low, the synthesis cost of the trifluoromethyl thioester compound is remarkably reduced, industrial production is facilitated, the synthesis conditions aremild and can also be carried out in a normal-pressure air atmosphere, the operation is simple and safe,metal participation is not needed, and environmental protection is achieved.
Owner:SHANDONG UNIV
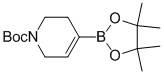
Method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester
InactiveCN102153579AShort process routeThe synthesis process is simpleGroup 3/13 element organic compoundsTrifluoromethanesulfonic anhydrideChemical synthesis
The invention provides a novel method for synthesizing N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester, which belongs to the technical field of chemical synthesis. The method comprises the following steps of: obtaining a target product, namely the N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridine-4-boronic acid pinacol ester through a three-step reaction by taking N-(tert-butoxycarbonyl)-4-piperidone, 4-aminopyridine, trifluoromethanesulfonic anhydride, n-butyl lithium, bis(pinacolato)diboron, triethylamine, diisopropylamine and potassium acetate as raw materials,dichloromethane, tetrahydrofuran and dioxane as solvents, and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) as a catalyst; and characterizing data through liquid chromatogram, nuclear magnetic spectrum and mass spectrum. By the method, the production period is short, the synthetic cost is low, a synthetic process is safe and reliable, and a post-treatment method is simple, convenient and quick; and the yield of a product is high (51 to 58 percent) and the purity of the product is high (98.2 to 99.6 percent).
Owner:LANZHOU MINUO BIOLOGICAL TECH
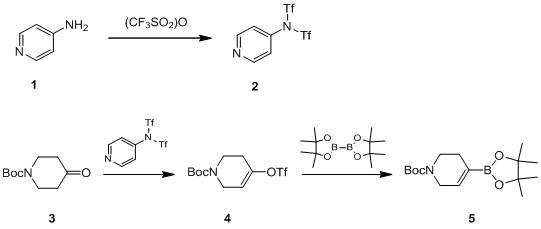
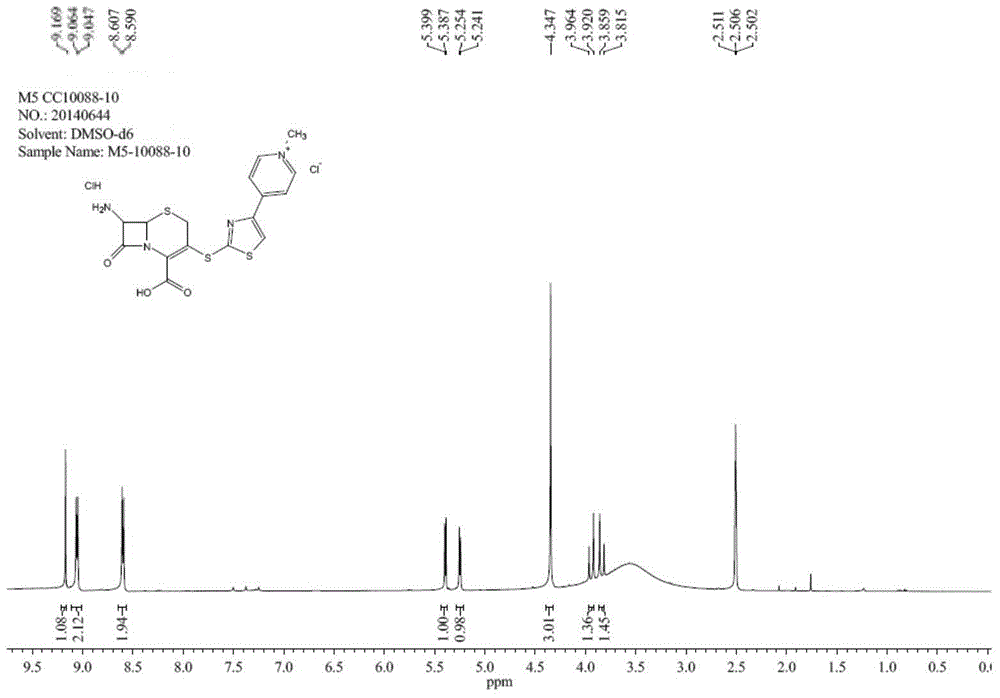
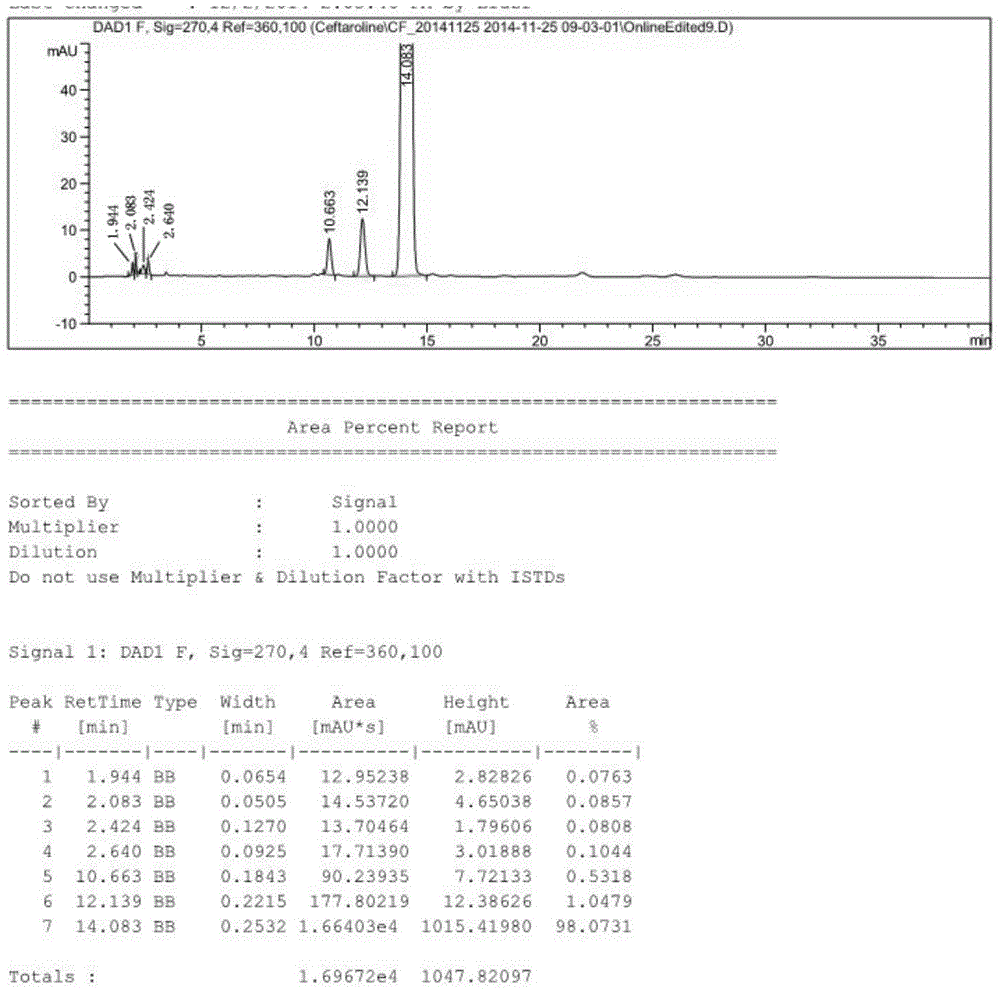
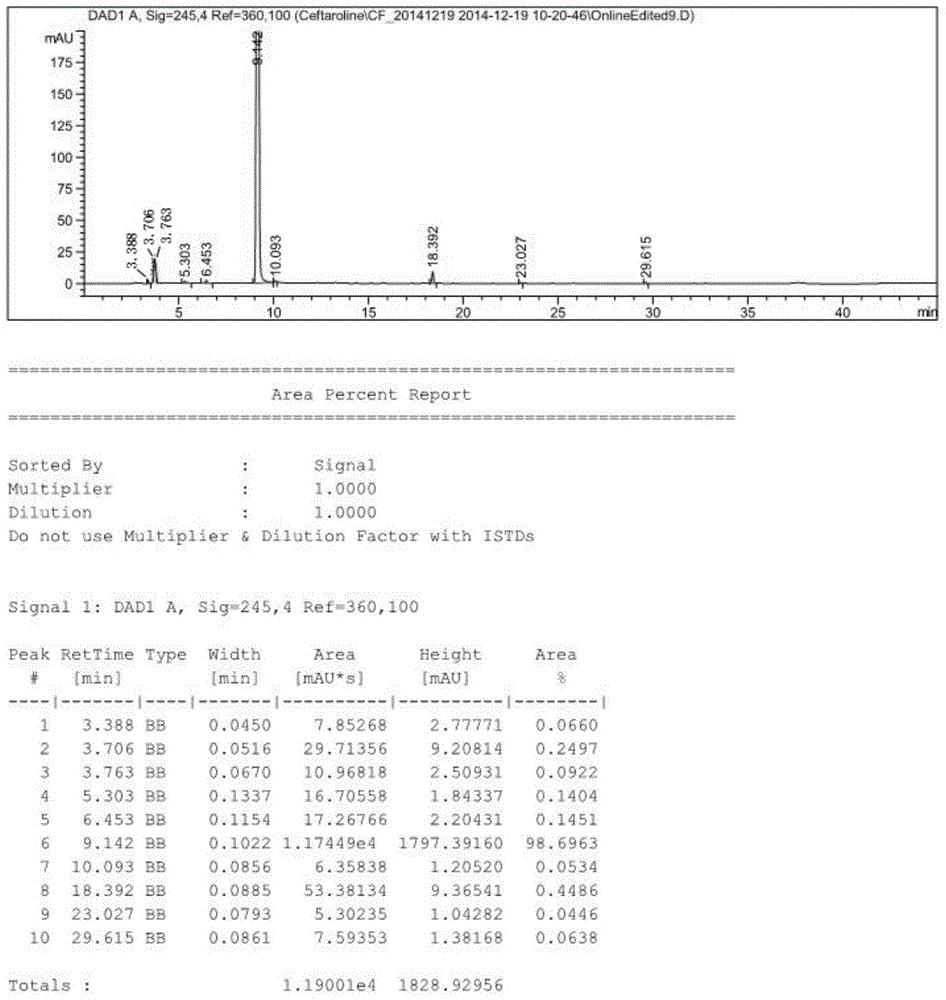
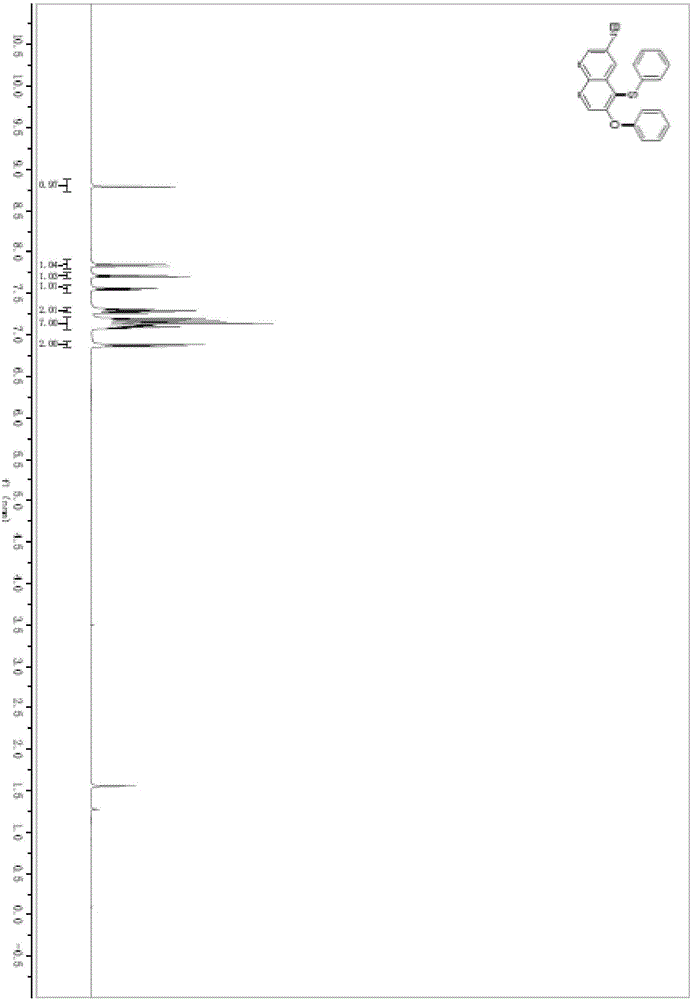
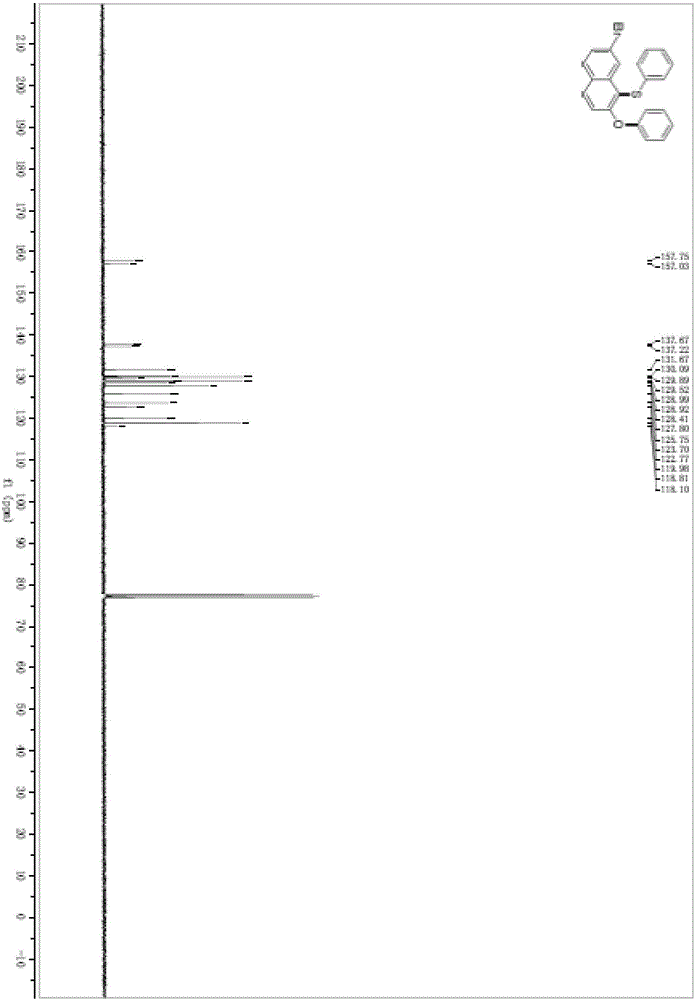
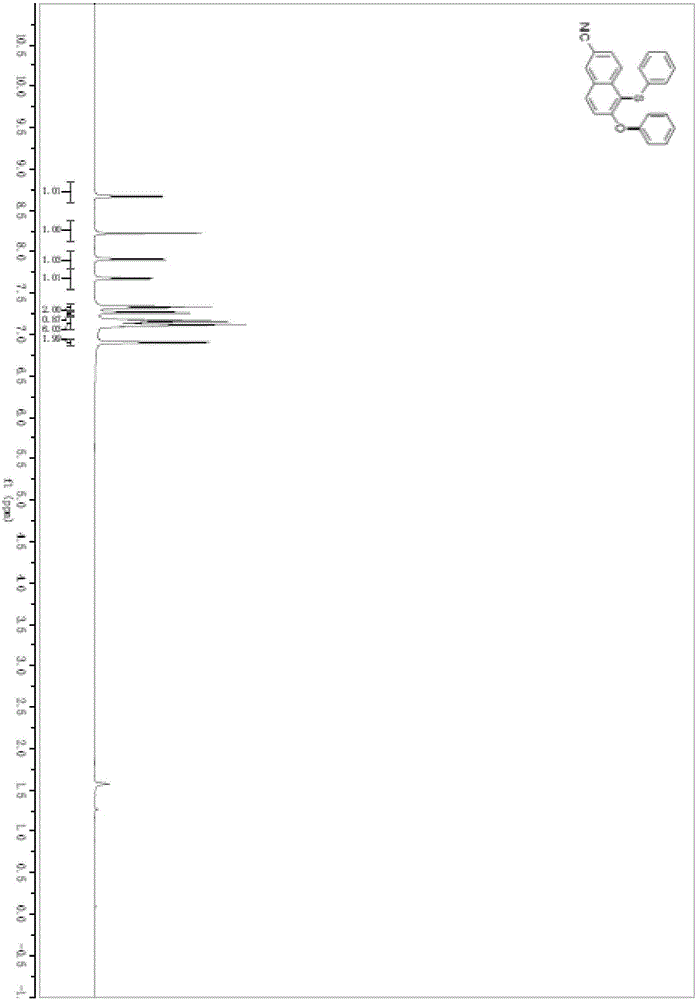
Preparation method of ceftaroline fosamil intermediate parent nucleus
ActiveCN104910185AOrganic chemistryBulk chemical productionTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of ceftaroline fosamil intermediate parent nucleus, which comprises the following steps: 3-hydroxycephem and an activation reagent are reacted under existence condition of an acid binding agent and an organic solvent to obtain an activated intermediate; the activation reagent comprises p-toluene sulfonyl chloride, benzenesulfonyl choride, 4-nitrobenzene sulfonyl chloride, trifluoroactic anhydride or trifluoromethanesulfonic anhydride; the activated intermediate and 4-(4-pyridyl)-2-mercaptothiazole are reacted, then is reacted to a quaternized reagent to obtain pyridinium salt; and then deprotection is carried out to obtain the ceftaroline fosamil intermediate parent nucleus. Compared with the prior art, The p-toluene sulfonyl chloride, benzenesulfonyl choride, 4-nitrobenzene sulfonyl chloride, trifluoroactic anhydride or trifluoromethanesulfonic anhydride are used for substituting the usage of a severe toxicity material, reaction security is increased, due to increasing steric hindrance of an activating group, rate for generating a delta-3 isomer is reduced, and purity and yield of the reaction products are increased.
Owner:国药集团致君(苏州)制药有限公司
Preparation method of thioether compound and product of preparation method
InactiveCN106748927AEliminate problems such as metal residueImprove biological activitySteroidsSulfide preparationTrifluoromethanesulfonic anhydrideAcetonitrile
The invention discloses a preparation method of a thioether compound and a product of the sulfoether compound. The method comprises the following steps: adding a sulfoxide compound into acetonitrile or dichloromethane under the protection of gas which does not react with a substrate, cooling the mixture, then adding trifluoromethanesulfonic anhydride or trifluoroacetic anhydride, adding an arylphenol compound, keeping the temperature and stirring for 2 to 4 hours, adding potassium phosphate, heating to 65 to 75 DEG C, stirring for 20 to 28 hours, and carrying out extraction and concentration chromatography to obtain a target product. The method for preparing the sulfoether compound has the characteristics of simplicity, efficiency, mildness and economy.
Owner:NANJING FORESTRY UNIV
Synthetic method applicable to industrial production of Abiraterone acetate
InactiveCN102558274ASuitable for mass industrial productionEasy to operateSteroidsTrifluoromethanesulfonic anhydrideHigh volume manufacturing
The invention discloses a synthetic method applicable to industrial mass production of Abiraterone acetate. According to the method, Abiraterone acetate is prepared through the steps of acylation, condensation and purification; in the step of acylation, dehydroepiandrosterone acetate is used as a raw material, the solvent of dichloromethane is added to dissolve dehydroepiandrosterone acetate under stirring, trifluromethanesulfonic anhydride is added at low temperature, a reaction at low temperature is maintained, then an alkaline catalyst is added at low temperature, and a reaction at room temperature is carried out. The method provided in the invention enables reaction byproducts to be reduced, operation to be simplified and purity of an HPLC test product to be more than 97% and is applicable to industrial mass production.
Owner:深圳万乐药业有限公司
Method for preparing Niraparib of PARP (poly-ADP-ribose polymerase) inhibitor
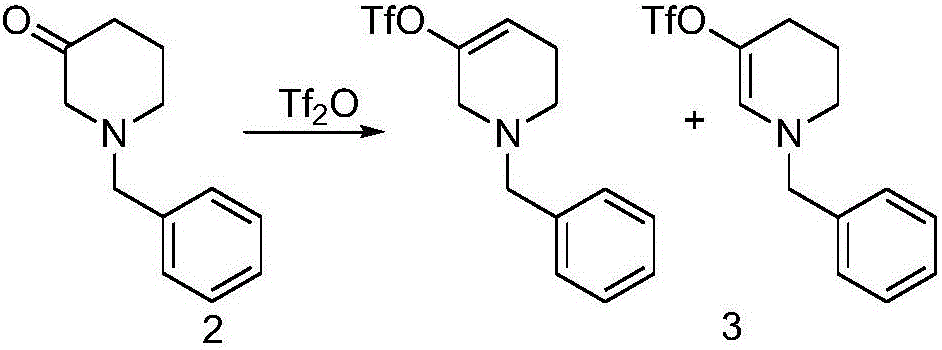
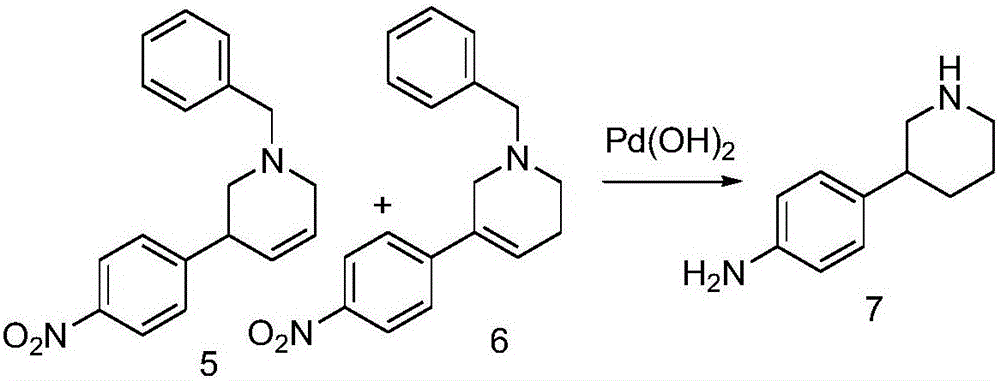
The invention discloses a method for preparing 2-(4-((3S)-3-piperidyl) phenyl)-2H-indazole-7-formamide of a compound. Benzyl protected-piperidone is generated into piperidone of 3-triflic anhydride under the action of triflic anhydride, the piperidone and nitrobenzoic acid are subjected to Suzuki reaction to obtain a coupled product, 3-(4-aminophenyl) piperidine is obtained under the action of a palladium reagent, (S)-3-(4-halogenated phenyl) piperidine is prepared with a chirality resolution reagent, the (S)-3-(4-halogenated phenyl) piperidine is condensed with 3-formyl-2-methyl nitrobenzoate to form pyrazolone ring under the action of sodium azide, and the Niraparid (with the molecular entity of 2-(4-((3S)-3-piperidyl) phenyl)-2H-indazole-7-formamide) is prepared via aminolysis.
Owner:NANJING CORE TECH CO LTD
One pot synthesis of tetrazole derivatives of rapamycin
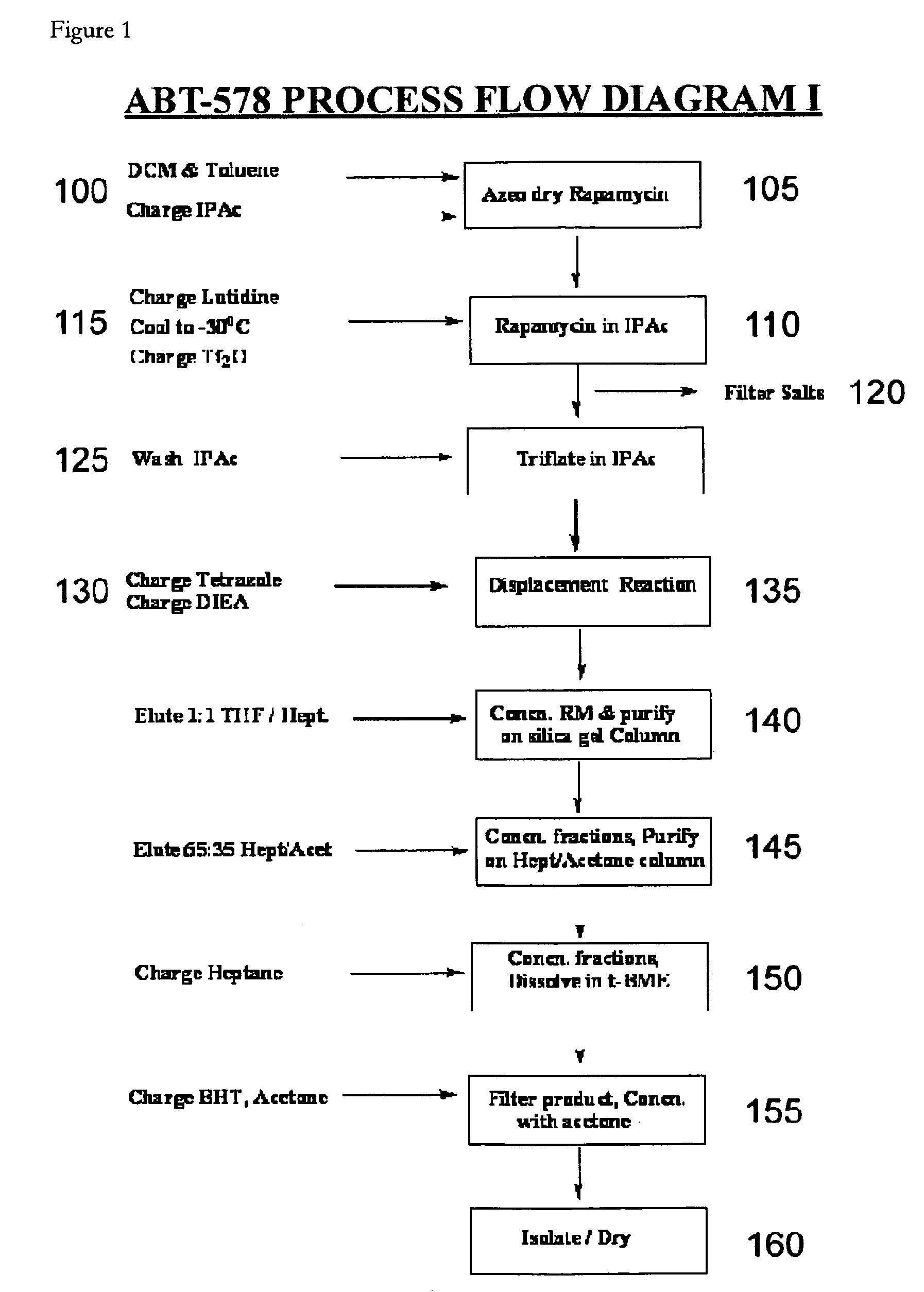
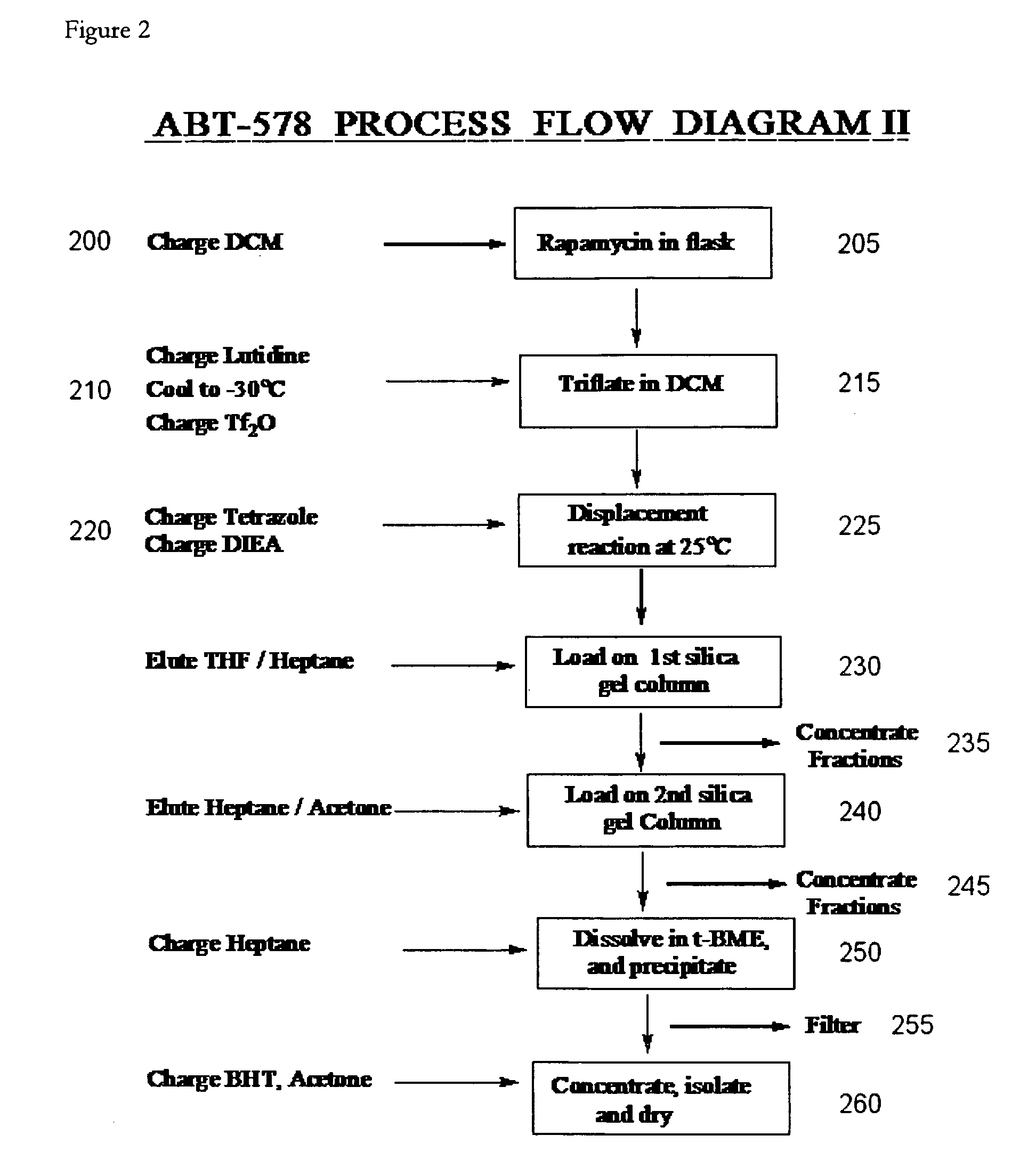
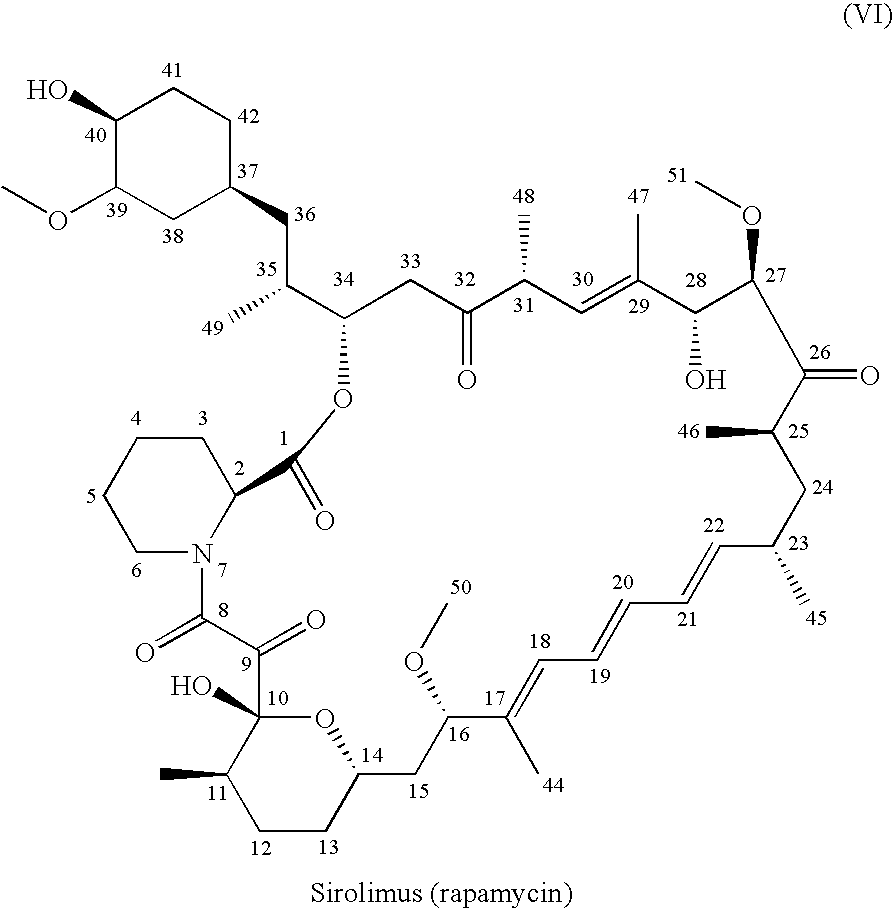
A single-step, one-pot process to obtain zotarolimus and other rapamycin derivatives on large scale is presented, which improves currently available synthesis schemes. In one embodiment, dried rapamycin is dissolved in isopropylacetate (IPAc). The solution is cooled, and 2,6-Lutidine is added, followed slowly adding triflic anhydride at −30° C. Salts are then removed by filtration. Tetrazole, followed by a tert-base diisopropylethylamine (DIEA) is added to the triflate solution. After incubation at room temperature, the product is concentrated and purified by a silica gel column using THF / heptane as eluant. The fractions containing the product are collected, concentrated, and purified again using an acetone / heptane column. The product containing fractions are concentrated. The product is dissolved in t-BME and precipitated with heptane. The solids are dissolved in acetone, treated with butylated-hydroxy toluene (BHT), and the solution concentrated. The process is repeated twice with acetone to remove solvents. At least one stabilizing agent is added, such as BHT at 0.5% before drying.
Owner:ABBOTT LAB INC
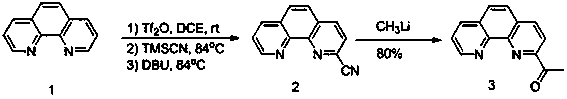
Method for synthesizing 2-acetyl-1, 10-phenanthroline (3)
ActiveCN110003203AHigh yieldReduce the impactOrganic chemistryTrifluoromethanesulfonic anhydrideLithium
The invention discloses a method for synthesizing 2-acetyl-1, 10-phenanthroline (3). The method comprises the following steps: using 1, 10-phenanthroline as a raw material to synthesize 2-cyano-1, 10-phenanthroline (2) by reacting with trimethylcyanosilane, trifluoromethanesulfonic anhydride, 1, 8-diazabicycloundec-7-ene; and then reacting 2-cyano-1, 10-phenanthroline (2) with methyl lithium to prepare 2-acetyl-1, 10-phenanthroline (3). The method provided by the invention prepares 2-acetyl-1, 10-phenanthroline (3) by adopting a two-step synthesis method, is beneficial to the increase of the yield, and purifies by quenching with a saturated sodium bicarbonate solution, extracting with dichloromethane and washing with a saturated sodium chloride solution after completing a first step, so that the influence of impurities generated in the first step on the reaction in a second step is minimized.
Owner:BEIJING YANLIAN CHEM TECH CO LTD
Composite fluorine removing agent for removing high-concentration fluorine-containing wastewater and application method thereof
InactiveCN102464394AReduce dosageLow running costWater contaminantsWater/sewage treatmentTrifluoromethanesulfonic anhydrideMagnesium salt
The invention discloses a composite fluorine removing agent for removing high-concentration fluorine-containing wastewater and an application method thereof, which belong to the field of sewage treatment in environment protection. The composite fluorine removing agent is prepared by compounding polyhydroxyl or polycarboxyl compounds and inorganic matters according to a certain proportion, wherein the polyhydroxyl or polycarboxyl compounds include one or more than two of trifluoromethanesulfonic anhydride, caprylyl chloride, 1R-(-)-camphorsulfonic acid, 3-furoic acid, pentanoyl chloride, and butanetetracarboxylic acid; and the inorganic matters include two or more than two of calcium salt, magnesium salt, phosphate, and ferric salt. The composite fluorine removing agent is added into the high-concentration fluorine-containing wastewater in a weight ratio of 1:100 to 1:1000 according to the concentration of fluorine in the wastewater; the pH value of the wastewater is adjusted to 9.0-11.0 with sodium hydroxide; and the wastewater is stirred for 15-30min, so that the yielding water can meet the national emission standard I. The composite fluorine removing agent and the application method thereof disclosed by the invention have the characteristics of simple technological process, smaller dosage of chemical agent, lower running expense, no sludge, high removal rate, and the like; and the removal rate can reach above 99.9%.
Owner:CHANGZHOU YAHUAN ENVIRONMENTAL PROTECTION TECH
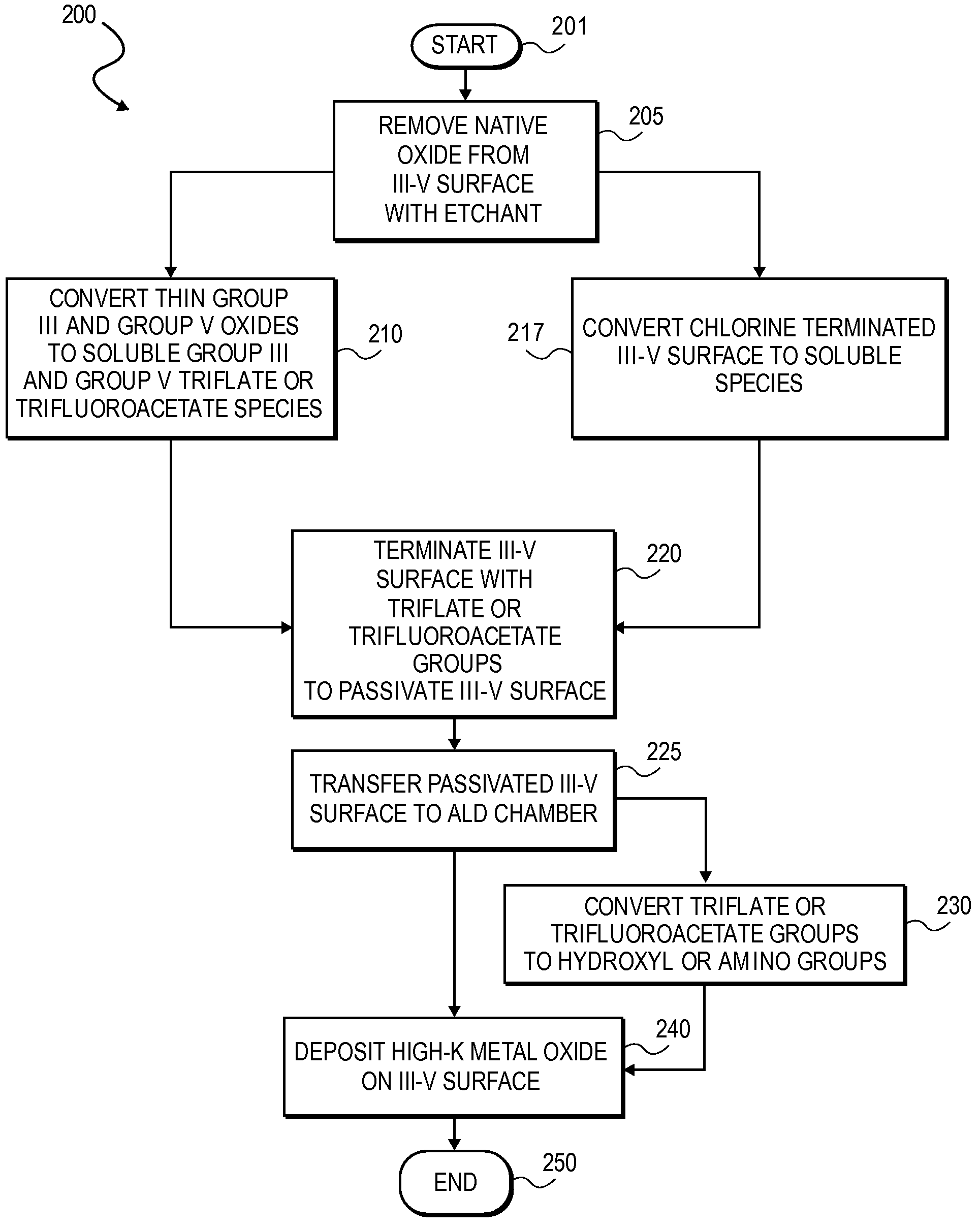
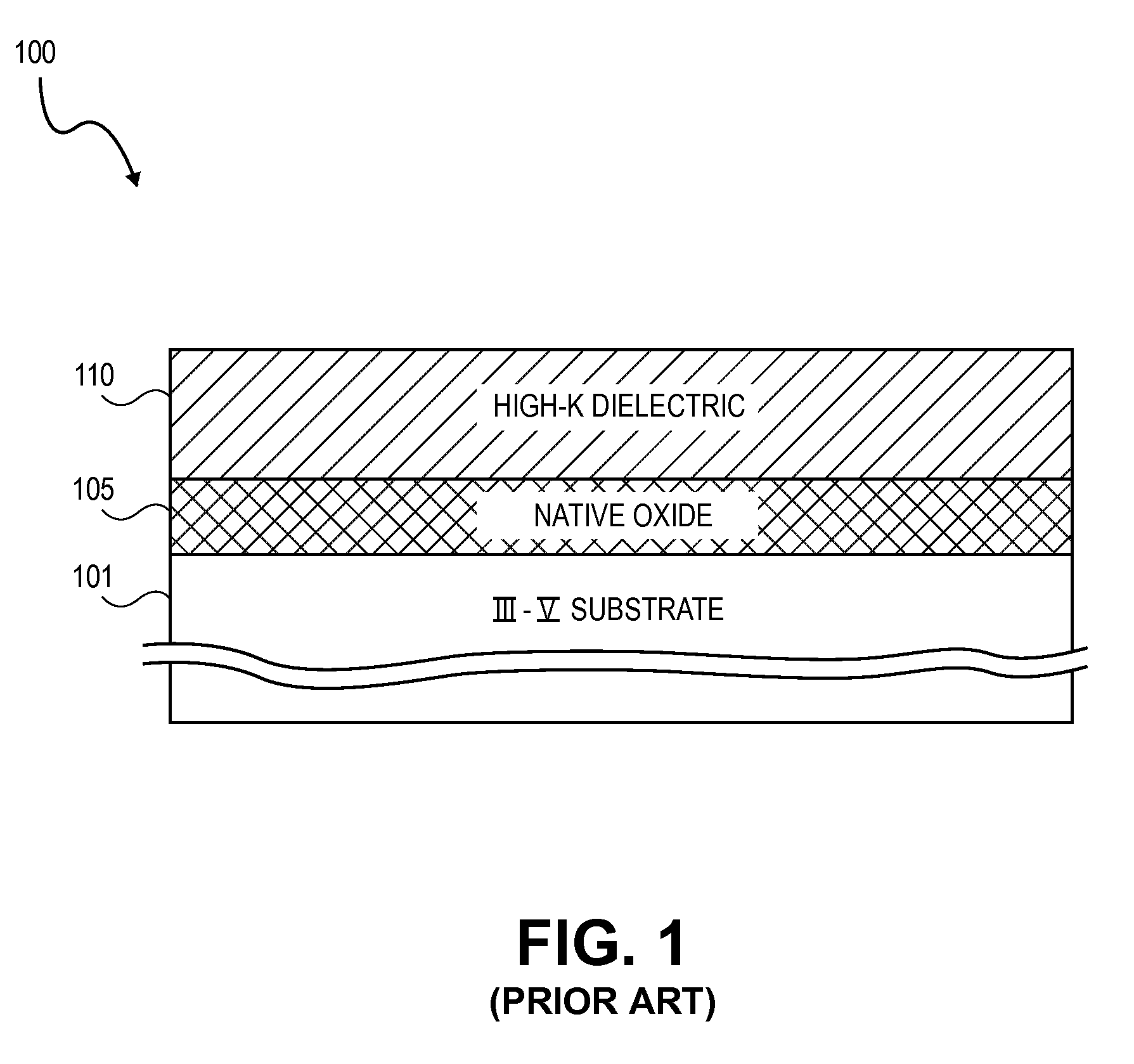
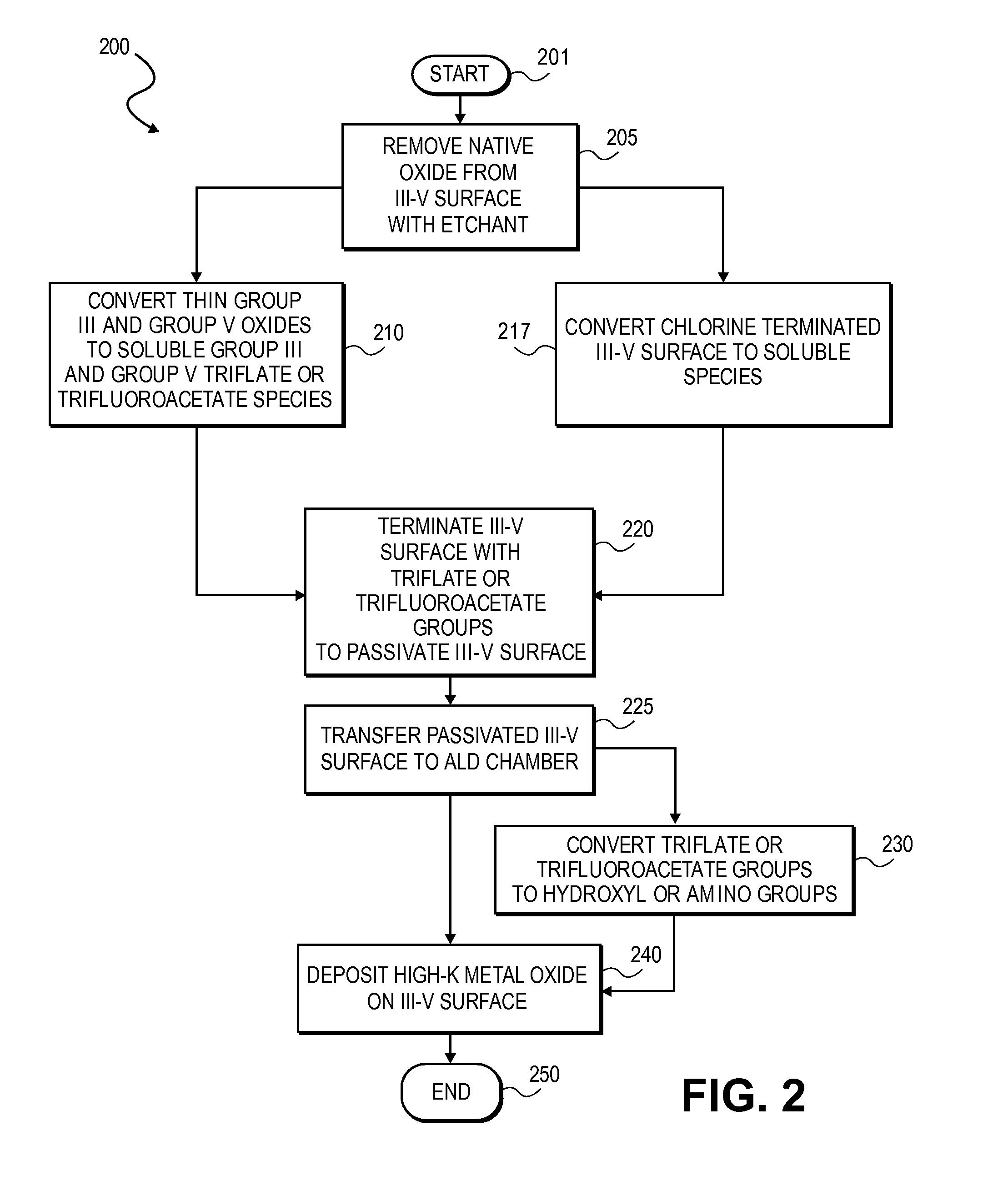
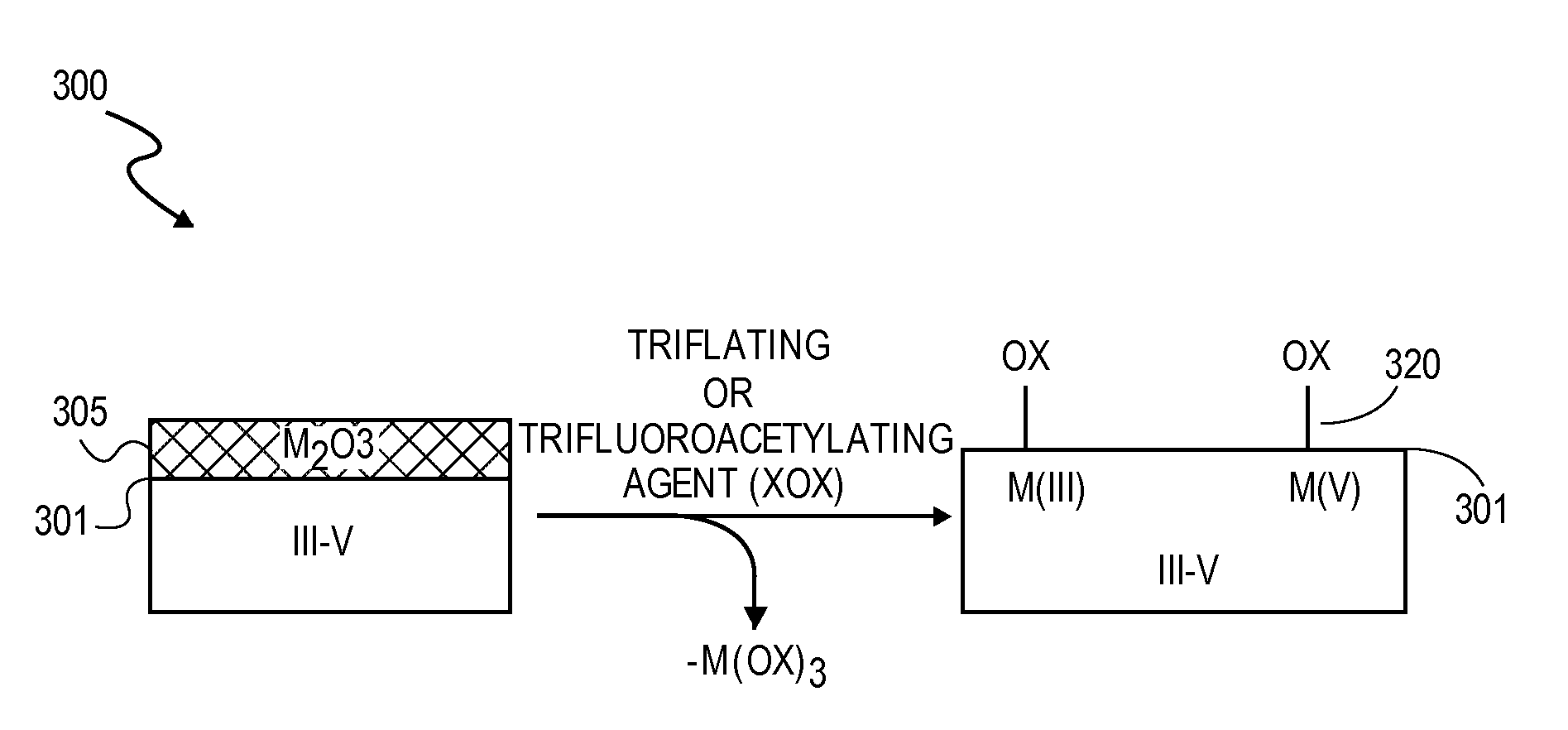
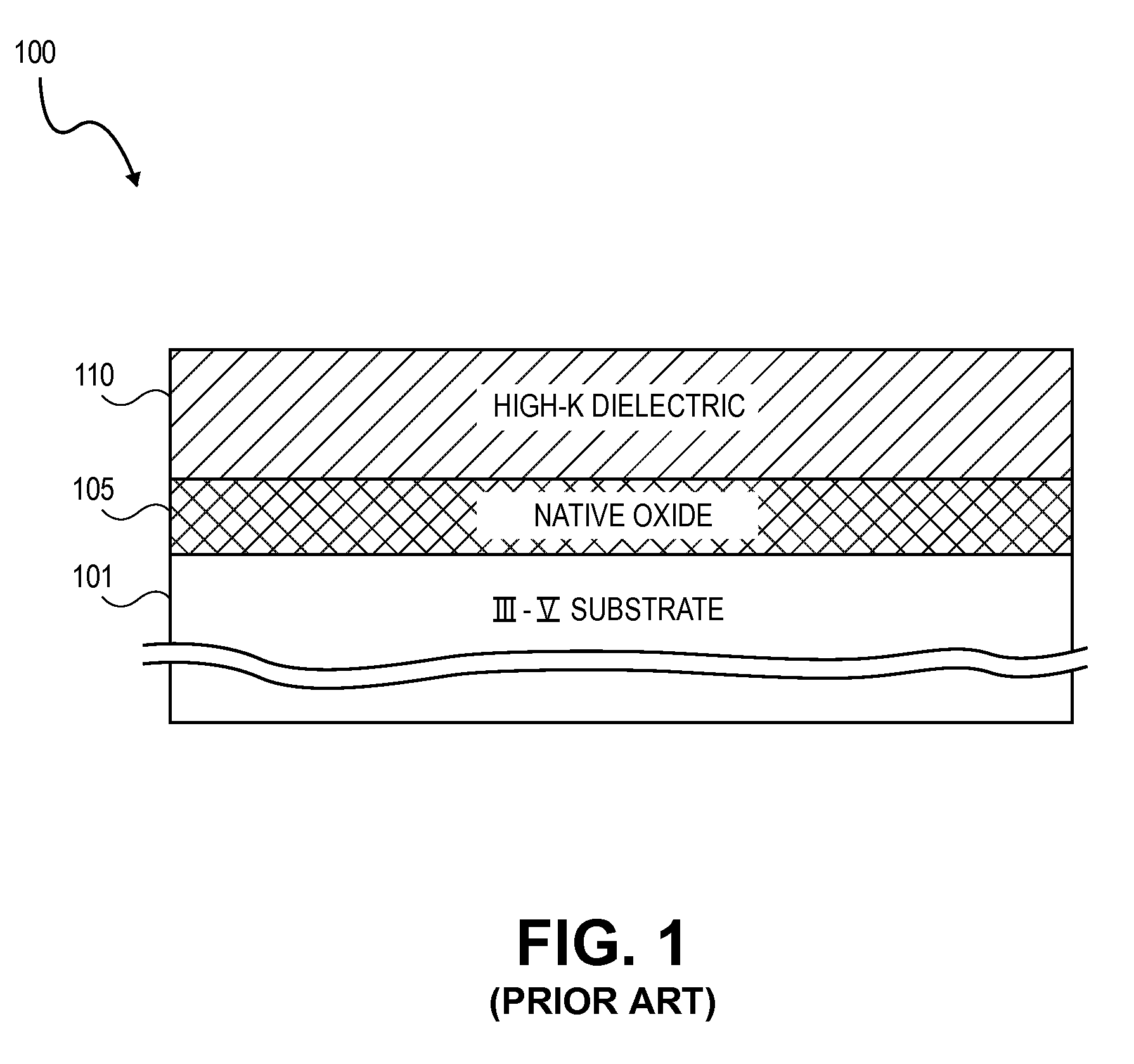
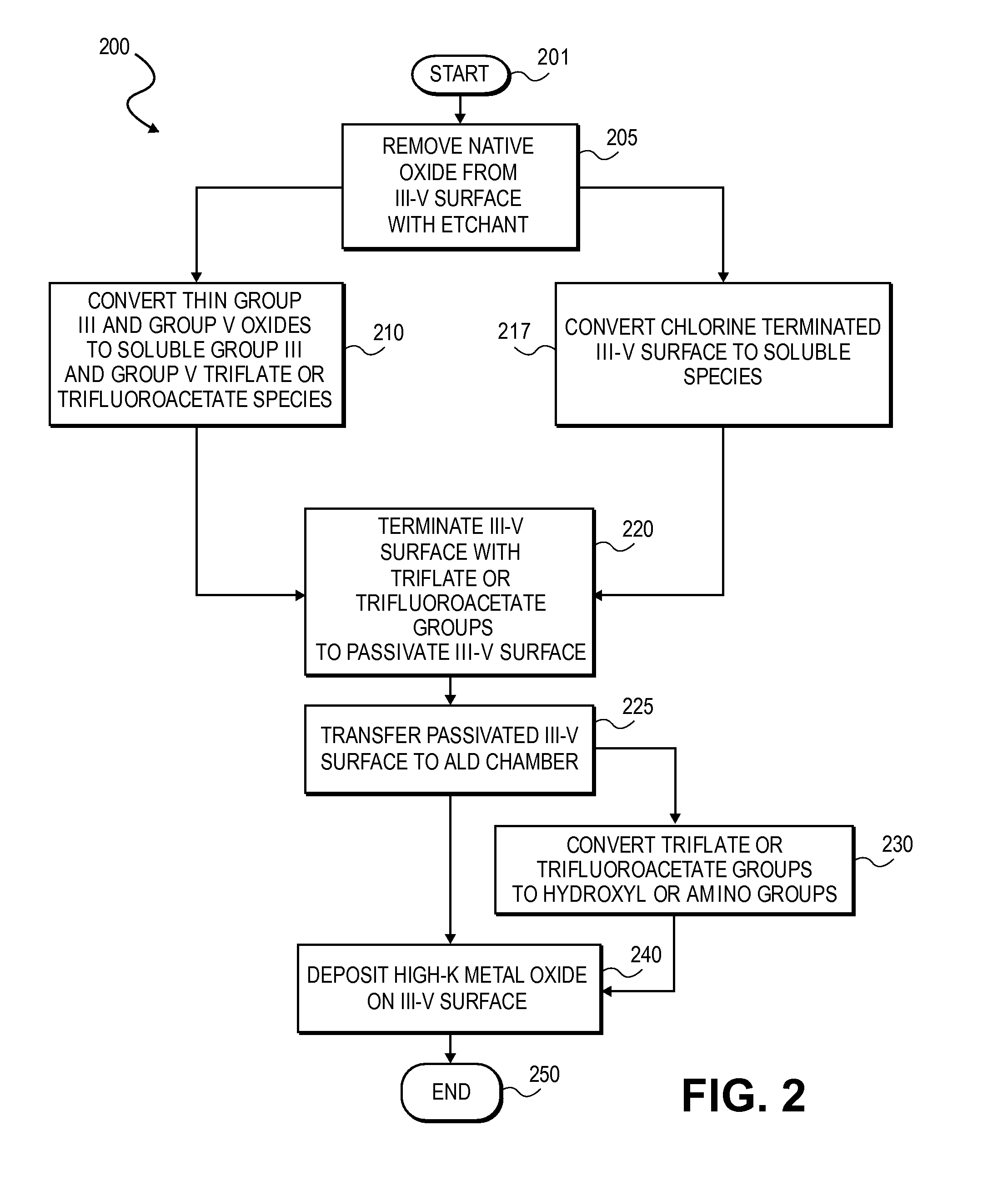
High K dielectric growth on metal triflate or trifluoroacetate terminated III-V semiconductor surfaces
InactiveUS7763317B2Polycrystalline material growthSemiconductor/solid-state device manufacturingTrifluoromethanesulfonic anhydrideTriflic acid
Surface preparation of a compound semiconductor surface, such as indium antimonide (InSb), with a triflating agent, such as triflic anhydride or a trifluoroacetylating agent, such as trifluoroacetic anhydride is described. In one embodiment, the triflating or trifluoroacetylating passivates the compound semiconductor surface by terminating the surface with triflate trifluoroacetate groups. In a further embodiment, a triflating agent or trifluoroacetylating agent is employed to first convert a thin native oxide present on a compound semiconductor surface to a soluble species. In another embodiment, the passivated compound semiconductor surface is activated in an ALD chamber by reacting the triflate or trifluoroacetate protecting groups with a protic source, such as water (H2O). Metalorganic precursors are then introduced in the ALD chamber to form a good quality interfacial layer, such as aluminum oxide (Al2O3), on the compound semiconductor surface.
Owner:INTEL CORP
Sulfonated polyetheretherketone/sulfonated chromium organic framework hybrid membrane, as well as preparation and applications thereof
InactiveCN103846024AHigh selectivityImprove solubility selectivitySemi-permeable membranesDispersed particle separationTrifluoromethanesulfonic anhydrideHydrofluoric acid
The invention discloses a sulfonated polyetheretherketone / sulfonated chromium organic framework hybrid membrane, as well as preparation and applications thereof. The hybrid membrane is composed of sulfonated polyetheretherketone and a sulfonated chromium metal organic framework. The preparation of the hybrid membrane comprises the following steps: reacting Cr(NO3)3.9H2O, terephthalic acid and hydrofluoric acid in water to obtain a chromium organic framework material; modifying by trifluoromethanesulfonic anhydride and concentrated sulfuric acid to obtain a sulfonated chromium organic framework material; mixing the sulfonated chromium organic framework material with a sulfonated polyetheretherketone solution to obtain a membrane casting solution, thereby preparing the hybrid membrane by tape casting. The hybrid membrane and the preparation of the hybrid membrane have the following advantages: raw materials are easy to obtain, the preparation process is simple and controllable, and the prepared sulfonated polyetheretherketone / sulfonated chromium organic framework hybrid membrane is applicable to CO2 / N2 gas separation and has high selectivity and high permeability.
Owner:TIANJIN UNIV
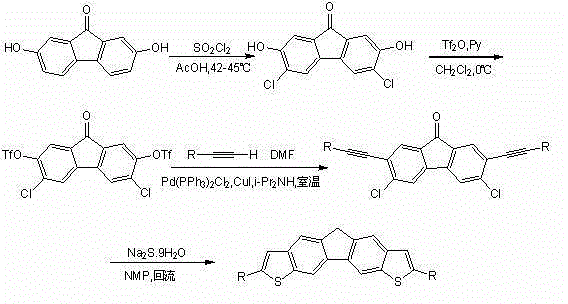
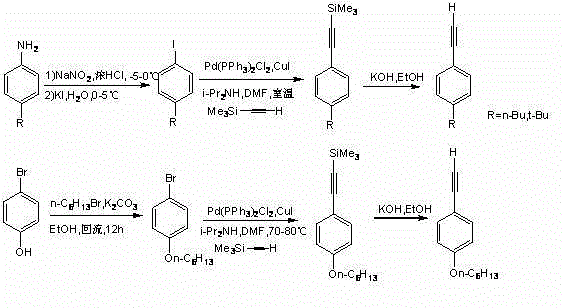
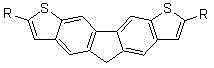
Bithienofluorene, its derivative and preparation method
The invention discloses bithienofluorene, its derivative and a preparation method. The compounds are characterized in that: a fluorene ring is in connection with thieno rings, and the 2- position of thiophene has a substituent group, which can be hydrogen, alkyl or aryl. The preparation method consists of: taking 2, 7-dyhydroxyl-9-fluorenone as a raw material, employing sulfonyl chloride to conduct chlorination so as to synthesize 3, 6-dichloro-2, 7-dyhydroxyl-9-fluorenone; and then adopting trifluoromethanesulfonic anhydride to carry out esterification, and performing a Sonogashira coupling reaction to obtain series of 2, 7-disubstituted ethynyl-3, 6-dichloro-9-fluorenone, and finally conducting ring closing by sodium sulfide and reduction to obtain bithienofluorene and its derivative. The series of compounds involved in the invention provides a new option for organic field effect transistor materials, and the preparation method provides a new method for aromatic ketone carbonyl reduction.
Owner:EAST CHINA NORMAL UNIV
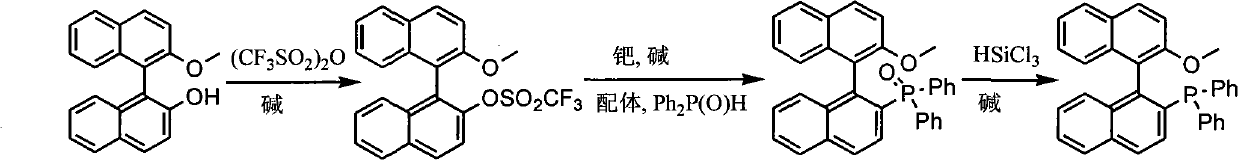
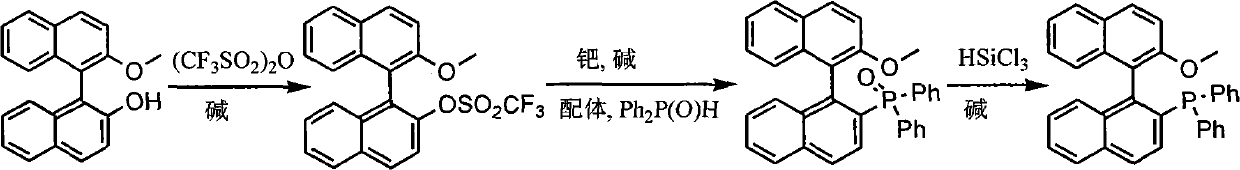
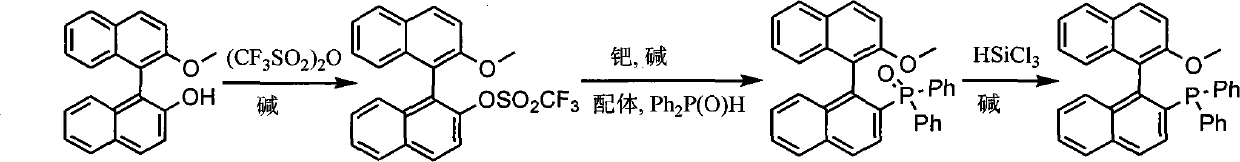
Synthesis method of chiral monophosphorus ligand
InactiveCN101885741AGroup 5/15 element organic compoundsTrifluoromethanesulfonic anhydrideMonomethyl ether
The invention discloses a synthesis method of chiral mono-phosphorus ligand 2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl. The chiral mono-phosphorus ligand is widely applied to the asymmetric catalysis field. The synthesis method comprises the following steps: allowing optically pure binaphthalene monomethyl ether to react with trifluoromethanesulfonic anhydride to obtain 2-trifluoromethyl sulfonyloxy-2'-methoxy-1,1'-binaphthyl; and allowing the obtained compound to react with diphenylphosphine oxide under the catalytic action of palladium to obtain 2-diphenylphosphine acyl-2'-methoxy-1,1'-binaphthyl, and then reducing with trichlorosilane to finally obtain the optically pure mono-phosphorus ligand 2-diphenylphosphine-2'-methoxy-1,1'-binaphthyl. The synthesis method has short synthetic route and high atom economy, thus greatly lowering cost; and the synthetic route is shown in the following reaction equation.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Method for producing trifluoromethanesulfonic anhydride
InactiveUS20090118543A1Avoid reactionImprove reaction efficiencyOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideTriflic acid
This method for producing trifluoromethanesulfonic anhydride reacting trifluoromethanesulfonic acid with phosphorus pentoxide to produce trifluoromethanesulfonic anhydride, wherein hardening of the reaction solution due to polyphosphoric acid, which is produced as a byproduct, is prevented by using an excess amount of trifluoromethanesulfonic acid with respect to the phosphorus pentoxide.
Owner:MITSUBISHI MATERIALS ELECTRONICS CHEM CO LTD +1
High k dielectric growth on metal triflate or trifluoroacetate terminated iii-v semiconductor surfaces
InactiveUS20080241423A1Polycrystalline material growthSemiconductor/solid-state device manufacturingTrifluoromethanesulfonic anhydrideTrifluoroacetic acid
Surface preparation of a compound semiconductor surface, such as indium antimonide (InSb), with a triflating agent, such as triflic anhydride or a trifluoroacetylating agent, such as trifluoroacetic anhydride is described. In one embodiment, the triflating or trifluoroacetylating passivates the compound semiconductor surface by terminating the surface with triflate trifluoroacetate groups. In a further embodiment, a triflating agent or trifluoroacetylating agent is employed to first convert a thin native oxide present on a compound semiconductor surface to a soluble species. In another embodiment, the passivated compound semiconductor surface is activated in an ALD chamber by reacting the triflate or trifluoroacetate protecting groups with a protic source, such as water (H2O). Metalorganic precursors are then introduced in the ALD chamber to form a good quality interfacial layer, such as aluminum oxide (Al2O3), on the compound semiconductor surface.
Owner:INTEL CORP
Purification method of trifluoromethanesulfonic anhydride
ActiveCN103450050AReduce contentEasy to operateOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydridePurification methods
The invention discloses a purification method of trifluoromethanesulfonic anhydride, and belongs to the field of fine chemicals. The method comprises the following steps: introducing a trifluoromethanesulfonic anhydride crude product into a rectification kettle with a rectification tower, vaccumizing a rectification system, starting to heat the rectification kettle after the vacuum degree is stable, and performing total reflux for 1-5 hours; collecting a front fraction at the reflux ratio of 10-15 when the temperature of the rectification kettle is 50-65 DEG C and the temperature at the tower top of the rectification tower is 40-60 DEG C; collecting a medium fraction at the reflux ratio of 4-6 when the temperature of the rectification kettle is 65-70 DEG C and the temperature at the tower top of the rectification tower is 60-68 DEG C; collecting a back fraction at the reflux ratio of 6-8 when the temperature of the rectification kettle increases by 2 DEG C and the temperature at the tower top of the rectification tower is 66-71 DEG C; stopping the collection of the back fraction, stopping heating, stopping vaccumizing and finishing rectification when the temperature of the rectification kettle is not less than 120 DEG C, wherein the medium fraction is a trifluoromethanesulfonic anhydride fine product. The method has the advantages of simplicity in operation and higher product purity.
Owner:PERIC SPECIAL GASES CO LTD
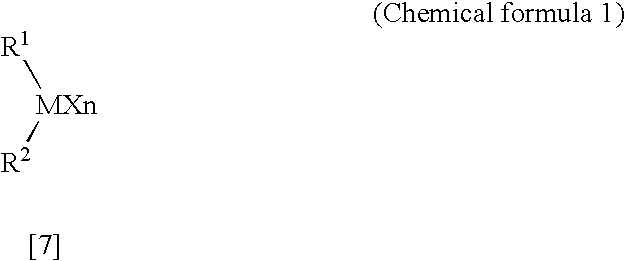
Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide
InactiveUS20080071113A1High yieldLow production costOrganic compound preparationSulfonic acid amide preparationTrifluoromethanesulfonic anhydrideAlkaline earth metal
A process for producing N-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide represented by formula [3]. The process includes the step of reacting trifluoromethanesulfonic anhydride with 1-bicyclo[2.2.1]hept-5-en-2-ylmethanamine in the presence of water and in the presence of a base selected from the group consisting of alkali metal hydroxides, alkaline-earth metal hydroxides and basic salts containing an alkali metal or an alkaline-earth metal.
Owner:CENT GLASS CO LTD
Preparation method and application for heterogeneous olefin polymerization catalyst
InactiveCN102942642ASimple preparationEasy to separate and purifySulfonic acid amide preparationTrifluoromethanesulfonic anhydrideAlpha-olefin
The invention relates to a preparation method for a heterogeneous olefin polymerization catalyst. The preparation method comprises the following steps: 1) mixing substituting arylamine with triethylamine, adding dichloromethane as a solvent, placing into a low-temperature reaction bath and cooling, and then dropwise adding a dichloromethane solution of trifluoromethanesulfonic anhydride and fully stirring, and after heating up, stirring under a backflow condition till fully reacting, and then washing, separating solution, drying, distilling and washing hexane, thereby obtaining a sulfonyl amine compound, and 2) adding a magnesium-containing compound into pre-cooled titanium tetrachloride, performing titanium loading reaction for the first time, heating up, and then adding the sulfonyl amine compound and reacting for a half hour, filtering out the supernatant, adding the titanium tetrachloride and performing the titanium loading reaction for the second time, filtering out the supernatant, washing and drying, thereby obtaining the heterogeneous olefin polymerization catalyst. The preparation method provided by the invention has the advantages that the preparation and reaction of the sulfonyl amine compound as an inner fed electron body are simple; the products are easily separated and purified; the catalyst has higher catalytic activity for olefin polymerization reaction; and the catalyzed alpha-olefin polymerization product has higher stereoregularity.
Owner:NANKAI UNIV
Arylbis (perfluoroalkylsulfonyl)methane and metallic salt thereof, and methods for producing the same
InactiveUS20050070741A1High yieldEasy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTrifluoromethanesulfonic anhydrideLithium
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis (trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dde1769a-9bcd-41c6-b4d5-53ceccaf618c/BDA0000080987530000021.PNG)
![A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dde1769a-9bcd-41c6-b4d5-53ceccaf618c/BDA0000080987530000022.PNG)
![A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound A kind of synthetic method of furo[3,2-c]pyridin-4(5h)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dde1769a-9bcd-41c6-b4d5-53ceccaf618c/BDA0000080987530000031.PNG)
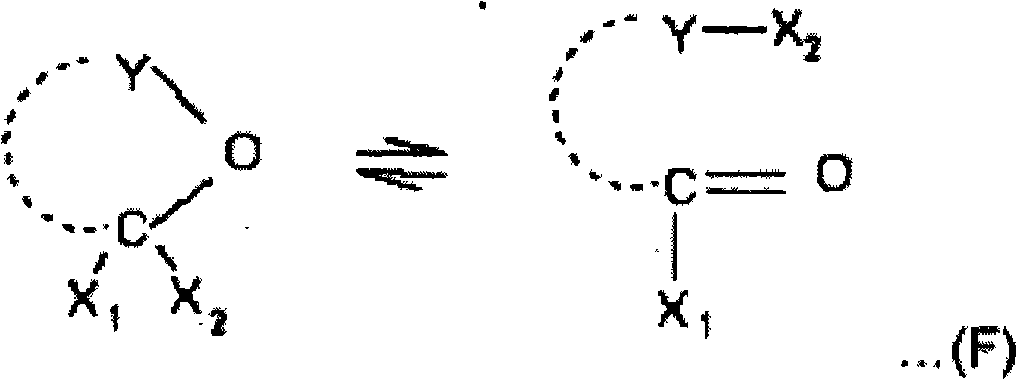




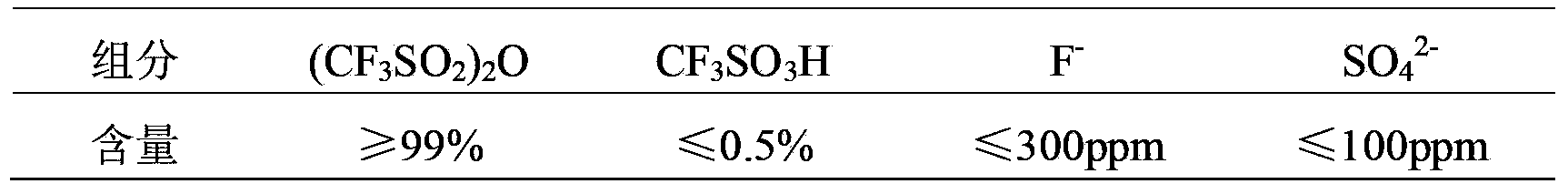
![Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02f72dd4-dae4-4a37-ae8d-3ef55e858618/US20080071113A1-20080320-C00001.png)
![Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02f72dd4-dae4-4a37-ae8d-3ef55e858618/US20080071113A1-20080320-C00002.png)
![Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide Process for producing n-(bicyclo[2.2.1]hept-5-en-2-ylmethyl)-1,1,1-trifluoromethanesulfonamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/02f72dd4-dae4-4a37-ae8d-3ef55e858618/US20080071113A1-20080320-C00003.png)